Abstract
The Eocene electric ray †Titanonarke Carvalho, 2010 from the Bolca Konservat-Lagerstätte, north-eastern Italy, is redescribed in detail based upon new material from recent excavations. This taxon exhibits a combination of features (large voids between the pectoral and the axial skeleton filled in life by electric organs, anteriorly directed fan-shaped antorbital cartilages, lack of dermal denticles, long prepelvic processes, and rounded basibranchial copula with a small caudal tab) that clearly supports its assignment to the order Torpediniformes. The analysis of new material also demonstrates that the previous apparent absence of typical narcinoid characters used to diagnose †Titanonarke was the result of taphonomic biases. †Titanonarke shares at least three synapomorphies (presence of a rostral fontanelle, low number of ribs, and rostral cartilage connected to the antorbital cartilage through lateral appendices) with the extant genera Benthobatis, Diplobatis, Discopyge and Narcine, with which it forms a clade (family Narcinidae) recognized herein as unquestionably monophyletic. Moreover, based upon a single specimen of †Titanonarke that exhibits a unique combination of morphometric and meristic features, a new species of Eocene numbfish †T. megapterygia sp. nov., is recognized. The presence of several specimens representing different ontogenetic stages of at least two species of numbfishes suggests a close association of this taxon with shallow-water habitats corresponding to coral reefs as hypothesized for the Monte Postale palaeoenvironment. The occurrence of a fossilized marine batoid embryo is reported here for the first time. Moreover, the analysis of the gut contents suggests that the dietary adaptations of †Titanonarke can be related, at least in part, to an opportunistic strategy in the context of abundant larger foraminifera in the Monte Postale palaeobiotope, suggesting that this kind of feeding mode, known to occur in present-day reefs, already was realized 50 million years ago.
Keywords: Chondrichthyes, Elasmobranchii, morphology, phylogeny, embryo, gut content
Introduction
Electric rays of the order Torpediniformes de Buen, 1926 are a well-defined monophyletic group within Batoidea that includes about 60 extant species in 11 genera (Compagno & Heemstra 2007; Claeson 2014; Nelson et al. 2016). Torpediniforms are typically characterized by a series of morphological traits that traditionally were used to distinguish them from all other batoids, including a rounded fleshy disc, a power-stroking tail without barbs, massive electric organs between the pectoral and axial skeleton, and the anteriorly directed fan- or antler-shaped antorbital cartilages (Davy 1829; Compagno 1973, 1977; Maisey 1984; Carvalho et al. 1999; McEachran & Carvalho 2002; Last et al. 2016). A recent comparative morphological analysis presented by Claeson (2014) recovered four additional synapomophies: the absence of dermal denticles or thorns, the suprascapular antimeres fused with a visible suture, long prepelvic processes, and a rounded basibranchial copula with a small caudal tab.
Several morphological and molecular studies agree with the hypothesis that torpediniforms form the sister group of all other batoids (e.g. McEachran et al. 1996; McEachran & Aschliman 2004; Rocco et al. 2007; Aschliman et al. 2012a), while other molecular-based phylogenies recovered electric rays as sister of Myliobatiformes Compagno, 1973 (Pavan-Kumar et al. 2013) or Platyrhinidae Jordan, 1923 (Aschliman et al. 2012b). The latter hypothesis appears to be more consistent with the first appearance data (Paleocene) of torpediniforms in the fossil record with respect to other batoid lineages (Underwood 2006; Claeson 2014). Within Torpediniformes, two monophyletic groups are traditionally recognized: the Torpedinoidea Jonet, 1968, and the Narcinoidea Compagno, 1973. Within Torpedinoidea, a single family, Torpedinidae Bonaparte, 1838, with two genera, Torpedo Houttuyn, 1764 and Hypnos Dumeril, 1852, is recognized. Narcinoidea includes the families Narcinidae Gill, 1862 with four genera (Benthobatis Alcock, 1898, Diplobatis Bigelow & Schroeder, 1948, Discopyge Heckel, 1846 and Narcine Henle, 1834) and Narkidae Fowler, 1934 with five genera (Electrolux Compagno & Heemstra, 2007, Typhlonarke Waite, 1909, Heteronarce Regan, 1921, Narke Kaup, 1826 and Temera Gray, 1831) (Carvalho 2010; Aschliman et al. 2012a; Nelson et al. 2016). The monophyletic status of the family Narcinidae was recently questioned based on a comprehensive morphological and phylogenetic analysis of all torpediniform genera, since the characters traditionally used to support the monophyly of narcinids were not recovered supportive of this condition (see Claeson 2014).
Although ghost lineages predict that torpediniforms should have been present in the Late Cretaceous (see Aschliman et al. 2012b; Guinot & Cavin 2016), fossil electric rays are known only from Cenozoic deposits. Very little is known about the evolutionary history of this group, because almost all fossil taxa (with the exception of †Titanonarke) are known from isolated teeth, which are phylogenetically not informative (Claeson 2014). The celebrated Eocene (late Ypresian, c. 50 Ma; Papazzoni et al. 2014) Bolca Konservat-Lagerstätte yielded the only fossil torpediniforms represented by complete articulated skeletons, which are of considerable relevance for identifying plesiomorphic morphological traits and understanding the early Palaeogene diversification of this group. The fossiliferous deposits from this locality have been known since the sixteenth century for their abundance of exquisitely preserved fishes, amongst which approximately 240 species, predominantly belonging to various teleost lineages, have been described to date (e.g. Carnevale & Pietsch 2009, 2010, 2011, 2012; Bannikov & Carnevale 2010; Carnevale et al. 2014; Marramà & Carnevale 2015a, b, 2016, 2017a, b; Pfaff et al. 2016; Davesne et al. 2017). Although several studies during the last few decades have contributed to our increased knowledge of the outstanding teleost palaeobiodiversity of this oldest example of a modern tropical reef fish assemblage (Marramà et al. 2016c; Bellwood et al. 2017), the taxonomic diversity, interrelationships and palaeoecology of the Bolca chondrichthyans remain poorly resolved when compared to those of the outstanding teleost assemblage from this celebrated Eocene locality. In fact, with the exception of a few recent revisions of selected selachians (e.g. Fanti et al. 2016; Marramà et al. 2017a), no other modern systematic studies have been carried out on the Bolca cartilaginous fishes (Marramà et al. in press).
Torpediniforms currently are represented at Bolca by a single species referred to †Titanonarke molini (Jaekel, 1894). The genus Titanonarke was erected by Carvalho (2010). This taxon is known from fossiliferous sediments of the Monte Postale site of the Bolca Konservat-Lagerstätte by several specimens representing different ontogenetic stages. Although †Titanonarke clearly exhibits the typical characters of the Narcinoidea, the diagnosis and the phylogenetic placement of this genus provided by Carvalho (2010) appear to be problematic, since the analysis of new material from recent excavations clearly shows that the apparent absence of typical narcinoid characters considered diagnostic of †Titanonarke is the result of taphonomic biases.
The goal of this paper is to provide a detailed morphological redescription of †Titanonarke from the Eocene Bolca Lagerstätte, Italy, test the hypothesis that only one species exists, and identify its relationships with the other torpediniforms. Palaeobiological and palaeoenvironmental implications based on an analysis of the gut contents provide new insights into the early Palaeogene rise of new feeding strategies in fishes associated with reefs.
Geological setting
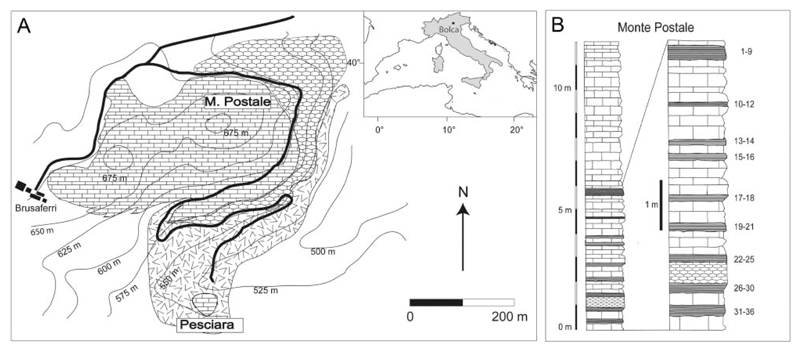
All specimens examined come from the fossiliferous layers of the Monte Postale site, one of the two main fossiliferous quarries of the Bolca Konservat-Lagerstätte, which is located about 2 km north-east of the village of Bolca (Verona Province, NE Italy) in the eastern part of the Lessini Mountains, southern Alps (Fig. 1). The Monte Postale site is about 300 m from the better known Pesciara site, from which most of the fossil Bolca specimens were recovered (Carnevale et al. 2014). Monte Postale is of almost the same age and exhibits similar sedimentological features, mostly comprising finely laminated micritic limestones with fish and plant remains. Papazzoni et al. (2017) recently investigated the stratigraphical relationships between the two fossiliferous deposits, suggesting that the uppermost productive sequence of Monte Postale should correlate with those of the Pesciara site, although the fossiliferous laminites of Pesciara appear to be slightly younger than those of Monte Postale. The Monte Postale succession includes the Cretaceous Scaglia Rossa Formation up to Ypresian fossiliferous limestone, containing larger benthic foraminifera of the genus †Alveolina d’Orbigny, 1826, and also marine and brackish molluscs in the uppermost part (Papazzoni et al. 2014). Fabiani (1914, 1915) was the first to conduct a detailed stratigraphical study of the Monte Postale site and assigned the entire succession to the Lutetian. Malaroda (1954) postulated the same age, whereas Hottinger (1960) suggested an Ypresian age based on the foraminifera content. Based on large benthic foraminifera and calcareous nannoplankton, the uppermost strata of the Monte Postale site was assigned to Shallow Benthic Zone 11 by Papazzoni et al. (2017) and, thus, corresponds to the late Cuisian (late Ypresian, around 50 Ma). Although the entire area of Bolca was historically regarded as belonging to a coral reef system (e.g. Bellwood 1996), solid evidence of a coralgal rim, lagoonal deposits, and a fore-reef system was only detected for the Monte Postale palaeobiotope (Vescogni et al. 2016). This interpretation was also supported by recent palaeoecological and taphonomic studies of the Monte Postale fish assemblage based on the abundance of marine and terrestrial plants, the large number of invertebrates (including abundant corals), and reef-associated fishes, which accumulated in a coral reef context close to an emerged coastal area (Marramà et al. 2016c). Disarticulation of fish skeletons, unimodal dispersion of the elements and bioturbation were the result of at least periodic oxic bottom conditions (Marramà et al. 2016c).
Figure 1.
A, location and geological map of the Bolca area. B, stratigraphical section of the uppermost part of the Monte Postale sequence. Modified from Marramà et al. (2016c).
Material and methods
The present study is based on six well-preserved specimens from the fossiliferous layers of the Monte Postale site. The fossils are currently housed in the collections of the Museo Civico di Storia Naturale di Verona (MCSNV), Museo dei Fossili di Bolca (technically part of the MCSNV), and Museo di Geologia e Paleontologia dell’Università degli Studi di Padova (MGP-PD). We examined new material including specimens from most recent controlled excavations carried out from 1999 to 2011 (MCSNV IG.135581; MCSNV IG.VR.67290; MCSNV IG.VR.91359), in addition to the three specimens examined by Carvalho (2010) (holotype MGP-PD 26275/6; MCSNV IG.135576; MCSNV IG.91128/9). Some of the specimens were mechanically prepared to reveal fine or hitherto obscured skeletal details. The holotype MGP-PD 26275/6 was examined with UV light to distinguish preserved soft tissues from grout or pigments used in historical reconstruction (see also Supplemental material). Measurements were taken to the nearest 0.1 mm. Standard length (SL; from the anterior tip of the rostral cartilage to the caudal fin origin) is used throughout instead of total length (TL), because most of the specimens lack the distal portion of the caudal fin.
A principal component analysis (PCA) was performed on the entire data set of log-transformed measurements (standardized for the head length) and meristic data to provide direct visual images of the spatial separation of specimens following Takács (2012) and Marramà et al. (2017a, b). The component loading values of the PCs were used to interpret the ‘meaning’ of the components (Hammer et al. 2001). The PCA was performed using the software package PAST (Hammer et al. 2001). Osteological terminology follows McEachran & Aschliman (2004), Aschliman et al. (2012a) and Claeson (2014). Morphometric terminology is adopted and modified from Compagno & Heemstra (2007). Extinct taxa are marked with a dagger (†) preceding their name.
The phylogenetic analysis is based on the recent morphological data set of Claeson (2014), which in turn is based on the matrices of McEachran et al. (1996), McEachran & Aschliman (2004) and Aschliman et al. (2012a). The original matrix of Claeson (2014) was rechecked and some states were corrected or recoded (see Supplemental material). The data matrix contains all 65 characters of Claeson (2014), to which we added five new dental characters (chars. 66, 67, 68, 69, 70) from Herman et al. (1995, 1997, 2002) and Compagno & Heemstra (2007). Based on the analysis of additional morphological and meristic features of extant and fossil taxa, we also included two new characters (71 and 72), describing two of the main synapomorphies of Narcinidae. The matrix includes all taxa considered by Claeson (2014), to which we added †Titanonarke and †Eotorpedo White, 1934, the latter only known by isolated teeth (see also the section ‘Palaeobiogeographical remarks’). The character matrix was compiled in Mesquite 3.03 (Maddison & Maddison 2008). The phylogenetic analysis was performed with TNT 1.5 using the branch-and-bound method (Goloboff et al. 2008). All characters are considered unordered and given equal weight. Tree length, consistency (CI), homoplasy (HI) and retention (RI) indices subsequently were calculated for the strict consensus tree and for each character individually (see Supplemental material). Two additional phylogenetic analyses using the same data matrix were excuted with WinClada 1.00.08 (Nixon 2002) and Mesquite 3.03 (Maddison & Maddison 2008) for cross-checking results obtained from the TNT analysis.
Institutional abbreviations
AMNH: American Museum of Natural History, New York, USA; ESB: Ecosystems Surveys Branch, Northeast Fisheries Science Center, Woods Hole, Massachusetts, USA; FMNH: Field Museum of Natural History, Chicago, Illinois, USA; MCSNV: Museo Civico di Storia Naturale di Verona, Italy; MCZ: Museum of Comparative Zoology, Cambridge, Massachusetts, USA; MGP-PD: Museo di Geologia e Paleontologia dell’Università degli Studi di Padova, Italy; MNHN: Muséum national d’Histoire naturelle, Paris, France; NHMUK: Natural History Museum, London, UK; SIO: Scripps Institution of Oceanography, San Diego, California, USA; ZMH: Museum für Naturkunde, Hamburg, Germany; TNHC: Texas Natural History Collection, University of Texas, Austin, Texas, USA; ZMB: Museum für Naturkunde, Berlin, Germany. (See comparative material in Supplemental material.)
Systematic palaeontology
Class Chondrichthyes Huxley, 1880
Superorder Batomorphii Cappetta, 1980
Order Torpediniformes de Buen, 1926
Superfamily Narcinoidea Compagno, 1973
Family Narcinidae Gill, 1862
Diagnosis (emended). Medium to large-sized narcinoid electric rays sharing three synapomorphies: presence of rostral fontanelle, reduced number of ribs (up to 10 pairs), and rostral cartilage connected to the antorbital cartilages through lateral rostral appendices. Additionally, narcinids possess the following combination of characters: palatoquadrate labiolingually compressed and tapered towards the symphysis; small subtriangular labial cartilages close to symphysis; rostral cartilage expanded and troughshaped; large and rounded basibranchial copula with small caudal tab; tooth cusps and roots narrow and high; long precaudal tail; two dorsal fins.
Included genera. Benthobatis Alcock, 1898; Diplobatis Bigelow & Schroeder, 1948; Discopyge Heckel, 1846; Narcine Henle, 1834; †Titanonarke Carvalho, 2010.
Genus †Titanonarke Carvalho, 2010
Diagnosis (emended). Narcinid electric ray unique in having the following two autapomorphic traits: large size (up to about 1 m of TL) and large number of vertebrae (133–155). Additionally, †Titanonarke has the following combination of characters: broadly branched antorbital cartilage, with a third smaller branch posteriorly directed and located at midlength; unfused hypobranchials; iliac process short and straight; prepelvic process wider towards tip than along the shaft; mesopterygium shorter than propterygium and metapterygium; tooth cusp length less than half the length of the blade-like cutting edges; tooth root high and narrow; precaudal tail reaching about 50% of TL; long claspers extending past posterior tips of pelvic fin lobes.
Type species. †Narcine molini Jaekel, 1894.
Included species. †Titanonarke molini (Jaekel, 1894); †Titanonarke megapterygia sp. nov.
Remarks. Jaekel (1894) was the first to document the presence of electric rays from the Monte Postale site in his comprehensive account of cartilaginous fishes from the Bolca Lagerstätte. In this work, he erected the species †Narcine molini based on a nearly complete specimen in part and counterpart housed in the collection of the Museo di Geologia e Paleontologia dell’Università di Padova (MGP-PD 26275/6). The specimen previously was assigned to †Narcine gigantea by Molin (1860). However, the holotype of †N. gigantea, housed in the Museum national d’Histoire naturelle, Paris (MNHN F.Bol567) later was recognized as a thornback and assigned to Platyrhina by Jaekel (1894). Both Eastman (1904, 1905) and Blot (1980) included †Narcine molini in their synoptic lists of chondrichthyans of Bolca. Recently, Carvalho (2010) undertook a re-examination of the holotype of †Narcine molini and two additional specimens recovered from the Monte Postale site in the second half of the twentieth century (MCSNV IG.135576 and MCSNV IG.91128/9), and erected the genus †Titanonarke containing †N. molini Jaekel, 1894. The diagnosis of this new genus was based on the presence of some derived characters within narcinids, including the large size, the long precaudal tail reaching about 50% of TL, the absence of dorsal fins, and absence of posteriorly directed branches of the antorbital cartilage. Carvalho (2010) also provided an interpretative phylogenetic analysis in which †Titanonarke was recovered as the basalmost genus within the Narcinidae. Although †Titanonarke clearly exhibits the diagnostic characters of this family, the diagnosis of this Eocene taxon provided by Carvalho (2010) and its relationships to other torpediniforms appear to be controversial. This is primarily due to the apparent absence of some typical narcinoid characters (dorsal fins and third branch of the antorbital cartilage), and to the ambiguous choice of characters used for the phylogenetic inference. Carvalho (2010) also hypothesized the presence of a possible new genus of numbfish in the Monte Postale site based on the observation of two juvenile specimens (MCSNV IG.135581 and MCSNV IG.VR.91359) indicating characters which were hypothesized to be absent in †T. molini (i.e. third small branch of the antorbital cartilage and a posteriorly directed scapular process). Our detailed revision of the historical and new material derived from recent controlled excavations reveals that the specimens used by Carvalho (2010) were only partially preserved and thus prevented the recognition of those elements whose apparent absence was erroneously regarded as diagnostic of †Titanonarke. Moreover, there are no substantial morphological differences to support the hypothesis that juvenile specimens should be recognized as a new genus because osteological, morphometric and meristic features identify these as belonging to †T. molini (see below). We also recognize the specimen MCSNV IG.135576 (but not MCSNV IG.135581 and MCSNV IG.VR.91359), previously ascribed to †T. molini by Carvalho (2010), as a new species of †Titanonarke. Finally, an integrated comprehensive phylogenetic analysis based on the data derived from the detailed comparative anatomy of the electric rays of Claeson (2014) has revealed new hypotheses about the relationships of Titanonarke within the Torpediniformes.
†Titanonarke molini (Jaekel, 1894) (Figs 2–16)
Figure 2.
