Abstract
Variants in the transcription factor-7–like 2 (TCF7L2/TCF4) gene, involved in Wnt signaling, are associated with type 2 diabetes. Loss of Tcf7l2 selectively from the β cell in mice has previously been shown to cause glucose intolerance and to lower β cell mass. Deletion of the tumor suppressor liver kinase B1 (LKB1/STK11) leads to β cell hyperplasia and enhanced glucose-stimulated insulin secretion, providing a convenient genetic model for increased β cell growth and function. The aim of this study was to explore the possibility that Tcf7l2 may be required for the effects of Lkb1 deletion on insulin secretion in the mouse β cell. Mice bearing floxed Lkb1 and/or Tcf7l2 alleles were bred with knockin mice bearing Cre recombinase inserted at the Ins1 locus (Ins1Cre), allowing highly β cell–selective deletion of either or both genes. Oral glucose tolerance was unchanged by the further deletion of a single Tcf7l2 allele in these cells. By contrast, mice lacking both Tcf7l2 alleles on this background showed improved oral glucose tolerance and insulin secretion in vivo and in vitro compared with mice lacking a single Tcf7l2 allele. Biallelic Tcf7l2 deletion also enhanced β cell proliferation, increased β cell mass, and caused changes in polarity as revealed by the “rosette-like” arrangement of β cells. Tcf7l2 deletion also increased signaling by mammalian target of rapamycin (mTOR), augmenting phospho-ribosomal S6 levels. We identified a novel signaling mechanism through which a modifier gene, Tcf7l2, lies on a pathway through which LKB1 acts in the β cell to restrict insulin secretion.
Keywords: pancreatic islet, insulin secretion, cell growth, liver kinase B1 (LKB1), T-cell factor (TCF), pancreatic β cell, TCF7L2
Introduction
Type 2 diabetes currently affects 415 million individuals worldwide, and this number is expected to rise to >600 million by 2040 (www.diabetesatlas.org).2 Pancreatic β cell failure is an essential, if still poorly understood, component of disease development and progression (1).
Genome-wide association studies have identified more than 100 loci associated with disease risk (2) with the majority affecting insulin secretion rather than the action of the hormone. Although in a few cases the likely effector transcript has been identified (3, 4), for most loci neither the causal gene nor its mechanism of action at the cellular level has been defined. Of the commonly inherited risk variants, those in the transcription factor-7–like 2 (TCF7L2/TCF4)3 gene, including rs7903146, display among the highest odds ratio for exaggerated type 2 diabetes risk (∼1.2/allele) (5). The identified single-nucleotide polymorphism (SNP) rs7903146 is located in the third intron of TCF7L2 and has been estimated to contribute to 10–25% of all cases of diabetes lean patients (6) TCF7L2 lies at the foot of the wingless (Wnt) signaling pathway activated both by Wnt ligands and by certain growth factors (e.g. insulin and IGF-1), which act through receptor tyrosine kinases (7). In the presence of Wnt ligands, a signaling cascade results in stabilization and nuclear localization of β-catenin, which interacts with T cell–specific factor/lymphoid enhancer–binding factor to control transcription of target genes. In the absence of Wnt ligands, β-catenin is degraded by protein complexes, including axin-2 and glycogen synthase kinase 3β (GSK3β) (8).
Several studies have explored the role of Tcf7l2 in insulin secretion in model systems. Thus, inhibition of TCF7L2 activity in a human or in rat insulinoma cell line (9, 10) inhibited insulin secretion in response to glucose. Likewise, deletion of the Tcf7l2 gene selectively in the β cell in mice (11, 12) reduced insulin production in older animals and impaired the expansion of β cell mass in response to a high-fat diet (11, 12). Finally, in a separate study (13), re-expression of TCF7L2 on a null background improved glucose tolerance. Importantly, the degree to which the action of disease-risk variants on the β cell may be context-dependent is unclear. Thus, TCF7L2 variants could have different pathophysiological effects among the five different subpopulations of diabetic patients identified in a recent study (14). The mechanisms, including the genetic drivers, behind these differences remain obscure.
Here, we have explored the impact of Tcf7l2 deletion in a model of β cell expansion driven by artificially enhanced growth factor signaling. Several earlier observations have suggested that a reciprocal relationship may exist between the tumor suppressor liver kinase B1 (LKB1/STK11) and TCF7L2 signaling in other systems. First, the LKB1/STK11 homologue XEEK1 is required for Wnt signaling in Xenopus laevis and acts by phosphorylating and inactivating GSK3 (15). Moreover, in Peutz-Jeghers syndrome, Wnt signaling activation is correlated to LKB1 expression (16). Similarly, in esophageal carcinoma patients, LKB1 is down-regulated and Wnt target genes are up-regulated through inhibition of GSK3β activity (17). We (18, 19) and others (20, 21) have shown previously that inactivation of LKB1 in the β cell leads to a substantial increase in insulin production and improved glucose tolerance. LKB1 is a tumor suppressor mutated in Peutz-Jeghers syndrome, a premalignant condition characterized by hamartomatous polyps and an increased risk of all cancers (22, 23). Although the mechanisms involved remain to be fully elucidated, increases in β cell mass (18), changes in the signaling pathways activated by glucose (19, 24), and alterations in cellular morphology and polarity (18, 20, 21) all appear to play a role in enhancing insulin secretion in the Lkb1-null β cell. Acting via the fuel-sensitive enzyme AMP-activated protein kinase (AMPK), and the tuberous sclerosis complex TSC1–TSC2, LKB1 also inhibits mammalian target of rapamycin (mTOR) signaling to restrict protein synthesis and cell division (25). This pathway may oppose β cell expansion in the adult because AMPK is likely to be active in these cells in the fasting state (26, 27).
To explore the above possibilities, we used an epistasis approach to examine the impact on the pancreatic β cell of deleting Tcf7l2 in the absence of Lkb1 alleles. We show that, in contrast to the action of Tcf7l2 ablation to impair insulin secretion in WT mice, loss of this transcription factor on an Lkb1-null background further increases insulin secretion, β cell size, and β cell mass and augments mTOR activity, consistent with a role for TCF7L2 as an inhibitor of mTOR signaling.
Results
Generation of β cell–specific Lkb1/Tcf7l2 double-knockout mice
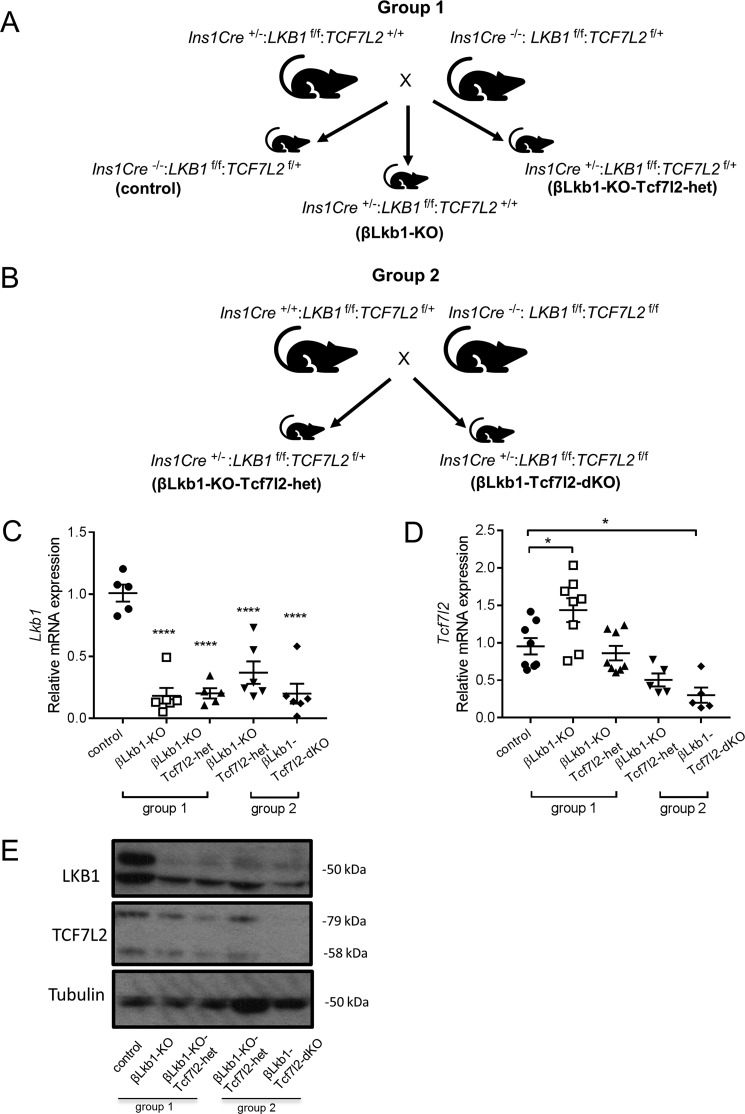
To study the impact of the deletion of Tcf7l2 and Lkb1 in the pancreatic β cell, we established breeding pairs on a mixed background (C57BL/6J, FVB/NJ, and 129sS1/SvlmJ) to produce offspring deleted for Lkb1 and/or Tcf7l2 selectively in the β cell using the highly selective Cre deleter strain Ins1Cre in which Cre recombinase is inserted into the Ins1 locus (28, 29) (Fig. 1, A and B). Deletion at other sites, including the brain, is minimal in this model, and, importantly, the transgene does not carry the human growth hormone minigene present in alternative Cre strains (e.g. RIP2.Cre) (30). Consequently, effects of Ins1Cre expression alone on glucose homeostasis are not observed. Because a strategy generating all possible genotypes would have produced mice homozygous for deletion of both alleles at a frequency of 1 per 64 pups, we designed instead two separate breeding colonies to reduce animal numbers in accordance with the 3Rs. The following offspring were produced and named as follows (group 1): control (Ins1Cre−/−:Lkb1f/f:Tcf7l2f/+),βLkb1-KO (Ins1Cre+/−:Lkb1f/f:Tcf7l2+/+), and βLkb1-KO-Tcf7l2-het (Ins1Cre+/−:Lkb1f/f:Tcf7l2f/+; Fig. 1A, group 1). The second breeding strategy (group 2) generated littermates βLkb1-KO-Tcf7l2-het (Ins1Cre+/−:Lkb1f/f:Tcf7l2f/+) and βLkb1-Tcf7l2-dKO (Ins1Cre +/−:Lkb1f/f:Tcf7l2f/f; Fig. 1B, group 2).
