Abstract
While obesity is associated with a variety of complications including diabetes, hypertension, cardiovascular disease and premature death, observational studies have also found that obesity and increasing body mass index (BMI) can be linked with improved survival in certain patient populations, including those with conditions marked by protein-energy wasting and dysmetabolism that ultimately lead to cachexia. The latter observations have been reported in various clinical settings including end-stage renal disease (ESRD) and have been described as the “obesity paradox” or “reverse epidemiology”, engendering controversy. While some have attributed the obesity paradox to residual confounding in an effort to “debunk” these observations, recent experimental discoveries provide biologically plausible mechanisms in which higher BMI can be linked to longevity in certain groups of patients. In addition, sophisticated epidemiologic methods that extensively adjusted for confounding have found that the obesity paradox remains robust in ESRD. Furthermore, novel hypotheses suggest that weight loss and cachexia can be linked to adverse outcomes including cardiomyopathy, arrhythmias, sudden death and poor outcomes. Therefore, the survival benefit observed in obese ESRD patients can at least partly be derived from mechanisms that protect against inefficient energy utilization, cachexia and protein-energy wasting. Given that in ESRD patients, treatment of traditional risk factors has failed to alter outcomes, detailed translational studies of the obesity paradox may help identify innovative pathways that can be targeted to improve survival. We have reviewed recent clinical evidence detailing the association of BMI with outcomes in patients with chronic kidney disease, including ESRD, and discuss potential mechanisms underlying the obesity paradox with potential for clinical applicability.
Keywords: Obesity, mortality, body mass index, cachexia, obesity paradox, end stage renal disease, chronic kidney disease
INTRODUCTION
Obesity, as defined by a body mass index (BMI) ≥30 kg/m2 (1), is a growing worldwide epidemic (2), which is associated with serious sequelae including higher risk of cardiovascular disease (CVD) and mortality (3-5). However, despite this markedly higher risk of adverse outcomes in the general population, elevated BMI may also be associated with improved survival in certain patient populations. This so-called “obesity paradox” has been observed in a variety of clinical settings including in patients suffering from obstructive pulmonary disease (6-8), chronic heart failure (HF) (9), acquired immunodeficiency disease syndrome (10, 11), advanced chronic kidney disease (CKD) and end-stage renal disease (ESRD) (12-39). Description of this phenomenon in patients with ESRD is particularly noteworthy given the disproportionately elevated risk of CVD and all-cause mortality in this patient population (5, 40-42). While treatment of traditional risk factors for CVD and mortality, such as hyperlipidemia, can be associated with improved outcomes in the general population, clinical trials targeting these pathways have failed to show a survival benefit in patients with ESRD. For instance, three large randomized clinical trials targeting serum low density lipoprotein (LDL) cholesterol levels have shown that treatment with 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors, i.e. statins, which have proven effective in other patient populations, does not improve survival in patients on hemodialysis (43-45). Therefore, it has been postulated that nontraditional risk factors, such as protein energy wasting (PEW) and cachexia (46-48), uremic toxins (49), inflammation (48, 50-54) and oxidative stress (55, 56) may play a more significant role in CVD-related deaths in CKD and ESRD patients than traditional markers of risk. Moreover, the latter pathways are interrelated and the pathogenesis of one begets development and progression of others thereby creating a vicious cycle, ultimately leading to adverse outcomes. Accordingly, understanding and addressing the pathogenic role of these pathways in the setting of ESRD may be more effective in improving CVD and overall outcomes rather than strategies which focus on more traditional risk factors commonly targeted in other patient populations. Therefore, while the scientific exercise of scrutinizing all findings including the obesity paradox is of importance, it is also critical that we remain open to the possibility that the paradoxical associations observed between BMI and mortality in ESRD may also have biologic underlying mechanisms that need to be determined. Furthermore, investigating and deciphering these mechanisms can provide important insights, which can be utilized to create novel therapies for risk factors such as cachexia and wasting. In the following sections, we will first describe the findings of prior studies which have assessed the association of BMI and body size with mortality in CKD and ESRD patients treated with hemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation (KT). Subsequently, we will review some of the potential mechanisms, which may account for the paradoxical associations reported in these studies.
MEASUREMENTS OF BODY SIZE AND BODY COMPOSITION
Most definitions of obesity rely on BMI; however, BMI does not reliably account for body composition. The use of BMI to define obesity can have major clinical implications since individuals with the same height and weight, and thus same BMI, can have significant differences in their composition and distribution of their body fat and muscle (57, 58). Accurate measurement of body composition in patients with CKD and ESRD is relevant given frequent muscle wasting (59) and changes in the type and distribution of adiposity (60) observed in this population. Hence, more detailed methods of evaluating body mass and composition have been developed and demonstrated as more accurate predictors of outcome risk. For instance, a systematic review and meta-analysis reported that waist-to-height ratio and waist circumference (WC) seem to be more precise estimates of cardio-metabolic risk as compared to BMI (61). Bioelectrical impedance analysis/spectroscopy (BIA/S) (59) is another commonly cited method used to estimate body composition in CKD, although its accuracy may be altered given the fluid retention associated with renal disease (62). Estimates of lean body mass (LBM) could also be obtained using mid-arm muscle circumference measurements (63) or serum creatinine-based formulas which utilize creatinine as a marker of muscle mass (64). Body fat percentage can be estimated using near-infrared interactance (NIR) (65, 66) and this may be a practical and accessible tool for objective assessment of body fat content in HD patients. Furthermore, the amount of the adipose tissue can also be measured by dual energy x-ray absorptiometry (DEXA) (67-69), which is considered a more accurate and reliable reference method (70, 71). However, the most reliable methods for obtaining detailed body composition data might be computed tomography (CT) and magnetic resonance imaging (MRI) (72-74) given that they allow for the assessment of total body adipose tissue distribution and also distinguish between tissue edema, visceral and subcutaneous adipose tissue. The importance of body composition and its relevance to outcomes has been demonstrated in a number of studies, which have utilized the mentioned techniques in patients with CKD and ESRD. A study by Postorino et al. (75) examined a prospective cohort of 537 ESRD patients using WC as a surrogate of intra-abdominal/visceral fat and reported that each 10-cm increase in WC was associated with a 23% higher risk for all-cause and 37% higher risk for CVD mortality, while BMI was an inverse predictor of these outcomes. The effect of fat and LBM on survival of maintenance HD patients was also evaluated by Noori et al. (76) who concluded that higher fat mass in male and female patients and higher LBM only in female patients appeared to be protective. Therefore, in discussing the association of BMI and outcomes in CKD and ESRD patients, it is essential to keep in mind notable limitations of these measures as a marker of obesity and body composition. Future studies will need to focus on delineating the impact of body composition on outcomes in patients with CKD and ESRD.
OBESITY AND MORTALITY IN CKD
While obesity and excess body fat are associated with a higher risk for development and progression of de novo CKD (77-79), a potential protective effect of higher BMI in terms of improved survival in CKD remains controversial (Table 1). Madero et al. (80) analyzed data of 1,759 CKD patients with a mean estimated glomerular filtration rate (eGFR) ± standard deviation (SD) 39±21 mL/min/1.73m2 and found no difference in all-cause mortality risk between higher BMI groups (BMI 25->40 kg/m2) compared to those with normal weight (BMI 18.5-24.9 kg/m2). Likewise, Dalrymple et al. (81) found that in 1,268 patients with eGFR <60 ml/min/1.73m2, the BMI-mortality association was not significant for higher BMI groups. In contrast, lower BMI was associated with higher mortality when compared to the reference BMI of 18.5-24.9 kg/m2 (Hazard Ratio [HR] 2.37, 95% confidence interval [95% CI] 1.37-4.10). In another study of individuals with eGFR<60 ml/min/1.73m2 or presence of microalbuminuria, a BMI of 18.5-<22 kg/m2 was associated with a higher risk of death (HR 1.30, 95% CI 1.03-1.64), while higher BMI was not significantly associated with mortality when compared to the reference group (BMI 22-<25 kg/m2) (82). In a cohort of CKD and non-CKD patients undergoing a surgical procedure, obese (BMI ≥35 kg/m2) patients with CKD (eGFR <60 mL/min/1.73 m2) had higher odds of 30-day mortality than non-obese non-CKD patients in unadjusted and fully adjusted models (Odds Ratio (OR) 5.51, 95% CI 4.48-6.79 and OR 1.49 95% CI 1.18-1.87, respectively) (83). Obermayr et al. (84) analyzed data from the Vienna Health Screening Initiative study and Austrian Death Registry that included patients with stage I-III CKD. Although the results were not statistically significant, the study observed an inverse trend between BMI and mortality risk in patients with moderate CKD (eGFR 45 ml/min/1.73m2). However, examining CVD risk as the primary outcome and BMI of 25 kg/m2 as the reference, the authors reported that the risk of CVD death was higher in participants with both low (BMI level 20 kg/m2, HR 1.35, 95% CI 0.82-2.20), and high BMI levels [BMI 30 kg/m2 (HR 1.37, 95% CI 1.07-1.75) and BMI 35 kg/m2 (HR 2.05, 95% CI 1.19-3.55)]. While most studies use all-cause mortality as the primary outcome, Navaneethan et al. (85) examined more refined causes of death (malignancy, non-CVD/non-malignancy-related death) to investigate the BMI-mortality association in 54,506 patients, the majority being in CKD stage III. They reported that CKD patients with BMI 25-39.9 kg/m2 had lower risk for all three death outcomes compared to BMI of 18.5-24.9 kg/m2.
Table 1.
Summary of studies with large sample size (>1,000 individuals) evaluating the association between BMI and mortality outcomes in CKD patients
| Study | Patients (n) | F/U* (y) | Results |
|---|---|---|---|
| Madero et al, 2007 (80) | 1,759 | 10 | No significant difference in all-cause mortality risk between higher BMI groups (BMI 25->40) compared to normal weight group (BMI 18.5-24.9 kg/m2) |
| Weiner et al, 2008 (86) | 1,678 | 9 | No significant association between increase in BMI (per 5 kg/m2) and all-cause mortality |
| Elsayed et al, 2008 (92) | 13,324 | up to 9.3 | Each SD increase in BMI reduced the risk of the composite outcome (HR 0.94, 95% CI 0.90-0.99) |
| Obermayr et al, 2009 (84) | 49,398 (392 Moderate CKD) | 5.5 | Compared to the BMI of 25 kg/m2, a higher risk of cardiovascular death in participants with BMI 20 kg/m2 (HR, 1.35), BMI 30 kg/m2 (HR, 1.37) and BMI 35 kg/m2 (HR, 2.05) |
| Kramer et al, 2011 (91) | 5,805 | 4 | Every 1-kg/m2 increase in BMI was related to a 3% reduction in mortality risk (95% CI, 0.94-0.99) and each 1-cm increase in waist circumference was associated with a 2% higher mortality risk (95% CI, 1.01-1.04) |
| Dalrymple et al, 2011 (81) | 1,268 | 9.7 | BMI <18.5 kg/m2 was associated with higher mortality, but higher BMI groups had similar mortality risk of the reference group (18.5-24.9 kg/m2) |
| De Nicola et al, 2012 (87) | 1,248 | 5.2 | No significant association between BMI (continuous parameter) and mortality |
| Bello et al, 2013 (83) | 393,659 (54,403 CKD patients) | up to 30 days | Higher odds for 30-day mortality following an eligible procedure in obese CKD (BMI ≥35 kg/m2 and eGFR <60 mL/min/1.73 m2) when compared to non-obese non-CKD patients in unadjusted and adjusted models (OR 5.51, 95% CI 4.48-6.79 and OR 1.49, 95%CI 1.18-1.87, respectively) |
| Babayev et al, 2013 (88) | 12,534 | up to 8 | BMI between 30-34.9 kg/m2 was associated with improved survival (HR, 0.74) when compared to CKD patients with BMI <30 kg/m2 |
| Ricardo et al, 2013 (82) | 2,288 | 13 | Participants with BMI 18.5-<22 kg/m2 had a higher mortality hazard rate (HR, 1.3). In contrast higher BMI was not associated with a significant different mortality risk when compared to BMI 22-<25 kg/m2 |
| Hanks et al, 2013 (95) | 4,374 | 4.5 | Metabolic healthy overweight (BMI 25-29.9 kg/m2) CKD patients had statistical significant lower all-cause mortality (HR, 0.74) in the fully adjusted model compared to metabolic healthy normal weight (BMI 18.5-24.9 kg/m2) |
| Lu et al, 2014 (89) | 453,946 | - | BMI had an U-shaped association (higher mortality in the lower and higher BMI groups) with all-cause mortality (reference group 30-<35 kg/m2) |
| Huang et al, 2015 (90) | 3,320 | 2.9 | Male participants with BMI <22.5 kg/m2 and BMI of 30.1-35 kg/m2 had higher risk of all-cause mortality (reference BMI 27.6-30 kg/m2) but not in females |
| Navaneetha n et al, 2016 (85) | 54,506 | 3.7 | CKD patients (mostly CKD stage III) with 25<BMI<39.9 kg/m2 had lower risk of cardiovascular, malignancy, non-cardiovascular/non-malignancy related death compared to the BMI of 18.5-24.9 kg/m2 |
| Sato et al, 2017 (93) | 27,978 | up to 4 | Increase of 1 SD in a body shape index associated with a higher risk for all-cause mortality in male CKD patients but not in females |
Duration of follow-up as reported by the authors. If not indicated mean or median reported.
