Abstract
Background
The allergic march describes the natural history of allergic conditions as they develop during childhood. Eosinophilic esophagitis (EoE) is a chronic allergic inflammatory disease that can be triggered by specific foods. Despite its allergic pathophysiology, the epidemiologic relationship between EoE and established members of the allergic march is unknown.
Objective
We sought to determine whether EoE meets epidemiologic criteria for being considered a member of the allergic march.
Methods
Utilizing a primary care birth cohort of 130,435 children, we determined the natural histories of atopic dermatitis (AD), IgE-mediated food allergy (IgE-FA), asthma, EoE, and allergic rhinitis (AR) in individual patients. We then performed case-control analyses to establish the extent that existing allergic conditions influence the rate of subsequent EoE diagnosis.
Results
139 children developed EoE during the observation period (prevalence of 0.11%). Peak age of EoE diagnosis was 2.6-years, as compared with 0.3-years, 1-year, 1.1-years, and 2.1-years for AD, IgE-FA, asthma, and AR, respectively. Presence of AD (hazard ratio [HR] 3.2, 95% confidence interval [CI] 2.2–4.6), IgE-FA (HR 9.1, 95% CI 6.5–12.6), and asthma (HR 1.9, 95% CI 1.3–2.7) were independently and cumulatively associated with subsequent EoE diagnosis. Presence of AR was associated with subsequent EoE diagnosis (HR 2.8, 95% CI 2.0–3.9), and presence of EoE was associated with subsequent AR diagnosis (HR 2.5, 95% CI 1.7–3.5).
Conclusion
Allergic comorbidities are positively associated with EoE diagnosis. Together, our findings suggest that EoE is a late manifestation of the allergic march.
Keywords: Allergic march, Atopic dermatitis, Food allergy, Allergic rhinitis, Asthma, Eosinophilic esophagitis
INTRODUCTION
The atopic march (otherwise known as the allergic march) refers to the natural history of allergic manifestations as they develop during infancy and childhood.(1) While the term “atopy” is often associated with immunoglobulin (Ig) E-mediated hypersensitivity, in the context of the march, IgE is a pathophysiologic mediator of some, but not all allergic diseases. With our improved understanding of allergic pathophysiology, it is now understood that the march is a stereotyped progression of conditions that have common genetic and environmental predisposing factors, share the immunologic feature of a “type 2” inflammatory response that can include development of antigen-specific T helper type 2 (TH2) responses, generation of allergen-specific IgE molecules, activation of granulocytes, and other features, such as mucous production and edema.(1)
Classically, the march begins with atopic dermatitis (AD), and progresses to IgE-mediated food allergy (IgE-FA), asthma, and allergic rhinitis (AR).(1) Though not present in all allergic individuals,(2) this characteristic progression has allowed numerous epidemiologic studies that have measured the associations between different allergic conditions. For example, prior work has shown that the presence and severity of AD is associated with higher rates of IgE-FA, asthma, and AR,(3, 4) while presence of IgE-FA is associated with the development of asthma and AR.(5, 6) In addition to exhibiting a characteristic progression, a second feature of the march is that members may impart an individual and cumulative allergic risk. For example, children with a history of AD and allergen sensitization have higher rates of asthma as compared to children with AD alone.(7) These epidemiologic observations are likely the result of common genetic and/or environmental risk factors, (8–10) as well as a shared type 2 inflammatory pathophysiology.(1)
Eosinophilic esophagitis (EoE) is a chronic allergic inflammatory disease of the esophagus that can be triggered by specific foods or pollens. If left untreated, EoE can result in significant impairment in quality of life due to odynophagia, esophageal stricture formation, and food impaction.(11, 12) There is evidence to suggest that EoE may be a member of the allergic march.(13) For example, EoE is characterized by allergic inflammation,(12) and is common in allergic individuals.(14–16) Additionally, EoE shares susceptibility loci with all of the other march members including polymorphisms in thymic stromal lymphopoietin (TSLP) and signal transducer and activator of transcription (STAT) 6.(8, 9, 17) Finally, epidemiologic associations have been made between IgE-FA and EoE,(18) and aeroallergens can exacerbate EoE in some individuals,(19) supporting pathophysiologic links between EoE and members of the march. Despite these observations, rigorous epidemiologic studies of EoE in the context of the allergic march are lacking, largely due to the fact that EoE is relatively rare.(13)
Here, we examine a large primary care birth cohort that includes patients seen in our Center for Pediatric Eosinophilic Disorders. We determine whether EoE fits the epidemiologic criteria of the allergic march including: 1) exhibiting a stereotyped incidence in relation to established march members, 2) having an increased prevalence in children with preceding allergic comorbidities, and 3) being associated with higher rates of developing subsequent allergic conditions. This study provides the first, comprehensive epidemiologic evaluation of EoE in the context of the allergic march.
