Abstract
We evaluated the in vitro activity of apramycin against clinical strains of vancomycin-intermediate and methicillin-resistant and -suseptible Staphylococcus aureus. Apramycin demonstrated an MIC50/MIC90 of 8/16 µg/mL. No strains had an MIC above the epidemiological cutoff value of 32 µg/mL, suggesting apramycin resistance mechanisms are rare in this strain population. The mounting evidence for broad-spectrum in vitro activity of apramycin against S. aureus and other bacterial species suggests that further exploration of apramycin or derivatives as repurposed human therapeutics is warranted.
Keywords: apramycin, Staphylococcus aureus, activity spectrum, repurposing, antibiotic, natural product, synergy, aminoglycoside, gentamicin
1. Introduction
Staphylococcus aureus is both a human skin commensal and an opportunistic pathogen. It is the leading cause of bacteremia and infective endocarditis, as well skin and soft tissue, osteoarticular, and surgical site infections (Akhi, et al., 2017; Deleo, et al., 2010; Tong, et al., 2015). Strains resistant to methicillin and by proxy, all β-lactams (so called methicillin resistant S. aureus or MRSA), are common in the United States (Tong, et al., 2015). Vancomycin is the first-line treatment for methicillin-resistant S. aureus (MRSA) infection. However, limitations of vancomycin include relatively lower bactericidal activity compared with β-lactams; nephrotoxicity, associated with high dosages and underlying risk factors; and a requirement for routine monitoring of drug levels to ensure adequate dosing (Hazlewood, et al., 2010). Furthermore, vancomycin tolerant and heteroresistant populations may emerge during treatment (Bamberger & Boyd, 2005; Dombrowski & Winston, 2008; Hawkins, et al., 2007; Lodise, et al., 2008; Steinkraus, et al., 2007).
Therefore, additional treatment options for S. aureus would be welcome, especially for those strains with resistance to newer agents such as daptomycin and linezolid. minoglycosides are not currently used to treat S. aureus infections as single agents. In particular, renal and ototoxic side effects are of significant concern (Jose, et al., 2010; Matt, et al., 2012). Therefore aminoglycoside therapy is generally reserved for treatment of Gram-negative infection, where pharmacodynamic considerations are considered more favorable (Tam, et al., 2006). Currently, gentamicin treamtent of S. aureus is advocated only in low dose (1mg/kg q8 h or 3–5mg/kg q 24h) in combination with vancomycin or a β-lactam and rifampin, during therapy of staphylococcal endocarditis where prosthetic material is present and in combination with daptomycin for persistent bactermia (Liu, et al., 2011). However, the relative benefit of gentamicin adjunctive therapy versus risk of kidney damage has been a subject of extensive debate (Bruss, 2009; Buchholtz, et al., 2009; Buchholtz, et al., 2011; Cosgrove, et al., 2009; Frippiat, et al., 2009). Therefore, a non-toxic aminoglycoside with predictable activity against S. aureus would therefore be especially welcome.
Apramycin is a structurally unique aminoglycoside used in veterinary medicine and is characterized by a bicyclic sugar moiety and a 4-monosubsituted 2-deoxystreptamine ring (Meyer, et al., 2014). In contrast to aminoglycosides currently used in human therapy, apramycin appears highly selective for bacterial ribosomes and on this basis thought devoid of significant ototoxic and renal toxic side effects (Matt, et al., 2012). Previous data from our group and others have shown broad-spectrum in vitro activity of apramycin against carbapenem-resistant Enterobacteriaceae and multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa (Kang, et al., 2017; Livermore, et al., 2011; K.P. Smith & J.E. Kirby, 2016a, 2016b). Furthermore, we recently demonstrated potent in vivo bactericidal activity against Acinetobacter baumannii in a murine thigh infection model (Kang, et al., 2018).
However, activity spectrum data for S. aureus is limited. Therefore, the goal of this study was to explore in vitro activity of apramycin against contemporary clinical isolates of S. aureus.
