Abstract
INTRODUCTION
Cerebrospinal fluid (CSF) protein analysis may facilitate detection and elucidate mechanisms of neurological consequences from repetitive head impacts (RHI), such as chronic traumatic encephalopathy (CTE). We examined CSF concentrations of total tau (t-tau), phosphorylated tau (p-tau), and Aβ1–42, and their association with RHI in former National Football League (NFL) players. The role of microglial activation (using sTREM2) was examined as a pathogenic mechanism of CTE.
METHODS
Sixty-eight former NFL players and 21 controls underwent lumbar puncture to quantify t-tau, p-tau181, Aβ1–42, and sTREM2 in the CSF using immunoassays. The cumulative head impact index (CHII) estimated RHI.
RESULTS
No between group differences for CSF analytes emerged. In the former NFL players, the CHII predicted higher t-tau concentrations (p=0.041) and higher sTREM2 levels were associated with higher t-tau concentrations (p=0.009).
DISCUSSION
In this sample of former NFL players, greater RHI and increased microglial activation were associated with higher CSF t-tau concentrations.
Keywords: Cerebrospinal fluid, Aβ, sTREM2, chronic traumatic encephalopathy, microglial activation, Alzheimer’s disease, repetitive head impacts, concussion, subconcussive
1. INTRODUCTION
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease associated with exposure to repetitive head impacts (RHI), such as those incurred through contact sports (e.g., tackle football, boxing) and combat military service involving blast exposures[1–4]. In a recent convenience sample of 202 deceased tackle football players, CTE was neuropathologically diagnosed in 177 participants, including 110 of 111 former National Football League (NFL) players[4]. CTE cannot yet be diagnosed during life and the mechanisms by which exposure to RHI transitions to neurodegeneration are unknown. To address these knowledge gaps, current research is focused on the development of in vivo biomarkers for CTE[5,6]. In vivo fluid biomarkers play a critical role in the clinical diagnosis and study of mechanistic pathways of other neurodegenerative diseases, such as Alzheimer’s disease (AD)[7]. Clinically useful fluid biomarkers for concussion and acute traumatic brain injury (TBI) are now emerging[8].
The pathognomonic diagnostic lesion of CTE is the perivascular deposition of abnormal phosphorylated tau (p-tau) in neurons and astroglia at the base of the cortical sulci[9]. If present, Aβ plaques are sparse and diffuse and related to age[4,10]. Positron emission tomography (PET) imaging of paired helical filament tau is currently under investigation as diagnostic biomarkers to identify and grade tau pathology in patients with suspected CTE and may have value for CTE diagnosis, particularly when used in conjunction with Aβ PET. PET imaging, however, is time-demanding and expensive. Although lumbar puncture is often viewed as invasive, cerebrospinal fluid (CSF) concentrations of tau (total tau [t-tau], p-tau) and Aβ represents a pragmatic biomarker that provides a direct assessment of the central nervous system (CNS). CSF concentrations of Aβ, t-tau, and p-tau are core biomarkers for AD[7,11–13]. As in AD[7], CSF p-tau181 may be a specific marker of intraneuronal tau pathology and CSF t-tau may be a marker of neurodegeneration in CTE. Because tau is predominantly expressed in neuronal axons, elevated CSF t-tau concentrations are usually interpreted to reflect general neuronal injury. However, CSF t-tau is non-specific (e.g., it can be temporarily increased following stroke or acute TBI) [14–16], particularly in the absence of neuropathological examination. Elevated CSF t-tau concentrations could reflect downstream CTE-related neurodegeneration and/or neuronal injury related to long-term neurological consequences associated with RHI (e.g., axonal degeneration). Nevertheless, the specific relationship between RHI and later life CSF tau, as well as Aβ is unknown.
There is evidence that RHI and CTE are associated with increases in microglial activation[17,18]. In 66 deceased American football players with autopsy-confirmed CTE, more years of football play predicted CD68 (a marker of microglial activation), which partially mediated the effect of RHI on p-tau[18]. Tackle football players with autopsy-confirmed CTE have been shown to exhibit elevated CSF CCL11 (a marker of neuroinflammation), which was related to RHI[19]. Molecular neuroimaging studies further demonstrate chronic microglial activation (e.g., PET measures of TSPO) in active, recently retired, and older former NFL players[20,21].
