Abstract
Chronic inflammation in inflammatory bowel disease (IBD) results from a breakdown of intestinal immune homeostasis and compromise of the intestinal barrier. Genome-wide association studies have identified over 200 genetic loci associated with risk for IBD, but the functional mechanisms of most of these genetic variants remain unknown. Polymorphisms at the TNFSF15 locus, which encodes the TNF superfamily cytokine commonly known as TL1A, are associated with susceptibility to IBD in multiple ethnic groups. In a wide variety of murine models of inflammation including models of IBD, TNFSF15 promotes immunopathology by signaling through its receptor DR3. Such evidence has led to the hypothesis that expression of this lymphocyte costimulatory cytokine increases risk for IBD. In contrast, here we show that the IBD-risk haplotype at TNFSF15 is associated with decreased expression of the gene by peripheral blood monocytes in both healthy volunteers and IBD patients. This association persists under various stimulation conditions at both the RNA and protein levels and is maintained after macrophage differentiation. Utilizing a “recall-by-genotype” bioresource for allele-specific expression measurements in a functional fine-mapping assay, we localize the polymorphism controlling TNFSF15 expression to the regulatory region upstream of the gene. Through a T cell costimulation assay, we demonstrate that genetically regulated TNFSF15 has functional relevance. These findings indicate that genetically enhanced expression of TNFSF15 in specific cell types may confer protection against the development of IBD.
Author summary
Crohn’s disease and ulcerative colitis, characterized by gut inflammation, are the two main subtypes of Inflammatory bowel disease (IBD). Over two hundred genetic loci have been identified that contribute to risk for IBD. However, functional studies are required to determine the mechanisms by which these genetic changes might affect disease risk. To date, only a few IBD loci have been functionally characterized. We focused on the IBD risk locus at TNFSF15, encoding the cytokine also known as TL1A. Previous work has shown that TNFSF15 enhances lymphocyte activation and promotes inflammation in animal models of disease. However, we here call into question the assumption that TNFSF15 is always a pro-inflammatory molecule by demonstrating that the IBD risk allele at TNFSF15 is associated with decreased production of TNFSF15 by monocytes and macrophages at rest and after stimulation. In addition, we narrow the list of potential variants at TNFSF15 responsible for controlling its expression, and we demonstrate that genetically controlled TNFSF15 can have functional impact on responding T cells. These findings both demonstrate a mechanism by which this IBD risk locus might drive disease predisposition and suggest a new protective role for TNFSF15 in maintaining the intestinal barrier.
Introduction
Maintaining immune homeostasis in the microbiome-rich environment of the intestine is a complex process, mediated by numerous mechanisms including the physical epithelial barrier, mucin secretion, antimicrobial peptides, anti-inflammatory cytokines, regulatory cells, and IgA responses [1, 2]. Inflammatory bowel disease (IBD, including Crohn’s disease, CD, and ulcerative colitis, UC) results from a breakdown in mucosal immune homeostasis [3], but the precise mechanisms by which barrier dysfunction begins remain largely unknown. To date, genome-wide association studies (GWASs) have identified approximately 200 distinct susceptibility loci for IBD, the majority of which are associated with both CD and UC [4, 5]. A fine-mapping study used Bayesian statistical methodology to find the most probable causal variants underlying the association signal at each locus [6]. However, to fully realize the benefit of GWAS discoveries, functional studies are required to move beyond statistical associations with genetic loci and uncover the biological mechanisms behind genetic predisposition to disease. Functional studies of IBD-associated genetic variants have been performed for several loci, demonstrating that risk variants can lead to a breakdown in the intestinal barrier through both reducing (e.g NOD2 [7–10] and ATG16L1 [11–13]) and enhancing (e.g. IL23R [14–17]) gene function. Expression quantitative trait locus (eQTL) studies have found genes whose expression may be increased or decreased by IBD risk-associated variants and have highlighted the impact of cell type on the effects of a genetic variant [18–20]. In a complementary approach, investigation of gene expression patterns in monocytes and monocyte-derived macrophages revealed enrichment for genes near IBD loci among those upregulated during macrophage differentiation or stimulation [21], suggesting the importance of this cellular lineage in IBD. Investigating how IBD-associated variants influence disease susceptibility should provide insight into disease etiology and enable design of improved therapeutic or preventive strategies.
The genomic locus encoding the tumor necrosis factor superfamily member TNFSF15 (also known as TL1A) is associated with both CD and UC in populations of multiple ethnic backgrounds [4, 5, 22, 23]. TNFSF15 is produced by a variety of tissues, the most studied of which include myeloid lineage cells, activated T cells, and endothelial cells [24]. At the cellular level, TNFSF15 costimulates both T cells and innate lymphoid cells (ILC) and promotes differentiation of IL-9-producing T cells [25–31]. Increased TNFSF15 expression has been observed systemically and at the site of inflammation in IBD and is particularly associated with active disease [32–37]. Such observations have led to the hypothesis that the allele associated with risk for IBD at TNFSF15 might increase its expression and thereby promote inflammation. Several studies have found associations between genetic variants tagging the IBD-associated locus at TNFSF15 and TNFSF15 mRNA and protein expression [18, 19, 38–47]. However, the population studied, the disease status of the subjects, the cell type considered and the observed direction of effect differ between studies, leaving the mechanism by which this genetic locus confers susceptibility to IBD unclear.
To shed light on the functional consequences of the IBD susceptibility locus at TNFSF15 we examined the association of single nucleotide polymorphism (SNP) genotype with mRNA expression in specific immune cell types in multiple cohorts of healthy individuals and patients with inflammatory diseases. We found that the IBD-associated locus is an eQTL for monocyte TNFSF15. The genetic signals underlying the associations with TNFSF15 expression and IBD colocalize, suggesting that disease risk is mediated though regulation of gene expression. Importantly, we show that the IBD-protective allele at TNFSF15 is strongly associated with increased monocyte TNFSF15 mRNA in both healthy individuals and patients with inflammatory diseases. To further investigate the mechanism of this protective haplotype, we used a “recall-by-genotype” bioresource of healthy individuals from the United Kingdom. This analysis demonstrated that the IBD-protective allele is associated with increased TNFSF15 mRNA and protein expression under a variety of stimulation contexts, as well as monocyte costimulatory capacity in acute lymphocyte activation. Through allele-specific expression measurements in individuals with breaks in the associated haplotype block, we functionally fine-mapped the expression-associated locus to upstream of the gene. Importantly, we found that association of the protective allele with increased TNFSF15 expression was maintained in monocyte-derived macrophages, which play an important role in mucosal immunology. Thus, our findings suggest that genetically elevated TNFSF15 from monocytes or monocyte-derived cells may protect healthy individuals from the development of IBD.
Results
The IBD protective genotype at TNFSF15 is associated with increased TNFSF15 expression in circulating monocytes
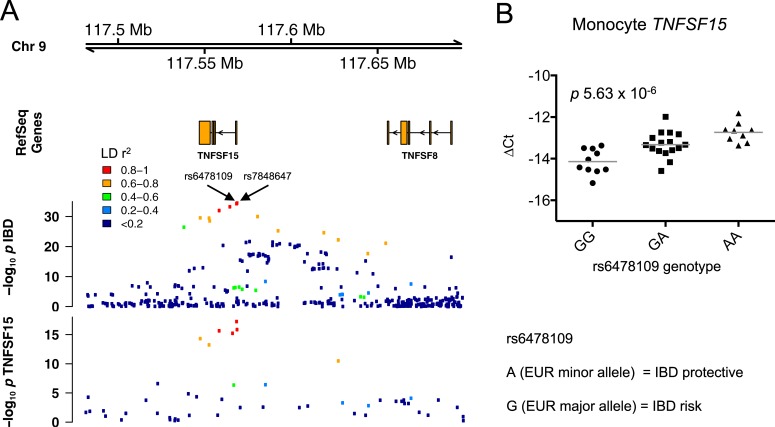
We previously identified an association between genotype at the TNFSF15 IBD susceptibility locus and TNFSF15 mRNA expression in peripheral blood monocytes in both healthy individuals and newly diagnosed IBD patients from the UK [18, 19] (Fig 1A, S1 Fig). We also confirmed this association in a cohort of British patients with another immune-mediated disease, anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis [18] (S1 Fig). The SNP most associated with TNFSF15 expression was rs6478109 [19], which is located 358 base pairs upstream of TNFSF15 and has a minor allele frequency (MAF) of 32.5% in individuals of European descent. An IBD GWAS trans-ancestry analysis by Liu et al indicated that the SNP most strongly associated with IBD in individuals of European descent at this locus is rs7848647 [4], which is 280 base pairs upstream of rs6478109 and in near complete linkage disequilibrium (LD) with it (r2 = 0.995 in 1000 Genomes Phase 3 European cohort). Comparison of the regional association plots for the TNFSF15 eQTL and IBD susceptibility revealed similar patterns of association (Fig 1A). In order to formally evaluate whether the eQTL and the disease association signal are driven by the same causal variant or two distinct variants in LD, we performed colocalization testing [48]. Our results indicated that the colocalization was highly likely (posterior probability of a shared underlying causal variant was 0.998).
Fig 1. The IBD-protective rs6478109:A allele is associated with increased TNFSF15 expression.
(A) Regional association plots for IBD (European ancestry cohort from Liu et al [4]) and TNFSF15 expression (n = 39 healthy individuals and 80 IBD patients). (B) TNFSF15 mRNA expression was measured in ex vivo monocytes relative to B2M by qPCR in a separate cohort of healthy individuals and plotted versus rs6478109 genotype (GG, n = 10; GA, n = 16; AA, n = 9). Expression is reported as ΔCt, each point represents an individual, lines represent mean values, and p value is from linear regression.
rs6478109 genotype is also associated with TNFSF8 expression in whole blood [49] and monocytes [50], and additional monocyte TNFSF8 eQTLs have been found in this genomic region [18–20, 51]. However, rs6478109 is not the SNP most associated with TNFSF8 expression in any of these studies. Comparison of the patterns of genetic association across this locus with IBD and TNFSF8 expression in our previous monocyte eQTL data [19] demonstrated stark differences (S2 Fig), suggesting that TNFSF8 is unlikely to be the causal gene at this locus. Colocalization testing confirmed that the IBD association and TNFSF8 eQTL signals were very unlikely to be driven by a shared causal variant (posterior probability 0.092).
These analyses indicate that IBD susceptibility may be mediated by changes in TNFSF15 expression. We therefore performed further functional examination of the locus using a recall-by-genotype study design in an independent bioresource of healthy individuals recruited from the region around Cambridge, UK. We confirmed the association of rs6478109 with TNFSF15 mRNA in peripheral blood monocytes (p 5.63 x 10−6, Fig 1B, S1 Table). Importantly, in both cohorts of healthy volunteers and the cohorts of IBD and ANCA-associated vasculitis patients, the IBD protective allele (A, the minor allele in the European population) was consistently associated with increased expression of TNFSF15 (Fig 1, S1 Fig). In contrast to previous speculation that TNFSF15 predisposes to inflammatory disease, this suggests a novel protective role for this cytokine in preventing the development of human IBD.