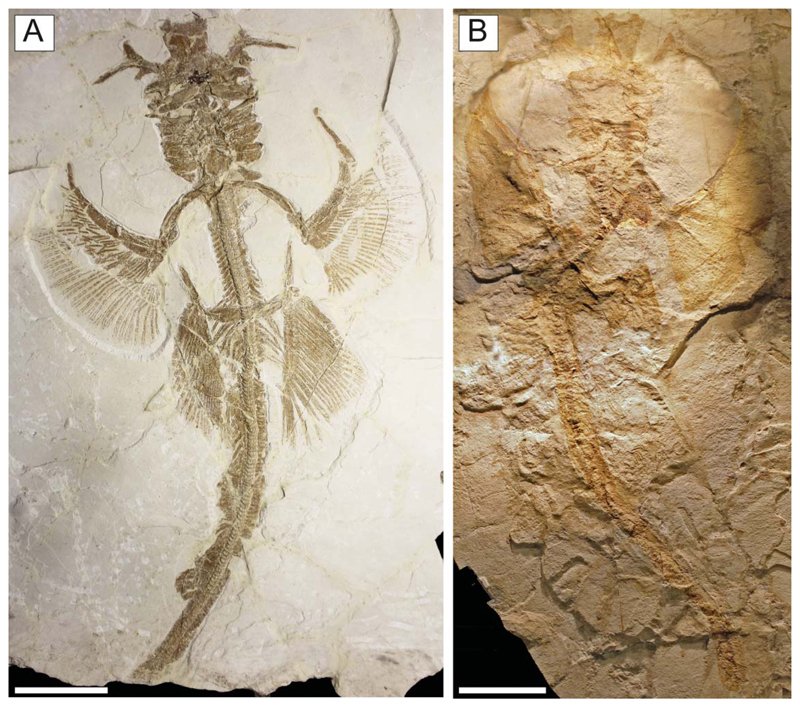
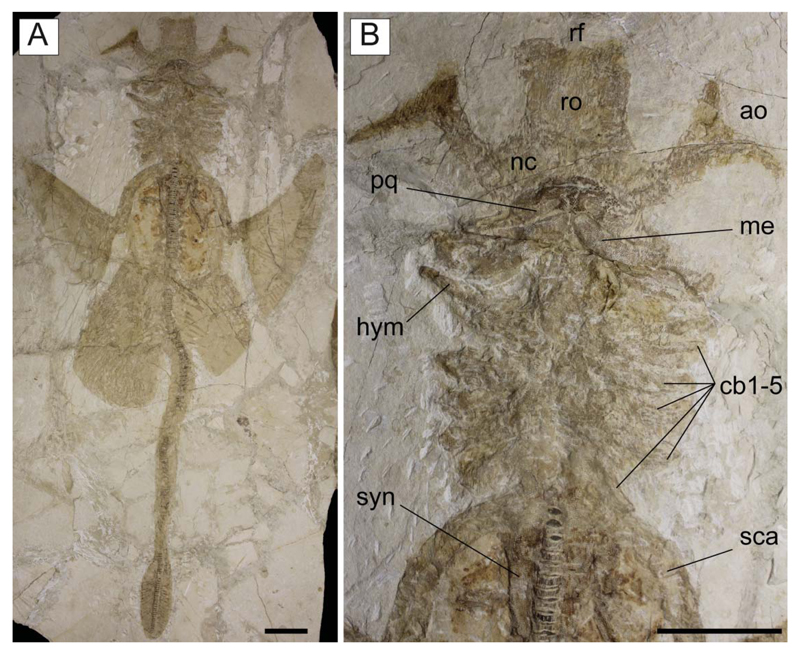
†Titanonarke molini (Jaekel, 1894) from the Eocene Monte Postale site. A, B, MGP-PD 26275/6, holotype in part and counterpart. Scale bars = 100 mm.
Figure 16.
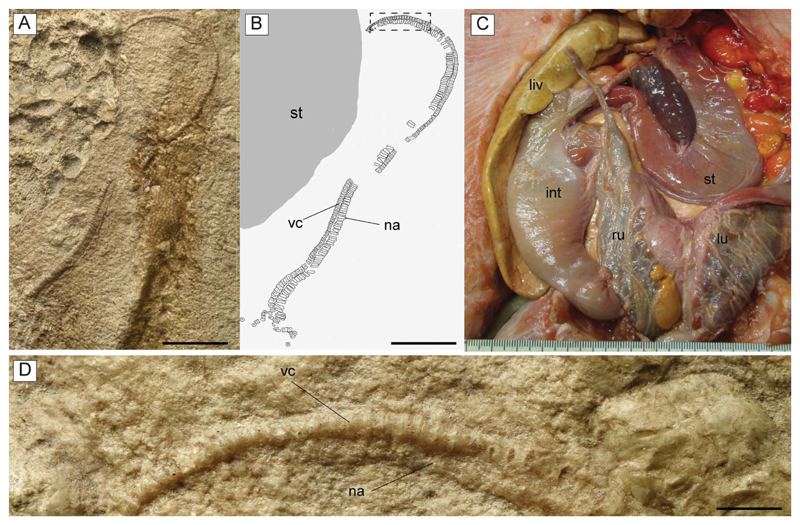
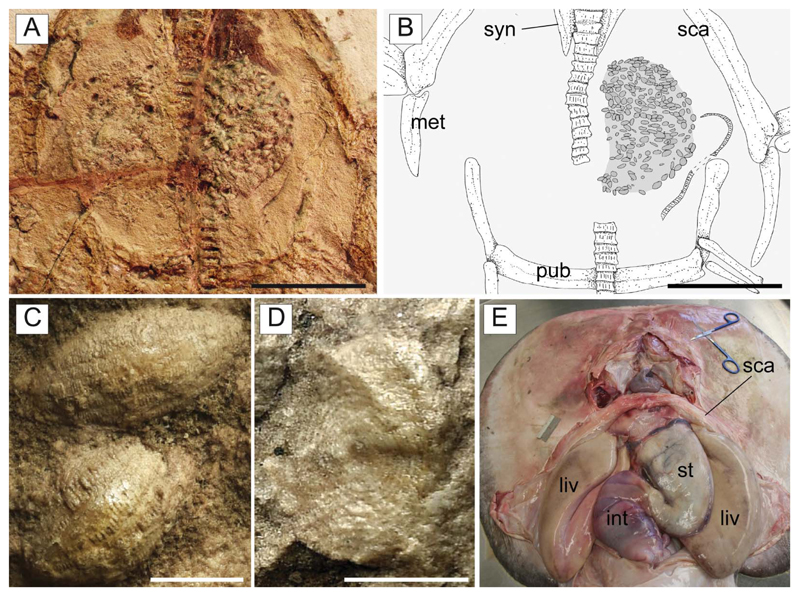
A, B, D, †Titanonarke molini (Jaekel, 1894) from the Eocene Monte Postale site; A, close-up of the abdominal region of MGP-PD 26275 showing the embryo; the anterior region of the body lies on the lower portion of the photo; B, reconstruction; D, detail of the vertebral column of the embryo indicated in B with a dotted rectangle. C, dissected specimen of Potamotrygon tigrina (IUWP 7361) showing the position of the left uterus, just next to the stomach. Abbreviations: int, intestine; liv, liver; lu, left uterus; na, neural arches; ru, right uterus; st, stomach; vc, vertebral centra. Scale bars: A, B = 10 mm; D = 1 mm.
1860 †Narcine gigantea Molin: 585 [pro parte].
1894 †Narcine molini Jaekel: 111, pl. 3 [original occurrence of name, photograph and outline reconstruction].
1904 †Narcine molini Jaekel, 1894; Eastman: 27.
1905 †Narcine molini Jaekel, 1894; Eastman: 351.
1979 †Narcine molini Jaekel, 1894; Stanghellini: 38 [misspelt ‘moloni’].
1980 †Narcine molini Jaekel, 1894; Blot: 344.
1987 †Narcine molini Jaekel, 1894; Cappetta: 161, fig. 138L.
1988 †Narcine molini Jaekel, 1894; Cappetta: 29.
1991 †Narcine molini Jaekel, 1894; Frickhinger: 211.
1993 †Narcine molini Jaekel, 1894; Cappetta et al.: 604.
1999 †‘Narcine’ molini Jaekel, 1894; Carvalho: 283, figs 107–111.
2010 †Titanonarke molini (Jaekel, 1894); Carvalho: 185, figs 2, 5A, 6, 7.
2012b †Titanonarke molini (Jaekel, 1894); Aschliman et al.: 33.
2012 †Narcine molini Jaekel, 1894; Cappetta: 410, fig. 401L.
2014 †Titanonarke molini (Jaekel, 1894); Carnevale et al.: 41.
2014 †Titanonarke molini (Jaekel, 1894); Claeson: 4.
2016c †Titanonarke molini (Jaekel, 1894); Marramà et al.: 232.
Holotype. MGP-PD 26275/6, nearly complete articulated skeleton in part and counterpart (Fig. 2), 816.8 mm SL.
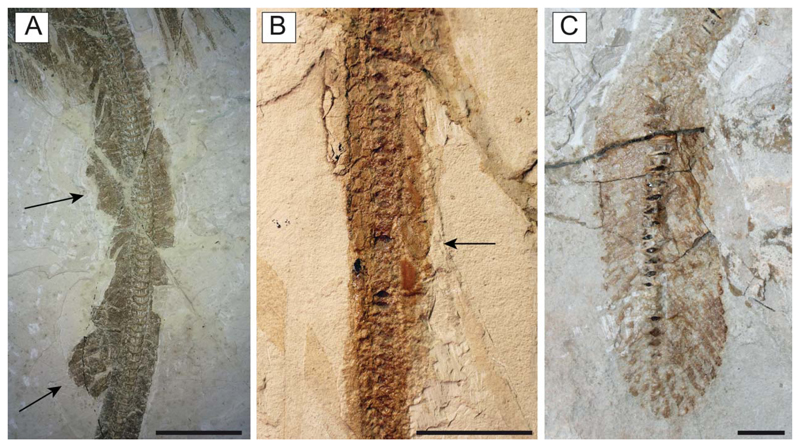
Referred material. MCSNV IG.VR.67290, nearly complete articulated skeleton in a single slab, 750.2 mm SL (Fig. 3A); MCSNV IG.91128/9, partially complete articulated specimen in part and counterpart, 850.4 mm SL (Fig. 3B); MCSNV IG.VR.91359, nearly complete articulated skeleton in a single slab, 99.0 mm SL (Fig. 4A); MCSNV IG.135581, incomplete articulated specimen in a single slab, lacking part of the cranial and caudal regions (Fig. 4B).
Figure 3.
†Titanonarke molini (Jaekel, 1894) from the Eocene Monte Postale site. A, MCSNV IG.VR.67290; B, MCSNV IG.91128/9. Scale bars = 100 mm.
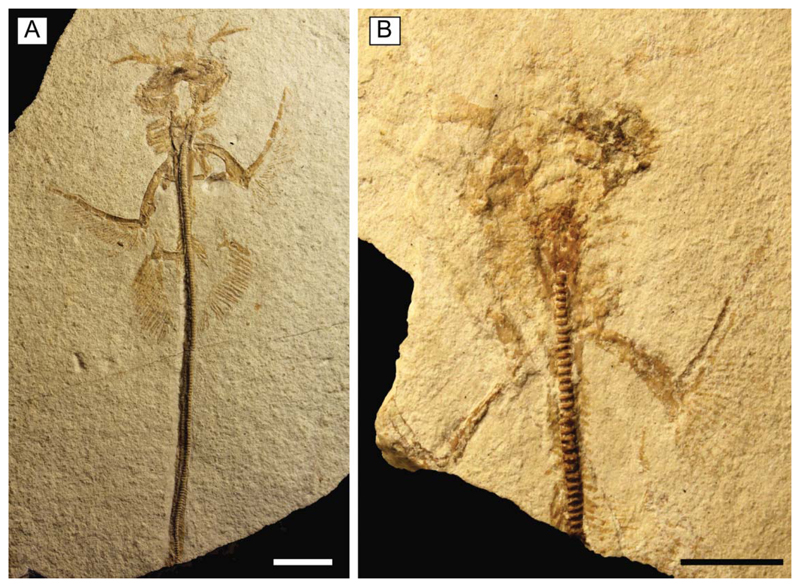
Figure 4.
Juvenile individuals of †Titanonarke molini (Jaekel, 1894) from the Eocene Monte Postale site. A, MCSNV IG.VR.91359; B, MCSNV IG.135581. Scale bars = 10 mm.
Type locality and horizon. Monte Postale site, Bolca Konservat-Lagerstätte, Italy; early Eocene, late Ypresian, middle Cuisian, SBZ 11, Alveolina dainelli Zone (see Papazzoni et al. 2017).
Diagnosis. Species of †Titanonarke characterized by the following combination of characters: subcircular disc length c. 44% SL and disc width c. 50% SL; greatly elongated precaudal tail of c. 60% SL; 153–155 vertebrae (27–30 trunk; 100–115 precaudal, 23–25 caudal); 15–16 tooth rows in upper jaw and 12–13 tooth rows in the lower jaw; c. 40 pectoral radials (12–16 propterygial, 9–12 mesopterygial, and 10–11 metapterygial); single-lobed pelvic fin with 21–24 basipterygial radials; first dorsal fin with 7–9 radials, and second dorsal fin with 6–7 radials; caudal fin with about 42 radials (20 dorsal and 22 ventral); width of pelvic fins about 50% of disc width; anterior pelvic fin margin length c. 24% of disc length.
Description. Specimens examined comprise different ontogenetic stages and range from 99 to 850.4 mm SL, with the largest specimen reaching 925 mm TL. Measurements and counts for †Titanonarke molini are summarized in Tables 1 and 2. Overall, the body is large and dorsoventrally compressed (Figs 2, 3). The disc is subcircular, slightly ovoid in outline, generally wider than long, and barely overlapping the pelvic fin origin. The largest width of the disc is slightly posterior to its midlength and its edges are continuously curved. The length and width of the disc are about 44% and 50% SL, respectively. The head is approximately 25% of SL and the preoral length is about 9% SL. The precaudal tail is elongated (about 60% SL). †Titanonarke molini has two dorsal fins; the predorsal distance at the first dorsal fin is about 62% SL, whereas the predorsal distance at the second dorsal fin is about 78% SL; the interdorsal distance is about 7% SL. The body is totally naked, lacking denticles and thorns. The skeleton is highly calcified and most of the skeletal elements, including parts of the rostral cartilage, jaws, hyomandibulae, antorbital cartilages, synarcual, and pectoral and pelvic girdles, show the typical prismatic calcification of elasmobranch fishes (Dean & Summers 2006).
Table 1.
Morphometric data for all examined specimens of †Titanonarke Carvalho, 2010 from the Eocene Monte Postale site, Bolca Lagerstätte. Abbreviations (see the morphological scheme in the Supplemental material): AOW, antorbital cartilage width; CFL, caudalfin length; CFD, caudal fin depth; CLO, clasper length; CPD, caudal peduncle depth; D1B, first dorsal fin base length; D2B, second dorsal fin base length; DCS, dorsal caudal space; DL, disc length; DW, disc width; HL, head length; IDS, interdorsal space; MOW, mouth width; P2A, pelvic fin anterior margin length; P2B, pelvic fin base length; P2S, pelvic fin span; PCS, space from pelvic fin insertion to the caudal fin origin; PD1, predorsal distance up to the first dorsal fin; PD2, predorsal distance up to the second dorsal fin; PDI, pelvic fin insertion to first dorsal fin origin; PDO, pelvic fin origin to first dorsal fin origin; PGW, snout tip to the level of the greatest disc width; PIW, body width at pectoral fin insertions; POR, preoral length; PP2, prepelvic length; SL, standard length; TAL, tail length; TBW, tail base at pelvic fin origin; TL, total length.
| †Titanonarke molini (Jaekel, 1894) | †Titanonarke megapterygia sp. nov. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MGP-PD 26275/6 |
MCSNV IG.VR.67290 |
MCSNV IG.91128/9 |
MCSNV IG.135581 |
MCSNV IG.VR.91359 |
MCSNV IG.135576 |
|||||||
| Measurements | mm | % SL | mm | % SL | mm | % SL | mm | % SL | mm | % SL | mm | % SL |
| AOW | 206.6 | 25.3 | 217.4 | 29.0 | 196.2 | 23.1 | 26.1 | – | 28.1 | 28.4 | 174.2 | 27.8 |
| CFL | 80.9 | 9.9 | – | – | 75.0 | 8.8 | – | – | – | – | 97.4 | 15.6 |
| CFD | 28.4 | 3.5 | – | – | 26.1 | 3.1 | – | – | – | – | 36.0 | 5.8 |
| CLO | – | – | – | – | 70.5 | 8.3 | – | – | – | – | – | – |
| CPD | 20.2 | 2.5 | 25.5 | 3.4 | 15.9 | 1.9 | – | – | 2.3 | 2.3 | 13.7 | 2.2 |
| D1B | – | – | 64.6 | 8.6 | 56.6 | 6.7 | – | – | – | – | – | – |
| D2B | 50.0 | 6.1 | 47.9 | 6.4 | 51.3 | 6.0 | – | – | – | – | – | – |
| DCS | 140.2 | 17.2 | 109.8 | 14.6 | 119.3 | 14.0 | – | – | – | – | – | – |
| DL | 353.1 | 43.2 | 341.2 | 45.5 | 371.6 | 43.7 | 33.8 | – | 42.8 | 43.2 | 334.5 | 53.4 |
| DW | 387.0 | 47.4 | 406.6 | 54.2 | 347.8 | 40.9 | – | – | 56.3 | 56.9 | 349.8 | 55.9 |
| HL | 178.1 | 21.8 | 172.5 | 23.0 | 204.4 | 24.0 | 22.2 | – | 23.6 | 23.8 | 171.8 | 27.4 |
| IDS | – | – | 53.6 | 7.1 | – | – | – | – | – | – | – | |
| MOW | 80.6 | 9.9 | 99.9 | 13.3 | 95.5 | 11.2 | 13.6 | – | 12.7 | 12.8 | 87.1 | 13.9 |
| P2A | 77.2 | 9.5 | 103.1 | 13.7 | 88.7 | 10.4 | – | – | 8.1 | 8.2 | 141.6 | 22.6 |
| P2B | 134.6 | 16.5 | 108.3 | 14.4 | 81.3 | 9.6 | – | – | 11.7 | 11.8 | 107.9 | 17.2 |
| P2S | 206.8 | 25.3 | 208.5 | 27.8 | – | – | – | – | 23.7 | 23.9 | 211.9 | 33.8 |
| PCS | 356.0 | 43.6 | 358.5 | 47.8 | – | – | – | – | 44.9 | 45.4 | 241.7 | 38.6 |
| PD1 | – | – | 468.1 | 62.4 | – | – | – | – | – | – | – | – |
| PD2 | 632.3 | 77.4 | 586.3 | 78.2 | – | – | – | – | – | – | – | – |
| PDI | – | – | 83.1 | 11.1 | – | – | – | – | – | – | – | – |
| PDO | – | – | 181.3 | 24.2 | – | – | – | – | – | – | – | – |
| PGW | 198.4 | 24.3 | 187.2 | 25.0 | 195.2 | 23.0 | – | – | 23.7 | 23.9 | 172.2 | 27.5 |
| PIW | 150.3 | 18.4 | 173.7 | 23.2 | 180.3 | 21.2 | – | – | 22.1 | 22.3 | 173.0 | 27.6 |
| POR | 73.0 | 8.9 | 62.6 | 8.3 | 85.4 | 10.0 | 7.7 | – | 9.6 | 9.7 | 56.2 | 9.0 |
| PP2 | 328.5 | 40.2 | 307.3 | 41.0 | 348.4 | 41.0 | 34.8 | – | 44.4 | 44.8 | 299.0 | 47.7 |
| SL | 816.8 | 100.0 | 750.2 | 100.0 | 850.4 | 100.0 | – | – | 99.0 | 100.0 | 626.2 | 100.0 |
| TAL | 493.5 | 60.4 | 436.8 | 58.2 | 502.0 | 59.0 | – | – | 55.4 | 56.0 | 318.3 | 50.8 |
| TBW | 94.8 | 11.6 | 109.7 | 14.6 | 132.7 | 15.6 | – | – | 13.2 | 13.3 | 103.5 | 16.5 |
| TL | 897.7 | 109.9 | – | – | 925.4 | 108.8 | – | – | – | – | 722.6 | 115.4 |
Table 2.
