Abstract
While fatigue is the most common and debilitating side effect of cancer and cancer treatment it is still poorly understood, partly because it is usually characterized by patient-reported outcomes. As patient-reports are inherently subjective, behavioral correlates of the symptom of fatigue are needed to increase our understanding of the symptom. We focused on motivational effort expenditure as a crucial behavior in cancer-related fatigue, using a validated computerized task contrasting high effort/high reward and low effort/low reward choices under different probabilities of success. Effort expenditure-choices were analyzed in 47 cancer patients differing by their status; current evidence for disease (n=17) or post-treatment survivors with no evidence for disease (n=30). In addition, patient-reported fatigue, negative and positive affect, and biomarkers of inflammation were assessed. Patient-reported general and motivational fatigue, negative affect, and plasma concentrations of pro-inflammatory biomarkers were related to higher effort expenditure while positive affect was associated with lower effort expenditure. As all four measures interacted with patient status, exploratory models were computed for patients and survivors separately. These analyses indicated that the effects of fatigue and negative affect were predominantly seen in survivors. In patients still under or shortly post treatment, general fatigue, but not motivational fatigue, was associated with lower effort expenditure but only in the most favorable reward condition. Negative affect did not have an effect. Thus, the effects observed seemed primarily driven by cancer survivors in whom both fatigue and negative affect were associated with higher effort expenditure. These findings are tentatively interpreted to suggest that a tendency to invest more effort despite feelings of fatigue is a vulnerability for developing chronic fatigue. Inflammation and negative affect might contribute to fatigue in some survivors through this effort investment pathway.
Keywords: fatigue, motivation, affect, inflammation, cytokines
1. Introduction
Fatigue is the most common, debilitating side effect of cancer and cancer-treatment. Up to 99% of patients report some fatigue during cancer therapy (Servaes et al., 2002) and between 44% and 66% report moderate to severe fatigue (de Jong et al., 2004; Servaes et al., 2002). Although fatigue usually abates after cessation of cancer therapy, it becomes chronic in 22 to 39% of cancer survivors (Goedendorp et al., 2013). Severe fatigue affects quality of life by hampering daily activities and interfering with return to work. Furthermore, it is strongly related to mood disturbances such as depression (Ho et al., 2015). Thus, the emotional and financial consequences of cancer-related fatigue are severe.
To date, no efficacious treatment options for cancer-related fatigue exist, although evidence is accumulating that some patients may benefit from physical, psychosocial, or mind-body interventions (Bower et al., 2014). The lack of evidence-based treatment options might be largely due to our poor understanding of the symptom of fatigue. Fatigue is a multi-dimensional construct including physical, mental, and motivational dimensions. These dimensions are usually quantified by patient-report, thereby inadvertently subjected to psychosocial influences on symptom experience. Subjective symptom reports do not always correlate with objective assessments as illustrated in chemotherapy-induced cognitive dysfunction where self-reported cognitive function does not correlate with objective cognitive tests (O'Farrell et al., 2016; Pullens et al., 2010). To our knowledge, the only used behavioral outcome in relation to fatigue is physical activity (assessed through actigraphy), which indeed shows only low correlations with reported fatigue (Ferriolli et al., 2012; Timmerman et al., 2015). Behavioral correlates of specific dimensions of cancer-related fatigue are thus far unknown.
Patients with cancer-related fatigue often report a lack of motivation. For example, in a qualitative study on cancer-related fatigue, lack of motivation was reported by over 80% of patients (Gledhill, 2005). Further, de Jong et al. showed consistently high scores on patient-reported lack of motivation during adjuvant chemotherapy for breast cancer (de Jong et al., 2005). For the present study, we therefore decided to focus on motivational effort expenditure as a possibly crucial element of cancer-related fatigue. Lack of motivation might express itself as an overall reduced willingness to exert effort, or as a reduced sensitivity to the reward obtained through the expenditure of effort (i.e., anhedonia), the latter being a component of depression. We hypothesized that cancer patients and survivors with higher fatigue but no depression would display decreased effort expenditure with intact reward sensitivity.
We used the Effort Expenditure for Reward Task (EEfRT), a validated computerized task designed to assess effort expenditure as well as the hedonic aspect of motivation (i.e., reward sensitivity) (Treadway et al., 2009) in cancer patients (with active disease) and in cancer survivors (post primary cancer therapy and with no evidence of disease) in a cross-sectional design. In the EEfRT, participants repeatedly choose between a high effort-high reward task and low effort-low reward task under varying reward probabilities and magnitudes. Performance on the EEfRT is related to self-reported anhedonia (Treadway et al., 2009). In addition, when compared to healthy controls, patients diagnosed with major depressive disorder show less willingness to select the high effort-high reward task and are less sensitive to changes in reward probability and magnitude (Treadway et al., 2012). The EEfRT is sensitive to inflammation as administration of endotoxin to volunteers resulted in a decrease in low effort-low reward choices when the probability to win was low and an increase in high effort-high reward choices when the probability to win was high (Lasselin et al., 2017). In this last study, the effect of inflammation was related to the level of sleepiness, suggesting a role for fatigue in inflammation-induced changes in motivated behavior. However, to our knowledge, the EEfRT has not yet been used to assess motivational changes in fatigued subjects.
Associations between cancer-related fatigue and personality characteristics related to a more negative mood as well as state negative mood have been repeatedly found (Shun et al., 2011; Wang et al., 2013). In addition, inflammation has repeatedly been associated with cancer-related fatigue (Bower, 2007; De Raaf et al., 2012). Therefore, we also measured affect and circulating levels of biomarkers of inflammation to assess their associations with motivational effort expenditure.
2. Materials and Methods
2.1. Participants
Patients were recruited at the Cancer-Related Fatigue Clinic and the Head and Neck Clinic at The University of Texas MD Anderson Cancer Center. The Cancer-Related Fatigue Clinic sees patients who are referred by oncologists at MD Anderson because of severe cancer-related fatigue. We included patients presenting for initial consult, at which stage they report high levels of fatigue, and for follow-up, at which stage fatigue is often of low or moderate severity. Patients recruited at this clinic could be under active cancer treatment, maintenance treatment, or no treatment. Patients recruited at the Head and Neck Clinic were between 6 weeks and 12 months post treatment for head and neck cancer and cancer free at the time of assessment. For both clinics, exclusion criteria were presence of major depressive disorder or severe depression (patients taking antidepressants and not showing signs of severe depressive symptoms were allowed), presence of pain, severe confusion, or cognitive impairments.
2.2. Study design
In a cross-sectional study design, participants were tested for incentive motivation and self-reported mood and fatigue. During testing, participants filled out questionnaires as described below, followed by the EEfRT (see below). A blood sample was drawn for plasma levels of inflammatory markers on the day of testing. Participants received a gift card of $10 plus their winnings on the EEfRT (see below) after completion of the assessments. The protocol was approved by the MD Anderson internal review board (2015-0500 and 2014-0511) and all patients provided written informed consent.
2.3. Self-report measures
Fatigue was measured with the General Fatigue subscale of the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SI), a 30-item questionnaire assessing five empirically derived dimensions of fatigue (i.e., general fatigue, physical fatigue, emotional fatigue, mental fatigue, and vigor) (Stein et al., 2004). The General Fatigue subscale included six items which were rated for the extent to which they were true on a 5-point Likert scale (range 0 “not true at all”- 4 “extremely”). Items included statements such as “I am worn out”, “I feel fatigued”, and “I feel run down”. Answers were summed resulting in score range of 0 – 24 with higher scores representing greater fatigue severity. The motivational aspect of fatigue was assessed with the motivation subscale of the Checklist Individual Strength (CIS), a 4-item scale with items answered on a 7-point Likert scale (range 1 “yes, that is true” – 7 “no, that is not true at all”). Items included statements as for example “I don’t feel like doing anything” and “I have a lot of plans”. Higher scores (after reversed scoring when necessary) represent greater motivational fatigue (range: 4 – 28) (Vercoulen et al., 1994).