Soluble CSF concentrations of the triggering receptor expressed on myeloid cells 2 (sTREM2) may serve as an additional in vivo biomarker of microglial activation in CTE. TREM2 is expressed on microglia in the CNS and modulates microglial activation[22,23]. TREM2 variants predict increased risk for other neurodegenerative diseases (e.g., frontotemporal dementia, AD)[24], with odds ratios for AD risk similar to apolipoprotein E, although the allele frequency in the population is low[25,26]. sTREM2 can be detected in the CSF and higher concentrations are believed to reflect increased microglial activation[27–31]. CSF sTREM2 has been the focus of several recent clinical research studies of AD. sTREM2 correlates with CSF markers of AD t-tau, p-tau[27–29,32], as well as with CSF glial protein YKL-40[27,28]. sTREM2 concentrations, however, vary with disease progression. AD cohorts have exhibited elevated[27,32], reduced[33], or normal levels[34] of sTREM2. When disease stage is considered, sTREM2 levels are higher in the early stages of the AD continuum[28,29,33] and can be increased five years before symptom onset[35].
Analysis of CSF t-tau, p-tau, and Aβ may be a pragmatic method to detect long-term neurological consequences associated with RHI, including early CTE pathology. Evaluation of sTREM2 levels, in conjunction with CSF t-tau, p-tau, and Aβ, may also provide insight into the role of microglial activation in the pathogenesis of CTE. This study compared CSF t-tau, p-tau181, Aβ1–42 and sTREM2 between 68 symptomatic former NFL players at high risk for CTE and 21 same-age asymptomatic controls without a history of contact sports or head trauma. The relationship between estimated exposure to RHI (using a cumulative head impact index [CHII][36]) and CSF t-tau, p-tau181, Aβ1–42, and sTREM2 was investigated in the former NFL players. The study used simultaneous equations regression models to examine the relationships among RHI, sTREM2, t-tau, p-tau, and Aβ1–42.
2. METHODS
2.1. Participants and Study Design
The current sample included 68 former NFL players who participated in the National Institutes of Health-funded study, known as, “Diagnosing and Evaluating Traumatic Encephalopathy using Clinical Tests” (DETECT). The present sample had complete data for all CSF analytes examined. The purpose of the DETECT study is to identify possible in vivo biomarkers for CTE. Inclusion criteria for the former NFL players were: male, ages 40–69, a minimum of two seasons in the NFL and a minimum of twelve years of organized football, and self-reported complaints of cognitive, behavior, and/or mood symptoms at the time of screening. A history of concussion within one year from study enrollment was an exclusion criterion and the overall mean (SD) of years since last concussion in the former NFL players was 21.80 (11.37). The sample also included 21 controls who were of the same-age as the former NFL players, had no history of TBI or participation in contact sports, and who reported being asymptomatic at the time of screening. Exclusion criteria for all participants included MRI and/or lumbar puncture contraindications, presence of another CNS disease, and/or a primary language other than English. Participants completed a single two- to three-day study visit, which involved administration of neuropsychological tests, neurological and psychiatric evaluations, lumbar puncture, and history interview, and other examinations not pertinent to the current study. Additional descriptions of the DETECT Study have been reported previously[5,6,37]. All study protocols were approved by the Boston University Medical Center Institutional Review Board. Participants provided written informed consent prior to participation.
2.2. Measures
2.2.1. CSF Analytes
CSF (15–20mL) was obtained by lumbar puncture (LP) in the morning after overnight fasting. LPs were performed by the study neurologist (S.F.) using an atraumatic 25-guage Sprotte needle at either L3/L4 or L4/L5. After aspiration, approximately 10mL of CSF was deposited into a polypropylene transfer tube and frozen at −80°C. Aliquots were shipped to the University of Pennsylvania for batch analysis of t-tau, p-tau181, and Aβ1–42. Methods of CSF analysis of t-tau, p-tau181, and Aβ1–42 are described elsewhere[38–40]. Briefly, p-tau181, t-tau, and Aβ1–42 levels were measured using the multiplex xMAP Luminex platform (Luminex Corp, Austin, TX) with Fujirebio (INNO-BIA AlzBio3; Ghent, Belgium; for research use–only reagents) immunoassay kit–based reagents. The Fujirebio kit reagents included well-characterized capture monoclonal antibodies specific for Aβ1–42 (4D7A3), t-tau(AT120), and p-tau181 (AT270), each chemically bonded to unique sets of color-coded beads, and analyte-specific detector antibodies (HT7, 3D6). Calibration curves were produced for each biomarker using aqueous buffered solutions that contained the combination of three biomarkers at concentrations ranging from 56 to 1,948 pg/ml for recombinant tau, 27 to 1,574 pg/ml for synthetic Aβ1–42 peptide, and 8 to 230pg/ml for a tau synthetic peptide phosphorylated at the threonine 181 position (i.e., p-tau181 standard).
