Abstract
Embryonic neurodevelopment involves inhibition of proliferation of multipotent neural stem cells followed by differentiation into neurons, astrocytes and oligodendrocytes to form the brain. We have identified a new neurotrophic factor, NF-α1, which inhibits proliferation and promotes differentiation of neural stem cell/progenitors derived from E13.5 mouse cortex. Inhibition of proliferation of these cells was mediated through negatively regulating the Wnt pathway and decreasing β-catenin. NF-α1 induced differentiation of neural stem cells to astrocytes by enhancing Glial Fibrillary Acidic Protein (GFAP) expression through activating the ERK1/2-Sox9 signaling pathway. Cultured E13.5 cortical stem cells from NF-α1-knockout mice showed decreased astrocyte numbers compared to wild-type mice, which was rescued by treatment with NF-α1. In vivo, immunocytochemistry of brain sections and western blot analysis of neocortex of mice showed a gradual increase of NF-α1 expression from E14.5 to P1 and a surge of GFAP expression at P1, the time of increase in astrogenesis. Importantly, NF-α1-KO mice showed ~49% fewer GFAP positive astrocytes in the neocortex compared to WT mice at P1. Thus, NF-α1 is critical for regulating anti-proliferation and cell fate determination, through differentiating embryonic stem cells to GFAP-positive astrocytes for normal neurodevelopment.
Keywords: Carboxypeptidase E, Neurotrophic factor - α1, astrocytes, stem cell, wnt pathway
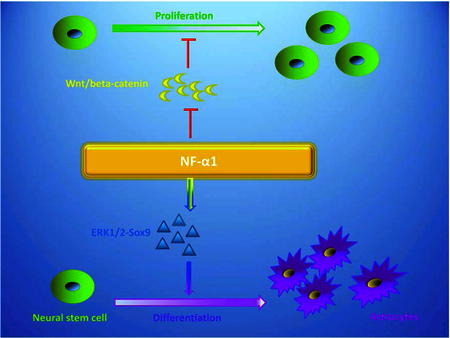
Legend for Graphical Abstract
NF-α1, a new neurotrophic factor, inhibits proliferation of neural stem cells and promotes differentiation of neural stem cell/progenitors into astrocytes. Inhibition of proliferation was mediated through negatively regulating the Wnt pathway and decreasing β-catenin. NF-α1 induced differentiation of neural stem cells to astrocytes by enhancing Glial Fibrillary Acidic Protein (GFAP) expression through activating the ERK1/2-Sox9 signaling pathway.
Introduction
Embryogenesis is a complex process involving multiple developmental events tightly regulated at various levels [1]. One important stage in embryogenesis is the neural development process that leads to the formation of the central nervous system, which is carefully orchestrated by a series of timed genetic events. At specific times, certain genes are activated and then turned off when not needed, and these landmarks are regulated by transiently expressed transcription factors. Numerous studies have identified growth factors and transcription factors and their signaling pathways that mediate neural induction and differentiation [2, 3, 4]. Recently, studies revealed a number of proteins and transcription factors that are responsible for controlling the proliferation of newly formed neural precursors and for specification of multipotent neural plate stem cells to yield neural progenitors, which ultimately differentiate into neurons and glia cells to form the brain [3].
Neural stem cells (NSCs) are usually located in the sub-ventricular zone of the developing neocortex. Some NSCs and neural progenitors are also present in the adult brain, primarily in the dentate gyrus of the hippocampus and the sub-ventricular zone of the lateral ventricle [3, 5]. Neural stem cells are multipotent self-renewing cells and give rise to a number of progenitors, which then differentiate into neurons, astrocytes or oligodendrocytes [6]. They first undergo inhibition of proliferation (cell cycle arrest) and then differentiate into the different cell types. Generally, inhibition of proliferation and differentiation are mediated by different factors, rather than a single protein. While many factors for the promotion of proliferation and differentiation of neural stem cells have been identified, the mechanisms underlying the inhibition of proliferation of these cells are less well explored.
To study the role of stem cells in embryonic development of the nervous system, efforts have focused on producing a population of NSCs in vitro that can be manipulated to yield differentiated cells found in the brain [5, 7]. Research has led to identification of many trophic factors (such as FGF, BDNF and TGFβ) that interact with their respective receptors on NSCs. This triggers signaling pathways such as Wnt, Notch, Sonic hedgehog, BMP, Ras/MAPK and JAK/STAT/FGF to promote proliferation and/or differentiation to specific cell types during embryonic development [8, 9, 10, 11, 12, 13, 14, 15]. Binding of the appropriate ligands to their signaling receptors leads to activation of transcription factors or co-activators, such as β-catenin, Hes, Sox, Gli, Smad and Stat proteins, that regulate the downstream genes involved in the induction and differentiation of NSCs [15, 16, 17]. Other factors derived from the NSC niche known to modulate the self-renewal, differentiation capacity, and survival of differentiated NSCs include BDNF, LIF or CNTF, and some vitamin derivatives e.g. retinoic acid and vitamin D3 (in vitro) [18, 19, 20, 21, 22, 23]. Molecules such as GDF11, IL-17, urocortin and homocysteine are involved in inhibiting the proliferation of neural stem cells during development but not in promoting differentiation [24, 25, 26, 27].
Recently, we found a new neurotrophic factor, neurotropic factor-α1 (NF-α1), also known as carboxypeptidase E (CPE) that is highly expressed in adult neural stem cells in the sub-ventricular niche of mice [28]. It has effects on inhibiting proliferation of neurospheres [28] and, because it is also expressed during early embryonic development, could be important in embryonic neural differentiation [29]. CPE/NF-α1-knock-out mice are obese, infertile, and have neurological disorders [30]. NF-α1 mRNA is differentially expressed in various regions at early embryonic stages of the rat and is especially high in brain and spinal cord [29, 31]. Various non-enzymatic roles of CPE/NF-α1 have also been found: it is a sorting receptor that targets proneuropeptides to the regulated secretory pathway and the cytoplasmic tail of the transmembrane form of CPE facilitates secretory vesicle movement in neuroendocrine cells [30, 32, 33]. Additionally, in vitro studies indicate that NF-α1 is a powerful neuroprotectant. Exogenous addition of recombinant NF-α1 rescued embryonic cortical rat neurons from oxidative stress in vitro [34]. Mice subjected to mild chronic restraint stress showed increased NF-α1 expression, leading to enhanced levels of BCL2, a pro-survival protein, in the hippocampus to protect neurons from stress-induced neurodegeneration [35]. While there has been a report indicating the expression and function of NF-α1/CPE in pro-neuropeptide processing [29] late rat embryos the function of NF-α1 in anti-proliferation and determining cell fate during early embryonic development has not been explored. In the present study, we investigated the role of NF-α1 as both an anti-proliferation and differentiation factor in NSCs.
Results
Temporal and spatial expression of NF-α1 in mice during embryonic development
Using qRT-PCR, NF-α1 mRNA expression was detected in all embryonic stages analyzed (E5.5 – E14.5 & E17.5 and P1). For clarity, since the earliest stage that we could dissect was E5.5, the levels of NF-α1 mRNA at all other embryonic stages were normalized to this level (Fig. 1A). NF-α1 expression increased from E6.5 - E8.5 and gradually fell to very low levels at E10.5 and E11.5. This was followed by a rapid increase from E12.5 to E14.5 and at E17.5 (whole body). Head alone samples also showed an increase from E12.5 to P1. In situ hybridization, using a 35S-UTP-labeled mouse NF-α1 probe, showed that at E10.5, NF-α1 mRNA was highly expressed in the telencephalon, diencephalon, and spinal cord regions (Fig. 1B). At E11.5 and E12.5, in addition to those brain regions seen at E10.5, there was expression in the mesencephalon of the brain, heart, and in somites (Fig. 1B). Western blot data confirmed the presence of NF-α1 protein in E8.5, E10.5, E11.5, E12.5 and E13.5 embryos (Fig. 1C). These data indicate that NF-α1 could play a role in neural development since it is expressed at the appropriate times in neural tissue in the embryo during development.
Figure 1. Temporal and spatial distribution of NF-α1 in embryos.
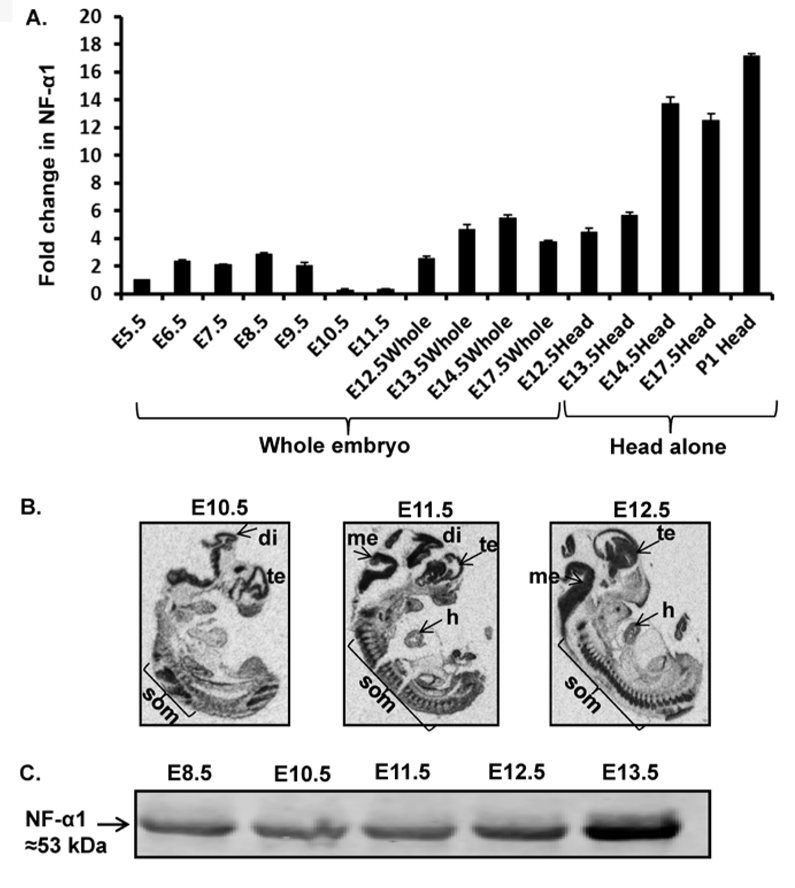
(A) Bar graphs show NF-α1 mRNA expression in E6.5 embryos to postnatal day1 (P1) (head only) relative to E5.5 embryos. Values are mean ± SEM; N=3, n=3 per embryo stage. (B) In situ hybridization indicates NF-α1 mRNA highly expressed in embryonic brain especially di (diencephalon), te (telencephalon), som (somites), me (mesencephalon), and h (heart) (N=3). (C) NF-α1 was immunoprecipitated with polyclonal rabbit anti-NF-α1 Ab from whole embryos (E8.5–11.5) or embryo head (E12.5& E13.5) and probed with mouse anti-NF-α1 Ab. NF-α1 protein is detectable at early embryonic stages (E8.5, 10.5, 11.5, 12.5 & 13.5).
