Abstract
Objective
Stereotactic radiation therapy is increasingly used to treat vestibular schwannomas (VSs) primarily and to treat tumor remnants following microsurgery. Little data are available regarding the effects of radiation on VS cells. Tyrosine nitrosylation is a marker of oxidative stress following radiation in malignant tumors. It is not known how long irradiated tissue remain under oxidative stress, and if such modifications occur in benign neoplasms such as VSs treated with significantly lower doses of radiation. We immunostained sections from previously radiated VSs with an antibody that recognizes nitrosylated tyrosine residues to assess for ongoing oxidative stress.
Study Design
Immunohistochemical analysis.
Methods
Four VSs, which recurred after excision, were treated with stereotactic radiation therapy. Ultimately each tumor required salvage re-resection for regrowth. Histologic sections of each tumor before and after radiation were immunolabeled with a monoclonal antibody specific to nitrotyrosine and compared. Two VSs that underwent re-resection of a growing tumor remnant without prior radiation therapy served as additional controls.
Results
Irradiated tumors enlarged in volume by 3.16-8.62 mL following radiation. Pre-radiation sections demonstrated little to no nitrotyrosine immunostaining. Three of four of irradiated VSs demonstrated increased nitrotyrosine immunostaining in the post-radiation sections compared to pre-radiation tumor sections. Non-irradiated VSs did not label with the anti-nitrotyrosine antibody.
Conclusions
VSs exhibit oxidative stress up to seven years after radiotherapy, yet these VSs continued to enlarge. Thus, VSs that grow following radiation appear to possess mechanisms for cell survival and proliferation despite radiation induced oxidative stress.
Keywords: vestibular schwannoma, acoustic neuroma, radiation, 3-nitrotyrosine, oxidative stress, reactive oxygen species
Introduction
Ionizing-radiation (IR) has been increasingly used in an effort to arrest tumor growth in select patients with vestibular schwannomas (VSs). Most commonly, IR is delivered in the form of stereotactic radiosurgery (SRS) or fractionated stereotactic radiotherapy (FRT). Outcomes of IR in the treatment of VSs have been widely reported and it is generally accepted that SRS and FRT yield high tumor control rates, relatively low associated morbidity and good quality of life outcomes (1,2). Despite the increasing use of IR in the treatment of VSs, the effects of IR on the VS cells themselves are poorly understood. Compared to malignant neoplasms, VS cells in culture possess radioresistant characteristics, consistent with their low proliferative capacity (3–6). Further, many studies that have examined the histopathological features of irradiated VSs fail to find evidence of significant radiation damage to the tumor cells (5,7). Such observations raise the possibility that the consequences of IR on VS growth involve indirect effects such as decreased tumor vascularity.
IR induced cell damage involves multiple mechanisms and processes. Beyond direct damage to DNA molecules, IR exposure leads to increased production of reactive nitrosative and oxidative free radical species (RONS) (8,9). Furthermore, ROS levels continue to rise beyond those that occur within milliseconds initial radiation exposure leading to cellular oxidative stress (10–12). Radiation-induced oxidative stress may also spread from targeted cells to bystander neighboring cells (13). Such highly reactive oxidizing free-radicals cause cellular damage by oxidizing and nitrosylating cellular macromolecules including nucleic acids, proteins and lipids (9). One of the byproducts of the oxidative and nitrosative stress, 3-nitrotyrosine (3-NT), has been used as a surrogate marker for radiation-induced oxidative cellular injury (14–16). In this study, we used 3-NT immunostaining to evaluate the presence of radiation-induced oxidative cellular stress in VSs before and after radiation in a rare series of four VSs which were initially resected, then irradiated for recurrent growth and ultimately underwent salvage re-resection for persistent growth after IR.
