Abstract
Most angiogenesis assays are performed using endothelial cells. However, blood vessels are composed of two cell types: endothelial cells and pericytes. Thus, co-culture of two vascular cells should be employed to evaluate angiogenic properties. Here, we developed an in vitro 3-dimensional angiogenesis assay system using spheroids formed by two human vascular precursors: endothelial colony forming cells (ECFCs) and mesenchymal stem cells (MSCs). ECFCs, MSCs, or ECFCs+MSCs were cultured to form spheroids. Sprout formation from each spheroid was observed for 24 h by real-time cell recorder. Sprout number and length were higher in ECFC+MSC spheroids than ECFC-only spheroids. No sprouts were observed in MSC-only spheroids. Sprout formation by ECFC spheroids was increased by treatment with vascular endothelial growth factor (VEGF) or combination of VEGF and fibroblast growth factor-2 (FGF-2). Interestingly, there was no further increase in sprout formation by ECFC+MSC spheroids in response to VEGF or VEGF+FGF-2, suggesting that MSCs stimulate sprout formation by ECFCs. Immuno-fluorescent labeling technique revealed that MSCs surrounded ECFC-mediated sprout structures. We tested vatalanib, VEGF inhibitor, using ECFC and ECFC+MSC spheroids. Vatalanib significantly inhibited sprout formation in both spheroids. Of note, the IC50 of vatalanib in ECFC+MSC spheroids at 24 h was 4.0 ± 0.40 μM, which are more correlated with the data of previous animal studies when compared with ECFC spheroids (0.2 ± 0.03 μM). These results suggest that ECFC+MSC spheroids generate physiologically relevant sprout structures composed of two types of vascular cells, and will be an effective pre-clinical in vitro assay model to evaluate pro- or anti-angiogenic property.
Keywords: Angiogenesis, Endothelial colony forming cells, Mesenchymal stem cells, Two-cell spheroid
INTRODUCTION
Blood vessel formation relies on a highly controlled sequence of cellular events. Two fundamental processes by which blood vessels are formed are vasculogenesis and angiogenesis. Vasculogenesis refers to the de novo process of new vessel formation by migration and differentiation of endothelial progenitor cells (EPCs) into endothelial cells (ECs), whereas angiogenesis refers to the extension of a pre-existing blood vessels through ECs sprouting and subsequent stabilization by mural cells (Carmeliet, 2000). If either one or both of these processes are dysregulated, a number of pathological conditions can arise (Carmeliet and Jain, 2000).
Drugs that modify angiogenesis hold great promise as potential treatment options for vascular malformation-associated diseases. Many pharmaceutical companies and research institutes have spent considerable effort, time, and money on discovering angiogenesis-modulating drugs. Irrespective of the efforts made, few drugs have entered into the clinical trials. This may be because the preclinical in vitro assay systems do not have sufficient sensitivity for identifying potential drug candidates that can effectively modify in vivo angiogenic events. Until now, only a handful of drugs, such as Bevacizumab (Avastin, Genentech-Roche, CA, USA) and Sunitinib (Sutent, Pfizer, NY, USA), have been approved for clinical use.
Pro- or anti-angiogenic properties are initially evaluated by in vitro assay systems that measure the degree of proliferation, invasion, migration, and tubular structure formation of ECs seeded in two-dimensional (2D) culture dishes. Although these assay systems have contributed significantly to the discovery of angiogenesis modulators, 2D culture systems have some limitations and drawbacks. One of the major limitations of 2D culture systems is loss of originality of cells. For example, 2D-cultured ECs progressively lose their differentiated phenotype as manifested by reduced expression of CD34 and several signals that govern cellular processes (Fina et al., 1990; Delia et al., 1993). Moreover, 2D-cultured cells cannot mimic the complex cascades involved in sprout formation in vivo (Lutolf et al., 2009). Thus, 2D culture assay systems could provide misleading results, which might be responsible for the discrepancies between the effects of angiogenic-modifying drugs in clinical trial and what was expected based on in vitro assays. To address the issues associated with 2D culture systems and to mimic closely the complex angiogenesis process in vivo, 3-dimensional (3D) assay system has been gaining more attention among researchers (Lee et al., 2016). 3D systems can provide an in vivo-like environment to the cells, which enable to generate data that bridge the gap between conventional 2D culture assay systems and in vivo animal models (Pampaloni et al., 2007; Hutmacher, 2010).
Recently, 3D spheroid assay model has been recommended for angiogenic modulator screening before activating animal protocols (Friedrich et al., 2009; Jaganathan et al., 2014). Current 3D spheroid angiogenesis assay model is utilized mature ECs, mainly human umbilical vein endothelial cells (HU-VECs), to focus on the behavior of ECs during angiogenesis. However, blood vessels are composed of two types of vascular cells: ECs and pericytes. During angiogenesis, ECs are responsible for sprout formation followed by the generation of new tubular structures, whereas pericytes are responsible for maturation of the nascent blood vessels by enveloping its surface. There needs to be a proper bi-directional interaction between the two cell types for the formation of functional blood vessels. To reflect the process of angiogenesis in vivo, some 3D angiogenesis assays have employed co-culture of ECs and vascular smooth muscle cells using scaffolds, micro-carriers or beads. The time required for assay varies from 4 to 15 days (Sanz-Nogues and O’Brien, 2016).