METHODS
Cohort generation and data extraction
The Children’s Hospital of Philadelphia (CHOP) network is both an international referral center and a provider of primary and sub-specialty care services to patients residing in the greater Delaware Valley. Our primary care network, consisting of 31 sites, has been validated as an accurate tool for estimating disease rates across our broader community population.(20) The Allergy Section at Children’s Hospital of Philadelphia (CHOP), which includes six care locations, is the largest provider of sub-specialty allergy care services in the region. In addition, it is an international referral center for eosinophilic esophagitis and has developed one of the largest pediatric cohorts for this condition through the Center for Pediatric Eosinophilic Diseases.
To examine the epidemiologic relationship between EoE and members of the allergic march, we extracted electronic medical record (EMR) data from 1/1/2001 to 12/31/2017 for patients seen in the CHOP primary care network. A single outpatient EMR system was used during this time period (EMR; Epic Systems, Verona, WI). Direct patient identifiers (e.g., medical record numbers and names) were removed to create a dataset with only limited identifiers (e.g. date of birth and dates of healthcare encounters). Data analyses were completed using R version 3.3.2. (R Foundation, Vienna, Austria). We identified an EMR-based virtual primary care birth cohort of 130,435 children who established care in our primary care network before their first birthday, and between 1/1/2001 and 12/31/2015. To ensure adequate follow-up to assess the outcomes of interest, we excluded individuals who received primary care from a CHOP-affiliated practice for less than 2 years (i.e., their last office visit occurred less than 2 years after their first office visit). Observation time for children was censored on the date of their last face-to-face outpatient healthcare encounter in our health system prior to their 17th birthday, and we assumed patients were observed continuously until they were censored.
Definitions of conditions studied
We ascertained presence or absence of allergic conditions of interest (AD, IgE-FA, asthma, AR, and EoE) within individuals using a combination of International Classification of Diseases Ninth or Tenth Revision diagnosis codes for AD (691.nn; L21.nn), asthma (493.n; J45.nn), AR (477.nn; J30.n), and EoE (530.13; K20.0), as well as allergen information and/or medication prescriptions consistent with previously validated methods.(5, 18) All diagnoses were made in accordance with established practice parameters.(21) To minimize inclusion of false diagnoses, we required patients to have diagnosis codes for each condition during at least two separate care visits occurring at least six months apart. Presence of a food allergen in the allergy module of our EMR was required for inclusion in our IgE-FA cohort.(5, 18) To maximize the specificity of our asthma cohort, we excluded diagnosis codes relating to reactive airway and post-viral wheeze, and any asthma diagnoses made before the age of 1 year. We also required prescriptions for asthma-specific medications (e.g., albuterol, inhaled corticosteroid) on at least two separate dates. Finally, to minimize the likelihood of including lactose intolerance and gluten sensitivity (celiac disease) in our analysis, we re-coded patients with diagnosis codes corresponding to these conditions as non-milk or non-wheat allergic, respectively.
