Abstract
Introduction
We describe an exploratory study of orofacial function in children with congenital myotonic dystrophy (CDM) versus healthy controls.
Methods
We evaluated 41 children with CDM and 29 healthy controls for speech and swallow function and for lingual and labial strength.
Results
The Iowa Oral Performance Instrument (IOPI), measuring tongue strength, and a lip force meter (LFM), measuring lip strength, had excellent inter-rater reliability with ICCs of 0.75 (N=19, p<.001) and 0.96 (N=20, p<.001), respectively. Mean overall lingual strength was 3.5-fold less and labial strength was about 7-fold less in CDM than in healthy controls. Eighteen of 24 children with CDM demonstrated dysarthria and an additional 11 participants were non-verbal. Dysarthria correlated moderately with lingual strength, age, and dysphagia. Strength measures correlated moderately with dysphagia.
Discussion
Children with CDM demonstrate impaired orofacial functioning that affects communication and swallowing. Reliability of strength measures may be useful for future therapeutic trials.
Search Terms: congenital myotonic dystrophy, CDM, orofacial, dysarthria, dysphagia, IOPI, lip force meter
Introduction
Congenital myotonic dystrophy (CDM) is a severe form of myotonic dystrophy type-1 (DM-1) characterized by significant impairment at birth in muscle tone, respiratory failure, and difficulty in sucking and swallowing.1 In addition to speech and swallow related disturbances, these individuals may have talipes equinovarus, sleep disturbances, cognitive impairment, and features of autism and attention deficit hyperactivity disorder (ADHD).1, 2, 3, 4 Mortality in these infants is 30-40% if they have required ventilation for 3 or more months.5
Limitations in communication are of significant concern and impact quality of life in myotonic dystrophy.6 Communication issues are particularly important in children with CDM who present in infancy with significant facial weakness and dysmorphia, both of which are contributors to dysphagia and dysarthria. Prior work has provided initial characterization of lip strength, orofacial mobility, and degree of dysarthria.7 This work is limited by validation of their quantitative measurements and limited qualitative descriptions of their speech and swallow evaluations.
Here we describe an exploratory study wherein we expand the description of bulbar involvement in CDM, describe a reproducible and quantitative measure of lingual strength, and determine areas of correlation between strength measures and functional measures.
Methods
Participants
Participants ages 0 to 13 years, 11 months with genetically confirmed congenital myotonic dystrophy and healthy controls in this same age range were recruited at the University of Utah and the University of Western Ontario. Inclusion for the study required a clinical diagnosis of CDM as evidenced by one of the following: 1) Symptom onset at birth requiring 72+ hours of hospitalization; 2) Documented symptoms including hypotonia, feeding difficulty, and/or respiratory failure; 3) CTG repeat length >200. Control participants had the following inclusion criteria: 1) No medical diagnoses; 2) Not taking any medication. Healthy controls may be unaffected siblings or cousins of participants with CDM, so long as the parent or participant was negative for the repeat expansion. Parents of all participants (children with CDM and healthy controls) provided informed consent after having all questions regarding the study procedures and outcomes answered by the principal investigator and/or the study coordinator. All study procedures were approved by the University of Utah and University of Western Ontario Institutional Review Boards.
All participants were divided by age into three cohorts for testing purposes: 0-2;11, 3.0 – 6;11, and 7.0 – 13;11 (years; months). Children in the youngest cohort were asked to only complete the feeding/swallowing measures. All measures were completed by all subjects in the other cohorts.
Strength measures
IOPI
The Iowa Oral Performance Instrument (IOPI Medical, LLC; Redmond, WA) is a device designed to measure applied pressure in a closed system using an air filled bulb attached to a manometer. The maximum pressure a participant could apply to a pressure filled bulb was recorded as a measure of anterior tongue strength. Previous work in healthy adults has shown a high degree of reliability.8 Briefly, the tongue bulb was placed on the anterior tongue at midline and the participant was asked to press the bulb to the hard palate as strongly as possible. The bulb was placed such that the entire air-filled portion of the bulb tip was posterior to the anterior mandibular teeth. This measure was repeated for a total of three measures once it was apparent the participant understood the task. This measure was obtained by the same evaluator on day 1 and day 2 to assess reliability.