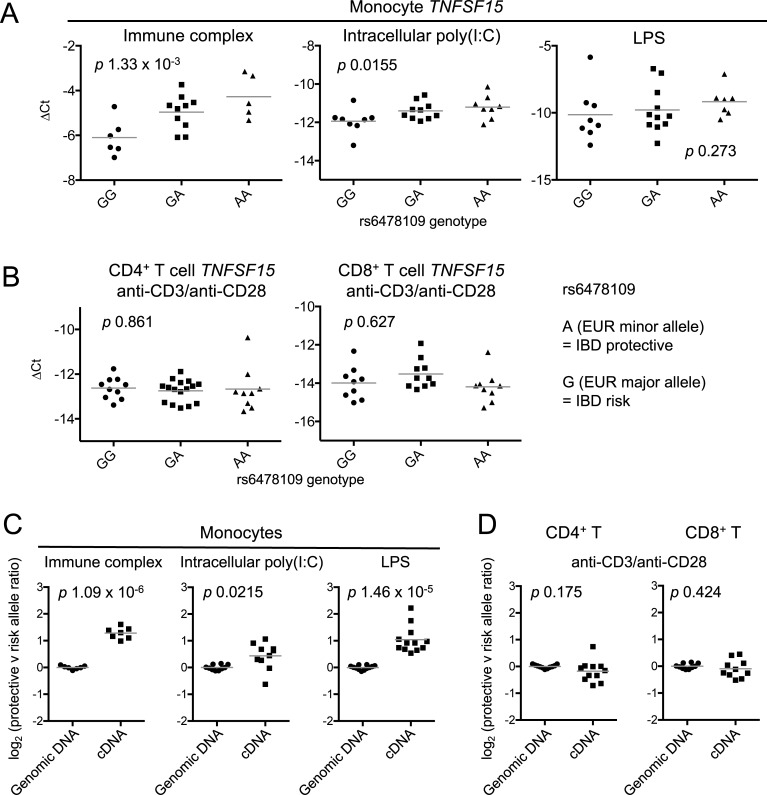
TNFSF15 expression is rapidly upregulated in myeloid cells by stimulation via pattern recognition receptors, such as toll-like receptor ligands, and Fc receptors (FcRs) [29, 52, 53]. Although resting T cells minimally express TNFSF15, T cell receptor (TCR) stimulation results in robust expression [29, 54]. To examine the effect of cellular stimulation on the association of genotype with TNFSF15 expression, we stimulated monocytes and T cells for 4 and 24 hours, respectively (time courses depicted in S3 Fig). In monocytes stimulated with immune complex and intracellular poly(I:C), the protective allele was associated with higher levels of TNFSF15 mRNA (p 1.33 x 10−3 and p 0.0155, respectively), similar to results in unstimulated cells, while LPS-stimulated monocytes showed a comparable trend but with more variability (p 0.273, Fig 2A). A formal comparison of the eQTL in unstimulated versus stimulated monocytes revealed no significant change in the magnitude of the effect of genotype on gene expression following stimulation (Methods, S4 Fig, S1 Table). In T cells stimulated via the TCR with anti-CD3 and anti-CD28, there was no association between rs6478109 genotype and TNFSF15 expression (CD4+ T cells p 0.861, CD8+ T cells p 0.627, Fig 2B). Thus rs6478109 is a monocyte eQTL in which the IBD risk allele is associated with reduced TNFSF15 expression both at baseline and after stimulation.
Fig 2. Monocyte but not T cell TNFSF15 expression is associated with genotype after stimulation.
(A) TNFSF15 mRNA expression was measured relative to B2M in monocytes stimulated for 4 hours with immune complex (rs6478109 genotype: GG, n = 6; GA, n = 10; AA, n = 5), intracellular poly(I:C) (GG, n = 8; GA, n = 10; AA, n = 8), or LPS (GG, n = 8; GA, n = 11; AA, n = 7). Expression is reported as ΔCt, each point represents an individual, lines represent mean values, and p values are from linear regression. (B) As (A) for CD4+ (GG, n = 10; GA, n = 16; AA, n = 9) and CD8+ (GG, n = 9; GA, n = 10; AA, n = 9) T cells stimulated for 24 hours. (C) Allelic ratios in cDNA from stimulated cells from heterozygous individuals are compared with those in genomic DNA from the same individuals: monocytes stimulated with immune complex (n = 7), intracellular poly(I:C) (n = 10) and LPS (n = 12), and (D) anti-CD3/anti-CD28-stimulated CD4+ (n = 11) and CD8+ (n = 10, genomic DNA as for panel (C) intracellular poly(I:C)) T cells. Ratios for immune complex- and LPS-stimulated monocytes and stimulated CD4+ T cells were measured using tag SNP rs4246905 (ratio of T/C reported), while ratios for poly(I:C)-stimulated monocytes and stimulated CD8+ T cells were measured using tag SNP rs4263839 (ratio of A/G reported). All individuals were heterozygous at both rs6478109 and the tag SNP. rs4263839:A and rs4246905:T are in phase with the IBD-protective allele rs6478109:A in 1000 Genomes EUR subjects. Lines represent mean values and p values are from Welch’s t-test.
Inter-individual differences apart from SNP genotype at the TNFSF15 locus might influence gene expression and thereby obscure genotype-dependent differences. Heterozygotes present a unique opportunity to control for this variability as the ratio of allelic expression can be measured within each individual using allele-specific expression (ASE) assays. rs6478109 is in LD with several intronic SNPs measurable in amplified pre-mRNA, two of which we used to examine ASE (S5 Fig). Due to low TNFSF15 pre-mRNA expression in unstimulated cells, allelic ratio measurements were only feasible in stimulated cells. In accordance with the allelic dosage effect observed in Fig 2A, such that TNFSF15 expression increased with more copies of the IBD-protective rs6478109:A allele, heterozygous monocytes stimulated with immune complex or intracellular poly(I:C) showed significant allelic imbalance favoring the protective allele (p 1.09 x 10−6, p 0.0215, respectively, Fig 2C left and middle panels). Although TNFSF15 expression in LPS-stimulated monocytes was not associated with allelic dosage by standard eQTL analysis (Fig 2A), in the internally controlled environment of heterozygous individuals, we did find significant ASE (p 1.46 x 10−5, Fig 2C right panel). In contrast, once again, stimulated T cells showed no allelic imbalance (CD4+ T cells p 0.175, CD8+ T cells p 0.424, Fig 2D and S2 Table), confirming the monocyte-specific nature of the TNFSF15 eQTL.
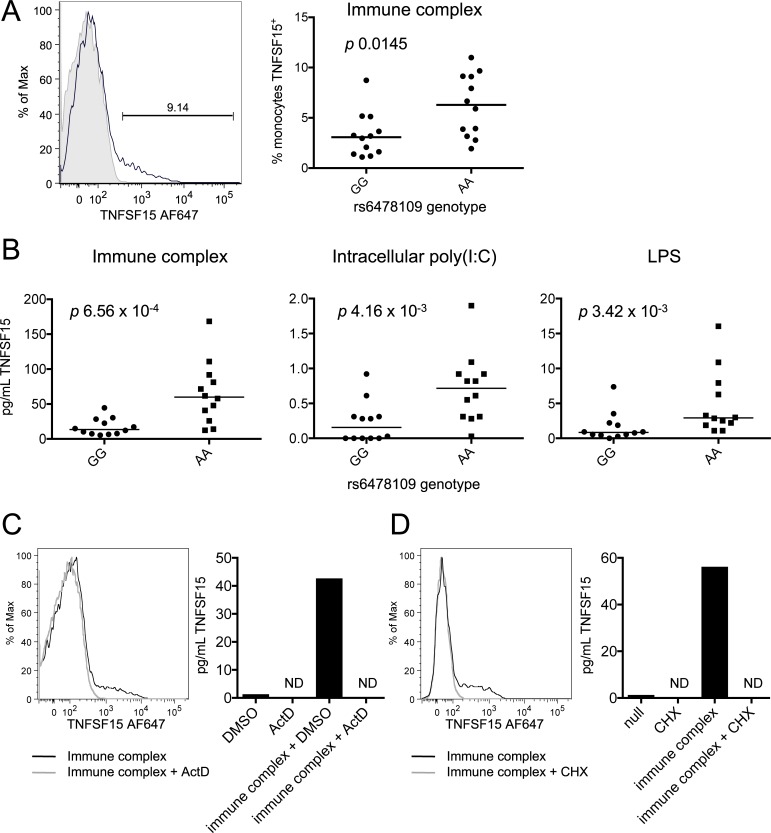
The most direct mechanism by which TNFSF15 mRNA expression could influence IBD susceptibility would be through controlling TNFSF15 protein levels. Stimulated monocytes express transmembrane TNFSF15 protein, but it is rapidly cleaved from their surfaces. TNFSF15 expression is thus measurable both on the cell surface and in the supernatant. To determine if the TNFSF15 eQTL extended to the protein level, we first measured surface TNFSF15 expression in immune-complex stimulated monocytes from homozygous individuals. Individuals homozygous for the IBD protective allele exhibited increased cell surface TNFSF15 (p 0.0145, Fig 3A). We next looked at soluble TNFSF15 in the supernatants of monocytes stimulated with immune complex, intracellular poly(I:C), or LPS. In the absence of stimulation, soluble TNFSF15 levels in monocyte supernatants were low (around the limit of detection) regardless of genotype. In contrast, under all stimulation conditions, soluble TNFSF15 was significantly increased in supernatants of cells from IBD protective allele homozygotes (immune complex p 6.56 x 10−4, intracellular poly(I:C) p 4.16 x 10−3, LPS p 3.42 x 10−3, Fig 3B). Thus, the levels of TNFSF15 both on the cell surface and in solution were associated with genotype. Of note, total serum TNFSF15 protein levels were not associated with genotype (p 0.962, S6A Fig), suggesting that the effect of genotype on monocyte TNFSF15 levels is relevant primarily in local cellular contexts. Secretion of other inflammatory cytokines from these monocytes was not associated with rs6478109 genotype at the same time-point, demonstrating the cis-specificity of the TNFSF15 eQTL (S6B–S6C Fig). To confirm that secreted TNFSF15 originated from newly synthesized protein, we stimulated monocytes with immune complex in the presence of either actinomycin D to block transcription (Fig 3C) or cycloheximide to block translation (Fig 3D). Both treatments resulted in loss of detectable protein, indicating that the effects of genotype on TNFSF15 protein expression were due to differences in de novo transcription and translation of TNFSF15.
Fig 3. Genotype is associated with de novo TNFSF15 protein production in stimulated monocytes.
(A) An example of gating for TNFSF15+ monocytes after 4 hours immune complex stimulation is depicted (left). Black line = monoclonal anti-TNFSF15; grey shading = isotype control. Percentages of TNFSF15+ monocytes after immune complex stimulation are quantified for GG (n = 12) and AA (n = 12) homozygous individuals (right). (B) Soluble TNFSF15 was measured in supernatants of monocytes from GG (n = 12) and AA (n = 12) homozygous individuals after the indicated 4-hour stimulations by custom anti-TNFSF15 Bio-Plex assay. p values are from Mann-Whitney test. (C) Monocytes were stimulated with immune complex for four hours in the presence of actinomycin D (ActD) or dimethyl sulfoxide (DMSO) control. ND indicates none detected. (D) As (C) in the presence or absence of cycloheximide (CHX). (C) and (D) are representative of two independent experiments each.