NF-α1 negatively regulates the proliferation of NSCs
The early developmental expression pattern of NF-α1 prompted us to investigate its role in neuronal proliferation and differentiation, particularly in NSCs using neurospheres as a model system. To study proliferation, neocortical cells isolated from E13.5 embryos were treated with or without recombinant NF-α1 protein for 5 days. Total neurospheres generated in NF-α1-treated cells were reduced by 41% compared to controls (Control: 215.0 ± 5.03; NFα−1: 126.3 ± 3.18; n = 6, p = 0.0001; Fig. 2A). Moreover, the number of neurospheres less than 100–149 µm in diameter was significantly reduced in the NF-α1-treated cells compared to the controls (Control, 42.67 ± 1.45; NF-α1, 19.00 ± 1.15; n = 6, p < 0.001; Fig. 2B). The EdU proliferation assay revealed a small but significant decrease in neural stem/progenitor proliferation on day 5 of neurosphere cultures treated with NF-α1 compared to control cultures (Control: 40.00 ± 0.94; NFα−1: 34.80 ± 0.91; n = 5, p = 0.004; Fig. 2C and 2D). These data collectively indicate that exogenously added NF-α1 inhibits proliferation of neural stem cells. As a control analysis, both the neocortical cells and neurospheres express NF-α1 protein (Fig. 2E).
Figure 2. NF-α1 negatively regulates neurosphere proliferation.
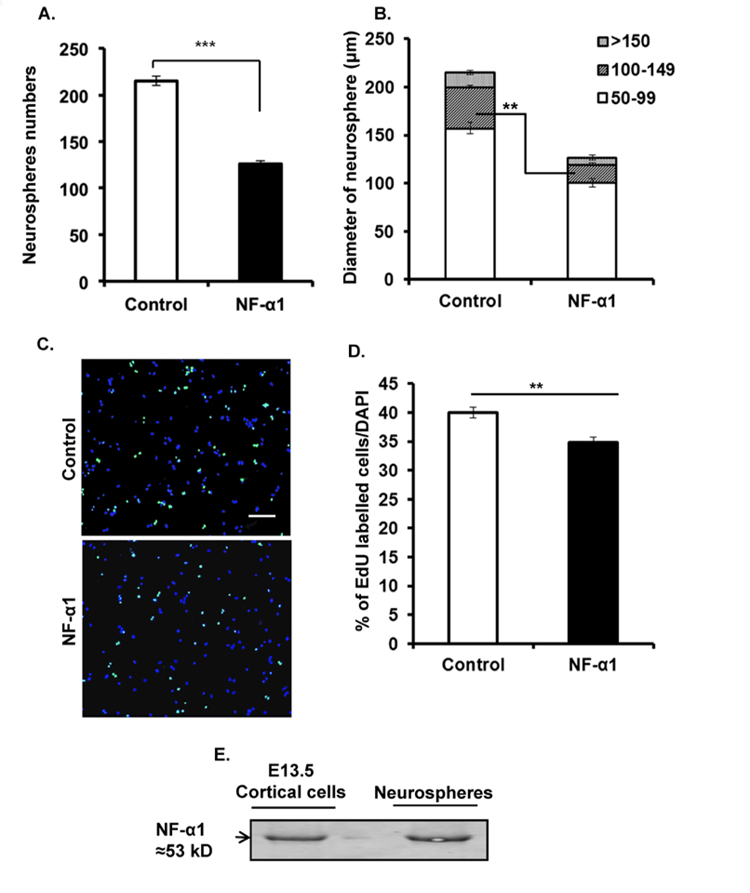
Neocortical cells from E13.5, were grown for 5 days in 96 well plate in the presence of growth factors (FGF2 and EGF) and treated with or without 200 nM recombinant NF-α1 at day 0. At the end of 5 days, images were taken and analyzed using ImageJ software. Exogenous addition of recombinant NF-α1 significantly reduced the numbers of neurospheres compared to control (A) and also affected the size of the neurospheres under 100–149 μm in diameter category (B) (N=3, **p<0.004, ***p<0.0007). (C) Representative ICC pictures (10X) of untreated and NF-α1 treated cells. Scale bar = 100 µm. (D) Bar graph shows the percentage of Edu labelled cells compared to total (DAPI). NF-α1 treatment on neural stem/progenitors significantly decreased proliferation of the cells (N=2, **p<0.004). (E) NF-α1 immunoprecipitation assay showed both the starting material (cortical cells) and the end product (neurosphere) express NF-α1 protein. The values represent the mean ± SEM, t test.
NF-α1 inhibits NSC proliferation through Wnt/β-Catenin signaling pathway
To examine the possibility that NF-α1 inhibits NSC proliferation through the Wnt/β-Catenin signaling pathway, neocortical cells were grown with NF-α1 treatment at day 0. Neurosphere lysates were collected at different time points (24 h, 72 h and 5 d) after NF-α1 treatment and β-Catenin protein levels were measured. After 24 h, neurospheres treated with NF-α1 showed slightly decreased levels of β-Catenin. By 72 h and 5 d, the levels of β-Catenin were significantly decreased (~30–35%) in NF-α1-treated cultures compared to controls (72 h Control, 100.00 ± 3.76; NF-α1, 64.71 ± 3.36; n = 12, p < 0.0001 and 5 d Control, 100.00 ± 2.72; NF-α1, 62.50 ± 3.27; n = 6, p < 0.0001) (Fig. 3).
Figure 3. NF-α1 decreases the neurosphere proliferation through Wnt3a/β-catenin signaling.
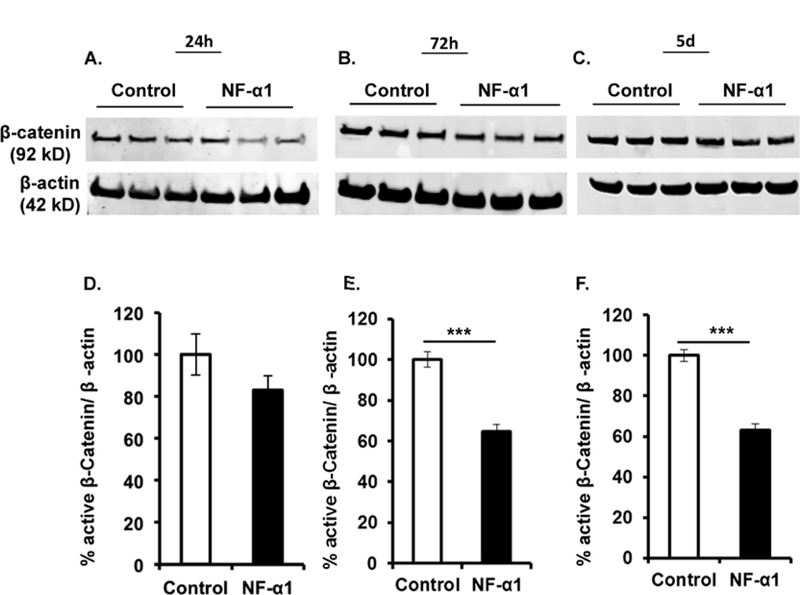
Cell lysates were prepared, at different time points (24 h, 72 h and 5 day), from neurospheres grown in the presence of growth factors and treated with or without NF-α1 on day 0. Upper panels (A-C) represent Western blots of β-Catenin and β-actin at each time point. Quantification of the Western blots (D-F) shows a significant decrease in β-Catenin levels in NF-α1 treated cultures at 72 h & 5 d compared to untreated controls (E & F). N=3, ***p<0.0001, t test.
NF-α1 promotes the differentiation of NSCs into astrocytes
Neurosphere-derived cells were cultured with or without NF-α1 and then immunostained for markers of astrocytes, neurons, and oligodendrocytes (Fig. 4A). There was a significant increase in the GFAP+ (astrocyte marker) population (~1.4-fold), and a trend towards a decrease in the Tuj1+ (neuronal marker) population in NF-α1-treated cells compared to the controls. No change in CNPase+ (oligodendrocyte) populations was observed between the two groups (% of GFAP+ cells: Control, 42.20 ± 2.95; NF-α1, 58.00 ± 1.78; n = 5, F(5, 24) = 179.3, p < 0.001; % of Tuj1+ cells: Control, 26.20 ± 0.86; NF-α1, 22.20 ± 1.31; n = 5, F(5, 24) = 179.3, p > 0.05; Fig. 4B). Similar results were seen when a non-enzymatically active form of NF-α1 (E300Q) [36] was used (% of GFAP+ cells: Control, 43.80 ± 2.74; E300Q-NF-α1, 61.20 ± 3.39; n = 5, F(5, 24) = 134.4, p < 0.05; % of Tuj1+ cells: Control, 28.60 ± 1.40; NF-α1, 22.20 ± 0.58; n = 5, F(5, 24) = 134.4, p > 0.05; Fig. 4C), confirming that the enzymatic activity of NF-α1 is not necessary for promoting the differentiation of NSCs into astrocytes.