Lip Force Meter
As a measure of lip strength, we used an Imada DS2 Digital Force Gauge (IMADA, Inc., Northbrook, IL) attached to a Vettex Doubleguard Mouthguard Pee Wee model PW22 (Markwort Sporting Goods Co.; St. Louis, MO) that had been trimmed to fit the participant’s mouth with the bite plate removed (Supplemental material). The mouthguard was placed in the mouth, anterior to the teeth and posterior to the lips and the DS2 was attached. The participants were instructed to hold the mouthguard in place with their lips without the use of suction. A continuous force was applied by the examiner directly away from the participant’s mouth until the lips were unable to hold onto the mouthguard. Ideally this was conducted with head in a natural position and the direction of pull parallel to the floor. This measure was repeated for a total of three measures once it was apparent the task had been learned by the participant. This measure was obtained on day 1 and day 2 to assess reliability and measures were taken by the same evaluator on both days.
Speech measures
Participants that were able to read were asked to read a short reading passage entitled “Limpy the Fuzzy Yellow Baby Duck” (Supplemental material). If participants were unable to read but were able to demonstrate expressive language, then they were asked to repeat this reading passage in 2-4 word increments. For participants who were non-verbal or pre-linguistic, they were engaged in looking at a children’s book such as Touch and Feel Farm (2008 DK Publishing, NY NY) or Animal Sounds (2005 Random House, NY NY). All speech and/or sound productions were video recorded with a Canon PowerShot ELPH 135 16.0 MP or a Canon PowerShot A810 HD16.0 MP camera (Canon U.S.A., Inc.) in a quiet clinic room. Tapes were reviewed later and dysarthria was rated on a 4 point scale where: 0 – 100% intelligible; 1 – reduced intelligibility, occasional repetitions required; 2 – reduced intelligibility, frequent repetitions required; and 3 – negligible speech or non-verbal, use of augmentative and alternative communication (AAC).7
Swallow assessment
The Oral-Motor Feeding Rating Scale (OMFRS, Pearson Education, Inc.) was used to document function of lips, cheeks, tongue, and jaw when eating and/or drinking. Observed function was rated on a scale of 0 to 5 where: 0, is normal function; 1, questionable dysfunction; 2, less than 25% dysfunction; 3, 25 – 49% dysfunction; 4, 50 – 75% dysfunction; and 5 more than 75% dysfunction. Participants that were able to eat orally were observed drinking thin liquid (e.g.: apple juice, water, etc.) from a cup and with a straw, eating a puree item (e.g.: applesauce, pudding, yogurt, etc.), a soft food item (e.g.: slice of untoasted bread, soft cookie, etc.), and a hard, crunchy item (e.g.: graham cracker, shortbread cookie, carrot, apple slice, etc.). All feeding was video recorded on a Canon PowerShot ELPH 135 16.0 MP or a Canon PowerShot A810 HD16.0 MP camera (Canon U.S.A., Inc.) for later verification. Descriptive statistics for the OMFRS include median values for level of function for each quartile where larger numbers indicate higher degree of dysfunction (Figure 1).
Figure 1.
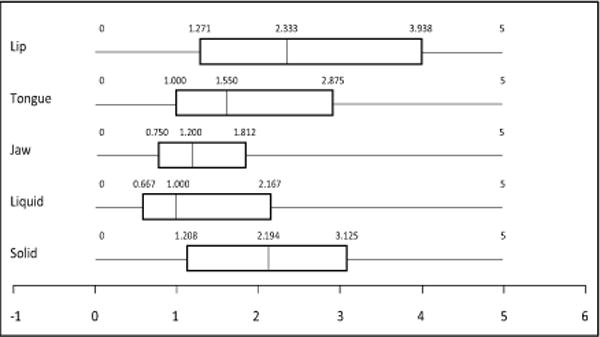
Box plots showing the median interquartile range of the Oral Motor Feeding Rating Scale swallow assessment ratings by orofacial region and food type.