Summary of selected meristic features used to discriminate fossil and living genera of the family Narcinidae. Includes new data from the examined material and data from Fechhelm & McEachran (1984), Rincon (1997), Carvalho et al. (1999, 2002a, b, 2003), Carvalho (2001, 2008), Rincon et al. (2001), Carvalho & Séret (2002), Carvalho & Randall (2003), Menni et al. (2008), Carvalho & White (2016), Last et al. (2016), Froese & Pauly (2017).
| Feature | Benthobatis | Diplobatis | Discopyge | Narcine | †Titanonarke |
|---|---|---|---|---|---|
| Trunk vertebrae | 13–20 | 23–25 | 20 | 15–31 | 27–30 |
| Precaudal vertebrae | 46–73 | 61–63 | 58–70 | 58–77 | 74–115 |
| Caudal vertebrae | 31–46 | 22–24 | ? | 18–32 | 23–32 |
| Total vertebrae | 96–118 | 97–112 | 85–91 | 100–127 | 133–155 |
| Rib pairs | 4 | 7–9 | 8 | 5–10 | 8–10 |
| Total pectoral radials | ? | 31 | ? | 27–41 | 35–40 |
| Pelvic fin radials | 12–13 | 16–21 | ? | 14–21 | 19–21 |
| First dorsal fin radials | 5–6 | 6–7 | ? | 6–10 | 7–9 |
| Second dorsal fin radials | 6–7 | 7–8 | ? | 6–11 | 6–7 |
| Total caudal fin radials | 34–35 | 33–36 | ? | 39–63 | 41–42 |
| Upper jaw tooth rows | 9–20 | 14–22 | 10–20 | 11–27 | 12–13 |
| Lower jaw tooth rows | 9–22 | 14–22 | 10–20 | 8–30 | 15–17 |
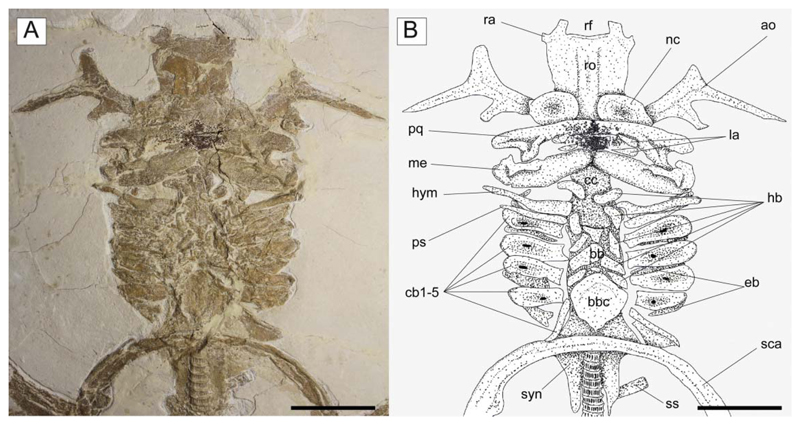
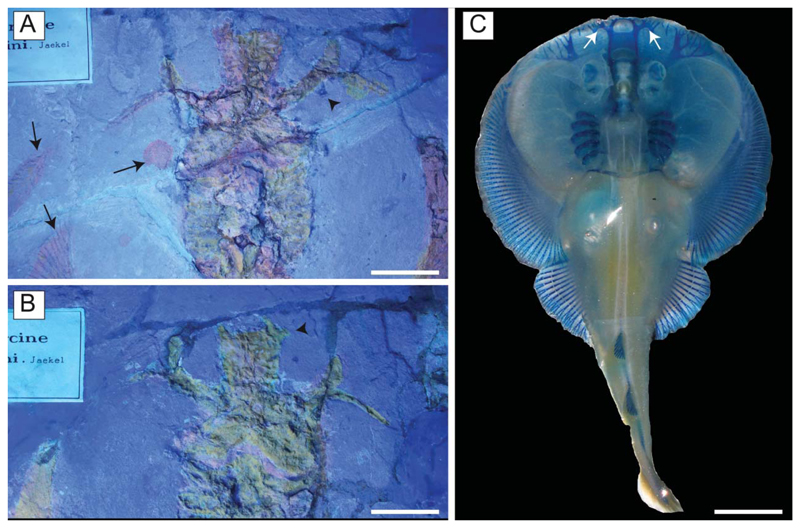
Chondrocranium. The rostral cartilage is expanded, dorsoventrally flattened and trough shaped (Figs 5, 6), resembling the typical condition of narcinids (Miyake et al. 1992). The rostrum is long, about one-half of the cranial length, wide anteriorly and tapering proximally towards the nasal capsules. The anterior margin of the rostral cartilage is concave, indicating the presence of a rostral fontanelle (= part of the precerebral fontanelle of Miyake et al. 1992), that is present also in Benthobatis, Diplobatis, Discopyge and Narcine (Claeson 2014) and which also is supported by the phylogenetic analysis of this study (see below). Small lateral projections (= rostral appendices of Holmgren 1941; lateral rostral cartilage of Carvalho 1999) off of the rostral fontanelle were indicated in the holotype by Carvalho (2010). Due to the grout and/or pigment used on the holotype MGP-PD 26275/6, UV light was not useful to distinguish further details of preserved tissues (see Fig. 7A, B). Ultraviolet light did highlight a possible lateral projection (Fig. 7B), but we cannot discern whether it was branching and connected to the antorbital cartilage as in Narcine brasiliensis (Olfers, 1831) (Fig. 7C). Evidence of a lateral projection off of the rostral fontanelle is, however, clearly present in MCSNV IG.VR.67290 (Fig. 5), but not in juvenile specimens of †Titanonarke that we examined, which thus resemble juveniles of Narcine brasiliensis (Miyake et al. 1992, fig. 14A). The connection of the rostral cartilage to the antorbital cartilage through the lateral projection was also detected in Diplobatis (Fechhelm & McEachran 1984, fig. 5), Benthobatis (Rincon et al. 2001, fig. 7) and all narcinids in general (Fechhelm & McEachran 1984, fig. 16; Carvalho 1999, 2010). This character, which is unique among torpediniforms, corroborates the monophyly and the systematic position of narcinids in this study. The juvenile specimen MCSNV IG.VR.91359 shows the early stage of the development of the rostral cartilage (Fig. 6), with at least one cartilaginous strip (= sensu Miyake et al. 1992) and two lateral rostral bars. The rostral cartilage of †Titanonarke lacks the basonasal fenestrae that characterize Diplobatis (Fechhelm & McEachran 1984). Although probably present on the dorsal surface of the chondrocranium, it is not possible to distinguish the presence of the anterior and frontoparietal fontanelle in the available material.
Figure 5.
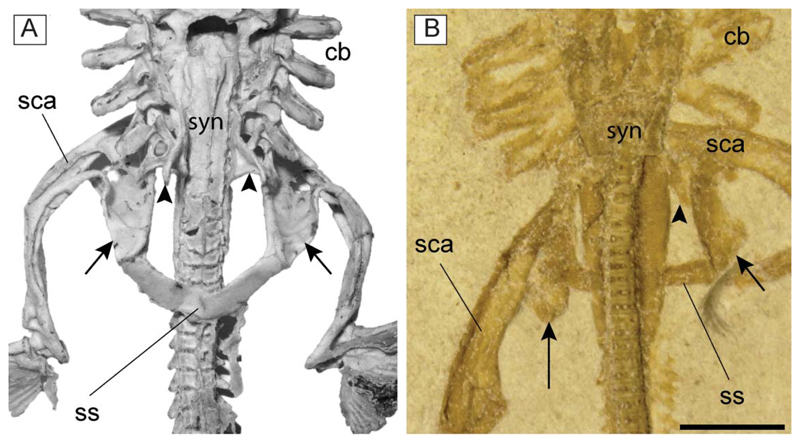
†Titanonarke molini (Jaekel, 1894) from the Eocene Monte Postale site. A, MCSNV IG.VR.67290, close-up of the head and hyoid apparatus; B, reconstruction. Abbreviations: ao, antorbital cartilage; bb, basibranchials; bbc, basibranchial copula; cb, ceratobranchials; cc, chondrocranium; eb, epibranchials; hb, hypobranchials; hym, hyomandibula; la, labial cartilages; me, Meckel’s cartilage; nc, nasal capsule; pq, palatoquadrate; ps, pseudohyoid; ra, rostral appendix; rf, rostral fontanelle; ro, rostral cartilage; sca, scapulocoracoid; ss, suprascapula; syn, synarcual. Scale bars = 50 mm.
Figure 6.
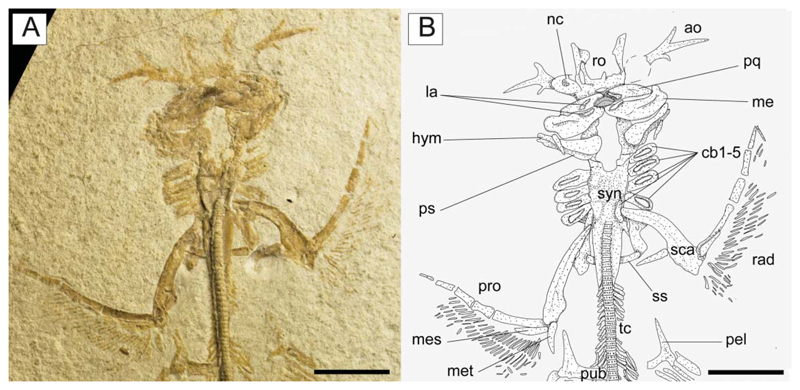
†Titanonarke molini (Jaekel, 1894) from the Eocene Monte Postale site. A, the juvenile individual MCSNV IG.VR.91359; B, reconstruction. Abbreviations: ao, antorbital cartilage; cb, ceratobranchials; hym, hyomandibula; la, labial cartilages; me, Meckel’s cartilage; mes, mesopterygium; met, metapterygium; nc, nasal capsule; pel, prepelvic process; pub, puboischiadic bar; pq, palatoquadrate; pro, propterygium; ps, pseudohyoid; rad, pectoral radials; ro, rostral cartilage; sca, scapulocoracoid; ss, suprascapula; syn, synarcual. Scale bars = 50 mm.
Figure 7.
†Titanonarke molini (Jaekel, 1894) from the Eocene Monte Postale site. A, B, UV images of the holotype MGP-PD 26275/6 showing that it was covered with a pigment reflecting an orange light (see also Supplementary material). The arrows in A indicate a leaf also covered with the pigment, a drop, and some rays that were not distally covered (they are blue/grey, as expected); the arrowhead in A indicates part of the third branch of the antorbital cartilage not covered by pigment; the arrowhead in B indicates the rostral appendix; C, Narcine brasiliensis (TNHC 18512); the arrows indicate the rostral appendices. Scale bars: A, B = 50 mm; C = 10 mm.
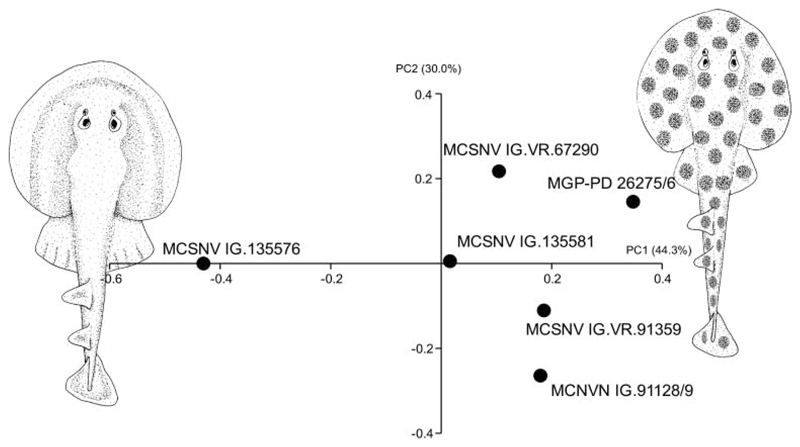
The antorbital cartilages in juveniles and adults are robust, well developed, directed anteriorly and broadly branching (Figs 5, 6). There are no distinct foramina on the anterior cartilages. Antorbitals are subdivided at about one-third of their length into two main branches; their stout bases articulate with the lateral aspect of the nasal capsules through an expanded posterior condyle. The distal end of the largest branch is broadly separated from the anterior extension of the propterygium. A third, smaller, posteriorly directed branch is located at midlength of the antorbital cartilages (Figs 5, 6). The absence of the posterior branch was considered diagnostic for †Titanonarke to the exclusion of Narcine and Discopyge by Carvalho (2010). However, the presence of this posterior branch in MCSNV IG.VR.67290, MCSNV IG. VR.91359, MCSNV IG.135581 and possibly, at least partially, in the holotype (Figs 7A), suggests that it was not discernible in the specimens described by Carvalho (2010). Moreover, since osteological, morphometric and meristic features are not useful to separate juvenile specimens from the holotype, there is no reason to assign this smaller specimens to a new genus, if only based on the presence of this character. In fact, the PCA performed in this study on the entire morphological data set of standardized and log-transformed measurements and counts (Fig. 8) shows no remarkable separation of the juvenile specimens MCSNV IG.VR.91359 and MCSNV IG.135581 from the holotype and other referred material.
Figure 8.
Visual image of the principal component analysis (PCA) performed on the entire set of log-transformed standardized morphometric and meristic features, showing the separation of the specimens referred to †Titanonarke molini (right side of morphospace) from those referred to †Titanonarke megapterygia sp. nov. (left side). The illustrations lying along the extreme values of PC1 represent the hypothetical reconstruction of the two species based on body proportions.
The nasal capsules are ovoid in shape, located at about midlength of the chondrocranium and projected laterally. The internasal plate between the two nasal capsules appears flat and narrow. As all specimens are preserved in dorsoventral view, it was not possible to detect the lateral aspect of the nasal capsules. The otic capsules and the posterior part of the chondrocranium are partially hidden by the jaws and hyoid arches. However, they appear to be approximately as wide as the widest part of the rostrum and much narrower than the nasal capsules. A large basicranial fenestra is recognizable in the juvenile MCSNVIG.VR.91359 (Fig. 6), which resembles in position and shape the condition shown in the early stages of chondrification of the otic region in Narcine brasiliensis (Miyake et al. 1992, fig. 14).
Jaws. Carvalho (2010) considered both palatoquadrate and Meckel’s cartilage of †T. molini broadly arched and not very stout when compared to those of other members of Narcinidae. Given that long, slender and curved jaws are diagnostic for the torpedinoids Torpedo and Hypnos whereas in narcinoid genera the jaws are short and stout (Claeson 2014), the description of the jaws of †Titanonarke is further clarified here. The palatoquadrate of †Titanonarke is labiolingually compressed, narrower than the Meckel’s cartilage, and tapers towards the symphysis (Fig. 5). Like the palatoquadrate of Narcine brasiliensis (see Dean & Motta 2004a), the palatoquadrate of †T. molini possesses a strong condyle that articulates with the Meckel’s cartilage at the mandibular articular fossa sensu Dean & Motta (2004a). The Meckel’s cartilages are stout, flat and broad. There are two pairs of small, slender and subtriangular labial cartilages situated near the symphysis of the jaws, surrounding the tooth bands (Fig. 5). Combined, upper and lower labial cartilages are less than the length of the Meckel’s cartilage. The triangular element displaced to the corner of the left jaw joint in MCSNV IG.135576, and interpreted by Carvalho (2010, fig. 5b) as a labial cartilage, instead appears to be a dorsal flange of the sustentanculum of the Meckel’s cartilage. Both jaws are not fused medially.
Hyoid and gill arches. The exquisite preservation of MCSNV IG.VR.67290 allows a detailed description of most of the hyoid arch (Fig. 5). The hyomandibulae are narrow and elongate, slightly stout at the proximal base, and tapering distally towards their articulation with the Meckel’s cartilage. The dorsal and ventral pseudohyoids are long and slender, and located just posterior to the articulation of the hyomandibula with the otic region of the chondrocranium. †Titanonarke lacks ceratohyals, resembling the condition of Narcine and Discopyge among narcinoids (Miyake & McEachran 1991).
There are five pairs of ceratobranchials. The anterior four pairs are large, and have a central depression (or fossa) with a small fenestra for the insertion of the depressor muscles (see Carvalho & Séret 2002). The fenestrae of †Titanonarke appear larger in small individuals (Fig. 6), as in Diplobatis (Miyake & McEachran 1991, fig. 6H; Claeson 2014, supplemental pl. 12). The fifth ceratobranchials are slender and posteriorly oriented, and articulate with the scapular process of the scapulocoracoid. The epibranchial elements are not clearly recognizable, being partially covered by the ceratohyals. At least three small basibranchials are recognizable. The basibranchial copula is large and rounded, with a small caudal tip or tab in its posterior margin. We counted five pairs of ovoid or bean-like hypobranchials. Hypobranchials are segmented, resembling the plesiomorphic condition of Benthobatis, Discopyge and Heteronarce among narcinoids (Miyake & McEachran 1991, fig. 6F, G; Claeson 2014, supplemental pl. 12). Pharyngobranchials, extrabranchials and branchial rays are not preserved in the available material.
Synarcual and vertebral column. The synarcual cartilage is strongly calcified in all mature specimens, though not in the embryo preserved inside the abdominal cavity of the holotype (see the section ‘Embryo’). In mature specimens the synarcual exhibits the typical prismatic tessellated cartilage found in elasmobranchs. The posteroventralmost portion of tessellated cartilage flanks several mineralized vertebral centra, which comprise densely packed areolar cartilage. Anteriorly, the synarcual cartilage contacts the occipital condyles of the chondrocranium via the occipital cotyles. The morphology of the anterior portion of the synarcual is difficult to discern, it being partially hidden by the hyoid arch (most of the specimens are ventrally exposed). Therefore, the position of the dorsal rim of the anterior neural canal opening (synarcual mouth) with respect to the occipital cotyle and the lips, as well as the shape of the ventral rim of the anterior neural canal opening (synarcual lip), are difficult to discern.
The synarcual possesses lateral stays, which are posteriorly displaced (Fig. 9) as in Benthobatis, Discopyge, Narcine, Heteronarce and Narke (Claeson 2014). The distal end of the lateral stays is apparently tab-like and their anterior margin forms an obtuse angle with the anterolateral margin of the synarcual. The long posterior flanges of the synarcual surround the anteriormost 8–12 free vertebral centra, and their posterior margins end posteriorly to the scapulocoracoid bar, resembling the condition of nonnarkid torpediniforms (Compagno 1999). It is not possible to detect the number of fused vertebrae that constitute the synarcual, or the foramina.
Figure 9.
A, synarcual and pectoral girdle of Narcine brasiliensis (AMNH 95343) in dorsal view; B, synarcual and pectoral girdle of †Titanonarke molini (MCSNV IG.VR.91359) in ventral view. The arrowheads indicate the posteriorly directed lateral stays of the synarcual. The arrows indicate the posteriorly directed scapular process of the scapulocoracoid. Abbreviations: cb, ceratocranchials; sca, scapulocoracoid; ss, suprascapula; syn, synarcual. Scale bar = 5 mm.
The vertebral column of †T. molini consists of about 153–155 vertebral centra; of these, 27–30 are trunk centra (18–20% of total, from the first distinguishable centrum to the anterior margin of the puboischiadic bar), 100–115 are precaudals (65–75%, from the anterior margin of the puboischiadic bars to the upper origin of the caudal fin), and 23–25 are caudals (15–16%, from the upper caudal fin origin to the end of the series). The number of vertebrae is by far the largest compared to all living torpediniforms and can be considered an autapomorphic condition of †Titanonarke (see Table 2).
The vertebral centra are strongly calcified, subrectangular in shape and anteroposteriorly short. Large basiventral processes are visible along most of the vertebral centra, from the posterior tip of the synarcual to the caudal fin base. There are eight to 10 pairs of ribs articulating with centra posterior to the puboischiadic bar (Fig. 11A). The low number of rib pairs is similar to that of Benthobatis, Discopyge, Diplobatis and Narcine and is considered a derived trait of the narcinids among torpediniforms (see the phylogenetic analysis in this study).
Figure 11.
