Abstract
Initiation and regulation of transcription by RNA polymerase II (RNAPII) in eukaryotes rely on the transcriptional regulatory elements. Promoters and enhancers share similar architectures and functions, and the prevailing view is that they can initiate bidirectional transcription. We summarize functional roles of enhancer transcription and possible mechanisms in enhancer–promoter communication. We discuss the potential roles of enhancer RNAs (eRNAs) in early elongation and highlight that transcriptional enhancers might modulate the release of paused RNAPII via 3D chromatin looping. Emerging evidence suggests that transcriptional enhancers regulate the promoter-proximal pausing of RNAPII, a key rate-limiting step required for productive elongation.
Keywords: chromatin, chromatin modification, chromatin regulation, gene transcription, nuclear organization, transcription enhancer
Mammalian promoters are predominantly bidirectional
Early biochemical studies defined the classical promoter as a DNA region required for the accurate initiation of gene expression (1). In yeast, most promoters contain at least one or several components needed for gene activation: an initiator element, a TATA box, and a downstream core promoter (2). Together, these elements ensure the precise assembly of the basal transcription machinery. Accordingly, the minimal DNA region surrounding the transcription start site (TSS)3 necessary for initiating transcription of a gene is called the core promoter. Before the genomic era, the terms promoter, core promoter, and TSS were used interchangeably, particularly to describe in vitro transcription initiation (3) and the unidirectional transcription of protein-coding genes.
Recent genomic studies have unexpectedly found that more than 60% of the human genome is transcribed, but less than 2% of that transcription originates from coding genes (4, 5). Some of this noncoding transcription can be accounted for by bidirectional transcription taking place near coding genes. It is now recognized that in mammals, the TSSs of genes encoding mRNA frequently generate a pair of oppositely oriented transcripts originating near the same region (6, 7). The unstable, noncoding transcripts originating upstream of the promoter are called promoter upstream transcripts, or PROMPTs (8–13). The paired, bidirectional transcripts are most often generated from genomically accessible regions that display DNase I hypersensitivity. These DNase I hypersensitive sites (DHSs) are typically depleted of nucleosomes, the fundamental subunit of chromatin, resulting in nucleosome-depleted regions (NDRs) or nucleosome-free regions (NFRs). Bidirectional transcripts from mammalian genomes typically originate from either end of an NDR but are not scattered throughout the NDR (7, 9, 13, 14). This led to a new understanding of mammalian promoters as NDRs that support bidirectional transcription initiated from two oppositely oriented core promoters (3, 15). Either side of the core promoter can facilitate the assembly of RNA polymerase II preinitiation complexes (PICs) (Fig. 1, upper panel).
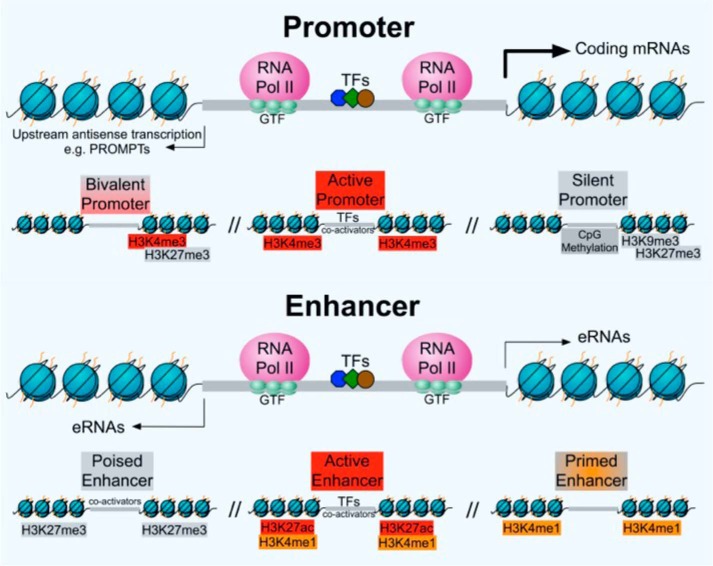
Figure 1.
Features of bidirectionally transcribed promoters and enhancers and their associated chromatin modification states. Both active promoters and distal enhancers are divergently transcribed. Core promoters within flanking nucleosomes recruit independent PICs formed by general transcription factors (GTFs) and RNAPII to initiate transcription in the sense and antisense direction. TFs facilitate the recruitment of PICs for transcription initiation. Transcription progresses from the initiation sites at core promoters and pauses at the boundary of the NDR. Bivalent promoters are normally associated with both activating (H3K4me3) and repressing (H3K27me3) epigenetic histone modifications, whereas H3K4me3 is highly enriched at active promoter regions. In contrast, silent/inactive promoters are marked by either short-term repressive histone modifications such as H3K9me3 and H3K27me3 or the long-term silencing mark of CpG DNA. The proximal pausing of RNAPII contributes to the productive elongation of both sense mRNA transcripts and upstream antisense transcripts (e.g. PROMPTs). Transcription at active enhancer regions is more prominently bidirectional, although the resulting eRNAs usually exhibit lower expression levels than mRNAs. Unlike mRNAs, eRNAs are subject to exosome-dependent degradation and therefore are short and unstable. Poised enhancers are bound by co-activators and are associated with H3K27me3 but not H3K27ac, whereas active enhancers are marked with H3K4me1 and H3K27ac and are bound by both TFs and co-activators. By contrast, primed enhancers are associated with H3K4me1 but not H3K27ac. In general, high levels of H3K27ac mark functionally active enhancers, whereas poised or inactive enhancers are flanked by nucleosomes marked with H3K27me3. Although not shown here, H3K36me3 and H3K79me2/3 at promoters and enhancers marked by H3K79me2/3 have also been observed. Pol II, polymerase II.
