Abstract
Cocaine- and amphetamine-regulated transcript (CART) peptide is a widely distributed neurotransmitter expressed in the central nervous systems. Previously, several reports demonstrated that nucleus accumbal-injected CART peptide positively modulated behavioral sensitization induced by psychostimulants and regulated the mesocorticolimbic dopaminergic pathway. It is confirmed that CART peptide exerted inhibitory effect on psychostimulant-enhanced dopamine receptors signaling, Ca2+/calmodulin-dependent kinase signaling and crucial transcription factors expression. Besides modulation of dopamine receptors-related pathways, CART peptide also exhibited elaborated interactions with other neurotransmitter receptors, such as glutamate receptors and γ-aminobutyric acid receptors, which further account for attribution of CART peptide to inhibition of psychostimulant-potentiated locomotor activity. Recently, CART peptide has been shown to have anxiolytic functions on the aversive mood and uncontrolled drug-seeking behaviors following drug withdrawal. Moreover, microinjection of CART peptide has been shown to have an anti-depressant effect, which suggests its potential utility in the mood regulation and avoidance of depression-like behaviors. In this review, we discuss CART pathways in neural circuits and their interactions with neurotransmitters associated with psychostimulant-induced depression.
Keywords: CART peptide, Addiction, Psychostimulant, Depression
INTRODUCTION
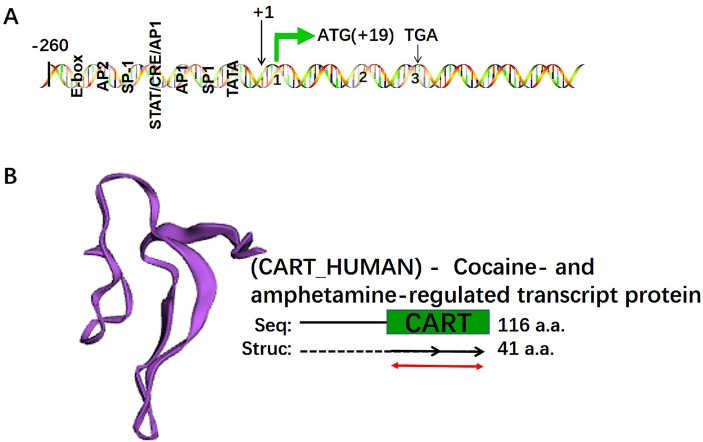
Fragment of cocaine- and amphetamine-regulated transcript (CART) peptide was first discovered by Spiess et al. (1981) in the extraction of hypothalamus in 1981. Douglass et al. identified increased CART mRNA expression within the striatum of psychostimulant-exposed rats (Douglass et al., 1995; Douglass and Daoud, 1996), suggesting the role of CART peptide on the drug abuse. The complete sequences of CART gene were available and showed highly conservation across species (Kuhar et al., 2000; Dallvechia-Adams et al., 2002). The CART gene is composed of 3 exons and 2 introns with alternatively splicing in rat and mouse (Kuhar et al., 2000). And the mouse CART promoter contains series of transcription factor binding site, such as E-box, SP1, overlapped STAT/cyclic adenosine 5′-monophosphate (cAMP) response element (CRE)/AP1, SP2 sites (Kuhar et al., 2000), in which transcription factors including cAMP response element binding protein (CREB), cJUN, SP1 and AP2 may regulate expression of CART gene expression (Fig. 1). Surprisingly, expression of CART peptide dominates in the mesocorticolimbic dopaminergic (DA) system that extends from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and includes other limbic areas (amygdala, hippocampus, and frontal cortex), and is also widely distributed in the central nervous system (CNS) (Kuhar and Yoho, 1999; Kuhar et al., 2000). Compelling evidences also shows that repeated administration of psychostimulants enhances expression of CART peptide (Jaworski et al., 2003a; Hubert et al., 2008), which is supported by a study in which microinjections of CART peptide into NAc that effectively attenuated the rewarding properties of psychostimulants (Jaworski et al., 2003b; Yoon et al., 2007; Peng et al., 2014; Fu et al., 2016). These observations suggested CART peptide plays a positive role in the regulation of behavioral sensitization induced by psychostimulants and led to thorough investigations of the modes of action of CART peptide with the object of identifying its potential use for the treatment of drug addiction. For example, microinjection of CART peptide into rat NAc significantly blocked psychostimulant-induced up-regulation of dopamine receptor (DR) and activation of downstream cAMP/protein kinase A (PKA)/cAMP response element binding protein (CREB) pathway (Peng et al., 2014; Fu et al., 2016; Xiong et al., 2018). Psychostimulant-induced Ca2+ influx and phosphorylated calcium/calmodulin-dependent protein kinase IIα (pCaMKIIα) expression have also been attenuated by CART peptide. In addition, interactions between pCaMKIIα and D3R blocked the inhibitory effect of D3R on the cAMP/PKA/CREB pathway and behavioral sensitization (Xiong et al., 2018). Recently, CART peptide has been suggested to positively and allosterically modulate γ-aminobutyric acid B receptors (GABAB R), based on the observation that it inhibited drug-depressed GABAB R-G-protein-coupled inwardly rectifying K+-channel (GIRK) signaling. Thus, it has been suggested CART peptide modulates psychostimulant-induced hyperlo-comotion through DR-related calcium signaling and GABA-R-associated pathways (Moffett et al., 2011; Upadhya et al., 2012; Cai et al., 2014; Hu et al., 2015; Fu et al., 2016; Xiong et al., 2018). However, our understanding of CART pathways in neuronal circuits is lacking.