Methods
All procedures for obtaining patient information and VS samples were approved by the Institutional Review Board. Patient demographics and case histories were obtained by retrospective chart review. 3D tumor volumes were calculated based on the axial post-contrast T1 magnetic resonance imaging (MRI) thin section images using Vitrea software [version 6.6.2, Vital images, Toshiba Medical Systems, Minnetonka, MN]. In all cases, the tumor was manually outlined on the axial post contrast images on all sections followed by computerized compilation of summated tumor volume and generation of a volumetric model. Segmented volumetric data was re-reviewed post analysis to ensure measurement accuracy. Tumor volumes were calculated from the MRI at the time of presentation, prior to radiation and prior to re-resection. For controls, we selected two patients with VSs who underwent subtotal resection of a VS with subsequent regrowth and re-resection of the VS without any IR therapy.
Samples from each specimen were fixed in 10% neutral buffered formalin and paraffin embedded. Five-micron thick sections of formalin fixed paraffin embedded tissue were stained with hematoxylin and eosin (H&E) and were also used for immunohistochemical assays.
3-Nitrotyrosine immunohistochemistry
Paraffin sections were deparaffinized with serial xylene washes and rehydrated with serial levels of ethanol. Slides were retrieved in citrate buffer (pH 6.0) using a Decloaking Chamber (Biocare, Concord CA). Endogenous peroxidase was quenched with 3% hydrogen peroxide for 8 min followed by incubation in 10% goat serum to block nonspecific binding. Anti-3-nitrotyrosine rabbit polyclonal antibody (Millipore; #06284, Billerica, MA, 1:2000) was applied for 1 h in Dako buffer (Carpinteria, CA) at room temperature. Dako Rabbit EnVision HRP System reagent was applied for 30 min and then slides were developed with Dako diaminobenzidine (DAB) plus for 5 min followed by DAB Enhancer for 3 min. Slides were counterstained with hematoxylin. Negative control slides were stained using the same procedure, omitting the primary antibody. 3-Nitrotyrosine immunohistochemical staining was scored by a pathologist (AW) blinded to the patient information and treatment conditions according to the following system: (-)=no staining, (+) = diffuse background staining of stroma, (++) = strong, specific cytoplasmic staining in cells. 3-NT immunohistochemical staining and scoring were repeated two times. Images were prepared for publication using Adobe PhotoShop and Illustrator software (Adobe, San Jose, CA).
Results
Table 1 provides demographic and tumor characteristics of the patients in this study. Four patients (A-D) had a subtotal resection of a VS with subsequent growth identified on serial imaging. Each patient received a single dose of LinAcSRS to treat the growing tumor remnant (Table 1 lists treatment specifications for each tumor). In each of these four cases, tumor growth continued following SRS prompting repeat tumor resection. Two patients (E,F) underwent subtotal resection of a VS with subsequent growth of the tumor remnant. In these patients, a second tumor resection was performed without intervening IR and thus these patients serve as a control of previously operated, unirradiated tumors. Figure 1 presents the MRI findings for the four patients (A-D) that received IR while Figure 2 presents the MRI finding for the two patients (E, F) that did not receive IR.
Table 1.
Patient demographic information and pertinent treatment-related data
| Radiated patients | Marginal radiation dose (gy) | Maximum radiation dose (gy) | Prescribed isodose | Initial tumor volume (mL) | Interval to radiation (months) | Pre-radiation tumor volume (mL) | Interval to salvage (months) | Pre-salvage tumor volume (mL) |
|---|---|---|---|---|---|---|---|---|
| A | 12 | 17.1 gy | 70% | 42.23 | 38 | 14.13 | 7 | 26.95 |
| B | 12.5 | 19 gy | 65% | MRI not available | 89 | 2.17 | 48 | 6.46 |
| C | 12 | 17.9 gy | 67% | 0.96 | 79 | 1.30 | 82 | 4.46 |
| D | 12 | 17.9 gy | 67% | 22.02 | 65 | 5.48 | 12 | 7.03 |
| Non-radiated patients | Gender | Age at presentation | Initial tumor volume (mL) | Interval to regrowth (months) | Tumor volume prior to 2nd resection (mL) |
|---|---|---|---|---|---|
| E | M | 64 | 15.41 | 79 | 9.72 |
| F | F | 46 | 5.67 | 35 | 2.25 |
Figure 1.