Statistics
We studied longitudinal disease diagnosis in individuals across our birth cohort, and performed case-control comparisons with adjustments for demographic covariates (birth year, race, ethnicity, gender, and payer type). Peak age of diagnosis was defined as the mode of the disease incidence curves and we used the paired Wilcoxon signed rank sum to test statistical differences in age of disease onset among children who experienced both diseases. In all comparisons, the exposure of interest (e.g., AD) was considered present if it preceded the outcome of interest (e.g., EoE) in a given individual. Similarly, we treated children who had the “exposure condition” after the “outcome condition” the same as children who did not have the exposure condition during our observation time. Due to the variable follow-up time, we chose cox proportional hazard ratios (HR) with 95% confidence intervals (CI) to present our results. Kaplan-Meier estimators were utilized to measure risk over time. After adjustment for the demographic covariates, p-values less than 0.05 were considered significant.
RESULTS
Cohort demographics
The demographic characteristics of our study cohorts are listed in Table I. Our overall cohort was evenly split between male and female patients, however, consistent with prior studies EoE was found to be more common in Caucasian males.(18, 22) The age distribution of patients in each of the disease cohorts were similar to prior studies, and were representative of the natural histories of the respective conditions (e.g., patients in the IgE-FA cohort were on average younger than those in the AR cohort).(5) Finally, private insurance was the most common payer type across our study cohorts.
Table I.
Demographic characteristics of birth and disease cohorts
| Characteristic | Cohort (n) | |||||
|---|---|---|---|---|---|---|
| Birth (130,435) |
AD (14,726) |
IgE-FA (11,725) |
Asthma (24,745) |
EoE (141) |
AR (22,272) |
|
| Gender, % (n) | ||||||
| Male | 51 (66,838) | 55 (8,118) | 57 (6,712) | 60 (14,800) | 72 (102) | 57 (12596) |
| Female | 49 (63,597) | 45 (6,608) | 43 (5,013) | 40 (9,945) | 28 (39) | 43 (9,676) |
| Race, % (n) | ||||||
| White | 49 (64,301) | 32 (4,692) | 51 (6,012) | 40 (9,797) | 62 (87) | 43 (9,642) |
| Black | 32 (42,821) | 52 (7,706) | 30 (3,590) | 47 (11,541) | 22 (31) | 44 (9,860) |
| Asian or Pacific Islander | 4 (4,539) | 5 (701) | 6 (678) | 2 (623) | 6 (8) | 3 (587) |
| Other | 2 (2,060) | 1 (186) | 2 (191) | 1 (372) | 2 (3) | 1 (268) |
| Unknown | 13 (16,714) | 10 (1,441) | 11 (1,254) | 10 (2,412) | 8 (12) | 9 (1915) |
| Ethnicity, % (n) | ||||||
| Hispanic or Latino | 6 (8200) | 6 (815) | 5 (550) | 6 (1,602) | 6 (9) | 6 (1,248) |
| Non-Hispanic or Latino | 94 (122,235) | 94 (13,911) | 95 (11,175) | 94 (23,143) | 94 (132) | 94 (21,024) |
| Birth year, % (n) | ||||||
| 2000 to 2004 | 10 (13,279) | 16 (2,445) | 10 (1,176) | 15 (3,654) | 11 (16) | 18 (4,008) |
| 2005 to 2009 | 46 (60,155) | 51 (7,462) | 50 (5,876) | 53 (13,228) | 58 (81) | 58 (13,017) |
| 2010 to 2014 | 44 (57,001) | 33 (4,819) | 40 (4,673) | 32 (7,863) | 31 (44) | 24 (5,247) |
| Payer type, % (n) | ||||||
| Medicaid | 33 (43,582) | 44 (6,419) | 26 (3,053) | 43 (10,536) | 23 (33) | 38 (8,527) |
| Non-Medicaid | 67 (86,853) | 56 (8,307) | 74 (8,672) | 57 (14,209) | 77 (108) | 62 (13,745) |
Peak EoE incidence is after that of AD, IgE-FA, asthma, and similar to AR
We first established population-level disease incidence patterns for AD, IgE-FA, asthma, AR, and EoE. Consistent with prior studies, we found that the peak age of diagnosis for AD, IgE-FA, asthma, and AR were 0.3-years, 1-year, 1.1-years, and 2.1-years, respectively.(5) Notably, the peak age of diagnosis for EoE was 2.6-years, placing its peak incidence after that of AD, IgE-FA, and asthma, and statistically co-incident with AR (Fig. 1). These findings indicate that the peak incidence of EoE is after that of AD, IgE-FA, and asthma, and coincident with AR, on a population-level.