Fig. 1.
Overview of CART gene structure and CART peptide 3D structure. (A) The schematic diagram of CART gene and its proximal promoter transcription factor binding sites. The diagram shown here is based on the genomic structure of mouse CART genes and adapted from works of Dominguez et al. The CART gene is composed of 3 exons and 2 introns, in which several transcription binding sites are presented, and the transcription initiation site is shown as +1. The diagram is not to scale. (B) The 3D structure of human CART peptide chain A, extending from residues 76–116 of C-terminal domain. The structure is downloaded from protein data bank (PBD, id: 1hy9).
On the other hand, repeated psychostimulant intake may increase the risk of persistent drug-relapse accompanied by irritability, anxiety, and dysphoria (Koob and Le Moal, 1997; Koob et al., 1998). Furthermore, these addiction-related anxious and aversive emotions can lead to depression-like behaviors that may ultimately facilitate suicidal actions possibly mediated by GABAergic pathways (Stanek, 2006; Wiehager et al., 2009; Yoon et al., 2014). Interestingly, CART peptide has been closely linked with corticotropin-releasing factor (CRF) in the hypothalamic-pituitary-adrenal (HPA) axis and amygdala, the latter of which provides the interface between stress and addiction (Koob, 2008a). We hope this review of the role of CART in psychostimulants-induced anxiety-like behaviors will provide new avenues for the development of effective drugs for addiction and/or depression disorders based on understanding of CART pathways in neuronal circuits.
RELATIONS BETWEEN CART CONTAINING NEURAL CIRCUITS AND DEPRESSION
Drug addiction is considered as a psychiatric disorder that progresses from impulsivity to compulsivity, during which people undergo transformation from sense of pleasure or gratification in a positive reinforcement toward relief of anxiety or stress in negative reinforcement (Koob et al., 2004; Koob, 2008a, 2008b). On the other hand, drug cessation triggers protracted anxiety and depression-like symptoms due to counter-adaptive processes that include diminished functions of neurotransmitters in the neuro-circuits associated with acute drug reinforcing effects, such as, the dopamine signaling pathway. Chronic drug exposure results in within-system neuro-adaptations including decreased function of the same neurotransmitter in the same neuro-circuits involved in the acute reinforcing effect of the drug, such as the dopamine signaling pathway (Koob et al., 2004; Koob, 2008a, 2008b). According to the self-medication hypothesis, drug-addicted individuals suffer from deficits in self-esteem and emotion regulation, and cope with aversive and painful emotions by binging (Koob et al., 2004; Koob, 2008a, 2008b). This dysregulation may be a manifestation of altered information-processing, decision-making and behavioral motivation, all of which are associated with functional deficits in brain-stress and anti-stress systems (Koob et al., 2004; Koob, 2008a, 2008b). We later discuss the molecular mechanisms underlying disrupted mood regulation following psychostimulant abstinence and the effects of CART peptide on depressive-like emotions induced by drug withdrawal.
The Hypothalamic-Pituitary-Adrenal (HPA) axis
Pathophysiologic studies showed that quantities of CART immune-reactivity were synthesized and secreted from the anterior pituitary gland (Stanley et al., 2004) and hypothalamus (Smith et al., 2004). Stress regulates CART expression via CRF and glucocorticoids (Stanley et al., 2004). Conversely, the administration of CART peptide up-regulates cirrculating levels of ACTH and corticosterone in the HPA axis through the CRF-dependent mechanism (Vrang et al., 2000; Smith et al., 2004). Furthermore, multiple line of evidence shows that CART peptide plays a key role in the HPA-axis associated stress response (Vrang et al., 2000; Smith et al., 2004; Stanley et al., 2004; Job et al., 2011).
The HPA axis is composed of three major structures: the paraventricular nucleus of the hypothalamus, the anterior lobe of the pituitary gland, and the adrenal gland (Turnbull and Rivier, 1997; Zuloaga et al., 2015). CRF is synthesized and released by the medial parvocellular subdivision of the paraventricular nucleus to the portal blood vessels and then binds to the CRF 1 receptor on the pituitary corticotrophin to induce adrenocorticotropic hormone (ACTH) release into the systemic circulation, which in turn stimulates cortical secretion of glucocorticoid from the adrenal gland (Herman et al., 2005). And the HPA axis is finely tuned by negative feedback from glucocorticoid, which activates glucocorticoid receptor within the paraventricular nucleus and the hippocampus (Turnbull and Rivier, 1997). Acute psychostimulants exposure behaves like stressors that can elicit the activation of HPA axis (Chartoff and Carlezon, 2014), and thus, induce the release of the above-mentioned molecules, which then exert immediate or delayed effects on the mesocorticolimbic system. However, acute withdrawal from chronic psychostimulants induces allosteric load in HPA axis, elevates secretion of CRF and ACTH, and decreases cortisol levels, which are hallmarks of depressive and anxiety spectrum disorders in man (Li et al., 2013a; Zuloaga et al., 2015). However, in depressed patients, regulation of HPA axis is disrupted, which leads to low level of cortisol concentration in serum via stress reactivity (Peng et al., 2015). Surprisingly, hypercortisolemia is present in about 40%–60% of depressed adults (Carroll et al., 2007), which may explain the cause of disrupted hippocampal integrity and impaired memory function in the pathogenesis of depression. Several groups have reported intracerebroventricular or intraamygdalar injection of CRF1 receptor antagonists could effectively attenuate the aversive states and anxiogenic effects induced by drug abstinence, and thus, inhibit drug self-administration (George et al., 2007; Specio et al., 2008; Greenwell et al., 2009).
