Abstract
BACKGROUND:
Adding modified FOLFOX6 (folinic acid, fluorouracil, oxaliplatin) after chemoradiotherapy and lengthening the chemoradiotherapy-to-surgery interval is associated with an increase in the proportion of rectal cancer patients with a pathological complete response.
OBJECTIVE:
To analyze disease-free and overall survival.
DESIGN:
Nonrandomized phase II trial.
SETTINGS:
Multi-institutional.
PATIENTS:
Four sequential study groups with stage II or III rectal cancer.
INTERVENTION:
All patients received 50 Gy of radiation with concurrent continuous infusion of fluorouracil for 5 weeks. Patients in each group received 0, 2, 4, or 6 cycles of modified FOLFOX6 after chemoradiation and before total mesorectal excision. Patients were recommended to receive adjuvant chemotherapy after surgery to complete a total of 8 cycles of modified FOLFOX6.
MAIN OUTCOME MEASURES:
The trial was powered to detect differences in pathological complete response, which was reported previously. Disease-free and overall survival are the main outcomes for the current study.
RESULTS:
Of 259 patients, 211 had a complete follow-up. Median follow-up was 59 months (range, 9–125). The mean number of total chemotherapy cycles differed between the four groups (p = 0.002), as one third of patients in the group assigned to no preoperative FOLFOX did not receive any adjuvant chemotherapy. Disease-free survival was significantly associated with study group, ypTNM stage, and pathological complete response (p = 0.004, <0.001 and 0.001, respectively). A secondary analysis including only patients who received at least one cycle of FOLFOX still showed differences in survival between study groups (p = 0.03).
LIMITATIONS:
The trial was not randomized and was not powered to show differences in survival. Survival data were not available for 19% of the patients.
CONCLUSIONS:
Adding modified FOLFOX6 after chemoradiotherapy and before total mesorectal excision increases compliance with systemic chemotherapy and disease-free survival in patients with locally advanced rectal cancer. Neoadjuvant consolidation chemotherapy may have benefits beyond increasing pathological complete response rates. See Video Abstract at http://links.lww.com/DCR/Axxx.
Keywords: Adjuvant chemotherapy, Chemotherapy, Chemotherapy compliance, Consolidation chemotherapy, Disease free survival, FOLFOX, Interval, Neoadjuvant chemotherapy, Neoadjuvant chemoradiation, Overall survival, Pathological response, Preoperative chemoradiation, Preoperative chemotherapy, Rectal cancer, Surgery, Survival, Time, Timing, Total mesorectal excision, Total neoadjuvant therapy, ypTNM stage
INTRODUCTION
The current treatment for patients with locally advanced rectal cancer consists of neoadjuvant fluorouracil-based chemoradiotherapy (CRT) followed by total mesorectal excision (TME) and postoperative systemic chemotherapy.1,2While this trimodal therapy provides excellent local tumor control and long-term survival,1,3,4 it is associated with significant morbidity and long-term impairment of function that compromises the quality of life.5
Meanwhile, rectal cancer response to neoadjuvant CRT is variable. Some tumors undergo minimal regression, while others seem to be fully eradicated by CRT.6 A complete tumor response to CRT has been associated with improved local and systemic tumor control and long-term survival.7,8 These findings call into question the added value of surgery in patients who achieve a clinical complete response (cCR) to CRT. Preliminary data suggests that a watch-and-wait approach may be safe for patients who achieve a cCR after neoadjuvant therapy.9
The proportion of patients achieving complete response after standard CRT is relatively low, ranging from 8 to 24%.8 Increasing the rate of tumor response, and therefore expanding the number of rectal cancer patients who could potentially benefit from a watch-and-wait strategy, has been an active area of research for years. The initiatives include radiation dose escalation,10 intensification of neoadjuvant treatment with induction or consolidation chemotherapy,11–17 and delaying the time of assessing tumor response after completion of neoadjuvant therapy.18 However, the effects of these approaches on tumor response and long-term survival are still controversial.10–18
The Timing of Rectal Cancer Response to Chemoradiation trial was designed to investigate the effect of adding an increasing number of cycles mFOLFOX6 (folinic acid, fluorouracil, oxaliplatin) after CRT and lengthening the CRT-to-surgery interval on the rate of pathological complete response (pCR) in patients with locally advanced rectal cancer.19 The results showed that adding systemic chemotherapy after CRT and delaying surgery increased the pCR rate without increasing surgical complications. In the current study, we analyzed the possible long-term benefit of neoadjuvant chemotherapy for disease-free survival (DFS) and overall survival (OS) in patients in the TIMING trial.
