Abstract
The repair of nerve gap injuries longer than 3 cm is limited by the need of sacrifice donor tissue and morbidity associated with the autograft gold standard, while decellularized grafts and biodegradable conduits are effective only in short nerve defects. The advantage of isogenic nerve implants seems to be the release of various growth factors by the denervated Schwann cells. We evaluated the effect of vascular endothelial growth factor, neurotrophins, and pleiotrophin (PTN) supplementation of multiluminal conduits, in the repair of 3 and 4 cm nerve gaps in the rabbit peroneal nerve. In vitro screening revealed a synergistic regenerative effect of PTN with glial-derived neurotrophic factor (GDNF) in promoting sensory axon density, and in motor axonal growth from spinal cord explants. In vivo, pleiotrophins were able to support nerve regrowth across a 3 cm gap. In the 4 cm lesions, PTNGDNF had a modest effect in the number of axons distal to the implant, while increasing the mean axon diameter (1 ± 0.4; p ≤ 0.001) over PTN or GDNF alone (0.80 ± 0.2, 0.84 ± 0.5; respectively). Some regenerated axons reinnervated muscle targets as indicated by neuromuscular junction staining. However, many were wrapped in Remak bundles, suggesting a delay in axonal sorting, explaining the limited electrophysiological function of the reinnervated muscle, and the modest recovery in toe spreading in the PTN-GDNF repaired animals. These results support the use of synergistic neurotrophic/pleiotrophic growth factors in long gap repair and underscore the need for remyelination strategies distal to the injury site.
Keywords: Long gap, agarose, micro-channels, neurotrophins, pleiotrophins, Remak bundles
1. Introduction
Despite the regenerative capacity of the adult peripheral nervous system and the routine repair of small nerve defects, critical lesions larger than 4 cm remain a significant challenge with limited expectations for functional recovery, even with the use of autologous grafts [1,2]. The poor success with long nerve gaps may be explained by limited neuronal intrinsic regenerative capacity, nonpermissive growth substrate, lack of trophic support, axon guidance errors, and re-innervation of dysfunctional targets [3,4]. Most repair alternatives that target some aspects of this multi-factorial process such as the use of hollow nerve conduits, processed allografts, or the incorporation of single growth factors, have shown only modest success [5]. Multi-channel conduits that increase the growth surface area, the addition of extracellular matrix molecules such as collagen or fibronectin, and added cellular and growth factor support, are reportedly more efficient compared to hollow nerve guides, and yet, unable to match the regenerative capacity offered by autologous grafts [6].
The advantage of isogenic nerve implants seems to be the release of several pleiotrophic and neurotrophic growth factors by the denervated Schwann cells [7]. In sensory nerves, these cells release insulin growth factor-1, vascular endothelial growth factor (VEGF), hepatocyte-derived growth factor, nerve growth factor (NGF), and brain-derived neurotrophic factor (BDNF), whereas the heparin-binding protein pleiotrophin (PTN) is expressed primarily in motor fibers. Other growth factors including glial-derived neurotrophic factor (GDNF), insulin growth factor-2, ciliary neurotrophic factor, fibroblast growth factor-2 (FGF-2) and neurotrophin 3 (NT-3), are upregulated after injury in all nerve types [8,9]. These growth factors contribute to the regeneration efficacy in the PNS, as exogenous NGF, BDNF, and NT-3 have been shown to increase axonal regeneration both in vitro and in vivo [10,11]. The neurotrophic factors bind to specific tyrosine receptor kinases (trk) and p75 receptors, and we have shown that activation of the extracellularly regulated kinase (Erk1/2) by the trk receptors in transgenic mice endow peripheral neurons with enhanced regenerative potential [12]. However, when recombinant proteins are applied to the adult transected peripheral nerve, the beneficial effect of individual growth factors is less clear. While some have observed functional regeneration by adding GDNF to nerve guides [13,14] or allografts [15], others have not seen superlative functional regeneration after BDNF or GDNF administration [16,17]. Furthermore, whereas the combination of GDNF-BDNF or NGF-GDNF treatments has shown to stimulate nerve regeneration, it does not match the growth enticed by autografts, suggesting the need for additional trophic support [16,18]. Overall, most of the regenerative strategies reported are focused primarily on stimulating axonal growth. We reasoned that a rich growth factor milieu that stimulates the proliferation, migration, and regeneration of both neuronal and non-neuronal cell populations at the injury site, would offer a more robust and efficient method for repairing critical gap nerve injuries.
To that end, we tested the effect of combined neurotrophins and pleiotrophins treatments aimed at increasing the migration and proliferation of Schwann cells, fibroblasts, monocytes, and endothelial cells, in their ability to mediate nerve growth across a long gap nerve injury [19,20]. Of these, PTN is of particular interest as it has been shown to mediate a number of cellular effects including mitogenicity, cell survival, oncogenicity, inflammation, differentiation and stem cell renewal. The different effects are mediated by the binding of PTN to cells expressing the anaplastic lymphoma kinase (ALK) receptor, the receptor protein tyrosine phosphatase beta/zeta (RPTPβ/ζ), integrins, neuroglycan, or syndecan (SDC) [21]. During nerve development, PTN plays a critical role in proliferation, migration and axonal growth [22], and while barely detectable in the adult, the expression of this pleiotrophin increases dramatically after injury or trauma [8,23]. This motivated the use of PTN in nerve regeneration, but the results are contradictory, as some indicate impaired muscle reinnervation with recombinant protein [24], while others observed improved regeneration by seeding PTN-expressing HEK-293 cells in the lumen of 15-mm-long nerve guides [25]. Here, we used multiluminal scaffolds filled with PLGA microparticles releasing VEGF or PTN to evaluate the regenerative capacity of pleiotrophins across 3 cm gap injuries in the rabbit peroneal nerve. Systematic in vitro screening of the effect of neurotrophins, glial-derived growth factors, and pleiotrophins, revealed synergistic effects of GDNF and PTN in both sensory and motor neurons. In vivo, this combination enticed the growth of larger diameter axons distal to a 4 cm critical gap, reinnervating the limb muscles and mediating moderate functional recovery.
2. Materials and Methods
2.1. Experimental Design
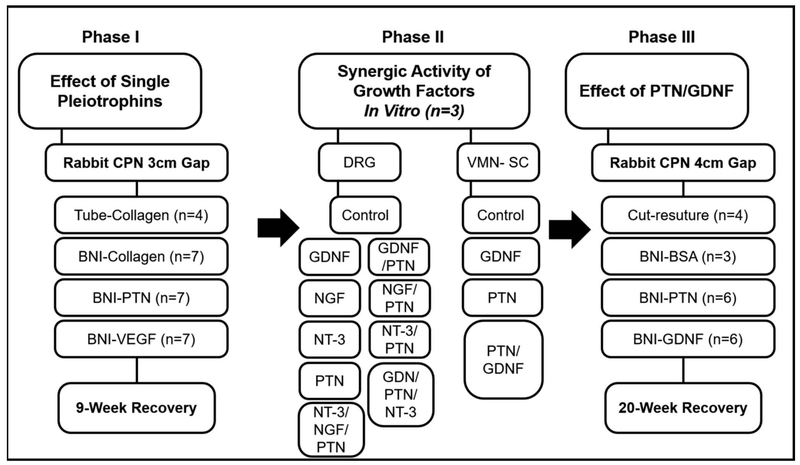
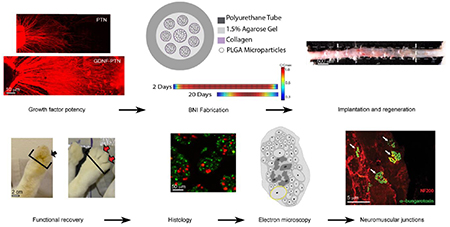
The study was designed in three phases. In the first phase, we tested the effect of pleiotrophins VEGF and PTN in supporting a 3 cm gap repair in the rabbit common peroneal nerve. In the second phase, we evaluated the synergistic effect of PTN combined with neurotrophins in the dorsal root ganglion assay for sensory neurons, the most effective combination was used to confirm the effect in motor axons using the ventral spinal cord assay in vitro, and then tested in a 4-cm gap repair in vivo (Phase III, Fig. 1).
Figure 1.
Experimental design. The flow chart indicates the three phases of the study, the groups, the sample size and timelines for the repair studies.
2.2. Dorsal Root Ganglia and Spinal Cord Explant Cultures
Neonatal (P0 - P4) dorsal root ganglia (DRG) and spinal cords (SC) were dissected from CD1 mice as previously described [26]. Individual DRG explants and SC slices were placed into separate poly-Dlysine (PDL) coated wells, immobilized with collagen (85 % type I, 15% type II; Millipore; Temecula, CA), and incubated at 37°C with 5% CO2 in Neurobasal-A media (Sigma Aldrich; St. Louis, MO) with 2% B27, 0.5% penicillin/streptomycin and 0.75% L-glutamine. Neurotrophins (BDNF, NGF, NT3), GDNF or pleiotrophin (PTN) were added individually or in combination 24 hours after plating at 5–100 ng/mL. Tissue cultured without growth factors were included as negative controls. After three days in vitro, the explants were fixed in 4% paraformaldehyde. Since isolated DRG explant cultures only have sensory neurons, these were labeled using a β tubulin III antibody (1:400; Sigma Aldrich, St. Louis, MO). Conversely, axons in the ventral SC explants are exclusively motor efferents and were labeled with a mouse anti RT97 antibody (1: 200, Santa Cruz Biotech, Dallas, TX) and visualized using 10X objectives on a Zeiss confocal microscope (Zeiss Axioplan 2 LSM 510 META). The axonal length was measured from the ganglia to the most distal end of the axons using Axiovision LM software (CarlZeiss, Axiocam version 4.2.0.1), and axon number estimated using optical densitometry and analyzed using the histogram tool in an average of 50 axons per explant in three separate experiments performed in triplicate (n=9).