Figure 1.
Breeding strategy for the generation of Lkb1/Tcf7l2 deletion mutants in the β cell and confirmation of the mouse model. A, littermate pups from group 1 display three different genotypes: control as Ins1Cre−/−:Lkb1f/f:Tcf7l2f/+, Lkb1 deletion only as βLkb1-KO (Ins1Cre+/−:Lkb1f/f:Tcf7l2+/+), and one single Tcf7l2 allele deleted in an Lkb1-null background as βLkb1-KO-Tcf7l2-het (Ins1Cre+/−:Lkb1f/f:Tcf7l2f/+). B, littermate pups from group 2 display two different genotypes; one single Tcf7l2 allele deleted in an Lkb1-null background as βLkb1-KO-Tcf7l2-het (Ins1Cre+/−:Lkb1f/f:Tcf7l2f/+) and two single Tcf7l2 alleles deleted in an Lkb1-null background as βLkb1-Tcf7l2-dKO (Ins1Cre+/−:Lkb1f/f:Tcf7l2f/f). C, RT-qPCR expression of Lkb1 mRNA in isolated islets (n = 5–6 mice/genotype). D, RT-qPCR expression of Tcf7l2 mRNA in isolated islets (n = 5–9 mice/genotype). E, Western blotting for TCF7L2 and LKB1 in isolated islets (n = 2 mice/genotype). Error bars represent the mean ±S.E.; *, p < 0.05; ****, p < 0.0001.
We first measured Lkb1 and Tcf7l2 gene expression in isolated islets using RT-qPCR analysis. The level of endogenous Lkb1 mRNA was strongly decreased in the presence of Cre transgene when one single or both Tcf7l2 alleles were floxed as expected. Likewise, the level of Tcf7l2 mRNA was decreased when both alleles were floxed (βLkb1-Tcf7l2-dKO) compared with control. Importantly, we observed no significant differences in the level of Lkb1 and Tcf7l2 mRNAs between βLkb1-KO-Tcf7l2-het mice from either group 1 or 2. Of note, deletion of Lkb1 significantly increased Tcf7l2 expression when both Tcf7l2 alleles were present in βLkb1-KO mice (Fig. 1, C and D). Likewise, we observed decreased LKB1 and TCF7L2 protein expression in isolated islets from βLkb1-KO, βLkb1-KO-Tcf7l2-het, and βLkb1-Tcf7l2-dKO compared with control islets (Fig. 1E).
Deletion in the β cell of two Tcf7l2 alleles in an Lkb1-null background improves oral glucose tolerance and insulin secretion
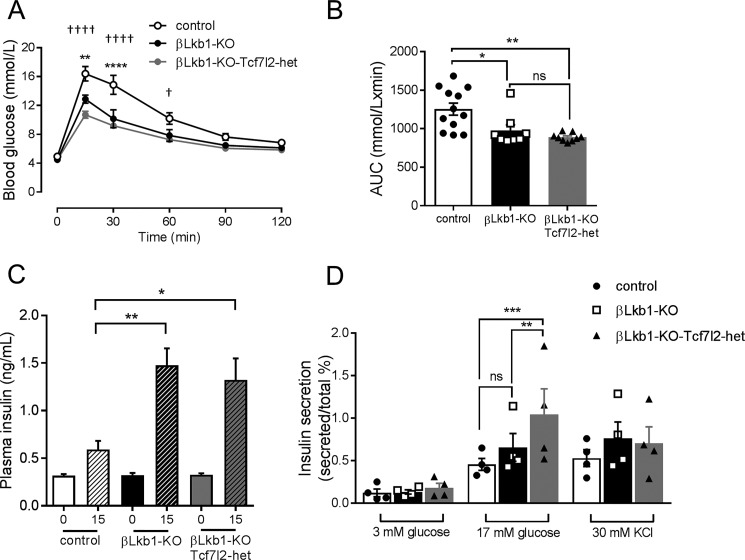
Consistent with previous findings (18–21, 28), deletion of both Lkb1 alleles in the β cell improved glucose tolerance in mice aged 8 weeks (Fig. 2, A and B). These changes were not associated with any alteration in body weight (Fig. S1A). Glucose tolerance was not further affected by the additional deletion of a single Tcf7l2 allele (Fig. 2, A and B). Deletion of Lkb1 alone also lowered fed glycemia, and this action was attenuated by the additional deletion of Tcf7l2 (Fig. S1B). Insulin sensitivity was unchanged by deletion of a single Tcf7l2 allele (Fig. S1D).
Figure 2.
Monoallelic deletion does not affect Lkb1 deletion-mediated improvements in glucose tolerance and augmented insulin secretion in males. A, oral glucose (2 g/kg) tolerance measurements were performed as described under “Experimental procedures” (n = 9–12 mice/genotype). p values were statistically determined by a two-way ANOVA test with a Bonferroni post-test (**, p < 0.01 and ****, p < 0.0001 βLkb1-KO versus control; †, p < 0.05 and ††††, p < 0.0001 βLkb1-KO-Tcf7l2-het versus control; p = 0.623 βLkb1-KO versus βLkb1-KO-Tcf7l2-het). B, area under the curve (AUC) for oral glucose tolerance tests (*, p < 0.05 βLkb1-KO versus control; **, p < 0.01 βLkb1-KO-Tcf7l2-het versus control). C, insulin plasma levels were measured in vivo 15 min after intraperitoneal injection of glucose (3 g/kg) (n = 7–9 mice/genotype; *, p < 0.05 and **, p < 0.01 versus 15-min control). D, insulin secretion in vitro was measured from groups of 10 size-matched isolated islets during static incubation (see “Experimental procedures”) and at the indicated glucose or KCl concentrations (n = 4 independent experiments; **, p < 0.01 βLkb1-KO-Tcf7l2-het versus βLkb1-KO; ***, p < 0.001 βLkb1-KO-Tcf7l2-het versus control). Error bars represent the mean ±S.E.; ns, not significant.