METHODS
Patients
The study was a multicenter, open-label, nonrandomized phase II trial (NCT00335816 on ClinicalTrials.gov). Details of the study design and methods were published previously.19 Eligible patients were 18 years of age or older, with clinical stage II (T3–4, N0) or III (any T, N1–2) invasive rectal adenocarcinoma within 12 cm from the anal verge. Endorectal ultrasonography or MRI was used for staging. Patients were required to have an Eastern Collaborative Oncology Group (ECOG) performance status score of 0 or 1 or a comparable Karnofsky score. Patients with a history of pelvic radiation, polyposis syndromes, inflammatory bowel disease, recurrent rectal cancer, metastatic disease, other primary tumors within the previous 5 years, substantial cardiac disease, neurological disease, renal, hepatic, or bone marrow dysfunction were ineligible.
A central institutional review board and the institutional review boards at each participating institution approved the study protocol. Memorial Sloan Kettering Cancer Center was responsible for overseeing all fiscal and administrative arrangements with the consortium. Patients provided written informed consent for the outcomes included in the original version of the study protocol. The original protocol was amended to include DFS and OS as secondary outcomes after accrual had already started. Long-term follow-up data could be collected only for patients who provided consent for these survival analyses.
Treatment
The trial consisted of four sequential study groups (SGs). The protocol schema is shown in Supplemental Figure 1. Patients in SG1 underwent fluorouracil-based CRT and TME. Patients in SG2–4 received two, four, or six cycles of mFOLFOX6 between CRT and TME, respectively. Patients in all SGs were recommended to receive additional adjuvant chemotherapy to complete a total of eight cycles of mFOLFOX6. Details of the CRT and mFOLFOX regimens were reported previously.19 The study recommended delivering adjuvant chemotherapy after surgery to complete a 4-month course of FOLFOX as recommended by the guidelines of the National Comprehensive Cancer Network.20 Because the initial study design did not include collecting information on adjuvant chemotherapy or long-term survival, some patients in SG1 were not consented for the survival analysis and were not included in this study. Surveillance was done according to the guidelines of the National Comprehensive Cancer Network.20
Outcomes
The primary endpoint of the study, the proportion of patients achieving pCR in each SG, has been reported previously.19 Here, we report DFS, counting local relapse, distant metastasis, or death as an event, whichever occurred first. We also report OS, counting death from any cause as an event. Time to event was calculated from the first day of CRT.
Statistical analysis
This phase II study was powered to evaluate pCR in sequentially accrued SGs.19 DFS and OS were secondary endpoints. All comparisons between SGs were based on intention to treat. A secondary survival analysis was done to exclude patients who did not receive any systemic chemotherapy before or after TME. Descriptive statistics were used to summarize the data overall and by SG. Comparisons were made using analysis of variance or the chi-square test. DFS and OS were analyzed separately using the Kaplan-Meier method and log-rank tests. A multivariate Cox proportional-hazards model was fit to each time-to-event endpoint. These models were based on results from univariate analyses and ensured that two highly correlated variables were not simultaneously included in a model. p-values less than 0.05 were deemed statistically significant. All analyses were conducted with SAS version 9.4 and R version 3.1.1 software.
RESULTS
A total of 259 eligible patients accrued between March 24, 2004, and November 16, 2012, were included in the primary analysis,19 but 48 patients (20 in SG1, 11 in SG2, 14 in SG3, 3 in SG4) were excluded from the survival analyses for various reasons (Fig. 1). We found no differences in demographics, tumor characteristics, or pCR rates between the patients who were included in the survival analyses (n = 211) and the patients who were excluded (n = 48) (Supplemental Table 1).
FIGURE 1.

Inclusion and exclusion of patients.
Table 1 lists the patient demographics and tumor characteristics by SG for the 211 patients included in the survival analyses. SG1 had a higher mean age compared to SG2–4, while sex, ECOG performance status, clinical stage, and distance from the anal verge were similar between the SGs. Early results on pathological response, neoadjuvant-chemotherapy-related adverse events, surgery, and postoperative complications were published previously.19
TABLE 1.