2.3. Biosynthetic Nerve Implants
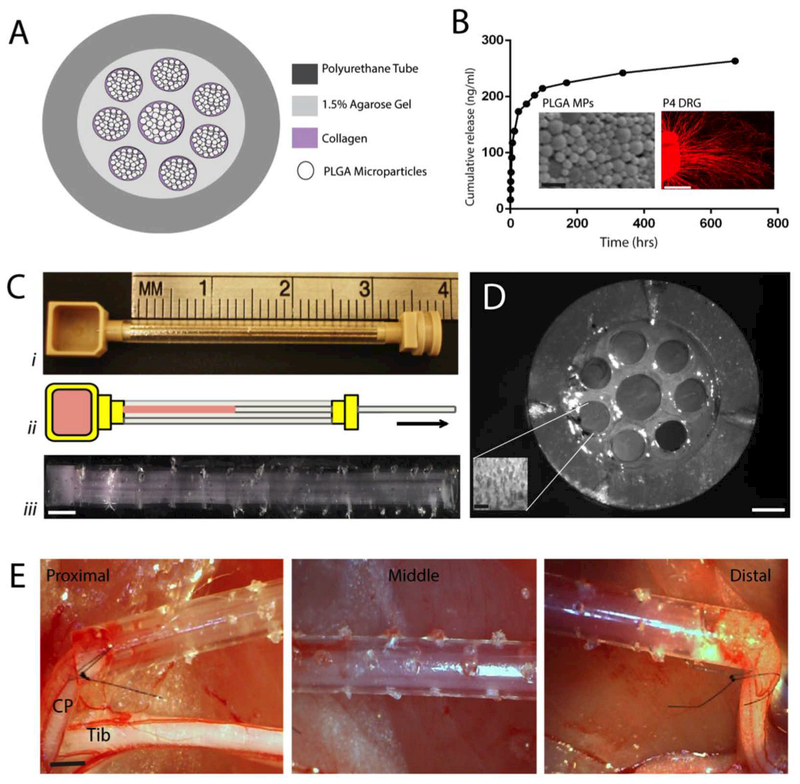
The biosynthetic nerve implant (BNI) conduits consisted of a transparent polyurethane conduit (3 mm OD, 1.7 mm ID; Micro-Renathane®; Braintree Scientific Inc.) 3 or 4 cm in length, with 8 microchannels casted in 1.5% agarose in the lumen of the conduit (7 of 0.4 mm and 1 in the center of 0.5 mm in diameter) providing a total of 0.9 mm2 or 36.2% of the nerve conduit lumen available for nerve regeneration. Each micro-channel was filled with growth factor-loaded PLGA microparticles (MP) suspended in type I collagen, releasing either bovine serum albumin (BSA), PTN, VEGF, GDNF or PTN-GDNF. The MPs were prepared using double emulsion as previously described [27]. Briefly, 10 μg/mL of BSA (Sigma-Aldrich, St Louis, MO), 20 μg/μL of PTN, 5 μg/μL of VEGF (Invitrogen, Carlsbad, CA), or 20 μg/μL of GDNF (PeproTech Inc., Rocky Hill, NJ) were mixed with 50% polylactic: 50% Co-glycolic acid, sonicated and lyophilized. The MP size was determined by scanning electron microscopy (SEM, Hitachi S-3000 N Variable Pressure) and a zeta potential size analyzer, and averaged 1 ± 0.1 μm in diameter. We used COMSOL 4.2 to model the release and diffusion of these proteins from the agarose microchannels in vivo during 20 days post-implantation, As reported before [26], The model considered 1.94 ng/μl of MP in the microchannels and used a combination of Brinkman and Carman-Kozeny models, and Fick’s diffusion law to estimate the growth factor diffusivity from the MPs in individual microchannels over time.
2.4. Animal surgery
A total of 49 adult female New Zealand White rabbits (Myrtle Rabbitry, TN) weighing between 1.5 to 1.8 kilograms were used in this study. The first cohort of 25 animals was used to evaluate the effect of BNI-VEGF and BNI-PTN in a 3 cm gap, compared to BNI-collagen (n=7 each), or collagen-filled nerve conduits (n=4; negative controls), while two rabbits from each group were used for neurofilament peptide (NFP) axon specific staining. The second cohort of 24 animals was used to compare the effect of BNIs filled with either GDNF (n=6), PTN (n=6) or a combination of PTN-GDNF (n=5) compared to BNI-BSA (n=3; negative control) and cut-resuture (n=4; positive control) in a 4 cm critical gap. The peroneal nerve was exposed through a muscle-sparing incision between the semitendinosus and the biceps muscles. A 20–40 mm segment was removed, and the proximal and distal nerve stumps were sutured to the conduit or BNI (either 3 or 4 cm long) and secured it to the underlying muscle with absorbable sutures. The muscle was then sutured, and the skin stapled. A topical antibiotic ointment was applied to the wound. All animals received antibiotic (trimethoprim-sulfa; 0.5 mg/kg oral) and analgesic (sustained release Buprenorphine; 1 mg/kg, sc) treatments post-surgery. The animals were randomly assigned to the treatment groups, and the study design was double-blind, as the samples were coded prior to implantation, and the investigators doing the behavioral testing and histomorphometric analysis were agnostic to the surgical procedures. All animal methods were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Texas at Arlington, according to the NIH guide for the care and use of laboratory animals.
2.5. Toe spread index
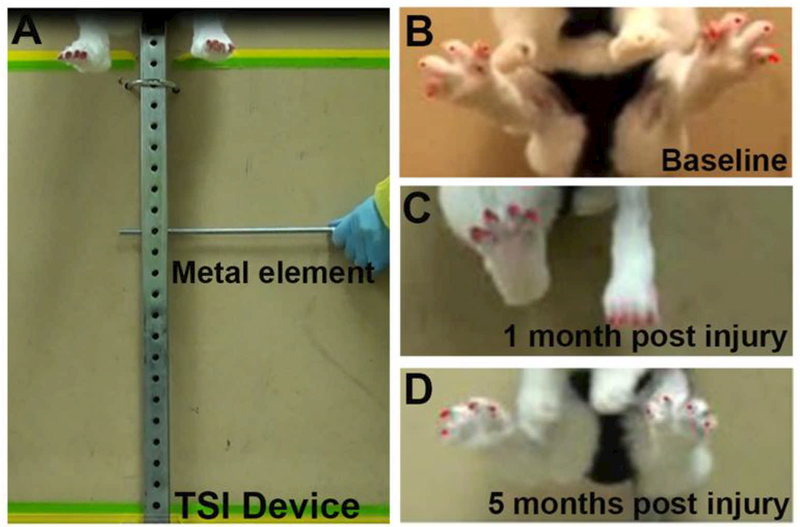
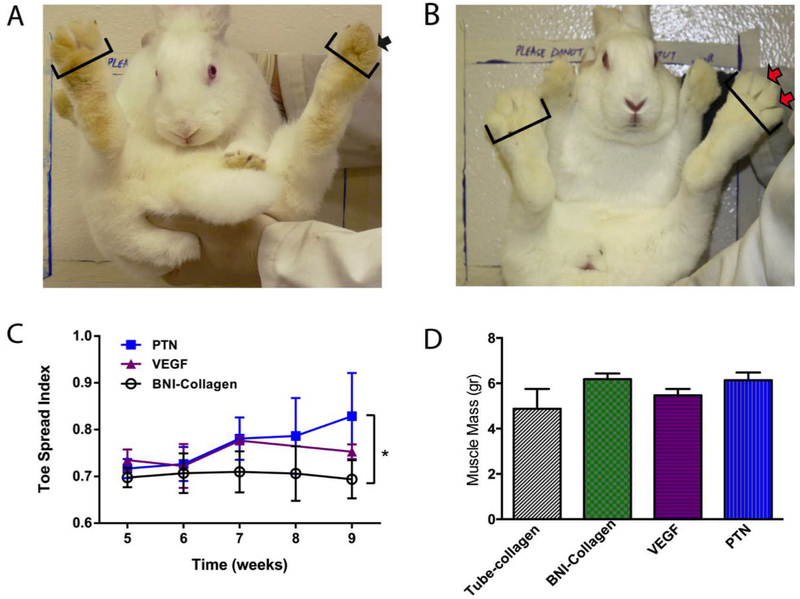
The toe-spread index (TSI) was used to evaluate the deep peroneal nerve reinnervation to the tibialis anterior, the extensor hallucis longus, the extensor digitorum longus, and the fibularis tertius muscles of the hindlimb. TSI is a sensitive indicator of the onset of motor recovery after peroneal injury where the animal spread its toes reflexively in an attempt to maximize the surface area of the foot for a safer landing [28]. For this test, the rabbits were held by the loose skin on their back and suddenly dropped 20–30 cm while providing weight support, to evoke a startle response characterized by toe extension. For the 4 cm gap study, the toe-spread index was measured using a custom-designed apparatus (Fig. 2A) to ensure stability and to eliminate extraneous variables related to human imprecision. The rabbit was secured to the hook via a harness placed at the top of a metal rail secured with stop pin on a support structure. By removing the stop pin, the rabbit was safely dropped evoking the toe-spread response. Multiple frames from video recordings were selected to measure the distance between the first and the fourth toes during the startle response using the Image J software. Measurements were taken before surgery and then monthly for the duration of the study. The toenails were colored to facilitate their visualization. The index was calculated as a ratio of the toe-spread of the injured to the healthy paw (Fig. 2B-D).
Figure 2. Toe spread apparatus.
(A) Photograph of the TSI custom device shows a rabbit secured to a sliding hook via a harness on the top of the device. Removing of the metal element causes the rabbit to free fall and then come to a halt resulting in a startle response. (B) Baseline shows left, and right foot has equal toe spreads. (C) One month after injury shows a loss of toe spread in the injured foot (D) Five months after injury shows the recovery of the toe spread in the left foot.