FIGURE 1.
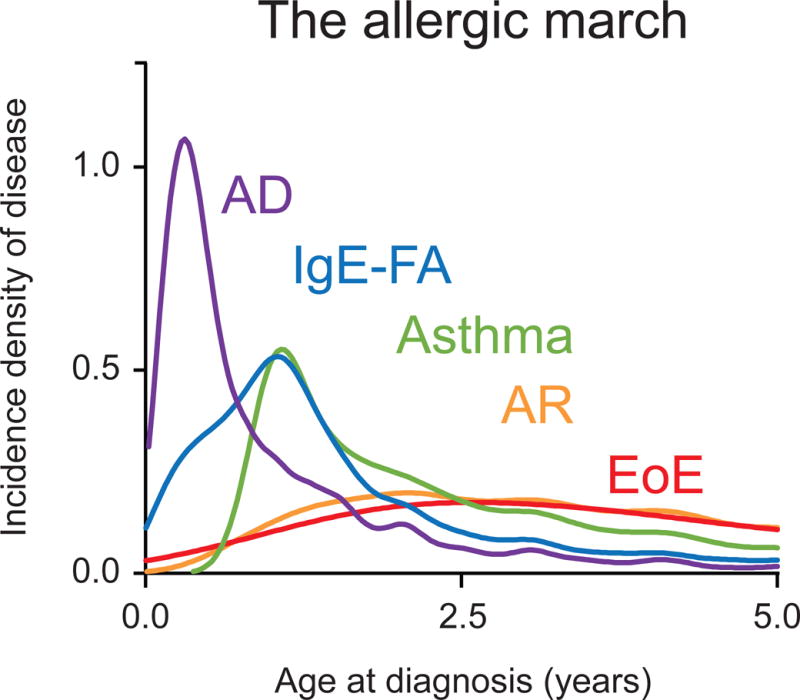
The allergic march. Density incidence of atopic dermatitis (AD), IgE-mediated food allergy (IgE-FA), asthma, allergic rhinitis (AR), and eosinophilic esophagitis (EoE) by age.
Allergic march members are associated with subsequent EoE diagnosis
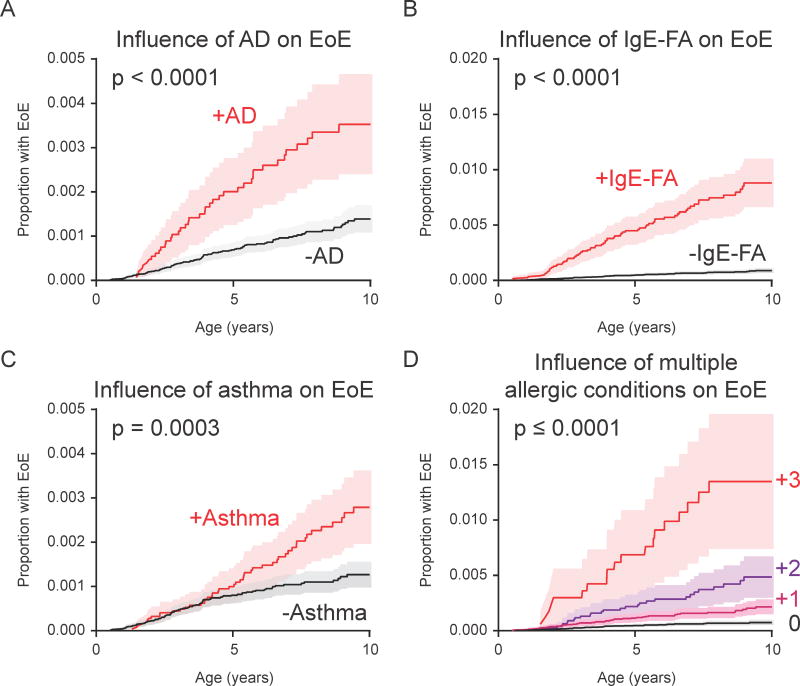
When studying disease acquisition patterns, population-level associations can miss inter-individual variability.(23, 24) As such, we studied longitudinal disease development in individuals across our birth cohort, and performed case-control comparisons with adjustments for demographic covariates. Consistent with prior studies, presence of AD, IgE-FA, or asthma were positively associated with development of subsequent allergic march members (as defined by our population-level incidence patterns) (Fig. 2).(5) Notably, presence of AD (HR 3.2, 95% CI 2.2–4.6), IgE-FA (HR 9.1, 95% CI 6.5–12.6), and asthma (HR 1.9, 95% CI 1.3–2.7) were independently associated with an increased rate of subsequent EoE diagnosis (Fig. 2 and Fig. 3A–C), with presence of IgE-FA having the strongest effect.(18)
FIGURE 2.