Among individuals with more advanced kidney disease (CKD stage III-IV [eGFR 15-60 ml/min/1.73m2]), several reports found no significant association between higher BMI levels (per 5 kg/m2) and all-cause mortality (86, 87). However, Babayev et al. (88) studied a cohort of 12,534 African American and Caucasian patients with CKD stage III-IV and reported that a BMI 30-34.9 kg/m2 was associated with better survival (HR 0.74, 95% CI 0.62-0.87) compared to patients with BMI <30 kg/m2. This effect was attenuated for CKD patients with a BMI >35 kg/m2 (HR 1.08, 95% CI 0.89-1.30). In addition, a study using a cohort of patients with CKD stage III-IV in the United States (US) Veterans Administration (VA) system, found a U-shaped association between BMI and all-cause mortality where low and high BMIs were both associated with worse outcomes (89). However, subsequent studies found that the latter findings may also be explained by gender differences given that the VA cohort was mostly comprised of male patients. Furthermore, a study from Taiwan found that in male patients with CKD, lower and higher BMI were associated with a higher risk of all-cause mortality, while in female patients, no association was observed (reference: BMI 27.6-30 kg/m2) (90). These studies highlight the potential role of gender as a modifying factor in the association of BMI with outcomes.
As mentioned earlier, another modifier of the association of obesity with outcomes is body composition and shape. In a US cohort consisting of 5,805 CKD patients (50.3% with CKD stage I-II and 49.7% with CKD stage III-IV), BMI ≥40 kg/m2 was associated with higher mortality risk after adjustment for age, sex and race (BMI reference group 25-29.9 kg/m2). However, after accounting for WC, higher BMI categories trended towards lower mortality (91). Another study evaluating 13,324 CKD patients found that each SD increase in BMI was associated with a lower risk of the composite outcome of incident CKD and mortality (HR 0.94, 95% CI 0.90-0.99). However, when waist-to-hip ratio was used as a measurement of body mass, increasing body size was associated with higher risk of the composite outcome of incident CKD and mortality (HR 1.12, 95% CI 1.06-1.18) (92). Likewise, a study (93) using a body shape index formula (94), which accounted for height, weight and BMI, reported that an increase of 1 SD in body shape index was associated with a higher all-cause mortality risk (HR 1.16, 95%CI 1.01-1.34) in male CKD patients, whereas in female CKD patients, body shape index showed no significant association with all-cause mortality (93).
Another consideration was the role of metabolic health in obese and non-obese patients. Given that metabolic abnormalities might alter the association of the BMI with outcomes in CKD patients, in another study investigators grouped patients into metabolic healthy and unhealthy categories and stratified them by BMI (reference group: metabolic healthy normal weight study participants). While no statistically significant differences were observed in mortality risk among metabolically unhealthy overweight (BMI 25-29.9 kg/m2) or obese (BMI ≥ 30 kg/m2) CKD patients, metabolically healthy overweight CKD patients had a lower risk of all-cause mortality (HR 0.74, 95% CI 0.57-0.96) in the fully adjusted model (95).
OBESITY AND MORTALITY IN ESRD TREATED WITH HD
One of the first studies which provided evidence of an altered BMI-mortality association in HD patients was published in 1982 by Degoulet et al. (12) who found that higher BMI was not associated with higher mortality (Table 2). Subsequently, Leavey et al. (13) also found no evidence of higher mortality risk among HD patients with higher BMI values using data from the US Renal Data System (USRDS). In addition, both investigators found that lower BMI was in fact associated with a higher risk of mortality (12, 13). Many subsequent studies have since observed similar findings. Fleischmann et al. (14) found that BMI >27.5 kg/m2 was associated with improved survival in HD patients when compared to a reference BMI of 20-27.5 kg/m2. In their study, each one-unit increase in BMI >27.5 kg/m2 was associated with a 30% decrease in the relative risk (RR) of mortality, while with each unit decrease in BMI below 20 kg/m2, the RR of death was 1.6-fold higher. Likewise, using the body weight-to-height relationship as an alternative metric for body size measurement, Kopple et al. (15) found a progressive decrease in the risk of mortality with increasing body size in HD-treated ESRD patients. In addition, higher body weight or body volume was also associated with decreased risk of mortality (16). The association of higher BMI with lower mortality was also reported by Pifer et al. (19) in a study of US patients in the international Dialysis Outcomes and Practice Patterns Study (DOPPS). A higher risk of mortality was observed in patients with lower BMI, while a high BMI was associated with improved survival in HD patients. Similarly, in another cohort including patients from the US, it was found that BMI ≥30 kg/m2 was associated with a higher survival rate in HD patients (HR 0.89, 95% CI 0.81-0.99) (96). Furthermore, Stack et al. (97) found that in a cohort of 117,309 HD patients, the adjusted RR of death was greatest for those with BMI ≤20.9 kg/m2 (RR 1.40, 95% CI 1.32-1.50 for diabetics and RR 1.27, 95% CI 1.21-1.34 for non-diabetics) and lowest for patients with BMI >30.0 kg/m2 (RR 0.97, 95% CI 0.96-0.99 for diabetic and RR 0.97, 95% CI 0.95-0.98 for non-diabetic patients) compared with the reference group (BMI 23.5-26.1 kg/m2).
Table 2.
Summary of studies with large sample size (>1,000 individuals) evaluating the association between BMI and mortality outcomes in HD patients
| Study | Patients (n) | F/U* (y) | Results |
|---|---|---|---|
| Degoulet et al, 1982 (12) | 1,453 | 2,063 person-years | A high BMI was not found to be associated with significantly higher all-cause and CV mortality |
| Leavey et al, 1998 (13) | 3,607 | up to 5 | Lower BMI was associated with higher mortality risk as an independent risk factor; whereas higher BMI was not associated with higher mortality risk |
| Fleischmann et al, 1999 (14) | 1,346 | up to 1 | With every one unit increase in BMI over 27.5 kg/m2 the relative risk of mortality was decreased by 30% , and with every one unit of BMI lower than 20 kg/m2, the relative risk was higher by 1.6-fold |
| Kopple et al, 1999 (15) | 12,965 | up to 1 | A progressive decrease in mortality with an increase in weight to height ratio |
| Wolfe et al, 2000 (16) | 9,165 | up to 2 | Larger body size was associated with lower mortality risk |
| Leavey et al. 2001 (17) | 9,714 | up to 4 | Increasing body size associated with a decreased mortality risk even in the healthier subgroups with mild to moderate obesity |
| Lowrie et al. 2002 (18) | 43,334 | - | There was a reversed J-shape association between mortality and weight to height ratio and BMI. |
| Pifer et al, 2002 (19) | 7,719 | up to 0.5 | A significantly higher risk of mortality was observed in patients with lower BMI and a high BMI seemed to have protective effect |
| Port et al, 2002 (20) | 45,967 | up to 2 | Mortality risk was 42% higher in group with the lowest BMI than the highest tertile. Higher dialysis dose; above the dialysis outcomes quality initiative guidelines (Urea Reduction Ratio >65%), was associated with lower mortality in all body-size groups |
| Beddhu et al, 2003 (21) | 70,028 | 105,042 person-years | Protective effect of high BMI was limited to those patients with normal or high muscle mass and high BMI patients with inferred high body fat had higher and not lower mortality |
| Glanton et al, 2003 (22) | 151,027 | 1.7 | Obesity was independently associated with reduced risk of mortality, particularly in African Americans |
| Johansen et al, 2004 (23) | 418,055 | 2 | High BMI was associated with greater survival even at extremely high BMI after adjustment for demographic, laboratory, and comorbidity data in all ethnic groups except Asian Americans |
| Abbott et al, 2004 (96) | 1,675 | up to 5.8 | 5-year survival for patients with BMI ≥ 30 kg/m2 was 39.8% vs. 32.3% for lower BMI |
| Stack et al, 2004 (97) | 117,309 | 1 | The adjusted RR of death was greatest for patients with BMI ≤ 20.9 kg/m2and lowest for patients with BMI >30.0 kg/m2 in HD patients compared with the reference (BMI 23.5-26.1 kg/m2) |
| Kalantar-Zadeh et al, 2005 (25) | 54,535 | up to 2 | Obesity, including morbid obesity, and progressive weight gain were associated with improved survival even after adjusting for variation in BMI and laboratory values over time |
| Johansen et al, 2006 [156] | 2,467 | - | Although higher BMI was associated with enhanced survival, physical function impairment by obesity adversely affected health status |
| Chazot et al, 2009 (26) | 5,592 | 2 | Confirmed existence of obesity paradox and showed reduced survival in those who were in the lower quintile of body weight variation |
| Kalantar-Zadeh et al, 2010 (27) | 121,762 | up to 5 | Higher BMI (up to 45 kg/m2) and higher serum creatinine concentration were progressively and independently associated with improved survival and muscle gain with weight loss over time may be associated with better survival in contrast to weight gain while losing muscle |
| Yen, et al, 2010 (28) | 959 | up to 3 | A higher mortality risk in HD patients who were underweight |
| Hall et al, 2011 (29) | 21,492 | up to 13 | Asians, Pacific Islanders and non-Hispanic Whites showed improved survival with higher BMI and HD patients waiting for kidney transplantation have better survival due to less severe co-morbidities |
| Molnar et al, 2011 (30) | 14,632 | 2.5 | Better survival in higher (>25 kg/m2) than lower (<22 kg/m2) BMI in transplant-waitlisted hemodialysis patients and higher risk of mortality in those with unintentional weight or muscle loss |
| Ricks et al, 2011 (31) | 109,605 | up to 6 | Higher BMI was associated with higher survival in all three racial/ethnic groups – non-Hispanic white, Hispanic, and African American. Hispanic and African American patients experienced greater survival than non-Hispanic whites across higher BMI categories and African American HD patients showing the largest consistent decrease in death HR with increasing BMI |
| Kalantar-Zadeh et al, 2012 (33) | 121,762 | up to 5 | A considerable proportion of the obesity paradox in dialysis patients might be explained by the amount of decline in muscle mass |
| Hoogeveen et al, 2012 (32) | 1,749 (1,087 HD) | up to 7 | Age-standardized mortality rate was higher in obese younger patients than those with normal BMI (OR 1.7, 95% CI 1.1-2.9) |
| Park et al 2013 (34) | 40,818 | 5.9 | Race (Asian, white, African American) does not affect the protective effect of larger body size and muscle mass in HD patients |
| Cabezas-Rodriguez et al, 2013 (99) | 6,797 | up to 3 | Weight loss or gain (<1% or >1% of body weight) was strongly associated with higher rates of mortality or survival, respectively. In obese patients, the association between weight loss and mortality was attenuated, no survival benefit of gaining weight was seen |
| Vashistha et al, 2014 (36) | 123,383 | up to 8 | Higher BMI was associated with lower death risk across all age groups and was more pronounced in those younger than 65 years |
| Calabia et al, 2015 (37) | 6,290 | up to 8 | No survival benefit for higher BMI values in young patients |
| Doshi et al, 2016 (38) | 123,624 | up to 6 | A BMI of <18 kg/m2 was associated with a 3.2-fold higher death risk; mortality risks declined with increasing BMI with the greatest survival advantage of 31% lower risk at a BMI of 40 to <45 kg/m2 |
Duration of follow-up as reported by the authors. If not indicated mean or median reported.
Finally, Doshi et al. (38) examined the obesity paradox with the application of causal modeling with a marginal structural model (MSM), which investigated the BMI-mortality relationship while controlling for time-varying confounders and informative censoring which may be influenced by prior BMI levels. The study assessed the association between BMI and all-cause mortality among 123,624 hemodialysis patients receiving treatment between 2001 and 2006. Compared with the reference (BMI 25 to <27.5 kg/m2), a BMI of <18 kg/m2 was associated with a 3.2-fold higher death risk (HR 3.17, 95% CI 3.05–3.29) and mortality risks declined with increasing BMI with the greatest survival advantage of a 31% lower risk (HR 0.69, 95% CI 0.64–0.75) observed for a BMI of 40 to <45 kg/m2. For this review, using the same cohort, we herein show the BMI-mortality relationship using the World Health Organization categorizations of obesity (1), which similarly show an inverse linear pattern across all analytical models (Figure 1). Hence, the linear inverse relationship between BMI and mortality was considered as robust across multiple models including MSM analyses that more completely account for time-varying confounders and other statistical sources of biases.
Figure 1.