The pervasiveness of bidirectional promoters in mammalian systems raises several crucial questions. Are there distinct biological functions for bidirectional promoters in contrast to unidirectional promoters? What are the specific and common features of bidirectional promoters compared with unidirectional promoters? Do certain classes of bidirectional promoters and their modularity have specific functional properties in terms of DNA sequence consensus and the general transcription factors that bind to them? For example, it will be important to determine the bidirectional modular differences, if any, between active promoter modules of SAGA and TFIID-dependent genes. TATA-binding protein (TBP) preferentially binds “TATA-less” promoters when it is part of the multisubunit TFIID complex (16), whereas the SAGA complex directs TBP to promoters with obvious TATA sequences when TBP is absent from the TFIID complex (17–19). In yeast, “TATA-less” promoters predominate (about 80–90% of all genes), and are more prevalent among ubiquitously expressed “housekeeping” genes (20–22). In contrast, obvious TATA boxes are present at only some promoters that switch between repressed and highly active states, a phenomenon seen in many “developmental genes” (23). Chromatin immunoprecipitation combined with exonuclease digestion (ChIP-exo) was used to map the sites engaged by PICs at high resolution in Saccharomyces cerevisiae (16). This study revealed that two distinct modes of mRNA transcription initiation may exist, depending upon the relationship to the +1 nucleosome near promoters. The first nucleosome in the transcribed region often blocks access to promoters containing obvious TATA boxes. Such promoters may then depend on the SAGA complex to facilitate nucleosome removal for the efficient binding of TBP, RNAPII, and its associated factors. In contrast, in TFIID-dependent promoters, the +1 nucleosome is located downstream of the TSSs. In this case, TBP-associated factors form TFIID with TBP, and interact with DNA sequences downstream of a noncanonical, TATA-like consensus sequence that differs from a TATA box by two or more bases (16). Despite these findings, more recent studies argue against distinct classes of SAGA- and TFIID-dependent genes (24, 25). Warfield et al. (25) found that nearly all yeast-coding genes strongly depend on TFIID at both TATA and TATA-less promoters. SAGA complexes were found at the regulatory regions of both SAGA- and TFIID-dominated genes (24), consistent with a role for SAGA as a general cofactor that works with TFIID at most coding genes. These findings suggest that the previously observed differences in regulation between SAGA- and TFIID-dependent genes are due to other properties (25). It is currently unclear whether these promoters have distinct features regulating their bidirectional transcription, and whether their associated bidirectional modularity has any biological/pathological significance during development. It also remains elusive how bidirectional promoters of highly paused and productively elongating genes are regulated, and how different types of promoters communicate with other types of regulatory DNA elements like enhancers. The underlying basis driving mammalian promoters to act bidirectionally or unidirectionally in key biological processes is not clear.
Enhancers as cis-regulatory DNA elements in gene activation
Enhancers are distal sequences that lie upstream or downstream of the core promoter, and can activate or regulate the level of transcriptional initiation by recruiting transcription factors necessary for PIC assembly at the core promoter (Fig. 1) (26–28). In yeast, upstream-activating sequences, also known as enhancer-like sequences, are required for transcription, and are typically positioned much closer to the core promoter (2, 29). Enhancers act independently of their orientation, and their genomic location is believed to be responsible for the accurate surveillance of spatiotemporal transcription patterns during development and/or in different cell types. For example, the first mammalian enhancer was discovered downstream of the immunoglobulin (Ig) heavy-chain gene, which is necessary for the proper expression of Ig, and only exhibits enhancer activity in lymphocyte-derived cell lines and during B lymphocyte differentiation (30, 31).
Enhancers help recruit RNAPII to promoters and can attract various chromatin-modifying enzymes to DNA to establish and/or maintain an active chromatin conformation via PICs (32). Enhancers can also recruit pioneer factors and lineage-specific transcription factors (TFs) as early as the ESC stage (17–22, 33). Promoters, on the other hand, are less likely to be occupied by developmentally important and lineage-specific TFs (34). Notably, enhancers are frequently marked with H3K4me1 and H3K27ac, but not H3K4me3, unless the enhancer is highly transcribed (35–38). Accordingly, putative enhancers are commonly annotated by comparing the ratio of H3K4me1 to H3K4me3, the presence of H3K27ac, the replacement of canonical histones with histone variants like H2A.Z, the binding of co-factors such as CBP/p300, and the clustered binding of multiple master TFs (20, 39–41). Enhancers can work with both homologous and heterologous promoters to increase the transcription of target genes, and can function independently of their position and orientation. Displaying DNase I hypersensitivity remains a primary criterion for identifying enhancers in mammalian genomes.
Enhancers and promoters share interchangeable properties
As early as 3 decades ago, researchers reported commonalities between promoters and enhancers. For example, when a tandem 72-bp repeat from SV40 polyomavirus was inserted into a plasmid lacking a promoter, this element–the first discovered enhancer–initiated a low level of transcription, indicating that it can recruit RNAPII through a promoter-like activity (23, 32). Later studies found that intragenic enhancers can serve as alternative tissue-specific gene promoters, producing a class of abundant, spliced, and multiexonic poly(A)+ mRNAs (42). Conversely, promoters also display enhancer-like functions. When stimulated by metal ion, an introduced mouse metallothionein I (Mt1) gene promoter acted as an enhancer by increasing the transcription of an upstream rabbit β-globin gene (43). In addition, recent genome-wide analysis using chromatin interaction analysis with paired-end–tag sequencing (ChIA-PET) detected interactions between promoters of different genes that typically resulted in gene co-expression. These findings implied that promoters behaving like enhancers may be common in transcriptional regulation (44). Notably, most promoter–promoter and enhancer–promoter interactions in mammalian genomes are restricted to megabase-sized local chromatin interaction domains, termed topologically associated domains (TADs) (45). However, Stunnenberg and co-workers (46) identified another class of promoter–promoter interactions, extremely long–range interactions, that result in the dynamic restructuring of chromatin as mouse embryonic stem cells (ESCs) shift between two states of pluripotency. These extremely long–range interactions form during the transition from the naive ground-state to the serum primed-like state, and provide yet another example of a promoter displaying enhancer-like function, this time to control the spatiotemporal regulation of Hox and other developmentally important genes.
It is evident that promoters and enhancers share many common features including their local chromatin architecture, their regulatory landscape, and their common mechanisms to control bidirectional transcription (28). However, promoters and their associated coding genes allow for the robust transcription of stable, spliced, and polyadenylated transcripts not seen in transcripts originating from enhancers. The majority of enhancer-templated RNAs (eRNAs) are short, unstable, unspliced, unpolyadenylated, and noncoding RNAs that are expressed at low levels (4, 5). The sequences used to signal for polyadenylation and splicing are absent in the transcribed enhancer regions, but are present in the coding region. Therefore, eRNA instability appears to be due to the lack of polyadenylation and early termination sites, such that eRNAs from both strands are subject to exosome-mediated degradation (10, 47). At bidirectional promoters, poly(A) sites (PASs) are enriched at the 3′ end of PROMPTs that lack 5′ splice sites or U1 small nuclear ribonucleoprotein recognition sites; consequently, these noncoding transcripts are also subject to exosome-dependent degradation. In contrast, coding transcripts contain 5′ splice sites that bind to the U1 splicing complex preventing PAS-mediated early termination (48, 49). It remains unclear whether the PAS-dependent mechanism mediates the degradation of eRNAs.