2.6. Immunohistochemistry
The animals were perfused via intracardial perfusion of 4% paraformaldehyde at the end of their respective treatments; the 3 cm cohort of animals at 9 weeks post-surgery, and the 4 cm cohort 20 weeks after implantation. The repaired nerves were dissected and post-fixed overnight, after which the polyurethane tubes were removed. The regenerated nerve tissue was divided into proximal, middle and distal segments, embedded in paraffin, and sliced in 10 μm coronal sections collected onto glass slides. The deparaffinized tissue was then labeled overnight at 4°C using antibodies specific for axons (NFP, β tubulin III; 1:400; Sigma Aldrich) and myelin (P0; 1:200; Millipore). After rinsing, the tissue was incubated with Cy2-and Cy3 conjugated secondary antibodies for one hour (1:400; Sigma Aldrich, St. Louis, MO). The slides were then counterstained and coverslipped. A Zeiss confocal microscope (Zeiss Axioplan 2 LSM 510 META) was used to evaluate the tissue. The number of axons was quantified using an automated image analysis software, CellC version 2.5 by setting a manual threshold of 0.4 for all the images.
For neuromuscular junction (NMJ) staining, the tibialis anterior muscle was harvested, wet weight recorded, fixed, and cryoprotected in 30% sucrose for 24–48 hours before embedding it in Tek OCT. Thin slices (40 μm) were sectioned in the cryostat and co-labeled with the NF200 antibody (1:200; Sigma Aldrich, St. Louis, MO) to visualize the heavy microfilament subunit in axons, and Cy3-labeled α-bungarotoxin (1: 1000, Invitrogen) to visualize the nicotinic acetylcholine receptor (AChR). Images were taken with a 63X oil immersion objective in the confocal microscope.
2.7. Electrophysiological Evaluation
Five months after implantation of the 4cm implants, the common peroneal nerves of the left (injured) and right (uninjured) legs were re-exposed under anesthesia. Bipolar hook electrodes were placed proximal to the BNI, and constant voltage biphasic pulses were applied using the AM Systems Isolated Stimulator. The motor recruitment from the tibialis anterior muscle was recorded using a second set of bipolar needle electrodes via the Biopack MP36 system. Signal amplitudes for the treatment groups were compared to the cut-resuture control to evaluate the strength of evoked responses.
2.8. Electron microscopy
A nerve segment 2.5 mm distal to the repair site was post-fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer. The tissue was then embedded in epoxy resin, and 1μm thin sections were cut and stained with osmium and examined at 6000x magnification using a JEOL LEM 1200 EX II system. Ten regions across each sample were randomly selected for quantifying axon number and axon diameter using the Image J software.
2.9. Statistical analysis
Analysis of Variance was used to test for statistical significance followed by post hoc Tukey’s or Dunn’s multiple comparison tests using Prism 7 software (GraphPad, Inc). For axon diameter, the Shapiro-Wilk test indicated that the data was not parametric and the Kruskal-Wallis test was used. Data collected from the study are represented as the average of the mean ± standard deviation unless otherwise noted. P values ≤ 0.05 were considered significant.
Results
We first investigated the regenerative effect of pleiotrophins PTN and VEGF released in the BNI, in the repair of a 3 cm long nerve gap (Fig. 3A). Quantification of PTN release from the MPs showed an initial burst at 24 hours, followed by sustained release of 85% of the load over 28 days as determined by ELISA assay (TSZ ELISA, HU9951), in agreement with our previously reported profiles from BSA, NGF [26], VEGF [27] and PTN [29] MPs (Fig. 3B). The bioactivity of the encapsulated protein was evaluated in vitro by determining the axonal growth of treated DRGs. The average axonal length increased when treated with PTN MPs (745 ± 1 μm) compared to cultures with no growth factors (542 ± 167 μm), or those with BSA MPs (553 ± 157 μm) (Fig. 3B). Computer simulation of growth factor concentration (C) over the maximal levels (Cmax) within the BNI at specific time points indicated that the concentration at the middle-distal segment of the BNI could be sustained over 20 days (Fig. 3C). As reported previously [30], a custom-made casting device was used to guide metal fibers in the lumen of the conduit, and liquefied agarose injected into the lumen covering the metal fibers (Fig. 3D-i). After agarose polymerization, the collagen-MPs were added into a loading chamber at one end of the device in which the fibers were previously inserted. Pulling the fibers out simultaneously cast the microchannels and filled the lumen with the collagen-MPs suspension by the generated negative pressure (Fig. 3D-E).
Figure 3. BNI with multi-luminal growth factor release for nerve gap repair.
(A) Cross section schematic of the BNI with MPs mixed in collagen. (B) Release profile of PTN-MPs. Inserts: SEM images of the PTN-MPs and confocal images of DRG axonal growth (β-tubulin; red) demonstrating the bioactivity of the encapsulated growth factors. C) COMSOL model of PTN diffusion in the BNI microchannels overtime. (D) BNI fabrication: (i) placement of metal rods in the silicone conduit and filled with agarose, (ii) rod removal and MPs/collagen loading, (iii) implantable device. (E) Cross section of the BNI showing the eight microchannels with luminal MP-collagen (insert). (F) BNI sutured to both ends of the injured common peroneal (CP) nerve. Tib = tibial nerve. Scale bars: B) SEM: 5 μm, DRG: 1 mm, D) 1 mm, E) Device: 250 μm, Insert: 10 μm. F) 2 mm.
3.1. Regenerative effect of pleiotrophins
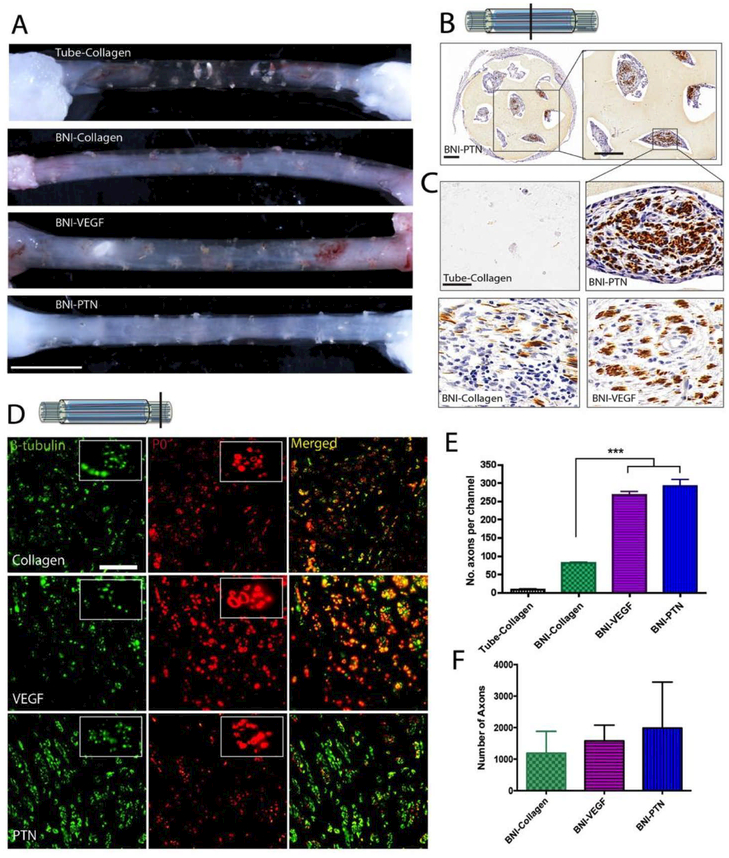
Gross evaluation of the nerves repaired with collagen-filled hollow conduits confirmed the failure of the nerve to grow spontaneously across the 3 cm critical gap, 9 weeks after implantation (Fig. 4A). In contrast, axonal regeneration was observed in animals implanted with BNIs, with some axonal growth in those with collagen (BNI-collagen), which improved when VEGF or PTN releasing MPs were added to the lumen of the BNI microchannels (Fig. 4A). Immunocytochemical visualization of the axonal marker neurofilament peptide (NFP), confirmed the regeneration of nerve tissue in the middle of the implants, where axons were observed in variable numbers in the lumen of most hydrogel microchannels, and more abundant in the VEGF and PTN groups (Fig. 4B). High magnification pictures of NFP+ fibers in representative microchannels with pleiotrophin release show a qualitative increase in the number of regenerating axons (Fig. 4C). In the distal segment, visualization of β-III tubulin (axons) and P0 (myelin) confirmed that regenerating axons in the BNIs grew across the critical gap (Fig. 4D). Quantification of the NFP+ fibers per microchannel in the middle of the BNI revealed a three-fold increase (p ≤ 0.001) in both the BNI-PTN (263 ± 71) and BNI-VEGF (248 ± 29) devices compared to BNI-collagen conduits (75 ± 2; Fig. 4E). While this trend was also observed 2 mm distal to the devices, it did not show statistical significance between the tube-collagen (1193 ± 1375), VEGF (1580 ± 1116) and PTN (1989 ± 3257), mainly due to the high variability within groups (Fig. 4F). The functional significance of the observed regeneration was assessed by toe-spread reflex function. Immediately after injury, all animals showed a TSI of 0.6 to 0.7, a functional deficit that remained for six weeks after nerve repair (Fig. 5A). By week 7, those implanted with BNI-VEGF or BNI-PTN began to show improvements in the ability for toe spreading, which reached significance only in those implanted with BNI-PTN at week 9 (Fig. 5B, 5C; p ≤ 0.05). However, the improvement was moderate, and none of the groups showed significant recovery of the total muscle mass (Fig. 5D), suggesting the possibility of further improvement if combined with neurotrophic support.
Figure 4. Effect of PTN and VEGF in nerve regeneration across a 3 cm-long nerve gap.
(A) Photographs of regenerated nerves 9 weeks after implantation. Collagen-filled conduits failed to mediate nerve growth. Conversely, BNIs with collagen, VEGF-MPs or PTN-MPs showed nerve regeneration. (B) Axonal growth in the BNI was confirmed by positive NFP staining. (C) Representative growth inside the microchannels at the middle of the conduit is shown at higher magnification. (D) Double labeling of axons (β-tubulin) and myelin (P0), confirmed nerve regeneration across the gap. (E) The number of axons per channel and (F) distal to the implant showed a mild effect of PTN. *** = p ≤ 0.001. Scale bars: A) 0.5 cm, (B) 350 μm, (C and D) 50 μm.
