Abstract
Cosmetics are primarily applied to the skin; therefore, the association of cosmetic dyes with skin diseases or inflammation is a topic of great interest. Thymic stromal lymphopoietin (TSLP) is an interleukin 7-like cytokine that activates dendritic cells to promote Th2 inflammatory immune responses. TSLP is highly expressed in keratinocytes under inflammatory conditions, which suggests that it may play a critical role in the development of skin diseases, such as atopic dermatitis. Therefore, we investigated whether cosmetic dyes influenced the production of TSLP by keratinocytes. Phloxine O, also known as D&C Red No.27, is one of the most common red synthetic pigments and is widely used in colored cosmetics. Our results showed that Phloxine O downregulated phorbol 12-myristate 13-acetate-induced production of TSLP in a murine keratinocyte cell line (PAM212). Phloxine O also suppressed TSLP expression in KCMH-1 cells, which are mouse keratinocytes that constitutively produce high levels of TSLP. To investigate the in vivo effects of Phloxine O, we induced TSLP expression in mouse ear skin by topically applying MC903, a vitamin D3 analogue that is a well-known inducer of atopic dermatitis-like symptoms. Topical application of Phloxine O prevented MC903-induced TSLP production in mouse ear skin, attenuated the acute dermatitis-like symptoms and decreased serum IgE and histamine levels in mice. Suppression of TSLP expression by Phloxine O correlated with reduced expression of OX40 ligand and Th2 cytokines in mouse ear skin. Our results showed that Phloxine O may be beneficial to prevent dermatitis by suppressing the expression of TSLP and Th2 cytokines in skin.
Keywords: Cosmetics, TSLP, Inflammation, Keratinocytes, Dermatitis
INTRODUCTION
Atopic eczema, also known as eczema, is an itchy inflammatory skin condition associated with significant morbidity (Johansson et al., 2004). Atopic eczema is a diagnostic term that is synonymous with atopic dermatitis (AD). AD is a chronic skin disease that affects a large portion of the world’s population. AD does not specifically affect a particular age group; rather, it can occur in people of all ages. Recent studies have shown that the prevalence of AD has increased steadily, affecting 15%–30% of urban children and 1%–3% of adults (Bieber, 2008; Brown et al., 2008; Odhiambo et al., 2009). AD is characterized by intense pruritus and chronic and recurrent skin irritation. AD is closely linked to immunomodulatory disorders and epidermal barrier defects, leading to increased susceptibility of suffering from allergic reactions and skin infections (Nutten, 2015). AD is known to be a multifactorial and heterogeneous disease characterized by a variety of clinical forms that result from the interaction of susceptibility genes, environmental factors, skin barrier integrity disorders and immunomodulatory disorders. Although both barrier damage and immunomodulatory disorders play important roles in its pathogenesis, the underlying sequence of events is still unclear (Leung, 2016).
Thymic stromal lymphopoietin (TSLP) is produced by various cells, such as fibroblasts, stromal cells and epithelial cells, and mediates the development and progression of skin inflammation. TSLP interconnects the innate and adaptive immune responses (Kashyap et al., 2011). In AD, TSLP is primarily produced by keratinocytes upon stimulation with an allergen. OX40 ligand (OX40L), which is preferentially expressed by dendritic cells stimulated with TSLP, transforms dendritic cells into promoters of atopic inflammation, stimulating a positive feedback loop that amplifies chronic Th2 inflammatory responses (Pulendran et al., 2010; Cianferoni and Spergel, 2014). TSLP promotes the interaction of dendritic cells and T cells, which is an essential step to induce the activation of inflammatory Th2 cells and ultimately cause allergic diseases (Murakami-Satsutani et al., 2014). Therefore, the TSLP pathway is a promising target for immunotherapy in AD (Wang et al., 2013).
Many dye products contain ingredients called “coal tar dyes” that are widely used in cosmetic products. Since cosmetics are applied primarily to the skin, the effects of cosmetic dyes on dermatitis are of great interest. However, the effects of cosmetic dyes on TSLP expression by the skin have not been clarified. Therefore, we investigated whether certain cosmetic dyes modulated the expression of TSLP in keratinocytes and mouse skin. In this study, we show that Phloxine O, also known as D&C Red No. 27, downregulates TSLP production by keratinocytes, thereby reducing skin inflammatory responses in mice.
