Abstract
Triclosan (TCS) and bisphenol A (BPA) are endocrine-disrupting chemicals that interfere with the hormone or endocrine system and may cause cancer. Kaempferol (Kaem) and 3,3′-diindolylmethane (DIM) are phytoestrogens that play chemopreventive roles in the inhibition of carcinogenesis and cancer progression. In this study, the influence of TCS, BPA, Kaem, and DIM on proliferation and apoptotic abilities of VM7Luc4E2 breast cancer cells were examined. MTT assay revealed that TCS (0.1–10 μM), BPA (0.1–10 μM) and E2 (0.01–0.0001 μM) induced significant cell proliferation of VM7Luc4E2 cells, which was restored to the control (0.1% DMSO) by co-treatment with Kaem (30 μM) or DIM (15 μM). Reactive oxygen species (ROS) production assays showed that TCS and BPA inhibited ROS production of VM7Luc4E2 cells similar to E2, but that co-treatment with Kaem or DIM on VM7Luc4E2 cells induced increased ROS production. Based on these results, the effects of TCS, BPA, Kaem, and DIM on protein expression of apoptosis and ROS production-related markers such as Bax and Bcl-xl, as well as endoplasmic reticulum (ER) stress-related markers such as eIF2α and CHOP were investigated by Western blot assay. The results revealed that TCS, and BPA induced anti-apoptosis by reducing ROS production and ER stress. However, Kaem and DIM effectively inhibited TCS and BPA-induced anti-apoptotic processes in VM7Luc4E2 cells. Overall, TCS and BPA were revealed to be distinct xenoestrogens that enhanced proliferation and anti-apoptosis, while Kaem and DIM were identified as natural chemopreventive compounds that effectively inhibited breast cancer cell proliferation and increased anti-apoptosis induced by TCS and BPA.
Keywords: Kaempferol; 3,3′-diindolylmethane; Triclosan; Bisphenol A; Apoptosis; Reactive oxygen species
INTRODUCTION
High levels of endogenous estrogen are considered a breast cancer risk factor in women (Missmer et al., 2004; Santen et al., 2007). Estrogen can induce overgrowth of cancer cells by stimulating cell proliferation and disrupting normal cellular processes such as apoptosis and DNA repair via estrogen receptor (ER)-mediated signaling (Lewis-Wambi and Jordan, 2009). In ER-positive breast cancer cells, 17β-estradiol (E2) increased cell growth by promoting cell cycle progression and induced anti-apoptosis by upregulating Bcl-2, an important anti-apoptotic protein (Sakamoto et al., 2010).
Endocrine disrupting chemicals (EDCs) that have similar structures to E2 may exert estrogenic functions and interfere with estrogen signaling, as well as cause cancer in estrogen-responsive organs (Park et al., 2011; Shanle and Xu, 2011; Kim et al., 2015a). Triclosan (TCS) is a broad-spectrum antibacterial compound commonly used in numerous consumer products (Darbre, 2006) that has been recently identified as a potential EDC (Feng et al., 2016). Previous studies have shown that TCS may play a role in cancer development through its estrogenicity (Dinwiddie et al., 2014), and that it inhibited death of breast cancer cells via regulation of cell cycle and apoptosis-related genes (Kim et al., 2016). Bisphenol A (BPA), which is a typical EDC, is a basic raw material for manufacturing polycarbonate plastics and epoxy resins that is commonly included in plastic, storage containers, and disposable products (Chen et al., 2002; Jeong et al., 2017). Bisphenol A is known to have cancer-related toxicity that leads to cell proliferation effects in estrogen-dependent breast and ovarian cancer cells (Lee et al., 2012, 2014) as well as to have significant effects on cancer development by increasing anti-apoptosis (Elswefy et al., 2016; Yin et al., 2017).
Plant-derived natural compounds have been shown to exert significant chemopreventive effects against cancers (Russo et al., 2016). Phytoestrogens are estrogenic chemical compounds derived from plants that have been shown to exert biological effects that prevent tumor development (Coelingh Bennink, 2004; Kim et al., 2014). Phytoestrogens have ER antagonistic actions in the classical ER signaling pathway in breast cancer cells and would be used as selective estrogen receptor modulators (SERMs) (Ahn et al., 2014). Moreover, some experimental studies have shown that phytoestrogens have anti-breast cancer activity (Hilakivi-Clarke et al., 1999; Kang et al., 2013; Hwang and Choi, 2015; Kim et al., 2016; Lee et al., 2016, 2017). As effective anti-cancer agents, phytoestrogens have been studied for their ability to suppress cancer progression by controlling cell proliferation, the cell cycle, and apoptosis (Fang et al., 2016). Phytoestrogens including polyphenols and flavonoids derived from plants such as soybeans and teas have been shown to have antioxidant activities (Santos et al., 2016). Kaempferol (Kaem) is a flavonoid agent found in fruits, teas, and ginkgo leaves that exerts antitumor and anti-oxidation effects in various cancer cells (Liao et al., 2016). Kaem was shown to suppress cell growth by inducing reactive oxygen species (ROS) production, endoplasmic reticulum (ER) stress, and apoptosis (Tatsimo et al., 2012; Lee and Kim, 2016; Liao et al., 2016). Indole-3-carbinol (I3C) is rich in Brassica vegetables such as cauliflower, turnips, kale, and broccoli and may exert protective functions against many types of cancer (Verhoeven et al., 1996). I3C is a precursor of 3,3′-diindolylmethane (DIM) that is converted into DIM via dehydration of acid-catalyzed reactions at low pH (Lee et al., 2016). DIM induces alterations in mitochondrial depolarization and hyperpolarization, which are the early stages of apoptosis (Gong et al., 2006).
Mitochondria-mediated apoptosis as an intrinsic apoptotic pathway is associated with cell death processes and tissue homeostasis (Shi, 2001; Stachon et al., 2015). When cells are stressed and the permeability of mitochondrial membranes is increased, mitochondrial proteins such as second mitochondria-derived activator of caspases (SMACs) are released into the cytosol, where they bind to proteins that inhibit apoptosis, thereby deactivating them and allowing apoptosis to proceed (Fesik and Shi, 2001). Currently, ER stress caused by exposure to reactive oxygen species (ROS) is considered one of the stresses that triggers apoptosis (Kitamura and Hiramatsu, 2010). The ER is an organelle that plays an essential role in the folding of protein molecules and the transport of synthesized proteins to the Golgi apparatus (Soltys et al., 1996). Production of ROS causes oxidative injury, which influences ER homeostasis and induces inappropriate protein folding in the ER (Yang et al., 2016). Under severe oxidative stress, accumulation of unfolded or misfolded proteins, namely the unfolded protein response (UPR), leads to ER stress. This further triggers the pro-apoptotic pathway via activation of transcription factor 6 (ATF6)-CCAAT/enhancer-binding protein-homologous protein (CHOP) (Kitamura and Hiramatsu, 2010). Therefore, CHOP is a major gene associated with ER stress-induced apoptosis (Galehdar et al., 2010).