Functional enhancers transcribe eRNAs as an active signature
Recent genome-wide studies have shown that RNA pol II recruitment to active enhancers initiates widespread transcription in mammalian genomes (50, 51). eRNAs were initially thought to arise from transcriptional noise due to abundant RNAPII activity, generating “nonspecific” transcripts in physically accessible genomic regions (52). This “nonspecific” transcription model suggests that eRNAs may be a by-product of random transcriptional activity at enhancer loci that are recognized and degraded via either nonsense-mediated decay or the exosome (53, 54). However, evidence for transcribed enhancers in recent genome-wide studies argues that enhancer transcription may be a regulated process that is specific to functionally active enhancers, rather than a random process caused by “background” RNAPII activity. For example, poised enhancers that are bound by co-activators and marked with H3K27me3, but not H3K27ac, lack transcriptional activity in mouse ESCs (Fig. 1, lower panel). These poised enhancers have been proposed to bookmark a limited number of regulatory elements in mouse ESCs, and to be activated in a timely and lineage-specific manner during differentiation (37, 55). By contrast, active enhancers marked with H3K4me1 and H3K27ac are highly transcribed, which also positively correlates with high mRNA levels of linked protein-coding genes (36, 37, 55). In addition, primed enhancers flanked by nucleosomes marked with H3K4me1, but not H3K27ac, are associated with intermediately expressed genes that are involved in a broad range of biological processes (36, 55). Collectively, enhancer transcription appears to be a regulated process that takes place only at functionally active enhancers (34, 36, 37, 39, 47, 50, 56).
Enhancer transcription and enhancer–promoter communication
Enhancers may use different but not mutually exclusive ways to communicate with their corresponding promoters depending upon the physical distances between these cis-elements. Possible models of communication include a linking model, a tracking/facilitated tracking or scanning model, and a looping model. In mouse ESCs, TADs contain most of the enhancer–promoter interactions, which range from several kilobases to ∼1 Mb, with a median length of 880 kb (57). In the linking model, a number of TFs are recruited sequentially following the binding of a first activator protein (such as pioneer TFs) that induces an open chromatin state at a promoter-proximal sequence during differentiation (58) (Fig. 2A). A chain of TFs then progressively extends along the chromatin fiber from the enhancer to the transcribed gene, and recruits the PIC to the core promoter for transcription initiation. This linking model may only apply to gene regulation between the proximal and core promoter, because this cascade of recruitment may not occur across very long distances.
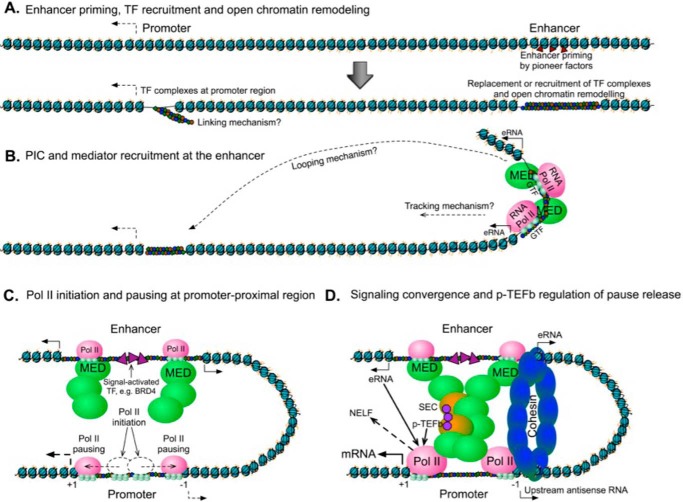
Figure 2.
Proposed steps of enhancer–promoter interaction and promoter-proximal pausing of RNAPII. A, cognate binding of pioneer factors disrupts the structure of closed, compact chromatin, together with chromatin-remodeling complexes, generating NFRs. NFRs then provide a platform for the replacement or recruitment of additional TFs. Enhancer priming by pioneer factors occurs in advance of promoter activation. The open chromatin structure resulting from enhancer priming enables the recruitment of large protein complexes to the NFRs of enhancer and promoter regions. A cascade of TF linking may occur at promoter-proximal sequences until the core promoter is bound by the recruited TFs, which provide platforms for the recruitment of PICs and Mediator. B, recruitment of large protein complexes, including the PIC and Mediator complexes occurs at the enhancer region and initiates bidirectional transcription from the enhancer. The activated enhancer may interact with the promoter via tracking or looping or a combined facilitated-tracking mechanism. The recruitment of PIC and Mediator at the promoter region may happen simultaneously with or after enhancer activation. These two situations lead to different models: 1) promoter activation may be an independent event from enhancer activation, or 2) enhancers may recruit the general transcription machinery and transfer it to the interacting promoter. C, enhancer–promoter interactions are mediated by RNAPII and the Mediator/Cohesin complex. The looped structure and RNAPII recruitment to the promoter-proximal region are associated with RNAPII pausing. RNAPII initiates transcription and progresses to the pause sites at nucleosomes flanking the promoter. Release of paused RNAPII results in divergent transcription elongation, which occurs at either the commencement of looping or thereafter. Although not shown, phosphorylation of Ser-5 in the RNAPII CTD promotes transcription initiation. In addition, subsequent to RNAPII progression to pause sites, several pausing factors, including NELF and 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) sensitivity-inducing factor, contribute to the stabilization of RNAPII pausing. D, promoter-proximal pausing of RNAPII is a rate-limiting step for productive transcriptional elongation. During signaling convergence, co-activators such as BRG4 are recruited by the TFs, resulting in the binding of elongation factors like the super-elongation complex (SEC) and Mediator. P-TEFb phosphorylates pausing factors and the CTD of RNAPII, leading to the release of paused RNAPII. Some eRNAs may facilitate the stable formation of enhancer–promoter looping through interaction with the Mediator and Cohesin complex and may also facilitate the transient release of NELF, leading to RNAPII pause release and stepping into a phase of productive elongation.
In the tracking model, the enhancer-bound transcription complexes, including active RNAPII, move toward the target promoter in a unidirectional manner (Fig. 2B) (59). Sometimes enhancer-bound proteins do not leave the enhancer, bringing the enhancer to the promoter during facilitated tracking. This results in progressive loop formation until the loop is stabilized at the target promoter. A classical example for this model stems from an ∼70-kb region containing five scattered DHS sites within the human β-globin gene locus (60). This region contains cis-regulatory sequences that confer position-independent activation of linked genes. Such regulatory regions were later named locus control regions (LCRs) (61). LCRs control the expression of a linked gene in a tissue-specific, copy-number-dependent manner. The key evidence for a tracking/facilitated tracking occurring at the β-globin LCR is that transcriptional activity extends across the β-globin LCR, its intervening regions, and into the globin genes (62–64). Similarly, other LCRs such as those that control activation of the human growth hormone (hGH) gene and major histocompatibility complex class II genes in their tissue-specific cell types have also shown RNAPII recruitment and transcriptional activity (65, 66). In the tracking model, the transcripts generated from the intervening sequence between enhancers and promoters are proposed to be passive products of active RNAPII ferried toward the target promoters (56, 67). Therefore, the tracking model requires that transcripts from both enhancers and intervening sequences be unidirectional and transiently expressed. However, genome-wide analyses have revealed that eRNA transcription at the majority of enhancers is bidirectional within confined flanking regions, and eRNA expression is often positively correlated with the expression level of the target gene. Therefore, the tracking model may not be a general mechanism in mammalian genomes, but reflective of a limited number of cases, for example, the regulation of different genes that must be expressed simultaneously within the same gene cluster.