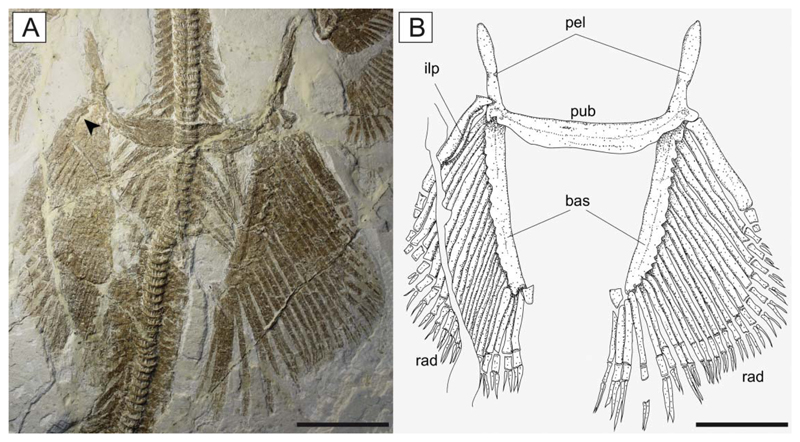
Pelvic fins of †Titanonarke molini (Jaekel, 1894) from the Eocene Monte Postale site. A, MCSNV IG.VR.67290; B, reconstruction. Abbreviations: bas, basipterygia; ilp, iliac process; pel, pelvic processes; pub, puboischiadic bar; rad, pelvic radials. The arrowhead indicates the iliac process. Scale bars = 50 mm.
Paired fins and girdles. The scapulocoracoid is the largest element of the pectoral girdle. It is robust and strongly arched, and its anteriormost margin is situated between the basibranchial copula and the posterior tips of the synarcual flanges. The scapulocoracoid articulates anteriorly with the fifth pair of ceratobranchials. The suprascapulae are slender and fused medially, forming a slightly bowed bar in MCSNV IG.VR.91359 (Fig. 6). In MCSNV IG.VR67290 the suprascapula appears to be inclined with respect to the vertebral column, confirming the interpretation of a bowed shape (Fig. 5). The suprascapular antimere is longer than the scapular process of the scapulocoracoid, which is posteriorly directed as in Narcine (Fig. 9). The fusion of the suprascapular antimere with a visible suture is considered a synapomorphy of Torpediniformes by Claeson (2014). Although MCSNV IG.VR.67290 and MCSNV IG.VR.91359 preserve this skeletal element (Figs 5, 6, 9), it is not possible to describe a visible suture because both specimens are exposed ventrally and this area is obscured by the vertebral column. However, it is expected that †Titanonarke shares this character with all other electric rays.
The juvenile MCSNV IG.VR.91359 shows a weak taphonomic displacement of the suprascapulae with respect to the vertebral column (Figs 6, 9), which supports the hypothesis that the suprascapulae in torpediniforms are completely separate from the vertebral column and that the only connection between pectoral girdle and postcranium is through the fifth ceratobranchial (Aschliman et al. 2012a; Claeson 2014). The same specimen shows that the suprascapular projection of †Titanonarke was lateral and that the suprascapula-scapulocoracoid articulation was loose and unforked.
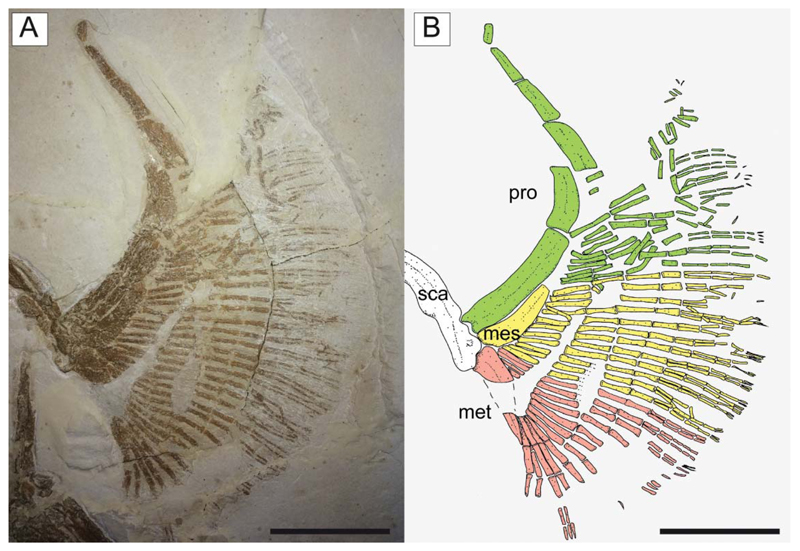
The propterygia are long and arched and extend well beyond the anterior margin of the scapulocoracoid (Fig. 10). They are composed of five or six propterygial segments. Two large voids, occupied in life by electric organs, are delimited by the propterygia, antorbital cartilages, hyoid archs and scapulocoracoids. The mesopterygium is flat, subtriangular in shape, and parallel and adjacent to the propterygium. The metapterygium is long and slender, is triangular in shape, and tapers posteriorly. The mesopterygium is shorter than the pro- and metapterygium in all specimens, although the apparent smaller size of the metapterygium in MCSNV IG.VR.67290 appears to be preservational (the metapterygium is weakly calcified in some narcinids; Carvalho & Séret 2002) or an artefact of the glue used to join pieces of the slab during preparation. There are about 40 pectoral radials (12–16 propterygial, 9–12 mesopterygial and 10–12 metapterygial), which bifurcate twice before reaching the edge of the pectoral fin margin. Each radial is composed of four segments before the first bifurcation, and another four elements before the second bifurcation (at least 9–10 segments in total; Fig. 10). The lower number of segments recognized by Carvalho (2010), and actually detected in some specimens, is probably related to the loss of distal elements due to taphonomic processes. The radials are covered with a continuous layer of small (less than 1 mm) tesserae, forming the so-called ‘crustal’ calcification that characterizes the radials of basalmost batoids having an axial-undulatory swimming style, including pristids and ‘rhinobatids’, other than torpedinids and narcinids (Schaefer & Summers 2005).
Figure 10.
Pectoral fin of †Titanonarke molini (Jaekel, 1894) from the Eocene Monte Postale site. A, MCSNV IG.VR.67290; B, reconstruction; different colours are used to distinguish the propterygial (green), mesopterygial (yellow) and metapterygial (red) radials (colours in the online version). Abbreviations: mes, mesopterygium; met, metapterygium; pro, propterygium; sca, scapulocoracoid. Scale bars = 50 mm.
The pelvic fins (Fig. 11) are small and single-lobed, and their anterior margin is straight and barely overlapped by the posterior margin of the pectoral disc. The maximum width of the pelvic fins is about 50% of the pectoral disc width, whereas their anterior margin is about 24% of the disc length. The puboischiadic bar is robust and wide, with a slightly concave anterior margin. The presence of the puboischiadic foramina is difficult to detect. The prepelvic processes are long and straight, extending anteriorly almost to the level of the scapulocoracoid. They are wider distally than along the shaft, resembling the condition of all narcinids (Rincon et al. 2001, fig. 8; Fechhelm & McEachran 1984, figs 7, 16). The distal end of the prepelvic process was described as spatulate by Carvalho (2010). However, the margin of this structure, identified with an arrowhead on the holotypic specimen MGP-PD 26275/6 by Carvalho (2010, fig. 7), is not part of the prepelvic process. The iliac process, preserved only on the left side of MCSNV IG.VR.67290, appears short, stout and straight (Fig. 11) if compared to the long and curved iliac process of Narcine and Discopyge (Menni et al. 2008; Claeson 2014). The basipterygia are slightly longer than the puboischiadic bar, and have a slightly concave inner margin. Each basipterygium supports about 21–24 pelvic fin radials, the first of which is enlarged, articulates with the lateral node of the puboischiadic bar and supports the anterior margin of the pelvic fin. Each pelvic fin radial bifurcates distally once and is composed of three segments. A single individual (MCSNV IG.91128/9) seems to show an elongate clasper, articulating with the distal tip of the basipterygium (Fig. 12). In length, the clasper appears to extend past the posterior tip of the pelvic fin lobe. However, it was not possible to analyse this structure in detail.
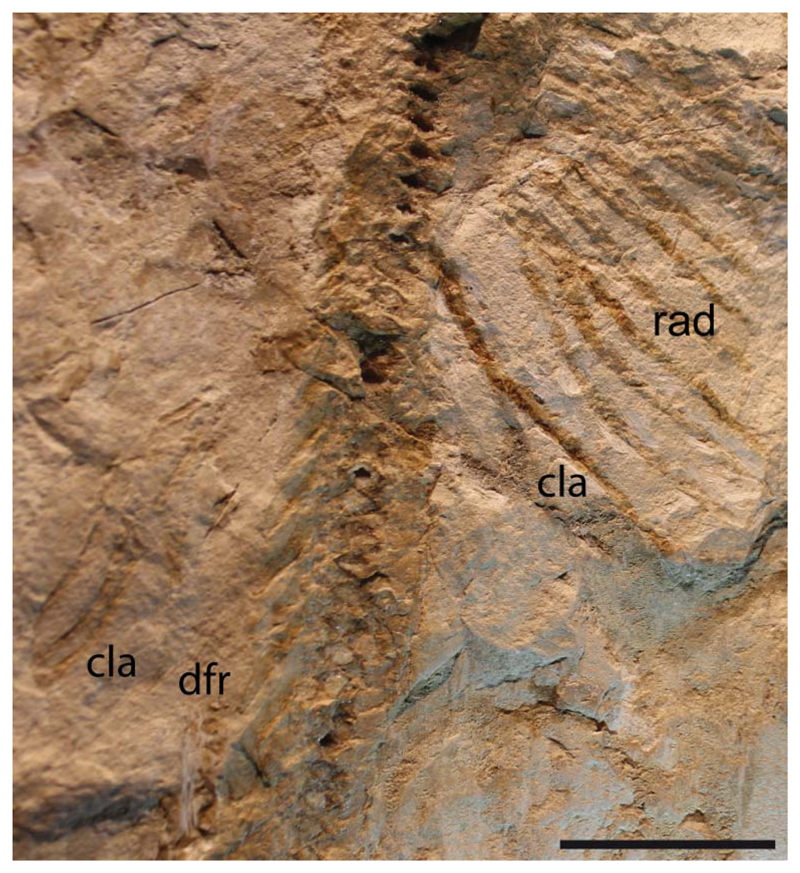
Figure 12.
†Titanonarke molini (Jaekel, 1894) from the Eocene Monte Postale site. Close-up of the distal end of pelvic fins of MCSNV IG.91128, which is supposed to be the unique male individual based on the presence of claspers. Abbreviations: cla, clasper; dfr, first dorsal-fin radials, pelvic radials. Scale bar = 50 mm.
Median fins. There are two dorsal fins (Fig. 13A). The first one originates at about 62% SL, is slightly larger than the second one and is supported by seven to nine radials. The second dorsal fin originates at about 78% SL and is supported by six or seven radials. The interdorsal distance measures about 7% SL. The obvious presence of two dorsal fins in MCSNV IG.VR.67290 supports the interpretation of Jaekel (1894) and Cappetta (2012) regarding the presence of at least one dorsal fin in the holotype MGP-PD 26275/6 (Fig. 13B). The inadequate preservation of the dorsal fins in the historical material prevented their recognition by Carvalho (2010), who erroneously regarded their absence as diagnostic for †Titanonarke. About 42 radials support the caudal fin, of which about 20 are dorsal and 22 ventral (Fig. 13C).
Figure 13.
†Titanonarke molini (Jaekel, 1894) from the Eocene Monte Postale site. Details of the precaudal tail of A, MCSNV IG. VR.67290 and B, MGP-PD 26276. The arrows mark the position of the two dorsal fins in MCSNV IG.VR.67290 and the second dorsal fin in MGP-PD 26276 already detected and figured by Jaekel (1894). C, Detail of the caudal fin of MCSNV IG.91129. Scale bars: A, B = 50 mm; C = 10 mm.
Dentition. The teeth of †Titanonarke are arranged in tooth bands medially across the jaw symphyses, forming a tessellated pavement (Fig. 14). The lower tooth band is wider than the upper one. It is not possible to detect the tooth formula, but the teeth of †T. molini appear to be arranged in at least 12–13 rows in the upper jaw and 15–16 rows in the lower jaw, counted on symphyseal tooth series. The dentition is gradient monognatic heterodont with lateral and posterior tooth crowns becoming slightly lower. However, both upper and lower jaws show very little heterodonty. Sexual heterodonty (studied via the analysis of the specimens with and without claspers) appears to be absent. It is not possible to detect any ontogenetic heterodonty due to the poor-quality preservation of this region in juveniles.
Figure 14.
†Titanonarke molini (Jaekel, 1894) from the Eocene of Monte Postale site. A, upper and lower tooth bands in MCSNV IG.VR.67290, with a close-up of some teeth in the area indicated. B, reconstruction. Abbreviations: la, labial cartilage; me, Meckel’s cartilage; pq, palatoquadrate. Scale bars 5 mm.
The tooth morphology is generally consistent with that of Narcine (see Herman et al. 2002, pls 10, 11). A single narrow, high and subtriangular cusp is present in each tooth. There are no accessory lateral cusplets. The crown base is broad and subcircular, and wider than the cusp length. The width of the cusp is less than half the length of the cutting edges. Cutting edges are blade-like. Lingual and labial ornamentations are absent and the tooth crown is completely smooth. Some teeth display a high and narrow root.
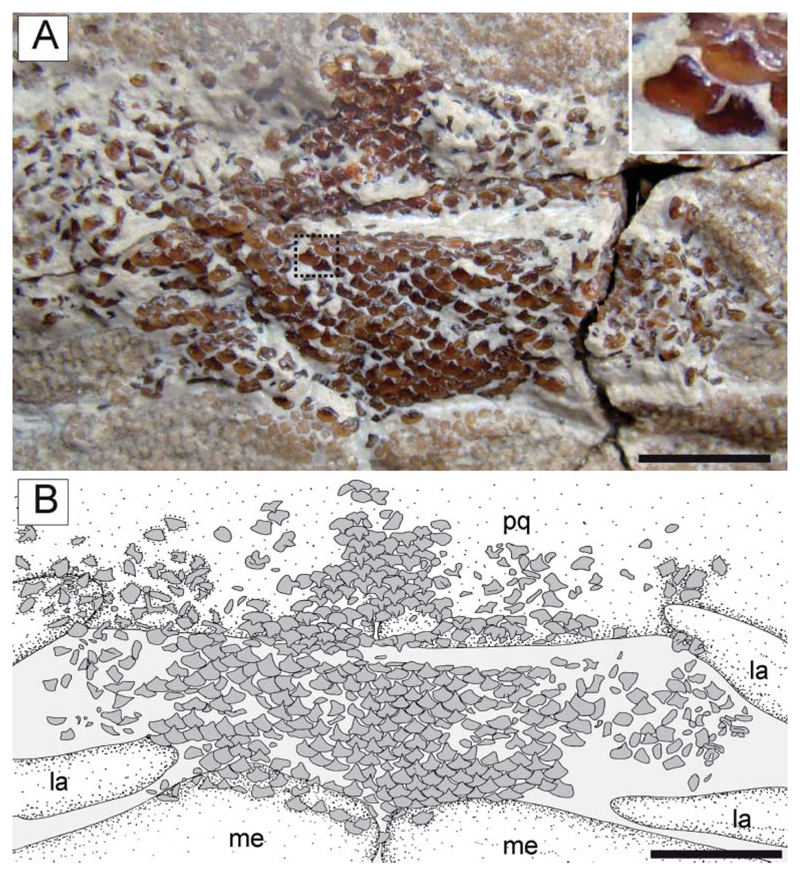
Gut contents. The holotype of †T. molini (MGP-PD 26275/6) shows abdominal contents consisting of a pellet-like accumulation of hundreds of specimens of †Alveolina (Fig. 15A–D), which is the most common foraminiferan genus in the Monte Postale sediments (Papazzoni et al. 2017). The individual foraminifera are grouped together and closely packed, forming an accumulation, which is ovoid in outline and anteroposteriorly elongate, measuring about 80.5 and 41.8 mm in length and width, respectively. The exceptionally preserved gut contents show little evidence of digestion, suggesting that consumption occurred shortly before the numbfish’s death. The accumulation is almost totally preserved in the abdominal cavity between pectoral and pelvic girdles, on one side of the vertebral axis, and just posteriorly to the flanges of the synarcual cartilage. This position is exactly comparable to that occupied by the gut-intestine tract in living electric rays (Fig. 15E), and in narcinids in particular (see Marinsek et al. 2017, fig. 1). Moreover, the general shape of the accumulation resembles fossilized gastric contents detected in other elasmobranchs (e.g. Hovestadt & Hovestadt-Euler 2002; Amalfitano et al. 2017). Additionally, there is no evidence of skeletal dispersal due to currents or bioturbation. Thus, we conclude that the accumulation is not the result of processes related to bottom tractive currents or bioturbation that sometimes can result in the accumulation of such elements (see Schäfer 1972). Consequently, this accumulation of foraminifera unquestionably represents gut contents.
Figure 15.
A, B, †Titanonarke molini (Jaekel, 1894) from the Eocene Monte Postale site; A, detail of the abdominal region in MGP-PD 26275 showing the stomach content; B, reconstruction; note also the embryo lying next to the stomach. C, D, close-up of some of the larger foraminifera of the genus †Alveolina in the stomach of MGP-PD 26275. E, dissected specimen of Torpedo nobiliana in ventral view (ESB tn200707_159) showing the position of the stomach, used to identify the accumulation in MGP-PD 26275 as gut contents. Abbreviations: int, intestine; liv, liver; met, metapterygium; pub, puboischiadic bar; sca, scapulocoracoid; st, stomach, syn synarcual. Scale bars: A, B = 50 mm; C, D = 2 mm.
Embryo. MGP-UP 26275/6 also shows a unique partially developed embryo whose length is estimated to range between 50 and 60 mm (Fig. 16), about 6–7% of the adult size. The embryo comprises an almost complete vertebral column having about 150 vertebrae, whose number is perfectly comparable to that of an adult individual of †T. molini. The vertebral column is partially disarticulated, with some elements scattered from their original position. Each vertebra consists of a vertebral centrum and associated neural arch. There is no trace of the synarcual cartilage. This is consistent with the pattern of mineralization present in extant developing batoid embryos (e.g. Myliobatis, Pristis, Raja; KMC pers. obs.), where the areolar cartilage of the free vertebrae is well mineralized earlier than the tessellated cartilage of the synarcual. The vertebral column is the only fossilized structure in this embryo. There are no cranial or girdle skeletal elements preserved. This condition is consistent with the late phases of skeletogenesis in elasmobranch fishes in which the mineralization of the cartilages involves only teeth, dermal denticles, vertebral centra and neural arches in very early stages (see e.g. Eames et al. 2007; Enault et al. 2016). However, teeth are not clearly recognizable in the embryo, whereas dermal denticles are expected to be absent, as in all torpediniforms.
The embryo is totally preserved in the abdominal cavity between the pectoral and pelvic girdle, on the left side of the vertebral axis, and just next to the stomach (see also Fig. 15). This position is totally comparable to that occupied by the left uterus in fossil (Carvalho et al. 2004, figs 2 and 13) and living batoids (Fig. 16C; but see also Spieler et al. 2013, fig. 6), and in narcinids in particular (see Nair & Soundararajan 1973, fig. 1; Devadoss 1998, fig. 5). The embryo lies externally to the stomach (whose outline is clearly delimited by its contents of larger foraminifera; see Fig. 16A, B) therefore excluding the hypothesis of a possible ingested prey. The absence of traces of egg case surrounding the embryo suggests that the reproductive mode of †T. molini was viviparous (probably yolk-sac), a condition that resembles that of most living batoids (Hamlett & Koob 1999; Kriwet et al. 2009), and narcinids in particular (Hoar & Randall 1988; Bruton 1990; Rincon 1997; McEachran & Carvalho 2002; Last et al. 2016), and is considered plesiomorphic in batoids (Cole 2010). Finally, although the general morphology, size and position of the embryo also resemble those already detected in fossil sharks (e.g. Hovestadt & Hovestadt-Euler 2010; Hovestadt et al. 2010) and extinct freshwater stingrays (Carvalho et al. 2004), the specimen described herein unquestionably represents to our knowledge the first occurrence of a fossilized embryo in situ in marine batoid fishes.
Parasites. The examination of the historical material also has shown that fossilized crustacean isopods are strictly associated with the body of two individuals of both species of †Titanonarke. Individual fishes appear to be infested by two to four isopods each, the analysis of which is beyond the scope of this paper and will be provided in a separate study (Robin et al. in prep.).
