Abstract
Terpenes constitute the largest class of natural products and serve as an important source for medicinal treatments. Despite constant progress in chemical synthesis, the construction of complex polycyclic sesqui- and diterpene scaffolds remains challenging. Natural cyclase enzymes, however, are able to form the whole variety of terpene structures from just a handful of linear precursors. Man-made catalysts able to mimic such natural enzymes are lacking. Here, we describe the examples of sesquiterpene cyclisations inside an enzyme-mimicking supramolecular catalyst. This strategy allowed the formation of the tricyclic sesquiterpene isolongifolene in only four steps. The mechanism of the catalysed cyclisation reaction was elucidated using 13C-labelling studies and DFT calculations.
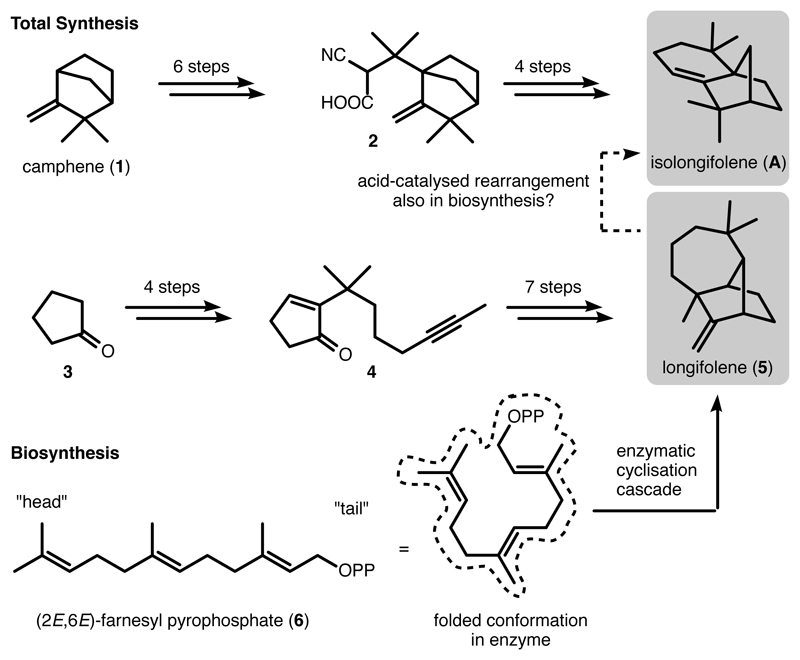
For decades, terpene natural products have been popular target compounds for total synthesis due to their biological functions and medicinal applications.1, 2 The syntheses of complex polycyclic sesqui- and diterpene skeletons routinely involve long linear sequences. Although step count is only one way to evaluate a synthetic route, a long synthetic route generally suffers from two main disadvantages: (1) A large effort in manpower is required and (2) the final yields of the natural products are usually low. For instance, in the shortest total synthesis of the sesquiterpene isolongifolene (A),3, 4 six steps were required to assemble the intermediate 2 containing all necessary carbon atoms, which was converted to isolongifolene in another four steps. Similarly, in the shortest synthesis of longifolene (5),5 an initial four-step sequence smoothly introduced the majority of the required carbon atoms. However, another seven steps were required for the completion of the longifolene skeleton. In contrast, nature utilizes a fundamentally different approach to access cyclic terpenes like longifolene and isolongifolene. The simple linear substrate farnesyl pyrophosphate 6 is cyclised by an enzyme, termed terpene cyclase, to directly produce the framework of longifolene.6 Longifolene is known to undergo facile acid-catalysed rearrangement to form isolongifolene.7, 8 A related mechanism could also be operational in natural enzymes. Isolongifolene has long been known to exist in some liverworts,9 but its biosynthesis has not been elucidated yet (Figure 1).
Figure 1. Comparison of the total synthesis and the biosynthesis6 of isolongifolene3, 4 and longifolene5.
In the total syntheses, long synthetic sequences were required for the stepwise construction of the skeleton of isolongifolene and longifolene. In contrast, by utilizing linear farnesyl pyrophosphate as the substrate, the natural enzyme directly accesses longifolene via cyclisation-rearrangement cascade reaction. Acid-catalysed rearrangement reaction of longifolene is known to produce isolongifolene. However, the biosynthesis of isolongifolene is unknown.
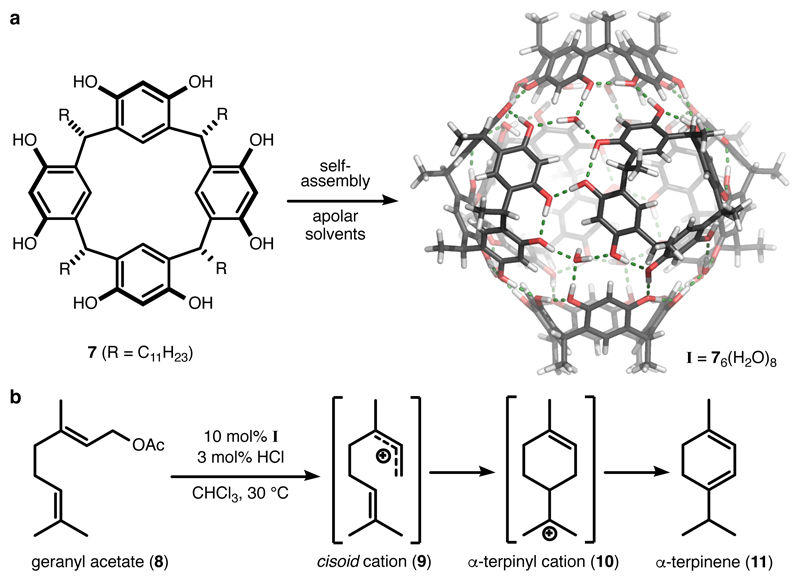
Fascinated by nature’s elegance and high efficiency, synthetic organic chemists have strived to mimic the biosynthesis of terpene natural products. Generally, the biosynthesis of terpenes occurs via two reaction pathways, which differ in the mechanism of the substrate activation. The head-to-tail (HT) terpene10 cyclisation is initiated via electrophilic activation at the prenyl end (head) of the substrate, which subsequently cyclises via a concerted mechanism. This class of cyclisation has been already extensively reproduced in bulk solution and applied to total synthesis.11 However, the HT terpene cyclisation only produces very limited structural variety, which in the case of sesquiterpenes is limited to decalin frameworks. A much larger structural variety is produced by the so-called tail-to-head (TH) terpene10 cyclisation. In this cyclisation type, the reaction is initiated by the formation of an allylic cation via the cleavage of the pyrophosphate group at the tail end of the acyclic terpene. Biomimetic TH terpene cyclisations employing man-made catalysts are challenging. There are a few literature examples describing the formation of polycyclic sesquiterpene structures in bulk solution by employing strong Brønsted or Lewis acids.12–16 Such strategies rely mainly on the