Abstract
Background.
Encapsulated papillary carcinoma (EPC) is a rare entity of breast cancer accounting for approximately 1–2% of all breast tumours. There are no evidence-based guidelines for the treatment of EPC.
Materials and methods.
From the database of the National Centre of Pathology (NCP), we obtained pathology reports of 19 patients with histologically confirmed EPC, who were treated at the National Cancer Institute (NCI) in Vilnius, Lithuania, between July 2009 and July 2015. Demographic, diagnostic and treatment data were collected from medical records retrospectively.
Results.
During the indicated period, 19 patients with EPC were treated at the NCI. Three of them had pure EPC, they were 74 to 81 years of age at the time of diagnosis (mean 76.7 years, median 75 years); all of them are still alive and no disease progression has been observed. Seven patients had EPC associated with carcinoma in situ. Nine patients had EPC associated with invasive breast ductal carcinoma. All patients underwent surgery, in most cases – wide local excision. Only one patient died.
Conclusions.
EPC is a rare form of breast cancer and usually presents with an invasive breast carcinoma or carcinoma in situ in postmenopausal women. Tumours have an excellent prognosis in the cases of pure EPC and in both EPC associated with carcinoma in situ (CIS) and invasive carcinoma.
Keywords: breast cancer, encapsulated papillary carcinoma, breast conserving surgery
Abstract
INKAPSULIUOTOS PAPILINĖS KARCINOMOS DIAGNOSTIKA, GYDYMAS, PROGNOZĖ: VIENOS ĮSTAIGOS PATIRTIS
Santrauka
Įvadas. Inkapsuliuota papilinė karcinoma (IPK) – tai reta krūties vėžio forma, sudaranti apie 1–2 % visų krūties navikų. Įrodymais pagrįstų duomenų apie šių navikų gydymą nėra.
Medžiaga ir metodai. Iš Valstybinio patologijos centro gauti 19 pacienčių, kurioms buvo nustatyta IPK ir kurios gydytos Nacionaliniame vėžio institute (NVI) (nuo 2009 m. liepos iki 2015 m. liepos mėn.), duomenys. Taip pat retrospektyviai surinkti demografiniai duomenys, atliktų tyrimų rezultatai ir duomenys apie gydymą.
Rezultatai. Minėtu periodu 19-ai pacienčių nustatyta IPK, jos gydytos NVI. Trims iš jų buvo nustatyta gryna IPK. Pacienčių amžius – 74–81 metai (vidurkis 76,7), visos jos buvo gyvos iki stebėjimo laikotarpio pabaigos, nė vienai nenustatytas ligos progresavimas. Septynioms pacientėms nustatyta IPK asocijuota su karcinoma in situ. Devynioms pacientėms kartu su IPK diagnozuota ir invazyvi duktalinė karcinoma. Visoms pacientėms taikytas chirurginis gydymas, daugiausia plati vietinė ekscizija. Tik viena iš tirtų pacienčių mirė.
Išvada. Inkapsuliuota papilinė karcinoma yra reta krūties navikų rūšis, dažniausiai nustatoma kartu su invazyvia ar in situ karcinoma. Dauguma atvejų diagnozuojami po menopauzės. Šiam navikui būdinga puiki prognozė, net jei nustatoma kartu su invazyvia karcinoma.
Raktažodžiai: krūties vėžys, inkapsuliuota papilinė karcinoma, krūtį tausojanti operacija
INTRODUCTION
EPC is a rare breast cancer accounting for approximately 1–2% of all breast carcinomas in women, with an excellent prognosis in its pure form (1). Usually this cancer presents in postmenopausal women between 55 and 67 years of age (2, 3). Cases of EPC have also been described in males. Encapsulated papillary carcinoma is also referred to by several synonyms: intracystic papillary carcinoma, encysted papillary carcinoma, and intracystic carcinoma not otherwise specified.