To monitor self-reported fatigue during the assessments, participants also filled out a fatigue visual analogue scale (VAS) before, in-between, and after the computerized tests indicating their momentary fatigue on a continuous 10-cm long scale from “not fatigued at all” to “very severely fatigued”.
Depression was assessed with the depression subscale of the Depression, Anxiety, and Stress Scale (DASS) (Antony et al., 1998; Lovibond and Lovibond, 1995) (for participants recruited at the Cancer-Related Fatigue Clinic) or the Hospital Anxiety and Depression scale (Zigmond and Snaith, 1983) (for participants recruited at the Head and Neck Clinic). Both subscales are comprised of seven items which are to be answered for the past week on a 0 – 3 Likert scale, resulting in sum scores with a range of 0 – 21.
Negative and positive affect were measured with the Positive and Negative Affect Scale (PANAS) (Watson et al., 1988). Both subscales (i.e., positive and negative affect) comprise 10 mood adjectives and the extent to which they are experienced during the last month is rated on a 5-point Likert scale.
Anhedonia was assessed with the Snaith-Hamilton Pleasure Scale (SHAPS) (Snaith et al., 1995). This 14-item scale assesses hedonic tone and its absence, anhedonia. Participants rate the degree to which they agree or disagree with statements describing situations that generate pleasure.
2.4. Markers of inflammation
Blood samples were typically drawn immediately after completion of assessments. Most participants were assessed between 12:00 and 6:00 P.M, except for ten participants who were assessed between 10:00 A.M. and 12:00 P.M. Blood samples were not drawn for three participants due to time constraints in the participant’s schedule. Blood was immediately spun down at 3,000 × g for 10 minutes and plasma was frozen at −80 °C until batch-wise analyses of inflammatory markers previously associated with cancer-related fatigue in both patients and survivors (Saligan and Kim, 2012; Xiao et al., 2017).
Plasma levels of IL-6, sIL-6r, TNF-α, sTNFRII, and IL-1ra were determined with enzyme linked immunosorbent assays (ELISAs) (R&D systems HS600B, DR600, HSTA00D, DRT200, and DRA00B, respectively). Mean minimum detectable levels were 0.039 pg/ml for IL-6, 6.5 pg/ml for sIL-6r, 0.106 pg/ml for TNF-α, 0.6 pg/ml for STNFRII, and 6.3 pg/ml for IL-1ra. One TNF-α sample that was below detection level was set at the mean minimum detectable dose. C-reactive protein (CRP) levels were determined by the MD Anderson Cancer Center core clinical laboratory using high sensitivity chemiluminescent immunometric assay run on a Siemen Immulite XPi. Standard range was 0.2 – 100 pg/mL. One sample exceeded the maximum range for CRP and was repeated at 1:10 dilution.
2.5. Motivation: Effort Expenditure for Reward Task (EEfRT)
The EEfRT is a multiple-trial task designed to assess both overall motivation to perform and reward sensitivity (Treadway et al., 2009). In this task, participants were repeatedly presented with a choice between performing a low effort/low reward (‘easy’) task or a high effort/high reward (‘hard’) task. The easy task required 30 button presses within 7 seconds with the index finger of the dominant hand for a $1 reward; the hard task required 100 button presses within 21 seconds with the little finger of the non-dominant hand for a reward varying between $1.24 and $4.30. The probability of being awarded the reward after completion of the task (either hard or easy) varied (12%, 50%, and 88%). At the beginning of each trial, the reward for the hard task and probability of being awarded the reward for both tasks were displayed for a maximum of 10 seconds on the screen and the participant had to choose which task to complete. If the participant did not choose within this time frame, the program randomly assigned one of the tasks. Thus, the sequence for one trial was as follows: (1) choosing a task using the information on reward magnitude and reward probability; (2) completing the task by repeatedly pressing the appropriate button; (3) receiving feedback on the completion and the total reward obtained for the task.
After reading the written instructions, participants were guided through four practice trials before the 15-minute actual test. Participants were informed that at the end of the task, two completed trials would be randomly chosen for which they would receive the actual monetary reward. Participants completed as many trials as fit within the allocated time frame. As the easy task takes less time to be completed, choosing the easy task more frequently resulted in completion of more trials.
The dependent variable in the EEfRT task is the ratio of high effort/low effort choices, which informs about the participant’s overall willingness to exert effort. Difference in high versus low effort task choices between the different reward and probability conditions inform about the participant’s sensitivity to reward. Behavior on the EEfRT has been related to depression and anhedonia (Treadway et al., 2012) and to systemic inflammation (Lasselin et al., 2017).
2.6. Statistical Analyses
All analyses were performed in SPSS version 23 (Corporation, 1989, 2016). As severe depression was an exclusion criterion, depressive symptom scores were, not surprisingly, low (range: 0-8). In addition, over 40% of the sample reported no depressive symptoms (sum score 0 or 1). Therefore, depression symptom scores were dichotomized into no symptoms (score 0-1) and some symptoms (score 2-8). Negative affect showed one outlier (score of 38) which was winsorized to the mean+2*standard deviation (new score = 29). Markers of inflammation were severely skewed and therefore log-transformed for all analyses. One outlier for TNF-α was detected even after log-transformation and winsorized to mean+2*standard deviation. Associations between self-reported measures were assessed with Pearson correlation coefficient r for continuous variables and Spearman’s rank correlation coefficient ρ for dichotomous variables. Associations between fatigue and markers of inflammation were assessed with multiple regression models in which standardized scores for biomarkers were entered as independent variables. Independent samples t-test were used for descriptive analyses regarding differences between patients and survivors in decision making reaction times, tap speed during trials, and high effort choices.
In line with previous reports on the EEfRT (Lasselin et al., 2017; Treadway et al., 2012; Treadway et al., 2009), the ratio of high effort/low effort choices on the EEfRT was analyzed with Generalized Estimating Equations (GEE) models, which allows for controlling for time-varying covariates such as trial number. Choice for high effort vs. low effort task (binary logistic) was entered as outcome variable. Trial number (continuous variable), reward probability (categorical variable), and reward magnitude (continuous variable) were entered as within-subject variables in all models using an unstructured working correlations matrix and model-based estimation of the covariance matrix. All GEE models included trial number, reward probability, and reward magnitude. Further covariates were selected in a preliminary model including demographic variables and medication use. Variables significantly contributing to the model with alpha at 0.01 were included in all subsequent models. The associations of high effort choice with general fatigue and motivational fatigue were analyzed in separate models. Associations of high effort choice with positive and negative affect were explored. Alpha was set at 0.0125 (0.05/4) for testing of associations of high effort choice with general and motivational fatigue and positive and negative affect. For subsequent exploratory models in survivors and patients separately, alpha was corrected for the number of models that were tested. Because of the small sample sizes in the subsamples, significant interactions between fatigue/affect and task conditions could not be followed-up by GEE models within each task condition. Thus, these interactions were further inspected by graphing high-effort choices per task condition for individuals scoring in the highest and the lowest tertile of the respective fatigue/affect measure. Associations between the biomarkers of inflammation and high effort choices were first tested in separate models for each biomarker. Markers identified in these models were then tested together in one model to account for possible overlap between the markers in their associations with high effort choices. We aimed for a sample size of 50, which allows for the detection of associations between fatigue and the ration of high effort/low effort choices with effect size of f2 = 0.30 (i.e., small effect size) in a multiple regression model including up to three covariates (alpha at 0.0125 and power of 90%). As we will use GEE models which allows for controlling of within subject variability, our estimate of detectable effect size is conservative.