iterative protonation of the formed monocyclic intermediates, and are low yielding and unselective. One example of a selective sesquiterpene cyclisation was reported by the Shenvi research group. Utilizing an unnaturally modified terpene precursor and stoichiometric amounts of aluminium Lewis acids, they were able to construct the highly strained funebrene skeleton.10 One major obstacle in the reproduction of TH terpene cyclisation using man-made catalysts is the instability of the highly reactive cationic intermediates involved in the reaction cascade, which are susceptible to exogenous nucleophiles or elimination reactions. Natural terpene cyclases circumvent this issue by enclosing the substrate within the enzymatic pocket and by stabilizing key carbocations with precisely positioned aromatic residues.17 Even more importantly, the uptake into the enzyme pocket facilitates the control over the substrate conformation. This conformation is then efficiently translated into the product and enables nature to produce the wide variety of structures with high fidelity. Regular Lewis or Brønsted acid catalysts inherently lack the ability to influence the flexible terpene precursor in a meaningful way. We reasoned that complex terpene cyclase enzymes may be mimicked by much simpler aromatic supramolecular capsules. Such systems were increasingly investigated as simple enzyme mimetics during the last decade.18–28 The hexameric resorcinarene capsule I, originally disclosed by the Atwood group in 1997,29 was identified by us30 and the groups of Scarso and Strukul31 as a supramolecular enzyme-like catalyst. Based on hydrogen-bond interactions, capsule I self-assembles from six resorcinarene monomers 7 and eight water molecules in apolar solvents to enclose an internal volume of approximately 1.4 nm3. Importantly, capsule I is known to stabilize cationic guests inside its confinement via cation-π interactions.32, 33 Recently, we reported that the resorcinarene capsule serves as an efficient catalyst for the TH cyclisation of monoterpenes.34, 35 The relatively selective cyclisation of geranyl acetate (8) to α-terpinene (11) is noteworthy (Figure 2). The preference for α-terpinene as the sole major product relies on the full propagation of the positive charge along the reaction pathways, which is likely due to the stabilisation of the cationic intermediates (for instance 9 and 10) by the capsule. This preliminary result encouraged us to probe the cyclisation of more complex substrates. Molecular modelling indicated that the precursor of cyclic sesquiterpenes, farnesol, easily fits the cavity of capsule I.
Figure 2. Selective cyclisation of the monoterpene geranyl acetate catalysed by the resorcinarene capsule.
a, The resorcinarene monomers 7 self-assemble in apolar solvents with eight water molecules to form the hexameric capsule I with an internal volume of 1.4 nm3. The capsule is capable of stabilizing cationic species via cation-π interactions. b, The resorcinarene capsule was employed as an artificial terpene cyclase mimic for the cyclisation of monoterpenes. The cyclisation reactions were likely facilitated by the stabilisation of the cationic intermediates/transition states by the capsule.
Here we report the biomimetic TH cyclisation of sesquiterpenes with I as the supramolecular catalyst. Using cyclisation precursors related to those of natural enzymes, capsule-catalysed cyclisations enable direct access to several bicyclic and tricyclic sesquiterpene natural products. Stabilisation of reaction intermediates was attributed to the encapsulation of the substrate into the cavity of the catalyst. Control on the substrate conformation was achieved by a combination of encapsulation and incorporating control elements into the substrate. In the case of the reaction of cyclofarnesyl acetate, the tricylic sesquiterpene isolongifolene was formed as the single main cyclisation product. This result raises new possibility for the efficient synthesis of terpene natural products. Additionally, the mechanism of the formation of isolongifolene was elucidated by 13C-labelling experiment and DFT-calculation.
Results
Cyclisation of linear sesquiterpenes
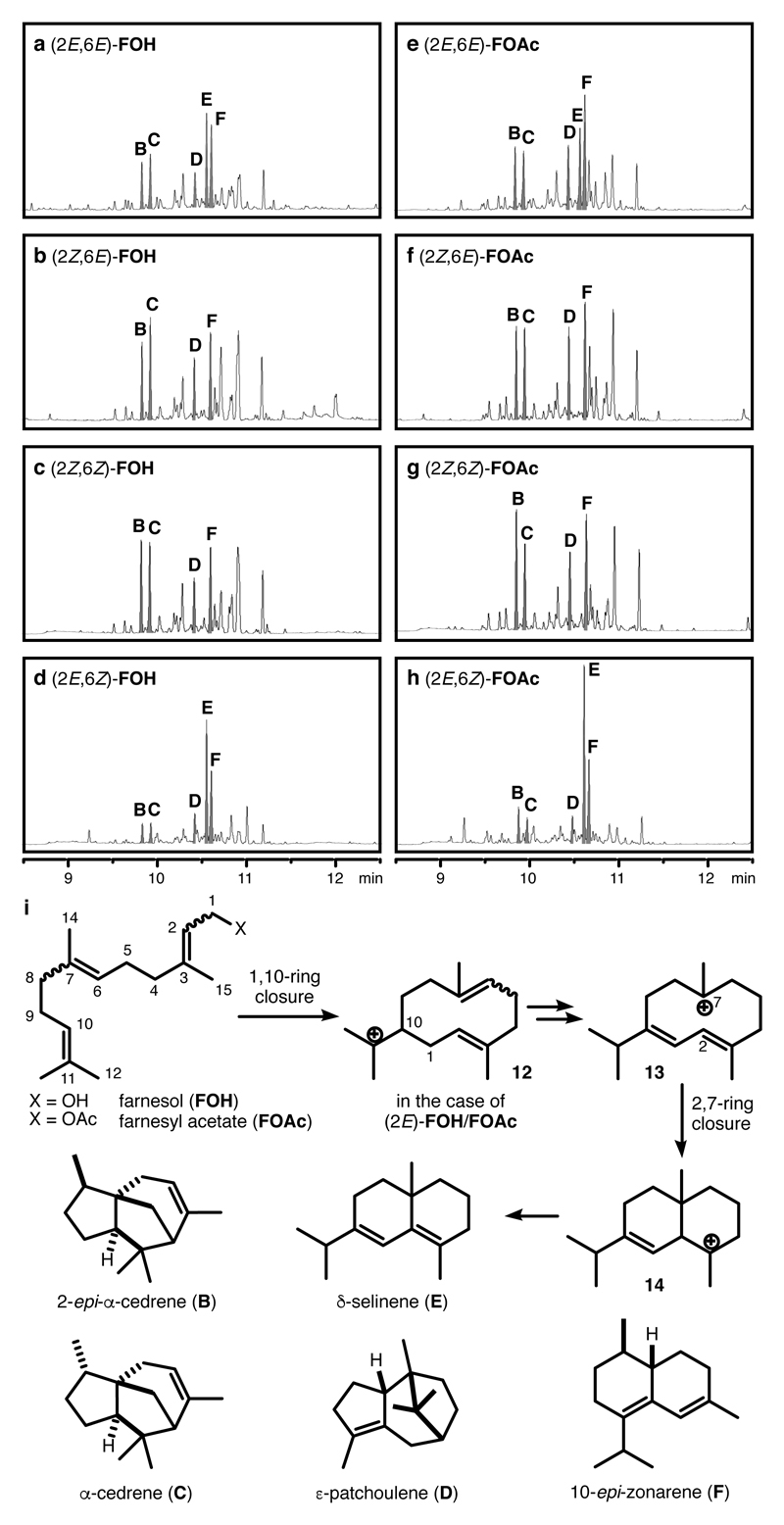
