Abstract
Adoptive cell transfer using chimeric antigen receptors (CARs) has emerged as one of the most promising new therapeutic modalities for patients with relapsed or refractory B-cell malignancies. Thus far, results in patients with advanced solid tumours have proven disappointing. Constitutive tonic signalling in the absence of ligand is an increasingly recognised complication when deploying these synthetic fusion receptors and can be a cause of poor anti-tumour efficacy, impaired survival and reduced persistence in vivo. In parallel, ligand-dependent tonic signalling can mediate toxicity and promote T-cell anergy, exhaustion and activation-induced cell death. Here, we review the mechanisms underpinning CAR tonic signalling and highlight the wide variety of effects that can emerge after making subtle structural changes or altering the methodology of CAR transduction. We highlight strategies to prevent unconstrained tonic signalling and address its deleterious consequences. We also frame this phenomenon in the context of endogenous TCR tonic signalling, which has been shown to regulate peripheral tolerance and facilitate the targeting of foreign antigens and suggest opportunities to co-opt ligand-dependent CAR tonic signalling in order to facilitate in vivo persistence and efficacy.
Keywords: Chimeric antigen receptor, CAR, Tonic signalling, Exhaustion, Terminal differentiation
Background
Adoptive cell transfer (ACT) utilising autologous T-cells engineered to express chimeric antigen receptors (CARs) has proven to be a highly efficacious strategy for the management of patients with relapsed or refractory B-cell malignancies [1–3]. Indeed, following the recent United States Food and Drug Administration (FDA) approvals of the second generation CD19-directed autologous CAR T-cell products: tisagenlecleucel (tradename Kymriah) for the management of paediatric and young adult patients with B-cell acute lymphoblastic leukaemia (ALL) [4, 5] and axicabtagene ciloleucel (tradename Yescarta) for adult patients with relapsed or refractory large B-cell lymphoma following two or more lines of systemic therapy [6], CAR T-cell therapy is now a standard of care and can no longer be regarded as a purely experimental therapeutic modality. However, the field remains in its infancy and these great strides are yet to be replicated in patients with advanced solid tumours [7–9]. Much work remains to be undertaken in order to more fully appreciate how CAR structure determines function and delineate the complexity of CAR intracellular signalling as well the web of interactions between CAR T-cells and other protagonist cells within the tumour microenvironment (TME) in vivo. Considerable effort continues to be applied to the optimisation of the CAR construct itself in order to enhance anti-tumour potency, metabolism, proliferative capacity and persistence [10, 11]. It is becoming increasingly apparent that subtle differences in CAR design can have amplified effects both in vitro and particularly in vivo and that the optimal selection of the CAR’s extracellular targeting moiety, hinge, spacer, transmembrane domain (TMD) and intracellular costimulatory domain(s) (ICD) is crucial.
It has become evident since the 1990s that non-activated basal state T-cells (and indeed B-cells) exhibit low level constitutive tonic signalling that is able to regulate their function and survival in a homeostatic manner [12–14]. More specifically, it is now understood that T-cell receptor (TCR)-mediated tonic signalling in non-engineered naïve endogenous T-cells, mediated by routine non-antigen-specific interactions with mature antigen presenting dendritic cells (DCs), is able to enhance their subsequent ability to react to foreign peptides (such as tumour neoantigens) [12, 13]. This is controlled, at least in part, by interactions between the TCRs of naïve T-cells and self-peptide presented on major histocompatibility complex (MHC) molecules expressed on the surface of DCs and appears to be an important physiological mechanism to ensure the homeostatic control of T-cell tolerance in the periphery [15, 16]. Despite considerable progress in understanding the molecular events involved in B-cell receptor (BCR)-mediated tonic signalling, which is a regulator of B-cell maturation and survival [14, 17], our understanding of TCR-mediated T-cell tonic signalling, which shares many of the hallmarks of the former, remains poorly defined [14].
CAR tonic signalling, however, may be defined as a non-coordinated and sustained activation of the T-cell in either a ligand-independent or dependent fashion. In the absence of spatial and/or temporal control of CAR cell surface expression, this constitutive or chronic cell signalling may have a substantial deleterious impact on CAR T-cell effector function and survival and may lead to a significant disparity between in vitro cytolytic capacity and in vivo anti-tumour efficacy [18–21]. This review highlights the current research being undertaken to identify and address CAR tonic signalling in all its forms, drawing attention to data that is at times conflicting and hypothesis generating. At least four major overlapping patterns of ligand-independent CAR tonic signalling are presented and a variety of strategies designed to ameliorate the negative consequences of these are expounded. Finally, through the prism of endogenous T-cell tonic signalling and its important regulatory role in immune tolerance and cell-mediated adaptive immunity, we posit a number of hypothetical strategies designed to harness the potential benefits of CAR tonic signalling in order to improve CAR T-cell anti-tumour efficacy and in vivo persistence.
CAR structure
Conventionally designed CARs exploit the specificity of an antibody-derived extracellular binding domain whilst harnessing the effector and memory capacity of T-cells in order to target tumours [22]. CAR T-cells may thus deliver the promise of “living drugs”, capable of targeting tumour-associated or tumour-specific antigens (TAAs or TSAs) over a prolonged period of time [23]. Given that CARs function in the absence of human leukocyte antigen (HLA) / TCR interactions, they have considerable applicability across patient groups and are ideally placed to address the growing problem of acquired resistance to immune checkpoint inhibition due to disrupted antigen processing and/or presentation [24]. Furthermore, with the advent of allogeneic HLA and TCR-edited CAR T-cells, the potential exists for scalable “off the shelf” delivery, potentially in combination for optimised TAA pattern recognition [25, 26].
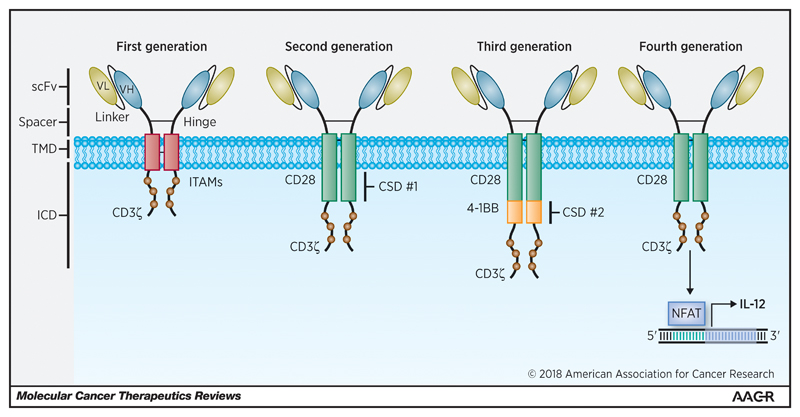
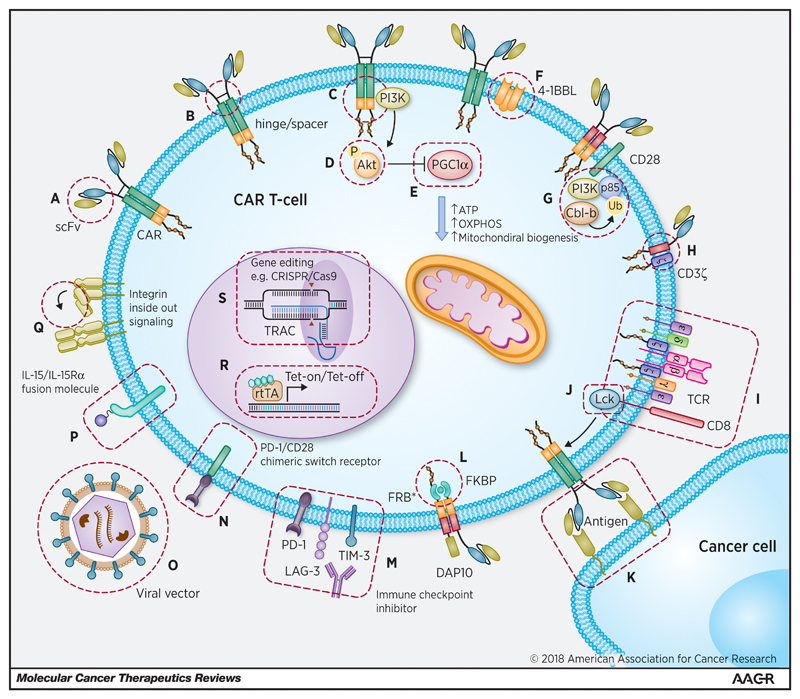
CAR design has undergone a number of iterative developments over the last two decades, with the aim of optimising CAR T-cell effector function and persistence [27]. First generation CARs or “T-bodies” linked an extracellular antibody-derived recognition moiety to a lymphocyte stimulating domain, such as the signal-transducing subunit of either the immunoglobulin receptor (FcγR) or the TCR CD3ε or CD3ζ chains [28]. First generation CAR T-cells tended to elicit only weak anti-tumour activity and were highly prone to anergy [29]. The fusion of costimulatory ICDs with the cytoplasmic tail of CD3ζ-containing first generation constructs has led to the emergence of second generation (comprising a single costimulatory ICD such as CD28 [30], 4-1BB (CD137) [31], inducible T-cell costimulator (ICOS) [32], OX40 [33], CD27 [34] or DNAX-activating protein 10 (DAP10) [35]) and third generation CARs (comprising multiple costimulatory ICDs, aligned in cis [36, 37]). Incorporation of costimulatory ICDs can recapitulate signal 2 required for T-cell activation, leading to enhanced effector function, proliferation, survival and ultimately enhanced tumour killing [38]. Fourth generation CAR T-cells (termed “TRUCKs”) containing CAR-inducible transgenes and “armoured CARs” capable of constitutively producing cytokines (such as IL-12, IL-15 and IL-18) in secreted or membrane-tethered form have been engineered to recapitulate signal 3 in an autocrine and paracrine manner [39–42]. These designs are illustrated in Figure 1. Further modifications have been explored with respect to the CAR TMD [43] and hinge/spacer region [20, 44]. The extracellular targeting moiety, which has typically constituted an antibody-derived single chain variable fragment (scFv) may alternatively comprise an endogenous receptor or ligand [9]. Anti-tumour efficacy relies upon optimal CAR binding to the target epitope and the formation of a cytolytic immune synapse between the CAR T-cell and the target cell. Spacer length, which impacts upon both the flexibility of the CAR [45] and the distance [46] between the target cell and the CAR T-cell membrane, is increasingly seen as critical in ensuring optimal immune synapse formation, particularly with regard to membrane-proximal epitopes [44].
Figure 1.
Iterative design of first, second, third and fourth generation CARs. CARs are modular fusion receptor dimers that comprise (from N-terminus to C-terminus) an extracellular targeting moiety (typically an scFv) fused to a spacer (such as an IgG1 hinge & CH2-CH3 domains), a transmembrane domain (such as CD8α or CD28) and a signalling endodomain. First generation CARs fused the scFv to a CD3ζ, CD3ε or FcγR activation domain. Second generation CARs contain an additional intracellular costimulatory domain (such as CD28, 4-1BB, OX40 or ICOS) to recapitulate signal 2 for T-cell activation. Third generation CARs combine two or more costimulatory domains in cis. Fourth generation CARs are engineered with an activation inducible element such as an NFAT-responsive expression cassette to facilitate secretion of a transgenic cytokine such as IL-12. CSD, costimulatory domain; ICD, intracellular domain; NFAT, nuclear factor of the activated T-cell; scFV, single chain variable fragment; TMD, trans-membrane domain.
Figure 1 and figures 3-5 are original and have been created specifically for this article.
Endogenous TCR tonic signalling
Maintenance of naïve T-cells in the periphery following their release from the thymus is maintained by tonic signalling via the TCR and common γ chain cytokine receptors [15, 16]. Specifically, the survival of naïve CD4+ and CD8+ T-cells in the periphery relies upon a combination of low to intermediate affinity binding of the TCR to MHC loaded with self-peptides presented on the surface of DCs and the presence of IL-7 on the surface of fibroblastic reticular cells (FRCs) in the T-cell zone of secondary lymphoid organs [16]. Naïve CD8+ T-cells are also partly reliant on JAK-STAT signalling mediated by the engagement of IL-15 receptors with DC-derived IL-15 [47]. Steady state DC-mediated TCR tonic signalling enhances T-cell responsiveness to MHC-associated foreign antigen [12], but does not necessarily induce a transition to a central memory phenotype [48]. T-cell hyporesponsiveness has been shown to be associated with lower baseline phosphorylation in proximal TCR events e.g. reduced basal phosphorylation of zeta-chain-associated protein kinase 70 (ZAP70)-associated CD3ζ [13]. Some baseline tonic signalling in naïve T-cells may reflect constitutive activation of Lck, a member of the SRC family kinase (SFK) that plays a pivotal role in TCR signalling [49], maintaining a basal level of phosphorylation on TCR-associated CD3ζ-chain immunoreceptor tyrosine-based activation motifs (ITAMs) [50]. This process is also regulated by a highly dynamic interplay between the receptor-like tyrosine phosphatase CD45 and the protein tyrosine kinase Csk [51, 52]). The importance of this interaction is illustrated by the fact that DC depletion results in rapid loss of T-cell responsiveness to cognate antigen, rapidly reversed with the restoration of T-cell / DC interactions [13]. Similarly, following the exposure of mice to MHC class II blocking antibodies, a loss of basal CD3ζ chain phosphorylation is observed [53]. In addition to complementary effects mediated by the engagement of leukocyte β integrins such as lymphocyte function-associated antigen 1 (LFA-1) with cell adhesion molecules such as intercellular adhesion molecule 1 (ICAM-1) [54, 55], low affinity interactions between the TCR and MHC (including monomeric MHC) appear to lower T-cell activation threshold by replenishing intracellular Ca2+ stores and increasing plasma membrane phosphatidylinositol 4,5-bisphosphate (PIP2) [56].
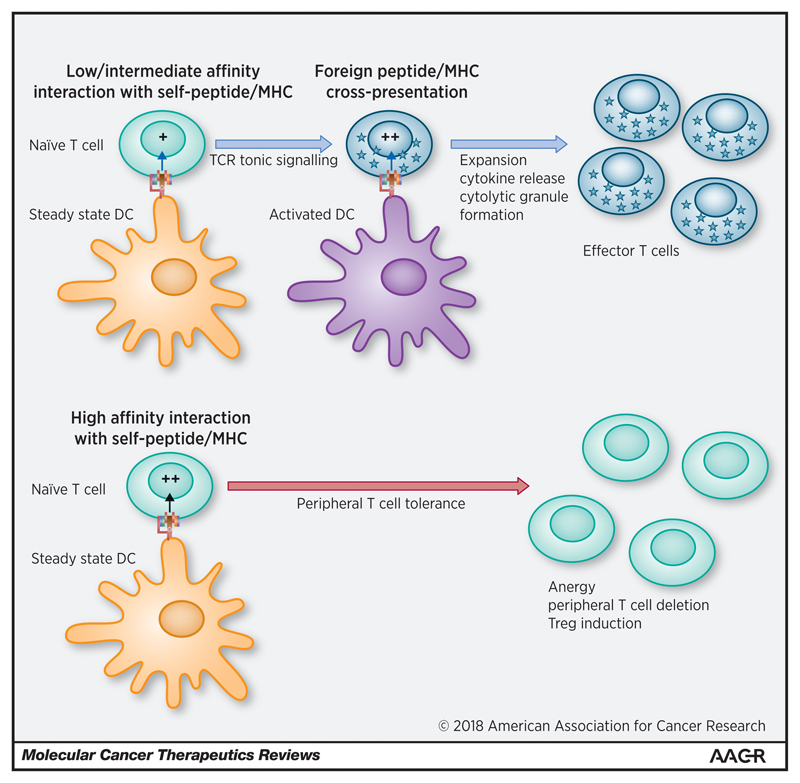
MHC class II interactions, in particular, have been linked to maintaining T-cell reactivity and proliferative capacity following activation to cognate antigen [50, 57]. This may occur through a two-step process in the lymphoid tissue, whereby naïve T-cells with TCRs exhibiting low to intermediate affinity for self-peptides presented on steady state DCs may induce TCR tonic signalling. This is enhanced further by cross-presentation of foreign peptide by activated DCs (accompanied by co-stimulatory interactions between B7/CD28 and CD70/CD27) leading to enhanced T-cell effector function [12]. This may be leveraged further by the “pseudodimer” effect (postulated for CD4+ T-cell / MHC class II interactions), whereby the concomitant recognition of MHC loaded self-peptides and foreign-peptides enhances T-cell responsiveness against the latter [58]. In parallel, peripheral tolerance is maintained by high affinity binding of TCR to self MHC leading to unconstrained tonic signalling, T-cell tolerance and exhaustion (mediated by upregulation of inhibitory checkpoints such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death 1 (PD-1)), anergy, apoptosis and/or enhanced regulatory T-cell (Treg) functionality. This model is illustrated in Figure 2 and demonstrates how TCR tonic signalling may contribute to a dynamic equilibrium between positive and negative regulators of T-cell efficacy and autoimmunity. Such a paradigm is analogous to thymic T-cell selection, whereby intermediate TCR and MHC affinity is positively selected and induces TCR tonic signalling required for subsequent reactivity to foreign peptides in the periphery [12].
Figure 2.