3. Results
3.1. Patient characteristics
A total of 50 participants was tested. Three participants did not comply with EEfRT task instructions and were therefore excluded from further analyses. As it is unclear if fatigue experienced acutely during cancer and cancer-treatment is the same as the persistent fatigue experienced by cancer survivors who are well past their primary cancer treatment, we categorized participants’ status as ‘patients’ and ‘survivors’ according to their disease and treatment status during testing. Approximately one-third of the sample (36%) consisted of patients either actively undergoing any type of cancer treatment (n = 15), having completed primary treatment less than three months prior (n = 2), or having stable metastatic disease for which they currently were not receiving treatment (n = 1). The remaining two-third (64%) was characterized as survivor: at least three months post any cancer treatment, with the exception of adjuvant endocrine treatment. Sample characteristics are presented in Table 1. Two patients (12%) had received chemoradiation less than 3 months before testing. Within the survivors group, six participants (20%) were receiving endocrine treatment at the time of testing, all whom had been receiving this treatment for over a year. Patients had higher general fatigue scores than survivors (independent sample t-test: p = 0.026). No other differences were found between the two subgroups. The plasma levels of inflammation biomarkers we observed here were somewhat lower when compared to previously reported data in cancer patients and survivors (Bower et al., 2009; Knobel et al., 2000), with the exception of sIL-6R, which was elevated compared to other studies (e.g., Collado-Hidalgo et al., 2006). Associations between inflammatory markers and demographic variables were found for IL-1ra with BMI (r = 0.43, p = .003) and for CRP with sex (rho = .41, p = .006).
Table 1.
Characteristics of patient sample (n = 47). Mean (SD), range unless otherwise indicated.
| Demographics | Complete sample | Patients (n = 17) | Survivors (n = 30) |
|---|---|---|---|
| Sex female n (%) | 25 (53) | 9 (53) | 16 (53) |
| Age | 57.13 (10.61), 26 – 82 | 55.06 (11.42), 32 – 6757.86 (10.83), 32 – 71 |
58.30 (10.14), 26 – 82 |
| BMI | 29.56 (7.08), 19.14 – 47.49 | 27.30 (5.84), 19.98 – 39.46 | 30.84 (7.48), 13.14 – 49.49 |
| Race/ethnicity n (%) | |||
| Caucasian | 41 (87) | 16 (94) | 25 (83) |
| Hispanic | 4 (9) | 1 (6) | 3 (10) |
| African American | 2 (4) | 0 (0) | 2 (7) |
| Clinical characteristics | |||
| Cancer diagnosis n (%) | |||
| Head & neck cancer | 14 (30) | 2 (12) | 12 (41) |
| Breast cancer | 12 (25) | 5 (29) | 7 (23) |
| Hematologic cancer | 13 (28) | 7 (41) | 6 (20) |
| Other | 8 (17) | 3 (18) | 5 (17) |
| Cancer treatment n (%) | - | Currently receiving: | Last received: |
| Chemo(radiation) | 10 (59) | 11 (37) | |
| Radiation | 1 (6) | 17 (57) | |
| Endocrine | 2 (12) | ||
| Other | 1 (6) | ||
| Use of psychotropic drugs a n (%) | |||
| anxiolytic/hypnotics | 15 (32) | 8 (47) | 7 (23) |
| antidepressants | 17 (36) | 7 (42) | 10 (33) |
| psychostimulants | 16 (34) | 8 (27) | 8 (47) |
| Self-report measures | |||
| General fatigue | 10.87 (5.80), 0 – 22 | 13.41 (4.80), 6 – 22 | 9.43 (5.89), 0 – 22 |
| Motivational fatigue | 13.81 (6.09), 4 – 26 | 15.88(6.31), 5 – 26 | 12.63 (5.73), 4 – 25 |
| Momentary fatigue (VAS) | |||
| Pre EEfRT | 3.59 (2.25), 0.35 – 8.80 | 3.51 (2.40), 0.40 – 8.00 | 3.63 (2.20), 0.35 – 8.80 |
| Post EEfRT | 4.52 (2.57), 0.30 – 9.25 | 4.53 (2.78), 0.36 – 9.00 | 4.42 (2.49), 0.30 – 9.25 |
| Negative affect | 16.15 (5.79), 10 – 29 | 17.41 (7.09), 10 – 29 | 15.43 (4.89), 10 – 28 |
| Positive affect | 31.38 (7.75), 15 – 45 | 29.42 (8.83), 15 – 43 | 32.50 (6.98), 18 – 45 |
| Anhedonia | 1.68 (2.31), 0 – 8 | 2.24 (2.82), 0 – 8 | 1.37 (1.96), 0 – 7 |
| Depressive symptoms b n (%) | 27 (57) | 10 (59) | 17 (57) |
| Inflammation biomarkersc Median, IQR | |||
| CRP (mg/l) | 2.50, 0.75 – 6.29 | 4.18, 0.73 – 5.74 | 2.47, 0.72 – 6.79 |
| TNF-α (pg/ml) | 0.93, 0.52 – 1.45 | 1.00, 0.78 – 2.00 | 0.86, 0.48 – 1.26 |
| sTNFRII (pg/ml) | 2983.33, 2331.04 – 3821.96 | 3584.17, 2155.06 – 4023.52 | 2912.09, 2368.22 – 3727.56 |
| IL-6 (pg/ml) | 1.49, 0.71 – 4.24 | 1.40, 0.71 – 1.87 | 2.02, 0.70 – 4.74 |
| sIL-6r (ng/mL) | 36.13, 27.98 – 44.01 | 36.46, 27.99 – 41.65 | 34.57, 27.77 – 45.96 |
| IL-1ra (pg/ml) | 274.70, 173.06 – 451.38 | 269.57, 211.55 – 415.38 | 279.83. 150.04 – 588.63 |
Several participants used medication from more than one category;
Questionnaire score ≥ 2; vs. no depressive symptoms (score 0 or 1);
n = 44. IQR: Interquartile range.
3.1.1 Fatigue
Self-reported general fatigue and motivational fatigue were positively correlated (r = 0.65, p < 0.001). Momentary fatigue scores showed a 26% increase between pre and post EEfRT (dependent sample t-test: p < 0.001) (29% within the patients group and 24% within the survivors group). General fatigue was not associated with change in momentary fatigue during the EEfRT (r = 0.07, p = .65), but higher motivational fatigue was associated with a stronger increase in momentary fatigue during the task (r = 0.33, p = 0.022). Self-reported general fatigue was not associated with age, gender, BMI, or race/ethnicity (p-values > 0.05), but was associated with use of anxiolytics (ρ = 0.37, p = 0.010), negative affect (r = 0.34, p = 0.018) and positive affect (r = −0.49, p 0.001). Motivational fatigue was not associated with gender, body mass index, or race/ethnicity (p-values > 0.05), and was associated with use of anxiolytics (ρ = 0.46), negative affect (r = 0.50, p < 0.001) and positive affect (r = −0.73, p < 0.001) as well as with use of stimulants (ρ = 0.37, p = 0.010), antidepressants (ρ = 0.41, p = 0.004), and age (r = −0.31, p = 0.033).
Univariate correlations showed a significant, but inverse association between motivational fatigue and CRP levels (r = −0.35, p = 0.021) and a marginally significant association with IL-6 concentrations (r = −.25, p = 0.099). All other correlations were non-significant (p > 0.05) (Table A.1).