We started our investigation by testing the commercially available (2E,6E)-farnesol (FOH) under the optimized reaction conditions for monoterpene cyclisations (10 mol% I and 3 mol% HCl as catalysts, 33.3 mM in chloroform, 30 °C).35 According to gas chromatography (GC) analysis, complete conversion of the starting material was reached after 4d. Subsequent analysis of the product mixture with 1H and 13C NMR spectroscopy (Supplementary Figure 9 and 10) and comparison with literature data (Supplementary Table 1 and 2), revealed the formation of polycyclic products (Figure 3a), including 2-epi-α-cedrene (B), α-cedrene (C), ε-patchoulene (D), δ-selinene (E) and 10-epi-zonarene (F).
Figure 3. Product analysis of the capsule-catalysed cyclisation reactions of farnesyl substrates.
a-h, GC-traces of the cyclisation reaction of (2E,6E)-FOH, (2Z,6E)-FOH, (2Z,6Z)-FOH, (2E,6Z)-FOH, (2E,6E)-FOAc, (2Z,6E)-FOAc, (2Z,6Z)-FOAc and (2E,6Z)-FOAc at full conversion of substrates. The intensity of the peaks in the GC-traces are normalized to an internal standard (For full spectra, see Supplementary Figure 1-8) i, the structures of the cyclisation precursors, the main cyclisation products and the proposed mechanism from (2E)-FOH/FOAc to δ–selinene (E). The double bond geometry in the substrate accounts for the different product selectivities observed. The higher selectivity of the cyclisation reaction of (2E,6Z)-FOAc is attributed to the conformational control of the substrate and the reaction intermediate.
The cyclisation conditions rely on catalytic amounts of capsule I and hydrochloric acid (HCl). To clarify if HCl alone could activate the substrate and promote the cyclisation reaction outside the capsule, a series of control experiments were performed. First, running the reaction without capsule I failed to effect any detectable formation of products under otherwise identical conditions. The same was true when omitting the catalytic amounts of HCl. Formation of cyclic products was only detected in the presence of catalytic amount of capsule I (10 mol%) and HCl (3 mol%). Third, when the capsule was blocked with a strongly binding inhibitor (1.5 eq nBu4NBr) in the presence of HCl, the cyclisation products were formed only in trace amounts (<0.22%, compare Supplementary Table 5 and 6). Additionally, 1H NMR studies clearly indicated that the cyclisation precursor is encapsulated to some extent, and is in slow exchange with the bulk solution (Supplementary Figure 11 and 12). Taken together, these results provided strong evidence that the cyclisation reactions indeed proceeded inside the confinement of the capsule. Furthermore, in line with our observations in the monoterpene cyclisation, the reaction relies on the synergistic interplay between both catalysts, capsule and HCl.35
It was demonstrated in the monoterpene cyclisations catalysed by capsule I that the double bond geometry of the substrate has a major influence on the product selectivity.34, 35 Therefore, the other double bond isomers of farnesol were synthesized according to literature procedures36 and subjected to the cyclisation conditions. The use of the 2Z-substrates did not improve product selectivity. More promiscuous product mixtures were formed in the reaction of (2Z,6E)- and (2Z,6Z)-farnesol (Figure 3b and 3c). However, the (2E,6Z)-isomer displayed a markedly improved product selectivity (Figure 3d). All four substrates were also tested with acetate as the leaving group (Fig. 3e-h).34, 35 The most selective cyclisation was achieved by employing (2E,6Z)-farnesyl acetate (FOAc) (Figure 3h) as the substrate. δ-Selinene (E) and 10-epi-zonarene (F) were formed as the main products in 18% and 10% yield, respectively (GC-yields, corrected by internal standard and response factors; Supplementary Table 3-5). Although the yield of δ-selinene (18%) may appear modest at first, it is comparable to that of the natural δ-selinene synthase (25%).37 In contrast to the monoterpene cyclisation, where the variation of the leaving group dramatically changes the cyclisation outcome,35 the use of the acetate leaving group only slightly increased the product yields in the case of sesquiterpene cyclisations. This may indicate that the intramolecular attack of a π-bond on the transiently formed carbocation prevents the premature quenching of cationic intermediates by the cleaved leaving group.