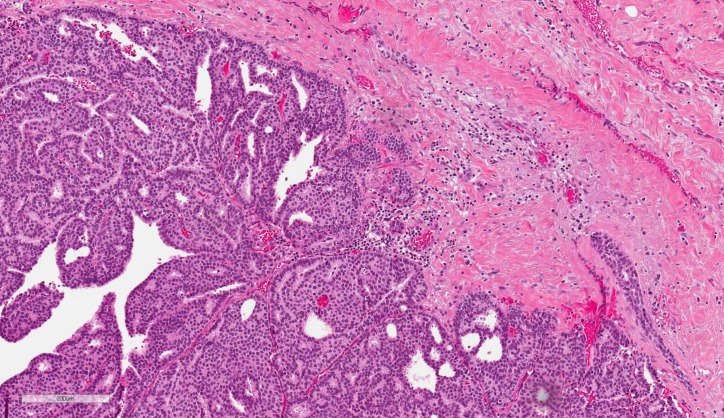
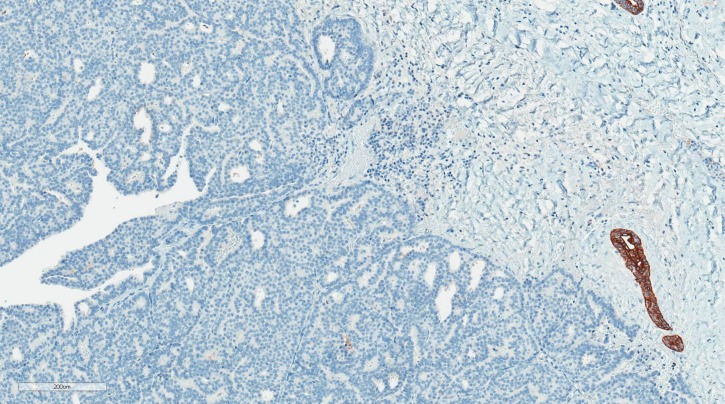
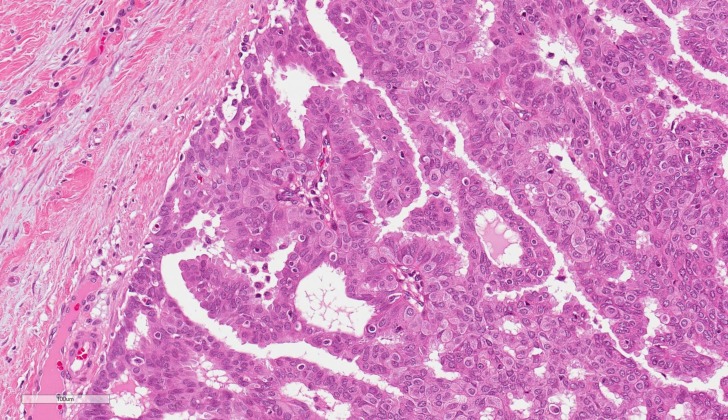
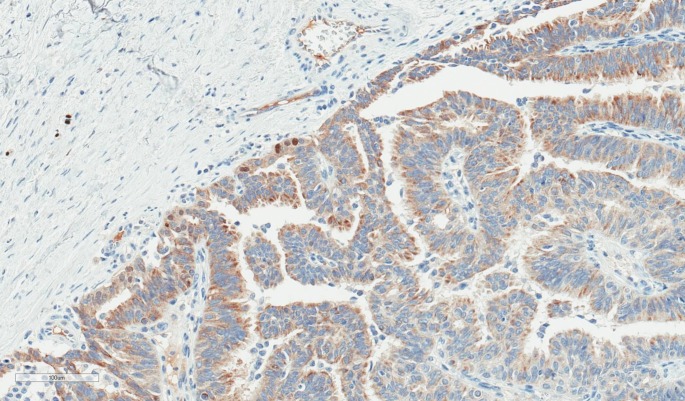
Histologically, EPC is characterised by a cystically dilated duct surrounded by a fibrous capsule with intraluminal arborization of the fibrovascular stroma covered by atypical epithelium with low or intermediate nuclear grade with no evidence of necrosis and rare mitoses (Figures 1–4). Immunohistochemically, EPC is strongly positive for oestrogen and progesterone receptors and negative for HER2. EPC usually lacks a myoepithelial cell layer both in papillary structures and in the fibrous capsule, which might suggest invasive behaviour of the lesion. Although histologically benign and malignant papillary lesions can be differentiated by the presence of the myoepithelial cell layer, not all the cases that lack a myoepithelial layer indicate an invasion, e.g., microglandular adenosis (4). There is still an ongoing discussion whether EPC is in situ or invasive carcinoma, and there is no clear agreement among different studies (5).
Fig. 1.
Encapsulated papillary carcinoma (H&E, ×100)
Fig. 2.
The same case of encapsulated papillary carcinoma (CK5, ×100). No CK5 positive mioepitelial cells at the periphery of the lesion
Fig. 3.
Encapsulated papillary carcinoma (H&E, ×200)
Fig. 4.