Fine-mapping narrows the functional variant in the TNFSF15 locus to upstream of the gene
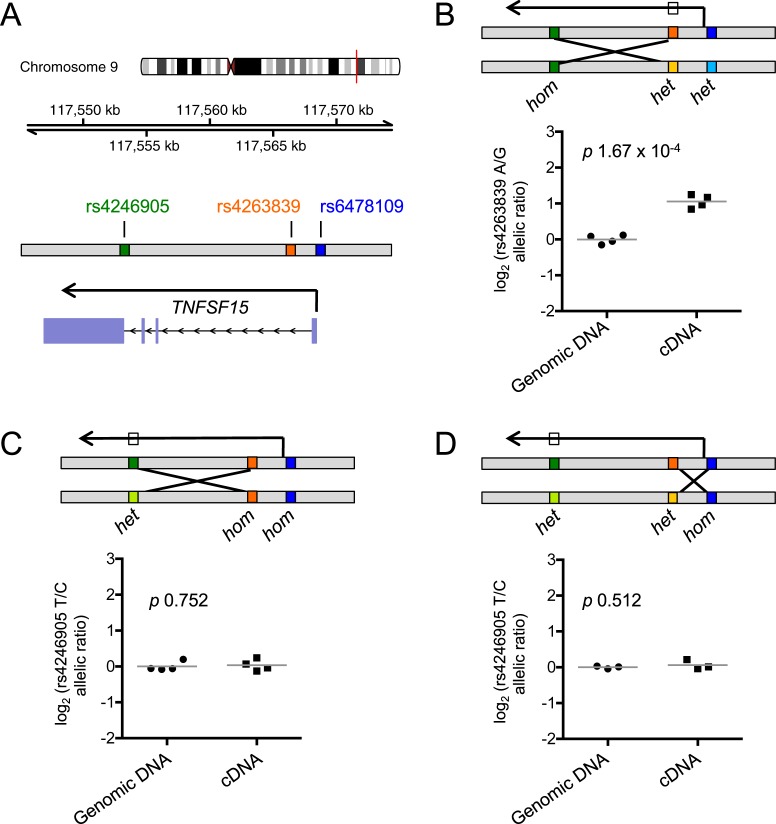
Fine-mapping of genotype-phenotype associations generally requires a large number of samples to achieve the power necessary to statistically infer probable causality for one SNP over another in high LD. In order to solve this problem with a limited number of samples, we again leveraged the power of the controlled environment within heterozygous individuals. We examined ASE in immune complex-stimulated monocytes from healthy volunteers recruited specifically for having genetic cross-over events in the TNFSF15 haplotype block, such that they were heterozygous for certain SNPs but homozygous for others (Fig 4A). An earlier IBD meta-analysis by Jostins et al identified rs4246905 in the third intron of TNFSF15 as the tag SNP for disease association at this locus in European individuals [5], whereas the more recent trans-ancestry analysis described above by Liu et al [4] identified the upstream SNP rs7848647 as the most associated in the same population. To narrow the location of the eQTL causal variant to either the upstream or downstream portion of the gene, we first examined ASE in individuals heterozygous at rs6478109 in the promoter and rs4263839 in the first intron but homozygous at rs4246905 in the third intron. These individuals maintained ASE of TNFSF15 (p 1.67 x 10−4, Fig 4B), indicating that rs4246905 is not causal and suggesting that the SNP influencing TNFSF15 expression is in greater LD with the upstream SNPs than with rs4246905. Confirming this finding, individuals heterozygous at rs4246905 but homozygous at the two upstream loci showed no ASE (p 0.752, Fig 4C).
Fig 4. The eQTL causal SNP resides upstream of TNFSF15.
(A) The TNFSF15 locus on chromosome 9 is depicted, marking SNPs used for functional fine-mapping. (B) Immune complex-stimulated monocytes from individuals heterozygous at rs6478109 and rs4263839 but homozygous at rs4246905 (n = 4) were examined for allele-specific expression. The box on the transcription arrow indicates the SNP used for measuring allelic imbalance. (C) As (B) with individuals homozygous at rs6478109 and rs4263839 but heterozygous at rs4246905 (n = 4). (D) As (B) with individuals homozygous at rs6478109 but heterozygous at rs4263839 and rs4246905 (n = 3). Lines represent mean values and p values are from Welch’s t-test.
Examining the LD structure of variants in the TNFSF15 locus, we found 17 variants that are in greater LD with the upstream SNPs rs6478109 and rs4263839 than with the downstream SNP rs4246905, and that are in higher LD with these upstream SNPs than is rs4246905 (S3 Table). To further distinguish between these potential causal variants, we identified individuals homozygous at the rs6478109 promoter SNP but heterozygous at rs4263839 in the first intron and measured TNFSF15 ASE in immune-complex-stimulated monocytes. These samples lacked ASE (p 0.512, Fig 4D), indicating that rs4263839 is not the causal variant. Only three variants identified in the 1000 Genomes Phase 3 European cohort in high LD with rs6478109 (r2 > 0.8) are in higher LD with rs6478109 than with the eliminated SNP rs4263839. These are rs6478109 itself, rs7848647 (the most significant IBD-associated variant in Europeans in Liu et al [4]) and rs10817678, all of which are located upstream of TNFSF15. Two of these, rs6478109 and rs7848647, are located close to the promoter of TNFSF15, in a region containing a cluster of transcription factor binding sites, active chromatin marks and enhancers, making them the leading candidates for the causal variant (S7 Fig). These two SNPs were completely linked in all individuals recruited for ASE measurement in Figs 2 and 4.
Genetically modulated TNFSF15 expression affects strength of costimulation
To examine the phenotypic consequences of variation in TNFSF15 expression, we performed comprehensive immunophenotyping of T cells, B cells, monocytes, dendritic cells, and NK cells from peripheral blood of individuals homozygous for the rs6478109 polymorphism. We found no association between genotype and cell population frequencies (S8 and S9 Figs).
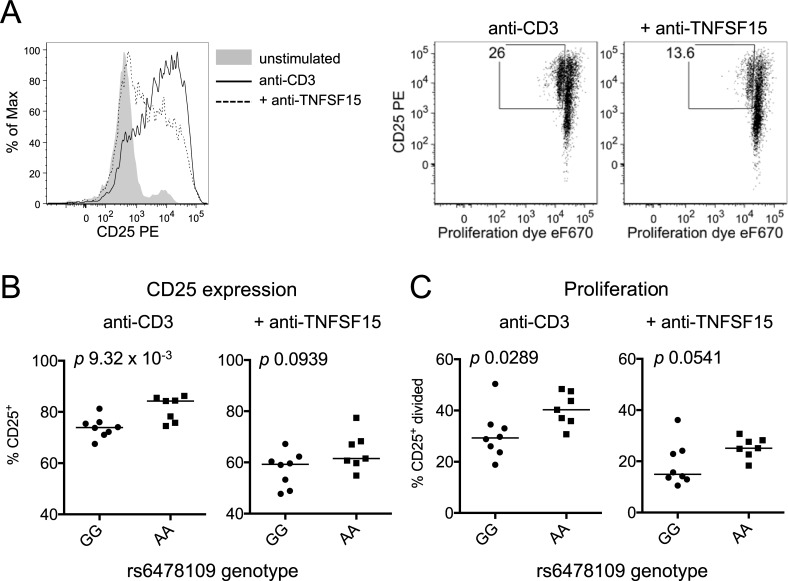
To test whether genetically driven variation in TNFSF15 expression under acute stimulation would result in differences in responding cell populations, we measured the effects of endogenous TNFSF15 on T cell activation. TNFSF15 costimulation promotes T cell proliferation and upregulation of the IL-2 receptor alpha chain (CD25), particularly under low levels of TCR stimulation [26, 29, 30]. We therefore examined CD4+ T cell proliferation in stimulated peripheral blood mononuclear cells from individuals homozygous for the TNFSF15 expression-associated variant rs6478109. CD8+ T cells make up a highly variable proportion of peripheral blood cells across individuals and these cells respond to TCR stimulation by making and consuming IL-2, which can affect CD4+ T cell proliferation. To avoid this confounding factor, we depleted peripheral blood mononuclear cells of CD8+ T cells before culturing the remaining cells (including CD4+ T cells and monocytes) with low level TCR stimulation for two days. Blocking TNFSF15 signaling with an antagonistic antibody resulted in decreased CD25 expression and proliferation of CD4+ T cells (Fig 5A), demonstrating the impact of endogenous TNFSF15 in this setting. Samples from individuals homozygous for the IBD protective allele that is associated with increased monocyte TNFSF15 exhibited significantly increased CD25 expression and proliferation of CD4+ T cells compared to individuals homozygous for the IBD risk allele (p 9.32 x 10−3, p 0.0289, respectively, Fig 5B and 5C). The differences in CD25 expression and proliferation were reduced with addition of anti-TNFSF15 such that these activation markers were no longer statistically significantly elevated in protective allele homozygotes (CD25 expression p 0.0939, proliferation p 0.0541, Fig 5B and 5C). While this system does not recapitulate the complex intestinal environment of the pre-morbid at-risk individual, it demonstrates that genetically regulated TNFSF15 expression can influence the ability of monocytes to costimulate responding lymphocytes, confirming the functional relevance of this TNFSF15 eQTL.
Fig 5. Genetically regulated TNFSF15 controls costimulatory capacity.
(A) CD8-depleted PBMC were stained with proliferation dye eFluor670 and stimulated with 1 μg/mL anti-CD3 with and without 10 μg/mL anti-TNFSF15 for 48 hours. Flow cytometry plots depict cells gated on live, CD4+ cells. (B) CD25+ cells were measured as in (A) in samples from rs6478109 genotyped individuals (GG, n = 8; AA, n = 7). (C) CD25+ divided cells were measured in the same samples as in (B). p values from Mann-Whitney test.
Monocyte-derived macrophages also exhibit allelic imbalance favoring the IBD protective allele
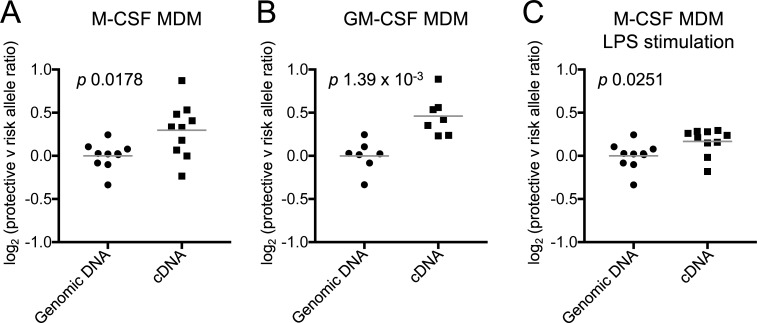
Intestinal CX3CR1+ mononuclear phagocytes derived from circulating monocytes [55–57] are likely to be the most relevant producers of TNFSF15 in the context of gut immune homeostasis [31]. To test whether phagocytic cells derived from peripheral blood monocytes maintain genotype-dependent TNFSF15 expression, we differentiated monocyte-derived macrophages (MDMs) from heterozygous individuals and measured ASE. MDM differentiated in the presence of M-CSF and GM-CSF both exhibited significant allelic imbalance favoring the minor, IBD-protective allele (p 0.0178 and p 1.39 x 10−3, respectively, Fig 6A and 6B). ASE was also maintained after stimulation of M-CSF-derived MDM with LPS for 4 hours (p 0.0251, Fig 6C). To confirm these findings in a second cohort, we mined publicly available data from an MDM eQTL study by Nedelec et al [46]. These data demonstrated a significant TNFSF15 eQTL at rs6478109 in resting MDM and after Salmonella infection (S10 Fig). The direction of effect was concordant with our monocyte and MDM data, such that expression of TNFSF15 increased with more copies of the IBD-protective allele rs6478109:A. These results demonstrate that the IBD-protective allele increases TNFSF15 expression in macrophages as well as monocytes, and is therefore likely to be relevant to the gut environment.