Serotonin (5-HT) neurotransmitter systems
5-HT is a widely distributed neurotransmitter in the CNS, and has been linked with arousal, anxiety, aversive affect and depressive disorders (Graeff et al., 1996; Pompili et al., 2010). The 5-HT pathway originates from the dorsal raphe nucleus (DRN) and it extends to the amygdala and frontal cortex to facilitate conditioned fear (Graeff et al., 1996). 5-HT synthesis is catalyzed by tyrosine hydroxylase and tryptophan hydroxylase, and synthesized 5-HT is stored in the presynaptic vesicles, where monoamine oxidase (MAO) metabolites can inhibit its release. 5-HT binds to guanine nucleotide triphosphate–binding protein–coupled receptors (GPCR) to activate (AC)/cAMP/PKA and PI/PLC/DAG/PKC signaling pathways, and ultimately promotes the expression of the CREB and induces profound antidepressant effects (Ruhe et al., 2007). Therefore, any abnormality that exists within the mechanisms of 5-HT secretion, receptor transportation and signal transduction may result in anxiogenic and depressive-like disorders. In particular, withdrawal from cocaine self-administration has been associated with a decrease in extracellular 5-HT in the NAc sub-regions (Parsons et al., 1996), whereas acute withdrawal from ethanol has been shown to increase in the sensitivity of 5-HT1A autoreceptor to modulate 5-HT synthesis (Esteban et al., 2002). Moreover, 5-HT2C receptor antagonist blocked the inhibitory dorsal raphe 5-HT2C receptor, and this potentially attenuated cocaine withdrawal exacerbated GABA activity, thereby prevented anxiety-like behaviors (Craige et al., 2015).
The serotonergic system interacts with CART system originated from the lateral hypothalamus to innervate other brain areas associated with stress (Ruhe et al., 2007). Light microscopic studies from Lee and Lee (2014) found reciprocal connections between CART-immunoreactive, hypothalamic paraventricular nucleus and the serotonergic dorsal raphe neurons. Ma et al. (2007) performed a microdialysis approach, which can increase 5-HT efflux made by infusions of CART peptide into the dorsal raphe nucleus and NAc. In addition, activation of 5-HT receptors by 5-HT might enhance dopaminergic signaling and further enhance anti-depressant effects. Interestingly, some studies also investigated that activation of 5-HT4 receptor upregulated CART mRNA expression in the NAc via cAMP/PKA signaling pathway (Jean et al., 2007; Jean et al., 2012) These observations support the protective role of CART peptide on the depression-deprived serotonin system during drug addiction (Ma et al., 2007).
GABA-ergic systems
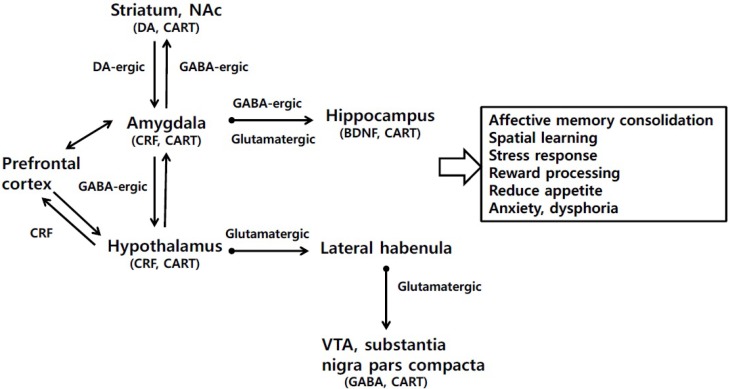
Immunoblotting results show CART peptide co-localizes with GABA in the nerve terminals in the VTA and substantia nigra (SN) (Dallvechia-Adams et al., 2002), suggesting the intricate relationship between CART peptide and GABA. GABAA R is a ligand-gated ion channel mediating fast inhibitory synaptic transmission, especially in the dopaminergic brain areas such as VTA and substantia nigra compacta (Jiao et al., 2015). On the other hand, GABAB R is a G-protein-coupled receptor composed of 2 subunits, GABAB1(a,b) and GABAB2, which are responsible for agonist or antagonist binding and G-protein activation, respectively (Kulik et al., 2003; Pin and Bettler, 2016). In addition, presynaptic GABAB R inhibits Ca2+ influx whereas postsynaptic GABAB R activates GIRK, which may cause neuron hyperpolarization leading to slow inhibitory post-synaptic current (IPSC) and a reduced synaptic activity (Padgett et al., 2012). It has been reported that withdrawal from chronic morphine exposure increases GABA release in the VTA, resulted in a short-lived (1–3 days) GABAA-mediated inhibition, and up-regulation of cAMP-dependent proteins (Bonci and Williams, 1996; Bonci and Williams, 1997), all of which drive conditioned place aversion (Tan et al., 2012) and diminished GABAB-mediated inhibition (Bonci and Williams, 1996). Chronic methamphetamine and cocaine exposure can depress both GABAA R and GABAB R expression, and weaken GABAB R-GIRK signaling in VTA GABA neurons including glutamatergic neurons within the medial prefrontal cortex (mPFC) (Filip et al., 2015; Jiao et al., 2015). Previous studies indicated that injection of GABAB R agonist