Figure 4. NF-α1 and E300Q-NF-α1 (non-enzymatic mutant of NF-α1) promotes the differentiation of neural stem/progenitors into astrocytes.
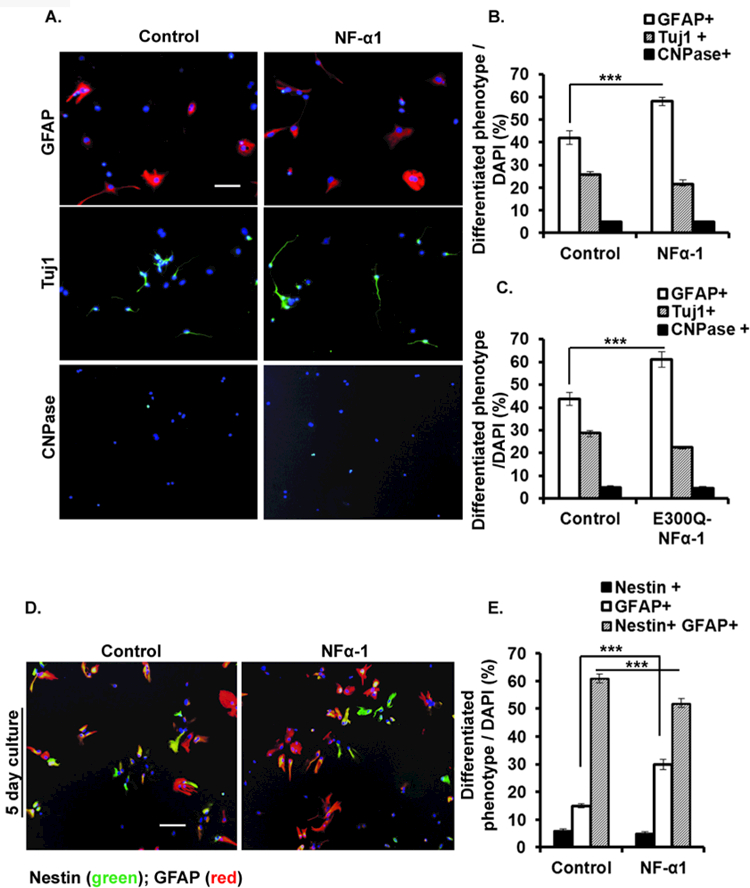
Neurospheres dissociated into single cells were grown for 5 days in the presence of 1% FBS (differentiation media) and treated with or without NF-α1 or E300Q-NF-α1 on day 0. At the end of day 5 single immunocytochemistry (ICC) staining for astrocytes (anti-GFAP), neurons (anti-β-III tubulin), and oligodendrocytes (CNPase), as well double immunostaining for astrocyte (anti-GFAP) and nestin (anti-Nestin) was carried out. (A) Representative ICC pictures (20X), of control (left) & NF-α1 treated neural stem/progenitor cells (right). Scale bar = 100 µm. (B) & (C) Bar graph shows the percentage of each cell phenotype population. Either NF-α1 or E300Q-NF-α1 treatment on neural stem/progenitor cells significantly increased the number of astrocytes (***p<0.0001) (open bars), without significantly altering the percentage of the neuron (shaded bars) and oligodendrocyte (solid bars) populations. (D) Representative ICC pictures of control (untreated) (left) & NF-α1 treated cells double immunostained for Nestin and GFAP (right). (E) Bar graph shows the percentage of differentiated phenotype populations. NF-α1 treatment on neural stem/progenitors significantly increases differentiation into GFAP+ alone, concomitant with a reduction in the Nestin+/GFAP+ cells population (***p<0.0001) without significantly altering the percentage of Nestin+ population. N=3, the values represent the mean ± SEM, one way ANOVA (Tukey’s multiple comparison test).
Neurosphere-derived cells cultured with or without NF-α1 were double immunostained for Nestin and GFAP. The majority of the NSCs stained for both Nestin and GFAP (Fig. 4D). A small portion of the stem cell population that stained for Nestin alone showed no difference between the NF-α1 treated and untreated groups in 5 d cultures. However, a significant increase (~2-fold) in GFAP+ alone (% of cells: Control, 15.17 ± 0.83; NF-α1, 29.50 ± 1.85; n = 6, F(5, 30) = 314.4, p < 0.0001) and a decrease (~1.18-fold) in Nestin+/GFAP+ cells (% of cells: Control, 60.50 ± 1.64; NF-α1, 51.50 ± 1.64; n = 6, F(5, 30) = 314.4, p < 0.0001) cells was observed in NF-α1-treated compared to untreated cultures (Fig. 4E). Similar results were observed in 10 d cultures (data not shown).
NF-α1 induces NSC differentiation to astrocytes during embryonic neural development
We performed loss-of-function and gain-of-function experiments in neurosphere-derived cells generated from NF-α1-KO and NF-α1-WT E13.5 embryos and subjected to the differentiation protocol. Neurosphere-derived cells from NF-α1-KO and NF-α1-WT embryos were cultured with or without NF-α1 and immunostained for markers of astrocytes, neurons, and oligodendrocytes. Fig. 5A, 5B (upper panels, representative ICC) and Fig. 5C–5E (bar graphs, quantification) show that NF-α1-KO mice had ~30% fewer GFAP+ cells than WT mice (Fig. 5C, open bars,% of GFAP+ cells: KO control, 34.33 ± 2.29; WT control, 49.17 ± 1.62; n = 6, F(5,30) = 121.2, p < 0.0001) and ~34% more Tuj1+ cells than WT mice (% of Tuj1+ cells: KO control, 52.17 ± 1.90; WT control, 34.67 ± 3.10; n = 6, F(5,30) =121.2, p < 0.0001; Fig. 5D, open bars). Moreover, there was a higher % of Tuj1+ neurons (52%) than GFAP+ cells (34%) in the KO mice (% of Tuj1+ cells: KO control, 52.17 ± 1.90 vs % of GFAP+ cells: KO control, 34.33 ± 2.29; n = 6, p < 0.0001, t test) (compare open bars in Fig. 5D versus 5C for KO), whereas this distribution appeared to be reversed in the WT cells. This differentiation profile was reversed upon treatment of the cells from NF-α1-KO mice with recombinant NF-α1, resulting in higher % of GFAP+ cells (48%) than Tuj1+ cells (40%), (% of GFAP+ cells: NF-α1 treated; 47.00 ± 2.25 and % of Tuj1+ cells: NF-α1 treated; 39.67 ± 1.961, n = 6, p = 0.03, t test; compare solid bars in Fig. 5C vs. 5D for KO). Irrespective of KO and WT derived neural progenitors, NF-α1 treatment showed a significant increase in GFAP+ cells and decrease in Tuj1+ cells.
Figure 5. NF-α1-KO/NF-α1-WT generated neural stem/progenitors indicate a modulatory role of NF-α1 in altering the differentiation cell phenotype.
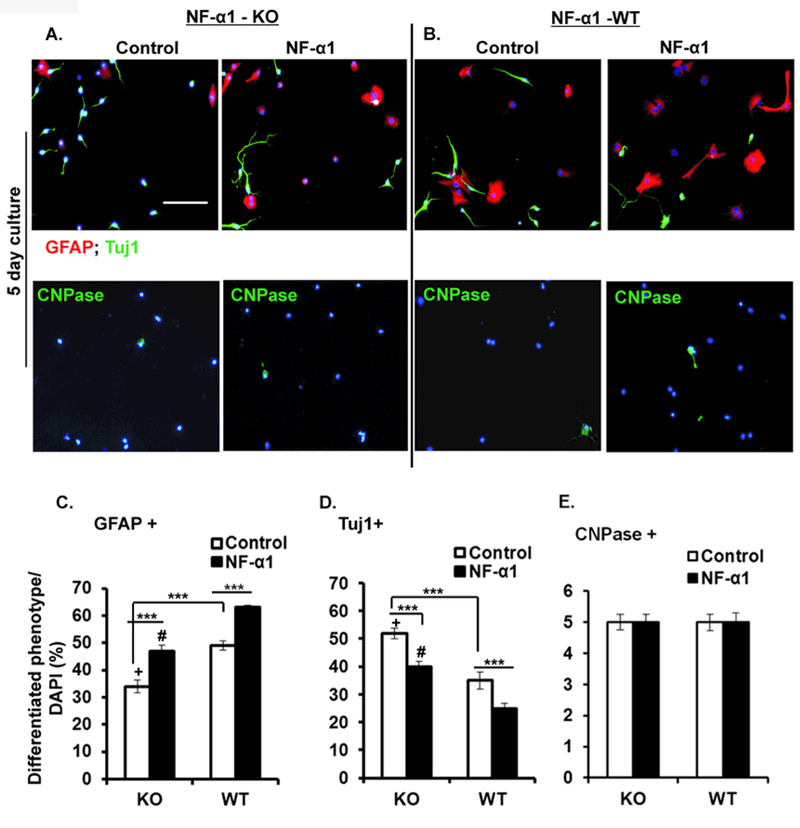
Neural stem/progenitors generated from NF-α1-KO and NF-α1-WT were grown for 5 days in differentiation media and treated with or without NF-α1 on day 0. At the end of day 5 the cells were stained and analyzed for GFAP, β-III tubulin and CNPase positive cells. Representative ICC pictures (x20) from NF-α1-KO (A) and NF-α1-WT (B) cells. Scale bar = 100 µm. Left and right side panels indicate controls and NF-α1 treated cells. C, D & E show bar graph analysis comparing between NF-α1-KO and NF-α1-WT for individual cell (GFAP+, Tuj1+ & CNPase+) phenotypes. Differentiated NPCs derived from NF-α1-KO (control) showed a significant ~30% decrease in GFAP+ cells (C) and ~34% increase in Tuj1+ cells (D) when compared to NF-α1-WT controls. Irrespective of NF-α1-KO and NF-α1-WT cultures, NF-α1 treatment increased GFAP+ cells and decreased Tuj1+ cells (C, D). +p=0.03, #p<0.001, ***p<0.0001, N=3, the values represent the mean ± SEM, one way ANOVA (Tukey’s multiple comparison test) and t test.