Baseline patient and tumor characteristicsa
| Characteristic | No. of patients (%) | P value | |||
|---|---|---|---|---|---|
| SG1 (n = 40) |
SG2 (n = 56) |
SG3 (n = 53) |
SG4 (n = 62) |
||
| Age (years; mean ± SD) | 61 ± 13 | 54 ± 10 | 56 ± 12 | 58 ± 9 | 0.02 |
| Sex | 0.82 | ||||
| Male | 24 (60) | 32 (57) | 29 (55) | 39 (63) | |
| Female | 16 (40) | 24 (43) | 24 (45) | 23 (37) | |
| ECOG performance status | 0.09 | ||||
| 0 | 38 (95) | 49 (88) | 44 (83) | 48 (77) | |
| 1 | 2 (5) | 7 (13) | 9 (17) | 14 (23) | |
| cT classification | 0.49 | ||||
| T2 | 1 (3) | 4 (7) | 7 (13) | 5 (8) | |
| T3 | 38 (95) | 51 (91) | 43 (81) | 52 (84) | |
| T4 | 1 (3) | 1 (2) | 3 (6) | 3 (5) | |
| Tx | 0 | 0 | 0 | 2 (3) | |
| cN classification | 0.07 | ||||
| N0 | 11 (28) | 9 (16) | 12 (23) | 18 (29) | |
| N1 | 27 (68) | 44 (79) | 34 (64) | 34 (55) | |
| N2 | 1 (3) | 3 (5) | 7 (13) | 10 (16) | |
| Nx | 1 (3) | 0 | 0 | 0 | |
| Clinical stage | 0.37 | ||||
| II | 11 (28) | 9 (16) | 12 (23) | 18 (29) | |
| III | 29 (73) | 47 (84) | 41 (77) | 44 (71) | |
| Distance from anal verge (cm; mean ± SD) | 6.5 ± 3.2 | 6.5 ± 3.2 | 7.4 ± 3.0 | 6.6 ± 3.3 | 0.43 |
| Procedure | 0.19 | ||||
| LAR | 30 (75) | 45 (80) | 41 (77) | 41 (66) | |
| APR | 10 (25) | 11 (20) | 12 (23) | 21 (34) | |
| Ileostomy | 24/30 | 38/45 | 38/41 | 35/41 | 0.74 |
| ypT classification | 0.84 | ||||
| T0 | 9 (23) | 17 (30)* | 14 (26) | 23 (37) | |
| Tis | 2 (5) | 1 (2) | 1 (2) | 1 (2) | |
| T1 | 2 (5) | 4 (7) | 5 (9) | 3 (5) | |
| T2 | 10 (25) | 18 (32) | 13 (25) | 15 (24) | |
| T3 | 17 (43) | 14 (25) | 19 (36) | 18 (29) | |
| T4 | 0 | 2 (4) | 1 (2) | 2 (3) | |
| ypN classification | 0.06 | ||||
| N0 | 30 (75) | 41 (73) | 45 (85) | 49 (79) | |
| N1 | 4 (10) | 9 (16) | 7 (13) | 12 (19) | |
| N2 | 6 (15) | 6 (11) | 1 (2) | 1 (2) | |
| ypTNM stage | 0.61 | ||||
| 0 | 10 (25) | 16 (29) | 15 (28) | 24 (39) | |
| I | 11 (28) | 17 (30) | 16 (30) | 14 (23) | |
| II | 9 (23) | 8 (14) | 14 (26) | 10 (16) | |
| III | 10 (25) | 15 (27) | 8 (15) | 14 (23) | |
| Pathological complete response | 0.04 | ||||
| Yes | 8 (20) | 13 (23) | 14 (26) | 23 (37) | |
| No | 32 (80) | 43 (77) | 39 (74) | 39 (63) | |
SG, study group; ECOG, Eastern Collaborative Oncology Group.
Three patients had residual adenoma after neoadjuvant chemoradiotherapy.