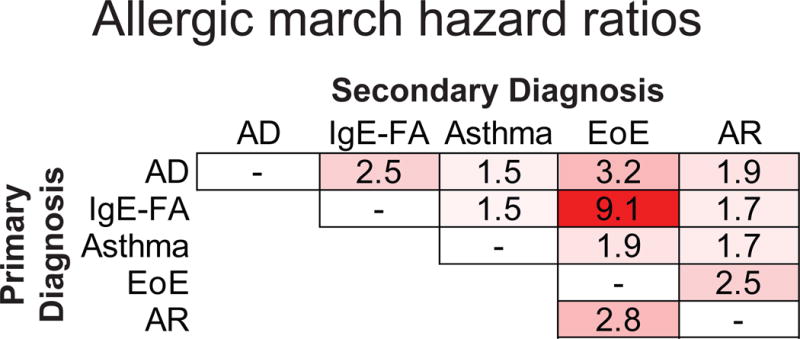
Cox hazard ratios indicating effect of primary allergic diagnosis on likelihood of secondary allergic diagnosis. White represents no correlation and red represents positive correlation, with darker color representing stronger relationship.
FIGURE 3.
Presence of allergic disease increases likelihood of EoE diagnosis. (A) Kaplan-Meier curve displaying influence of AD on EoE diagnosis by age. (B) Influence of IgE-FA on EoE diagnosis by age. (C) Influence of asthma on EoE diagnosis by age. (D) Influence of one or more atopic conditions on EoE diagnosis by age.
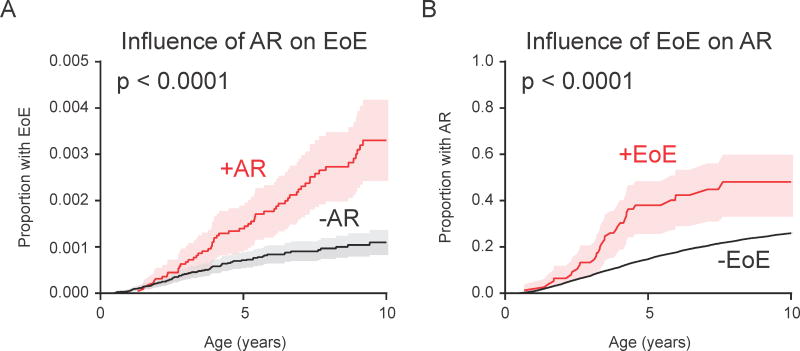
We next sought to ascertain whether there was a cumulative effect of multiple preceding allergic conditions on the rate of subsequent EoE diagnosis. Consistent with this hypothesis, the rate of EoE diagnosis was higher among individuals with more than one preceding allergic condition (Fig. 3D; HR [95% CI] of 2.45 [1.56–3.87], 5.60 [3.52–8.90], 9.13 [5.22–15.96] for 1, 2, or 3 allergic conditions, respectively). Finally, as AR and EoE were co-incident, we examined the association between these conditions in a bidirectional manner. First, we found that the presence of AR was associated with an increased rate of subsequent EoE diagnosis (Fig. 2 and Fig. 4A; HR 2.8, 95% CI 2.0–3.9). In addition, the presence of EoE was associated with an increased rate of subsequent AR diagnosis (Fig. 2 and Fig. 4B; HR 2.5, 95% CI 1.7–3.5). Together, these findings indicate that presence of one or more established allergic march members is individually and cumulatively associated with the subsequent development of EoE, and that AR and EoE are closely associated with each other.