Endogenous TCR tonic signalling facilitates T-cell differentiation & effector function. Circulating naive T-cells interact with steady state dendritic cells (DCs) in secondary lymphoid organs. High affinity interactions between the TCR and MHC presenting self-peptide mediate peripheral T-cell tolerance, clonal editing, anergy and Treg induction. Low to intermediate affinity interactions enhance basal TCR tonic signalling via CD3ζ and ZAP70 phosphorylation leading to a reduction in the T-cell activation threshold prior to encountering foreign antigen. Subsequent encounters with activated DCs result in enhanced clonal proliferation, cytokine release, cytotoxic granule formation (via hedgehog signalling and upregulation of RAC1) and differentiation to an effector phenotype. Non-MHC-mediated T-cell / DC interactions, such as the binding of adhesion molecules (not illustrated) further facilitates tonic signalling by inducing a transient increase in intracellular Ca2+, cAMP and ERK phosphorylation, strengthening T-cell responses to foreign antigen. Adapted from Garbi, N. et al. Tonic T-cell signalling and T-cell tolerance as opposite effects of self-recognition on dendritic cells, Current Opinion in Immunology 22, 601–608 (2010) [12], with permission from Elsevier.
In contrast to CAR-mediated tonic signalling, low level TCR tonic signalling appears to be spatially compartmentalised to the lymphoid tissue. T-cells isolated from peripheral blood fail to demonstrate basal CD3ζ chain phosphorylation [53] and it has been proposed that TCR tonic signalling may occur in a cyclical fashion as T-cells enter and exit lymphoid organs from the circulation [14]. Furthermore, TCR tonic signalling appears to have a differential impact upon maintenance and survival of CD4+ versus CD8+ T-cells. Specifically, self-recognition of MHC class I by peripheral CD8+ T-cells appears to be far more crucial for their survival (supported by their significantly reduced half-life following TCR ablation [59]) than recognition of MHC class II by peripheral CD4+ T-cells, which are able to undergo homeostatic proliferation as measured by bromodeoxyuridine (Brdu) incorporation in recombination activating gene 2 (Rag2)-/- class II-/- transgenic mice [60]. With the emergence of validated surrogate markers of TCR tonic signalling, we are gaining greater insight into the mechanistic basis of this process, illuminating the multitude of downstream pathways that impact upon T-cell function, differentiation and survival [14]. Examples include T-cell surface expression of CD5 (a negative regulator of TCR signalling), which correlates with self-MHC interactions and basal TCR tonic signalling; or expression of nuclear receptor subfamily 4 group A member 1 (Nr4a1) (an immediate-early transcription factor encoding nuclear hormone receptor 77 (Nur77)), which is rapidly upregulated by TCR stimulation in thymocytes and T-cells. Similar approaches are likely to prove insightful when applied to CAR tonic signalling pathways.
Revealing a further layer of complexity to T-cell tonic signalling, it has been shown that TCR expression (specifically the TCRα chain) may itself be subject to tonic signalling mediated by basal activity through the linker for activation of T-cells (LAT) - diacylglycerol (DAG) - RAS guanyl-releasing protein 1 (Rasgrp1) pathway [61]. Studies involving transgenic mice with Rasgrp1 deficiency or mutation (Rasgrp1Anaef) have revealed evidence of basal tonic signalling via the mammalian target of rapamycin (mTOR) pathway in the absence of TCR-ligand binding [14]. Rasgrp1Anaef mice were found to uniformly express elevated levels of the activation marker CD44 on all CD4+ T-cells (irrespective of CD62L expression, a marker of naïve, stem cell memory (TSCM) and central memory T-cells (TCM)) and exhibited enhanced basal phosphorylation of the ribosomal protein S6, a downstream target of mTOR. These animals exhibited enhanced T-cell autoreactivity and autoimmunity and it is interesting to speculate whether this impact of elevated tonic signalling can be co-opted for the development of effective CAR T-cells with a non-terminally differentiated phenotype.
Ligand-independent CAR tonic signalling
A number of CAR constructs have been shown to elicit prolonged exponential expansion, constitutive cytokine release and progressive differentiation to an effector phenotype in the absence of ligand, exogenous cytokines or feeder cells [21]. This appears, at least in part, due to the level of CAR surface expression achieved as well as the specific characteristics of the individual scFvs utilised, with those designed to target the disialoganglioside GD2, c-mesenchymal-epithelial transition (c-Met) and mesothelin featuring repeatedly in the literature concerning ligand-independent expansion [18–21], whereas CD19 targeting scFvs, such as FMC63, appear to be relatively resistant to this phenomenon [18, 21]. In a study by Frigault et al., ligand-independent tonic signalling leading to continuous T-cell expansion ex vivo was shown to be dependent upon the integration of the CD28 transmembrane and cytosolic domain within the CAR construct [21]. Other members of the CD28 immunoglobulin superfamily, such as ICOS, did not appear capable of inducing constitutive expansion when substituted for CD28 in otherwise similar CAR constructs. And whilst utilisation of a 4-1BB costimulatory ICD appears to confer enhanced ligand-independent proliferation [62], continuous expansion and constitutive cytokine release has not been demonstrated [11], although more recent reports described later in this review muddy the water somewhat by highlighting alternative mechanisms for 4-1BB-mediated tonic signalling, characterised by cell death rather than proliferation [19, 63].
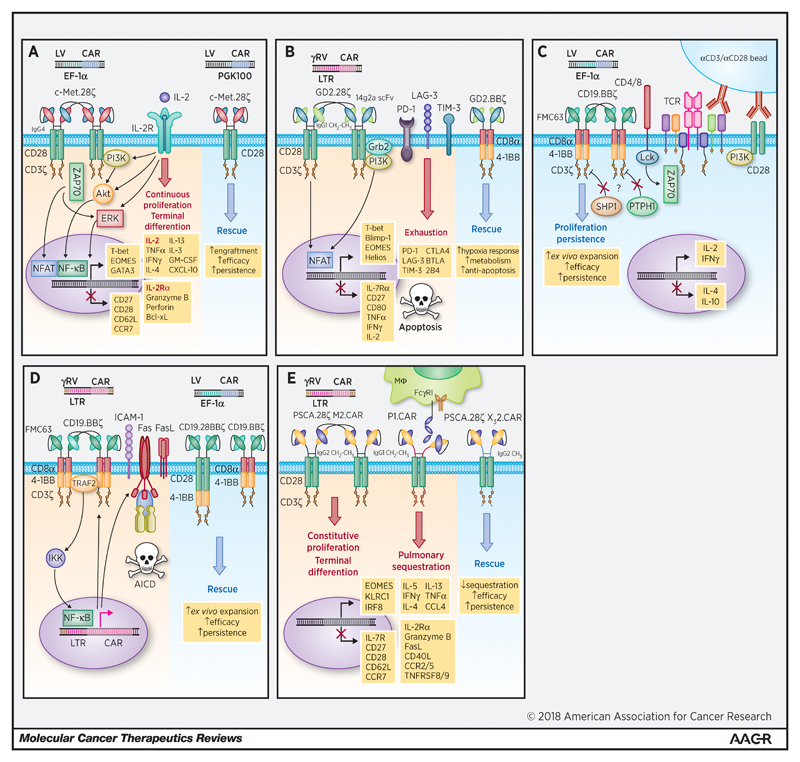
Frigault et al. evaluated a set of 12 CARs designed to target c-Met, mesothelin, and CD19 [21]. These contained either an immunoglobulin G4 (IgG4) hinge or CD8α stalk coupled with CD28, ICOS or CD8α transmembrane domains. The intracellular signalling domains comprised either CD28, 4-1BB or ICOS bound in cis with CD3ζ. A lentiviral vector was utilised with an elongation factor 1 alpha (EF-1α) promoter. As expected, following in vitro activation with anti-CD3/anti-CD28-loaded beads and subsequent viral transduction, the majority of CAR T-cells demonstrated a predictable pattern of rapid initial proliferation followed by a return to a resting state in the absence of exogenous IL-2. Intriguingly, however, certain CAR constructs demonstrated continued expansion for up to 60-90 days in the absence of IL-2 or target ligand. These included a c-Met-directed IgG4 28ζ CAR and both mesothelin-directed SS1 IgG4 and CD8α 28ζ CARs. Of note, of the c-Met-directed CARs neither the CD8α 28ζ, IgG4 BBζ, IgG4 ICOSζ or first generation IgG4 CD3ζ CARs exhibited this continuous activation phenotype. Continuous expansion of both CD4+ and CD8+ T-cells transduced with c-Met IgG4 28ζ CARs was observed and was associated with a 100 to 1000-fold increase in various cytokines (including interferon gamma (IFNγ), tumour necrosis factor alpha (TNFα), IL-2, IL-4, IL-13, IL-3 and granulocyte-macrophage colony-stimulating factor (GM-CSF)) as well as elevated levels of granzyme B and perforin. “Continuous” CARs were also characterised by significantly enhanced expression of the master transcription factors T-box transcription factor 21 (TBX21) (encoding T-bet), EOMES (encoding eomesodermin) and GATA-3, as well as early enhanced expression of anti-apoptotic proteins such as B-cell lymphoma-extra large (Bcl-xL). A pattern of sustained signal transduction protein activation was also identified, involving Akt (pS473), ERK1/2 (pT202 and pY204) and nuclear factor (NF)-κB (p65 (RelA) pS529) as well as reciprocal downregulation of endogenous CD28. High CAR surface expression also appeared key, as use of a cytomegalovirus (CMV) or variably truncated phospho-glycerate kinase (PGK) promoters led to both reduced CAR surface expression and a non-continuous phenotype. While alloreactivity may have been a confounding factor in their in vivo model, c-Met IgG4 28ζ CARs encoded downstream of the shortest PGK promoter (PGK100) outperformed their more highly expressed EF-1α counterparts in terms of anti-tumour efficacy and persistence in NOD SCID γcnull (NSG) mice implanted with human ovarian cancer cell line (SK-OV3)-derived xenografts. This is reminiscent of later results published by Eyquem et al. [64] and Hale et al. [65] regarding the targeted expression of CARs to the T-cell receptor alpha constant (TRAC) locus. This phenotype of CAR tonic signalling is summarised in Figure 3(a).
Figure 3.
(a): Tonic signalling correlates with CAR surface expression and can be addressed by optimal selection of the CAR promoter during lentiviral transduction. Frigault et al. found that c-Met or mesothelin-directed second generation CARs comprising an IgG4-derived hinge, CD28 CSD and CD3ζ underwent continuous proliferation during ex vivo expansion in the absence of ligand or exogenous growth factors [21]. Continuous proliferation correlated with CAR surface expression and required CD28 costimulation. A diverse array of cytokines and chemokines were significantly upregulated, including IL-2. Also upregulated were the transcription factors T-bet, GATA3 and EOMES (a hallmark of terminal effector differentiation) as well as the pro-survival protein Bcl-xL. CAR surface expression was reduced using a truncated PGK promoter during lentiviral transduction, reducing tonic signalling and improving anti-tumour efficacy and persistence in vivo.
(b): CAR tonic signalling can induce T-cell exhaustion mediated by the upregulation of inhibitory molecules and can be reversed by substitution of the intracellular costimulatory domain. Utilising a GD2-directed second generation CAR comprising an IgG1-derived hinge and CH2-CH3 spacer, CD28 TMD/CSD fused to CD3ζ, Long et al. were able to demonstrate that ligand-independent tonic signalling during ex vivo expansion relied upon scFv interactions, causing CAR aggregation in cell surface punctae and the upregulation of cell surface inhibitory receptors including PD-1, LAG-3 and TIM-3 leading to an exhausted phenotype and increased apoptosis [18]. The deleterious impact of this tonic signalling could be reversed by substituting the CD28 CSD with 4-1BB. GD2.BBζ CAR T-cells exhibited reduced expression of exhaustion-associated molecules and an upregulation of pathways implicated in response to hypoxia, cellular metabolism and negative regulation of apoptosis.
(c): 4-1BB costimulation can mediate tonic signalling and enhanced proliferation during ex vivo expansion. Milone et al. have demonstrated that during ex vivo expansion using anti-CD3/anti-CD28 coated magnetic beads, CD19.BBζ CAR T-cells exhibited a prolonged blast phase associated with higher rates of proliferation than corresponding 28ζ and 28BBζ CARs [62]. Enhanced proliferative capacity (but not persistence) was lost approximately 2 weeks following bead expansion. BBζ CARs produced both IL-2 and IFNγ (albeit at a lower level than 28ζ CARs) and significantly reduced levels of IL-4 and IL-10, consistent with skewing to a Th1-like phenotype. The picture is suggestive of an interaction between the 4-1BB costimulatory ICD and downstream mediators of TCR activation. The authors suggest that dysregulation of CD3ζ ITAM phosphatases (such as SHP1 or PTPH1) may be playing a role. The possibility of scFv domain swapping in this CD19 FMC63 model also remains uncertain.
(d): 4-1BB costimulation can facilitate CAR tonic signalling via TRAF2 and NF-κB leading to Fas-related AICD, exacerbated by self-amplification at the level of the CAR promoter. Contrary to Long et al. [18], Gomes-Silva et al. have reported that a second generation CD19-directed CAR comprising a CD8α stalk and TMD, 4-1BB and CD3ζ ICDs expanded poorly ex vivo due to tonic signalling mediated by an interaction between the 4-1BB ICD and TRAF2 [73]. This led to activation of NF-κB, upregulation of Fas and Fas ligand and ICAM-1, ultimately causing caspase-8-mediated AICD. An additional effect on the γ-retroviral LTR promoter was also noted, causing a positive feedback loop via CAR self-amplification. This phenotype could be eliminated by mutating the TRAF2 binding site on 4-1BB at the expense of effective costimulation. Interestingly, the addition of a CD28 CSD was able to restore ex vivo expansion, overcoming the adverse effects of 4-1BB tonic signalling. Likewise, the insertion of an IRES element upstream of the LTR or transducing the CAR with a lentiviral vector and the EF-1α promoter reduced tonic signalling and restored function.
(e): Alterations to the hinge and spacer domain can exacerbate tonic signalling, causing constitutive ligand-independent proliferation, terminal differentiation and poor migration in vivo. Watanabe et al. demonstrated that a second generation anti-PSCA CAR containing an IgG1 hinge and CH2-CH3 spacer linked to a CD28 CSD and CD3ζ was liable to bind to FcγRI and FcγRII expressed on monocytes and macrophages, resulting in pulmonary sequestration in vivo and poor trafficking into implanted tumours in NSG mice [20]. Substituting the spacer framework to IgG2 abrogated FcγR binding and improved CAR T-cell trafficking in vivo. However, the CH2-CH3 spacer was found to mediate CAR tonic signalling independent of ligand during ex vivo expansion, leading to constitutive proliferation, terminal differentiation to an effector memory phenotype and senescence. Utilisation of a shorter spacer could ameliorate tonic signalling without compromising cytotoxicity and improved in vivo efficacy.
Figure 1 and figures 3-5 are original and have been created specifically for this article.
