Abstract
The α7 nicotinic receptor subunit and its partially duplicated human-specific dupα7 isoform are coexpressed in neuronal and non-neuronal cells. In these cells, α7 subunits form homopentameric α7 nicotinic acetylcholine receptors (α7-nAChRs) implicated in numerous pathologies. In immune cells, α7-nAChRs are essential for vagal control of inflammatory response in sepsis. Recent studies show that the dupα7 subunit is a dominant-negative regulator of α7-nAChR activity in Xenopus oocytes. However, its biological significance in mammalian cells, particularly immune cells, remains unexplored, as the duplicated form is indistinguishable from the original subunit in standard tests. Here, using immunocytochemistry, confocal microscopy, coimmunoprecipitation, FRET, flow cytometry, and ELISA, we addressed this challenge in GH4C1 rat pituitary cells and RAW264.7 murine macrophages transfected with epitope- and fluorescent protein-tagged α7 or dupα7. We used quantitative RT-PCR of dupα7 gene expression levels in peripheral blood mononuclear cells (PBMCs) from patients with sepsis to analyze its relationship with PBMC α7 mRNA levels and with serum concentrations of inflammatory markers. We found that a physical interaction between dupα7 and α7 subunits in both cell lines generates heteromeric nAChRs that remain mainly trapped in the endoplasmic reticulum. The dupα7 sequestration of α7 subunits reduced membrane expression of functional α7-nAChRs, attenuating their anti-inflammatory capacity in lipopolysaccharide-stimulated macrophages. Moreover, the PBMC's dupα7 levels correlated inversely with their α7 levels and directly with the magnitude of the patients' inflammatory state. These results indicate that dupα7 probably reduces human vagal anti-inflammatory responses and suggest its involvement in other α7-nAChR–mediated pathophysiological processes.
Keywords: nicotinic acetylcholine receptors (nAChR), inflammation, sepsis, macrophage, protein assembly, α-nicotinic subunit, dupα7 nicotinic subunit, human sepsis, RAW264.7 macrophages, GH4C1 cells
Introduction
The human α7 nicotinic acetylcholine receptor (α7-nAChR)7 is a ligand-gated cation channel with a homomeric structure composed of five identical α7 subunits (1, 2). Each α7 subunit has a long N-terminal extracellular region that contains the ligand-binding domain, four transmembrane segments (M1–M4) with the M2 segment lining the ion-conducting pore, two cytoplasmic loops between M1–M2 and M3–M4, and a C-terminal extracellular region (3). The expression and function of α7-nAChR were initially circumscribed to the nervous system. Consequently, this receptor has been associated with physiological processes in the CNS that affect neuronal plasticity, survival, learning, and memory, whereas impaired expression or function of α7-nAChRs has been implicated in many disorders, including schizophrenia, bipolar disorder, Alzheimer's disease, or epilepsy (4, 5).
However, recent experimental evidence has revealed that α7-nAChRs are also distributed in non-neuronal cells, including a wide variety of immune cell types (see Ref. 6 and references therein). With respect to these latter cells, there is a generalized consensus that α7-nAChRs in spleen-resident macrophages are a key element in regulating the host's inflammatory response to infection or injury by the vagus nerve. This anti-inflammatory protective mechanism, known as the “cholinergic anti-inflammatory pathway” (CAP), is anatomically located in the efferent arm of a neuronal circuit of vagal fibers whose activation counteracts excessive inflammation via a reflex mechanism (7–11). It is noteworthy that CAP stimulation and subsequent ACh-mediated activation of α7-nAChRs in splenic macrophages not only attenuate systemic inflammation but also improve survival in animal models of sepsis (12–14). In this context, our group conducted a pilot study in patients with sepsis that revealed, for the first time in humans with this pathology, the connection between α7-nAChRs and CAP after demonstrating that the α7 gene expression level in patients' peripheral blood mononuclear cells (PBMC) is a clinically relevant marker for CAP activity (15).
The human CHRNA7 gene is located on the long arm of chromosome 15 (15q13-q14 region) and has 10 exons that encode for a protein of around 57 kDa, the α7-nAChR subunit. A new hybrid gene (CHRFAM7A) resulting from a fusion of a partial duplication of CHRNA7 (from exons 5 to 10) with the FAM7A gene has been identified at 1.6 Mb upstream from the parent gene and in the opposite orientation (16, 17). Thus, CHRNA7 and CHRFAM7A are highly homologous (>99%) from exon 5 to the 3′-UTR region. The acquisition of this duplication seems to be a recent evolutionary event because it only appears in humans after divergence from other higher primates (18). Furthermore, CHRFAM7A is polymorphic with more than 95% of the population carrying one or two copies of the gene (see Ref. 19 and references therein). The CHRFAM7A transcript is translated to a protein of around 41–45 kDa (depending on glycosylation), the dupα7 subunit, which shares all the structural elements of the full-length α7 subunit, except for a substantial part of the N-terminal region containing the signal peptide and the agonist-binding domain. Interestingly, dupα7 and α7 subunits are naturally expressed in the same human cell types (see Ref. 19 and references therein), including neurons and immune cells, although dupα7 mRNA levels differ between these two cell types, being relatively low in brain and very high in immune cells (20, 21). Several genomic analyses have associated a 2-bp mutation of CHRFAM7A with certain neuropsychiatric disorders (22–24). However, a specific function for the dupα7 subunit was long unidentified until our group found that it acted as a dominant-negative regulator of the α7-nAChR function in a pioneering electrophysiological study carried out on Xenopus oocytes (21). This finding was corroborated shortly afterward in a study also performed in oocytes (25), but not in a subsequent study in mouse Neuro2A neuroblastoma cells (26). Whether or not, as occurs in oocytes, dupα7 has a biological role that interferes with α7-nAChR function in mammalian cell types other than neuroblastoma cells, particularly immune cells, has not yet been evaluated. The main difficulty in approaching this type of study lies in the high homology of the peptide sequences for the dupα7 and α7 subunits that cause the commercially available antibodies to cross-react with both subunits in immunoblotting, immunocytochemistry, and immunoprecipitation experiments.
To circumvent the above limitation, we prepared several epitope- and fluorescent protein-tagged α7 and dupα7 constructs to be transfected into GH4C1 rat pituitary cells and RAW264.7 murine macrophages. Combining coimmunoprecipitation, Föster resonance energy transfer (FRET), immunocytochemistry, confocal microscopy, flow cytometry (FC), and ELISAs, we have investigated whether dupα7 interacts with α7 subunits in these mammalian cell types, as well as the possible functional repercussion of this interaction by assessing its effects on the α7-nAChR–mediated anti-inflammatory response in RAW264.7 macrophages upon lipopolysaccharide (LPS) challenge. Finally, we have translated our experimental findings to human sepsis, a disorder accompanied by immune dysregulation and excessive activation of the inflammatory response. Thus, in the context of this last pathology, we have studied whether the dupα7 subunit would be able to interfere with the control of inflammation by α7-nAChRs. To address this issue, we have used quantitative PCR (qPCR) to determine the absolute dupα7 mRNA expression level in peripheral blood mononuclear cells (PBMCs) from patients with sepsis with the aim of analyzing its relationship with PBMC α7 mRNA levels and with the inflammatory state of patients.
Results
Coexpression of dupα7 reduces the α7 subunit expression levels in the cytosolic membrane of GH4C1 cells
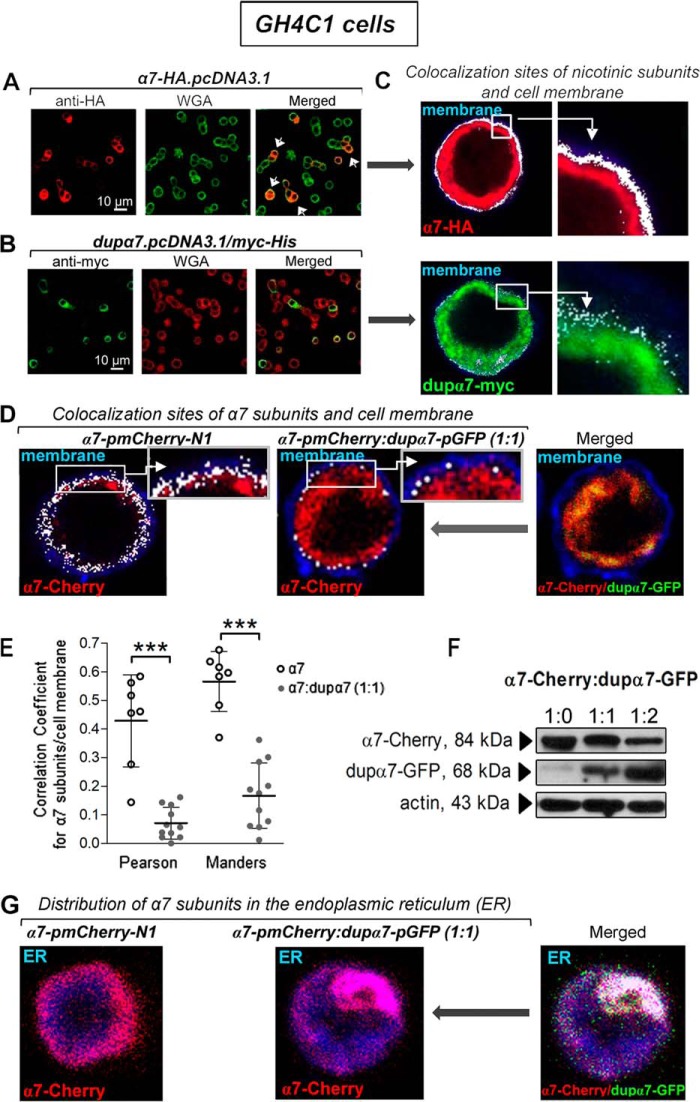
These cells do not naturally express α7 or dupα7 subunits; therefore, they were chosen at the beginning of our study to analyze the cellular distribution of each subunit after separate or combined transfection of the α7-HA.pcDNA3.1 and dupα7.pcDNA3.1/Myc-His constructs. Representative confocal images in Fig. 1, A and B, left panels, show the expression efficiency of α7-HA (red) or dupα7-Myc (green) subunits after immunostaining their respective epitopes. Images in Fig. 1, A and B, middle panels, of the same cells also show their plasma membrane (green and red, respectively) labeled with Alexa Fluor 633-conjugated wheat germ agglutinin (WGA). Pooled results obtained from three different cultures reveal that 17 ± 3 and 11 ± 2% of transfected cells efficiently expressed the α7 or dupα7 subunits, respectively. When confocal images obtained from monitoring α7 or dupα7 were superimposed over fluorescent WGA-stained cell membranes, both signals only colocalized in the cells expressing α7 subunits but not in those expressing dupα7 (Fig. 1A, white arrows in the merged image). This latter finding is corroborated by using the “colocalization function” of the Leica software (see under “Experimental procedures”) to generate an image of double-positive structures (white dots) that identifies the coinciding labeling region of nAChR subunits and the plasma membrane (Fig. 1C). The double-labeled pixels show that a significant proportion of α7 subunits reach the cell membrane, in contrast to the poor ability of the dupα7 subunits to migrate to this cellular structure.
Figure 1.