Figure 5. Motor functional recovery after a 3 cm gap repair with BNI-PTN.
(A) All injured animals were unable to show digit abduction (black arrow) up to 6 weeks after injury repair. (B, C) By week 9, those implanted with PTN MPs showed significant improvement in toe spreading (red arrows) compared to those with collagen filled conduits. (D) None of the groups showed a significant recovery in the total mass of the tibialis anterior muscle at this time. * = p ≤ 0.05.
3.2. PTN-GDNF synergistic effect on axonal regeneration in vitro
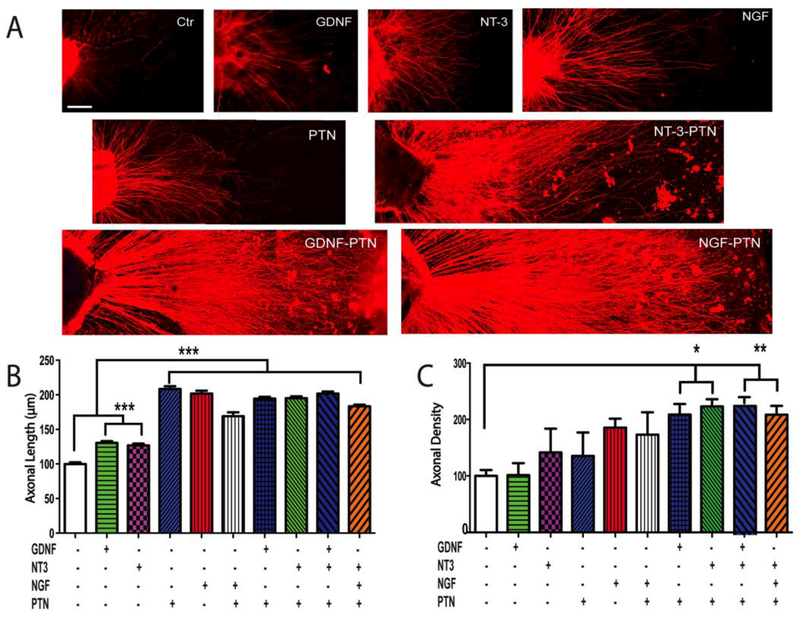
In order to evaluate the possibility of increasing the modest recovery of function of PTN by combining it with other neurotrophic and glial-derived growth factors, we first determine their relative regenerative potency in sensory (DRG) in vitro. We observed that the number of axons elongating from the sensory ganglia and their maximum axonal length was influenced by the growth factor treatments. Qualitatively DRGs with GDNF, NT3 and NGF showed enhanced axonal growth, which was further increased when combined with PTN (Fig. 6A). Quantitative analysis of axonal length in the DRG showed that the maximal growth observed in the absence of growth factors (100 ± 2 μm), was significantly stimulated by PTN (208 ± 4 μm) and NGF (202 ± 4 μm), and the one mediated by GDNF (131 ± 3 μm) and NT-3 (137 ± 4 μm), was potentiated by the addition of the pleiotrophin as seen in those with PTN-NT-3 (195 ± 3 μm) and PTN-GDNF (195 ± 3 μm; p ≤ 0.001; Fig. 6B). The addition of three growth factors did not increase significantly the effect further as indicated by the mean axonal length observed in the PTN-GDNF-NT-3 (202 ± 3 μm). Since axonal length can represent the growth of different populations of DRG neurons, we also evaluated axonal density to estimate the number sensory neurons responsive to the growth factors. This analysis showed a lack of effect of GDNF compared to the negative control, and moderate effects of NT-3 (142 ± 42), PTN (135 ± 42), NGF (186 ± 16), and PTN-NGF (173 ± 40), whereas the combination of PTN-GDNF (209 ± 19) and PTNNT-3 (223 ± 13) showed a statistically significant increase in axonal density compared to that induced by the individual growth factor treatments (Fig. 6C; p ≤ 0.05). The addition of three factors in combination yielded no further increase in axonal number in these cells.
Figure 6. Synergistic growth factor effects on sensory axons in vitro.
(A) Confocal images of neonatal DRG with neurotrophin/pleiotrophin support for axonal growth. (B) Quantitative analysis revealed that PTN, alone or in combination, doubled the axonal length, and that (C) the combination of PTN with GDNF or NT-3 increased the axonal density (number of regenerating axons) compared to control. Axonal density is reported as a percentage of the negative control. *** = p ≤ 0.001, ** = p ≤ 0.01, * = p ≤ 0.05. Scale bar 10 μm.
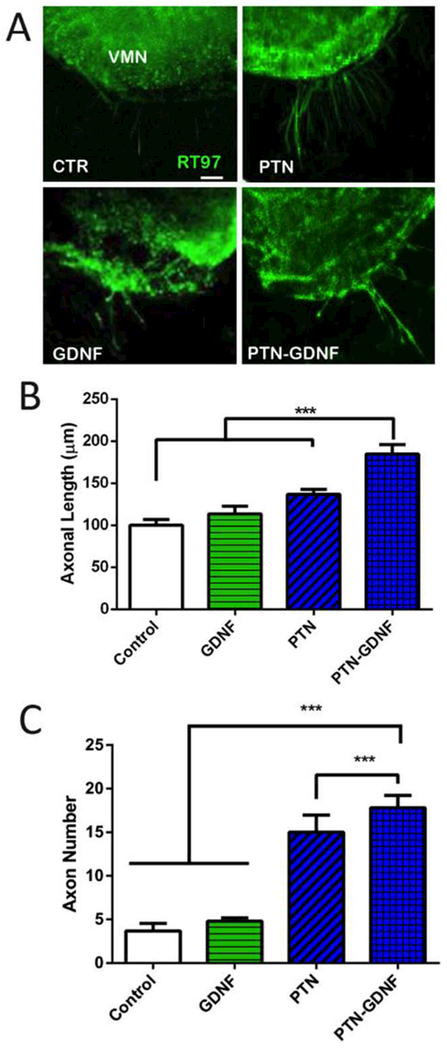
While both GNDF and NT-3 synergized with PTN, given the recognized benefit of GDNF on motor axons, we decided to test whether the PTN-GDNF synergistic effect could be reproduced in ventral spinal cord explants. Control explants with no growth factors showed limited axonal growth in vitro (100 ± 7 μm) which was not different from those with PTN (136 ± 6 μm) or GDNF (113 ± 9 μm; Figure 7A-B). However, the PTN-GDNF combination showed a significant increase (Fig. 7C; p ≤ 0.001) in the number of regenerating axons (185 ± 11 μm). This effect was also observed in the number of growing axons from the spinal cord explants, which were limited in the control (4 ± 1) and GDNF (5 ± 0.4), and significantly improved by PTN (15 ± 2), and enhanced by the PTN-GDNF combination (18 ± 1; p ≤ 0.001). The lack of response of GDNF was surprising which indicated that the assay was insensitive to this growth factor alone but sufficient to reveal a synergistic effects of the combined treatments. Taken together, the in vitro data on motor and sensory neuron growth indicated that a combination of PTN-GDNF would provide a synergistic effect on nerve regeneration, particularly on critical nerve gaps.
Figure 7. Enhanced motor nerve growth in SC slices by PTN-GDNF.
(A) Axon growth from the ventral SC explants. (B) PTN-GDNF showed a significant improvement on axon length compared to control, or PTN and GDNF alone. (C) Compared to control and GDNF, PTN was able to show a significant effect in the number of extending axons, which was further improved in the PTN-GDNF group. The mean represents the average of 20 axons per SC slice and four separate experiments per group. ***=p ≤ 0.001. Scale Bar 50 μm
3.3. PTN-GDNF mediates nerve regeneration across a 4 cm critical gap.
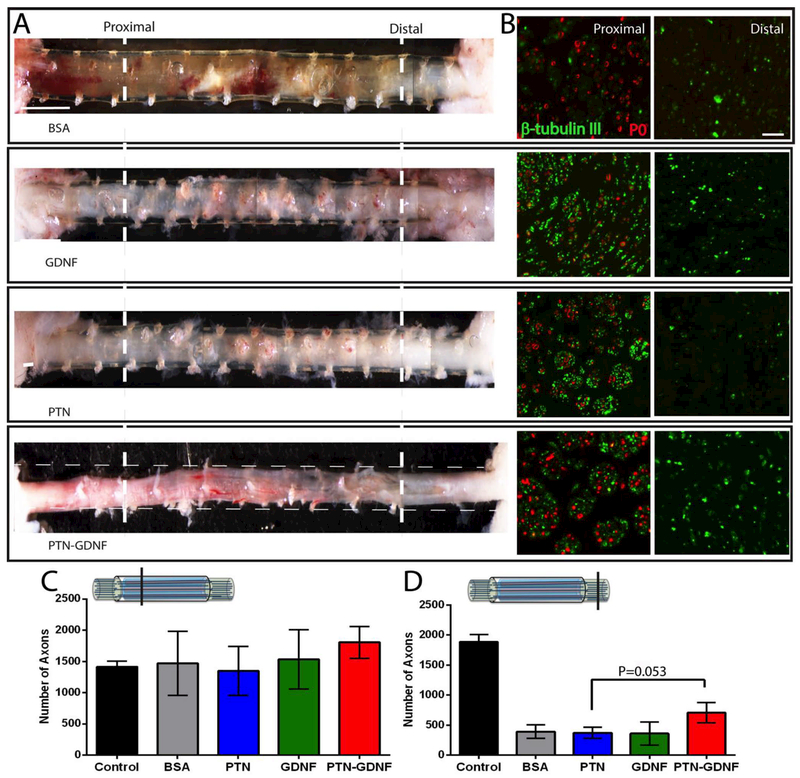
The release of PTN in the BNI provided only a moderate effect on the 3 cm gap, and the systematic in vitro screening for synergistic effects between PTN and other growth factors indicated that PTNGDNF stimulates the growth of both motor and sensory neurons. We then tested the regenerative effect of GDNF, PTN or a PTN-GDNF combination in a 4 cm critical nerve gap lesion, and compared it to cut and re-suture controls (i.e., idealized positive control not clinically available), and BNI-BSA as a negative control. Five months after implantation, the gross inspection of the regenerated tissue confirmed that BNI repaired nerves were able to regenerate in all groups, connecting the proximal and distal nerve stumps. The regenerated nerve appeared denser in those with growth factor support compared to the BSA control (Fig. 8A). Double immunofluorescence of tissue sections taken at 1 cm from the proximal end, showed that most of the β-tubulin positive axons in the BSA group were not remyelinated (P0), and that all growth factors were able to improve in the apparent number of regenerating axons. However, evaluation of the tissue 1 cm from the distal end, showed a qualitative reduction in the number of axons, particularly in the BSA and PTN groups (Fig. 8B). Quantification of the number of axons in the proximal end showed similar growth on all the BNI groups ranging from 1350 to1806, compared to the cut-resuture group (1412 ± 186; Fig. 8C). However, the number of axons distal to the repair site revealed a reduction in the number of axons in all the BNI groups. While the cut-resuture group maintained the similar number of axons that were counted proximally (1885 ± 247), those with BSA, PTN and GDNF showed less than 25% of those in the positive control group (361 ± 394). However, those with a PTN-GDNF doubled the number of axons distally (708 ± 385) compared to the other BNI treatments to approximately 37.55% of those in the positive control group, and different compared to the PTN group (372 ± 231; p ≤ 0.053; Fig. 8D).