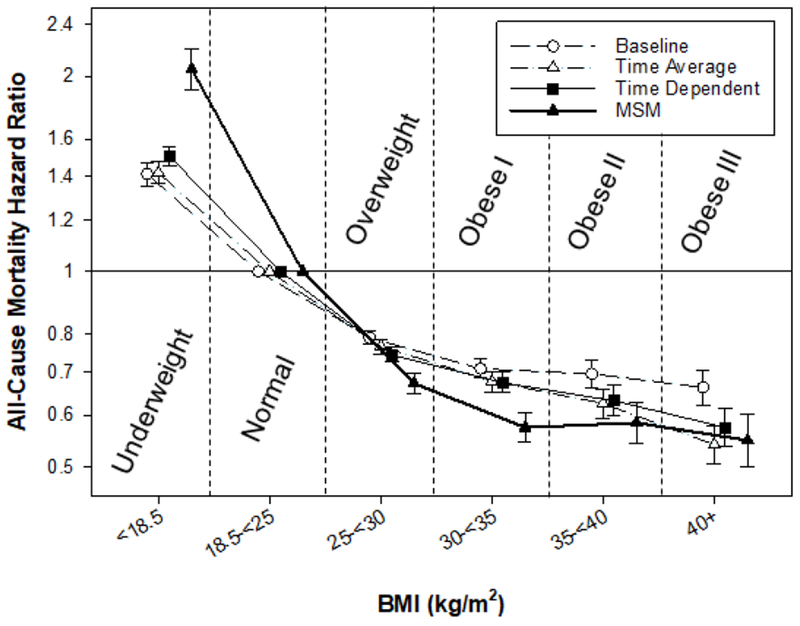
Associations of body mass index categories with all-cause mortality from baseline, time-average, time-varying and marginal structural models in a cohort of 123,624 hemodialysis patients. Models adjusted for case-mix covariates and markers of malnutrition and inflammation.
The findings reported in US patients have been also been replicated globally. Leavey et al. (17) analyzed data from an international cohort including 9,714 HD patients from the US and Europe and again found a decrease in the relative mortality risk with increasing BMI. When using BMI of 23-24.9 kg/m2 as the reference group, the RR of mortality was 0.84 in overweight (BMI 25-29.9 kg/m2), 0.73 in mildly obese (BMI 30-34.9 kg/m2), and 0.76 in moderately obese (BMI 35-39.9 kg/m2) HD patients, while a BMI <20 kg/m2 was associated with the highest risk of mortality. In another cohort of HD patients from Southern Europe, which was distinguished by a lower prevalence of diabetes mellitus, Chazot et al. (26) found a lower mortality risk for overweight and obese HD patients when compared to those with a normal BMI.
Additionally, the impact of race, ethnicity and place of origin on the association of obesity with outcomes has also been extensively studied in HD patients. In a study of 151,027 patients receiving either HD and PD using data from the USRDS, Glanton et al. (22) found that while obesity was independently associated with a reduced risk of mortality in the overall patient population (HR 0.75, 95% CI 0.72-0.78), this relationship was more pronounced in African Americans. Furthermore, Ricks et al. (31) examined a cohort of 109,605 maintenance HD patients that comprised of 39,090 African Americans, 17,417 Hispanics, and 53,098 non-Hispanic whites. Although higher BMI was associated with a survival benefit in Hispanic and African American patients when compared to non-Hispanic whites, African American patients showed the largest decrease in mortality risk with increasing BMI. Similarly, Wang et al. (98) sought to determine differences among racial/ethnic groups using LBM as predictor of mortality. Higher LBM was not only associated with lower mortality risk, but this association was also particularly evident among non-Hispanic white and African American HD patients. Hispanic HD patients, however, did not benefit from higher LBM, showing a U-shaped association with higher mortality in lower and higher LBM categories. Meanwhile, the association of obesity with survival in patients of Asian ancestry has been less consistent and less studied. Johansen et al. (23) found that high BMI was associated with higher survival in whites, African Americans, and Hispanics but not in Asians even after adjustment for LBM and estimates for adiposity using the Benn index. However, Park et al. (34) matched 20,818 HD patients from South Korea to 20,000 patients from the US (10,000 non-Hispanic whites and 10,000 African Americans) and found a consistent association between higher baseline BMI and lower mortality across all three racial groups. Finally, Hall et al. (29) reported that in a cohort of 21,492 Asian, Pacific Islander and non-Hispanic white dialysis patients, higher BMI was associated with better survival across almost all races and ethnicities.
A number of studies have attempted to evaluate the interaction between obesity, age and long-term survival in HD patients, but have reported conflicting results (32, 36, 37). Hoogeveen et al. (32) prospectively followed patients from a European cohort of HD patients and examined the association of age (<65 or ≥65 years) and baseline BMI (<20, 20-24 [reference], 25-29, and ≥30 kg/m2) with mortality. They found that the age-standardized mortality rate was higher in younger obese patients than those with normal BMI (OR 1.70, 95% CI 1.10-2.90). Meanwhile, Calabia et al. (37) did not observe a survival benefit for higher BMI in young patients in a cohort of 6,290 adult incident HD patients. Finally, Vashistha et al. (36) analyzed the data of 123,383 maintenance HD patients in a US cohort and found that while higher BMI was associated with lower death risk across all age groups, the degree of this association was more pronounced in those younger than 65 years old.
There also have been studies conducted to account for the limitations of BMI as a surrogate for body size by using alternative metrics. This was done by using parameters of muscle mass as demonstrated by Beddhu et al. (21) who examined mortality risk in 70,028 HD patients using creatinine clearance as a proxy for muscle mass. They found that the association of elevated BMI with improved survival was limited to patients with normal or high serum creatinine (suggesting normal or higher muscle mass); and high BMI patients who have lower creatinine levels (suggesting lower muscle mass) had a higher mortality risk. Kalantar-Zadeh et al. (27) also examined 121,762 HD patients and found that higher BMI (up to 45 kg/m2) and higher serum creatinine concentrations were progressively and independently associated with better survival, even after multivariate adjustment for surrogates of nutritional status and inflammation. They also found evidence suggesting that muscle gain with loss of total body weight over time may be associated with better survival in contrast to weight gain while losing muscle mass. Using the same cohort in a separate study, Kalantar-Zadeh et al. (33) applied composite ranking scores to further clarify the BMI-mortality association and reported that a decrease in muscle mass (using serum creatinine as a surrogate) but an increase in weight (measured as BMI) was associated with higher mortality. The impact of weight loss, which is one of the key indicators of cachexia, has also been evaluated in patients on maintenance HD. Molnar et al. (30) found that unintentional weight loss was associated with a higher risk of mortality in a relatively healthy subset of HD patients who were waitlisted for a renal transplantation. These findings are also supported by another study by Kalantar-Zadeh et al. (25), who found that progressive weight loss over time was associated with higher CVD and all-cause mortality risk in HD patients. Cabezas-Rodriguez et al. (99) also sought to determine the effects of weight gain and loss (>1% or <1% of body weight) in HD patients and found that while weight gain was strongly associated with higher rates of survival, weight loss had the opposite effect. Interestingly, after stratification by BMI, this association was not observed in obese patients indicating a potential resistance to the development of cachexia in obese HD patients. Given that cachexia has been shown to be associated with a higher risk of mortality in numerous chronic conditions, it is possible that the association of improved survival with obesity may be at least partly related to mechanisms which prevent the development of wasting and its adverse effects in these patients.
OBESITY AND MORTALITY IN ESRD TREATED WITH PD
While it has been debated that obesity is a relative contraindication for initiation of PD therapy (100-103), the effect of obesity on survival rates among PD patients also remains controversial (104) (Table 3). Comparison between the different studies done so far in this area has proven difficult given the heterogeneous methodology and BMI categories used in these studies. There are studies which found that obesity was associated with worse outcomes in patients undergoing PD. McDonald et al. (105) analyzed data from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry that included 9,679 patients with ESRD who underwent PD treatment. In multivariate analyses, they found that BMI ≥30 kg/m2 was associated with higher mortality (HR 1.36, 95% CI 1.14-1.54) and higher risk of technique failure (HR 1.17, 95% CI 1.07-1.26) in most patients, except in those of Maori/Pacific Islander origin. Furthermore, a J-shaped association was reported between BMI and mortality where BMI values close to 20 kg/m2 were associated with the lowest risk of death. In addition, a study investigating 1,263 Chinese PD patients found that obesity was associated with a higher risk of CVD and all-cause mortality, in the unadjusted model (referent BMI, 18.5-22.9 kg/m2) (106). However, in the multivariate model, this association only persisted for CVD, but not all-cause mortality.
Table 3.
Summary of studies with large sample size (>500 individuals) evaluating the association between BMI and mortality outcomes in PD patients
| Study | Patients (n) | F/U* (y) | Results |
|---|---|---|---|
| McDonald et al, 2003 (105) | 9,679 | 17,973 person-years | BMI≥30 kg/m2 was associated with higher mortality and technique failure; there was a J-shaped association between BMI and mortality with lowest mortality risk for patients with BMI close to 20 kg/m2 |
| Snyder et al, 2003 (111) | 41,197 | up to 3 | Overweight and obese participants had a survival benefit compared to those with lower BMI |
| Abbott et al, 2004 (96) | 1,662 | up to 5.8 | BMI ≥30 kg/m2 was not related to improved 5-year survival |
| Stack et al, 2004 (97) | 17,419 | 1 | No survival advantage with higher BMI values |
| Ramkumar et al, 2005 (114) | 10,140 | 17,500 patient-years | Patients with a BMI≥ 25 kg/m2 and high muscle mass, had a 10% lower hazard ratio of all-cause mortality |
| Pliakogiannis et al, 2007 (108) | 4,054 | 4.3 | BMI >30 kg/m2 had neither higher nor lower mortality risk than the reference group (BMI 19-24.9 kg/m2) |
| de Mutsert et al, 2009 (109) | 688 | up to 5 | BMI≥30 kg/m2 did not have statistically significant difference in mortality risk than normal BMI (18.5-25 kg/m2) |
| Mehrotra et al, 2009 (112) | 66,381 | up to 10 | Higher BMI quartiles were related to lower mortality but higher technique failure (reference BMI<21.88 kg/m2) |
| Fernandes et al, 2013 (113) | 1,911 | up to 2.8 | BMI <18.5 kg/m2 had a higher death risk while a BMI >30 kg/m2 was associated with a survival benefit, also weight reduction in the first year of dialysis of <−3.1% was associated with significant higher mortality |
| Park et al, 2013 (117) | 10,896 | 5.9 | Patients with serum creatinine levels≥ 10 mg/dL had lower mortality risk compared to serum creatinine levels of 8-9.9 mg/dL, accordingly higher muscle mass might be associated with better survival |
| Kim et al, 2014 (107) | 900 | 2 | Increased BMI was not associated with higher mortality |
| Badve et al, 2014 (35) | 6,162 | 2.3 | Survival advantage was seen only for patients with time-varying BMI between 28.1-31 kg/m2 (reference group time-varying BMI 25.1-28 kg/m2) |
| Xiong et al, 2015 (106) | 1,263 | 2.1 | Obesity was associated with a higher cardiovascular mortality risk in multivariate analysis in Chinese patients and BMI decline >0.8% during the first year after PD initiation had higher cardiovascular and all-cause mortality hazard ratios (reference group Δ −0.8% to 2.69%) |
| Obi et al, 2017 (110) | 15,573 | up to 2 | U-shaped association between BMI and mortality with best survival in 30-<35 kg/m2 strata |
Duration of follow-up as reported by the authors. If not indicated mean or median reported.
There are also studies which found that lower BMI was associated with worse outcomes, but observed no association between mortality and obesity in patients being treated with PD. A study of 1,662 PD patients from the USRDS Dialysis Morbidity and Mortality Wave II Study (DMMS) found that BMI ≥30 kg/m2 was not associated with a change in survival (96). Furthermore, a cohort study of 900 prevalent PD patients from Korea showed that higher BMI was not associated with increased mortality (107). Meanwhile, in another investigation in patients on PD, Stack et al. (97) found that the RR of death for participants with BMI <20.9 kg/m2 was higher (referent BMI 23.5-26.1 kg/m2) but there was no association between higher BMI and improved survival. Data from the Canadian Organ Replacement Registry (CORR) also suggested that underweight incident PD patients (BMI <18.5 kg/m2) suffered from a higher death risk (HR 1.3, 95% CI 1.1-1.6) when compared to the reference group of BMI 19-24.9 kg/m2. The risk of mortality for incident PD patients with BMI >30 kg/m2 compared to the reference group was null (HR 1.01, 95% CI 0.89-1.14) (108). Furthermore, in a prospective PD cohort from the Netherlands (Netherlands Co-operative Study on the Adequacy of Dialysis-2 (NECOSAD)), de Mutsert et al. (109) included 688 PD patients with a follow-up period of 5 years. After adjustment for age, sex, tobacco use, comorbidities and primary cause of CKD, PD patients with a BMI≥30 kg/m2 did not have a statistically significant difference in mortality risk (HR 0.8, 95% CI 0.5-1.3) than those with a normal BMI (reference 18.5-25 kg/m2); and the similar relationship was observed in time-dependent models (HR 0.7, 95% CI 0.4-1.2). In contrast, those patients who were underweight (BMI<18.5 kg/m2) at the initiation of PD therapy suffered from a higher mortality risk than those with a normal BMI (HR 1.3, 95% CI 0.4-3.2). In a recent study using a large US cohort of PD patients, Obi et al. (110) found a U-shaped association between all-cause mortality and BMI, where the highest and lowest BMIs were associated with worse outcomes while patients with a BMI 30-<35 kg/m2 had the lowest mortality risk.
