Abstract
Viperin is a radical SAM enzyme that has been shown to possess antiviral activity against a broad spectrum of viruses; however, its molecular mechanism is unknown. We report here that recombinant fungal and archaeal viperin enzymes catalyze the addition of the 5′-deoxyadenosyl radical (5′-dA•) to the double bond of isopentenyl pyrophosphate (IPP), producing a new compound we named adenylated isopentyl pyrophosphate (AIPP). The reaction is specific for IPP, as other pyrophosphate compounds involved in the mevalonate biosynthetic pathway did not react with 5′-dA•. Enzymatic reactions employing IPP derivatives as substrates revealed that any chemical change in IPP diminishes its ability to be an effective substrate of fungal viperin. Mutational studies disclosed that the hydroxyl group on the side chain of Tyr-245 in fungal viperin is the likely source of hydrogen in the last step of the radical addition, providing mechanistic insight into the radical reaction catalyzed by fungal viperin. Structure-based molecular dynamics (MD) simulations of viperin interacting with IPP revealed a good fit of the isopentenyl motif of IPP to the active site cavity of viperin, unraveling the molecular basis of substrate specificity of viperin for IPP. Collectively, our findings indicate that IPP is an effective substrate of fungal and archaeal viperin enzymes and provide critical insights into the reaction mechanism.
Keywords: enzyme, enzyme mechanism, radical, antiviral agent, isoprenoid, adenylation, isopentenyl pyrophosphate, radical addition, radical SAM enzyme, viperin, interferon-stimulated protein, antiviral cellular factor, antiviral protein, hydrogen abstraction
Introduction
The interferon (IFN)3 response is the first line of defense against invading pathogens, eliciting many interferon-stimulated genes (1, 2). On the other hand, enzymes of the radical SAM superfamily carry out some of the most novel and challenging chemical transformations in organisms (3–5). Viperin is uniquely positioned at the intersection of these two research disciplines; it is an interferon-stimulated gene product (6, 7), and it is a radical SAM enzyme (8). Among eight conical radical SAM enzymes found in humans (9), viperin is the only one possessing antiviral activities against a broad spectrum of viruses. Viperin (also known as RSAD2 and Cig5) was first identified as a gene induced by human cytomegalovirus in fibroblasts (6). Subsequent analysis of the IFN-γ response elements in human macrophages also revealed the same gene, and it was renamed viperin (7). Since its discovery, viperins from diverse vertebrates, ranging from humans to fish, have been studied for their antiviral activity. In addition to IFN-γ, viperin is also induced by IFN-α, IFN-β, dsDNA, dsRNA, lipopolysaccharide, and infection with viruses. To date, viperin has been shown to inhibit a broad range of viruses, including two dsDNA viruses (7, 10–12), 10 positive-sense single-stranded RNA viruses (13–23), nine negative-sense single-stranded RNA viruses (24–32), and one retrovirus (26, 33, 34). Some of these viruses, such as HIV-1, Zika virus, West Nile virus, hepatitis C virus, and influenza A virus, pose a great threat to human health.
Despite extensive studies over the past 16 years, however, the molecular mechanism underlying viral inhibition by viperin is unknown. The study that has come closest to revealing the mechanism of action was carried out by Wang et al. (24) and suggested that viperin physically interacts with farnesyl diphosphate synthase (FPPS) and inhibits its enzymatic activity. But a recent study by Makins et al. (35) indicates that viperin might not be involved in regulating FPPS activity. The recently published crystal structure of viperin from Mus musculus (MmuViperin) provides molecular insight into viperin as a radical SAM enzyme (36), but the study did not reveal possible biological substrate(s) of viperin. More recently, two studies described the in vitro reconstitution of viperin-catalyzed reactions and resulted in two different conclusions (37, 38). Based on our study presented here, we have disagreements with both studies, and we will discuss them below.
Several studies over the years have provided insight into the roles of different regions of viperin in its antiviral activity. First, the motifs required for coordinating [4Fe-4S] and SAM binding are essential for the antiviral activity of viperin (14, 17, 21, 22), indicating that the radical reaction catalyzed by viperin is likely responsible for the activity. Second, the C-terminal extension is also essential for the antiviral activity of viperin, as even a single deletion or mutation of the very last C-terminal residue abolishes its antiviral activity (14, 15, 21). Third, although the N-terminal domain, which is responsible for anchoring viperin on the surface of the endoplasmic reticulum (ER) and lipid droplets (39, 40), is important for the effectiveness of viral inhibition by viperin, it is not essential, as the enzyme lacking the N-terminal ER-associated domain still possesses detectable antiviral activity (14, 21).
To illuminate the mechanism of the enzymatic reaction carried out by viperin, we have carried out extensive bioinformatic, biochemical, and structural analyses of viperin. We demonstrate here that fungal viperin specifically catalyzes the radical addition of 5′-dA• to the double bond of IPP, resulting in the formation of a new compound, AIPP.
Results
Bioinformatic analysis of viperin
To provide insight into the mode of action of human viperin (HsaViperin), we performed a Blastp search based on the HsaViperin sequence against the Protein Data Bank. At the time we started our work on viperin a few years ago, the only hit of our search using the default setting of Blastp (E = 10) was the structure of MoaA from Staphylococcus aureus (SauMoaA) (41), which shares modest sequence identities (23%) with HsaViperin in the radical SAM domain (Fig. S1). MoaA catalyzes the first step of biosynthesis of the essential molybdopterin co-factor using GTP as the substrate (42). We noticed that several residues in MoaA responsible for binding the triphosphate motif of GTP are conserved in HsaViperin (Fig. S1), implying that the substrate(s) of viperin might also contain a phosphate motif(s). This observation, together with the knowledge from a previous study indicating that viperin inhibits enzymatic activity of FPPS (24), led us to hypothesize that, instead of FPPS, the substrate(s) or product(s) of FPPS might be the target(s) of viperin.
To test this hypothesis, we first constructed the sequence similarity network (SSN) of viperin (43), which revealed the presence of viperin beyond the reported animal kingdom (Fig. 1A). Briefly, in addition to viperin found in eukaryota (magenta nodes), viperin homologs are also present in a dozen archaea (green nodes) as well as ∼60 bacteria (blue nodes). Furthermore, the eukaryotic viperin are predominantly from two distinct kingdoms, with 117 sequences from Animalia and 74 sequences from Fungi. Guided by SSN of viperin, we selected 12 viperin (four each from bacteria, archaea, and fungi) for cloning to obtain recombinant proteins for in vitro reconstitution and structural characterization. Among the 12 cloned viperin, only viperin from fungal Trichoderma virens (TviViperin) and archaeal Methanofollis liminatans (MliViperin) produced soluble proteins in Escherichia coli (Fig. S2A). In addition, we also obtained the recombinant protein of HsaViperin with the N-terminal 50 aa truncated (HsaViperin-ΔN50; Fig. S2A), which has previously been shown to express in E. coli (8, 44). As expected, the eukaryotic HsaViperin and TviViperin are more homologous with each other than the archaeal MliViperin, which is reflected both in the SSN (Fig. 1A) and the degrees of sequence identities among them (Fig. 1B). Among the three, archaeal MliViperin has the highest level of expression in E. coli (Fig. S2A, lane 4), but the purified recombinant fungalTviViperin exhibits the highest solubility in solution.HsaViperin-ΔN50 ranks last both in the level of expression in E. coli and the solubility of the purified protein, consistent with the previous observations (8, 44). Accordingly, the recombinant fungal TviViperin and archaeal MliViperin were used for most experiments reported here.
Figure 1.
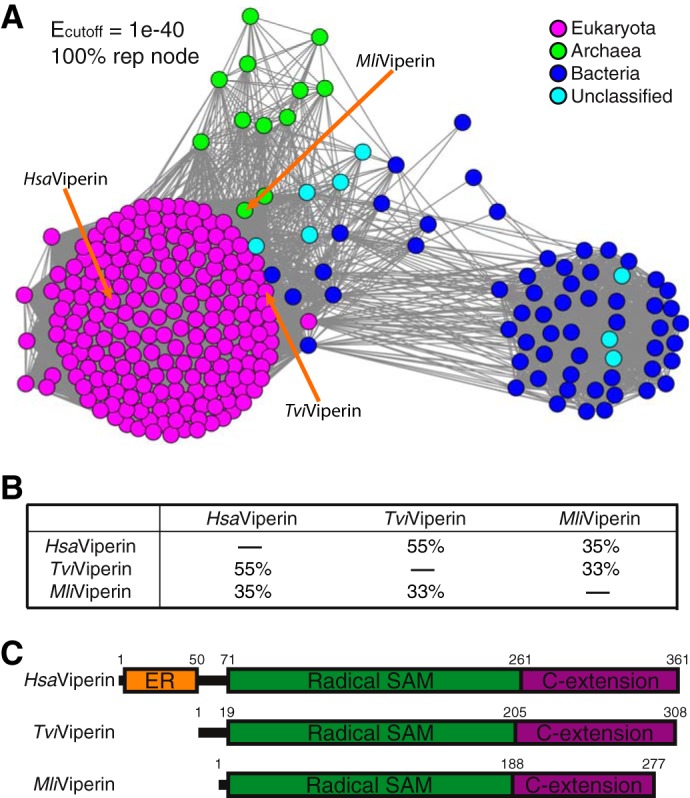
Distribution and domain structure of viperin. A, SSN of viperin based on Blastp of HsaViperin. Three viperins employed in this study are marked with arrows. Tvi, Trichoderma virens; Mli, Methanofollis liminatans. B, pairwise amino acid sequence identities among the three viperins employed in this study. C, domain structures of these three viperins. The ER-associated domain (colored orange) present in HsaViperin is missing in both fungal TviViperin and archaeal MliViperin. C-extension, C-terminal extension.