Figure 8. Effect of PTN-GDNF on nerve regeneration across a 4 cm nerve gap.
(A) Photographs of the regenerated nerves in the conduits or with the conduit indicated by longitudinal broken lines. (B) β-tubulin/P0 immunofluorescence at 1 cm (proximal) and 3 cm (distal) from the proximal end. Enhanced axonal regeneration is evidenced in the PTN-GDNF group. The number of axons proximally (C) is comparable among the groups, but (D) reduced distally in those with BNI implants, where PTN-GDNF showed a trend towards significance over the PTN group (p=0.053). Scale Bar: A) 600 μm, B) 50 μm.
Despite the effect on nerve regeneration in the PTN-GDNF group, only a small number of axons were myelinated distally in the BNI repaired nerves (Fig. 8B).
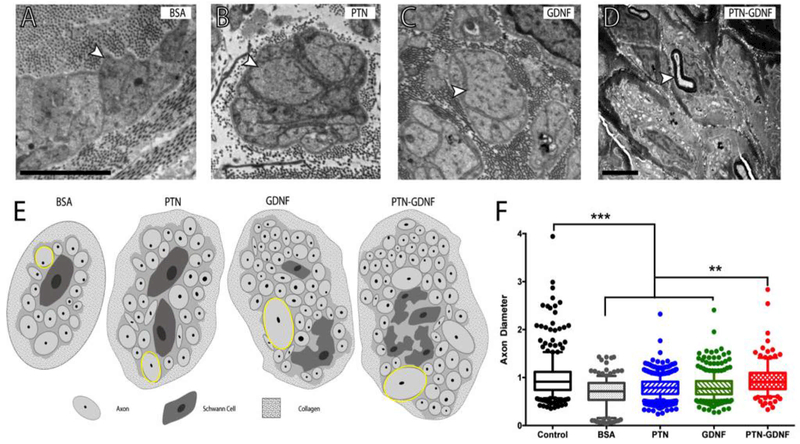
To better understand this phenomenon we evaluated the tissue distal to the implants with electron microscopy. We observed that the regenerated tissue in the BSA group was composed of small diameter unmyelinated axons, mostly located within Remak bundles (Fig. 9A), with an apparent increase in axon diameter in those with GDNF and PTN (Fig. 9B-D). Large diameter axons were also found in the PTN-GDNF group within Remak ensheathing as well as some re-myelination of large axons (Fig. 9E). Evaluation of the axon diameter distribution revealed that the mean fiber size in the cut-resuture control was 1 ± 0.5 μm, with large axons up to 7 μm in diameter. Those with BSA averaged 0.7 ± 0.3 μm in diameter, and no axons larger than 2 μm were observed, whereas GDNF and PTN induced a moderate increment in axon diameter to 0.8 ± 0.5 μm and 0.8 ± 0.2 μm; respectively. In contrast, animals implanted with PTN-GDNF BNIs showed average axon diameter (1 ± 0.4 μm) similar to the cut-resuture control and statistically different compared to the BSA and individual growth factor treatments (Fig. 9F; p ≤ 0.01). This result suggested that while axons regenerated across the BNI, the resident Schwann cells failed to support axon sorting and remyelination, underscoring a limitation in the growth-promoting milieu provided by the distal stump to the regenerating axons.
Figure 9. Radial sorting delay.
Electron micrographs of axons in Remak bundles. (A) Compared to those with BSA, larger axons were observed in the (B) PTN and (C) GDNF implants (arrowheads). (D) Re-myelinated axons were only present in the PTN-GDNF group. (E) Schematic representation of axons in the Remak bundles per treatment. (F) Axon diameter distribution showed significantly smaller axons in the BSA, PTN and GDNF groups compared to the PTN-GDNF. Scale bar: 5 μm. *** = p ≤ 0.001, ** = p ≤ 0.01.
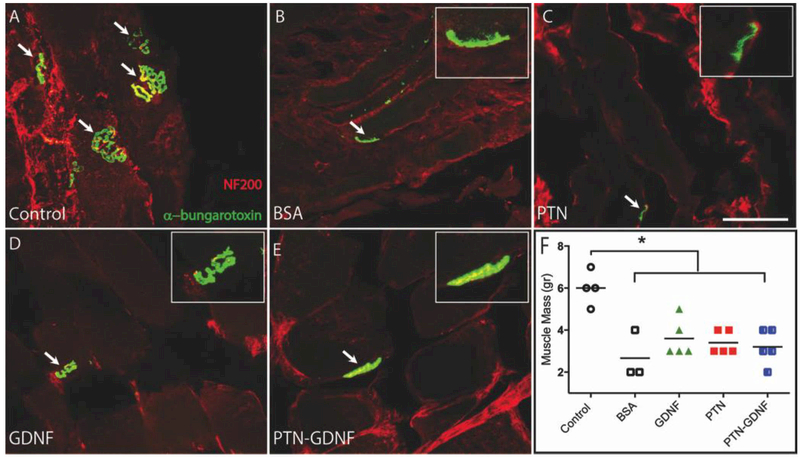
To determine if the axons in these groups were able to regrow to their muscle targets, we labeled the neuromuscular junctions (NMJ) in the tibialis anterior muscle with double immunofluorescence for NF200 and α-bungarotoxin which binds to acetylcholine receptors (AchR). The NMJs on the cutresuture group were large and abundant (5–7 per section; Fig 10A) and those with BSA and PTN showed fewer (2 per section) and smaller clustered AchR, mostly negative for NF200 (Fig. 10B, C). In contrast, those with GDNF and PTN-GDNF showed a range of 3–5 NMJ per section, slightly larger than those in the BSA and PTN groups, with evident NF200/AchR co-labeling, indicating that these were re-innervated muscles (Fig. 10D, E). However, quantification of the tibialis anterior muscle mass was significantly larger in the cut-resuture control (6 ± 0.4 g) compared to all others with a growth factor (3 ± 0.4 g) or BSA (3 ± 0.7 g; Fig. 10F). The data suggested that the PTN-GDNF treatment was able to mediate some muscle reinnervation but less efficiently compared to the re-sutured positive control.
Figure 10. Re-innervation of the tibialis anterior muscle.
(A) Co-labeling of axons (NF200) and AchR clustering (α-bungarotoxin) demonstrated the successful target re-innervation. The amount of axon/NMJs overlap was larger in the GDNF (D) and PTN-GDNF (E) compared to the BSA (B) and PTN (C) groups, but not as elaborate as the (cut-resuture) control. (F) Muscle mass did not improve significantly compared to the positive control. Scale bar: 5 μm. * = p ≤ 0.05.
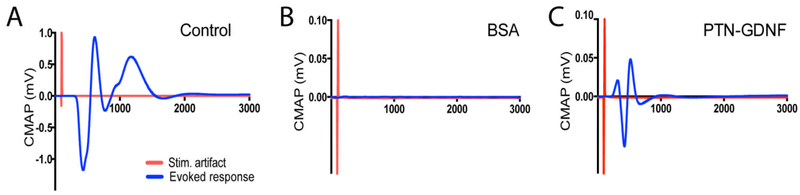
To determine whether these axons were functional, we conducted an electrophysiological evaluation of compound motor action potentials (CMAPs) on the tibialis anterior muscle evoked from stimulating electrodes placed proximal to the repair site. This test confirmed that electrical stimulation elicited CMAP with a peak amplitude of 0.7 ± 0.2 mV at 3X threshold in the cut-resuture group (Figure 11A). Conversely, no activity was evoked in animals implanted with BSA, GDNF or PTN, and only 1 of 5 rabbits repaired with PTN-GDNF showed evoked CMAP, with just 10 % (0.05 mV) of the amplitude compared to cut-resuture controls (Figure 11B-C). This result was inconsistent with the number of axons observed to regenerate across the 4 cm gap in the BNI-implanted animals, which in all cases were more than 10%, but in agreement with the limited re-myelination of the regenerated nerves.
Figure 11. Evoked compound action potentials from regenerated nerves.
(A) Compound action potentials were evoked in the cut-resuture control but failed in all animals implanted with (B) BSA, GDNF or PTN BNIs. (C) Only 1 of 5 rabbits repaired with PTN-GDNF showed a CMAP with an approximately 10% of the amplitude generated in the control group.