The looping model has been proposed to allow for direct contact of promoters and enhancers over long distances. In this model, the enhancer and promoter make contact by looping out the intervening chromatin (Fig. 2, B and C). The resulting chromatin loops are stabilized by protein–protein interactions. A number of large proteins and protein complexes have been proposed to bridge and direct physical contact between enhancers and promoters, to facilitate both chromatin looping and promoter-proximal pausing of RNAPII. These complexes and proteins include chromatin-remodeling complexes, Mediator, CCCTC-binding factor (CTCF), Cohesin, and many lineage-determining transcription factors (68). eRNAs might also physically participate in establishing or stabilizing enhancer–promoter looping by interacting with either the Cohesin or Mediator complexes (69–71). It is not clear how boundary elements or insulators might restrict the action of enhancers on target promoters. Thus, enhancer–promoter communication is likely a complex and highly regulated process, involving mechanisms from a combination of several working models with different spatial, temporal, and physiological contexts.
Enhancer transcription and eRNAs in gene regulation
Although it is generally agreed that the act of enhancer transcription may have an important biological function in gene regulation, the role of the eRNAs themselves remains controversial. The main debate lies in whether enhancer transcripts have an active role in gene regulation or are merely the by-products of RNAPII transcription. Recruitment of RNAPII to chromatin can itself lead to the formation of accessible genomic regions by the “piggybacking” of histone-modifying enzymes via the CTD of RNAPII (65, 72). This may enable RNAPII-mediated transcription to induce active chromatin modifications, or to increase the levels of enhancer-specific histone marks while altering the eRNA transcripts. However, this function of RNAPII does not preclude a direct role for eRNA transcripts in transcriptional regulation.
Initial observations of genome-wide features of active enhancers revealed a number of stimulation-regulated eRNAs in many different mammalian cell types, including neurons, macrophages, and embryonic stem cells (70, 71, 73–75). Interestingly, the levels of these eRNAs often correlate with the expression levels of nearby protein-coding genes. It has been proposed that eRNA synthesis is one of the earliest events relative to mRNA transcription in response to a variety of environmental or developmental stimuli (51, 71, 76–78). Importantly, a number of studies indicate that knockdown of eRNAs results in a substantial down-regulation of their enhancer-targeted genes, suggesting that eRNA transcripts might function in transcriptional activation (70, 71, 74). eRNAs may also participate in chromosomal looping by recruiting Cohesin or Mediator to enhancer regions upon stimulation (70, 71, 79). Support for this idea stems from the finding that a number of long-noncoding RNAs (lncRNAs), including HOTTIP, CCAT1-L, and LUNAR1, activate their corresponding genes by interacting with their own protein partners that participate in chromosomal looping (80–82). It is noteworthy that a substantial number of lncRNAs are eRNAs. A recent study analyzed functional lncRNAs from the ENCODE project, and identified that 28% of annotated lncRNAs (2,695 of 9,505) overlapped with PreSTIGE database-predicted cell-type–specific enhancers (83), suggesting a subset of eRNAs may be subcategorized as lnc-eRNAs with the potential to interact with distinct proteins involved in chromosomal looping (84). However, a primary difference between eRNA and lncRNA is that lncRNA are stable transcripts and eRNA are not. Some studies suggest eRNAs have roles other than mediating enhancer–promoter looping. These studies indicate that there is a substantial reduction of eRNAs and correlated coding gene expression without significant changes in DNA looping in distinct cell models, including transcription from estrogen receptor–binding sites in MCF7 breast cancer cells and a depolarized neuron model (76, 77). These studies raise the possibility that eRNAs may facilitate RNAPII transcription by increasing chromatin accessibility, or by participating in the process of releasing paused RNAPII without changes in enhancer–promoter interactions (76, 77).
Emerging roles of eRNAs in promoter-proximal pausing of RNAPII
A number of recent genome-wide studies have shown that pausing of RNAPII in promoter-proximal regions is a common regulatory step in the productive transcription of many important genes responsive to a variety of developmental or environmental stimuli (85, 86). The pause and release of RNAPII in promoter-proximal DNA regions are essential regulatory steps in early elongation (Fig. 2, C and D). A role for eRNAs in this process has been suggested by Schaukowich et al. (77), who reported that eRNA transcript-dependent regulation during early transcriptional elongation provides a mechanism by which eRNAs, in response to early neuronal induction, directly bind to the NELF-E subunit of negative elongation factor (NELF). NELF mediates RNAPII pausing, and thus facilitates the efficient release of NELF from the target promoter (77). In this activity-regulated neuronal model, transient release of NELF, but not enhancer–promoter interactions or RNAPII recruitment, was impaired by eRNA knockdown, suggesting eRNA transcripts may act as “lure” molecules to facilitate NELF release from paused RNAPII during early transcriptional elongation (Fig. 2D).
Transcriptional elongation by RNAPII is a highly regulated process that requires RNAPII pausing for efficient transcription. The role of eRNAs in pausing and early elongation may depend largely on different developmental or environmental contexts. A recent study from Lai et al. (79) reported that Integrator, a multisubunit complex associated with the CTD of RNAPII, has a role in RNAPII pause and release, and is also required for the biogenesis of eRNAs at enhancers and super-enhancers. Super-enhancers are clusters of multiple transcriptional enhancers in large domains, which often show high levels of RNAPII occupancy and are highly transcribed (87–89). Integrator binds to enhancers and super-enhancers in a tissue- and temporal-specific manner. The catalytic subunit of Integrator has a core RNA endonuclease activity that catalyzes the 3′-end processing of eRNAs required for transcriptional termination of eRNAs upon stimulation (79). Depletion of Integrator subunits reduces the signal-dependent induction of eRNA transcripts and abolishes the associated enhancer–promoter chromatin looping. Interestingly, Integrator depletion also results in an accumulation of eRNA primary transcripts (unprocessed, polyadenylated levels) that bind to transcribing RNAPII at enhancers and super-enhancers upon epidermal growth factor induction, suggesting a possible role of eRNAs in early elongation at Integrator-regulated enhancers and super-enhancers. Multiple eRNAs generated within the same super-enhancers may act as single regulatory modules to control cell identity in development and disease. Accordingly, chromatin looping and promoter-proximal pausing of RNAPII provide a key platform and a potential regulatory step for the convergence of various signaling pathways during early elongation. Additional studies will be required to fully define the functional and biological significance of eRNAs, their in vivo inter-relationship with bidirectional promoters and enhancer transcription, the associated large protein complexes as mediators, and their structural and functional roles in early transcriptional steps, including chromatin looping and promoter-proximal pausing of RNAPII.