Cellular distribution of α7 and dupα7 subunits expressed separately or in combination in GH4C1 cells. A and B, left panels show confocal images representative of cells expressing either α7-HA (red) or dupα7-Myc (green) subunits after the respective epitopes were immunostained. The center panels show the plasma membrane of the same cells (green and red, respectively) labeled with Alexa Fluor 633–WGA. The panels on right (merged) show the cells with successful expression of α7 subunits (indicated by white arrows) reaching the cell membrane (yellow/orange), in contrast to the expression of dupα7-Myc subunits mostly located in the cytosol. C, image of double-positive structures (white dots) of the coinciding labeling region of α7 or dupα7 subunits and the plasma membrane generated by using the Leica software “colocalization function” in the above cells. D, same software was employed to qualitatively analyze the colocalization sites (white dots) of α7-Cherry subunits and plasma membrane (blue) in two WGA-stained cells transfected with the α7-pmCherry construct, alone or in combination with the dupα7-GFP construct; higher magnifications of colocalization sites in boxed areas (insets) are shown. E, evaluation of the dupα7 effect on α7 expression in the cytosolic membrane of cells transfected as described in D using the Pearson's correlation and Manders' overlap coefficients to quantitatively analyze the colocalization of α7-Cherry subunits and Alexa Fluor 633–WGA-stained membranes. The scatter plots show the distribution of the values found in single cells from three different cultures and the mean ± S.D. for each group. ***, p < 0.001 compared with cells transfected only with the α7-pmCherry–N1 construct by using the Student's t test. F, representative immunoblots from the same cell culture of the protein extracts from cells expressing α7-Cherry, combined or not with dupα7-GFP, at the indicated proportions. Specific bands corresponding to α7-Cherry or dupα7-GFP subunits were detected with the appropriate primary (anti-Cherry and anti-GFP) and secondary (HRP)-conjugated antibodies. G, confocal image of the homogeneous distribution of α7-Cherry subunits throughout the ER (blue) in a cell transfected with the indicated construct (left panel). The coexpression of dupα7-GFP together with α7-Cherry in a different cell causes the entrapment of α7 subunits in the form of aggregates in a localized region of the ER, probably hindering the migration to the cell membrane of these subunits conveniently assembled into homomeric α7-nAChRs (center and right images). Images like those obtained in these two cells were also found in other cells from two different cell cultures.
The next experiments were designed to qualitatively analyze the effect of dupα7 transfection on the expression level of α7 subunits at the GH4C1 cell surface using the Leica software “colocalization function.” The cells were transfected with the α7-pmCherry construct, alone or in combination with the dupα7-pGFP construct (1:1 ratio). Fig. 1D shows representative confocal images of two WGA-stained cells (blue) transfected as mentioned above. The double-positive structures (Fig. 1D, white dots) that reflect the expression level of α7 subunits in the cell membrane are drastically reduced after coexpression of dupα7 (left versus middle panels). This reduction could be derived from the intracellular entrapment of α7 subunits by dupα7 subunits (Fig. 1D, yellow color in the merged image on right). This entrapment takes place in the endoplasmic reticulum (ER), as can be deduced from the confocal image of the cell coexpressing α7-Cherry and dupα7-GFP subunits, after staining (blue) this intracellular structure (Fig. 1G, white color in the merged image). To quantitatively evaluate the effect of dupα7 on the expression level of α7-Cherry subunits in the cytosolic membrane of WGA-stained GH4C1 cells transfected as described above, we proceeded to analyze the colocalization between pixels generated by the two fluorophores (Cherry and Alexa Fluor 633–WGA) using the Pearson's correlation and the Manders's overlap coefficients. These two coefficients, which are available in the ImageJ JACoP plugging software, measure correlation and co-occurrence, respectively, and they are the metrics most widely used to quantify the degree of colocalization in fluorescence microscope images (27–29). Results reveal that both coefficients were significantly reduced by dupα7 coexpression (Fig. 1E). Finally, the immunoblots from the same cell culture (representative from two independent experiments) of protein extracts from cells transfected with α7-Cherry alone, or combined with different proportions of dupα7-GFP, and probed with the anti-Cherry or anti-GFP antibodies allow us to exclude the possibility that, at a 1:1 ratio, the dupα7 effect on cell-surface expression of α7 subunits was due to interference in the α7 mRNA translation to the corresponding protein (Fig. 1F). Therefore, this α7/dupα7 ratio was the one used whenever the double transfection of the α7 and dupα7 constructs was required to analyze either the cell-surface expression level or the functional activity of the α7-nAChR in the rest of the study.
Physical interaction of α7 and dupα7 subunits in GH4C1 cells
Using three complementary experimental approaches (confocal imaging of colocalization, coimmunoprecipitation, and FRET) in GH4C1 cells cotransfected with several pairs of α7 and dupα7 constructs, we have evaluated whether α7 and dupα7 subunits physically interact to form heteromeric nAChRs in mammalian cells. The pair α7-HA/dupα7-Myc was used for colocalization and coimmunoprecipitation assays, whereas α7-GFP/dupα7-Cherry or dupα7-GFP/α7-Cherry were the two pairs selected for FRET studies.
Fig. 2A shows confocal images of α7-HA (red) or dupα7-Myc (green) subunits expressed in cells doubly transfected with the pair α7-HA.pcDNA3.1/dupα7.pcDNA3.1/Myc-His (ratio 1:1); each subunit was detected after immunostaining its corresponding epitope. The image at the right on Fig. 2A shows the overlap of the two signals with the arrows indicating the colocalization sites (yellow) of both subunits in the cells in which the joint expression has occurred. The ability of dupα7 to assemble with α7 subunits in the same receptor was evaluated in the cell lysates from the above doubly-transfected cells subjected to immunoprecipitation (IP) of the dupα7-Myc subunit with the anti-Myc antibody. In parallel, total lysates from cells transfected separately with α7-HA.pcDNA3.1 or dupα7.pcDNA3.1/Myc-His and not subjected to immunoprecipitation were used as references. Fig. 2B shows two typical blots from the same cell culture corresponding to the above cell lysates probed with the anti-HA or anti-Myc antibodies. As expected, single bands corresponding to the predicted sizes for the α7 (57 kDa) or dupα7 (41 kDa) subunits were found in the total lysates from cells respectively transfected with either α7-HA or dupα7-Myc constructs. Analysis of the IP lysate from cells doubly-transfected with both constructs allowed the simultaneous identification of the two bands corresponding to α7-HA and dupα7-Myc, providing strong evidence for coassembly of both subunits in a heteropentameric receptor. Data are representative of two independent experiments.
Figure 2.
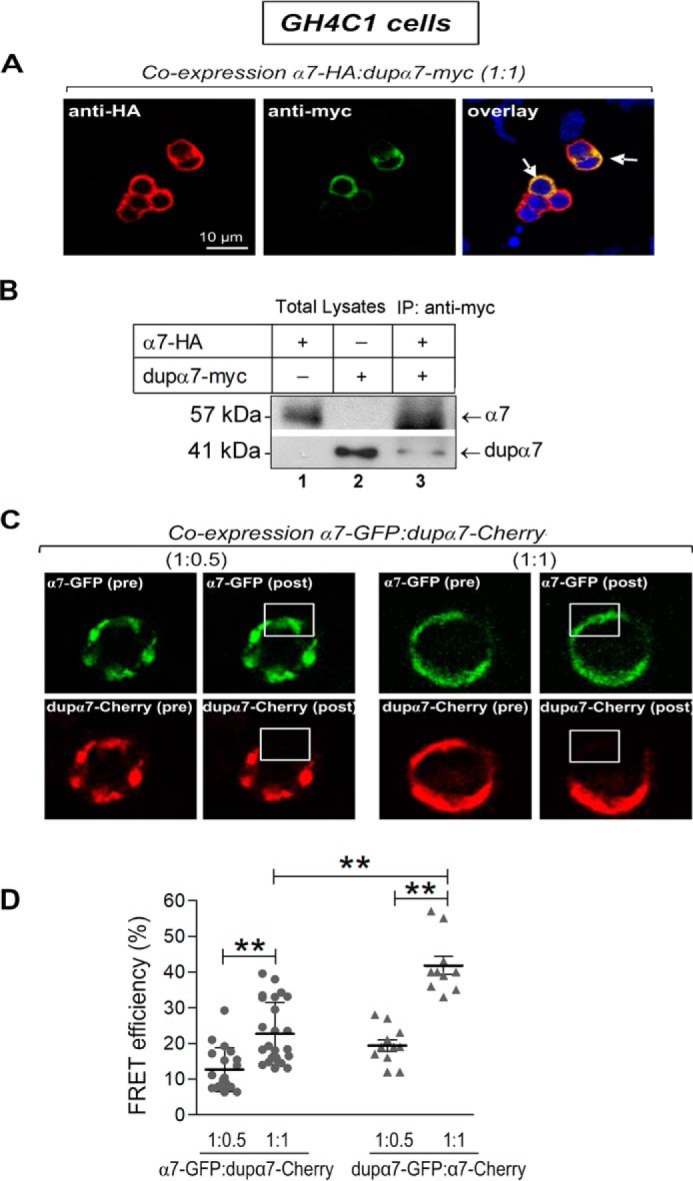
Association of α7 with dupα7 subunits identified by immunostaining, coimmunoprecipitation, and FRET in GH4C1 cells transfected with several pairs of α7 and dupα7 constructs. A, confocal images representative of cells transfected with the pair of constructs α7-HA.pcDNA3.1/dupα7.pcDNA3.1/Myc-His. Expression of α7-HA (red) or dupα7-Myc (green) subunits was detected after the respective epitopes were immunostained; colocalization of both subunits is clearly recognizable (yellow) in those cells in which joint expression has occurred (white arrows). B, two representative blots from the same cell culture probed with anti-HA or anti-Myc antibodies. Lanes 1 and 2 correspond to nonimmunoprecipitated cell lysates (Total Lysates) from cells transfected separately with α7-HA.pcDNA3.1 or dupα7.pcDNA3.1/Myc-His, respectively. Lane 3 corresponds to the cell lysate from cells doubly transfected with the above pair of constructs and subjected to IP of the dupα7-Myc subunits with the Myc antibody. C, representative FRET experiment in two cells cotransfected with the pair α7-pGFP/dupα7-pmCherry at the indicated ratios; confocal images were acquired before (pre) or after (post) photobleaching of the acceptor (dupα7-Cherry) in the framed area. The increase in the emission at 488 nm of the donor (α7-GFP (post)) in both cells is worth noting. D, scatter plots representing the FRET efficiency values, expressed as a percentage of maximal efficiency, determined in the region of interest for acceptor photobleaching in single cells from three independent experiments. These cells were transfected with the pairs of constructs α7-pGFP/dupα7-pmCherry or dupα7-pGFP/α7-pmCherry at 1:0.5 or 1:1 ratios. The horizontal bars show the mean ± S.D. for the group; **, p < 0.01 after comparing the indicated groups.