Finally, there are also studies that have found that obesity is associated with improved outcomes in patients undergoing PD. Snyder et al. (111) performed a retrospective study of 41,197 PD patients and found that overweight and obese participants had a survival benefit compared to those with lower BMI. The adjusted mortality HR for overweight patients (BMI 25-29.9 kg/m2), were 0.84, 0.89 and 0.98 for the first, second and third years of follow-up, respectively. For obese patients (BMI ≥30 kg/m2), adjusted mortality HR for the first, second and third years of follow-up were 0.89, 0.99 and 1.00. Except for the higher mortality risk after three years of follow-up in obese patients, these findings remained robust after accounting for any modality switch to HD or transplantation. Moreover, Mehrotra et al. (112) analyzed data from the USRDS using multivariate piecewise exponential survival models to examine all-cause mortality and technique failure in 66,381 incident PD patients and concluded that higher BMI quartiles were related to lower mortality risk but higher technique failure risk compared to the reference BMI group <21.88 kg/m2. Another study by Fernandes et al. (113) examined baseline BMI and weight change with all-cause mortality in 1,911 Brazilian incident PD patients and found that PD patients with BMI <18.5 kg/m2 had a higher death risk while a BMI >30 kg/m2 was associated with a survival benefit. Accordingly, in a study using time-varying analyses Badve and colleagues (35) found lower mortality risk in PD patients with BMI between 28.1-31 kg/m2 (time-varying BMI reference group 25.1-28 kg/m2) and a higher mortality risk in those with lower BMI (BMI <25 kg/m2) values.
There are also numerous studies, which have attempted to account for muscle mass in the evaluation of the obesity and outcomes in PD patients. These investigations mostly relied on serum and urinary creatinine concentrations as a surrogate of total body muscle mass. In a study of 10,140 incident PD patients, Ramkumar et al. (114) used urinary creatinine to estimate muscle mass and stratified patients into subgroups of BMI (BMI reference group 18.5-24.9 kg/m2) and low vs. normal/high muscle mass, based on the 25th percentile of 24-hour urinary creatinine. PD patients classified with high BMI and high muscle mass had a 10% lower risk of all-cause mortality compared to those with normal BMI and normal/high muscle mass. In contrast, high BMI with low muscle mass was associated with a higher risk of all-cause (HR 1.29, 95% CI 1.17-1.42) and CV (HR 1.21, 95% CI 1.06-1.39) mortality. Also using creatinine as surrogate of LBM, the Canada-US (CANUSA) Peritoneal Dialysis Study Group reported that 1% lower LBM was associated with 3% higher RR for death (115, 116). Similarly, Park et al. (117) evaluated the association of change in baseline serum creatinine concentration during the first three months of treatment with all-cause mortality in a large cohort of patients undergoing PD therapy. They found that in the fully adjusted model, patients with serum creatinine <4 mg/dL and 4-5.9 mg/dL had a higher mortality risk (HR 1.36, 95% CI 1.19-1.55 and HR 1.19, 95% CI 1.08-1.31, respectively) when compared to PD patients with serum creatinine levels of 8-9.9 mg/dL. Accordingly, PD patients with serum creatinine levels of 10-11.9 mg/dL, 12-13.9 mg/dL and ≥14 mg/dL had lower mortality risk (HR 0.88 [95% CI 0.79-0.97], 0.71 [0.62-0.81] and 0.64 [0.55-0.75], respectively). While the authors concluded that muscle mass may partly explain these associations, they also noted the limitation that residual kidney function and delivered dialysis dose may also be confounders in these findings.
OBESITY AND MORTALITY IN ESRD TREATED WITH KT
Transplantation is the preferred method of treatment for ESRD since it is associated with a survival benefit in those who meet the eligibility criteria (118-121). While we have enumerated many studies, which indicate a paradoxical association between BMI and outcomes in most HD and PD patients, BMI is also a factor that may determine eligibility for receipt of a KT in ESRD patients. In fact, a survey conducted by the American Society of Transplant Surgeons (ASTS) indicated that 66 of 67 kidney transplant centers used a BMI cutoff value of 35-45 kg/m2 in order to exclude those with obesity for potential KT evaluation (122). However, there are several studies, which have addressed the impact of pre-KT body size on post-KT outcomes, including graft and patient survival (Table 4). In a US cohort of 10,090 HD patients, Streja et al. (123) evaluated the association between pre-KT three-month averaged BMI and three-month averaged serum creatinine, as a surrogate for muscle mass, with mortality. Pre-KT averaged serum creatinine levels of 12-<14 and ≥14 mg/dl in renal recipients were associated with 44% and 54% lower risks of mortality, respectively. However, pre-KT BMI neither as a continuous nor as a categorical parameter was significantly associated with mortality. Further adjustment for markers of malnutrition and inflammation did not alter these associations. Likewise, in a Spanish cohort of 3,365 KT recipients without graft loss in the first year after transplantation, pre-KT BMI measurement had no significant effect on post-KT mortality (124). Published data from Australia and New Zealand (BMI reference group 18.5-24.9 kg/m2) (125), the Netherlands (BMI reference group 20.1-25 kg/m2) (126) and Canada (BMI reference group 20-24.9 kg/m2) (127) also reported no association between pre-KT BMI and post-KT mortality. Notably, morbid obesity (BMI >35-40 kg/m2) was not an independent predictor of post-KT mortality (128). However, Aalten et al. (129) did find that that pre-KT BMI as a continuous parameter was an independent predictor of post-KT CVD events (HR 1.03, 95% CI 1.00-1.05), although not all-cause mortality. Furthermore, Meier-Kriesche et al. (130) also reported a U-shaped association between BMI and post-KT mortality. Ahmadi et al. (131) conducted a meta-analysis including four studies (125-127, 132) and reported that compared to normal BMI (BMI, 18.5-24.9 kg/m2), underweight (BMI <18.5 kg/m2) (HR 1.09 95% CI 1.02-1.20), overweight (BMI 25-29.9 kg/m2) (HR 1.07, 95% CI 1.04-1.12) and obese (BMI ≥30kg/m2) (HR 1.20, 95% CI 1.14-1.23) categories were associated with higher mortality in KT recipients. Furthermore, Lafranca et al. (133) and Sood et al. (134) in two different meta-analyses concluded that obesity in KT recipients prior to surgery may be associated with worse outcomes after KT. Moreover, there is also evidence that age may impact the latter observations as elderly renal transplant recipients ≥75 years old might be more affected by negative sequela of obesity, since in these patients a pre-KT BMI >30 kg/m2 (BMI reference group ≤30 kg/m2) was associated with a 50% higher all-cause mortality risk (132). It should also be noted that analyzing data from the United Network for Organ Sharing (UNOS) database showed that a pre-KT BMI of 30-34.9 kg/m2 might have a slightly reduced mortality risk (HR 0.92, 95% CI 0.86-0.99) in comparison to the referent group of non-obese patients with BMI <30 kg/m2 (135). However, the findings of these studies have not been replicated in the other investigations mentioned. Therefore, future studies are needed to further assess the association of pre-KT BMI with post-transplant outcomes. Since net state of immunosuppression and chronic inflammatory state from chronic immunosuppression in kidney transplant recipients plays a role in several outcomes in kidney transplantation (136-138), potential confounders involving in the interplay between immunologic and non-immunologic factors need to be taken into account in these studies.
Table 4.
Summary of studies with large sample size (>1,000 subjects) evaluating the association between BMI and mortality outcomes in kidney transplant patients
| Study | Patients (n) | F/U* (y) | Results |
|---|---|---|---|
| Meier-Kriesche et al, 2002 (130) | 51,927 | - | BMI was strongly associated with renal post-transplant outcomes. BMI<18 or>36 kg/m2 were associated with worse patient and graft survival |
| Gonzales-Posada et al, 2006 (124) | 3,365 | up to 3 | Pre-KT BMI had no significant effect on post-transplant mortality |
| Aalten et al, 2006 (186) | 2,067 | 2 | Pre-KT BMI greater than 28 kg/m2 was associated with increased mortality risk |
| Chang et al, 2007 (125) | 5,684 | up to 5 | Obesity was not associated with higher risk of graft loss or patient death in multivariate model |
| Aalten et al, 2008 (129) | 2,187 | 8 | Pre-KT BMI was an independent predictor for post-transplantation cardiovascular events but not for all-cause mortality |
| Hoogeveen et al, 2011 (126) | 1,810 | 8.3 | One year post-transplant obesity and BMI increment had higher relative risk of death and death censored graft failure (reference BMI 20.1-25 kg/m2) but no association between pre-KT BMI and death |
| Streja et al, 2011 (123) | 10,090 | up to 5 | Higher pre-KT serum creatinine level (larger muscle mass) was associated with better graft and patient survival; whereas BMI was not significantly associated with higher or lower mortality |
| Cannon et al, 2013 (135) | 74,983 | 5 | 30≤BMI<35 kg/m2 was associated with reduced hazard of death (reference BMI<30 kg/m2) |
| Hatamizadeh et al, 2013 (132) | 145,470 (15,667 >65y) | 3.9 | Pre-KT BMI>30 kg/m2 in elderly (≥75y) was associated with higher all-cause mortality (reference BMI≤30 kg/m2) |
| Curran et al, 2014 (127) | 1,151 | 4405 patient-years | BMI>35 kg/m2 was associated with higher all cause graft failure (reference BMI 20-24.9 kg/m2) but not significantly associated with death with graft function |
| Pieloch et al, 2014(128) | 30,132 | up to 3 | Morbid obesity (BMI 35-40 kg/m2) did not independently predict higher graft failure or mortality rates |
Duration of follow-up as reported by the authors. If not indicated mean or median reported.
POTENTIAL MECHANISMS UNDERLYING THE OBESITY PARADOX
The observations that increased BMI and obesity can be associated with improved survival in patients with ESRD and especially those treated with HD have been met with some degree of skepticism. Some have attributed these observations to confounding and questioned the biologic plausibility of obesity being associated with protective mechanisms in this patient population. However, there is accumulating evidence that the observations collectively described as the obesity paradox remain robust even after extensive adjustment for various confounders. In addition, there is some evidence that obesity and increased body fat can provide potential protective mechanisms in the setting of inflammation and hemodynamic instability. More importantly, there is now a growing body of experimental and clinical evidence, which links cachexia and wasting to poor outcomes including CVD and mortality. The findings of these studies have shed light on underlying basic and molecular mechanisms which link cachexia and weight loss to worse outcomes thereby raising the possibility that patients with increasing BMI and obesity are resistant to these mechanisms and their deleterious sequelae. Nevertheless, the obesity-mortality associations and their underlying mechanisms remain complex and not yet fully understood. Here we will present some of the theories that have been proposed to contribute to the obesity paradox (Figure 2).
Figure 2.
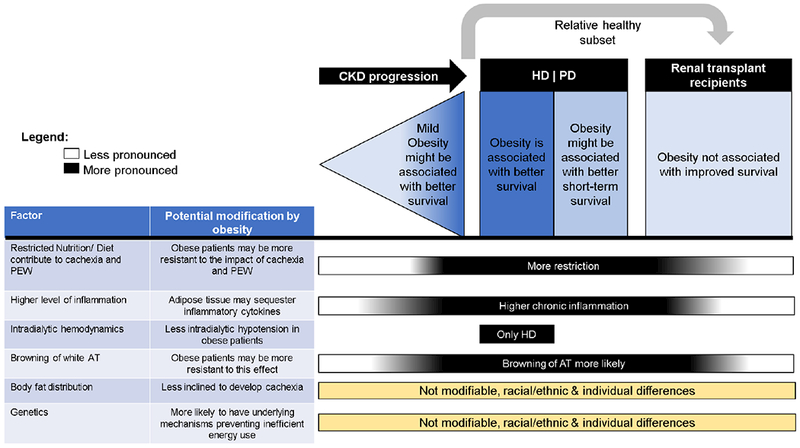
Changes in the obesity-mortality association and possible underlying mechanisms (factors) in different stages of CKD/ESRD.
Abbreviations:
AT – adipose tissue, CKD – chronic kidney disease, ESRD – end-stage renal disease, HD – hemodialysis, PD – peritoneal dialysis, PEW – protein-energy wasting
CACHEXIA AND INEFFICENT ENERGY METABOLISIM
Cachexia is a metabolic syndrome characterized by an imbalance in energy storage and expenditure which commonly manifests clinically as weight loss and loss of muscle and fat tissue (139). The cachectic state has been described in numerous chronic inflammatory conditions including cancer, severe chronic obstructive pulmonary disease, chronic HF, rheumatologic disorders and advanced CKD and ESRD. It is well established that the presence of cachexia is associated with poor quality of life and higher risk of mortality (140). Although many factors including anorexia, malnutrition, oxidative stress and inflammation have been implicated in the pathogenesis of cachexia, it is recognized that ultimately this condition arises due to a severe alteration of energy balance (141). There is a large body of evidence which indicate that elevated resting energy expenditure (REE) is a major determinant in the development of energy wasting and consequently cachexia in patients at risk for this condition (47, 141). Further evidence supporting this mechanism is provided by data indicating that the complications associated with cachexia cannot be overcome by nutritional supplementation and appetite stimulants (142). Although the main tissues affected by cachexia are fat and skeletal muscle, several other organ systems including the liver, heart, and brain are also negatively impacted by this condition (143). In fact, there is now evidence describing the mechanisms by which cancer cachexia leads to cardiomyopathy and thereby cardiac dysfunction and higher mortality (144, 145). In addition, recent investigations in animal experimental models of cancer cachexia have indicated that preventing muscle and adipose tissue loss is effective in prolonging survival (146-148). Therefore, the biologic plausibility of a link between cachexia and development of multiorgan dysfunction leading to higher mortality and the potential efficacy of targeting this condition in order to improve survival is becoming more established.