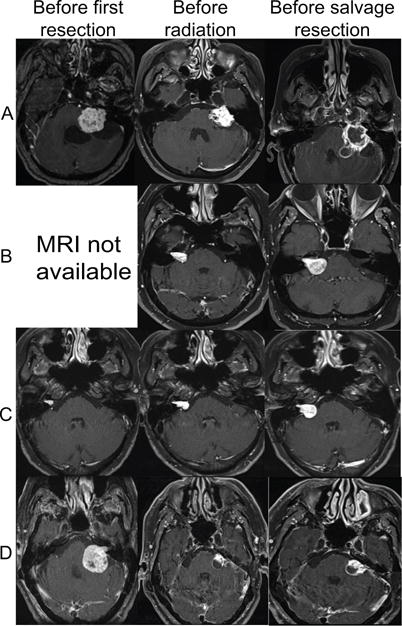
Representative T1 post gadolinium magnetic resonance imaging results from four patients (A-D) that underwent a second resection of a vestibular schwannoma following radiation of a growing tumor remnant.
Figure 2.
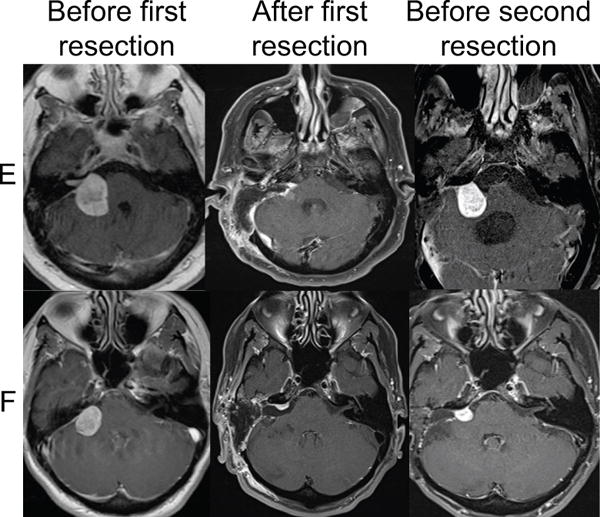
Representative T1 post gadolinium magnetic resonance imaging results from two patients (E,F) that underwent a second resection of a vestibular schwannoma after growth of a tumor remnant. These patients did not receive any radiation therapy.
Immunohistochemical staining for 3-NT was performed on sections from both the initial resection and from the subsequent resection of the recurrent tumor. Three of the four irradiated tumors (A-C) demonstrated a dramatic increase in 3-NT immunostaining compared to their initial tumor counterparts resected prior to radiation (Fig. 3). There was little to no 3-NT immunostaining in any of the sections from the initial tumor specimens (Fig. 3). Likewise, there was little to no 3-NT immunostaining in sections from recurrent tumors that had not received prior radiation (E,F) (Fig. 3). In contrast to specimens from patients A-C, sections from the irradiated tumor from patient D failed reveal significant 3-NT immunostaining. Additional sections from each tumor were stained with H&E to examine overall pathological characteristics. Two of the four tumors demonstrated histopathological changes consistent with prior radiation including vessel hyalinization and luminal narrowing and fibrinoid necrosis (Fig. 4).
Figure 3.