In further agreement with the above findings, confocal FRET imaging analysis in cells transfected with the pair of constructs α7-pGFP/dupα7-pmCherry or dupα7-pGFP/α7-pmCherry (1:0.5 or 1:1 ratio) provides additional evidence that both nAChR subunits were in sufficient proximity to interact with each other as part of a heteropentameric receptor. Fig. 2C shows confocal images acquired before (pre) and after (post) acceptor (dupα7-Cherry) photobleaching at 561 nm in the framed area; the increase in emission at 488 nm of the donor (α7-GFP (post)) in the two cells analyzed after coexpression of the α7-GFP/dupα7-Cherry pair in the indicated proportions is worth noting. The lower panel of Fig. 2D shows mean ± S.D. of the FRET efficiency values, expressed as a percentage of the maximum efficiency, obtained in the region selected for acceptor photobleaching in individual cells transfected with the two pairs of constructs assayed in this study at the 1:0.5 or 1:1 ratios. The significant increase in FRET efficiency when dupα7 was the donor of the pair or when the proportion of the acceptor increased in the pair is also worth noting. The increase in FRET efficiency when dupα7 is the donor of the pair agrees with previous observations in mouse neuroblastoma cells (Neuro2A) after analyzing the dupα7/α7 interaction by FRET using several pairs of fluorescent protein-fused α7 and dupα7 constructs (26). The authors of the previous study justified their results by the low expression of dupα7 subunits compared with that of α7 subunits considering that a dupα7 subunit (acting as a donor) was more likely to lie adjacent to one α7 subunit in the heteromeric nAChR.
dupα7 also interacts physically with α7 subunits reducing their expression in the plasma membrane of mouse RAW264.7 macrophages
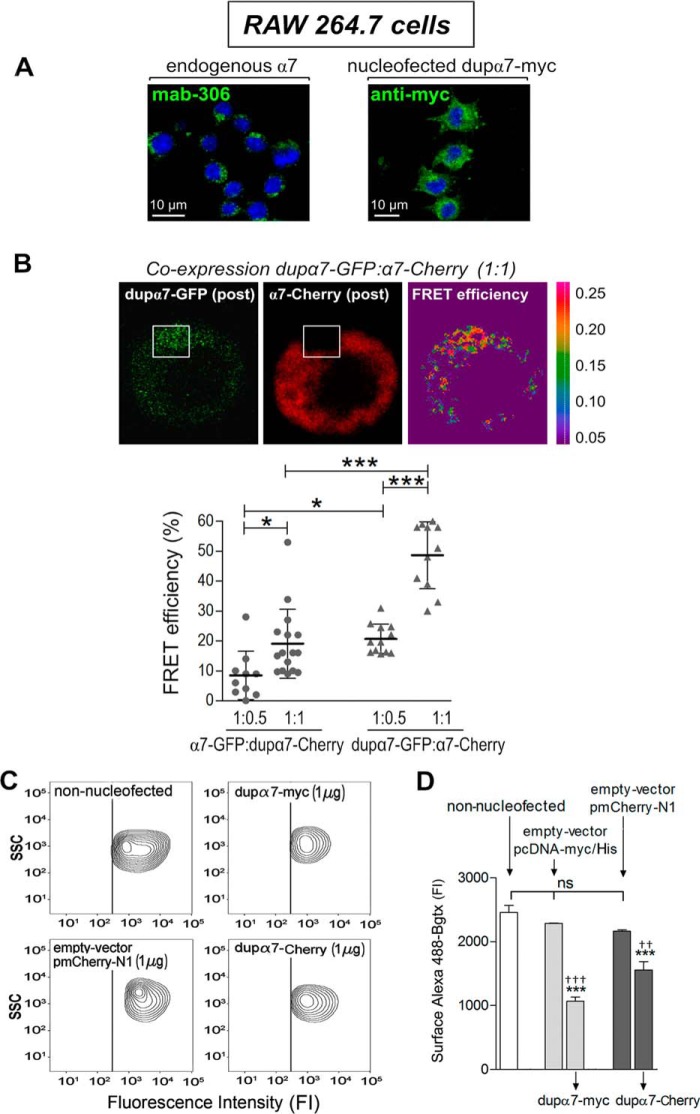
The next experiments were designed to evaluate whether physical interaction of dupα7 with the α7 subunits found in GH4C1 cells also occurs in another mammalian cell type that naturally expresses functional α7-nAChRs, the murine macrophage RAW264.7 cell line. If so, we would proceed to evaluate whether the dupα7 interaction with endogenous α7 subunits interfered with the cell-surface expression and function of α7-nAChRs expressed by these immune cells. The representative confocal image of a group of RAW264.7 cells (Fig. 3A, left panel) shows the endogenous α7 subunits (green) immunostained with the primary antibody Mab306. Fig. 3A (right panel) corroborates the efficient expression of foreign dupα7 subunits (green) after cell nucleofection with the dupα7-Myc construct.
Figure 3.
Physical interaction of dupα7 with α7 subunits reduces endogenous expression of functional α7-nAChRs in the plasma membrane of RAW264.7 cells. A, confocal images of the cells revealing the native expression of α7 subunits (left panel) or the successful and abundant expression of foreign dupα7-Myc subunits (right panel) after cell nucleofection with the dupα7.pcDNA3.1/Myc-His construct. The subunits α7 (green) and dupα7-Myc (green) were immunostained, respectively, with either Mab306 or the anti-Myc antibody, followed by the Alexa Fluor 488-conjugated secondary antibody in both cases. Nuclei (blue) appear stained with DAPI. B, upper panels: representative confocal images of FRET in a cell doubly-nucleofected with the pair dupα7-pGFP/α7-pmCherry at a 1:1 ratio; images reveal the increase in the emission at 488 nm of the donor (dupα7-GFP (post)) after photobleaching of the acceptor (α7-Cherry (post)) in the framed area. Right, false-color image showing higher FRET efficiency (red) in the selected area. Lower panel: scatter graph reflecting the FRET efficiency values, expressed as percentage of maximal efficiency, determined in the region of interest for acceptor photobleaching in single cells from three independent cell cultures. The cells were transfected with the pairs of constructs α7-pGFP/dupα7-pmCherry or dupα7-pGFP/α7-pmCherry at 1:0.5 or 1:1 ratios. The horizontal bars show the mean ± S.D. for the group; *, p < 0.05, and ***, p < 0.001, after comparing the indicated groups. C and D, flow cytometry analysis of native expression of functional α7-nAChRs labeled with Alexa Fluor 488–Bgtx in cells nucleofected with the dupα7.pcDNA3.1/Myc-His or pmCherry-tagged dupα7 constructs or with their corresponding empty vectors; non-nucleofected cells were used as a reference. C, representative contour plots showing SCC and cell-surface expression of α7-nAChRs in non-nucleofected cells or in cells nucleofected with the indicated constructs (positive for dupα7-Myc, dupα7-Cherry, or Cherry). D, histogram representing pooled results of the fluorescence intensity corresponding to the Alexa Fluor 488–Bgtx bound to the α7-nAChRs on the cell surface of each cell population. The bars represent means ± S.E. from three independent cell cultures. ***, p < 0.001 compared with non-nucleofected cells; †††, p < 0.001, and ††, p < 0.01 compared with cells nucleofected with the corresponding empty vector. ns is nonsignificant.
FRET efficiency analysis in cells nucleofected with the two pairs of constructs α7-pGFP/dupα7-pmCherry or dupα7-pGFP/α7-pmCherry-N1 (1:0.5 or 1:1 ratio) again showed that both nAChR subunits are in sufficient proximity so as to interact with each other as part of a heteropentameric receptor in this immune cell type. Thus, Fig. 3B (upper panel) shows a representative confocal image acquired after photobleaching α7-Cherry in the framed area, which produces a marked increase in dupα7-GFP fluorescence; at the right, a false color image shows the increase in FRET efficiency (yellow/red colors in the selected area) under the experimental conditions just described. Fig. 3B (bottom panel) represents mean ± S.D. of the FRET efficiency values obtained in single cells from three different cultures nucleofected with the two pairs of constructs mentioned above at the 1:05 or 1:1 ratios. As in the GH4C1 cells, FRET efficiency in the RAW264.7 cells increased significantly when the proportion of the acceptor (Cherry) was raised in the pair of transfected constructs, as well as when dupα7 acted as the donor in the pair.
Once the physical interaction of dupα7/α7 in RAW264.7 cells was corroborated, we proceeded to evaluate whether, as occurred in GH4C1 cells, this interaction affected the membrane expression of α7-nAChRs and, consequently, modified the functional capacity of these receptors. The following experiments sought to explore both proposals. To investigate the first proposal, we combined immunofluorescence and flow cytometry analysis to determine the expression levels of α7-nAChRs at the cell surface of the following populations of RAW264.7 cells: 1) non-nucleofected; 2) positive for dupα7-Myc or dupα7-Cherry after nucleofection of the appropriate constructs (1 μg); and 3) positive for Myc or Cherry after the nucleofection of the corresponding empty vectors (1 μg). The expression level of native homomeric α7-nAChRs in the plasma membrane of each of the above cell groups was determined through the fluorescence intensity generated by α-bungarotoxin conjugated to Alexa Fluor 488 (Alexa Fluor 488–Bgtx), a toxin that selectively recognizes functional α7-nAChRs expressed in the cell membrane. Fig. 3C displays the representative contour plots showing side scatter (SSC) and cell-surface expression of α7-nAChRs in non-nucleofected cells or in cells nucleofected with the indicated constructs. Although the SSC data reflect a similar complexity of all the cell populations analyzed, the fluorescence intensity generated by the toxin labeling the receptors that are expressed on the cell surface is lower in cells nucleofected with either of the two dupα7 constructs. Fig. 3D shows the mean ± S.E. of the above fluorescence intensity values obtained in all experimental conditions assayed. The results again reveal that the expression of dupα7, whatever the dupα7 construct used for nucleofection, significantly reduces cell-surface expression of functional α7-nAChRs with respect to the expression observed in non-nucleofected cells or in cells nucleofected with the corresponding empty vectors.
Expression of dupα7 markedly reduces the α7-nAChR–mediated anti-inflammatory effect in RAW264.7 macrophages
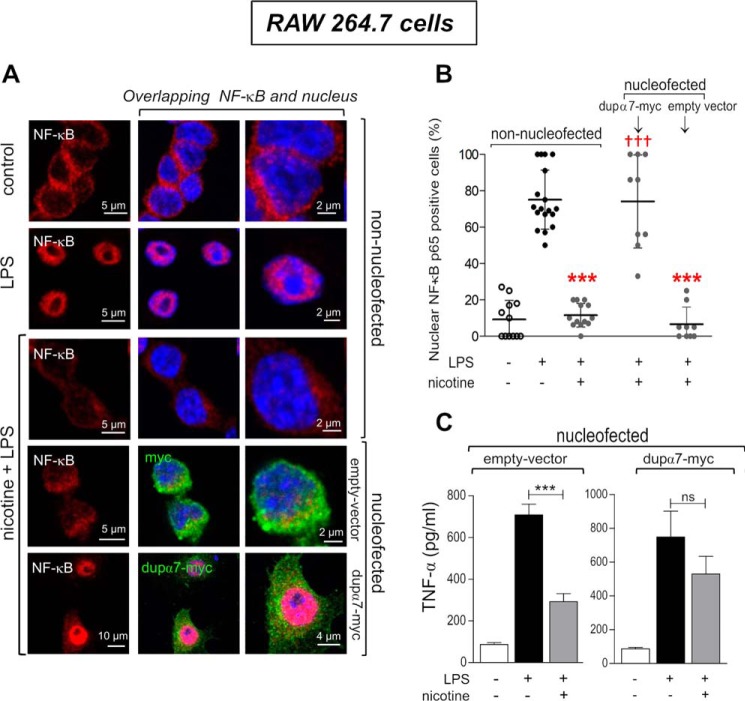
Next, we investigated whether the reduction in the number of α7-nAChRs expressed on the cell surface elicited by dupα7 overexpression had functional repercussions by interfering with the anti-inflammatory effect mediated by these receptors. The effect of this overexpression was evaluated on the nicotine-mediated blockade of NF-κB activation and TNFα production induced by LPS in the above immune cells. Fig. 4A shows representative confocal images of cell distribution of NF-κB (red) in non-nucleofected cells or in cells nucleofected with either dupα7-Myc or the corresponding empty vector. The images in Fig. 4A also show the cell nuclei (blue) stained with DAPI and the confirmation of the successful overexpression of dupα7-Myc or the empty vector after cell nucleofection with the corresponding constructs and immunostaining of the expressed Myc epitope (green).