To improve current 3D assay model, we developed two-cell spheroid system by combining two vascular progenitors: endothelial colony forming cells (ECFCs) and mesenchymal stem cells (MSCs). ECFCs, also called late-EPCs, are circulating precursors of ECs. ECFCs have been reported to have robust proliferation capacity compared to mature ECs such as human umbilical vein endothelial cells (Melero-Martin et al., 2007). MSCs belongs to the perivascular niche and are closely related to pericytes (Shi and Gronthos, 2003; Crisan et al., 2008; Ayala-Cuellar et al., 2018). Previous studies have reported that co-implantation of ECFCs and MSCs in immune-deficient mice results in the formation of perfused functional blood vessels in vivo (Foubert et al., 2008; Melero-Martin et al., 2008; Reinisch et al., 2009). In the present study, ECFC+MSC spheroids were generated by the hanging drop spheroid formation method, which is relatively easy and do not require complex technique or materials to perform. This assay can be performed within two days. For effective tracking of complex-spout-formation events, we utilized a real-time cell recorder for continuous 24 h monitoring. Results demonstrated that sprouts from the two-cell spheroids were stable and durable, and may closely mimic the angiogenic sprouting in vivo compared with other angiogenesis assays based on one cell type.
MATERIALS AND METHODS
Isolation and culture of human ECFCs and MSCs
The study protocol was approved by the institutional review board of Duksung Women’s University (IRB No. 2017-002-001). Human peripheral blood was provided from the national biobank. ECFCs were isolated from the adherent mononuclear cell (MNC) fraction using CD31-coated magnetic beads (Invitrogen, MA, USA) as described in the previous report (Melero-Martin et al., 2008). The isolated ECFCs were expanded on 1% gelatin-coated plates (BD Biosciences, NJ, USA) using endothelial growth medium-2 (EGM-2; Lonza, MD, USA) without hydrocortisone supplemented with 10% fetal bovine serum (FBS; Atlas Biologicals, CO, USA) and 1% glutamine-penicillin-streptomycin (GPS; Gibco, MA, USA). In all experiments, ECFCs from passages 7 to 10 were used.
MSCs were obtained from the MNC fraction of human adult bone marrow (Lonza). MSCs were cultured in MSC growth medium (Lonza) containing 10% FBS and 1% GPS until 80% confluence was achieved. MSCs from passage numbers 5 to 8 were used.
Generation of ECFC-only, MSC-only, and ECFC+MSC spheroids
ECFCs and MSCs were trypsinized and suspended in the appropriate culture medium containing 20% methocel (Sigma, MO, USA). One-cell spheroids consisting of 600 cells/spheroid, and two-cell spheroids consisting of 500 ECFCs and 100 MSCs per spheroid were generated by the hanging drop spheroid formation method described previously (Korff and Augustin, 1998, 1999). In brief, 25 μL of cell suspension was deposited on the lid of a 150 p (150×20 mm) culture dish followed by inversion of the lid onto the PBS-filled bottom chamber. Drops were incubated for 24 h at 37°C in a 5% CO2 atmosphere with 95% relative humidity. Under these conditions, suspended cells were molded into standard spheroids with a defined size and cell number. These spheroids were harvested for experiments.
In vitro 3D angiogenesis assay using ECFC, MSC, and ECFC+MSC spheroids
Spheroids comprising ECFCs, MSCs, or ECFCs+MSCs were collected, suspended in the corresponding basal medium with 5% FBS (Atlas Biologicals) and 40% methocel (Sigma, MO, USA) to avoid sedimentation of the spheroids. The spheroid suspension was mixed with neutralized collagen solution (Corning, NY, USA) and quickly seeded into pre-warmed 24-well plates followed by polymerization in an incubator. In some experiments, vatalanib was added to the spheroid-containing collagen gel just before seeding. After 30 min of polymerization, 0.1 mL of corresponding basal medium in the presence and absence of pro-angiogenic factors (VEGF or VEGF+FGF-2) was added to the top of the gel. The plate was placed on a real-time cell recorder (JuLI stage; NanoEnTek, Seoul, Korea), which took microscope images of spheroids every 1 h for 24 h automatically. Sprout formation was analyzed by the average number and cumulative length of sprouts from at least five spheroids per group.
Live cell immuno-fluorescent labeling
Immuno-fluorescent labeling of live cell surfaces was performed as per the manufacturer’s instructions (Sigma) with slight modifications. Prior to spheroid generation, single cell suspensions of ECFCs and MSCs were washed once with the corresponding basal medium. ECFCs and MSCs were incubated with PKH67 (green) or PKH26 (red), respectively, for 5 min at room temperature. Dye uptake by cells was terminated by addition of FBS, and then cells were washed twice in their corresponding medium. Labeled ECFCs and MSCs were used for spheroid generation as described above. Fluorescent-labeled sprouting from spheroids were observed using a real-time cell recorder every hour for 24 h.
Statistical analysis
Values are expressed as means ± SEM of at least three independent experiments. Statistically significant differences were determined by one-way ANOVA followed by Fisher’s least significant difference (LSD) post hoc test for multiple comparisons or Student’s t-test for paired comparisons (OriginLab, MA, USA). A value of p≤0.05 was considered statistically significant.