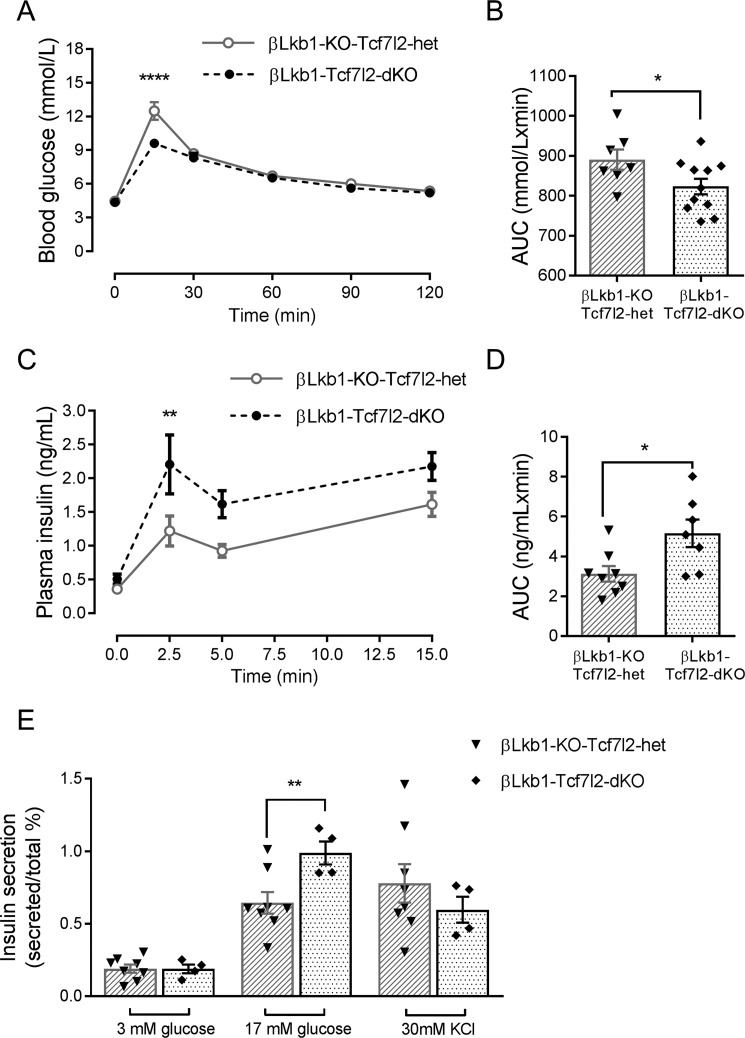
As described previously (18–21, 28), Lkb1 deletion substantially increased insulin release in response to glucose in vivo (Fig. 2C). Interestingly, glucose-stimulated insulin secretion in vitro only tended to increase on an Lkb1-null background compared with control (Fig. 2D). Monoallelic Tcf7l2 deletion had little further impact on these changes such that the glycemic phenotype of βLkb1-KO-Tcf7l2-het did not differ from βLkb1-KO mice in vivo, but insulin release was enhanced in vitro (Fig. 2, C and D). In contrast, when βLkb1-KO-Tcf7l2-het mice were compared with homozygous βLkb1-Tcf7l2-dKO animals deleted for both Tcf7l2 alleles, we observed a further improvement in glucose tolerance (Fig. 3, A and B) but unchanged, body weight, fed glycemia, and insulin sensitivity (Fig. S2, A, C, and E). A substantial (∼2-fold) increase in acute insulin release in response to glucose injection was also observed in vivo (Fig. 3, C and D) when comparing βLkb1-Tcf7l2-dKO with βLkb1-KO-Tcf7l2-het littermates. Likewise, comparing islets isolated from mice deleted for both versus a single Tcf7l2 allele, insulin secretion was significantly increased in response to elevated glucose but not to depolarization with KCl (Fig. 3E). Thus, deletion of Tcf7l2 on an Lkb1-null background exerts an effect whose direction is opposite to that seen in control islets (11, 12). In females, deletion of one or two Tcf7l2 alleles on an Lkb1-null background did not affect oral glucose tolerance, insulin sensitivity, fed glycemia, and body weight compared with Lkb1 deletion only (βLkb1-KO; Fig. S2).
Figure 3.
Deletion of both Tcf7l2 alleles potentiates the effects of Lkb1 deletion on glucose tolerance and insulin secretion in males. Oral glucose tolerance experiments were performed as described in Fig. 2 legend (A and B) (n = 7–11 mice/genotype). Insulin plasma levels were measured in vivo 2.5, 5, and 15 min after intraperitoneal injection of glucose (3 g/kg) (C and D) (n = 7–8 mice/genotype). E, insulin secretion in vitro was measured from groups of 10 size-matched isolated islets during static incubation (see “Experimental procedures”) and at the indicated glucose or KCl concentrations (n = 4–6 mice/genotype; *, p < 0.05; **, p < 0.01; ****, p < 0.0001). Gray open circles, βLkb1-KO-Tcf7l2-het; black filled circles, βLkb1-Tcf7l2-dKO. Error bars represent the mean ±S.E. AUC, area under the curve.
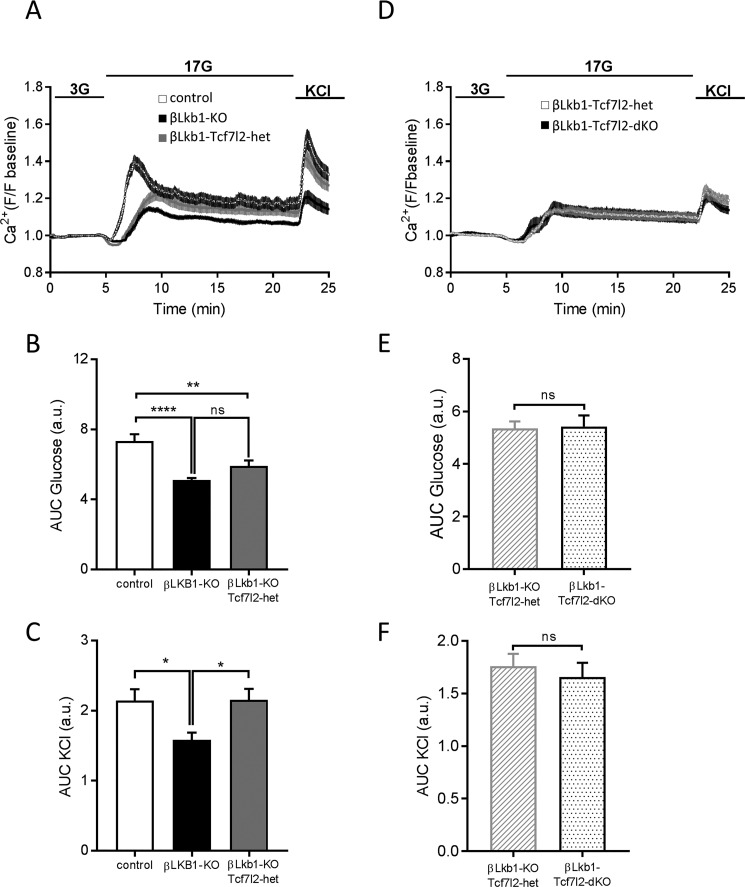
Next, we sought to explore intracellular free calcium (Ca2+) dynamics to elucidate whether these may be altered and contribute to the enhanced insulin secretion. Islets derived from βLkb1-KO mice displayed a delayed and decreased response to high glucose in free cytosolic Ca2+ increases compared with control animals (Fig. 4, A and B). A similar degree of impairment was observed after the additional deletion of a single Tcf7l2 allele. Interestingly, islets from βLkb1-KO mice showed a decreased response to depolarization with KCl compared with control mice, whereas βLkb1-KO-Tcf7l2-het islets displayed a similar response to KCl compared with control islets (Fig. 4, A and C). In group 2, no difference in response to high glucose or KCl was noted between islets from βLkb1-KO-Tcf7l2-het and βLkb1-Tcf7l2-dKO mice (Fig. 4, D, E, and F).
Figure 4.
Deletion of one or two Tcf7l2 alleles did not further alter Ca2+ dynamics in response to glucose but restored responses to KCl in males. A, changes in free cytosolic Ca2+ in response to 3 mmol/liter glucose (3G), 17 mmol/liter glucose (17G), and 20 mmol/liter KCl in group 1. B, quantification of area under the curve (AUC) for glucose responses. C, quantification of area under the curve for KCl responses. D, free cytosolic Ca2+ changes in response to 3 mmol/liter glucose (3G), 17 mmol/liter glucose (17G), and 20 mmol/liter KCl in group 2. E, quantification of area under the curve for glucose responses. F, quantification of area under the curve for KCl responses. Each plot represents the average of 16–29 islets (n = 3 per genotype; *, p < 0.05; **, p < 0.01; ****, p < 0.0001). Error bars represent the mean ±S.E.; ns, not significant. a.u., arbitrary unit.