Statistical Analyses
Data were summarized as mean or median, standard deviation, minimum, maximum, frequency and percentage as appropriate. Independent samples t-tests were used to examine the difference in measurement between the control and the CDM groups. Test-retest reliability of the day 1 and 2 measures were analyzed by computing the intraclass correlation (ICC). Analyses of the association between measures used the Pearson or Spearman correlation. The level of statistical significance was set at p<0.05 for all tests. Corrections for multiple comparisons were not undertaken in this exploratory study, however mean differences and corresponding 95% confidence intervals were calculated.
Results
This study enrolled a total of 41 children with CDM, ages 0.5 – 13.22 years, and 29 healthy controls, ages 1.34 – 13.93 years. Mean age was 6.8 years (SD 3.3) and 9.1 years (SD 3.1), respectively. There was a significant age difference between children with CDM and healthy controls (p=0.008). Detailed demographic data is provided in Table 1. Not all affected children were able to complete all measures secondary to behavioral issues or reduced cognitive functioning limiting understanding of task.
Table 1.
Demographic data
| Demographic | Participants with Congenital Myotonic Dystrophy (n=41) | Control Participants(n=29) |
|---|---|---|
| Mean age (years) (SD) | 6.8 (3.3) | 9.1 (3.1) |
| Female (%) | 49 | 59 |
| Ethnicity | 12% Hispanic, 88% non-Hispanic | 7% Hispanic, 93% non-Hispanic |
| Race | 98% Caucasian, 2% Asian | 100% Caucasian |
| Mean CTG repeat length (SD) | 1245.97 (474.91) | NA |
| Mean duration of respiratory support at birth (range) | 25.9 weeks (1 week-156 weeks) | 0 |
| Current respiratory support (mean duration of use during day) | 7.0% BiPAP (13.3h), 7.0% supplemental oxygen (9.6h) | 0 |
Twenty children with CDM completed IOPI measures (49%), 21 completed LFM measures (51%), 35 completed speech measures (85%), and 40 participated in feeding assessment (98%). All healthy controls were able to complete all measures applicable to their cohort. Not all affected children were able to complete repeated strength measures on both days. Test-retest reliability (intraclass correlation coefficient, ICC) was excellent for both strength measures with IOPI ICC = 0.75 (N=19, p<.001) and LFM ICC = 0.96 (N=20, p<.001).
Tongue pressure versus age is shown in figure 2 as is labial resistive force versus age. CDM patients and controls both showed increasing lingual and labial strength with age. On average, the affected children had an increase of 0.59 kPa/year for IOPI and 0.22 N/year for LFM. Healthy controls demonstrated an increase of 1.43 kPa/year for IOPI and 1.65 N/year for LFM.
Figure 2.
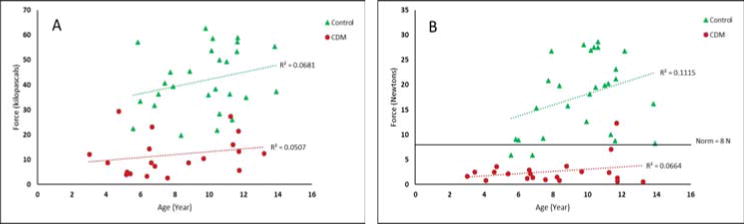
Scatterplots showing average maximum labial and lingual strength in children with CDM and healthy controls. (A) Tongue strength in kilopascals (kPa) measured with Iowa Oral Performance Instrument. (B) Lip strength in Newtons (N) measured with lip force meter. Cut off at 8 N for normative performance indicated.
Median and mean values for tongue strength were calculated (Table 2) and the 95% confidence interval (CI) around the mean is provided in Table 2.