FIGURE 4.
AR and EoE are bi-directionally associated. (A) Kaplan-Meier curve displaying influence of AR on EoE diagnosis by age. (B) Influence of EoE on AR diagnosis by age.
DISCUSSION
The allergic march is a long standing pillar of the allergy field that has acted as a framework to explain both the pathophysiologic links among allergic conditions, and the concurrent global increase in the prevalence and severity of allergic diseases in recent decades. Importantly, the march also has considerable relevance to patient care as it provides a mechanism to estimate risk for the development of additional allergic sequela in allergic individuals.(1) However, the concept of the allergic march has come under recent criticism stemming from the fact that initial studies utilized epidemiologic evidence from cross-sectional populations, as opposed to studying longitudinal disease development in individual patients.(23, 24) As such, progression through all allergic manifestations on the march may not be as common as previously thought.(2)
To avoid this potential confounder, and ensure that our associations reflect natural covariance of conditions within individuals (as opposed to population-level patterns), we adopted two analytic approaches with our birth cohort. First, we performed traditional, population-level examinations of disease incidence patterns to establish peak ages of disease onset for each condition of interest. This approach established that on a population-level, the peak incidence of EoE is after that of AD, IgE-FA, and asthma, and is statistically co-incident with that of AR. To our knowledge, this is the first head-to-head comparison of population-level EoE disease incidence rates to those of other allergic conditions.
In a second approach, we retrospectively examined longitudinal disease acquisition patterns within individual patients. In this manner, we were able to estimate individual disease risk relationships, and power these associations across our birth cohort. Via this second approach, we found that history of AD, IgE-FA, and asthma were independently and cumulatively associated with an increased rate of EoE diagnosis. In addition, we found that AR and EoE were significantly associated with each other. This bi-directional association is consistent with AR and EoE being co-incident in our cohort, and with prior studies that have suggested these conditions share a close pathophysiologic relationship.(19)
There are some limitations of our study that should be noted. First, this study is a secondary analysis of health records at a single institution collected as part of routine care. We relied primarily on diagnosis codes to identify conditions of interest, and choice of diagnosis codes result in potential biases in our data collection, and may be affected by billing or administrative constraints. Similarly, there may have been changes in the use of specific diagnosis codes over the study period that could influence our population-level disease incidence estimates. Second, it is possible that an additional peak age of disease onset was not detected in our analysis due to the variable observation time among children in our cohort. Third, EoE can go undiagnosed for a period of time as symptoms are non-specific and diagnostic endoscopy can lag behind initial presentation. As such, our peak incidence estimate of 2.6-years may be later than actual disease onset. Fourth, it should be noted that ours is an open birth cohort. Thus, even though we took care to extract data on patents followed in our primary care clinics, patients may have established diagnoses outside of our healthcare system. Similarly, we treated children who had the “exposure condition” after the “outcome condition” the same as children who did not have the exposure condition during our observation time. Both of these assumptions could have the effect of biasing our results towards the null hypothesis. Therefore, the measurements reported here may be underestimates of actual risk associations.
Together, our findings suggest that EoE is a late manifestation of the allergic march in some individuals. These findings have broad implications for our understanding of EoE pathophysiology, and suggest that sensitization to foods and/or aeroallergens early in life may predispose to EoE development. Clinically, our results support the need for more active screening of allergic patients for EoE symptoms.
Highlights.
What is already known about this topic?
Despite its allergic nature, the epidemiologic relationship between EoE and classical members of the allergic march is not known.
What does this article add to our knowledge?
We measure the rate of allergic march members’ subsequent diagnosis of EoE. Our findings suggest that EoE is a late manifestation of the allergic march.