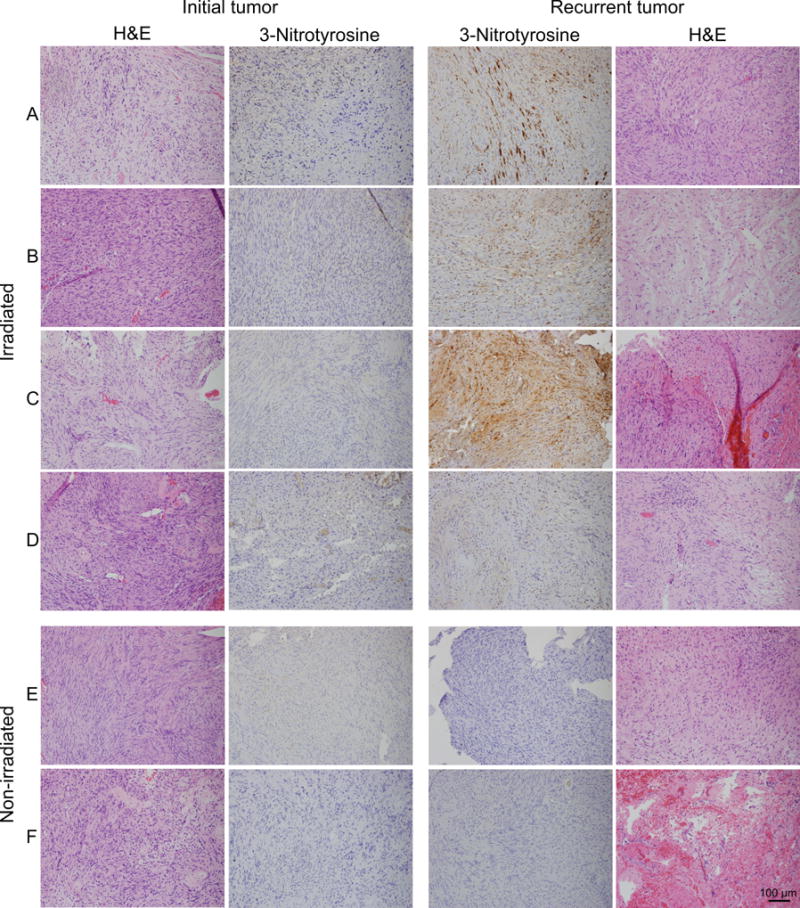
Radiation increases 3-nitrotyrosine (3-NT) immunostaining in vestibular schwannomas (VSs). Sections of VSs were immunostained with an antibody that detects 3-NT. For four VSs (A-D) sections were derived from the initial tumor resection and from the recurrent tumor that had previously been irradiated. For two VSs (E,F) sections were derived from the initial tumor resection and from the recurrent tumor that had not previously been irradiated. Representative hematoxylin and eosin (H&E) stains are shown for each tumor. Scale bar=100 μm.
Figure 4.
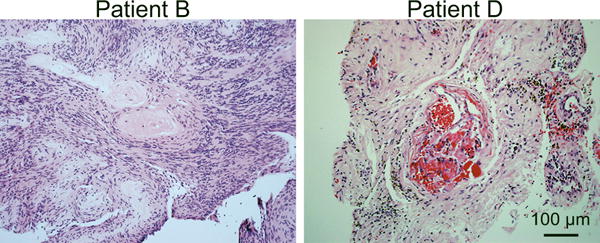
Histopathological radiation changes to vestibular schwannomas. Hematoxylin and eosin stained sections from the post-irradiated tumors from patients B and D demonstrating vessel hyalinization and luminal narrowing and fibrinoid necrosis consistent with radiation changes. Scale bar=100 μm.
A pathologist blinded to the specimen treatment scored the 3-NT immunostaining and the results are presented in Table 2. On this scale only ++ is considered a true-positive whereas + likely represents non-specific background staining. Three of the four tumors (A-C) that had previously been irradiated demonstrated a significant increase in 3-NT immunostaining (++) whereas all tumors that had not been previously irradiated (both initial and recurrent tumors) and one irradiated tumor (patient D) were scored as having no (-) or only background (+) staining.
Table 2.
3-nitrotyrosine immunohistochemistry scoring
| Radiated patients | First resection | Second Resection |
|---|---|---|
| A | + | ++ |
| B | − | ++ |
| C | − | ++ |
| D | − | + |
| Non-irradiated patients | ||
| E | + | + |
| F | − | − |
Discussion
Accumulating evidence suggests that SRS and FRT are safe and effective treatments for appropriately selected VSs (1,2); accordingly, increasing numbers of VSs are being treated with SRS/FRT. While the prolific evaluation of clinical outcomes following IR for VSs continues, there is a paucity of research on the radiobiology of VSs. This is likely due, at least in part, to the low numbers of tumors that have been resected following IR and the challenges of growing large numbers of primary VS cells in culture (17,18). Here we had a unique opportunity to analyze IR-induced cellular damage in a series of four paired VS specimens that progressed or recurred after microsurgical excision, were treated with IR, progressed again and ultimately required salvage re-resection. The decision of what constituted tumor progression and whether or not to resect the tumors after radiation treatment was a complex decision process that involved surgeons, radiation-oncologists, and the patient. Given the retrospective nature of the study it was not possible to determine the specific criteria and decision processes that led to surgery in these cases. It is important to note that different surgeons and services were involved in these cases so the decision-making process was not necessarily uniform. Further, we were able to analyze paired specimens from two other patients that underwent an initial tumor resection followed by re-resection of a recurrent tumor but without receiving any intervening IR. These later specimens provide an excellent control for potential effects of surgery on the histopathological findings.