Long et al. have subsequently shown that antigen-independent clustering of CAR scFvs is seen in second generation γ-retrovirally-transduced GD2 28ζ CARs incorporating a 14g2a-derived scFv with an IgG1-derived hinge and CH2-CH3 spacer, leading to chronic CAR CD3ζ domain phosphorylation, CAR T-cell exhaustion and increased rates of apoptosis [18]. This was shown to occur during anti-CD3/anti-CD28 bead-based ex vivo CAR T-cell expansion and was associated with an increase in cellular volume, CD25 upregulation and an exhausted phenotype indistinguishable from exhausted non-engineered T-cells in the context of chronic viral infection and cancer. An important mechanism appears to relate to the propensity for 14g2a (and, to a greater or lesser degree, other scFvs and antibody fragments studied, e.g. targeting CD22 and ErbB2) to oligomerize, resulting in cell surface CAR clustering, visualised using functional CAR-fluorescent protein fusion constructs. The effect was found to be related specifically to the non-antigen binding framework regions within the 14g2a scFv rather than the CAR’s linker peptide or spacer domain. GD2-directed CAR tonic signalling-mediated T-cell exhaustion was found to be associated with a transcriptional profile favouring the expression of numerous inhibitory receptors, including PD-1, lymphocyte-activation gene 3 (LAG-3), T-cell immunoglobulin and mucin-domain containing-3 (TIM-3), CTLA-4, B- and T-lymphocyte attenuator (BTLA) and 2B4 (CD244); the helix-loop-helix (HLH) apoptosis-associated protein ID-1; as well as recognised exhaustion-associated transcription factors, such as T-bet, EOMES, Blimp-1 and Helios. These CAR T-cells demonstrated poor proliferative capacity, cytokine production and antitumour efficacy in vivo. Elaborating on the data of Frigault et al. [21], Long et al. were able to demonstrate that costimulation with CD28 augmented CAR T-cell exhaustion, mediated by tonic signalling, whereas 4-1BB costimulation was able to limit it [18]. This finding, alongside data highlighting differences between CD28 and 4-1BB ICDs with regard to CAR T-cell responsiveness to hypoxia, oxidative metabolism and negative regulation of apoptosis as well as data generated by a number of groups highlighting 4-1BB-mediated mitochondrial biogenesis, persistence and central memory differentiation [10, 66, 67], have direct relevance to future optimal CAR design. Indeed, analogous differences in CAR persistence have already been demonstrated in clinical trials evaluating CD19-directed CARs containing CD28 versus 4-1BB [68–70]. Recent data, however, from Klein Geltink et al. highlight the complexity of CD28-mediated costimulation, which, at least during the initial phase of T-cell activation, has been shown to prime mitochondria with latent metabolic capacity that is essential for future T-cell responses [71]. Thus, the timing and duration of CD28 and 4-1BB signalling may be crucial to optimise CAR T-cell metabolism and differentiation. Of relevance to subsequently described studies evaluating the contribution of the hinge and spacer to tonic signalling, modified GD2 BBζ CARs lacked the IgG1 hinge-CH2-CH3 spacer and utilised a CD8α TMD with an scFv peptide linker derived from the CD19 FMC63 scFv. The relative contribution of these changes to the amelioration of CAR exhaustion appears limited, based upon subsequent experiments utilising a GD2 28ζ CAR incorporating a CD19 FMC63-derived peptide linker and lacking the IgG1 hinge-CH2-CH3 spacer. This adapted CAR demonstrated no improvement in exhaustion and no anti-tumour efficacy in vivo. This exhaustion-predominant CAR tonic signalling phenotype is illustrated in Figure 3(b)
Interestingly, the Penn group, utilising the same anti-CD19 FMC63 scFv had previously demonstrated that incorporation of a 4-1BB (rather than CD28) costimulatory ICD could mediate ligand-independent tonic signalling and enhanced proliferation during ex vivo expansion using anti-CD3/anti-CD28-coated beads [62]. However, as these cells later lost their proliferative advantage (albeit not their persistence) following removal from the bead-containing culture medium, this is markedly different from the continuous expansion phenotype described by Frigault et al. with regard to their c-Met and mesothelin-directed 28ζ CARs [21]. Milone et al. compared a number of CD19-directed lentiviral-transduced CARs utilising the potent EF-1α promoter. FMC63 scFvs were fused to a CD8α stalk/TMD and various costimulatory ICDs (namely 4-1BB, CD28 and both in tandem). As mentioned, 4-1BB-containing single ICD CARs, unlike the other constructs, continued to proliferate during in vitro expansion with anti-CD3/anti-CD28-coated beads in the absence of CD19 antigen or exogenous 4-1BBL. This enhanced proliferation was observed in both CD4+ and CD8+ T-cells and was associated with a prolonged period of increased cellular volume akin to a more durable blast phase. This initial period of enhanced proliferation appears to recapitulate the findings seen in other models with the continuous administration of a 4-1BB agonist antibody or the ectopic in trans expression of 4-1BBL [72]. The authors postulated that CAR oligomerisation or impaired dephosphorphylation of CD3ζ ITAMs (by SRC homology region 2 domain-containing phosphatase 1 (SHP-1) and protein tyrosine phosphatase 1 (PTPH1) for example) may be playing a role in this regard (summarised in Figure 3(c)). The absence of tonic signalling seen with both the 28ζ and third generation 28BBζ CARs would imply that scFv oligomerisation may not be playing a particular role here, particularly as Long et al. have clearly demonstrated that anti-GD2 scFv oligomerization can induce CD28 ICD-mediated ligand-independent proliferation, whereas this was not witnessed with CD19-directed CARs [18]. It is conceivable that differences between lentiviral and retroviral promoters used by the two groups may also have been relevant, particularly with regard to promoter strength, CAR surface expression and potential unforeseen interactions between CAR intracellular signalling and the promoter itself (see discussion below regarding recent work published by Gomes-Silva et al. [73]). Furthermore, tonic signalling has been rarely reported with anti-CD19 CARs other than those containing a single 4-1BB ICD [11, 19, 63]. Indeed, the fact that 4-1BB-mediated tonic signalling was only seen during endogenous TCR-mediated activation implies a direct interaction with TCR-related adapter proteins and/or signalling molecules. Given that the introduction of the CD28 TMD and ICD upstream of 4-1BB was able to abrogate ligand-independent tonic signalling (also seen more recently in a third generation ICOSBB construct [74]), this implies that the aforementioned interaction between the 4-1BB ICD and endogenous TCR activation may depend upon the relative position of the 4-1BB ICD with respect to the cell membrane or that CD28-associated proteins may block this interaction. Indeed, geometric constraints that emerge during the trimeric engagement of TNF receptor family members with their corresponding ligands are thought to facilitate recruitment of signal adapter proteins such as TNF receptor-associated factors (TRAFs) that activate downstream signalling pathways [75, 76]. It is intriguing to posit that the fusion of the 4-1BB costimulatory ICD into a dimerizing synthetic receptor may alter the natural recruitment and/or disengagement of TRAFs involved in downstream signalling [11]. Importantly, in this model, CAR tonic signalling did not appear to compromise in vitro or in vivo efficacy and was, in fact, associated with considerable efficacy and persistence, with anti-CD19 scFvs being detectable in the splenic tissue of mice at 6 months [62].
Intriguingly, recent reports from the Baylor group highlight some important parallels with regard to 4-1BB costimulation, but also reveal some key differences [19, 63, 73]. Whilst structurally their CAR is identical to the Penn group CD19-directed BBζ CAR (comprising the FMC63 scFv, CD8α stalk and TMD [21, 62]), Gomes-Silva et al. use a non-self-inactivating (non-SIN) γ-retrovirus with an long terminal repeats (LTR) promoter for CAR transduction [73], whereas the Penn group have utilised lentiviral transduction and a variety of promoters with EF-1α most commonly associated with tonic signalling. During ex vivo expansion, Gomes-Silva et al. have demonstrated that CARs containing the 4-1BB ICD alone proliferated 70% more slowly, exhibited a 4-fold increase in apoptosis and were characterised by a gradual downregulation of CAR expression [63]. Further analysis revealed evidence of constitutive CD3ζ ITAM phosphorylation as well as 4-1BB-associated tonic signalling via TRAF2, leading to phosphorylation of the IκB kinase (IKK) complex (containing IKKα/β subunits), non-canonical NF-κB pathway activation, upregulation of Fas (CD95) and Fas ligand (CD95L) (which were seen to co-localise on the surface membrane) and, ultimately, caspase 8-dependent activation-induced cell death (AICD) [73]. Tonic signalling appeared to be further increased via a self-amplifying positive feedback loop acting at the level of the retroviral LTR promoter, which is positively regulated by host NF-κB. There was also an upregulation of cell surface ICAM-1, which is also known to be activated by NF-κB and the authors postulate that ICAM-1 overexpression facilitated the cell clustering seen in their model, causing trans-engagement of Fas and Fas ligand between neighbouring CAR T-cells. This 4-1BB-dependent tonic signalling phenotype is illustrated in Figure 3(d). Subsequent work revealed that by disrupting the TRAF2-binding site in the 4-1BB domain, Fas upregulation could be prevented, restoring T-cell function, albeit at the expense of costimulation [19, 73]. Whilst the finding that 4-1BB costimulation could induce AICD appears to contradict the data from Penn and other groups (not least in the clinical domain, where Penn’s 4-1BB-containing CD19 CAR, tisagenlecleucel, is now FDA-approved), the different viral vectors and promoters may confer different levels of CAR surface expression, which appears to be a crucial factor for ligand-independent tonic signalling. The interaction between 4-1BB-mediated tonic signalling and the retroviral LTR promoter also appears to be important. Similar outcomes were noted using 14G2a GD2-directed CARs and therefore the results differ markedly from those seen by Long et al. Although exhaustion markers were not evaluated by Gomes-Silva et al., these differences are likely to be occurring at the level of the CAR promoter. Whilst both groups made use of retroviral vectors and LTR sequences, Gomes-Silva utilised an SFG vector whereas Long et al. transduced with a murine stem cell virus-based splice-gag (MSVG) vector, which utilises the murine stem cell virus LTR with an extended gag region and Kozak sequence [77] and may not be regulated by host NF-κB in the same manner. Indeed, enforced reduction of CAR expression using an internal ribosome entry site (IRES) element upstream of the CAR transgene reduces tonic signalling in Gomes-Silva et al.’s model. A similar restorative effect was seen using lentiviral transduction and an EF-1α promoter, exactly replicating the experimental model utilised by Frigault et al. Of note, the use of the IRES element does not appear to have inhibited the continuous expansion of CD19 and GD2 CD28ζ CARs, which is also redolent of the findings seen by Frigault et al. using non-CD19 CARs. However, differences in ex vivo expansion may have played a role with the former being expanded in the presence of continuous IL-7 and IL-15.
Intriguingly, a third generation construct combining a CD28 ICD / TMD upstream of 4-1BB was able to overcome or avoid this deleterious 4-1BB tonic signalling despite utilising the same γ-retrovirus and LTR promoter and, following delivery to a small number of patients in combination with a 28ζ second generation CAR, was able to demonstrate a 23-fold greater level of expansion and correspondingly longer persistence in vivo [63]. The differing effects may parallel models of acute viral infection, whereby 4-1BB appears to have a biphasic role [78]. Early 4-1BB activation has been shown to have a deleterious impact on anti-viral T-cell effector function by inducing AICD through prolonged upregulation of TNF and Fas [79]. Thus, the precise timing and duration of 4-1BB costimulation may be key [11] and it is interesting to speculate that the relative position of the 4-1BB ICD and its preferential access to TRAF2 rather than TRAF1 or TRAF3, which are both known to exert a negative regulatory role on non-canonical NF-κB activation by preventing activation of the NF-κB inducing kinase (NIK) [80, 81]), may be playing a role. Indeed, TRAF1 is also known to activate ERK, upregulate Bcl-xL and downregulate the pro-apoptotic protein BIM [82] and loss of TRAF1 has been associated with CD8+ T-cell dysfunction during human and murine chronic infection [83]. These data, while currently only hypothesis generating, appear to emphasise the considerable importance of optimally positioning costimulatory ICD(s) to facilitate interactions with cell membrane-localised adapter and signal transduction molecules (such as members of the TRAF family, which are likely to have pleiomorphic roles in different contexts) in the CAR’s activated conformational state, as well as the hitherto relatively underexplored impact of using different hinges, spacers and TMDs.
The mainstay of available data with regard to the impact of the CAR hinge and spacer domain relates to potential FcγR-mediated interactions with immune cells causing ligand-independent CAR tonic signalling, chronic activation and AICD [20, 44, 84]. A commonly utilised spacer domain comprises an IgG-derived hinge (usually IgG1 or IgG4), and a variable length IgG Fc CH2-CH3 domain. However, CARs comprising an IgG1 Fc spacer domain are prone to ligand-independent activation by binding to bystander immune cells expressing FcγR. Substitution of an IgG1-derived CH2 sequence with IgG2 (which has a lower affinity for FcγR) has been shown reduce this effect in vitro [85]. IgG4 has been shown to bind to FcγRI and other FcγRs with an equivalent or lower affinity than IgG2. However, Hudecek et al. have shown that the use of a full-length IgG4 Fc motif (containing the hinge, CH2 and CH3 modules) in CD19 and receptor tyrosine kinase-like orphan receptor 1 (ROR1)-directed CARs was associated with significant tumour-independent trapping of CAR T-cells in the lungs of NSG mice, and reduced anti-tumour efficacy and persistence compared to CARs with a truncated IgG4 spacer lacking CH2 and CH3 [44]. The authors postulate that CARs with a full length IgG4 Fc spacer are sequestered by lung-resident Ly6C+ mononuclear cells expressing FcγR, highlighting the finding that the few CAR-T-cells able to escape to the periphery have a highly activated phenotype with a significant propensity to undergo AICD. For patients, this may be particularly relevant in cases of B-cell lymphodepletion or hypogammaglobulinaemia where immune cell FcγRs may be relatively under-occupied, accentuating the interaction with IgG Fc-containing CARs. Aside from causing AICD, the cross-activation of FcγR+ immune cells may activate the innate immune system contributing to macrophage activation syndrome (MAS) and/or cytokine release syndrome (CRS). Targeting of the myeloid compartment and/or natural killer (NK) cells (depending upon the spacer’s IgG subclass) would also be liable to have repercussions for anti-tumour efficacy and, with regard to myeloid cells, may have positive or negative effects in different tumour models.
The contribution of the hinge / spacer domain to ligand-independent CAR tonic signalling has been investigated further by Watanabe et al. [20]. Starting with a γ-retrovirus-transduced second generation prostate stem cell antigen (PSCA)-directed CAR comprising an IgG1-derived hinge and CH2-CH3 spacer bound to a CD28 TMD / endodomain and CD3ζ chain (termed P1.CAR), they proceeded to evaluate how modifications to the spacer could impact in vitro expansion and cytotoxicity as well as CAR performance in vivo using NSG mice engrafted with human PSCA-expressing tumour cell lines. In keeping with other reports of FcγR-mediated pulmonary trapping, intravenous delivery of P1.CARs resulted in poor trafficking to the tumour or lymphoid tissue and significant accumulation in the lungs. This was found to be mediated by interactions with monocytes and macrophages expressing FcγR I and II and could be abrogated by making residue alterations to the IgG1 CH2 region or, optimally, by substituting the IgG1 framework for IgG2. Despite far superior migration and an absence of significant pulmonary trapping these modified CARs continued to perform poorly in vivo. Subsequent analysis revealed that all of these CARs (bar a truncated control) exhibited continuous expansion and cytokine production in vitro in the absence of ligand, consistent with other reports of constitutive tonic signalling. Continuous CAR expansion was associated with progressive differentiation towards a terminal effector phenotype with elevated expression of EOMES, FASL (encoding Fas ligand) and GZMB (encoding granzyme B) and loss of CD27, CD28 and CD62L (encoded by SELL) (illustrated in Figure 3(e)). However, unlike the exhausted phenotype identified by Long et al. [18], these CARs did not exhibit an upregulation of PD-1 or other inhibitory molecules. Accelerated cell senescence, however, was a feature, although telomere length following expansion was not evaluated in this study. Deletion of the IgG2 CH2-CH3 spacer prevented tonic signalling, allowing CARs to maintain an undifferentiated phenotype (high CCR7:CD45RO ratio), but at the expense of cytotoxicity (particularly in the face of low target surface expression). Re-insertion of an IgG2-derived hinge and CH3 domain to create an intermediate length spacer (X32.CAR) could restore cytolytic capacity without the re-emergence of tonic signalling and demonstrated significantly improved in vivo performance. Interestingly, alteration of spacer length in both a first generation MUC1 CAR and a second generation CD19 CAR resulted in a similarly undifferentiated phenotype. Taken together, these data suggest that a different pattern of tonic signalling can occur with different hinge / spacer domains and that this is likely to be occurring at the level of scFv oligomerisation, which may be facilitated by the flexibility and length of these domains.
CARs containing murine scFvs have, unsurprisingly, been found to be immunogenic when used in humans [86] and can cause anaphylaxis [87]. Whilst the former would not be anticipated to be a significant long-term problem with CARs targeting B-cell antigens (such as CD19, CD20 or CD22) or indeed in the aftermath following the administration of a lymphodepleting conditioning regimen; however, in the longer-term this is anticipated to be a problem, particularly if using these scFvs to target solid tumours. Indeed, antibodies directed to a murine scFv targeting carbonic anhydrase 9 (CAIX) were detected in patients receiving a first generation CAR [86]. While binding of host immunoglobulin to murine scFVs in this manner would be expected to elicit CAR cross-linking and cell surface clustering in the absence of ligand, the detrimental impact of antibody-induced tonic signalling is likely to be considerably outweighed by the targeting of antibody-bound CAR T-cells for destruction by antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
Ligand-independent tonic signalling may also induce constitutive systemic production of cytokines outside of the TME, with potentially deleterious effects, including CRS, MAS, multi-organ toxicity and the expansion of immunosuppressive cells. This issue may be further magnified when utilising TRUCKs or armoured CARs, capable of secreting transgenic cytokines at a high level in an inducible or constitutive manner. Indeed, a phase I clinical trial evaluating ACT with inducible IL-12-engineered tumour infiltrating lymphocytes (TILs) in patients with advanced melanoma revealed high serum levels of IL-12 and significant hepatotoxicity [88]. CAR tonic signalling acting on the nuclear factor of the activated T-cell (NFAT) promoter may exacerbate this further. A method of potentially constraining this, albeit without addressing tonic signalling itself, would be to link cytokine production to an inducible switch promoter and a background reduction signal (BRS). Uchibori et al. have developed such a system by delivering a switch cassette comprised of two modified Simian virus 40 early polyA sequences (serving as a BRS), four NFAT-responsive elements, a minimal IL-2 promoter, a ZsGreen1 reporter, and a bovine growth hormone polyadenylation (BGH polyA) sequence, to CD19-directed CAR Jurkat cells [89]. Jurkat cell ZsGreen1 expression was only seen when co-cultured with CD19-positive target cells in this model.
Finally, unconstrained activation caused by tonic signalling may lead to impaired trafficking of CAR T-cells into the TME, mediated by the downregulated expression of relevant chemokine receptors. It has been shown, for example that ex vivo activation of CAR T-cells using anti-CD3 and anti-CD28 antibodies can lead to a concomitant reduction in the surface expression of both C-C motif chemokine receptor 9 (CCR9) and α4β7 integrin [90], which may, for example, be predicted to impair trafficking to the small intestine [91]. However, this area remains relatively underexplored and as such is subject to conflicting reports. For example, in Watanabe et al.’s model of CAR tonic signalling, significant upregulation of the chemokine receptors CCR2 and CCR5 is noted [20]. In a non-CAR context, CCR2+ CCR5+ CD4+ T-cells derived from healthy human donors have been shown to harbour a central memory and effector memory phenotype and are capable of migrating to a number of inflammatory chemokines (including the C-X-C motif chemokine ligands CXCL-9 and CXCL-12; and the C-C motif chemokine ligands CCL-2 and CCL-20) [92]. Furthermore, ligand-dependent CAR tonic signalling may be anticipated to enhance cell adhesion molecule interactions, diapedesis and trafficking via inside-out signalling to integrins (such as LFA-1). This is known to be dependent, in part, on the activation of TCR downstream signalling shared with CARs, and specifically the activation of membrane-derived DAG via the LAT-phospholipase Cγ1 (PLCγ1) signalosome complex causing knock-on activation of the small GTPase Rap1 [93].