How does this study impact current management guidelines?
This observation expands our understanding of a pillar concept in the field of allergy, and supports more active screening of allergic children for EoE symptoms.
Acknowledgments
We thank the Reviewers for critical contributions to this manuscript. DAH, RWG, and JMS made contributions to conception and design of the work; DAH, RWG, and MR made contributions to the acquisition and analysis of the data; DAH was primarily responsible for interpretation of the data and writing of the manuscript. All authors made intellectual contributions during drafting and revision of the work, approved the final version to be published, and agree to be accountable for all aspects of the work, including accuracy and integrity.
Sources of Funding: DAH is supported by the CHOP NRSA Institutional Training in Pediatric Research Grant (T32 HD043021) and a Children’s Hospital of Philadelphia Senior Fellow K-readiness grant. JMS is supported by the Stuart Starr Endowed Chair of Pediatrics, The Children’s Hospital of Philadelphia Eosinophilic Esophagitis Fund, a Food Allergy Research & Education, Inc. Clinical Network grant, and the Consortium of Eosinophilic Gastrointestinal Disease Researchers (U54 AI117804).
Abbreviations
- AD
atopic dermatitis
- IgE-FA
IgE-mediated food allergy
- AR
allergic rhinitis
- EoE
eosinophilic esophagitis
- HR
hazard ratio
- CI
confidence interval.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Availability of data and materials
The dataset supporting the conclusions of this article is available in the Zenodo repository (http://doi.org/10.5281/zenodo.1248931).
Ethical and regulatory oversight
The CHOP Institutional Review Board reviewed our study and determined that it was exempt from requiring ethics approval or subject consent, as it did not meet the definition of “human subject” research.
Author Contributions and Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Hill DA, Spergel JM. The atopic march: Critical evidence and clinical relevance. Ann Allergy Asthma Immunol. 2018 Feb;120(2):131–7. doi: 10.1016/j.anai.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belgrave DC, Granell R, Simpson A, Guiver J, Bishop C, Buchan I, et al. Developmental profiles of eczema, wheeze, and rhinitis: Two population-based birth cohort studies. PLoS Med. 2014 Oct 21;11(10):e1001748. doi: 10.1371/journal.pmed.1001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsakok T, Marrs T, Mohsin M, Baron S, du Toit G, Till S, et al. Does atopic dermatitis cause food allergy? A systematic review. J Allergy Clin Immunol. 2016 Apr;137(4):1071–8. doi: 10.1016/j.jaci.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson D, Sjoberg O, Foucard T. Development of allergies and asthma in infants and young children with atopic dermatitis–a prospective follow-up to 7 years of age. Allergy. 2000 Mar;55(3):240–5. doi: 10.1034/j.1398-9995.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 5.Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: A retrospective cohort study. BMC Pediatr. 2016 Aug 20;16 doi: 10.1186/s12887-016-0673-z. 133,016-0673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alduraywish SA, Lodge CJ, Campbell B, Allen KJ, Erbas B, Lowe AJ, et al. The march from early life food sensitization to allergic disease: A systematic review and meta-analyses of birth cohort studies. Allergy. 2016 Jan;71(1):77–89. doi: 10.1111/all.12784. [DOI] [PubMed] [Google Scholar]
- 7.Illi S, von Mutius E, Lau S, Nickel R, Gruber C, Niggemann B, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004 May;113(5):925–31. doi: 10.1016/j.jaci.2004.01.778. [DOI] [PubMed] [Google Scholar]