In the present study, the roles associated with apoptosis of two EDCs, TCS and BPA, as well as two phytoestrogens, Kaem and DIM, in VM7Luc4E2 breast cancer cells, which are estrogen-responsive ER positive cells, were investigated by examining their effects on the interactions between ROS-induced ER stress and apoptosis. The results of this study will improve understanding of the opposite roles of the EDCs and phytoestrogens in regulation of breast cancer progression.
MATERIALS AND METHODS
Reagents and chemicals
17β-estradiol (E2), Irgasan (TCS), bisphenol A (BPA), 3,3′diindolylmethane (DIM) and kaempferol (Kaem) were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). All chemicals were dissolved in 100% dimethyl sulfoxide (DMSO; Junsei Chemical Co., Tokyo, Japan). The culture media used for cell treatment contained a final concentration of DMSO of 0.1%.
Cell culture and media
VM7Luc4E2 human breast carcinoma cells (VM7Luc4E2 cells), which were recently identified as variant MCF-7 human breast cancer cells (Li et al., 2014), were obtained from ATCC (American Type Culture Collection, Manassas, VA, USA). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone Laboratories, Inc., Logan, UT, USA) supplemented with 10% heat inactivated fetal bovine serum (FBS; Hyclone Laboratories, Inc., Logan, UT, USA), 200 U/ml penicillin G, and 200 mg/ml streptomycin (A&E Scientific, Logan, UT, USA) at 37°C in a humidified atmosphere of 5% CO2 containing air. To prevent the estrogenic effects of some components in DMEM and FBS, phenol red-free DMEM (Sigma Aldrich) supplemented with 5% charcoal-dextran treated FBS was used for detection of the diverse effects of reagents and chemicals on VM7Luc4E2 cells. The cells were detached with 0.05% Trypsin-EDTA (PAA Laboratories, Dartmouth, MA, USA).
Cell viability assays
VM7Luc4E2 cells were seeded at a density of 3×103 cells per well in 96-well plates. After 2-days of incubation, the culture medium was replaced with new medium containing E2 (0.01, 0.001, 0.0001 μM), TCS (0.1, 1, 10 μM), BPA (0.1, 1, 10 μM), and each combination of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and Kaem (10, 20, 30, 40 μM) or DIM (5, 10, 15, 20 μM). The cells were then incubated for an additional 8 days, during which time the medium was changed every three days. The culture medium was then removed, after which 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma Aldrich) solution was added for 3 h. Following removal of the culture medium, 200 μl DMSO (Junsei Chemical Co., Tokyo, Japan) was added per well and the absorbance was measured at 540 nm using an ELISA reader (Epoch, BioTek, VT, USA).
Western blot assay
After treatment of the VM7Luc4E2 cells with E2 (0.001 μM), TCS (1 μM), BPA (1 μM), Kaem (30 μM), DIM (15 μM), a combination of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and Kaem (30 μM), and a combination of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and DIM (15 μM), the proteins from VM7Luc4E2 cells were harvested with RIPA lysis buffer (50 mM Tris-HCl, pH 8.0; 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, and 0.1% SDS). Bicinchoninc acid (BCA; Sigma-Aldrich Corp.) was used for determining protein concentrations. Total cell proteins (50 μg) were separated on 10% SDS-PAGE gel, then transferred to a polyvinylidene fluoride (PVDF) membrane (BioRad Laboratories Inc., Hercules, CA, USA). The membrane was subsequently blocked with 5% skim milk (BioRad Laboratories Inc.) blocking buffer to inhibit the nonspecific interaction with primary antibody for 2 h, then washed four times in 1× TBS buffer (adjusted to pH 7.6 with HCl) containing 0.1% (v/v) Tween 20 (BioRad Laboratories Inc.). Next, the membrane was incubated with mouse monoclonal antibody specific for Bax (1:1000 dilution, Santa Cruz Biotechnology, Dallas, TX, USA), Bcl-xl (1:1000 dilution, Cell Signaling Technology, Danvers, MA, USA), CHOP (1:1000 dilution, Cell Signaling Technology), p-eIF2α (1:1000 dilution, Cell Signaling Technology) or GAPDH (1:12000 dilution, Abcam) in 1× TBS with 3% (w/v) BSA (Sigma-Aldrich Corp.) and 0.1% (v/v) Tween 20 overnight at 4°C. The membranes were subsequently probed with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG or anti-mouse IgG (1:2000, Thermo Scientific Corp., Rockford, IL, USA) for 2 h at room temperature. Target proteins were detected with a West-Q Chemilu-minescent Substrate Plus kit (GenDEPOT, Barker, TX, USA). Quantification of each protein was accomplished by scanning the densities of bands on a transfer membrane using Lumino graph II (ATTO, Amberst, NY, USA).
Dichloro-dihydro-fluorescein diacetate (H2DCF-DA) assay
Intracellular ROS generation in VM7Luc4E2 cells was measured by 20,70-dichlorofluorescein diacetate (DCF-DA) assay. VM7Luc4E2 cells were seeded at a density of 1×105 cells per well in a 6 well plate with 200 μl phenol-free DMEM media containing 5% CD-FBS. After 24 h, the medium was replaced with new medium containing E2 (0.001 μM), TCS (1 μM), BPA (1 μM), Kaem (30 μM), DIM (15 μM), a combination of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and Kaem (30 μM), and a combination of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and DIM (15 μM), after which cells were incubated for an additional 72 h. Moreover, H2O2 was used as a positive control to detect ROS formation in VM7Luc4E2 cells. Briefly, 3% H2O2 solution was added to the wells with 1×105 cells of VM7Luc4E2 cells at 1% concentration and incubated for 30 min. Following incubation, the culture medium was removed, and each well was treated with 200 μl H2DCF-DA solution (10 mM in PBS) for 30 min. This plate was then placed in a dark room at room temperature for 30 min, after which the fluorescence intensity of DCF (an oxidized form of H2DCF) was measured and samples were photographed using a fluorescence microscope (IX-73 Inverted Microscopy, Olympus, Japan) to detect ROS production. The ROS production induced by each treatment was quantified using the Cell Sens Dimension software (Olympus).
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling assay
A terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick-end labeling (TUNEL) assay was processed to detect the fluorescence of apoptotic cells using TdT enzyme with the fluorometric TUNEL assay kit (Promega, Madison, WI, USA). Briefly, VM7Luc4E2 cells were seeded at 1×105 cells per well in a 6 well plate with DMEM media. After 24 h, the medium was replaced with new medium containing E2 (0.001 μM), TCS (1 μM), BPA (1 μM), Kaem (30 μM), DIM (15 μM), a combination of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and Kaem (30 μM), and a combination of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and DIM (15 μM). After 48 h, cells were fixed with 4% methanol-free formaldehyde (pH 7.4) for 1 h, permeabilized using lysis buffer (1% Triton X-100 in 1% sodium citrate) for 15 min, and then treated with 50 μL TdT enzyme buffer, which attached to DNA strand breaks. Finally, labeled strand breaks were visualized by the attachment of fluorescein isothiocyanate-5-dUTP. All slides were counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen Life Technologies, Carlsbad, CA, USA), then viewed using a fluorescence microscope (IX-73 Inverted Microscopy, Olympus). Fluorescence by each treatment was quantified with the Cell Sens Dimension software (Olympus).