The formation of δ-selinene (E) is noteworthy. Mechanistically, it arises from an initial 1,10-cyclisation followed by a reaction cascade37 (Figure 3i and Supplementary Figure 13). The relatively useful yield (18%) of δ-selinene in the reaction of (2E,6Z)-FOAc obviously indicates the preference for this reaction pathway (Figure 3h). However, in the cyclisation of (2E,6E)-FOAc (Figure 3e), the selectivity for δ-selinene is greatly attenuated. The preference for the 1,10-cyclisation in the case of (2E,6Z)-FOAc likely stems from the suitable substrate conformation caused by the 6Z-double bond. Intriguingly, the formation of δ-selinene was not observed in the cyclisation reactions of (2Z,6E)- and (2Z,6Z)-FOAc (Figure 3f-g). This indicates that, with the substrates containing the 2Z-double bond moiety, the alternative 1,6-ring closure mechanism is operational. When comparing the GC-traces of all substrates, it is evident that the 1,10-cyclisation path ((2E,6Z)-FOAc, Figure 3h) displays higher product selectivity than the 1,6-pathway ((2Z,6E)-FOAc and (2Z,6Z)-FOAc, Figure 3f-g). In the former mechanism, a cyclodecadiene structure is formed as the first intermediate, whereas the 1,6-ring closure leads to the formation of a cyclohexene intermediate with a pendent octyl residue. It is likely that the higher strain in the cyclodecadiene intermediate limits its conformational freedom, which reduces the available reaction pathways and ultimately leads to higher selectivity.
These results demonstrated that conformational control of the substrate and intermediates is required for good selectivity in the cyclisation reaction. As a long-term goal, we desire to effect this conformational control by utilizing less symmetric supramolecular containers as the catalysts. For the time being, however, we explored an alternative way to limit the conformational flexibility of the substrate: Incorporation of one ring into the cyclisation precursor.
Cyclisation of monocyclic sesquiterpenes
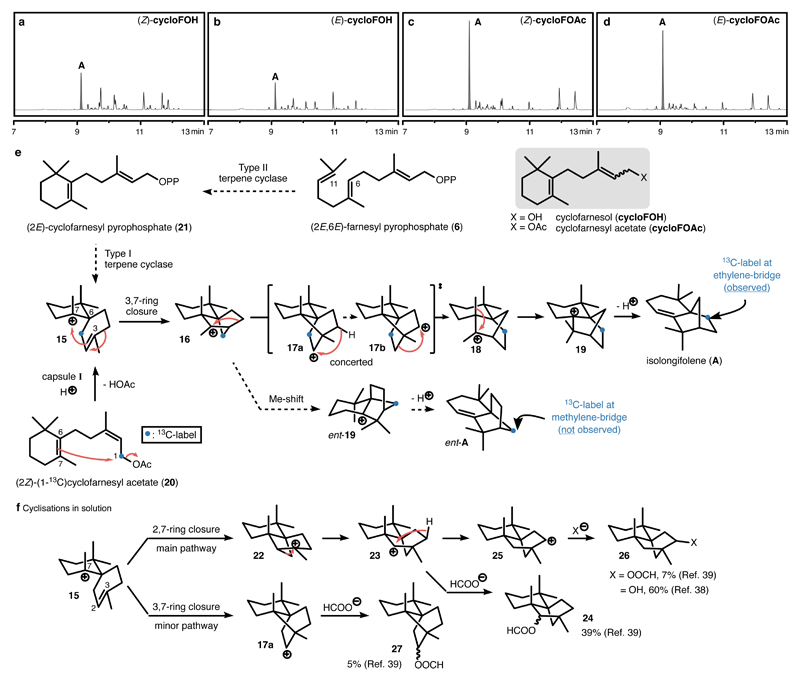
The cyclofarnesyl substrates (Figure 4, grey box) were synthesized and investigated under the cyclisation conditions. Indeed, the reactions of the cyclofarnesyl alcohols (cycloFOH, Figure 4a and 4b) exhibited a higher degree of product selectivity than the corresponding acyclic farnesols. Moreover, the selectivity was further improved when the alcohol leaving group was replaced by an acetate moiety: A single major species was observed in the reactions of cyclofarnesyl acetates (cycloFOAc, Figure 4c and 4d). With the aid of GC-MS and NMR spectroscopy (Supplementary Figure 18-20), the major product (GC yield of 29% and 23% starting from (Z)- and (E)-cyclofarnesyl acetate, respectively; Supplementary Table 7 and 8) was identified to be the tricyclic sesquiterpene isolongifolene (A), a natural product that was found in some liverworts.9 Cyclisations of related substrates were reported in the literature using chlorosulfonic and formic acid.38, 39 Interestingly, the reactions in solution followed an alternative pathway (see discussion below).
Figure 4. Product analysis of the capsule-catalysed cyclisation reactions of cyclofarnesyl substrates.
a-d, GC-traces of the cyclisation reaction of (Z)-cycloFOH, (E)-cycloFOH, (Z)-cycloFOAc and (E)-cycloFOAc at full conversion of the substrates. The intensities of the peaks in the GC-traces are normalized to an internal standard (For full spectra, see Supplementary Figure 14-17). e, The proposed mechanism for the biosynthesis of isolongifolene. f, Literature results for the cyclisation of cyclofarnesyl derivatives in solution utilizing chlorosulfonic acid or formic acid.38, 39
Mechanistic investigations
The formation of isolongifolene potentially follows a mechanism related to the biosynthesis of the monoterpene camphene40 (Figure 4e). Initially, a 1,6-cyclisation yields the spirocyclic intermediate 15. In the case of (2E)-cyclofarnesyl acetate (cycloFOAc), an isomerization of the allylic moiety has to occur prior to the cyclisation. This also explains the convergence of the reactivity from the E- and Z-isomers. Intermediate 15 undergoes a 3,7-ring closure that according to DFT calculations (see below) is concerted with a Wagner-Meerwein [1,2]-shift to form intermediate 16. From cation 16, two alternative pathways are conceivable. The first mechanism is more straightforward and involves a [1,2]-methyl shift, followed by proton elimination to yield compound ent-A. Alternatively, a sequence consisting of an [1,2]-alkyl shift, a 1,3-hydride shift and a second [1,2]-alkyl shift (a concerted reaction according to DFT calculations, see below) would deliver cation 18. Intermediate 18 would then deliver isolongifolene (A) via methyl shift and proton elimination. Although the second possibility initially seemed unlikely, recent experimental work on the cyclisation mechanisms of the diterpenes cyclooctat-9-en-7-ol and tsukubadiene using isotopically labelled precursors resulted in the discovery of unexpected carbon backbone rearrangements.41, 42 This prompted us to experimentally differentiate between these two possibilities. For this purpose, the cyclisation of (2Z)-(1-13C)cyclofarnesyl acetate (20), synthesized based on a known route to labelled farnesols (Supplementary Method),43 was investigated. The more direct first mechanism would lead to the incorporation of the 13C-label at the methylene bridge. In contrast, the second mechanism would deliver the label at the ethylene bridge. Surprisingly, the isolongifolene formed contained the 13C-label exclusively on the ethylene bridge, ruling out the more direct route via ent-19(Supplementary Figure 21-26). The reported cyclization studies of cyclofarnesyl derivatives in solution produced different products (Figure 4f).38, 39 An initial 2,7-ring closure and Wagner-Meerwein [1,2]-alkyl shift produced intermediate 23 that was either directly quenched by a nucleophile (product 24) or underwent a 1,3-hydride shift to form product 26. The 3,7-ring closure pathway observed inside capsule I is only occurring to a very small extent and more importantly, the cation produced was immediately quenched by the nucleophile present to produce 27. It is evident that in solution cationic intermediates are much more susceptible to quenching by nucleophiles present. In addition, an alternative cyclization mechanism seems operational.