MATERIALS AND METHODS
Animals
BALB/c mice were obtained from Orient Bio (Seoul, Korea). The mice were housed under specific pathogen-free conditions in a room where the optimal temperature (23 ± 3°C) and relative humidity (40–60%) were controlled. Mice were acclimated in the animal facility for at least one week before the experiments. Animal care and the experimental protocols were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Catholic University of Korea (permission #2016-006) and the ARRIVE guidelines. Mice in the different experimental groups were of similar age and weight and were randomly allocated to each group. Investigators were blinded to the treatment of mice in all experiments.
Cells
The murine keratinocyte cell line PAM212, which was derived from BALB/c mouse skin, was generously provided by Dr. Stuart H. Yuspa from the National Cancer Institute (Bethesda, MD, USA) (Yuspa et al., 1980). KCMH-1, a mouse keratinocyte-derived squamous cell carcinoma cell line derived from CBA/j mouse skin, was generously provided by Dr. Noriyasu Hirasawa at Tohoku University (Segawa et al., 2014). PAM212 and KCMH-1 cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA) containing 10% (v/v) fetal bovine serum, 10,000 units/ml of penicillin, and 10,000 μg/ml of streptomycin. The cells were plated in 48-well plates at a density of 5×104 cells/well and were incubated overnight before the treatments.
Reagents
Phorbol 12-myristate 13-acetate (PMA) and MC903 (calcipotriol) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Phloxine O (CAS, 13473-26-2; IUPAC name, 2′,4′,5′,7′-tetrabromo-4,5,6,7-tetrachloro-3′,6′-dihydroxyspiro[2-benzofuran-3,9′-xanthene]-1-one; molecular formula, C20H4Br4Cl4O5) was obtained from LG Household and Health Care Co (Daejeon, Korea).
Acute dermatitis mouse model
BALB/c mice were randomly divided into five groups (7-8 animals/group): vehicle, Phloxine O (1% in a 3:1 mix ture of acetone:olive oil), MC903 (5 nmole in ethanol), MC903 (5 nmole)+Phloxine O (1%), and MC903 (5 nmole)+dexamethasone (0.1% in a 3:1 mixture of acetone:olive oil). Acute dermatitis was induced by repeated topical application of MC903 according to a published protocol with slight modification (Li et al., 2006). Briefly, 25 μl of Phloxine O, vehicle (3:1 mixture of acetone:olive oil), or dexamethasone was topically applied to mouse ear skin. After 4 hours, 25 μl of MC903 was topically applied to the ear skin. The application of Phloxine O, dexamethasone, and MC903 was repeated once per day for 5 days. Ear thickness was recorded every day. On day 6, mice were sacrificed to obtain ear skin tissues and blood. Serum was collected after centrifugation of blood at 8,000 g for 30 min. Samples were stored at −80°C pending further analysis.
Enzyme-linked immunosorbent assays
Enzyme-linked immunosorbent assays (ELISA) were performed as previously described (Lee et al., 2017). TSLP levels were determined using a DuoSet enzyme-linked immunosorbent assay (ELISA) kit (R&D systems, Minneapolis, MN, USA) following the manufacturer’s instructions. The serum levels of IgE and histamine were measured using ELISA kits (IgE, eBioscience, San Diego, CA, USA; histamine, Enzo life science, Farmingdale, NY, USA) according to the manufacturer’s protocols.
Reverse transcription and quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was extracted from cells or mouse ear tissues using WelPrep reagent (Welgene, Korea). Reverse transcription and qRT-PCR analysis was performed as described previously (Lee et al., 2016). Primers used were as follows: Tslp, 5′-GGACCACTG GTGTTTATTCT-3′ and 5′-CAGGGTTTAG ATGCTGTCAT-3′; Tslpr, 5′-TTCGCAGGGTGAAAGATG-3′ and 5′-CTTAGCCTTGGTGTGGATG-3′; Ox40l, 5′-GTCGAAGGATTGTCTTCACT-3′ and 5′-TGGAATTCACAGTGGTACT-3′; Il-4, 5′-AGATGGATGTGCCAAACGTCCTCA-3′ and 5′-AATATGCGAAGCACCTTGGAAGCC-3′; Il-5, 5′-CAAAAAGAGA AGTGTGGCGA GG-3′ and 5′-TAGATAGGAG CAGGAAGCCC-3′; Il-13, 5′-GCAACGGCAG CATGGTATGG A-3′ and 5′-TGGTATAGGG GAGGCTGGAG AC3′; Actin, 5′-TCATGAAGTGTGACGTTGACATCCGT-3′ and 5′-TTGCGGTGCACGATGGAGGGGCCGGA-3′. Specificity of the amplified PCR products was assessed by melting curve analysis. Fold change was calculated using 2−ΔΔCt relative to the internal reference gene (actin) levels (Kim et al., 2017).