baclofen or GABAB R positive allosteric modulators can attenuate self-administration and drug craving in ethanol-dependent rats and human alcoholics (Addolorato et al., 2006; Knapp et al., 2007). Recently, we have shown the modulatory effect of intra-accumbally injected CART peptide on methamphetamine-induced depression of GABAB R-GIRK signaling and concomitant internalization and reduction of membrane GABAB R and GIRK receptors in the VTA and mPFC neurons (Hu et al., 2015), which suggests the regulatory targets of CART peptide are similar to those of the positive allosteric modulator GABAB R on GABAB1 R and GABAB2 R in the context of rescue from drug-depressed GABAB R-GIRK signaling (Fig. 2).
Fig. 2.
Outline of key CART-containing neural circuits underlying the pathogenesis of depression. The role of CART in the brain regions such as frontal cortex, striatum, nucleus accumbens, amygdala, and hypothalmus are involved in the development of neuro-pathogenesis. DA and CART peptide could increase the hippocampal BDNF secretion, which may rescue the disrupted function of hippocampus. Additionally, GABAergic and glutaminergic pathways are also associated with reward process, anxiety and dysphoria. The stress-depressed interaction between the amygdala and hippocampus was also augmented by CART peptide, which may play an important role in the modulation of the anxious and depressive-like behaviors.
Glutamatergic pathway
The excitatory neurotransmitter glutamate is essential for the excitation propagation and neuronal transmission (Javitt et al., 2011). There are two main classes of glutamate receptors, that is, metabotropic glutamate receptors (mGluRs) and ionotropic glutamate receptors (iGluRs) (Javitt et al., 2011). mGluRs, are classified into group I, II and III subfamilies, and are seven transmembrane G-protein-coupled receptors (GP-CRs) associated with protein kinase B (PKB), nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), CaM signaling cascades, or coupled Ca2+, K+ and Na+ channels (Javitt et al., 2011). Conversely, iGluRs are composed of N-methyl-D-aspartate (NMDA), a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors and share a voltage-gated ion channel function to enable cation influx (Javitt et al., 2011).
The lateral habenula (LHb) has recently attracted attention due to its modulatory role on depressive behaviors and drug abuse (Lecca et al., 2014). Anatomical studies indicated that LHb, which is positioned near the midline and surrounded by the thalamus, receives prominent glutamatergic afferents from the lateral hypothalamus, and cortical inputs, for example, from the high AMPA-R but low synaptic NMDA-R expressing neurons in medial prefrontal cortex (Lecca et al., 2014). Investigators have reported that aberrant LHb activity over-activates βCaMKII and contributes to aversive and depressive disorders (Li et al., 2013b), whereas the co-release of GABA and glutamate controls LHb activity and can be used to treat depression (Shabel et al., 2014). Cui et al. (2018) found the upregulation of astroglial Kir4.1 in the LHb depressed neuronal bursts in a rat model of depression, and Yang et al. (2018) found LHb burst required both NMDARs and low-voltage-sensitive T-type calcium channels (T-VSCCs) and that blockade of NMDAR or T-VSCCs by ketamine (a NMDAR antagonist) in the LHb induced a rapid antidepressant effect.
Previous reports on CART peptide showed similar pathways to those associated with glutamate receptors. Intra-accumbally injected CART peptide decreased the expression of αCaMKII (Fu et al., 2016; Xiong et al., 2018), which after Ca2+ channel activation couples with NMDAR to enhance neuronal excitability (Liu and Murray, 2012). As discussed above, our recent experiments showed CART peptide has a significant positive effect on GIRK signaling pathways, whereby GIRK channels exert modulatory effects on depressive-like behaviors (Hu et al., unpublished data). In addition, NMDA receptors also activate GIRK channels. Taken together, it is apparent more efforts are required to identify the role of CART peptide on the depression-related LHb area and to determine whether the glutamatergic system is a key target for the inhibitory effect of CART peptide on rewarding properties and anxiety-like behaviors.