2. Materials and Methods
2.1 Bacterial Strains and Antimicrobials
We evaluated a collection of 109 strains of S. aureus for their susceptibility to apramycin. Fourteen S. aureus strains obtained from the FDA-CDC Antimicrobial Resistance Isolate Bank (https://www.cdc.gov/drugresistance/resistance-bank/) were part of the vancomycin intermediate S. aureus panel with vancomycin MIC values of 4–8 µg/mL. 95 additional strains of de-identified S. aureus clinical isolates were obtained from the Beth Israel Deaconess Medical Center (Boston, MA) clinical microbiology laboratory. Our strain collection was comprised of 38.5% methicillin-resistant S. aureus (MRSA), 48.6% methicillin-suseptible S. aureus (MSSA) and 12.9% vancomycin-intermediate S. aureus (VISA). All strains were stored frozen at −80°C in a stock solution of 50% glycerol and 50% cation-adjusted Mueller-Hinton broth (BD Diagnostics, Franklin Lakes, NJ) until use in experiments.
Apramycin sulfate was obtained from Alfa Aesar (Tewksbury, MA) and was dissolved in deionized water at 32 mg/ml and stored at −20°C in aliquots that were used only once. For synergy studies, apramycin, gentamicin, daptomycin, and vancomycin were dissolved at 100, 100, 10, and 10 mg/ml in water supplemented with 0.3% polysorbate 20 (P-20; Sigma–Aldrich), respectively. Linezolid was dissolved at 30 mg/ml in DMSO (Signma-Aldrich). Solvents used reflect requirements for liquid handling by the HP D300 Digital Dispensing System (HP Inc, Palo Alto, CA) (Brennan-Krohn, et al., 2017; K. P. Smith & J. E. Kirby, 2016).
2.2 Suseptibility Testing
The Clinical Laboratory and Standards Institute (CLSI) broth microdilution reference method was used for MIC testing of apramycin (CLSI, 2015). MIC panels were created by serial dilution of stock apramycin with cation-adjusted Mueller-Hinton broth in round bottom, 96-well plates (Evergreen Scientific, Los Angeles, CA). Dilutions were prepared at 2× the final concentration in volumes of 50 µL with final concentrations ranging from 1–256 µg/mL after an equal volume of bacterial inoculum (5 × 105 cfu/ml final concentration) was added. Stock solutions were quality controlled against S. aureus ATCC 29213 on three separate days. All MIC values for ATCC 29213 were consistently 4 µg/ml, which was in the middle of the 2–8 µg/mL acceptable quality control range suggested by CLSI. Bacterial inocula were prepared by passaging previously frozen bacterial strains on trypticase soy agar containing 5% sheep’s blood at 37°C. Isolated colonies were then suspended in cation-adjusted Mueller-Hinton broth for a final inoculum concentration of 5×105 CFU/mL. After inoculation, broth microdilution plates were incubated at 35°C in ambient air for 16–20 hours. Each experiment also included both a positive (S. aureus ATCC 25923) and a negative control to which no organisms were added. S. aureus 25923 always showed an MIC of 4 or 8 µg/ml supporting consistency of assay readout.
2.3 MIC Interpretation
The MIC of each strain from our collection was determined in duplicate on separate days. If duplicate MICs were within one doubling dilution of each other but were not the same, the higher MIC was used. If duplicate MICs were not within one doubling dilution of each other, a third replicate was performed, and the MIC was defined as the modal MIC of the three replicates. Categorical breakpoints for apramycin are not available either from the CLSI or the European Committee for Antimicrobial Susceptibility Testing and, therefore, categorical intepretation was not performed.
2.4 Time-Kill Studies
Time-kill studies were performed according to CLSI guidelines (Clinical and Laboratory Standards Institute, 1999). To prepare a starting inoculum for the time-kill studies, 100 µL of a 0.5 McFarland suspension of colonies from an overnight plate was added to 5 mL of CAMHB and incubated on a shaker in ambient air at 35°C until it reached log phase growth. The log phase culture was then adjusted to a turbidity of 1.0 McFarland in CAMHB, and 100 µL of this suspension was added to 10 mL volumes of antimicrobial solutions in cation-adjusted Mueller-Hinton broth (BD Diagnostics, Franklin Lakes, NJ) at time 0. Antibiotic concentrations selected were based on multiples of each isolate’s MIC as determined through broth microdilution assays. A growth control and a negative control were run in parallel with each experiment. Cultures were incubated on a shaker in ambient air at 35°C.