Figure 4.
Loss of the anti-inflammatory effect of nicotine in LPS-stimulated RAW264.7 macrophages due to dupα7 overexpression. The anti-inflammatory activity of nicotine acting on α7-nAChRs of macrophages exposed to LPS was analyzed by measuring the drug effect on nuclear translocation of NF-κB or TNFα production assayed by immunostaining/confocal microscopy or ELISA, respectively. A, representative confocal images of the cellular distribution of NF-κB (red) after staining with primary anti-NF-κB–p65 antibody and Alexa Fluor 555-conjugated secondary antibody in non-nucleofected cells or in cells nucleofected with dupα7.pcDNA3.1/Myc-His or with the corresponding empty vector. Successful expression of the dupα7-Myc subunit or empty vector in nucleofected cells was ensured by staining of the Myc epitope (green) with the primary anti-Myc antibody and the Alexa Fluor 488-conjugated secondary antibody. DAPI was used for nuclear staining (blue). In non-nucleofected cells, NF-κB was distributed throughout the cytosol in the absence of stimulation and was translocated to the nucleus in response to LPS (100 ng/ml; 1 h). Incubation of cells with nicotine prevents the NF-κB activation induced by endotoxin. This anti-inflammatory effect of nicotine was preserved in cells nucleofected with the empty vector while it is markedly reduced in cells overexpressing dupα7 subunits. B, percentage of cells with respect to total cells visualized in individual microscope fields from three independent experiments in which the NF-κB–p65 translocation to the nucleus has occurred (positive cells) in response to each experimental condition described above. The horizontal bars show the mean ± S.D. for the group; ***, p < 0.001 compared with non-nucleofected cells exposed to LPS. †††, p < 0.001 compared with non-nucleofected cells exposed to LPS and nicotine. C, histogram representing the effect of nicotine on TNFα production induced by LPS (100 ng/ml; 4 h) in cells nucleofected with dupα7.pcDNA3.1/Myc-His or with the empty vector. Once again, the anti-inflammatory effect of nicotine in cells nucleofected with the empty vector practically disappears after dupα7 overexpression. The bars show the mean ± S.E. from three independent experiments. ***, p < 0.001 after comparing the indicated bars. ns is nonsignificant.
Results in non-nucleofected cells reveal that, in the absence of stimulation, NF-κB is distributed throughout the cytosol and, in response to LPS (100 ng/ml, 1 h), NF-κB translocates to the nucleus (Fig. 4A, magenta). This latter effect is blocked by cell exposure to nicotine (10 μm) 30 min before and during the LPS treatment. Interestingly, the anti-inflammatory effect of nicotine on NF-κB activation is preserved in cells nucleofected with the empty vector but is significantly blocked by dupα7-Myc overexpression. Fig. 4B shows the scatter diagram for individual microscope fields showing the percentage of cells, with respect to total cells visualized, in which the NF-κB translocation to the nucleus has occurred (positive cells) in response to each experimental condition just described; the means ± S.D. of the values obtained in each experimental condition are also represented. These last data confirm the representative experiment in Fig. 4A showing that the anti-inflammatory effect of nicotine on NF-κB activation induced by LPS is markedly inhibited by the overexpression of dupα7 but remains unchanged after nucleofection of the empty vector. Fig. 4C shows the anti-inflammatory effect of nicotine with respect to the production of TNFα induced by LPS as determined by ELISA in the supernatant of RAW264.7 cells nucleofected with dupα7-Myc or with the corresponding empty vector. It is remarkable that exposure to LPS (100 ng/ml, 4 h) induced a significant and similar increase in cytokine production in the two cell groups. However, although incubation with nicotine (10 μm, 30 min before and during LPS treatment) significantly reduced the pro-inflammatory effect of LPS in cells overexpressing the empty vector, it failed to interfere with the LPS effect in those nucleofected with dupα7-Myc. Taken together, the results of these latter experiments indicate that dupα7 overexpression prevents the anti-inflammatory effect of nicotine in RAW264.7 cells stimulated with LPS, as regards both the activation of NF-κB and the production of TNFα.
Absolute dupα7 mRNA expression in PBMCs from patients with sepsis and its correlation with PBMC α7 mRNA expression, inflammatory state, and disease severity
The results described above demonstrate that dupα7 behaves as a negative regulator of the α7-nAChR–mediated anti-inflammatory effect in LPS-stimulated RAW264.7 cells. However, whether dupα7 could play any role in human pathologies with excessive inflammatory response, such as sepsis, was still unknown. In a previous pilot study, we demonstrated that normalized expression of the α7 mRNA level in PBMC from 33 nonsmokers with sepsis, determined by qPCR within the first 24 h of their sepsis diagnosis, was a good marker of CAP activity in these patients (15). In that pilot study, the inflammatory status and disease severity in each patient were also assessed on the day of diagnosis by measuring the serum concentrations of various inflammatory markers (TNFα, IL-1β, and C-reactive protein (CRP)) as well as by calculating the “Acute Physiology Evaluation and Chronic Health Assessment (APACHE) II score,” which is a very useful classification system to establish disease severity and estimate mortality risk (30). The APACHE II scoring index is calculated based on 12 physiological and 2 disease-related variables; the higher the scoring index, the greater the severity of the disease and the risk of death.
In this study, we have gone further to determine whether there was any type of correlation between the expression levels of dupα7 mRNA in PBMCs from the above patients and their PBMC α7 mRNA levels or inflammatory state. To be able to compare PBMC α7 and dupα7 gene expression levels, the absolute expression values of both messengers (number of mRNA copies) were determined by qPCR as described under “Experimental procedures.” The same control group of 33 healthy nonsmoker subjects included in the above pilot study was incorporated as a reference in this study.
We found an inverse and significant correlation between the absolute expression levels of dupα7 and α7 mRNAs (ρ = −0.40; p < 0.05) in the patients. However, because the variability of the absolute α7 mRNA expression in PBMC was so great between patients, they were distributed into tertiles (11 patients/tertile) on the basis of their absolute α7 expression levels (1st tertile, high; 2nd tertile, medium; 3rd tertile, low levels). According to the aforementioned distribution, the mean number of copies (±S.E.) in α7 mRNA was 112.20 ± 34.18, 24.76 ± 4.52, and 13.77 ± 1.36 for the 1st, 2nd, and 3rd tertiles, respectively. Meanwhile, the absolute α7 expression levels in PBMC from the control group was low and showed very little variability (13.50 ± 1.25 copies). Significant differences (p < 0.001) were found in absolute α7 expression between the first and second or third tertiles or with respect to the control group. Fig. 5 shows the box-whisker plots of the number of copies of dupα7 mRNA (A) or the α7/dupα7 mRNA ratio (B) determined in PBMCs from the three tertiles. Fig. 5A reveals that the median value of dupα7 mRNA expression is significantly higher in the third tertile (low α7 levels) than in the other tertiles (p < 0.01) or the control group (p < 0.001). Fig. 5B shows the significant differences between tertiles with respect to the median of the α7/dupα7 mRNA ratio; it is interesting to note that this last value is significantly lower in patients from the third tertile (low α7 and high dupα7 expression levels) than in patients from the other tertiles. Finally, we found a direct and significant correlation between the absolute dupα7 mRNA levels assessed in this study and the serum concentrations of some of the inflammatory markers determined in the previous pilot study using the same set of patients (15): for TNFα (ρ = 0.46; p < 0.01); IL-1β (ρ = 0.37; p < 0.05); CRP (ρ = 0.39; p < 0.05). In contrast, there was a significant inverse correlation between the α7/dupα7 mRNA ratio and the above serum marker concentrations or APACHE II score: for TNFα (ρ = −0.41; p < 0.05); IL-1β (ρ = −0.60; p < 0.001); CRP (ρ = −0.61; p < 0.001); APACHE II score (ρ = −0.69; p = 0.001). It is interesting to note that no significant differences were found with respect to age or gender between the groups of patients and controls. Furthermore, our data on absolute expression levels of α7 or dupα7 in PBMCs from healthy subjects (control group) show very little variability, ruling out the possibility that age or gender can be considered confounding variables in relation to the gene expression of α7 and dupα7.
Figure 5.
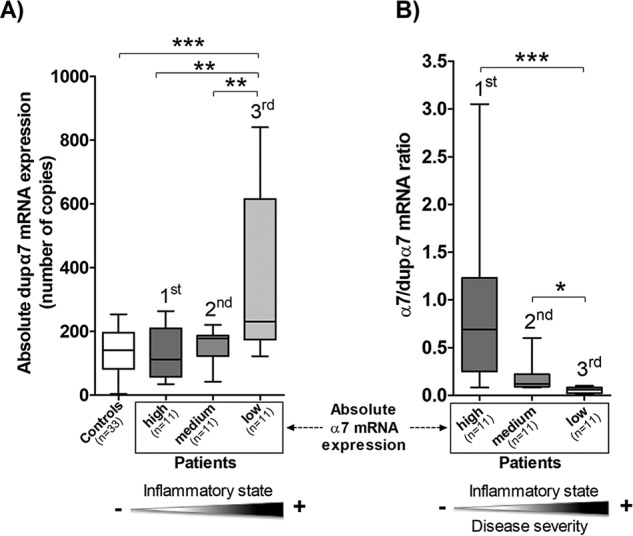
Expression analysis of dupα7 and α7 subunit genes in PBMCs from patients with sepsis. Absolute expression values for each gene transcript (number of mRNA copies) in PBMCs from 33 patients with sepsis and 33 healthy individuals (Controls) were determined by qPCR on the basis of a standard six-point curve as described under “Experimental procedures.” Each value, obtained in triplicate, represents an average of three separate determinations. A, expression of dupα7 mRNA in the control group and in patients distributed into tertiles (11 patients/tertile) according to their absolute α7 mRNA expression level (1st tertile, high; 2nd tertile, medium; 3rd tertile, low levels). B, α7/dupα7 ratio calculated on the basis of the number of copies of both transcripts. Data are represented as box-and-whisker plots; the line within each box shows the median expression of dupα7 or α7/dupα7 ratio, upper and lower edges of the box represent the 75th and 25th percentiles, respectively, and the ends of the whisker the maximum and minimum value of the series. The ANOVA test followed by the Bonferroni post hoc test (A) or the Kruskal-Wallis test followed by the Dunn post hoc test (B) was used for data analysis. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 after comparing the indicated boxes. The shaded triangles at the bottom panel reflect the existence of significant correlations between dupα7 mRNA levels or α7/dupα7 ratio and the inflammatory state or disease severity of the patients.