Histological analysis
Ear tissue specimens were fixed in 10% buffered formalin, embedded in paraffin, cut at a thickness of 5-μm and stained with hematoxylin and eosin for histological examination, as described previously (Yang et al., 2016a).
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA). All data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test and were expressed as the means ± SEM. Values of p<0.05 were considered to be significant. Graphs or figures are representative of at least two or three independent experiments.
RESULTS
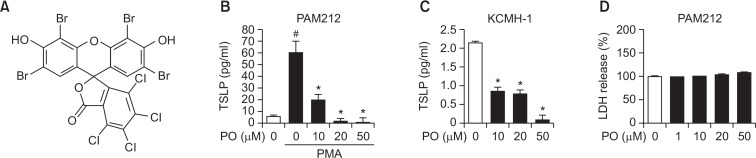
To investigate whether Phloxine O affected the production of TSLP by keratinocytes (Fig. 1A), murine keratinocytes (PAM212) were treated with Phloxine O and stimulated with phorbol 12-myristate 13-acetate (PMA) to induce TSLP expression. Phloxine O treatment inhibited the production of TSLP by PAM212 cells in a dose-dependent manner (Fig. 1B). To confirm the inhibitory effects of Phloxine O on TSLP expression, KCMH-1 cells, which are mouse keratinocytes that constitutively produce high levels of TSLP (Segawa et al., 2014), were treated with Phloxine O. Phloxine O treatment decreased the production of TSLP by KCMH-1 cells (Fig. 1C). To determine whether Phloxine O affected cell viability, PAM212 cells were treated with Phloxine O for 24 hours, and an LDH release assay was performed. Up to 50 μM of Phloxine O did not induce cytotoxicity in PAM212 cells (Fig. 1D).
Fig. 1.
Phloxine O inhibits TSLP production in keratinocytes. (A) Chemical structure of phloxine O. (B) PAM212 cells were treated with phorbol 12-myristate 13-acetate (PMA, 30 nM) or vehicle (0.2% ethanol) in the presence or absence of Phloxine O (PO) for 16 hours. (C) KCMH-1 cells were treated with Phloxine O (PO) or vehicle (0.2% ethanol) for 16 hours. TSLP protein levels in culture media were determined by ELISA. For (B) and (C), data are shown as the mean ± SEM (n=3). #Significantly different from vehicle alone, p<0.05. *Significantly different from PMA alone, p<0.05. (D) PAM212 cells were treated with Phloxine O for 24 hours, and the cytotoxicity was determined by LDH release assay. The LDH release of vehicle group was set up as 100%. Data are shown as the mean ± SEM (n=6).
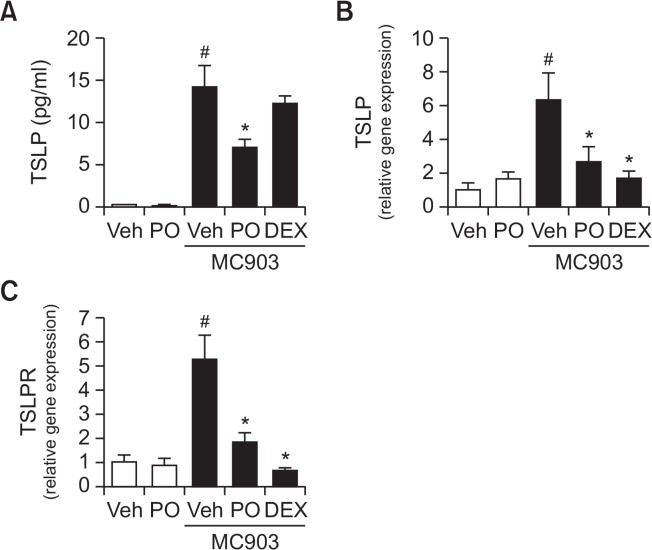
Next, we investigated whether Phloxine O suppressed the production of TSLP in vivo. MC903 (calcipotriol) was used as an in vivo inducer of TSLP expression in mouse skin, which results in the development of an atopic dermatitis-like syndrome (Li et al., 2006). To examine whether Phloxine O inhibited MC903-induced TSLP expression, Phloxine O (1%) was topically applied to mouse ear skin. After 4 hours, MC903 was topically applied to the same area of ear skin. The application of Phloxine O and MC903 was repeated for 5 days. Topical treatment with Phloxine O reduced the levels of TSLP protein, induced by MC903 in mouse ear skin tissues (Fig. 2A). Increased levels of TSLP mRNA following MC903 application in mouse ear skin were also reduced by Phloxine O (Fig. 2B). In addition, Phloxine O decreased TSLP receptor (TSLPR) mRNA levels, also increased as a result of the topical application of MC903 to mouse ear skin (Fig. 2C). Dexamethasone, which we used as a positive control, was effective in reducing TSLP and TSLPR mRNA levels, but it did not reduce TSLP protein levels (Fig. 2). These results demonstrate that Phloxine O inhibits the expression of TSLP in keratinocytes and in skin tissue.