3.1.2. EEfRT
Trials for which no choice was made and trials for which choices were made with a reaction time < 100 milliseconds were omitted from analyses (deleted trials n = 66; less than 3% of the total number of trials). Analyses included all eligible trials (n = 2,269). Each participant completed between 30 and 71 trials (M = 26.5, SD = 15.85) with an average of 3.2% ineligible trials (range: 0%–18%), resulting in 27 – 71 eligible trials (M = 48.3, SD = 9.0) per participant. On average, 81% (range: 0% – 100%) of high effort trials were successfully completed (i.e., key press goal was obtained), with a success rate of less than 10% for four participants (three survivors and one patient). Neither the percentage of ineligible trials nor the percentage of successfully completed high effort trials was associated with general fatigue (r = −.03, p = .82; r = 0.18, p = .24) or motivational fatigue (r = −.24, p = .11; r = .24, p = .10). In addition, tap speed during the trials was not related to general fatigue (r = 0.05, p = 0.722) or motivational fatigue (r = 0.007, p = 0.96).
In line with previous reports (Treadway et al., 2012; Treadway et al., 2009), reward probability (p < 0.001), reward magnitude (p < 0.001), and trial number (p < 0.001) were all found to affect high effort choices (Table A.2; see also Figure 1, panel A). An older age (B = .025, p < .001), being male (B = −0.752, p < 0.001), and use of anti-depressants (B = 0.0.323, p < 0.001), anxiolytics (B = −0.431, p < 0.001), and psychostimulants (B = 0.339, p = 0.009) were associated with high effort choices (Table A.2). Patient status (patient or survivor) was also associated with high effort choices (B = 0.520, p < 0.001), indicating that patients currently undergoing treatment chose the high effort task more often than survivors. These variables were thus included as covariates in all subsequent models. As both depression and anhedonia scores were extremely low for the sample, these measures were not considered as covariates.
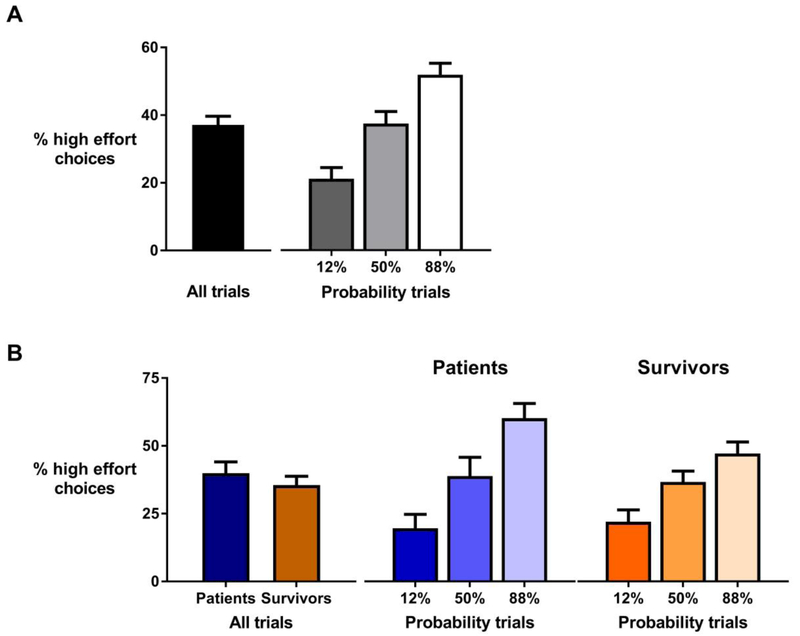
Figure 1.
Percentage of high effort choices across all trials and separated for reward probability conditions. In the complete sample (panel A), participants choose the high effort task 37% of the time and showed sensitivity to changes in reward probability as evident from more high effort choices in the trials with higher probabilities. Patients made more high effort choices than survivors (panel B), but both groups were equally sensitive to changes in reward probability.
Although patients choose the high effort task more often than survivors did (Figure 1, panel B), both groups showed the same sensitivity to changes in reward probability (F(2,44) = 0.057, p = 0.28) and reward magnitude (F(2,44) = 0.069, p = .93). Compared to previously reported results on healthy subjects with similar age ranges i.e., (McCarthy et al., 2016; Treadway et al., 2012), the ratio of high effort task choices was markedly lower in both patients and survivors.
3.2. Association of Fatigue with Effort Expenditure
Within the sample as a whole, both general fatigue and motivational fatigue were significantly associated with increased odds for high-effort choices (Table 2 for GEE models and Table A.3 for bivariate correlations). More interestingly, both fatigue measures also showed an interaction with patient status in their association with high effort choices. Negative affect was likewise associated with increased odds for high effort choices, while positive affect was associated with a decrease in high effort choices. Both affect measures also interacted with patient status. Exploration of other fatigue dimensions showed that higher physical fatigue was associated with more high effort choices (B = 0.124, p < 0.001). In addition, an increase in momentary fatigue during the task was also associated with more high effort choices (B = 0.428, p < 0.001) (Table A.4).
Table 2.
Results for associations of fatigue and affectivity measures with high-effort choices in total sample. Each GEE model included task variables (trial no., reward probability and magnitude) and the selected covariates (age, sex, race/ethnicity, patient status, use of anxiolytics, use of antidepressants, and use of psychostimulants). Main and interaction effects significant at p<0.0125 are depicted in bold.
| Model | B | SE | 95% CI | P | |
|---|---|---|---|---|---|
| 1. | General fatigue | 0.060 | 0.013 | 0.034 – 0.085 | <0.001 |
| Fatigue × patient status | −0.065 | 0.024 | −0.110 – −0.019 | 0.006 | |
| 2. | Motivational fatigue | 0.054 | 0.015 | 0.026 – 0.083 | <0.001 |
| Fatigue × patient status | −0.057 | 0.021 | −0.099 – −0.016 | 0.007 | |
| 3. | Negative affectivity | 0.073 | 0.015 | 0.043 – 0.102 | <0.001 |
| NA × patient status | −0.090 | 0.022 | −0.133 – −0.046 | <0.001 | |
| 4. | Positive affectivity | −0.034 | 0.013 | −0.058 – −0.009 | 0.008 |
| PA × patient status | 0.046 | 0.016 | 0.014 – 0.078 | 0.005 |
3.3. Exploration of the Effects of Acute or Chronic Fatigue
As both fatigue measures and both affect measures showed an interaction with patient status, we repeated all main models within the subgroups to explore differences in the observed effects between patients and survivors. Herein, we assume that fatigue experienced by patients is more acutely related to the cancer treatment, while fatigue in survivors has a more chronic nature.
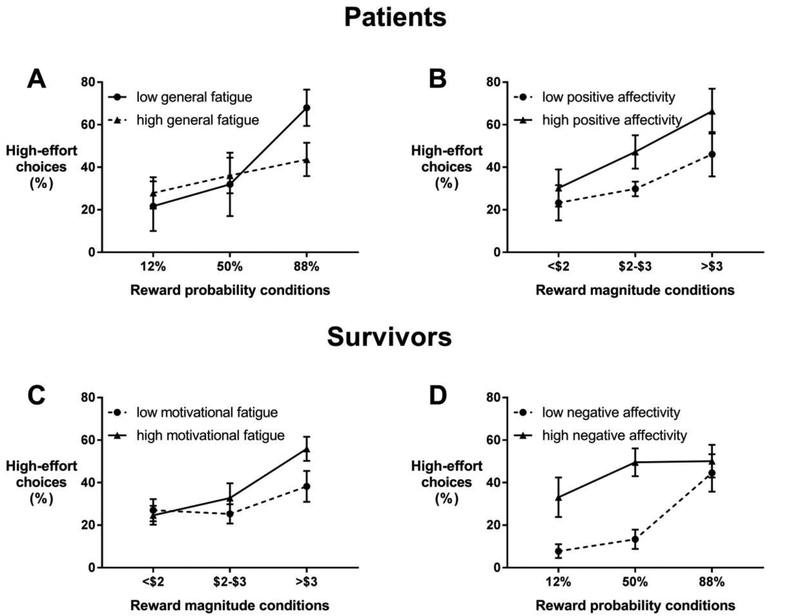
In patients, general fatigue interacted with reward probability (Table 3, model 1). As shown in figure 2 (panel A), whereas patients with low fatigue markedly increased their high-effort choices when the probability to win was at its highest, patients with high fatigue did not show this pronounced increase. Higher motivational fatigue was associated with overall decreased odds for high-effort choices, although this effect was not significant after adjusting for the multiple tests (adjusted p = .072) (Table 3, model 2). Motivational fatigue did not interact with reward probability or magnitude (corrected p-values > 0.05). Positive affect showed an interaction with reward magnitude but not with probability (Table 3, model 3) . Figure 2 (panel B) shows that patients with high positive affect showed a more pronounced increase in high-effort choices with increasing reward magnitude. Negative affect was not associated with high effort choices (B = −0.034, p = 0.10).