CART PEPTIDE AND DEPRESSION THERAPY
Interest in the pathophysiological mechanisms of depression and treatment strategies is increasing, and in view of the many addiction and depression patterns exhibited, the anxiolytic effects of CART peptide might be useful in this context. Currently, the treatment of depression involves the prolonged use of high doses of antidepressants, such as, selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs) or monoamine oxidase inhibitors (MAOIs), or electroconvulsive therapy (ECT) (Kupfer et al., 2012). Furthermore, evidence indicates the effects of these drugs might be augmented by CART peptide (Job et al., 2011; Mao, 2011). It has been reported CART peptide levels are low in the cerebrospinal fluid of major depression disease patients, which suggests a close relationship between CART peptide and depression (Yoon et al., 2018), which is supported by marked reductions of CART-immunoreactive fibers in the paraventricular thalamic nucleus and locus coeruleus of socially isolated rats (Dandekar et al., 2009; Choudhary et al., 2018). Recently, it was reported CART-ergic neurons in the lateral hypothalamus innervated neurons of the paraventricular nucleus communicated with glutamatergic fibers in the NAc shell to modulate psychostimulant-induced reward behaviors, whereas intra-paraventricular hypothalamus infusions of CART antibody or intra-accumbal NMDA receptor antagonist (MK-801) injection blunted the modulatory effect of CART peptide (Choudhary et al., 2018). We have observed pretreatment of basal CaMKIIα-overexpressing NAc neurons with CART peptide injection decreased cocaine or amphetamine (stress)-enhanced pCaMKIIα expression (Fu et al., 2016; Xiong et al., 2018) and that these reductions were coupled with the activations of NMDA receptors, which suggests the inhibitory and modulatory effects of CART peptide on the glutamate receptor-Ca2+/CAM-pCaMKII signaling pathway influences learning and memory formation. Furthermore, we previously showed CART peptide can promote hippocampal neuron survival by upregulating BDNF (Wu et al., 2006; Pae et al., 2007). In addition, some reports have demonstrated the availability of CART-specific binding sites within brain stress regions, suggesting selective CART peptide receptors can induce anxiolytic and antidepressant effects (Nagelova et al., 2014). These findings and their implications indicate more efforts are required to identify other regions influenced by the anxiolytic and antidepressant CART pathways.
CONCLUSION
Recent experimental findings and reports of associations between neural circuits and disrupted neurotransmissions and psychostimulant addiction and depression suggest CART peptide might be therapeutically useful for the treatment of addiction and depression (Job et al., 2011; Mao et al., 2012). Briefly, we summarize the pathways involved in CART peptide-induced amelioration of anxiety-like behaviors during drug withdrawal as follows: (1) CRF-dependent up-regulation of a disrupted HPA axis, (2) CART-induced increase 5HT efflux in the dorsal raphe nucleus and NAc and the subsequent activations of 5-HT autoreceptors and dopaminergic signaling, and (3) CART-induced rescue of the psychostimulant-depressed GABA R signaling pathway within the VTA. (4) CART peptide-induced modulation of glutamate pathways in LHb.
However, some studies have reported adverse side effects when CART peptide was used to alleviate anxious and depressive-related behaviors (Stanek, 2006; Job et al., 2011; Mao et al., 2012). Therefore, we suggest CART related pathways should be further investigated to elucidate the molecular mechanisms responsible for the anxiolytic and antidepressant effects of CART during drug withdrawal.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of Jiangxi Province (Grant no. 20161BBG70065 to Z. Hu) and the National Research Foundation (NRF) of Korea funded by the Ministry of Science, ICT & Future Planning (MRC 2010-0029355 to S. Oh).
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
REFERENCES
- Addolorato G, Leggio L, Agabio R, Colombo G, Gasbarrini G. Baclofen: a new drug for the treatment of alcohol dependence. Int J Clin Pract. 2006;60:1003–1008. doi: 10.1111/j.1742-1241.2006.01065.x. [DOI] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Cattaneo A, Carvalho LA, Garabedian MJ, Thuret S, Pariante CM. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Molecular Psychiatry. 2011;16(7):738–750. doi: 10.1038/mp.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos-Fuentes A, Albarrán-Bravo S, Loya-Lopéz S, Cortés H, Recillas-Morales S, Magaña JJ, et al. Dopaminergic denervation switches dopamine D3 receptor signaling and disrupts its ca(2+) dependent modulation by camkii and calmodulin in striatonigral projections of the rat. Neurobiology of Disease. 2015;74:336–346. doi: 10.1016/j.nbd.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Bonci A, Williams JT. A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron. 1996;16:631–639. doi: 10.1016/S0896-6273(00)80082-3. [DOI] [PubMed] [Google Scholar]