Table 2.
Comparisons between CDM and control patients on strength measures, age, and performance on the Oral Motor Feeding Rating Scale.
| Variable | Median (Control) | Median (CDM) | Mean (Control) | Mean (CDM) | Mean Difference | Confidence Interval |
|---|---|---|---|---|---|---|
| IOPI | 39.33 | 9.50 | 41.81 | 11.88 | 29.93 | 23.86 - 40.00 |
| LFM | 19.50 | 2.10 | 17.81 | 2.58 | 15.23 | 12.02 – 18.45 |
| Age | 10.14 | 6.63 | 9.14 | 7.04 | 2.09 | 0.57 – 3.61 |
| Lip | 0.00 | 2.33 | 0.00 | 2.61 | −2.61 | −3.18 – −2.044 |
| Tongue | 0.00 | 1.67 | 0.00 | 1.99 | −1.99 | −2.50 – −1.48 |
| Jaw | 0.00 | 1.20 | 0.00 | 1.67 | −1.67 | −2.20 – −1.14 |
| Liquid | 0.00 | 1.00 | 0.00 | 1.54 | −1.54 | −2.10 – −0.97 |
| Solid | 0.00 | 2.33 | 0.00 | 2.24 | −2.24 | −2.74 – −1.74 |
Thirty-five affected children participated in speech assessments, however 11 of 35 children were essentially non-verbal or used augmentative or alternative communication (31%). Of the verbal children, 6 demonstrated speech that was developmentally appropriate (17%). Ten affected children exhibited mild flaccid dysarthria with occasional repetitions required (29%). The remaining eight children presented with more moderate to severe dysarthria requiring frequent repetitions (23%). (Figure 3) The presence of any dysarthria showed a moderate negative correlation with IOPI performance and a moderate negative correlation with age (Table 3). Dysarthria also had a moderate positive correlation with all swallow measures. None of our healthy controls demonstrated any dysarthria.
Figure 3.
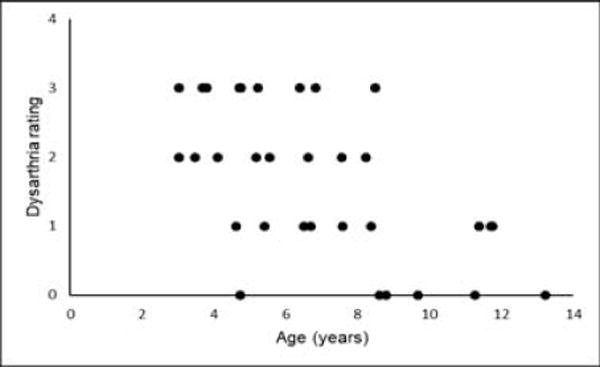
Dysarthria rating during speech task versus age. (0-3; 0=100% intelligible, 1=reduced intelligibility with occasional repetitions, 2=reduced intelligibility with frequent repetitions, 3=non-verbal or use of augmentative or alternative communication).
Table 3.
Correlations of strength measures, IQ, repeat expansion, age, and degree of dysarthria with functional assessment on the OMFRS and with the degree of dysarthria.
| Comparison | IOPI | LFM | IQ | CTG | Age | Dysarthria |
|---|---|---|---|---|---|---|
| OMFRS | ||||||
| Lip | −0.387 | −0.488 | −0.269 | 0.314 | −0.215 | 0.466 |
| Tongue | −0.482 | −0.379 | −0.356 | 0.322 | −0.214 | 0.572 |
| Jaw | −0.418 | −0.364 | −0.324 | 0.393 | −0.232 | 0.581 |
| Liquid | −0.405 | −0.267 | −0.268 | 0.379 | −0.248 | 0.527 |
| Solid | −0.463 | −0.612 | −0.33 | 0.273 | −0.190 | 0.537 |
| Dysarthria | ||||||
| Dysarthria | −0.538 | −0.134 | −0.378 | 0.090 | −0.592 | – |
Abbreviations: IOPI, Iowa Oral Performance Instrument; LFM, lip force meter; IQ, intelligence quotient; CTG, repeat length of CTG expansion. Bold and italicized data indicates p<0.05.