IPP, but not other pyrophosphate compounds involved in the mevalonate pathway, is the substrate of viperin
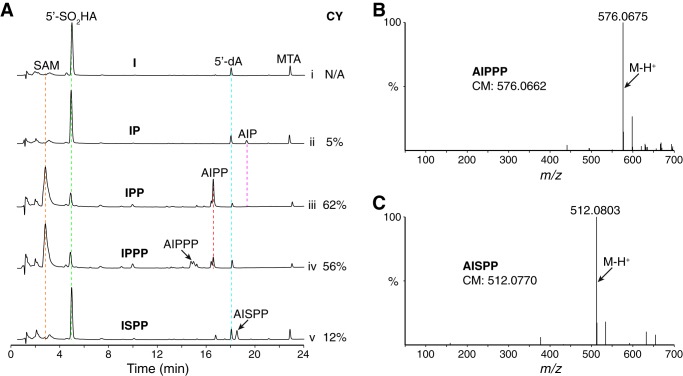
We carried out enzymatic assays with recombinant viperin using the pyrophosphate compounds involved in the mevalonate biosynthetic pathway as substrates. The chemical structures of two of these compounds, dimethylallyl pyrophosphate (DMAPP) and IPP, are shown in Fig. 2A. For the reaction catalyzed by fungal TviViperin, only SAM was observed in the absence of the reducing agent sodium dithionite (Na2S2O4), which serves as the electron donor (Fig. 2B, trace i). The addition of Na2S2O4 resulted in formation of 5′-dA•, which is subsequently quenched to 5′-adenosine sulfinic acid (5′-SO2HA) and 5′-deoxyadenosine (5′-dA) in the absence of a substrate (Fig. 2B, trace ii). A small amount of methylthioadenosine (MTA), the major decomposing product of SAM, was also produced with the radical reaction for a reason we do not understand (Fig. 2B, trace ii; compare with trace i). The addition of DMAPP produced similar results (Fig. 2B, trace iii; compare with trace ii), indicating that DMAPP is not a substrate of fungal TviViperin. However, when IPP was employed as the substrate, a major new product, which is shown as two partially separated peaks, was formed in addition to the minor abortive products of 5′-SO2HA and 5′-dA (Fig. 2B, trace iv). As described below, this new product results from the radical addition of 5′-dA• to the double bond of IPP, and it is therefore named adenylated isopentyl pyrophosphate (AIPP). The selectivity of the reaction is remarkable, as DMAPP and IPP share the same chemical connection and only differ in the location of the double bond (Fig. 2A). We also carried out similar reactions with geranyl pyrophosphate (GPP), farnesyl pyrophosphate (FPP), and geranylgeranyl pyrophosphate (GGPP), and the results indicate that these three compounds are also not the substrates of fungal TviViperin (Fig. S3).
Figure 2.
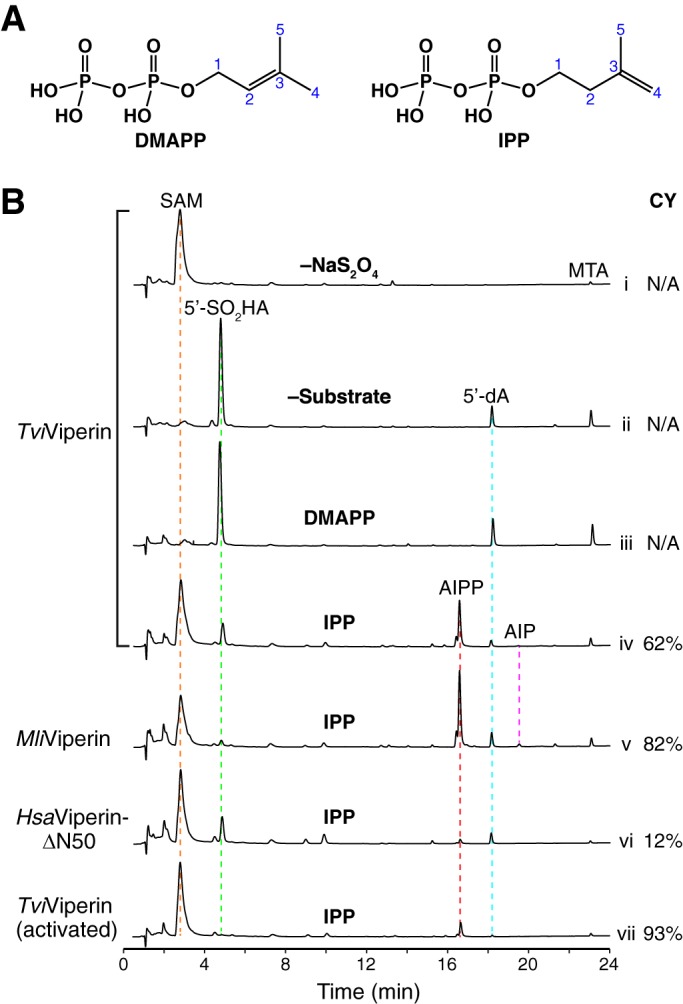
In vitro activity of viperin. A, chemical structures of DMAPP and IPP, two compounds tested as potential substrates of viperin. B, UPLC analysis of the viperin-catalyzed reactions. Viperin (50 μm) was incubated with SAM (100 μm) at room temperature under anaerobic conditions in the absence of substrate (traces i and ii), with DMAPP (100 μm) as substrate (trace iii), and with IPP (100 μm) as substrate (traces iv-vii). The reaction was initiated with the addition of 0.5 mm Na2S2O4 (except for the reaction in trace i). After 1 h of incubation, an equal volume of 8 m guanidine hydrochloride was added to the reaction sample, which denatures viperin and releases the enzyme-bound small molecules into solution. The sample was then filtrated, and the flow-through was analyzed by a UPLC system equipped with a C18 reverse phase column. UPLC analysis was monitored by a UV detector at a wavelength of 260 nm. MTA, methylthioadenosine; CY, coupling yield.
We carried out the same reactions using the recombinant archaeal MliViperin instead of fungal TviViperin, and similar results were observed (Fig. 2B, trace v; compare with trace iv). The minor differences between these two enzymes are the relative ratio of the abortive products 5′-SO2HA and 5′-dA and the extent of adenylated isopentyl phosphate (AIP), which is presumably produced via slow dephosphorylation of AIPP.
We also performed the reaction with the recombinant HsaViperin-ΔN50 (Fig. 2B, trace vi). AIPP was also produced, but the efficiency of the reaction was significantly lower when compared with the ones carried out by fungal TviViperin and archaeal MliViperin (Fig. 2B, trace vi; compare with traces iv and v). The low activity of the recombinant HsaViperin-ΔN50 observed here is also comparable with the study by Nelp et al. (45), who showed the production of less than 0.5 μm of 5′-dA from a 1-h reaction containing 2 mm SAM and 2 μm recombinant HsaViperin-ΔN44 fused to the maltose-binding protein at the N terminus.
To evaluate the likelihood of IPP as an effective substrate of fungal viperin, we introduce here a new term of the coupling yield. The coupling yield is defined here as the percentage of 5′-dA• converting to the product. Unlike most reactions carried out by radical SAM enzymes, the calculation of the coupling yield of a reaction catalyzed by viperin is straightforward, as both the coupled (AIPP and AIP) and abortive (5′-SO2HA and 5′-dA) products can be measured directly via UV integration of their corresponding peaks. Based on our calculations, the coupling yields of the reactions catalyzed by fungal TviViperin, archaeal MliViperin, and HsaViperin-ΔN50 are 62, 82, and 12%, respectively (Fig. 2B, traces iv–vi).
We regard the coupling yield of 82% (for the reaction catalyzed by archaeal MliViperin) as reasonably good. On the other hand, the number is a bit low with the reaction catalyzed by fungal TviViperin. This is mainly caused by the formation of significant amount of abortive product 5′-SO2HA. 5′-SO2HA is probably the product of radical coupling between 5′-dA• and NaSO2 radical, the decomposing product of Na2S2O4 in solution. Such a reaction should not occur in vivo because Na2S2O4 is absent in cells. To mimic what might have occurred in vivo, we carried out a modified experiment by first activating fungal TviViperin with Na2S2O4, followed by removing excess Na2S2O4 via a NAP-10 gel filtration column. Indeed, the peak corresponding to 5′-SO2HA was no longer present in our ultraperformance LC (UPLC) analysis (Fig. 2B, trace vii). Although the majority of fungal TviViperin appeared to be inactive with this approach (compare the extent of the reaction in trace vii with the one in trace iv), the coupling yield is 93% for the reaction carried out by the active fraction of fungal TviViperin (Fig. 2B, trace vii).
The coupling yield of the reaction catalyzed by the recombinant HsaViperin-ΔN50 is low. This may indicate that the reaction carried out in solution might not be ideal for HsaViperin, which may require a reaction environment similar to the surface of ER and lipid droplets, where HsaViperin resides in vivo. Alternatively, the presence of the N-terminal ER-associated domain (Fig. 1C), which is missing in our construct, might increase the efficiency of the enzymatic reaction of HsaViperin. It is also possible that HsaViperin might have a different biological substrate when compared with fungal and archaeal viperin.