The data provide evidence for ongoing oxidative stress months and years following IR in three of four irradiated VSs, evidenced by robust 3-NT immunostaining up to 7 years following IR. Despite this oxidative stress, there was ongoing tumor growth indicating that the tumor cells continued to proliferate under this stress. The reason(s) for lack of 3-NT immunostaining in patient D following IR are unknown. One possibility is that the portion of the tumor encompassed in the histopathology specimen represents areas of de novo tumor growth and did not include areas that were previously irradiated. VS control rates are uniformly good with IR therapy and some studies have even demonstrated varying degrees of VS regression following IR therapy in a subset of patients (19). However, in contrast to many malignant tumors, rather than inducing tumor regression, IR typically only arrests tumor growth in most responsive VSs(2). These clinical observations are consistent with the fact that IR doses much higher than those currently employed in treatment of VSs (e.g. ≥30 Gy) are required to induce cell cycle arrest and cell death in cultured VS cells (3,6). Amazingly, even after 150 Gy of IR, some cultured VS cells survive and remain viable (6). Others have demonstrated that VSs resected after IR retain viable VS cells with the typical histologic appearance of non-radiated VS cells and a proliferative capacity (4,5,7,20). Consistent with these prior reports, two of the four post-radiation specimens in our study demonstrated no post-radiation changes. Taken together these observations suggest that, compared to malignant neoplasms, VS cells are relatively radioresistant.
There are several potential mechanisms that may contribute to the relative radioresistance in VS cells (4). For instance, radiation-induced cell death depends in part on the proliferation rate of the targeted cells and VS cells have a very low proliferative capacity (18). Indeed, stimulating cultured VS cells to proliferate increases radiosensitivity while inhibiting proliferation protects VS cells from radiation-induced apoptosis (3).
Another mechanism that appears to contribute to the relative radioresistance of VS cells relates to their capacity to mitigate oxidative stress. IR indirectly inflicts molecular and cellular damage through the formation of highly reactive RONS. IR ionizes H2O molecules into free radicals and induces the action of nitric oxide synthase, mitochondrial oxidase and cytoplasmic NADPH synthase, all of which result in the generation of RONS (11,21,22). Due to their lack of merlin protein expression, VS cells display persistent activation of several intracellular kinase signaling cascades, including phosphatidyl-inositol 3- kinase/Akt/mTORC1, extracellular regulated kinase/mitogen-activated protein kinase (ERK/MAPK), p21-activated kinase, and c-Jun N-terminal kinase (JNK), among others (23–29). Of these, activation of JNK appears to protect VS cells from cell death by inhibiting accumulation of mitochondrial ROS (23). Indeed, inhibition of JNK increases VS cell radiosensitivity correlated with an increase in mitochrondrial oxidative stress following IR (30). Thus, persistent activation of JNK due to a lack of merlin protein expression reduces oxidative stress and decreases sensitivity of VS cells to IR.
RONS result in several molecular modifications including carbohydrate oxidation, lipoprotein oxidation, DNA hydroxylation, and protein oxidation (9,15,16,31). Many of these molecular alterations are detectable and are used as surrogate biomarkers for oxidative cellular stress. One of these markers, 3-NT, is a frequently used marker of cellular oxidative stress (31). 3-NT is the byproduct of the oxidation of tyrosine residues in proteins by peroxynitrite. Using 3-NT, the presence of oxidative cellular stress has been demonstrated acutely in radiated tissues and cellular cultures including lung, cornea, skin, intestine and kidney as well as cultures of hepatocytes, squamous cell carcinoma of the head and neck, and gliomas (15,16,21). However, to our knowledge, no prior studies have determined whether 3-NT persists in the long-term in these tissues following IR. Thus, our unique access to paired tumors specimens prior to and following IR provides the first evidence of persistent 3-NT for several years after exposure to IR. These results indicate ongoing oxidative stress in tissues for months and years following IR.