RESULTS
Comparison of the sprouting potential of ECFC-only and ECFC+MSC spheroids
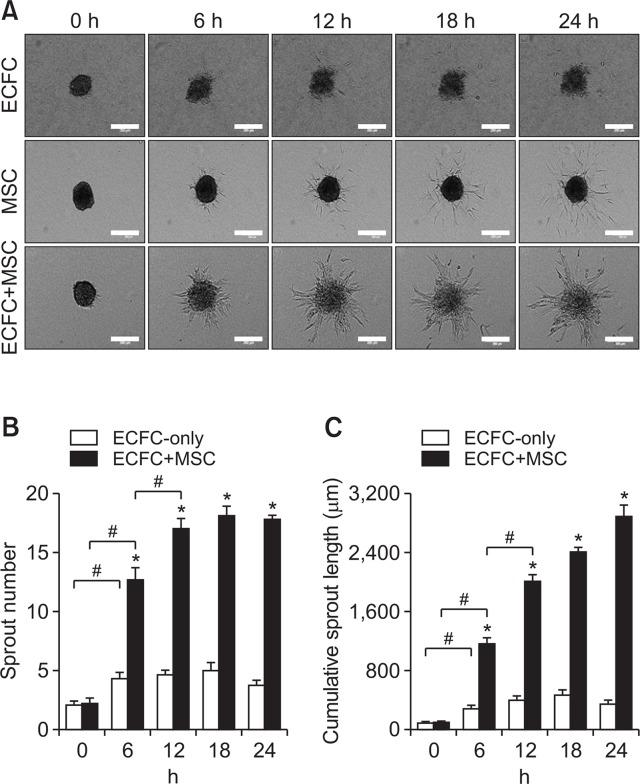
Spheroids comprised of ECFCs and MSCs at the ratio of 5:1 were generated for the purpose of developing in vitro two-cell spheroid angiogenesis assay system. To compare sprout formation, ECFC-only spheroids and MSC-only spheroids were also generated using the same harvested cells. Sprout formation from spheroids was monitored for 24 h by real-time cell recorder, which could capture the progressions of cellular angiogenic sprouting from tip cells to capillary-like sprout structures. As shown in Fig. 1, numerous capillary-like sprouts were formed by ECFC+MSC spheroids. The number and cumulative length of the sprouts from ECFC+MSC spheroids were significantly greater than those of ECFC spheroids (Fig. 1A, 1B, 1C). Sprouts formed by ECFC+MSC spheroids appeared to be thicker and longer than sprouts from ECFC spheroids. Moreover, the number and length of ECFC+MSC sprouts increased for 12 h in a time-dependent manner (Fig. 1B, 1C). This suggests that MSCs may improve the stability and durability of newly-formed sprouts in ECFC+MSC spheroids. MSC spheroids did not produce sprouts but some individual cells migrated (Fig. 1A).
Fig. 1.
Comparison of sprout formation between ECFC-only, MSC-only, and ECFC+MSC spheroids. ECFC-only spheroids, MSC-only spheroids, or ECFC+MSC spheroids were embedded in collagen gel and sprout formation was observed by real-time cell recorder every 1 h for 24 h. (A) Representative images of sprouts formed by ECFC-only, MSC-only, or ECFC+MSC spheroids at 0, 6, 12, 18, and 24 h time points (scale bar=250 μm). (B) Analysis of sprout number of ECFC-only or ECFC+MSC spheroids. (C) Analysis of cumulative sprout length of ECFC-only or ECFC+MSC spheroids. *Significant difference (p≤0.05) between groups. #Significant difference (p≤0.05) between groups indicated by a bracket (n=3).