AIPP is the product of the radical addition of 5′-dA• at the terminal carbon of the double bond of IPP
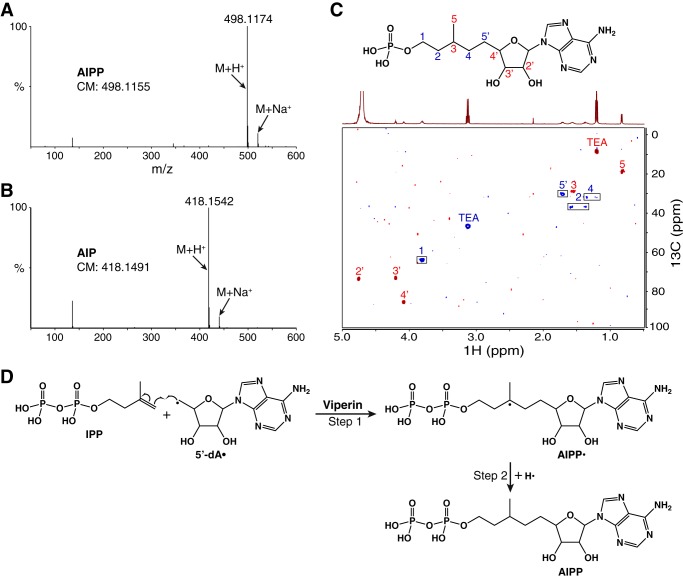
The first evidence of a novel radical reaction catalyzed by fungal viperin stemmed from the observation that the product was UV-observable with a λmax of 260 nm (Fig. 2B, trace iv), indicating that 5′-dA is part of the product. This was confirmed by high-resolution MS, which showed the presence of the protonated molecular ion at m/z = 498.1174 (Fig. 3A). The number matches, within the experimental error, the calculated protonated molecular ion of 5′-dA + IPP (498.1155), indicating a product of the radical addition of 5′-dA• to the double bond of IPP. In addition, the minor product from the archaeal MliViperin-catalyzed reaction is also consistent with the formation of AIP (Fig. 3B).
Figure 3.
Chemical structures of AIPP and AIP. A, high-resolution LC-MS analysis (in positive mode) of the major product from the reaction catalyzed by fungal TviViperin with IPP. CM, calculated mass. B, the same analysis of the minor product from the reaction catalyzed by archaeal MliViperin. C, 2D 1H-13C HSQC spectrum of AIP, with cross-peaks of CH2 colored blue and those of CH and CH3 colored red. The cross-peaks were assigned to the chemical structure of AIP depicted above the spectrum. AIP employed for the spectroscopic study was produced by the overnight reaction catalyzed by archaeal MliViperin and purified by HPLC. TEA, triethylammonium cation. D, proposed mechanism of the radical reaction catalyzed by viperin with IPP as the substrate.
Despite the observed product inhibition of the viperin-catalyzed reaction in vitro (described below), we managed to obtain sufficient quantities of AIPP and AIP for 1D and 2D NMR (Fig. 3C and Figs. S4 and S5). This was made possible with the overnight reaction carried out by archaeal MliViperin, resulting in production of both AIPP and AIP in approximately equal amount. Production of AIP was critical for the structural assignment by NMR because the proton peaks in 1D NMR of AIPP are significantly broader (Fig. S4A; compare with Fig. S4B). The radical addition of 5′-dA• to the double bond of IPP could occur at C3 or at C4 (Fig. 2A), and the lack of the singlet proton peak of CH3 in 1D NMR eliminates the possible addition at C3 because such a reaction would have produced a compound having two methyl groups connected to C3. This is consistent with the observation that DMAPP is not a substrate of fungal viperin (Fig. 2B, trace iii). Assisted by 2D 1H-1H COSY and 1H-13C HSQC NMR, the chemical structure of AIP, and by extension AIPP, has been unequivocally assigned as shown in Fig. 3C. Therefore, we propose the mechanism of the reaction catalyzed by fungal viperin with IPP as the substrate as shown in Fig. 3D; the radical addition of 5′-dA• at C4 of IPP forms a carbon–carbon bond between 5′-dA and IPP and creates a new radical at C3 of IPP (Fig. 3D, Step 1); abstraction of a nearby hydrogen by the newly created radical results in the formation of product AIPP (Fig. 3D, Step 2). A chemical search on SciFinder indicates that AIPP and its two dephosphorylated derivatives, AIP and adenylated isopentanol, are new compounds that have not been observed or synthesized before.
The reaction of step 2 shown in Fig. 3D creates a new chiral center at C3 of AIPP. If the reaction of step 2 is not strictly stereospecific, the reaction should produce two diastereomers of AIPP. This appears to be what we have observed with two overlapping peaks in our UPLC analysis (Fig. 2B, traces iv–vii). Future 2D NMR analyses of the samples from each individual peak are required to validate whether they are indeed diastereomers. Because one diastereomer of AIPP is significantly more abundant than the other, the viperin-catalyzed reaction exhibits a certain degree of stereoselectivity.
To provide insight into the mechanism of the step 2 reaction, we carried out the reaction catalyzed by fungal TviViperin in D2O, followed by LC-MS analysis of the product. Compared with the reaction carried out in H2O (Fig. 4A), most AIPP produced from the reaction carried out in D2O results in a 1-Da increase of the protonated molecular ion (Fig. 4B). This indicates that the source of the hydrogen in the step 2 reaction is either the solvent or the solvent-exchangeable side chain(s) of viperin.
Figure 4.
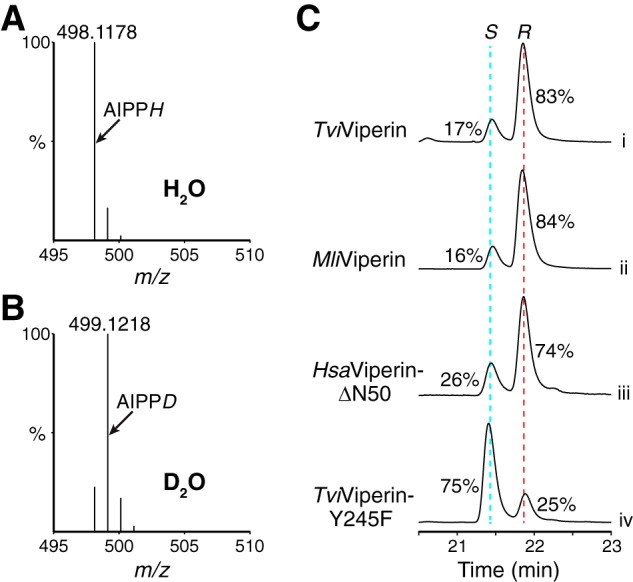
Mechanistic insight into the step 2 reaction. A and B, comparison of the protonated molecular ion of AIPP from the reactions carried out in H2O and D2O. C, UPLC analysis of the viperin-catalyzed reactions focusing on the peaks of two diastereomers of AIPP. The relative abundances of the two diastereomers of AIPP, tentatively assigned as S and R chirality at C3 of AIPP, were marked based on the integration of the corresponding peaks.
To integrate the two diastereomers of AIPP more accurately, we modified the gradient of UPLC, resulting in better separation of the two peaks representing the two diastereomers of AIPP (Fig. 4C). Thus, the ratio of the two diastereomers from the reaction catalyzed by fungal TviViperin and archaeal MliViperin is ∼1:5, and the number is 1:3 from the reaction catalyzed by HsaViperin-ΔN50. Structure-based MD simulations of viperin interacting with IPP, which we will discuss below, revealed that Tyr-302 of MmuViperin is the primary candidate to provide the hydrogen for the step 2 reaction. We therefore mutated the equivalent residue Tyr-245 to Phe-245 in fungal TviViperin. The purified recombinant TviViperin-Y245F is enzymatically active, but the activity and the efficiency of the reaction are significantly diminished when compared with the WT enzyme (data not shown). Surprisingly, TviViperin-Y245F essentially flips the ratio of the two diastereomers of AIPP produced (Fig. 4C, trace iv; compare with trace i), indicating that the hydroxyl group on the side chain of Tyr-245 is the likely source of hydrogen for the step 2 reaction.
Most AIPP stays within viperin and inhibits subsequent rounds of reaction
We noticed that, unlike in the reactions without a substrate or with DMAPP, the majority of SAM was not consumed in the reaction employing IPP (Fig. 2B, trace iv; compare with traces ii and iii). We suspected that the product AIPP might inhibit subsequent rounds of the reaction. To shed light on the slow reaction with IPP, we carried out a time course of the reaction catalyzed by fungal TviViperin using DMAPP and IPP as substrates (Fig. 5A). With the reactions employing DMAPP, production of 5′-SO2HA and 5′-dA increased continuously over time, concurrent with the reduction of SAM (Fig. 5A, green and blue curves). When IPP was employed as the substrate, AIPP was produced rapidly at the beginning and subsequently leveled off over the course of 30 min to 2 h (Fig. 5A, red curve).
Figure 5.