Swallow assessments were grouped by region of function and food type: lips, tongue, jaw, liquid, and solid. Mean and median data indicate that labial function was more impaired than either lingual or mandibular function. Children with CDM demonstrated greater difficulty ingesting solids than liquids (Figure 1).
Discussion
Our cross-sectional, exploratory study in CDM demonstrates the intra-rater reliability of a described measure of lip strength.1,7 Additionally, we demonstrate the intra-rater reliability for use of a pressure measuring device used in dysphagia therapy to assess lingual strength.9 The measures of labial and lingual strength show significant differences exist between children with CDM and healthy controls.
Communication difficulties have previously been identified as the highest area of concern for parents of children with CDM.6 We report 17% of our study population as having developmentally appropriate speech and almost one third as being non-verbal and/or using AAC. Additionally, our data suggest that dysarthria improves with age. Children with CDM demonstrate improvement in precision of speech at a markedly later age than for typically developing children.10
Dysphagia, and other concerns with feeding are likely related to several deficits. These include reduced lingual and labial strength and dysfunctional jaw movement. If children are prescribed swallowing therapy, specific attention should be given to these aspects.
Limitations to this study include that genetic testing was not completed as part of the study visit and CTG repeat length was obtained through clinical sources which may result in minor discrepancies in repeat length measurement. Participants also predominantly lived far from our study centers and the financial ability to travel may have inadvertently skewed our population. The average age and age range of control participants was higher than for our affected participants, limiting direct comparison with our younger affected participants. Cognitive deficits and/or sensory concerns limited the number of children with CDM willing to participate in oral strength testing and/or eating.
Further studies to assess longitudinal orofacial performance in children with CDM would be of interest. We know that parents perceive that children with CDM tend to have a period of decreasing abilities until approximately age 6 and then make some developmental progress.6 As children with CDM enter puberty, adult-onset phenotypes typical of DM1 are thought to become more evident.
Anterior orofacial structures are the most affected in children with CDM leading to significant dysarthria and oral phase dysphagia. This study highlights the unique phenotype of CDM and provides validity for use of quantification measures in this population. Further, this study serves to help guide clinical care with particular attention to speech and swallow concerns in this population.
The strength measures described herein may be useful as endpoint measures that are sensitive and specific for lingual and labial strength for disease progression in future studies. They reflect a correlative and quantifiable means to assess the functional measures of speech and swallowing in CDM.
Supplementary Material
Acknowledgments
Kiera N. Berggren had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Grant Support: NIH(1K23NS091511-01), The Muscular Dystrophy Association, and Valerion Therapeutics.
Abbreviations
- AAC
augmentative and alternative communication
- ADHD
attention deficit hyperactivity disorder
- CDM
congenital myotonic dystrophy
- CTG
repeat length of CTG expansion
- ICC
intraclass correlation
- IOPI
Iowa Oral Performance Instrument
- IQ
intelligence quotient
- LFM
lip force meter
- OMFRS
oral motor feeding rating scale
Footnotes
Author contributions
Kiera Berggren: Design of study, analysis of data, drafting of manuscript
Man Hung: Design of study, analysis of data, revision of manuscript
Melissa Dixon: Design of study, analysis of data, revision of manuscript
Jerry Bounsanga: Analysis of data, revision of manuscript
Becky Crockett: Analysis of data, revision of manuscript
Yushan Gu: Analysis of data, revision of manuscript
Mary Foye: Analysis of data, revision of manuscript
Craig Campbell: Analysis of data, revision of manuscript
Russell Butterfield: Analysis of data, revision of manuscript
Nicholas E Johnson: Design of study, analysis of data, revision of manuscript
Ethical publication statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosures
Dr. Johnson serves as an Associate Editor for Neurology: Genetics. He is funded by the NIH, grant #1K23NS091511-01. He has received research support from the Muscular Dystrophy Association, Myotonic Dystrophy Foundation, Valerion Therapeutics, Ionis Pharmaceuticals, and Biogen Idec.