Effect of exogenous angiogenic factors on sprout formation in ECFC-only and ECFC+MSC spheroids
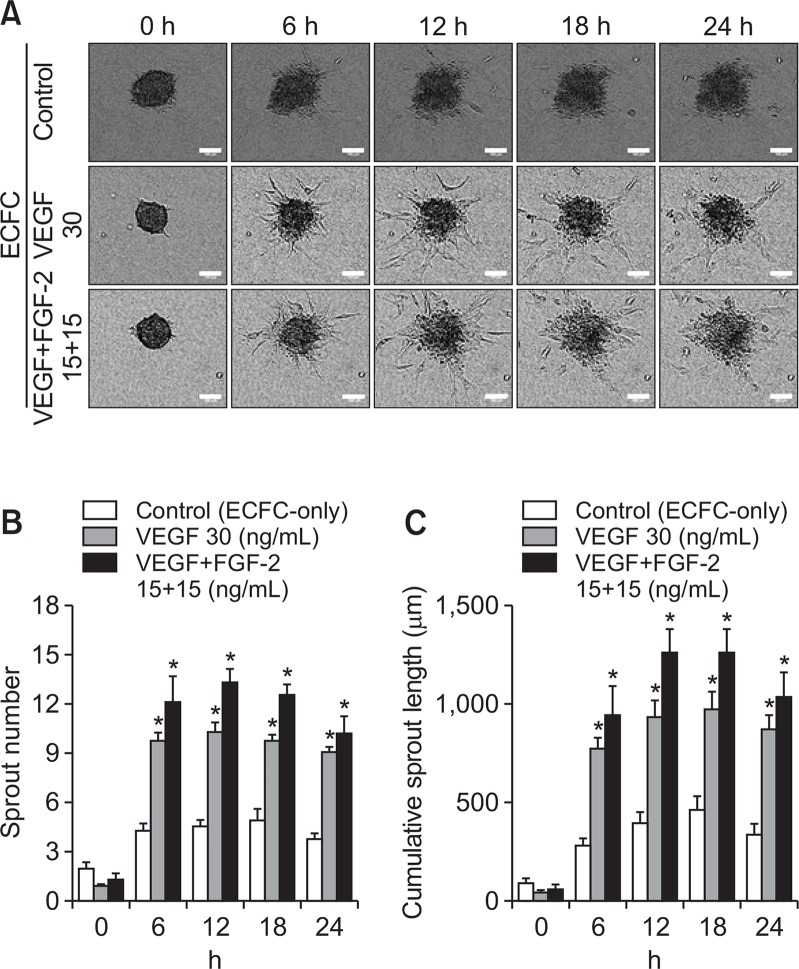
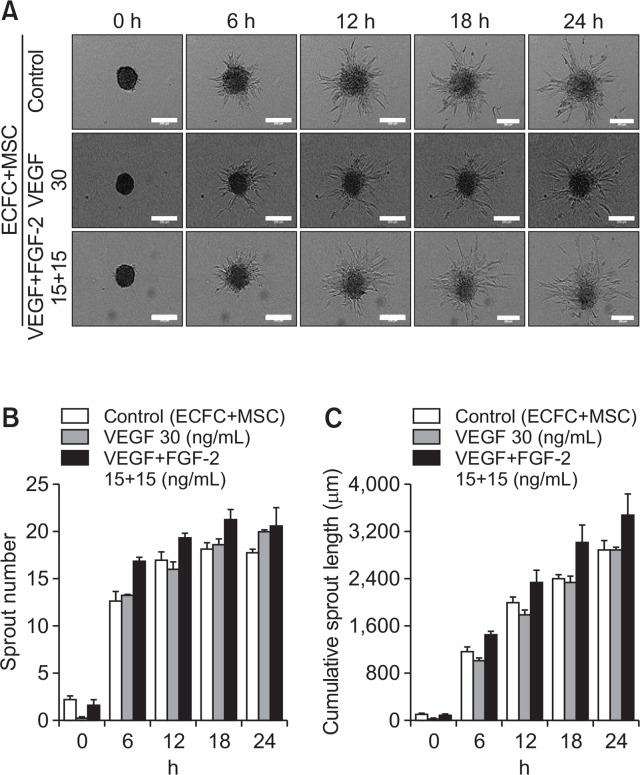
To determine the sprout formation ability of spheroids, spheroids were treated with angiogenic growth factor, vascular endothelial growth factor (VEGF), and fibroblast growth factor-2 (FGF-2). In ECFC spheroids, the number and length of sprouts increased significantly upon treatment with VEGF as well as VEGF+FGF-2 compared with the control group (Fig. 2A, 2B, 2C). ECFC+MSC spheroids were also treated with angiogenic growth factors. As shown in Fig. 3, VEGF or VEGF+FGF-2 treatment did not result in a further increase in sprout number or length compared with the control group (Fig. 3A, 3B, 3C). This suggests that ECFCs and MSCs interact, thereby MSCs induce robust sprout formation by ECFCs.
Fig. 2.
Effect of exogenous angiogenic factors on sprout formation by ECFC-only spheroids. ECFC-only spheroids were treated with VEGF (30 ng/mL) or VEGF (15 ng/mL)+FGF-2 (15 ng/mL) followed by seeding in a plate. (A) Representative images of sprouts formed by ECFC-only spheroids in the presence and absence of angiogenic factors at 0, 6, 12, 18, and 24 h (scale bar=100 μm). (B) Analysis of sprout numbers of ECFC-only spheroids with/without angiogenic factors. (C) Analysis of cumulative sprout length of ECFC-only spheroids with/without angiogenic factors. *Significant difference (p≤0.05) from control group (not treated with angiogenic factors) (n=3).
Fig. 3.
Effect of exogenous angiogenic factors on sprout formation by ECFC+MSC spheroids. ECFC+MSC spheroids were treated with VEGF (30 ng/mL) or VEGF (15 ng/mL)+FGF-2 (15 ng/mL). (A) Representative images of sprouts formed by ECFC+MSC spheroids in the presence and absence of angiogenic factors at 0, 6, 12, 18, and 24 h (scale bar=250 μm). (B) Analysis of sprout number of ECFC+MSC spheroids with/without angiogenic factors. (C) Analysis of cumulative sprout length of ECFC+MSC spheroids with/without angiogenic factors (n=3).