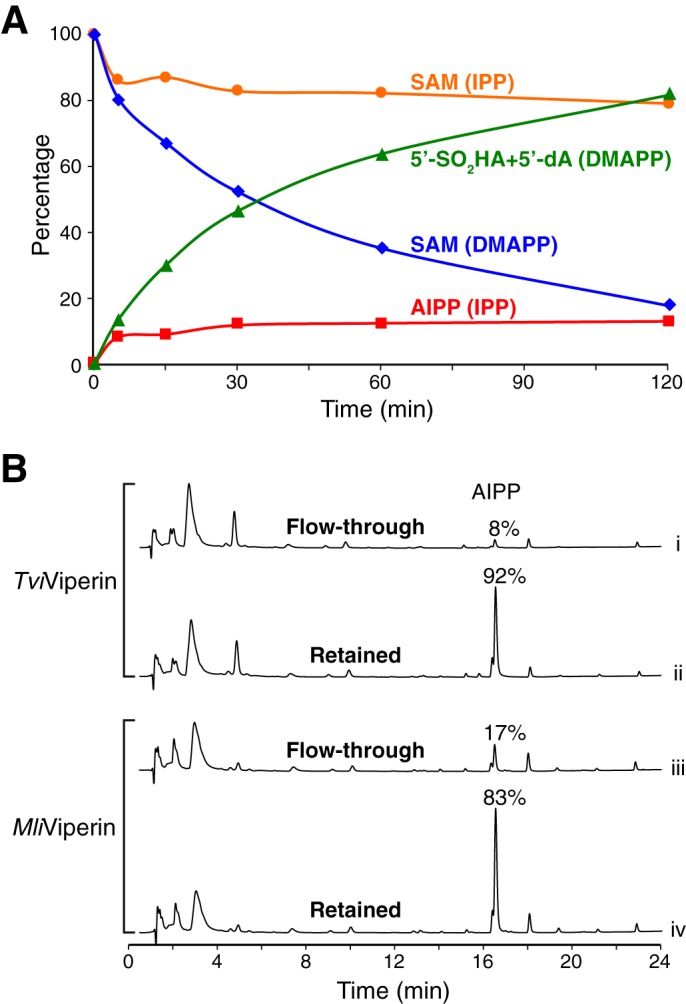
Product inhibition of the viperin-catalyzed reaction in vitro. A, time course of the TviViperin-catalyzed reaction. The concentrations of SAM and products are plotted against the reaction time for the reactions using DMAPP and IPP as substrates. Each reaction contains 50 μm TviViperin and 200 μm both SAM and the substrate. The measurement of the iron content of the purified TviViperin indicates that ∼50% protein contains [4Fe-4S]. Therefore, the molar ratio of the active enzyme and AIPP produced is ∼1:1. B, UPLC analysis of the samples from the partition experiments. Under the nondenaturing conditions, a 3-kDa centrifugal filter was used to separate small molecules free in solution (flow-through) from those bound to the enzyme (retained). An equal volume of 8 m guanidine hydrochloride was then added to both the flow-through and the retained fractions, followed by the second round of filtration. The filtrates of the second round of filtration were analyzed by UPLC.
We also performed the partition experiment to provide further insight into production inhibition. Briefly, the reaction sample was first filtered with a 3-kDa centrifugal filter under nondenaturing conditions to separate small molecules free in solution from the ones bound to the enzyme. This was followed by the standard post-treatment of the reaction sample for both the flow-through and the retained fractions (e.g. the addition of an equal volume of 8 m guanidine hydrochloride to the sample, followed by filtration). UPLC analysis of the two fractions of the TviViperin-catalyzed reaction revealed that the overwhelming majority of AIPP was found in the retained fraction, whereas other small-molecule components were approximately evenly distributed between the two fractions (Fig. 5B, traces i and ii). This indicates that, after its formation, AIPP remains bound to fungal TviViperin instead of being released into solution. Similar results were obtained with the sample of the reaction catalyzed by archaeal MliViperin, but the difference of AIPP distributed in two fractions is less pronounced (Fig. 5B, traces iii and iv). Taken together, these experiments are consistent with the conclusion that, at least for the reaction catalyzed by fungal TviViperin, the overwhelming majority of AIPP produced from the initial round of the reaction stays within the enzyme, which prevents the subsequent rounds of the reaction from occurring. Because the issue of product release is less severe with the reaction catalyzed by archaeal MliViperin (Fig. 5B, traces iii and iv), the overnight reactions carried out by archaeal MliViperin with excess IPP and SAM were used for preparing NMR samples. According to our calculation based on the results of overnight reaction, archaeal MliViperin catalyzes multiple turnovers, with 1 eq of enzyme producing approximately 3 eq of combined AIPP and AIP.
Viperin is discriminative in selecting molecules to be its substrates
To provide further insight into the substrate specificity of viperin, we carried out the reaction catalyzed by fungalTviViperin using four IPP derivatives as substrates (Fig. 6A). Isoprenol (I), which has the same isopentenyl motif as IPP but lacks the pyrophosphate group, is not a substrate (Fig. 6A, trace i). On the other hand, isopentenyl phosphate (IP), which is one phosphate group less than IPP, is a substrate, but the reaction was inefficient and has only 5% of the coupling yield (Fig. 6A, trace ii).
Figure 6.
The discriminative nature of the viperin-catalyzed reaction. A, UPLC analysis of the TviViperin-catalyzed reactions employing four IPP derivatives as substrates. The UPLC trace of the reaction with IPP as the substrate in Fig. 2B was used here for comparison. The reaction conditions were the same as shown in Fig. 2B (50 μm TviViperin, 100 μm SAM, 100 μm each substrate, 0.5 mm Na2S2O4, and 1-h incubation at room temperature). B, high-resolution LC-MS analysis (in negative mode) of a product (marked with an arrow in trace iv) from the reaction using IPPP as the substrate. C, high-resolution LC-MS analysis (in negative mode) of the product (marked with an arrow in trace v) from the reaction using ISPP as the substrate.
The reaction with isopentenyl triphosphate (IPPP), which is one phosphate group more than IPP, was more complicated, as both adenylated isopentyl triphosphate (AIPPP) and AIPP were produced in approximately equal amounts (Fig. 6, A (trace iv) and B). The combined coupling yield (AIPPP + AIPP) of the reaction is 56%, slightly lower than the one with IPP (62%). Furthermore, the stereoselectivity of the reaction is significantly worse, as the reaction produces approximately equal amounts of two diastereomers of AIPPP and a 1:2 ratio of two diastereomers of AIPP (Fig. 6A, trace iv). Therefore, IPPP is a poorer substrate of fungal viperin than IPP. The reaction with IPPP is also likely to have the issue of product release because the majority of SAM remains unconsumed (Fig. 6A, trace iv).
In addition to probing the pyrophosphate motif of IPP, we also investigated the effect of the spacing between the pyrophosphate and the double bond by employing isopentenyl thiopyrophosphate (ISPP) as the substrate. The reaction with ISPP results in the formation of adenylated isopentyl thiopyrophosphate (AISPP), the first synthetic derivative of AIPP (Fig. 6, A (trace v) and C). The coupling yield of the reaction is only 12%, which is significantly worse than that of the reaction with IPP. It is interesting to observe that fungal viperin is sensitive to such a small change in its substrates (the difference between IPP and ISPP is 0.4 Å in length). Experiments with IPP derivatives provide further support for the argument that IPP is an effective substrate of fungal viperin.
Molecular basis of IPP recognition by viperin
We crystallized fungal TviViperin under aerobic conditions and solved the structure at 2.8 Å resolution (Fig. S6 and Table S1), which does not contain a [4Fe-4S]. Because the crystal structure of MmuViperin has been described in detail (36), here we only focus on two observations based on our structural analysis. First, we interpret that the boundary between the radical SAM domain and the C-terminal extension is likely to be somewhere within the peptide segment that links β6 and α6 (named loop 6 here), which is slightly different from the one by Fenwick et al. (36). This interpretation is based on the observations that part of loop 6 is disordered in our structure, and loop 6 is one of the least conserved parts of viperin in terms of both the sequence identities and the length (Fig. S7). Second, structural similarity between fungal TviViperin and MmuViperin, which share 54% sequence identities with each other, is unevenly distributed over the entire viperin. The root mean square deviation of the overall structures is 1.3 Å, but the number for the radical SAM domain is 0.9 Å, and that for the C-terminal extension is 1.9 Å (Fig. S6, C and D). This unevenness is not caused by the different degrees of sequence identities, because the numbers are 56 and 53% for the radical SAM domain and the C-terminal extension, respectively. This may have implications in potential roles of the C-terminal extension, such as [4Fe-4S] insertion (21, 46), activation of viperin for the reaction, and even product release.
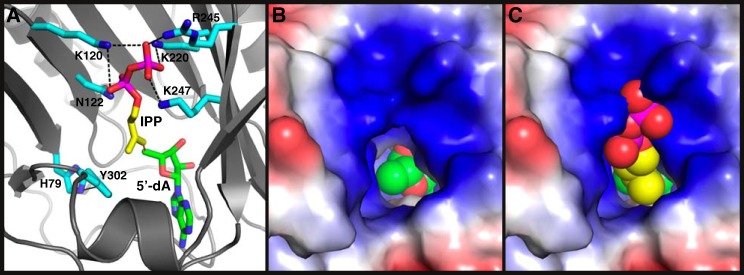
To gain insight into IPP recognition and the mechanism of the viperin-catalyzed reaction, we carried out MD simulations of viperin interacting with IPP. Because the MmuViperin structure includes 5′-dA, Met, and [4Fe-4S] (36), it was therefore chosen for the simulations. The simulations revealed that the side chains of Lys-120, Asn-122, Lys-220, Arg-245, and Lys-247 are probably responsible for the binding of the pyrophosphate motif of IPP (Fig. 7A and Movie S1). Extensive interactions of most these residues with the terminal phosphate may explain why IP is such a poor substrate of fungal viperin (Fig. 6A, trace ii). Whereas the methylene group of IPP points to 5′-dA for the potential carbon–carbon formation, the methyl group of IPP stacks on top of the phenyl ring of Tyr-302, whose position is restricted via its hydrogen bonding to the side chain of His-79 (Fig. 7A). Viperin–IPP interaction results in significant conformational changes of most residues described above (Fig. S8). Because of its closeness to C3 of IPP, Tyr-302 is the primary candidate for providing the hydrogen in the step 2 reaction. As discussed before, Tyr → Phe mutation of the corresponding residue in fungal TviViperin (Tyr-245) reduces its enzymatic activity but essentially flips the relative ratio of the two diastereomers of AIPP (Fig. 4C).