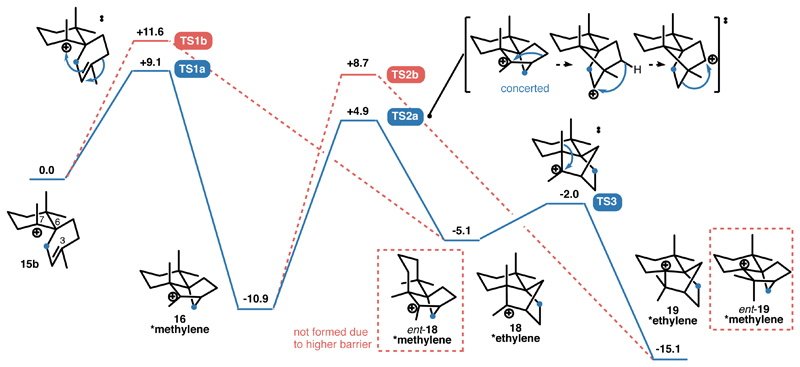
To learn more about the mechanism operational inside capsule I, DFT-computations were performed (Supplementary Table 9). The reactions depicted in Figure 5 were calculated at the mPW1PW91/6-311+G(d,p)//B3LYP/6-311+G(d,p)-GD3BJ level of theory. The suitability of this computational method for carbocation rearrangements was demonstrated before.44, 45 The energy barrier for the cyclisation of 15 (i.e. the most stable conformer 15b) to 16 (TS1a, +9.1 kcal/mol) is favoured by 2.5 kcal/mol over an alternative cyclisation leading to ent-18 (TS1b, +11.6 kcal/mol). Cation 16 rearranges via a concerted 1,2-alkyl/1,3-hydride/1,2-alkyl shift sequence (TS2a, +4.9 kcal/mol) to 18. A subsequent 1,2-Me-shift (TS3, -2.0 kcal/mol, shift of the exo-methyl group) rearranges 18 to the protonated isolongifolene 19, which exhibits the 13C-label on its ethylene bridge. An alternative rearrangement of 16 (TS2b, +8.7 kcal/mol, shift of the endo-methyl group) yielding ent-19 with a 13C-labeled methylene group is prevented by a 3.8 kcal/mol higher barrier. This 3.8 kcal/mol higher barrier is due to the unfavourable endo-position of the 1,2-migrating methyl group in 16. In contrast, 18 exhibits the 1,2-migrating methyl group in exo-position, resulting in a smooth 1,2-Me rearrangement via TS3 to produce 19 (Figure 5).
Figure 5. Computed energy profile for the formation of protonated isolongifolene 19.
The reactions were calculated at the mPW1PW91/6-311+G(d,p)//B3LYP/6-311+G(d,p)-GD3BJ level of theory.44–46 In accordance with the result obtained from the 13C-labelling experiment, DFT calculation indicated that the reaction pathway involving the additional 1,3-hydride shift step (blue) is energetically favourable.
The mechanism proposed may also be of relevance for the biosynthesis of isolongifolene. The acid-catalysed rearrangement of the sesquiterpene natural product longifolene is known to generate isolongifolene as the major product.8 In principle, such a rearrangement could account for the biosynthetic origin of isolongifolene. The selective conversion of cyclofarnesyl acetate to isolongifolene catalysed by capsule I raises another mechanistic possibility which involves cyclofarnesyl pyrophosphate 21 as the key intermediate (see Figure 4e). Although 21 has not yet been described as naturally occurring, the cyclofarnesyl formate ester is a known natural product.47 A type II terpene cyclase48 may convert farnesyl pyrophosphate 6 to 21 via protonation on the distal double bond and nucleophilic attack of the internal double bond on the C11-position, followed by deprotonation at C6. Subsequently, the monocyclic intermediate 21 would be converted inside a type I terpene cyclase48 in analogy to the reaction observed in capsule I. Such a synergistic operation of type I and type II terpene cyclase enzymes is known for instance in the biosynthesis of the diterpene natural product abietadiene and other labdane related diterpenes.17, 49
Application in total synthesis
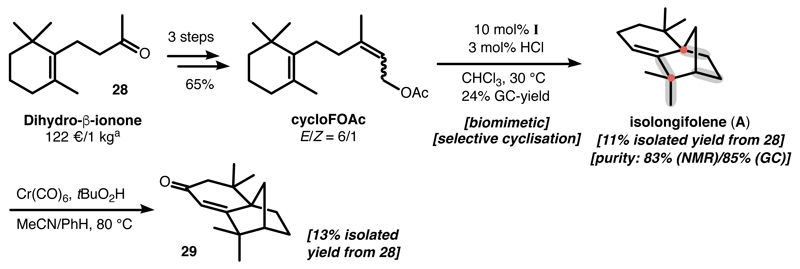
The selective cyclisation of cyclofarnesyl acetates also enables the shortest total synthesis of isolongifolene to date (Figure 6). Starting from the inexpensive bulk chemical dihydro-β-ionone (28), cyclofarnesyl acetate (cycloFOAc) was synthesized in a scalable three-step sequence. The capsule-catalysed cyclisation of cycloFOAc serves as the key step, which allows the formation/rearrangement of four C-C bonds (highlighted in grey in Figure 6) and the construction of two quaternary carbon centres (highlighted by dots) in one step. In total, racemic isolongifolene was synthesized in 11% overall yield (83% NMR purity, 85% GC purity, Supplementary Figure 27-30). This sequence provides isolongifolene (A) in only four steps and in preparatively useful yields (previously reported total syntheses: 10-19 steps).3, 50Although the isolongifolene obtained by separation using regular column chromatography is not analytically pure, it can serve as readily available material for further functionalization. Allylic oxidation of isolongifolene to the natural product isolongifolenone (29)51 facilitated the isolation as a pure compound. It was obtained in 13% isolated yield from 28 (Supplementary Figure 31).