Localization of ECFCs and MSCs in sprout structures
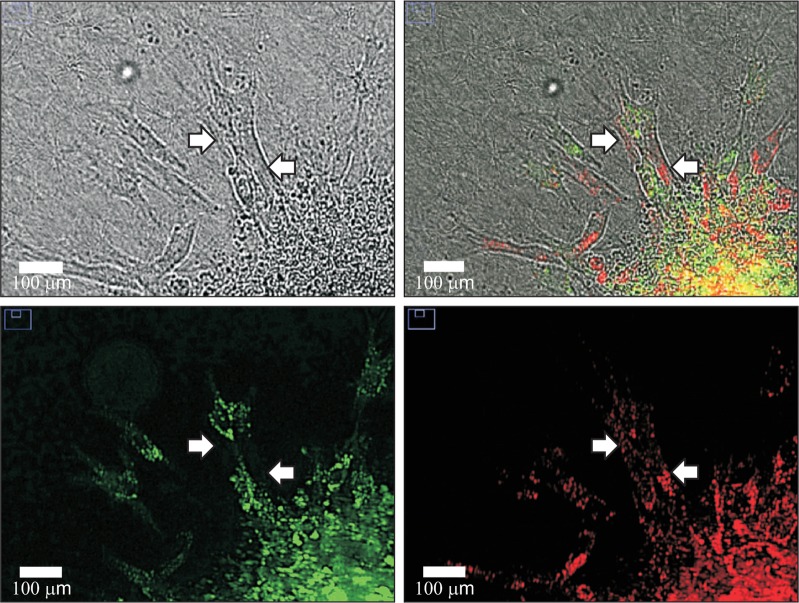
ECFCs are endothelial progenitors, while MSCs are multipotent stem cells that can differentiate into pericytes during angiogenesis. Live cell immuno-fluorescent labeling of ECFCs with green dye and MSCs with red dye was conducted followed by two-cell spheroid generation to determine the location of ECFCs and MSCs in sprouts. Fluorescent images revealed that MSCs surrounded the ECFC-mediated sprout structures (Fig. 4). This result is consistent with a previous study that human MSCs were found in regions adjacent to ECFC-mediated luminal structures when these two cells were co-injected subcutaneously into immune-deficient mice (Melero-Martin et al., 2008). This suggests that MSCs function as perivascular cells and may contribute to sprout stability and durability.
Fig. 4.
Localization of ECFCs and MSCs in sprout structures. Live cell fluorescent staining of ECFCs and MSCs was performed before spheroid formation using PKH67 (green) and PKH26 (red), respectively. Representative image was taken at the 24 h time point using a real-time cell recorder. Arrows indicate MSCs beside ECFCs in sprout structures (scale bar=100 μm).
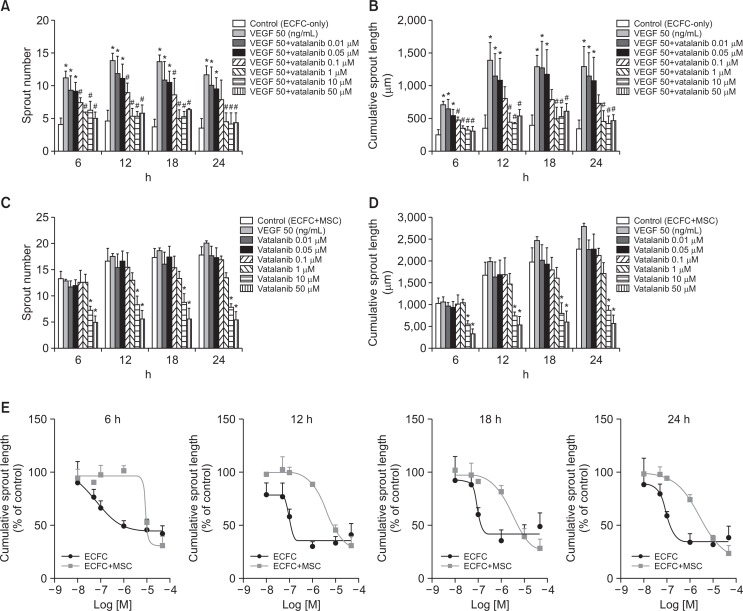
Inhibitory effect of vatalanib on sprout formation by ECFC-only and ECFC+MSC spheroids
To examine the ability of our newly-developed two-cell spheroid assay system to screen anti-angiogenic drug candidates, spheroids were treated with vatalanib, a VEGF inhibitor. ECFC spheroids were treated with vatalanib followed by stimulation with VEGF (50 ng/mL), which is required for sprout formation from ECFC-only spheroids. Vatalanib significantly decreased ECFC spheroid sprout number and length starting at 0.1 μM compared with VEGF-treated group (Fig. 5A, 5B). Vatalanib also treated to ECFC+MSC spheroids without VEGF stimulation. Vatalanib significantly inhibited sprout number and length of ECFC+MSC spheroids starting at 10 μM compared with control group (Fig. 5C, 5D). We assessed IC50 values of vatalanib for inhibition of sprout length in both spheroids (Table 1 and Fig. 5E). Of note, IC50 values of vatalanib was significantly greater for ECFC+MSC spheroids than ECFC-only spheroids at all-time points (Table 1). These results suggest that two-cell spheroid forms physiologically relevant sprouts composed of two vascular cells, thereby two-cell spheroid system may be more reasonable assay model to evaluate pro- or anti-angiogenic efficiency on in vivo angiogenic events.
Fig. 5.