Ligand-dependent CAR tonic signalling
Thus far, CARs designed to target TAAs in solid tumours have been reliant upon the existence of an expression differential between tumour cells and normal tissue [22, 94]. Whilst adjustments to CAR expression levels and target binding affinity and avidity can help discriminate between low level and high level antigen expression [95, 96], difficulties with physiological low level antigen expression have been encountered with both first and second generation constructs evaluated in clinical trials [27]. Unfortunately, outcomes can be dire, as illustrated by the case of a colorectal cancer patient who developed an acute respiratory distress syndrome (ARDS)-type picture followed by fatal multi-organ failure after the intravenous delivery of 1010 CD8+ T-cells expressing a potent third generation HER2-directed CAR containing both CD28 and 4-1BB costimulatory ICDs [97]. Subsequent post-mortem analysis provided credence to the hypothesis that physiological low level HER2 expression on lung epithelium and/or microvasculature resulted in rapid cytokine release syndrome (CRS), exacerbated by first pass sequestration of CARs in the lungs following intravenous administration. On-target, off-tumour toxicity has been witnessed in other models, including a first generation CAR targeting CAIX, a TAA commonly expressed by clear cell renal cell carcinoma. However, clinical trials revealed multiple cases of cholangitis due to the targeting of low level CAIX expression on biliary epithelium [98]. Below the threshold of cytotoxicity, however, chronic engagement of CARs with low level off-tumour antigen may induce chronic ligand-dependent tonic signalling, anergy (particularly with first generation constructs) and exhaustion prior to their entry into the TME.
Theoretically, antigen shedding (e.g. soluble carcinoembryonic antigen (CEA)) could induce low level CAR tonic signalling in the TME or systemic vasculature. However, there is little evidence in the published literature to support this phenomenon in vivo. In vitro studies, thus far, have demonstrated that CEA-directed CARs are not inhibited by high concentrations of soluble CEA (up to level 10-fold higher than usually found in the sera of cancer patients) [99].
Constitutional ligand-dependent tonic signalling is a problem that might be anticipated in the specific scenario of targeting T-cell lineage leukaemias or lymphomas. CD5 and CD7-directed CAR T-cells have been developed and a variable degree of fratricide has been noted [100, 101]. Following CAR engagement, surface expression of CD5 on transduced T-cells is lost due to complete ligand-dependent internalisation, leading to acceptable levels of fratricide. The loss of CD5, which is known to exert inhibitory effects on TCR activation [102] (at least partly due to negative regulation of ZAP70 via a reduction in the kinase activity of Fyn [103]), may also confer a beneficial effect on CAR T-cell effector function. In the case of CD7, however, there is incomplete loss of expression leading to high levels of fratricide that was capable of impairing CAR expansion. This could be abrogated by using CRISPR/Cas9-mediated targeted disruption of the CD7 gene prior to CAR expression. Both unedited CD5 and edited CD7 CARs have been successfully expanded long-term ex vivo and both were able to effectively eliminate malignant T-cell acute lymphoblastic leukaemia (T-ALL) and T-cell lymphoma cell lines in vitro as well as inhibit disease progression in xenograft mouse models [100, 101]. Separately, we have found that the transmembrane glycoprotein mucin 1 (MUC1) may be expressed on activated T-cells, giving rise to ligand-dependent tonic signalling by MUC1-directed CAR T-cells comprising scFvs derived from either the SM3 or HMFG2 antibodies [104]. Ex vivo expansion was associated with increased activation, cytokine release and fratricide. A possible method to circumvent this may be to culture the CAR T-cells in the presence of a peptide epitope capable of blocking the CAR without downregulating it.
Finally, both ligand-independent and ligand-dependent tonic signalling may theoretically be enhanced by non-specific T-cell adhesion, for example via ICAM-1 / LFA-1 interactions, which are able to lower the threshold for TCR-mediated T-cell activation [56]. This process is known to contribute to the priming of naïve T-cells in lymphoid structures by mature DCs, which readily express and modulate cell surface adhesion molecules [105]. These interactions may also facilitate TCR-mediated tonic signalling following binding of self-peptide presenting MHC and, due to the impact on downstream signal transduction pathways shared with CARs, it is plausible that a similar phenomenon may occur following CAR ACT in vivo. As ICAM-1, ICAM-2, VCAM-1 and other adhesion molecules are often overexpressed on both tumour cells and the TME [106], the impact of these interactions on CAR signalling may be particularly beneficial when targeting solid tumours. Interestingly, tumour cell surface expression of adhesion molecules can be induced by exposure to activated CAR T-cells. For example, following exposure of low mesothelin expressing A549 lung cancer cells to cytokines secreted by activated mesothelin-directed 28ζ CARs, A549 cells upregulated ICAM-1 and were susceptible to enhanced bystander cytotoxicity by CAR T-cells that also demonstrated upregulation of cell surface LFA-1 [107].
Potential strategies to address CAR tonic signalling
Engineering or altering the targeting moiety
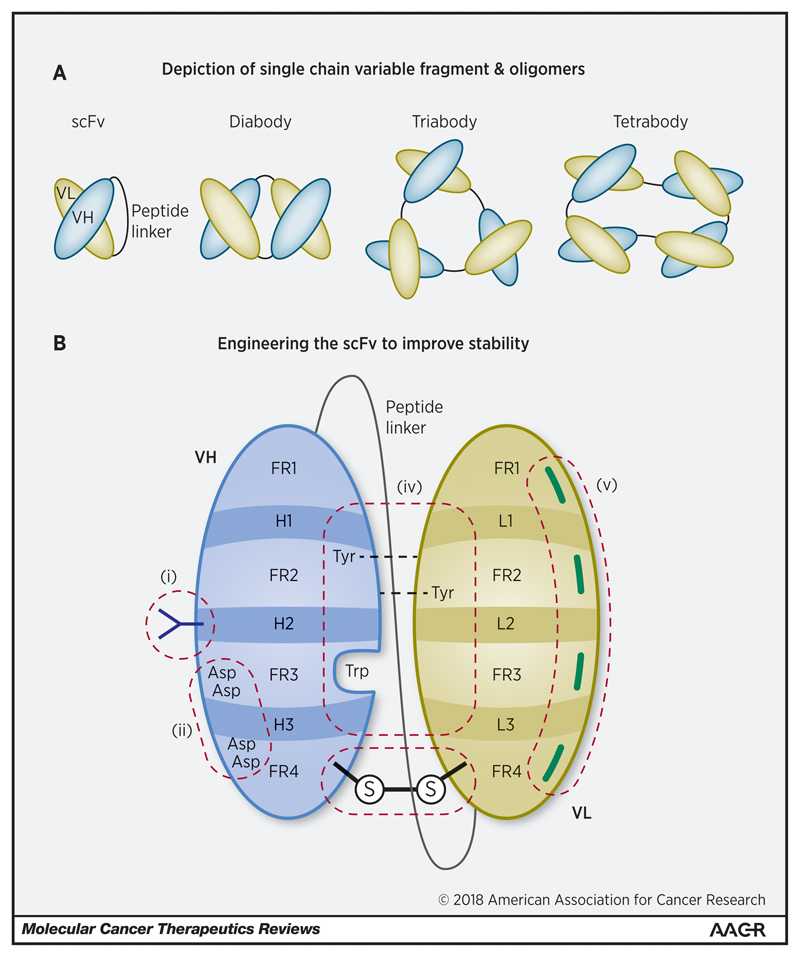
With the absence of structural support provided by the IgG constant regions, scFv stability and/or folding properties can render CARs susceptible to oligomerization, clustering and ligand-independent tonic signalling. Variable heavy (VH) and light (VL) immunoglobulin chains are typically joined by a flexible peptide linker resistant to endopeptidase degradation [108]. Nevertheless, most employed scFvs still demonstrate a tendency to unfold at the VH:VL interface, leading to sub-optimal stability of the two immunoglobulin domains. This type of unfolding can permit “protein domain swapping”, whereby complementary domains from adjacent scFv molecules can interact with one another leading to scFv oligomerization [109]. Depending upon the length of the peptide linker, which may impede the rotation of the complementary Ig domains, oligomers comprising two, three or even four scFv molecules can form (illustrated in Figure 4(a)) [108]. Allowing for steric hindrance caused by the attached CARs, such oligomers can also be envisaged on the cell surface, potentially causing tonic signalling. The use of highly flexible and/or long extracellular spacers may be expected to facilitate oligomerization of intrinsically unstable scFvs. Likewise, the construction of tandem CARs with two scFvs per CAR monomer [110] may be at greater risk of oligomerization, clustering and tonic signalling.
Figure 4.
(a): Depiction of single chain variable fragment & oligomers. scFvs are inherently unstable structures due to non-covalent interactions between the heavy and light chains. They are liable to form oligomers, particularly at extremes of pH and temperature, due to domain swapping and framework interactions. Outside of their use in CARs a variety of conformations have been demonstrated, dependent upon the relative length of the peptide linker, with shorter linkers conducive to multimer formation.
(b): Engineering the scFv to improve stability. The scFv lends itself to protein engineering to optimise stability and prevent oligomerisation. The primary objective is to strengthen the VH:VL interface. Options include (i) glycosylation to counter hydrophobic motifs and improve solubility; (ii) addressing the net charge of the antibody scaffold by substituting residues on either side of the CDRs; (iii) adding disulphide bridges; (iv) utilising computational modelling to improve the stability of the VH:VL interface (e.g. by substituting residues to add hydrogen bonds or to fill gaps); and (v) reverting hypermutations in framework regions to germline. VH, heavy chain; VL, light chain; FR, framework region; H1-3 & L1-3 represent complementary determining regions in the heavy & light chains respectively; Asp, aspartic acid; Trp, tryptophan; Tyr, tyrosine.
Figure 1 and figures 3-5 are original and have been created specifically for this article.
These issues may be addressed by optimising the orientation of heavy and light chains; selecting VH and VL consensus master gene sequences [111]; by engineering disulphide bonds between the VH and VL domains, either in the absence of a peptide linker or in combination to ensure maximal stability [112]; by introducing charged mutations within the VH and VL domains [113]; by complementarity-determining region (CDR) grafting [114]; or by using a combination of these strategies. Longer peptide linkers may reduce the likelihood of multivalent oligomerization, although potentially at the cost of increased proteolysis or weak domain association. A linker of 15-20 residues is generally regarded as thermodynamically most stable [109]. In the absence of covalent bonds, VH and VL interactions are dependent upon electrostatic interactions, hydrophobic repulsion, hydrogen bonds and van der Waals forces. They are thus subject to local temperature, protein concentration, ionic strength, and above all pH, which in the TME may be detrimental to stable folding [108]. These issues and the impact of scFv engineering upon antigen binding affinity and avidity as well as immune synapse formation remain to be characterised.
Single chain variable fragment aggregation may also occur in the absence of domain swapping due to the hydrophobic nature of residues within their CDRs, which mediate binding to target antigens. Various techniques have been deployed to resist aggregation without reducing binding affinity. Examples include inserting two or more negatively charged residues at each edge of the scFv’s third CDR (CDR3) [115] or introducing a glycosylation site inside the second CDR to compensate for the presence of hydrophobic residues within the third CDR [116].
Further improvements in scFv stability, particularly at the VH:VL interface can also be achieved using advanced computational modelling. Ultimately, the biophysical characteristics of scFvs are determined by their germline sequence but influenced by somatic hypermutations in the framework regions. Computational modelling has been used to revert these hypermutations to germline consensus and optimise stability at the VH:VL interface. For example, outside of the CDRs additional hydrogen bonds can be introduced between the VH and VL domains by replacing phenylalanine with tyrosine residues and by filling in pockets within the topological structure of the VH domain by substituting phenylalanine with tryptophan [117]. Improvements in CAR stability translated to improved CAR surface expression and enhanced in vitro cytotoxicity. Furthermore, a reduction in tonic signalling was noted in comparison to the original scFv-derived CARs. Beyond dissociation at the VH:VL interface, disparities may exist between the relative thermal stability of the VH and VL domains. Such a scenario may lead to an accumulation in equilibrium of an unfolding intermediate, where one domain completely unfolds and the other remains native, leading to enhanced aggregation. By simulating the molecular dynamics in silico, particularly with regard to the less stable of the two domains, one can systematically engineer scFvs to improve intrinsic stability and minimize aggregation [118]. These strategies are summarised in Figure 4(b).
Alternative strategies may include selecting a target epitope localised on the membrane-anchored part of antigen to avoid tonic signalling mediated by shed, oligomerised soluble antigen, or by utilising scFvs with low to intermediate binding affinity to enhance discrimination between membrane-bound and soluble antigens [119]. And, in general terms, the use of a low affinity scFv is likely not only to provide a means of discriminating between tumour cells with high level antigen expression and normal cells with low level expression (thus improving safety) [120] but may also minimise ligand-dependent tonic signalling caused by the presence of more widespread low level antigen expression on normal tissues.
Alternative targeting moieties, such as camelid single-domain antibodies (VHHs) termed “nanobodies”, which share a high degree of homology with human VH sequences and are the smallest known single chain antibodies [121] may also avoid tonic signalling by being intrinsically unable to domain swap, although their efficacy in CARs remains to be fully elucidated in experimental models. Interestingly, due to their small size and the length of their CDRs, which form extended loops, they are able to access cryptic epitopes (such as catalytic sites in enzymes) or large structures that typically escape immunosurveillance [122]. Like murine scFvs, the potential immunogenicity of camelid nanobodies is being addressed using sequence humanisation techniques.
In addition, centyrin-based CARs have also been designed, with properties that may limit ligand-independent tonic signalling. Centyrins represent a novel class of alternative scaffold protein based on a consensus tenascin fibronectin domain. They are smaller than scFvs and are monomeric. A human B-cell maturation antigen (BCMA)-directed centyrin CAR transduced using the Super piggyBac™ transposon / transposase system has shown excellent in vitro cytotoxicity with a predominantly stem cell memory phenotype [123].
The issue of scFv clustering may also be circumvented by utilising endogenous receptors or ligands as CAR extracellular targeting moieties. A wide variety of such constructs have been designed with several already undergoing clinical evaluation [9, 124]. Those that have progressed furthest along clinical development include interleukin 13 (IL-13)-zetakine CARs incorporating membrane-tethered IL-13 to target the interleukin 13 receptor subunit alpha-2 (IL13Rα2) decoy receptor, a glioma-restricted cell-surface epitope [125, 126]; CARs armed with an epidermal growth factor (EGF) / transforming growth factor alpha (TGFα) fusion molecule capable of targeting pan-ErbB homo- and heterodimers expressed on a plethora of solid tumours [127, 128]; CARs armed with the natural killer group 2D (NKG2D) protein fused to CD3ζ alone [129]or in combination with an intracellular costimulatory domain [130] to target a wide variety of haematological malignancies and solid tumours overexpressing NKG2D stress inducible ligands (such as MHC class I chain-related protein A (MICA), MHC class I chain-related protein B (MICB) and UL16 binding proteins 1 to 6 (ULBP1-6) in humans) [131]; and CARs utilising CD27 to target CD70 [132], an antigen aberrantly expressed by a broad range of haematological malignancies and some solid tumours including renal cell carcinoma and glioma. Thus far, no target-independent tonic signalling has been reported, but due to the non-restricted expression of many of their targets these constructs may be liable to encounter chronic low-level ligand-dependent tonic signalling, which may have positive or negative effects in different contexts.
Adjusting the hinge / spacer
Hinge and spacer domains have proved particularly beneficial for the targeting of membrane proximal epitopes and are able to relieve spatial constraints that may hinder interactions between tumour antigens and CARs [9, 45, 133]. The CD8α hinge is typically used with the CD8α TMD and plays an important role in maintaining the flexibility of the CAR binding domain and the ability to form an efficient immunologic synapse with the target cell. The substitution of cysteine residues normally involved in CD8α/α and CD8α/β dimerization can permit both homo- and hetero-dimerization of the CAR, enhancing its transport out of the endoplasmic reticulum (ER) to the cell surface [134] and increasing the level of productive dimerization resulting in more effective target-cell killing in a transduced NK cell model [135]. In the context of a molar excess of endogenous CD3ζ, enhanced heterodimerization would be expected to lower the threshold for CAR tonic signalling.
Experiments undertaken by Watanabe et al. whereby an intermediate length IgG2 hinge/spacer was shown to abrogate CAR tonic signalling without compromising cytolytic capacity have already been discussed [20]. Separately, experiments conducted with second generation lentiviral-transduced 28ζ CARs directed to a variety of antigen targets (including CD19, mesothelin, PSCA, HER2 and MUC1) either with or without an IgG4-CH3 hinge/spacer domain have demonstrated that, in all cases, the presence of the hinge conferred increased expansion (and particularly late expansion beyond Day 15) in a ligand-independent manner during in vitro culture following prior exposure to anti-CD3/anti-CD2/anti-CD28-loaded microbeads [136]. Enhanced hinge-containing CAR T-cell expansion appeared to depend upon proliferation of the CD4+ subfraction, but was abrogated if CD4+ and CD8+ T-cells were cultured separately, suggesting that tonic signalling may have a differential role in CD4+ and CD8+ populations and that cross-talk between the two lineages may also be occurring. Interestingly, utilising a chemoattractant assay the researchers were also able to show that the hinge-containing CAR T-cells had inherently enhanced migratory and invasive capabilities, reinforcing the likelihood of tonic signalling playing a decisive role here.