Fig 6. Monocyte-derived macrophages also favor expression of the IBD-protective allele.
(A) Peripheral blood monocytes from rs6478109 heterozygotes were differentiated into macrophages in the presence of M-CSF (n = 10) or (B) GM-CSF (n = 7) before examining allele-specific expression. (C) Macrophages derived in (A) were stimulated with LPS for 4 hours before examining allele-specific expression (n = 10, genomic DNA as for panel (A)). ASE was measured at rs4263839 (ratio of A/G reported) in the first intron of TNFSF15. All individuals were heterozygous at both rs4263839 and rs6478109. rs4263839:A is in phase with the IBD-protective allele rs6478109:A in 1000 Genomes EUR subjects.
Discussion
Understanding the mechanisms by which disease susceptibility variants influence disease risk is a key challenge of the post-GWAS era. Exploring the consequences of disease risk variants in healthy individuals allows examination of their effects in the pre-disease state and avoids the potential confounding effects of treatment and disease itself. Use of a “recall-by-genotype” bioresource enables investigation of specific polymorphisms with balanced experimental designs and facilitates in-depth investigation of causal quantitative trait loci by specific recruitment of individuals with breaks in common LD blocks. Here we used such a bioresource to investigate the functional consequences of an IBD-associated genetic variant and perform functional fine-mapping. We have demonstrated that the IBD-associated locus at TNFSF15 harbors an expression-associated polymorphism in which the rs6478109:A IBD protective allele is associated with increased monocyte TNFSF15 expression and lymphocyte costimulatory activity. Interestingly, TNFSF15 expression in stimulated T cells was not significantly associated with SNP genotype at this locus (discussed further in S1 Text), suggesting the importance of cellular context in utilization of the particular cis regulatory element that this polymorphism affects. Through ASE assays in cells from individuals specifically recruited for haplotype cross-over events, we refined the location of the causal variant for gene expression to the upstream region of the gene. Colocalization testing indicated that the eQTL and IBD association are very likely to be due to the same causal variant. This suggests that the mechanism by which SNP genotype at TNFSF15 influences IBD susceptibility is through altering TNFSF15 expression. Our work provides an example of how functional studies not only uncover the phenotypic effects of genetic variation but can also complement statistical methods for mapping disease association.
The IBD fine-mapping study by Huang et al examined the TNFSF15 locus, detailing technical difficulties with their genotyping of an indel in the region [6]. The three typed variants that interrogated the indel (chr9:117571294, chr9:117571293, and rs59418409) were assigned posterior probabilities of being causal 0.40, 0.40, and 0.11, respectively. On the basis that these three variants in fact represent a single indel (now annotated as rs35396782), the authors then summed the probabilities and concluded that this indel is the likely causal variant for IBD risk. The rs35396782 indel is 2885 base pairs upstream of TNFSF15 and in LD (r2 = 0.817) with rs6478109 (S3 Table and S11 Fig). Our analysis cannot exclude the possibility that this indel is causal for the eQTL, but the LD patterns in our functional fine-mapping and the presence of active chromatin marks in the region of the promoter SNPs rs6478109 and rs7848647 favor these candidates. Robust genotyping of the indel will be necessary to draw conclusions about its association with both IBD and TNFSF15 expression.
Previous studies describing gene expression association with genotype at TNFSF15 have yielded conflicting results [38–45] (discussed further in S1 Text). Our data unequivocally show that the IBD risk allele is associated with decreased monocyte TNFSF15 expression. Use of three genotyping platforms, two mRNA measurement technologies, and two protein measurement methods ensures the robustness of our results. In support of our findings, mining of the GTEx project database [45, 47] reveals a significant TNFSF15 eQTL at rs6478109 in whole blood with the same direction of effect that we observed. Of relevance to IBD, the GTEx database also includes a nominally significant eQTL (p <10−3) with the same direction of effect in sigmoid colon.
In the gut, peripheral blood monocytes differentiate into macrophages, which are critical for maintaining mucosal immune homeostasis [58]. A recent study by Baillie et al posited that monocyte maladaptation to macrophage differentiation and activation in the gut environment is an important driver of IBD [21]. Examination of the supporting information for their study reveals that TNFSF15 is strongly upregulated during this process. A key question is whether the TNFSF15 monocyte eQTL is maintained in macrophages and is therefore likely to be relevant to IBD pathogenesis. A previous study by Hedl et al reported association of the TNFSF15 risk haplotype SNP rs6478108:A (in phase with rs6478109:G, LD r2 0.917, S1 Fig) with increased TNFSF15 expression in M-CSF-differentiated MDM [41]. In contrast, we observed the opposite result in these cells (Fig 6), demonstrating ASE that was directionally concordant with our findings in monocytes. Through further ASE assays in macrophages from multiple differentiation and stimulation conditions, we demonstrated that the IBD protective allele is consistently preferentially expressed. We corroborated our findings by mining publicly-available data from a recent eQTL study in MDM with and without Salmonella infection, confirming that the IBD risk allele is associated with lower MDM TNFSF15 expression [46]. We thus clearly establish that, in both monocytes and macrophages, genetic predisposition to lower TNFSF15 expression is associated with IBD risk.
The association we have identified may at first seem counterintuitive given the known functions of TNFSF15. In IBD, TNFSF15 is generally considered an inflammatory marker, with TNFSF15 expression levels increasing with IBD activity [32–37]. However, such observational studies cannot distinguish causal effects from associations arising from confounding factors or the consequences of IBD (reverse causation). In animal models of inflammatory disease including colitis, asthma, arthritis, and experimental autoimmune encephalomyelitis, genetic or antibody-mediated disruption of TNFSF15 signaling through its cognate receptor TNFRSF25 (also known as DR3) generally leads to reduced pathology [29, 54, 59–63], but it is important to remember that these animal studies usually measure disease course and are poor models for disease susceptibility. At the cellular level, TNFSF15 generally promotes cytokines associated with inflammation, such as IL-2 and IFNγ from T cells, and IL-13 and IL-5 from ILC2 [26–28, 52, 64]. Indeed, we find that genetically-driven TNFSF15 enhances T cell activation in our in vitro assay. Despite this inflammatory role, studies have highlighted that TNFSF15 may be more pleiotropic than originally thought, costimulating lymphocytes that control both pro- and anti-inflammatory activities. Jia et al demonstrated a protective role for TNFSF15-TNFRSF25 interaction in acute DSS colitis and clearance of gut Salmonella enterica infection via maintenance of regulatory T cells [65], suggesting that TNFSF15 may be protective in certain contexts of intestinal inflammation. Additionally, as well as T cells and ILC2, TNFSF15 can also costimulate group 3 innate lymphocytes (ILC3), which reside in the gut and respond to TNFSF15 with enhanced IL-22 production [31, 66]. IL-22 promotes gut barrier maintenance in both infectious and non-infectious contexts [67–69]. Thus, there is also the potential for TNFSF15 to play a protective role in the gut through costimulation of ILC3.
Reduced gene expression driven by the IBD risk allele at TNFSF15 is in line with several other IBD risk variants that reduce protein function and lead to intestinal barrier disruption. For example, the T300A variant of ATG16L1 reduces autophagy in intestinal Paneth cells, dampening antimicrobial activity [11–13]. Likewise, while multiple mechanisms have been posited for the association of variants in the NOD2 locus with CD [70], disease-associated coding polymorphisms were found to reduce cellular responsiveness to peptidoglycan ligands [9] and were associated with decreased expression of Paneth cell antimicrobial peptides [10] and defective anti-bacterial responses by dendritic cells [8]. The reduction in monocyte and macrophage expression of TNFSF15 that we find associated with the IBD risk allele suggests that TNFSF15 may also promote intestinal homeostasis in the pre-morbid state.
Materials and methods
Additional methods are included in S1 Text.
Presentation of previous eQTL data
We present peripheral blood monocyte eQTLs for TNFSF15 from a previous study using samples from 39 healthy individuals and 80 patients with IBD [19], as well as a study using 45 patients with anti-neutrophil cytoplasmic antibody-associated vasculitis [18]. Gene expression data was measured on the Affymetrix Human Gene 1.1 ST Array. Microarray mRNA expression was normalized by robust multiarray averaging using the oligo package [71] and adjusted with PEER [72]. IBD patient and healthy control genotypes were measured using the Illumina Human OmniExpress12v1.0 BeadChip, and vasculitis patients were genotyped using the Affymetrix SNP6.0 Array. eQTL testing was performed using a score test implemented in the GGtools Bioconductor package [73]. Genomic locus plots were generated using the Gviz Bioconductor package [74] with RefSeq annotation for genes in the hg19 genome build.
Colocalization testing
Colocalization testing was performed using the coloc R package v2.3–1 [48]. We used the coloc.abf function and the default priors (prior probability that a SNP is associated with trait 1 = 1 x 10−4, prior probability that a SNP is associated with trait 2 = 1 x 10−4, prior probability that a SNP is associated with both traits = 1 x 10−5). Summary statistics for association of TNFSF15 and TNFSF8 expression with genotype (regression coefficients and variances) were calculated through linear regression (lm function in R) using the genotype and expression data from our previous eQTL study of healthy controls and patients with IBD [19]. For the IBD GWAS data, we used summary statistics for the European cohort from the GWAS plus Immunochip trans-ancestry MANTRA meta-analyses by Liu et al [4] (downloaded from the International Inflammatory Bowel Disease Genetics Consortium’s website, url https://www.ibdgenetics.org/, link “Latest combined GWAS and Immunochip trans-ancestry summary statistics”, file “IBD_trans_ethnic_association_summ_stats_b37.txt.gz”).
Sample collection
Peripheral blood samples from healthy volunteers were obtained through the Cambridge BioResource. Ninety-six blood samples were taken from 90 separate volunteers recruited based on relevant genotype at rs6478109 and/or rs4246905. All recruited individuals were Caucasian, between 18 and 65 years of age. Volunteers self-declared that they were free from autoimmune disease, cancer and human immunodeficiency virus. No individuals took regular systemic immunomodulatory therapy for at least one year before recruitment. During the recruitment process, volunteer samples were grouped by genotype, and investigators were blinded as to which group corresponded to which genotype. Derived numeric data from experiments utilizing Cambridge BioResource samples are included in S1 Data.