Data analysis
All experiments were conducted at least three times to ensure consistent results. All data were analyzed with the Graph-pad Prism software (San Diego, CA, USA). In vitro data were expressed as the means ± standard deviation (SD). Data were analyzed by one-way analysis of variance (ANOVA) followed by post hoc Dunnett’s multiple comparison test or by Student’s t-tests for two-pair comparisons. p-values <0.05 were considered statistically significant.
RESULTS
Effect of Kaem and DIM on TCS or BPA induced-VM7Luc4E2 breast cancer cell growth
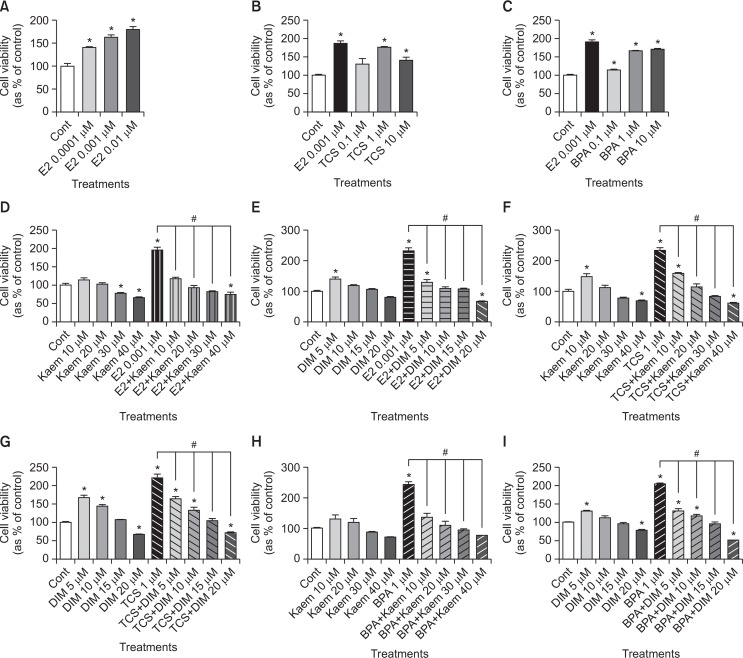
To evaluate the effects of E2, TCS or BPA on VM7LUC4E2 cell growth, VM7LUC4E2 cells were cultured with E2 (a positive control), TCS or BPA for 8 days. As shown in Fig. 1A–1C, increased VM7LUC4E2 cell viability was observed in response to treatment with E2, TCS or BPA when compared to 0.1% DMSO (a control). The highest increase in cell viability was observed in response to E2 at 0.001 μM in the concentration range tested (Fig. 1A). The cell viability of VM7LUC4E2 cells was the highest at 1 μM TCS, and it was lowered at 10 μM TCS than at 1 μM TCS as shown in Fig. 1B. For BPA, cell viability showed the greatest increase in response to 1 μM BPA in the concentration range of 0.1 μM to 10 μM, and there was no change at two concentrations between 1 μM and 10 μM of BPA (Fig. 1C). These findings indicate that TCS and BPA can induce proliferation of VM7LUC4E2 cells that are ER positive in a fashion similar to that of E2. In addition, the effects of Kaem and DIM on the viability of VM7LUC4E2 cells was identified for the treatment with these compounds alone and in combination with E2, TCS or BPA. When VM7LUC4E2 cells were treated with Kaem or DIM alone, a slight increase in cell viability was observed in response to low doses, while decreased cell viability was observed in response to high doses (Fig. 1D–1I). When cells were co-treated Kaem and DIM and E2, TCS or BPA, E2, TCS or BPA-induced cell proliferation was significantly suppressed in response to all doses (Fig. 1D–1I), indicating that Kaem and DIM exert anti-proliferative activity to abrogate E2, TCS or BPA-induced cell proliferation.
Fig. 1.
Cell viability of VM7Luc4E2 cells following treatment with E2, TCS, BPA, Kaem, and DIM. VM7Luc4E2 cells were plated at 3,000 cells/well in 200 μl of phenol red-free DMEM with 5% CD–FBS medium and then cultured in phenol red-free DMEM with 0.1% DMSO (a control), (A–C) E2 (0.0001-0.01 μM), TCS (0.1–10 μM) or BPA (0.1–10 μM), (D, E) E2 (0.001 μM) in the presence or absence of Kaem (10–40 μM) or DIM (5–20 μM), (F, G) TCS (1 μM) in the presence or absence of Kaem (10–40 μM) or DIM (5–20 μM), and (H, I) BPA (1 μM) in the presence or absence of Kaem (10–40 μM) or DIM (5–20 μM) for 8 days. The cell viability was measured using MTT assay and replicated at least three times to ensure consistent results. Values shown are the means ± SD. *Mean values were significantly different from that of control, p<0.05 (Dunnett’s multiple comparison test). #Mean values were significantly different from that of a single treatment of E2, TCS or BPA, p<0.05 (Student’s t-test).
Effects of Kaem and DIM on E2, TCS or BPA-induced anti-apoptosis of VM7LUC4E2 breast cancer cells
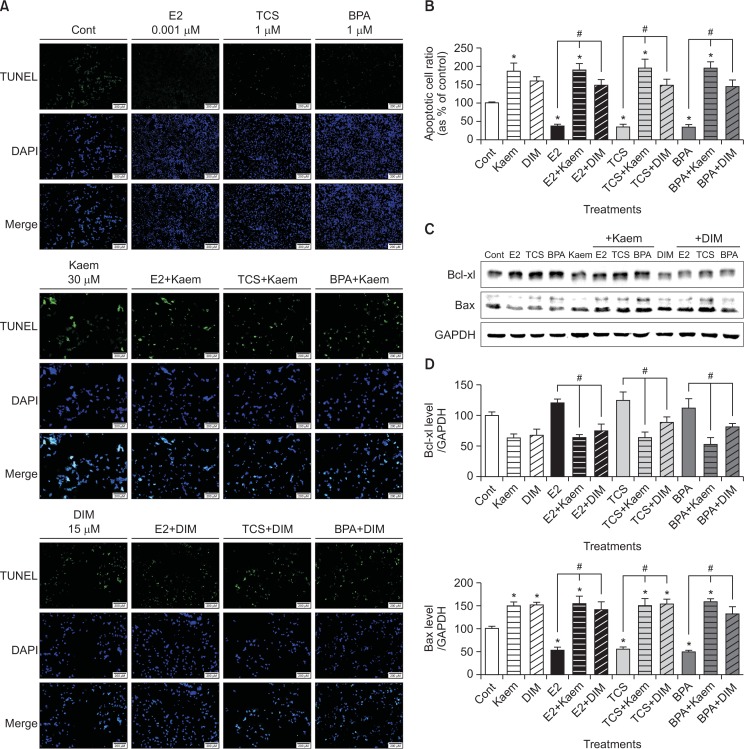
After VM7LUC4E2 cells were exposed to EDCs and phytoestrogens, apoptotic cells were measured using TUNEL assay and fluorescence microscopy. The merged images of E2, TCS or BPA-treated VM7LUC4E2 cells subjected to TUNEL reaction and DAPI staining revealed a decreased number of apoptotic cells compared to the control. TCS and BPA were shown to exert anti-apoptotic effects similar to E2. However, an increased number of apoptotic cells with green fluorescence were detected in cases of co-treatment with Kaem or DIM and E2, TCS or BPA compared to treatment with E2, TCS or BPA alone (Fig. 2).