The same case of encapsulated papillary carcinoma (p63, ×200). There are only a few faintly positive nuclei at the periphery of the lesion
There exist several classifications of EPC. According to Carter, EPC is classified as either invasive or non-invasive EPC; and diffuse or a localized encysted form, which is a solitary tumour in the cyst or a dilated duct (6). Also, EPC can be classified into three main subtypes: EPC alone (pure form), EPC with surrounding ductal carcinoma in situ (DCIS), and EPC associated with invasive carcinoma (7). Usually EPC is classified as a non-invasive form of breast cancer and a variant subtype of low-grade DCIS. But it is known that EPC occurs with DCIS or invasive breast cancer in about 40% of cases (8). Classifying EPC under invasive or ductal carcinoma in situ is still a matter of debate. According to the WHO Classification of Tumours of the Breast (2012), EPC is classified into encapsulated papillary carcinoma and encapsulated papillary carcinoma with invasion (9). There have been a few studies that investigated the biology of encapsulated papillary carcinoma. Using markers of invasion, Rakha et al. found that EPC exhibited an expression pattern of invasion-associated markers between ductal carcinoma in situ and invasive ductal carcinoma, concluding that this tumour has unique biological features (10). Encysted papillary carcinoma is genetically closer to ductal carcinoma in situ than to invasive ductal carcinoma, which may explain the indolent behaviour of this tumour (11). The WHO recommends encapsulated papillary carcinoma to be staged and treated like a ductal carcinoma in situ as the behaviour of this tumour is usually indolent.
The aim of our retrospective study was to collect data on EPC, describe its main characteristics, and analyze treatment and outcomes.
MATERIALS AND METHODS
A total of 19 patients with histologically confirmed EPC treated at the NCI, Lithuania, between July 2009 and July 2015 were included in the study. Pathology reports were obtained from the NCP. Demographic data and clinical characteristics, the extent of the disease, the type of surgery, chemotherapy, radiotherapy, and hormonal therapy were collected from medical records. The date of the last follow-up was July 2016.
RESULTS
According to the literature, we divided all EPCs into three categories: pure EPC, EPC with CIS, and EPC with invasive carcinoma. We identified three patients in the first group, seven in the second, and nine in the third. All patients were followed at the NCI outpatient department, except for the three patients who did not arrive after the surgery. One patient has died recently; the reason is unknown. Demographic and clinical characteristics, treatment, and the current status are presented in tables. The data on pure EPC are presented in Table 1; the data on EPC associated with DCIS are given in Table 2, and the data on EPC associated with invasive carcinoma are presented in Table 3.
Table 1.
Pure EPC
| Age | Clinical data | Radiological data | Surgery | Immunophenotype | Further treatment | Status at the last follow up |
|---|---|---|---|---|---|---|
| 81 | 2003 WLE for the EPC. RT after surgery. In 2009 after the injury of the same breast, the deformation appeared | MG – 3 cm mass | WLE | EPC 19 mm; ER, PR positive | Refused | Alive. Not attending |
| 74 | Palpable tumour for 2 weeks | MG – 32 × 24 mm mass, BI-RADS 4a US – 20 × 22 mm mass, BIRADS 5 | WLE | EPC 15 mm; ER, PR positive | HT given for 3 years | Alive. Last visit Oct 2015, without progression |
| 75 | Palpable tumour for several years | MG – 20 mm microcalcifications, BIRADS 4 US – 25 mm mass, BIRADS 4 | Mastec tomy | EPC 27 mm G2; ER, PR positive | HT | Alive. Last visit Jan 2016, without progression |
HT – hormonal therapy; RT – radiotherapy; WLE – wide local excision; MG – mammography; US – ultrasound.
Table 2.
EPC with DCIS
| Age | Clinical data | Radiological data | Surgery | Imonophenotype | Further treatment | Status at the last follow up |
|---|---|---|---|---|---|---|
| 80 | Not available | MG – right mass with mcc; left breast spiculated mass 26 × 35 mm and satellites 5 mm and 7 mm. Bilateral BI-RADS 5 | Right WLE, left mastectomy | Right breast: EPC 60 mm (ER positive, PR negative) and LCIS. Left breast: Ductal CA (ER, PR positive, HER2 1+) | Refused RT, HT given | Alive, last visit July 2015, without progression |
| 54 | Palpable tumour for 3 months, injury of the breast in anamnesis | MG – several masses up to 17 mm, BIRADS 3; US – several cystic masses BIRADS 3 | WLE | EPC 2 nodes both 15 mm; ER, PR positive and LCIS | RT after surgery and HT given | Alive, last visit May 2016, refused tamoxifen, without progression |
| 61 | No complains | MG – masses BIRADS 3 in both breasts; US – right 5 mm mass, BIRADS 4 | WLE | EPC 3 mm; ER, PR positive and low grade DCIS | RT after surgery and HT given | Alive, last visit March 2016, without progression |
| 70 | Palpable tumour for 7 months | MG mass 20 mm left, BI-RADS 4; US mass 20 × 14 mm, BI-RADS 4 | WLE | 17 mm and DCIS; ER, PR positive | RT after surgery and HT given | Alive, continues tamoxifen. Last visit Apr 2016, without progression |
| 64 | No complaints | MG BI-RADS 3, right mass 25 × 20 mm, left mass 20 × 15 mm; US right mass 25 mm, left mass 17 mm, BI-RADS 4 | WLE of both breasts. In the right - EPC, DCIS in the left | 18 mm and DCIS; ER, PR positive DCIS bilateral, EPC in the right breast | RT after surgery and HT given | Alive, continues tamoxifen. Last visit June 2016, without progression |
| 74 | Palpable tumour for 3 months, hemorrhagic discharges from nipple | MG left masses up to 30 mm, BIRADS 4b | WLE | 21 mm, ER, PR positive | No further treatment given | Alive, last visit Dec 2010 |
| 79 | Palpable tumour for 2 months | MG right lobulated mass and microcalcifications, BI-RADS 4 | WLE | 10 mm, IH not specified | Did not arrive after surgery | Died, last visit - released from surgical department |
HT – hormonal therapy; RT – radiotherapy; WLE – wide local excision; MG – mammography; US – ultrasound.