There is growing evidence that IR results in a chronic oxidative stress. The mechanisms for these chronic oxidative changes following radiation have not been fully elucidated. IR-induced mitochondrial dysfunction likely contributes to chronic overproduction of mitochondrial RONS (22,32–34). 3-NT immunostaining in VSs up to 7 years after IR supports the theory that IR results in long-term chronic oxidative stress; yet, VSs may continue to grow in these conditions.
One aspect of the four irradiated patients in this study that differs from many in the radiotherapy literature is that these tumors had all been observed for tumor growth prior to receiving IR. Significantly, a recent study of large VSs that underwent subtotal resection found that over 30% of VSs that received IR for growth of tumor remnants demonstrated persistent growth and ultimately required further resection (35). These findings are similar to our patients and highlight the differences in clinical success of IR for VSs when IR use is restricted to tumors that have already been observed and found to have documented growth.
One could argue that the tumors analyzed in this study could be considered “more aggressive” or “less responsive to radiation” than typical vestibular schwannomas based on the fact that they recurred/progressed after prior surgical removal. However, other vestibular schwannomas that are controlled with radiation seem as, or perhaps even more, likely to demonstrate 3-NT labeling, indicative of ongoing oxidative stress, than tumors that grow after radiation. Ultimately data from a larger number of vestibular schwannomas will be required to verify chronic oxidative stress following radiation.
Our findings also highlight the importance of long-term follow-up for VS patients following IR to assess for delayed tumor progression. While studies demonstrating long-term tumor control with microsurgery and IR are emerging (36–38), most of the data in the literature are based on more limited follow-up periods. In two recent systematic reviews of VS outcomes, mean follow-up was reported as ≤5 years in at least 23 studies (1,2). Two of the VSs in this study demonstrated chronic oxidative stress 4 and 7 years after IR and still progressed. The potential for delayed malignant transformation following IR also warrants long-term follow-up. IR induces immediate genetic damage along with a host of long-term indirect cellular carcinogenic effects (22,39). Related to the findings in these VS specimens is the observation that IR-induced mitochondrial dysfunction leads to further genomic instability secondary to chronic overproduction of RONS (32,33). Furthermore, chronic IR-induced oxidative stress appears to promote mutagenesis and tumorigenesis in human thyrocytes (34).
Conclusions
The data reported here demonstrate chronic oxidative stress in VSs up to 7 years after radiation. Further, VSs may continue to progress in spite of these effects suggesting that VS cells possess mechanisms for cell survival and proliferation despite radiation induced oxidative stress and highlighting the need for long-term follow-up in patients treated with IR.
Acknowledgments
This work was supported in part by CDMRP NF130072 from the Department of Defense.