Fig. 2.
Topical application of Phloxine O suppresses TSLP production induced by MC903 in mouse ear skin. Twenty-five μl of vehicle (3:1 mixture of acetone/olive oil), Phloxine O (PO, 1%), or dexamethasone (DEX, 0.1%) was topically applied to mouse ear skin. After 4 hours, 25 μl of MC903 (5 nmole in ethanol) or vehicle was treated. The application of Phloxine O, DEX and MC903 was repeated for 5 days (n=7–8 animals/group). (A) TSLP protein levels in ear tissue homogenates were determined by ELISA. (B, C) The mRNA levels of TSLP or TSLPR in ear tissue homogenates were determined by RT-real time quantitative-PCR analysis. The mRNA levels for each gene were normalized with β-actin mRNA levels. Data are shown as the mean ± SEM (n=7–8). #Significantly different from vehicle alone, p<0.05. *Significantly different from MC903 alone, p<0.05. Veh, vehicle.
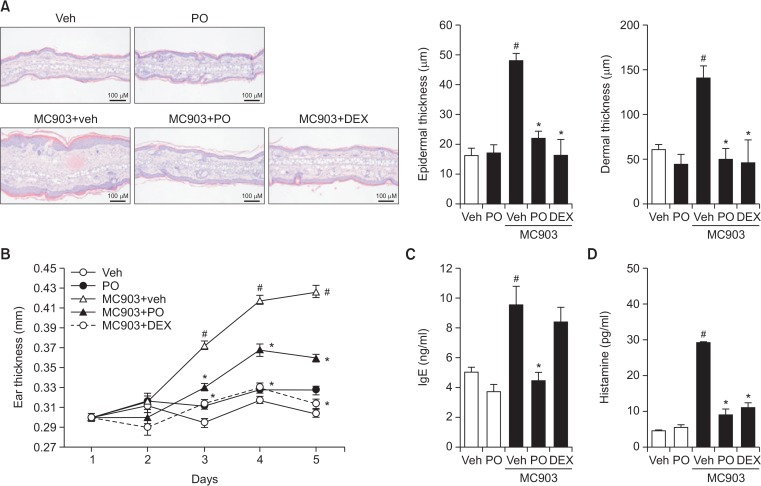
To investigate whether the in vivo inhibition of TSLP production by Phloxine O resulted in an attenuation of TSLP-mediated dermatitis symptoms, we analyzed the inflammatory responses in mouse ear skin tissues. Topical application of MC903 on mouse ear skin once per day for 5 days led to the development of dermatitis symptoms, such as epidermal hyperplasia, edema, and accumulation of inflammatory cells in the dermis/epidermis layer, as shown by histological assessment of ear skin tissues (Fig. 3A). In contrast, pretreatment with Phloxine O suppressed MC903-induced dermatitis symptoms, with an efficacy comparable to that of dexamethasone (Fig. 3A). The increase in ear skin thickness induced by MC903 was reduced by Phloxine O treatment (Fig. 3B). The atopic dermatitis (AD)-like skin inflammation is accompanied by an increase in serum IgE and histamine levels (Gao et al., 2004). While topical application of MC903 increased the levels of IgE and histamine in serum, treatment with Phloxine O significantly reduced these levels (Fig. 3C, 3D). Interestingly, dexamethasone treatment did not reduce the levels of IgE (Fig. 3C), although it did reduce histamine levels (Fig. 3D). These results indicate that dermal application of Phloxine O reduced the AD-like skin allergic inflammatory responses, most likely due to inhibition of TSLP production by keratinocytes.