Figure 7.
Molecular recognition of IPP by viperin. A, side view of the structure of MmuViperin in complex with IPP and 5′-dA, based on one of the frames of MD simulations. The carbon atoms of the side chains of viperin, IPP, and 5′-dA are colored cyan, yellow, and green, respectively. The heteroatoms are colored individually, with nitrogen in blue, oxygen in red, and phosphate in magenta. The hydrogen atoms are omitted for clarity. Hydrogen bonds are depicted with black dashed lines. The potential radical addition of 5′-dA• to the double bond of IPP is marked with a red dashed line. All of the residues from viperin depicted here, together with three more in Movie S1, are strictly conserved in viperin (Fig. S7). B, top view of the structure of MmuViperin looking into the active site. Viperin is shown in a surface representation and colored by electrostatic potential, with the positively charged surface in blue and the negatively charged surface in red. Part of 5′-dA, which is in spheres and colored the same as in A, can be seen at the bottom. C, same view as in B except for the presence of IPP, which is also in spheres and colored the same as in A.
The structures of viperin revealed that the entrance toward the active site is narrow, and it fits the isopentenyl motif of IPP relatively well (Fig. 7 (B and C) and Fig. S9). As expected, the surrounding areas of the entrance are overwhelmingly positively charged (Fig. 7, B and C), consistent with their roles of interacting with the negatively charged pyrophosphate group. The mode of interaction between the side chains of viperin and the pyrophosphate motif (Fig. 7 (A and C) and Movie S1) indicates that one more phosphate group in the substrate might not be beneficial, consistent with the experimental data showing that IPPP is a poorer substrate than IPP (Fig. 6A, trace iv).
Discussion
In this study, we have provided four lines of experimental evidence to support the conclusion that IPP is an effective substrate of fungal viperin. First, we demonstrated that, among the pyrophosphate compounds involved in the mevalonate biosynthetic pathway, IPP is the only substrate of fungal viperin based on in vitro assays. It is striking to observe the different outcomes of the reactions with IPP and DMAPP, as these two compounds share the same chemical connection and only differ in the location of the double bond. Second, the efficiency of the reaction with IPP is high. If we eliminate the effect of the artificial reducing agent Na2S2O4 employed for the reaction in vitro, the coupling yield of the reaction catalyzed by fungal TviViperin is 93%. Third, the reactions with IPP and its derivatives revealed that IPP is the best substrate of fungal TviViperin among them, demonstrating that any chemical change in IPP diminishes its ability to be an efficient substrate of fungal viperin. It is remarkable to observe that the addition of one more phosphate to IPP in fact makes it a worse substrate of fungal TviViperin. Fourth, the structure-based MD simulations revealed the good fit of the isopentenyl motif of IPP to the active site cavity of viperin. MD simulations also provided insight into recognition of the pyrophosphate motif of IPP by the side chains of several strictly conserved residues in viperin.
From a mechanistic point of view, the reaction catalyzed by fungal viperin is unusual among the reactions catalyzed by radical SAM enzymes. In almost all cases, 5′-dA• generated by a radical SAM enzyme abstracts a hydrogen from a substrate or from an enzyme, which then performs subsequent step(s) of radical-based transformation. Fungal viperin catalyzes the radical addition of 5′-dA• to the double bond of IPP, resulting in 5′-dA being a part of the product. To the best of our knowledge, this is only the second case of radical addition of 5′-dA• to the double bond of a biological substrate (assuming IPP is a biological substrate of fungal viperin), preceded only by the radical addition catalyzed by MqnE (46). The study carried out by Wagner et al. (47) provided the first example of the radical addition of 5′-dA• to a double bond, but the substrate is nonbiological. Recently, Ji et al. (48) demonstrated the NosL-catalyzed radical addition of not only 5′-dA•, but also other radicals generated from SAM analogues, to the double bond of a NosL substrate analogue. Although acting on a single bond, the HydE-catalyzed radical attack of 5′-dA• on the sulfur atom of a thioester may also be regarded as the same class of reaction due to 5′-dA becoming part of the product (49).
Our work presented here reached different conclusions when compared with two recent studies of in vitro reconstitution of the viperin-catalyzed reactions (37, 38). First, Honarmand Ebrahimi et al. (37) reported an in vitro study of the recombinant viperin from fungal Thielavia terrestris (TteViperin), indicating the radical addition of 5′-dA• to UDP-glucose. To test whether this result can be reproduced with our system, we carried out similar reactions catalyzed by fungal TviViperin, which shares 71% sequence identity with TteViperin. The radical reaction only produced 5′-SO2HA and 5′-dA, and UDP-glucose remained intact (Fig. S10A, trace ii; compare with trace i). Furthermore, we failed to detect any protonated molecular ion in the range of m/z = 818.1–818.2, which should cover the molecular ion of the hypothetical 5′-dA-UDP-glucose covalent adduct if it were formed.
The second recent study, based on the in vitro reaction catalyzed by the truncated recombinant HsaViperin (with 45- and 60-aa N-terminal deletion), reached a different conclusion (38). Mikulecky et al. (38) concluded that GPP and FPP are the substrates of HsaViperin, mainly based on the following two observations. First, the production of 5′-dA is significantly more pronounced in the presence of GPP and FPP when compared with IPP and DMAPP. Second, the hydrogen abstracted by 5′-dA• to form the 5′-dA byproduct is from a source(s) that is not solvent-exchangeable. Regarding the 5′-dA production, we have also observed similar results with the reactions catalyzed by fungal TviViperin (Fig. S3 and Fig. 2B). In our case, however, the different level of 5′-dA production can be explained if the productions of AIPP (in the case of using IPP as the substrate) and another byproduct, 5′-SO2HA, are taken into consideration. It is possible that mammalian and fungal viperin might have different biological substrates, resulting in different observations discussed here.
While this manuscript was under revision, a paper was published describing the conversion of CTP to 3′-deoxy-3′,4′-didehydro-CTP (ddhCTP) by a mammalian viperin and the biological function of ddhCTP as a viral RdRp inhibitor (47). We therefore revisited our TviViperin-catalyzed reaction using CTP as the substrate and found that TviViperin also converts CTP to ddhCTP (Fig. S10). In our FPLC systems, CTP, CDP, ddhCTP, and ddhCDP were not separated (Fig. S10, traces iii and iv), resulting in our previously incorrect conclusion that CTP is not a substrate of TviViperin. We further carried out the reactions with recombinant MliViperin and HsaViperin and found that both of these two enzymes are also able to convert CTP to ddhCTP (data not shown). These new data, together with the data shown in Fig. 2B, indicate that, at least in vitro, mammalian, fungal, and archaeal viperin carry out the same two reactions discovered to date. Further studies are required, however, to evaluate whether IPP is a biological substrate of viperin and whether AIPP possesses antiviral activity.
Experimental procedures
General materials and methods
Reagents used for molecular biology experiments were purchased from New England BioLabs (Ipswich, MA) and Thermo Fisher Scientific (Waltham, MA). IPP, DMAPP, GPP, FPP, GGPP, and ISPP were purchased from Echelon Bioscience Inc. I, IP, IPPP, UDP-glucose, UTP, and CTP were purchased from Sigma-Aldrich. SAM was also purchased from Sigma-Aldrich but was further purified by HPLC. Other chemicals were purchased from Sigma-Aldrich or Thermo Fisher Scientific. Plasmid inserts were sequenced at the University of Illinois core sequencing facility. The sequence similarity network of viperin was generated according to the published protocol (43).
Cloning, expression, and purification of viperin
Viperin genes from T. virens, M. liminatans, and humans (codon-optimized for Escherichia coli) were ordered from IDT. For structural studies, primers were designed to delete the first 10 aa of TviViperin from T. virens, and the gene was amplified from the codon-optimized gene by PCR. The synthetic genes were enzymatically digested and inserted into the petDuet-1 vector (Novagen).
E. coli BL21 (DE3) transformed with an expression plasmid encoding viperin were grown in LB at 37 °C until the A600 reached 0.4. The cells were then cooled to 18 °C, 1 mm supplementary iron was added, and the expression was induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside. After overnight growth at 18 °C, cells were collected by centrifugation and stored in a −80 °C freezer. Cells containing His-tagged viperin were first thawed inside an anaerobic chamber and resuspended in Ni Binding Buffer (20 mm Tris-HCl, pH 8.0, 100 mm NaCl). Cells were briefly removed from the chamber and aerobically lysed using a French press. Cell lysate was returned to the chamber, and viperin was manually purified using a 1-ml HisTrap column (GE Healthcare). The 1-ml fraction containing the highest concentration of protein was buffer-exchanged with Reaction Buffer (50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 5% glycerol) using a NAP-10 column (GE Healthcare), and the purified viperin was immediately used for the reactions in the anaerobic chamber. Using this protocol, ∼3, 4, and 2 mg of TviViperin, MliViperin, and HsaViperin were obtained, respectively, from 1 liter of cell culture.