Impact of Lkb1 and Tcf7l2 deletion on islet morphology
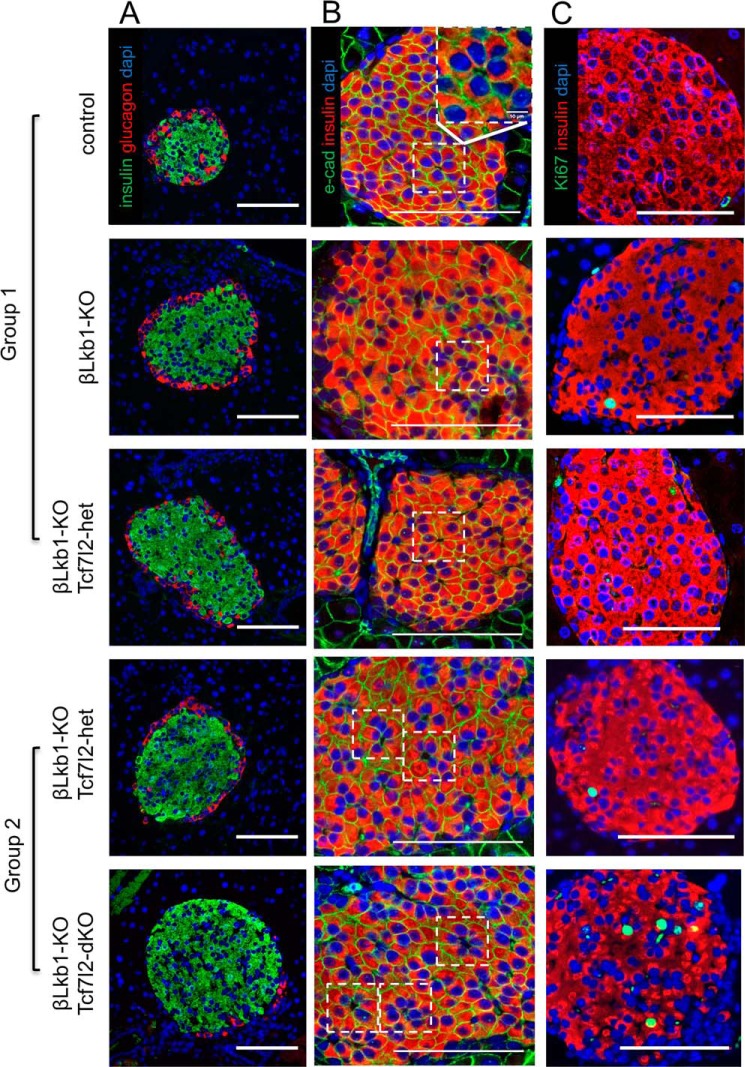
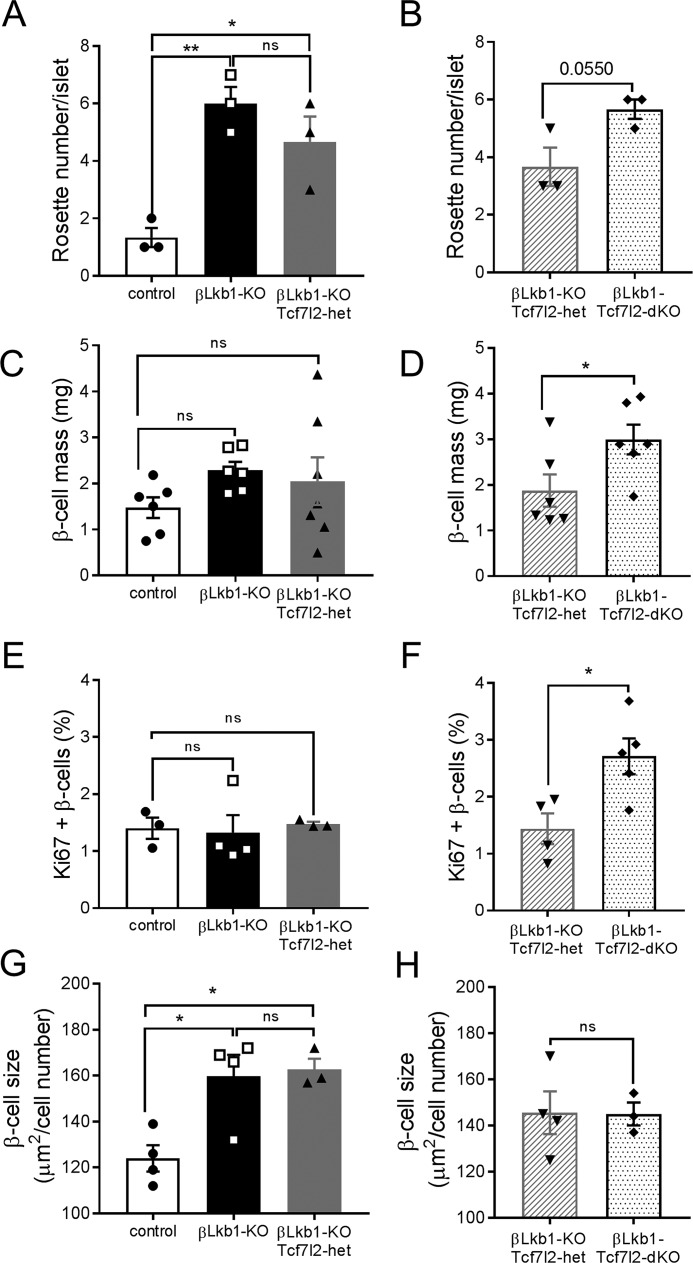
We next examined β cell size and the distribution of β cells within the islet in pancreatic slices (Fig. 5, A and B). Cellular proliferation was also assessed through Ki-67 staining (Fig. 5C). As reported previously (18–21, 28), deletion of Lkb1 increased the number of “rosette-like” structures within each islet, as identified using the adherens junction marker E-cadherin, likely reflecting a change in cellular polarity (Fig. 6, A and B) (see (18, 21). The number of rosette structures was not significantly affected by deletion of a single Tcf7l2 allele, whereas the deletion of both alleles tended (p = 0.055) to increase this number, a change that may also contribute to the enhanced secretion observed (18, 21).
Figure 5.
Impact of Lkb1 or Tcf7l2 deletion on islet topography in males. Representative immunohistochemistry results of pancreatic sections stained for β cell mass (A; insulin, 1:200, green; glucagon, 1:1000, red), an adherens junction marker (B; E-cadherin, 1:100, green), and a proliferation marker (C; Ki-67, 1:100, green) are shown. Rosette-like structures are localized in the white dotted-line square, and a representative image of an enlarged rosette-like structure is shown in column B in control mouse. Scale bars, 100 μm.
Figure 6.
Effects of Lkb1 or Tcf7l2 deletion on β cell size and mass in males. A and B, rosette-like structure count per islet (n = 3 mice/genotype) in group 1 (A) and in group 2 (B). C and D, β cell mass is the ratio of insulin-positive staining to the total pancreatic surface and pancreas weight (n = 6–7 mice/genotype). E and F, quantification of Ki-67–positive and insulin-positive cells based on 10–15 islets per pancreas (n = 3–4 mice/genotype). G and H, mean β cell size measured as the ratio of the insulin-positive staining surface area to the number of β cells (n = 3–4 mice/genotype) in group 1 (G) and in group 2 (H) (*, p < 0.05; **, p < 0.01). White bars, control; black bars, βLkb1-KO; gray bars, βLkb1-KO-Tcf7l2-het (group 1); gray hatched bars, βLkb1-KO-Tcf7l2-het (group 2); black dotted bars, βLkb1-Tcf7l2-dKO. Error bars represent the mean ±S.E.; ns, not significant.
β cell mass did not show any significant differences after Lkb1 deletion, and deletion of a single Tcf7l2 allele had no further effect (Fig. 6C). In contrast, deletion of both Tcf7l2 alleles caused a substantial (>30%) and significant increase in β cell mass as examined in βLkb1-Tcf7l2-dKO versus βLkb1-KO-Tcf7l2-het littermates. Correspondingly, β cell proliferation, examined by Ki-67 staining, was not affected by Lkb1 deletion alone or the loss of a single Tcf7l2 allele but significantly increased when two Tcf7l2 alleles were deleted (Figs. 5C and 6, E and F). β cell size, as assessed by comparing islet volume with the number of DAPI-labeled nuclei/islet, was significantly increased by Lkb1 deletion but not further affected by either mono- or biallelic deletion of Tcf7l2 (Fig. 6, G and H).
Impact of Tcf7l2 deletion on mTOR signaling
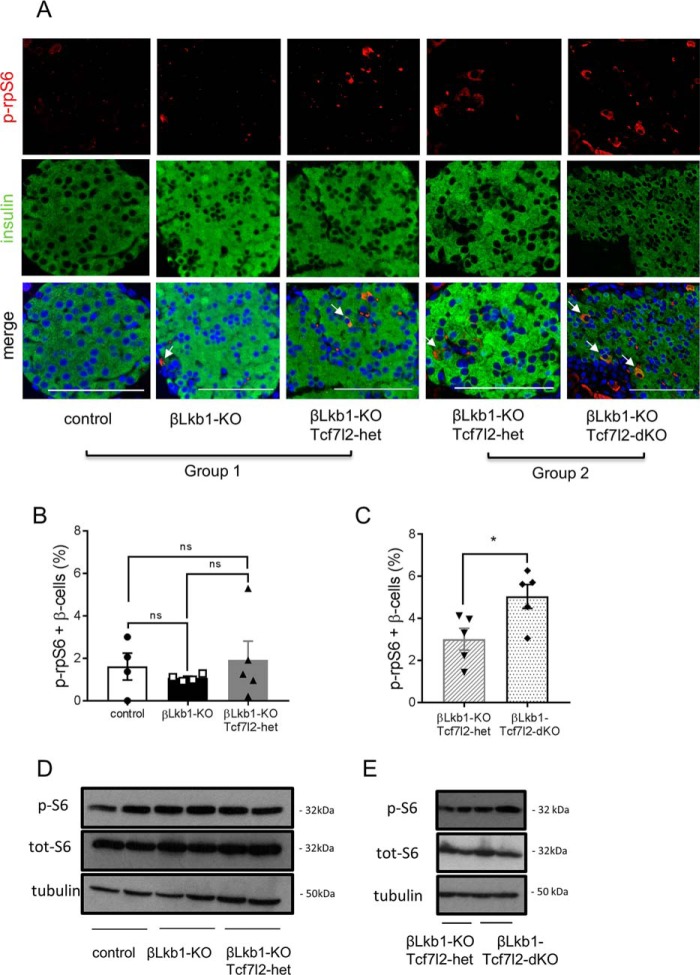
As described previously (18, 20), mTOR signaling is implicated in β cell hypertrophy when Lkb1 is deleted. We therefore examined whether Tcf7l2 deletion may impact mTOR signaling. Whereas deletion of Lkb1 alone had no effect on the levels of phospho-ribosomal protein subunit S6 (rpS6) (Fig. 7, A, B, and D), a significant increase was observed in βLkb1-Tcf7l2-dKO versus βLkb1-Tcf7l2-het islets by immunostaining of pancreatic slices (Fig. 7, A and C) and confirmed by Western (immuno)blotting (Fig. 7E).