Table 3.
GEE models for effects of self-reported general fatigue, motivational fatigue, and positive affectivity in association with high effort choices in patients. Interactions with task-related variables were non-significant for motivational fatigue (p > 0.006) and therefore not included in model 2. Associations significant at p ≤ 0.006 are depicted bold.
| Model | 1.General fatigue | 2.Motivational fatigue | 3.Positive affect | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | 95% CI | p | B | SE | 95% CI | p | B | SE | 95% CI | p | ||||
| Intercept | − 6.768 |
1.339 | −9.393 – − 4.143 |
<0.001 | −3.373 | 0.931 | −5.198- − 1.547 |
0.001 | −1.683 | 1.441 | −4.508 – 1.142 |
0.24 | |||
| Age | 0.042 | 0.012 | 0.020 – 0.065 |
<0.001 | 0.028 | 0.012 | 0.004 – 0.052 |
0.024 | 0.028 | 0.013 | 0.003 – 0.053 |
0.027 | |||
| Gender 1 | − 1.213 |
0.455 | −2.105 – − 0.322 |
0.008 | −0.687 | 0.482 | 1.632 – 0.257 |
0.15 | −0.865 | 0.476 | −1.797 – 0.068 |
0.069 | |||
| Race/ethnicity 2 | − 1.652 |
0.442 | −2.519 – − 0.785 |
<0.001 | −0.990 | 0.463 | −1.898 – − 0.081 |
0.033 | -0.998 | 0.473 | −1.924 – − 0.072 |
0.035 | |||
| Anxiolytics 3 | − 0.347 |
0.474 | −1.275 – 0.581 |
0.46 | 0.183 | 0.496 | −0.790 – 1.155 |
0.71 | −0.154 | 0.467 | −1.070 – 0.764 |
0.74 | |||
| Antidepressants 3 | 0.967 | 0.214 | 0.549 – 1.385 |
<0.001 | 1.100 | 0.229 | 0.650 – 1.549 |
<0.001 | 0.922 | 0.205 | 0.520 – 1.325 |
<0.001 | |||
| Psychostimulants 3 |
− 0.130 |
0.199 | −0.520 – 0.260 |
0.51 | 0.051 | 0.195 | −0.332 – 0.434 |
0.79 | 0.038 | 0.191 | −0.337 – 0.412 |
0.84 | |||
| Trialnr | − 0.023 |
0.006 | −0.034 – − 0.012 |
<0.001 | −0.022 | 0.006 | −0.033 – − 0.011 |
<0.001 | −0.022 | 0.006 | −0.033 – − 0.011 |
<0.001 | |||
| Probability | 0.053 | 0.009 | 0.035 – 0.072 |
<0.001 | 0.027 | 0.003 | 0.021 – 0.033 |
<0.001 | 0.016 | 0.011 | −0.005 – 0.037 |
0.125 | |||
| Magnitude | 0.935 | 0.301 | 0.345 – 1.525 |
0.002 | 0.475 | 0.100 | 0.279 – 0.670 |
<0.001 | −0.541 | 0.341 | −1.209 – 0.127 |
0.11 | |||
| Fatigue/Affect | 0.159 | 0.073 | 0.016 – 0.303 |
0.029 | −0.067 | 0.025 | −0.117 – − 0.018 |
0.008 | −0.086 | 0.042 | −0.168 – − 0.003 |
0.041 | |||
| x Magnitude | − 0.033 |
0.021 | −0.073 – 0.007 |
0.106 | - | - | - | - | 0.035 | 0.002 | 0.013 – 0.058 |
0.002 | |||
| x Probability | − 0.002 |
0.0006 | −0.003 – − 0.001 |
0.002 | - | - | - | - | 0.0001 | 0.0004 | 0.000 – 0.001 |
0.26 | |||
Reference = female.
Reference = Caucasian.
Reference = no use.
Figure 2.
Graphic display of the interactions between fatigue/affect measures and high-effort choices within the patient sample (panels A and B) and the survivor sample (panels C and D). Markers display the mean (±SE) percentage of high-effort choices per reward condition for patients/survivors within the lowest and the highest tertile of the respective fatigue or affect score.
In survivors, general fatigue was associated with overall increased odds for high-effort choices (Table 4, model 1). In other words, survivors with higher general fatigue choose the high-effort task more often than survivors with lower fatigue. No interactions were observed with reward conditions. Motivational fatigue showed an interaction with reward magnitude (Table 4, model 2). Survivors with higher motivational fatigue choose the high-effort task more often than survivors with lower motivational fatigue, only when the reward for the high-effort task was high (Figure 2, panel C). Negative affect interacted with reward probability (Table 4, model 3). Survivors with higher negative affect choose the high-effort task more often than survivors with lower negative affect when the probability for winning was not optimal (i.e., 12% and 50%) (Figure 2, panel D). Associations for positive affect were not significant (b = −0.034, adjusted p = 0.088).
Table 4.
GEE models for effects of self-reported general fatigue, motivational fatigue, and positive affectivity in association with high effort choices in survivors. Interactions with task-related variables were not significant for general fatigue (p-values > 0.006) therefore not included in model 1. Associations significant at p ≤ 0.006 are depicted bold.