NF-α1 promotes NSC differentiation via MAPK/MEK-Sox9 signaling pathway
Signaling pathways were examined in neurosphere-derived cultures treated with or without NF-α1 which included notch, hedgehog, SMAD (data not shown) and MAPK/MEK. Only MAPK/MEK showed a response to NF-α1 treatment (Fig. 6A). NF-α1 treatment led to increased phosphorylation of ERK1/2, a component of the MAPK/MEK signaling pathway, in a time-dependent manner with a maximum increase at 15 min compared to the control cells (% protein expression: Control (15 min), 100.00 ± 13.10; NF-α1, 951.2 ± 83.45; n = 5, F(4,20) = 69.54, p < 0.0001) (Fig. 6B). Furthermore, we detected maximum up-regulation of the expression of the ERK-targeted downstream transcription factor Sox9 at 15 and 30 min, concomitant with the increase of pERK1/2, in NF-α1-treated cells (% protein expression: Control (15 min), 100.00 ± 7.85; NF-α1, 242.6 ± 46.88; n = 5, F(4,20) =3.857, p < 0.05) (Fig. 6C, 6D). In the presence of the ERK inhibitor, U0126, NF-α1-induced pERK was reduced by ~3-fold (NF-α1, 140.5 ± 6.65; U0126 + NF-α1, 47.75 ± 6.84; n = 4, F(3,12) = 59.03, p < 0.0001), confirming that NF-α1 signals through the ERK pathway. NF-α1 increased Sox9 protein levels by ~1.3-fold compared to untreated control, whereas U0126 decreased Sox9 levels by ~2.4-fold compared to the NF-α1 treated cells (NF-α1, 130.10 ± 7.59; U0126 + NF-α1, 53.42 ± 5.48; n = 4, F(3,12) =36.42, p < 0.0001; Fig. 6E compare Sox9 lanes 2 and 4). RT-PCR data from Sox9 knockdown experiments (Fig. 6F, upper panel) showed that Sox9 mRNA levels were significantly downregulated in si-Sox9 RNA transfected NSCs compared to si-scrambled RNA (control) (si-scrambled, 1.00 ± 0.16; si-Sox9, 0.63 ± 0.15; n = 8, F(3,28)=74.23, p < 0.05). A significant increase in fold change of Sox9 and GFAP expression was found in control samples by treatment with NF-α1, (Sox9 mRNA fold change: si-scrambled, 1.00 ± 0.16; si-scrambled + NF-α1, 1.63 ± 0.10; n = 8, F(3,28)=74.23, p < 0.0001 & GFAP mRNA fold change: si-scrambled, 1.00 ± 0.13; si-scrambled + NF-α1, 1.86 ± 0.12; n = 8, F(3,28) = 33.17, p < 0.0001), but this effect of NF-α1 was absent in the si-Sox9 RNA transfected cells (Fig. 6F, lower panel).
Figure 6. NF-α1 increases Sox9 mRNA and protein levels in NPCs signaling via the ERK pathway.
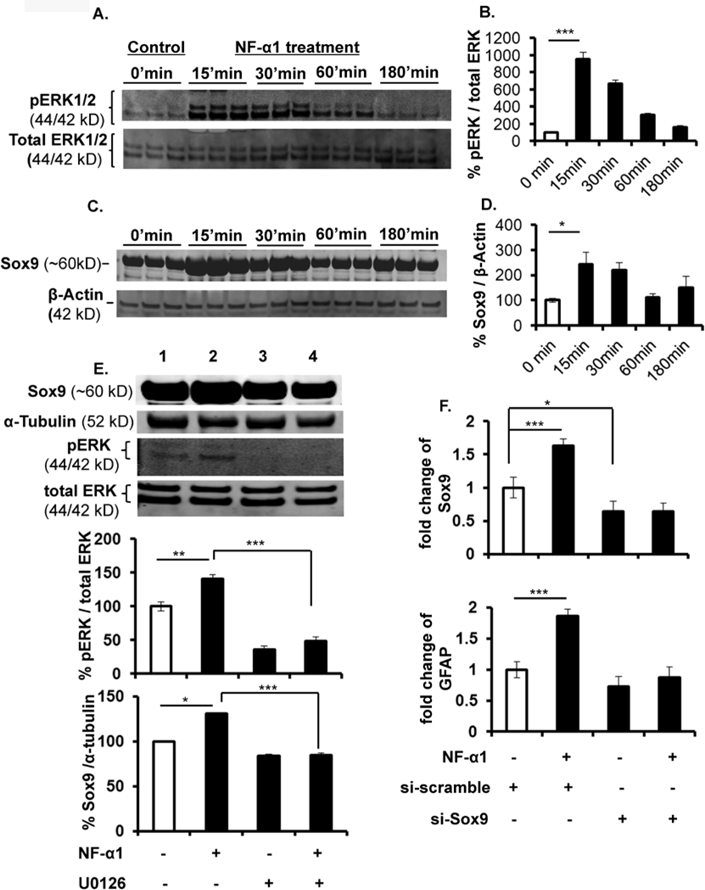
(A & C) Neural stem/progenitors were cultured for the indicated periods of time in the presence or absence of 200 nM NF-α1. Total cell lysates were examined by Western blot analysis using pERK and total ERK antibody. NF-α1 increased pERK and Sox9 protein ~9 and ~2.4-fold respectively at 15 min and decreased with time compared with the Control group. N=3; values are mean ±SEM, *p<0.05, ***p<0.0001. (B & D) represents the bar graph of the Western blot results (A & C). (E) ERK inhibitor U0126 treatment of neural stem/progenitors for 30 min blocked the NF-α1 induced pERK signaling by ~3-fold. NF-α1 increased Sox9 protein levels by ~1.3-fold in the absence of U0126. U0126 prevented the increase and reduced the levels of Sox9 by ~2.4-fold. N=2; values are mean ±SEM, *p<0.05, **p<0.001, ***p<0.0001. (F) Neural stem/progenitors were transfected with si-scrambled & si-Sox9 RNA, followed by NF-α1 treatment and cultured for 48h. Sox9 mRNA expression was significantly reduced in si-Sox9 treated cells compared to si-scrambled. Sox9 and GFAP mRNA levels were significantly increased in NF-α1 treated scrambled cells compared to untreated. N=2; values are mean ±SEM, ****p<0.0001, one way ANOVA (Tukey’s multiple comparison test).
Expression of NF-α1 in GFAP+ in brain during development from E14.5 to P1
Western blot analysis (Fig.7A) showed that the expression of NF-α1 in brain gradually increased from E14.5 to P1 (Fig 7B). GFAP protein (Figs. 7A, C) expression was at a low level between E14.5 −17.5, with a surge occurring at P1 at time of increased astrogenesis. Immunohistochemical staining of expression of NF-α1 (Fig.7D) and GFAP (Fig.7E) from E14.5 to P1 mice showed similar pattern of changes as the Western blot analysis (see images in supplementary Fig. 1). Expression of NF-α1 from immunostained sections showed gradual increase in intensity from E14.5 to P1 (Fig.7D), while GFAP-immunoreactive staining showed low intensity from E14.5 - E17.5 and a surge at P1 (Fig.7E). High magnification images showed co-localization of GFAP with NF-α1 in astrocytes at P1 (Fig. 7F).
Figure 7. Developmental expression of NF-α1 and GFAP and reduced astrocyte population observed in NF-α1-KO compared to NF-α1-WT in neocortex.
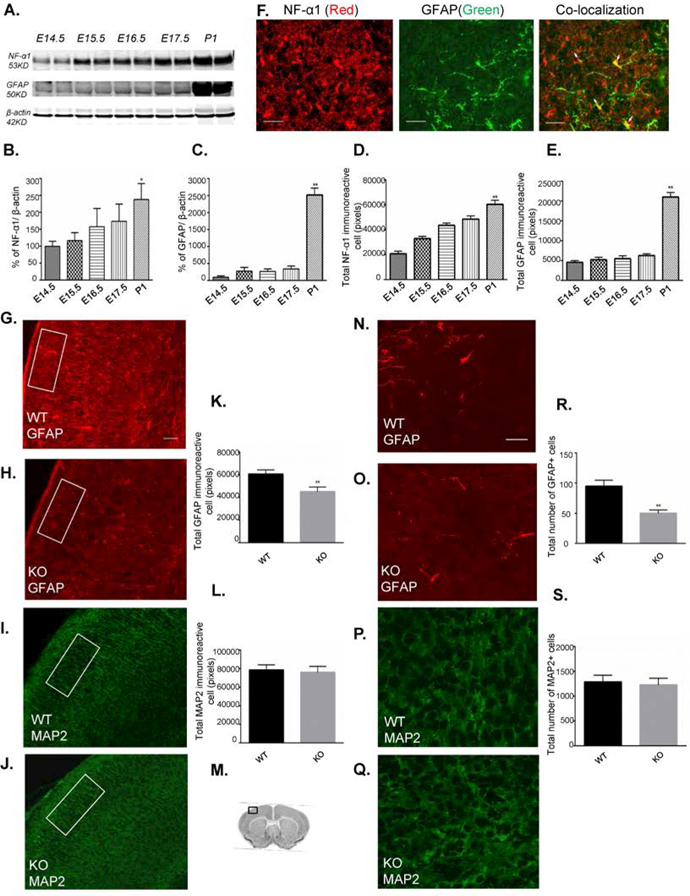
Brains from E14.5 to P1 mice were extracted and protein analyzed by Western blot. Neocortex coronal sections (16µm) from E14.5-P1 mice and NF-α1-KO & -WT mice at P1 stage were immunostained for NF-α1, GFAP (astrocytes) or MAP2 (neuron). (A) Western blot and bar graphs of NF-α1 (B) and GFAP (C) showing quantification of protein expression in brain during development. Each lane contains 2 brains pooled. NF-α1 expression increased gradually from E14.5 to P1 (B), while GFAP showed a major increase at P1(C), corresponding to the time of increased astrocyte differentiation. F(4,5)= 5.27 for (B) and F(4,5)=152.2 for (C), values are mean±SD, *p<0.05 and **p<0.01, one way ANOVA (Tukey’s multiple comparison test).