Figure 7.
Deletion of Tcf7l2 increases mTOR activity in Lkb1-null islets from males. A, representative immunofluorescence staining of pancreatic sections from random-fed 10-week-old males using rabbit anti-phospho-ribosomal protein S6 (Ser-235/236) (p-rpS6; 1:100; red) and guinea pig anti-insulin antibodies (1:200; red). White arrows represent phospho-rpS6 and insulin colocalization. Scale bars, 100 μm. B and C, quantification of phospho-rpS6–positive staining of total β cells per islet based on 15–20 islets per pancreas from n = 4–5 mice/genotype. White bars, control; black bars, βLkb1-KO; gray bars, βLkb1-KO-Tcf7l2-het (B), gray hatched bars, βLkb1-KO-Tcf7l2-het (C); and black dotted bars, βLkb1-Tcf7l2-dKO. D and E, mouse pancreatic islets were isolated from animals of group 1 (D) and group 2 (E). After an overnight incubation in 11 mmol/liter glucose in RPMI 1640 medium, isolated islets were collected, and lysates from 125 islets were analyzed by immunoblotting with anti-phosphorylated (p-S6) and total ribosomal protein S6 (tot-S6) (Ser-235/236) and anti-tubulin for group 1 (D) and group 2 (E). Error bars represent the mean ±S.E.; *, p < 0.05; ns, not significant.
Regulation of Wnt signaling
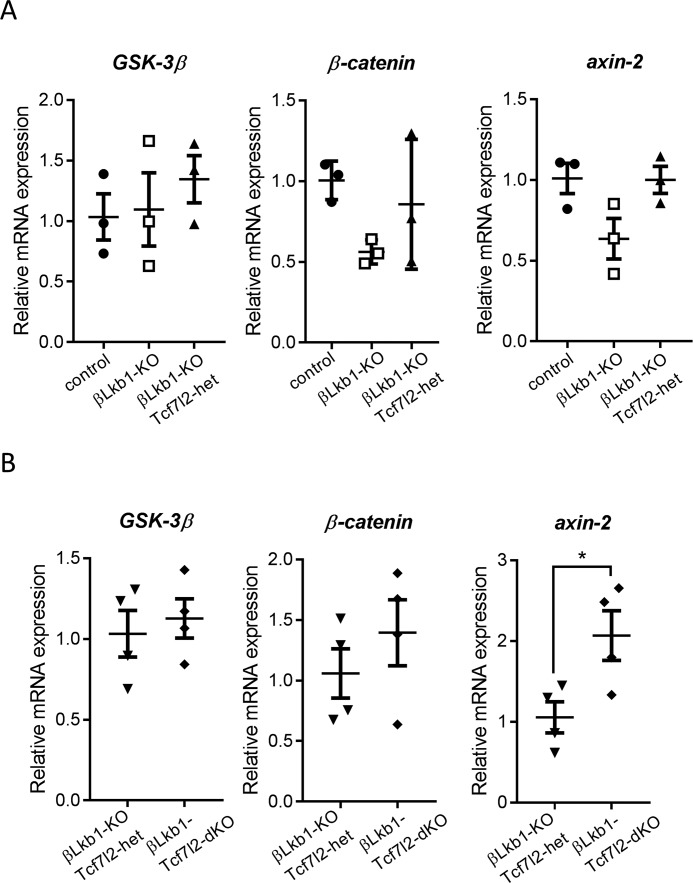
Finally, we explored the effects of LKB1 and TCF7L2 deletion on genes in the Wnt/β-catenin pathway. We found that β-catenin, the transcriptional activator for the T cell–specific factor family of transcription factors, and axin-2, a negative loop regulator of Wnt signaling, tend to be down-regulated in the absence of LKB1 (Fig. 8A). However, axin-2 was up-regulated by LKB1 and TCF7L2 deletion (Fig. 8B). Therefore, it is possible that a cross-talk exists between LKB1 and Wnt/TCF7L2 signaling in pancreatic islets and that this could be involved in controlling β cell proliferation. Furthermore, it is possible that Lkb1 could be a regulator of Wnt signaling and that TCF7L2 could contribute to loop regulation of Wnt signaling involved in proliferative signaling induced by Wnt ligands.
Figure 8.
Wnt signaling is partly dependent on LKB1 and TCF7L2 in males. Wnt signaling target gene (GSK-3β, β-catenin, and axin-2) expression was assessed by RT-qPCR in isolated islets from group 1 (A) and group 2 (B) (n = 2–3 mice/genotype). Error bars represent the mean ±S.E.; *, p < 0.05.
Discussion
The overall aim of the present study was to determine whether, under conditions of exaggerated β cell proliferation, the role of Tcf7l2 may differ from that previously described in animals placed under metabolic stress imposed by aging or by a high-fat diet (11, 12). To this end, we used a mouse model in which Lkb1 was deleted selectively in β cells, mimicking, at least in part, changes during early development (31, 32), pregnancy (33, 34), and insulin resistance (“compensation”) prior to the onset of type 2 diabetes (35, 36). This seemed an important question given that apparent differences in action have previously been described for other genome-wide association study–identified type 2 diabetes genes, such as SLC30A8 (37–39), when modeled in mice.
Strikingly, we demonstrated that the direction of the effect of Tcf7l2 deletion is reversed under these conditions (β cell hyperfunction) versus those seen under metabolic stress (11, 12). Given that TCF7L2 is normally considered to be a positive regulator of the cell cycle and thus proproliferative (40, 41), this result was unexpected. We therefore considered carefully the possibility that this might be due to alterations elsewhere in the genome given that the Lkb1 alleles (FVB/N/129S1) were carried by animals with a slightly different genetic background from the floxed Tcfl72 strain (C57BL/6J) used (see Table S1). Although this possibility cannot be excluded absolutely, we believe it is unlikely given that both FVB/N (42) and 129S1 (43) animals display similar glucose tolerance on a regular chow diet as C57BL/6 mice.
As an alternative explanation, we speculated that Tcf7l2 acts as a negative regulator of mTOR signaling. This view was supported by the data shown in Fig. 7, which demonstrated increased mTOR signaling after deletion of both, but not a single, Tcf7l2 allele. Interestingly, in the present study, we saw relatively little effect of Lkb1 deletion on mTOR signaling in the presence of Tcf7l2 despite the predicted activation of the downstream TSC1–TSC2 complex in the absence of AMPK activity (25). Nevertheless and interestingly, loss of TCF7L2 impacted the alterations in β cell apical–basolateral polarity observed after Lkb1 ablation (18–21, 28), which led to alterations in the number of “rosette” structures. The latter changes have previously been ascribed to alterations in signaling by the AMPK-related kinase MARK2/Par1b (21).
Interestingly, increased Tcf7l2 and decreased β-catenin mRNA levels were also observed after Lkb1 deletion in the present study, providing evidence for an interaction between these genes in the β cell wherein LKB1 represses Tcf7l2 expression (Fig. 8). Moreover, we found that axin-2 was regulated positively and negatively, respectively, by Lkb1 and Tcf7l2.
We also noted that deletion of Tcf7l2 on an Lkb1-null background resulted in changes in β cell growth (i.e. hypertrophy and hyperplasia) but also increased β cell function (secretion of insulin as normalized to total insulin content). Although increased mTOR signaling provides a likely mechanism for the former, the mechanisms driving increased insulin secretion remain unclear. In recent studies, we (19) and others (24) demonstrated that loss of LKB1 signaling resulted in marked alterations in glucose signaling to ATP generation and calcium dynamics such that the so-called “amplifying” pathway of insulin secretion (44, 45), possibly mediated by enhanced synthesis of glutamate and other amino acids, became the predominant means through which hormone release was activated in response to the sugar. The present study confirmed these findings (Fig. 4A). Importantly, we observed no evident improvement in Ca2+ dynamics in response to elevated glucose or KCl after deletion of a single or both Tcf7l2 alleles. This observation argues against the view that Tcf7l2 deletion leads to a reversion to a more conventional route for glucose-stimulated insulin secretion, chiefly reliant on the closure of ATP-dependent K+ channels (46) and calcium influx. Instead, the new findings point toward a further enhancement of the amplifying pathways for insulin secretion in β cells lacking both Tcf7l2 alleles in the absence of Lkb1.