Figure 6. Short synthesis of isolongifolene and isolongifolenone.
a, Online price quote from Sigma Aldrich. Starting from Dihydro-ß-ionone, isolongifolene was synthesized in a scalable four-step sequence involving the capsule-catalysed TH terpene cyclisation as the key step. The minor impurities contained in the isolated isolongifolene could be completely eliminated after an allylic oxidation reaction, yielding the natural product isolongifolenone as an analytically pure compound.
Conclusions
We report examples of a selective tail-to-head sesquiterpene cyclisation catalysed by an artificial enzyme mimic. In nature, the control over the conformation of farnesyl pyrophosphate is known to be a critical determinant for the ultimate product specificity.17 In this study, this was achieved by a combination of encapsulating the substrate into the confined molecular capsule and incorporating control elements (Z-double bond, cyclohexene ring) into the substrate structure. The capsule-catalysed cyclisation reactions may serve as a promising tool for the efficient construction of complex terpene natural products in the future. As a first proof of principle, a four-step synthesis of the complex tricyclic sesquiterpene natural product isolongifolene was achieved by using a biomimetic TH terpene cyclisation as the key step. The mechanism of the cyclisation/rearrangement cascade was elucidated through 13C-labelling experiments and DFT calculations. The proposed mechanism indicated that cyclofarnesyl pyrophosphate potentially could serve as a key intermediate in the biosynthesis of isolongifolene. In the future, we aim at achieving more efficient control of the substrate conformation inside supramolecular capsules by tailoring the cavity shape. To this end, efforts will be devoted to modification of the present system or developing new catalysts, which are better suited to influence the conformation of the bound substrate.
Methods
General procedure for the cyclisation reaction
Chloroform was filtered through basic aluminium oxide prior to usage. To a solution of the resorcinarene capsule I (11.1 mg, 1.67 μmol, 0.10 eq) in chloroform (200 μL) were added successively HCl stock solution in chloroform (0.50 μmol, 0.03 eq) and n-decane stock solution in chloroform (20 μL, 167 mmol/L, 3.34 μmol, 0.2 eq). Then additional chloroform was added to reach a total volume of 500 μL for the reaction mixture. After substrate (16.7 μmol, 1.00 eq) was added, the reaction mixture was briefly agitated. An aliquot (approx. 10 μL) of the reaction mixture was diluted with 0.2 mL n-hexane and subjected to GC analysis (initial sample). Meanwhile, the reaction was kept at 30 °C. After the indicated time, the reactions were sampled as described above and analyzed by GC. Conversions and yields were calculated as described in our previous work.34
Preparation and titration of HCl-concentrated chloroform solution
HCl-concentrated chloroform solution was prepared by passing HCl-gas (prepared by dropwise addition of concentrated H2SO4 to dry NaCl) through chloroform for ca. 30min. The concentration of HCl in chloroform was determined as follows: to a solution of phenol red in EtOH (0.002wt%, 2.5 mL) was added HCl-saturated chloroform (100 μL) via a microman M1 pipette equipped with plastic tips. Upon addition, the solution turned from yellow (neutral) to pink (acidic). The resulting solution was then titrated with 0.1 M ethanolic solution of triethylamine (NEt3). At the equivalence point, the solution turned from pink to yellow.
Computational method
Localization of stationary points and their characterization as minima (no imaginary frequency) or as transition structures (one imaginary frequency) was performed using GAUSSIAN16 (A.03)46 with the B3LYP/6-311+G(d,p)52 method including Grimme’s dispersion with Becke-Johnson damping.53 For all localized species, mPW1PW91/6-311+G(d,p)//B3LYP/6-311+G(d,p)-GD3BJ54 single point computations provided electronic energies. The suitability of mPW1PW91//B3LYP computations for carbocation rearrangements was demonstrated recently.44, 45 Intrinsic reaction coordinate computations (IRC) were performed for all transition structures to verify correct interconnections with their related reactants and products.
Supplementary Material
Acknowledgements
This work was supported by funding from the European Research Council Horizon 2020 Programme [ERC Starting Grant 714620-TERPENECAT], the Swiss National Science Foundation as part of the NCCR Molecular Systems Engineering and the Bayerische Akademie der Wissenschaften (Junges Kolleg)
Footnotes
Data availability. The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Information files. The data that support the plots within the paper and other findings of this study are available from the corresponding author upon reasonable request.
Author contributions
K.T. conceived and supervised the project. K.T. and Q.Z. planned the project. Q.Z. carried out all the experiments except the synthesis of 13C-labeled substrates, which were synthesized by J.R. J.D. conceived the investigations concerning the 13C-labeled substrates. The 13C-labeled products were analysed by J.D and J.R who elucidated the proposed mechanism for the formation of isolongifolene. B.G. performed the DFT calculations. Q.Z. and K.T. compiled the first draft of the manuscript. All authors contributed to the final version of the manuscript.
Competing interests
The authors declare no competing interests.