Aliquots from the culture were removed at 0, 1, 2, 4, 6, and 24 hours. A serial 10-fold dilution in 0.9% sodium chloride was prepared and a 10 µL drop from each dilution was transferred to a Mueller Hinton plate (Thermo Fisher, Waltham, MA) (Chen, et al., 2003; Herigstad, et al., 2001; Naghili, et al., 2013) and incubated overnight in ambient air at 35°C. Colonies within each drop were counted. For drops containing 3 to 30 colonies, the cell density of the sample was calculated; if more than one dilution for a given sample had a countable number of colonies, the cell density of the two dilutions was averaged. If no drops had a countable number of colonies, the two drops above and below the countable range were averaged. The limit of detection was 300 CFU mL−1. Bactericidal activity was defined as a reduction of ≥3 log10 CFU mL−1 at 24 hours compared to the starting inoculum (Leber, 2016; Pillai, et al., 2005).
2.5 Synergy studies
Checkboard synergy arrays consisting of orthogonal two-fold serial dilutions of antibiotic combinations were set up using the HPD300 as previously described (Brennan-Krohn, et al., 2017). Combinatorial fractional inhibitory concentrations ≤ 0.5 were considered synergistic; > 0.5 and < 4 were considered indifferent; and ≥ 4 were considered antagonistic (Odds, 2003).
2.6 Statistical Analysis
R was used to plot MIC distributions and for statistical analysis (RStudio, 2017; Team, 2017). P < 0.05 was considered to be statistically significant.
3. Results
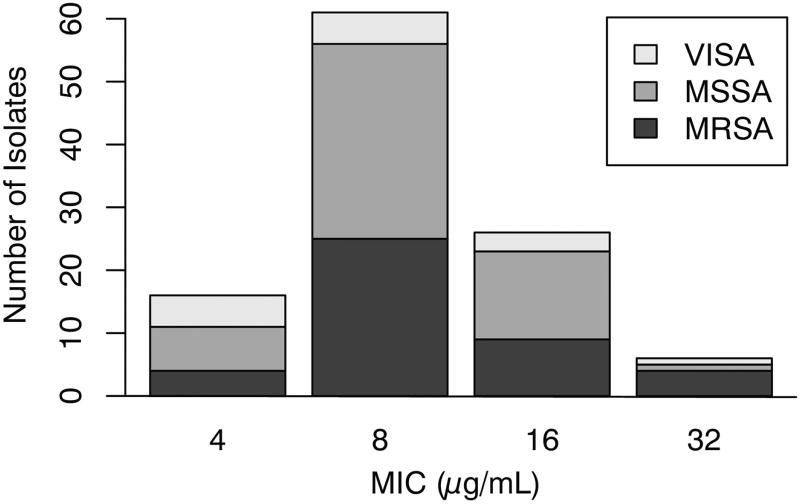
The S. aureus collection had a narrow apramycin MIC range of 4 to 32 µg/mL (see Fig. 1). The MIC50 and the MIC90 were 8 µg/mL and 16 µg/mL, respectively. Based on the visual inspection method, an apramycin epidemiological cutoff value of 32 µg/mL was assigned (Turnbridge & Patterson, 2007). A chi-square test of independence detected no significant relationship between MSSA, MRSA and VISA strain phenotype and apramycin MIC distribution (P = 0.17).
Figure 1. Apramycin MIC distribution for S. aureus clinical isolates.
MSSA (methicillin-susceptible S. aureus); MRSA (methicillin-resistant S. aureus); VISA (vancomycin-intermediate S. aureus).