Footnotes
Level of Evidence: Level 3b- Case controlled study
References
- 1.Wolbers JG, Dallenga AH, Mendez Romero A, et al. What intervention is best practice for vestibular schwannomas? A systematic review of controlled studies. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-001345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthurs BJ, Fairbanks RK, Demakas JJ, et al. A review of treatment modalities for vestibular schwannoma. Neurosurg Rev. 2011;34:265–77. doi: 10.1007/s10143-011-0307-8. discussion 77-9. [DOI] [PubMed] [Google Scholar]
- 3.Hansen MR, Clark JJ, Gantz BJ, et al. Effects of ErbB2 signaling on the response of vestibular schwannoma cells to gamma-irradiation. Laryngoscope. 2008;118:1023–30. doi: 10.1097/MLG.0b013e318163f920. [DOI] [PubMed] [Google Scholar]
- 4.Yeung AH, Sughrue ME, Kane AJ, et al. Radiobiology of vestibular schwannomas: mechanisms of radioresistance and potential targets for therapeutic sensitization. Neurosurg Focus. 2009;27:E2. doi: 10.3171/2009.9.FOCUS09185. [DOI] [PubMed] [Google Scholar]
- 5.Lee F, Linthicum F, Jr, Hung G. Proliferation potential in recurrent acoustic schwannoma following gamma knife radiosurgery versus microsurgery. Laryngoscope. 2002;112:948–50. doi: 10.1097/00005537-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Anniko M, Arndt J, Noren G. The human acoustic neurinoma in organ culture. II Tissue changes after gamma irradiation. Acta oto-laryngologica. 1981;91:223–35. doi: 10.3109/00016488109138503. [DOI] [PubMed] [Google Scholar]
- 7.Jacob A, Igarashi S, Platto T, et al. The Solid Component of Radiographically Non-Growing, Post-Radiated Vestibular Schwannoma Retains Proliferative Capacity: Implications for Patient Counseling. Ann Otol Rhinol Laryngol. 2015;124:834–40. doi: 10.1177/0003489415588128. [DOI] [PubMed] [Google Scholar]
- 8.Biaglow JE, Mitchell JB, Held K. The importance of peroxide and superoxide in the X-ray response. Int J Radiat Oncol Biol Phys. 1992;22:665–9. doi: 10.1016/0360-3016(92)90499-8. [DOI] [PubMed] [Google Scholar]
- 9.Hall E. Radiobiology for the radiologist. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 10.Du C, Gao Z, Venkatesha VA, et al. Mitochondrial ROS and radiation induced transformation in mouse embryonic fibroblasts. Cancer Biol Ther. 2009;8:1962–71. doi: 10.4161/cbt.8.20.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao Z, Sarsour EH, Kalen AL, et al. Late ROS accumulation and radiosensitivity in SOD1-overexpressing human glioma cells. Free Radic Biol Med. 2008;45:1501–9. doi: 10.1016/j.freeradbiomed.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60. doi: 10.1016/j.canlet.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hei TK, Zhou H, Chai Y, et al. Radiation induced non-targeted response: mechanism and potential clinical implications. Curr Mol Pharmacol. 2011;4:96–105. doi: 10.2174/1874467211104020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji C, Shioya S, Hirota Y, et al. Increased production of nitrotyrosine in lung tissue of rats with radiation-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;278:L719–25. doi: 10.1152/ajplung.2000.278.4.L719. [DOI] [PubMed] [Google Scholar]
- 16.Coleman MC, Olivier AK, Jacobus JA, et al. Superoxide mediates acute liver injury in irradiated mice lacking sirtuin 3. Antioxid Redox Signal. 2014;20:1423–35. doi: 10.1089/ars.2012.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schularick NM, Clark JJ, Hansen MR. Primary culture of human vestibular schwannomas. Journal of visualized experiments : JoVE. 2014 doi: 10.3791/51093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen MR, Roehm PC, Chatterjee P, et al. Constitutive neuregulin-1/ErbB signaling contributes to human vestibular schwannoma proliferation. Glia. 2006;53:593–600. doi: 10.1002/glia.20316. [DOI] [PubMed] [Google Scholar]
- 19.Slane BG, Goyal U, Grow JL, et al. Radiotherapeutic management of vestibular schwannomas using size- and location-adapted fractionation regimens to maximize the therapeutic ratio. Pract Radiat Oncol. 2017;7:e233–e41. doi: 10.1016/j.prro.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Lee DJ, Westra WH, Staecker H, et al. Clinical and histopathologic features of recurrent vestibular schwannoma (acoustic neuroma) after stereotactic radiosurgery. Otology & Neurotology. 2003;24:650–60. doi: 10.1097/00129492-200307000-00020. discussion 60. [DOI] [PubMed] [Google Scholar]