- Bonci A, Williams JT. Increased probability of GABA release during withdrawal from morphine. J Neurosci. 1997;17:796–803. doi: 10.1523/JNEUROSCI.17-02-00796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Zhang D, Ying Y, Yan M, Yang J, Xu F, Oh K, Hu Z. Inhibitory modulation of CART peptides in accumbal neuron through decreasing interaction of CaMKIIalpha with dopamine D3 receptors. Brain Res. 2014;1557:101–110. doi: 10.1016/j.brainres.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Cassidy F, Naftolowitz D, Tatham NE, Wilson WH, Iranmanesh A, Liu PY, Veldhuis JD. Pathophysiology of hypercortisolism in depression. Acta Psychiatr Scand Suppl. 2007;(433):90–103. doi: 10.1111/j.1600-0447.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Carlezon WA., Jr Drug withdrawal conceptualized as a stressor. Behav Pharmacol. 2014;25:473–492. doi: 10.1097/FBP.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary AG, Somalwar AR, Sagarkar S, Rale A, Sakharkar A, Subhedar NK, Kokare DM. CART neurons in the lateral hypothalamus communicate with the nucleus accumbens shell via glutamatergic neurons in paraventricular thalamic nucleus to modulate reward behavior. Brain Struct Funct. 2018;223:1313–1328. doi: 10.1007/s00429-017-1544-6. [DOI] [PubMed] [Google Scholar]
- Craige CP, Lewandowski S, Kirby LG, Unterwald EM. Dorsal raphe 5-HT(2C) receptor and GABA networks regulate anxiety produced by cocaine withdrawal. Neuropharmacology. 2015;93:41–51. doi: 10.1016/j.neuropharm.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, Ma S, Sang K, Tang S, Li Y, Shen Y, Berry H, Wu S, Hu H. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature. 2018;554:323–327. doi: 10.1038/nature25752. [DOI] [PubMed] [Google Scholar]
- Dallvechia-Adams S, Kuhar MJ, Smith Y. Cocaine- and amphetamine-regulated transcript peptide projections in the ventral midbrain: colocalization with gamma-aminobutyric acid, melanin-concentrating hormone, dynorphin, and synaptic interactions with dopamine neurons. J Comp Neurol. 2002;448:360–372. doi: 10.1002/cne.10268. [DOI] [PubMed] [Google Scholar]
- Dandekar MP, Singru PS, Kokare DM, Subhedar NK. Cocaine- and amphetamine-regulated transcript peptide plays a role in the manifestation of depression: social isolation and olfactory bulbectomy models reveal unifying principles. Neuropsychopharmacology. 2009;34:1288–1300. doi: 10.1038/npp.2008.201. [DOI] [PubMed] [Google Scholar]
- Douglass J, Daoud S. Characterization of the human cDNA and genomic DNA encoding CART: a cocaine- and amphetamine-regulated transcript. Gene. 1996;169:241–245. doi: 10.1016/0378-1119(96)88651-3. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban S, Moranta D, Sastre-Coll A, Miralles A, Garcia-Sevilla JA. Withdrawal from chronic ethanol increases the sensitivity of presynaptic 5-HT(1A) receptors modulating serotonin and dopamine synthesis in rat brain in vivo. Neurosci Lett. 2002;326:121–124. doi: 10.1016/S0304-3940(02)00313-0. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Sadakierska-Chudy A, Suder A, Szumiec L, Mierzejewski P, Bienkowski P, Przegalinski E, Cryan JF. GABAB receptors as a therapeutic strategy in substance use disorders: focus on positive allosteric modulators. Neuropharmacology. 2015;88:36–47. doi: 10.1016/j.neuropharm.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Fu Q, Zhou X, Dong Y, Huang Y, Yang J, Oh KW, Hu Z. Decreased caffeine-induced locomotor activity via microinjection of CART peptide into the nucleus accumbens is linked to inhibition of the pCaMKIIa-D3R interaction. PLoS ONE. 2016;11:e0159104. doi: 10.1371/journal.pone.0159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O’Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA. 2007;104:17198–17203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav. 1996;54:129–141. doi: 10.1016/0091-3057(95)02135-3. [DOI] [PubMed] [Google Scholar]
- Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC, Zorrilla EP, Koob GF. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol. 2009;14:130–143. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hu Z, Oh EH, Chung YB, Hong JT, Oh KW. Predominant D1 receptors involvement in the over-expression of CART peptides after repeated cocaine administration. Korean J Physiol Pharmacol. 2015;19:89–97. doi: 10.4196/kjpp.2015.19.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert GW, Jones DC, Moffett MC, Rogge G, Kuhar MJ. CART peptides as modulators of dopamine and psychostimulants and interactions with the mesolimbic dopaminergic system. Biochem Pharmacol. 2008;75:57–62. doi: 10.1016/j.bcp.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate C, Merchant K, Bear MF, Umbricht D, Hajos M, Potter WZ, Lee CM. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011;3:102mr2. doi: 10.1126/scitranslmed.3002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther. 2003a;307:1038–1044. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Vicentic A, Hunter RG, Kimmel HL, Kuhar MJ. CART peptides are modulators of mesolimbic dopamine and psychostimulants. Life Sci. 2003b;73:741–747. doi: 10.1016/S0024-3205(03)00394-1. [DOI] [PubMed] [Google Scholar]