Additional samples of CSF were shipped to University College London (UK) for batch analysis of sTREM2. Methods for analysis of CSF sTREM2 analysis were adapted from Kelinberger et al.[33] The antibodies validated in Kleinberger et al.[33] were used (i.e., goat polyclonal against the N-terminus of human TREM2; 1:1,000 – 1:2,000, R&D Systems, AF1828). Streptavidin-coated 96-well plates (Meso-Scale discovery (MSD) Maryland, U.S.) were blocked for 1 hour at 4°C in block buffer (0.5% bovine serum albumin (BSA) and 0.05% Tween 20 in PBS (pH 7.4). The plates were incubated with the biotinylated polyclonal goat anti-human TREM2 capture antibody (0.25 μg/ml R&D Systems, Minnesota U.S) diluted in block buffer for 1 hour at room temperature. They were subsequently washed four times with wash buffer (0.05% Tween 20 in PBS) and incubated overnight at 4°C with CSF diluted 1:4 in assay buffer, 0.025% BSA and 0.05% Tween 20 in PBS (pH 7.4) or a standard curve constructed from recombinant human TREM2 protein (4000 −62.5pg/ml (Sino Biological Inc. Beijing, China) diluted in assay buffer. Plates were again washed three times with wash buffer before incubation for 1 hour at room temperature with the detector antibody monoclonal mouse anti-human TREM2 antibody (1μg/ml Santa Cruz Biotechnology, Texas, U.S). After three additional washing steps, plates were incubated with the secondary antibody (SULFO-TAG–labeled anti-mouse secondary antibody, MSD) and incubated for 1 hour in the dark. Lastly, plates were washed three times with wash buffer followed by two washing steps in PBS alone. The electrochemical signal was developed by adding MSD Read buffer (1 in 2) and the light emission measured using the MSD SECTOR Imager 6000. The concentration of sTREM2 was calculated using a five-parameter logistic curve fitting method with the MSD Workbench software package. Intra-assay cvs were < 10%, and all samples were measured on the same day using the same reagents.
2.2.2. Cumulative Head Impact Index (CHII)
The CHII quantified estimated exposure to RHI[36]. The CHII is based on reported number of football seasons played, position[s] played, and levels played (e.g., youth, high school, college), as well as estimated head impact frequencies derived from published helmet accelerometer studies. The CHII was initially developed in former high school and college football players because helmet accelerometer studies at the professional football level have not been published or made available. For the current sample of former NFL players, college level estimates of head impact frequencies were applied for estimation of professional level head impact frequencies. A higher CHII reflects greater exposure to RHI. Previous research in the DETECT sample supports the sensitivity of the CHII to neurological outcomes[5,41].
2.2.3. Cognitive and Neuropsychiatric Function
A neuropsychological test battery and semi-structured interviews and self-report measures of neuropsychiatric function were administered to participants on a separate day from the LP. For a full list of the tests administered as part of DETECT, see Alosco et al.[37] To limit the number of analyses, a subset of measures was selected a priori to include tests routinely used in the clinical evaluation of neurodegenerative disease, as well as those that assess clinical functions presumed to be impaired in CTE and included in provisional clinical research diagnostic criteria for CTE[42]. This included measures of depression (Center for Epidemiologic Studies Depression Scale [CES-D]), behavioral regulation (Behavior Rating Inventory of Executive Function-Adult version [BRIEF-A] Behavioral Regulation Index [BRI]), episodic memory (Neuropsychological Assessment Battery [NAB] List Learning [LL] Delayed Free Recall), and executive function (Trail Making Test Part B [TMT B]). Raw scores from the neuropsychological tests were transformed to standard scores using normative data that accounted for age, sex, and/or education. For analyses that included clinical measures, the sample size was reduced to 66 former NFL players due to exclusion of two participants for evidence of intentional symptom exaggeration; sample size for TMT B was reduced to 65 former NFL players due to missing data.