To crystallize the N-terminal 10-amino acid deletion of TviViperin, His-tagged protein was purified to homogeneity using an AKTA FPLC under aerobic conditions. Protein was first purified using a HisTrap column (GE Healthcare). Fractions were pooled, diluted with DEAE Binding Buffer (20 mm Tris-HCl pH 8.0, 10 mm NaCl, 2% glycerol), and purified with a MonoQ column (GE Healthcare) (DEAE Binding Buffer and DEAE Elution Buffer (20 mm Tris-HCl, pH 8.0, 1 m NaCl, 2% glycerol)). Last, the protein was purified using a Superdex 75 size-exclusion column (GE Healthcare) (buffer: 10 mm HEPES, pH 7.0, 200 mm NaCl, 2% glycerol).
Iron content of viperin
The ferene method was used to determine the amount of iron in viperin (48). First, standard solutions (200 μl each) of ammonium iron(II) sulfate (Mohr's salt), concentration range 0–200 μm, were prepared in 0.5% (v/v) HCl (49). Viperin solutions of known concentration were then prepared analogous to the standards. 0.5% (v/v) HCl solution was used as blank. All of the solutions were incubated at 100 °C for 10 min. 500 μl of ammonium acetate (15% w/v), 100 μl of ascorbic acid (4% w/v), and 100 μl of SDS (2.5%, w/v) were successively added to each sample. Finally, to chelate the iron, a 100-μl solution of ferene (5,5′(3-(2-pyridyl)-1,2,4-triazine-5,6-diyl)-bis-2-furansulfonate) (1.5%, w/v) was added. Samples were centrifuged at 9,000 rpm for 5 min, and their absorbance was then measured at 593 nm. The amount of iron bound to viperin was calculated using the standard curve based on ammonium iron(II) sulfate standard solutions. Based on our measurements, we calculated that purified TviViperin, MliViperin, and HsaViperin contain 1.9, 2.0, and 1.7 eq of iron. Assuming that iron is from [4Fe-4S], we conclude that ∼50% of TviViperin and MliViperin is active.
In vitro viperin activity
All of the in vitro reactions were carried out in an anaerobic chamber. Vacuum-dried SAM and substrates, as well as Na2S2O4 powder, were resuspended in Reaction Buffer to prepare their stock solutions. 50 μm protein was first incubated with 100 μm substrate and 100 μm SAM in 1× Reaction Buffer at room temperature for 10 min. 0.5 mm Na2S2O4 was then added to initiate the radical reaction. Following a 1-h incubation at room temperature, the reaction was stopped by adding an equal volume of 8 m guanidine hydrochloride. The denatured sample was filtered with a 3-kDa centrifuge filter, and the flow-through fraction was analyzed by UPLC and LC-MS.
For the time course of the TviViperin-catalyzed reaction, the reaction conditions were the same as described above, except the concentrations of both SAM and substrates were doubled. The reactions were stopped at various time points by adding 8 m guanidine hydrochloride, followed by UPLC analysis and quantitation of peaks corresponding to SAM, 5′-SO2HA, 5′-dA, and AIPP.
For the partition experiments, the TviViperin- and MliViperin-catalyzed reactions were first filtered with a 3-kDa centrifuge filter. An equal volume of 8 m guanidine hydrochloride was then added to both the retained and flow-through fractions. The resulting solutions were filtered for the second time, each with a 3-kDa centrifuge filter. The flow-through fractions from the second filtration were analyzed by UPLC.
UPLC and LC-MS analyses
UPLC analysis was carried out on a Waters Acquity H-class UPLC system with a reversed phase Luna Omega C18 column (150 × 1.0 mm; Phenomenex). The column was equilibrated with 100% solvent A (10 mm sodium phosphate, pH 7.0), and the following gradient was applied with solvent B (40% CH3CN in H2O): 0 min, 0% B; 1 min, 0% B; 5 min, 2% B; 15 min, 20% B; 20 min, 40% B; 25 min, 70% B; 26 min, 100% B; 28 min, 100% B; 29 min, 0% B; 30 min, 0% B.
The LC component of the LC-MS analysis is the same as the one described above except 1 mm (Et)3NHCO3 (Sigma) was used as buffer A. The UPLC system is connected to a Waters Synapt G2-Si high-definition mass spectrometer (Thermo Fisher Scientific), and the reaction samples were analyzed both in positive and negative modes, depending on the requirement of the individual sample.
Preparation and purification of AIPP and AIP for NMR
For the preparative scale of AIPP and AIP, we employed MliViperin for the synthesis. Although the results of enzymatic reaction with TviViperin and MliViperin were comparable, MliViperin is significantly more expressed in E. coli. In addition, MliViperin slowly dephosphorylates AIPP to produce AIP, allowing us to purify both AIPP and AIP simultaneously from a prolonged reaction. Thus, cells harvested from 3 liters of growth expressing His-tagged MliViperin were thawed, lysed, and purified analogous to the procedures described above. After purification, a large-scale enzymatic reaction (typically with a reaction volume of 0.6–0.9 ml, depending on the yield of the purified protein) was carried out containing 100 μm protein, 600 μm SAM, 600 μm IPP, and 3 mm NaS2O4. Following overnight incubation at room temperature, the reaction was stopped by adding an equal volume of 8 m guanidine hydrochloride. The denatured sample was filtered with a 3-kDa centrifuge filter, and the flow-through fraction was purified by HPLC as described below. It was necessary to repeat the purification eight times to obtain a sufficient quantity of AIPP and AIP for NMR.
Purification of AIPP and AIP was carried out in two steps. In the first step, the reaction sample was cleaned up by HPLC with the Waters 1525 HPLC system on a C18 XTerra column (150 × 4.6 mm; Waters) with a flow rate of 2 ml/min. Solvent A was 10 mm triethylammonium bicarbonate (pH 8), and solvent B was 40% acetonitrile in water. The eluent from a single peak containing a mixture of AIPP, AIP, and 5′-dA was collected. The collected sample was dried and resuspended in H2O, and the suspended sample was subjected to the second step of HPLC purification, which separated AIPP, AIP, and 5′-dA from each other. The second step of HPLC purification employed a C18(2) Luna column (250 × 2 mm, Phenomenex) and 1 mm triethylammonium bicarbonate (pH 8.0) as Buffer A. This purification process was repeated many times to obtain a sufficient quantity of AIPP and AIP for NMR. The final concentrations of NMR samples of AIPP and AIP are 0.16 and 0.15 mm, respectively.
1D and 2D NMR
The NMR experiments were performed on the Bruker Avance III 500-MHz spectrometer with a 5-mm cryoprobe. The 1D 1H (128 scans), 2D 1H-1H COSY (∼3 h), and 2D 1H-13C HSQC (∼7 h) were collected at 25 °C. The 1H and 13C chemical shifts were referenced to the external TMS peaks at 0 ppm, respectively. 1H NMR of AIPP and AIP revealed an essentially identical pattern of proton peaks (Fig. S4, A and B). Because 1) the proton peaks from AIP are significantly sharper than the ones from AIPP and 2) the AIP sample has half as much triethylammonium cation as the one in AIPP sample, AIP sample was employed for subsequent 2D NMR experiments (Fig. 3C and Fig. S5). 1H NMR (500 MHz, D2O) of AIP: 8.24 (s, 1H), 8.19 (s, 1H), 5.98 (d, 3JHH = 5.6 Hz, 1H), 4.77 (1H, under water peak), 4.20 (t, 3JHH = 4.9 Hz, 1H), 4.07 (m, 1H), 3.80 (m, 2H), 1.71 (m, 2H), 1.57 (m, 2H), 1.37 (m, 2H), 1.21 (1H, under CH3 of TEA), 0.83 (d, 3JHH = 6.5 Hz, 3H).
Crystallization, data collection, and structural determination
Crystals of the His-tagged TviViperin with the N-terminal 10-aa deletion were grown by the hanging-drop vapor diffusion method at 4 °C. The purified protein (∼7 mg/ml) was mixed with reservoir solution containing 1.6–1.8 m ammonium sulfate and 100 mm sodium citrate (pH 5.5–5.7). Crystals appeared after 3–7 days. Crystals were soaked briefly in a cryoprotection solution containing all of the components of the reservoir solution supplemented with 35% glycerol. The cryoprotected crystals were mounted on a nylon loop and flash-frozen in liquid nitrogen. Data were collected at the Advanced Photon Source (APS) and processed using the HKL2000 program (50). Phase was determined based on selenomethionine single-wavelength anomalous diffraction data using the Phenix program (51). A partial model was automatically built using Phenix. The remaining model was manually built using the Coot program (52). Refinement was done using Phenix.
MD simulations
The SOLVATE and AUTOIONIZE plug-ins in VMD (53) were used respectively to solvate the system in a water box of dimensions 120 × 120 × 120 Å and to ionize it to 150 mm concentration of NaCl. IPP was first manually placed in the substrate-binding pocket of viperin. The extra bonds feature of NAMD (54) was used to bring C4 of IPP closer to C5′ of 5′-dA in five steps, from a distance of 4.0 Å to 2.5 Å, with 0.5 Å at a time. For each step, the system was first minimized for 5000 fs and then equilibrated for 1 ns. During all of these initial simulations, the protein backbone atoms were kept fixed. After the desired distance between C4 of IPP and C5′ of 5′-dA was satisfied, the protein backbone atoms were allowed to move under harmonic constraints for 2 ns. Another 2-ns equilibration simulation was followed with only Cα of the protein subject to harmonic constraints. Finally, a production run of 10 ns was performed with harmonic constraints on Cα (see Movie S1 for the result from the production run).