Decrease in GFAP+ cells in NF-α1-KO versus NF-α1-WT in P1 brains
NF-α1-KO and NF-α1-WT brain sections (P1) were immunostained for GFAP and MAP2. A significant ~25.6% decrease in GFAP immunostaining intensity measured over the whole neocortex was observed in NF-α1-KO embryos compared to WT embryos (GFAP intensity/brain: NF-α1-WT, 60428 ± 2154; NF-α1-KO, 44988 ± 2376; mean±SEM, n = 3, p < 0.01, Figs. 7G,H,K). Counting GFAP+ cell numbers in randomly selected areas of the neocortex (M) revealed ~49.4% fewer cells (GFAP cell numbers/brain: NF-α1-WT, 94.67 ± 5.6; NF-α1-KO, 50 ± 3.2; mean±SEM, n = 3, p < 0.01) in the NF-α1-KO embryos compared to WT mice (Fig. 7 N,O,R). There was no difference in the intensity of MAP2 staining in the neocortex close to the same regions where the cells were counted between NF-α1-WT and NF-α1-KO mice (Figs.7 I,J,L). This was confirmed by counting MAP2 stained neurons in similar areas where GFAP+ cells were counted (Figs. 7P,Q, S).
Discussion
In this study, we report a new stem cell anti-proliferation/differentiation factor, NF-α1, which functions independent of its previously identified carboxypeptidase activity, (CPE, Fig. 4). We showed that NF-α1 mRNA is expressed as early as E5.5, primarily in neural primordium and continues to be expressed in the telencephalon and diencephalon of mouse embryos at later developmental stages. Moreover, we demonstrated that NF-α1 is expressed in NSCs derived from E13.5 mouse neocortex, indicating that NF-α1 is poised to act as an intrinsic neural cell fate determinant.
Neural cell fate determination involves multifaceted steps and is influenced by various extrinsic and intrinsic factors that control the initiation and inhibition of proliferation and differentiation of cells [37]. Our in vitro data indicates that NF-α1 inhibits NSC proliferation, a prerequisite step to differentiation. Other proteins such as PTEN and MD20 are transiently expressed in select neural cell populations of embryos and regulate proliferation of NSCs [38, 39]. We propose that in vivo at a specific time during neural development, NF-α1 could be transiently expressed to down-regulate NSC proliferation in an autocrine/paracrine fashion. Several signaling studies have shown that the Wnt/β-catenin pathway plays a critical role in regulating neural stem cell proliferation and fate determination processes in embryonic development [40, 41, 42]. Our study show that NF-α1 regulates neurospheres proliferation by controlling the expression of β-Catenin, a molecule normally found to enhance proliferation [43, 44]. Indeed, we have previously reported that NF-α1 negatively regulates the canonical Wnt signaling pathway via interaction with its receptor, frizzled [45]. Thus, based on our findings, we propose that NF-α1 mediates inhibition of NSC proliferation through decreasing β-catenin levels via the Wnt signaling pathway.
Our NSC differentiation data indicate that the majority of differentiated cells were astrocytes, followed by neurons and very low numbers of oligodendrocytes. This is consistent with neural progenitor differentiation profile data reported by others [46]. The differentiation of NSCs depends on the trophic factors and growth factors secreted by the NSC niche [47, 48, 49]. Our study show that addition of NF-α1 to NSCs significantly increased the differentiation of NCSs to astrocytes without affecting the differentiation to neurons and oligodendrocytes. Likewise, EGF-responsive stem cells (cerebellar-derived) in the presence of CNTF have been shown to differentiate into more astrocytes rather than neurons and oligodendrocytes [75]. In contrast, addition of BDNF to stem cells derived from cortical and /or striata showed an increase in neurons and oligodendrocytes rather than astrocytes [76, 10]. In another study, neocortex-derived stem cells subjected to Neurotorphin-3 treatment promoted more neuronal differentiation [77, 78], Similarly, addition of GDNF to mesencephalon-derived neurosphere cultures showed an increase in the number of neuronal cells rather than astrocytes [79]. These studies indicate that some trophic factors such as BDNF, GDNF and Neurotrophin-3 promote neurogenesis, while others such as CNTF and NF-α1 promote astrogenesis.
NF-α1/CPE is secreted at the germinal zones of mouse brain and by NSCs, astrocytes and neurons [50, 51, 28], and we showed that NF-α1 is expressed and presumably secreted by the embryonic NSCs (neurospheres). Therefore, NF-α1 is present in the right location in vivo to regulate the differentiation of NSC during neural development in an autocrine/paracrine fashion. Interestingly, exogenous addition of NF-α1 to neurospheres generated from adult mouse sub-ventricular zone had no effect on differentiation to astrocytes, neurons, or oligodendrocytes [28]. This difference in response to NF-α1 suggests that NF-α1 may be primarily acting as a cell fate determinant during embryonic development.
Our data also show that astrocytes are primarily derived from a population of NSCs immunopositive for both Nestin and GFAP protein and these in fact represent the majority of cells differentiated from the neurospheres. Evidence in support of this comes from our observation that treatment with NF-α1 decreased the number of Nestin+/GFAP+ cells and increased cells that were positive for GFAP alone, which are the mature astrocytes, to a similar extent. Increase in GFAP+ cells during differentiation of multipotent neural precursors is considered as an indication of maturation to the astrocyte phenotype [52, 53, 54].
In vivo, neural development is regulated by a complex environment under the influence of various growth factors and transcription factors that switch on and off in a coordinated manner to reprogram the cells and determine their fate [55, 56, 57]. These factors activate different signaling pathways such as NOTCH, BMP, ERK1/2, TGFβ1, known to regulate differentiation of NSCs into astrocytes [58, 9, 14, 59, 13, 60]. Here we showed that NF-α1 activated the Raf/MEK/ERK pathway, which then up-regulated the expression of Sox9. Sox9 is an important transcription factor that plays a central role in transmitting the signals for major signaling pathways that regulate astrogliogenesis/astrocyte differentiation [11, 61, 62] and its expression could be suppressed by ERK1/2 inhibition [63, 64]. Indeed, we showed that in the presence of the ERK1/2 inhibitor (U0126) and knockdown of Sox9 gene expression by siRNA, NF-α1 induced GFAP mRNA expression was blocked. These results demonstrate that NF-α1 regulates the differentiation of NSCs into astrocytes by signaling through the ERK1/2 pathway, which enhances expression of the Sox9 transcription factor and GFAP.
Our in vitro data on NF-α1-KO embryos showed a significant difference in differentiated cell phenotype. There were less astrocytes among the cells derived from the NF-α1-KO neurospheres compared to those from NF-α1-WT mice. In contrast, there were more neurons derived from the NSCs in the NF-α1-KO compared to NF-α1-WT embryos. These results reaffirm that NF-α1 plays an important role in inducing differentiation of NSCs to astrocytes. This was further confirmed by rescue experiments showing that addition of exogenous NF-α1 to the NSCs from KO embryos increased the astrocyte population and decreased the neuronal population. To demonstrate that NF-α1 plays a role in the induction of astrogenesis in vivo, we examined its developmental expression together with GFAP in mouse embryos and P1 pups, as well as the number of GFAP+ astrocytes in NF-α1-KO versus WT mice. Our data showed a steady increase in expression of NF-α1 in the brain of E14.5 to P1 mice. At P1, the time of significant astrogenesis in mice, [80], there was a surge in the expression of GFAP and an increase in numbers of GFAP+ astrocytes which also showed co-expression of NF-α1 in these cells. The presence of NF-α1 in these astrocytes suggests that it could have played a regulatory role in the differentiation of neural stem cells to astrocytes. More importantly, we demonstrated that the lack of NF-α1 perturbed normal astrogenesis in mice at P1 in vivo. Brain sections from the neocortex of NF-α1-KO mice revealed a significant (49%) decrease in the number of GFAP+ cells and a reduction in their staining intensity compared to the NF-α1-WT P1 mice. The extent of the decrease was even greater than the decrease in GFAP+ astrocytes observed in E13.5 cortex derived NSCs in the KO versus WT embryos. This finding together with the in vitro evidence showing decreased GFAP+ astrocytes in neocortex neurosphere cultures which could be rescued with addition of exogenous NF-α1, strongly indicate a regulatory role of NF-α1 in astrocyte differentiation. Unlike the in vitro studies, MAP2 staining was similar in intensity, suggesting no difference in the neuronal population between KO and WT mice in this brain region. This could be due to compensatory mechanisms that exist in vivo. Given the importance of astrocytes in supplying neurons with substrates for energy metabolism, modulating the immune response and synaptic transmission and control of extracellular water and electrolyte homeostasis [65, 81], NF-α1’s role in promoting differentiation of NSCs to astrocytes during development is pivotal in building an optimally functioning CNS. Indeed, decreased astrocyte numbers in the cortex may contribute to cognitive deficits in CPE/ NF-α1 knockout mice [30].