Fig. 2.
Effects of E2, TCS, BPA, Kaem, and DIM on apoptosis of VM7Luc4E2 breast cancer cells. To detect the apoptotic cells, TUNEL assay was examined for VM7Luc4E2 cells treated with (A) 0.1% DMSO (a control), E2 (0.001 μM), TCS (1 μM), and BPA (1 μM), Kaem (30 μM) and a mixture of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and Kaem (30 μM), and DIM (15 μM) and a mixture of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and DIM (15 μM) for 48 h. (B) Fluorescence in response to each treatment was quantified using the Cell Sens Dimension software. (C) VM7Luc4E2 cells were treated with 0.1% DMSO (a control), E2 (0.001 μM), TCS (1 μM), BPA (1 μM), Kaem (30 μM), DIM (15 μM), a mixture of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and Kaem (30 μM), and a mixture of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and DIM (15 μM), respectively, for 72 h. After protein extraction from the cells, each protein band corresponding to Bax and Bcl-xl was detected by Western blot assay. (D) Quantification of each protein was accomplished by scanning the densities of bands on a transfer membrane using Lumino graph II. TUNEL and Western blot assays were replicated at least three times. *Mean values were significantly different from that of 0.1% DMSO (a control), p<0.05 (Dunnett’s multiple comparison test). #Mean values were significantly different from that of a single treatment of E2, TCS or BPA, p<0.05 (Student’s t-test).
To investigate the effects of E2, TCS, BPA, Kaem and DIM on protein expression of apoptosis-related markers, a Western blot assay was conducted to measure the proteins extracted from VM7LUC4E2 breast cancer cells treated with E2 (0.001 μM), TCS (1 μM), BPA (1 μM), Kaem (30 μM) or DIM (15 μM) for 72 h. The protein expression of Bcl-xl, an anti-apoptotic protein, was decreased by Kaem or DIM compared to the control, while co-treatment of Kaem or DIM and E2, TCS or BPA significantly decreased Bcl-xl expression compared to treatment with E2, TCS or BPA alone (Fig. 2C, 2D). On the contrary, the protein expression of Bax, a pro-apoptotic protein, was decreased by E2, TCS or BPA compared to the control. However, treatment with Kaem and DIM increased the Bax protein expression, and co-treatment with Kaem or DIM and E2, TCS or BPA significantly increased Bax expression compared to treatment with E2, TCS or BPA alone (Fig. 2C, 2D). These results indicate that E2, TCS, and BPA suppressed the apoptosis of VM7LUC4E2 breast cancer cells, but that Kaem and DIM can induce apoptosis and inhibit the pro-apoptotic effects of E2, TCS or BPA in VM7LUC4E2 cells.
Generation of ROS by treatment with E2, TCS, BPA, Kaem, and DIM
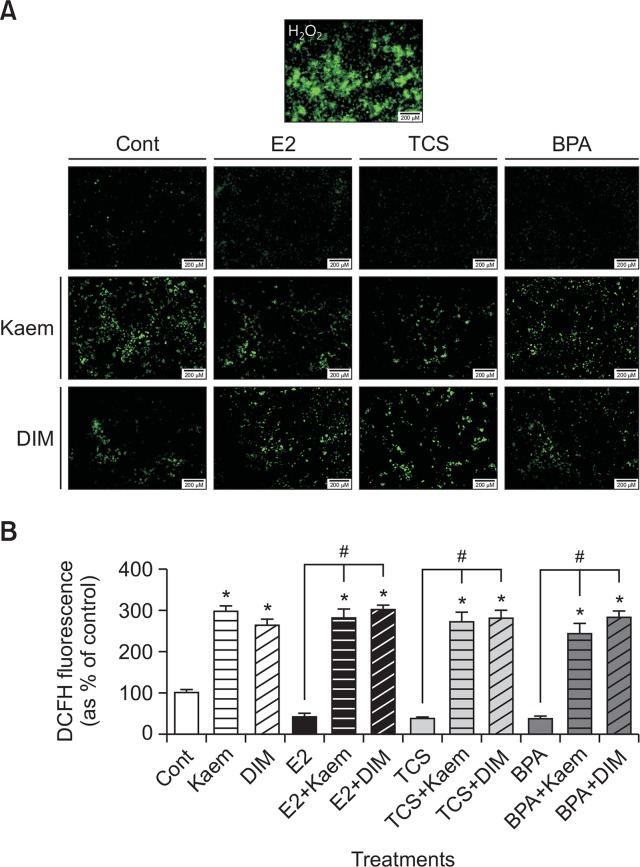
To investigate the intracellular ROS generation in VM7LUC4E2 breast cancer cells a H2DCF-DA assay was conducted to detect ROS based on the appearance of green fluorescence after treatment with E2, TCS, BPA, Kaem, and DIM alone as well as after co-treatment with E2, TCS or BPA and Kaem or DIM for 72 h. We first identified ROS formation from the image of cells treated with H2O2 as a positive control for detection of ROS formation. When VM7LUC4E2 cells were treated with E2, TCS or BPA, a small amount of ROS was detected less than that of observed in the control (0.1% DMSO). On the other hand, treatment with Kaem or DIM alone, as well as cotreatment with Kaem or DIM and E2, TCS or BPA significantly increased the ROS formation in VM7LUC4E2 cells compared to the control or treatment with E2, TCS or BPA alone (Fig. 3A, 3B). These results indicate that E2, TCS, and BPA inhibit ROS formation, but Kaem and DIM promote ROS formation in VM7LUC4E2 cells.
Fig. 3.
ROS formation in VM7Luc4E2 cells following treatment with E2, TCS, BPA, Kaem, and DIM. (A) VM7Luc4E2 cells were treated with 0.1% DMSO (a control), E2 (0.001 μM), TCS (1 μM), BPA (1 μM), Kaem (30 μM), DIM (15 μM), a mixture of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and Kaem (30 μM), and a mixture of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and DIM (15 μM), respectively, for 72 h. Cells were treated with H2O2 (a positive control for ROS production) for 30 min, after which H2DCF-DA was added to each well for 30 min, and the fluorescence intensity of DCF (an oxidized form of H2DCF) was measured and photographed using a fluorescence microscopy to detect ROS production. H2DCF-DA assay was replicated at least three times. (B) ROS production by each treatment was quantified using the Cell Sens Dimension software. *Mean values were significantly different from that of 0.1% DMSO (a control), p<0.05 (Dunnett’s multiple comparison test). #Mean values were significantly different from that of a single treatment of E2, TCS or BPA, p<0.05 (Student’s t-test). *Mean values were significantly different from that of 0.1% DMSO (control), p<0.05 (Dunnett’s multiple comparison test). #Mean values were significantly different from that of a single treatment of E2, TCS or BPA, respectively, p<0.05 (Student’s t-test).