As already discussed, in cases where CARs are utilising full length IgG Fc-containing spacers, interactions with FcγR-expressing mononuclear or NK cells are expected to induce “off target” activation and AICD. Although myelodepleting conditioning regimens may limit these interactions in the immediate period following CAR T-cell infusion, this problem would be expected to re-emerge following recovery of the myeloid compartment. Likewise, saturating immune cell FcγRs with exogenous human Ig prior to CAR T-cell administration provides only a short-term solution. In a ROR1-targeting CAR incorporating the R11 scFV, IgG4 Fc spacer, CD28 and 4-1BB costimulatory ICDs with CD3ζ, Hudacek et al. have shown that modification of the spacer in order to limit FcγR-mediated activation and AICD promotes enhanced effector function and persistence in a NSG mouse model [44]. Whilst CARs designed to target non-proximal cell surface epitopes (such as CD19) can be optimised with shortened spacers that omit the entire IgG4 CH2 domain (thereby eliminating binding by FcγRI), CARs designed to target a transmembrane proximal epitope (such as the ROR1 kringle domain) require a full-length spacer to optimise immune synapse formation and reduce steric hindrance. Hudacek et al. were able to maintain function and address pan-FcγR activation, sequestration and AICD in this model by swapping the CH2 sequences of the IgG4 spacer with those of IgG2 and replacing the crucial N-glycosylation site Asn297 with a conserved residue not amenable to N-linked glycosylation.
Optimal selection of the transmembrane domain
Although few reports exist regarding the role of the CAR TMD in contributing to tonic signalling, it is abundantly clear that the TMD plays a vital role in CAR cell surface expression and stability as well as its ability to interact with other cell surface molecules that may contribute to signal transduction [137]. Utilising an unedited CD3ζ TMD may facilitate heterodimerization with endogenous CD3ζ chains, potentially lowering the threshold of antigen binding required to elicit a cytotoxic response [138] and, as an anticipated corollary, enhanced tonic signalling. However, cell surface expression of CD3ζ TMD-containing CARs appears to be lower than those containing CD28 or CD8α TMDs [139]. The optimal selection of TMD to mitigate tonic signalling remains to be elucidated and is likely to be impacted or subsumed by the many other factors outlined in this review.
Optimal selection of costimulatory intracellular domains
When ligand-independent tonic signalling occurs due to scFv clustering, particularly negative effects appear to be mediated by constitutive CD28 signalling, leading in some scenarios to IL-2 gene expression and a positive feedback loop of unconstrained proliferation and activation [21, 64]. Uncontrolled IL-2 production may also have the unintended consequence of attracting and enhancing the proliferation of immunosuppressive Tregs [140]. Deletion of the CD28 Lck-binding moiety in this model could abrogate enhanced IL-2 production, without compromising IFNγ secretion, proliferation, and cytolysis. Greater complexity may exist in certain tumour models due to endogenous receptor interactions (e.g. between CD2 (LFA-2) on CAR T-cells and CD58 (LFA-3) on tumour cells) potentially recapitulating CD28-mediated IL-2 production [141]. In most cases where scFv clustering has been implicated, the CD28 domain appears to be detrimental and the 4-1BB domain beneficial. Nevertheless, there appear to be at least two distinct phenotypes – one that is characterised by continuous expansion, terminal differentiation and senescence (seen in studies by Frigault et al. [21], Watanabe et al. [20] and Qin et al. [136]); and one characterised by T-cell exhaustion (seen in studies by Long et al. [18]). In the case of the latter, the production of activating cytokines (such as IL-2 and TNFα) appears to be significantly curtailed and the use of a 4-1BB costimulatory domain could rescue these cells from exhaustion and was associated with a memory T-cell metabolic phenotype [18]. In the case of the former, a reduction in CAR surface expression or a shortened spacer could ameliorate the negative consequences of tonic signalling both in vitro and in vivo. In Frigault et al.’s experiments the use of a 4-1BB or ICOS costimulatory domain also could alleviate continuous expansion. Watanabe et al. did not explore the use of a 4-1BB costimulatory ICD.
However, when using scFvs that are not typically prone to clustering (e.g. anti-CD19 FMC63 scFv), the use of a 4-1BB costimulatory domain may confer ligand-independent 4-1BB tonic signalling that appears to require T-cell activation (mediated by CD3 and CD28 binding) [62]. Again, there appear to be at least two phenotypes – one that is non-continuous and characterised by improved expansion, in vivo persistence and anti-tumour efficacy (Milone et al. [62]); and another associated with poor expansion, upregulation of Fas and Fas ligand and AICD (Mamonkin et al. [19]). In the case of the latter, the negative consequences of tonic signalling could be ameliorated by adding a CD28 costimulatory domain upstream of 4-1BB to construct a third generation CAR [63] or by reducing CAR expression by adding an IRES element between the retroviral promoter and the CAR transgene. A highly vector-specific amplification loop involving the LTR promoter appears to explain this unusual phenomenon. Therefore, the negative or positive consequences of 4-1BB-mediated tonic signalling are likely to result from differences in quantitative and qualitative 4-1BB activation and from differences in the temporal and spatial interaction of the 4-1BB ICD with membrane-associated signal transduction molecules that are also involved more broadly in T-cell activation.
Additional techniques that have been associated with improved CAR surface expression, such as mutating CD28 non-canonical di-leucine internalization motifs (albeit in a murine CD28 model) [142] may also be expected to exacerbate the consequences of tonic signalling occurring in certain CARs.
Finally, there may be scope to utilise alternative strategies to recapitulate the benefits of 4-1BB costimulation, while preventing the possibility of 4-1BB-mediated tonic signalling. Zhao et al. have found that the expression of a second generation SJ25C1 CD19 28ζ CAR in trans with constitutively expressed transgenic 4-1BBL (thus providing paracrine costimulation following inducible 4-1BB upregulation) resulted in considerably improved performance (and significant IFNβ production) compared to an equivalent third generation 28BBζ CAR [10]. However, care may be needed with this approach based upon reports that 4-1BBL crosslinking in the absence of available 4-1BB may foster suboptimal CD4+ T-cell activation [143]. Whether this would have consequences for CAR, rather than TCR activation remains to be seen as there may be differences in the degree of 4-1BB upregulation, which if more potent following CAR activation may more easily reverse the suppressive effects of 4-1BBL through T-cell intrinsic 4-1BB-regulated 4-1BBL internalization.
Controlling CAR expression
With regard to gene transduction using viral vectors, numerous strategies have been adopted to improve safety by minimizing the risk of producing replication-competent virus and reducing the potential to cause insertional mutagenesis. Self-inactivating (SIN) vectors have been developed using both retroviruses and lentiviruses by deleting/replacing LTR elements. Non-integrative lentiviruses (NILVs) have also been designed by mutating the integrase gene or by modifying the attachment sequences of the LTR [144–146]. By limiting high level CAR expression, these methods may reduce the likelihood of tonic signalling being caused by CAR clustering. As previously discussed, Frigault et al. have demonstrated continuous ligand-independent CAR T-cell proliferation with lentiviral vectors using the EF-1α promoter but not when driven by the CMV or variably truncated PGK promoters [21]. More recently, Gomes-Silva et al. have demonstrated that 4-1BB-mediated tonic signalling was highly dependent upon CAR surface expression and that a γ-retroviral LTR promoter was liable to amplify CAR expression in a positive feedback loop mediated by 4-1BB induced NF-κB activation [73]. The use of an IRES element upstream of the LTR promoter could curtail CAR expression, thereby reducing tonic signalling. A similar improvement was also seen following transduction with a SIN lentiviral vector.
In addition, by ensuring transient or self-limiting CAR expression utilising plasmid or mRNA electroporation the risks of both genotoxicity and tonic signalling-induced T-cell exhaustion may be addressed. Following RNA transfection the transgene is typically expressed for approximately one week [147]. Such a system, therefore, is likely to require repeated CAR T-cell administration at multiple time points [148]. Constitutive T-cell proliferation caused by tonic signalling has not yet been reported when CARs are expressed by electroporation of mRNA or plasmids encoding the Sleeping Beauty transposon/transposase system [149, 150], in contrast to lentiviral transduction [21]. The impact on tonic signalling of newer NILVs (e.g. those containing a scaffold/matrix attachment region (S/MAR) element) with the capacity to confer long-lasting episomal CAR expression on par with that of integrative lentiviral vectors [151] remains to be seen.
Regulated on/off switches, designed primarily to mitigate CAR toxicity, may also have a dual role in reducing tonic signalling by ensuring that CAR surface expression is tightly controlled in a temporal manner following antigen exposure. One such setup incorporates a single vector tetracycline (Tet)-On inducible gene expression system, whereby the CAR gene is located downstream of a reverse Tet transactivator (rtTA) fusion protein, which is able to activate its promotor only in the presence of doxycycline [152]. Extrapolating from the supposition that CAR tonic signalling, terminal differentiation and/or exhaustion are, at least in part, due to unconstrained CAR cell surface expression, one may hypothesise that the intermittent withdrawal of doxycycline using this model (particularly during the initial phase following CAR T-cell delivery) may avert these negative consequences and enhance anti-tumour efficacy. Naturally, this approach would rely upon the pharmacokinetics of doxycycline being conducive to ensuring a kinetically optimal CAR transcription profile that could minimise tonic signalling. Separately, Mamonkin et al. have reported that the negative consequences of 4-1BB-mediated tonic signalling could be prevented by utilising a small molecule to regulate CAR expression at the level of their γ-retroviral promoter [19].
Likewise, separating the CAR into two functional entities and/or utilising a dimerizing agent such as a rapamycin analogue (rapalog) [153] could limit the likelihood of both ligand-independent and ligand-dependent tonic signalling. The latter could also be limited by divorcing the CAR scFv from the intended epitope by utilising an exogenous targeting module as per the UniCAR system [154]. Wu et al. have constructed a split ON-switch CAR triggered only in the presence of target ligand and a small molecule dimerizing agent (using either the rapalog AP21967 or the plant hormone gibberellin) [155]. For the rapalog-gated CAR one component comprises the scFv extracellular targeting moiety linked to a CD8α hinge / TMD, a 4-1BB costimulatory ICD and a distal FK506 Binding Protein (FKBP) domain; the second component comprises a DAP10 ectodomain, CD8α hinge / TMD, 4-1BB ICD, mutant FKBP-rapamycin binding domain (FRB*) and CD3ζ ICD. The DAP10 ectodomain was selected to aid homodimerization, doubling the potential number of CD3ζ ITAM domains per assembled CAR. For the gibberellin-gated CAR, FKBP and FRB* were substituted with gibberellin insensitive dwarf 1 (GID1) and gibberellic-acid insensitive (GAI). These CARs demonstrated titratable cytotoxicity in the presence of the dimerizing agent and similar in vivo efficacy.
The design of customisable logic-gated circuits may also alleviate the negative consequences of unconstrained tonic signalling by rendering CAR surface expression controllable, temporally and/or spatially. The synNotch system couples CAR transcription to the signalling of a synthetic Notch receptor, engineered to engage with a second TAA [156–158]. Subsequent proteolytic cleavage of the receptor induces the release of a synthetic transcription factor able to induce (or suppress) CAR transcription. Due to the orthogonal nature of these synthetic gene circuits it is conceivable that a single CAR T-cell may be controlled by multiple synNotch receptors. The authors have not commented on the potential for tonic signalling to occur in this model. However, tonic signalling of endogenous Notch has been reported in a variety of contexts, such as in mouse myoblasts [159] and epidermal keratinocytes where the metalloprotease ADAM17 has been implicated in maintaining a basal level of Notch1 activity in a ligand-independent fashion [160]. In addition, in T-cells Notch has been shown to undergo spontaneous cleavage in the absence of Notch ligands following TCR engagement, where it may augment signal 1 and 2-induced proliferation [161]. Separately, the CAR product may exhibit tonic signalling due to the choice of promoter or due to intrinsic structural issues. SynNotch may also be liable to induce immunogenicity as well as theoretical off-target effects due to persistent CAR transcription in synNotch-controlled T-cells that have exited the TME.
Eyquem et al. have demonstrated that targeting a CD19-specific 28ζ CAR to the T-cell receptor α constant (TRAC) locus using CRISPR/Cas9 results in superior performance and persistence compared to conventionally generated CAR T-cells using a SFG γ-retroviral vector, independent of TCR disruption [64]. Interestingly, targeting the CAR to the TRAC locus reduced tonic signalling and enhanced CAR internalisation and re-expression following repeated exposure to antigen, delaying effector T-cell differentiation and exhaustion. Unlike CD19-specific CARs utilising the FMC63 scFv, γ-retrovirally transfected SJ25C1 scFv CARs (RV CARs) demonstrated constitutive activation and tonic signalling in the absence of ligand evidenced by baseline ITAM phosphorylation. Furthermore, by localising the CAR to the β2-microglobulin (B2M) locus or to TRAC using either the EF-1α constitutive promoter or the LTR retroviral promoter, they were able to show that the degree of antigen-independent tonic signalling correlated with CAR surface expression. In contrast to endogenous promotor TRAC CAR T-cells, following repeated stimulation by antigen, RV CARs (and TRAC EF-1α CARs) rapidly differentiated into an effector T-cell phenotype with loss of CD62L expression, potent secretion of IL-2 and expression of T-bet, EOMES and GATA3. A critical difference was identified in the level of CAR expression following repeated antigen exposure in endogenous promotor TRAC CARs versus EF-1α or RV LTR CARs. Whilst the latter group demonstrated a rapid step-wise increase in CAR surface expression following each antigen exposure, endogenous promotor TRAC CAR cell surface expression conversely reduced following each exposure and remained below baseline after 48 hours, mediated by CAR internalisation and degradation. The implication of this work is that by constraining both baseline and dynamic CAR tonic signalling (and mimicking endogenous TCR expression), CAR T-cell exhaustion can be delayed or avoided and anti-tumour efficacy enhanced.
Pharmacological strategies
Certainly, one may conceive of using pharmacological agents to inhibit or reshape the negative consequences of CAR tonic signalling such as exhaustion and terminal differentiation. The former can potentially be reversed utilising monoclonal antibody inhibitors of upregulated immune checkpoints such as PD-1, LAG-3 or TIM-3 [162]. Attempts have been made to address the latter using various strategies. These include activating the canonical Wnt/β-catenin pathway (which has been shown to promote a TSCM phenotype) by inhibiting glycogen synthase kinase 3 beta (GSK3β), a serine/threonine kinase implicated in β-catenin degradation, either alone [163] or during ex vivo culture with IL-7, IL-21 and CD8+/CD62L+/CD45RA+ streptamer-based serial-positive selection [164]; inhibiting glycolysis using 2-deoxyglucose [165]; and remodelling mitochondrial function to replicate a memory T-cell metabolic phenotype characterised by oxidative phosphorylation (OXPHOS) and fatty acid oxidation (FAO) [166]. Separately, the phosphatidylinositol-3-kinase (PI3K)/Akt pathway has been implicated in T-cell memory formation. For example, Akt has been shown to phosphorylate and sequester forkhead box O (FOXO) transcription factors, blocking the transcription of molecules associated with less differentiated T-cells (such as CD62L, CCR7 and interleukin-7 receptor-α (IL7Rα or CD127) [167]. In parallel, Akt inhibitors have been demonstrated to improve the in vitro expansion of minor histocompatibility antigen-specific CD8+ T-cells with a minimally differentiated early memory phenotype, correlating with improved long-term persistence and a superior graft-versus-tumour effect in mice following adoptive transfer [168]. More recent work by Klebanoff et al. has demonstrated that the inhibition of Akt using an allosteric kinase inhibitor during the ex vivo expansion of CAR and TCR retroviral transduced T-cells decouples differentiation from expansion, enhancing the intranuclear localization of FOXO1. These cells exhibited a CD62L+ early memory phenotype, suppressed glycolysis and superior anti-tumour efficacy [169]. Likewise, inhibiting the PI3Kδ catalytic subunit p110δ using the small molecule selective inhibitor Idelalisib (formerly CAL-101), can promote a strong undifferentiated memory phenotype in both murine and human CD8+ mesothelin-directed CAR T-cells (as well as pmel-1-directed transgenic TCR T-cells) characterized by the upregulation of transcription factor 7 (Tcf7) and elevated surface expression of CD62L, CCR7 and CD127 [170]. In vivo, Idelalisib-exposed CAR and transgenic TCR T-cells persisted longer following ACT and induced greater tumour regression compared to traditionally expanded CD8+ controls. Inhibition of Akt may also be anticipated to reverse metabolic dysfunction caused by CAR tonic signalling by blocking the negative regulation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) caused by chronic Akt activation [171]. PGC-1α is known to enhance T-cell mitochondrial oxidative metabolism and promote mitochondrial biogenesis. Indeed, inhibiting Akt during ex vivo TIL expansion has also been shown to confer a memory T-cell metabolic profile with increased rates of OXPHOS and FAO, leading to enhanced in vivo persistence and improved anti-tumor immunity [172]. Engineering T-cells to overexpress PGC-1α may be an alternative strategy to optimise its beneficial effects on effector T-cell metabolism [171]. Finally, CAR activation has been shown to induce expression of the adenosine A2A receptor (A2AR) [173], which is able to exert potent negative feedback on CAR function via an interaction with adenosine, which is upregulated in the TME of many tumours [174]. Inhibition of A2AR either with a small molecule antagonist or using a short hairpin RNA (shRNA) could reverse CAR suppression, and was found to be particularly synergistic with anti-PD-1 therapy in a HER2+ breast cancer model [173].
Other T-cell engineering strategies
In addition to the insertion of a CAR, T-cells can be further engineered to optimise downstream signalling, express costimulatory molecules or secrete cytokines. All these approaches may be utilised to ameliorate the negative consequences of tonic signalling. Possible strategies include overexpressing 4-1BBL in combination with a 28ζ CAR [10]; knocking out Cbl-b, an E3 ubiquitin-protein ligase that promotes anergy by regulating PI3K access to CD28 [175, 176], in combination with a BBζ CAR; overexpressing PGC-1α to enhance OXPHOS and mitochondrial biogenesis [171]; or expressing a cell surface tethered IL-15/IL-15R fusion protein, which has been shown to encourage a CD45RO- CCR7+ CD95+ TSCM phenotype [42].