In conclusion, this study has uncovered a novel neurotrophic factor, NF-α1, which has the unique ability to perform the dual role of switching off proliferation and activating differentiation of embryonic NSCs, offering greater efficiency in this process. We propose that by up-regulating NF-α1 expression in specified NSCs at a specific time during neurodevelopment, this switch from proliferation to differentiation could be activated. Moreover, NF-α1 is a cell fate determinant, promoting NSC differentiation into astrocytes. Its importance in normal brain development in vivo was evident in NF-α1-KO mice which exhibited decreased astrocyte numbers in the neocortex. Indeed, deficits in astrocyte numbers and function during neurodevelopment have been linked to human neurological diseases [66].
MATERIALS AND METHODS
Animals
All animals were given food and water ad libitum in a humidity and temperature controlled room under a 12 h light: dark cycle. Mice (3–12 weeks old) were purchased from Taconic Farms, Inc., (Derwood, MD). Timed pregnant mice were generated by mating C57BL6 mice in our animal facility and embryonic 13.5 or P1 NF-α1-wild type (WT)/NF-α1-knock out (KO) pups were produced by mating male and female heterozygote mice (since homozygotes are infertile [67]) at the NIH animal facility. All animal procedures were approved by the Animal Care and Use Committee, NICHD, NIH.
Recombinant NF-α1
Purified recombinant wild type NF-α1 and enzymatically inactive NF-α1 (E300Q) mutant were custom generated by GenScript USA Inc, Piscataway, NY, as previously described [34].
In Situ hybridization
Animals and tissue:
All embryos were generated from timed-pregnant C57BL6 female mice. Data reported are from at least one litter for each age group and at least 3 embryos at each age. The embryos (E10, E11, and E12) were collected and freshly frozen, or embedded directly in OCT compound without prior fixation. Sagittal cryostat sections were prepared as described [68, 29].
In situ Hybridization:
A cRNA probe was generated using linearized pGEM-T-Easy plasmid vector containing 980 bp corresponding to nucleotides 540–1520 of murine CPE mRNA (NM_013494) and labeled with 35S-UTP. The details of the hybridization procedure are described elsewhere [69, 70]. The slides from all animals were processed together to eliminate differences from inter-assay variation.
Quantitative RT-PCR of NF-α1
Total RNA isolation, cDNA synthesis and qPCR was performed as described previously [71]. The qPCR cycling conditions were: 10 min denaturation at 95°C and 40 cycles of DNA synthesis at 95°C for 15 seconds and 60°C for 1 min and the results analyzed using SDS 1.9.1 software (Applied Biosystems). Primer sequences for mouse CPE/NF-α1 and 18S RNA can be found in Supplementary Table S1. All qPCRs were performed in triplicates and the relative amount of CPE/NF-α1 mRNA was normalized to 18S rRNA.
Preparation of mouse E13.5 cortical cells
Preparation of E13.5 cortical cells has been described previously [72]. The final cell pellet was re-suspended in DMEM/F12 (Sigma Aldrich) containing B27 (1X) (Gibco), Penicillin-Streptomycin (1X) (Gibco), 2 μg/ml heparin (Sigma Aldrich) and 15 mM HEPES (Gibco), and used in proliferation and differentiation studies.
Neurosphere culture preparation, proliferation and differentiation assays
Cortical cells were cultured as described previously [7,72]. Cells were grown for 5–7 days at 37°C in the presence of 5% CO2, with supplementation of additional EGF (20 ng/ml)/bFGF2 (10 ng/ml) every 2 days. Proliferation Assay: cells were cultured in 96 well plates at a density of 500 cells per 100µl in the presence of growth factors (EGF/bFGF), treated with or without 200 nM recombinant NF-α1 on day 0 and grown for 5 days. At the end of 5 days, images were taken to count the number and size of the neurospheres using imageJ software. Ethynyldeoxyuridine (Edu) incorporation method: Five day neurosphere cultures were treated with or without NF-α1 (200 nM), incubated with 10 µM EdU for 1 h and immediately dissociated to single cells using Accutase (StemPro® Accutase®, Life Technologies, USA) followed by neutralization by trypsin inhibitor from Glycine max (soybean) (Sigma Aldrich). Cells were plated on poly-L-lysine coated chamber slides and grown in DMEM/F12 media with 1% FBS (no growth factors) overnight at 37°C. The cells were then processed according to the manufacturer’s instructions to visualize nuclear EdU (Click-it EdU Imaging Kit, Invitrogen, Life Technologies, USA). Slides were examined using a fluorescence microscope (Nikon Eclipse 80i, Tokyo, Japan). Nine images (10× magnification) from randomly selected fields containing ~55–60 cells were assessed for EdU positive staining. Differentiation Assay: 7 day neurosphere cultures were dissociated into single cells, plated at the density of 50,000 cells/ well in a 2 well chamber slide coated with poly-L-lysine, and cultured in DMEM/F12 media with 1% FBS (no growth factors). Cells were then treated with or without 200 nM NF-α1 and grown for an additional 5 or 10 days and then analyzed by immunocytochemistry.
Phenotypic characterization of differentiated cells by immunocytochemistry
Immunocytochemistry was performed using standard protocols and examined using a fluorescence microscope (Nikon Eclipse 80i, Tokyo, Japan). Cell types analyzed were astrocytes (GFAP), neurons (Tuj1 or βIII Tubulin), oligodendrocytes (CNPase) and nestin (Nestin, a stem cell marker). For quantification, cells in 8–10 images taken at 20x magnification from randomly selected fields (~45–50 cells/field) were counted and averaged.
Treatment of neural stem/progenitor cells (NSC) with NF-α1 with or without ERK inhibitor
NSCs from dissociated neurospheres were plated on poly-l-lysine coated dishes and incubated with 200 nM NF-α1 for 0, 15, 30, 60 and 180 min, after which the cells were harvested and lysates analyzed by Western blot for p-ERK and total-ERK. Alternatively, cultured NSCs were pre-incubated with or without the ERK inhibitor, U0126 (5 µM) (Sigma Aldrich), for 30 min after which 200 nM NF-α1 was added and incubated for a further 30 min. The cells were then harvested and the cell lysates analyzed by Western blot for p-ERK and total-ERK.
Inhibition of Sox9 in NSCs using RNA interference
Double-stranded small-interfering RNAs (siRNAs) and control (scramble) RNA for mouse sox9 were obtained commercially (Santa Cruz). Using the RNAiMAX (Life Technologies) transfection procedure, neural stem/progenitor cells plated on poly-l-lysine coated cells, were transfected with Sox9/scramble siRNA and treated with or without NF-α1. After 48 h, the cells were harvested and extracted for RNA which was used to prepare cDNA. RT-PCR was performed to study mRNA levels of Sox9 and Gfap. The primer sequences for mouse sox9 and Gfap mRNA can be found in Supplementary Table S1. The relative amount of Sox9 and Gfap mRNA was normalized to 18S rRNA. For detailed RT-PCR protocol and cycling conditions please refer to the above method section.
Immunoprecipitation of embryonic tissue
To check whether NF-α1 was expressed at the protein level during early embryonic development, we harvested embryos from pregnant mice at different developmental stages. We pooled 4–5 embryos/stage for immunoprecipition (IP) and Western blot. Briefly lysates from each embryonic stage (whole embryos: E8.5, E10.5 & E11.5 and head alone: E12.5 & E13.5) were immunprecipitated [73] using rabbit polyclonal anti-NFα−1 (generated in our laboratory) and analyzed by Western blot with mouse monoclonal anti-NF-α1 (1:2000) (BD bioscience).
Western Blot
Cell lysates were prepared from E13.5, E14.5, E15.5, E16.5, E17.5, P1 cortex, neurospheres and differentiated cells as described previously [34]. Twenty μg of protein from the supernatants were analyzed by standard western blotting procedures using nitrocellulose. Protein bands were visualized and quantified by the Odyssey infrared imaging system and software v2.1 (LI-COR Inc.). The protein expression level for each sample was normalized to β-actin. A complete list of the antibodies used can be seen in Supplementary Table S2.
Immunohistochemistry (IHC) of mouse brains
For immunohistochemistry of E14.5, E15.5, E16.5, E17.5 and P1 mouse brains, pups were sacrificed by decapitation and processed as described previously [74]. The sections were processed also as described previously [74] and incubated with primary antibodies listed in Supplementary Table S2. Slides were examined using a fluorescence microscope (Nikon Eclipse 80i, Tokyo, Japan). The cortical layer was examined for astrocyte (GFAP), NF-α1 and neuron (MAP2) immunostaining intensity. Coronal sections (4 consecutive sections, 16 µm thick from each embryo were used for quantification, totaling 12 sections per phenotype from 3 NF-α1-WT and 3 NF-α1-KO pups or 3 WT mice at each developmental stage, from 3 independent litters). The intensity of the GFAP, NF-α1 and MAP2 immunostaining within 8 rectangular boxed fields (400 × 166 µm) (8 random areas/section, 4 sections/embryo, N=3 embryo/phenotype) in each section representing the whole neocortex was analyzed and quantified using imageJ software. Low magnification (×10) images were used to show immunostained cell pictures and high magnification (×20) images were used for quantification. The same number of coronal sections was used for counting individual GFAP+ and MAP2+ cells in 3 NF-α1-WT and 3 NF-α1-KO pups. For GFAP- and MAP2- immunopositive cell counting, 2 images/section, in the neocortical region [squared area, 140 × 140 µm] were captured by a confocal microscope (Zeiss LSM 510 Inverted Meta) with ×60 magnification and oil immersion lens. High magnification (×60) images were used for counting the immunostained cells manually in each square area.
Statistical Analysis
Data were analyzed by Student’s paired, unpaired t-test and one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons tests where noted. Significance was set at p<0.05.