2.3. Statistical Analyses
Multivariate linear mixed-effects models examined differences between former NFL players and controls in t-tau, p-tau181, p-tau181/t-tau, Aβ1–42, and sTREM2. Linear mixed-effect models reduce type I error, as they account for correlations between groups and outcomes from the same participant. The models were adjusted for age and body mass index (BMI). Race was not included as a covariate in the between group analyses because only one control was African American, precluding the ability to obtain reliable estimates of the differential effects of race between the study groups. Bootstrap analysis was performed on 500 replicates to control for type I error, account for potential non-linear relationships, and increase statistical power. The between group results presented are those from the bootstrap analysis.
Additional statistical analyses were conducted only on data from the former NFL player group. Independent regression analyses examined the relationship between exposure to RHI (using the CHII) and the CSF analytes of t-tau, p-tau181, p-tau181/t-tau, and Aβ1–42. These analyses were adjusted for age, BMI, and race. For relationships that emerged significant, the role of sTREM2 was examined using simultaneous equations regression models. Simultaneous equations regression modeling is a form of structural equation modeling that limits endogeneity and evaluates for the presence of potential feedback loops between multiple outcomes[43]. The predictor variables included age, BMI, race, and the CHII. Outcome variables included sTREM2 and CSF t-tau, p-tau181, p-tau181/t-tau, and Aβ1–42; again, only the analyte(s) that demonstrated a significant relationship with the CHII in the independent regression model was (were) examined as an outcome, along with sTREM2, in the model. The model tested for direct and total effects (i.e., direct + indirect) of the predictor variables on the outcomes (i.e., the direct and total effects of age, BMI, race, and CHII on the CSF analytes), as well as for direct effects between the outcomes. In initial models, age, BMI, race, and the CHII were estimated to predict all outcome variables. To maintain model parsimony, only pathways with a p<0.10 were retained. Finally, independent regressions controlling for age, BMI, and race were used to examine the effects of t-tau, p-tau181, p-tau181/t-tau, Aβ1–42, and sTREM2 on each of the clinical measures, i.e., NAB LL Delayed Recall, TMT-B, BRIEF-A BRI, and CES-D.
3. RESULTS
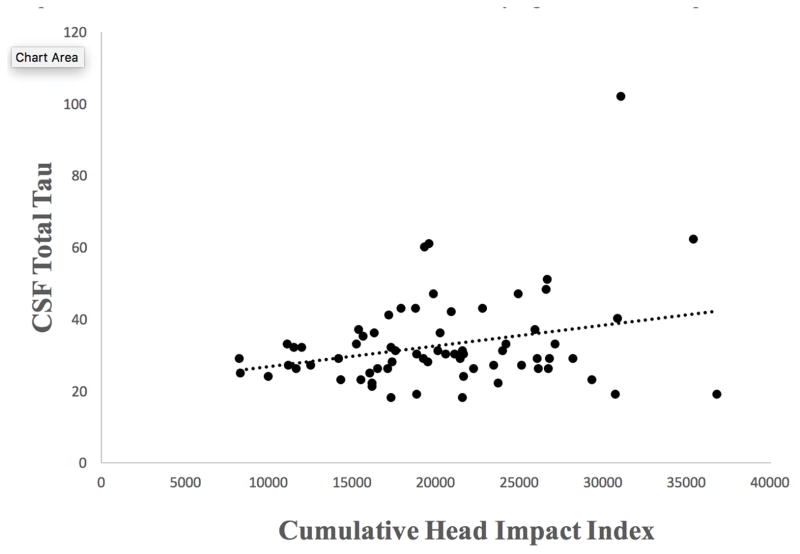
Table 1 summarizes sample characteristics, and Table 2 provides descriptive statistics for CSF concentrations in the sample. The multivariate linear mixed-effect models showed no differences between the former NFL players and controls in t-tau (p=0.18), p-tau181 (p=0.79), p-tau181/t-tau (p=0.06), Aβ1–42 (p=0.08), or sTREM2 (p=0.50). In the former NFL players, independent regressions showed a statistically significant association between the CHII and t-tau (p=0.041). See Figure 1. Higher CHII scores were associated with higher levels of t-tau (Table 3). There were no effects between the CHII and p-tau181 (p=0.13), p-tau181/t-tau (p=0.61), Aβ1–42 (p=0.75), or sTREM2 (p=0.54).