†Titanonarke megapterygia sp. nov. (Fig. 17)
Figure 17.
†Titanonarke megapterygia sp. nov. from the Eocene Monte Postale site. A, MCSNV IG.135576; B, detail of the head and hyoid apparatus. Abbreviations: ao, antorbital cartilage; cb, ceratobranchials; hym, hyomandibula; me, Meckel’s cartilage; nc, nasal capsule; pq, palatoquadrate; sca, scapulocoracoid; syn, synarcual; rf, rostral fontanelle; ro, rostral cartilage. Scale bars = 50 mm.
2010 †Titanonarke molini (Jaekel, 1894); Carvalho: 188, figs 3, 5b–c, 8 [pro parte].
Derivation of name. After the Greek words méga, meaning ‘large’, and ptérygia, meaning ‘fins’, referring to the proportionally larger pectoral and pelvic fins compared to those of the type species.
Holotype. MCSNV IG.135576, nearly complete articulated skeleton in a single slab (Fig. 17), 626.2 mm SL.
Type locality and horizon. Monte Postale site, Bolca Konservat-Lagerstätte, Italy; early Eocene, late Ypresian, middle Cuisian, SBZ 11, Alveolina dainelli Zone (see Papazzoni et al. 2017).
Diagnosis. †Titanonarke with large subcircular disc of length c. 53% SL and width 56% SL; precaudal tail c. 51% SL; 136 total vertebrae (27 trunk; 74 precaudal, 32 caudal); total tooth row count c. 32 (15 rows in the upper and 17 in the lower jaw); 35 total pectoral radials (12 propterygial, eight mesopterygial and 15 metapterygial); greatly enlarged single-lobed pelvic fins containing c. 19 basipterygial radials; width of pelvic fins c. 61% of disc width; anterior pelvic fin margin length c. 42% disc length; caudal fin with 41 radials (20 dorsal and 21 ventral).
Remarks. Originally, MCSNV 135576 was considered a holomorphic specimen of †T. molini by Carvalho (2010), although that assignment to the type species was tentative. The new species of †Titanonarke differs from the type species †T. molini (Jaekel, 1894) in a combination of morphometric and meristic characters. The differences mostly include those associated with the number of precaudal vertebrae (74 in †T. megapterygia sp. nov. vs. 100–115 in †T. molini). Consequently, the comparably reduced vertebral number in †T. megapterygia sp. nov. (133 in †T. megapterygia sp. nov. vs. 153–155 in †T. molini) results in different body proportions (see also Table 1). †Titanonarke megapterygia sp. nov. also differs from †T. molini by having a greater head length (c. 27 vs. 23% SL), disc length (53 vs. 44% SL), disc width (56 vs. 50%) and shorter tail (51 vs. 58% SL). However, the main difference in body proportions is in the size of the pelvic fins, which have a larger span (34 vs. 26% SL), anterior margin (23 vs. 11% SL) and base length (17 vs. 13% SL) in †T. megapterygia sp. nov. than in †T. molini. Moreover, the caudal fin also is longer in †T. megapterygia sp. nov. (16% SL) than in †T. molini (9% SL), with a higher number of caudal vertebral centra (32 vs. 23–25, respectively). The PCA performed on the entire morphological data set of standardized and log-transformed measurements and counts (Fig. 8) shows a remarkable separation of specimen MCSNV IG.135576 from all others along PC1 (PCA loading values indicate that this axis is mainly related to the variation in tail length, pelvic fin span, and the number of precaudal vertebrae), thereby suggesting that morphometric and meristic data are useful to separate †T. megapterygia sp. nov. from †T. molini.
Phylogenetic analysis
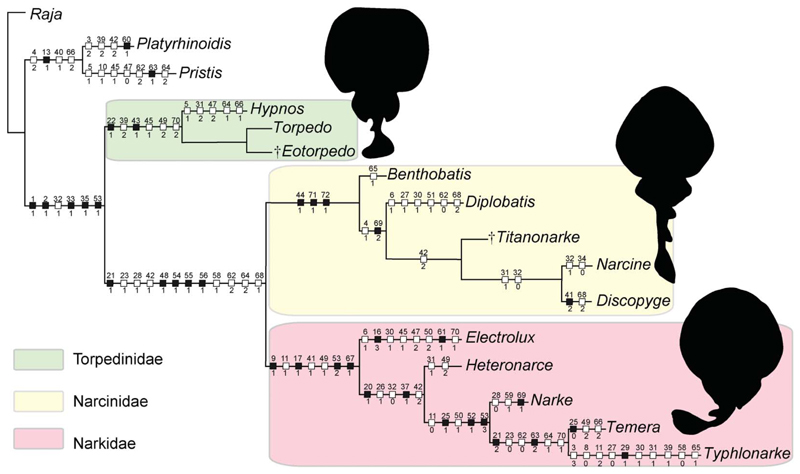
The analysis of 71 morphological characters coded for 16 taxa produced a single most parsimonious tree (MPT) with a length of 170 steps (Fig. 18). The phylogenetic hypothesis of taxa is supported by the following indices: CI = 0.62, RI = 0.68; HI = 0.38. The relationships among torpediniforms are mostly consistent with those of Claeson (2014), although our analysis recovered the family Narcinidae as monophyletic. The monophyly of torpediniforms as recognized by McEachran et al. (1996), McEachran & Aschliman (2004), Aschliman et al. (2012a) and Claeson (2014) is confirmed and supported herein by six synapomorphies: presence of electric organs (char. 1[1]); absence of dermal denticles or thorns (char. 2[1]); iliac process straight (char. 32[1]); long prepelvic process (char. 33[1]); suprascapular antimere fused with visible suture (char. 35[1]); basibranchial copula rounded with a small caudal point/tab (char. 53[1]). Two main clades can be recognized within Torpediniformes: the Torpedinoidea (solely including the family Torpedinidae) and the Narcinoidea (including the sister taxa Narcinidae and Narkidae).
Figure 18.
The single tree retrieved in TNT 1.5 based on 72 morphological characters and 16 taxa, showing the hypothetic relationships of †Titanonarke and †Eotorpedo within the Torpediniformes. Black squares indicate consistency index (CI) = 1.00; white squares CI < 1.00.
The monophyly of Torpedinoidea is supported by the five characters proposed by Claeson (2014): long, slender, flexible jaw cartilages (char. 22[1]); suprascapular antimere shorter than scapular process (char. 39[2]); antorbitals articulate on anterior aspect of nasal capsules (char. 43[1]); frontoparietal fontanelle absent (char. 45[1]); median rostral cartilage inconspicuous or absent (char. 49 [2]). An additional dental character, tooth root low and broad (char. 70[2]), represents a further synapomorphy of the group, and is based on previous descriptions of †Eotorpedo, Torpedo and Hypnos among torpediniforms (Cappetta 1988, 2012; Noubhani & Cappetta 1997; Herman et al. 2002). The absence of labial cartilages does not support the clade because it is considered plesiomorphic for elasmobranchs (see also Claeson 2014). †Eotorpedo in particular was recovered as sister to Torpedo because they share a single tooth cusp, contrary to Hypnos, the only torpediniform characterized by multicuspidate teeth (char. 66[1]) (see also Herman et al. 2002).
The monophyly of all remaining torpediniforms (the clade Narcinoidea) is supported by 12 characters: small labial cartilages that combined are less than the length of the Meckel’s cartilage (char. 21[1]); palatoquadrate labiolingually compressed (narrower than Meckel’s cartilage) and tapering towards symphysis (char. 23[1]); medial margin of pelvic fin lobes attached to precaudal tail (char. 28[1]); antorbital cartilage bifurcating at least once (char. 42[1]); nasal capsules project ventrally (char. 48[1]); dorsal marginal clasper cartilage possesses distomedial extension/medial flange (char. 54[1]); presence of a ligamentous sling on Meckel’s cartilage (char. 55[1]); coracobranchialis consisting of a single component (char. 56[1]); dorsal rim of anterior neural canal opening (synarcual mouth posterior to occipital cotyle) (char. 58[1]); lateral stay located in the posterior third of the synarcual length (char. 62[2]); anterior margin on lateral stay describing an obtuse angle to axis (char. 64[2]); tooth cusp length less than half the length of the cutting edge (char. 68[1]).
We recovered monophyletic Narkidae (Electrolux, Typhlonarke, Heteronarce, Narke and Temera) sensu Claeson (2014). Unlike Claeson (2014), we also recovered a monophyletic family Narcinidae (including Benthobatis, Diplobatis, Discopyge, Narcine and †Titanonarke) that is sister to Narkidae. In this study, the monophyly of the Narcinidae conversely is supported by three unambiguous synapomorphies (CI = 1): presence of a rostral fontanelle (char. 44[1]); low number (up to 10) of rib pairs (char. 71 [1]); and rostral cartilage connected to the antorbital cartilage through a lateral appendix (char. 72[1]).
In the analyses of McEachran et al. (1996), McEachran & Aschliman (2004) and Aschliman et al. (2012a), the monophyly of Narcinidae was not specifically addressed, since they used Narcine as the only representative of the family. The monophyly of the family Narcinidae was tentatively recognized by Carvalho (2010) based on the presence of an expanded and trough-shaped rostral cartilage, presence of rostral fontanelle, and antorbital cartilages articulating with the lateral aspect of nasal capsulae. Compagno (1973) and Fechhelm & McEachran (1984) also considered the Narcinidae to be monophyletic based on a set of synapomorphies including a broad, expanded and trough-shaped rostral cartilage, ventrolaterally directed nasal capsules, forked antler-shaped antorbital cartilages, large precerebral fossa, transverse jaw with labial cartilages, anterior hypobranchials large and meeting midventrally, and large basibranchial copula. Most of these characters in our analysis are considered plesiomorphies shared with outgroups and/or with narkids (see also Claeson 2014). The precerebral fossa and the hyoid and gill arches, however, require further investigation for all those taxa, which was beyond the scope of the present study.
The phylogenetic placement of †Titanonarke within the Narcinidae is evident in our analysis, although its relationships with living numbfishes are not consistent with the hypothesis of Carvalho (2010), who recovered †Titanonarke as the most basal narcinid. The sister-group relationship between †Titanonarke and the monophyletic grouping formed by the most derived narcinids (Narcine + Discopyge) is supported herein by a unique autapomorphy, antorbital cartilage broadly branched, with a third small branch posteriorly directed (char. 42[2]), which Carvalho (2010) hypothesized was absent in †Titanonarke. The sister-group relationship between Narcine and Discopyge detected herein is consistent with that proposed in the analysis of Fechhelm & McEachran (1984), because these two genera also share a long and slender (char. 31 [1]), and curved (char. 32[0]), iliac process of the pelvic girdle, a morphological character not present in †Titanonarke.
An analysis of the data matrix with WinClada 1.00.08 using the same settings resulted in exactly the same phylogenetic hypothesis as TNT. The single MPT has the same length (170 steps) and indices (CI = 0.62; RI = 0.68). The Mesquite analysis produced 87 MPTs; in the strict consensus tree, however, all branches are collapsed, resulting in an extended polytomy. The majority rule tree, conversely, displays the same systematic arrangement of taxa, but required more steps (180). The RI is identical (0.68) and the results for the CI are slightly improved (0.64). These results indicate that the characters employed in our TNT analysis are quite robust and the resulting systematic arrangement is very stable.
Discussion
Comparison and relationships
The analysis of the skeletal morphology of †Titanonarke has revealed the presence of several characters that unquestionably support the inclusion of this genus within the Torpediniformes, including large voids between the axial and pectoral skeleton that suggest the accommodation of massive electric organs, skin without dermal denticles, anteriorly directed antorbital cartilages, long prepelvic processes, and rounded basibranchial copula with a small caudal tab (see Davy 1829; Compagno 1973, 1977; Claeson 2014). The suprascapula with a visible median suture, regarded as a further torpediniform synapomorphy (Claeson 2014), is not exposed in the examined material. Additional features that align †Titanonarke with torpediniforms, although considered plesiomorphic within Batoidea (Compagno 1977; Maisey 1984; McEachran et al. 1996; Carvalho 2010), include some aspects of the branchial arch structure, the presence of a power-stocking precaudal tail, a posteriorly arched scapulocoracoid, and the propterygium being longer than the metapterygium.
The assignment of †Titanonarke to the superfamily Narcinoidea is supported by a number of features (see Herman et al. 2002; Claeson 2014), including the presence of labial cartilages, palatoquadrate labiolingually compressed and tapered towards the symphysis, bifurcated antorbital cartilage, lateral stays located on posterior third of synarcual length, anterior margin of the lateral stay approximately forming an obtuse angle with axis, and tooth cusp length less than half the cutting edge length. Furthermore, †Titanonarke is excluded from Torpedinoidea (†Eotorpedo, Hypnos and Torpedo) because of the lack of several unambiguous characters diagnostic for this superfamily, including long, slender and flexible jaw cartilages, suprascapular antimere shorter than scapular process, antorbital cartilages articulating on anterior aspect of nasal capsules, inconspicuous or absent rostral cartilage, and low and broad tooth root (see Herman et al. 2002; Claeson 2014).
†Titanonarke is a member of a monophyletic Narcinidae, which is supported by three unambiguous synapomorphies: the presence of a rostral fontanelle, the reduced number of ribs, and rostral appendices connected to the antorbital cartilage. Other plesiomorphic features characterizing the Narcinidae (Carvalho et al. 1999; McEachran & Carvalho 2002; Last et al. 2016) and observed in †Titanonarke include a large subcircular disc; a tail longer than the disc; two dorsal fins, with the first one originating posterior to the anterior half of the body length; and single-lobed pelvic fins. †Titanonarke also is characterized by a mesopterygium that is shorter than the pro- and metapterygia, a trough-shaped and expanded rostral cartilage, and a rounded basibranchial copula with a small caudal tip. †Titanonarke is excluded from Narkidae (Electrolux, Heteronarce, Narke, Temera and Typhlonarke), which shows instead a higher number of ribs, absence of rostral fontanelle and appendix, rostral cartilage slender, inconspicuous or absent, mesopterygium longer than the metapterygium, and a heart-shaped basibranchial copula (Claeson 2014). Within the Narcinidae, †Titanonarke shares with the most derived Narcine and Discopyge at least one synapomorphy (broadly branched antorbital cartilage with a posteriorly directed third branch). Therefore, we can exclude the hypothesis that †Titanonarke represents a basal narcinid (see Carvalho 2010).
†Titanonarke is by far the largest narcinid (up to about 1 m TL, compared to living narcinids that usually have an average body size of less than 50 cm TL; Carvalho et al. 1999; Carvalho 2010; McEachran & Carvalho 2002; Last et al. 2016). Moreover, †Titanonarke differs from other narcinid genera in its unique combination of osteological features. It can be separated from Benthobatis by the position of the anteriormost free vertebral centrum, which is posterior to the synarcual in this living genus, but surrounded by posterior flanges of the synarcual in †Titanonarke. Discopyge possesses a mesopterygium that is longer than the pro- and metapterygia, a condition significantly different from that observed in †Titanonake, in which the mesopterygium is shorter than both pro- and metapterygia. Specimens of †Titanonake can be separated from Diplobatis and Narcine by the hypobranchial configuration (fused in Narcine and Diplobatis, unfused in †Titanonarke), and the position of the lateral stay on the synarcual (midway along its length in Diplobatis, posterior in †Titanonarke). †Titanonarke can be further separated from Narcine and Discopyge by the absence of a long, slender and curved iliac process.
Moreover, †Titanonarke differs from other narcinid genera in its unique combination of meristic features (Table 2). It can be distinguished from all living numbfishes by the largest number of vertebrae (133–155 vs. 96–127). In particular, †Titanonarke can be separated from Benthobatis, Diplobatis and Discopyge because of its higher number of trunk (27–30 vs. 13–25, respectively) and precaudal (74–115 vs. 46–73, respectively) vertebral centra. The number of rib pairs is useful to separate †Titanonarke (8–10) from Benthobatis (four). †Titanonarke differs from Diplobatis in having a higher total number of pectoral radials (35–42 vs. 31), from Diplobatis and Benthobatis in the higher number of caudal fin radials (41–42 vs. 33–36), and from Benthobatis in having a higher number of pelvic radials (19–24 vs. 12–13).
Palaeobiogeography of Torpediniformes
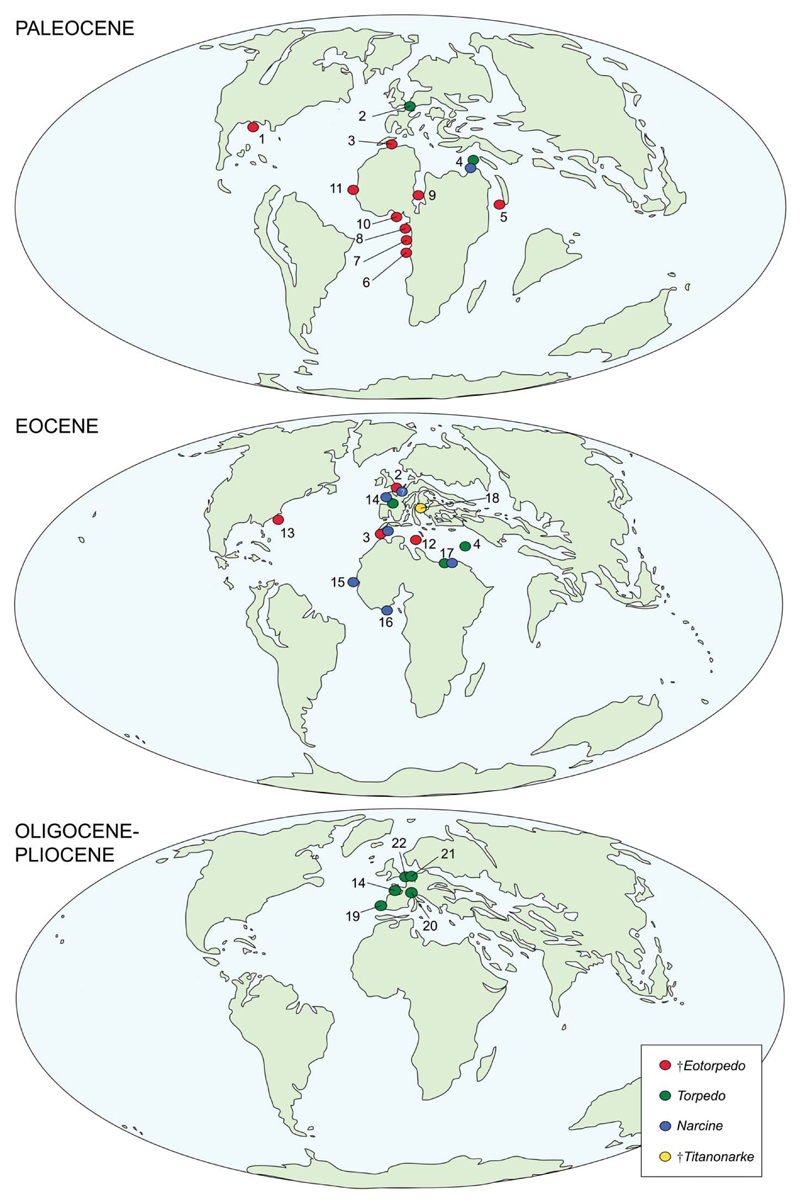
Except for †Titanonarke, the fossil record of torpediniforms (Fig. 19) is heavily biased towards isolated teeth. The oldest electric ray appears to be †Eotorpedo White, 1934, based on teeth from the upper Paleocene of Gada, Nigeria, whose morphology resembles that of Torpedo. The torpedinoid †Eotorpedo (including the species †E. jaekeli White, 1934, †E. hilgendorfi (Jaekel, 1904) and †E. zennaroi Cappetta, 1988) appears to have been wide-spread during the Paleocene, with several occurrences from the Danian and Thanetian of North and western Africa, Saudi Arabia and Texas (Jaekel 1904; White 1934; Dartevelle & Casier 1943; Arambourg 1952; Cappetta 1972, 1987, 1988, 2012; Madden et al. 1995; Noubhani & Cappetta 1997; Siguendibo Sambou et al. 2017). During the Paleocene the extant genera Torpedo and Narcine are scarcely represented, with only a few occurrences from Belgium and Jordan (Smith 1999; Cappetta 2012).