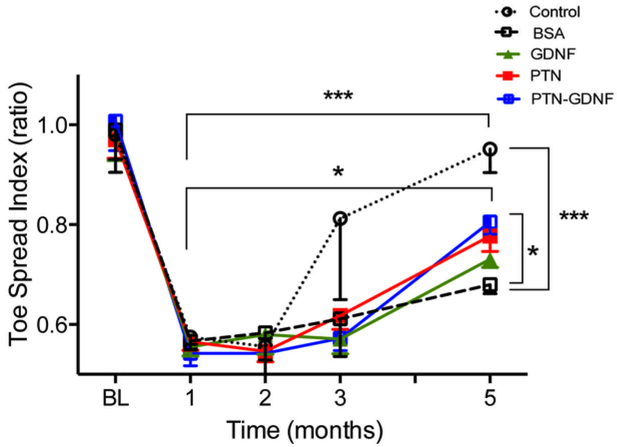
Evaluation of the recovery of function by toe-spread index (TSI) during five months after the 4 cm gap repair revealed 40% loss of function immediately after injury compared to baseline, which improved over the course of 5 months (Fig. 12). Signs of functional recovery were first observed in the cutresuture group after three months, whereas those with BNI showed signs of improving two months later. This was confirmed by two-way ANOVA with repetitive measurements which showed significant effects of treatment (F4,84 = 2.9; p ≤ 0.03) and time (F4,84 = 68.4; p ≤ 0.0001). Five months after the repair surgery, a 76% improvement in TSI was observed in animals with PTN-GDNF, which was significant compared to the BSA (p ≤ 0.05), but failed to reach the 95% regain of function observed in the cut-resuture control group (p ≤ 0.0001) (Fig. 12). This data confirmed that the PTN-GDNF combination was able to entice some functional nerve regeneration to the distal targets compared to the negative BSA control and those with individual growth factor treatments.
Figure 12. PTN-GDNF mediates moderate functional recovery after critical nerve gap repair.
Evaluation of toe-spread index after five months confirmed that animals implanted with PTN-GDNF BNIs were significantly better compared to those implanted with BSA, but not compared to the positive control. *** = p ≤ 0.001, * = p ≤ 0.05.
Together, our findings demonstrated that the topographical multiluminal design of the BNI supplemented with sustained release of PTN-GDNF allowed the moderate regeneration of axons across a critical 4 cm critical gap and mediated partial restoration of function after common peroneal nerve lesion in rabbits.
3. Discussion
Multi-channel conduits are recognized as a viable strategy to entice linear regeneration in injured peripheral nerves and mimic the multi-fascicular anatomy of this tissue [31]. We and others have demonstrated that multi-channel conduits are effective in nerve regeneration across 1 cm gaps in the rat sciatic nerve mode [32,33] and this approach has also been reported beneficial in repairing a 1.5 cm defect in the rat [34]. It is also recognized that long critical lesion in the rabbit and cat models require growth factor support such as GDNF for optimal regeneration and target reinnervation [35–37]. Despite this progress, the type of growth factors needed, and whether they should be neurotropic or stimulate additional cellular targets including Schwann cells, fibroblasts, and endothelial cells, have not been fully elucidated [11]. Recent studies in the rat using a 1 cm injury gap model reported that NGF induced a modest improvement in the number of re-myelinated axons compared to BDNF, NT-3, GDNF, and FGF [38]. Others have shown that co-releasing GDNF and NGF from collagen nerve guides coated with PLGA have a beneficial effect [39]. However, the early effect in stimulating axonal growth did not translate into increased myelination or functional recovery [40]. Additional studies have tested the synergistic effect of granulocyte colony stimulating factor (GCSF) and VEGF using gene transfection into the murine sciatic nerve prior to a very short (0.3 cm) gap repair using a hollow conduit and reported improved recovery of function. These reports indicated that a combined growth factor therapy might offer distinct advantages over single neurotrophic support, and raised the possibility of an improved functional outcome from a broad range of cellular targets during nerve repair. In the first phase of this study, we tested the effect of VEGF and PTN in a 3 cm gap, with the objective of comparing the regenerative potency between these pleiotrophins, and to confirm that PTN will provide neurotrophic support without inducing abnormal growth. The results indicated that both growth factors stimulate nerve growth, which was observed unevenly distributed in the microchannels, but the regenerative effect was modest. Since both PTN and GDNF are significantly up-regulated in Schwann cells after injury [9], it seemed plausible that a combination of neurotrophins, glial derived growth factors, and PTN would offer synergistic regenerative effects. We confirmed this possibility by measuring the regenerative potency of several growth factors in both sensory and motor neurons, in vitro. Our results are consistent with an additive effect of PTN and GDNF in stimulating a larger number of responding neurons with long axon extensions.
In the second repair study, we compared the regenerative efficacy of multi-luminal BNIs filled with PTN, GDNF or PTN-GDNF and compared to optimal regenerative control, the cut-resutured approach and to BSA negative implants, in a 4 cm critical nerve injury. This approach enticed nerve regeneration across the critical gap as indicated by the number of axons distal to the BNI which in the PTN-GDNF group was estimated to be 37% of that in the cut-resuture idealistic positive control, and supported by the significant in increased in the diameter of those axons (Fig. 9F), mediating partial reinnervation of the anterior tibialis muscle, and moderate, but significant recovery in toe spreading compared to the single growth factor treatments (Fig. 12). Together, the results indicate a modest, but a significant regenerative benefit of combining pleiotrophic and glial derived growth factor sustained release in multi-channel nerve scaffolds, for the regeneration of critical gap injuries
The synergistic PTN-GDNF effect may be explained by an increase in regenerative neural capacity and the recruitment of non-neuronal cells. These possibilities are supported by previous reports indicating that GDNF binds to the receptor tyrosine kinase Ret receptor which functions as a multifunctional co-receptor for guidance molecules [41] and to GFRα co-receptors in both motor neurons and non-peptidergic DRG sensory neurons [42]. It is also known that the Ret receptor is modulated by NGF during development [43] and after injury [44]. Conversely, PTN binds to ALK receptors in SC motor neurons and DRG cells, which are up-regulated after injury [25,45]. DRG neurons also express the Syndecan-1 PTN receptor with antiapoptotic effects during development [46], and contributes to the survival of injured neurons [47,48]. Sensory neurons also express the RPTPα/ζ PTN [49], which stimulates the phosphorylation of Fyn, a member of the Src family involved in oligodendrocyte differentiation, myelination, and re-myelination [50–52], suggesting a similar role in Schwann cells. These studies indicate that GDNF and PTN are synergistic by increasing the growth capacity of single cells, and by activating different cell types in the injured nerve including Schwann cells, fibroblasts, endothelial cells, DRG sensory axons and SC motor neurons [25,45,53,54]. However, more studies are necessary to define the precise cellular and molecular mechanisms that mediate the synergistic effects observed with PTN and GDNF in the regeneration of adult peripheral nerves.
Despite the observed effect of PTN-GDNF in axon regeneration, most of these axons were associated with Remak bundles and showed limited re-myelination distal to the implant. A similar defect occurs in mice with loss of Grb2-associated binder 2, a scaffolding protein involved in Neuregulin 1 type III (NRG1)/ErbB receptor signaling in Schwann cells [55,56]. NRG1 is known to be up-regulated by Schwann cells after losing contact with the axons due to Wallerian degeneration and is required for later stages of Schwann cell re-differentiation and re-myelination [57]. Thus, it is possible that regenerative axons produce insufficient NRG1 necessary for re-myelination, needed to proceed through radial sorting [58]. Alternatively, the data can be interpreted to indicate that regeneration supported by PTN-GDNF, albeit delayed compared to the re-sutured control, may proceed normally with additional time. This notion is suggested by the recovery in toe-spreading function, first observed 3 months after injury in the cut-resuture animals, and 5 months post-injury in the PTN-GDNF group. This might explain the significant number of axons distal to the implant, but insufficient muscle reinnervation and minimal CMAP activity. However, axonal regeneration is expected to proceed at 1–2 mm a day, and five months should be enough time for axons to regenerate 2 mm distal to the injury site. An additional scenario might be the retention of axons in the middle of the BNI due to the sustained release of growth factors, although this possibility seems less likely due to the expected depletion of the growth factor release by 8 weeks. It is also possible that the regenerative capacity of the neurons diminished overtime despite the growth factor support. This possibility could be addressed by incorporating methods for priming the regenerative potential of injured nerves such as electrical stimulation for 1 hour prior to the repair [59–61]. Also, the uneven axonal growth in the micro-channels likely represents microdomains of a focal release of growth factors by the MPs, which are also randomly loaded into the lumen. This can also be improved by methods that provide a more precise control of growth factor release, particularly forming a controllable chemotactic gradient [62–64] To that end, we previously reported that polymeric coils placed in the walls of the agarose microchannels, can be used to form chemotactic gradients that improved the linear growth of axons in vitro [26]. It remains to be determined if the combination of methods that accelerate axonal growth can improve the functional regeneration of long critical nerve defects.
In summary, our results demonstrate that the topographical multiluminal structural design of the BNI with sustained PTN-GDNF release successfully entice moderate functional nerve regeneration across a 4 cm long gap defect. This result is significant not only because this effect was compared to an idealized best positive cut-suture control, not clinically available, but also because this growth was achieved with only 38% of the luminal area in the BNI (0.9 mm2) compared to collagen filled conduits (2.3 mm2) that fail at 3 cm. Future devices will benefit from strategies in which the structural support provided by agarose in the BNI could be replaced or degraded over time in order to further increase the total growth area for nerve repair in these devices.