| Model | 1. General fatigue | 2. Motivational fatigue | 3. Negative affect | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | 95% CI | p | B | SE | 95% CI | p | B | SE | 95% CI | p | ||||
| Intercept | − 4.142 |
0.573 | −5.266 – − 3.018 |
<0.001 | −2.447 | 0.867 | −4.147 – − 0.748 |
0.005 | − 5.054 |
0.642 | −6.313 – − 3.796 |
<0.001 | |||
| Age | 0.025 | 0.007 | 0.010 – 0.039 |
0.001 | 0.025 | 0.007 | 0.011 – 0.039 |
0.001 | 0.028 | 0.007 | 0.014 – 0.042 |
<0.001 | |||
| Gender 1 | − 0.413 |
0.170 | −0.746 – − 0.079 |
0.015 | −0.523 | 0.169 | −0.855 – − 0.191 |
0.002 | −0.465 | 0.168 | −0.794 – − 0.137 |
0.006 | |||
| Race/ethnicity2 | − 0.330 |
0.230 | −0.780 – 0.120 |
0.15 | −0.138 | 0.226 | −0.580 – 0.305 |
0.54 | −0.063 | 0.220 | −0.494 – 0.368 |
0.77 | |||
| Anxiolytics 3 | − 0.819 |
0.153 | −1.112 – − 0.518 |
<0.001 | −0.709 | 0.151 | −1.006 – − 0.413 |
<0.001 | −0.665 | 0.148 | −0.954 – − 0.376 |
<0.001 | |||
| Antidepressants 3 | 0.062 | 0.163 | −0.257 – 0.381 |
0.70 | −0.076 | 0.173 | −0.415 – 0.263 |
0.66 | 0.234 | 0.162 | −0.084 – 0.552 |
0.15 | |||
| Psychostimulants 3 |
0.475 | 0.176 | 0.129 – 0.820 |
0.007 | 0.405 | 0.183 | 0.046 – 0.765 |
0.027 | 0.749 | 0.174 | 0.409 – 1.089 |
<0.001 | |||
| Trialnr | − 0.037 |
0.004 | −0.046 – − 0.029 |
<0.001 | −0.038 | 0.004 | −0.047 – − 0.030 |
<0.001 | −0.038 | 0.004 | −0.047 – 0.030 |
<0.001 | |||
| Probability | 0.018 | 0.003 | 0.013 – 0.023 |
<0.001 | 0.014 | 0.006 | 0.002 – 0.026 |
0.022 | 0.018 | 0.003 | 0.013 – 0.023 |
<0.001 | |||
| Magnitude | 0.558 | 0.078 | 0.406 – 0.711 |
<0.001 | 0.030 | 0.186 | −0.333 – 0.394 |
0.87 | 0.573 | 0.079 | 0.419 – 0.727 |
<0.001 | |||
| Fatigue/Affect | 0.063 | 0.015 |
0.034 – 0.091 |
<0.001 | −0.083 | 0.055 |
−0.190 – 0.023 |
0.13 | 0.143 | 0.058 |
0.029 – 0.257 |
0.014 | |||
| x Magnitude | 0.042 | 0.014 | 0.015 – 0.070 |
0.002 | 0.0002 | 0.016 | −0.030 – 0.031 |
0.99 | |||||||
| x Probability | 0.0003 | 0.0005 | −0.001 – 0.001 |
0.49 | −0.001 | 0.0005 | −0.002 – − 0.0004 |
0.006 | |||||||
Reference = female.
Reference = Caucasian.
Reference = no use.
Thus, these exploratory models indicate that the effects for fatigue observed for the sample as a whole appear to be driven primarily by survivors, in whom fatigue has a more chronic character. In contrast, patients with more acute cancer-related fatigue, display the expected inverse relation between fatigue and high effort choices, predominantly under favorable reward conditions.
3.4. Association of Biomarkers of Inflammation with Effort Expenditure
GEE models for each biomarker separately indicated significant associations with high-effort choices for all markers except sTNFRII (Table A.5). A GEE model with all biomarkers identified in the separate models entered simultaneously confirmed significant main effects for all five markers (Table 5, model 1) (alpha adjusted to 0.008). A pro-inflammatory profile (i.e., increased CRP, IL-6, sIL-6R, and TNF-α and decreased IL-1Ra) was associated with more high-effort choices. Repeating the model for survivors and patients separately showed that the observed associations were apparent in both groups, although associations were less often significant for patients, probably due to the small sample size (Table 5, models 2 and 3). These findings indicate that inflammation could contribute to the experience of fatigue by inducing increased effort expenditure. Indeed, higher plasma levels of TNF-α were associated with stronger increases in fatigue during the task (r = 0.33, p = 0.027), although associations were not found for the other biomarkers.
Table 5.
GEE model including the inflammation biomarkers (standardized log-transformed plasma concentrations) that were identified in individual models to be associated with high-effort choices. Each model included task variables (trial no., reward probability and magnitude) as well as the selected covariates (age, gender, race/ethnicity, use of anxiolytics, use of antidepressants, and use of psychostimulants). Model 1 additionally included patient status as covariate. Associations with high effort choices significant at p < .008 (.05/6 biomarkers) are depicted in bold.
| Model | 1. total sample | 2. Patients | 3. Survivors | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | 95% CI | p | B | SE | 95% CI | p | B | SE | 95% CI | p | |||
| CRP | 0.196 | 0.060 | 0.079 – 0.314 |
0.001 | 0.3 94 |
0.1 73 |
0.055 – 0.733 |
0.02 3 |
0.262 | 0.6 85 |
0.128 – 0.396 |
<0. 001 |
||
| IL-6 | 0.210 | 0.064 | 0.085 – 0.336 |
0.001 | 0.8 58 |
0.1 89 |
0.488 – 1.229 |
<0.0 01 |
0.264 | 0.0 85 |
0.098 – 0.430 |
0.00 2 |
||
| sIL-6R | 0.281 | 0064 | 0.156 – 0.406 |
<0.0 01 |
− 0.1 79 |
0.1 93 |
−0.558 – 0.200 |
0.36 | 0.384 | 0.0 71 |
0.246 – 0.522 |
<0. 001 |
||
| TNF-α | 0.183 | 0.058 | 0.069 – 0.297 |
0.002 | 0.4 44 |
0.2 55 |
−0.056 – 0.944 |
0.08 2 |
0.083 | 0.0 77 |
−0.068 – 0.234 |
0.28 | ||
| IL-1ra | −0.341 | 0.072 | −0.481 – − 0.201 |
<0.0 01 |
− 1.4 70 |
0.4 43 |
−2.339 – − 0.602 |
0.00 1 |
−0.341 | 0.0 96 |
−0.529 – − 0.154 |
<0. 001 |
||
4. Discussion
We show here that contrary to our expectations, fatigue experience was associated with increased effort expenditure in our sample. Exploratory models for patients and survivors separately indicated that this association was primarily driven by the cancer survivors who completed all primary cancer treatment at least three months ago. Survivors, making up two-thirds of the study sample, showed an association of fatigue with increased effort expenditure. In contrast, patients who were actively undergoing or had recently finished cancer treatment showed the expected association of cancer-related fatigue with reductions in effort expenditure, although only in the highest reward probability condition; patients with high fatigue did not show the strong increase in effort expenditure for high reward probability as compared to patients with low fatigue. Negative affect, a reflection of the experience of aversive emotions or feelings of emotional distress (Watson, Clark & Tellegen, 1988), was likewise associated with increased effort expenditure in survivors, but not in patients. Inflammation was associated with increased effort expenditure in the whole sample as well as in both groups separately, indicating that inflammation could act as a risk factor for fatigue.
There are several ways of interpreting these findings. We tentatively propose that a tendency to engage in the high effort-high reward task while experiencing fatigue, such as what we observed in survivors, is a reflection of an inability to effectively manage effort expenditure. As fatigue signals a need to conserve energy, the inability to adjust effort expenditure accordingly may serve as a contributing factor to the ongoing experience of fatigue. This interpretation is strengthened by the finding that negative affect, a tendency to experience negative emotions, was also associated with increased effort expenditure in cancer survivors. Interestingly, the association for negative affect was predominantly seen for the less rewarding conditions, suggesting that negative affect interferes with the ability to adjust behavior to external cues. As trait negative affect was also strongly associated with fatigue, this personality measure might contribute to fatigue by increasing an already present tendency to exert more effort. However, it has to be noted that the small sample size did not allow for any formal testing of these suggested mediation pathways. Patients still under treatment or shortly after its cessation did not show any association between negative affect and effort expenditure and showed the expected association of increased fatigue with decreased effort expenditure. Thus, the tendency to exert more effort while fatigued might specifically underlie chronic and not acute fatigue experience.