Accordingly, patients with advanced CKD and ESRD have been found to have a significantly high prevalence of cachexia and its presence is associated with a higher risk of mortality (47). In addition, there is evidence that patients with ESRD have an increased REE (149-151). Furthermore, many of the risk factors identified in the causal pathway of poor energy metabolism and cachexia including PEW, inflammation, oxidative stress, insulin resistance and anorexia are also present in the CKD and ESRD population. More interestingly, there is also recent data indicating that CKD and ESRD are associated with browning of white adipose tissue which has been implicated in the inefficient expenditure of energy commonly found in many different patient populations with cachexia. In this regard, Cheung et al. (152) demonstrated that subtotally nephrectomized mice developed cachexia as indicated by an increased metabolic rate, loss of LBM, along with increased expression and abundance of uncoupling protein-1 (UCP1) in the fat tissue indicating a transition from energy preservation and storage to energy expenditure. Furthermore, it was noted that pair-feeding the animals in order to restore their energy intake to the level of control mice did not improve weight gain in the uremic animals, further confirming the role of abnormally elevated energy expenditure rather than poor energy intake in uremia-associated cachexia. More recently, Kir et al. demonstrated that the browning of white adipose tissue observed in uremia is at least partly mediated by the secondary hyperparathyroidism which is commonly encountered in this condition (153, 154). This is also consistent with the findings of a small clinical study which showed that severe hyperparathyroidism in HD patients was associated with higher REE (150).
In regards to the potential impact of obesity in the pathogenesis and progression of cachexia, it can be postulated that obese patients may have underlying mechanisms (genetic or environmental) which allow them to be more resistant to the energy dysmetabolism and increased REE that is prevalent in chronic conditions such as ESRD. It is known that a major cause of obesity is increased energy surplus and decreased energy expenditure (155). Therefore, obese patients may have factors that protect them from inefficient energy loss and thereby cachexia and its complications. Furthermore, obese patients may have more energy reserves and hence more resilience against the deleterious impact of CKD and ESRD-associated REE. There is evidence, which indicates that fat tissue wasting can be the critical turning point in the cachectic process by stimulating skeletal muscle wasting (156). Therefore, patients with large adipose tissue reserves and underlying mechanisms that prevent lipolysis and loss of fat may be protected against the consequences of cachexia such as muscle loss. Future studies will need to examine the potential links between obesity, cachexia and mortality in the setting of CKD and ESRD.
INFLAMMATION
Chronic inflammation is commonly observed among CKD and ESRD patients, and may be a cause and consequence of CKD and its many different complications including cachexia (157, 158). Pro-inflammatory cytokines are linked to reduced appetite (159), but they can also facilitate muscle breakdown. For instance, tumor necrosis factor-α can induce muscle breakdown by inhibition of nuclear factor kappa-B kinase subunit beta/nuclear factor kappa-B pathway (160-164). In addition, inflammation can play a role in impaired insulin signaling and insulin resistance observed in ESRD (163). Furthermore, inflammation is considered a major contributor to CVD and mortality observed in patients with CKD and ESRD (41, 165-168). In this regard, there is evidence that adipose tissue may temper the deleterious effects of inflammatory mediators by sequestering them. For instance, it has been shown that adipose tissue can synthesize and release soluble tumor necrosis factor-α receptors which can bind tumor necrosis factor-α and prevent its proinflammatory activity (169).
THE IMPACT OF FAT DISTRIBUTION
Besides the total body mass of adipose tissue, the distribution of fat tissue might also influence survival. It has been shown that a pronounced central fat distribution may be associated with a higher risk of CVD, cancer and metabolic disorders (170-173). Moreover, the endocrine function of visceral and subcutaneous fat might markedly differ based on their distribution in the body. For instance, in HD patients it has been shown that waist circumference as a surrogate of visceral fat, directly correlated with C-reactive protein and interleukin-6 levels, whereas a proxy of subcutaneous fat inversely correlated with C-reactive protein and interleukin-6 (174). Therefore, regional fat distribution could impact the relationship between obesity and outcomes, details which may not be captured by evaluation of BMI alone.
On a side note, regional fat distribution, at least in the seemingly healthy population, varies between racial/ethnic groups, which could partly explain the racial/ethnic differences within the obesity paradox observed among HD patients. In a study of African American women, it was found that they had less visceral fat in comparison to Caucasian women, even though their BMI measurements were comparable (175). In addition, some Asian populations have been shown to be prone to higher visceral fat per total body fat content when compared to Europeans (176). Future studies will need to examine the role of fat distribution in the relationship between BMI and outcomes in patients with CKD and ESRD.
MORE STABLE HEMODYNAMICS
A study in patients with HF (177) reported that overweight and obese study participants had higher systolic blood pressure values, while there was no difference in pulmonary capillary wedge pressure and cardiac indices. Furthermore, obese hypertensive subjects in contrast to lean hypertensive individuals had lesser activation of the catecholamine and renin-angiotensin-aldosterone system when exposed to stress (178). These findings may also be relevant to patients with ESRD being treated with HD who are at risk for intra-dialytic hypotension and its deleterious consequences including cardiomyopathy (179-181) and higher mortality (182-184). Extrapolation of the evidence reported in the HF patients to the HD population would suggest that obese HD patients are more resistant to the deleterious hemodynamic effects of HD therapy. However, future studies are needed to assess these hypotheses and address the potential role of BMI in hemodynamic stability of HD patients.
EPIDEMIOLOGICAL LIMITATIONS WITHIN THE OBESITY PARADOX
It is possible that lower BMI is not a cause but rather a consequence of illness or poor health conditions, which leads to poor outcomes in ESRD patients. Illness and poor health may lead to loss of appetite and muscle wasting which promote a decrease in BMI and higher mortality risk. This opposition of general presumptions of the causal direction is known as reverse causation, and has been posited previously in a similar review by this group (185). However, if observational studies took full account of severity of illness and other clinical characteristics, reverse causation would not fully explain why higher BMI is associated with better outcomes in ESRD patients (41).
Another possible explanation for the obesity paradox is that the change of BMI is not in the causal pathway of the outcomes of ESRD patients, but rather an epiphenomenon that occurs when there is an alteration in a patient’s health status (41). In this case, clinical studies would have limited ability to prove the causality of BMI and the survival rate in ESRD patients. However, the obesity paradox does not only occur in ESRD patients, but also exists in other chronic diseases with a preponderance of inflammation (41). Moreover, as shown in Tables 1-3, inverse associations between BMI and mortality were more likely observed in studies with longer follow up time, whereas associations were not as strong or did not exist under shorter follow up times. The long follow up time between BMI and the mortality may mitigate assumptions that the obesity paradox is an epiphenomenon derived from reverse causality. Recurring consistency and other emerging experimental evidence strengthen a potential causal biologic link between BMI and outcomes in ESRD.
The ESRD population represents a unique group of patients with a certain phenotype. It is possible that selection bias in the ESRD population may explain the difference in outcomes observed for obese patients in this population compared to the general population. Typically, CKD patients succumb to complications and mortality sooner than that of the general population. However, among CKD patients who reach ESRD, this group is considered more resilient than their counterparts, and thus may have a survival advantage. Additional studies are needed to more fully understand the obesity paradox in light of these selection biases.
Moreover, studies that have looked at the obesity paradox are observational in design, and thus, causality cannot be established between obesity and survival. Nevertheless, Kalantar-Zadeh et al. (41) used the Bradford Hill criteria as a framework to evaluate the association between obesity and outcomes in CKD patients. Applying Hill’s criteria to the obesity paradox, the authors concluded that despite the observational nature of the studies on the obesity paradox, the increasing body of scientific evidence and considerations from Hill’s criteria have contributed to a stronger picture of the association between higher body weight and outcomes.
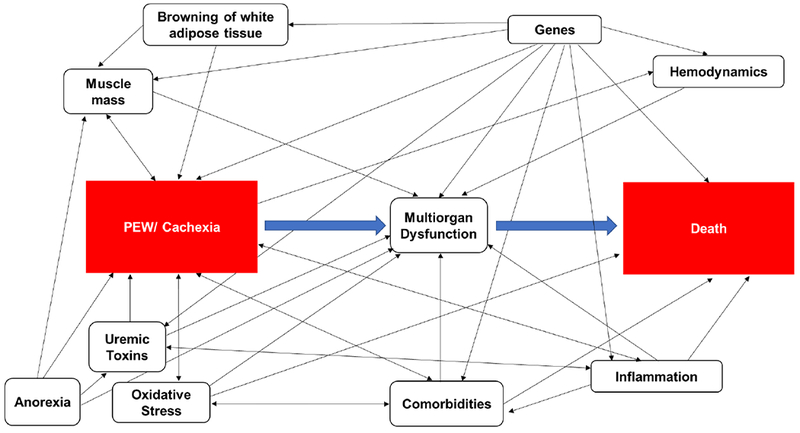
Using a causal diagram or a directed acyclic graph (DAG), we sought to describe the underlying relations between the main exposures of obesity and PEW/cachexia and primary outcomes of survival and death, respectively. Based on existing literature, we accounted for potential confounders of the associations between obesity and survival and PEW/cachexia and death in the DAG models. Figure 3A-B shows the DAGs for the possible causal pathways between (A) obesity and survival and (B) PEW/cachexia and death and the potential confounders. These figures represent the complexity of the relationship between BMI and mortality and call for additional studies using causal modeling to further understand the obesity paradox.
Figure 3.
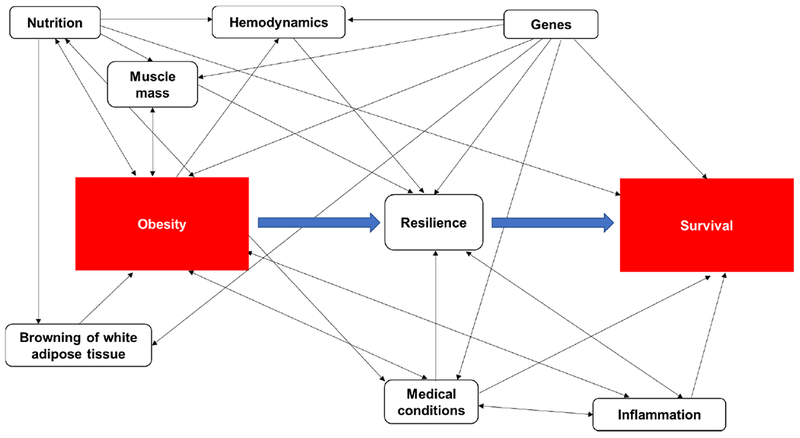
(A) Directed acyclic graph (DAG) explaining the relationship between obesity (exposure) and survival (outcome). A head-to-tail arrow indicates a possible association from one variable to another. A bidirectional arrow represents an association between two variables with an unknown common cause. Variables (i.e., nutrition, muscle mass, hemodynamics, genes, resilience, browning of white adipose tissue, medical conditions and inflammation) in the DAG are potential confounders.
(B) Directed acyclic graph (DAG) explaining the relationship between PEW/cachexia (exposure) and death (outcome). A head-to-tail arrow indicates a possible association from one variable to another. A bidirectional arrow represents an association between two variables with an unknown common cause. Variables (i.e., muscle mass, anorexia, uremic toxins, oxidative stress, medical conditions, inflammation, multiorgan dysfunction, browning of white adipose tissue, genes and hemodynamics) in the DAG are potential confounders.
CONCLUSION
While obesity is a well-established risk factor for the development of CVD and poor outcomes in the general population, there is also considerable evidence that its presence is associated with improved outcomes in select patient populations including some with CKD and ESRD. However, the limitations of the studies evaluating the obesity paradox including the use of BMI as a marker of body mass and the potential for residual confounding needs to be acknowledged. It is also important to recognize that obesity may be an indication of underlying mechanisms which are protective against some of the deleterious effects of CKD/ESRD including impaired energy utilization, cachexia and wasting. It is interesting to note that the association of obesity with improved outcomes is most consistent in patients with ESRD being treated with HD. It is also noteworthy that patients on maintenance HD have been shown to have an increased REE and are at great risk for cachexia and PEW. This is in light of mounting evidence linking cachexia to multiorgan dysfunction including cardiomyopathy and higher mortality. There is also experimental evidence, which indicates that treatment and prevention of cachexia can be associated with improved outcomes. Therefore, the findings described as the obesity paradox may be providing investigators with clues, which can be leveraged into novel therapies. The latter point is especially important in the ESRD population where CVD remains the major cause of mortality, and currently there are no therapies, which have proven to be effective in improving survival.
Acknowledgements:
HM and ES are employees of the Department of Veterans Affairs but the content in this manuscript are the sole responsibility of the authors and in no way should be seen as official policy or interpretation by the US Department of Veterans Affairs or the United States government.