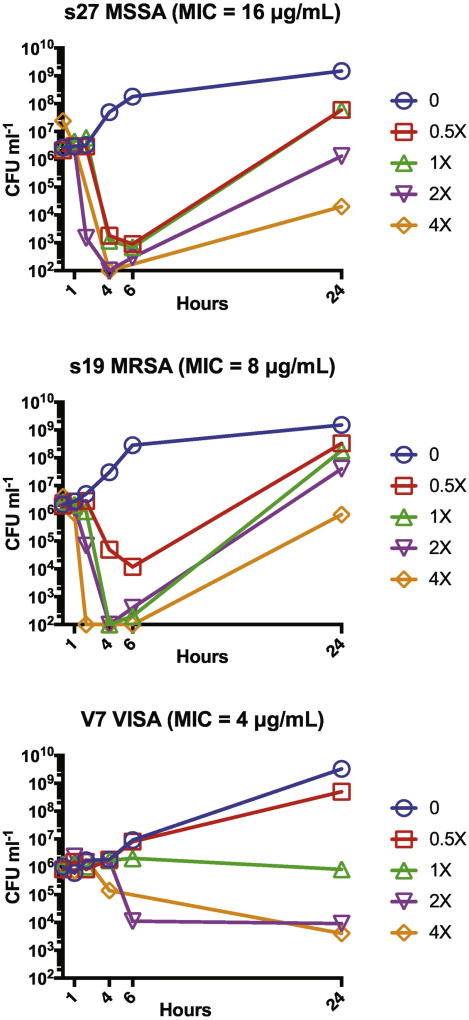
Apramycin exposure led to rapid rapid killing of clinical MSSA strain S27 and MRSA strain S19 at 1×–4× the broth microdilution MIC in time-kill experiments. However, regrowth was observed bewteen 6 and 24 hours (Fig. 2). In contrast, VISA strain, FDA-CDC AR Bank #226, showed bacteriostatic activity at 1×–4× the broth microdilution MIC at all time points examined.
Figure 2. Time-kill experiments.
Macrobroth time-kill analysis was performed against representative MSSA, MRSA, and VISA strains. Data points plotted at 102 cfu indicate no growth and correspond to the assay detection limit. Panel titles list the broth microdilution MIC associated with each isolate.
During checkerboard synergy testing of the same strains use for time-kill studies, both apramycin and gentamicin demonstrated indifference when tested in combination with vancomycin, daptomycin, and linezolid.
4. Discussion
In our examination of human clinical isolates of S. aureus, we observed a very narrow distribution of MIC values. No isolate had an MIC above an epidemiological cutoff value of 32 µg/mL, suggesting near to complete absence of apramycin modifying enzymes in our geographic region and in the smaller number of FDA-CDC VISA strains examined. This observation contrasted with a low prevalence of apramycin resistance in MRSA strains isolated from dairy cattle with mastitis and from diseased swine (Fessler, et al., 2011; Kadlec, et al., 2009). It is possible that selective pressure led to somewhat increased prevalence of apramycin resistance in these veterinary isolates. In the current study, we also found that apramycin demonstrated rapid, early, time-kill properties against MSSA and MRSA isolates with later regrowth, similar to previous reports for gentamicin (Schafer, et al., 2006), with static activity against a VISA isolate.
Apramycin is currently used as an orally administered, non-absorbable antibiotic to treat diarrheal diseases in poultry and livestock, as well as an intravenous treatment for pneumonia in calves and mastitis in cows and sheep (Livermore, et al., 2011; Ziv, et al., 1985; Ziv, et al., 1995). S. aureus is the most frequently isolated pathogen in bovine mastitis specimens (Bradley, et al., 2007), suggesting potential therapeutic efficacy against one type of S. aureus infection in large mammals. In addition, efficacy of apramycin against a single MRSA strain was demonstrated in an immunocompromised murine septicemia model (Meyer, et al., 2014). Here, apramycin decreased bacterial burden in a dose-dependent manner by 2- to 3-log10 in the blood and up to 4-log10 in the kidneys. Therefore, there is evidence supporting therapeutic effect of apramycin aginst Staphylococcal aureus infection.
Notably, aminoglycosides are not used currently as single agents for treatment of S. aureus infections in humans based on limiting toxicities. Further, risk-benefit of short term use in combination with vancomycin and other agents for treat of persistent bacteremia and prosthetic valve endocarditis, although recommended by some guidelines (Liu, et al., 2011) has been a matter of debate (Deresinski, 2009). It is possible that apramycin, based on a putatively more compelling side effect profile, may offer an alternative treatment for S. aureus infections as a single agent or in combination. However, basic pharmacokinetic and pharmacodynamic parameters still need to be defined in humans. Therefore use of apramycin for treatment of human Staphylococcus aureus infection as yet remains speculative.