Fig. 3.
Topical application of Phloxine O suppresses atopic dermatitis-like allergic inflammatory responses in mouse ear skin stimulated with MC903. (A–D) Samples were obtained from animals in Fig. 2. (A) Representative pictures of the histopathological features of ear skin tissues (200×, scale bar: 100 μm). (B) The thickness of ear skin was measured every day before MC903 treatment. (C, D) The serum levels of IgE and histamine were determined by ELISA. Data are shown as the mean ± SEM (n=7–8). #Significantly different from vehicle alone, p<0.05. *Significantly different from MC903 alone, p<0.05. Veh, vehicle; DEX, dexamethasone; Phloxine O, PO.
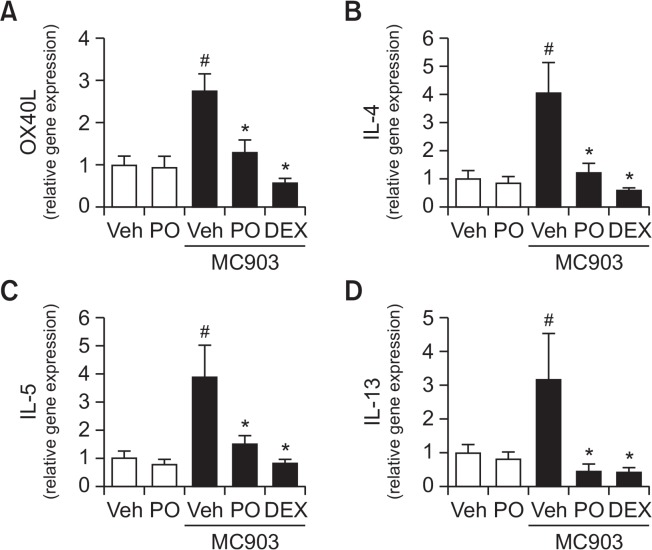
TSLP induces the expression of OX40L in dendritic cells which, in turn, stimulates the differentiation of naive CD4+ T cells into Th2 cells, thereby promoting Th2-type allergic immune responses (Leyva-Castillo et al., 2013). Therefore, we investigated whether Phloxine O treatment would affect the expression of OX40L and Th2 cytokines in mouse ear skin tissues exposed to MC903. Topical application of Phloxine O inhibited OX40L expression induced by MC903 in mouse ear skin tissues, a similar outcome to that seen with dexamethasone treatment (Fig. 4A). Consistently, Phloxine O significantly reduced the expression of Th2 cytokines, such as IL-4, IL-5, and IL-13, in mouse ear skin stimulated with MC903 (Fig. 4B–4D). These results show that Phloxine O inhibits the activation of dendritic cells, thereby blocking the activation of Th2 immune responses, possibly as a result of the inhibition of TSLP production by skin keratinocytes.
Fig. 4.
Topical application of Phloxine O decreases the expression of OX40L and Th2 cytokines in mouse ear skin stimulated with MC903. (A–D) Mouse ear skin tissues in Fig. 2 were used for the analysis. The mRNA levels of OX40L, IL-4, IL-5, and IL-13 in ear tissue homogenates were determined by RT-real time quantitative-PCR analysis. The mRNA levels of each gene were normalized with β-actin mRNA levels. Data are shown as the mean ± SEM (n=7–8). #Significantly different from vehicle alone, p<0.05. *Significantly different from MC903 alone, p<0.05. Veh, vehicle; DEX, dexamethasone; Phloxine O, PO.
Collectively, our results show that Phloxine O downregulates the expression of TSLP and Th2 cytokines in skin, resulting in the attenuation of acute dermatitis symptoms.
DISCUSSION
Although persons are frequently exposed to cosmetic dyes on a daily basis, there are few studies investigating the effects of cosmetic dyes on TSLP expression. To the best of our knowledge, this study is the first to show inhibitory effects of a cosmetic dye on TSLP production by the skin. Our results showed that Phloxine O suppressed TSLP expression both in vitro using murine keratinocyte cell lines and in vivo in a murine acute dermatitis model. TSLP expression correlated well with the acute dermatitis symptoms, since application of MC903, an in vivo inducer of TSLP, to mouse skin, resulted in an inflammatory response. In contrast, Phloxine O alleviated the acute dermatitis symptoms induced by MC903 in mice by reducing TSLP production.