(D&E) Developmental expression of NF-α1 (D) and GFAP (E) was quantified by immunohistochemistry in brain sections from E14.5 to P1 mice (see supplementary Fig 1 and methods). Eight random areas/section in the neocortex from 4 sections per brain and 3 brains for each stage were quantified. The rectangular box in the immunostained panels is representative of the size of the box (400 × 166 µm) used to measure the intensity of NF-α1 and GFAP staining (supplementary Fig 1). Expression of NF-α1 from immunostained sections showed a gradual increase in intensity from E14.5 to P1 (D), while GFAP immunoreactive staining showed low intensity from E14.5 to E17.5 and a surge at P1 (E). F(4,10)=39.73 for (D) and F(4,10)= 289.4 for (E) respectively, values are mean±SEM, *p<0.05 and **p<0.01, N=3, one way ANOVA (Tukey’s multiple comparison test). (F) Immunohistochemistry showing the co-localization of NF-α1 (red) and GFAP (green) in astrocytes (see arrows in merged image) at P1. Scale bar = 20 μm (G-L) Eight random areas/section from four consecutive sections/brain, 12 total sections per phenotype from 3 independent litters (P1 mice) were used to quantify GFAP and MAP2 staining in the neocortex of WT and KO mice. The rectangular box in the immunostained panels is representative of the size of the area in the neocortex used to measure the intensity of GFAP and MAP2 staining (see methods). (G & H) GFAP+ immunostaining in WT and KO mice Scale bar = 100 µm. (K) Bar graph showing quantification of GFAP+ immunoreactive staining intensity in WT and KO neocortex represented in panels G and H. A significant decrease in GFAP+ expression was found in KO compared to WT mice. *p<0.05, N=3.
(I & J) MAP2+ immunostaining intensity was observed in NF-α1-KO & NF-α1-WT mice. (L) Bar graph showing MAP2+ immunoreactive staining in WT and KO neocortex represented in panel I and J. No significant difference was observed between WT and KO mice.
(M) Schematic representative of P1 coronal section; the square box indicates the neocortex region which was used to count the GFAP and MAP2 stained cells. Two square areas/section, four sections/ brain and 3 brains /phenotype which came from 3 NF-α1-WT and 3 NF-α1-KO pups from 3 independent litters were quantified. (N&O) GFAP+ immunostained astrocytes in WT and KO neocortex respectively. GFAP+ astrocytes were significantly decreased in NF-α1-KO compared to NF-α1-WT. Scale bar = 20µm. (R) Bar graph showing GFAP+ astrocyte numbers represented in panels N and O. There was a significant decrease in astrocyte numbers in NF-α1-KO embryos compared to NF-α1-WT. The values represent the mean ± SEM, *p<0.05, N=3, t test. Scale bar = 20 µm). (P&Q) MAP2+ immunostained neurons in WT and KO neocortex respectively. No significant difference in MAP2+ immunostaining was observed between NF-α1-KO & NF-α1-WT. (S) Bar graph showing MAP2+ neurons represented in panels P and Q. The values represent the mean ± SEM, N=3, t test. Scale bar = 20 µm.
Supplementary Material
Acknowledgements
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health, USA. We would like to thank Dr. Marianne Bronner (CALTECH, Pasadena, Ca.) for her critical reading of this manuscript, and Lynn Holtzclaw and Dr. Vincent Schram (NICHD Microscopy Core Facility) for their assistance in microscopy.
Footnotes
Conflict of interest disclosure
The authors have no conflict of interest to declare.
References:
- 1.Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development 2004;131(13):3021–34. [DOI] [PubMed] [Google Scholar]
- 2.Kuroda H, Fuentealba L, Ikeda A et al. Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Gene Dev 2005;19(9):1022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang X, Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge. Dev Biol 2004;275(1):1–11. [DOI] [PubMed] [Google Scholar]
- 4.Lee HK, Lee HS, Moody SA. Neural transcription factors: from embryos to neural stem cells. Mol Cells 2014;37(10):705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roitbak T, Thomas K, Martin A et al. Moderate fetal alcohol exposure impairs neurogenic capacity of murine neural stem cells isolated from the adult subventricular zone. Exp Neurol 2011;229(2):522–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temple S The development of neural stem cells. Nature 2001. November 01;414(6859):112–7. [DOI] [PubMed] [Google Scholar]
- 7.Groszer M, Erickson R, Scripture-Adams DD et al. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci U S A 2006;103(1):111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guentchev M, McKay RDG . Notch controls proliferation and differentiation of stem cells in a dose-dependent manner. Europ J Neuroscience 2006; 23: 2289–2296. [DOI] [PubMed] [Google Scholar]
- 9.Stipursky J, Francis D, Gomes FC. Activation of MAPK/PI3K/SMAD pathways by TGF-β(1) controls differentiation of radial glia into astrocytes in vitro. Dev Neurosci 2012;34(1):68–81. [DOI] [PubMed] [Google Scholar]
- 10.Chen BY, Wang X, Wang ZY et al. Brain-derived Neurotrophic Factor stimulates proliferation and differentiation of neural stem cells, possibly by triggering the Wnt/β-catenin signaling pathway. J Neurosci Res 2013;91(1):30–41. [DOI] [PubMed] [Google Scholar]
- 11.Martini S, Bernoth K, Main H et al. A Critical Role for Sox9 in Notch-Induced Astrogliogenesis and Stem Cell Maintenance. Stem Cells 2013;31(4):741–51. [DOI] [PubMed] [Google Scholar]
- 12.Slawny NA, O’Shea KS. Dynamic changes in Wnt signaling are required for neuronal differentiation of mouse embryonic stem cells. Mol Cell Neurosci 2011;48(3):205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srikanth M, Kim J, Das S et al. BMP Signaling Induces Astrocytic Differentiation of Clinically Derived Oligodendroglioma Propagating Cells Mol Cancer Res 2014;12(2):283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda S, Abematsu M, Mori H et al. Potentiation of astrogliogenesis by STAT3-mediated activation of bone morphogenetic protein-Smad signaling in neural stem cells. Mol Cell Biol 2007;27(13):4931–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faigle R, Song HJ. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta 2013;1830(2):2435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai C, Grabel L. Directing the differentiation of embryonic stem cells to neural stem cells. Dev Dyn 2007. December;236(12):3255–66. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed S, Gan HT, Lam CS et al. Transcription factors and neural stem cell self-renewal, growth and differentiation. Cell Adh Migr 2009;3(4):412–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed S, Reynolds BA, Weiss S. BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors. J Neurosci 1995;15(8):5765–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benoit BO, Savarese T, Joly M et al. Neurotrophin channeling of neural progenitor cell differentiation. J Neurobiol 2001;46(4):265–80. [PubMed] [Google Scholar]
- 20.Majumder A, Banerjee S, Harrill JA et al. Neurotrophic Effects of Leukemia Inhibitory Factor on Neural Cells Derived from Human Embryonic Stem Cells. Stem Cells 2012. November;30(11):2387–99. [DOI] [PubMed] [Google Scholar]
- 21.He Z, Li JJ, Zhen CH et al. Effect of leukemia inhibitory factor on embryonic stem cell differentiation: implications for supporting neuronal differentiation. Acta Pharmacol Sin 2006;27(1):80–90. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi J, Palmer TD, Gage FH. Retinoic acid and neurotrophins collaborate to regulate neurogenesis in adult-derived neural stem cell cultures. J Neurobiol 1999. January;38(1):65–81. [PubMed] [Google Scholar]
- 23.Shirazi HA, Rasouli J, Ciric B et al. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp Mol Pathol 2015;98(2):240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams G, Zentar MP, Gajendra S et al. Transcriptional basis for the inhibition of neural stem cell proliferation and migration by the TGFβ-family member GDF11. PLoS One 2013. 7;8(11) :e78478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Z, Li K, Zhu L et al. Inhibitory effect of IL-17 on neural stem cell proliferation and neural cell differentiation. BMC Immunol 2013;14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang HY, Liu DD, Chang HF et al. Histone deacetylase inhibition mediates urocortin-induced anti-proliferation and neuronal differentiation in neural stem cells. Stem Cells 2012;30(12):2760–73. [DOI] [PubMed] [Google Scholar]
- 27.Lin N, Qin S, Luo S et al. Homocysteine induces cytotoxicity and proliferation inhibition in neural stem cells via DNA methylation in vitro. FEBS J 2014;281(8):2088–96. [DOI] [PubMed] [Google Scholar]
- 28.Lee C, Hu J, Ralls S et al. The molecular profiles of neural stem cell niche in the adult subventricular zone. PLoS One 2012;7(11):e50501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng M, Streck RD, Scott RE et al. The developmental expression in rat of proteases furin, PC1, PC2, and carboxypeptidase E: implications for early maturation of proteolytic processing capacity. J Neurosci 1994;14(8):4656–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cawley NX, Wetsel WC, Murthy SR et al. New roles of carboxypeptidase E in endocrine and neural function and cancer. Endocr Rev 2012;33(2):216–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birch NP, Rodriguez C, Dixon JE et al. Distribution of carboxypeptidase H messenger RNA in rat brain using in situ hybridization histochemistry: implications for neuropeptide biosynthesis. Brain Res Mol Brain Res 1990;7(1):53–9. [DOI] [PubMed] [Google Scholar]
- 32.Cool DR, Normant E, Shen FS et al. Carboxypeptidase E is a regulated secretory pathway sorting receptor: Genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell 1997;88(1):73–83. [DOI] [PubMed] [Google Scholar]