The present data may also provide a mechanistic underpinning for other findings in the literature that have pointed to a possible interaction between nutrient levels and TCF7L2 action. For example, the action of TCF7L2 risk (T) allele rs7903146 depended on plasma glucose levels during oral glucose tolerance tests (47) with deleterious actions being most apparent at high glucose and a tendency to be protective at low glucose. We would note that, although the direction of this effect might appear to be the reverse of that reported here in mice, the above study chiefly interrogated the actions of incretins on insulin secretion; incretins were not examined here. Nevertheless, glucose-dependent suppression of AMPK activity, likely to mimic the effect of Lkb1 deletion on mTOR activity, may provide a means through which changes in glycemia modulate the direction of the effect of TCF7L2 variants on type 2 diabetes risk.
In summary, we demonstrate here that Tcf7l2 acts as a modifier gene for Lkb1 in the β cell, affecting islet polarity, cellular proliferation, and mass via mTOR signaling. These findings may be relevant for our understanding of the actions of human TCF7L2 variants on type 2 diabetes risk in different individuals and settings (14).
Experimental procedures
Generation of mutant mice lacking LKB1 and TCF7L2 selectively in pancreatic β cells
Mice homozygous for the floxed Lkb1/Stk11 gene (mixed FVB/129S1 and C57BL/6 background) (18) were crossed to mice homozygous for floxed (f/f) Tcf7l2 alleles (C57BL/6 background) (12). The resulting double heterozygotes (Lkb1f/+:Tcf7l2f/+) were crossed with double heterozygous mice, and the latter were then bred with mice expressing Cre recombinase at the insulin 1 locus (Ins1Cre) (28, 29). Subsequently, two separate breeding colonies were established to produce the following offspring and named as follows (group 1): control (Ins1Cre−/−:Lkb1f/f:Tcf7l2f/+), βLkb1-KO (Ins1Cre+/−:Lkb1f/f:Tcf7l2+/+), and βLkb1-KO-Tcf7l2-het (Ins1Cre+/−:Lkb1f/f:Tcf7l2f/+; Fig. 1A). The second breeding strategy generated littermates βLkb1-KO-Tcf7l2-het (Ins1Cre+/−:Lkb1f/f:Tcf7l2f/+) and βLkb1-Tcf7l2-dKO (Ins1Cre +/−:Lkb1f/f:Tcf7l2f/f; Fig. 1B, group 2). The genetic background of the resulting crosses was quantified by SNP genome scanning analysis (The Jackson Laboratory; Table S1).
Mouse maintenance and diet
Animals were housed two to five per individually ventilated cage in a pathogen-free facility with 12-h light/dark cycle and had free access to standard mouse chow diet. Unless otherwise stated, data presented are those obtained using male mice. All in vivo procedures described were performed at the Imperial College Central Biomedical Service and approved by the UK Home Office Animals Scientific Procedures Act, 1986 (HO License PPL PA03F7F07 to I. L.).
Measurement of metabolic parameters in vivo
Glucose tolerance was performed on 15-h–fasted mice after an oral gavage of glucose (2 g/kg of body weight). Tail venous blood glucose was monitored at 0, 15, 30, 60, 90, and 120 min after glucose administration. Insulin tolerance was performed on 5-h–fasted mice after an intraperitoneal injection of insulin (0.75 unit/kg of body weight; Humulin® S; Lilly). Tail venous blood glucose was monitored at 0, 15, 30, and 60 min. In vivo glucose-stimulated insulin secretion was assessed after intraperitoneal injection of glucose (3 g/kg), and blood was collected at 0, 2.5, 5, and 15 postinjection. Plasma insulin levels were measured using a homogenous time-resolved fluorescence (HTRF) mouse insulin kit (Cisbio, France).
Isolation of mouse islets
Islets were isolated by digestion with collagenase as described (48). In brief, pancreata were inflated with a collagenase solution (1 mg/ml) and placed in a water bath at 37 °C for 12 min. After several washes, the islets were purified on a Histopaque gradient (Sigma-Aldrich), and isolated islets were cultured for 24 h in RPMI 1640 medium containing 11.1 mm glucose, 10% fetal bovine serum, and l-glutamine (Sigma-Aldrich) and allowed to recover overnight.
Ex vivo glucose-stimulated insulin secretion
Insulin secretion assays on isolated mouse islets were performed as described previously (18). In brief, 10 size-matched islets per condition were incubated for 1 h in Krebs-HEPES-bicarbonate (KHB) solution (130 mm NaCl, 3.6 mm KCl, 1.5 mm CaCl2, 0.5 mm MgSO4, 0.5 mm KH2PO4, 2 mm NaHCO3, 10 mm HEPES, and 0.1% (w/v) BSA, pH 7.4) containing 3 mm glucose. Subsequently, islets were incubated for 30 min in KHB solution with either 3 mm glucose, 17 mm glucose, or 30 mm KCl. Secreted insulin and total insulin were quantified using an HTRF insulin kit (Cisbio) in a PHERAstar reader (BMG Labtech, UK) following the manufacturer's guidelines.
RNA extraction and quantitative real-time PCR analysis
RNA was isolated and purified from fresh isolated islets (50–200) with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. RNA purity and concentration were measured by spectrophotometry (Nanodrop, Thermo Fisher), and only RNA samples with an absorption ratio between 1.8 and 2.0 for 260/280 nm were used. cDNA was synthesized using 200 ng of RNA using the High-capacity cDNA Reverse Transcription kit (Applied Biosystems) including random primers. For quantitative real-time PCR, we used SYBR Green PCR Master Mix (Life Technologies) and the primers sequences in Table S2.
Immunohistochemistry and islet morphology
Isolated pancreata were removed from euthanized mice, fixed overnight in 10% (v/v) formalin, and subsequently embedded in paraffin wax. Sections (5 μm) were cut and fixed in Superfrost slides. Slides were prepared as detailed previously (28). For antigen retrieval before specific antigen detection, sections were treated with Tris-EDTA buffer, pH 9.0, at 95 °C for 20 min. Primary antibodies used were anti-guinea pig insulin (1:200; Dako), anti-mouse glucagon (1:1000; Sigma-Aldrich), anti-E-cadherin (1:100; Cell Signaling Technology), and anti-Ki-67 (1:200; Abcam, UK). Slides were visualized using an Axiovert 200 M microscope (Zeiss, Germany) with Alexa Fluor 488 goat anti-guinea pig IgG, Alexa Fluor 568 donkey anti-mouse IgG, Alexa Fluor 488 goat anti-rabbit IgG, or Alexa Fluor 568 goat anti-guinea pig IgG (Invitrogen). ImageJ software (Wayne Rasband, National Institute of Mental Health) was used to calculate the β cell mass and size. We determined the percentage of pancreatic surface that was insulin- or glucagon-positive as measured in four sections separated by 75 μm in the z axis from six to seven mice of each genotype. For Ki-67 and E-cadherin detection, pancreata from three 10-week-old mice in each genotype were examined. At least three 5-μm sections per mouse at least 150 μm apart were analyzed. To quantify the number of rosette-like structures (i.e. 8–10 cells arranged concentrically around an identifiable central “core”) in islets (18), we used E-cadherin and DAPI staining of pancreatic sections. Structures were included where the void at the center was negative for DAPI. Ten islets per mouse and three mice per genotype were assessed.
Western (immuno)blotting
After isolation, islets were collected and lysed in ice-cold buffer (150 mm NaCl, 10 mm Tris-HCl, pH 7.2, 0.1% SDS, 1% deoxycholate, 5 mm EDTA, and 1% Triton X-100) containing protease inhibitor mixture (Roche) and phosphatase inhibitors (Sigma-Aldrich). Lysates from 125 islets were denatured for 5 min at 95 °C in Laemmli buffer, resolved by 10% SDS-PAGE, and transferred to polyvinylidene difluoride membranes before immunoblotting. Intensities were quantified using ImageJ.
Antibodies
The following antibodies were used in Western (immuno) blot analysis and immunohistochemistry: rabbit anti-phospho-S6 ribosomal protein (Ser-235/236) (Cell Signaling Technology), mouse anti-α-tubulin (Sigma-Aldrich), rabbit anti-E-cadherin (Cell Signaling Technology), guinea pig anti-insulin (Dako), mouse anti-glucagon (Sigma-Aldrich), and rabbit anti-Ki-67 (Abcam).