Providing ligand for ligand-dependent tonic signalling
Finally, a common finding when utilising CARs in vivo (particularly in the case of solid tumours) is that they fail to persist over time. Aside from issues of sequestration, AICD or exhaustion, one reason is that, unlike the case in haematological malignancies where CARs and target antigen are in close proximity, they may simply fail to encounter ligand in sufficient quantity or frequency to expand. Whilst repetitive CAR delivery can be feasible in certain circumstances, issues with immunogenicity and/or the toxicities associated with lymphodepleting conditioning can complicate matters. Regional or intra-tumoural CAR T-cell injection [127, 177] may also be tried but is unlikely to foster a systemic response in the case of metastatic disease. Various groups have attempted to utilise virus-specific (e.g. CMV or EBV) T-cells for CAR transduction [178, 179], with the dual aims of reducing GvHD following allogeneic use and enhancing in vivo expansion and persistence following interactions with DCs presenting viral epitopes in previously infected patients. However, T-cells dually activated via a CAR and their endogenous TCR may be liable to become anergic and exhausted, undergo AICD and exhibit poor persistence in vivo. This has been highlighted in a murine allogeneic haematopoietic stem cell transplantation model using CD28-costimulated CD19-directed CAR T-cells [180] and more recently in an immunocompetent syngeneic mouse model of CD19+ B-cell ALL where engagement of the TCR with target antigen was found to have a deleterious impact on CD8+ (but not CD4+) CAR T-cell efficacy, mediated by exhaustion and apoptosis [181].
Other groups are exploring the delivery of autologous T-cell antigen presenting cells (T-APCs) expressing truncated ligands (such as CD19) to mimic antigen presentation and engender persistence. This technique is already being evaluated within the phase I PLAT-02 trial at Seattle Children's Hospital [182]. Conceivably, autologous DCs or irradiated engineered autologous tumour cells (EATCs) [183] could be loaded with ligand and introduced intradermally or intranodally to enhance CAR expansion and persistence. APCs are particularly promising as they provide a whole gamut of signals that could stimulate and co-ordinate CAR (or indeed endogenous non-engineered) T-cell anti-tumour efficacy. Indeed, one could potentially conceive of CAR “service stations” utilising APCs modified to upregulate surface adhesion molecules (e.g. ICAM-1) or co-stimulatory ligands (CD80/86, 4-1BBL or CD40L) and secrete cytokines (IL-7 or IL-15) or chemokines (such as CXCL-9 or CXCL-10). All these strategies are summarised together in Figure 5.
Figure 5.
Potential strategies to address the negative effects of CAR tonic signalling. (a) Optimal selection of the extracellular targeting moiety +/- engineering of the scFv or substitution with camelid-derived nanobodies or non-immunoglobulin based scaffolds; (b) optimisation of the hinge and spacer; (c) optimal selection of costimulatory endodomains; (d) utilising pharmacological agents to reverse or prevent negative consequences of tonic signalling (e.g. Akt inhibitors to prevent terminal effector differentiation +/- metabolic features of T-cell exhaustion); (e) engineering CAR T-cell metabolism (e.g. overexpressing PGC1α or impairing its degradation; (f) reconfiguring costimulation by overexpressing costimulatory molecules or ligands such as 4-1BBL or (g) CD28, potentially optimised by knocking down expression of Cbl-b, an E3 ubiquitin-protein ligase, that promotes anergy by regulating PI3K access to CD28; (h) optimising interactions with endogenous TCR components, which may contribute to CAR tonic signalling; (i) recapitulating or enhancing T-cell / DC interactions to lower the activation threshold for cytotoxicity; (j) preventing constitutive IL-2 production and Treg induction by mutating the CD28 binding site for Lck; (k) optimal selection of target ligand, autologous APCs, T-APCs or EATCs expressing target ligand may also facilitate CAR T-cell expansion and persistence in vivo; (l) utilising small molecule gated CARs e.g. by incorporating an FKBP/FRB* heterodimerizing module in the presence of a rapamycin analogue; (m) utilising blocking monoclonal antibodies to target inhibitory immune checkpoints; (n) utilising switch CARs (e.g. PD-1/CD28); (o) optimal selection of the expression vector and promoter, e.g. using non-LTR (SIN) lentiviruses, mRNA or transposon delivery; (p) co-expressing tethered cytokine fusion molecules (such as IL-15/IL-15Rα); (q) exploiting inside-out signalling to integrins to facilitate T-cell migration & bystander tumour cell targeting; (r) utilising Tet-off systems for temporal control of CAR expression; and (s) utilising CRISPR Cas9 to direct CAR expression specifically to the T-cell receptor α constant (TRAC) locus.
Figure 1 and figures 3-5 are original and have been created specifically for this article.
Outstanding questions & conclusion
The preclinical data regarding CAR tonic signalling is at times conflicting and contradictory. Whilst ligand-dependent tonic signalling can potentially be co-opted to mimic endogenous T-cell/DC interactions and improve in vivo expansion and persistence, ligand-independent signalling appears to be far less benevolent. However, is the latter always detrimental? Certainly, data generated from the majority of experiments using non-4-1BB containing CARs would suggest so [18, 20, 21]. However, the picture is undoubtedly more complex with at least two reports suggesting that 4-1BB or CD28-mediated tonic signalling may confer improved ex vivo expansion and enhanced in vivo efficacy and persistence [62, 136]. With regard to 4-1BB, several other reports suggest the contrary [19, 63, 73] and it appears that at least a portion of the blame can be attributed to the viral vector and promoter. It may also be the case that 4-1BB tonic signalling is occurring at different time points and/or spatial compartments. Indeed, other models have suggested that early acute 4-1BB signalling can have a deleterious impact on T-cell function and survival.
In addition, whilst scFv domain swapping is highly likely to be a common initial event in almost all cases of ligand-independent tonic signalling, it remains to be seen whether this may be triggering both phenotypes of 4-1BB-mediated tonic signalling. Indeed, numerous experiments utilising identical anti-CD19 (FMC63) scFvs with either a CD28 or 4-1BB costimulatory domain have not demonstrated ligand-independent tonic signalling [18, 63].
Based upon the available literature, we have highlighted four overlapping models of ligand-independent CAR tonic signalling. Model (i) is characterised by continuous proliferation, terminal effector differentiation and cell senescence and appears to rely upon high CAR surface expression (see Figures 3(a) and 3(e)). Changes to the promoter or the spacer have reversed this phenotype. Model (ii) is characterised by CAR T-cell exhaustion and may be reversed by the substitution of CD28 with a 4-1BB ICD (see Figure 3(b)). The role of the CAR spacer and TMD is less clear here. Model (iii) appears to be mediated by 4-1BB costimulation and is characterised by enhanced (but not continuous) proliferation during ex vivo expansion and greater persistence and efficacy in vivo, without evidence of AICD (see Figure 3(c)). Model (iv) also appears 4-1BB mediated but is characterised by poor expansion ex vivo, the upregulation of death receptors and their ligands and enhanced AICD (see Figure 3(c)). This phenotype appears to be due primarily to a 4-1BB-mediated positive feedback loop occurring at the level of the γ-retroviral LTR promoter. Interestingly, however, reversal was also seen following the introduction of a CD28 ICD upstream of 4-1BB suggesting that localisation of the latter with respect to the cell surface membrane and associated signalling molecules may also be playing a role.
Clearly, considerable work remains to be untaken to uncover the precise function of second and third generation CARs at the molecular level, with emphasis placed upon determining how factors such as the costimulatory ICD’s relative distance to the cell membrane and their accessibility to downstream adapter and signalling transduction proteins may impact function. The precise contribution of other structural components (such as the spacer and TMD) to tonic signalling also remains to be elucidated, as does the relative importance of scFv instability, promoter strength and dysfunctional CAR recycling. Furthermore, the use of multiple CARs in parallel for logic gated signalling or the combined use of chimeric costimulatory receptors (CCRs) may be anticipated to increase the relative risk of encountering ligand-independent tonic signalling. A further question is whether CARs targeting membrane proximal epitopes confer greater risk of tonic signalling due to the requirement for longer spacers, increasing the promiscuity of scFv domain swapping? Protein engineering using computational modelling may prove highly effective at stabilising the scFv VH:VL interface. Alternative strategies employing endogenous ligands, using camelid-derived nanobodies or fibronectin-based targeting moieties may also prevent ligand-independent oligomerisation.
If low level ligand-dependent tonic signalling can be beneficial, it may be beneficial in a CAR context to recapitulate the model whereby endogenous naïve peripheral T-cell TCR / DC self-peptide MHC interactions are able to elicit more efficacious killing of foreign peptide-containing target cells. An important question is how can this be optimised to minimise toxicity, CAR T-cell exhaustion and/or AICD? If the latter is unavoidable when targeting solid tumours due to a lack of available TSAs, how might this process be minimised or reversed? Certainly, the use of a constitutively expressed 4-1BBL may provide a degree of tonic signalling for activated CAR T-cells in a juxtacrine manner. Likewise, the use of blocking antibodies targeting inhibitory molecules, dominant negative inhibitory receptors [184] or switch CARs [185] to reverse inhibitory signalling may temper tonic signalling induced exhaustion. Metabolic dysfunction may be targeted with small molecule inhibitors of signal transduction proteins or by engineering the CAR T-cell itself to function with a TCM or TSCM metabolic phenotype. Recapitulating endogenous TCR expression and recycling by targeting CAR expression to the TRAC locus appears to be a highly promising and efficacious method of CAR transduction. It also facilitates the clean engineering of allogenic CARs lacking functional TCR. These, however, may perform less well in the absence of endogenous self-peptide MHC/TCR interactions conferring a lower activation threshold, particularly when targeting epitopes with low cell surface density. However, by utilising scFvs the majority of CARs currently in development have binding affinities several orders of magnitude greater than TCR/MHC binding [186], so although more CARs are required to bind to mediate cytotoxicity, basal tonic signalling may not be so crucial for CAR versus TCR-mediated killing and may worsen on-target, off-tumour toxicity. Other interactions with APCs (e.g. via CD40L/CD40, ICAM-1/LPA-1) will also likely prove useful for CAR efficacy and persistence in vivo and could be recapitulated using T-APCs, engineered APCs or irradiated EATCs. All these hypotheses remain to be supported by empirical evidence.
The use of engineered cellular therapies to target cancer is rapidly evolving yet clearly, we remain in a nascent period of development. With the emergence of powerful gene editing techniques that can help uncover the hidden mechanisms of CAR function and aid precise engineering, this process is likely to accelerate considerably.
Financial support
A.A. Ajina is supported by the Medical Research Council (Grant Reference Number: MR/R001936/1). J. Maher is supported by: the Wellcome Trust (Grant Reference Number: 104802/Z/14/Z); the Medical Research Council (Grant Reference Number: MR/M024733/1); Cancer Research UK (Grant Reference Number: C11499/A21623); the British Lung Foundation (Grant Reference Number: MPG16-6); and Pancreatic Cancer UK. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Abbreviations
- A2AR
adenosine A2A receptor, 31
- ADCC
antibody-dependent cell-mediated cytotoxicity, 17
- AICD
activation-induced cell death, 14
- ALL
acute lymphoblastic leukaemia, 4
- ARDS
acute respiratory distress syndrome, 19
- Bcl-xL
B-cell lymphoma-extra large, 10
- BCMA
B-cell maturation antigen, 23
- BCR
B-cell receptor, 5
- BGH polyA
bovine growth hormone polyadenylation, 18
- Brdu
bromodeoxyuridine, 8
- BRS
background reduction signal, 18
- BTLA
B- and T-lymphocyte attenuator, 11
- CAIX
carbonic anhydrase 9, 17
- CAR
chimeric antigen receptor, 2, 4
- CCL
C-C motif chemokine ligand, 18
- CCR
-
C-C motif chemokine receptor, 18
chimeric costimulatory receptor, 34
- CDC
complement-dependent cytotoxicity, 17
- CEA
carcinoembryonic antigen, 19
- c-Met
c-mesenchymal-epithelial transition, 9
- CMV
cytomegalovirus, 10
- CRS
cytokine release syndrome, 19
- CTLA-4
cytotoxic T-lymphocyte-associated protein 4, 8
- CXCL
C-X-C motif chemokine ligand, 18
- DAG
diacylglycerol, 9
- DAP10
DNAX-activating protein 10, 6
- DC
dendritic cell, 4
- EATC
engineered autologous tumour cells, 33
- EF-1α
elongation factor 1 alpha, 10, 30
- EGF
epidermal growth factor, 23
- ER
endoplasmic reticulum, 24
- FAO
fatty acid oxidation, 31
- FKBP
FK506 Binding Protein, 29
- FOXO
forkhead box O, 31
- FRB*
FKBP-rapamycin binding domain, 29
- FRC
fibroblastic reticular cells, 7
- GAI
gibberellic-acid insensitive, 29
- GID1
gibberellin insensitive dwarf 1, 29
- GM-CSF
granulocyte-macrophage colony-stimulating factor, 10
- GSK3β
glycogen synthase kinase 3 beta, 30
- HLA
human leukocyte antigen, 5
- HLH
helix-loop-helix, 11
- ICAM-1
intercellular adhesion molecule 1, 7
- ICD
intracellular costimulatory domain, 4
- ICOS
inducible T-cell costimulator, 6
- IFNγ
interferon gamma, 10, 26
- IgG
immunoglobulin G, 10
- IKK
IκB kinase, 14
- IL-13
interleukin 13, 23
- IL13Rα2
interleukin 13 receptor subunit alpha-2, 23
- IRES
internal ribosome entry site, 14
- ITAM
immunoreceptor tyrosine-based activation motif, 7
- LAG-3
lymphocyte-activation gene 3, 11
- LAT
linker for activation of T-cells, 9
- LFA-1
lymphocyte function-associated antigen 1, 7
- LTR
long terminal repeats, 13
- MAS
macrophage activation syndrome, 16
- MHC
major histocompatibility complex, 4
- MICA
MHC class I chain-related protein A, 23
- MICB
MHC class I chain-related protein B, 23
- mTOR
mammalian target of rapamycin, 9
- NFAT
nuclear factor of the activated T-cell, 17, 48
- NK
natural killer, 16, 24
- NKG2D
natural killer group 2D, 23
- Nr4a1
nuclear receptor subfamily 4 group A member 1, 8
- NSG
NOD SCID γcnull, 10
- Nur77
nuclear hormone receptor 77, 8
- OXPHOS
oxidative phosphorylation, 31
- PD-1
programmed death 1, 8
- PGC-1α
Peroxisome proliferator-activated receptor gamma coactivator 1-alpha, 31
- PGK
phospho-glycerate kinase, 10
- PIP2
phosphatidylinositol 4,5-bisphosphate, 7
- PLCγ1
phospholipase Cγ1, 18
- PSCA
prostate stem cell antigen, 16
- PTHP1
protein tyrosine phosphatase 1, 12
- Rag2
recombination activating gene 2, 8
- Rasgrp1
RAS guanyl-releasing protein 1, 9
- ROR1
receptor tyrosine kinase-like orphan receptor 1, 15
- rtTA
reverse Tet transactivator, 28
- S/MAR
scaffold/matrix attachment region, 28
- SFK
SRC family kinase, 7
- SHP-1
SRC homology region 2 domain-containing phosphatase 1, 12
- shRNA
short hairpin RNA, 32
- SIN
Self-inactivating, 27
- TAA
tumour-associated antigen, 5
- T-ALL
T-cell acute lymphoblastic leukemia, 20
- T-APC
T-cell antigen presenting cell, 33
- TBX21
T-box transcription factor 21, 10
- Tcf7
transcription factor 7, 31
- TCM
central memory T-cells, 9
- TCR
T-cell receptor, 4, 30
- TGFα
transforming growth factor alpha, 23
- TIM-3
T-cell immunoglobulin and mucin-domain containing-3, 11
- TMD
transmembrane domain, 4, 34
- TME
tumour microenvironment, 4
- TNFα
tumour necrosis factor alpha, 10
- TRAC
T-cell receptor alpha constant, 11
- Treg
regulatory T-cell, 8, 48
- TSA
tumour-specific antigen, 5
- TSCM
stem cell-like memory T-cells, 30, 32
- ULBP
UL16 binding proteins, 23
- ZAP70
zeta-chain-associated protein kinase 70, 7
Footnotes
Conflict of interest disclosure:
JM is chief scientific officer of Leucid Bio, a spinout company focussed on CAR T-cell and gamma delta T-cell immunotherapies for malignant disease. AA does not have any conflicts of interest to declare.
References
- 1.Maher J. Clinical immunotherapy of B-cell malignancy using CD19-targeted CAR T-cells. Curr Gene Ther. 2014;14:35–43. doi: 10.2174/1566523213666131223130554. [DOI] [PubMed] [Google Scholar]
- 2.Davila ML, Brentjens RJ. CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2016;14:802–808. [PMC free article] [PubMed] [Google Scholar]
- 3.Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–9. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United States Food and Drug Administration Approval Letter 30th August 2017 - Kymriah. 2017.
- 5.US regulator signs off on new $475,000 cancer therapy. 2017.
- 6.United States Food and Drug Administration Approval Letter 18th October 2017 - Yescarta. 2017.