Conclusions
In this study, we found that apramycin shows consistent in vitro activity against contemporary S. aureus strains including MSSA, MRSA and VISA. Furtheremore, apramycin demonstrated rapid bactericidal activity against MSSA and MRSA. Ultimate utility against human S. aureus infection will depend on pharmacokinetic and pharmacodynamic parameters that have yet to be fully established in animal models and investigated in humans. Nevertheless, the broad-spectrum in vitro activity of apramycin against multidrug-resistant S. aureus strains in combination with prior descriptions of activity against carbapenem-resistant Enterobacteriaceae, and multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa, suggests that further exploration of apramycin and/or derivatives as repurposed human therapeutics may be warranted.
Highlights.
Apramycin is an aminoglycoside approved for veterinary use
Activity against highly drug resistant S. aureus was studied
Frank apramycin resistance was not found in MSSA, MRSA and VISA strains studied
Apramycin could potentially be repurposed against highly drug-resistant pathogens
Acknowledgments
J.E.K. received support from a Chief Academic Officer’s Pilot Award from Beth Israel Deaconess Medical Center. T.B.-K. and K.P.S. were supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers K08AI132716 and T32AI007061, and F32 AI124590, respectively. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The HP D300 digital dispenser was provided by Tecan (Morrisville, NC). Tecan had no role in study design, data collection/interpretation, manuscript preparation, or decision to publish.
References
- Akhi MT, Ghotaslou R, Alizadeh N, Pirzadeh T, Beheshtirouy S, Memar MY. High frequency of MRSA in surgical site infections and elevated vancomycin MIC. Wound Medicine. 2017;17:7–10. doi: 10.1016/j.wndm.2017.01.002. [DOI] [Google Scholar]
- Bamberger DM, Boyd SE. Management of Staphylococcus aureus infections. American Family Physician. 2005;72:2474. [PubMed] [Google Scholar]
- Bradley AJ, Leach KA, Breen JE, Green LE, Green MJ. Survey of the incidence and aetiology of mastitis on dairy farms in England and Wales. Veterinary Record. 2007;160:253–257. doi: 10.1136/vr.160.8.253. [DOI] [PubMed] [Google Scholar]
- Brennan-Krohn T, Truelson KA, Smith KP, Kirby JE. Screening for synergistic activity of antimicrobial combinations against carbapenem-resistant Enterobacteriaceae using inkjet printer-based technology. Journal of Antimicrobial Chemotherapy. 2017;72:2775–2781. doi: 10.1093/jac/dkx241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss JB. Lack of evidence associating nephrotoxicity with low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis. Clinical Infectious Diseases. 2009;49:806. doi: 10.1086/605287. author reply 807–808. [DOI] [PubMed] [Google Scholar]
- Buchholtz K, Larsen CT, Hassager C, Bruun NE. Severity of gentamicin's nephrotoxic effect on patients with infective endocarditis: a prospective observational cohort study of 373 patients. Clinical Infectious Diseases. 2009;48:65–71. doi: 10.1086/594122. [DOI] [PubMed] [Google Scholar]
- Buchholtz K, Larsen CT, Schaadt B, Hassager C, Bruun NE. Once versus twice daily gentamicin dosing for infective endocarditis: a randomized clinical trial. Cardiology. 2011;119:65–71. doi: 10.1159/000329842. [DOI] [PubMed] [Google Scholar]
- Chen CY, Nace GW, Irwin PL. A 6 × 6 drop plate method for simultaneous colony counting and MPN enumeration of Campylobacter jejuni, Listeria monocytogenes, and Escherichia coli. Journal of Microbiological Methods. 2003;55:475–479. doi: 10.1016/s0167-7012(03)00194-5. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute. Methods for determining the bactericidal activity of antimicrobial agents; Approved guideline. CLSI document M-26A. Wayne, PA: Clinical and Laboratory Standards Institute; 1999. [Google Scholar]
- CLSI. CLSI document M07-A10. Wayne, PA: Clinical and Laboratory Standards Institute; 2015. Methods for Dilution Antimicrobial Suceptibility Tests for Bacteria that Grow Aerobically; Approved Standard—10th Edition. [Google Scholar]