Table 2 lists the data on chemotherapy received in different SGs. Consistent with the trial design, the mean number of neoadjuvant mFOLFOX6 cycles received per patient increased across SGs. Only 2 patients in SG4 did not receive any consolidation mFOLFOX6 after CRT: 1 patient refused additional chemotherapy after he achieved clinical complete response, and the other patient developed fluorouracil-related grade 4 adverse events during CRT. Also consistent with the study design, the mean interval from completion of CRT to TME increased across SGs, from 8.6 ± 4.5 weeks in SG1 to 12.9 ± 4.5 weeks in SG2, 15.4 ± 2.4 weeks in SG3, and 19.3 ± 4.1 weeks in SG4. Adjuvant chemotherapy information was missing for 17 patients (8%): 8 in SG1, 5 in SG2, 2 in SG3, and 2 in SG4. Of the 194 patients with complete information on adjuvant chemotherapy, 130 patients (67%) started adjuvant chemotherapy. Although the study recommended FOLFOX as the preferred postoperative adjuvant chemotherapy, some patients received different treatment regimens: FOLFOX (116 patients), fluorouracil with folinic acid (5 patients), FOLFIRI (folinic acid, fluorouracil, irinotecan; 3 patients), XELOX (capecitabine, oxaliplatin; 3 patients), capecitabine (2 patients), or oxaliplatin (1 patient). The remaining 64 patients (33%) did not receive any adjuvant chemotherapy due to postoperative complications, adverse reactions to neoadjuvant chemotherapy, and/or patient refusal. The mean number of total chemotherapy cycles (neoadjuvant and adjuvant) per patient differed significantly between the SGs, since 31% of patients in SG1 did not receive any adjuvant chemotherapy as planned (p = 0.002; Table 2).
TABLE 2.
Chemotherapy by study group
| Study group (n) | Neoadjuvant chemotherapy | Adjuvant chemotherapy | Any chemotherapy | |||
|---|---|---|---|---|---|---|
| No. of patients (%) | No. of cyclesa | No. of patients (%)b | No. of cyclesa | No. of patients (%)b | Total no. of cyclesa | |
| SG1 (40) | NA | 0 | 22 (69) | 4.9 ± 4.4 | 22 (69) | 4.9 ± 4.4 |
| SG2 (56) | 50 (89) | 1.8 ± 0.7 | 41 (80) | 5.5 ± 3.4 | 51 (100) | 7.3 ± 3.4 |
| SG3 (53) | 50 (94) | 3.6 ± 1 | 34 (67) | 3 ± 2.6 | 51 (100) | 6.5 ± 2.4 |
| SG4 (62) | 54 (87) | 5.1 ± 2 | 33 (55) | 1.9 ± 2.4 | 58 (97) | 7.1 ± 2.4 |
Mean ± SD.
Data on adjuvant chemotherapy were not available for 17 patients: 8 in SG1, 5 in SG2, 2 in SG3, and 2 in SG4. Therefore, the percentages are based on the total number of patients with complete data on adjuvant chemotherapy in each SG. NA, not available.
With a median follow-up of 59 months (range, 9 to 125 months), a total of 42 (20%) patients experienced tumor recurrence (13 [6%] local, 39 [18%] distant, and 10 [5%] both local and distant), 21 patients died from rectal cancer, and 5 patients died from causes unrelated to rectal cancer. Only 5 patients (2%) had a positive circumferential resection margin. Age, gender, clinical stage, and sphincter-preserving surgery were not correlated with DFS or OS. ypTNM stage was associated with both DFS and OS (p<0.0001; Fig. 2 and Table 3). Of the 211 patients, 58 (27.5%) had a pCR. Patients with a pCR had higher rates of DFS (p = 0.0001) and OS (p = 0.005) than patients without a pCR (Fig. 2 and Table 3). DFS differed significantly between the SGs (p = 0.004; Fig. 3 and Table 3), due to a lower DFS rate in SG1 than in SG2–4. There were no differences in DFS rates between SG2, SG3, and SG4 (Fig. 3). OS did not differ between the SGs (p = 0.37; Fig. 3 and Table 3). A multivariable Cox regression model found that both ypTNM and SG were independently correlated with DFS (p < 0.0001 and p = 0.002, respectively; Table 4).
FIGURE 2.
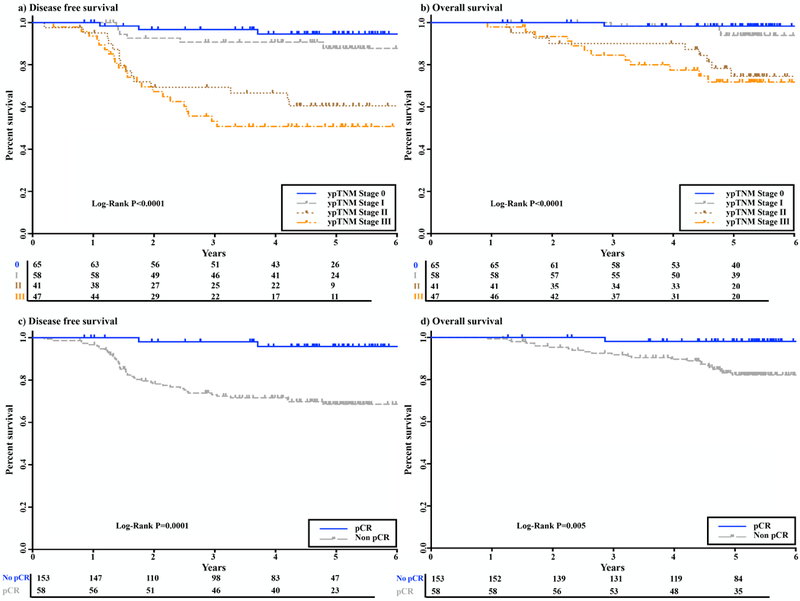
Kaplan-Meier curves of disease-free (DFS) and overall survival (OS) are shown, with numbers of subjects at risk indicated. a, b) DFS and OS in relation to ypTNM stage. c, d) DFS and OS in relation to pathological complete response (pCR).