Table 1.
Sample Characteristics
| NFL (n = 68) | Controls (n = 21) | p-value | |
|---|---|---|---|
| Demographic/Athletic | |||
| Age, mean (SD) years | 54.50 (7.95) | 57.57 (7.10) | 0.12 |
| Education, mean (SD) years | 16.44 (0.97) | 17.38 (2.22) | 0.07 |
| a African American, n (%) | 29 (42.65) | 1 (4.76) | 0.001 |
| Duration of football play, mean (SD) years | 18.67 (3.56) | -- | -- |
| Years in the NFL, mean (SD) | 8.30 (2.89) | -- | -- |
| Cumulative Head Impact Index, mean (SD) | 20,304.87 (6195.14) | -- | -- |
| Primary Position Group, n (%) | -- | ||
| Offensive line | 20 (29.41) | -- | -- |
| Running back | 5 (7.35) | -- | -- |
| Tight end | 5 (7.35) | -- | -- |
| Offensive skill | 0 | -- | -- |
| Defensive line | 12 (17.65) | -- | -- |
| Linebacker | 14 (20.59) | -- | -- |
| Defensive Back | 12 (17.65) | -- | -- |
| Body mass index, mean (SD) kg/m2 | 32.67 (4.43) | 28.36 (3.84) | <0.001 |
The control group was required to be asymptomatic and have no history of head trauma.
Independent sample t-tests were performed for continuous outcomes and Fisher’s Exact Test was used to compare group differences in race.
Table 2.
Cerebrospinal Fluid Biomarker Concentrations
| t-tau (pg/ml) | p-tau181 (pg/ml) | Aβ1–42 (pg/ml) | p-tau181/t-tau Ratio | sTREM2 (ng/ml) | |
|---|---|---|---|---|---|
| NFL Players (n = 68) | |||||
| Median | 29.50 | 17.00 | 362.00 | 0.57 | 3.03 |
| Mean (SD) | 32.81 (12.97) | 18.34 (9.46) | 362.75 (8.73) | 0.59 (0.27) | 3.50 (1.91) |
| 95% CI | 29.67, 35.95 | 16.05, 20.63 | 345.32, 380.18 | 0.52, 0.65 | 3.03, 3.96 |
| Controls (n = 21) | |||||
| Median | 36.00 | 22.00 | 425.00 | 0.69 | 4.11 |
| Mean (SD) | 36.57 (14.12) | 22.62 (9.51) | 409.05 (78.74) | 0.66 (0.24) | 4.50 (1.74) |
| 95% CI | 30.15, 43.00 | 18.29, 26.95 | 373.20, 444.89 | 0.55, 0.76 | 3.71, 5.30 |
Abbreviations: t-tau = total tau, p-tau181 = hyperphosphorylated tau, Aβ = beta-amyloid.
Figure 1.
Relationship Between Exposure to Repetitive Head Impacts and CSF Total Tau in Former National Football League Players. As shown in Table 3, independent regressions showed a statistically significant association between the cumulative head impact index and t-tau after controlling for age, BMI, and race (p = 0.041). Figure 1 shows the unadjusted relationship between the cumulative head impact index and CSF total tau, which was also statistically significant: r = 0.27, p = 0.024.
Table 3.