Funding Source: KKZ is supported by NIH (NIDDK) grants K24-DK091419, R01-DK078106, and philanthropic grants from Mr. Harold Simmons. HM is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs 1 IK CX 001043-01A2.
Abbreviations:
- 95% CI
95% Confidence Interval
- ANZDATA Registry
Australia and New Zealand Dialysis and Transplant Registry
- ASTS
American Society of Transplant Surgeons
- BIA/S
bioelectrical impedance analysis/spectroscopy
- BMI
body mass index
- CANUSA
Canada-US Peritoneal Dialysis Study Group
- CKD
chronic kidney disease
- CORR
Canadian Organ Replacement Register
- CT
computed tomography
- CVD
cardiovascular disease
- DAG
directed acyclic graph
- DEXA
dual energy x-ray absorptiometry
- DMMS
Dialysis Morbidity and Mortality Wave II Study
- DOPPS
Dialysis Outcomes and Practice Patterns Study
- eGFR
estimated glomerular filtration rate
- ESRD
end-stage renal disease
- HD
hemodialysis
- HF
heart failure
- HMG-CoA
3-hydroxy-3-methyl-glutaryl-coenzyme A
- HR
Hazard Ratio
- KT
kidney transplant
- LBM
lean body mass
- LDL
low density lipoprotein
- MRI
magnetic resonance imaging
- MSM
Marginal Structural Model
- NECOSAD
Co-operative Study on the Adequacy of Dialysis-2
- NIR
near-infrared interactance
- OR
Odds Ratio
- PD
peritoneal dialysis
- PEW
protein energy wasting
- REE
resting energy expenditure
- RR
Relative Risk
- SD
standard deviation
- UCP1
uncoupling protein-1
- UNOS database
United Network for Organ Sharing
- US
United States of America
- USRDS
United States Renal Data System
- VA
Veterans Administration
- WC
waist circumference
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflict of Interest: KKZ has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra-Zeneca, AVEO, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor, ZS-Pharma. HM has received grant funding from the NIH, VA ORD and Novartis.
References
- 1.World Health Organization Europe. Body Mass Index - BMI. http://wwweurowhoint/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi accessed 06/26/2018.
- 2.Mitchell NS, Catenacci VA, Wyatt HR, Hill JO. Obesity: overview of an epidemic. Psychiatr Clin North Am. 2011;34(4):717–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet (London, England). 2009;373(9669):1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Progress in cardiovascular diseases. 2014;56(4):369–81. [DOI] [PubMed] [Google Scholar]
- 5.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–80. [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi Y, Hasegawa W, Yasunaga H, Sunohara M, Jo T, Takami K, et al. Paradoxical association between body mass index and in-hospital mortality in elderly patients with chronic obstructive pulmonary disease in Japan. International journal of chronic obstructive pulmonary disease. 2014;9:1337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lainscak M, von Haehling S, Doehner W, Sarc I, Jeric T, Ziherl K, et al. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. Journal of cachexia, sarcopenia and muscle. 2011;2(2):81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Divo MJ, Cabrera C, Casanova C, Marin JM, Pinto-Plata VM, de-Torres JP, et al. Comorbidity Distribution, Clinical Expression and Survival in COPD Patients with Different Body Mass Index. Chronic obstructive pulmonary diseases (Miami, Fla). 2014;1(2):229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. Journal of the American College of Cardiology. 2004;43(8):1439–44. [DOI] [PubMed] [Google Scholar]
- 10.Hanrahan CF, Golub JE, Mohapi L, Tshabangu N, Modisenyane T, Chaisson RE, et al. Body mass index and risk of tuberculosis and death. AIDS (London, England). 2010;24(10):1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Sande MA, Schim van der Loeff MF, Aveika AA, Sabally S, Togun T, Sarge-Njie R, et al. Body mass index at time of HIV diagnosis: a strong and independent predictor of survival. Journal of acquired immune deficiency syndromes (1999). 2004;37(2):1288–94. [DOI] [PubMed] [Google Scholar]
- 12.Degoulet P, Legrain M, Reach I, Aime F, Devries C, Rojas P, et al. Mortality risk factors in patients treated by chronic hemodialysis. Report of the Diaphane collaborative study. Nephron. 1982;31(2):103–10. [DOI] [PubMed] [Google Scholar]
- 13.Leavey SF, Strawderman RL, Jones CA, Port FK, Held PJ. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis. 1998;31(6):997–1006. [DOI] [PubMed] [Google Scholar]
- 14.Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney international. 1999;55(4):1560–7. [DOI] [PubMed] [Google Scholar]
- 15.Kopple JD, Zhu X, Lew NL, Lowrie EG. Body weight-for-height relationships predict mortality in maintenance hemodialysis patients. Kidney Int. 1999;56(3):1136–48. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe RA, Ashby VB, Daugirdas JT, Agodoa LY, Jones CA, Port FK. Body size, dose of hemodialysis, and mortality. Am J Kidney Dis. 2000;35(1):80–8. [DOI] [PubMed] [Google Scholar]
- 17.Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW. Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2001;16(12):2386–94. [DOI] [PubMed] [Google Scholar]
- 18.Lowrie EG, Li Z, Ofsthun N, Lazarus JM. Body size, dialysis dose and death risk relationships among hemodialysis patients. Kidney Int. 2002;62(5):1891–7. [DOI] [PubMed] [Google Scholar]
- 19.Pifer TB, McCullough KP, Port FK, Goodkin DA, Maroni BJ, Held PJ, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney international. 2002;62(6):2238–45. [DOI] [PubMed] [Google Scholar]
- 20.Port FK, Ashby VB, Dhingra RK, Roys EC, Wolfe RA. Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. Journal of the American Society of Nephrology : JASN. 2002;13(4):1061–6. [DOI] [PubMed] [Google Scholar]
- 21.Beddhu S, Pappas LM, Ramkumar N, Samore M. Effects of body size and body composition on survival in hemodialysis patients. Journal of the American Society of Nephrology : JASN. 2003;14(9):2366–72. [DOI] [PubMed] [Google Scholar]
- 22.Glanton CW, Hypolite IO, Hshieh PB, Agodoa LY, Yuan CM, Abbott KC. Factors associated with improved short term survival in obese end stage renal disease patients. Annals of epidemiology. 2003;13(2):136–43. [DOI] [PubMed] [Google Scholar]
- 23.Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. The American journal of clinical nutrition. 2004;80(2):324–32. [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Kilpatrick RD, Kopple JD, Stringer WW. A matched comparison of serum lipids between hemodialysis patients and nondialysis morbid controls. Hemodial Int. 2005;9(3):314–24. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, McAllister CJ, Shinaberger CS, Gjertson DW, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005;46(3):489–500. [DOI] [PubMed] [Google Scholar]
- 26.Chazot C, Gassia JP, Di Benedetto A, Cesare S, Ponce P, Marcelli D. Is there any survival advantage of obesity in Southern European haemodialysis patients? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(9):2871–6. [DOI] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Streja E, Kovesdy CP, Oreopoulos A, Noori N, Jing J, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85(11):991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yen TH, Lin JL, Lin-Tan DT, Hsu CW. Association between body mass and mortality in maintenance hemodialysis patients. Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. 2010;14(4):400–8. [DOI] [PubMed] [Google Scholar]
- 29.Hall YN, Xu P, Chertow GM. Relationship of body size and mortality among US Asians and Pacific Islanders on dialysis. Ethnicity & disease. 2011;21(1):40–6. [PMC free article] [PubMed] [Google Scholar]
- 30.Molnar MZ, Streja E, Kovesdy CP, Bunnapradist S, Sampaio MS, Jing J, et al. Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(4):725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricks J, Molnar MZ, Kovesdy CP, Kopple JD, Norris KC, Mehrotra R, et al. Racial and ethnic differences in the association of body mass index and survival in maintenance hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;58(4):574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoogeveen EK, Halbesma N, Rothman KJ, Stijnen T, van Dijk S, Dekker FW, et al. Obesity and mortality risk among younger dialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(2):280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalantar-Zadeh K, Streja E, Molnar MZ, Lukowsky LR, Krishnan M, Kovesdy CP, et al. Mortality prediction by surrogates of body composition: an examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. American journal of epidemiology. 2012;175(8):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park J, Jin DC, Molnar MZ, Dukkipati R, Kim YL, Jing J, et al. Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clinic proceedings. 2013;88(5):479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badve SV, Paul SK, Klein K, Clayton PA, Hawley CM, Brown FG, et al. The association between body mass index and mortality in incident dialysis patients. PloS one. 2014;9(12):e114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vashistha T, Mehrotra R, Park J, Streja E, Dukkipati R, Nissenson AR, et al. Effect of age and dialysis vintage on obesity paradox in long-term hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;63(4):612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calabia J, Arcos E, Carrero JJ, Comas J, Valles M. Does the obesity survival paradox of dialysis patients differ with age? Blood purification. 2015;39(1-3):193–9. [DOI] [PubMed] [Google Scholar]
- 38.Doshi M, Streja E, Rhee CM, Park J, Ravel VA, Soohoo M, et al. Examining the robustness of the obesity paradox in maintenance hemodialysis patients: a marginal structural model analysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2016;31(8):1310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenvinkel P, Gillespie IA, Tunks J, Addison J, Kronenberg F, Drueke TB, et al. Inflammation Modifies the Paradoxical Association between Body Mass Index and Mortality in Hemodialysis Patients. Journal of the American Society of Nephrology : JASN. 2016;27(5):1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodkin DA, Mapes DL, Held PJ. The dialysis outcomes and practice patterns study (DOPPS): how can we improve the care of hemodialysis patients? Seminars in dialysis. 2001;14(3):157–9. [DOI] [PubMed] [Google Scholar]
- 41.Kalantar-Zadeh K, Rhee CM, Chou J, Ahmadi SF, Park J, Chen JL, et al. The Obesity Paradox in Kidney Disease: How to Reconcile it with Obesity Management. Kidney international reports. 2017;2(2):271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, et al. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016;67(3 Suppl 1):Svii, S1–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395–407. [DOI] [PubMed] [Google Scholar]
- 44.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–48. [DOI] [PubMed] [Google Scholar]
- 46.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney international. 2008;73(4):391–8. [DOI] [PubMed] [Google Scholar]
- 47.Obi Y, Qader H, Kovesdy CP, Kalantar-Zadeh K. Latest consensus and update on protein-energy wasting in chronic kidney disease. Current opinion in clinical nutrition and metabolic care. 2015;18(3):254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheung WW, Paik KH, Mak RH. Inflammation and cachexia in chronic kidney disease. Pediatric nephrology (Berlin, Germany). 2010;25(4):711–24. [DOI] [PubMed] [Google Scholar]
- 49.Moradi H, Sica DA, Kalantar-Zadeh K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. American journal of nephrology. 2013;38(2):136–48. [DOI] [PubMed] [Google Scholar]
- 50.Ross R. Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340(2):115–26. [DOI] [PubMed] [Google Scholar]
- 51.Bologa RM, Levine DM, Parker TS, Cheigh JS, Serur D, Stenzel KH, et al. Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1998;32(1):107–14. [DOI] [PubMed] [Google Scholar]
- 52.Ikizler TA, Wingard RL, Harvell J, Shyr Y, Hakim RM. Association of morbidity with markers of nutrition and inflammation in chronic hemodialysis patients: a prospective study. Kidney international. 1999;55(5):1945–51. [DOI] [PubMed] [Google Scholar]
- 53.Yilmaz MI, Solak Y, Saglam M, Cayci T, Acikel C, Unal HU, et al. The relationship between IL-10 levels and cardiovascular events in patients with CKD. Clinical journal of the American Society of Nephrology : CJASN. 2014;9(7):1207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soriano S, Gonzalez L, Martin-Malo A, Rodriguez M, Aljama P. C-reactive protein and low albumin are predictors of morbidity and cardiovascular events in chronic kidney disease (CKD) 3-5 patients. Clinical nephrology. 2007;67(6):352–7. [DOI] [PubMed] [Google Scholar]
- 55.Vaziri ND. Role of dyslipidemia in impairment of energy metabolism, oxidative stress, inflammation and cardiovascular disease in chronic kidney disease. Clinical and experimental nephrology. 2014;18(2):265–8. [DOI] [PubMed] [Google Scholar]
- 56.Fujii H, Nakai K, Fukagawa M. Role of oxidative stress and indoxyl sulfate in progression of cardiovascular disease in chronic kidney disease. Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. 2011;15(2):125–8. [DOI] [PubMed] [Google Scholar]
- 57.Cornier MA, Despres JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, et al. Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011;124(18):1996–2019. [DOI] [PubMed] [Google Scholar]
- 58.Buss J. Limitations of body mass index to assess body fat. Workplace health & safety. 2014;62(6):264. [DOI] [PubMed] [Google Scholar]
- 59.Carrero JJ, Johansen KL, Lindholm B, Stenvinkel P, Cuppari L, Avesani CM. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016;90(1):53–66. [DOI] [PubMed] [Google Scholar]