It is noteworthy that there is no consensus regarding the function of eRNA transcripts in transcriptional activation. Furthermore, the mechanism underlying the strong correlation between eRNA production and enhancer activity remains unclear. Current studies reporting the functional significance of eRNAs have relied on RNA interference (RNAi) to knock down eRNA transcripts in different human cell lines. However, RNAi approaches are not entirely suitable for these studies. The majority of eRNAs are nuclear, and although RNAi works well in the cytoplasm, it is not efficient in the nucleus (33). Instead, using a polyadenylation signal to cause premature termination might provide a more rigorous way to interrogate eRNA function (90, 91). Indeed, when a poly(A) cassette is inserted near the TSS of an eRNA, it triggers premature transcription termination. A recent study used this approach to investigate how truncation of the lncRNA encoded by Lockd influences transcription of the adjacent Cdkn1b gene in an erythroid cell line (91). They found that Lockd truncation, caused by the inserted poly(A) signal, had no effect on Cdkn1b transcription, whereas CRISPR–Cas9-mediated deletion of the Lockd locus significantly reduced Cdkn1b expression, suggesting an enhancer-like cis-acting mechanism. The eRNA generated from the enhancer-like Lockd locus appeared to be a by-product of local transcriptional activity. Likewise, both promoter deletions and poly(A) cassette insertion into the Blustr lncRNA locus (formerly linc1319) substantially influenced the expression of the nearby Sfmbt2 gene. This influence was dependent upon the transcription and splicing of Blustr (90). Yet, not all lncRNAs are likely to have such specific functions. In a separate study, Lander and co-workers (90) systematically analyzed 12 lncRNA loci in mouse ESCs using a genetic approach based on the classic cis-trans test. They found that five of the 12 lncRNAs significantly affected the expression of a neighboring gene in cis (90). Notably, all five lncRNAs appeared to influence nearby gene expression via general processes associated with lncRNA production, rather than a sequence-specific function of the lncRNA transcripts. Therefore, more rigorous methods such as the insertion of a poly(A) cassette, in addition to the RNAi approaches, are required to further clarify the cis-trans regulatory roles of eRNA in transcriptional activation. Regardless, it is fair to say that a consensus on the functional significance of eRNAs is lacking, and the relationship between eRNA and enhancer transcriptional activity remains unclear.
Acknowledgment
We thank Dr. Briana Dennehey for help editing.
This work was supported by the National Institutes of Health R01 Grant 048413 (to B. B.). This is the second article in the Thematic Minireview series “Chromatin and Transcription.” The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- TSS
- transcription start site
- DHS
- DNase I hypersensitivity
- NFR
- nucleosome-free region
- NDR
- nucleosome-depleted region
- TBP
- TATA-binding protein
- ESC
- embryonic stem cell
- eRNA
- enhancer RNA
- PAS
- poly(A) site
- lncRNA
- long-noncoding RNA
- NELF
- negative elongation factor
- RNAPII
- RNA polymerase II
- TF
- transcription factor
- CTD
- C-terminal domain
- PIC
- preinitiation complex
- LCR
- locus control region
- PROMPT
- promoter upstream transcript
- pol II
- polymerase II
- TAD
- topologically associated domain.
References
- 1. Smale S. T., and Kadonaga J. T. (2003) The RNA polymerase II core promoter. Annu. Rev. Biochem. 72, 449–479 10.1146/annurev.biochem.72.121801.161520 [DOI] [PubMed] [Google Scholar]
- 2. Struhl K. (1987) Promoters, activator proteins, and the mechanism of transcriptional initiation in yeast. Cell 49, 295–297 10.1016/0092-8674(87)90277-7 [DOI] [PubMed] [Google Scholar]
- 3. Andersson R., Chen Y., Core L., Lis J. T., Sandelin A., and Jensen T. H. (2015) Human gene promoters are intrinsically bidirectional. Mol. Cell 60, 346–347 10.1016/j.molcel.2015.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ENCODE Project Consortium. (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Djebali S., Davis C. A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F., Xue C., Marinov G. K., Khatun J., Williams B. A., Zaleski C., et al. (2012) Landscape of transcription in human cells. Nature 489, 101–108 10.1038/nature11233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andersson R., Refsing Andersen P., Valen E., Core L. J., Bornholdt J., Boyd M., Heick Jensen T., and Sandelin A. (2014) Nuclear stability and transcriptional directionality separate functionally distinct RNA species. Nat. Commun. 5, 5336 10.1038/ncomms6336 [DOI] [PubMed] [Google Scholar]
- 7. Core L. J., Martins A. L., Danko C. G., Waters C. T., Siepel A., and Lis J. T. (2014) Analysis of nascent RNA identifies a unified architecture of initiation regions at mammalian promoters and enhancers. Nat. Genet. 46, 1311–1320 10.1038/ng.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Almada A. E., Wu X., Kriz A. J., Burge C. B., and Sharp P. A. (2013) Promoter directionality is controlled by U1 snRNP and polyadenylation signals. Nature 499, 360–363 10.1038/nature12349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Core L. J., Waterfall J. J., and Lis J. T. (2008) Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science 322, 1845–1848 10.1126/science.1162228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Flynn R. A., Almada A. E., Zamudio J. R., and Sharp P. A. (2011) Antisense RNA polymerase II divergent transcripts are P-TEFb dependent and substrates for the RNA exosome. Proc. Natl. Acad. Sci. U.S.A. 108, 10460–10465 10.1073/pnas.1106630108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ntini E., Järvelin A. I., Bornholdt J., Chen Y., Boyd M., Jørgensen M., Andersson R., Hoof I., Schein A., Andersen P. R., Andersen P. K., Preker P., Valen E., Zhao X., Pelechano V., et al. (2013) Polyadenylation site-induced decay of upstream transcripts enforces promoter directionality. Nat. Struct. Mol. Biol. 20, 923–928 10.1038/nsmb.2640 [DOI] [PubMed] [Google Scholar]
- 12. Preker P., Nielsen J., Kammler S., Lykke-Andersen S., Christensen M. S., Mapendano C. K., Schierup M. H., and Jensen T. H. (2008) RNA exosome depletion reveals transcription upstream of active human promoters. Science 322, 1851–1854 10.1126/science.1164096 [DOI] [PubMed] [Google Scholar]