Roles of E2, TCS, BPA, Kaem, and DIM on protein expression of ER stress- and apoptosis-related genes
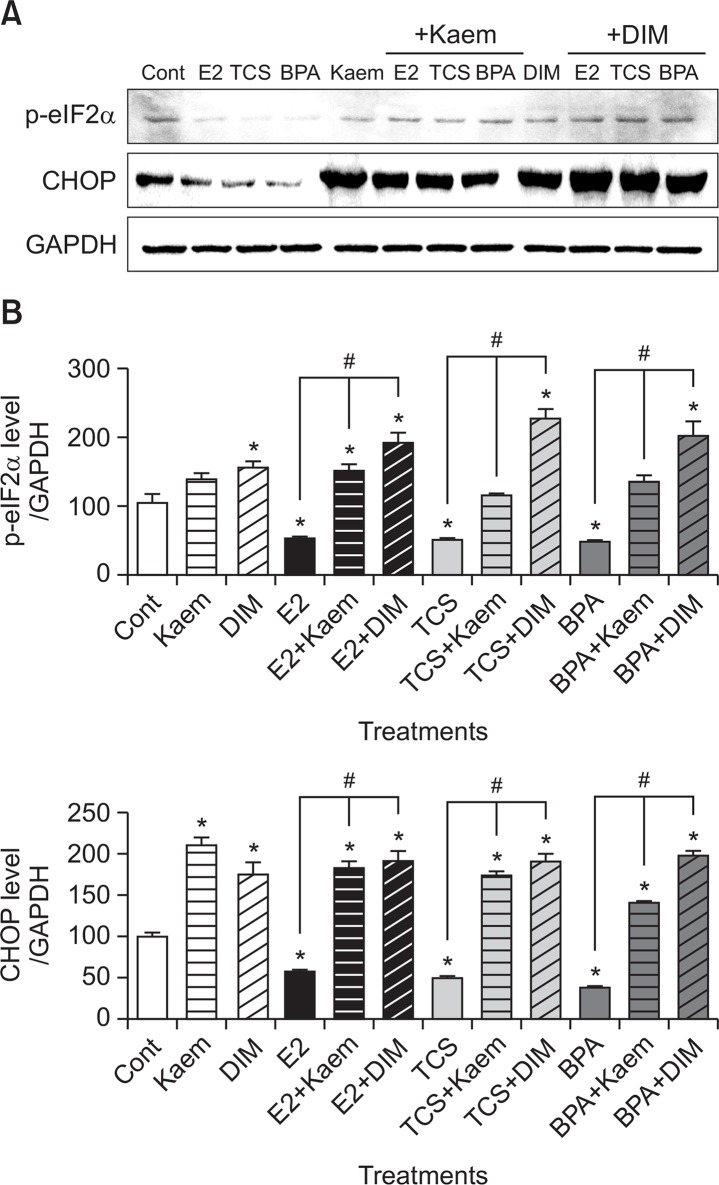
To examine the effects of E2, TCS, BPA, Kaem, and DIM on ER stress-related apoptotic process, we examined the alterations in protein expression of p-eIF2α and CHOP in VM7LUC4E2 cells in response to treatment with these reagents. As shown in Fig. 4, E2, TCS, and BPA suppressed the protein expression of p-eIF2α and CHOP compared to the control. However, co-treatment of Kaem and DIM significantly increased their expression compared to treatment with E2, TCS, and BPA alone. These results indicated that E2, TCS, and BPA inhibited ER stress-related apoptosis, but Kaem and DIM promoted the apoptotic process by enhancing ER-stress while also inhibiting E2, TCS, and BPA-induced anti-apoptosis.
Fig. 4.
Protein expression of ER stress-related markers following treatment with E2, TCS, BPA, Kaem, and DIM. (A) VM7Luc4E2 cells were treated with 0.1% DMSO (a control), E2 (0.001 μM), TCS (1 μM), BPA (1 μM), Kaem (30 μM), DIM (15 μM), a mixture of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and Kaem (30 μM), and a mixture of E2 (0.001 μM), TCS (1 μM) or BPA (1 μM) and DIM (15 μM) for 72 h. After protein extraction from the cells, the protein band corresponding to CHOP and p-eIF2α, respectively, was detected by Western blot assay. Western blot assay was replicated at least three times. (B) Quantification of the protein was accomplished by scanning the densities of bands on a transfer membrane using Lumino graph II. *Mean values were significantly different from that of 0.1% DMSO (a control), p<0.05 (Dunnett’s multiple comparison test). #Mean values were significantly different from that of a single treatment of E2, TCS or BPA, p<0.05 (Student’s t-test).
DISCUSSION
Apoptosis is a highly regulated programmed cell death for development processes of embryos and homeostasis; however, insufficient apoptosis is closely associated with uncontrolled cell proliferation and cancer (Fuchs and Steller, 2011). Mitochondria-mediated apoptosis is an intrinsic apoptotic pathway, and ER stress is considered to induce this type of apoptosis via UPR and CHOP production (Wang and Kaufman, 2012; Bhat et al., 2017). Stress to the ER generally causes damage to cellular protein-folding, which is responsible for the biosynthesis and modification of many proteins (Gafar et al., 2016). During induction of apoptosis by ER stress via UPR caused by oxidative stress, activated protein kinase R-like endoplasmic reticulum kinase (PERK) phosphorylates eIF2α, and p-eIF2α causes selective induction of activating transcription factor 4 (ATF4), leading to the activation of pro-apoptotic CHOP (Kitamura and Hiramatsu, 2010). Eventually, CHOP induces intrinsic apoptosis by regulating BCL2 family protein expression as well as by activating caspase-4 (Bhat et al., 2017). Bax and caspase play crucial roles in mitochondrial permeacilization and apoptosis and they control cytochrome c in mitochondrial inner membrane (Park et al., 2017). And annexin V has been used for detection of apoptosis directly (Kwon et al., 2013).
In the present study, the effects of two EDCs, TCS and BPA, as well as two phytoestrogens, Kaem and DIM, on cell viability of VM7LUC4E2 breast cancer cells were investigated via MTT assay. The results revealed that TCS and BPA significantly induced proliferation of VM7LUC4E2 cells at concentrations of 0.1–10 μM similar to E2 as a positive control. The cell proliferation inducing activities of TCS and BPA in ER-positive cancer cells were also identified in our previous studies, in which TCS and BPA were displayed as xenoestrogenic EDCs that can induce breast cancer or ovarian cancer cell proliferation via an ER-dependent pathway in a similar fashion as E2 (Kim et al., 2015b; Lee et al., 2017). These findings support the fact that EDCs have intrinsic estrogenic behavior in estrogen dependent caners (Dinwiddie et al., 2014). Conversely, Kaem (10–40 μM) and DIM (5–20 μM) applied in conjunction with E2, TCS, and BPA abrogated the cell proliferation effect of E2, TCS, and BPA, suggesting they have chemopreventive effects against the cancer progression effect of E2 and EDCs.