- Cosgrove SE, Vigliani GA, Fowler VG, Jr, Abrutyn E, Corey GR, Levine DP, Rupp ME, Chambers HF, Karchmer AW, Boucher HW. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clinical Infectious Diseases. 2009;48:713–721. doi: 10.1086/597031. [DOI] [PubMed] [Google Scholar]
- Deleo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated methicillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deresinski S. Vancomycin in combination with other antibiotics for the treatment of serious methicillin-resistant Staphylococcus aureus infections. Clinical Infectious Diseases. 2009;49:1072–1079. doi: 10.1086/605572. [DOI] [PubMed] [Google Scholar]
- Dombrowski JC, Winston LG. Clinical failures of appropriately-treated methicillin-resistant Staphylococcus aureus infections. Journal of Infection. 2008;57:110–115. doi: 10.1016/j.jinf.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler AT, Kadlec K, Schwarz S. Novel apramycin resistance gene apma in bovine and porcine methicillin-resistant Staphylococcus aureus ST398 isolates. Antimicrobial Agents & Chemotherapy. 2011;55:373. doi: 10.1128/AAC.01124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frippiat F, Chandrikakumari K, Moutschen M. Gentamicin in infective endocarditis: how to use it? Clinical Infectious Diseases. 2009;49:320–321. doi: 10.1086/600063. author reply 321. [DOI] [PubMed] [Google Scholar]
- Hawkins C, Huang J, Jin N, Noskin GA, Zembower TR, Bolon M. Persistent Staphylococcus aureus bacteremia: an analysis of risk factors and outcomes. JAMA Intern Med. 2007;167:1861. doi: 10.1001/archinte.167.17.1861. [DOI] [PubMed] [Google Scholar]
- Hazlewood KA, Brouse SD, Pitcher WD, Hall RG. Vancomycin-associated nephrotoxicity: Grave concern or death by character assassination? American Journal of Medicine. 2010;123:182.e181–182.e187. doi: 10.1016/j.amjmed.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herigstad B, Hamilton M, Heersink J. How to optimize the drop plate method for enumerating bacteria. J Microbiol Methods. 2001;44:121–129. doi: 10.1016/s0167-7012(00)00241-4. [DOI] [PubMed] [Google Scholar]
- Jose ML-N, Yaremi Q, Laura V, Ana IM, Francisco JL-H. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2010;79:33. doi: 10.1038/ki.2010.337. [DOI] [PubMed] [Google Scholar]
- Kadlec K, Ehricht R, Monecke S, Steinacker U, Kaspar H, Mankertz J, Schwarz S. Diversity of antimicrobial resistance pheno- and genotypes of methicillin-resistant Staphylococcus aureus ST398 from diseased swine. Journal of Antimicrobial Chemotherapy. 2009;64:1156. doi: 10.1093/jac/dkp350. [DOI] [PubMed] [Google Scholar]
- Kang AD, Smith KP, Berg AH, Truelson KA, Eliopoulos GM, McCoy C, Kirby JE. Efficacy of apramycin against multidrug-resistant Acinetobacter baumannii in the murine neutropenic thigh model. Antimicrobial Agents & Chemotherapy. 2018;62:e02585–02517. doi: 10.1128/AAC.02585-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang AD, Smith KP, Eliopoulos GM, Berg AH, McCoy C, Kirby JE. In vitro apramycin activity against multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Diagnostic Microbiology and Infectious Disease. 2017;88:188–191. doi: 10.1016/j.diagmicrobio.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Leber AL. Time-Kill Assay for Determining Synergy. In: Leber AL, editor. Clinical Microbiology Procedures Handbook, Fourth Edition. Washington, D.C.: ASM Press; 2016. pp. 5.14.03.01–15.14.03.06. [Google Scholar]
- Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, M JR, Talan DA, Chambers HF. Infectious Diseases Society of, A. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clinical Infectious Diseases. 2011;52:e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- Livermore DM, Mushtaq S, Warner M, Zhang JC, Maharjan S, Doumith M, Woodford N. Activity of aminoglycosides, including ACHN-490, against carbapenem-resistant Enterobacteriaceae isolates. Journal of Antimicrobial Chemotherapy. 2011;66:48–53. doi: 10.1093/jac/dkq408. [DOI] [PubMed] [Google Scholar]
- Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrobial Agents & Chemotherapy. 2008;52:1330. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt T, Ng CL, Lang K, Sha S-H, Akbergenov R, Shcherbakov D, Meyer M, Duscha S, Xie J, Dubbaka SR, Perez-Fernandez D, Vasella A, Ramakrishnan V, Schacht J, Böttger EC. Dissociation of antibacterial activity and aminoglycoside ototoxicity in the 4-monosubstituted 2-deoxystreptamine apramycin. Proceedings of the National Academy of Sciences, USA. 2012;109:10984. doi: 10.1073/pnas.1204073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Freihofer P, Scherman M, Teague J, Lenaerts A, Böttger EC. In vivo efficacy of apramycin in murine infection models. Antimicrobial Agents & Chemotherapy. 2014;58:6938. doi: 10.1128/AAC.03239-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghili H, Tajik H, Mardani K, Razavi Rouhani SM, Ehsani A, Zare P. Validation of drop plate technique for bacterial enumeration by parametric and nonparametric tests. Vet Res Forum. 2013;4:179–183. [PMC free article] [PubMed] [Google Scholar]
- Odds FC. Synergy, antagonism, and what the chequerboard puts between them. Journal of Antimicrobial Chemotherapy. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- Pillai SK, Moellering RC, Jr, Eliopoulos GM. Antimicrobial Combinations. In: Lorian V, editor. Antibiotics in Laboratory Medicine, Fifth Edition. Philadelphia, PA: Williams and Wilkins; 2005. pp. 365–440. [Google Scholar]
- RStudio. RStudio. 1.0.143. Boston, MA: 2017. [Google Scholar]
- Schafer JA, Hovde LB, Rotschafer JC. Consistent rates of kill of Staphylococcus aureus by gentamicin over a 6-fold clinical concentration range in an in vitro pharmacodynamic model (IVPDM) Journal of Antimicrobial Chemotherapy. 2006;58:108–111. doi: 10.1093/jac/dkl216. [DOI] [PubMed] [Google Scholar]
- Smith KP, Kirby JE. Evaluation of apramycin activity against carbapenem-resistant and -susceptible strains of Enterobacteriaceae. Diagnostic Microbiology and Infectious Disease. 2016a;86:439–441. doi: 10.1016/j.diagmicrobio.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Smith KP, Kirby JE. Validation of a high-throughput screening assay for identification of adjunctive and directly acting antimicrobials targeting carbapenem-resistant Enterobacteriaceae. Assay and Drug Development Technologies. 2016b;14:194. doi: 10.1089/adt.2016.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KP, Kirby JE. Verification of an Automated, Digital Dispensing Platform for At-Will Broth Microdilution-Based Antimicrobial Susceptibility Testing. Journal of Clinical Microbiology. 2016;54:2288–2293. doi: 10.1128/JCM.00932-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-vancomycin-intermediate Staphylococcus aureus (VISA), vancomycin-susceptible clinical methicillin-resistant S. aureus (MRSA) blood isolates from 2001–05. Journal of Antimicrobial Chemotherapy. 2007;60:788–794. doi: 10.1093/jac/dkm258. [DOI] [PubMed] [Google Scholar]
- Tam VH, Kabbara S, Vo G, Schilling AN, Coyle EA. Comparative pharmacodynamics of gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrobial Agents & Chemotherapy. 2006;50:2626–2631. doi: 10.1128/AAC.01165-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEAM, RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clinical Microbiology Reviews. 2015;28:603. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbridge J, Patterson DL. Setting and revising antibacterial susceptibility breakpoints. Clinical Microbiology Reviews. 2007;20:391. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv G, Bor A, Sobagk S, Elad D, Nouws JFM. Clinical pharmacology of apramycin in calves. J Vet Pharmacol Ther. 1985;8:95–104. doi: 10.1111/j.1365-2885.1985.tb00930.x. [DOI] [PubMed] [Google Scholar]
- Ziv G, Kurtz B, Risenberg R, Glickman A. Serum and milk concentrations of apramycin in lactating cows, ewes and goats. J Vet Pharmacol Ther. 1995;18:346–351. doi: 10.1111/j.1365-2885.1995.tb00602.x. [DOI] [PubMed] [Google Scholar]