- 13. Seila A. C., Calabrese J. M., Levine S. S., Yeo G. W., Rahl P. B., Flynn R. A., Young R. A., and Sharp P. A. (2008) Divergent transcription from active promoters. Science 322, 1849–1851 10.1126/science.1162253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scruggs B. S., Gilchrist D. A., Nechaev S., Muse G. W., Burkholder A., Fargo D. C., and Adelman K. (2015) Bidirectional transcription arises from two distinct hubs of transcription factor binding and active chromatin. Mol. Cell 58, 1101–1112 10.1016/j.molcel.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaplan C. D. (2016) Pairs of promoter pairs in a web of transcription. Nat. Genet. 48, 975–976 10.1038/ng.3649 [DOI] [PubMed] [Google Scholar]
- 16. Basehoar A. D., Zanton S. J., and Pugh B. F. (2004) Identification and distinct regulation of yeast TATA box-containing genes. Cell 116, 699–709 10.1016/S0092-8674(04)00205-3 [DOI] [PubMed] [Google Scholar]
- 17. Mohibullah N., and Hahn S. (2008) Site-specific cross-linking of TBP in vivo and in vitro reveals a direct functional interaction with the SAGA subunit Spt3. Genes Dev. 22, 2994–3006 10.1101/gad.1724408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dudley A. M., Rougeulle C., and Winston F. (1999) The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes Dev. 13, 2940–2945 10.1101/gad.13.22.2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhaumik S. R., and Green M. R. (2002) Differential requirement of SAGA components for recruitment of TATA-box-binding protein to promoters in vivo. Mol. Cell. Biol. 22, 7365–7371 10.1128/MCB.22.21.7365-7371.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huisinga K. L., and Pugh B. F. (2004) A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol. Cell 13, 573–585 10.1016/S1097-2765(04)00087-5 [DOI] [PubMed] [Google Scholar]
- 21. Lee T. I., Causton H. C., Holstege F. C., Shen W. C., Hannett N., Jennings E. G., Winston F., Green M. R., and Young R. A. (2000) Redundant roles for the TFIID and SAGA complexes in global transcription. Nature 405, 701–704 10.1038/35015104 [DOI] [PubMed] [Google Scholar]
- 22. Tirosh I., and Barkai N. (2008) Two strategies for gene regulation by promoter nucleosomes. Genome Res. 18, 1084–1091 10.1101/gr.076059.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benoist C., and Chambon P. (1981) In vivo sequence requirements of the SV40 early promotor region. Nature 290, 304–310 10.1038/290304a0 [DOI] [PubMed] [Google Scholar]
- 24. Baptista T., Grünberg S., Minoungou N., Koster M. J. E., Timmers H. T. M., Hahn S., Devys D., and Tora L. (2018) SAGA is a general cofactor for RNA polymerase II transcription. Mol. Cell 70, 1163–1164 10.1016/j.molcel.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 25. Warfield L., Ramachandran S., Baptista T., Devys D., Tora L., and Hahn S. (2017) Transcription of nearly all yeast RNA polymerase II-transcribed genes is dependent on transcription factor TFIID. Mol. Cell 68, 118–129.e5 10.1016/j.molcel.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akbari O. S., Bae E., Johnsen H., Villaluz A., Wong D., and Drewell R. A. (2008) A novel promoter-tethering element regulates enhancer-driven gene expression at the bithorax complex in the Drosophila embryo. Development 135, 123–131 10.1242/dev.010744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maniatis T., Goodbourn S., and Fischer J. A. (1987) Regulation of inducible and tissue-specific gene expression. Science 236, 1237–1245 10.1126/science.3296191 [DOI] [PubMed] [Google Scholar]
- 28. Kim T. K., and Shiekhattar R. (2015) Architectural and functional commonalities between enhancers and promoters. Cell 162, 948–959 10.1016/j.cell.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guarente L. (1988) UASs and enhancers: common mechanism of transcriptional activation in yeast and mammals. Cell 52, 303–305 10.1016/S0092-8674(88)80020-5 [DOI] [PubMed] [Google Scholar]
- 30. Banerji J., Olson L., and Schaffner W. (1983) A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell 33, 729–740 10.1016/0092-8674(83)90015-6 [DOI] [PubMed] [Google Scholar]
- 31. Gillies S. D., Morrison S. L., Oi V. T., and Tonegawa S. (1983) A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell 33, 717–728 10.1016/0092-8674(83)90014-4 [DOI] [PubMed] [Google Scholar]
- 32. Moreau P., Hen R., Wasylyk B., Everett R., Gaub M. P., and Chambon P. (1981) The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 9, 6047–6068 10.1093/nar/9.22.6047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roux B. T., Lindsay M. A., and Heward J. A. (2017) Knockdown of nuclear-located enhancer RNAs and long ncRNAs using locked nucleic acid GapmeRs. Methods Mol. Biol. 1468, 11–18 10.1007/978-1-4939-4035-6_2 [DOI] [PubMed] [Google Scholar]
- 34. Hah N., Danko C. G., Core L., Waterfall J. J., Siepel A., Lis J. T., and Kraus W. L. (2011) A rapid, extensive, and transient transcriptional response to estrogen signaling in breast cancer cells. Cell 145, 622–634 10.1016/j.cell.2011.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heintzman N. D., Stuart R. K., Hon G., Fu Y., Ching C. W., Hawkins R. D., Barrera L. O., Van Calcar S., Qu C., Ching K. A., Wang W., Weng Z., Green R. D., Crawford G. E., and Ren B. (2007) Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 39, 311–318 10.1038/ng1966 [DOI] [PubMed] [Google Scholar]
- 36. Creyghton M. P., Cheng A. W., Welstead G. G., Kooistra T., Carey B. W., Steine E. J., Hanna J., Lodato M. A., Frampton G. M., Sharp P. A., Boyer L. A., Young R. A., and Jaenisch R. (2010) Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. U.S.A. 107, 21931–21936 10.1073/pnas.1016071107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rada-Iglesias A., Bajpai R., Swigut T., Brugmann S. A., Flynn R. A., and Wysocka J. (2011) A unique chromatin signature uncovers early developmental enhancers in humans. Nature 470, 279–283 10.1038/nature09692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whyte W. A., Bilodeau S., Orlando D. A., Hoke H. A., Frampton G. M., Foster C. T., Cowley S. M., and Young R. A. (2012) Enhancer decommissioning by LSD1 during embryonic stem cell differentiation. Nature 482, 221–225 10.1038/nature10805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaikkonen M. U., Spann N. J., Heinz S., Romanoski C. E., Allison K. A., Stender J. D., Chun H. B., Tough D. F., Prinjha R. K., Benner C., and Glass C. K. (2013) Remodeling of the enhancer landscape during macrophage activation is coupled to enhancer transcription. Mol. Cell 51, 310–325 10.1016/j.molcel.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shlyueva D., Stampfel G., and Stark A. (2014) Transcriptional enhancers: from properties to genome-wide predictions. Nat. Rev. Genet. 15, 272–286 10.1038/nrg3682 [DOI] [PubMed] [Google Scholar]