Table 3.
EPC with invasive BC
| Age | Clinical data | Radiological data | Surgery | Imunophenotype | Further treatment | Status at the last follow up |
|---|---|---|---|---|---|---|
| 85 | Palpable tumour for several years, started growing | Not performed | Mastectomy | G1 invasive carcinoma and EPC 60 mm; ER, PR positive, HER2 0 | Refused | Alive, last visit July 2014, not attending |
| 62 | Injury of the right breast several years ago, palpable tumour for several years, started growing, exulcerated | Not performed | Mastectomy | G1 invasive carcinoma and EPC 30 mm; ER, PR positive, HER2 1 + | Chemotherapy 6 cycles (FAC regimen), HT given | Alive. Last visit Aug 2015, continues tamoxifen, without progression |
| 34 | Palpable tumour for 1 month, from fine needle biopsy carcinoma suspected | MG – BI-RADS 4; microcalcifications and mass | WLE | EPC 4 mm, ER, PR positive, HER2 0 and G1 ductal CA (ER, PR positive, HER2 1+) | RT after surgery and HT given | Alive, last visit March 2016 without progression; continues tamoxifen |
| 55 | Palpable tumour for 2 months, lots of years of replacing hormonal therapy | MG – BI-RADS 4; mass 20 mm; US – 22 × 12 mm polycyclic hypoechogenic mass, vascularised with cystic zones | Mastectomy | EPC 13 mm (IH not specified) and G1 ductal CA (ER, PR positive, HER2 1+) | HT given | Alive, last visit Feb 2016 without progression; continues tamoxifen |
| 48 | Palpable tumour for 6 months, cyanotic skin, large 9 cm diameter mass in the left breast | MG – BI-RADS 5 dex ~ 100 mm (sarcoma?); US – BIRADS 6; mass 70 × 90 mm with fluid | Mastectomy | EPC (size and IH not specified) and G2 ductal CA, ER, PR negative, HER2 3+ | Chemotherapy 4 cycles AC plus 4 cycles Paclitaxel with biological therapy with trastuzumab | Alive, 2015 FA from the right breast removed. Last visit Mar 2016, without progression |
| 73 | Palpable tumour for 1 month in the left breast. 36 years ago mastectomy of the right breast due to III stage carcinoma. 6 years ago surgery and RT due to uterus carcinoma | MG – 80 × 55 mm infiltration with microcalcifications and 20 mm mass; BI-RADS 5; US – 20 mm vascularised mass and infiltration zone with microcalcifications 15 × 40 mm | WLE | EPC 16 mm (IH not specified) and G3 ductal CA, ER, PR positive, HER2 3+ | Chemotherapy with Capecitabine | Alive, last visit Apr 2015 after the first chemotherapy cycle. Not attending |
| 63 | No complains. Accidental findings during chest CT. In 2007 carcinoma recti I stage | MG – BI-RADS 4, 16 mm mass; US – BI-RADS 5, 15 mm mass | WLE | EPC 8 mm (IH not specified) and G1 ductal CA (ER, PR positive, HER2 1+) | RT after surgery and HT given | Alive. Last visit Jun 2016, without progression, finished 5 years of tamoxifen |
| 64 | Palpable tumour for several years, carcinoma suspected 2 years ago, refused from treatment | US – BI-RADS 5, 7 × 4 mm mass and cysts up to 4 mm | Mastectomy | EPC 25 mm (IH not specified) and G2 ductal CA (ER, PR positive, HER2 1+) | Chemotherapy 5 cycles (FAC regimen), refused from RT, HT given | Alive, Last visit Nov 2015, without progression; continues tamoxifen |
| 50 | Palpable tumour for 1 month | MG – BIRADS 4, 20 mm mass | WLE | EPC 22 mm, ER, PR positive, HER2 0 and G2 ductal CA | RT after surgery and HT given | Alive, last visit Jan 2016; continues tamoxifen |
HT – hormonal therapy; RT – radiotherapy; WLE – wide local excision; MG – mammography; US – ultrasound.