TABLE 3.
Univariable analysis of survivala
| Variable | No. of patients (%) | 5-year DFS | 5-year OS | ||
|---|---|---|---|---|---|
| % (95% CI) | p | % (95% CI) | P | ||
| Clinical stage | |||||
| II | 50 (24) | 77 (60–87) | 0.58 | 89 (74–95) | 0.57 |
| III | 161 (76) | 76 (68–82) | 86 (79–91) | ||
| Procedure | |||||
| LAR | 157 (74) | 77 (69–83) | 0.80 | 89 (82–93) | 0.28 |
| APR | 54 (26) | 73 (57–84) | 81 (66–90) | ||
| ypTNM | |||||
| 0 | 65 (31) | 94 (83–98) | <0.0001 | 98 (89–100) | <0.0001 |
| I | 58 (27) | 88 (74–94) | 94 (82–98) | ||
| II | 41 (19) | 60 (42–74) | 75 (56–86) | ||
| III | 47 (22) | 51 (35–64) | 72 (55–83) | ||
| Pathological complete response | |||||
| No | 153 (73) | 69 (60–76) | 0.0001 | 82 (75–88) | 0.005 |
| Yes | 58 (27) | 96 (83–99) | 98 (88–100) | ||
| Total number of chemotherapy cyclesb | |||||
| ≤4 cyclesc | 49 (25) | 73 (61–84) | 0.9 | 87 (73–94) | 0.81 |
| >4 cyclesc | 145 (75) | 78 (69–84) | 87 (80–92) | ||
| Study group | |||||
| SG1 | 40 (19) | 50 (30–68) | 0.004 | 79 (60–89) | 0.37 |
| SG2 | 56 (27) | 81 (67–89) | 92 (81–97) | ||
| SG3 | 53 (25) | 86 (73–93) | 88 (76–95) | ||
| SG4 | 62 (29) | 76 (62–85) | 84 (70–92) | ||
| Trial arm | |||||
| Control (SG1) | 40 (19) | 50 (30–68) | 0.0005 | 79 (60–89) | 0.20 |
| Experimental (SG2–4) | 171 (81) | 81 (74–86) | 88 (82–92) | ||
DFS, disease-free survival; OS, overall survival; CI, confidence interval; LAR, low anterior resection; APR, abdominoperineal resection.
Only patients with complete adjuvant chemotherapy data (n = 194) were included.
A cutoff of 4 cycles was used, as it represents 50% of the recommended total number of cycles. The total number of cycles did not affect survival with different quartile cutoff numbers.
FIGURE 3.

a, b) Kaplan-Meier curves of disease-free survival (DFS) and overall survival (OS) are shown in different study groups (SGs), with numbers of subjects at risk indicated. SG1, CRT + TME; SG2, CRT + mFOLFOX6 (two cycles) + TME; SG3, CRT + mFOLFOX6 (four cycles) + TME; SG4, CRT + mFOLFOX6 (six cycles) + TME. CRT, chemoradiotherapy; TME, total mesorectal excision.
TABLE 4.
Multivariable analysis of disease-free survivala
| Variable | Hazard ratio (95% CI) | p |
|---|---|---|
| Study group | ||
| SG1 | 1 | 0.002 |
| SG2 | 0.30 (0.13–0.67) | |
| SG3 | 0.23 (0.10–0.58) | |
| SG4 | 0.40 (0.19–0.84) | |
| ypTNM stage | ||
| 0 | 1 | <0.0001 |
| I | 2.10 (0.52–8.40) | |
| II | 9.80 (2.83–33.93) | |
| III | 13.39 (3.99–44.91) | |
The overall p-values were based on a Type III test. CI, confidence interval.