Measurement of intracellular free calcium
Whole isolated islets were incubated with Fura-8AM (Invitrogen) for 45 min at 37 °C in KHB containing 3 mmol/liter glucose. Fluorescence imaging was performed using a Nipkow spinning disk head, allowing rapid scanning of islet areas for prolonged periods of time with minimal phototoxicity. Velocity software (PerkinElmer Life Sciences) provided the interface while islets were kept at 37 °C and constantly perifused with KHB containing 3 or 17 mmol/liter glucose or 20 mmol/liter KCl. For each experiment, we used 16–29 islets. Imaging data were analyzed with ImageJ software using an in-house macro (49).
Statistical analysis
GraphPad Prism 7.0 was used for statistical analysis. Statistical significance was evaluated by two-tailed paired Student's t test and one- or two-way ANOVA with a Bonferroni or Tukey post hoc test when appropriate. All data are shown as means ± S.E. p values of <0.05 were considered statistically significant.
Author contributions
M.-S. N.-T. data curation; M.-S. N.-T. formal analysis; M.-S. N.-T., I. L., and G. A. R. methodology; M.-S. N.-T. and G. A. R. writing-original draft; G. d. S. X. and I. L. writing-review and editing; G. A. R. conceptualization; G. A. R. funding acquisition; G. A. R. investigation.
Supplementary Material
This work was supported by Medical Research Council (MRC) Program Grants MR/J0003042/1, MR/N00275X/1, and MR/L020149/1 (DIVA); Wellcome Trust Senior Investigator Grant WT098424AIA; Royal Society Wolfson Research Merit Awards; and Diabetes UK Project Grants BDA11/0004210 and BDA/15/0005275 (to G. A. R.). The authors declare that they have no conflicts of interest with the contents of this article.
This article was selected as one of our Editors' Picks.
This article contains Figs. S1 and S2 and Tables S1 and S2.
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party-hosted site.
- TCF7L2
- transcription factor-7–like 2
- AMPK
- AMP-activated protein kinase
- GSK3
- glycogen synthase kinase 3
- LKB1
- liver kinase B1
- mTOR
- mammalian target of rapamycin
- TSC
- tuberous sclerosis complex
- qPCR
- quantitative PCR
- KO
- knockout
- dKO
- double knockout
- DAPI
- 4′,6-diamidino-2-phenylindole
- rpS6
- ribosomal protein subunit S6
- HTRF
- homogenous time-resolved fluorescence
- KHB
- Krebs-HEPES-bicarbonate
- ANOVA
- analysis of variance.
References
- 1. Kahn S. E., Zraika S., Utzschneider K. M., and Hull R. L. (2009) The β cell lesion in type 2 diabetes: there has to be a primary functional abnormality. Diabetologia 52, 1003–1012 10.1007/s00125-009-1321-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fuchsberger C., Flannick J., Teslovich T. M., Mahajan A., Agarwala V., Gaulton K. J., Ma C., Fontanillas P., Moutsianas L., McCarthy D. J., Rivas M. A., Perry J. R. B., Sim X., Blackwell T. W., Robertson N. R., et al. (2016) The genetic architecture of type 2 diabetes. Nature 536, 41–47 10.1038/nature18642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van de Bunt M., Manning Fox J. E., Dai X., Barrett A., Grey C., Li L., Bennett A. J., Johnson P. R., Rajotte R. V., Gaulton K. J., Dermitzakis E. T., MacDonald P. E., McCarthy M. I., and Gloyn A. L. (2015) Transcript expression data from human islets links regulatory signals from genome-wide association studies for type 2 diabetes and glycemic traits to their downstream effectors. PLoS Genet. 11, e1005694 10.1371/journal.pgen.1005694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mehta Z. B., Fine N., Pullen T. J., Cane M. C., Hu M., Chabosseau P., Meur G., Velayos-Baeza A., Monaco A. P., Marselli L., Marchetti P., and Rutter G. A. (2016) Changes in the expression of the type 2 diabetes-associated gene VPS13C in the β cell are associated with glucose intolerance in humans and mice. Am. J. Physiol. Endocrinol. Metab. 311, E488–E507 10.1152/ajpendo.00074.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grant S. F., Thorleifsson G., Reynisdottir I., Benediktsson R., Manolescu A., Sainz J., Helgason A., Stefansson H., Emilsson V., Helgadottir A., Styrkarsdottir U., Magnusson K. P., Walters G. B., Palsdottir E., Jonsdottir T., et al. (2006) Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 38, 320–323 10.1038/ng1732 [DOI] [PubMed] [Google Scholar]
- 6. Zeggini E., and McCarthy M. I. (2007) TCF7L2: the biggest story in diabetes genetics since HLA? Diabetologia 50, 1–4 10.1007/s00125-007-0809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brantjes H., Barker N., van Es J., and Clevers H. (2002) TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling. Biol. Chem. 383, 255–261 10.1515/BC.2002.027 [DOI] [PubMed] [Google Scholar]
- 8. Habener J. F., and Liu Z. (2014) Wnt signalling in pancreatic islets, in Islets of Langerhans (Islam M., ed) pp. 391–419, Springer, Dordrecht, Netherlands [Google Scholar]
- 9. da Silva Xavier G., Loder M. K., McDonald A., Tarasov A. I., Carzaniga R., Kronenberger K., Barg S., and Rutter G. A. (2009) TCF7L2 regulates late events in insulin secretion from pancreatic islet β-cells. Diabetes 58, 894–905 10.2337/db08-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou Y., Park S. Y., Su J., Bailey K., Ottosson-Laakso E., Shcherbina L., Oskolkov N., Zhang E., Thevenin T., Fadista J., Bennet H., Vikman P., Wierup N., Fex M., Rung J., et al. (2014) TCF7L2 is a master regulator of insulin production and processing. Hum. Mol. Genet. 23, 6419–6431 10.1093/hmg/ddu359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. da Silva Xavier G., Mondragon A., Sun G., Chen L., McGinty J. A., French P. M., and Rutter G. A. (2012) Abnormal glucose tolerance and insulin secretion in pancreas-specific Tcf7l2 null mice. Diabetologia 55, 2667–2676 10.1007/s00125-012-2600-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitchell R. K., Mondragon A., Chen L., Mcginty J. A., French P. M., Ferrer J., Thorens B., Hodson D. J., Rutter G. A., and Da Silva Xavier G. (2015) Selective disruption of Tcf7l2 in the pancreatic β cell impairs secretory function and lowers β cell mass. Hum. Mol. Genet. 24, 1390–1399 10.1093/hmg/ddu553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Savic D., Ye H., Aneas I., Park S. Y., Bell G. I., and Nobrega M. A. (2011) Alterations in TCF7L2 expression define its role as a key regulator of glucose metabolism. Genome Res. 21, 1417–1425 10.1101/gr.123745.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahlqvist E., Storm P., Käräjämäki A., Martinell M., Dorkhan M., Carlsson A., Vikman P., Prasad R. B., Aly D. M., Almgren P., Wessman Y., Shaat N., Spégel P., Mulder H., Lindholm E., et al. (2018) Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 6, 361–369 10.1016/S2213-8587(18)30051-2 [DOI] [PubMed] [Google Scholar]
- 15. Ossipova O., Bardeesy N., DePinho R. A., and Green J. B. (2003) LKB1 (XEEK1) regulates Wnt signalling in vertebrate development. Nat. Cell Biol. 5, 889–894 10.1038/ncb1048 [DOI] [PubMed] [Google Scholar]
- 16. Ma Y., Zhang G., Fu X., Xia O., Zhan C., Li L., Wang Z., and Wu B. (2010) Wnt signaling may be activated in a subset of Peutz-Jeghers syndrome polyps closely correlating to LKB1 expression. Oncol. Rep. 23, 1569–1576 [DOI] [PubMed] [Google Scholar]
- 17. Liu K., Luo Y., Tian H., Yu K. Z., He J. X., and Shen W. Y. (2014) The tumor suppressor LKB1 antagonizes WNT signaling pathway through modulating GSK3β activity in cell growth of esophageal carcinoma. Tumour Biol. 35, 995–1002 10.1007/s13277-013-1133-0 [DOI] [PubMed] [Google Scholar]