Summary of Linear Regression Examining the Direct Effects of Exposure to Repetitive Head Impacts on Cerebrospinal Fluid Markers of Tau, Amyloid, and sTREM2
| t-tau | p-tau181 | p-tau181/t-tau | Aβ1–42 | sTREM2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| beta | SE | P-value | beta | SE | P-value | beta | SE | P-value | beta | SE | P-value | beta | SE | P-value | |
| Age | 0.36 | 0.19 | 0.056 | 0.09 | 0.15 | 0.552 | −0.003 | 0.004 | 0.423 | −0.49 | 1.17 | 0.68 | 0.08 | 0.03 | 0.002 |
| BMI | −0.73 | 0.38 | 0.055 | −0.23 | 0.31 | 0.461 | 0.003 | 0.01 | 0.684 | 0.08 | 2.36 | 0.973 | −0.04 | 0.05 | 0.388 |
| Race | −3.28 | 3.37 | 0.333 | 4.01 | 2.79 | 0.154 | 0.198 | 0.07 | 0.009 | −35.51 | 21.11 | 0.098 | −1.35 | 0.46 | 0.004 |
| CHII | 0.54 | 0.26 | 0.041 | 0.33 | 0.22 | 0.127 | 0.003 | 0.01 | 0.61 | 0.52 | 1.64 | 0.750 | 0.02 | 0.04 | 0.541 |
Abbreviations: t-tau = total tau, p-tau181 = hyperphosphorylated tau, Aβ = beta-amyloid, BMI = body mass index, CHII = cumulative head impact index, SE = standard error. Beta is unstandardized and bolded values highlight statistical significance. For race, 1 = African American and 0 = Caucasian.
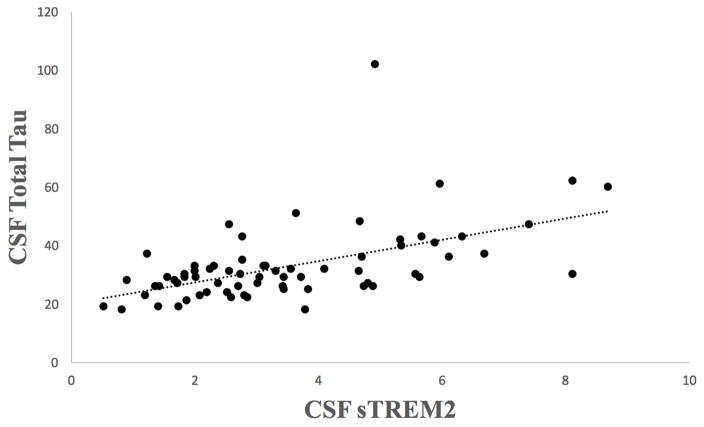
To examine the role of microglial activation (sTREM2) in the relationship between RHI and t-tau, a simultaneous equations regression model with t-tau and sTREM2 as the outcome variables was conducted in the former NFL players. Age, BMI, race, and the CHII were included as predictors. In the initial model, the p-values for paths between age and t-tau, as well as between BMI and sTREM2 were greater than 0.10 and were removed from the final model. Based on the independent regression models (see above), the CHII was modeled to have a direct effect on t-tau. The model fit was excellent (Goodness of Fit=0.9995). All direct and total effects from the model are presented in Table 4. There was a statistically significant direct effect of sTREM2 on t-tau (p=0.009), such that higher concentrations of sTREM2 were associated with higher concentrations of t-tau (Figure 2). There was a statistically significant total effect for the CHII on t-tau (p=0.030), meaning that the relationship between the CHII and t-tau was strengthened by sTREM2.
Table 4.