Discussion
This study reports the first experimental evidence that dupα7 expression in RAW264.7 macrophages produces a loss of anti-inflammatory activity mediated by native α7-nAChRs. It also shows that this effect on inflammation is the result of the physical interaction between dupα7 and α7 subunits forming heteromeric nAChRs that are mostly trapped in the ER, thus negatively affecting the expression level of functional α7-nAChRs in the cytosolic membrane. This mechanism of dupα7/α7 interaction seems to be a generalized phenomenon because it occurs in more cell types than just immune cells, including GH4C1 neuroendocrine cells. Finally, our data in patients with sepsis also reveal that the dupα7 gene expression level determined in their PBMCs correlated inversely with their PBMC α7 gene expression level and directly with the magnitude of their inflammatory state.
Although it is well-established that α7 subunits are co-assembled to form homomeric α7-nAChRs that regulate a wide variety of pathophysiological processes affecting the nervous and immune systems in mammals, it is unknown whether the partially duplicated subunit is involved in any of these α7-nAChR–controlled processes. Despite the lack of information in relation to a possible role for the dupα7 subunit, several observations support the hypothesis: 1) dupα7 subunits heterologously expressed in Xenopus oocytes act as dominant-negative regulators of the α7-nAChR function (21, 25); 2) dupα7 and α7 subunits are naturally expressed in the same cell types in humans (see Ref. 19 and references therein); and 3) the insertion of dupα7 subunits in the pentameric structure of nAChRs containing α7 subunits is feasible because the duplicated form shares most of the structural elements of the original subunit, specifically the two M1–M2 and M3–M4 loops that participate in the interaction, stabilization, and assembly of subunits into the receptor complex, although the duplicated subunit lacks a part of the N-terminal domain involved in the initial association of subunits to form the receptor (16, 31–33).
To investigate whether dupα7 expression modifies α7-nAChR function in mammalian cells, as occurs in amphibian cells, we prepared several constructs of α7 and dupα7 subunits tagged with different epitopes or fused to various fluorescent proteins for their subsequent expression in the two mammalian cell lines analyzed in this study. Thus, we used rat GH4C1 cells that do not naturally express either of the two nAChR subunits but have the full capacity to express both foreign subunits (21), and we used murine RAW264.7 macrophages that endogenously express α7-nAChRs with a well-verified anti-inflammatory activity. Analysis of nAChR subunit colocalization sites in the cytosolic membrane of GH4C1 cells revealed substantial expression differences between α7-HA and dupα7-Myc when the two constructs were transfected separately (Fig. 1, A and B). Whereas α7 subunits reached high expression levels in the cell membrane, dupα7 subunits were barely detected in this cellular structure. This finding corroborates our previous data in Xenopus oocytes (21) and could be explained by the loss of the signal peptide of the duplicated subunit with respect to its parent subunit (34), which would hinder its transport from the ER. Interestingly, coexpression of dupα7-GFP and α7-Cherry (1:1 ratio) in GH4C1 cells significantly reduces the number of α7 subunits expressed in the cell membrane (Fig. 1, D and E) without modifying the translation of α7 mRNA to the corresponding protein, as seen in the immunoblot of protein extracts from cells doubly transfected with the pair of constructs indicated above at the 1:1 ratio (Fig. 1F).
The dupα7 effect on membrane expression of α7 subunits in these cells is the result of the physical interaction of both subunits generating mixed α7/dupα7-nAChRs, as inferred from the immunoprecipitation and FRET data (Fig. 2). It is to be expected that the insertion of dupα7 subunits within the pentameric structure of the nAChR reduces its capacity of being transported to the cytosolic membrane due, in part, to the loss of signal peptide sites. This proposal is confirmed by confocal images of GH4C1 cells expressing α7-Cherry, alone or in combination with dupα7-GFP, after ER staining with the corresponding antibody (Fig. 1G); the image shows how the vast majority of α7 subunits expressed by the cell are sequestered by dupα7 subunits in this intracellular structure. Our data do not allow us to exclude the possibility that some dupα7 subunits could migrate toward the cell membrane forming part of the mixed nAChRs, as has been reported previously in Neuro2A cells (26). However, the number of dupα7 subunits that makes this trip as part of mixed nAChRs appears to be very low in GH4C1 cells, as inferred from confocal images of cells doubly-transfected with dupα7-GFP and α7-Cherry (Fig. 1G, merge). The different capacity to migrate to the cell surface of the nAChRs containing dupα7 subunits reported in Neuro2A and in GH4C1 cells could be explained by the different proportion of dupα7 subunits that make up the nAChR in each cell type; the higher this proportion the lower the probability that the receptor reaches the cell membrane.
Once the dupα7/α7 interaction and its impact on membrane expression of α7-nAChRs has been established in neuroendocrine cells, the next question is to know whether this entire sequence of cellular events has consequences on α7-nAChR function. A dupα7/α7 interaction forming mixed nAChRs has been previously reported by FRET analysis in Neuro2A cells (26). However, that study failed to find a clear effect from the duplicated subunit on ACh-induced currents in neuroblastoma cells, in contrast to the strong inhibitory effect on α7-nAChR–mediated currents found by us and others in oocytes (21, 25). The above authors ascribe the discrepancy of differences in the subunit assembly that forms nAChRs in the two expression systems and/or the extremely low expression level of dupα7 subunits achieved in Neuro2A cells; however, they do not exclude the possibility that transfection of the RiC-3 chaperone in the cells used in all their electrophysiological recordings could have affected the inhibitory capacity of dupα7 subunits with regard to ACh-mediated currents.
Here, we investigated the dupα7 effect on α7-nAChR function in a non-neuronal cell type, RAW264.7 macrophages, due to their native expression of α7-nAChRs with well-defined anti-inflammatory activity and their ability to reach high expression levels of foreign dupα7 subunits (Fig. 3A, right panel). The signaling pathways downstream from the α7-nAChR that inhibit pro-inflammatory cytokine production (TNFα, high mobility group box 1 protein (HMGB1), and IL-1β, IL-6, and IL-8) in LPS-stimulated macrophages require collaboration by NF-κB and JAK2/STAT3 to interfere with the signaling pathway triggered by Toll-like receptors (35, 36). Therefore, this study has evaluated the inflammatory activity of these macrophages by measuring the degree of NF-κB activation and TNFα production in response to each experimental situation tested.
Our data from FRET efficiency analysis, FC, and immunofluorescence staining of functional α7-nAChRs labeled with Alexa Fluor 488–Bgtx in RAW264.7 cells nucleofected with different dupα7 constructs demonstrate the physical interaction of dupα7/α7 subunits and its negative impact on membrane expression of functional α7-nAChRs (Fig. 3, B–D). As expected, this latter dupα7 effect leads to the loss of the α7-nAChR–mediated anti-inflammatory response in LPS-stimulated macrophages, as inferred from confocal microscopy and ELISA data showing that dupα7 expression significantly reverses the anti-inflammatory effect of nicotine acting on α7-nAChRs in the above cells, both at the level of nuclear translocation of NF-κB as well as of TNFα production (Fig. 4). None of the above dupα7 effects on α7-nAChR function is reproduced by the empty vector expression. Additionally, it is worth highlighting the fact that dupα7-Myc subunits were detected on the cell surface of a reduced group of cells nucleofected with the dupα7-Myc construct after their isolation by FC using the anti-Myc antibody that recognizes the corresponding epitope located in the extracellular C-terminal domain of the duplicated subunit (Fig. 3C). This finding indicates that some dupα7 subunits are assembled with α7 subunits in a mixed nAChR that does reach the cell membrane. This receptor would have a lower capacity to respond to nicotine or ACh, and this could also contribute to the reduction in anti-inflammatory response mediated by α7-nAChRs in RAW264.7 macrophages.
If dupα7 subunits have a role in vivo in interfering with the human inflammatory response controlled by α7-nAChRs, their expression levels must be susceptible to regulation by external stimuli that require greater or lesser α7-nAChR activity. Results of several in vitro studies performed in human monocytes or monocytic cell lines show that this is what actually happens. Thus, exposure of the above cells to LPS, IL-1β, or nicotine down-regulates the dupα7 mRNA expression level (21, 37). Moreover, the same negative regulation of dupα7/CHRFAM7A expression has been reported in peripheral blood lymphocytes of individuals who are smokers (38). Having previously reported that the PBMC α7 gene expression level in patients with sepsis is a clinically relevant marker of their CAP activity (15), here we have tried to extend our experimental data from RAW264.7 cells to patients with sepsis by evaluating the absolute expression levels of dupα7 mRNA determined in their PBMCs. Our data in these patients indicate that dupα7 levels correlate inversely with α7 levels and directly with the patient's inflammatory state. We have previously reported that the greater the expression of α7, the better the control of inflammation and the prognosis (15). Here, we have found that the group of patients with the best prognosis (1st tercile) is the one with the highest α7 and lowest dupα7 mRNA levels. In contrast, the group of patients with the worst prognosis (3rd tercile) has the lowest α7 and the highest dupα7 mRNA levels. These two findings explain why the higher the α7/dupα7 ratio, the better the control of inflammation and the lower the severity of the disease. The negative correlation between α7 and dupα7 mRNA levels found in PBMCs from the patients in this study is not restricted to sepsis but has also been found in colon biopsies from patients with ulcerative colitis and Crohn's disease (39). Furthermore, in a recent study performed in paired tumor and nontumor lung specimens from patients with squamous cell carcinoma, we have found that, compared with normal lung, tumor samples showed significant up-regulation of CHRNA7 and down-regulation of CHRFAM7A (40). This last finding suggests that deficient dupα7 expression may facilitate the oncogenic process mediated by α7-nAChR, the main nAChR subtype responsible for the nicotine-mediated proliferative, pro-angiogenic and pro-metastatic effects in human smoking-related lung cancers (41–44).
An inherent weakness of our clinical study was the relatively small sample size. Another sample-associated shortcoming, which we have tried to minimize here, is the presence of confounding factors that might influence gene expression measurements in PBMCs from the patients. Yet another challenge not resolved by our study is the stoichiometry of the α7 and dupα7 subunits that make up the heteromeric nAChR after cell transfection with different proportions (1:0.5 or 1:1) of the corresponding cDNAs.
In summary, despite the limitations outlined above, our data not only identify the mechanism by which dupα7 interferes with α7 subunits in immune and neuroendocrine mammalian cells, it also highlights the role of the duplicated subunit in counteracting the excessive brake of the inflammatory response mediated by vagal activation of α7-nAChRs in immune cells. This dupα7 effect on inflammation could have pathophysiological repercussions in human sepsis, as can be deduced from our data in patients with sepsis showing how the subgroup of patients with better control of the inflammatory response and better prognosis is the one with the lowest gene expression level of dupα7 and highest α7 level in their PBMCs. Given that the acquisition of CHRFAM7A seems to be a recent evolutionary event that only appears in humans, it is likely that the partial duplicate gene may confer an evolutionary advantage by regulating several of the α7-nAChR–mediated functions not only in relation to inflammation but also to tumor progression in smoking-related tumors or to neurotransmitter release in CNS. Future studies are necessary to evaluate these possibilities.