- 7.Scarfò I, Maus MV. Current approaches to increase CAR T cell potency in solid tumors: targeting the tumor microenvironment. J Immunother Cancer. 2017;5:28. doi: 10.1186/s40425-017-0230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newick K, Moon E, Albelda SM. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther Oncolytics. 2016;3:16006. doi: 10.1038/mto.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilham DE, Maher J. 'Atypical' CAR T cells: NKG2D and Erb-B as examples of natural receptor/ligands to target recalcitrant solid tumors. Immunotherapy. 2017;9:723–733. doi: 10.2217/imt-2017-0045. [DOI] [PubMed] [Google Scholar]
- 10.Zhao Z, Condomines M, van der Stegen SJC, Perna F, Kloss CC, Gunset G, et al. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell. 2015;28:415–428. doi: 10.1016/j.ccell.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Stegen SJ, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov. 2015;14:499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garbi N, Hammerling GJ, Probst HC, van den Broek M. Tonic T cell signalling and T cell tolerance as opposite effects of self-recognition on dendritic cells. Curr Opin Immunol. 2010;22:601–8. doi: 10.1016/j.coi.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Hochweller K, Wabnitz GH, Samstag Y, Suffner J, Hammerling GJ, Garbi N. Dendritic cells control T cell tonic signaling required for responsiveness to foreign antigen. Proc Natl Acad Sci U S A. 2010;107:5931–6. doi: 10.1073/pnas.0911877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers DR, Zikherman J, Roose JP. Tonic Signals: Why Do Lymphocytes Bother? Trends Immunol. 2017 doi: 10.1016/j.it.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–84. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Rowland SL, DePersis CL, Torres RM, Pelanda R. Ras activation of Erk restores impaired tonic BCR signaling and rescues immature B cell differentiation. J Exp Med. 2010;207:607–21. doi: 10.1084/jem.20091673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21:581–90. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamonkin M, da Silva DG, Mukherjee M, Sharma S, Srinivasan M, Orange JS, et al. Tonic 4-1BB signaling from chimeric antigen receptors (CARs) impairs expansion of T cells due to Fas-mediated apoptosis. J Immunol. 2016;196:143–147. [Google Scholar]
- 20.Watanabe N, Bajgain P, Sukumaran S, Ansari S, Heslop HE, Rooney CM, et al. Fine-tuning the CAR spacer improves T-cell potency. Oncoimmunology. 2016;5:e1253656. doi: 10.1080/2162402X.2016.1253656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frigault MJ, Lee J, Basil MC, Carpenito C, Motohashi S, Scholler J, et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol Res. 2015;3:356–67. doi: 10.1158/2326-6066.CIR-14-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whilding LM, Maher J. CAR T-cell immunotherapy: The path from the by-road to the freeway? Mol Oncol. 2015;9:1994–2018. doi: 10.1016/j.molonc.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Priceman SJ, Forman SJ, Brown CE. Smart CARs engineered for cancer immunotherapy. Curr Opin Oncol. 2015;27:466–74. doi: 10.1097/CCO.0000000000000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375:819–29. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Themeli M, Riviere I, Sadelain M. New cell sources for T cell engineering and adoptive immunotherapy. Cell Stem Cell. 2015;16:357–66. doi: 10.1016/j.stem.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcus A, Waks T, Eshhar Z. Redirected tumor-specific allogeneic T cells for universal treatment of cancer. Blood. 2011;118:975–83. doi: 10.1182/blood-2011-02-334284. [DOI] [PubMed] [Google Scholar]
- 27.Sadelain M, Brentjens R, Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–98. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–4. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brocker T, Karjalainen K. Signals through T cell receptor-zeta chain alone are insufficient to prime resting T lymphocytes. J Exp Med. 1995;181:1653–9. doi: 10.1084/jem.181.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–5. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 31.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–84. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 32.Shen CJ, Yang YX, Han EQ, Cao N, Wang YF, Wang Y, et al. Chimeric antigen receptor containing ICOS signaling domain mediates specific and efficient antitumor effect of T cells against EGFRvIII expressing glioma. J Hematol Oncol. 2013;6:33. doi: 10.1186/1756-8722-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hombach AA, Heiders J, Foppe M, Chmielewski M, Abken H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4(+) T cells. Oncoimmunology. 2012;1:458–466. doi: 10.4161/onci.19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song DG, Powell DJ. Pro-survival signaling via CD27 costimulation drives effective CAR T-cell therapy. Oncoimmunology. 2012;1:547–549. doi: 10.4161/onci.19458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang YH, Connolly J, Shimasaki N, Mimura K, Kono K, Campana D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013;73:1777–86. doi: 10.1158/0008-5472.CAN-12-3558. [DOI] [PubMed] [Google Scholar]
- 36.Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A. 2009;106:3360–5. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hombach AA, Abken H. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. Int J Cancer. 2011;129:2935–44. doi: 10.1002/ijc.25960. [DOI] [PubMed] [Google Scholar]
- 38.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–6. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeku OO, Brentjens RJ. Armored CAR T-cells: utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochem Soc Trans. 2016;44:412–8. doi: 10.1042/BST20150291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15:1145–54. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 41.Hu B, Ren J, Luo Y, Keith B, Young RM, Scholler J, et al. CAR T Cells Secreting IL18 Augment Antitumor Immunity and Increase T Cell Proliferation and Costimulation. bioRxiv. 2017 [Google Scholar]
- 42.Hurton LV, Singh H, Najjar AM, Switzer KC, Mi T, Maiti S, et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci U S A. 2016;113:E7788–E7797. doi: 10.1073/pnas.1610544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willemsen RA, Debets R, Chames P, Bolhuis RL. Genetic engineering of T cell specificity for immunotherapy of cancer. Hum Immunol. 2003;64:56–68. doi: 10.1016/s0198-8859(02)00730-9. [DOI] [PubMed] [Google Scholar]
- 44.Hudecek M, Sommermeyer D, Kosasih PL, Silva-Benedict A, Liu L, Rader C, et al. The nonsignaling extracellular spacer domain of chimeric antigen receptors is decisive for in vivo antitumor activity. Cancer Immunol Res. 2015;3:125–35. doi: 10.1158/2326-6066.CIR-14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkie S, Picco G, Foster J, Davies DM, Julien S, Cooper L, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180:4901–9. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- 46.Hombach AA, Schildgen V, Heuser C, Finnern R, Gilham DE, Abken H. T cell activation by antibody-like immunoreceptors: the position of the binding epitope within the target molecule determines the efficiency of activation of redirected T cells. J Immunol. 2007;178:4650–7. doi: 10.4049/jimmunol.178.7.4650. [DOI] [PubMed] [Google Scholar]
- 47.Jabri B, Abadie V. IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction. Nat Rev Immunol. 2015;15:771–83. doi: 10.1038/nri3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiehagen KR, Corbo E, Schmidt M, Shin H, Wherry EJ, Maltzman JS. Loss of tonic T-cell receptor signals alters the generation but not the persistence of CD8+ memory T cells. Blood. 2010;116:5560–70. doi: 10.1182/blood-2010-06-292458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766–77. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–34. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 51.Tan YX, Zikherman J, Weiss A. Novel tools to dissect the dynamic regulation of TCR signaling by the kinase Csk and the phosphatase CD45. Cold Spring Harb Symp Quant Biol. 2013;78:131–139. doi: 10.1101/sqb.2013.78.020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schoenborn JR, Tan YX, Zhang C, Shokat KM, Weiss A. Feedback circuits monitor and adjust basal Lck-dependent events in T cell receptor signaling. Sci Signal. 2011;4:ra59. doi: 10.1126/scisignal.2001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stefanova I, Hemmer B, Vergelli M, Martin R, Biddison WE, Germain RN. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–54. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 54.Van Seventer GA, Bonvini E, Yamada H, Conti A, Stringfellow S, June CH, et al. Costimulation of T cell receptor/CD3-mediated activation of resting human CD4+ T cells by leukocyte function-associated antigen-1 ligand intercellular cell adhesion molecule-1 involves prolonged inositol phospholipid hydrolysis and sustained increase of intracellular Ca2+ levels. J Immunol. 1992;149:3872–80. [PubMed] [Google Scholar]
- 55.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 56.Randriamampita C, Boulla G, Revy P, Lemaitre F, Trautmann A. T cell adhesion lowers the threshold for antigen detection. Eur J Immunol. 2003;33:1215–23. doi: 10.1002/eji.200323844. [DOI] [PubMed] [Google Scholar]
- 57.Fischer UB, Jacovetty EL, Medeiros RB, Goudy BD, Zell T, Swanson JB, et al. MHC class II deprivation impairs CD4 T cell motility and responsiveness to antigen-bearing dendritic cells in vivo. Proc Natl Acad Sci U S A. 2007;104:7181–6. doi: 10.1073/pnas.0608299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krogsgaard M, Juang J, Davis MM. A role for "self" in T-cell activation. Semin Immunol. 2007;19:236–44. doi: 10.1016/j.smim.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Polic B, Kunkel D, Scheffold A, Rajewsky K. How alpha beta T cells deal with induced TCR alpha ablation. Proc Natl Acad Sci U S A. 2001;98:8744–9. doi: 10.1073/pnas.141218898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 1996;5:217–28. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 61.Markegard E, Trager E, Yang CW, Zhang W, Weiss A, Roose JP. Basal LAT-diacylglycerol-RasGRP1 signals in T cells maintain TCRalpha gene expression. PLoS One. 2011;6:e25540. doi: 10.1371/journal.pone.0025540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomes da Silva D, Mukherjee M, Srinivasan M, Dakhova O, Liu H, Grilley B, et al. Direct Comparison of In Vivo Fate of Second and Third-Generation CD19-Specific Chimeric Antigen Receptor (CAR)-T Cells in Patients with B-Cell Lymphoma: Reversal of Toxicity from Tonic Signaling. Blood. 2016;128:1851. [Google Scholar]
- 64.Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hale M, Lee B, Honaker Y, Leung WH, Grier AE, Jacobs HM, et al. Homology-Directed Recombination for Enhanced Engineering of Chimeric Antigen Receptor T Cells. Mol Ther Methods Clin Dev. 2017;4:192–203. doi: 10.1016/j.omtm.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kawalekar OU, O'Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD, Jr, et al. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44:380–90. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 67.Song DG, Ye Q, Carpenito C, Poussin M, Wang LP, Ji C, et al. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB) Cancer Res. 2011;71:4617–27. doi: 10.1158/0008-5472.CAN-11-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Klein Geltink RI, O'Sullivan D, Corrado M, Bremser A, Buck MD, Buescher JM, et al. Mitochondrial Priming by CD28. Cell. 2017 doi: 10.1016/j.cell.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3:682–5. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 73.Gomes-Silva D, Mukherjee M, Srinivasan M, Krenciute G, Dakhova O, Zheng Y, et al. Tonic 4-1BB Costimulation in Chimeric Antigen Receptors Impedes T Cell Survival and Is Vector-Dependent. Cell Reports. 2017;21:17–26. doi: 10.1016/j.celrep.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guedan S, Posey AD, Jr, Shaw C, Wing A, Da T, Patel PR, et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight. 2018;3 doi: 10.1172/jci.insight.96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chattopadhyay K, Lazar-Molnar E, Yan Q, Rubinstein R, Zhan C, Vigdorovich V, et al. Sequence, structure, function, immunity: structural genomics of costimulation. Immunol Rev. 2009;229:356–86. doi: 10.1111/j.1600-065X.2009.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 77.Kochenderfer JN, Feldman SA, Zhao Y, Xu H, Black MA, Morgan RA, et al. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Humphreys IR, Lee SW, Jones M, Loewendorf A, Gostick E, Price DA, et al. Biphasic role of 4-1BB in the regulation of mouse cytomegalovirus-specific CD8(+) T cells. Eur J Immunol. 2010;40:2762–8. doi: 10.1002/eji.200940256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang B, Zhang Y, Niu L, Vella AT, Mittler RS. Dendritic cells and Stat3 are essential for CD137-induced CD8 T cell activation-induced cell death. J Immunol. 2010;184:4770–8. doi: 10.4049/jimmunol.0902713. [DOI] [PubMed] [Google Scholar]
- 80.McPherson AJ, Snell LM, Mak TW, Watts TH. Opposing roles for TRAF1 in the alternative versus classical NF-kappaB pathway in T cells. J Biol Chem. 2012;287:23010–9. doi: 10.1074/jbc.M112.350538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hauer J, Puschner S, Ramakrishnan P, Simon U, Bongers M, Federle C, et al. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-kappaB pathway by TRAF-binding TNFRs. Proc Natl Acad Sci U S A. 2005;102:2874–9. doi: 10.1073/pnas.0500187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH. ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J Immunol. 2008;180:8093–101. doi: 10.4049/jimmunol.180.12.8093. [DOI] [PubMed] [Google Scholar]
- 83.Wang C, McPherson AJ, Jones RB, Kawamura KS, Lin GH, Lang PA, et al. Loss of the signaling adaptor TRAF1 causes CD8+ T cell dysregulation during human and murine chronic infection. J Exp Med. 2012;209:77–91. doi: 10.1084/jem.20110675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jonnalagadda M, Mardiros A, Urak R, Wang X, Hoffman LJ, Bernanke A, et al. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol Ther. 2015;23:757–68. doi: 10.1038/mt.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hombach A, Hombach AA, Abken H. Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc 'spacer' domain in the extracellular moiety of chimeric antigen receptors avoids 'off-target' activation and unintended initiation of an innate immune response. Gene Ther. 2010;17:1206–13. doi: 10.1038/gt.2010.91. [DOI] [PubMed] [Google Scholar]
- 86.Lamers CH, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 87.Maus MV, Haas AR, Beatty GL, Albelda SM, Levine BL, Liu X, et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol Res. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L, Morgan RA, Beane JD, Zheng Z, Dudley ME, Kassim SH, et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res. 2015;21:2278–88. doi: 10.1158/1078-0432.CCR-14-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Uchibori R, Ninomiya S, Tsukahara T, Ido H, Teruya T, Omine K, et al. Development of Inducible Switch Promoters That Drive Exogenous Gene Expression Upon the Recognition of CD19 by Chimeric Antigen Receptors. Molecular Therapy. 2014;22:S165–S166. [Google Scholar]
- 90.Gilham D, Abken H. Cellular Therapy of Cancer. WORLD SCIENTIFIC; 2014. Gene Transfer into T Cells; pp. 19–48. [Google Scholar]
- 91.Eksteen B, Mora JR, Haughton EL, Henderson NC, Lee-Turner L, Villablanca EJ, et al. Gut homing receptors on CD8 T cells are retinoic acid dependent and not maintained by liver dendritic or stellate cells. Gastroenterology. 2009;137:320–9. doi: 10.1053/j.gastro.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang HH, Song K, Rabin RL, Hill BJ, Perfetto SP, Roederer M, et al. CCR2 identifies a stable population of human effector memory CD4+ T cells equipped for rapid recall response. J Immunol. 2010;185:6646–63. doi: 10.4049/jimmunol.0904156. [DOI] [PubMed] [Google Scholar]
- 93.Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nat Rev Immunol. 2013;13:257–69. doi: 10.1038/nri3403. [DOI] [PubMed] [Google Scholar]
- 94.Beatty GL, O'Hara M. Chimeric antigen receptor-modified T cells for the treatment of solid tumors: Defining the challenges and next steps. Pharmacol Ther. 2016;166:30–9. doi: 10.1016/j.pharmthera.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Watanabe K, Terakura S, Martens AC, van Meerten T, Uchiyama S, Imai M, et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. J Immunol. 2015;194:911–20. doi: 10.4049/jimmunol.1402346. [DOI] [PubMed] [Google Scholar]
- 96.Caruso HG, Hurton LV, Najjar A, Rushworth D, Ang S, Olivares S, et al. Tuning Sensitivity of CAR to EGFR Density Limits Recognition of Normal Tissue While Maintaining Potent Antitumor Activity. Cancer Res. 2015;75:3505–18. doi: 10.1158/0008-5472.CAN-15-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–2. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 99.Hombach A, Koch D, Sircar R, Heuser C, Diehl V, Kruis W, et al. A chimeric receptor that selectively targets membrane-bound carcinoembryonic antigen (mCEA) in the presence of soluble CEA. Gene Ther. 1999;6:300–4. doi: 10.1038/sj.gt.3300813. [DOI] [PubMed] [Google Scholar]
- 100.Mamonkin M, Rouce RH, Tashiro H, Brenner MK. A T-cell-directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood. 2015;126:983–92. doi: 10.1182/blood-2015-02-629527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gomes-Silva D, Srinivasan M, Sharma S, Lee CM, Wagner DL, Davis TH, et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood. 2017;130:285–296. doi: 10.1182/blood-2017-01-761320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tabbekh M, Mokrani-Hammani M, Bismuth G, Mami-Chouaib F. T-cell modulatory properties of CD5 and its role in antitumor immune responses. Oncoimmunology. 2013;2:e22841. doi: 10.4161/onci.22841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bamberger M, Santos AM, Goncalves CM, Oliveira MI, James JR, Moreira A, et al. A new pathway of CD5 glycoprotein-mediated T cell inhibition dependent on inhibitory phosphorylation of Fyn kinase. J Biol Chem. 2011;286:30324–36. doi: 10.1074/jbc.M111.230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maher J, Wilkie S, Davies DM, Arif S, Picco G, Julien S, et al. Targeting of Tumor-Associated Glycoforms of MUC1 with CAR T Cells. Immunity. 2016;45:945–946. doi: 10.1016/j.immuni.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 105.Comrie WA, Li S, Boyle S, Burkhardt JK. The dendritic cell cytoskeleton promotes T cell adhesion and activation by constraining ICAM-1 mobility. J Cell Biol. 2015;208:457–73. doi: 10.1083/jcb.201406120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farahani E, Patra HK, Jangamreddy JR, Rashedi I, Kawalec M, Rao Pariti RK, et al. Cell adhesion molecules and their relation to (cancer) cell stemness. Carcinogenesis. 2014;35:747–59. doi: 10.1093/carcin/bgu045. [DOI] [PubMed] [Google Scholar]