- 18. Sun G., Tarasov A. I., McGinty J. A., French P. M., McDonald A., Leclerc I., and Rutter G. A. (2010) LKB1 deletion with the RIP. Cre-transgene modifies pancreatic β-cell morphology and enhances insulin secretion in vivo. Am. J. Physiol. Endocrinol. Metab. 298, E1261–E1273 10.1152/ajpendo.00100.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swisa A., Granot Z., Tamarina N., Sayers S., Bardeesy N., Philipson L., Hodson D. J., Wikstrom J. D., Rutter G. A., Leibowitz G., Glaser B., and Dor Y. (2015) Loss of liver kinase B1 (LKB1) in β cells enhances glucose-stimulated insulin secretion despite profound mitochondrial defects. J. Biol. Chem. 290, 20934–20946 10.1074/jbc.M115.639237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fu A., Ng A. C., Depatie C., Wijesekara N., He Y., Wang G. S., Bardeesy N., Scott F. W., Touyz R. M., Wheeler M. B., and Screaton R. A. (2009) Loss of Lkb1 in adult β cells increases β cell mass and enhances glucose tolerance in mice. Cell Metab. 10, 285–295 10.1016/j.cmet.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 21. Granot Z., Swisa A., Magenheim J., Stolovich-Rain M., Fujimoto W., Manduchi E., Miki T., Lennerz J. K., Stoeckert C. J. Jr., Meyuhas O., Seino S., Permutt M. A., Piwnica-Worms H., Bardeesy N., and Dor Y. (2009) LKB1 regulates pancreatic β cell size, polarity, and function. Cell Metab. 10, 296–308 10.1016/j.cmet.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boardman L. A., Thibodeau S. N., Schaid D. J., Lindor N. M., McDonnell S. K., Burgart L. J., Ahlquist D. A., Podratz K. C., Pittelkow M., and Hartmann L. C. (1998) Increased risk for cancer in patients with the Peutz-Jeghers syndrome. Ann. Intern. Med. 128, 896–899 10.7326/0003-4819-128-11-199806010-00004 [DOI] [PubMed] [Google Scholar]
- 23. Jenne D. E., Reimann H., Nezu J., Friedel W., Loff S., Jeschke R., Müller O., Back W., and Zimmer M. (1998) Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 18, 38–43 10.1038/ng0198-38 [DOI] [PubMed] [Google Scholar]
- 24. Fu A., Robitaille K., Faubert B., Reeks C., Dai X. Q., Hardy A. B., Sankar K. S., Ogrel S., Al-Dirbashi O. Y., Rocheleau J. V., Wheeler M. B., MacDonald P. E., Jones R., and Screaton R. A. (2015) LKB1 couples glucose metabolism to insulin secretion in mice. Diabetologia. 58, 1513–1522 10.1007/s00125-015-3579-7 [DOI] [PubMed] [Google Scholar]
- 25. Inoki K., Zhu T., and Guan K. L. (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115, 577–590 10.1016/S0092-8674(03)00929-2 [DOI] [PubMed] [Google Scholar]
- 26. Salt I. P., Johnson G., Ashcroft S. J., and Hardie D. G. (1998) AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic β cells, and may regulate insulin release. Biochem. J. 335, 533–539 10.1042/bj3350533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. da Silva Xavier G., Leclerc I., Varadi A., Tsuboi T., Moule S. K., and Rutter G. A. (2003) Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem. J. 371, 761–774 10.1042/bj20021812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kone M., Pullen T. J., Sun G., Ibberson M., Martinez-Sanchez A., Sayers S., Nguyen-Tu M. S., Kantor C., Swisa A., Dor Y., Gorman T., Ferrer J., Thorens B., Reimann F., Gribble F., et al. (2014) LKB1 and AMPK differentially regulate pancreatic β-cell identity. FASEB J. 28, 4972–4985 10.1096/fj.14-257667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thorens B., Tarussio D., Maestro M. A., Rovira M., Heikkilä E., and Ferrer J. (2015) Ins1 knock-in mice for β cell-specific gene recombination. Diabetologia 58, 558–656 10.1007/s00125-014-3468-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brouwers B., de Faudeur G., Osipovich A. B., Goyvaerts L., Lemaire K., Boesmans L., Cauwelier E. J., Granvik M., Pruniau V. P., Van Lommel L., Van Schoors J., Stancill J. S., Smolders I., Goffin V., Binart N., et al. (2014) Impaired islet function in commonly used transgenic mouse lines due to human growth hormone minigene expression. Cell Metab. 20, 979–990 10.1016/j.cmet.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bouwens L., and Rooman I. (2005) Regulation of pancreatic β-cell mass. Physiol. Rev. 85, 1255–1270 10.1152/physrev.00025.2004 [DOI] [PubMed] [Google Scholar]
- 32. Ackermann A. M., and Gannon M. (2007) Molecular regulation of pancreatic β-cell mass development, maintenance, and expansion. J. Mol. Endocrinol. 38, 193–206 10.1677/JME-06-0053 [DOI] [PubMed] [Google Scholar]
- 33. Rieck S., and Kaestner K. H. (2010) Expansion of β-cell mass in response to pregnancy. Trends Endocrinol. Metab. 21, 151–158 10.1016/j.tem.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Butler A. E., Cao-Minh L., Galasso R., Rizza R. A., Corradin A., Cobelli C., and Butler P. C. (2010) Adaptive changes in pancreatic β cell fractional area and β cell turnover in human pregnancy. Diabetologia 53, 2167–2176 10.1007/s00125-010-1809-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sachdeva M. M., and Stoffers D. A. (2009) Minireview: meeting the demand for insulin: molecular mechanisms of adaptive postnatal β-cell mass expansion. Mol. Endocrinol. 23, 747–758 10.1210/me.2008-0400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mezza T., Muscogiuri G., Sorice G. P., Clemente G., Hu J., Pontecorvi A., Holst J. J., Giaccari A., and Kulkarni R. N. (2014) Insulin resistance alters islet morphology in nondiabetic humans. Diabetes 63, 994–1007 10.2337/db13-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pound L. D., Sarkar S. A., Benninger R. K., Wang Y., Suwanichkul A., Shadoan M. K., Printz R. L., Oeser J. K., Lee C. E., Piston D. W., McGuinness O. P., Hutton J. C., Powell D. R., and O'Brien R. M. (2009) Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem. J. 421, 371–376 10.1042/BJ20090530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nicolson T. J., Bellomo E. A., Wijesekara N., Loder M. K., Baldwin J. M., Gyulkhandanyan A. V., Koshkin V., Tarasov A. I., Carzaniga R., Kronenberger K., Taneja T. K., da Silva Xavier, Libert S., Froguel P., Scharfmann R., et al. (2009) Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58, 2070–2083 10.2337/db09-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rutter G. A., and Chimienti F. (2015) SLC30A8 mutations in type 2 diabetes. Diabetologia 58, 31–36 10.1007/s00125-014-3405-7 [DOI] [PubMed] [Google Scholar]
- 40. Muncan V., Faro A., Haramis A. P., Hurlstone A. F., Wienholds E., van Es J., Korving J., Begthel H., Zivkovic D., and Clevers H. (2007) T-cell factor 4 (Tcf7l2) maintains proliferative compartments in zebrafish intestine. EMBO Rep. 8, 966–973 10.1038/sj.embor.7401071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clevers H. (2006) Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 42. Montgomery M. K., Hallahan N. L., Brown S. H., Liu M., Mitchell T. W., Cooney G. J., and Turner N. (2013) Mouse strain-dependent variation in obesity and glucose homeostasis in response to high-fat feeding. Diabetologia 56, 1129–1139 10.1007/s00125-013-2846-8 [DOI] [PubMed] [Google Scholar]
- 43. Cruciani-Guglielmacci C., Bellini L., Denom J., Oshima M., Fernandez N., Normandie-Levi P., Berney X. P., Kassis N., Rouch C., Dairou J., Gorman T., Smith D. M., Marley A., Liechti R., Kuznetsov D., et al. (2017) Molecular phenotyping of multiple mouse strains under metabolic challenge uncovers a role for Elovl2 in glucose-induced insulin secretion. Mol. Metab. 6, 340–351 10.1016/j.molmet.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Henquin J. C. (2000) Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49, 1751–1760 10.2337/diabetes.49.11.1751 [DOI] [PubMed] [Google Scholar]
- 45. Rutter G. A., Pullen T. J., Hodson D. J., and Martinez-Sanchez A. (2015) Pancreatic β cell identity, glucose sensing and the control of insulin secretion. Biochem. J. 466, 203–218 10.1042/BJ20141384 [DOI] [PubMed] [Google Scholar]
- 46. Ashcroft F. M., and Rorsman P. (2013) KATP channels and islet hormone secretion: new insights and controversies. Nat. Rev. Endocrinol. 9, 660–669 10.1038/nrendo.2013.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heni M., Ketterer C., Thamer C., Herzberg-Schäfer S. A., Guthoff M., Stefan N., Machicao F., Staiger H., Fritsche A., and Häring H. U. (2010) Glycemia determines the effect of type 2 diabetes risk genes on insulin secretion. Diabetes 59, 3247–3252 10.2337/db10-0674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ravier M. A., and Rutter G. A. (2010) Isolation and culture of mouse pancreatic islets for ex vivo imaging studies with trappable or recombinant fluorescent probes. Methods Mol. Biol. 633, 171–184 10.1007/978-1-59745-019-5_12 [DOI] [PubMed] [Google Scholar]
- 49. Mitchell R. K., Nguyen-Tu M. S., Chabosseau P., Callingham R. M., Pullen T. J., Cheung R., Leclerc I., Hodson D. J., and Rutter G. A. (2017) The transcription factor Pax6 is required for pancreatic β cell identity, glucose-regulated ATP synthesis, and Ca2+ dynamics in adult mice. J. Biol. Chem. 292, 8892–8906 10.1074/jbc.M117.784629 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.