Phloxine O is one of the commonest synthetic red pigments and is widely used in colored cosmetics. Phloxine O is approved by the FDA as an ingredient in pharmaceuticals and cosmetics and is primarily used to manufacture lipsticks and blushers. Lakes containing Phloxine O are used in lipsticks, blushers, makeup preparations, hair dyes and colors, rouges, face powders, bath oils, tablets and salts (Qi et al., 2012). An evaluation of its dermal toxicity has been reported (FDA, 2001). In these dermal toxicity studies, Phloxine O (0.1%, 1%) was topically applied daily to the intact or abraded skin of rabbits for 28 days or 91 days. Body weight and the healing time for abraded skin were not affected by Phloxine O treatment. There were no gross or histopathological changes in the internal organs after topical treatment with Phloxine O (FDA, 2001). Another study showed that 1% Phloxine O applied to the dorsal skin of ICR mice twice weekly for 18 months had no effect on survival and did not produce any skin lesions (FDA, 2001). These studies show that dermal application of Phloxine O does not produce any systemic effects or local skin lesions. However, these studies did not assess the potentially beneficial effects of applying Phloxine O on the dermis. In our study, 1% Phloxine O was topically applied for 5 days to MC903treated mouse skin, and we observed an anti-inflammatory effect. Our study highlights the beneficial effects that certain cosmetic dyes can have on skin inflammation. Furthermore, these results suggest that Phloxine O may be safely used on compromised and inflamed skin.
TSLP is a well-known inducer of Th2 immune responses. Therefore, the modulation of TSLP expression may be an effective strategy to prevent atopic dermatitis symptoms, balancing Th1 and Th2 immune responses. Our previous study showed that dieckol, a phlorotannin isolated from Ecklonia cava, suppressed TSLP production in mouse skin, reduced Th2-type immunity, and alleviated atopic dermatitis-like symptoms in the NC/Nga mouse model of AD (Yang et al., 2016b). In this study topical application of Phloxine O, a cosmetic colorant, inhibited TSLP production and downregulated Th2 cytokines, such as IL-4, IL-5 and IL-13, resulting in decreased IgE and histamine levels. These results suggest that Phloxine O can suppress exacerbated Th2-type responses and restore the Th1/Th2 immune balance.
Cultrone et al. (2013). showed that treatment of polarized epithelial cell lines with TLR agonists significantly upregulated TSLP expression in an NF-κB-dependent manner. After in silico analysis, the authors identified several putative binding sites for NF-κB and AP-1 within the 4 kilobase length region of the TSLP promoter (Cultrone et al., 2013). Similarly, our previous study showed that the suppression of TSLP production by dieckol in keratinocytes was mediated by the NF-κB pathway. This finding suggests that the downregulation of TSLP production induced by Phloxine O may also be mediated by NF-κB signaling. To the best of our knowledge, there are no published studies investigating the anti-dermatitis effects of Phloxine O or the underlying cellular signaling events. Our study is the first to investigate the cytokine modulatory effects after Phloxine O treatment of keratinocytes. Additional studies are needed to understand how Phloxine O interacts with the NF-κB signaling pathway.