- 33.Park JJ, Loh YP. How Peptide Hormone Vesicles Are Transported to the Secretion Site for Exocytosis. Mol Endocrinol 2008;22(12):2583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng Y, Cawley NX, Loh YP. Carboxypeptidase E/NFalpha1: a new neurotrophic factor against oxidative stress-induced apoptotic cell death mediated by ERK and PI3-K/AKT pathways. PLoS One 2013;8(8):e71578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murthy SR, Thouennon E, Li WS et al. Carboxypeptidase E protects hippocampal neurons during stress in male mice by up-regulating prosurvival BCL2 protein expression. Endocrinology 2013;154(9):3284–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian Y, Varlamov O, Fricker LD. Glu300 of rat carboxypeptidase E is essential for enzymatic activity but not substrate binding or routing to the regulated secretory pathway. J Biol Chem 1999;274(17):11582–6. [DOI] [PubMed] [Google Scholar]
- 37.Pfister S, Steiner KA, Tam PP. Gene expression pattern and progression of embryogenesis in the immediate post-implantation period of mouse development. Gene Expr Patterns 2007;7(5):558–73. [DOI] [PubMed] [Google Scholar]
- 38.Ross ME, Carter ML, Lee JH. MN20, a D2 cyclin, is transiently expressed in selected neural populations during embryogenesis. J Neurosci 1996;16(1):210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stiles B, Groszer M, Wang SY et al. PTENless means more. Developmental Biology 2004;273(2):175–84. [DOI] [PubMed] [Google Scholar]
- 40.Hirsch C, Campano LM, Wohrle S et al. Canonical Wnt signaling transiently stimulates proliferation and enhances neurogenesis in neonatal neural progenitor cultures. Exp Cell Res 2007;313(3):572–87. [DOI] [PubMed] [Google Scholar]
- 41.Lyashenko N, Winter M, Migliorini D et al. Differential requirement for the dual functions of beta-catenin in embryonic stem cell self-renewal and germ layer formation. Nat Cell Biol 2011. July;13(7):753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miki T, Yasuda SY, Kahn M. Wnt/beta-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogramming. Stem Cell Rev 2011;7(4):836–46. [DOI] [PubMed] [Google Scholar]
- 43.Cui XP, Xing Y, Chen JM et al. Wnt/beta-catenin is involved in the proliferation of hippocampal neural stem cells induced by hypoxia. Ir J Med Sci 2011;180(2):387–93. [DOI] [PubMed] [Google Scholar]
- 44.Braunschweig L, Meyer AK, Wagenführ L et al. Oxygen regulates proliferation of neural stem cells through Wnt/β-catenin signalling. Mol Cell Neurosci 2015;67:84–92. [DOI] [PubMed] [Google Scholar]
- 45.Skalka N, Caspi M, Caspi E et al. Carboxypeptidase E: a negative regulator of the canonical Wnt signaling pathway. Oncogene 2013;32(23):2836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shanley DK, Sullivan AM. Alterations in cellular phenotypes differentiating from embryonic rat brain neurosphere cultures by immunoselection of neuronal progenitors. Brain Res 2006;1067(1):85–94. [DOI] [PubMed] [Google Scholar]
- 47.Decimo I, Bifari F, Krampera M et al. Neural Stem Cell Niches in Health and Diseases. Curr Pharm Design 2012;18(13):1755–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silva A, Pereira J, Oliveira CR et al. BDNF and Extracellular Matrix Regulate Differentiation of Mice Neurosphere-Derived Cells into a GABAergic Neuronal Phenotype. J Neurosci Res 2009;87(9):1986–96. [DOI] [PubMed] [Google Scholar]
- 49.Lin R, Iacovitti L. Classic and novel stem cell niches in brain homeostasis and repair. Brain Res 2015. pii: S0006–8993(15)00325-X. [DOI] [PubMed] [Google Scholar]
- 50.Easterday MC, Dougherty JD, Jackson RL et al. Neural progenitor genes. Germinal zone expression and analysis of genetic overlap in stem cell populations. Dev Biol 2003;264(2):309–22. [DOI] [PubMed] [Google Scholar]
- 51.Vilijn MH, Das B, Kessler JA et al. Cultured astrocytes and neurons synthesize and secrete carboxypeptidase E, a neuropeptide-processing enzyme. J Neurochem 1989;53(5):1487–93. [DOI] [PubMed] [Google Scholar]
- 52.Hajos F, Kalman M. Distribution of glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes in the rat brain. II. Mesencephalon, rhombencephalon and spinal cord. Exp Brain Res 1989;78(1):164–73. [DOI] [PubMed] [Google Scholar]
- 53.Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res 1995;84(1):109–29. [DOI] [PubMed] [Google Scholar]
- 54.Lee JA, Cole GJ. Localization of transitin mRNA, a nestin-like intermediate filament family member, in chicken radial glia processes. J Comp Neurol 2000;418(4):473–83. [DOI] [PubMed] [Google Scholar]
- 55.Zhou ZD, Kumari U, Xiao ZC et al. Notch as a Molecular Switch in Neural Stem Cells. Iubmb Life 2010;62(8):618–23. [DOI] [PubMed] [Google Scholar]
- 56.Vong KI, Leung CK, Behringer RR et al. Sox9 is critical for suppression of neurogenesis but not initiation of gliogenesis in the cerebellum. Mol Brain 2015;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morrison SJ, Perez SE, Qiao Z et al. Transient notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell 2000;101(5):499–510. [DOI] [PubMed] [Google Scholar]
- 58.Stipursky J, Gomes FC. TGF-beta1/SMAD signaling induces astrocyte fate commitment in vitro: implications for radial glia development. Glia 2007;55(10):1023–33. [DOI] [PubMed] [Google Scholar]
- 59.Li S, Mattar P, Dixit R et al. RAS/ERK signaling controls proneural genetic programs in cortical development and gliomagenesis. J Neurosci 2014;34(6):2169–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomes FC, Paulin D, Moura Neto V. Glial fibrillary acidic protein (GFAP): modulation by growth factors and its implication in astrocyte differentiation. Braz J Med Biol Res 1999;32(5):619–31. [DOI] [PubMed] [Google Scholar]
- 61.Stolt CC, Lommes P, Sock E et al. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev 2003;17(13):1677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kang P, Lee HK, Glasgow SM et al. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron 2012;74(1):79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ling S, Chang X, Schultz L et al. An EGFR-ERK-SOX9 signaling cascade links urothelial development and regeneration to cancer. Cancer Res 2011;71(11):3812–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li XL, Chen XQ, Zhang MN et al. SOX9 was involved in TKIs resistance in renal cell carcinoma via Raf/MEK/ERK signaling pathway. Int J Clin Exp Patho 2015;8(4):3871–81. [PMC free article] [PubMed] [Google Scholar]
- 65.De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci 2008;267(1–2):3–16. [DOI] [PubMed] [Google Scholar]
- 66.Molofsky AV, Krencik R, Ullian EM et al. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev 2012;26(9):891–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cawley NX, Zhou J, Hill JM et al. The carboxypeptidase E knockout mouse exhibits endocrinological and behavioral deficits. Endocrinology 2004. December;145(12):5807–19. [DOI] [PubMed] [Google Scholar]
- 68.Lugo DI, Roberts JL, Pintar JE. Analysis of Proopiomelanocortin Gene-Expression during Prenatal Development of the Rat Pituitary-Gland. Mol Endocrinol 1989;3(8):1313–24. [DOI] [PubMed] [Google Scholar]
- 69.Shughrue PJ, Komm B, Merchenthaler I. The distribution of estrogen receptor-beta mRNA in the rat hypothalamus. Steroids 1996;61(12):678–81. [DOI] [PubMed] [Google Scholar]
- 70.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. Journal of Comparative Neurology 1999;403(2):261–80. [DOI] [PubMed] [Google Scholar]
- 71.Qin XY, Cheng Y, Murthy SR et al. Carboxypeptidase E-ΔN, a neuroprotein transiently expressed during development protects embryonic neurons against glutamate neurotoxicity. PLoS One 2014; 26;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rietze RL, Reynolds BA. Neural stem cell isolation and characterization. Methods Enzymol 2006;419:3–23. [DOI] [PubMed] [Google Scholar]
- 73.Bonifacino JS, Dell’Angelica EC, Springer TA. Immunoprecipitation. Curr Protoc Mol Biol 2001. Chapter 10:Unit 10.16. [DOI] [PubMed] [Google Scholar]
- 74.Holtzclaw LA, Pandhit S, Bare DJ et al. Astrocytes in adult rat brain express type 2 inositol 1,4,5-trisphosphate receptors. Glia 2002;39(1):69–84. [DOI] [PubMed] [Google Scholar]
- 75.Okano-Uchida T, Naruse M, Ikezawa T, et al. Cerebellar neural stem cells differentiate into two distinct types of astrocytes in response to CNTF and BMP2. Neurosci Lett 2013; 552:15–20. doi: 10.1016/j.neulet.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 76.Ahmed S, Reynolds BA, Weiss S. BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors. J Neurosci 1995; 15(8):5765–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lim MS, Nam SH, Kim SJ, et al. Signaling pathways of the early differentiation of neural stem cells by neurotrophin-3. Biochem Biophys Res Commun 2007; 357(4):903–9. [DOI] [PubMed] [Google Scholar]
- 78.Willerth SM, Sakiyama-Elbert SE. Kinetic analysis of neurotrophin-3- mediated differentiation of embryonic stem cells into neurons. Tissue Eng Part A 2009; 15(2):307–18. doi: 10.1089/ten.tea.2008.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roussa E, Krieglstein K. GDNF promotes neuronal differentiation and dopaminergic development of mouse m esencephalicneurospheres. Neurosci Lett 2004; 361(1–3):52–5. [DOI] [PubMed] [Google Scholar]
- 80.Chung WS, Allen NJ, Eroglu C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol 2015;7(9):a020370. doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mallamaci A Developmental control of cortico-cerebral astrogenesis. Int J Dev Biol 2013;57(9–10):689–706. doi: 10.1387/ijdb.130148am. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