Whole blood processing
Whole blood was collected in CPDA tubes and passed over a Histopaque-1077 (Sigma Aldrich) gradient to separate peripheral blood mononuclear cells (PBMC). Where indicated, cell types were separated first into the CD14+ monocyte fraction and CD14- fraction by positive selection with human CD14 MicroBeads and LS columns (Miltenyi Biotec), according to the manufacturer’s protocol. The negative fraction was then enriched for CD4+ T cells or CD8+ T cells with human CD4 or CD8 MicroBeads (Miltenyi Biotec) in the same manner. For CD8-depleted PBMC, cells were separated with CD8 MicroBeads (Miltenyi Biotec) and the negative fraction collected. Purity of separated cell subsets was examined by flow cytometry as described in the “Cell subset purity QC” section. For eQTL and ASE measurements, eighty monocyte samples were sorted for use in various assays, all with over 60% purity and a median purity of 74%; thirty-five CD4+ T cell samples were sorted, all with over 92% purity and a median purity of 97%; thirty-four CD8+ T cell samples were sorted, all with purity over 70% and a median purity of 90%; sixteen PBMC samples were depleted of CD8+ T cells, all demonstrating less than 7% CD8+ T cells remaining. One CD8-depleted PBMC sample was excluded on the basis of a CD8-intermediate CD3+ population composing 13% of the PBMC population after depletion.
Cell subset purity QC
Purity of cell subsets was determined by flow cytometry. Cells were blocked with FcR Blocking Reagent (Miltenyi) and stained with anti-human CD14-PE (BD Biosciences); anti-human CD3-AmCyan, -FITC or–PE (clone SK7 or UCHT1, BD Biosciences); anti-human CD4-FITC (clone RPA-T4, BD Biosciences); and/or anti-human CD8-APC (clone RPA-T8, BD Biosciences) or -eFluor 450 (clone SK1, eBioscience). Flow cytometry was performed on a BD LSR Fortessa (BD Biosciences) and data analyzed in FlowJo (FlowJo, LLC).
In vitro cell stimulations
All stimulations took place in complete medium, composed of RPMI with 10% FCS, 10 mM HEPES, 1x MEM non-essential amino acids, 1 mM sodium pyruvate, 1x GlutaMAX, 100 U/mL penicillin and 0.1 mg/mL streptomycin (Sigma or Gibco). Monocytes were stimulated with 100 ng/mL LPS (Sigma), plate-bound immune complex (as previously described [52]), or 100 μg/mL intracellular polyinosinic:polycytidylic acid (poly(I:C)), prepared with 1 mg/mL high molecular weight poly(I:C) mixed 1:1 with LyoVec transfection reagent (Invivogen) at RT for 15 minutes. Where indicated, 1 μg/mL cycloheximide or 5 μg/mL actinomycin D (Sigma) was added to cell cultures. T cells were stimulated with Dynabeads Human T-Activator CD3/CD28 (Life Technologies) at a 1:1 ratio of beads:cells. For the PBMC proliferation assay, five million CD8- cells were stained with Cell Proliferation Dye eFluor 670 (eBioscience), according to the manufacturer’s instructions. These cells were stimulated for 48 hours with 1 μg/mL anti-CD3 (OKT3), with addition of 10 μg/mL blocking anti-TNFSF15 monoclonal antibody (1A9, described under Soluble cytokine measurements) where indicated.
Monocytes were differentiated into monocyte-derived macrophages in the presence of M-CSF or GM-CSF. For M-CSF macrophages, cells were grown in 10 ng/mL recombinant human M-CSF (R and D Systems) for 7 days, adding half the volume of media with 30 ng/mL M-CSF to replenish the cytokine on day 5. For GM-CSF macrophages, monocytes were grown in 50 U/mL recombinant human GM-CSF (Peprotech) for 5 days. Where indicated, macrophages were stimulated with 10 ng/mL LPS for 4 hours. One sample recruited for monocyte-derived macrophage studies was excluded due to poor RNA yield before any measurements were made.
Nucleic acid extraction and qPCR for samples
RNA and DNA from ex vivo and cultured cells was extracted using the AllPrep DNA/RNA Mini Kit (or RNeasy Mini Kit for extracting RNA only), using on-column DNase digestion with the RNase-Free DNase Set (Qiagen). RNA was reverse-transcribed to cDNA using the SuperScript VILO cDNA Synthesis Kit (Life Technologies). qPCR reactions were performed with Taqman Gene Expression Master Mix and Taqman Gene Expression Assays for TNFSF15 (Hs00270802_s1) or Beta-2-Microglobulin (B2M, Hs00984230_m1) (Life Technologies) on an Applied Biosystems 7900HT Fast Real-Time PCR System (Life Technologies) or CFX384 Touch Real-Time PCR Detection System (BioRad). All reactions were performed in triplicate, and the median TNFSF15 Ct value was subtracted from the median B2M Ct value for each sample to generate ΔCt values representing log expression relative to B2M.
qPCR eQTL analysis
Linear regression test statistics were calculated in R to estimate the effect of each additional copy of the minor (IBD protective) allele on gene expression. To test for an interaction between genotype and stimulation condition, a linear model with coefficients for genotype, stimulation and genotype x stimulation was fitted.
Genotyping
Samples were genotyped using Taqman SNP Genotyping Assays (rs6478109, C___1305297_10; rs4263839, C____120268_10; rs4246905, C____363307_20; rs7848647, C__11277159_10; rs6478108, C____170492_10) and Taqman Genotyping Master Mix (Life Technologies) according to the manufacturer’s protocol on an Applied Biosystems 7900HT Fast Real-Time PCR System (Life Technologies) or CFX384 Touch Real-Time PCR Detection System (BioRad).
Allele-specific expression (ASE)
ASE in heterozygous genomic DNA or reverse-transcribed cDNA from the same individual was measured by qPCR. Where ASE was measured in multiple conditions using cells from the same individuals, one genomic DNA sample from each individual was measured concurrently with cDNA samples from multiple conditions. First, the intronic region containing the target SNP was amplified from DNA using the primers and PCR conditions listed in S4 Table. Amplified regions were gel purified. A standard curve was constructed by mixing amplified genomic DNA samples from homozygous individuals 8:1, 4:1, 2:1, 1:1, 1:2, 1:4, and 1:8. Samples were then measured by qPCR in triplicate reactions using Taqman SNP Genotyping Assays for rs4263839 (C____120268_10) or rs4246905 (C____363307_20) and Taqman Gene Expression Master Mix on an Applied Biosystems 7900HT Fast Real-Time PCR System (Life Technologies) or CFX384 Touch Real-Time PCR Detection System (BioRad). Allelic ratios were calculated from VIC and FAM Ct values as described in S1 Text. Unpaired Welch's t-test statistics were calculated using GraphPad Prism Software or R. We used two intronic SNPs, rs4246905 and rs4263839 (the latter of which is in LD r2 = 0.977 with rs6478109 and is the most linked of all transcribed SNPs) to examine ASE of TNFSF15. Examination of the effect size estimates obtained from assays with each of the two SNP probes on a subset of samples from Fig 2C revealed similar detection of ASE but a slightly lower estimate by the rs4263839 probe (S5B Fig). For this reason, we do not recommend directly comparing effect sizes between samples measured with different probes.
Soluble cytokine measurements
A mouse monoclonal antibody against human TNFSF15 (clone 1A9) was generated through immunizing mice with human TNFSF15 and screening supernatants for binding TNFSF15 transfected 293T cells. The 1A9 clone also blocks soluble TNFSF15 binding to TNFRSF25 and TNFSF15 costimulation of human T cell activation ex vivo [75].
Soluble TNFSF15 in supernatants of stimulated cells was measured by custom Bio-Plex assay. Capture beads were created by conjugating anti-human TNFSF15 antibody (clone 1A9) to Bio-Plex Pro Magnetic COOH beads, region 27, using the Bio-Plex Amine Coupling Kit (BioRad) with 10 μg antibody per reaction, according to the manufacturer’s instructions. Cell culture supernatant assays were performed with 2500 beads per well for capture and 1 μg/mL biotinylated polyclonal rabbit anti-human TNFSF15 (Peprotech) for detection, using the Bio-Plex Pro Reagent Kit (BioRad) and following the manufacturer’s protocol. Data was collected on a Bio-Plex 200 or Bio-Plex MAGPIX Multiplex Reader (BioRad). Cell culture supernatant TNFSF15 concentrations were calculated using a standard curve of recombinant human TNFSF15 (Peprotech).
Serum TNFSF15 levels were also measured by custom Bio-Plex assay. Capture beads were created exactly as described for supernatant cytokine measurements but polyclonal rabbit anti-human TNFSF15 antibody (Peprotech) was used for conjugation to Bio-Plex Pro Magnetic COOH beads, region 27, with 8.5 μg antibody per reaction. Assays were run with samples diluted 1 in 4 in Sample Diluent from the Bio-Plex Pro Reagent Kit (BioRad). Values below the detection limit of the assay were set to 0. Values above the detection limit of the assay were excluded, and this is indicated in the figure legend. For genotype association testing, serum TNFSF15 protein levels were inverse-rank normalized prior to linear regression.
Inflammatory cytokines in the supernatants of stimulated monocytes were measured using the Human ProInflammatory 9-Plex Tissue Culture Kit (MesoScale Diagnostics) at the Core Biochemical Assay Laboratory in Cambridge University Hospitals or using Bio-Plex Pro reagents (BioRad), according to the manufacturer’s protocol. For plotting on a log-scale graph, 2 undetectable IL-10 measurements were set to positive values below the lowest value detected.
Mann-Whitney test statistics were calculated using GraphPad Prism Software.
Cell surface TNFSF15 measurements
Monocyte surface TNFSF15 expression was measured by flow cytometry. Cells were treated with human FcR Blocking Reagent (Miltenyi) for 5 minutes in PBS and then stained with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen) and either anti-TNFSF15 (clone 1A9) Alexa-Fluor 647 or mouse IgG2a Alexa-Fluor 647 (BD Biosciences) as an isotype control. Cells were analyzed on a BD LSRFortessa. Mann-Whitney test statistics were calculated using GraphPad Prism Software.
Ethics statement
The use of genotyped human peripheral blood samples in this study was approved by the National Research Ethics Service, Cambridgeshire 2 Research Ethics Committee (08/H0308/176). All subjects provided written informed consent prior to inclusion in the study.
Data mining
The data used for corroborating eQTL evidence described in this manuscript were obtained from the GTEx Portal on 10 February 2018 and from the IMMUNPOP browser (http://132.219.138.157/nedelec/eQTL/ and [46]) on 7 March 2018. Regulatory information about the TNFSF15 promoter region was visualized in the UCSC genome browser (http://genome.ucsc.edu/ and [76], hg19 build) on 17 Feb 2018. Tracks included transcription factor binding sites from ENCODE transcription factor ChIP-seq of 161 factors across a variety of cell types, DNase hypersensitivity signal measured by ENCODE/UW, and CTCF and histone ChIP-seq profiles from ENCODE/BROAD [77]. CAGE data for enhancer identification were downloaded from the ZENBU browser (http://fantom.gsc.riken.jp/zenbu/gLyphs/#config=dXO5cTaJBZiiw73fJq2oGD;loc=hg19::chr9:117566249..117571251+ and [21, 78]), on 17 February 2018.