A total of 182 of the 194 patients (94%) with complete systemic chemotherapy data received at least 1 chemotherapy cycle, either as consolidation chemotherapy after CRT, as adjuvant chemotherapy after TME, or both. Twelve patients (6%) did not receive any chemotherapy cycles after CRT: 10 patients in SG1 and 2 in SG4. Patients who did not receive any chemotherapy cycles had reduced DFS compared to those who received at least 1 chemotherapy cycle (p = 0.02; Supplemental Figure 2). To investigate whether the differences in DFS between SGs were exclusively attributable to compliance with chemotherapy, we performed a secondary survival analysis only for patients who received any systemic chemotherapy after CRT (n = 182). The mean numbers of chemotherapy (neoadjuvant and/or adjuvant) cycles were not different across the four SGs (7 ± 3 cycles in SG1, 7 ± 3 cycles in SG2, 7 ± 2 cycles in SG3, and 7 ± 2 cycles in SG4; p = 0.31). SG1 still had worse DFS compared to SG2–4 within the subset of patients who received at least 1 chemotherapy cycle after CRT (p = 0.03; Figure 4).
FIGURE 4.
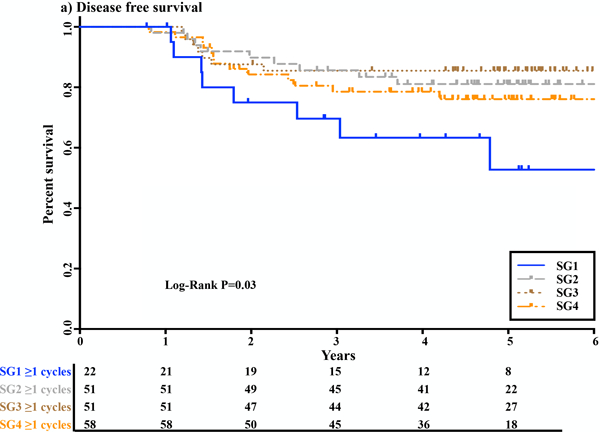
Kaplan-Meier curves of disease-free survival, with numbers of subjects at risk shown in different study groups (SGs) for patients who received at least 1 chemotherapy cycle, either as consolidation chemotherapy after chemoradiotherapy (CRT), as adjuvant chemotherapy after total mesorectal excision (TME), or both. SG1, CRT + TME + ≥1 cycle of adjuvant chemotherapy; SG2, CRT + mFOLFOX6 (two cycles) + TME +/− adjuvant chemotherapy; SG3, CRT + mFOLFOX6 (four cycles) + TME +/− adjuvant chemotherapy; SG4, CRT + mFOLFOX6 (six cycles) + TME +/− adjuvant chemotherapy.
DISCUSSION
The results of this phase II trial indicate that adding neoadjuvant mFOLFOX6 after CRT and increasing the time interval between CRT and surgery not only increases the rate of pCR response but also improves DFS. The study also shows that the proportion of patients receiving any systemic chemotherapy and the number of cycles of chemotherapy were higher for the SGs scheduled to receive chemotherapy before surgery (p = 0.002). This could be explained by the fact that 31% of patients in SG1 did not receive any adjuvant chemotherapy. This rate is similar to the proportion of patients not receiving any scheduled adjuvant chemotherapy in phase III rectal cancer trials.21,22
Lower compliance with adjuvant chemotherapy could explain at least in part the differences in survival between SGs.23 However, our study also shows that the differences in DFS between study groups persisted when patients who received no systemic chemotherapy after CRT were excluded. Since the mean numbers of total chemotherapy cycles were similar in all SGs in this subset of patients, our data suggest that delivering systemic chemotherapy after CRT and before surgery may be more effective than conventional postoperative chemotherapy beyond compliance with the planned regimen.
Our study also confirms previous observations that pCR is a strong predictor of survival and that ypTNM stage is also associated with survival in multivariable analysis.7,8 Considering the close relationship between SG and pCR, we were surprised to find no differences in survival in SG 2 to 4. However, the design of the TIMING trial did not allow determining whether the increase in pCR observed in a study group was secondary to the lengthening of the CRT-to-surgery interval or the number of cycles of FOLFOX added during the waiting period. While our current results could be interpreted as an indication that pCR depends more on the CRT-to-surgery interval and that survival depends more on adding some cycles of FOLFOX during the waiting period, it is possible that the differences in pCR between groups were too small to impact survival.