- Jean A, Conductier G, Manrique C, Bouras C, Berta P, Hen R, Charnay Y, Bockaert J, Compan V. Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci USA. 2007;104:16335–16340. doi: 10.1073/pnas.0701471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A, Laurent L, Bockaert J, Charnay Y, Dusticier N, Nieoullon A, Barrot M, Neve R, Compan V. The nucleus accumbens 5-HTR(4)-CART pathway ties anorexia to hyperactivity. Transl Psychiatry. 2012;2:e203. doi: 10.1038/tp.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao D, Liu Y, Li X, Liu J, Zhao M. The role of the GABA system in amphetamine-type stimulant use disorders. Front Cell Neurosci. 2015;9:162. doi: 10.3389/fncel.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job MO, McNamara IM, Kuhar MJ. cart peptides regulate psychostimulants and may be endogenous antidepressants. Curr Neuropharmacol. 2011;9:12–16. doi: 10.2174/157015911795017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Baclofen blocks expression and sensitization of anxiety-like behavior in an animal model of repeated stress and ethanol withdrawal. Alcohol Clin Exp Res. 2007;31:582–595. doi: 10.1111/j.1530-0277.2007.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Hedonic homeostatic dysregulation as a driver of drug-seeking behavior. Drug Discov. Today Dis Models. 2008a;5:207–215. doi: 10.1016/j.ddmod.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008b;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/S0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O’Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Yoho LL. CART peptide analysis by Western blotting. Synapse. 1999;33:163–171. doi: 10.1002/(SICI)1098-2396(19990901)33:3<163::AID-SYN1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Adams LD, Hunter RG, Vechia SD, Smith Y. CART peptides. Regul Pept. 2000;89:1–6. doi: 10.1016/S0167-0115(00)00096-3. [DOI] [PubMed] [Google Scholar]
- Kulik A, Vida I, Lujan R, Haas CA, Lopez-Bendito G, Shigemoto R, Frotscher M. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci. 2003;23:11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379:1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca S, Meye FJ, Mameli M. The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. Eur J Neurosci. 2014;39:1170–1178. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- Lee JS, Lee HS. Reciprocal connections between CART-immunoreactive, hypothalamic paraventricular neurons and serotonergic dorsal raphe cells in the rat: light microscopic study. Brain Res. 2014;1560:46–59. doi: 10.1016/j.brainres.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Li K, Zhou T, Liao L, Yang Z, Wong C, Henn F, Malinow R, Yates JR, 3rd, Hu H. betaCaMKII in lateral habenula mediates core symptoms of depression. Science. 2013a;341:1016–1020. doi: 10.1126/science.1240729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Yan SY, Bao YP, Lian Z, Qu Z, Wu YP, Liu ZM. Depression and alterations in hypothalamic-pituitary-adrenal and hypothalamic-pituitary-thyroid axis function in male abstinent methamphetamine abusers. Hum Psychopharmacol. 2013b;28:477–483. doi: 10.1002/hup.2335. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD. Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: location, location, location. Epilepsia. 2012;53(Suppl 1):45–52. doi: 10.1111/j.1528-1167.2012.03474.x. [DOI] [PubMed] [Google Scholar]
- Ma Z, Pearson E, Tao R. CART peptides increase 5-hydroxytryptamine in the dorsal raphe and nucleus accumbens of freely behaving rats. Neurosci Lett. 2007;417:303–307. doi: 10.1016/j.neulet.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao P. Potential antidepressant role of neurotransmitter CART: implications for mental disorders. Depress Res Treat. 2011;2011:762139. doi: 10.1155/2011/762139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao PZ, Meshul CK, Thuillier P, Goldberg NRS, Reddy PH. CART Peptide Is a Potential Endogenous Antioxidant and Preferentially Localized in Mitochondria. PLoS ONE. 2012;7:e29343. doi: 10.1371/journal.pone.0029343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Song J, Kuhar MJ. CART peptide inhibits locomotor activity induced by simultaneous stimulation of D1 and D2 receptors, but not by stimulation of individual dopamine receptors. Synapse. 2011;65:1–7. doi: 10.1002/syn.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelova V, Pirnik Z, Zelezna B, Maletinska L. CART (cocaine- and amphetamine-regulated transcript) peptide specific binding sites in PC12 cells have characteristics of CART peptide receptors. Brain Res. 2014;1547:16–24. doi: 10.1016/j.brainres.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Padgett CL, Lalive AL, Tan KR, Terunuma M, Munoz MB, Pangalos MN, Martinez-Hernandez J, Watanabe M, Moss SJ, Lujan R, Luscher C, Slesinger PA. Methamphetamine-evoked depression of GABA(B) receptor signaling in GABA neurons of the VTA. Neuron. 2012;73:978–989. doi: 10.1016/j.neuron.2011.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae CU, Lee C, Paik IH. Therapeutic implication of cocaine- and amphetamine-regulated transcript (CART) in the treatment of depression. Med Hypotheses. 2007;69:132–135. doi: 10.1016/j.mehy.2006.11.009. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Extracellular serotonin is decreased in the nucleus accumbens during withdrawal from cocaine self-administration. Behav Brain Res. 1996;73:225–228. doi: 10.1016/0166-4328(96)00101-5. [DOI] [PubMed] [Google Scholar]