Supporting information
(PDF)
(A) Plots depict monocyte TNFSF15 mRNA expression versus rs6478109 genotype in healthy controls and IBD patients (genotyping by Illumina Human OmniExpress12v1.0 BeadChip, gene expression by Affymetrix Human Gene ST 1.1 microarrays). (B) Table depicts results of LD calculation and haplotype phasing using 1000 Genomes Phase 3 European (EUR) and East Asian (EAS) cohorts for rs6478109 and rs6478108. Phasing is written as rs6478109 allele 1 in phase with rs6478108 allele 1 / rs6478109 allele 2 in phase with rs6478108 allele 2. (C) As (A), plots depict TNFSF15 mRNA expression versus rs6478108 genotype in healthy controls (n = 39) and IBD patients (n = 80) (genotyping by Illumina Human OmniExpress12v1.0 BeadChip) and patients with ANCA-associated vasculitis (n = 45) (genotyping by Affymetrix SNP 6.0). rs6478109 was not present on the Affymetrix SNP 6.0 genotyping platform, and thus rs6478108 was used as a proxy SNP for examining the eQTL in the AAV cohort. (D) Ex vivo monocyte TNFSF15 expression values from Fig 1B plotted by rs6478108 genotype (TT, n = 9; CT, n = 17; CC, n = 9).
(EPS)
Regional association plots for IBD (European ancestry cohort from Liu et al [4]) and TNFSF8 expression (n = 39 healthy individuals and 80 IBD patients). LD is colored with reference to the most associated SNP within each plot.
(EPS)
(A) Human peripheral blood monocytes were left unstimulated (null), or were stimulated with 100 ng/mL LPS, cross-linked immune complex, LyoVec transfection reagent control, or LyoVec with 100 μg/mL poly(I:C) for the indicated times. TNFSF15 mRNA was measured by qPCR relative to B2M with expression reported as ΔCt (left); secreted protein was measured in the supernatant by custom Bio-Plex assay (right). Plots are representative of at least 2 independent experiments each. (B) As (A) for CD4+ and CD8+ T cells stimulated with anti-CD3/anti-CD28-coated beads at a ratio of 1:1, beads:cells. The plot on the right shows a longer stimulation time-course for both T cell subsets.
(EPS)
Effect sizes estimated by linear regression (beta coefficients) and their standard errors are plotted for monocyte eQTLs from Fig 1B and Fig 2A.
(EPS)
(A) The TNFSF15 gene region was visualized in the UCSC genome browser (hg19 genome build). TNFSF15 gene position is from RefSeq. SNPs of interest are underlined in the same colors as Fig 4: rs6478109 eQTL SNP in blue, and rs4246905 and rs4263839 SNPs used for allele-specific expression (ASE) measurements in green and orange, respectively. Binding sites for primers used to amplify pre-mRNA for ASE measurements are indicated. (B) Probes for rs4246905 and rs4263839 were used to measure ASE in the same four samples of immune complex-stimulated monocytes (samples included in Fig 2C). 95% confidence interval for difference in mean log2(allelic ratio) between genomic DNA and cDNA calculated from Welch’s t-test.
(EPS)
(A) Serum TNFSF15 was measured in rs6478109 genotyped individuals (GG, n = 22; GA, n = 26; AA, n = 19; two additional AA individuals were excluded due to measurements above the detectable range of the standard curve). Line denotes the median; p value from linear regression on inverse-rank normalized values. (B) Inflammatory cytokines were measured in supernatants of stimulated monocytes from rs6478109 homozygotes (immune complex, n = 4 per genotype; LPS, n = 7 per genotype). No significant differences between genotypes by Mann-Whitney test. (C) Selected cytokines from (B) were further examined in a second cohort (n = 12 per genotype). No significant differences between genotypes by Mann-Whitney test.
(EPS)
The promoter region of TNFSF15 was visualized in the UCSC genome browser (http://genome.ucsc.edu/ and [76], hg19 build). TNFSF15 gene position is from RefSeq. SNPs of interest are indicated. Genome regulatory marks from ENCODE [77] are shown. Transcription factor binding sites are from ENCODE transcription factor ChIP-seq of 161 factors across a variety of cell types with consensus motifs marked in green. Histogram tracks represent measurements in primary human monocytes. “DHS” denotes raw DNase hypersensitivity signal measured by ENCODE/UW. CTCF and histone ChIP-seq profiles highlighted in orange are from ENCODE/BROAD. “Control” indicates sequencing of ChIP input control DNA. Enhancers and transcriptional activity identified by Cap Analysis of Gene Expression (CAGE) were downloaded from the ZENBU browser view associated with Baillie et al [21] (http://fantom.gsc.riken.jp/zenbu/gLyphs/#config=dXO5cTaJBZiiw73fJq2oGD;loc=hg19::chr9:117566249..117571251+), which includes FANTOM5 consortium data [78]. CAGE-identified enhancers across multiple human cell types and monocyte-derived macrophages, as well as transcriptional activity measurements by CAGE, are highlighted in turquoise.
(EPS)
Immunophenotyping gating strategies are depicted for (A) basic T cell subsets, (B) Th effector cell subsets, (C) Treg, (D) B cells, and (E) monocytes, dendritic cells, and NK cells.
(EPS)
Age- and sex-matched rs6478109 homozygotes were immunophenotyped for 39 different peripheral blood parameters. Between 6 and 19 pairs were examined for each subset. No immune cell subsets were found to be differentially abundant between genotypes by Wilcoxon test.
(EPS)
Plots were generated using the IMMUNPOP browser at http://132.219.138.157/nedelec/eQTL/
(EPS)
Regional linkage disequilibrium (r2) plot of genetic variants +/- 3 kbp from rs6478109 calculated using 1000 Genomes Phase 3 EUR cohort.
(EPS)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(ZIP)
Acknowledgments
We thank the flow cytometry group of the Office of Science and Technology in NIAMS, and John Reid, John Ferdinand and Alejandro Villarino for insightful discussions. We gratefully acknowledge the participation of all NIHR Cambridge BioResource volunteers, and thank the NIHR Cambridge BioResource centre and staff for their contribution. We thank the National Institute for Health Research and NHS Blood and Transplant. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS.
Data Availability
Genotype data for the eQTL analyses shown in Fig 1A, S1A and S1C Fig, and S2 Fig are available in EGA https://www.ebi.ac.uk/ega (accession number EGAS00001001251); access is controlled to protect personal genetic data of study volunteers and is available for scientific researchers via requests to the data access committee (EGAC00001000338). Gene expression data for these same eQTL analyses are available through Array Express (accession number E-MTAB-3554). Publicly available datasets were obtained as follows: summary statistics for the European cohort from the GWAS plus Immunochip trans-ancestry MANTRA meta-analyses by Liu et al (URL ftp://ftp.sanger.ac.uk/pub/consortia/ibdgenetics/iibdgc-trans-ancestry-filtered-summary-stats.tgz, file IBD_trans_ethnic_association_summ_stats_b37.txt.gz); corroborating data for a monocyte-derived macrophage TNFSF15 eQTL at rs6478109 by Nédélec et al in the IMMUNPOP browser (URL http://132.219.138.157/nedelec/eQTL/, search term rs6478109); regulatory information about the TNFSF15 promoter from ENCODE in the UCSC genome browser (URL http://genome-euro.ucsc.edu/cgi-bin/hgTracks?db=hg19&lastVirtModeType=default&lastVirtModeExtraState=&virtModeType=default&virtMode=0&nonVirtPosition=&position=chr9%3A117566800-117570000&hgsid=228207134_vgkDNh2jKZKgDRQr38TDxd4l3YSF); CAGE data for enhancer identification associated with Baillie et al in the ZENBU browser (URL http://fantom.gsc.riken.jp/zenbu/gLyphs/#config=dXO5cTaJBZiiw73fJq2oGD;loc=hg19::chr9:117566249..117571251+). All other relevant data are within the paper and its Supporting Information files.