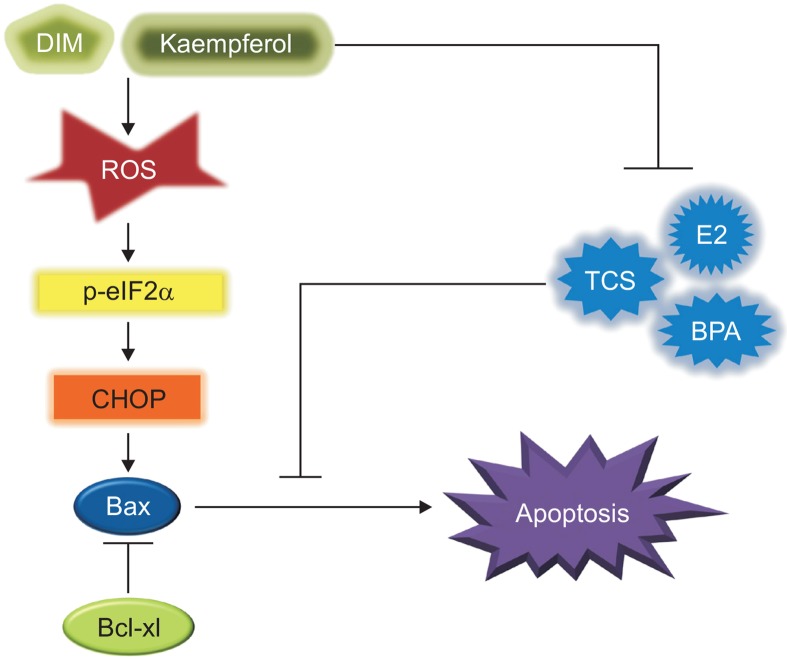
To elucidate the mechanisms underlying the opposite effects of E2, TCS, and BPA as well as Kaem and DIM on cell viability, we examined their roles in the induction of the apoptotic process in VM7LUC4E2 cells. As shown in Fig. 5, E2, TCS, and BPA inhibited the sequential apoptotic process associated with ROS-induced ER stress in VM7LUC4E2 cells. Conversely, Kaem and DIM promoted ROS formation and apoptosis of VM7LUC4E2 cells in the presence or absence of E2, TCS, and BPA via the induction of ER stress, of which activation was confirmed by increased formation of the phosphorylated form of eIF2α. As described above, p-eIF2α induces the activation of pro-apoptotic CHOP by activating ATF4 (Kitamura and Hiramatsu, 2010). The increase in apoptosis by Kaem and DIM was verified by the increased number of apoptotic cells, as well as upregulation of the apoptotic protein BAX via decreased protein expression of Bcl-xl gene. The Bcl-2/Bcl-xl family members controlling the release of pro-apoptotic proteins from the mitochondria intermembrane inhibits programmed cell death by downregulating Bax/Bak, which induce apoptosis (Gross et al., 1999).
Fig. 5.
Suppression effect of Kaem and DIM on E2, TCS or BPA-induced anti-ROS formation and anti-ER stress induced apoptosis in VM7Luc4E2 breast cancer cells. As xenoestrogenic EDCs, TCS and BPA inhibited ROS production, ER stress response and apoptosis of VM7Luc4E2 breast cancer cells similar to E2 and down-regulated the expression of ER stress response-related genes such as CHOP and p-eIF2α. As a result, the protein expression of Bax, a pro-apoptotic gene, was decreased, but the protein expression of Bcl-xl, an anti-apoptotic gene, was increased by E2 and EDCs. Conversely, Kaem and DIM displayed chemopreventive effects against breast cancer progression by effectively producing ROS and ER stress-associated apoptosis in VM7Luc4E2 cells exposed to E2 and EDCs such as TCS and BPA.
Previous studies have also reported apoptosis-inducing anticancer activity via the stimulation of ER stress by Kaem and DIM. Kaem promoted apoptosis via induction of the ER stress-CHOP signaling pathway in HepG2 hepatocellular carcinoma cells (Guo et al., 2016). Highly increased production of intracellular ROS and induction of apoptosis were also identified in MCF-7 breast cancer cells via H2DCF-DA assay (Kim et al., 2008). Moreover, DIM and its derivatives induced apoptosis in Panc-1 and Panc-28 pancreatic cancer cells through ER stress-dependent upregulation of DR5, a CHOP-dependent induction of death receptor (Abdelrahim et al., 2006). Additionally, DIM toxicity toward tumor cells in vitro was enhanced when the cells were undergoing nutritional deprivation and ER stress (Sun et al., 2004).
ER stress-associated apoptosis is considered an encouraging chemopreventive approach to treatment of cancer, and potential ER stress-inducing agents have been introduced as an attractive strategy for novel cancer therapy (Bhat et al., 2017). Resveratrol, which is a crucial phytoestrogen derived from grapes and peanuts, was recently found to induce autophagy-mediated apoptosis by triggering ER stress in PC3 and DU145 prostate cancer (Selvaraj et al., 2016). In addition, β-phenethyl isothiocyanate (PEITC) promoted ROS accumulation and UPR-mediated apoptosis in SKOV-3 ovarian cancer cells, while it did not cause any effects in normal ovarian epithelial cells (Hong et al., 2015).
In summary, TCS and BPA, which are considered xenoestrogenic EDCs, were investigated to induce breast cancer progression via the stimulation of cell proliferation, anti-ROS production, anti-ER stress response, and anti-apoptosis by regulating the expression of ROS induced ER stress-associated genes, including p-eIF2α, CHOP, Bcl-xl and Bax. Conversely, Kaem and DIM significantly inhibited E2, TCS or BPA-induced breast cancer cell proliferation and anti-apoptosis effects by regulating the protein expression of pro- and anti-apoptosis, ROS production, and ER stress response-related genes as shown in Fig. 5. Therefore, Kaem and DIM are effective chemopreventive compounds capable of inducing apoptosis of breast cancer that have the potential for use to prevent tumor aggressiveness and as alternatives to conventional therapeutic agents in environments consistently exposed to diverse EDCs such as TCS and BPA.
Acknowledgments
The authors appreciate Ms. Jae-Rim Heo, Soo-Min Kim, and Mr. Gyu-Sik Kim at Chungbuk National University for their technical assistance with experiments.
Footnotes
CONFLICT OF INTEREST
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science and Technology (MEST) of the Republic of Korea (2017R1D1A1A09000663). In addition, this work was also supported by the intramural research grant of Chungbuk National University in 2015.