- 8.Weidinger S, Willis-Owen SA, Kamatani Y, Baurecht H, Morar N, Liang L, et al. A genome-wide association study of atopic dermatitis identifies loci with overlapping effects on asthma and psoriasis. Hum Mol Genet. 2013 Dec 1;22(23):4841–56. doi: 10.1093/hmg/ddt317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marenholz I, Esparza-Gordillo J, Ruschendorf F, Bauerfeind A, Strachan DP, Spycher BD, et al. Meta-analysis identifies seven susceptibility loci involved in the atopic march. Nat Commun. 2015 Nov 6;6:8804. doi: 10.1038/ncomms9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, et al. Commensal bacteria-derived signals regulate basophil hematopoiesis and allergic inflammation. Nat Med. 2012 Mar 25;18(4):538–46. doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBrosse CW, Franciosi JP, King EC, Butz BK, Greenberg AB, Collins MH, et al. Long-term outcomes in pediatric-onset esophageal eosinophilia. J Allergy Clin Immunol. 2011 Jul;128(1):132–8. doi: 10.1016/j.jaci.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill DA, Spergel JM. The immunologic mechanisms of eosinophilic esophagitis. Curr Allergy Asthma Rep. 2016 Jan;16(2) doi: 10.1007/s11882-015-0592-3. 9,015-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill DA, Spergel JM. Is eosinophilic esophagitis a member of the atopic march? Ann Allergy Asthma Immunol. 2018 Feb;120(2):113–4. doi: 10.1016/j.anai.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammad AA, Wu SZ, Ibrahim O, Bena J, Rizk M, Piliang M, et al. Prevalence of atopic comorbidities in eosinophilic esophagitis: A case-control study of 449 patients. J Am Acad Dermatol. 2017 Mar;76(3):559–60. doi: 10.1016/j.jaad.2016.08.068. [DOI] [PubMed] [Google Scholar]
- 15.Hill DA, Shuker M, Cianferoni A, Wong T, Ruchelli E, Spergel JM, et al. The development of IgE-mediated immediate hypersensitivity after the diagnosis of eosinophilic esophagitis to the same food. J Allergy Clin Immunol Pract. 2015 Jan-Feb;3(1):123–4. doi: 10.1016/j.jaip.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maggadottir SM, Hill DA, Ruymann K, Brown-Whitehorn TF, Cianferoni A, Shuker M, et al. Resolution of acute IgE-mediated allergy with development of eosinophilic esophagitis triggered by the same food. J Allergy Clin Immunol. 2014 May;133(5):1487,1489.e1. doi: 10.1016/j.jaci.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Hirota T, Nakayama T, Sato S, Yanagida N, Matsui T, Sugiura S, et al. Association study of childhood food allergy with GWAS-discovered loci of atopic dermatitis and eosinophilic esophagitis. J Allergy Clin Immunol. 2017 Jun 16; doi: 10.1016/j.jaci.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 18.Hill DA, Dudley JW, Spergel JM. The prevalence of eosinophilic esophagitis in pediatric patients with IgE-mediated food allergy. J Allergy Clin Immunol Pract. 2017 Mar-Apr;5(2):369–75. doi: 10.1016/j.jaip.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ram G, Lee J, Ott M, Brown-Whitehorn TF, Cianferoni A, Shuker M, et al. Seasonal exacerbation of esophageal eosinophilia in children with eosinophilic esophagitis and allergic rhinitis. Ann Allergy Asthma Immunol. 2015 Jul 30; doi: 10.1016/j.anai.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Feemster KA, Li Y, Grundmeier R, Localio AR, Metlay JP. Validation of a pediatric primary care network in a US metropolitan region as a community-based infectious disease surveillance system. Interdiscip Perspect Infect Dis. 2011;2011:219859. doi: 10.1155/2011/219859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011 Jul;128(1):3,20.e6. doi: 10.1016/j.jaci.2011.02.040. quiz 21-2. [DOI] [PubMed] [Google Scholar]
- 22.Straumann A, Simon HU. Eosinophilic esophagitis: Escalating epidemiology? J Allergy Clin Immunol. 2005 Feb;115(2):418–9. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Belgrave DC, Simpson A, Buchan IE, Custovic A. Atopic dermatitis and respiratory allergy: What is the link. Curr Dermatol Rep. 2015;4(4):221–7. doi: 10.1007/s13671-015-0121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busse WW. The atopic march: Fact or folklore? Ann Allergy Asthma Immunol. 2018 Feb;120(2):116–8. doi: 10.1016/j.anai.2017.10.029. [DOI] [PubMed] [Google Scholar]