About half of the cases (9/19) were associated with invasive ductal carcinoma, seven patients had EPC and carcinoma in situ, and only three patients had pure EPC. The majority of the patients were treated with wide local excision and none of them had lymph nodes metastases or distant spread.
In the first group of patients with pure EPC, mean tumour size was 20.3 mm, median 19 mm (range 15–27 mm). In the second group of patients with EPC associated with CIS, mean tumour size was 20.5 mm, median 17 mm (range 3–60), and in the last group – EPC associated with invasive ductal carcinoma – mean tumour size was 22.25 mm, median 22 mm (range 4–60 mm).
The majority of EPCs (13/19 cases) were immunohistochemically tested for hormone receptors. All of the tested EPC cases were positive for oestrogen and progesterone receptors, except for one which was ER positive and PR negative. HER2 was negative (0 or 1+) in all tested cases.
DISCUSSION
EPC usually occurs in postmenopausal women, when the average age of occurrence, according to literature, is around 65 (range 34–92 years). According to our data, the average age of patients with pure EPC was 76.67 years, for EPC associated with carcinoma in situ 68.86 years, and the youngest group of patients was EPC associated with invasive breast cancer with the average age of 59.33 years at the time of diagnosis.
Usually EPC manifests as a painless lump in the breast, which could be present for several years. EPC is usually a large size tumour (mean 2 cm) located within a large cystic duct. In our cases, many EPCs were about 2 cm, the biggest was 6 cm. Clinically, EPC usually presents like a benign mass, it can often be asymptomatic and found by screening mammography. A common symptom is a bloody nipple discharge.
Clinical and radiological findings of EPC are not very specific. The tumour may be seen in mammograms as oval or lobulated, a well circumscribed mass (Figures 1–4). On ultrasound, EPC is usually heterogeneous and shows cystic and solid components and may have an indistinct border or a polycyclic contour, which may suggest malignancy. The cystic part may have septations, internal echoes. The use of a Doppler may help to identify the vascularity in the solid component (12, 13). Magnetic resonance imaging (MRI) is not specific for these tumours. Only in the cases of extent papillomatosis it may help to find malignant tumours (14). Radiological findings of four of our patients are shown in figures 5–8. Fine needle aspiration may be useful – aspiration of bloody content and/or residual palpable mass after aspiration is an indicator for a suggestion of invasion. A core needle biopsy may help distinguish benign and malignant papillary lesions, but it has a low accuracy for differentiation of invasive and non-invasive tumours (3). A core needle biopsy that targets the central part of the solid component is recommended for morphological investigation, because only a fragmented component without complete solid mass may be obtained for cytology. Some authors propose surgical excision of such lesions, sometimes without a biopsy (15). In all cases, after a core needle biopsy, a surgical excisional biopsy is recommended for papillary lesions, because invasion is usually found not in the central, but in the peripheral part of the tumour; also, EPC can often be associated with DCIS. The histological examples of our patients showing the main specificity of EPC are shown in figures 1–4.
Fig. 5.
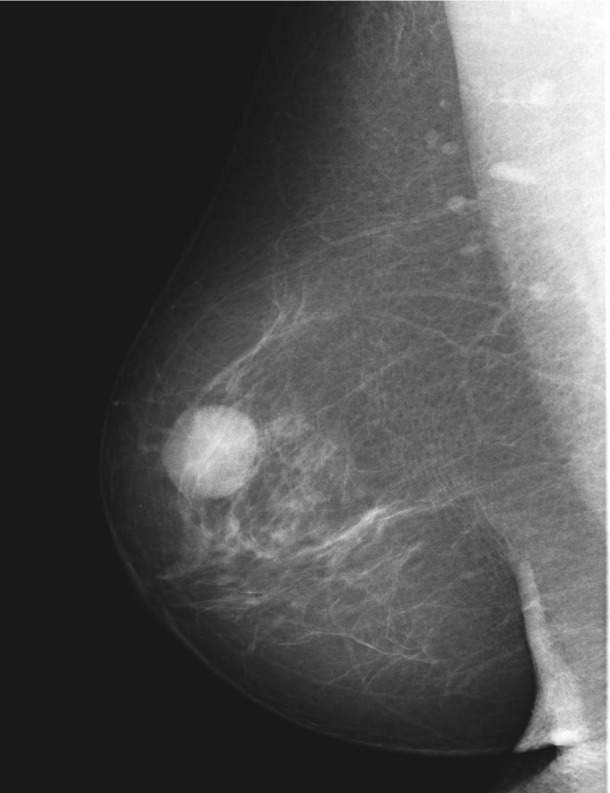
BI-RADS 2 on MG: an oval-shaped mass. G1 EPC non-invasive carcinoma, 15 mm
Fig. 6.