Statement of significance:
Nerve injuries due to trauma or tumor resection often result in long gaps that are challenging to repair. The best clinical option demands the use of autologous grafts that are associated with serious side effects. Bioengineered nerves are considered a good alternative, particularly if supplemented with growth factors, but current options do not match the regenerative capacity of autografts. This study revealed the synergistic effect of neurotrophins and pleiotrophins designed to achieve a broad cellular regenerative effect, and reports that GDNF- PTN are able to mediated axonal growth and partial functional recovery in a 4 cm nerve gap injury, albeit delays in remyelination. This report underscores the need for defining an optimal growth factor support for biosynthetic nerve implants.
Acknowledgments
We thank Lokesh Patil for assistance in the computer modeling and Dr. Bryan Black for suggestions on the preparation of the manuscript. We would like to thank Vinit Sheth for his help in video screening for the toe spread index. This work was supported by NIH/NINDS R21NS072955–01A1 grant (MIR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing Interests
The authors declare that they have no competing interests.
REFERENCES
- [1].Höke A, Brushart T, Introduction to special issue: Challenges and opportunities for regeneration in the peripheral nervous system, Exp. Neurol 223 (2010) 1–4. doi: 10.1016/j.expneurol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Grinsell D, Keating CP, Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies, Biomed Res. Int 2014 (2014). doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fu SY, Gordon T, Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation., J. Neurosci 15 (1995) 3886–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Krarup C, Archibald SJ, Madison RD, Factors that influence peripheral nerve regeneration: An electrophysiological study of the monkey median nerve, Ann. Neurol 51 (2002) 69–81. doi: 10.1002/ana.10054. [DOI] [PubMed] [Google Scholar]
- [5].Moore AM, Kasukurthi R, Magill CK, Farhadi FH, Borschel GH, Mackinnon SE, Limitations of conduits in peripheral nerve repairs, Hand 4 (2009) 180–186. doi: 10.1007/s11552-008-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pabari A, Lloyd-Hughes H, Seifalian AM, Mosahebi A, Nerve conduits for peripheral nerve surgery, Plast. Reconstr. Surg 133 (2014) 1420–1430. doi: 10.1097/PRS.0000000000000226. [DOI] [PubMed] [Google Scholar]
- [7].Gordon T, Tyreman N, Raji MA, The Basis for Diminished Functional Recovery after Delayed Peripheral Nerve Repair, J. Neurosci 31 (2011) 5325–5334. doi: 10.1523/JNEUROSCI.6156-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hoke A, Schwann Cells Express Motor and Sensory Phenotypes That Regulate Axon Regeneration, J. Neurosci 26 (2006) 9646–9655. doi: 10.1523/JNEUROSCI.1620-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Brushart TM, Aspalter M, Griffin JW, Redett R, Hameed H, Zhou C, Wright M, Vyas A, Höke A, Schwann cell phenotype is regulated by axon modality and central-peripheral location, and persists in vitro, Exp. Neurol 247 (2013) 272–281. doi: 10.1016/j.expneurol.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Romero MI, Rangappa N, Garry MG, Smith GM, Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy., J. Neurosci 21 (2001) 8408–8416. doi:21/21/8408 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gordon T, The role of neurotrophic factors in nerve regeneration, Neurosurg. Focus 26 (2009) E3. doi: 10.3171/FOC.2009.26.2.E3. [DOI] [PubMed] [Google Scholar]
- [12].Romero MI, Lin L, Lush ME, Lei L, Parada LF, Zhu Y, Deletion of Nf1 in Neurons Induces Increased Axon Collateral Branching after Dorsal Root Injury, J. Neurosci 27 (2007) 2124–2134. doi: 10.1523/JNEUROSCI.4363-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Patel M, Mao L, Wu B, VandeVord PJ, GDNF-chitosan blended nerve guides: A functional study, J. Tissue Eng. Regen. Med 1 (2007) 360–367. doi: 10.1002/term.44. [DOI] [PubMed] [Google Scholar]
- [14].Wood MD, Kim H, Bilbily A, Kemp SWP, Lafontaine C, Gordon T, Shoichet MS, Borschel GH, GDNF released from microspheres enhances nerve regeneration after delayed repair, Muscle and Nerve 46 (2012) 122–124. doi: 10.1002/mus.23295. [DOI] [PubMed] [Google Scholar]
- [15].Tajdaran K, Gordon T, Wood MD, Shoichet MS, Borschel GH, A glial cell line-derived neurotrophic factor delivery system enhances nerve regeneration across acellular nerve allografts, Acta Biomater 29 (2015) 1–9. doi: 10.1016/j.actbio.2015.10.001. [DOI] [PubMed] [Google Scholar]
- [16].Boyd JG, Gordon T, Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo, Exp. Neurol 183 (2003) 610–619. doi: 10.1016/S0014-4886(03)00183-3. [DOI] [PubMed] [Google Scholar]
- [17].Magill CK, Moore AM, Yan Y, Tong AY, MacEwan MR, Yee A, Hayashi A, Hunter DA, Ray WZ, Johnson PJ, Parsadanian A, Myckatyn TM, Mackinnon SE, The differential effects of pathway- versus target-derived glial cell line–derived neurotrophic factor on peripheral nerve regeneration, J. Neurosurg 113 (2010) 102–109. doi: 10.3171/2009.10.JNS091092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Madduri S, Feldman K, Tervoort T, Papaloïzos M, Gander B, Collagen nerve conduits releasing the neurotrophic factors GDNF and NGF, J. Control. Release 143 (2010) 168–174. doi: 10.1016/j.jconrel.2009.12.017. [DOI] [PubMed] [Google Scholar]
- [19].Fang W, Hartmann N, Chow DT, Riegel AT, Wellstein A, Pleiotrophin stimulates fibroblasts and endothelial and epithelial cells and is expressed in human cancer, J. Biol. Chem 267 (1992) 25889–25897. [PubMed] [Google Scholar]
- [20].Blondet B, Carpentier G, Lafdil F, Courty J, Pleiotrophin Cellular Localization in Nerve Regeneration after Peripheral Nerve Injury, J. Histochem. J. Histochem. Cytochem 53 (2005) 971–977. doi: 10.1369/jhc.4A6574.2005. [DOI] [PubMed] [Google Scholar]
- [21].Xu C, Zhu S, Wu M, Han W, Yu Y, Functional receptors and intracellular signal pathways of midkine (MK) and pleiotrophin (PTN)., Biol. Pharm. Bull 37 (2014) 511–20. doi: 10.1248/bpb.b13-00845. [DOI] [PubMed] [Google Scholar]
- [22].Kadomatsu K, Muramatsu T, Midkine and pleiotrophin in neural development and cancer, Cancer Lett 204 (2004) 127–143. doi: 10.1016/S0304-3835(03)00450-6. [DOI] [PubMed] [Google Scholar]
- [23].Ochiai K, Muramatsu H, Yamamoto S, Ando H, Muramatsu T, The role of midkine and pleiotrophin in liver regeneration, Liver Int 24 (2004) 484–491. doi: 10.1111/j.1478-3231.2004.0990.x. [DOI] [PubMed] [Google Scholar]
- [24].Blondet B, Carpentier G, Ferry A, Courty J, Exogenous pleiotrophin applied to lesioned nerve impairs muscle reinnervation, Neurochem. Res 31 (2006) 907–913. doi: 10.1007/s11064-006-9095-x. [DOI] [PubMed] [Google Scholar]
- [25].Mi R, Chen W, Höke A, Pleiotrophin is a neurotrophic factor for spinal motor neurons., Proc. Natl. Acad. Sci. U. S. A 104 (2007) 4664–4669. doi: 10.1073/pnas.0603243104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Alsmadi N, Patil LS, Hor EM, Lofti P, Razal JM, Chuong C, Wallace GG, Romero-ortega MI, Coiled polymeric growth factor gradients for multi-luminal neural chemotaxis, Brain Res 1619 (2015) 72–83. doi: 10.1016/j.brainres.2015.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dawood AF, Lotfi P, Dash SN, Kona SK, Nguyen KT, Romero-Ortega MI, VEGF Release in Multiluminal Hydrogels Directs Angiogenesis from Adult Vasculature In Vitro, Cardiovasc. Eng. Technol 2 (2011) 173–185. doi: 10.1007/s13239-011-0048-4. [DOI] [Google Scholar]
- [28].Schmitz HC, Beer GM, The toe-spreading reflex of the rabbit revisited--functional evaluation of complete peroneal nerve lesions., Lab. Anim 35 (2001) 340–345. doi: 10.1258/0023677011911930. [DOI] [PubMed] [Google Scholar]
- [29].Anand S, Desai V, Alsmadi N, Kanneganti A, Nguyen DHT, Tran M, Patil L, Vasudevan S, Xu C, Hong Y, Cheng J, Keefer E, Romero-Ortega MI, Asymmetric Sensory-Motor Regeneration of Transected Peripheral Nerves Using Molecular Guidance Cues, Sci. Rep 7 (2017). doi: 10.1038/s41598-017-14331-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tansey KE, Seifert JL, Botterman B, Delgado MR, Romero MI, Peripheral nerve repair through multi-luminal biosynthetic implants, Ann. Biomed. Eng 39 (2011) 1815–1828. doi: 10.1007/s10439-011-0277-6. [DOI] [PubMed] [Google Scholar]
- [31].Wieringa PA, Gonçalves de Pinho AR, Micera S, van Wezel RJA, Moroni L, Biomimetic Architectures for Peripheral Nerve Repair: A Review of Biofabrication Strategies, Adv. Healthc. Mater 1701164 (2018) 1–19. doi: 10.1002/adhm.201701164. [DOI] [PubMed] [Google Scholar]
- [32].Yao L, de Ruiter GCW, Wang H, Knight AM, Spinner RJ, Yaszemski MJ, Windebank AJ, Pandit A, Controlling dispersion of axonal regeneration using a multichannel collagen nerve conduit, Biomaterials 31 (2010) 5789–5797. doi: 10.1016/j.biomaterials.2010.03.081. [DOI] [PubMed] [Google Scholar]