Only one previous study reported long-term outcomes in patients with rectal cancer treated with CRT and consolidation chemotherapy. That retrospective study included patients with clinically staged T3 rectal cancer treated with a standard neoadjuvant regimen (50.4 Gy of radiation and two cycles of infusional fluorouracil/leucovorin) and an extended neoadjuvant regimen (54 Gy of radiation and three cycles of infusional fluorouracil/leucovorin, followed by three additional cycles of fluorouracil/leucovorin).14 The study found no difference in distant-metastasis-free survival between the standard-regimen group and the extended-regimen group. However, the doses of infusional fluorouracil used during and after radiation were significantly lower than in our trial. Specifically, the total dose of fluorouracil scheduled after CRT was less than one-half of the dose scheduled during a single cycle of FOLFOX. In addition, the study did not use oxaliplatin after CRT, whereas oxaliplatin is now commonly combined with fluorouracil in neoadjuvant or adjuvant chemotherapy regimens for locally advanced rectal cancer23. Finally, tumors in the extended-regimen group were larger than and twice as likely to have nodal metastasis as tumors in the standard-regimen group.
Few studies have reported survival outcomes in patients treated with short-course radiation (SCR) and consolidation chemotherapy for locally advanced rectal cancer. Myerson et al. found that a regimen of five fractions of pelvic radiation (5 Gy/day for 5 consecutive days) followed by four cycles of FOLFOX resulted in a 25% pCR rate and an 87% rate of absence of disease relapse after a median follow-up of 30 months.15 A subsequent study from the same institution compared the outcomes of patients treated with total neoadjuvant therapy (5 Gy/day for 5 consecutive days followed by four cycles of FOLFOX) and patients treated with standard CRT (45 Gy of radiation in 25 fractions with concurrent fluorouracil or capecitabine and scheduled adjuvant FOLFOX).16 Similar to our study, patients who underwent total neoadjuvant therapy received more cycles of chemotherapy, achieved higher pCR rates (28 vs. 16%), and had a higher rate of 3-year DFS (85% vs. 68%) than matched controls treated with CRT. Although the dose and fractionation of radiation were different, these studies lend support to the idea that delivering chemotherapy after radiation improves tumor response and DFS.
In a recently completed Polish Colorectal Study Group trial, patients with cT3 or cT4 rectal cancer were randomized to either preoperative 5 × 5 Gy irradiation followed by three cycles of FOLFOX-4 or 50.4 Gy of radiation in 28 fractions of 1.8 Gy each concomitantly with oxaliplatin, boluses of fluorouracil, and folinic acid.17 These regimens were chosen to ensure equivalent treatment time and interval from initiation of radiation to surgery. The proportion of patients with an R0 resection or a pCR was greater among patients who received SCR and consolidation chemotherapy than among patients who received long-course radiation and concomitant chemotherapy, but the differences did not reach statistical significance. Toxicity was lower and the overall survival rate was higher in patients who received SCR and consolidation chemotherapy. While the cumulative rates of local failure, distant failure, and death from non-cancer-related causes at 3 years were similar between groups, cumulative incidence of death in patients with tumor relapse was lower in the group treated with SCR and consolidation chemotherapy than in the group treated with long-course radiation and concomitant chemotherapy (23% versus 31%; p = 0.049). The reasons for those differences were unclear, and the authors indicated that a longer follow-up was needed to clarify the issue. The ongoing phase III RAPIDO trial, with patients randomized to either SCR and consolidation chemotherapy before TME or CRT, TME, and adjuvant chemotherapy, will provide additional information about the effects of consolidation chemotherapy on tumor response and patient survival.
Our study’s strengths include its prospective design with predefined inclusion and exclusion criteria and uniform CRT in all SGs. Among the limitations are the fact that because the study was powered to detect differences in pCR rates, it may have been underpowered to detect differences in survival between SGs. Patient demographics and tumor characteristics were similar in all SGs, but due to the lack of randomization the possibility of selection bias between SGs cannot be completely excluded. Other potential limitations are the lack of data on MRI-identified circumferential resection margins, MRI-identified extramural venous invasion, TME quality, and lymphatic or perineural invasion (MRI was not routinely used for locoregional staging of rectal cancer at the time the study opened to accrual, TME procedures in all groups were performed by the same highly experienced surgeons, and lymphatic and perineural invasion cannot be reliably detected in pretreatment biopsies). Lastly, almost one-third of the patients in SG1 were not consented for survival analysis and could not be included. Other patients were excluded for a variety of reasons. Although patient demographics and tumor characters did not differ between patients who were included and patients who were excluded, the impact of the exclusion on the study outcomes cannot be fully ascertained.