- 41. Rivera C. M., and Ren B. (2013) Mapping human epigenomes. Cell 155, 39–55 10.1016/j.cell.2013.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kowalczyk M. S., Hughes J. R., Garrick D., Lynch M. D., Sharpe J. A., Sloane-Stanley J. A., McGowan S. J., De Gobbi M., Hosseini M., Vernimmen D., Brown J. M., Gray N. E., Collavin L., Gibbons R. J., Flint J., et al. (2012) Intragenic enhancers act as alternative promoters. Mol. Cell 45, 447–458 10.1016/j.molcel.2011.12.021 [DOI] [PubMed] [Google Scholar]
- 43. Serfling E., Lübbe A., Dorsch-Häsler K., and Schaffner W. (1985) Metal-dependent SV40 viruses containing inducible enhancers from the upstream region of metallothionein genes. EMBO J. 4, 3851–3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li G., Ruan X., Auerbach R. K., Sandhu K. S., Zheng M., Wang P., Poh H. M., Goh Y., Lim J., Zhang J., Sim H. S., Peh S. Q., Mulawadi F. H., Ong C. T., Orlov Y. L., et al. (2012) Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 148, 84–98 10.1016/j.cell.2011.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dixon J. R., Selvaraj S., Yue F., Kim A., Li Y., Shen Y., Hu M., Liu J. S., and Ren B. (2012) Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 485, 376–380 10.1038/nature11082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Joshi O., Wang S. Y., Kuznetsova T., Atlasi Y., Peng T., Fabre P. J., Habibi E., Shaik J., Saeed S., Handoko L., Richmond T., Spivakov M., Burgess D., and Stunnenberg H. G. (2015) Dynamic reorganization of extremely long-range promoter–promoter interactions between two states of pluripotency. Cell Stem Cell 17, 748–757 10.1016/j.stem.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 47. Andersson R., Gebhard C., Miguel-Escalada I., Hoof I., Bornholdt J., Boyd M., Chen Y., Zhao X., Schmidl C., Suzuki T., Ntini E., Arner E., Valen E., Li K., Schwarzfischer L., et al. (2014) An atlas of active enhancers across human cell types and tissues. Nature 507, 455–461 10.1038/nature12787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Berg M. G., Singh L. N., Younis I., Liu Q., Pinto A. M., Kaida D., Zhang Z., Cho S., Sherrill-Mix S., Wan L., and Dreyfuss G. (2012) U1 snRNP determines mRNA length and regulates isoform expression. Cell 150, 53–64 10.1016/j.cell.2012.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kaida D., Berg M. G., Younis I., Kasim M., Singh L. N., Wan L., and Dreyfuss G. (2010) U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature 468, 664–668 10.1038/nature09479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim T. K., Hemberg M., Gray J. M., Costa A. M., Bear D. M., Wu J., Harmin D. A., Laptewicz M., Barbara-Haley K., Kuersten S., Markenscoff-Papadimitriou E., Kuhl D., Bito H., Worley P. F., Kreiman G., and Greenberg M. E. (2010) Widespread transcription at neuronal activity-regulated enhancers. Nature 465, 182–187 10.1038/nature09033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. De Santa F., Barozzi I., Mietton F., Ghisletti S., Polletti S., Tusi B. K., Muller H., Ragoussis J., Wei C. L., and Natoli G. (2010) A large fraction of extragenic RNA pol II transcription sites overlap enhancers. PLoS Biol. 8, e1000384 10.1371/journal.pbio.1000384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Struhl K. (2007) Transcriptional noise and the fidelity of initiation by RNA polymerase II. Nat. Struct. Mol. Biol. 14, 103–105 10.1038/nsmb0207-103 [DOI] [PubMed] [Google Scholar]
- 53. Wyers F., Rougemaille M., Badis G., Rousselle J. C., Dufour M. E., Boulay J., Régnault B., Devaux F., Namane A., Séraphin B., Libri D., and Jacquier A. (2005) Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121, 725–737 10.1016/j.cell.2005.04.030 [DOI] [PubMed] [Google Scholar]
- 54. LaCava J., Houseley J., Saveanu C., Petfalski E., Thompson E., Jacquier A., and Tollervey D. (2005) RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 121, 713–724 10.1016/j.cell.2005.04.029 [DOI] [PubMed] [Google Scholar]
- 55. Zentner G. E., Tesar P. J., and Scacheri P. C. (2011) Epigenetic signatures distinguish multiple classes of enhancers with distinct cellular functions. Genome Res. 21, 1273–1283 10.1101/gr.122382.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hatzis P., and Talianidis I. (2002) Dynamics of enhancer–promoter communication during differentiation-induced gene activation. Mol. Cell 10, 1467–1477 10.1016/S1097-2765(02)00786-4 [DOI] [PubMed] [Google Scholar]
- 57. Noonan J. P., and McCallion A. S. (2010) Genomics of long-range regulatory elements. Annu. Rev. Genomics Hum. Genet. 11, 1–23 10.1146/annurev-genom-082509-141651 [DOI] [PubMed] [Google Scholar]
- 58. Chen J., Zhang Z., Li L., Chen B. C., Revyakin A., Hajj B., Legant W., Dahan M., Lionnet T., Betzig E., Tjian R., and Liu Z. (2014) Single-molecule dynamics of enhanceosome assembly in embryonic stem cells. Cell 156, 1274–1285 10.1016/j.cell.2014.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Blackwood E. M., and Kadonaga J. T. (1998) Going the distance: a current view of enhancer action. Science 281, 60–63 10.1126/science.281.5373.60 [DOI] [PubMed] [Google Scholar]
- 60. Grosveld F., van Assendelft G. B., Greaves D. R., and Kollias G. (1987) Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell 51, 975–985 10.1016/0092-8674(87)90584-8 [DOI] [PubMed] [Google Scholar]
- 61. Orkin S. H. (1990) Globin gene regulation and switching: circa 1990. Cell 63, 665–672 10.1016/0092-8674(90)90133-Y [DOI] [PubMed] [Google Scholar]
- 62. Ashe H. L., Monks J., Wijgerde M., Fraser P., and Proudfoot N. J. (1997) Intergenic transcription and transinduction of the human β-globin locus. Genes Dev. 11, 2494–2509 10.1101/gad.11.19.2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tuan D., Kong S., and Hu K. (1992) Transcription of the hypersensitive site HS2 enhancer in erythroid cells. Proc. Natl. Acad. Sci. U.S.A. 89, 11219–11223 10.1073/pnas.89.23.11219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Routledge S. J., and Proudfoot N. J. (2002) Definition of transcriptional promoters in the human β globin locus control region. J. Mol. Biol. 323, 601–611 10.1016/S0022-2836(02)01011-2 [DOI] [PubMed] [Google Scholar]
- 65. Masternak K., Peyraud N., Krawczyk M., Barras E., and Reith W. (2003) Chromatin remodeling and extragenic transcription at the MHC class II locus control region. Nat. Immunol. 4, 132–137 10.1038/ni883 [DOI] [PubMed] [Google Scholar]
- 66. Ho Y., Elefant F., Liebhaber S. A., and Cooke N. E. (2006) Locus control region transcription plays an active role in long-range gene activation. Mol. Cell 23, 365–375 10.1016/j.molcel.2006.05.041 [DOI] [PubMed] [Google Scholar]