Figure 19.
Palaeobiogeographical distribution of the Torpediniformes during the Cenozoic: 1, Texas; 2, Belgium; 3, Morocco; 4, Jordan; 5, Saudi Arabia; 6, Enclave of Cabinda; 7, Nigeria; 8, Niger; 9, Cameroun; 10, Senegal; 11, Tunisia; 12, South Carolina; 13, France; 14, Guinea-Bissau; 15, Togo; 16, Egypt; 17, Italy; 18, Portugal; 19, Switzerland; 20, Germany; 21, Netherlands. Data from Hasse (1879), Jaekel (1904), White (1934), Dartvelle & Casier (1943), Arambourg (1952), Cappetta et al. (1967, 2000), Cappetta (1972, 1987, 1988, 2012), Herman (1974), Banks (1978), Cappetta & Traverse (1988), Cappetta & Nolf (1991), Bolliger et al. (1995), Madden et al. (1995), Noubhani & Cappetta (1997), Antunes et al. (1999), Smith (1999), Bracher (2005), Reinecke et al. (2005), Adnet (2006), Knight et al. (2007), Adnet et al. (2010), Carvalho (2010), Mollen (2010), Underwood et al. (2011), Case et al. (2015), Reinecke (2015) and Siguendibo Sambou et al. (2017). Maps are modified from Scotese (2002).
Although torpedinoids are well represented in the Eocene, with the most recent records of †Eotorpedo (†E. hilgendorfi, †E. nolfi), and some occurrences of Torpedo from northern Africa, Belgium, France, Jordan, and South Carolina, USA (Arambourg 1952; Herman 1974; Banks 1978; Cappetta 1988; Noubhani & Cappetta 1997; Cappetta et al. 2000; Adnet 2006; Cahuzac et al. 2007; Knight et al. 2007; Underwood et al. 2011; Case et al. 2015), the Eocene also marks the first major radiation of narcinoids. In addition to †Titanonarke molini and †T. megapterygia from the Ypresian of Italy, a broad distribution of Narcine is documented by several Ypresian to Priabonian occurrences in France, Guinea-Bissau, Togo, Morocco and Egypt (Cappetta 1987, 1988, 2012; Cappetta & Traverse 1988; Noubhani & Cappetta 1997; Adnet 2006; Adnet et al. 2010; Underwood et al. 2011). A single vertebra from the Eocene of Belgium referred by Hasse (1879) to Narcine requires further investigation before assignment to this genus can be confirmed (Carvalho 2010).
The fossil record of the Torpediniformes is scarce from the Oligocene to the Pliocene and is only represented by Torpedo from the Chattian to the Zanclean of Portugal, Switzerland, Germany, France and the Netherlands (Cappetta et al. 1967; Cappetta 1987, 2012; Cappetta & Nolf 1991; Bolliger et al. 1995; Antunes et al. 1999; Bracher 2005; Reinecke et al. 2005; Mollen 2010; Reinecke 2015). Teeth referred to Narcine from the Miocene of Portugal (Jonet 1968) and India (Sahni & Mehrotra 1981) cannot be referrred to any member of the Torpediniformes according to Cappetta (2012). The scarcity of torpediniforms in the Oligocene and Neogene fossil record may be related to sampling and/or taphonomic biases rather than a genuine biological and/or ecological signal, because extant electric rays have a worldwide distribution from tropical to temperate seas (Carvalho et al. 1999; McEachran & Carvalho 2002; Last et al. 2016).
Although torpediniforms are known in the fossil record only in Cenozoic deposits, several lines of evidence indicate that electric rays should have been present at least since the Late Cretaceous (see Aschliman et al. 2012b; Guinot & Cavin 2016). It has been suggested that the appearance and rapid diversification of nectobenthic durophagous/opportunistic predators such as dasyatoids, myliobatoids, rajoids and torpediniforms in the aftermath of the K–Pg boundary was related to the ecological replacement of extinct prey with similar adaptations, including demersal durophagous benthic dwellers such as †hypsobatids, †parapalaeobatids and †rhombodontids (Kriwet & Benton 2004; Guinot & Cavin 2016). Electric rays, particularly Torpedinidae, together with carcharhinid and isurid selachians, played a fundamental role during the recovery of the full diversity of elasmobranchs in the aftermath of the end-Cretaceous extinction event (Kriwet & Benton 2004).
Palaeobiological remarks
The potential palaeobiological and palaeoecological role of †Titanonarke has been poorly investigated up to now, considering the presence of several individuals of different ontogenetic stages in the Monte Postale palaeobiotope. Extant numbfishes of the family Narcinidae are typically inshore to deep-water torpediniforms, with a worldwide distribution, inhabiting warm-temperate to tropical waters of continental and insular shelves. They occur down to about 1000 m depth, although they usually are found within 250 m, mostly occurring off soft sandy beaches and in muddy enclosed bays, often associated with coral reefs (Carvalho et al. 1999; McEachran & Carvalho 2002; Last et al. 2016). From this perspective, the presence of at least two species of †Titanonarke, the relative abundance of specimens and a gravid female suggest close affinities of this taxon with shallow-water habitats associated with coral reefs, as hypothesized for the Monte Postale palaeobiotope (Marramà et al. 2016c; Vescogni et al. 2016).
The stomach contents of MGP-PD 26275/6 provide a rare example of feeding relationships at a mid-high level of the trophic network in a reef-associated fish community in the context of the Early Eocene Climatic Optimum. The stomach contents of the holotype of †T. molini indicate that this numbfish fed on larger benthic foraminifera of the genus †Alveolina, at least occasionally, representing the first unambiguous and direct evidence of feeding behaviour in extinct electric rays. Modern narcinoids prey upon benthic invertebrates (mostly polychaetes and crustaceans, rarely molluscs) and small bony fishes using their highly specialized protrusible feeding apparatus (Carvalho et al. 1999; McEachran & Carvalho 2002; Last et al. 2016; Froese & Pauly 2017). Narcinids, in particular, are gape-limited polychaete specialists that use their highly calcified and protractile jaws as an excavation tool (Bigelow & Schroeder 1953; Dean & Motta 2004a, b). Gut content analyses of extant Narcine species have shown that polychaete annelids are the dominant prey item, whereas soft-bodied invertebrates and small bony fishes make up only a minor percentage of the diet (McKay 1966; Amaral & Migotto 1980; Rudloe 1989; Goitein et al. 1998; Carvalho 1999; Bornatowski et al. 2006; Cerqueira Ferreira & Vooren 2012). The labial cartilages play a fundamental role in limiting the circular gape of jaw protrusion during the suction phase, therefore enhancing the buccal pressure to suck in polychaetes (Dean & Motta 2004a, b). The specialized jaw apparatus morphology of narcinids is unique among batoids and it is probably related to a specialization that strongly constrains their ecological niche (Dean & Motta 2004a). The morphology of the jaw apparatus of †Titanonarke is similar to that of other narcinids, although it has a more slender hyomandibula. However, to the best of our knowledge, no evidence of feeding activity on benthic foraminifera has been reported in extant torpediniforms (data on food items are also available in Froese & Pauly 2017) or in other fossil or extant cartilaginous fishes. Among bony fishes the only known genus specialized in feeding on foraminifera is the living leopard wrasse Macropharyngodon (see Randall 1978) whose lineage and unique specialized feeding mode evolved apparently in the early Miocene (Cowman et al. 2009). Although fossil polychaetes have been reported in the Bolca Lagerstäatte (Alessandrello 1990), and we do not exclude that this giant electric ray also fed on them, the abundance of larger foraminifera as stomach contents seems to suggest a selective predation of this kind of food rather than accidental ingestion that sometimes can occur in coral reef fishes (e.g. Daniels & Lipps 1978; Lipps 1988; Debenay et al. 2011). From this perspective, this peculiar and unique occurrence of predator-prey relationship between †Titanonarke and †Alveolina might represent a further feeding specialization among torpediniforms early in their evolutionary history, or at least it can be considered an opportunistic strategy in a context of remarkable abundance of this trophic resource in the Tethys realm during the Eocene (see e.g. Renema et al. 2008; Scheibner & Speijer 2008; Papazzoni et al. 2017).
The large size of †Titanonarke in relation to the small size of its prey is very striking, especially if compared to the generally smaller size of extant narcinids (see Carvalho 1999; McEachran & Carvalho 2002; Last et al. 2016), which are able to ingest polychaetes that may be as long as their total body length (Dean & Motta 2004a, b). The relative size difference between †Titanonarke as predator and its prey might support the hypothesis that larger marine fish predators could gain a competitive advantage by feeding on both small and large prey, with the latter being unavailable to smaller predators (Scharf et al. 2000). Moreover, it has been demonstrated that marine predators tend to select small prey instead of larger prey when possible (Gillen et al. 1981; Hoyle & Keast 1987; Hart & Hamrin 1990; Juanes 1994). Thus, the consistent inclusion of small prey in the diet of large predators might represent an opportunistic strategy in terms of benefit-cost ratio (Scharf et al. 2000), since the energetic costs of an active predatory lifestyle are less sustainable with an increase in size, and these predators show a clear tendency to prefer less-active feeding strategies, such as filtering or scavenging (Ferrón et al. 2017). Variation in size-based feeding strategies among predators can be related to differences in foraging tactics, predator gape allometry and morphological specializations, as well as individual behaviour and morphology of prey (Scharf et al. 2000). Marine fish predators seemingly become more successful with size due to a variety of factors including an increase in sustained and burst swimming speed (Keast & Webb 1966; Webb 1976; Beamish 1978; Blaxter 1986). The high number of precaudal vertebrae and the consequent proportionally longer tail of †Titanonarke with respect to extant narcinoids might suggest an increased swimming performance, because in torpediniforms, contrary to most batoids, the precaudal tail and the caudal fin are the primary propellers (Roberts 1969; Rosenberger 2001; Claeson 2014).
It is well established that the early Palaeogene is marked by high origination rates of bony and cartilaginous fish lineages, related at least in part to opportunistic ecological niche-filling scenarios in pelagic and benthic realms (Walker & Brett 2002; Kriwet & Benton 2004; Friedman 2009, 2010; Guinot & Cavin 2016; Marramá et al. 2016a, b; Bellwood et al. 2017). Moreover, the appearance of modern coral reef ecosystems during the early Palaeogene allowed the exploitation of new ecological resources, as highlighted by the development and expansion of piscine herbivory, high-precision benthic feeding, nocturnal feeding and ambush predation (Bellwood 2003; Goatley et al. 2010; Schmitz & Wainwright 2011; Bellwood et al. 2014; Floeter et al. 2017; Marramá & Carnevale 2017b). A second wave of innovations in feeding mode, including coral feeding, foraminifera feeding, particulate feeding and fish cleaning, arose during the Oligocene/Miocene transition, and were linked to the expansion of scleractinian-dominated reefs (Cowman et al. 2009; Bellwood et al. 2017; Floeter et al. 2017). However, larger foraminifera of the genus †Alveolina might have represented an abundant and unexploited food source for fishes of the Bolca palaeobiotope well before the Oligocene/Miocene boundary. From this perspective, the Eocene †Titanonarke might be considered the first documented evidence of a novel feeding strategy experimented with by the Torpediniformes in the context of the massive adaptive fish radiation in the aftermath of the end-Cretaceous extinction.
Conclusions
The excellent preservation of the †Titanonarke specimens from the Bolca Lagerstätte allowed a detailed reinterpretation of the morphology of this Eocene numbfish. A new species of †Titanonarke also has been recognized, based on several morphological differences with respect to the type species. A monophyletic Narcinidae has been unquestionably recovered based on parsimony analyses, with †Titanonarke occupying a more derived position compared to previous hypotheses. The presence of a fossilized embryo in situ in a marine batoid fish is reported here for the first time, providing evidence of viviparity in narcinids already in the early Eocene. Moreover, the analysis of the gut contents and the large size of †Titanonarke indicate that the early Palaeogene radiation of torpediniforms was characterized by the exploration of novel feeding strategies, remarkably different from those of extant polychaete-specialized numbfishes. The emergence of a novel feeding adaptation in the Eocene is particularly intriguing if considered in the context of the coeval extensive adaptive radiation in several bony and cartilaginous fish lineages that took place to fill the ecological roles left unoccupied by the biodiversity lost via the end-Cretaceous extinction (see Kriwet & Benton 2004; Friedman 2009, 2010; Guinot & Cavin 2016; Marramà et al. 2016a, b; Bellwood et al. 2017).
Supplemental data
Supplemental material for this article can be accessed at: https://doi.org/10.1080/14772019.2017.1371257.
Acknowledgements
The authors thank Roberto Zorzin and Anna Vaccari (MCSNV), Massimo Cerato (Museo dei Fossili di Bolca) and Mariagabriella Fornasiero (MGP-PD) for access to the facilities and material under their care. Special thanks are due to Stefano Castelli (Università degli Studi di Padova) for some of the photographs, Letizia Del Favero (MGP-PD) and Davide Quagliotto (Padova) for technical support in using the UV light, Luca Giusberti (Università degli Studi di Padova) for useful suggestions, and Erika Giacomel (Mortara) for her help in the search of some taxonomic authorships. The manuscript was improved by the constructive reviews provided by Todd D. Cook (Penn State Behrend) and an anonymous reviewer, and editorial comments from Zerina Johanson. The research was supported by the Austrian Federal Ministry of Science, Research and Economy (OeAD; Ernst Mach Grant ICM-2016-03318 to GM), and by grants (ex-60% 2016 and 2017 to GC) from the Università degli Studi di Torino.
Footnotes
ORCID
Giuseppe Marramà http://orcid.org/0000-0002-7856-5605
Jürgen Kriwet http://orcid.org/0000-0002-6439-8455
References
- Adnet S. Nouvelles faunes de selaciens (Elasmobranchii, Neoselachii) de l’Eocene moyen des Landes (Sud-Ouest, France). Implication dans la connaissance des communautes d’eaux profondes. Palaeo Ichthyologica. 2006;10:1–128. [Google Scholar]
- Adnet S, Cappetta H, Tabuce R. A Middle–Late Eocene vertebrate fauna (marine fish and mammals) from southwestern Morocco; preliminary report: age and palaeobiogeographical implications. Geological Magazine. 2010;147:860–870. [Google Scholar]
- Alcock A. A note on the deep-sea fishes, with descriptions of some new genera and species, including another probably vivparous Ophidioid. Annals and Magazine of Natural History, (Series 7) 1898;2:136–156. [Google Scholar]
- Alessandrello A. A revision of the annelids from the Eocene of Monte Bolca (Verona, Italy) Studi e ricerche sui giacimenti terziari di Bolca. 1990;6:175–214. [Google Scholar]
- Amalfitano J, Dalla Vecchia FM, Giusberti L, Fornaciari E, Luciani V, Roghi G. Direct evidence of trophic interaction between a large lamniform shark, Cretodus sp., and a marine turtle from the Cretaceous of northeastern Italy. Palaeogeography, Palaeoclimatology, Palaeoecology. 2017;469:104–121. [Google Scholar]
- Amaral ACZ, Migotto AE. Importância dos anelídeos poliquetas na alimentaçao da macrofauna demersal e epibentônica da região de Ubatuba. Boletim do Instituto Oceanográfico. 1980;29:31–35. [Google Scholar]
- Antunes MT, Balbino AC, Cappetta H. Selaciens du Miocene terminal du Bassin d’Alvalade (Portugal). Essai de synthese. Ciencias da Terra (Universidade Nova de Lisboa) 1999;13:115–129. [Google Scholar]
- Arambourg C. Les vertébrés fossiles des gisements de phosphates (Maroc-Algeérie-Tunisie) Notes et Mémoires du Service Géologique du Maroc. 1952;92:1–372. [Google Scholar]
- Aschliman NC, Claeson KM, McEachran JD. Phylogeny of Batoidea. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. 2nd edition. CRC Press; Boca Raton: 2012a. pp. 57–96. [Google Scholar]
- Aschliman NC, Nishida M, Miya M, Inoue JG, Rosana KM, Naylor GJP. Body plan convergence in the evolution of skates and rays (Chondrichthyes: Batoidea) Molecular Phylogenetics and Evolution. 2012b;63:28–42. doi: 10.1016/j.ympev.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Banks RS. Stratigraphy of the Eocene Santee Limestone in three quarries of the Coastal Plain of South Carolina. Geological Notes. 1978;21(3):85–149. [Google Scholar]
- Bannikov AF, Carnevale G. Bellwoodilabrus landinii, a new genus and species of labrid fish (Teleostei: Perciformes) from the Eocene of Bolca. Geodiversitas. 2010;32:201–220. [Google Scholar]
- Beamish FWH. Swimming capacity. In: Hoar WS, Randall DJ, editors. Fish physiology. Academic Press; New York: 1978. pp. 101–187. [Google Scholar]
- Bellwood DR. The Eocene fishes of Monte Bolca: the earliest coral reef fish assemblage. Coral Reefs. 1996;15:11–19. [Google Scholar]
- Bellwood DR. Origins and escalation of herbivory in fishes: a functional perspective. Paleobiology. 2003;29(1):71–83. [Google Scholar]
- Bellwood DR, Goatley CHR, Bellwood O. The evolution of fishes and corals on reefs: form, function and interdependence. Biological Reviews. 2017;92(2):878–901. doi: 10.1111/brv.12259. [DOI] [PubMed] [Google Scholar]
- Bellwood DR, Goatley CHR, Brandl SJ, Bellwood O. Fifty million years of herbivory on coral reefs: fossils, fish and functional innovations. Proceedings of the Royal Society, Series B. 2014;281:20133046. doi: 10.1098/rspb.2013.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow HB, Schroeder WC. New genera and species of batoid fishes. Journal of Marine Research. 1948;7:543–566. [Google Scholar]
- Bigelow HB, Schroeder WC. Fishes of the Western North Atlantic. Part 2. Sawfishes, guitarfishes, skates, rays, and chimaeroids. Memoirs of the Sears Foundation for Marine Research. 1953;1:1–514. [Google Scholar]
- Blaxter JHS. Development of sense organs and behavior of teleost larvae with special reference to feeding and predator avoidance. Transactions of the American Fisheries Society. 1986;115:98–114. [Google Scholar]
- Blot J. La faune ichthyologique des gisements du Monte Bolca (Province de Verone, Italie). Catalogue systématique présentant l’état actuel des 160 recherches concernant cette faune. Bulletin du Muséum national d’Histoire naturelle, Paris. 1980;2:339–396. [Google Scholar]
- Bolliger T, Kindlimann R, Wegmüller U. Die marinen Sedimente (jüngere OMM, St. Galler-Formation) am Südwestrand der Hörnlischüttung (Ostschweiz) und die palökologische Interpretation ihres Fossilinhaltes. Eclogae Geologicae Helvetiae. 1995;88(3):885–909. [Google Scholar]
- Bonaparte CL. Selachorum tabula analytica. Nuovi Annali della Science Naturali Bologna. 1838;1(2):195–214. [Google Scholar]
- Bornatowski H, Abilhoa V, Freitas MO. Sobre a alimentacão de Narcine brasiliensis na Baía de Ubatuba-Enseada, São Francisco do Sul, SC, Brasil. Revista Estudos de Biologia. 2006;28:57–60. [Google Scholar]
- Bracher H. Untermiozäne Haie und Rochen. Privately published; Altheim: 2005. p. 183. [Google Scholar]
- Bruton MN. Alternative life-history styles of fishes. Developments in environmental biology of fishes 10. Kluwer Academic Publishers; Dordrecht: 1990. p. 327. [Google Scholar]
- de Buen F. Catalogo ictiologico del Mediterraneo espanol y de Marruecos recopilando 10 publicado sobre peces de las costas mediterranea y proximas del Atlantico (Mar de Espana) Resultado de las campañas realizadas por acuerdos internacionales. 1926;2:1–221. [Google Scholar]
- Cahuzac B, Adnet S, Cappetta H, Vullo R. The fossil species and genera of Selachians fishes (Cretaceous, Tertiary) created in the Aquitaine Basin; inventory, taxonomics. Bulletin de la Société, Linnéenne de Bordeaux. 2007;142(35):3–43. [Google Scholar]
- Cappetta H. Les poissons crétacés et tertiaires du bassin des Iullemmeden (République du Niger) Palaeovertebrata. 1972;5(5):179–251. [Google Scholar]
- Cappetta H. Les selaciens du Cretace superieur du Liban. II: Batoides. Palaeontographica, Abteilung A. 1980;168:149–229. [Google Scholar]
- Cappetta H. Handbook of paleoichthyology, 3B – Chondrichthyes II – Mesozoic and Cenozoic Elasmobranchii. Gustav Fischer Verlag; Stuttgart; 1987. p. 193. [Google Scholar]
- Cappetta H. Les Torpediniformes (Neoselachii, Batomorphii) des phosphates du Maroc. Observations sur la denture des genres actuels. Tertiary Research. 1988;10(1):21–52. [Google Scholar]
- Cappetta H. Handbook of Paleoichthyology – Chondrichthyes – Mesozoic and Cenozoic Elasmobranchii: Teeth. Verlag Dr. Friedrich Pfeil; Munich: 2012. p. 512. [Google Scholar]
- Cappetta H, Nolf D. Les sélaciens du Pliocène inférieur de Le-Puget-sur-Argens (Sud-Est de la France) Palaeontographica, Abteilung A. 1991;218(1–3):49–67. [Google Scholar]