Inhibitory effect of vatalanib on sprout formation by ECFC-only spheroids or ECFC+MSC spheroids. ECFC-only spheroids or ECFC+MSC spheroids were treated with vatalanib, a VEGF inhibitor, followed by seeding in a plate. (A) Analysis of number of sprouts formed by ECFC-only spheroids with/without vatalanib treatment. (B) Analysis of cumulative length of sprouts formed by ECFC spheroids with/without vatalanib treatment. (C) Analysis of number of sprouts formed by ECFC+MSC spheroids with/without vatalanib treatment. (D) Analysis of cumulative length of sprouts formed by ECFC+MSC spheroids with/without vatalanib treatment. (E) Comparison of IC50 values of vatalanib for inhibition of sprout length of ECFC-only spheroids and ECFC+MSC spheroids at 6, 12, 18 and 24 h. *Significant difference (p≤0.05) from control group (not treated with angiogenic factors), #Significant difference (p≤0.05) from VEFG-treated group (n=3).
Table 1.
IC50 values of vatalanib for inhibition of cumulative sprout length of either ECFC or ECFC+MSC spheroids at 6, 12, 18, and 24 h
| Time (h) | IC50 (µM) of vatalanib | p-value | |
|---|---|---|---|
|
| |||
| ECFC spheroid | ECFC+MSC spheroid | ||
| 6 | 0.6 ± 0.23 | 14.80 ± 0.70 | 0.00005 |
| 12 | 0.2 ± 0.10 | 8.9 ± 1.57 | 0.02200 |
| 18 | 0.1 ± 0.02 | 5.9 ± 0.29 | 0.00060 |
| 24 | 0.2 ± 0.03 | 4.0 ± 0.40 | 0.00073 |
ECFC spheroids were treated with vatalanib followed by stimulation with VEGF (50 ng/mL), which is required for sprout formation from ECFC spheroids. ECFC+MSC spheroids were treated with vatalanib without VEGF stimulation. Both spheroids embedded into the collagen gel, and sprout formation from each spheroid was observed for 24 h by real time cell recorder. Data are represented as mean ± SEM (n=3).
DISCUSSION
All blood vessels are formed by two types of vascular cells, namely endothelial cells and pericytes. During angiogenesis, precise cell-cell interactions between these two cell types are needed for functional blood vessels to form. To mimic vascular sprout formation in vivo, we created spheroids comprising two vascular progenitors: ECFCs and MSCs. The one-cell angiogenesis assays commonly use human umbilical vein endothelial cells or human dermal microvascular endothelial cells because of their ready availability (Arnaoutova and Kleinman, 2010). However, these mature ECs progressively lose their proliferative phenotype as well as differentiation potential, which dampens their angiogenic responses (Delia et al., 1993). Mature ECs also possess considerable organ- and tissue-specific heterogeneity (Gumkowski et al., 1987). This weakness can be overcome by using ECFCs, also known as late-EPCs. EPCs were first recognized by Asahara et al (1997). Since then, several subpopulations of EPCs have been identified. The consensus so far is that early-EPCs promote neovascularization by secreting pro-angiogenic factors rather than differentiating into endothelium (Fadini et al., 2012). In contrast, late-EPCs, here called ECFCs, have been defined in in vitro and in vivo assays to have proliferative and functional potential for neovascularization (Ingram et al., 2004). It is now recognized that ECFCs contribute to new blood vessel formation in many post-natal pathophysiological conditions (Kwon et al., 2012). For instance, circulating ECFCs are recruited into sites such as ischemic tissues for vascular regeneration, where they are incorporated into the vascular endothelial lining and differentiate in situ into endothelial cells (Murasawa and Asahara, 2005). It has also been reported that 40% of endothelial cells in tumor tissue are derived from ECFCs that originate from bone marrow (Rafii et al., 2002). Therefore, use of ECFCs in in vitro angiogenesis assay systems can provide valuable insights into the pro- and anti-angiogenic properties of drug candidates. As a source of pericytes, MSCs are multipotent stem cells that have self-renewal and differentiation capacity and that participate in angiogenesis. ECFCs and MSCs were found to have a synergistic effect on new vessel formation (Melero-Martin et al., 2008) and ischemic tissue repair (Kang et al., 2013). Moreover, promising results were obtained when these cells were used in several clinical studies (Lara-Hernandez et al., 2010; Gupta et al., 2013). Based on these previous findings, we employed two vascular progenitors, ECFCs and MSCs, to generate two-cell spheroid model to closely mimic in vivo sprouting angiogenesis.
Most in vitro angiogenesis assays developed to date are based on monoculture of ECs grown in 2D cell culture dishes. However, ECs grown on artificial plastic surfaces do not have sufficiently cell-cell and cell-matrix interactions, thereby yielding less physiologically relevant information. In addition, 2D in vitro assay systems reflect part of angiogenesis such as proliferation, migration, or invasion (Gautier et al., 2011; Hada et al., 2012). Thus, in vitro assay systems that can reflect the complete cascade of angiogenesis would be more appropriate systems for screening pro- or anti-angiogenic compounds. To generate an in vivo-like 3D environment for the cells, we embedded ECFC+MSC spheroids in collagen type I gels. The extracellular matrix has been shown to play a crucial role in regulating cell behavior (Lu et al., 2012). Collagen is one of the main constituents of the matrix around ECs (Detry et al., 2011; Paupert et al., 2011).