Experimental procedures
Reagents and cell lines
Primary mouse anti-Myc and rabbit anti-HA monoclonal antibodies were purchased from Roche Applied Science (Mannheim, Germany) and Sigma, respectively. Primary mouse monoclonal anti-α7 (Mab306) and rabbit polyclonal anti-calnexin antibodies were from Sigma. Primary rabbit anti-NF-κB p65 antibody was from Cell Signaling. Alexa Fluor 488 goat anti-mouse IgG, Alexa Fluor 546 goat anti-mouse IgG, Alexa Fluor 555 goat anti-rabbit IgG, Alexa Fluor 647 goat anti-rabbit IgG, Alexa Fluor 633–WGA, Alexa Fluor 488-conjugated α-bungarotoxin (Alexa Fluor 488–Bgtx), and ProLong Gold Antifade Reagent were purchased from Molecular Probes (Invitrogen). Primary mouse anti-Cherry and mouse anti-GFP monoclonal antibodies were from Abcam (Cambridge, UK) and Roche Applied Science, respectively. The secondary (HRP)-conjugated anti-mouse IgG and anti-rabbit IgG antibodies were from Jackson ImmunoResearch (Suffolk, UK). Lipopolysaccharide (LPS) and the remaining unspecified products were purchased from Sigma. The GH4C1 rat pituitary and the RAW264.7 murine macrophage cell lines were purchased from American Type Culture Collection ATCC (Manassas, VA).
Generation of several α7 and dupα7 constructs
All constructs were generated according to standard molecular biology techniques to insert the epitope or the fluorescent protein of interest in-frame with the C-terminal domain of the ORF in the human α7 or dupα7 cDNA sequences. The α7-HA.pcDNA3.1 construct was prepared by two successive PCRs using as a template the plasmid α7.pcDNA3.1 containing the full-length cDNA sequence of the α7 subunit and the following primers: forward 5′-TAATACGACTCACTATAGGG-3′ and reverse 5′-AACATCGTATGGGTAGGATCCCGCAAAGTCTTTGGACACGCC-3′ containing the BamHI site. The hemagglutinin (HA) epitope was subsequently inserted by performing a second PCR using the pair of primers: forward 5′-CCACCATGCGCTGCTCGCCG-3′ and reverse 5′-GTCTAGATCAAGCGTAATCTGGAACATCGTATGGGTAGGATCCCGCA-3′ containing the HA sequence flanked by the XbaI site at 5′. The resulting amplified product containing the α7-HA cDNA sequence was ligated to pGEM-T Easy vector (Promega) and later subcloned into the NotI/XbaI sites of pcDNA3.1 (Invitrogen) yielding the final α7-HA.pcDNA3.1 construct. The construct dupα7.pcDNA3.1/Myc-His was generated by PCR using the dupα7.pSP64T plasmid as a template and the following pair of primers: forward 5′-GATTTAGGTGACACT-ATAG-3′ and reverse 5′-TCTAGACGCAAAGTCTTTGGACACGGC-3′ containing the XbaI site. The resulting PCR product was ligated to pGEM-T Easy vector and subcloned into the NotI/XbaI sites of the pcDNA3.1/Myc-His (Invitrogen), yielding the final dupα7.pcDNA3.1/Myc-His construct containing the full-length human dupα7 cDNA sequence in-frame with the Myc-His tag.
The GFP- and Cherry-tagged constructs were prepared by PCR using the α7.pSP64T and dupα7.pSP64T plasmids as templates and the following pairs of primers: α7-GFP forward 5′-TACCGAAGCCGCTAGCCCACCATGCGCTGCTCGC-3′, dupα7-GFP forward 5′-TACCGAAGCCGCTAGCATGCAAAAATATTGCATCTACC-3′, and for both α7-GFP and dupα7-GFP reverse 5′-GACCGGTAGCGCTAGCCCCGCAAAGTCTTTGGACAC-3′ containing the NheI sites; α7-Cherry forward 5′-GAATTCTGCAGTCGACACCACCATGCGCTGCTCGC-3′, dupα7-Cherry forward 5′-GAATTCTGCAGTCGACATGCAAAAATATTGCATCTACC-3′, and for both α7-Cherry and dupα7-Cherry reverse 5′-CCGCGGTACCGTCGACGCAAAGTCTTTGGACACGG-3′ containing the SalI sites. The amplified products containing the full-length α7 and dupα7 cDNA sequences were cloned at the NheI/NheI sites in the pTurboGFP (Evrogen, Moscow, Russia) and the SalI/SalI sites in the pmCherry-N1 (Clontech, France), using the In-Fusion cloning kit from Clontech and following the manufacturer's instructions. All constructs were verified by DNA sequencing.
GH4C1 cell culture and transfection
Cells were grown and maintained in Ham's F-10 medium supplemented with 15% horse serum and 2.5% FBS in a humidified atmosphere containing 5% CO2 at 37 °C. Cells were plated on 12-mm poly-l-lysine–coated glass coverslips at the density required to reach 50–80% confluence 24 h after plating. Next, transient transfection of the constructs of interest was performed using Lipofectamine 2000 (Invitrogen) following the manufacturer's instructions. Nontransfected cells were used in parallel as controls. Transfected and nontransfected cells were incubated for an additional 48 h at 37 °C before starting the experiments.
RAW264.7 cell culture and nucleofection
Cells were grown and maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS under 5% CO2 at 37 °C. Nucleofection was performed 24 h later using the specific Amaxa cell line Nucleofector® kit V (Amaxa Biosystems, Germany) for this cell line following the manufacturer's instructions and using the Amaxa Nucleofector II device (Lonza, Cologne, Germany). Briefly, 2 × 106 cells suspended in 100 μl of nucleofection solution were nucleofected with the appropriate construct using the D-032 software of the nucleofector. Next, the cells were seeded in 24-well plates allowing their adhesion to the plate for a period of 48 h before starting the experiments.
Immunofluorescent staining, confocal imaging, and colocalization analysis
The confocal images of the endoplasmic reticulum (blue) of GH4C1 cells transfected with α7-Cherry, alone or in combination with dupα7-GFP, were obtained after the cells were fixed and processed for immunocytochemistry using a rabbit anti-calnexin antibody (1:50; 2 h) detected by a goat anti-rabbit Alexa Fluor 647 antibody (1:400; 1 h). In a different set of experiments, cell membranes of living GH4C1 cells transfected with α7-HA or dupα7-Myc were stained by incubation with Alexa Fluor 633–WGA for 10 min at 37 °C. After thorough washing, cells were fixed and processed for immunocytochemistry using a rabbit anti-HA or a mouse anti-Myc antibody (1:200; 2 h) detected by a goat anti-rabbit Alexa Fluor 555 (red) or a goat anti-mouse Alexa Fluor 488 (green) antibody (1:400; 1 h), respectively. Finally, coverslips rinsed with PBS were mounted using the ProLong Gold Antifade Reagent. All images were captured using the Leica TCS SP5 spectral confocal laser-scanning microscope at the same adjustments of laser intensity and photomultiplier sensitivity and were later analyzed by the Leica LAS AF software as reported elsewhere (21, 45, 46). Colocalization analysis of the α7 or dupα7 subunits and the cell membrane was performed on cells transfected with separate or combined constructs of these subunits (α7-HA, dupα7-Myc, α7-Cherry, and dupα7-GFP), using the “LAS AF Process Tool Colocation Function” of the above software as has been described elsewhere (45). Briefly, one horizontal confocal plane (Z-scan) located in a central region of the cell was monitored simultaneously for both fluorescent probes labeling the nAChR subunit and the cytosolic membrane; then, the images registered for both signals were saved and superimposed. For every pixel in the original image, the system plots a corresponding point related to the intensities of each of the two channels. On the resulting cytofluorogram, the x axis represents the intensity of pixels in one channel, and the y axis represents the intensity of pixels in the other channel. If a pixel has equal intensity in both channels, then that pixel point will fall on the diagonal of the graph. The further away the points are from the diagonal line, the higher the intensity is for one channel compared with the other. By selecting a constant elliptical area in the cytofluorogram located around the 45° line, it is possible to exclude background signals; pixels that have high intensities in both channels, which represent the colocalization sites for the nAChR subunit and plasma membrane, were captured and visualized as white points.
Coimmunoprecipitation and immunoblots
GH4C1 cells transfected or not with the appropriate α7 and/or dupα7 construct were homogenized in a specific lysis buffer as described elsewhere (47). Protein concentration in supernatant was quantified using the BCA assay kit (Pierce BCA Protein Assay kit, ThermoFisher Scientific). Dynabead protein G immunoprecipitation kit (Invitrogen Dynal, Oslo, Norway) was used for coimmunoprecipitation experiments following the manufacturer's instructions. For dupα7-Myc subunit immunoprecipitation, the cells were harvested and solubilized; 800 μg of total protein from each sample was reacted with the mouse anti-Myc antibody bound to the Dynabeads protein G. The isolated proteins resulting from immunoprecipitation as well as those from total lysates not subjected to immunoprecipitation (50–80 μg) were resolved by denaturing 10% SDS-polyacrylamide gel, transferred to a polyvinylidene difluoride membrane (Millipore Corp., Billerica, MA), and immunoblotted as described elsewhere with the appropriate antibodies (47). The following primary antibodies and dilutions were used for immunoblotting in this study: mouse anti-GFP (1:1000; 1.5 h); anti-Cherry (1:2000; 1.5 h); anti-Myc (1:1000; 1 h); or rabbit anti-HA (1:1000; 2 h). The secondary (HRP)-conjugated antibodies, anti-mouse IgG or anti-rabbit IgG (1:5000), were incubated at room temperature for 1 h. The resulting bands were detected using ECL Plus reagents (Amersham Biosciences, GE Healthcare, UK).
FRET experiments
GH4C1 and RAW264.7 cells grown on glass coverslips treated with poly-l-lysine were transfected with the pairs of constructs α7-pGFP/dupα7-pmCherry-N1 or dupα7-pGFP/α7-pmCherry-N1 to perform FRET experiments. Forty eight hours after transfection, cells were fixed, rinsed, and mounted using citifluor AF1. Images of selected regions in each cell were taken to determine the FRET efficiency using the acceptor photobleaching technique and the Leica TCS SP2 spectral confocal microscope as described previously (48). Efficiency was calculated using the equation: FRET efficiency = 1 − (Dpre/Dpost), where Dpre and Dpost represent the fluorescence emitted by the donor (GFP) before and after photobleaching of the acceptor (Cherry) using the 561 laser. The data were presented as scatter plots of individual cells, with the mean ± S.D. for each experimental condition. The Kruskal-Wallis test, followed by the Dunn post hoc test, was used for statistical analysis.
Flow cytometry (FC)
The FC methods used in this study to detect the surface expression of endogenous α7-nAChRs in RAW264.7 cells using immunofluorescence staining and the specific labeled toxin for this receptor subtype (Alexa Fluor 488–Bgtx) are similar to those described elsewhere (47). Briefly, cells nucleofected or not with the appropriate dupα7 construct or their corresponding empty vectors were washed with PBS and detached from the plate using 5 mm EDTA/PBS solution. Next, cells expressing dupα7-Myc or the Myc epitope were processed for immunocytochemistry using the mouse anti-Myc antibody (1:200; 1 h) detected by the goat anti-mouse Alexa Fluor 546 (1:600; 30 min). Both non-nucleofected and nucleofected cells were incubated for 1 h at 4 °C with a 2 mm EDTA, 0.5% FBS/PBS solution containing Alexa Fluor 488–Bgtx (1 μm). After staining, the cells were washed thoroughly, and their nuclei were labeled with DAPI for further selection of living cells (DAPI-negative) by FC. Finally, cell fluorescence was processed in a FACS Canto II flow cytometer equipped with the FACS Diva 6.1.2 software (BD Biosciences). Fluorescence intensity provided by Alexa Fluor 488–Bgtx labeling of α7-nAChRs expressed at the cell surface in non-nucleofected cells or in cells expressing each of the assayed constructs are depicted as contour diagrams using the FLOW JO 7.6.5 software. Data for the fluorescence intensity corresponding to cell-surface expression of α7-nAChRs in each experimental condition are given as means ± S.E. One-way ANOVA followed by Bonferroni's post hoc comparison tests were used for the statistical analysis.