Funding Statement
ACR was supported by the NIH-Oxford-Cambridge Scholars Program. JEP was supported by a Wellcome Trust Clinical PhD Fellowship and a Research Fellowship through the BHF Cambridge Centre of Excellence [RE/13/6/30180]. JCL was supported by the Wellcome Trust Clinical PhD Programme. This work was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health, the Wellcome Trust [083650/Z/07/Z], the Medical Research Council [MR/L19027/1], and the National Institute for Health Research Cambridge Biomedical Research Centre. KGCS is an NIHR Senior Investigator. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Geuking MB, Koller Y, Rupp S, McCoy KD. The interplay between the gut microbiota and the immune system. Gut Microbes. 2014;5(3):411–8. 10.4161/gmic.29330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longman RS, Littman DR. The functional impact of the intestinal microbiome on mucosal immunity and systemic autoimmunity. Curr Opin Rheumatol. 2015;27(4):381–7. 10.1097/BOR.0000000000000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cader MZ, Kaser A. Recent advances in inflammatory bowel disease: mucosal immune cells in intestinal inflammation. Gut. 2013;62(11):1653–64. 10.1136/gutjnl-2012-303955 [DOI] [PubMed] [Google Scholar]
- 4.Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–86. 10.1038/ng.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang H, Fang M, Jostins L, Umicevic Mirkov M, Boucher G, Anderson CA, et al. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature. 2017;547(7662):173–8. 10.1038/nature22969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411(6837):603–6. 10.1038/35079114 [DOI] [PubMed] [Google Scholar]
- 8.Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16(1):90–7. 10.1038/nm.2069 [DOI] [PubMed] [Google Scholar]
- 9.Chamaillard M, Philpott D, Girardin SE, Zouali H, Lesage S, Chareyre F, et al. Gene-environment interaction modulated by allelic heterogeneity in inflammatory diseases. Proc Natl Acad Sci U S A. 2003;100(6):3455–60. 10.1073/pnas.0530276100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wehkamp J, Harder J, Weichenthal M, Schwab M, Schaffeler E, Schlee M, et al. NOD2 (CARD15) mutations in Crohn's disease are associated with diminished mucosal alpha-defensin expression. Gut. 2004;53(11):1658–64. 10.1136/gut.2003.032805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456(7219):259–63. 10.1038/nature07416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lassen KG, Kuballa P, Conway KL, Patel KK, Becker CE, Peloquin JM, et al. Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc Natl Acad Sci U S A. 2014;111(21):7741–6. 10.1073/pnas.1407001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murthy A, Li Y, Peng I, Reichelt M, Katakam AK, Noubade R, et al. A Crohn's disease variant in Atg16l1 enhances its degradation by caspase 3. Nature. 2014;506(7489):456–62. 10.1038/nature13044 [DOI] [PubMed] [Google Scholar]
- 14.Sivanesan D, Beauchamp C, Quinou C, Lee J, Lesage S, Chemtob S, et al. IL23R (Interleukin 23 Receptor) Variants Protective against Inflammatory Bowel Diseases (IBD) Display Loss of Function due to Impaired Protein Stability and Intracellular Trafficking. J Biol Chem. 2016;291(16):8673–85. 10.1074/jbc.M116.715870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Meglio P, Di Cesare A, Laggner U, Chu CC, Napolitano L, Villanova F, et al. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PLoS One. 2011;6(2):e17160 10.1371/journal.pone.0017160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pidasheva S, Trifari S, Phillips A, Hackney JA, Ma Y, Smith A, et al. Functional studies on the IBD susceptibility gene IL23R implicate reduced receptor function in the protective genetic variant R381Q. PLoS One. 2011;6(10):e25038 10.1371/journal.pone.0025038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarin R, Wu X, Abraham C. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc Natl Acad Sci U S A. 2011;108(23):9560–5. 10.1073/pnas.1017854108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters JE, Lyons PA, Lee JC, Richard AC, Fortune MD, Newcombe PJ, et al. Insight into Genotype-Phenotype Associations through eQTL Mapping in Multiple Cell Types in Health and Immune-Mediated Disease. PLoS Genet. 2016;12(3):e1005908 10.1371/journal.pgen.1005908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richard AC, Peters JE, Lee JC, Vahedi G, Schaffer AA, Siegel RM, et al. Targeted genomic analysis reveals widespread autoimmune disease association with regulatory variants in the TNF superfamily cytokine signalling network. Genome Med. 2016;8(1):76 10.1186/s13073-016-0329-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairfax BP, Makino S, Radhakrishnan J, Plant K, Leslie S, Dilthey A, et al. Genetics of gene expression in primary immune cells identifies cell type-specific master regulators and roles of HLA alleles. Nat Genet. 2012;44(5):502–10. 10.1038/ng.2205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baillie JK, Arner E, Daub C, De Hoon M, Itoh M, Kawaji H, et al. Analysis of the human monocyte-derived macrophage transcriptome and response to lipopolysaccharide provides new insights into genetic aetiology of inflammatory bowel disease. PLoS Genet. 2017;13(3):e1006641 10.1371/journal.pgen.1006641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada Y, Yamazaki K, Umeno J, Takahashi A, Kumasaka N, Ashikawa K, et al. HLA-Cw*1202-B*5201-DRB1*1502 haplotype increases risk for ulcerative colitis but reduces risk for Crohn's disease. Gastroenterology. 2011;141(3):864–71 e1-5. 10.1053/j.gastro.2011.05.048 [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki K, Umeno J, Takahashi A, Hirano A, Johnson TA, Kumasaka N, et al. A genome-wide association study identifies 2 susceptibility Loci for Crohn's disease in a Japanese population. Gastroenterology. 2013;144(4):781–8. 10.1053/j.gastro.2012.12.021 [DOI] [PubMed] [Google Scholar]
- 24.Richard AC, Ferdinand JR, Meylan F, Hayes ET, Gabay O, Siegel RM. The TNF-family cytokine TL1A: from lymphocyte costimulator to disease co-conspirator. J Leukoc Biol. 2015;98(3):333–45. 10.1189/jlb.3RI0315-095R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richard AC, Tan C, Hawley ET, Gomez-Rodriguez J, Goswami R, Yang XP, et al. The TNF-family ligand TL1A and its receptor DR3 promote T cell-mediated allergic immunopathology by enhancing differentiation and pathogenicity of IL-9-producing T cells. J Immunol. 2015;194(8):3567–82. 10.4049/jimmunol.1401220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16(3):479–92. [DOI] [PubMed] [Google Scholar]
- 27.Meylan F, Hawley ET, Barron L, Barlow JL, Penumetcha P, Pelletier M, et al. The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol. 2014;7(4):958–68. 10.1038/mi.2013.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu X, Pappu R, Ramirez-Carrozzi V, Ota N, Caplazi P, Zhang J, et al. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7(3):730–40. 10.1038/mi.2013.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meylan F, Davidson TS, Kahle E, Kinder M, Acharya K, Jankovic D, et al. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008;29(1):79–89. 10.1016/j.immuni.2008.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slebioda TJ, Rowley TF, Ferdinand JR, Willoughby JE, Buchan SL, Taraban VY, et al. Triggering of TNFRSF25 promotes CD8(+) T-cell responses and anti-tumor immunity. Eur J Immunol. 2011;41(9):2606–11. 10.1002/eji.201141477 [DOI] [PubMed] [Google Scholar]
- 31.Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER, et al. CX(3)CR1(+) mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J Exp Med. 2014;211(8):1571–83. 10.1084/jem.20140678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bamias G, Kaltsa G, Siakavellas SI, Papaxoinis K, Zampeli E, Michopoulos S, et al. High intestinal and systemic levels of decoy receptor 3 (DcR3) and its ligand TL1A in active ulcerative colitis. Clin Immunol. 2010;137(2):242–9. 10.1016/j.clim.2010.07.001 [DOI] [PubMed] [Google Scholar]
- 33.Bamias G, Martin C 3rd, Marini M, Hoang S, Mishina M, Ross WG, et al. Expression, localization, and functional activity of TL1A, a novel Th1-polarizing cytokine in inflammatory bowel disease. J Immunol. 2003;171(9):4868–74. [DOI] [PubMed] [Google Scholar]
- 34.Bamias G, Kaltsa G, Siakavellas SI, Gizis M, Margantinis G, Zampeli E, et al. Differential expression of the TL1A/DcR3 system of TNF/TNFR-like proteins in large vs. small intestinal Crohn's disease. Dig Liver Dis. 2012;44(1):30–6. 10.1016/j.dld.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 35.Prehn JL, Mehdizadeh S, Landers CJ, Luo X, Cha SC, Wei P, et al. Potential role for TL1A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin Immunol. 2004;112(1):66–77. [DOI] [PubMed] [Google Scholar]
- 36.Jin S, Chin J, Seeber S, Niewoehner J, Weiser B, Beaucamp N, et al. TL1A/TNFSF15 directly induces proinflammatory cytokines, including TNFalpha, from CD3+CD161+ T cells to exacerbate gut inflammation. Mucosal Immunol. 2013;6(5):886–99. 10.1038/mi.2012.124 [DOI] [PubMed] [Google Scholar]
- 37.Song L, Zhou R, Huang S, Zhou F, Xu S, Wang W, et al. High intestinal and systemic levels of interleukin-23/T-helper 17 pathway in Chinese patients with inflammatory bowel disease. Mediators Inflamm. 2013;2013:425915 10.1155/2013/425915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kakuta Y, Ueki N, Kinouchi Y, Negoro K, Endo K, Nomura E, et al. TNFSF15 transcripts from risk haplotype for Crohn's disease are overexpressed in stimulated T cells. Hum Mol Genet. 2009;18(6):1089–98. 10.1093/hmg/ddp005 [DOI] [PubMed] [Google Scholar]
- 39.Michelsen KS, Thomas LS, Taylor KD, Yu QT, Mei L, Landers CJ, et al. IBD-associated TL1A gene (TNFSF15) haplotypes determine increased expression of TL1A protein. PLoS One. 2009;4(3):e4719 10.1371/journal.pone.0004719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zucchelli M, Camilleri M, Andreasson AN, Bresso F, Dlugosz A, Halfvarson J, et al. Association of TNFSF15 polymorphism with irritable bowel syndrome. Gut. 2011;60(12):1671–7. 10.1136/gut.2011.241877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hedl M, Abraham C. A TNFSF15 disease-risk polymorphism increases pattern-recognition receptor-induced signaling through caspase-8-induced IL-1. Proc Natl Acad Sci U S A. 2014;111(37):13451–6. 10.1073/pnas.1404178111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee MN, Ye C, Villani AC, Raj T, Li W, Eisenhaure TM, et al. Common genetic variants modulate pathogen-sensing responses in human dendritic cells. Science. 2014;343(6175):1246980 10.1126/science.1246980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raj T, Rothamel K, Mostafavi S, Ye C, Lee MN, Replogle JM, et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science. 2014;344(6183):519–23. 10.1126/science.1249547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Irwanto A, Toyo-Oka L, Hong M, Liu H, Andiappan AK, et al. Fine-mapping analysis revealed complex pleiotropic effect and tissue-specific regulatory mechanism of TNFSF15 in primary biliary cholangitis, Crohn's disease and leprosy. Sci Rep. 2016;6:31429 10.1038/srep31429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–60. 10.1126/science.1262110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nedelec Y, Sanz J, Baharian G, Szpiech ZA, Pacis A, Dumaine A, et al. Genetic Ancestry and Natural Selection Drive Population Differences in Immune Responses to Pathogens. Cell. 2016;167(3):657–69 e21. 10.1016/j.cell.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 47.Consortium GTEx, et al. Genetic effects on gene expression across human tissues. Nature. 2017;550(7675):204–13. 10.1038/nature24277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10(5):e1004383 10.1371/journal.pgen.1004383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westra HJ, Peters MJ, Esko T, Yaghootkar H, Schurmann C, Kettunen J, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45(10):1238–43. 10.1038/ng.2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fairfax BP, Humburg P, Makino S, Naranbhai V, Wong D, Lau E, et al. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science. 2014;343(6175):1246949 10.1126/science.1246949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeller T, Wild P, Szymczak S, Rotival M, Schillert A, Castagne R, et al. Genetics and beyond—the transcriptome of human monocytes and disease susceptibility. PLoS One. 2010;5(5):e10693 10.1371/journal.pone.0010693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prehn JL, Thomas LS, Landers CJ, Yu QT, Michelsen KS, Targan SR. The T cell costimulator TL1A is induced by FcgammaR signaling in human monocytes and dendritic cells. J Immunol. 2007;178(7):4033–8. [DOI] [PubMed] [Google Scholar]
- 53.Shih DQ, Kwan LY, Chavez V, Cohavy O, Gonsky R, Chang EY, et al. Microbial induction of inflammatory bowel disease associated gene TL1A (TNFSF15) in antigen presenting cells. Eur J Immunol. 2009;39(11):3239–50. 10.1002/eji.200839087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang L, Adkins B, Deyev V, Podack ER. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008;205(5):1037–48. 10.1084/jem.20072528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bain CC, Bravo-Blas A, Scott CL, Gomez Perdiguero E, Geissmann F, Henri S, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15(10):929–37. 10.1038/ni.2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varol C, Vallon-Eberhard A, Elinav E, Aychek T, Shapira Y, Luche H, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31(3):502–12. 10.1016/j.immuni.2009.06.025 [DOI] [PubMed] [Google Scholar]
- 57.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31(3):513–25. 10.1016/j.immuni.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260(1):102–17. 10.1111/imr.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bull MJ, Williams AS, Mecklenburgh Z, Calder CJ, Twohig JP, Elford C, et al. The Death Receptor 3-TNF-like protein 1A pathway drives adverse bone pathology in inflammatory arthritis. J Exp Med. 2008;205(11):2457–64. 10.1084/jem.20072378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meylan F, Song YJ, Fuss I, Villarreal S, Kahle E, Malm IJ, et al. The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal Immunol. 2011;4(2):172–85. 10.1038/mi.2010.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pappu BP, Borodovsky A, Zheng TS, Yang X, Wu P, Dong X, et al. TL1A-DR3 interaction regulates Th17 cell function and Th17-mediated autoimmune disease. J Exp Med. 2008;205(5):1049–62. 10.1084/jem.20071364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takedatsu H, Michelsen KS, Wei B, Landers CJ, Thomas LS, Dhall D, et al. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135(2):552–67. 10.1053/j.gastro.2008.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Hu Y, Charpentier T, Lamarre A, Qi S, Wu J, et al. TNF-like ligand 1A (TL1A) gene knockout leads to ameliorated collagen-induced arthritis in mice: implication of TL1A in humoral immune responses. J Immunol. 2013;191(11):5420–9. 10.4049/jimmunol.1301475 [DOI] [PubMed] [Google Scholar]
- 64.Papadakis KA, Prehn JL, Landers C, Han Q, Luo X, Cha SC, et al. TL1A synergizes with IL-12 and IL-18 to enhance IFN-gamma production in human T cells and NK cells. J Immunol. 2004;172(11):7002–7. [DOI] [PubMed] [Google Scholar]
- 65.Jia LG, Bamias G, Arseneau KO, Burkly LC, Wang EC, Gruszka D, et al. A Novel Role for TL1A/DR3 in Protection against Intestinal Injury and Infection. J Immunol. 2016;197(1):377–86. 10.4049/jimmunol.1502466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahn YO, Weeres MA, Neulen ML, Choi J, Kang SH, Heo DS, et al. Human group3 innate lymphoid cells express DR3 and respond to TL1A with enhanced IL-22 production and IL-2-dependent proliferation. Eur J Immunol. 2015;45(8):2335–42. 10.1002/eji.201445213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14(3):282–9. 10.1038/nm1720 [DOI] [PubMed] [Google Scholar]
- 68.Cox JH, Kljavin NM, Ota N, Leonard J, Roose-Girma M, Diehl L, et al. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol. 2012;5(1):99–109. 10.1038/mi.2011.54 [DOI] [PubMed] [Google Scholar]
- 69.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29(6):947–57. 10.1016/j.immuni.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caruso R, Warner N, Inohara N, Nunez G. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity. 2014;41(6):898–908. 10.1016/j.immuni.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carvalho BS, Irizarry RA. A framework for oligonucleotide microarray preprocessing. Bioinformatics. 2010;26(19):2363–7. 10.1093/bioinformatics/btq431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stegle O, Parts L, Durbin R, Winn J. A Bayesian framework to account for complex non-genetic factors in gene expression levels greatly increases power in eQTL studies. PLoS Comput Biol. 2010;6(5):e1000770 10.1371/journal.pcbi.1000770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carey VJ, Davis AR, Lawrence MF, Gentleman R, Raby BA. Data structures and algorithms for analysis of genetics of gene expression with Bioconductor: GGtools 3.x. Bioinformatics. 2009;25(11):1447–8. 10.1093/bioinformatics/btp169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hahne F, Ivanek R. Visualizing Genomic Data Using Gviz and Bioconductor. Methods Mol Biol. 2016;1418:335–51. 10.1007/978-1-4939-3578-9_16 [DOI] [PubMed] [Google Scholar]
- 75.Siegel RM, Meylan F, Song Y-J, inventors; The United States of America, as represented by the Secretary, Dept. of Health and Human Services (Washington, DC, US), assignee. Antibodies that bind to TL1A and methods of treating inflammatory or autoimmune disease comprising administering such antibodies. United States patent US 9068003 B2. 2015.