The simulations were performed using NAMD 2.12 and under periodic boundary conditions. For van der Waals and short-range electrostatic interactions, a smooth cutoff of 12 Å was chosen with a switching function starting at 10 Å. The long-range electrostatic interactions were calculated by the PME method with a grid point density of 1 per Å. The constant temperature of 300 K for the NVT ensemble was provided by coupling the system to a heat bath and through Langevin dynamics for all of the nonhydrogen atoms with a Langevin coupling coefficient of 5 ps−1. CHARMM36 force field (55) was used for the protein and ions, and CHARMM general force field (56) was used for IPP. The TIP3P model (57) was utilized for the water molecules.
Author contributions
A. C., K. S., and R. H. H. designed the research; A. C., K. S., R. S., H. L., S. F., and R. H. H. performed the research; A. C., K. S., R. S., H. L., E. T., and R. H. H. analyzed the data; R. H. H. wrote the manuscript.
Supplementary Material
Acknowledgments
We thank F. Sun for assistance with LC-MS, J. Gerlt and K. Whalen for the initial sequence similarity network of the radical SAM superfamily, Z. Wawrzak and D. Smith for help with data collection, and L. Zhu for performing 1D and 2D NMR. We also acknowledge computing resources provided by Blue Waters at the National Center for Supercomputing Applications and the Extreme Science and Engineering Discovery Environment (Grant TG-MCA06N060 to E. T.).
This work was supported in part by National Institutes of Health Grants GM107533 (to R. H. H.) and P41 GM104601 (to E. T.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S10, Table S1, and Movie S1.
The atomic coordinates and structure factors (code 6B4C) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- IFN
- interferon
- SAM
- S-adenosyl-l-methionine
- FPPS
- farnesyl diphosphate synthase
- ER
- endoplasmic reticulum
- IPP
- isopentenyl pyrophosphate
- AIPP
- adenylated isopentyl pyrophosphate
- SSN
- sequence similarity network
- aa
- amino acid(s)
- DMAPP
- dimethylallyl pyrophosphate
- 5′-SO2HA
- 5′-adenosine sulfinic acid
- 5′-dA
- 5′-deoxyadenosine
- GPP
- geranyl pyrophosphate
- FPP
- farnesyl pyrophosphate
- GGPP
- geranylgeranyl pyrophosphate
- AIP
- adenylated isopentyl phosphate
- 1D and 2D
- one- and two-dimensional, respectively
- IPPP
- isopentenyl triphosphate
- ISPP
- isopentenyl thiopyrophosphate
- MD
- molecular dynamics
- ddhCTP and ddhCDP
- 3′-deoxy-3′,4′-didehydro-CTP and -CDP, respectively
- I
- isoprenol
- IP
- isopentenyl phosphate
- UPLC
- ultraperformance LC.
References
- 1. Kim B.-H., Chee J. D., Bradfield C. J., Park E.-S., Kumar P., and MacMicking J. D. (2016) Interferon-induced guanylate-binding proteins in inflammasome activation and host defense. Nat. Immunol. 17, 481–489 10.1038/ni.3440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schneider W. M., Chevillotte M. D., and Rice C. M. (2014) Interferon-stimulated genes: a complex web of host defenses. Annu. Rev. Immunol. 32, 513–545 10.1146/annurev-immunol-032713-120231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frey P. A. (2001) Radical mechanisms of enzymatic catalysis. Annu. Rev. Biochem. 70, 121–148 10.1146/annurev.biochem.70.1.121 [DOI] [PubMed] [Google Scholar]
- 4. Sofia H. J., Chen G., Hetzler B. G., Reyes-Spindola J. F., and Miller N. E. (2001) Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 29, 1097–1106 10.1093/nar/29.5.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Broderick J. B., Duffus B. R., Duschene K. S., and Shepard E. M. (2014) Radical S-adenosylmethionine enzymes. Chem. Rev. 114, 4229–4317 10.1021/cr4004709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu H., Cong J. P., and Shenk T. (1997) Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc. Natl. Acad. Sci. U.S.A. 94, 13985–13990 10.1073/pnas.94.25.13985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chin K. C., and Cresswell P. (2001) Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus. Proc. Natl. Acad. Sci. U.S.A. 98, 15125–15130 10.1073/pnas.011593298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duschene K. S., and Broderick J. B. (2010) The antiviral protein viperin is a radical SAM enzyme. FEBS Lett. 584, 1263–1267 10.1016/j.febslet.2010.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Landgraf B. J., McCarthy E. L., and Booker S. J. (2016) Radical S-adenosylmethionine enzymes in human health and disease. Annu. Rev. Biochem. 85, 485–514 10.1146/annurev-biochem-060713-035504 [DOI] [PubMed] [Google Scholar]
- 10. Seo J.-Y., Yaneva R., Hinson E. R., and Cresswell P. (2011) Human cytomegalovirus directly induces the antiviral protein viperin to enhance infectivity. Science 332, 1093–1097 10.1126/science.1202007 [DOI] [PubMed] [Google Scholar]
- 11. Seo J.-Y., and Cresswell P. (2013) Viperin regulates cellular lipid metabolism during human cytomegalovirus infection. PLoS Pathog. 9, e1003497 10.1371/journal.ppat.1003497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lei M., Liu H., Liu S., Zhang Y., and Zhang S. (2015) Identification and functional characterization of viperin of amphioxus Branchiostoma japonicum: implications for ancient origin of viperin-mediated antiviral response. Dev. Comp. Immunol. 53, 293–302 10.1016/j.dci.2015.07.008 [DOI] [PubMed] [Google Scholar]
- 13. Helbig K. J., Lau D. T.-Y., Semendric L., Harley H. A. J., and Beard M. R. (2005) Analysis of ISG expression in chronic hepatitis C identifies viperin as a potential antiviral effector. Hepatology 42, 702–710 10.1002/hep.20844 [DOI] [PubMed] [Google Scholar]
- 14. Jiang D., Guo H., Xu C., Chang J., Gu B., Wang L., Block T. M., and Guo J.-T. (2008) Identification of three interferon-inducible cellular enzymes that inhibit the replication of hepatitis C virus. J. Virol. 82, 1665–1678 10.1128/JVI.02113-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helbig K. J., Eyre N. S., Yip E., Narayana S., Li K., Fiches G., McCartney E. M., Jangra R. K., Lemon S. M., and Beard M. R. (2011) The antiviral protein viperin inhibits hepatitis C virus replication via interaction with nonstructural protein 5A. Hepatology 54, 1506–1517 10.1002/hep.24542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van der Hoek K. H., Eyre N. S., Shue B., Khantisitthiporn O., Glab-Ampi K., Carr J. M., Gartner M. J., Jolly L. A., Thomas P. Q., Adikusuma F., Jankovic-Karasoulos T., Roberts C. T., Helbig K. J., and Beard M. R. (2017) Viperin is an important host restriction factor in control of Zika virus infection. Sci Rep. 7, 4475 10.1038/s41598-017-04138-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang D., Weidner J. M., Qing M., Pan X.-B., Guo H., Xu C., Zhang X., Birk A., Chang J., Shi P.-Y., Block T. M., and Guo J.-T. (2010) Identification of five interferon-induced cellular proteins that inhibit west nile virus and dengue virus infections. J. Virol. 84, 8332–8341 10.1128/JVI.02199-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szretter K. J., Brien J. D., Thackray L. B., Virgin H. W., Cresswell P., and Diamond M. S. (2011) The interferon-inducible gene viperin restricts West Nile virus pathogenesis. J. Virol. 85, 11557–11566 10.1128/JVI.05519-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cho H., Proll S. C., Szretter K. J., Katze M. G., Gale M. Jr., and Diamond M. S. (2013) Differential innate immune response programs in neuronal subtypes determine susceptibility to infection in the brain by positive-stranded RNA viruses. Nat. Med. 19, 458–464 10.1038/nm.3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan Y.-L., Chang T.-H., Liao C.-L., and Lin Y.-L. (2008) The cellular antiviral protein viperin is attenuated by proteasome-mediated protein degradation in Japanese encephalitis virus-infected cells. J. Virol. 82, 10455–10464 10.1128/JVI.00438-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Upadhyay A. S., Vonderstein K., Pichlmair A., Stehling O., Bennett K. L., Dobler G., Guo J.-T., Superti-Furga G., Lill R., Överby A. K., and Weber F. (2014) Viperin is an iron-sulfur protein that inhibits genome synthesis of tick-borne encephalitis virus via radical SAM domain activity. Cell. Microbiol. 16, 834–848 10.1111/cmi.12241 [DOI] [PubMed] [Google Scholar]
- 22. Teng T.-S., Foo S.-S., Simamarta D., Lum F.-M., Teo T.-H., Lulla A., Yeo N. K. W., Koh E. G. L., Chow A., Leo Y.-S., Merits A., Chin K. C., and Ng L. F. P. (2012) Viperin restricts chikungunya virus replication and pathology. J. Clin. Invest. 122, 4447–4460 10.1172/JCI63120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y., Burke C. W., Ryman K. D., and Klimstra W. B. (2007) Identification and characterization of interferon-induced proteins that inhibit alphavirus replication. J. Virol. 81, 11246–11255 10.1128/JVI.01282-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang X., Hinson E. R., and Cresswell P. (2007) The interferon-inducible protein viperin inhibits influenza virus release by perturbing lipid rafts. Cell Host Microbe 2, 96–105 10.1016/j.chom.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 25. Severa M., Coccia E. M., and Fitzgerald K. A. (2006) Toll-like receptor-dependent and -independent viperin gene expression and counter-regulation by PRDI-binding factor-1/BLIMP1. J. Biol. Chem. 281, 26188–26195 10.1074/jbc.M604516200 [DOI] [PubMed] [Google Scholar]