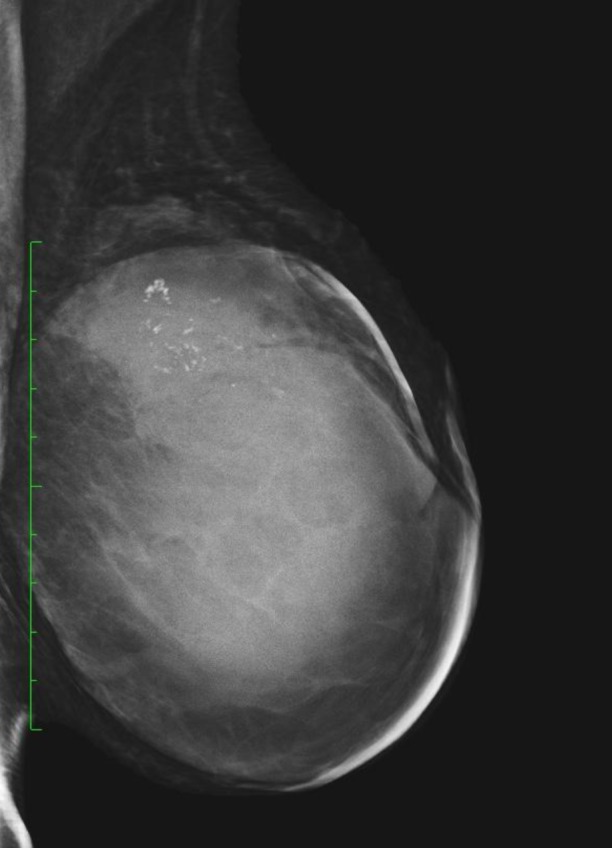
Cystic mass on MG with microcalcifications – 70 mm G2 EPC. Invasive ductal carcinoma
Therapeutic management of EPC is controversial. There are no evidence-based guidelines for the treatment of EPC. No randomized trials investigated different surgery types in EPC. Surgical excision is a golden standard in the treatment of papillary lesions and is recommended if the core needle biopsy shows atypia or invasion and in the presence of imaging-histological discordance. Surgery is recommended in all cases when a solid mass in the cyst is seen.
Treatment of EPC consists of surgery (wide local excision or mastectomy) ± radiotherapy (RT) ± hormonal treatment (16). The question of an axillary procedure remains open, some investigations report the metastatic potential of EPC and recommend sentinel lymph node biopsy (SLNB) (17).
Adjuvant treatment is chosen if DCIS or invasion is associated with EPC. Systemic adjuvant therapy has focused on endocrine manipulation, principally the use of tamoxifen as EPC tends to be ER/PR positive and HER2 negative (18), although there are no clear indications for adjuvant endocrine therapy.
Fig. 7.
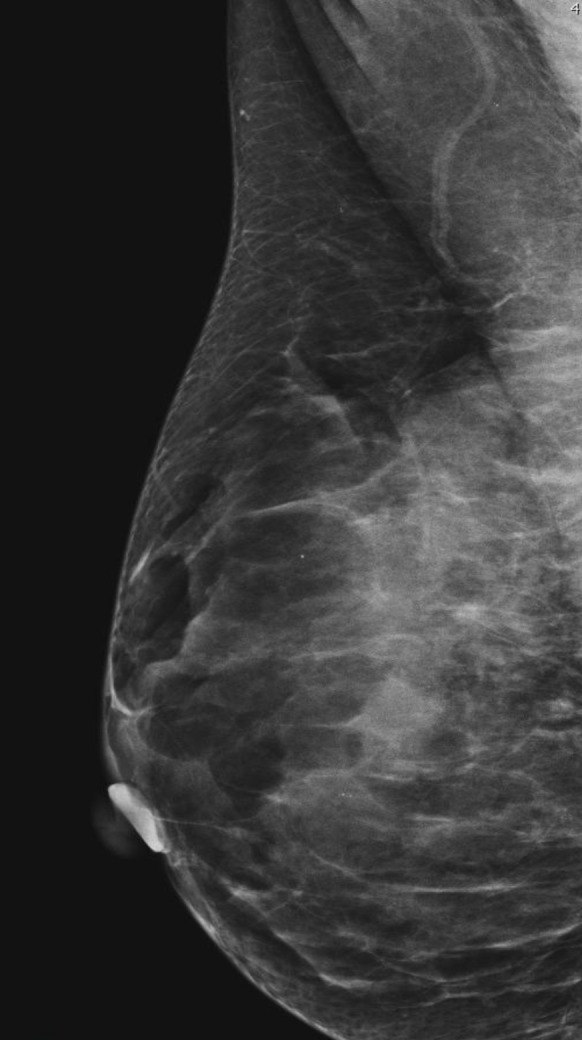
Non-typical presentation of intracystic carcinoma. Microcalcifications on MG. G1 3 mm invasive ductal carcinoma and 4 mm EPC
Many centres use RT and hormonal therapy, but their role in prognosis improvement is still inadequate. It is not clear if adjuvant radiotherapy significantly reduces the risk of local recurrence in EPC patients undergoing breast conserving surgery. Radiotherapy plays an important role in the treatment of young EPC patients.