REFERENCES
- Abdelrahim M, Newman K, Vanderlaag K, Samudio I, Safe S. 3,3′-diindolylmethane (DIM) and its derivatives induce apoptosis in pancreatic cancer cells through endoplasmic reticulum stress-dependent upregulation of DR5. Carcinogenesis. 2006;27:717–728. doi: 10.1093/carcin/bgi270. [DOI] [PubMed] [Google Scholar]
- Ahn HN, Jeong SY, Bae GU, Chang M, Zhang D, Liu X, Pei Y, Chin YW, Lee J, Oh SR, Song YS. Selective estrogen receptor modulation by larrea nitida on MCF-7 cell proliferation and immature rat uterus. Biomol. Ther (Seoul) 2014;22:347–354. doi: 10.4062/biomolther.2014.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat TA, Chaudhary AK, Kumar S, O’Malley J, Inigo JR, Kumar R, Yadav N, Chandra D. Endoplasmic reticulum-mediated unfolded protein response and mitochondrial apoptosis in cancer. Biochim. Biophys Acta. 2017;1867:58–66. doi: 10.1016/j.bbcan.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MY, Ike M, Fujita M. Acute toxicity, mutagenicity, and estrogenicity of bisphenol-A and other bisphenols. Environ Toxicol. 2002;17:80–86. doi: 10.1002/tox.10035. [DOI] [PubMed] [Google Scholar]
- Coelingh Bennink HJ. Are all estrogens the same? Maturitas. 2004;47:269–275. doi: 10.1016/j.maturitas.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Darbre PD. Environmental oestrogens, cosmetics and breast cancer. Best Pract Res Clin Endocrinol Metab. 2006;20:121–143. doi: 10.1016/j.beem.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Dinwiddie MT, Terry PD, Chen J. Recent evidence regarding triclosan and cancer risk. Int. J. Environ. Res Public Health. 2014;11:2209–2217. doi: 10.3390/ijerph110202209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elswefy SE, Abdallah FR, Atteia HH, Wahba AS, Hasan RA. Inflammation, oxidative stress and apoptosis cascade implications in bisphenol A-induced liver fibrosis in male rats. Int J Exp Pathol. 2016;97:369–379. doi: 10.1111/iep.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Zhang Q, Wang X, Yang X, Wang X, Huang Z, Jiao Y, Wang J. Quantitative phosphoproteomics reveals genistein as a modulator of cell cycle and DNA damage response pathways in triple-negative breast cancer cells. Int J Oncol. 2016;48:1016–1028. doi: 10.3892/ijo.2016.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Zhang P, Zhang Z, Shi J, Jiao Z, Shao B. Endocrine disrupting effects of triclosan on the placenta in pregnant rats. PLoS ONE. 2016;11:e0154758. doi: 10.1371/journal.pone.0154758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesik SW, Shi Y. Structural biology. Controlling the caspases. Science. 2001;294:1477–1478. doi: 10.1126/science.1062236. [DOI] [PubMed] [Google Scholar]
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafar AA, Draz HM, Goldberg AA, Bashandy MA, Bakry S, Khalifa MA, AbuShair W, Titorenko VI, Sanderson JT. Lithocholic acid induces endoplasmic reticulum stress, autophagy and mitochondrial dysfunction in human prostate cancer cells. PeerJ. 2016;4:e2445. doi: 10.7717/peerj.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galehdar Z, Swan P, Fuerth B, Callaghan SM, Park DS, Cregan SP. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J Neurosci. 2010;30:16938–16948. doi: 10.1523/JNEUROSCI.1598-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Sohn H, Xue L, Firestone GL, Bjeldanes LF. 3,3′-Diindolylmethane is a novel mitochondrial H(+)-ATP synthase inhibitor that can induce p21(Cip1/Waf1) expression by induction of oxidative stress in human breast cancer cells. Cancer Res. 2006;66:4880–4887. doi: 10.1158/0008-5472.CAN-05-4162. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Guo H, Ren F, Zhang L, Zhang X, Yang R, Xie B, Li Z, Hu Z, Duan Z, Zhang J. Kaempferol induces apoptosis in HepG2 cells via activation of the endoplasmic reticulum stress pathway. Mol Med Rep. 2016;13:2791–2800. doi: 10.3892/mmr.2016.4845. [DOI] [PubMed] [Google Scholar]
- Hilakivi-Clarke L, Cho E, Onojafe I, Raygada M, Clarke R. Maternal exposure to genistein during pregnancy increases carcinogen-induced mammary tumorigenesis in female rat off-spring. Oncol Rep. 1999;6:1089–1095. doi: 10.3892/or.6.5.1089. [DOI] [PubMed] [Google Scholar]
- Hong YH, Uddin MH, Jo U, Kim B, Song J, Suh DH, Kim HS, Song YS. ROS accumulation by PEITC Selectively Kills Ovarian Cancer Cells via UPR-mediated apoptosis. Front Oncol. 2015;5:167. doi: 10.3389/fonc.2015.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang KA, Choi KC. Anticarcinogenic effects of dietary phytoestrogens and their chemopreventive mechanisms. Nutr Cancer. 2015;67:796–803. doi: 10.1080/01635581.2015.1040516. [DOI] [PubMed] [Google Scholar]
- Jeong JS, Nam KT, Lee B, Pamungkas AD, Song D, Kim M, Yu WJ, Lee J, Jee S, Park YH, Lim KM. Lowdose bisphenol A increases bile duct proliferation in juvenile rats: a possible evidence for risk of liver cancer in the exposed population? Biomol. Ther (Seoul) 2017;25:545–552. doi: 10.4062/biomolther.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang NH, Hwang KA, Lee HR, Choi DW, Choi KC. Resveratrol regulates the cell viability promoted by 17β-estradiol or bisphenol A via down-regulation of the cross-talk between estrogen receptor α and insulin growth factor-1 receptor in BG-1 ovarian cancer cells. Food Chem Toxicol. 2013;59:373–379. doi: 10.1016/j.fct.2013.06.029. [DOI] [PubMed] [Google Scholar]
- Kim BW, Lee ER, Min HM, Jeong HS, Ahn JY, Kim JH, Choi HY, Choi H, Kim EY, Park SP, Cho SG. Sustained ERK activation is involved in the kaempferol-induced apoptosis of breast cancer cells and is more evident under 3-D culture condition. Cancer Biol Ther. 2008;7:1080–1089. doi: 10.4161/cbt.7.7.6164. [DOI] [PubMed] [Google Scholar]
- Kim CW, Go RE, Choi KC. Treatment of BG-1 ovarian cancer cells expressing estrogen receptors with lambda-cyhalothrin and cypermethrin caused a partial estrogenicity via an estrogen receptor-dependent pathway. Toxicol Res. 2015a;31:331–337. doi: 10.5487/TR.2015.31.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hwang KA, Choi KC. Treatment with kaempferol suppresses breast cancer cell growth caused by estrogen and triclosan in cellular and xenograft breast cancer models. J Nutr Biochem. 2016;28:70–82. doi: 10.1016/j.jnutbio.2015.09.027. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim CW, Jeon SY, Go RE, Hwang KA, Choi KC. Chemopreventive and chemotherapeutic effects of genistein, a soy isoflavone, upon cancer development and progression in preclinical animal models. Lab Anim Res. 2014;30:143–150. doi: 10.5625/lar.2014.30.4.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Choi KC, Hwang KA. Genistein suppressed epithelial-mesenchymal transition and migration efficacies of BG-1 ovarian cancer cells activated by estrogenic chemicals via estrogen receptor pathway and downregulation of TGF-β signaling pathway. Phytomedicine. 2015b;22:993–999. doi: 10.1016/j.phymed.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Kitamura M, Hiramatsu N. The oxidative stress: endoplasmic reticulum stress axis in cadmium toxicity. Biometals. 2010;23:941–950. doi: 10.1007/s10534-010-9296-2. [DOI] [PubMed] [Google Scholar]