Dexamethasone was used as a positive anti-inflammatory control in our MC903-induced skin inflammation experiments. In our study, dexamethasone suppressed MC903-induced ear swelling and thickness, as shown in Fig. 3A and 3B, demonstrating its anti-inflammatory activity. However, it is unclear whether the anti-inflammatory effects of dexamethasone involved the regulation of TSLP production, since although TSLP mRNA levels decreased, TSLP protein levels were not suppressed by dexamethasone (Fig. 2A, 2B). There are few reports addressing the regulation of TSLP production by dexamethasone. In a 2,4,6-trinitro-1-chlorobenzene (TNCB) induced mouse atopic dermatitis model mediated by TNF-α, topical application of 0.12% dexamethasone suppressed both TSLP mRNA and protein levels in the atopic lesion (Mizuno et al., 2015). Dexamethasone has also been reported to suppress TSLP mRNA expression in keratinocytes induced after 6 hours by double-stranded RNA (Le et al., 2010). In contrast, dexamethasone alone increased the expression of the TSLP receptor (TSLPR), and even enhanced IL-1/TNF-induced TSLPR expression in mast cells (MCs) and CD34+ cells (Allakhverdi et al., 2011). Dexamethasone reportedly increased IL-5 and IL-13 production in MCs, while it suppressed the production of Th2 cytokines in CD34+ cells, paradoxically suggesting that dexamethasone stimulates Th2 responses in MCs, promoting the cellular mechanisms that drive allergic inflammation (Allakhverdi et al., 2011). Our results suggest that dexamethasone may not be able to suppress all the cellular events induced by MC903. In our study, dexamethasone did not attenuate IgE production after applying MC903 to mouse ear skin (Fig. 3C), although histamine levels did decrease (Fig. 3D). There are several reports describing the paradoxical effects of glucocorticoids on allergic inflammation. Wu et al. (1991) showed that hydrocortisone enhanced the production of IgE in human lymphocytes stimulated with IL-4. Another study reported an increase of serum IgE levels in asthma patients treated with prednisone for 7 days, although prednisone still showed clinical efficacy against asthma (Zieg et al., 1994). Similarly, our results showed that dexamethasone exhibited anti-inflammatory effects against MC903-induced ear skin inflammation, despite its inability to suppress IgE production in vivo. Taken together, these results suggest that the in vivo significance of dexamethasone effects on TSLP and TSLPR expression in atopic dermatitis warrants further investigation.
Acknowledgments
This research was supported by a grant (14172MFDS975) from the Ministry of Food and Drug Safety in 2016.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Allakhverdi Z, Comeau MR, Delespesse G. Dexamethasone regulation of thymic stromal lymphopoietin receptor expression on mast cells and their precursors. J Allergy Clin Immunol. 2011;127:523–524.e2. doi: 10.1016/j.jaci.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Relton CL, Liao H, Zhao Y, Sandilands A, Wilson IJ, Burn J, Reynolds NJ, McLean WH, Cordell HJ. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940–946.e3. doi: 10.1016/j.jaci.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianferoni A, Spergel J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev Clin Immunol. 2014;10:1463–1474. doi: 10.1586/1744666X.2014.967684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cultrone A, de Wouters T, Lakhdari O, Kelly D, Mulder I, Logan E, Lapaque N, Dore J, Blottiere HM. The NF-κB binding site located in the proximal region of the TSLP promoter is critical for TSLP modulation in human intestinal epithelial cells. Eur J Immunol. 2013;43:1053–1062. doi: 10.1002/eji.201142340. [DOI] [PubMed] [Google Scholar]
- FDA . The Code of Federal Regulations of the United States of America, Title 21, Part 74.1328. U.S. Government Printing Office; 2001. [Google Scholar]
- Gao XK, Nakamura N, Fuseda K, Tanaka H, Inagaki N, Nagai H. Establishment of allergic dermatitis in NC/Nga mice as a model for severe atopic dermatitis. Biol Pharm Bull. 2004;27:1376–1381. doi: 10.1248/bpb.27.1376. [DOI] [PubMed] [Google Scholar]