In our cases, nearly all the patients after conserving surgery were admitted to radiotherapy (except for those, who refused such treatment). Also, if it was an ER positive tumour, tamoxifen was prescribed. In the cases of invasive carcinoma with an aggressive phenotype, chemotherapy was applied.
Fig. 8.
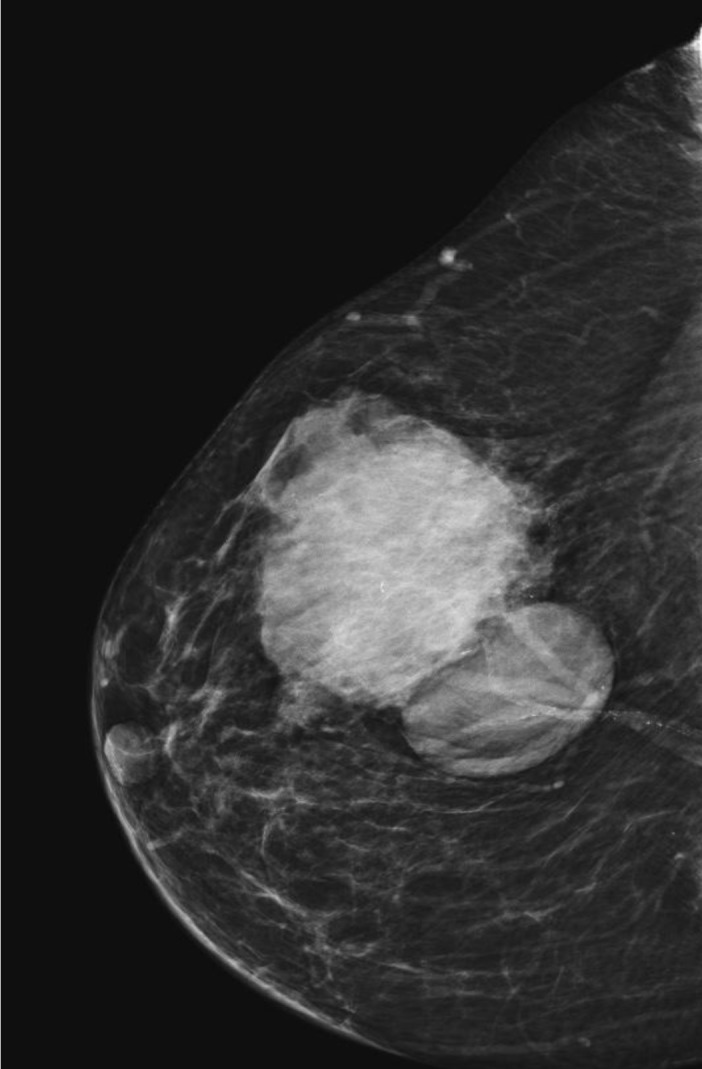
Two mass lesions on MG – multicystic intracystic G1 60 mm carcinoma and invasive ductal carcinoma
It seems that this kind of tumour has an excellent clinical prognosis: since it grows slowly, 10-year survival reaches about 100%, and 10-year disease-free survival 91%. The largest reported study of 917 cases carried out on EPC patients from California demonstrated that the relative cumulative survival rate was almost the same in both groups: of EPC alone or EPC associated with invasive cancer, which were 96.8% and 94.4%, respectively (P = 0.18), followed up at ten years (19). Usually, in the absence of concomitant DCIS or invasive carcinoma EPC has a very favourable prognosis with only a few reported cases of lymph node metastases (17) and no disease-related deaths. Our study supports this statement: 18 patients are alive and without progression, and only one patient died for an unknown reason.
CONCLUSIONS
EPC is a rare form of breast cancer that usually manifests in postmenopausal women. EPC is often associated with DCIS or invasive breast cancer. Clinically and radiologically, EPC may appear like a benign lump. A histological classification remains an object of debate. In the opinion of many authors, EPC must be treated like DCIS. We believe that the experience of our institution will contribute the available data on this rare tumour, and supplement to a better understanding of this unique subgroup.