- Peng GJ, Tian JS, Gao XX, Zhou YZ, Qin XM. Research on the pathological mechanism and drug treatment mechanism of depression. Curr Neuropharmacol. 2015;13:514–523. doi: 10.2174/1570159X1304150831120428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Q, Sun X, Liu Z, Yang J, Oh KW, Hu Z. Micro-injection of CART (cocaine- and amphetamine-regulated transcript) peptide into the nucleus accumbens inhibits the cocaine-induced upregulation of dopamine receptors and locomotor sensitization. Neurochem Int. 2014;75:105–111. doi: 10.1016/j.neuint.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Pin JP, Bettler B. Organization and functions of mGlu and GABAB receptor complexes. Nature. 2016;540:60–68. doi: 10.1038/nature20566. [DOI] [PubMed] [Google Scholar]
- Pompili M, Serafini G, Innamorati M, Moller-Leimkuhler AM, Giupponi G, Girardi P, Tatarelli R, Lester D. The hypothalamic-pituitary-adrenal axis and serotonin abnormalities: a selective overview for the implications of suicide prevention. Eur Arch Psychiatry Clin Neurosci. 2010;260:583–600. doi: 10.1007/s00406-010-0108-z. [DOI] [PubMed] [Google Scholar]
- Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol. Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- Shabel SJ, Proulx CD, Piriz J, Malinow R. Mood regulation. GABA/glutamate co-release controls habenula output and is modified by antidepressant treatment. Science. 2014;345:1494–1498. doi: 10.1126/science.1250469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Vaughan JM, Donaldson CJ, Rivier J, Li C, Chen A, Vale WW. Cocaine- and amphetamine-regulated transcript activates the hypothalamic-pituitary-adrenal axis through a corticotropin-releasing factor receptor-dependent mechanism. Endocrinology. 2004;145:5202–5209. doi: 10.1210/en.2004-0708. [DOI] [PubMed] [Google Scholar]
- Specio SE, Wee S, O’Dell LE, Boutrel B, Zorrilla EP, Koob GF. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology. 2008;196:473–482. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J, Villarreal J, Vale W. Isolation and sequence analysis of a somatostatin-like polypeptide from ovine hypothalamus. Biochemistry. 1981;20:1982–1988. doi: 10.1021/bi00510a038. [DOI] [PubMed] [Google Scholar]
- Stanek LM. Cocaine- and amphetamine related transcript (CART) and anxiety. Peptides. 2006;27:2005–2011. doi: 10.1016/j.peptides.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Stanley SA, Murphy KG, Bewick GA, Kong WM, Opacka-Juffry J, Gardiner JV, Ghatei M, Small CJ, Bloom SR. Regulation of rat pituitary cocaine- and amphetamine-regulated transcript (CART) by CRH and glucocorticoids. Am J Physiol Endocrinol Metab. 2004;287:E583–E590. doi: 10.1152/ajpendo.00576.2003. [DOI] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, Deisseroth K, Tye KM, Luscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc Soc Exp Biol Med. 1997;215:1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- Upadhya MA, Nakhate KT, Kokare DM, Singh U, Singru PS, Subhedar NK. CART peptide in the nucleus accumbens shell acts downstream to dopamine and mediates the reward and reinforcement actions of morphine. Neuropharmacology. 2012;62:1823–1833. doi: 10.1016/j.neuropharm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Kristensen P, Tang-Christensen M. Central administration of cocaine-amphetamine-regulated transcript activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 2000;141:794–801. doi: 10.1210/endo.141.2.7295. [DOI] [PubMed] [Google Scholar]
- Wiehager S, Beiderbeck DI, Gruber SH, El-Khoury A, Wamsteeker J, Neumann ID, Petersen A, Mathe AA. Increased levels of cocaine and amphetamine regulated transcript in two animal models of depression and anxiety. Neurobiol Dis. 2009;34:375–380. doi: 10.1016/j.nbd.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Wu B, Hu S, Yang M, Pan H, Zhu S. CART peptide promotes the survival of hippocampal neurons by upregulating brain-derived neurotrophic factor. Biochem Biophys Res Commun. 2006;347:656–661. doi: 10.1016/j.bbrc.2006.06.117. [DOI] [PubMed] [Google Scholar]
- Xiong L, Meng Q, Sun X, Lu X, Fu Q, Peng Q, Yang J, Oh KW, Hu ZZ. CART peptide in the nucleus accumbens shell inhibits cocaine-induced locomotor sensitization to transient overexpression of alpha-Ca2+/Calmodulin-dependent Protein Kinase II. J Neurochem. 2018 doi: 10.1111/jnc.14289. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, Hu H. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–322. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Adachi N, Kunugi H. Microinjection of cocaine- and amphetamine-regulated transcript 55–102 peptide into the nucleus accumbens could modulate anxiety-related behavior in rats. Neuropeptides. 2014;48:319–325. doi: 10.1016/j.npep.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Kim S, Park HK, Kim JH. Microinjection of CART peptide 55–102 into the nucleus accumbens blocks both the expression of behavioral sensitization and ERK phosphorylation by cocaine. Neuropharmacology. 2007;53:344–351. doi: 10.1016/j.neuropharm.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Yoon HS, Hattori K, Sasayama D, Kunugi H. Low cocaine- and amphetamine-regulated transcript (CART) peptide levels in human cerebrospinal fluid of major depressive disorder (MDD) patients. J Affect Disord. 2018;232:134–138. doi: 10.1016/j.jad.2018.02.039. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Jacobskind JS, Raber J. Methamphetamine and the hypothalamic-pituitary-adrenal axis. Front Neurosci. 2015;9:178. doi: 10.3389/fnins.2015.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]