- [33].Tansey KE, Seifert JL, Botterman B, Delgado MR, Romero MI, Peripheral nerve repair through multi-luminal biosynthetic implants, Ann. Biomed. Eng 39 (2011) 1815–1828. doi: 10.1007/s10439-011-0277-6. [DOI] [PubMed] [Google Scholar]
- [34].Hu X, Huang J, Ye Z, Xia L, Li M, Lv B, Shen X, Luo Z, A novel scaffold with longitudinally oriented microchannels promotes peripheral nerve regeneration., Tissue Eng. Part A 15 (2009) 3297–3308. doi: 10.1089/ten.tea.2009.0017. [DOI] [PubMed] [Google Scholar]
- [35].Young RC, Terenghi G, Wiberg M, Poly-3-hydroxybutyrate (PHB): A resorbable conduit for long-gap repair in peripheral nerves, Br. J. Plast. Surg 55 (2002) 235–240. doi: 10.1054/bjps.2002.3798. [DOI] [PubMed] [Google Scholar]
- [36].Mohanna PN, Young RC, Wiberg M, Terenghi G, A composite pol-hydroxybutyrate-glial growth factor conduit for long nerve gap repairs, J. Anat 203 (2003) 553–565. doi: 10.1046/j.1469-7580.2003.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Deumens R, Bozkurt A, Meek MF, Marcus MAE, Joosten EAJ, Weis J, Brook GA, Repairing injured peripheral nerves: Bridging the gap, Prog. Neurobiol 92 (2010) 245–276. doi: 10.1016/j.pneurobio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- [38].Santos D, Giudetti G, Micera S, Navarro X, Del Valle J, Focal release of neurotrophic factors by biodegradable microspheres enhance motor and sensory axonal regeneration in vitro and in vivo, Brain Res 1636 (2016) 93–106. doi: 10.1016/j.brainres.2016.01.051. [DOI] [PubMed] [Google Scholar]
- [39].Madduri S, di Summa P, Papaloïzos M, Kalbermatten D, Gander B, Effect of controlled co-delivery of synergistic neurotrophic factors on early nerve regeneration in rats, Biomaterials 31 (2010) 8402–8409. doi: 10.1016/j.biomaterials.2010.07.052. [DOI] [PubMed] [Google Scholar]
- [40].de Boer R, Borntraeger A, Knight AM, Hebert-Blouin MN, Spinner RJ, Malessy MJ, Yaszemski MJ, Windebank AJ, Short- and long-term peripheral nerve regeneration using a polylactic-co-glycolic-acid scaffold containing nerve growth factor and glial cell line-derived neurotrophic factor releasing microspheres, J Biomed Mater Res A 100 (2012) 2139–2146. doi: 10.1002/jbm.a.34088. [DOI] [PubMed] [Google Scholar]
- [41].Bonanomi D, Chivatakarn O, Bai G, Abdesselem H, Lettieri K, Marquardt T, Pierchala BA, Pfaff SL, Ret is a multifunctional coreceptor that integrates diffusible- and contact-axon guidance signals, Cell 148 (2012) 568–582. doi: 10.1016/j.cell.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Baudet C, Mikaels a, Westphal H, Johansen J, Johansen TE, Ernfors P, Positive and negative interactions of GDNF, NTN and ART in developing sensory neuron subpopulations, and their collaboration with neurotrophins., Development 127 (2000) 4335–4344. [DOI] [PubMed] [Google Scholar]
- [43].Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD, A Hierarchical NGF Signaling Cascade Controls Ret-Dependent and Ret-Independent Events during Development of Nonpeptidergic DRG Neurons, Neuron 54 (2007) 739–754. doi: 10.1016/j.neuron.2007.04.027. [DOI] [PubMed] [Google Scholar]
- [44].Fontana X, Hristova M, Da Costa C, Patodia S, Thei L, Makwana M, Spencer-Dene B, Latouche M, Mirsky R, Jessen KR, Klein R, Raivich G, Behrens A, C-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling, J. Cell Biol 198 (2012) 127–141. doi: 10.1083/jcb.201205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Degoutin J, Brunet-De Carvalho N, Cifuentes-Diaz C, Vigny M, ALK (Anaplastic Lymphoma Kinase) expression in DRG neurons and its involvement in neuron-Schwann cells interaction, Eur. J. Neurosci 29 (2009) 275–286. doi: 10.1111/j.1460-9568.2008.06593.x. [DOI] [PubMed] [Google Scholar]
- [46].Paveliev M, Hienola A, Jokitalo E, Planken A, Bespalov MM, Rauvala H, Saarma M, Sensory neurons from N-syndecan-deficient mice are defective in survival, Neuroreport 19 (2008) 1397–1400. doi: 10.1097/WNR.0b013e32830d1486. [DOI] [PubMed] [Google Scholar]
- [47].Murakami K, Yoshida S, Nerve injury induces the expression of syndecan-1 heparan sulfate proteoglycan in peripheral motor neurons, Neurosci. Lett 527 (2012) 28–33. doi: 10.1016/j.neulet.2012.08.043. [DOI] [PubMed] [Google Scholar]
- [48].Murakami K, Tanaka T, Bando Y, Yoshida S, Nerve injury induces the expression of syndecan-1 heparan sulfate proteoglycan in primary sensory neurons, Neuroscience 300 (2015) 338–350. doi: 10.1016/j.neuroscience.2015.05.033. [DOI] [PubMed] [Google Scholar]
- [49].Meng K, Rodriguez-Peña a, Dimitrov T, Chen W, Yamin M, Noda M, Deuel TF, Pleiotrophin signals increased tyrosine phosphorylation of beta beta-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase beta/zeta., Proc. Natl. Acad. Sci. U. S. A 97 (2000) 2603–2608. doi: 10.1073/pnas.020487997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Harroch S, Furtado GC, Brueck W, Rosenbluth J, Lafaille J, Chao M, Buxbaum JD, Schlessinger J, A critical role for the protein tyrosine phosphatase receptor type Z in functional recovery from demyelinating lesions, Nat. Genet 32 (2002) 411–414. doi: 10.1038/ng1004. [DOI] [PubMed] [Google Scholar]
- [51].Pariser H, Ezquerra L, Herradon G, Perez-Pinera P, Deuel TF, Fyn is a downstream target of the pleiotrophin/receptor protein tyrosine phosphatase β/ζ-signaling pathway: Regulation of tyrosine phosphorylation of Fyn by pleiotrophin, Biochem. Biophys. Res. Commun 332 (2005) 664–669. doi: 10.1016/j.bbrc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- [52].Fujikawa A, Noda M, Role of pleiotrophin-protein tyrosine phosphatase receptor type Z signaling in myelination, Neural Regen. Res 11 (2016) 549–551. doi: 10.4103/1673-5374.180761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Stoica GE, Kuo A, Powers C, Bowden ET, Sale EB, Riegel AT, Wellstein A, Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types, J. Biol. Chem 277 (2002) 35990–35998. doi: 10.1074/jbc.M205749200. [DOI] [PubMed] [Google Scholar]
- [54].Muramatsu T, Midkine and pleiotrophin: Two related proteins involved in development, survival, inflammation and tumorigenesis, J. Biochem 132 (2002) 359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- [55].Shin YK, Jang SY, Park SY, Park JY, Kim JK, Kim JP, Suh DJ, Lee HJ, Park HT, Grb2-Associated Binder-1 Is Required for Neuregulin-1-Induced Peripheral Nerve Myelination, J. Neurosci 34 (2014) 7657–7662. doi: 10.1523/JNEUROSCI.4947-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Feltri ML, Poitelon Y, Previtali SC, How Schwann Cells Sort Axons New Concepts, Neurosci 1 (2015) 1–14. doi: 10.1177/1073858415572361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Stassart RM, Fledrich R, Velanac V, Brinkmann BG, Schwab MH, Meijer D, Sereda MW, Nave KA, A role for Schwann cell-derived neuregulin-1 in remyelination, Nat. Neurosci 16 (2013) 48–54. doi: 10.1038/nn.3281. [DOI] [PubMed] [Google Scholar]
- [58].Fricker FR, Lago N, Balarajah S, Tsantoulas C, Tanna S, Zhu N, Fageiry SK, Jenkins M, Garratt AN, Birchmeier C, Bennett DLH, Axonally Derived Neuregulin-1 Is Required for Remyelination and Regeneration after Nerve Injury in Adulthood, J. Neurosci 31 (2011) 3225–3233. doi: 10.1523/JNEUROSCI.2568-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Al-Majed AA, Siu LT, Gordon T, Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons, Cell. Mol. Neurobiol 24 (2004) 379–402. doi: 10.1023/B:CEMN.0000022770.66463.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].a Al-Majed A, Neumann CM, Brushart TM, Gordon T, Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration., J. Neurosci 20 (2000) 2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Brushart TM, Jari R, Verge V, Rohde C, Gordon T, Electrical stimulation restores the specificity of sensory axon regeneration, Exp. Neurol 194 (2005) 221–229. doi: 10.1016/j.expneurol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- [62].Tang S, Zhu J, Xu Y, Peng A, Hua M, Biomaterials The effects of gradients of nerve growth factor immobilized PCLA scaffolds on neurite outgrowth in vitro and peripheral nerve regeneration in rats, Biomaterials 34 (2013) 7086–7096. doi: 10.1016/j.biomaterials.2013.05.080. [DOI] [PubMed] [Google Scholar]
- [63].Roam JL, Nguyen PK, Elbert DL, Controlled release and gradient formation of human glial-cell derived neurotrophic factor from heparinated poly(ethylene glycol) microsphere-based scaffolds, Biomaterials 35 (2014) 6473–6481. doi: 10.1016/j.biomaterials.2014.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Johnson BN, Lancaster KZ, Zhen G, He J, Gupta MK, Kong YL, Engel EA, Krick KD, Ju A, Meng F, Enquist LW, Jia X, McAlpine MC, 3D Printed Anatomical Nerve Regeneration Pathways, Adv. Funct. Mater 25 (2015) 6205–6217. doi: 10.1002/adfm.201501760. [DOI] [PMC free article] [PubMed] [Google Scholar]