- 60.Kramer H, Tuttle KR, Leehey D, Luke A, Durazo-Arvizu R, Shoham D, et al. Obesity management in adults with CKD. Am J Kidney Dis. 2009;53(1):151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2012;13(3):275–86. [DOI] [PubMed] [Google Scholar]
- 62.Kamimura MA, Avesani CM, Cendoroglo M, Canziani ME, Draibe SA, Cuppari L. Comparison of skinfold thicknesses and bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body fat in patients on long-term haemodialysis therapy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18(1):101–5. [DOI] [PubMed] [Google Scholar]
- 63.Noori N, Kopple JD, Kovesdy CP, Feroze U, Sim JJ, Murali SB, et al. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2010;5(12):2258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Noori N, Kovesdy CP, Bross R, Lee M, Oreopoulos A, Benner D, et al. Novel equations to estimate lean body mass in maintenance hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57(1):130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kalantar-Zadeh K, Block G, Kelly MP, Schroepfer C, Rodriguez RA, Humphreys MH. Near infra-red interactance for longitudinal assessment of nutrition in dialysis patients. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2001;11(1):23–31. [DOI] [PubMed] [Google Scholar]
- 66.Kalantar-Zadeh K, Dunne E, Nixon K, Kahn K, Lee GH, Kleiner M, et al. Near infra-red interactance for nutritional assessment of dialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1999;14(1):169–75. [DOI] [PubMed] [Google Scholar]
- 67.Ding WQ, Liu JT, Shang YX, Gao B, Zhao XY, Zhao HP, et al. DXA-measured visceral fat mass and lean body mass reflect abnormal metabolic phenotypes among some obese and nonobese Chinese children and adolescents. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2018. [DOI] [PubMed] [Google Scholar]
- 68.Bross R, Chandramohan G, Kovesdy CP, Oreopoulos A, Noori N, Golden S, et al. Comparing body composition assessment tests in long-term hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010;55(5):885–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Olarescu NC, Jorgensen AP, Godang K, Jurik AG, Froslie KF, Bollerslev J. Dual-energy X-ray absorptiometry is a valid method to estimate visceral adipose tissue in adult patients with Prader-Willi syndrome during treatment with growth hormone. The Journal of clinical endocrinology and metabolism. 2014;99(9):E1727–31. [DOI] [PubMed] [Google Scholar]
- 70.Ravindranath J, Pillai PP, Parameswaran S, Kamalanathan SK, Pal GK. Body Fat Analysis in Predialysis Chronic Kidney Disease: Multifrequency Bioimpedance Assay and Anthropometry Compared With Dual-Energy X-Ray Absorptiometry. J Ren Nutr. 2016;26(5):315–9. [DOI] [PubMed] [Google Scholar]
- 71.Kim J, Heshka S, Gallagher D, Kotler DP, Mayer L, Albu J, et al. Intermuscular adipose tissue-free skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in adults. Journal of applied physiology (Bethesda, Md : 1985). 2004;97(2):655–60. [DOI] [PubMed] [Google Scholar]
- 72.Machann J, Thamer C, Schnoedt B, Haap M, Haring HU, Claussen CD, et al. Standardized assessment of whole body adipose tissue topography by MRI. Journal of magnetic resonance imaging : JMRI. 2005;21(4):455–62. [DOI] [PubMed] [Google Scholar]
- 73.Kullberg J, Brandberg J, Angelhed JE, Frimmel H, Bergelin E, Strid L, et al. Whole-body adipose tissue analysis: comparison of MRI, CT and dual energy X-ray absorptiometry. The British journal of radiology. 2009;82(974):123–30. [DOI] [PubMed] [Google Scholar]
- 74.Fang H, Berg E, Cheng X, Shen W. How to best assess abdominal obesity. Current opinion in clinical nutrition and metabolic care. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Postorino M, Marino C, Tripepi G, Zoccali C. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. Journal of the American College of Cardiology. 2009;53(15):1265–72. [DOI] [PubMed] [Google Scholar]
- 76.Noori N, Kovesdy CP, Dukkipati R, Kim Y, Duong U, Bross R, et al. Survival predictability of lean and fat mass in men and women undergoing maintenance hemodialysis. The American journal of clinical nutrition. 2010;92(5):1060–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. Jama. 2004;291(7):844–50. [DOI] [PubMed] [Google Scholar]
- 78.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O. Obesity and risk for chronic renal failure. Journal of the American Society of Nephrology : JASN. 2006;17(6):1695–702. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney international. 2008;73(1):19–33. [DOI] [PubMed] [Google Scholar]
- 80.Madero M, Sarnak MJ, Wang X, Sceppa CC, Greene T, Beck GJ, et al. Body mass index and mortality in CKD. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2007;50(3):404–11. [DOI] [PubMed] [Google Scholar]
- 81.Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, et al. Chronic kidney disease and the risk of end-stage renal disease versus death. Journal of general internal medicine. 2011;26(4):379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ricardo AC, Madero M, Yang W, Anderson C, Menezes M, Fischer MJ, et al. Adherence to a healthy lifestyle and all-cause mortality in CKD. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(4):602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bello A, Padwal R, Lloyd A, Hemmelgarn B, Klarenbach S, Manns B, et al. Using linked administrative data to study periprocedural mortality in obesity and chronic kidney disease (CKD). Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28 Suppl 4:iv57–64. [DOI] [PubMed] [Google Scholar]
- 84.Obermayr RP, Temml C, Gutjahr G, Kainz A, Klauser-Braun R, Fugger R, et al. Body mass index modifies the risk of cardiovascular death in proteinuric chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(8):2421–8. [DOI] [PubMed] [Google Scholar]
- 85.Navaneethan SD, Schold JD, Arrigain S, Kirwan JP, Nally JV Jr. Body mass index and causes of death in chronic kidney disease. Kidney international. 2016;89(3):675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, et al. The relationship between nontraditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis. 2008;51(2):212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Nicola L, Minutolo R, Chiodini P, Borrelli S, Zoccali C, Postorino M, et al. The effect of increasing age on the prognosis of non-dialysis patients with chronic kidney disease receiving stable nephrology care. Kidney international. 2012;82(4):482–8. [DOI] [PubMed] [Google Scholar]
- 88.Babayev R, Whaley-Connell A, Kshirsagar A, Klemmer P, Navaneethan S, Chen SC, et al. Association of race and body mass index with ESRD and mortality in CKD stages 3-4: results from the Kidney Early Evaluation Program (KEEP). American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61(3):404–12. [DOI] [PubMed] [Google Scholar]
- 89.Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP. Association of body mass index with outcomes in patients with CKD. Journal of the American Society of Nephrology : JASN. 2014;25(9):2088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang JC, Lin HY, Lim LM, Chen SC, Chang JM, Hwang SJ, et al. Body mass index, mortality, and gender difference in advanced chronic kidney disease. PloS one. 2015;10(5):e0126668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kramer H, Shoham D, McClure LA, Durazo-Arvizu R, Howard G, Judd S, et al. Association of waist circumference and body mass index with all-cause mortality in CKD: The REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;58(2):177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elsayed EF, Sarnak MJ, Tighiouart H, Griffith JL, Kurth T, Salem DN, et al. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;52(1):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sato Y, Fujimoto S, Konta T, Iseki K, Moriyama T, Yamagata K, et al. Body shape index: Sex-specific differences in predictive power for all-cause mortality in the Japanese population. PloS one. 2017;12(5):e0177779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PloS one. 2012;7(7):e39504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hanks LJ, Tanner RM, Muntner P, Kramer H, McClellan WM, Warnock DG, et al. Metabolic subtypes and risk of mortality in normal weight, overweight, and obese individuals with CKD. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(12):2064–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abbott KC, Glanton CW, Trespalacios FC, Oliver DK, Ortiz MI, Agodoa LY, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney international. 2004;65(2):597–605. [DOI] [PubMed] [Google Scholar]
- 97.Stack AG, Murthy BV, Molony DA. Survival differences between peritoneal dialysis and hemodialysis among “large” ESRD patients in the United States. Kidney international. 2004;65(6):2398–408. [DOI] [PubMed] [Google Scholar]
- 98.Wang J, Streja E, Rhee CM, Soohoo M, Feng M, Brunelli SM, et al. Lean Body Mass and Survival in Hemodialysis Patients and the Roles of Race and Ethnicity. Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2016;26(1):26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cabezas-Rodriguez I, Carrero JJ, Zoccali C, Qureshi AR, Ketteler M, Floege J, et al. Influence of body mass index on the association of weight changes with mortality in hemodialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2013;8(10):1725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thamer M, Hwang W, Fink NE, Sadler JH, Wills S, Levin NW, et al. US nephrologists’ recommendation of dialysis modality: results of a national survey. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2000;36(6):1155–65. [DOI] [PubMed] [Google Scholar]
- 101.Stack AG. Determinants of modality selection among incident US dialysis patients: results from a national study. Journal of the American Society of Nephrology : JASN. 2002;13(5):1279–87. [DOI] [PubMed] [Google Scholar]
- 102.Little J, Irwin A, Marshall T, Rayner H, Smith S. Predicting a patient’s choice of dialysis modality: experience in a United Kingdom renal department. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2001;37(5):981–6. [DOI] [PubMed] [Google Scholar]
- 103.Mehrotra R, Marsh D, Vonesh E, Peters V, Nissenson A. Patient education and access of ESRD patients to renal replacement therapies beyond in-center hemodialysis. Kidney international. 2005;68(1):378–90. [DOI] [PubMed] [Google Scholar]
- 104.Abbott KC, Oliver DK, Hurst FP, Das NP, Gao SW, Perkins RM. Body mass index and peritoneal dialysis: “exceptions to the exception” in reverse epidemiology? Seminars in dialysis. 2007;20(6):561–5. [DOI] [PubMed] [Google Scholar]
- 105.McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. Journal of the American Society of Nephrology : JASN. 2003;14(11):2894–901. [DOI] [PubMed] [Google Scholar]
- 106.Xiong L, Cao S, Xu F, Zhou Q, Fan L, Xu Q, et al. Association of Body Mass Index and Body Mass Index Change with Mortality in Incident Peritoneal Dialysis Patients. Nutrients. 2015;7(10):8444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim YK, Kim SH, Kim HW, Kim YO, Jin DC, Song HC, et al. The association between body mass index and mortality on peritoneal dialysis: a prospective cohort study. Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis. 2014;34(4):383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pliakogiannis T, Trpeski L, Taskapan H, Shah H, Ahmad M, Fenton S, et al. Reverse epidemiology in peritoneal dialysis patients: the Canadian experience and review of the literature. International urology and nephrology. 2007;39(1):281–8. [DOI] [PubMed] [Google Scholar]
- 109.de Mutsert R, Grootendorst DC, Boeschoten EW, Dekker FW, Krediet RT. Is obesity associated with a survival advantage in patients starting peritoneal dialysis? Contributions to nephrology. 2009;163:124–31. [DOI] [PubMed] [Google Scholar]
- 110.Obi Y, Streja E, Mehrotra R, Rivara MB, Rhee CM, Soohoo M, et al. Impact of Obesity on Modality Longevity, Residual Kidney Function, Peritonitis, and Survival Among Incident Peritoneal Dialysis Patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney international. 2003;64(5):1838–44. [DOI] [PubMed] [Google Scholar]
- 112.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Vonesh E. The outcomes of continuous ambulatory and automated peritoneal dialysis are similar. Kidney international. 2009;76(1):97–107. [DOI] [PubMed] [Google Scholar]
- 113.Fernandes NM, Bastos MG, Franco MR, Chaoubah A, Lima Mda G, Divino-Filho JC, et al. Body size and longitudinal body weight changes do not increase mortality in incident peritoneal dialysis patients of the Brazilian peritoneal dialysis multicenter study. Clinics (Sao Paulo, Brazil). 2013;68(1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ramkumar N, Pappas LM, Beddhu S. Effect of body size and body composition on survival in peritoneal dialysis patients. Peritoneal dialysis international : journal of the International Society for Peritoneal Dialysis. 2005;25(5):461–9. [PubMed] [Google Scholar]
- 115.Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Journal of the American Society of Nephrology : JASN. 1996;7(2):198–207. [DOI] [PubMed] [Google Scholar]
- 116.McCusker FX, Teehan BP, Thorpe KE, Keshaviah PR, Churchill DN. How much peritoneal dialysis is required for the maintenance of a good nutritional state? Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Kidney international Supplement. 1996;56:S56–61. [PubMed] [Google Scholar]
- 117.Park J, Mehrotra R, Rhee CM, Molnar MZ, Lukowsky LR, Patel SS, et al. Serum creatinine level, a surrogate of muscle mass, predicts mortality in peritoneal dialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28(8):2146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sezer S, Ozdemir FN, Akcay A, Arat Z, Boyacioglu S, Haberal M. Renal transplantation offers a better survival in HCV-infected ESRD patients. Clinical transplantation. 2004;18(5):619–23. [DOI] [PubMed] [Google Scholar]