- Kwon YJ, Jung JJ, Park NH, Ye DJ, Kim D, Moon A, Chun YJ. Annexin a5 as a new potential biomarker for Cisplatin-induced toxicity in human kidney epithelial cells. Biomol. Ther (Seoul) 2013;21:190–195. doi: 10.4062/biomolther.2013.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GA, Choi KC, Hwang KA. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ Toxicol Pharmacol. 2017;49:48–57. doi: 10.1016/j.etap.2016.11.016. [DOI] [PubMed] [Google Scholar]
- Lee GA, Hwang KA, Choi KC. Roles of dietary phytoestrogens on the regulation of epithelial-mesenchymal transition in diverse cancer metastasis. Toxins (Basel) 2016;8 doi: 10.3390/toxins8060162. pii: E162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HR, Hwang KA, Park MA, Yi BR, Jeung EB, Choi KC. Treatment with bisphenol A and methoxychlor results in the growth of human breast cancer cells and alteration of the expression of cell cycle-related genes, cyclin D1 and p21, via an estrogen receptor-dependent signaling pathway. Int J Mol Med. 2012;29:883–890. doi: 10.3892/ijmm.2012.903. [DOI] [PubMed] [Google Scholar]
- Lee HS, Park EJ, Oh JH, Moon G, Hwang MS, Kim SY, Shin MK, Koh YH, Suh JH, Kang HS, Jeon JH, Rhee GS, Hong JH. Bisphenol A exerts estrogenic effects by modulating CDK1/2 and p38 MAP kinase activity. Biosci Biotechnol Biochem. 2014;78:1371–1375. doi: 10.1080/09168451.2014.921557. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim JH. Kaempferol inhibits pancreatic cancer cell growth and migration through the blockade of EGFR-related pathway in vitro. PLoS ONE. 2016;11:e0155264. doi: 10.1371/journal.pone.0155264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Wambi JS, Jordan VC. Estrogen regulation of apoptosis: how can one hormone stimulate and inhibit? Breast Cancer Res. 2009;11:206. doi: 10.1186/bcr2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Arao Y, Hall JM, Burkett S, Liu L, Gerrish K, Cavailles V, Korach KS. Research resource: STR DNA profile and gene expression comparisons of human BG-1 cells and a BG-1/MCF-7 clonal variant. Mol Endocrinol. 2014;28:2072–2081. doi: 10.1210/me.2014-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Chen L, Ma X, Jiao R, Li X, Wang Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur J Med Chem. 2016;114:24–32. doi: 10.1016/j.ejmech.2016.02.045. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96:1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- Park JE, Piao MJ, Kang KA, Shilnikova K, Hyun YJ, Oh SK, Jeong YJ, Chae S, Hyun JW. A benzylidene-acetophenone derivative induces apoptosis of radiation-resistant human breast cancer cells via oxidative stress. Biomol. Ther (Seoul) 2017;25:404–410. doi: 10.4062/biomolther.2017.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Hwang KA, Choi KC. Diverse animal models to examine potential role(s) and mechanism of endocrine disrupting chemicals on the tumor progression and prevention: Do they have tumorigenic or anti-tumorigenic property? Lab Anim Res. 2011;27:265–273. doi: 10.5625/lar.2011.27.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo M, Russo GL, Daglia M, Kasi PD, Ravi S, Nabavi SF, Nabavi SM. Understanding genistein in cancer: The “good” and the “bad” effects: a review. Food Chem. 2016;196:589–600. doi: 10.1016/j.foodchem.2015.09.085. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Horiguchi H, Oguma E, Kayama F. Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells. J Nutr Biochem. 2010;21:856–864. doi: 10.1016/j.jnutbio.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Boyd NF, Chlebowski RT, Cummings S, Cuzick J, Dowsett M, Easton D, Forbes JF, Key T, Hankinson SE, Howell A, Ingle J. Critical assessment of new risk factors for breast cancer: considerations for development of an improved risk prediction model. Endocr. Relat Cancer. 2007;14:169–187. doi: 10.1677/ERC-06-0045. [DOI] [PubMed] [Google Scholar]
- Santos UP, Campos JF, Torquato HF, Paredes-Gamero EJ, Carollo CA, Estevinho LM, de Picoli Souza K, Dos Santos EL. Antioxidant, antimicrobial and cytotoxic properties as well as the phenolic content of the extract from hancornia speciosa gomes. PLoS ONE. 2016;11:e0167531. doi: 10.1371/journal.pone.0167531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S, Sun Y, Sukumaran P, Singh BB. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol Carcinog. 2016;55:818–831. doi: 10.1002/mc.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanle EK, Xu W. Endocrine disrupting chemicals targeting estrogen receptor signaling: identification and mechanisms of action. Chem Res Toxicol. 2011;24:6–19. doi: 10.1021/tx100231n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. A structural view of mitochondria-mediated apoptosis. Nat Struct Biol. 2001;8:394–401. doi: 10.1038/87548. [DOI] [PubMed] [Google Scholar]
- Soltys BJ, Falah M, Gupta RS. Identification of endoplasmic reticulum in the primitive eukaryote Giardia lamblia using cryoelectron microscopy and antibody to Bip. J Cell Sci. 1996;109:1909–1917. doi: 10.1242/jcs.109.7.1909. [DOI] [PubMed] [Google Scholar]
- Stachon T, Wang J, Song X, Langenbucher A, Seitz B, Szentmary N. Impact of crosslinking/riboflavin-UVA-photo-dynamic inactivation on viability, apoptosis and activation of human keratocytes in vitro. J Biomed Res. 2015;29:321–325. doi: 10.7555/JBR.29.20130173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Han J, Ralph WM, Jr, Chandrasekaran A, Liu K, Auborn KJ, Carter TH. Endoplasmic reticulum stress as a correlate of cytotoxicity in human tumor cells exposed to diindolylmethane in vitro. Cell Stress Chaperones. 2004;9:76–87. doi: 10.1379/1466-1268(2004)009<0076:ERSAAC>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsimo SJ, Tamokou Jde D, Havyarimana L, Csupor D, Forgo P, Hohmann J, Kuiate JR, Tane P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res Notes. 2012;5:158. doi: 10.1186/1756-0500-5-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733–748. [PubMed] [Google Scholar]
- Wang S, Kaufman RJ. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Xu Y, Hu Y, Luo Y, Lu X, Tsui CK, Lu L, Liang X. Madecassic acid protects against hypoxia-induced oxidative stress in retinal microvascular endothelial cells via ROS-mediated endoplasmic reticulum stress. Biomed Pharmacother. 2016;84:845–852. doi: 10.1016/j.biopha.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Yin L, Dai Y, Cui Z, Jiang X, Liu W, Han F, Lin A, Cao J, Liu J. The regulation of cellular apoptosis by the ROS-triggered PERK/EIF2α/chop pathway plays a vital role in bisphenol A-induced male reproductive toxicity. Toxicol Appl Pharmacol. 2017;314:98–108. doi: 10.1016/j.taap.2016.11.013. [DOI] [PubMed] [Google Scholar]