- 107.Morello A, Zeltsman M, Bograd A, Jones D, Adusumilli P. MA04.11 Mechanistic Insights into CAR T-Cell Efficacy in the Treatment of Heterogenous Antigen Expressing Lung Adenocarcinoma. Journal of Thoracic Oncology. 2017;12:S363–S364. [Google Scholar]
- 108.Gil D, Schrum AG. Strategies to stabilize compact folding and minimize aggregation of antibody-based fragments. Adv Biosci Biotechnol. 2013;4:73–84. doi: 10.4236/abb.2013.44A011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wörn A, Plückthun A. Stability engineering of antibody single-chain Fv fragments. J Mol Biol. 2001;305:989–1010. doi: 10.1006/jmbi.2000.4265. [DOI] [PubMed] [Google Scholar]
- 110.Schneider D, Xiong Y, Wu D, Nlle V, Schmitz S, Haso W, et al. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. J Immunother Cancer. 2017;5:42. doi: 10.1186/s40425-017-0246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Monsellier E, Bedouelle H. Improving the stability of an antibody variable fragment by a combination of knowledge-based approaches: validation and mechanisms. J Mol Biol. 2006;362:580–93. doi: 10.1016/j.jmb.2006.07.044. [DOI] [PubMed] [Google Scholar]
- 112.Young NM, MacKenzie CR, Narang SA, Oomen RP, Baenziger JE. Thermal stabilization of a single-chain Fv antibody fragment by introduction of a disulphide bond. FEBS Lett. 1995;377:135–9. doi: 10.1016/0014-5793(95)01325-3. [DOI] [PubMed] [Google Scholar]
- 113.Miller BR, Demarest SJ, Lugovskoy A, Huang F, Wu X, Snyder WB, et al. Stability engineering of scFvs for the development of bispecific and multivalent antibodies. Protein Eng Des Sel. 2010;23:549–57. doi: 10.1093/protein/gzq028. [DOI] [PubMed] [Google Scholar]
- 114.Kügler M, Stein C, Schwenkert M, Saul D, Vockentanz L, Huber T, et al. Stabilization and humanization of a single-chain Fv antibody fragment specific for human lymphocyte antigen CD19 by designed point mutations and CDR-grafting onto a human framework. Protein Eng Des Sel. 2009;22:135–47. doi: 10.1093/protein/gzn079. [DOI] [PubMed] [Google Scholar]
- 115.Perchiacca JM, Ladiwala AR, Bhattacharya M, Tessier PM. Aggregation-resistant domain antibodies engineered with charged mutations near the edges of the complementarity-determining regions. Protein Eng Des Sel. 2012;25:591–601. doi: 10.1093/protein/gzs042. [DOI] [PubMed] [Google Scholar]
- 116.Wu SJ, Luo J, O'Neil KT, Kang J, Lacy ER, Canziani G, et al. Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng Des Sel. 2010;23:643–51. doi: 10.1093/protein/gzq037. [DOI] [PubMed] [Google Scholar]
- 117.Zhou L, Abel T, Schneider IC, Beghelli S, Labbal F, David M, et al. Designing the next generation Chimeric Antigen Receptors for Regulatory T cell therapy through in silico modeling-guided single chain Fv engineering. CAR-TCR Summit 2017; Boston, USA: 2017. [Google Scholar]
- 118.Wang T, Duan Y. Probing the stability-limiting regions of an antibody single-chain variable fragment: a molecular dynamics simulation study. Protein Eng Des Sel. 2011;24:649–57. doi: 10.1093/protein/gzr029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bridgeman J, Hombach AA, Gilham D, Eshhar Z, Abken H. Cellular Therapy of Cancer. WORLD SCIENTIFIC; 2014. T-Bodies: Antibody-Based Engineered T-Cell Receptors; pp. 83–129. [Google Scholar]
- 120.Liu X, Jiang S, Fang C, Yang S, Olalere D, Pequignot EC, et al. Affinity-Tuned ErbB2 or EGFR Chimeric Antigen Receptor T Cells Exhibit an Increased Therapeutic Index against Tumors in Mice. Cancer Res. 2015;75:3596–607. doi: 10.1158/0008-5472.CAN-15-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jamnani FR, Rahbarizadeh F, Shokrgozar MA, Mahboudi F, Ahmadvand D, Sharifzadeh Z, et al. T cells expressing VHH-directed oligoclonal chimeric HER2 antigen receptors: Towards tumor-directed oligoclonal T cell therapy. Biochimica et Biophysica Acta (BBA) - General Subjects. 2014;1840:378–386. doi: 10.1016/j.bbagen.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 122.Wesolowski J, Alzogaray V, Reyelt J, Unger M, Juarez K, Urrutia M, et al. Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med Microbiol Immunol. 2009;198:157–74. doi: 10.1007/s00430-009-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barnett BE, Hermanson DL, Smith JB, Wang X, Tan Y, Martin CE, et al. piggyBac™-Produced CAR-T Cells Exhibit Stem-Cell Memory Phenotype. Blood. 2016;128:2167–2167. [Google Scholar]
- 124.Bezverbnaya K, Mathews A, Sidhu J, Helsen CW, Bramson JL. Tumor-targeting domains for chimeric antigen receptor T cells. Immunotherapy. 2017;9:33–46. doi: 10.2217/imt-2016-0103. [DOI] [PubMed] [Google Scholar]
- 125.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med. 2016;375:2561–9. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, et al. Bioactivity and Safety of IL13Ralpha2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin Cancer Res. 2015;21:4062–72. doi: 10.1158/1078-0432.CCR-15-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Papa S, van Schalkwyk M, Maher J. Clinical Evaluation of ErbB-Targeted CAR T-Cells, Following Intracavity Delivery in Patients with ErbB-Expressing Solid Tumors. Methods Mol Biol. 2015;1317:365–82. doi: 10.1007/978-1-4939-2727-2_21. [DOI] [PubMed] [Google Scholar]
- 128.Whilding LM, Maher J. ErbB-targeted CAR T-cell immunotherapy of cancer. Immunotherapy. 2015;7:229–41. doi: 10.2217/imt.14.120. [DOI] [PubMed] [Google Scholar]
- 129.Zhang T, Barber A, Sentman CL. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res. 2006;66:5927–33. doi: 10.1158/0008-5472.CAN-06-0130. [DOI] [PubMed] [Google Scholar]
- 130.Lehner M, Gotz G, Proff J, Schaft N, Dorrie J, Full F, et al. Redirecting T cells to Ewing's sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PLoS One. 2012;7:e31210. doi: 10.1371/journal.pone.0031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sentman CL, Meehan KR. NKG2D CARs as cell therapy for cancer. Cancer J. 2014;20:156–9. doi: 10.1097/PPO.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shaffer DR, Savoldo B, Yi Z, Chow KK, Kakarla S, Spencer DM, et al. T cells redirected against CD70 for the immunotherapy of CD70-positive malignancies. Blood. 2011;117:4304–14. doi: 10.1182/blood-2010-04-278218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guest RD, Hawkins RE, Kirillova N, Cheadle EJ, Arnold J, O'Neill A, et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J Immunother. 2005;28:203–11. doi: 10.1097/01.cji.0000161397.96582.59. [DOI] [PubMed] [Google Scholar]
- 134.Nolan KF, Yun CO, Akamatsu Y, Murphy JC, Leung SO, Beecham EJ, et al. Bypassing immunization: optimized design of "designer T cells" against carcinoembryonic antigen (CEA)-expressing tumors, and lack of suppression by soluble CEA. Clin Cancer Res. 1999;5:3928–41. [PubMed] [Google Scholar]
- 135.Schonfeld K, Sahm C, Zhang C, Naundorf S, Brendel C, Odendahl M, et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol Ther. 2015;23:330–8. doi: 10.1038/mt.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Qin L, Lai Y, Zhao R, Wei X, Weng J, Lai P, et al. Incorporation of a hinge domain improves the expansion of chimeric antigen receptor T cells. J Hematol Oncol. 2017;10:68. doi: 10.1186/s13045-017-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bridgeman JS, Hawkins RE, Hombach AA, Abken H, Gilham DE. Building better chimeric antigen receptors for adoptive T cell therapy. Curr Gene Ther. 2010;10:77–90. doi: 10.2174/156652310791111001. [DOI] [PubMed] [Google Scholar]
- 138.Oldham RAA, Medin JA. Practical considerations for chimeric antigen receptor design and delivery. Expert Opin Biol Ther. 2017;17:961–978. doi: 10.1080/14712598.2017.1339687. [DOI] [PubMed] [Google Scholar]
- 139.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–26. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kofler DM, Chmielewski M, Rappl G, Hombach A, Riet T, Schmidt A, et al. CD28 costimulation Impairs the efficacy of a redirected t-cell antitumor attack in the presence of regulatory t cells which can be overcome by preventing Lck activation. Mol Ther. 2011;19:760–7. doi: 10.1038/mt.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cheadle EJ, Rothwell DG, Bridgeman JS, Sheard VE, Hawkins RE, Gilham DE. Ligation of the CD2 co-stimulatory receptor enhances IL-2 production from first-generation chimeric antigen receptor T cells. Gene Ther. 2012;19:1114–20. doi: 10.1038/gt.2011.192. [DOI] [PubMed] [Google Scholar]
- 142.Nguyen P, Moisini I, Geiger TL. Identification of a murine CD28 dileucine motif that suppresses single-chain chimeric T-cell receptor expression and function. Blood. 2003;102:4320–5. doi: 10.1182/blood-2003-04-1255. [DOI] [PubMed] [Google Scholar]
- 143.Eun SY, Lee SW, Xu Y, Croft M. 4-1BB ligand signaling to T cells limits T cell activation. J Immunol. 2015;194:134–41. doi: 10.4049/jimmunol.1401383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sarkis C, Philippe S, Mallet J, Serguera C. Non-integrating lentiviral vectors. Curr Gene Ther. 2008;8:430–7. doi: 10.2174/156652308786848012. [DOI] [PubMed] [Google Scholar]
- 145.Morgan RA, Boyerinas B. Genetic Modification of T Cells. Biomedicines. 2016;4 doi: 10.3390/biomedicines4020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shaw A, Cornetta K. Design and Potential of Non-Integrating Lentiviral Vectors. Biomedicines. 2014;2:14–35. doi: 10.3390/biomedicines2010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang X, Riviere I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics. 2016;3:16015. doi: 10.1038/mto.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, et al. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–20. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao Y, Moon E, Carpenito C, Paulos CM, Liu X, Brennan AL, et al. Multiple injections of electroporated autologous T cells expressing a chimeric antigen receptor mediate regression of human disseminated tumor. Cancer Res. 2010;70:9053–61. doi: 10.1158/0008-5472.CAN-10-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Singh H, Manuri PR, Olivares S, Dara N, Dawson MJ, Huls H, et al. Redirecting specificity of T-cell populations for CD19 using the Sleeping Beauty system. Cancer Res. 2008;68:2961–71. doi: 10.1158/0008-5472.CAN-07-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jin C, Fotaki G, Ramachandran M, Nilsson B, Essand M, Yu D. Safe engineering of CAR T cells for adoptive cell therapy of cancer using long-term episomal gene transfer. EMBO Mol Med. 2016;8:702–11. doi: 10.15252/emmm.201505869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sakemura R, Terakura S, Watanabe K, Julamanee J, Takagi E, Miyao K, et al. A Tet-On Inducible System for Controlling CD19-Chimeric Antigen Receptor Expression upon Drug Administration. Cancer Immunol Res. 2016;4:658–68. doi: 10.1158/2326-6066.CIR-16-0043. [DOI] [PubMed] [Google Scholar]
- 153.Hartmann J, Schussler-Lenz M, Bondanza A, Buchholz CJ. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol Med. 2017;9:1183–1197. doi: 10.15252/emmm.201607485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cartellieri M, Feldmann A, Koristka S, Arndt C, Loff S, Ehninger A, et al. Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J. 2016;6:e458. doi: 10.1038/bcj.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wu CY, Roybal KT, Puchner EM, Onuffer J, Lim WA. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350:aab4077. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Morsut L, Roybal KT, Xiong X, Gordley RM, Coyle SM, Thomson M, et al. Engineering Customized Cell Sensing and Response Behaviors Using Synthetic Notch Receptors. Cell. 2016;164:780–91. doi: 10.1016/j.cell.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Roybal KT, Rupp LJ, Morsut L, Walker WJ, McNally KA, Park JS, et al. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell. 2016;164:770–9. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roybal KT, Williams JZ, Morsut L, Rupp LJ, Kolinko I, Choe JH, et al. Engineering T Cells with Customized Therapeutic Response Programs Using Synthetic Notch Receptors. Cell. 2016;167:419–432 e16. doi: 10.1016/j.cell.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bozkulak EC, Weinmaster G. Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol Cell Biol. 2009;29:5679–95. doi: 10.1128/MCB.00406-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Murthy A, Shao YW, Narala SR, Molyneux SD, Zuniga-Pflucker JC, Khokha R. Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity. 2012;36:105–19. doi: 10.1016/j.immuni.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 161.Thauland TJ, Butte MJ. Taking T cell priming down a Notch: signaling through Notch receptors enhances T cell sensitivity to antigen. Immunity. 2015;42:6–8. doi: 10.1016/j.immuni.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 162.Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–13. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sabatino M, Hu J, Sommariva M, Gautam S, Fellowes V, Hocker JD, et al. Generation of clinical-grade CD19-specific CAR-modified CD8+ memory stem cells for the treatment of human B-cell malignancies. Blood. 2016;128:519–28. doi: 10.1182/blood-2015-11-683847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J Clin Invest. 2013;123:4479–88. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Buck MD, O'Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hedrick SM, Hess Michelini R, Doedens AL, Goldrath AW, Stone EL. FOXO transcription factors throughout T cell biology. Nat Rev Immunol. 2012;12:649–61. doi: 10.1038/nri3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van der Waart AB, van de Weem NM, Maas F, Kramer CS, Kester MG, Falkenburg JH, et al. Inhibition of Akt signaling promotes the generation of superior tumor-reactive T cells for adoptive immunotherapy. Blood. 2014;124:3490–500. doi: 10.1182/blood-2014-05-578583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Klebanoff CA, Crompton JG, Leonardi AJ, Yamamoto TN, Chandran SS, Eil RL, et al. Inhibition of AKT signaling uncouples T cell differentiation from expansion for receptor-engineered adoptive immunotherapy. JCI Insight. 2017;2 doi: 10.1172/jci.insight.95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bowers JS, Majchrzak K, Nelson MH, Aksoy BA, Wyatt MM, Smith AS, et al. PI3Kδ inhibition supports memory T cells with enhanced antitumor fitness. bioRxiv. 2017 [Google Scholar]
- 171.Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, et al. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity. 2016;45:374–88. doi: 10.1016/j.immuni.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015;75:296–305. doi: 10.1158/0008-5472.CAN-14-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Beavis PA, Henderson MA, Giuffrida L, Mills JK, Sek K, Cross RS, et al. Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J Clin Invest. 2017;127:929–941. doi: 10.1172/JCI89455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Leone RD, Lo YC, Powell JD. A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J. 2015;13:265–72. doi: 10.1016/j.csbj.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wallner S, Gruber T, Baier G, Wolf D. Releasing the brake: targeting Cbl-b to enhance lymphocyte effector functions. Clin Dev Immunol. 2012;2012:692639. doi: 10.1155/2012/692639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Stromnes IM, Blattman JN, Tan X, Jeevanjee S, Gu H, Greenberg PD. Abrogating Cbl-b in effector CD8(+) T cells improves the efficacy of adoptive therapy of leukemia in mice. J Clin Invest. 2010;120:3722–34. doi: 10.1172/JCI41991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6:261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Terakura S, Yamamoto TN, Gardner RA, Turtle CJ, Jensen MC, Riddell SR. Generation of CD19-chimeric antigen receptor modified CD8+ T cells derived from virus-specific central memory T cells. Blood. 2012;119:72–82. doi: 10.1182/blood-2011-07-366419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ghosh A, Smith M, James SE, Davila ML, Velardi E, Argyropoulos KV, et al. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat Med. 2017;23:242–249. doi: 10.1038/nm.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yang Y, Kohler ME, Chien CD, Sauter CT, Jacoby E, Yan C, et al. TCR engagement negatively affects CD8 but not CD4 CAR T cell expansion and leukemic clearance. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aag1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Pilot Study of T-APCs Following CAR T Cell Immunotherapy for CD19+ Leukemia. 2017 Available from: https://clinicaltrials.gov/ct2/show/NCT03186118.
- 183.Ghisoli M, Barve M, Mennel R, Lenarsky C, Horvath S, Wallraven G, et al. Three-year Follow up of GMCSF/bi-shRNA(furin) DNA-transfected Autologous Tumor Immunotherapy (Vigil) in Metastatic Advanced Ewing's Sarcoma. Mol Ther. 2016;24:1478–83. doi: 10.1038/mt.2016.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest. 2016;126:3130–44. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu X, Ranganathan R, Jiang S, Fang C, Sun J, Kim S, et al. A Chimeric Switch-Receptor Targeting PD1 Augments the Efficacy of Second-Generation CAR T Cells in Advanced Solid Tumors. Cancer Res. 2016;76:1578–90. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oren R, Hod-Marco M, Haus-Cohen M, Thomas S, Blat D, Duvshani N, et al. Functional comparison of engineered T cells carrying a native TCR versus TCR-like antibody-based chimeric antigen receptors indicates affinity/avidity thresholds. J Immunol. 2014;193:5733–43. doi: 10.4049/jimmunol.1301769. [DOI] [PubMed] [Google Scholar]