Determination of NF-κB nuclear translocation
To determine the nuclear translocation of NF-κB p65 in RAW264.7 cells, the cells in suspension were nucleofected or not (control) with the construct dupα7.pcDNA3.1/Myc-His or with its corresponding empty vector. Subsequently, the cells were seeded on coverslips to be subjected to pretreatment or not with nicotine followed by exposure to LPS in the absence or presence of nicotine. After being fixed and permeabilized (Triton X-100, 0.2%), the cells were initially incubated with rabbit anti-NF-κB p65 antibody (1:50; 2 h) and, in the case of nucleofected cells, with mouse anti-Myc antibody (1:200; 2 h). These primary antibodies were respectively detected by a goat anti-rabbit Alexa Fluor 555 (red) or a goat anti-mouse Alexa Fluor 488 (green) antibody (1:400; 1 h). After staining, the cells were washed thoroughly and their nuclei labeled with DAPI. The coverslips were rinsed with PBS and mounted using ProLong Gold Antifade Reagent. All images were captured and analyzed using the same confocal microscope, conditions, and software reported above. The data were presented as scatter plots of individual microscope fields, with the mean ± S.D. for each experimental condition. One-way ANOVA followed by Bonferroni's post hoc comparison tests were used for the statistical analysis.
TNFα determination
The quantification of TNFα in the supernatant of cultures of RAW264.7 cells seeded in P24 multiwell plates was determined by ELISA. The cells had previously been nucleofected with the dupα7.pcDNA3.1/Myc-His construct or with its corresponding empty vector. Cells from either group were preincubated or not with nicotine followed by exposure to LPS in the presence or absence of nicotine; untreated cells were used to determine basal cytokine release. The concentration of TNFα in the supernatant was determined with the murine TNFα ELISA development kit from PreproTech (UK) following the manufacturer's instructions. Data are represented as mean ± S.E. One-way ANOVA followed by Bonferroni's post hoc comparison tests were used for the statistical analysis.
Study population and absolute quantification of mRNAs by qPCR in PBMCs from the participating subjects
The study population is the same included in a previous pilot study conducted by our group (15); it consists of a cohort of individuals diagnosed with sepsis and a control group of healthy volunteer blood donors. Thus, the experiments contained in this section are a continuation and extension of those of the pilot study, and consequently, they were also planned respecting the ethical principles for medical research in humans (Declaration of Helsinki, 2008) and approved by the Committee of Medical Research Ethics of University Hospital La Paz (Madrid, Spain). All participants signed agreement forms. The cohort group consisted of 33 nonsmoking white patients (13 males and 20 females; age range, 38–80 years (mean value ± S.D., 60.3 ± 12.7)) admitted to La Paz University Hospital from March to October, 2012, after meeting the diagnostic criteria for sepsis. The demographic and clinical characteristics, exclusion criteria, and precautions taken to minimize the impact of confounding factors in the patients' group have been detailed in the previous pilot study (15). The control group consisted of 33 healthy white nonsmoking volunteer blood donors (14 males, 19 females; age range 35–78 years (mean value ± S.D., 58.3 ± 11.6)), who had not taken medications in the month prior to enrollment.
To measure the number of dupα7 and α7 mRNA copies by qPCR in PBMCs from the participants, total RNA was extracted from the cells isolated from each subject as we have reported in the previous pilot study (15). Techniques for gene expression analysis by qPCR from reverse-transcribed RNA, using the SYBR Green-based assays (Bio-Rad) and the ABI Prism 7500 Sequence Detector (Applied Biosystems, Foster City, CA), have been described elsewhere (15, 21, 47). The set of primers and PCR cycling conditions were as follows: dupα7 forward 5′-CAATTGCTAATCCAGCATTTGTGG-3′ and reverse 5′-CCCAGAAGAATTCACCAACACG-3′; α7 forward 5′-GCTGCAAATGTCTTGGACAGAT-3′ and reverse 5′-AACAGTTTCACCCCTGGATAT-3′; 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s and 60 °C for 30 s. The number of dupα7 and α7 mRNA molecules expressed in PBMCs was determined as described elsewhere (40) on the basis of a standard six-point curve prepared with the plasmids dupα7.pcDNA3.1/Myc-His and α7-HA.pcDNA3.1 (our own laboratory constructs) as templates and applying the following formula: number = (ng·number/mol)/(bp·ng/g·g/mol of bp) (http://cels.uri.edu/gsc/cndna.html).8 The Spearman correlation coefficient was used to analyze correlations between the absolute dupα7 mRNA expression levels or the α7/dupα7 mRNA ratio in PBMCs determined in the actual study, with serum concentrations of inflammatory markers or APACHE II scores determined in the previous study (15). Additionally, the same analysis was applied to investigate the correlation between the absolute dupα7 and α7 mRNA expression levels in the PBMCs from the patients.
Author contributions
M. C. M., C. M.-S., F. A., F. Z., C. G., and C. M. data curation; M. C. M., C. M.-S., F. A., and C. M. formal analysis; M. C. M., C. M.-S., G. A., J. L. C., F. A., A. B., F. Z., C. G., M. E., J. R., and C. M. investigation; M. C. M., C. M.-S., G. A., J. L. C., F. A., A. B., F. Z., C. G., M. E., J. R., and C. M. methodology; M. C. M., C. M.-S., F. A., and C. M. writing-original draft; G. A. project administration; F. A. and C. M. conceptualization; F. A. and C. M. supervision; F. A. and C. M. funding acquisition; F. A. and C. M. validation; F. A. and C. M. writing-review and editing; C. M. resources.
Acknowledgments
We thank Prof. Henk Sipma (Johnson & Johnson Pharmaceutical Research & Development, Division of Janssen Pharmaceutica N. V., Beerse, Belgium) for donating the α7.pSP64T plasmid and Prof. Juan José de la Cruz (Department of Preventive Medicine and Public Health, Medical School, Universidad Autónoma de Madrid) for advice in conducting statistical analysis. We also thank the patients who participated, the Internal Medicine Service, and Intensive Care Unit nurses, residents, and senior staff attending physicians of the University Hospital La Paz for their cooperation in making this study possible.
This work was supported in part by Ministry of Economy, Industry and Competitiveness, Government of Spain Grants SAF2011-23575, SAF2014-56623-R, and SAF2017-82689-R (to C. M. and F. A.). The authors declare that they have no conflicts of interest with the contents of this article.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party hosted site.
- α7-nAChR
- α7 subtype of the nicotinic acetylcholine receptor
- ACh
- acetylcholine
- APACHE
- Acute Physiology and Chronic Health Evaluation
- Bgtx
- α-bungarotoxin
- CAP
- cholinergic anti-inflammatory pathway
- CNS
- central nervous system
- CRP
- C-reactive protein
- dupα7
- partial duplicated isoform of the human α7 subunit
- ER
- endoplasmic reticulum
- FBS
- fetal bovine serum
- FC
- flow cytometry
- HA
- influenza hemagglutinin
- IL
- interleukin
- IP
- immunoprecipitation
- LPS
- lipopolysaccharide
- PBMC
- peripheral blood mononuclear cells
- qPCR
- real-time quantitative PCR
- SSC
- side scatter
- TNF-α
- tumor necrosis factor-α
- WGA
- wheat germ agglutinin
- HRP
- horseradish peroxidase
- DAPI
- 4′,6-diamidino-2-phenylindole
- ANOVA
- analysis of variance.
References
- 1. Albuquerque E. X., Pereira E. F., Alkondon M., and Rogers S. W. (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol. Rev. 89, 73–120 10.1152/physrev.00015.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fasoli F., and Gotti C. (2015) Structure of neuronal nicotinic receptors. Curr. Top. Behav. Neurosci. 23, 1–17 10.1007/978-3-319-13665-3_1 [DOI] [PubMed] [Google Scholar]
- 3. Unwin N. (2005) Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 346, 967–989 10.1016/j.jmb.2004.12.031 [DOI] [PubMed] [Google Scholar]
- 4. Dajas-Bailador F., and Wonnacott S. (2004) Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 25, 317–324 10.1016/j.tips.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 5. Dineley K. T., Pandya A. A., and Yakel J. L. (2015) Nicotinic ACh receptors as therapeutic targets in CNS disorders. Trends Pharmacol. Sci. 36, 96–108 10.1016/j.tips.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arias H. R., Richards V. E., Ng D., Ghafoori M. E., Le V., and Mousa S. A. (2009) Role of non-neuronal nicotinic acetylcholine receptors in angiogenesis. Int. J. Biochem. Cell Biol. 41, 1441–1451 10.1016/j.biocel.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 7. Borovikova L. V., Ivanova S., Zhang M., Yang H., Botchkina G. I., Watkins L. R., Wang H., Abumrad N., Eaton J. W., and Tracey K. J. (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- 8. Wang H., Yu M., Ochani M., Amella C. A., Tanovic M., Susarla S., Li J. H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C. J., and Tracey K. J. (2003) Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 421, 384–388 [DOI] [PubMed] [Google Scholar]
- 9. Wang H., Liao H., Ochani M., Justiniani M., Lin X., Yang L., Al-Abed Y., Wang H., Metz C., Miller E. J., Tracey K. J., and Ulloa L. (2004) Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 10, 1216–1221 10.1038/nm1124 [DOI] [PubMed] [Google Scholar]
- 10. Rosas-Ballina M., Ochani M., Parrish W. R., Ochani K., Harris Y. T., Huston J. M., Chavan S., and Tracey K. J. (2008) Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. U.S.A. 105, 11008–11013 10.1073/pnas.0803237105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosas-Ballina M., Olofsson P. S., Ochani M., Valdés-Ferrer S. I., Levine Y. A., Reardon C., Tusche M. W., Pavlov V. A., Andersson U., Chavan S., Mak T. W., and Tracey K. J. (2011) Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101 10.1126/science.1209985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tracey K. J. (2007) Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 117, 289–296 10.1172/JCI30555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huston J. M., Gallowitsch-Puerta M., Ochani M., Ochani K., Yuan R., Rosas-Ballina M., Ashok M., Goldstein R. S., Chavan S., Pavlov V. A., Metz C. N., Yang H., Czura C. J., Wang H., and Tracey K. J. (2007) Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit. Care Med. 35, 2762–2768 10.1097/01.CCM.0000288102.15975.BA [DOI] [PubMed] [Google Scholar]