- 76.Casper J, Zweig AS, Villarreal C, Tyner C, Speir ML, Rosenbloom KR, et al. The UCSC Genome Browser database: 2018 update. Nucleic Acids Res. 2018;46(D1):D762–D9. 10.1093/nar/gkx1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosenbloom KR, Sloan CA, Malladi VS, Dreszer TR, Learned K, Kirkup VM, et al. ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic Acids Res. 2013;41(Database issue):D56–63. 10.1093/nar/gks1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arner E, Daub CO, Vitting-Seerup K, Andersson R, Lilje B, Drablos F, et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science. 2015;347(6225):1010–4. 10.1126/science.1259418 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(A) Plots depict monocyte TNFSF15 mRNA expression versus rs6478109 genotype in healthy controls and IBD patients (genotyping by Illumina Human OmniExpress12v1.0 BeadChip, gene expression by Affymetrix Human Gene ST 1.1 microarrays). (B) Table depicts results of LD calculation and haplotype phasing using 1000 Genomes Phase 3 European (EUR) and East Asian (EAS) cohorts for rs6478109 and rs6478108. Phasing is written as rs6478109 allele 1 in phase with rs6478108 allele 1 / rs6478109 allele 2 in phase with rs6478108 allele 2. (C) As (A), plots depict TNFSF15 mRNA expression versus rs6478108 genotype in healthy controls (n = 39) and IBD patients (n = 80) (genotyping by Illumina Human OmniExpress12v1.0 BeadChip) and patients with ANCA-associated vasculitis (n = 45) (genotyping by Affymetrix SNP 6.0). rs6478109 was not present on the Affymetrix SNP 6.0 genotyping platform, and thus rs6478108 was used as a proxy SNP for examining the eQTL in the AAV cohort. (D) Ex vivo monocyte TNFSF15 expression values from Fig 1B plotted by rs6478108 genotype (TT, n = 9; CT, n = 17; CC, n = 9).
(EPS)
Regional association plots for IBD (European ancestry cohort from Liu et al [4]) and TNFSF8 expression (n = 39 healthy individuals and 80 IBD patients). LD is colored with reference to the most associated SNP within each plot.
(EPS)
(A) Human peripheral blood monocytes were left unstimulated (null), or were stimulated with 100 ng/mL LPS, cross-linked immune complex, LyoVec transfection reagent control, or LyoVec with 100 μg/mL poly(I:C) for the indicated times. TNFSF15 mRNA was measured by qPCR relative to B2M with expression reported as ΔCt (left); secreted protein was measured in the supernatant by custom Bio-Plex assay (right). Plots are representative of at least 2 independent experiments each. (B) As (A) for CD4+ and CD8+ T cells stimulated with anti-CD3/anti-CD28-coated beads at a ratio of 1:1, beads:cells. The plot on the right shows a longer stimulation time-course for both T cell subsets.
(EPS)
Effect sizes estimated by linear regression (beta coefficients) and their standard errors are plotted for monocyte eQTLs from Fig 1B and Fig 2A.
(EPS)
(A) The TNFSF15 gene region was visualized in the UCSC genome browser (hg19 genome build). TNFSF15 gene position is from RefSeq. SNPs of interest are underlined in the same colors as Fig 4: rs6478109 eQTL SNP in blue, and rs4246905 and rs4263839 SNPs used for allele-specific expression (ASE) measurements in green and orange, respectively. Binding sites for primers used to amplify pre-mRNA for ASE measurements are indicated. (B) Probes for rs4246905 and rs4263839 were used to measure ASE in the same four samples of immune complex-stimulated monocytes (samples included in Fig 2C). 95% confidence interval for difference in mean log2(allelic ratio) between genomic DNA and cDNA calculated from Welch’s t-test.
(EPS)
(A) Serum TNFSF15 was measured in rs6478109 genotyped individuals (GG, n = 22; GA, n = 26; AA, n = 19; two additional AA individuals were excluded due to measurements above the detectable range of the standard curve). Line denotes the median; p value from linear regression on inverse-rank normalized values. (B) Inflammatory cytokines were measured in supernatants of stimulated monocytes from rs6478109 homozygotes (immune complex, n = 4 per genotype; LPS, n = 7 per genotype). No significant differences between genotypes by Mann-Whitney test. (C) Selected cytokines from (B) were further examined in a second cohort (n = 12 per genotype). No significant differences between genotypes by Mann-Whitney test.
(EPS)
The promoter region of TNFSF15 was visualized in the UCSC genome browser (http://genome.ucsc.edu/ and [76], hg19 build). TNFSF15 gene position is from RefSeq. SNPs of interest are indicated. Genome regulatory marks from ENCODE [77] are shown. Transcription factor binding sites are from ENCODE transcription factor ChIP-seq of 161 factors across a variety of cell types with consensus motifs marked in green. Histogram tracks represent measurements in primary human monocytes. “DHS” denotes raw DNase hypersensitivity signal measured by ENCODE/UW. CTCF and histone ChIP-seq profiles highlighted in orange are from ENCODE/BROAD. “Control” indicates sequencing of ChIP input control DNA. Enhancers and transcriptional activity identified by Cap Analysis of Gene Expression (CAGE) were downloaded from the ZENBU browser view associated with Baillie et al [21] (http://fantom.gsc.riken.jp/zenbu/gLyphs/#config=dXO5cTaJBZiiw73fJq2oGD;loc=hg19::chr9:117566249..117571251+), which includes FANTOM5 consortium data [78]. CAGE-identified enhancers across multiple human cell types and monocyte-derived macrophages, as well as transcriptional activity measurements by CAGE, are highlighted in turquoise.
(EPS)
Immunophenotyping gating strategies are depicted for (A) basic T cell subsets, (B) Th effector cell subsets, (C) Treg, (D) B cells, and (E) monocytes, dendritic cells, and NK cells.
(EPS)
Age- and sex-matched rs6478109 homozygotes were immunophenotyped for 39 different peripheral blood parameters. Between 6 and 19 pairs were examined for each subset. No immune cell subsets were found to be differentially abundant between genotypes by Wilcoxon test.
(EPS)
Plots were generated using the IMMUNPOP browser at http://132.219.138.157/nedelec/eQTL/
(EPS)
Regional linkage disequilibrium (r2) plot of genetic variants +/- 3 kbp from rs6478109 calculated using 1000 Genomes Phase 3 EUR cohort.
(EPS)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(ZIP)
Data Availability Statement
Genotype data for the eQTL analyses shown in Fig 1A, S1A and S1C Fig, and S2 Fig are available in EGA https://www.ebi.ac.uk/ega (accession number EGAS00001001251); access is controlled to protect personal genetic data of study volunteers and is available for scientific researchers via requests to the data access committee (EGAC00001000338). Gene expression data for these same eQTL analyses are available through Array Express (accession number E-MTAB-3554). Publicly available datasets were obtained as follows: summary statistics for the European cohort from the GWAS plus Immunochip trans-ancestry MANTRA meta-analyses by Liu et al (URL ftp://ftp.sanger.ac.uk/pub/consortia/ibdgenetics/iibdgc-trans-ancestry-filtered-summary-stats.tgz, file IBD_trans_ethnic_association_summ_stats_b37.txt.gz); corroborating data for a monocyte-derived macrophage TNFSF15 eQTL at rs6478109 by Nédélec et al in the IMMUNPOP browser (URL http://132.219.138.157/nedelec/eQTL/, search term rs6478109); regulatory information about the TNFSF15 promoter from ENCODE in the UCSC genome browser (URL http://genome-euro.ucsc.edu/cgi-bin/hgTracks?db=hg19&lastVirtModeType=default&lastVirtModeExtraState=&virtModeType=default&virtMode=0&nonVirtPosition=&position=chr9%3A117566800-117570000&hgsid=228207134_vgkDNh2jKZKgDRQr38TDxd4l3YSF); CAGE data for enhancer identification associated with Baillie et al in the ZENBU browser (URL http://fantom.gsc.riken.jp/zenbu/gLyphs/#config=dXO5cTaJBZiiw73fJq2oGD;loc=hg19::chr9:117566249..117571251+). All other relevant data are within the paper and its Supporting Information files.