- 26. Rivieccio M. A., Suh H.-S., Zhao Y., Zhao M.-L., Chin K. C., Lee S. C., and Brosnan C. F. (2006) TLR3 ligation activates an antiviral response in human fetal astrocytes: a role for viperin/cig5. J. Immunol. 177, 4735–4741 10.4049/jimmunol.177.7.4735 [DOI] [PubMed] [Google Scholar]
- 27. Stirnweiss A., Ksienzyk A., Klages K., Rand U., Grashoff M., Hauser H., and Kröger A. (2010) IFN regulatory factor-1 bypasses IFN-mediated antiviral effects through viperin gene induction. J. Immunol. 184, 5179–5185 10.4049/jimmunol.0902264 [DOI] [PubMed] [Google Scholar]
- 28. Carlton-Smith C., and Elliott R. M. (2012) Viperin, MTAP44, and protein kinase R contribute to the interferon-induced inhibition of Bunyamwera Orthobunyavirus replication. J. Virol. 86, 11548–11557 10.1128/JVI.01773-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGillivary G., Jordan Z. B., Peeples M. E., and Bakaletz L. O. (2013) Replication of respiratory syncytial virus is inhibited by the host defense molecule viperin. J. Innate Immun. 5, 60–71 10.1159/000342473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rabbani M. A. G., Ribaudo M., Guo J.-T., and Barik S. (2016) Identification of interferon-stimulated gene proteins that inhibit human parainfluenza virus type 3. J. Virol. 90, 11145–11156 10.1128/JVI.01551-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang H.-B., Lu Z.-L., Wei X.-K., Zhong T.-Z., Zhong Y.-Z., Ouyang L.-X., Luo Y., Xing X.-W., Liao F., Peng K.-K., Deng C.-Q., Minamoto N., and Luo T. R. (2016) Viperin inhibits rabies virus replication via reduced cholesterol and sphingomyelin and is regulated upstream by TLR4. Sci Rep. 6, 30529 10.1038/srep30529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hinson E. R., Joshi N. S., Chen J. H., Rahner C., Jung Y. W., Wang X., Kaech S. M., and Cresswell P. (2010) Viperin is highly induced in neutrophils and macrophages during acute and chronic lymphocytic choriomeningitis virus infection. J. Immunol. 184, 5723–5731 10.4049/jimmunol.0903752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lim E. S., Wu L. I., Malik H. S., and Emerman M. (2012) The function and evolution of the restriction factor Viperin in primates was not driven by lentiviruses. Retrovirology 9, 55 10.1186/1742-4690-9-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nasr N., Maddocks S., Turville S. G., Harman A. N., Woolger N., Helbig K. J., Wilkinson J., Bye C. R., Wright T. K., Rambukwelle D., Donaghy H., Beard M. R., and Cunningham A. L. (2012) HIV-1 infection of human macrophages directly induces viperin which inhibits viral production. Blood 120, 778–788 10.1182/blood-2012-01-407395 [DOI] [PubMed] [Google Scholar]
- 35. Makins C., Ghosh S., Román-Meléndez G. D., Malec P. A., Kennedy R. T., and Marsh E. N. G. (2016) Does viperin function as a radical S-adenosyl-l-methionine-dependent enzyme in regulating farnesylpyrophosphate synthase expression and activity? J. Biol. Chem. 291, 26806–26815 10.1074/jbc.M116.751040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fenwick M. K., Li Y., Cresswell P., Modis Y., and Ealick S. E. (2017) Structural studies of viperin, an antiviral radical SAM enzyme. Proc. Natl. Acad. Sci. U.S.A. 114, 6806–6811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Honarmand Ebrahimi K., Carr S. B., McCullagh J., Wickens J., Rees N. H., Cantley J., and Armstrong F. A. (2017) The radical-SAM enzyme viperin catalyzes reductive addition of a 5′-deoxyadenosyl radical to UDP-glucose in vitro. FEBS Lett. 591, 2394–2405 10.1002/1873-3468.12769 [DOI] [PubMed] [Google Scholar]
- 38. Mikulecky P., Andreeva E., Amara P., Weissenhorn W., Nicolet Y., and Macheboeuf P. (2018) Human viperin catalyzes the modification of GPP and FPP potentially affecting cholesterol synthesis. FEBS Lett. 592, 199–208 10.1002/1873-3468.12941 [DOI] [PubMed] [Google Scholar]
- 39. Hinson E. R., and Cresswell P. (2009) The N-terminal amphipathic α-helix of viperin mediates localization to the cytosolic face of the endoplasmic reticulum and inhibits protein secretion. J. Biol. Chem. 284, 4705–4712 10.1074/jbc.M807261200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hinson E. R., and Cresswell P. (2009) The antiviral protein, viperin, localizes to lipid droplets via its N-terminal amphipathic α-helix. Proc. Natl. Acad. Sci. U.S.A. 106, 20452–20457 10.1073/pnas.0911679106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hänzelmann P., and Schindelin H. (2006) Binding of 5′-GTP to the C-terminal FeS cluster of the radical S-adenosylmethionine enzyme MoaA provides insights into its mechanism. Proc. Natl. Acad. Sci. U.S.A. 103, 6829–6834 10.1073/pnas.0510711103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hover B. M., Tonthat N. K., Schumacher M. A., and Yokoyama K. (2015) Mechanism of pyranopterin ring formation in molybdenum cofactor biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 112, 6347–6352 10.1073/pnas.1500697112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gerlt J. A., Bouvier J. T., Davidson D. B., Imker H. J., Sadkhin B., Slater D. R., and Whalen K. L. (2015) Enzyme function initiative-enzyme similarity tool (EFI-EST): a web tool for generating protein sequence similarity networks. Biochim. Biophys. Acta 1854, 1019–1037 10.1016/j.bbapap.2015.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shaveta G., Shi J., Chow V. T. K., and Song J. (2010) Structural characterization reveals that viperin is a radical S-adenosyl-l-methionine (SAM) enzyme. Biochem. Biophys. Res. Commun. 391, 1390–1395 10.1016/j.bbrc.2009.12.070 [DOI] [PubMed] [Google Scholar]
- 45. Nelp M. T., Young A. P., Stepanski B. M., and Bandarian V. (2017) Human viperin causes radical SAM-dependent elongation of Escherichia coli, hinting at its physiological role. Biochemistry 56, 3874–3876 10.1021/acs.biochem.7b00608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Upadhyay A. S., Stehling O., Panayiotou C., Rösser R., Lill R., and Överby A. K. (2017) Cellular requirements for iron-sulfur cluster insertion into the antiviral radical SAM protein viperin. J. Biol. Chem. 292, 13879–13889 10.1074/jbc.M117.780122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gizzi A. S., Grove T. L., Arnold J. J., Jose J., Jangra R. K., Garforth S. J., Du Q., Cahill S. M., Dulyaninova N. G., Love J. D., Chandran K., Bresnick A. R., Cameron C. E., and Almo S. C. (2018) A naturally occurring antiviral ribonucleotide encoded by the human genome. Nature. 558, 610–614 10.1038/s41586-018-0238-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hennessy D. J., Reid G. R., Smith F. E., and Thompson S. L. (1984) Ferene: a new spectrophotometric reagent for iron. Can. J. Chem. 62, 721–724 10.1139/v84-121 [DOI] [Google Scholar]
- 49. Honarmand Ebrahimi K., Bill E., Hagedoorn P.-L., and Hagen W. R. (2012) The catalytic center of ferritin regulates iron storage via Fe(II)-Fe(III) displacement. Nat. Chem. Biol. 8, 941–948 10.1038/nchembio.1071 [DOI] [PubMed] [Google Scholar]
- 50. Otwinowski Z., and Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 10.1016/S0076-6879(97)76066-X [DOI] [PubMed] [Google Scholar]
- 51. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L.-W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Emsley P., Lohkamp B., Scott W. G., and Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 10.1107/S0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Humphrey W., Dalke A., and Schulten K. (1996) VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38, 27–28 10.1016/0263-7855(96)00018-5 [DOI] [PubMed] [Google Scholar]
- 54. Phillips J. C., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., Chipot C., Skeel R. D., Kalé L., and Schulten K. (2005) Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 10.1002/jcc.20289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Best R. B., Zhu X., Shim J., Lopes P. E. M., Mittal J., Feig M., and Mackerell A. D. Jr. (2012) Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ(1) and χ(2) dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 10.1021/ct300400x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vanommeslaeghe K., Hatcher E., Acharya C., Kundu S., Zhong S., Shim J., Darian E., Guvench O., Lopes P., Vorobyov I., and Mackerell A. D. (2010) CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 31, 671–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jorgensen W. L., Chandrasekhar J., Madura J. D., Impey R. W., and Klein M. L. (1983) Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 10.1063/1.445869 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.