References
- 1.Maimone TJ, Baran PS. Modern synthetic efforts toward biologically active terpenes. Nat Chem Biol. 2007;3:396–407. doi: 10.1038/nchembio.2007.1. [DOI] [PubMed] [Google Scholar]
- 2.Urabe D, Asaba T, Inoue M. Convergent Strategies in Total Syntheses of Complex Terpenoids. Chem Rev. 2015;115:9207–9231. doi: 10.1021/cr500716f. [DOI] [PubMed] [Google Scholar]
- 3.Sobti RR, Dev S. Synthesis of (±)-isolongifolene. Tetrahedron Lett. 1967;8:2893–2895. [Google Scholar]
- 4.Sobti RR, Dev S. Studies in sesquiterpenes—XLIII: Isolongifolene (part 4): Synthesis. Tetrahedron. 1970;26:649–655. [Google Scholar]
- 5.Volkmann RA, Andrews GC, Johnson WS. Novel synthesis of longifolene. J Am Chem Soc. 1975;97:4777–4779. [Google Scholar]
- 6.Arigoni D. Stereochemical aspects of sesquiterpene biosynthesis. Pure Appl Chem. 1975;41:219–245. [Google Scholar]
- 7.Berson JA, et al. Chemistry of methylnorbornyl cations. VI. The stereochemistry of vicinal hydride shift. Evidence for the nonclassical structure of 3-methyl-2-norbornyl cations. J Am Chem Soc. 1967;89:2590–2600. [Google Scholar]
- 8.Yadav JS, Nayak UR, Dev S. Studies in sesquiterpenes—LV: Isolongifolene(part 6): Mechanism of rearrangement of longifolene to isolongifolene-I. Tetrahedron. 1980;36:309–315. [Google Scholar]
- 9.Svensson L, Bendz G. Essential oils from some liverworts. Phytochemistry. 1972;11:1172–1173. [Google Scholar]
- 10.Pronin SV, Shenvi RA. Synthesis of highly strained terpenes by non-stop tail-to-head polycyclization. Nat Chem. 2012;4:915–920. doi: 10.1038/nchem.1458. [DOI] [PubMed] [Google Scholar]
- 11.Yoder RA, Johnston JN. A Case Study in Biomimetic Total Synthesis: Polyolefin Carbocyclizations to Terpenes and Steroids. Chem Rev. 2005;105:4730–4756. doi: 10.1021/cr040623l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohta Y, Hirose Y. Electrophile induced cyclization of farnesol. Chem Lett. 1972;1:263–266. [Google Scholar]
- 13.Andersen NH, Syrdal DD. Chemical simulation of the biogenesis of cedrene. Tetrahedron Lett. 1972;13:2455–2458. [Google Scholar]
- 14.Polovinka MP, et al. Cyclization and Rearrangements of Farnesol and Nerolidol Stereoisomers in Superacids. J Org Chem. 1994;59:1509–1517. [Google Scholar]
- 15.Gutsche CD, Maycock JR, Chang CT. Acid-catalyzed cyclization of farnesol and nerolidol. Tetrahedron. 1968;24:859–876. [Google Scholar]
- 16.Susumu K, Mikio T, Teruaki M. Biogenetic-like cyclization of farnesol and nerolidol to bisabolene by the use of 2-fluorobenzothiazolium salt. Chem Lett. 1977;6:1169–1172. [Google Scholar]
- 17.Christianson DW. Structural Biology and Chemistry of the Terpenoid Cyclases. Chem Rev. 2006;106:3412–3442. doi: 10.1021/cr050286w. [DOI] [PubMed] [Google Scholar]
- 18.Pluth MD, Bergman RG, Raymond KN. Proton-Mediated Chemistry and Catalysis in a Self-Assembled Supramolecular Host. Acc Chem Res. 2009;42:1650–1659. doi: 10.1021/ar900118t. [DOI] [PubMed] [Google Scholar]
- 19.Yoshizawa M, Fujita M. Development of Unique Chemical Phenomena within Nanometer-Sized, Self-Assembled Coordination Hosts. Bull Chem Soc Jpn. 2010;83:609–618. [Google Scholar]
- 20.Ronson TK, Zarra S, Black SP, Nitschke JR. Metal-organic container molecules through subcomponent self-assembly. Chem Commun. 2013;49:2476–2490. doi: 10.1039/c2cc36363a. [DOI] [PubMed] [Google Scholar]
- 21.Han M, Engelhard DM, Clever GH. Self-assembled coordination cages based on banana-shaped ligands. Chem Soc Rev. 2014;43:1848–1860. doi: 10.1039/c3cs60473j. [DOI] [PubMed] [Google Scholar]
- 22.Zhang G, Mastalerz M. Organic cage compounds - from shape-persistency to function. Chem Soc Rev. 2014;43:1934–1947. doi: 10.1039/c3cs60358j. [DOI] [PubMed] [Google Scholar]
- 23.Leenders SHAM, Gramage-Doria R, de Bruin B, Reek JNH. Transition metal catalysis in confined spaces. Chem Soc Rev. 2015;44:433–448. doi: 10.1039/c4cs00192c. [DOI] [PubMed] [Google Scholar]
- 24.Hof F, Craig SL, Nuckolls C, Rebek JJ. Molecular Encapsulation. Angew Chem Int Ed. 2002;41:1488–1508. doi: 10.1002/1521-3773(20020503)41:9<1488::aid-anie1488>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 25.Rebek J. Molecular Behavior in Small Spaces. Acc Chem Res. 2009;42:1660–1668. doi: 10.1021/ar9001203. [DOI] [PubMed] [Google Scholar]
- 26.Ajami D, Rebek J. More Chemistry in Small Spaces. Acc Chem Res. 2012;46:990–999. doi: 10.1021/ar300038r. [DOI] [PubMed] [Google Scholar]
- 27.Ajami D, Liu L, Rebek J., Jr Soft templates in encapsulation complexes. Chem Soc Rev. 2015;44:490–499. doi: 10.1039/c4cs00065j. [DOI] [PubMed] [Google Scholar]
- 28.Jordan JH, Gibb BC. Molecular containers assembled through the hydrophobic effect. Chem Soc Rev. 2014;44:547–585. doi: 10.1039/c4cs00191e. [DOI] [PubMed] [Google Scholar]
- 29.MacGillivray LR, Atwood JL. A chiral spherical molecular assembly held together by 60 hydrogen bonds. Nature. 1997;389:469–472. [Google Scholar]
- 30.Zhang Q, Tiefenbacher K. Hexameric Resorcinarene Capsule is a Brønsted Acid: Investigation and Application to Synthesis and Catalysis. J Am Chem Soc. 2013;135:16213–16219. doi: 10.1021/ja4080375. [DOI] [PubMed] [Google Scholar]