- 14. de Jonge W. J., and Ulloa L. (2007) The α7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br. J. Pharmacol. 151, 915–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cedillo J. L., Arnalich F., Martín-Sánchez C., Quesada A., Rios J. J., Maldifassi M. C., Atienza G., Renart J., Fernández-Capitán C., García-Rio F., López-Collazo E., and Montiel C. (2015) Usefulness of α7 nicotinic receptor messenger RNA levels in peripheral blood mononuclear cells as a marker for cholinergic antiinflammatory pathway activity in septic patients: results of a pilot study. J. Infect. Dis. 211, 146–155 10.1093/infdis/jiu425 [DOI] [PubMed] [Google Scholar]
- 16. Gault J., Robinson M., Berger R., Drebing C., Logel J., Hopkins J., Moore T., Jacobs S., Meriwether J., Choi M. J., Kim E. J., Walton K., Buiting K., Davis A., Breese C., et al. (1998) Genomic organization and partial duplication of the human α7 neuronal nicotinic acetylcholine receptor gene (CHRNA7). Genomics 52, 173–185 10.1006/geno.1998.5363 [DOI] [PubMed] [Google Scholar]
- 17. Riley B., Williamson M., Collier D., Wilkie H., and Makoff A. (2002) A 3-Mb map of a large segmental duplication overlapping the α7-nicotinic acetylcholine receptor gene (CHRNA7) at human 15q13-q14. Genomics 79, 197–209 10.1006/geno.2002.6694 [DOI] [PubMed] [Google Scholar]
- 18. Locke D. P., Archidiacono N., Misceo D., Cardone M. F., Deschamps S., Roe B., Rocchi M., and Eichler E. E. (2003) Refinement of a chimpanzee pericentric inversion breakpoint to a segmental duplication cluster. Genome Biol. 4, R50 10.1186/gb-2003-4-8-r50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Costantini T. W., Dang X., Coimbra R., Eliceiri B. P., and Baird A. (2015) CHRFAM7A, a human-specific and partially duplicated α7-nicotinic acetylcholine receptor gene with the potential to specify a human-specific inflammatory response to injury. J. Leukoc. Biol. 97, 247–257 10.1189/jlb.4RU0814-381R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Luca V., Likhodi O., Van Tol H. H., Kennedy J. L., and Wong A. H. (2006) Regulation of α7-nicotinic receptor subunit and α7-like gene expression in the prefrontal cortex of patients with bipolar disorder and schizophrenia. Acta Psychiatr. Scand. 114, 211–215 10.1111/j.1600-0447.2006.00785.x [DOI] [PubMed] [Google Scholar]
- 21. de Lucas-Cerrillo A. M., Maldifassi M. C., Arnalich F., Renart J., Atienza G., Serantes R., Cruces J., Sánchez-Pacheco A., Andrés-Mateos E., and Montiel C. (2011) Function of partially duplicated human α7 nicotinic receptor subunit CHRFAM7A gene: potential implications for the cholinergic anti-inflammatory response. J. Biol. Chem. 286, 594–606 10.1074/jbc.M110.180067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freedman R., Leonard S., Gault J. M., Hopkins J., Cloninger C. R., Kaufmann C. A., Tsuang M. T., Farone S. V., Malaspina D., Svrakic D. M., Sanders A., and Gejman P. (2001) Linkage disequilibrium for schizophrenia at the chromosome 15q13–14 locus of the α7-nicotinic acetylcholine receptor subunit gene (CHRNA7). Am. J. Med. Genet. 105, 20–22 10.1002/1096-8628(20010108)105:1%3C20::AID-AJMG1047%3E3.0.CO%3B2-C [DOI] [PubMed] [Google Scholar]
- 23. Hong C. J., Lai I. C., Liou L. L., and Tsai S. J. (2004) Association study of the human partially duplicated α7 nicotinic acetylcholine receptor genetic variant with bipolar disorder. Neurosci. Lett. 355, 69–72 10.1016/j.neulet.2003.10.043 [DOI] [PubMed] [Google Scholar]
- 24. Casey J. P., Magalhaes T., Conroy J. M., Regan R., Shah N., Anney R., Shields D. C., Abrahams B. S., Almeida J., Bacchelli E., Bailey A. J., Baird G., Battaglia A., Berney T., Bolshakova N., et al. (2012) A novel approach of homozygous haplotype sharing identifies candidate genes in autism spectrum disorder. Hum. Genet. 131, 565–579 10.1007/s00439-011-1094-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Araud T., Graw S., Berger R., Lee M., Neveu E., Bertrand D., and Leonard S. (2011) The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant-negative regulator of α7*nAChR function. Biochem. Pharmacol. 82, 904–914 10.1016/j.bcp.2011.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang Y., Xiao C., Indersmitten T., Freedman R., Leonard S., and Lester H. A. (2014) The duplicated α7 subunits assemble and form functional nicotinic receptors with the full-length α7. J. Biol. Chem. 289, 26451–26463 10.1074/jbc.M114.582858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bolte S., and Cordelières F. P. (2006) A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 10.1111/j.1365-2818.2006.01706.x [DOI] [PubMed] [Google Scholar]
- 28. Adler J., and Parmryd I. (2010) Quantifying colocalization by correlation: the Pearson correlation coefficient is superior to the Mander's overlap coefficient. Cytometry A 77, 733–742 [DOI] [PubMed] [Google Scholar]
- 29. Dunn K. W., Kamocka M. M., and McDonald J. H. (2011) A practical guide to evaluating colocalization in biological microscopy. Am. J. Physiol. Cell Physiol. 300, C723–C742 10.1152/ajpcell.00462.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Knaus W. A., Draper E. A., Wagner D. P., and Zimmerman J. E. (1985) APACHE II: a severity of disease classification system. Crit. Care Med. 13, 818–829 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 31. García-Guzmán M., Sala F., Sala S., Campos-Caro A., and Criado M. (1994) Role of two acetylcholine receptor subunit domains in homomer formation and intersubunit recognition, as revealed by α3 and α7 subunit chimeras. Biochemistry 33, 15198–15203 10.1021/bi00254a031 [DOI] [PubMed] [Google Scholar]
- 32. Mukherjee J., Kuryatov A., Moss S. J., Lindstrom J. M., and Anand R. (2009) Mutations of cytosolic loop residues impair assembly and maturation of α7 nicotinic acetylcholine receptors. J. Neurochem. 110, 1885–1894 10.1111/j.1471-4159.2009.06285.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kracun S., Harkness P. C., Gibb A. J., and Millar N. S. (2008) Influence of the M3–M4 intracellular domain upon nicotinic acetylcholine receptor assembly, targeting and function. Br. J. Pharmacol. 153, 1474–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gault J., Hopkins J., Berger R., Drebing C., Logel J., Walton C., Short M., Vianzon R., Olincy A., Ross R. G., Adler L. E., Freedman R., and Leonard S. (2003) Comparison of polymorphisms in the α7 nicotinic receptor gene and its partial duplication in schizophrenic and control subjects. Am. J. Med. Genet. B Neuropsychiatr. Genet. 123, 39–49 10.1002/ajmg.b.20061 [DOI] [PubMed] [Google Scholar]
- 35. Kim T. H., Kim S. J., and Lee S. M. (2014) Stimulation of the α7 nicotinic acetylcholine receptor protects against sepsis by inhibiting Toll-like receptor via phosphoinositide 3-kinase activation. J. Infect. Dis. 209, 1668–1677 10.1093/infdis/jit669 [DOI] [PubMed] [Google Scholar]
- 36. Yang Y. H., Li D. L., Bi X. Y., Sun L., Yu X. J., Fang H. L., Miao Y., Zhao M., He X., Liu J. J., and Zang W. J. (2015) Acetylcholine inhibits LPS-induced MMP-9 production and cell migration via the α7 nAChR-JAK2/STAT3 pathway in RAW264.7 cells. Cell. Physiol. Biochem. 36, 2025–2038 10.1159/000430170 [DOI] [PubMed] [Google Scholar]
- 37. Benfante R., Antonini R. A., De Pizzol M., Gotti C., Clementi F., Locati M., and Fornasari D. (2011) Expression of the α7 nAChR subunit duplicate form (CHRFAM7A) is down-regulated in the monocytic cell line THP-1 on treatment with LPS. J. Neuroimmunol. 230, 74–84 10.1016/j.jneuroim.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 38. Severance E. G., Dickerson F. B., Stallings C. R., Origoni A. E., Sullens A., Monson E. T., and Yolken R. H. (2009) Differentiating nicotine- versus schizophrenia-associated decreases of the α7 nicotinic acetylcholine receptor transcript, CHRFAM7A, in peripheral blood lymphocytes. J. Neural Transm. 116, 213–220 10.1007/s00702-008-0164-y [DOI] [PubMed] [Google Scholar]
- 39. Baird A., Coimbra R., Dang X., Eliceiri B. P., and Costantini T. W. (2016) Up-regulation of the human-specific CHRFAM7A gene in inflammatory bowel disease. BBA Clin. 5, 66–71 10.1016/j.bbacli.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bordas A., Cedillo J. L., Arnalich F., Esteban-Rodriguez I., Guerra-Pastrián L., de Castro J., Martín-Sánchez C., Atienza G., Fernández-Capitan C., Rios J. J., and Montiel C. (2017) Expression patterns for nicotinic acetylcholine receptor subunit genes in smoking-related lung cancers. Oncotarget 8, 67878–67890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Singh S., Pillai S., and Chellappan S. (2011) Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J. Oncol. 2011, 456743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pillai S., and Chellappan S. (2012) α7 nicotinic acetylcholine receptor subunit in angiogenesis and epithelial to mesenchymal transition. Curr. Drug Targets 13, 671–679 10.2174/138945012800398847 [DOI] [PubMed] [Google Scholar]
- 43. Schuller H. M. (2012) Regulatory role of the α7 nAChR in cancer. Curr. Drug Targets 13, 680–687 10.2174/138945012800398883 [DOI] [PubMed] [Google Scholar]
- 44. Zhang C., Ding X. P., Zhao Q. N., Yang X. J., An S. M., Wang H., Xu L., Zhu L., and Chen H. Z. (2016) Role of α7-nicotinic acetylcholine receptor in nicotine-induced invasion and epithelial-to-mesenchymal transition in human non-small cell lung cancer cells. Oncotarget 7, 59199–59208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Solís-Garrido L. M., Pintado A. J., Andrés-Mateos E., Figueroa M., Matute C., and Montiel C. (2004) Cross-talk between native plasmalemmal Na+/Ca2+ exchanger and inositol 1,4,5-trisphosphate-sensitive Ca2+ internal store in Xenopus oocytes. J. Biol. Chem. 279, 52414–52424 10.1074/jbc.M408872200 [DOI] [PubMed] [Google Scholar]
- 46. Serantes R., Arnalich F., Figueroa M., Salinas M., Andrés-Mateos E., Codoceo R., Renart J., Matute C., Cavada C., Cuadrado A., and Montiel C. (2006) Interleukin-1β enhances GABAA receptor cell-surface expression by a phosphatidylinositol 3-kinase/Akt pathway: relevance to sepsis-associated encephalopathy. J. Biol. Chem. 281, 14632–14643 10.1074/jbc.M512489200 [DOI] [PubMed] [Google Scholar]
- 47. Maldifassi M. C., Atienza G., Arnalich F., López-Collazo E., Cedillo J. L., Martín-Sánchez C., Bordas A., Renart J., and Montiel C. (2014) A new IRAK-M-mediated mechanism implicated in the anti-inflammatory effect of nicotine via α7 nicotinic receptors in human macrophages. PLoS ONE 9, e108397 10.1371/journal.pone.0108397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li H., Sánchez-Torres J., del Carpio A. F., Nogales-González A., Molina-Ortiz P., Moreno M. J., Török K., and Villalobo A. (2005) The adaptor Grb7 is a novel calmodulin-binding protein: functional implications of the interaction of calmodulin with Grb7. Oncogene 24, 4206–4219 10.1038/sj.onc.1208591 [DOI] [PubMed] [Google Scholar]