- 119.Mange KC, Weir MR. Preemptive renal transplantation: why not? American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2003;3(11):1336–40. [DOI] [PubMed] [Google Scholar]
- 120.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England journal of medicine. 1999;341(23):1725–30. [DOI] [PubMed] [Google Scholar]
- 121.Bunnapradist S, Danovitch GM. Evaluation of adult kidney transplant candidates. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2007;50(5):890–8. [DOI] [PubMed] [Google Scholar]
- 122.Pondrom S. The AJT report: news and issues that affect organ and tissue transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(7):1663–4. [DOI] [PubMed] [Google Scholar]
- 123.Streja E, Molnar MZ, Kovesdy CP, Bunnapradist S, Jing J, Nissenson AR, et al. Associations of pretransplant weight and muscle mass with mortality in renal transplant recipients. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(6):1463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gonzalez-Posada JM, Hernandez D, Genis BB, Tamajon LP, Perez JG, Maceira B, et al. Increased cardiovascular risk profile and mortality in kidney allograft recipients with post-transplant diabetes mellitus in Spain. Clinical transplantation. 2006;20(5):650–8. [DOI] [PubMed] [Google Scholar]
- 125.Chang SH, Coates PT, McDonald SP. Effects of body mass index at transplant on outcomes of kidney transplantation. Transplantation. 2007;84(8):981–7. [DOI] [PubMed] [Google Scholar]
- 126.Hoogeveen EK, Aalten J, Rothman KJ, Roodnat JI, Mallat MJ, Borm G, et al. Effect of obesity on the outcome of kidney transplantation: a 20-year follow-up. Transplantation. 2011;91(8):869–74. [DOI] [PubMed] [Google Scholar]
- 127.Curran SP, Famure O, Li Y, Kim SJ. Increased recipient body mass index is associated with acute rejection and other adverse outcomes after kidney transplantation. Transplantation. 2014;97(1):64–70. [DOI] [PubMed] [Google Scholar]
- 128.Pieloch D, Dombrovskiy V, Osband AJ, Lebowitz J, Laskow DA. Morbid obesity is not an independent predictor of graft failure or patient mortality after kidney transplantation. J Ren Nutr. 2014;24(1):50–7. [DOI] [PubMed] [Google Scholar]
- 129.Aalten J, Hoogeveen EK, Roodnat JI, Weimar W, Borm GF, de Fijter JW, et al. Associations between pre-kidney-transplant risk factors and post-transplant cardiovascular events and death. Transplant international : official journal of the European Society for Organ Transplantation. 2008;21(10):985–91. [DOI] [PubMed] [Google Scholar]
- 130.Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation. 2002;73(1):70–4. [DOI] [PubMed] [Google Scholar]
- 131.Ahmadi SF, Zahmatkesh G, Streja E, Molnar MZ, Rhee CM, Kovesdy CP, et al. Body mass index and mortality in kidney transplant recipients: a systematic review and meta-analysis. American journal of nephrology. 2014;40(4):315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hatamizadeh P, Molnar MZ, Streja E, Lertdumrongluk P, Krishnan M, Kovesdy CP, et al. Recipient-related predictors of kidney transplantation outcomes in the elderly. Clinical transplantation. 2013;27(3):436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lafranca JA, IJ JN, Betjes MG, Dor FJ. Body mass index and outcome in renal transplant recipients: a systematic review and meta-analysis. BMC Med. 2015;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sood A, Hakim DN, Hakim NS. Consequences of Recipient Obesity on Postoperative Outcomes in a Renal Transplant: A Systematic Review and Meta-Analysis. Exp Clin Transplant. 2016;14(2):121–8. [PubMed] [Google Scholar]
- 135.Cannon RM, Jones CM, Hughes MG, Eng M, Marvin MR. The impact of recipient obesity on outcomes after renal transplantation. Annals of surgery. 2013;257(5):978–84. [DOI] [PubMed] [Google Scholar]
- 136.Vazquez MA, Jeyarajah DR, Kielar ML, Lu CY. Long-term outcomes of renal transplantation: a result of the original endowment of the donor kidney and the inflammatory response to both alloantigens and injury. Current opinion in nephrology and hypertension. 2000;9(6):643–8. [DOI] [PubMed] [Google Scholar]
- 137.Cottone S, Palermo A, Vaccaro F, Mule G, Guarneri M, Arsena R, et al. Inflammation and endothelial activation are linked to renal function in long-term kidney transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2007;20(1):82–7. [DOI] [PubMed] [Google Scholar]
- 138.Abedini S, Holme I, Marz W, Weihrauch G, Fellstrom B, Jardine A, et al. Inflammation in renal transplantation. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(7):1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kalantar-Zadeh K, Rhee C, Sim JJ, Stenvinkel P, Anker SD, Kovesdy CP. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J Cachexia Sarcopenia Muscle. 2013;4(2):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle. 2016;7(5):507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Argiles JM, Fontes-Oliveira CC, Toledo M, Lopez-Soriano FJ, Busquets S. Cachexia: a problem of energetic inefficiency. J Cachexia Sarcopenia Muscle. 2014;5(4):279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89(2):381–410. [DOI] [PubMed] [Google Scholar]
- 143.Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14(11):754–62. [DOI] [PubMed] [Google Scholar]
- 145.Belloum Y, Rannou-Bekono F, Favier FB. Cancer-induced cardiac cachexia: Pathogenesis and impact of physical activity (Review). Oncol Rep. 2017;37(5):2543–52. [DOI] [PubMed] [Google Scholar]
- 146.Johnston AJ, Murphy KT, Jenkinson L, Laine D, Emmrich K, Faou P, et al. Targeting of Fn14 Prevents Cancer-Induced Cachexia and Prolongs Survival. Cell. 2015;162(6):1365–78. [DOI] [PubMed] [Google Scholar]
- 147.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142(4):531–43. [DOI] [PubMed] [Google Scholar]
- 148.Segatto M, Fittipaldi R, Pin F, Sartori R, Dae Ko K, Zare H, et al. Epigenetic targeting of bromodomain protein BRD4 counteracts cancer cachexia and prolongs survival. Nat Commun. 2017;8(1):1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ikizler TA, Wingard RL, Sun M, Harvell J, Parker RA, Hakim RM. Increased energy expenditure in hemodialysis patients. J Am Soc Nephrol. 1996;7(12):2646–53. [DOI] [PubMed] [Google Scholar]
- 150.Cuppari L, de Carvalho AB, Avesani CM, Kamimura MA, Dos Santos Lobao RR, Draibe SA. Increased resting energy expenditure in hemodialysis patients with severe hyperparathyroidism. Journal of the American Society of Nephrology : JASN. 2004;15(11):2933–9. [DOI] [PubMed] [Google Scholar]
- 151.Skouroliakou M, Stathopoulou M, Koulouri A, Giannopoulou I, Stamatiades D, Stathakis C. Determinants of resting energy expenditure in hemodialysis patients, and comparison with healthy subjects. J Ren Nutr. 2009;19(4):283–90. [DOI] [PubMed] [Google Scholar]
- 152.Cheung WW, Kuo HJ, Markison S, Chen C, Foster AC, Marks DL, et al. Peripheral administration of the melanocortin-4 receptor antagonist NBI-12i ameliorates uremia-associated cachexia in mice. Journal of the American Society of Nephrology : JASN. 2007;18(9):2517–24. [DOI] [PubMed] [Google Scholar]
- 153.Thomas SS, Mitch WE. Parathyroid hormone stimulates adipose tissue browning: a pathway to muscle wasting. Current opinion in clinical nutrition and metabolic care. 2017;20(3):153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kir S, Komaba H, Garcia AP, Economopoulos KP, Liu W, Lanske B, et al. PTH/PTHrP Receptor Mediates Cachexia in Models of Kidney Failure and Cancer. Cell metabolism. 2016;23(2):315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hill JO, Wyatt HR, Peters JC. Energy balance and obesity. Circulation. 2012;126(1):126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333(6039):233–8. [DOI] [PubMed] [Google Scholar]
- 157.Jankowska M, Cobo G, Lindholm B, Stenvinkel P. Inflammation and Protein-Energy Wasting in the Uremic Milieu. Contrib Nephrol. 2017;191:58–71. [DOI] [PubMed] [Google Scholar]
- 158.Dai L, Golembiewska E, Lindholm B, Stenvinkel P. End-Stage Renal Disease, Inflammation and Cardiovascular Outcomes. Contrib Nephrol. 2017;191:32–43. [DOI] [PubMed] [Google Scholar]
- 159.Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. The American journal of clinical nutrition. 2004;80(2):299–307. [DOI] [PubMed] [Google Scholar]
- 160.Cai D, Frantz JD, Tawa NE Jr., Melendez PA, Oh BC, Lidov HG, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119(2):285–98. [DOI] [PubMed] [Google Scholar]
- 161.Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science (New York, NY). 2000;289(5488):2363–6. [DOI] [PubMed] [Google Scholar]
- 162.Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. The Journal of biological chemistry. 2003;278(4):2294–303. [DOI] [PubMed] [Google Scholar]
- 163.Hu Z, Wang H, Lee IH, Du J, Mitch WE. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. The Journal of clinical investigation. 2009;119(10):3059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mak RH, Ikizler AT, Kovesdy CP, Raj DS, Stenvinkel P, Kalantar-Zadeh K. Wasting in chronic kidney disease. Journal of cachexia, sarcopenia and muscle. 2011;2(1):9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kalantar-Zadeh K, Kopple JD, Humphreys MH, Block G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19(6):1507–19. [DOI] [PubMed] [Google Scholar]
- 166.Qureshi AR, Alvestrand A, Divino-Filho JC, Gutierrez A, Heimburger O, Lindholm B, et al. Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. Journal of the American Society of Nephrology : JASN. 2002;13 Suppl 1:S28–36. [PubMed] [Google Scholar]
- 167.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney international. 1999;55(2):648–58. [DOI] [PubMed] [Google Scholar]
- 168.Kim JK, Kim SG, Oh JE, Lee YK, Noh JW, Kim HJ, et al. Impact of sarcopenia on long-term mortality and cardiovascular events in patients undergoing hemodialysis. The Korean journal of internal medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mohamed-Ali V, Goodrick S, Bulmer K, Holly JM, Yudkin JS, Coppack SW. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. The American journal of physiology. 1999;277(6 Pt 1):E971–5. [DOI] [PubMed] [Google Scholar]
- 170.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiological reviews. 1994;74(4):761–811. [DOI] [PubMed] [Google Scholar]
- 171.Sardinha LB, Teixeira PJ, Guedes DP, Going SB, Lohman TG. Subcutaneous central fat is associated with cardiovascular risk factors in men independently of total fatness and fitness. Metabolism: clinical and experimental. 2000;49(11):1379–85. [DOI] [PubMed] [Google Scholar]
- 172.Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS. Regional fat distribution in women and risk of cardiovascular disease. The American journal of clinical nutrition. 1997;65(3):855–60. [DOI] [PubMed] [Google Scholar]
- 173.Britton KA, Massaro JM, Murabito JM, Kreger BE, Hoffmann U, Fox CS. Body fat distribution, incident cardiovascular disease, cancer, and all-cause mortality. Journal of the American College of Cardiology. 2013;62(10):921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Delgado C, Chertow GM, Kaysen GA, Dalrymple LS, Kornak J, Grimes B, et al. Associations of Body Mass Index and Body Fat With Markers of Inflammation and Nutrition Among Patients Receiving Hemodialysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017;70(6):817–25. [DOI] [PubMed] [Google Scholar]
- 175.Conway JM, Yanovski SZ, Avila NA, Hubbard VS. Visceral adipose tissue differences in black and white women. The American journal of clinical nutrition. 1995;61(4):765–71. [DOI] [PubMed] [Google Scholar]
- 176.Lear SA, Humphries KH, Kohli S, Chockalingam A, Frohlich JJ, Birmingham CL. Visceral adipose tissue accumulation differs according to ethnic background: results of the Multicultural Community Health Assessment Trial (M-CHAT). The American journal of clinical nutrition. 2007;86(2):353–9. [DOI] [PubMed] [Google Scholar]
- 177.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. Journal of the American College of Cardiology. 2001;38(3):789–95. [DOI] [PubMed] [Google Scholar]
- 178.Weber MA, Neutel JM, Smith DH. Contrasting clinical properties and exercise responses in obese and lean hypertensive patients. Journal of the American College of Cardiology. 2001;37(1):169–74. [DOI] [PubMed] [Google Scholar]
- 179.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clinical journal of the American Society of Nephrology : CJASN. 2009;4(5):914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.McIntyre CW. Recurrent circulatory stress: the dark side of dialysis. Seminars in dialysis. 2010;23(5):449–51. [DOI] [PubMed] [Google Scholar]
- 181.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clinical journal of the American Society of Nephrology : CJASN. 2008;3(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Flythe JE, Xue H, Lynch KE, Curhan GC, Brunelli SM. Association of mortality risk with various definitions of intradialytic hypotension. Journal of the American Society of Nephrology : JASN. 2015;26(3):724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney international. 2004;66(3):1212–20. [DOI] [PubMed] [Google Scholar]
- 184.Tisler A, Akocsi K, Borbas B, Fazakas L, Ferenczi S, Gorogh S, et al. The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18(12):2601–5. [DOI] [PubMed] [Google Scholar]
- 185.Park J, Ahmadi SF, Streja E, Molnar MZ, Flegal KM, Gillen D, et al. Obesity paradox in end-stage kidney disease patients. Progress in cardiovascular diseases. 2014;56(4):415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Aalten J, Christiaans MH, de Fijter H, Hene R, van der Heijde JH, Roodnat J, et al. The influence of obesity on short- and long-term graft and patient survival after renal transplantation. Transplant international : official journal of the European Society for Organ Transplantation. 2006;19(11):901–7. [DOI] [PubMed] [Google Scholar]