We also observed an association between inflammation and an increased tendency for effort expenditure. While counter to our expectations, this finding is in line with the results of an earlier study showing that experimental induction of low-grade inflammation was associated with increased effort expenditure when the probability of reward was high (Lasselin et al., 2017). Inflammation (measured by CRP plasma levels) was further negatively associated with motivational fatigue, while no associations were found with general fatigue. The lack of association between general fatigue and inflammation is not surprising considering that previously reported associations are generally modest and inconsistent, especially in cross-sectional studies (Saligan and Kim, 2012). Furthermore, blood samples were not all drawn at the same time of day, adding variability to the data which could have obscured potential associations. However, the negative association between CRP and motivational fatigue is unexpected considering that when associations are found, they are generally positive, i.e., higher fatigue relates to more inflammation (Bower, 2014; Saligan et al., 2015). De Raaf and colleagues showed that associations with inflammation depend on the dimension of fatigue that is assessed (De Raaf et al., 2012). Thus, it is well possible that motivational fatigue entails a dimension of fatigue that responds to inflammation differently from other dimensions, although it has to be noted that at least in patients with diabetes, motivational fatigue showed a positive association with inflammation (Lasselin et al., 2012). As we are the first to report on associations between inflammation and motivational fatigue in cancer patients, this finding needs replication before strong inferences can be made. In light of the experimental study of Lasselin and colleagues (Lasselin et al., 2017) and previous findings on positive associations between fatigue and inflammation (e.g., Bower et al., 2002; Orre et al., 2009; Orre et al., 2011), our finding that inflammation is associated with increased effort expenditure points to a behavioral pathway possibly explaining how inflammation contributes to the experience of general fatigue. Indeed, we found some evidence for an association between inflammation and increased momentary fatigue during the task. Further studies are needed to formally assess the notion that effort expenditure mediates the association between inflammation and fatigue.
From an ecological perspective, fatigue and fatigue-related reduced motivation can be seen as having a functional role during cancer-treatment by inhibiting energy demanding activities, which enables preservation of energy for dealing with the damaging effects of cancer treatment. It is now well-known that acute inflammation leads to changes in behavior and mood which are all associated with decreased activity (Dantzer, 2001; Dantzer et al., 2008). Furthermore, preclinical studies have convincingly shown that these reductions in activity can be reversed or overwritten when an immediate action is necessitated (Aubert et al., 1997; Vichaya et al., 2014). Personality characteristics associated with negative mood such as neuroticism, its related construct trait negative affect, and trait anxiety are generally associated with increased effort expenditure (Andreassen et al., 2014; Castonguay et al., 2017; Powell et al., 2009). Thus, while negative affect assessed here was a state and not a trait, its effects could explain the discrepancy we observed in survivors between fatigue experience and behavior: If reduced motivation contributes to the recovery from cancer treatment by temporarily reducing effort expenditure and thus saving energy for more pressing needs, then the failure to adhere to this need for energy conservation could lead to an ongoing experience of fatigue as a continuous signal of insufficient energy supply. This could be particularly true during survivorship, when the acute reasons for experience of fatigue (i.e., cancer treatment) have dissolved and individuals expect to return to a normal level of functioning.
Some limitations of the current study warrant mentioning. The cross-sectional design of our study does not allow for testing of causal relationships. Further, the sample size is appropriate for revealing an effort expenditure aspect of fatigue in cancer patients and survivors but too small for more in-depth analyses of the differences between patients and survivors or for investigating possible mediation pathways between negative affect, fatigue, and effort expenditure. While it was assumed that fatigue reported by survivors was more chronic in character, we did not formally test this and thus, our interpretation of fatigue as being chronic is tentative. Also, patients and survivors with diverse cancer diagnoses and treatment histories were included in the study, which could have obscured associations. Replications of our findings in larger and more homogenous populations of cancer patients and survivors are warranted. However, finding an association between fatigue and effort expenditure in such a heterogeneous group indicates that this is a robust effect that warrants further studies. Our findings on negative affect indicate that some aspects of personality could explain the controversial association between fatigue and effort expenditure observed in survivors. However, no data on other personality characteristics was collected in the current study. Future studies should include several other characteristics such as neuroticism and trait anxiety to assess their effect on the association between effort expenditure and fatigue.
In conclusion, cancer-related fatigue is associated with increased effort expenditure in cancer patients actively undergoing treatment but not in cancer survivors after cessation of immediate therapy. We propose that it is this inability to continue to conserve effort after the immediate reasons for fatigue are gone that leads to the development of persistent fatigue in survivorship. As inflammation and negative affect were also associated with increased effort expenditure, these factors might further contribute to chronic fatigue via an effort pathway.
Supplementary Material
Highlights.
We assessed motivational effort expenditure in cancer patients and survivors;
Fatigue was associated with more effort expenditure, especially in survivors;
Inflammation was also associated with increased effort expenditure;
Investing more effort while fatigued might predispose for developing chronic fatigue;
Inflammation might contribute to fatigue via an increased effort pathway.
Acknowledgements
We would like to thank Dr. M. Treadway for sharing the EEfRT program with us and for his advice on analyzing the EEfRT outcomes. We would also like to thank D. Estrada and S. Sherwani for their help with the data collection.
Funding
This work was supported by funds from the University Cancer Foundation and the Duncan Family Institute for Cancer Prevention and Risk Assessment via the Cancer Survivorship Research Seed Money Grants at the University of Texas MD Anderson Cancer Center. Additional support came from the University of Texas MD Anderson Cancer Center and the National Institutes of Health MD Anderson Cancer Center Support Grant [CA016672]. The content is solely the responsibility of the authors and does not necessarily represent the official view of the funding sources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
References
- 1.Andreassen CS, Griffiths MD, Hetland J, Kravina L, Jensen F, Pallesen S, 2014. The prevalence of workaholism: a survey study in a nationally representative sample of Norwegian employees. PLoS One 9, e102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antony M, Bieling P, Cox B, Enns M, Swinson R.,1998Psychometric properties of the 42-item and 21-item version of the Depression Anxiety Stress Scales (DASS) in clinical groups and a community sample. Psychol. Assess 10, 176–181. [Google Scholar]
- 3.Aubert A, Goodall G, Dantzer R, Gheusi G, 1997. Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain. Behav. Immun 11, 107–118. [DOI] [PubMed] [Google Scholar]
- 4.Bower JE, 2007. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain. Behav. Immun 21, 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bower JE, 2014. Cancer-related fatigue--mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol 11, 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bower JE,Bak K,Berger A,Breitbart W,Escalante CP, Ganz PA, Schnipper HH, Lacchetti C, Ligibel JA, Lyman GH, Ogaily MS, Pirl WF, Jacobsen PB, 2014. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J. Clin. Oncol 32, 1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bower JE, Ganz PA, Aziz N, Fahey JL, 2002. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom. Med 64, 604–611. [DOI] [PubMed] [Google Scholar]