Dr. Butterfield serves on the scientific advisory boards for Bamboo Therapeutics, Sarepta Therapeutics, Marathon Pharmaceuticals, and Avexis. He is the site principal investigator for clinical trials sponsored by Marathon Pharmaceuticals, Sarepta Therapeutics, Pfizer, Eli Lilly, PTC Therapeutics, and aTyr Pharma.
Dr. Campbell is on advisory boards for PTC Therapeutics and voluntary consultative meetings for Biomarin and Acceleron. He is a site PI for clinical trials sponsored by Biogen, Ionis, PTC Therapeutics, Eli Lily, and Pfizer.
References
- 1.Johnson NE, Butterfield R, Berggren K, Hung M, Chen W, DiBella D, et al. Disease burden and functional outcomes in congenital myotonic dystrophy: A cross-sectional study. Neurology. 2016;87(2):160–167. doi: 10.1212/WNL.0000000000002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ekström AB, Hakenäs-Plate L, Tulinius M, Wentz E. Cognition and adaptive skills in myotonic dystrophy type 1: A study of 55 individuals with congenital and childhood forms. Dev Med Child Neurol. 2009;51(12):982–990. doi: 10.1111/j.1469-8749.2009.03300.x. [DOI] [PubMed] [Google Scholar]
- 3.Ekström AB, Hakenäs-Plate L, Samuelsson L, Tulinius M, Wentz E. Autism spectrum conditons in myotonic dystrophy type 1: A study on 57 individuals with congenital and childhood forms. Am J Med Genet Part B Neuropsychiatr Genet. 2008;147(6):918–926. doi: 10.1002/ajmg.b.30698. [DOI] [PubMed] [Google Scholar]
- 4.Ho G, Cardamone M, Farrar M. Congenital and childhood myotonic dystrophy: Current aspects of disease and future directions. World J Clin Pediatr Novemb World J Clin Pediatr. 2015;8(44):66–80. doi: 10.5409/wjcp.v4.i4.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell C, Sherlock R, Jacob P, Blayney M. Congenital myotonic dystrophy: assisted ventilation duration and outcome. Pediatrics. 2004;113(4):811–816. doi: 10.1542/peds.113.4.811. [DOI] [PubMed] [Google Scholar]
- 6.Johnson NE, Ekstrom AB, Campbell C, Hung M, Adams HR, Chen W, et al. Parent-reported multi-national study of the impact of congenital and childhood onset myotonic dystrophy. Dev Med Child Neurol. 2016;58(7):698–705. doi: 10.1111/dmcn.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjogreen L, Engvall M, Ekstrom A-B, Lohmander A, Kiliaridis S, Tulinius M. Orofacial dysfunction in children and adolescents with myotonic dystrophy. Dev Med Child Neurol. 2007;49(1):18–22. doi: 10.1111/j.1469-8749.2007.0060a.x. [DOI] [PubMed] [Google Scholar]
- 8.Youmans SR, Stierwalt JAG. Measures of tongue function related to normal swallowing. Dysphagia. 2006;21(2):102–111. doi: 10.1007/s00455-006-9013-z. [DOI] [PubMed] [Google Scholar]
- 9.Steele CM, Bayley MT, Peladeau-Pigeon M, Nagy A, Namasivayam AM, Stokely SL, et al. A Randomized Trial Comparing Two Tongue-Pressure Resistance Training Protocols for Post-Stroke Dysphagia. Dysphagia. 2016;31(3):452–461. doi: 10.1007/s00455-016-9699-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sander EK. When are speech sounds learned? Journal of Speech and Hearing Disorders. 1972;37(1):55–63. doi: 10.1044/jshd.3701.55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