In the present study, we found that both the number and length of sprouts from ECFC+MSC spheroids were significantly greater than those of sprouts from ECFC-only spheroids. Previous studies have shown that MSCs provide a strong angiogenic stimulus to ECFCs by secreting growth factors such as VEGF-A (Melero-Martin et al., 2008; Grellier et al., 2009). ECFCs can also function as paracrine mediators prior to the establishment of blood perfusion, modulating the regenerative potential of MSCs (Lin et al., 2014). Therefore, bi-directional angiogenic stimulation of ECFCs by MSCs in our two-cell spheroids might be sufficient for sprout formation. Angiogenic cross-stimulations between ECFCs and MSCs is supported by the observation of no significant differences in the sprouting potential of ECFC+MSC spheroids when treated with VEGF and FGF-2 or not. In contrast, ECFC spheroids required exogenous growth factors for sprout formation. Results from live cell fluorescent labeling of ECFCs and MSCs also suggest that MSCs contribute to the sprout formation process in a pericyte-like manner because they were found in the surrounding area of ECFC-mediated sprout structures. Future studies will focus on the integrated cross-talk between ECFCs and MSCs, which will provide the novel insight into the pro- and anti-angiogenic drug discovery.
We verified our newly developed two-cell spheroid assay system by performing an inhibitor study using vatalanib. Vatalanib is a potent inhibitor of VEGF receptor tyrosine kinases (Banerjee et al., 2009). We found that the IC50 value of vatalanib for inhibition of sprout length was 4.0 ± 0.40 μM for ECFC+MSC spheroids and 0.2 ± 0.03 μM for ECFC spheroids after 24 h. IC50 values of vatalanib was significantly greater in two-cell spheroid assay system compared with conventional one-cell spheroid system (Table 1). ECFC+MSC spheroids generate stable and durable sprouts which are composed of two vascular cell types. Interestingly, IC50 value from two-cell spheroid assay system is closer to the previous in vivo data. In the previous report, oral administration of vatalanib 50 mg/kg/day inhibited more than 50% of DUI45 carcinoma tumor growth, and plasma concentration of vatalanib was >1 μM 8 h after administration of 50 mg/kg (Wood et al., 2000). Further studies are necessary to prove whether ECFC+MSC spheroid assay system have considerable sensitivity to provide predictive data for in vivo studies.
To summarize, we developed an advanced 3D angiogenesis assay system by utilizing spheroids comprising two types of vascular cells, ECFCs and MSCs, to mimic physiological vascular formation. We used a real-time cell recorder to observe angiogenic sprouting over time. The results from our new assay system confirm that MSCs play an important support role in angiogenesis by activating endothelial cells and by covering the outer surface of newly-formed sprout structures. Moreover, our new assay system provided more relevant inhibitory concentration data to that obtained from in vivo studies than one-cell assay system. Therefore, the two-cell spheroid assay system described here may be a useful in vitro assay system for more accurately evaluating pro- or anti-angiogenic property.
Acknowledgments
This research was supported by a grant (17172MFDS215) from Ministry of Food and Drug Safety in 2017, the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2017R1A2B4005463), and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1A6A1A03007648).
REFERENCES
- Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat. Protoc. 2010;5:628–635. doi: 10.1038/nprot.2010.6. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Ayala-Cuellar AP, Kang JH, Jeung EB, Choi KC. Roles of mesenchymal stem cells in tissue regeneration and immunomodulation. Biomol. Ther. (Seoul) 2018 doi: 10.4062/biomolther.2017.260. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Zvelebil M, Furet P, Mueller-Vieira U, Evans DB, Dowsett M, Martin LA. The vascular endothelial growth factor receptor inhibitor PTK787/ZK222584 inhibits aromatase. Cancer Res. 2009;69:4716–4723. doi: 10.1158/0008-5472.CAN-08-4711. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen C-W, Corselli M, Park TS, andriolo G, Sun B, Zheng B, Zhang L. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Delia D, Lampugnani M, Resnati M, Dejana E, Aiello A, Fontanella E, Soligo D, Pierotti M, Greaves M. CD34 expression is regulated reciprocally with adhesion molecules in vascular endothelial cells in vitro. Blood. 1993;81:1001–1008. [PubMed] [Google Scholar]
- Detry B, Bruyère F, Erpicum C, Paupert J, Lamaye F, Maillard C, Lenoir B, Foidart J-M, Thiry M, Noël A. Digging deeper into lymphatic vessel formation in vitro and in vivo. BMC Cell Biol. 2011;12:29. doi: 10.1186/1471-2121-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF. Expression of the CD34 gene in vascular endothelial cells. Blood. 1990;75:2417–2426. [PubMed] [Google Scholar]
- Foubert P, Matrone G, Souttou B, Leré-Déan C, Barateau V, Plouët J, Le Ricousse-Roussanne S, Lévy BI, Silvestre J-S, Tobelem G. Coadministration of endothelial and smooth muscle progenitor cells enhances the efficiency of proangiogenic cell-based therapy. Circ Res. 2008;103:751–760. doi: 10.1161/CIRCRESAHA.108.175083. [DOI] [PubMed] [Google Scholar]
- Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Spheroid-based drug screen: considerations and practical approach. Nat Protoc. 2009;4:309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- Gautier B, Miteva MA, Goncalves V, Huguenot F, Coric P, Bouaziz S, Seijo B, Gaucher J-F, Broutin I, Garbay C. Targeting the proangiogenic VEGF-VEGFR protein-protein interface with drug-like compounds by in silico and in vitro screening. Chem. Biol. 2011;18:1631–1639. doi: 10.1016/j.chembiol.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Grellier M, Ferreira-Tojais N, Bourget C, Bareille R, Guillemot F, Amédée J. Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. J Cell Biol. 2009;106:390–398. doi: 10.1002/jcb.22018. [DOI] [PubMed] [Google Scholar]
- Gumkowski F, Kaminska G, Kaminski M, Morrissey LW, Auerbach R. Heterogeneity of mouse vascular endothelium. In vitro studies of lymphatic, large blood vessel and microvascular endothelial cells. Blood Vessels. 1987;24:11–23. [PubMed] [Google Scholar]
- Gupta PK, Chullikana A, Parakh R, Desai S, Das A, Gottipamula S, Krishnamurthy S, Anthony N, Pherwani A, Majumdar AS. A double blind randomized placebo controlled phase I/II study assessing the safety and efficacy of allogeneic bone marrow derived mesenchymal stem cell in critical limb ischemia. J Transl Med. 2013;11:143. doi: 10.1186/1479-5876-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hada K, Suda A, Asoh K, Tsukuda T, Hasegawa M, Sato Y, Ogawa K, Kuramoto S, Aoki Y, Shimma N. Angiogenesis inhibitors identified by cell-based high-throughput screening: Synthesis, structure-activity relationships and biological evaluation of 3-[(E)-styryl] benzamides that specifically inhibit endothelial cell proliferation. Bioorg Med Chem. 2012;20:1442–1460. doi: 10.1016/j.bmc.2011.12.058. [DOI] [PubMed] [Google Scholar]
- Hutmacher DW. Biomaterials offer cancer research the third dimension. Nat Mater. 2010;9:90–93. doi: 10.1038/nmat2619. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- Jaganathan H, Gage J, Leonard F, Srinivasan S, Souza GR, Dave B, Godin B. Three-dimensional in vitro co-culture model of breast tumor using magnetic levitation. Sci. Rep. 2014;4:6468. doi: 10.1038/srep06468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KT, Coggins M, Xiao C, Rosenzweig A, Bischoff J. Human vasculogenic cells form functional blood vessels and mitigate adverse remodeling after ischemia reperfusion injury in rats. Angiogenesis. 2013;16:773–784. doi: 10.1007/s10456-013-9354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112:3249–3258. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Lee JH, Jung SY, Kim JW, Lee SH, Lee DH, Lee KS, Lee BY, Kwon SM. Phloroglucinol inhibits the in vitro differentiation potential of CD34 positive cells into endothelial progenitor cells. Biomol. Ther (Seoul) 2012;20:158–164. doi: 10.4062/biomolther.2012.20.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Hernandez R, Lozano-Vilardell P, Blanes P, Torreguitart-Mirada N, Galmes A, Besalduch J. Safety and efficacy of therapeutic angiogenesis as a novel treatment in patients with critical limb ischemia. Ann Vasc Surg. 2010;24:287–294. doi: 10.1016/j.avsg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Lee JH, Han YS, Lee SH. Long-duration three-dimensional spheroid culture promotes angiogenic activities of adipose-derived mesenchymal stem cells. Biomol. Ther (Seoul) 2016;24:260–267. doi: 10.4062/biomolther.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RZ, Moreno-Luna R, Li D, Jaminet SC, Greene AK, Melero-Martin JM. Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci USA. 2014;111:10137–10142. doi: 10.1073/pnas.1405388111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero-Martin JM, De Obaldia ME, Kang S-Y, Khan ZA, Yuan L, Oettgen P, Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood–derived progenitor cells. Circ. Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- Murasawa S, Asahara T. Endothelial progenitor cells for vasculogenesis. Physiology (Bethesda) 2005;20:36–42. doi: 10.1152/physiol.00033.2004. [DOI] [PubMed] [Google Scholar]
- Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839–845. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- Paupert J, Sounni NE, Noël A. Lymphangiogenesis in post-natal tissue remodeling: lymphatic endothelial cell connection with its environment. Mol Aspects Med. 2011;32:146–158. doi: 10.1016/j.mam.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat. Rev Cancer. 2002;2:826–835. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- Reinisch A, Hofmann NA, Obenauf AC, Kashofer K, Rohde E, Schallmoser K, Flicker K, Lanzer G, Linkesch W, Speicher MR. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood. 2009;113:6716–6725. doi: 10.1182/blood-2008-09-181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Nogues C, O’Brien T. In vitro models for assessing therapeutic angiogenesis. Drug Discov Today. 2016;21:1495–1503. doi: 10.1016/j.drudis.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18:696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- Wood JM, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O’Reilly T. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60:2178–2189. [PubMed] [Google Scholar]