- 31.Bianchini G, La Sorella G, Canever N, Scarso A, Strukul G. Efficient isonitrile hydration through encapsulation within a hexameric self-assembled capsule and selective inhibition by a photo-controllable competitive guest. Chem Commun. 2013;49:5322–5324. doi: 10.1039/c3cc42233j. [DOI] [PubMed] [Google Scholar]
- 32.Shivanyuk A, Rebek J. Reversible encapsulation by self-assembling resorcinarene subunits. Proc Natl Acad Sci USA. 2001;98:7662–7665. doi: 10.1073/pnas.141226898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avram L, Cohen Y. Spontaneous Formation of Hexameric Resorcinarene Capsule in Chloroform Solution as Detected by Diffusion NMR. J Am Chem Soc. 2002;124:15148–15149. doi: 10.1021/ja0272686. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Q, Tiefenbacher K. Terpene cyclization catalysed inside a self-assembled cavity. Nat Chem. 2015;7:197–202. doi: 10.1038/nchem.2181. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Catti L, Pleiss J, Tiefenbacher K. Terpene Cyclizations inside a Supramolecular Catalyst: Leaving-Group-Controlled Product Selectivity and Mechanistic Studies. J Am Chem Soc. 2017;139:11482–11492. doi: 10.1021/jacs.7b04480. [DOI] [PubMed] [Google Scholar]
- 36.Snyder SA, Treitler DS, Brucks AP. Simple Reagents for Direct Halonium-Induced Polyene Cyclizations. J Am Chem Soc. 2010;132:14303–14314. doi: 10.1021/ja106813s. [DOI] [PubMed] [Google Scholar]
- 37.Steele CL, Crock J, Bohlmann J, Croteau R. Sesquiterpene Synthases from Grand Fir (Abies grandis): comparison of constitutive and wound-induced activities, and cDNA isolation, characterization, and bacterial expression of δ-selinene synthase and γ-humulene synthase. J Biol Chem. 1998;273:2078–2089. doi: 10.1074/jbc.273.4.2078. [DOI] [PubMed] [Google Scholar]
- 38.Tanimoto H, Kiyota H, Oritani T, Matsumoto K. Stereochemistry of a Unique Tricarbocyclic Compound Prepared by Superacid-Catalyzed Cyclization. Synlett. 1997;1:121–122. [Google Scholar]
- 39.Fráter G, Müller U, Kraft P. Synthesis of Tricyclic Ketones with Sesquiterpene Skeletons by Acid-Catalyzed Rearrangement of β-Monocyclofarnesol. Helv Chim Acta. 1999;82:522–530. [Google Scholar]
- 40.Croteau R, Satterwhite DM, Cane DE, Chang CC. Biosynthesis of monoterpenes. Enantioselectivity in the enzymatic cyclization of (+)- and (-)-linalyl pyrophosphate to (+)- and (-)-pinene and (+)- and (-)-camphene. J Biol Chem. 1988;263:10063–10071. [PubMed] [Google Scholar]
- 41.Meguro A, et al. An Unusual Terpene Cyclization Mechanism Involving a Carbon–Carbon Bond Rearrangement. Angew Chem Int Ed. 2015;54:4353–4356. doi: 10.1002/anie.201411923. [DOI] [PubMed] [Google Scholar]
- 42.Rabe P, et al. Mechanistic Investigations of Two Bacterial Diterpene Cyclases: Spiroviolene Synthase and Tsukubadiene Synthase. Angew Chem Int Ed. 2017;56:2776–2779. doi: 10.1002/anie.201612439. [DOI] [PubMed] [Google Scholar]
- 43.Rabe P, et al. Conformational Analysis, Thermal Rearrangement, and EI-MS Fragmentation Mechanism of (1(10)E,4E,6S,7R)-Germacradien-6-ol by 13C-Labeling Experiments. Angew Chem Int Ed. 2015;54:13448–13451. doi: 10.1002/anie.201507615. [DOI] [PubMed] [Google Scholar]
- 44.Matsuda SPT, Wilson WK, Xiong Q. Mechanistic insights into triterpene synthesis from quantum mechanical calculations. Detection of systematic errors in B3LYP cyclization energies. Org Biomol Chem. 2006;4:530–543. doi: 10.1039/b513599k. [DOI] [PubMed] [Google Scholar]
- 45.Tantillo DJ. Biosynthesis via carbocations: Theoretical studies on terpene formation. Nat Prod Rep. 2011;28:1035–1053. doi: 10.1039/c1np00006c. [DOI] [PubMed] [Google Scholar]
- 46.Frisch MJ, et al. Gaussian 16. Wallingford, CT: 2016. [Google Scholar]
- 47.Shimomura O, Johnson FH. The structure of Latia luciferin. Biochemistry. 1968;7:1734–1738. doi: 10.1021/bi00845a017. [DOI] [PubMed] [Google Scholar]
- 48.Wendt KU, Schulz GE. Isoprenoid biosynthesis: manifold chemistry catalyzed by similar enzymes. Structure. 1998;6:127–133. doi: 10.1016/s0969-2126(98)00015-x. [DOI] [PubMed] [Google Scholar]
- 49.Peters RJ. Two rings in them all: The labdane-related diterpenoids. Nat Prod Rep. 2010;27:1521–1530. doi: 10.1039/c0np00019a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosal M, Karpha TK, Pal SK, Mukherjee D. Facile synthesis of (±)-isolongifolene and (±)-isolongifolenedione involving Ar1-5 cyclisations. Tetrahedron Lett. 1995;36:2527–2528. [Google Scholar]
- 51.Zhang A, Klun JA, Wang S, Carroll JF, Debboun M. Isolongifolenone: A Novel Sesquiterpene Repellent of Ticks and Mosquitoes. J Med Entomol. 2009;46:100–106. doi: 10.1603/033.046.0113. [DOI] [PubMed] [Google Scholar]
- 52.Becke AD. Density - functional thermochemistry. III. The role of exact exchange. J Chem Phys. 1993;98:5648–5652. [Google Scholar]
- 53.Grimme S, Ehrlich S, Goerigk L. Effect of the damping function in dispersion corrected density functional theory. J Comput Chem. 2011;32:1456–1465. doi: 10.1002/jcc.21759. [DOI] [PubMed] [Google Scholar]
- 54.Adamo C, Barone V. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models. J Chem Phys. 1998;108:664–675. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.