- 67. Wang Q., Carroll J. S., and Brown M. (2005) Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol. Cell 19, 631–642 10.1016/j.molcel.2005.07.018 [DOI] [PubMed] [Google Scholar]
- 68. Vernimmen D., and Bickmore W. A. (2015) The hierarchy of transcriptional activation: from enhancer to promoter. Trends Genet. 31, 696–708 10.1016/j.tig.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 69. Lai F., Orom U. A., Cesaroni M., Beringer M., Taatjes D. J., Blobel G. A., and Shiekhattar R. (2013) Activating RNAs associate with mediator to enhance chromatin architecture and transcription. Nature 494, 497–501 10.1038/nature11884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li W., Notani D., Ma Q., Tanasa B., Nunez E., Chen A. Y., Merkurjev D., Zhang J., Ohgi K., Song X., Oh S., Kim H. S., Glass C. K., and Rosenfeld M. G. (2013) Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 498, 516–520 10.1038/nature12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hsieh C. L., Fei T., Chen Y., Li T., Gao Y., Wang X., Sun T., Sweeney C. J., Lee G. S., Chen S., Balk S. P., Liu X. S., Brown M., and Kantoff P. W. (2014) Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proc. Natl. Acad. Sci. U.S.A. 111, 7319–7324 10.1073/pnas.1324151111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gribnau J., Diderich K., Pruzina S., Calzolari R., and Fraser P. (2000) Intergenic transcription and developmental remodeling of chromatin subdomains in the human β-globin locus. Mol. Cell 5, 377–386 10.1016/S1097-2765(00)80432-3 [DOI] [PubMed] [Google Scholar]
- 73. Melo C. A., Drost J., Wijchers P. J., van de Werken H., de Wit E., Oude Vrielink J. A., Elkon R., Melo S. A., Léveillé N., Kalluri R., de Laat W., and Agami R. (2013) eRNAs are required for p53-dependent enhancer activity and gene transcription. Mol. Cell 49, 524–535 10.1016/j.molcel.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 74. Mousavi K., Zare H., Dell'orso S., Grontved L., Gutierrez-Cruz G., Derfoul A., Hager G. L., and Sartorelli V. (2013) eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol. Cell 51, 606–617 10.1016/j.molcel.2013.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. IIott N. E., Heward J. A., Roux B., Tsitsiou E., Fenwick P. S., Lenzi L., Goodhead I., Hertz-Fowler C., Heger A., Hall N., Donnelly L. E., Sims D., and Lindsay M. A. (2014) Long noncoding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nat. Commun. 5, 3979 10.1038/ncomms4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hah N., Murakami S., Nagari A., Danko C. G., and Kraus W. L. (2013) Enhancer transcripts mark active estrogen receptor binding sites. Genome Res. 23, 1210–1223 10.1101/gr.152306.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schaukowitch K., Joo J. Y., Liu X., Watts J. K., Martinez C., and Kim T. K. (2014) Enhancer RNA facilitates NELF release from immediate early genes. Mol. Cell 56, 29–42 10.1016/j.molcel.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Arner E., Daub C. O., Vitting-Seerup K., Andersson R., Lilje B., Drabløs F., Lennartsson A., Rönnerblad M., Hrydziuszko O., Vitezic M., Freeman T. C., Alhendi A. M., Arner P., Axton R., Baillie J. K., et al. (2015) Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science 347, 1010–1014 10.1126/science.1259418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lai F., Gardini A., Zhang A., and Shiekhattar R. (2015) Integrator mediates the biogenesis of enhancer RNAs. Nature 525, 399–403 10.1038/nature14906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Trimarchi T., Bilal E., Ntziachristos P., Fabbri G., Dalla-Favera R., Tsirigos A., and Aifantis I. (2014) Genome-wide mapping and characterization of Notch-regulated long noncoding RNAs in acute leukemia. Cell 158, 593–606 10.1016/j.cell.2014.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang K. C., Yang Y. W., Liu B., Sanyal A., Corces-Zimmerman R., Chen Y., Lajoie B. R., Protacio A., Flynn R. A., Gupta R. A., Wysocka J., Lei M., Dekker J., Helms J. A., and Chang H. Y. (2011) A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature 472, 120–124 10.1038/nature09819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xiang J. F., Yin Q. F., Chen T., Zhang Y., Zhang X. O., Wu Z., Zhang S., Wang H. B., Ge J., Lu X., Yang L., and Chen L. L. (2014) Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 24, 513–531 10.1038/cr.2014.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vučićević D., Corradin O., Ntini E., Scacheri P. C., and Ørom U. A. (2015) Long ncRNA expression associates with tissue-specific enhancers. Cell Cycle 14, 253–260 10.4161/15384101.2014.977641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li W., Notani D., and Rosenfeld M. G. (2016) Enhancers as noncoding RNA transcription units: recent insights and future perspectives. Nat. Rev. Genet. 17, 207–223 10.1038/nrg.2016.4 [DOI] [PubMed] [Google Scholar]
- 85. Jonkers I., and Lis J. T. (2015) Getting up to speed with transcription elongation by RNA polymerase II. Nat. Rev. Mol. Cell Biol. 16, 167–177 10.1038/nrm3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Adelman K., and Lis J. T. (2012) Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat. Rev. Genet. 13, 720–731 10.1038/nrg3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hnisz D., Abraham B. J., Lee T. I., Lau A., Saint-André V., Sigova A. A., Hoke H. A., and Young R. A. (2013) Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 10.1016/j.cell.2013.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lovén J., Hoke H. A., Lin C. Y., Lau A., Orlando D. A., Vakoc C. R., Bradner J. E., Lee T. I., and Young R. A. (2013) Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell 153, 320–334 10.1016/j.cell.2013.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Whyte W. A., Orlando D. A., Hnisz D., Abraham B. J., Lin C. Y., Kagey M. H., Rahl P. B., Lee T. I., and Young R. A. (2013) Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell 153, 307–319 10.1016/j.cell.2013.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Engreitz J. M., Haines J. E., Perez E. M., Munson G., Chen J., Kane M., McDonel P. E., Guttman M., and Lander E. S. (2016) Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 539, 452–455 10.1038/nature20149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Paralkar V. R., Taborda C. C., Huang P., Yao Y., Kossenkov A. V., Prasad R., Luan J., Davies J. O., Hughes J. R., Hardison R. C., Blobel G. A., and Weiss M. J. (2016) Unlinking an lncRNA from its associated cis element. Mol. Cell 62, 104–110 10.1016/j.molcel.2016.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]