- Cappetta H, Traverse M. Une riche fauna de selaciens dans le bassin a phosphate de Kpogame-Hahotoe (Eocene moyen du Togo): note preliminaire et precisions sur la structure et l’age du gisement. Geobios. 1988;21:359–365. [Google Scholar]
- Cappetta H, Duffin C, Zidek J. Chondrichthyes. In: Benton MJ, editor. The Fossil Record 2. Chapman & Hall; London: 1993. pp. 593–609. [Google Scholar]
- Cappetta H, Granier J, Ledoux J-C. Deux faunes de selaciens du Miocene mediterraneen de France et leur signification bathymetrique. Compte rendu sommaire des séances de la Société géologique de France. 1967;1967:292–293. [Google Scholar]
- Cappetta H, Pfeil F, Schmidt-Kittler N. New biostratigraphical data on the marine Upper Cretaceous and Palaeogene of Jordan. Newsletters on Stratigraphy. 2000;38(1):81–95. [Google Scholar]
- Carnevale G, Pietsch TW. An Eocene frogfish from Monte Bolca, Italy: the earliest skeletal record for the family. Palaeontology. 2009;52:745–752. [Google Scholar]
- Carnevale G, Pietsch TW. Eocene handfishes from Monte Bolca, with description of a new genus and species, and a phylogenyof the family Brachionichthyidae (Teleostei: Lophiiformes) Zoological Journal of the Linnean Society. 2010;160:621–647. [Google Scholar]
- Carnevale G, Pietsch TW. Batfishes from the Eocene of Bolca. Geological Magazine. 2011;148:461–472. [Google Scholar]
- Carnevale G, Pietsch TW. †Caruso, a new genus of anglerfishes from the Eocene of Bolca, Italy, with a comparative osteology and phylogeny of the teleost family Lophiidae. Journal of Systematic Palaeontology. 2012;10:47–72. [Google Scholar]
- Carnevale G, Bannikov AF, Marramà G, Tyler JC, Zorzin R. The Pesciara-Monte Postale Fossil-Lagerstätte: 2. Fishes and other vertebrates. In: Papazzoni CA, Giusberti L, Carnevale G, Roghi G, Bassi D, Zorzin R, editors. The Bolca Fossil-Lagerstätte: a window into the Eocene world. Rendiconti della Società Paleontologica Italiana, 4. Società Paleontologica Italiana; Modena: 2014. pp. 37–63. [Google Scholar]
- de Carvalho MR. A systematic revision of the electric ray genus Narcine Henle, 1834 (Chondrichthyes: Torpediniformes: Narcinidae), and the higher-level phylogenetic relationships of the orders of elasmobranch fishes (Chondrichthyes) City University of New York; 1999. p. 735. Unpublished PhD thesis. [Google Scholar]
- de Carvalho MR. A new species of electric ray, Narcine leoparda, from the tropical eastern Pacific Ocean (Chondrichthyes: Torpediniformes: Narcinidae) Proceedings of the Biological Society of Washington. 2001;114(3):561–573. [Google Scholar]
- de Carvalho MR. New species of numbfishes from Australia, with a key to Australian electric rays of the genus Narcine Henle, 1834 (Chondrichthyes: Torpediniformes: Narcinidae) In: Last PR, White WT, Pogonoski JJ, editors. Description of new Australian chondrichthyans. CSIRO Marine and Atmospheric Research; Hobart: 2008. pp. 241–260. [Google Scholar]
- de Carvalho MR. Morphology and phylogenetic relationships of the giant electric ray from the Eocene of Monte Bolca, Italy (Chondrichthyes: Torpediniformes) In: Elliot DK, Maisey JG, Yu X, Miao D, editors. Morphology, phylogeny and paleobiogeography of fossil fishes. Verlag Dr. Friedrich Pfeil; Munich: 2010. pp. 183–198. [Google Scholar]
- de Carvalho MR, Randall JE. Numbfishes from the Arabian Sea and surrounding gulfs, with the description of a new species from Oman (Chondrichthyes: Torpediniformes: Narcinidae) Ichthyological Research. 2003;50:59–66. [Google Scholar]
- de Carvalho MR, Séret B. Narcine lasti, a new species of numbfish from Western Australia and Indonesia (Chondrichthyes: Torpediniformes: Narcinidae) Records of the Western Australian Museum. 2002;20:393–408. [Google Scholar]
- de Carvalho MR, White WT. Narcine baliensis, a new species of electric ray from southeast Asia (Chondrichthyes: Torpediniformes) Zootaxa. 2016;4127:149–160. doi: 10.11646/zootaxa.4127.1.8. [DOI] [PubMed] [Google Scholar]
- de Carvalho MR, Compagno LJV, Ebert DA. Benthobatis yangi, a new species of blind electric ray from Taiwan (Chondrichthyes: Torpediniformes: Narcinidae) Bulletin of Marine Science. 2003;72(3):923–939. [Google Scholar]
- de Carvalho MR, Compagno LJV, Last PR. Torpediniformes: Narcinidae, Numbfishes. In: Carpenter KE, Niem VH, editors. FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. Volume 3. Batoid fishes, chimaeras and bony fishes part 1 (Elopidae to Linophrynidae) FAO; Rome: 1999. pp. 1397–2068. [Google Scholar]
- de Carvalho MR, Compagno LJV, Mee JKL. Narcine oculifera: a new species of electric ray from the gulfs of Oman and Aden (Chondrichthyes: Torpediniformes: Narcinidae) Copeia. 2002a;2002:137–145. [Google Scholar]
- de Carvalho MR, Maisey JC, Grande L. Freshwater stingrays of the Green River Formation of Wyoming (Early Eocene), with the description of a new genus and species and an analysis of its phylogenetic relationships (Chondrichthyes: Myliobatiformes) Bulletin of the American Museum of Natural History. 2004;284:1–136. [Google Scholar]
- de Carvalho MR, Séret B, Compagno LJV. A new species of electric ray of the genus Narcine Henle, 1834 from the South-Western Indian Ocean (Chondrichthyes: Torpediniformes: Narcinidae) South African Journal of Marine Science. 2002b;24:135–149. [Google Scholar]
- Case GR, Cook TD, Wilson MVH. A new elasmobranch assemblage from the early Eocene (Ypresian) Fishburne Formation of Berkeley County, South Carolina, USA. Canadian Journal of Earth Sciences. 2015;52:1–16. [Google Scholar]
- Cerqueira Ferreira L, Vooren CM. Diet of the lesser electric ray Narcine brasiliensis (Olfers, 1831) (Elasmobranchii, Narcinidae) in southern Brazil. Pan-American Journal of Aquatic Sciences. 2012;7(1):37–44. [Google Scholar]
- Claeson KM. The impacts of comparative anatomy of electric rays (Batoidea: Torpediniformes) on their systematic hypotheses. Journal of Morphology. 2014;275:597–612. doi: 10.1002/jmor.20239. [DOI] [PubMed] [Google Scholar]
- Cole KS. Reproduction and sexuality in marine fishes: patterns and processes. University California Press; Berkeley: 2010. p. 432. [Google Scholar]
- Compagno LJV. Interrelationships of living elasmobranchs. In: Greenwood PH, Miles RS, Patterson C, editors. Interrelationships of fishes. Academic Press; New York: 1973. pp. 15–61. [Google Scholar]
- Compagno LJV. Phyletic relationships of living sharks and rays. American Zoologist. 1977;17:303–322. [Google Scholar]
- Compagno LJV. Chapter 3. Endoskeleton. In: Hamlett WC, editor. Sharks, skates and rays. The biology of elasmobranch fishes. Johns Hopkins Press; Baltimore: 1999. pp. 69–92. [Google Scholar]
- Compagno LJV, Heemstra PC. Electrolux addisoni, a new genus and species of electric ray from the east coast of South Africa (Rajiformes: Torpedinoidei: Narkidae), with a review of torpedinoid taxonomy. Smithiana Bulletin. 2007;7:15–49. [Google Scholar]
- Cowman PF, Bellwood DR, van Herwerden L. Dating the evolutionary origin of wrasse lineages (Labridae) and the rise of trophic novelty on coral reefs. Molecular Phylogenetics and Evolution. 2009;52:621–631. doi: 10.1016/j.ympev.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Daniels RA, Lipps JH. Predation on foraminifera by Antarctic fish. Journal of Foraminiferal Research. 1978;8(2):110–113. [Google Scholar]
- Dartevelle E, Casier E. Les poissons fossiles du Bas-Congo et des regions voisines. Annales du Musée du Congo Beige, Séries A (Mineralogie, Geologie, Paleontologie), série III. 1943;2(1):1–200. [Google Scholar]
- Davesne D, Carnevale G, Friedman M. Bajaichthys elegans from the Eocene of Bolca (Italy) and the overlooked morphological diversity of Zeiformes (Teleostei, Acanthomorpha) Palaeontology. 2017;2017:1–14. [Google Scholar]
- Davy H. An account of some experiments on the Torpedo. Philosophical Transactions of the Royal Society of London. 1829;119:15–18. [Google Scholar]
- Dean MN, Motta PJ. Anatomy and functional morphology of the feeding apparatus of the lesser electric ray, Narcine brasiliensis (Elasmobranchii: Batoidea) Journal of Morphology. 2004a;262:462–483. doi: 10.1002/jmor.10245. [DOI] [PubMed] [Google Scholar]
- Dean MN, Motta PJ. Feeding behavior and kinematics of the lesser electric ray, Narcine brasiliensis (Elasmobranchii: Batoidea) Zoology. 2004b;107:171–189. doi: 10.1016/j.zool.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Dean MN, Summers AP. Mineralized cartilage in the skeleton of chondrichthyan fishes. Zoology. 2006;109:164–168. doi: 10.1016/j.zool.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Debenay J-P, Sigura A, Justine J-L. Foraminifera in the diet of coral reef fish from the lagoon of the New Caledonia: predation, digestion, dispersion. Revue de Micropaléontologie. 2011;54:87–103. [Google Scholar]
- Devadoss P. Observations on the breeding and development in some batoid fishes. Indian Journal of Fisheries. 1998;45(3):271–283. [Google Scholar]
- Dumeril AHA. Monographie de la famille des torpédiniens, ou poissons plagiostomes électriques, comprenant la description d’un genre nouveau, de 3 espèces nouvelles, et de 2 espèces nommées dans le Musée de Paris, mais non encore décrites. Revue et Magasin de Zoologie (Ser. 2) 1852;4:176–285. [Google Scholar]
- Eames BF, Allen N, Young J, Kaplan A, Helms JA, Schneider RA. Skeletogenesis in the swell shark Cephaloscyllium ventriosum. Journal of Anatomy. 2007;210:542–554. doi: 10.1111/j.1469-7580.2007.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CR. Description of Bolca fishes. Bulletin of the Museum of Comparative Zoology. 1904;46:1–36. [Google Scholar]
- Eastman CR. Les types de poissons fossiles du Monte Bolca au Muséum d’Histoire Naturelle de Paris. Mémoires de la Société géologique de France. 1905;34:1–33. [Google Scholar]
- Enault S, Adnet S, Debiais-Thibaud M. Skeletogenesis during the late embryonic development of the catshark Scyliorhinus canicula (Chondrichthyes, Neoselachii) MorphoMuseuM. 2016;1(4):e2. doi: 10.18563/m3.1.4.e2. [DOI] [Google Scholar]
- Fabiani R. La serie stratigrafica del Monte Bolca e dei suoi dintorni. Memorie dell’Istituto di Geologia della Regia Università di Padova. 1914;2:223–235. [Google Scholar]
- Fabiani R. Il Paleogene del Veneto. Memorie dell’Istituto di Geologia della Regia Università di Padova. 1915;3:1–336. [Google Scholar]
- Fanti F, Minelli D, Larocca Conte G, Miyashita T. An exceptionally preserved Eocene shark and the rise of modern predatory-prey interaction in the coral reef food web. Zoological Letters. 2016;2:9. doi: 10.1186/s40851-016-0045-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechhelm JD, McEachran JD. A revision of the electric ray genus Diplobatis with notes on the interrelationships of Narcinidae (Chondrichthyes, Torpediniformes) Bulletin of the Florida State Museum Biological Sciences. 1984;29:171–209. [Google Scholar]
- Ferrón HG, Martínez-Pérez C, Botella H. The evolution of gigantism in active marine predators. Historical Biology. 2017 doi: 10.1080/08912963.2017.1319829. [DOI] [Google Scholar]
- Floeter SR, Bender MG, Siqueira AC, Cowman PF. Phylogenetic perspectives on reef fish functional traits. Biological Reviews. 2017 doi: 10.1111/brv.12336. [DOI] [PubMed] [Google Scholar]
- Fowler HW. Descriptions of new fishes obtained 1907 to 1910, chiefly in the Philippine Islands and adjacent seas. Proceedings of the Academy of Natural Sciences of Philadelphia. 1934;85:233–367. [Google Scholar]
- Frickhinger KA. Fossilien Atlas: Fische. Mergus; Melle: 1991. p. 1088. [Google Scholar]
- Friedman M. Ecomorphological selectivity among marine teleost fishes during the end-Cretaceous extinction. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5218–5223. doi: 10.1073/pnas.0808468106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. Explosive morphological diversification of spiny-finned teleost fishes in the aftermath of the end-Cretaceous extinction. Proceedings of the Royal Society, Series B. 2010;277:1675–1683. doi: 10.1098/rspb.2009.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froese R, Pauly D. FishBase. World Wide Web electronic publication version (02/2017) [accessed 13 April 2017];2017 Updated at: http://www.fishbase.org.
- Gill T. Analytical synopsis of the order of Squali and revision of the nomenclature of the genera. Annals of the Lyceum of Natural History of New York. 1862;7(32):367–408. [Google Scholar]
- Gillen AL, Stein RA, Carline RF. Predation by pellet-reared tiger muskellunge on minnows and bluegills in experimental systems. Transactions of the American Fisheries Society. 1981;110:197–209. [Google Scholar]
- Goatley CHR, Bellwood DR, Bellwood O. Fishes on coral reefs: changing roles over the past 240 million years. Paleobiology. 2010;36:415–427. [Google Scholar]
- Goitein R, Torres FS, Signorini CE. Morphological aspects related to feeding of two marine skates Narcine brasiliensis Ölfers and Rhinobatus horkelli Müller & Henle. Acta Scientiarum. 1998;20:165–169. [Google Scholar]
- Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008;24:774–786. [Google Scholar]
- Gray JE. Description of twelve new genera of fish, discovered by Gen. Hardwicke, in India, the greater part in the British Museum. Zoological Miscellany. 1831;1831:7–9. [Google Scholar]
- Guinot G, Cavin L. ‘Fish’ (Actinopterygii and Elasmobranchii) diversification patterns through deep time. Biological Reviews. 2016;91:950–981. doi: 10.1111/brv.12203. [DOI] [PubMed] [Google Scholar]
- Hamlett WC, Koob TJ. Chapter 15. Female reproductive system. In: Hamlett WC, editor. Sharks, skates and rays. The biology of elasmobranch fishes. Johns Hopkins Press; Baltimore: 1999. pp. 398–443. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica. 2001;4:1–9. [Google Scholar]
- Hart PJB, Hamrin SF. The role of behaviour and morphology in the selection of prey by pike. In: Hughes RN, editor. Behavioural mechanisms of food selection. Springer-Verlag; Berlin: 1990. pp. 235–253. [Google Scholar]
- Hasse JCF. Das Natürliche System der Elasmobranchier auf Grundlage des Baues und der Entwicklung ihrer Wirbelsäule. Verlag von Gustav Fischer; Jena: 1879. [Google Scholar]
- Heckel JJ. Ichthyologie. In: Von Tschudi JJ, editor. Untersuchungen über die Fauna Peruana. Scheitlin & Zollikofer; St. Gallen: 1846. pp. 1–35. [Google Scholar]
- Henle FGJ. Ueber Narcine, eine neue Gattung electrischer Rochen nebst einer Synopsis der electrischen Rochen. G. Eichler; Berlin: 1834. p. 44. [Google Scholar]
- Herman J. Compléments palaeoichthyologiques à la faune éocène de la Belgique. 2. Présence du genre Eotorpedo White, E.I., 1935 dans les Sables de Forest (Yprésien supérieur belge) Bulletin de la Société Belge de Géologié. 1974;83(1):33–34. [Google Scholar]
- Herman J, Hovestadt-Euler M, Hovestadt DC, Stehmann M. Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living supra-specific taxa of Chondrichthyan fishes. Part B: Batomorphii No. 1b: Order Rajiformes – Suborder Rajoidei – Family: Rajidae – Genera and Sub genera: Bathyraja (with a deep-water, shallow-water and transitional morphotype), Psammobatis, Raja (Amblyraja), Raja (Dipturus), Raja (Leucoraja), Raja (Raja), Raja (Rajella) (with two morphotypes), Raja (Rioraja), Raja (Rostroraja), Raja lintea, and Sympterygia. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Biologie. 1995;65:237–307. [Google Scholar]
- Herman J, Hovestadt-Euler M, Hovestadt DC, Stehmann M. Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living supra-specific taxa of Chondrichthyan fishes. Part B: Batomorphii No. 2: Order Rajiformes – Suborder: Pristoidei – Family: Pristidae – Genera: Anoxypristis and Pristis No. 3: Suborder Rajoidei – Superfamily Rhinobatoidea – Families: Rhinidae – Genera Rhina and Rhynchobatus and Rhinobatidae – Genera: Aptychotrema, Platyrhina, Platyrhinoidis, Rhinobatos, Trygonorrhina, Zanobatus and Zapteryx. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Biologie. 1997;67:107–162. [Google Scholar]
- Herman J, Hovestadt-Euler M, Hovestadt DC, Stehmann M. Contributions to the study of the comparative morphology of teeth and other relevant ichthyodorulites in living supraspecific taxa of chondrichthyan fishes. Part B: Batomorphii. No. 4: Order Torpediniformes – Family Narcinidae – Subfamily Narcininae – Genera: Benthobatis, Diplobatis, Discopyge and Narcine, Subfamily Narkinae – Genera: Bengalichthys, Crassinarke, Heteronarce, Narke, Temera, and Typhlonarke, Family Torpedinidae – Subfamily Torpedininae – Genus: Torpedo – Subgenus: T. (Tetronarke) and T. (Torpedo) and Subfamily Hypninae – Genus: Hypnos. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Biologie. 2002;72:5–45. [Google Scholar]
- Hoar WS, Randall DJ. Fish physiology. Volume XI. The physiology of developing fish – Part B Viviparity and posthatching juveniles. Academic Press; San Diego: 1988. p. 436. [Google Scholar]
- Holmgren N. Studies of the head in fishes. Part 2. Comparative anatomy of the adult selachian skull with remarks on the dorsal fins in sharks. Acta Zoologica. 1941;22:1–100. [Google Scholar]
- Hottinger L. Recherches sur les Alvéolines du Paléocène et de l’Eocène. Schweizerische Paläontologische Abhandlungen. 1960;75–76:1–243. [Google Scholar]
- Houttuyn M. Natuurlyke historie of uitvoerige beschryving der dieren, planten en mineraalen, volgens het samenstel van den Heer Linnaeus. Met naauwkeurige afbeeldingen. 1764;1–3:1761–85. [Google Scholar]
- Hovestadt DC, Hovestadt-Euler M. The remains of a carcharhinid shark with a new triakid species in its digestive tract from the Oligocene of Germany. Tertiary Research. 2002;21(1–4):171–182. [Google Scholar]
- Hovestadt DC, Hovestadt-Euler M. A partial skeleton of Carcharias gustrowensis (Winkler, 1875) (Chondrichthyes, Odontaspididae) including embryos, a chimaeroid dorsal fin spine and a myliobatoid tail spine from the Oligocene of Germany. Cainozoic Research. 2010;7(1–2):83–97. [Google Scholar]
- Hovestadt DC, Hovestadt-Euler M, Micklich N. A review of the chondrichthyan fauna of Grube Unterfeld (Frauenweiler) clay pit. Kaupia. 2010;17:57–71. [Google Scholar]
- Hoyle JA, Keast A. The effect of prey morphology and size on handling time in a piscivore, the largemouth bass (Micropterus salmoides) Canadian Journal of Zoology. 1987;65:1972–1977. [Google Scholar]
- Huxley TH. On the application of the laws of evolution to the arrangement of the Vertebrata, and more particularly of the Mammalia. Proceedings of the Zoological Society, London. 1880;43:649–662. [Google Scholar]
- Jaekel O. Die eocänen Selachier vom Monte Bolca: ein Beitrag zur Morphogenie der Wirbelthiere. Springer; Berlin: 1894. p. 177. [Google Scholar]
- Jaekel O. Ueber einen Torpediniden und andere Fischreste aus dem Tertiiir von Kamerun. In: Esch E, Solger F, Oppenheim P, Jaekel O, editors. Beitrage zur Geologie von Kamerun. E. Schweizerbartsche Verlagshandlung (E. Nagele); Stuttgart: 1904. pp. 287–291. [Google Scholar]
- Jonet S. Notes d’ichthyologie Miocene Portugaise; V, Queleques batoides. Revista de Faculdade de Ciencias 2a Ser. C. Ciencias Naturais. 1968;15:233–257. [Google Scholar]
- Jordan DS. A classification of fishes including families and genera as far as known. Stanford University Publications, University Series, Biological Sciences. 1923;3:77–243. [Google Scholar]
- Juanes F. What determines prey size selectivity in piscivorous fishes? In: Stouder DJ, Fresh KL, Feller RJ, editors. Theory and application in fish feeding ecology. Carolina University Press; Columbia: 1994. pp. 79–100. [Google Scholar]
- Kaup JJ. Beiträge zu Amphibiologie und Ichthiyologie. Isis (Oken) 1826;19(1):87–90. [Google Scholar]
- Keast A, Webb D. Mouth and body form relative to feeding ecology in the fish fauna of a small lake, Lake Opinicon, Ontario. Journal of the Fisheries Research Board of Canada. 1966;23:1845–1874. [Google Scholar]
- Knight JL, Cicimurri DJ, Purdy RW. New western hemisphere occurrences of Schizorhiza Weiler, 1930 and Eotorpedo White, 1934 (Chondrichthyes, Batomorphii) Paludicola. 2007;6(3):87–93. [Google Scholar]
- Kriwet J, Benton MJ. Neoselachian (Chondrichthyes, Elasmobranchii) diversity across the Cretaceous–Tertiary boundary. Palaeogeography, Palaeoclimatology, Palaeoecology. 2004;214:181–194. [Google Scholar]
- Kriwet J, Kiessling W, Klug S. Diversification trajectories and evolutionary life-history traits in early sharks and batoids. Proceedings of the Royal Society, Series B. 2009;276:945–951. doi: 10.1098/rspb.2008.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last P, White W, de Carvalho MR, Séret B, Stehmann M, Naylor G. Rays of the world. CSIRO Publishing; Clayton North: 2016. p. 800. [Google Scholar]
- Lipps JH. Predation on foraminifera by coral reef fish: taphonomic and evolutionary implications. Palaios. 1988;3:315–326. [Google Scholar]
- Madden CT, Whitmore FC, Schmidt DL, Naqvi IM. The Umm Himar Formation (Paleocene) of Saudi Arabia and associated strata: stratigraphy, vertebrate fauna, and paleoenvironment. In: Whitmore FC, Madden CT, editors. Paleocene vertebrates from Jabal Umm Himar, Kingdom of Saudi Arabia. Vol. 2093. US Geological Survey; Bulletin: 1995. pp. 1–19. [Google Scholar]
- Maddison WP, Maddison DR. [accessed 5 March 2017];Mesquite: a modular system for evolutionary analysis. Version 3.03. 2008 Updated at: http://mesquiteproject.org.