Laura Steponavičienė, Daiva Gudavičienė, Rūta Briedienė, Donatas Petroška, Aušra Garnelytė
References
- Al Reefy S, Kameshki R, Al Sada Dh, El Elewah A, Awadhi A, Al Awadhi K.. Intracystic papillary breast cancer: a clinical update. Ecancermedicalscience. 2013; 7: 286 Published online 2013. January 3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem A, Mrad K, Driss M.. Carcinome papillaire mammaire intrakystique. Journal of Radiology. 2009; 90: 515–8. [DOI] [PubMed] [Google Scholar]
- Muttarak M, Samwangprasert A, Chaiwun B.. Intracystic papillary carcinoma of the breast. Biomed Imag Interv J. 2005; 1(1): 52–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A, Gage I, Evans S, Shaffer M, Fleury T, Smith FP, et al. . Sentinel lymph node positivity of patients with ductal carcinoma in situ or microinvasive breast cancer. Am J Surg. 2006; 191: 761–6. [DOI] [PubMed] [Google Scholar]
- Wynveen CA, Nehhozina T, Akram M, Hassan M, Norton L, Van Zee KJ, Brogi E.. Intracystic papillary carcinoma of the breast: An in situ or invasive tumor? Results of immunohistochemical analysis and clinical follow-up. Am J Surg Pathol. 2011. January; 35(1): 1–14. [DOI] [PubMed] [Google Scholar]
- Carter D, Orr SL, Merino MJ. Intracystic papillary carcinoma of the breast. After mastectomy, radiotherapy or excisional biopsy alone. Cancer. 1983; 52(1): 14–9. [DOI] [PubMed] [Google Scholar]
- Baykara M, Coskun U, Demirci U, Yildiz R, Benekli M, Cakir A, Buyukberber S.. Intracystic papillary carcinoma of the breast: one of the youngest patients in the literature. Med Oncol. 2010; 4: 1427–8. [DOI] [PubMed] [Google Scholar]
- Calderaro J, Espie M, Duclos J, Giachetti S, Wehrer D, Sandid W, et al. . Breast intracystic papillary carcinoma: an update. Breast J. 2009; 15(6): 639–44. [DOI] [PubMed] [Google Scholar]
- Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Breast. IARC Press: Lyon, France, 2012, pp. 106–107. [Google Scholar]
- Rakha EA, Tun M, Junainah E, Elis IO, Green A.. Encapsulated papillary carcinoma of the breast: a study of invasion associated markers. J Clin Pathol. 2012; 65: 710–4. [DOI] [PubMed] [Google Scholar]
- Khoury Th, Hu Q, g Liu, Wang J.. Intracystic Papillary Carcinoma of Breast: Interrelationship With in Situ and Invasive Carcinoma and a Proposal of Pathogenesis.Array Comparative Genomic Hybridization Study of 14 Cases. Mod Pathol. 2014; 27(2): 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan BE, Whitman GJ, Middleton LP, Phelps M.. Intracystic papillary carcinoma of the breast. AJR Am J Roentgenol. 2003; 181(1): 186. [DOI] [PubMed] [Google Scholar]
- Cox KL, Korourian S, Klimberg VS. Unusual tumors of the breast. Curr Probl in Surg. 2009; 46(7): 514–90. [DOI] [PubMed] [Google Scholar]
- Lam W, Tang A, Tse G, Chu W.. Radiology-pathology conference: papillary carcinoma of the breast. Clin Imaging. 2005; 29(6): 396–400. [DOI] [PubMed] [Google Scholar]
- Reefy S, Osman H, Chao C, Perry N, Mokbel K.. Surgical excision for B3 breast lesions diagnosed by vacuum-assisted core biopsy. Anticancer Res. 2010; 30(6): 2287–90. [PubMed] [Google Scholar]
- Solorzano CC, Middleton LP, Hunt KK, Mirza N, Meric F, Kuerer HM, et al. . Treatment and outcome of patients with intracystic papillary carcinoma of the breast. Am J Surg. 2002; 184(4): 364–8. [DOI] [PubMed] [Google Scholar]
- Mulligan AM, O’Malley FP. Metastatic potential of encapsulated (intracystic) papillary carcinoma of the breast: a report of 2 cases with axillary lymph node micrometastases. Int J Surg Pathol. 2007; 15(2): 143–7. [DOI] [PubMed] [Google Scholar]
- Fayanju OM, Ritter J, Gillanders WE, Eberlein TJ, Dietz JR, Aft R, Margenthaler JA. Therapeutic management of intracystic papillary carcinoma of the breast: the roles of radiation and endocrine therpy. Am J Surg. 2007; 194(4): 497–500. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Salzstein SL, Sadler GR Blair S.. Intracystic papillary carcinoma: a review of 917 cases. Cancer. 2008; 113(5): 916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]