- 8.Bower JE, Ganz PA, May LT, Hu W, Belin TR, Sepah S, Cole S, Aziz N, 2009. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin. Cancer Res 15, 5534–5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castonguay AL, Wrosch C, Sabiston CM, 2017. The roles of negative affect and goal adjustment capacities in breast cancer survivors: Associations with physical activity and diurnal cortisol secretion. Health Psychol. 36, 320–331. [DOI] [PubMed] [Google Scholar]
- 10.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR, 2006. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin. Cancer Res 12, 2759–2766. [DOI] [PubMed] [Google Scholar]
- 11.Corporation, I., 1989, 2016. IBM; SPSS Statistics, 24.0.0.0 ed. [Google Scholar]
- 12.Dantzer R, 2001. Cytokine-induced sickness behavior: Mechanisms and implications, in: Sorg BA, Bell IR (Eds.), Role of Neural Plasticity in Chemical Intolerance, pp. 222–234. [DOI] [PubMed] [Google Scholar]
- 13.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW, 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci 9, 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong N, Candel MJ, Schouten HC, Abu-Saad HH, Courtens AM, 2004. Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Ann. Oncol 15, 896–905. [DOI] [PubMed] [Google Scholar]
- 15.de Jong N, Candel MJJM, Schouten HC, Abu-Saad HH, Courtens AM, 2005. Course of mental fatigue and motivation in breast cancer patients receiving adjuvant chemotherapy. Ann. Oncol 16, 372–382. [DOI] [PubMed] [Google Scholar]
- 16.De Raaf PJ, Sleijfer S, Lamers CHJ, Jager A, Gratama JW, Van Der Rijt CCD, 2012Inflammation and fatigue dimensions in advanced cancer patients and cancer survivors: An explorative study. Cancer 118, 6005–6011. [DOI] [PubMed] [Google Scholar]
- 17.Ferriolli E, Skipworth RJ, Hendry P, Scott A, Stensteth J, Dahele M, Wall L, Greig C, Fallon M, Strasser F, Preston T, Fearon KC, 2012. Physical activity monitoring: a responsive and meaningful patient-centered outcome for surgery, chemotherapy, or radiotherapy? J. Pain Symptom Manage 43, 1025–1035. [DOI] [PubMed] [Google Scholar]
- 18.Gledhill J, 2005. A qualitative study of the characteristics and representation of fatigue in a French speaking population of cancer patients and healthy subjects. Eur. J. Oncol. Nurs 9, 294–312. [DOI] [PubMed] [Google Scholar]
- 19.Goedendorp MM, Gielissen MFM, Verhagen CAHHVM, Bleijenberg G, 2013. Development of fatigue in cancer survivors: A prospective follow-up study from diagnosis into the year after treatment. J. Pain Symptom Manage 45, 213–222. [DOI] [PubMed] [Google Scholar]
- 20.Ho SY, Rohan KJ, Parent J, Tager FA, McKinley PS, 2015. A longitudinal study of depression, fatigue, and sleep disturbances as a symptom cluster in women with breast cancer. J. Pain Symptom Manage 49, 707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knobel H, Loge JH, Nordøy T, Kolstad AL, Espevik T, Kvaløy S, Kaasa S, 2000. High Level of Fatigue in Lymphoma Patients Treated With High Dose Therapy. J. Pain Symptom Manage 19, 446–456. [DOI] [PubMed] [Google Scholar]
- 22.Lasselin J, Layé S, Dexpert S, Aubert A, Gonzalez C,Gin H,Capuron L,2012Fatigue symptoms relate to systemic inflammation in patients with type 2 diabetes. Brain. Behav. Immun 26, 1211–1219. [DOI] [PubMed] [Google Scholar]
- 23.Lasselin J, Treadway MT, Lacourt TE, Soop A Olsson J, Karshikoff B, Paues-Goranson S, Axelsson J, Dantzer R, Lekander M, 2017. Lipopolysaccharide Alters Motivated Behavior in a Monetary Reward Task: a Randomized Trial. Neuropsychopharmacology 42, 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovibond S, Lovibond P, 1995. Manual for the Depression Anxiey Stress Scales, 2nd Ed. ed. Psychology Foundation, Sydney. [Google Scholar]
- 25.McCarthy JM, Treadway MT, Bennett ME, Blanchard JJ, 2016. Inefficient effort allocation and negative symptoms in individuals with schizophrenia. Schizophr. Res 170, 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Farrell E, Smith A, Collins B, 2016. Objective-subjective disparity in cancer-related cognitive impairment: does the use of change measures help reconcile the difference? Psychooncology. [DOI] [PubMed] [Google Scholar]
- 27.Orre IJ, Murison R, Dahl AA, Ueland T, Aukrust P, Fosså SD, 2009. Levels of circulating interleukin-1 receptor antagonist and C-reactive protein in long-term survivors of testicular cancer with chronic cancer-related fatigue. Brain. Behav. Immun 23, 868–874. [DOI] [PubMed] [Google Scholar]
- 28.Orre IJ, Reinertsen KV, Aukrust P, Dahl AA, Fossa SD, Ueland T, Murison R, 2011. Higher levels of fatigue are associated with higher CRP levels in disease-free breast cancer survivors. J. Psychosom. Res 71, 136–141. [DOI] [PubMed] [Google Scholar]
- 29.Powell R, Allan JL, Johnston DW, Gao C, Johnston M, Kenardy J, Pollard B, Rowley DI, 2009. Activity and Affect: Repeated Within-Participant Assessment in People After Joint Replacement Surgery. Rehabil. Psychol 54, 83–90. [DOI] [PubMed] [Google Scholar]
- 30.Pullens MJ, De Vries J, Roukema JA, 2010. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology 19, 1127–1138. [DOI] [PubMed] [Google Scholar]
- 31.Saligan LN, Kim HS, 2012. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain. Behav. Immun 26, 830–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saligan LN, Olson K, Filler K, Larkin D, Cramp F, Sriram Y, Escalante CP, del Giglio A, Kober KM, Kamath J, Palesh O, Mustian K, 2015. The biology of cancer-related fatigue: a review of the literature. Support. Care Cancer 23, 2461–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Servaes P, Verhagen C, Bleijenberg G, 2002. Fatigue in cancer patients during and after treatment: Prevalence, correlates and interventions. Eur. J. Cancer 38, 27–43. [DOI] [PubMed] [Google Scholar]
- 34.Shun SC, Hsiao FH, Lai YH, Liang JT, Yeh KH, Huang J, 2011. Personality trait and quality of life in colorectal cancer survivors. Oncol. Nurs. Forum 38, E221–E228. [DOI] [PubMed] [Google Scholar]
- 35.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwill P, 1995. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. The British Journal of Psychiatry 167, 99–103. [DOI] [PubMed] [Google Scholar]
- 36.Stein KD, Jacobsen PB, Blanchard CM, Thors CT, 2004. Further validation of the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF). J. Pain Symptom Manage 27, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Timmerman JG, Dekker-van Weering MG, Tonis TM, Hermens HJ, Vollenbroek-Hutten MM, 2015. Relationship between patterns of daily physical activity and fatigue in cancer survivors. Eur. J. Oncol. Nurs 19, 162–168. [DOI] [PubMed] [Google Scholar]
- 38.Treadway MT, Bossaller NA, Shelton RC, Zald DH, 2012. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J. Abnorm. Psychol 121, 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH, 2009. Worth the ‘EEfRT’? The Effort Expenditure for Rewards Task as an Objective Measure of Motivation and Anhedonia. PLoS One 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vercoulen JH, Swanink CM, Fennis JF, Galama JM, van der Meer JW, Bleijenberg G, 1994Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res 38, 383–392. [DOI] [PubMed] [Google Scholar]
- 41.Vichaya EG, Hunt SC, Dantzer R, 2014. Lipopolysaccharide reduces incentive motivation while boosting preference for high reward in mice. Neuropsychopharmacology 39, 2884–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang SH, He GP, Jiang PL, Tang LL, Feng XM, Zeng C, Wang GF, 2013. Relationship between cancer-related fatigue and personality in patients with breast cancer after chemotherapy. Psychooncology 22, 2386–2390. [DOI] [PubMed] [Google Scholar]
- 43.Watson D, Clark LA, Tellegen A, 1988. Development and validation of brief measures of positive and negative affect - The PANAS scales. J. Pers. Soc. Psychol 54, 1063–1070. [DOI] [PubMed] [Google Scholar]
- 44.Xiao C, Miller AH, Felger J, Mister D, Liu T, Torres A, 2017. Depressive symptoms and inflammation are independent risk factors of fatigue in breast cancer survivors. Psychol. Med, 1–11. [DOI] [PubMed] [Google Scholar]
- 45.Zigmond AS, Snaith RP, 1983. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand 67,361–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.