- Maisey JG. Chondrichthyan phylogeny: a look at the evidence. Journal of Vertebrate Paleontology. 1984;4:359–371. [Google Scholar]
- Malaroda R. Il Luteziano di Monte Postale (Lessini medi) Memorie degli Istituti di Geologia e Mineralogia dell’Università di Padova. 1954;19:3–107. [Google Scholar]
- Marinsek GP, Viliod MCL, Mari RB. Ecomorphology of the digestive tract of the brazilian electric ray Narcine brasiliensis (Olfers, 1831) (Torpediniformes: Narcinidae) Acta Zoologica. 2017;98:229–236. [Google Scholar]
- Marramà G, Carnevale G. Eocene round herring from Monte Bolca, Italy. Acta Palaeontologica Polonica. 2015a;60:701–710. [Google Scholar]
- Marramà G, Carnevale G. The Eocene sardine †Bolcaichthys catopygopterus (Woodward, 1901) from Monte Bolca, Italy: osteology, taxonomy and paleobiology. Journal of Vertebrate Paleontology. 2015b;35:e1014490. [Google Scholar]
- Marramà G, Carnevale G. An Eocene anchovy from Monte Bolca, Italy: the earliest known record for the family Engraulidae. Geological Magazine. 2016;153:84–94. [Google Scholar]
- Marramà G, Carnevale G. Eoalosa janvieri gen. et sp. nov., a new clupeid fish (Teleostei, Clupeiformes) from the Eocene of Monte Bolca, Italy. Paläontologische Zeitschrift. 2017a doi: 10.1007/s12542-017-0378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marramà G, Carnevale G. Morphology, relationships, and paleobiology of the Eocene barracudina †Holosteus esocinus (Aulopiformes, Paralepididae) from Monte Bolca, Italy. Zoological Journal of the Linnean Society. 2017b doi: 10.1093/zoolinnean/zlw029. [DOI] [Google Scholar]
- Marramà G, Carnevale G, Engelbrecht A, Claeson KM, Zorzin R, Fornasiero M, Kriwet J. A synoptic review of the Eocene (Ypresian) cartilaginous fishes (Chondrichthyes: Holocephali, Elasmobranchii) of the Bolca Konservat-Lagertätte, Italy. Paläontologische Zeitschrift. doi: 10.1007/s12542-017-0387-z. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marramà G, Garbelli C, Carnevale G. A morphospace for the Eocene fish assemblage of Bolca, Italy: a window into the diversification and ecological rise to dominance of modern tropical marine fishes. Bollettino della Società Paleontologica Italiana. 2016a;55(1):11–21. [Google Scholar]
- Marramà G, Garbelli C, Carnevale G. A clade-level morphospace for the Eocene fishes of Bolca: patterns and relationships with modern tropical marine fish assemblages. Bollettino della Società Paleontologica Italiana. 2016b;55(2):139–156. [Google Scholar]
- Marramà G, Engelbrecht A, Carnevale G, Kriwet J. Eocene sand tiger sharks (Lamniformes, Odontaspididae) from the Bolca Konservat-Lagerstätte, Italy: palaeobiology, palaeobiogeography and evolutionary significance. Historical Biology. 2017a doi: 10.1080/08912963.2017.1341503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marramà G, Lombardo C, Tintori A, Carnevale G. Redescription of ‘Perleidus’ (Osteichthyes, Actinopterygii) from the Early Triassic of northwestern Madagascar. Rivista Italiana di Paleontologia e Stratigrafia. 2017b;123(2):219–242. [Google Scholar]
- Marramà G, Bannikov AF, Tyler JC, Zorzin R, Carnevale G. Controlled excavations in the Pesciara and Monte Postale sites provide new insights about the paleoecology and taphonomy of the fish assemblages of the Eocene Bolca Konservat-Lagerstätte, Italy. Palaeogeography, Palaeoclimatology, Palaeoecology. 2016c;454:228–245. [Google Scholar]
- McEachran JD, Aschliman N. Phylogeny of Batoidea. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. CRC Press; Boca Raton: 2004. pp. 79–113. [Google Scholar]
- McEachran JD, Carvalho MR. Batoid fishes. In: Carpenter K, editor. The living marine resources of the Western Central Atlantic. FAO species identification guide for fishery purposes and American Society of Ichthyologists and Herpetologists. Vol. 5. FAO; Rome: 2002. pp. 508–589. [Google Scholar]
- McEachran JD, Dunn KA, Miyake T. Interrelationships of the Batoid fishes (Chondrichthyes: Batoidea) In: Stassney MLJ, Parenti LR, Johnson GD, editors. Interrelationships of fishes. Academic Press; San Diego: 1996. pp. 63–84. [Google Scholar]
- McKay RJ. Studies on western Australian sharks and rays of the families Scyliorhinidae, Urolophidae, and Torpedinidae. Journal of the Royal Society of Western Australia. 1966;49:65–82. [Google Scholar]
- Menni RC, Rincón G, García ML. Discopyge castelloi sp. nov. (Torpediniformes, Narcinidae), una nueva especie de raya eléctrica del Mar Argentino. Revista del Museo Argentino de Ciencias Naturales. 2008;10(1):161–171. [Google Scholar]
- Miyake T, McEachran JD. The morphology and evolution of the ventral gill arch skeleton in batoid fishes (Chondrichthyes: Batoidea) Zoological Journal of the Linnean Society. 1991;102:75–100. [Google Scholar]
- Miyake T, McEachran JD, Walton PJ, Hall BK. Development and morphology of rostral cartilages in batoid fishes (Chondrichthyes: Batoidea), with comments on homology within vertebrates. Biological Journal of the Linnean Society. 1992;46:259–298. [Google Scholar]
- Molin R. Primitiae Musei Archigymnasii patavini. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften. Mathematisch-Naturwissenschaftliche Klasse. 1860;40:582–588. [Google Scholar]
- Mollen FH. A partial rostrum of the porbeagle shark Lamna nasus (Lamniformes, Lamnidae) from the Miocene of the North Sea basin and the taxonomic importance of rostral morphology in extinct sharks. Geologica Belgica. 2010;13(1–2):61–76. [Google Scholar]
- Nair RV, Soundararajan R. On an instance of hermaphroditism in the electric ray, Narcine timlei (Bloch and Schneider) Indian Journal of Fisheries. 1973;20(1):260–264. [Google Scholar]
- Nelson JS, Grande TC, Wilson MVH. Fishes of the world. 5th edition. John Wiley & Sons; Hoboken: 2016. p. 752. [Google Scholar]
- Nixon KC. WinClada ver. 1.00.08. Ithaca, New York: 2002. Published by the author. [Google Scholar]
- Noubhani A, Cappetta H. Les Orectolobiformes, Carcharhiniformes et Myliobatiformes (Elasmobranchii, Neoselachii) des bassins phosphate du Maroc (Maastrichtien–Lutetien basal). Systematique, biostratigraphie, evolution et dynamique des faunes. Palaeo Ichthyologica. 1997;8:1–327. [Google Scholar]
- Olfers JFM. Die Gattung Torpedo in ihren naturhistorischen und antiquarischen Beziehungen erläutert. Berlin: 1831. p. 35. [Google Scholar]
- d’ Orbigny AD. Tableau méthodique de la classe des Céphalopodes. Annales des Sciences Naturelles. 1826;7:96–169. 245–314. [Google Scholar]
- Papazzoni CA, Fornaciari E, Giusberti L, Vescogni A, Fornaciari B. Integrating shallow benthic and calcareous nannofossil zones: the lower Eocene of the Monte Postale section (northern Italy) Palaios. 2017;32:6–17. [Google Scholar]
- Papazzoni CA, Bassi D, Fornaciari E, Giusberti L, Luciani V, Mietto P, Roghi G, Trevisani E. The Pesciara-Monte Postale Fossil-Lagerstätte: 3. Geological and stratigraphical setting of the Bolca area. In: Papazzoni CA, Giusberti L, Carnevale G, Roghi G, Bassi D, Zorzin R, editors. The Bolca Fossil-Lagerstätte: a window into the Eocene world. Rendiconti della Società Paleontologica Italiana 4. Società Paleontologica Italiana; Modena: 2014. pp. 19–28. [Google Scholar]
- Pavan-Kumar A, Gireesh-Babu P, Babu PP, Jaiswar AK, Hari Krishna V, Prasasd KP, Chaudhari A, Raje SG, Chakraborty SK, Krishna G, Lakra WS. Molecular phylogeny of elasmobranchs inferred from mitochondrial and nuclear markers. Molecular Biology Reports. 2013;41:447–457. doi: 10.1007/s11033-013-2879-6. [DOI] [PubMed] [Google Scholar]
- Pfaff C, Zorzin R, Kriwet J. Evolution of the locomotory system in eels (Teleostei: Elopomorpha) BMC Evolutionary Biology. 2016;16:159. doi: 10.1186/s12862-016-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall JE. A revision of the Indo-Pacific labrid fish genus Macropharyngodon, with descriptions of five new species. Bulletin of Marine Science. 1978;28:742–770. [Google Scholar]
- Regan CT. New fishes from deep water off the coast of Natal. Annals and Magazine of Natural History, (Series 9) 1921;7:412–420. [Google Scholar]
- Reinecke T. Batoids (Rajiformes, Torpediniformes, Myliobatiformes) from the Sülstorf Beds (Chattian, Late Oligocene) of Mecklenburg, northeastern Germany: a revision and description of three new species. Palaeovertebrata. 2015;39(2):e2. [Google Scholar]
- Reinecke T, Moths H, Grant A, Breitkreuz H. Die Elasmobranchier des norddeutschen Chattiums, insbesondere des Sternberger Gesteins (Eochattium, Oligozan) Palaeontos. 2005;8:1–135. [Google Scholar]
- Renema W, Bellwood DR, Braga JC, Bromfield K, Hall R, Johnson KG, Lunt P, Meyer CP, McMonagle LB, Morley RJ, O’Dea A, et al. Hopping hotspots: global shifts in marine biodiversity. Science. 2008;321:654–657. doi: 10.1126/science.1155674. [DOI] [PubMed] [Google Scholar]
- Rincon G. Taxonomia, alimentação e reprodução da raia elétrica Benthobatis sp. (Torpediniformes: Narcinidae) no sul do Brasil. Fundação Universidade de Rio Grande-FURG; 1997. p. 95. Unpublished MSc thesis. [Google Scholar]
- Rincon G, Stehmann MFW, Vooren CM. Results of the research cruises of FRV Walther Herwig to South America. LXXIV. Benthobatis kreffti sp. nov. (Chondrichthyes, Torpediniformes, Narcinidae), a new deep-water electric ray from off South Brazil and the third species of the genus. Archive of Fishery Marine Research. 2001;49(1):45–60. [Google Scholar]
- Roberts BL. The buoyancy and locomotory movements of electric rays. Journal of the Marine Biological Association of the United Kingdom. 1969;49:621–640. [Google Scholar]
- Rocco L, Liguori I, Costagliola D, Morescalchi MA, Tinti F, Stingo V. Molecular and karyological aspects of Batoidea (Chondrichthyes, Elasmobranchii) phylogeny. Gene. 2007;389:80–86. doi: 10.1016/j.gene.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Rosenberger LJ. Pectoral fin locomotion in batoid fishes: undulation versus oscillation. Journal of Experimental Biology. 2001;204:379–394. doi: 10.1242/jeb.204.2.379. [DOI] [PubMed] [Google Scholar]
- Rudloe A. Captive maintenance of the lesser electric ray, with observations of feeding behavior. Progressive Fish-Culturist. 1989;51:37–41. [Google Scholar]
- Sahni A, Mehrotra DK. The elasmobranch fauna of coastal Miocene sediments of peninsular India. Biological Memoirs Lucknow. 1981;5(2):83–121. [Google Scholar]
- Schaefer JT, Summers AP. Batoid wing skeletal structure: novel morphologies, mechanical implications, and phylogenetic patterns. Journal of Morphology. 2005;264:298–313. doi: 10.1002/jmor.10331. [DOI] [PubMed] [Google Scholar]
- Schäfer W. Ecology and palaeoecology of marine environments. Chicago University Press; Chicago: 1972. p. 568. [Google Scholar]
- Scharf FS, Juanes F, Rountree RA. Predator size–prey size relationships of marine fish predators: interspecific variation and effects of ontogeny and body size on trophic-niche breadth. Marine Ecology Progress Series. 2000;208:229–248. [Google Scholar]
- Scheibner C, Speijer RP. Late Paleocene–early Eocene Tethyan carbonate platform evolution – a response to long- and short-term paleoclimatic change. Earth-Science Reviews. 2008;90:71–102. [Google Scholar]
- Schmitz L, Wainwright PC. Nocturnality constrains morphological and functional diversity in the eyes of reef fishes. BMC Evolutionary Biology. 2011;11:338. doi: 10.1186/1471-2148-11-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotese CR. [accessed 5 March 2017];Paleomap. 2002 Updated at: http://www.scotese.com.
- Siguendibo Sambou B, Sarr R, Hautier L, Cappetta H, Adnet S. The selachian fauna (sharks and rays) of the phosphate series of Ndendouri-Ouali Diala (Matam, Western Senegal): Dating and paleoenvironmental interests. Neues Jahrbuch für Geologie und Paläontologie – Abhandlungen. 2017;283(2):205–219. [Google Scholar]
- Smith R. Elasmobranches nouveaux de la transition Paléocène-Eocène de Dormaal (Belgique) Bulletin de l’Institut royal des Sciences naturelles de Belgique, Sciences de la Terre. 1999;69:173–185. [Google Scholar]
- Spieler RE, Fahy DP, Sherman RL, Sulikowski JA, Quinn TP. The yellow stingray, Urobatis jamaicensis (Chondrichthyes: Urotrygonidae): a synoptic review. Caribbean Journal of Science. 2013;47(1):67–97. [Google Scholar]
- Stanghellini E. Bolca e i suoi fossili. La Grafica, Verona: 1979. p. 96. [Google Scholar]
- Takács P. Morphometric differentiation of gudgeon species inhabiting the Carpathian Basin. International Journal of Limnology. 2012;48:53–61. [Google Scholar]
- Underwood CJ. Diversification of the Neoselachii (Chondrichthyes) during the Jurassic and Cretaceous. Paleobiology. 2006;32:215–235. [Google Scholar]
- Underwood CJ, Ward DJ, King C, Antar SM, Zalmout IS, Gingerich PD. Shark and ray faunas in the Middle and Late Eocene of the Fayum Area, Egypt. Proceedings of the Geologists’ Association. 2011;122:47–66. [Google Scholar]
- Vescogni A, Bosellini FR, Papazzoni CA, Giusberti L, Roghi G, Fornaciari E, Dominici S, Zorzin R. Coralgal buildups associated with the Bolca Fossil-Lager-stätten: new evidence from the Ypresian of Monte Postale (NE Italy) Facies. 2016;62:21. [Google Scholar]
- Waite ER. Pisces. Part I. In: Scientific results of the New Zealand government trawling expedition, 1907. Records of the Canterbury Museum. 1909;1(2):131–155. [Google Scholar]
- Walker SE, Brett CE. Post-Paleozoic patterns in marine predation: was there a Mesozoic and Cenozoic marine predatory revolution? Paleontological Society Papers. 2002;8:119–194. [Google Scholar]
- Webb PW. The effect of size on the fast-start performance of rainbow trout, Salmo gardneri, and a consideration of piscivorous predator-prey interactions. Journal of Experimental Biology. 1976;65:157–177. doi: 10.1242/jeb.65.1.157. [DOI] [PubMed] [Google Scholar]
- White EI. Fossil fishes of Sokoto province. Bulletin of the Geological Survey of Nigeria. 1934;14:1–78. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.