- 21.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80:251–9. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 22.Szumiel I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: the pivotal role of mitochondria. Int J Radiat Biol. 2015;91:1–12. doi: 10.3109/09553002.2014.934929. [DOI] [PubMed] [Google Scholar]
- 23.Yue WY, Clark JJ, Fernando A, et al. Contribution of persistent C-Jun N-terminal kinase activity to the survival of human vestibular schwannoma cells by suppression of accumulation of mitochondrial superoxides. Neuro-oncology. 2011;13:961–73. doi: 10.1093/neuonc/nor068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James MF, Han S, Polizzano C, et al. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29:4250–61. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi C, Wilker EW, Yaffe MB, et al. Validation of the p21-activated kinases as targets for inhibition in neurofibromatosis type 2. Cancer Res. 2008;68:7932–7. doi: 10.1158/0008-5472.CAN-08-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flaiz C, Chernoff J, Ammoun S, et al. PAK kinase regulates Rac GTPase and is a potential target in human schwannomas. Exp Neurol. 2009;218:137–44. doi: 10.1016/j.expneurol.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rong R, Tang X, Gutmann DH, et al. Neurofibromatosis 2 (NF2) tumor suppressor merlin inhibits phosphatidylinositol 3-kinase through binding to PIKE-L. Proc Natl Acad Sci U S A. 2004;101:18200–5. doi: 10.1073/pnas.0405971102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giovannini M, Bonne NX, Vitte J, et al. mTORC1 inhibition delays growth of neurofibromatosis type 2 schwannoma. Neuro-oncology. 2014;16:493–504. doi: 10.1093/neuonc/not242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob A, Lee TX, Neff BA, et al. Phosphatidylinositol 3-kinase/AKT pathway activation in human vestibular schwannoma. Otology & Neurotology. 2008;29:58–68. doi: 10.1097/mao.0b013e31816021f7. [DOI] [PubMed] [Google Scholar]
- 30.Yue WY, Clark JJ, Telisak M, et al. Inhibition of c-Jun N-terminal kinase activity enhances vestibular schwannoma cell sensitivity to gamma irradiation. Neurosurgery. 2013;73:506–16. doi: 10.1227/01.neu.0000431483.10031.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teixeira D, Fernandes R, Prudencio C, et al. 3-Nitrotyrosine quantification methods: Current concepts and future challenges. Biochimie. 2016 doi: 10.1016/j.biochi.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Dayal D, Martin SM, Owens KM, et al. Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation. Radiat Res. 2009;172:737–45. doi: 10.1667/RR1617.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimura T, Kunugita N. Mitochondrial reactive oxygen species-mediated genomic instability in low-dose irradiated human cells through nuclear retention of cyclin D1. Cell Cycle. 2016:1–5. doi: 10.1080/15384101.2016.1170271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ameziane-El-Hassani R, Talbot M, de Souza Dos Santos MC, et al. NADPH oxidase DUOX1 promotes long-term persistence of oxidative stress after an exposure to irradiation. Proc Natl Acad Sci U S A. 2015;112:5051–6. doi: 10.1073/pnas.1420707112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monfared A, Corrales E, Theodosopoulos P, et al. Facial Nerve Outcome and Tumor Control Rate as a Function of Degree of Resection in Treatment of Large Acoustic Neuromas: Preliminary Report of the Acoustic Neuroma Subtotal Resection Study. Neurosurgery. 2015 doi: 10.1227/NEU.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 36.Frischer JM, Gruber E, Schoffmann V, et al. Long-term outcome after Gamma Knife radiosurgery for acoustic neuroma of all Koos grades: a single-center study. J Neurosurg. 2018:1–10. doi: 10.3171/2017.8.JNS171281. [DOI] [PubMed] [Google Scholar]
- 37.Hasegawa T, Kida Y, Kobayashi T, et al. Long-term outcomes in patients with vestibular schwannomas treated using gamma knife surgery: 10-year follow up. J Neurosurg. 2013;119(Suppl):10–6. doi: 10.3171/jns.2005.102.1.0010. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa T, Kida Y, Kato T, et al. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg. 2013;118:557–65. doi: 10.3171/2012.10.JNS12523. [DOI] [PubMed] [Google Scholar]
- 39.Kadhim M, Salomaa S, Wright E, et al. Non-targeted effects of ionising radiation–implications for low dose risk. Mutat Res. 2013;752:84–98. doi: 10.1016/j.mrrev.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