CONCLUSIONS
The findings of our study indicate that adding neoadjuvant consolidation chemotherapy after CRT is a safe approach that can lead to higher pCR rates, increased compliance with systemic chemotherapy regimens, and longer DFS. This neoadjuvant regimen may be particularly attractive for patients with very distal rectal cancer who may be interested in organ preservation via a watch-and-wait approach.
Supplementary Material
Trial protocol.
Disease-free survival.
Follow-up availability.
Acknowledgments:
The authors will like to thank Dr. Theodore Coustoftides for helping in data acquisition.
Funding:NCI grants R01 CA090559 and P30 CA008748.
Footnotes
Disclaimer: none of the authors had a conflict of interest.
Author contributions: MRM, JEM, MGV, SO, PAC, SRH, AK, DOH, AF, BNP, NHH, CAT, MJS, AP, DD, and JG-A designed the study. SP did the statistical analysis. MRM and JG-A wrote the manuscript and prepared the figures. All authors contributed toward data acquisition, data interpretation, and critical revision of the content of the manuscript and approved the final version of the manuscript.
REFERENCES
- 1.Sauer R, Becker H, Hohenberger W, et al. ; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 2.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–460. [DOI] [PubMed] [Google Scholar]
- 3.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926–1933. [DOI] [PubMed] [Google Scholar]
- 4.Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124–5130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams NS, Johnston D. The quality of life after rectal excision for low rectal cancer. Br J Surg. 1983;70:460–462. [DOI] [PubMed] [Google Scholar]
- 6.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. [DOI] [PubMed] [Google Scholar]
- 8.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. [DOI] [PubMed] [Google Scholar]
- 9.Smith JJ, Chow OS, Gollub MJ, et al. ; Rectal Cancer Consortium. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiltshire KL, Ward IG, Swallow C, et al. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer: effect of dose escalation on pathologic complete response, local recurrence-free survival, disease-free survival, and overall survival. Int J Radiat Oncol Biol Phys. 2006;64:709–716. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859–865. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial. Ann Oncol. 2015;26:1722–1728. [DOI] [PubMed] [Google Scholar]
- 13.Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.São Julião GP, Habr-Gama A, Vailati BB, et al. Is neoadjuvant chemoradiation with dose-escalation and consolidation chemotherapy sufficient to increase surgery-free and distant metastases-free survival in baseline cT3 rectal cancer? Eur J Surg Oncol. 2018;44:93–99. [DOI] [PubMed] [Google Scholar]
- 15.Myerson RJ, Tan B, Hunt S, et al. Five fractions of radiation therapy followed by 4 cycles of FOLFOX chemotherapy as preoperative treatment for rectal cancer. Int J Radiat Oncol Biol Phys. 2014;88:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markovina S, Youssef F, Roy A, et al. Improved metastasis- and disease-free survival with preoperative sequential short-course radiation therapy and FOLFOX chemotherapy for rectal cancer compared with neoadjuvant long-course chemoradiotherapy: results of a matched pair analysis. Int J Radiat Oncol Biol Phys. 2017;99:417–426. [DOI] [PubMed] [Google Scholar]
- 17.Bujko K, Wyrwicz L, Rutkowski A, et al. ; Polish Colorectal Study Group. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27:834–842. [DOI] [PubMed] [Google Scholar]
- 18.Du D, Su Z, Wang D, Liu W, Wei Z. Optimal interval to surgery after neoadjuvant chemoradiotherapy in rectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer. 2018;17:13–24. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Aguilar J, Chow OS, Smith DD, et al. ; Timing of Rectal Cancer Response to Chemoradiation Consortium. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer. www.nccn.org. [DOI] [PubMed]
- 21.Bosset J-F, Calais G, Mineur L, et al. ; EORTC Radiation Oncology Group. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–190. [DOI] [PubMed] [Google Scholar]
- 22.Rödel C, Graeven U, Fietkau R, et al. ; German Rectal Cancer Study Group. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16:979–989. [DOI] [PubMed] [Google Scholar]
- 23.Ludmir EB, Palta M, Willett CG, Czito BG. Total neoadjuvant therapy for rectal cancer: an emerging option. Cancer. 2017;123:1497–1506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
Disease-free survival.
Follow-up availability.