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega Martell JA, Platts-Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J. Allergy Clin. Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol. 2011;187:1207–1211. doi: 10.4049/jimmunol.1100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Yun JW, Shin K, Cho Y, Yang M, Nam KT, Lim KM. Expression levels of GABA-A receptor subunit alpha 3, Gabra3 and lipoprotein lipase, Lpl are associated with the susceptibility to acetaminophen-induced hepatotoxicity. Biomol. Ther (Seoul) 2017;25:112–121. doi: 10.4062/biomolther.2016.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TA, Takai T, Vu AT, Kinoshita H, Ikeda S, Ogawa H, Okumura K. Glucocorticoids inhibit double-stranded RNA-induced thymic stromal lymphopoietin release from keratinocytes in an atopic cytokine milieu more effectively than tacrolimus. Int Arch Allergy Immunol. 2010;153:27–34. doi: 10.1159/000301576. [DOI] [PubMed] [Google Scholar]
- Lee DY, Hwang CJ, Choi JY, Park MH, Song MJ, Oh KW, Son DJ, Lee SH, Han SB, Hong JT. Inhibitory effect of carnosol on phthalic anhydride-induced atopic dermatitis via inhibition of STAT3. Biomol. Ther (Seoul) 2017;25:535–544. doi: 10.4062/biomolther.2017.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HE, Yang G, Kim ND, Jeong S, Jung Y, Choi JY, Park HH, Lee JY. Targeting ASC in NLRP3 inflammasome by caffeic acid phenethyl ester: a novel strategy to treat acute gout. Sci Rep. 2016;6:38622. doi: 10.1038/srep38622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DY. Clinical implications of new mechanistic insights into atopic dermatitis. Curr Opin Pediatr. 2016;28:456–462. doi: 10.1097/MOP.0000000000000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyva-Castillo JM, Hener P, Michea P, Karasuyama H, Chan S, Soumelis V, Li M. Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat Commun. 2013;4:2847. doi: 10.1038/ncomms3847. [DOI] [PubMed] [Google Scholar]
- Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci USA. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Morizane S, Takiguchi T, Iwatsuki K. Dexamethasone but not tacrolimus suppresses TNF-α-induced thymic stromal lymphopoietin expression in lesional keratinocytes of atopic dermatitis model. J Dermatol Sci. 2015;80:45–53. doi: 10.1016/j.jdermsci.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Murakami-Satsutani N, Ito T, Nakanishi T, Inagaki N, Tanaka A, Vien PT, Kibata K, Inaba M, Nomura S. IL-33 promotes the induction and maintenance of Th2 immune responses by enhancing the function of OX40 ligand. Allergol Int. 2014;63:443–455. doi: 10.2332/allergolint.13-OA-0672. [DOI] [PubMed] [Google Scholar]
- Nutten S. Atopic dermatitis: global epidemiology and risk factors. Ann. Nutr. Metab. 2015;66(Suppl 1):8–16. doi: 10.1159/000370220. [DOI] [PubMed] [Google Scholar]
- Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251–1258.e23. doi: 10.1016/j.jaci.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Pulendran B, Tang H, Manicassamy S. Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol. 2010;11:647–655. doi: 10.1038/ni.1894. [DOI] [PubMed] [Google Scholar]
- Qi H, Zhu B, Abe N, Shin Y, Murata Y, Nakamura Y. Involvement of intracellular oxidative stress-sensitive pathway in phloxine B-induced photocytotoxicity in human T lymphocytic leukemia cells. Food Chem Toxicol. 2012;50:1841–1847. doi: 10.1016/j.fct.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Segawa R, Yamashita S, Mizuno N, Shiraki M, Hatayama T, Satou N, Hiratsuka M, Hide M, Hirasawa N. Identification of a cell line producing high levels of TSLP: advantages for screening of anti-allergic drugs. J. Immunol Methods. 2014;402:9–14. doi: 10.1016/j.jim.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Wang WL, Li HY, Zhang MS, Gao PS, He SH, Zheng T, Zhu Z, Zhou LF. Thymic stromal lymphopoietin: a promising therapeutic target for allergic diseases. Int Arch Allergy Immunol. 2013;160:18–26. doi: 10.1159/000341665. [DOI] [PubMed] [Google Scholar]
- Wu CY, Sarfati M, Heusser C, Fournier S, Rubio-Trujillo M, Peleman R, Delespesse G. Glucocorticoids increase the synthesis of immunoglobulin E by interleukin 4-stimulated human lymphocytes. J Clin Invest. 1991;87:870–877. doi: 10.1172/JCI115092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Lee HE, Lee JY. A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet. Sci Rep. 2016a;6:24399. doi: 10.1038/srep24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Oh JW, Lee HE, Lee BH, Lim KM, Lee JY. Topical application of dieckol ameliorates atopic dermatitis in NC/Nga mice by suppressing thymic stromal lymphopoietin production. J Invest Dermatol. 2016b;136:1062–1066. doi: 10.1016/j.jid.2015.12.046. [DOI] [PubMed] [Google Scholar]
- Yuspa SH, Hawley-Nelson P, Koehler B, Stanley JR. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980;40:4694–4703. [PubMed] [Google Scholar]
- Zieg G, Lack G, Harbeck RJ, Gelfand EW, Leung DY. In vivo effects of glucocorticoids on IgE production. J Allergy Clin Immunol. 1994;94:222–230. doi: 10.1053/ai.1994.v94.a54936. [DOI] [PubMed] [Google Scholar]