Summary of Direct and Total Effects from Simultaneous Equations Regression Model Examining sTREM2 and Total Tau as Outcomes
| Standardized Direct Effects | Standardized Total Effects | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sTREM2 | t-tau | sTREM2 | t-tau | |||||||||||||
| Beta | SE | t | P | Beta | SE | t | P | Beta | SE | t | P | Beta | SE | t | P | |
| Age | 0.28 | 0.12 | 2.43 | 0.0149 | -- | -- | -- | -- | 0.35 | 0.10 | 3.59 | 0.0003 | 0.18 | 0.08 | 2.14 | 0.0323 |
| BMI | -- | -- | -- | -- | −0.20 | 0.12 | −1.65 | 0.10 | −0.09 | 0.10 | −0.88 | 0.38 | −0.24 | 0.12 | −1.97 | 0.0489 |
| Race | −0.28 | 0.14 | −2.02 | 0.0432 | -- | -- | -- | -- | −0.35 | 0.11 | −3.15 | 0.0016 | −0.18 | 0.09 | −2.04 | 0.0411 |
| CHII | -- | -- | -- | -- | 0.20 | 0.11 | 1.80 | 0.07 | 0.09 | 0.10 | 0.90 | 0.37 | 0.25 | 0.12 | 2.17 | 0.0300 |
| sTREM2 | -- | -- | -- | -- | 0.52 | 0.20 | 2.61 | 0.0091 | -- | -- | -- | -- | 0.64 | 0.37 | 1.74 | 0.08 |
| t-tau | 0.36 | 0.36 | 0.99 | 0.32 | -- | -- | -- | -- | 0.44 | 0.55 | 0.80 | 0.43 | -- | -- | -- | -- |
Note. In the initial model, the p-values for paths between age and t-tau, as well as between BMI and sTREM2 were greater than 0.10 and were therefore removed from the final model. Based on independent regression, the CHII was only estimated to have a direct effect on t-tau. BMI = body mass index, CHII = cumulative head impact index, t-tau = total tau, SE = standard error. Beta is standardized and bolded values highlight statistical significance. For race, 1 = African American and 0 = Caucasian.
Figure 2.
Relationship Between Exposure to CSF TREM2 and CSF Total Tau in Former National Football League Players. Figure shows a scatter plot of the relationship between CSF sTREM2 and total tau. Bivariate correlations showed this to be a statistically significant relationship: r = 0.54, p < 0.001.
See Table 5 for clinical test scores in the former NFL players and asymptomatic controls. Independent regressions showed that t-tau and sTREM2 were not associated with any of the cognitive or neuropsychiatric function tests (ps>0.10). Therefore, simultaneous equations regression models with clinical measures included as additional outcomes were not performed. However, lower Aβ1–42 was associated with worse performance on the NAB LL Delayed Recall (beta=0.06, t=2.59, p=0.012). Higher levels of p-tau181 also correlated with worse depression severity on the CES-D (beta=0.33, t=2.04, p=0.046).
Table 5.
Cognitive and Neuropsychiatric Test Performance in the Former NFL Players and Controls
| Former NFL Players (n = 65–66)* | Controls (n = 21) | P-value | |
|---|---|---|---|
| Cognitive Function, mean (SD) T-score | |||
| NAB List Learning Delayed Recall | 41.92 (13.77) | 50.38 (12.64) | 0.0415 |
| Trail Making Test Part B | 45.12 (15.01) | 52.14 (15.45) | 0.1076 |
| Neuropsychiatric Status | |||
| CES-D, mean (SD) raw score | 21.14 (13.76) | 3.71 (4.15) | <0.0001 |
| BRIEF-A BRI, mean (SD) T-score | 62.62 (11.49) | 47.24 (9.43) | <0.0001 |
Note. The control group was required to be asymptomatic and have no history of head trauma at the time of recruitment. Mixed-effect models controlling for age and body mass index compered the former NFL players and controls on each of the clinical measures.
The sample size was reduced to 66 former NFL players due to exclusion of two participants for evidence of intentional symptom exaggeration; sample size for TMT B was further reduced to 65 former NFL players due to missing data.
Abbreviations: NAB: Neuropsychological Assessment Battery; CES-D: Center for Epidemiologic Studies Depression Scale; BRIEF-A: Behavior Rating Inventory of Executive Function-Adult version; BRI: Behavioral Regulation Index
4. DISCUSSION
In this sample of 68 symptomatic former NFL players, greater exposure to RHI was associated with higher CSF concentrations of t-tau, but not p-tau181, p-tau181/t-tau, or Aβ1–42. Higher sTREM2 concentrations were associated with higher t-tau concentrations, and sTREM2 strengthened the relationship between exposure to RHI and t-tau. Although sTREM2 and t-tau were not associated with neurobehavioral test performance, lower Aβ1–42 levels were associated with worse episodic memory and higher levels of p-tau181 correlated with greater symptoms of depression. There were no statistically significant differences in concentrations for any of the CSF analytes between former NFL players and same-age asymptomatic controls without a history of contact sports or head trauma. Overall, this study provides evidence that exposure to RHI is associated with later life CSF t-tau concentrations in former professional football players and that microglial activation may play a role in that relationship.
Exposure to RHI and CSF t-tau and p-tau181 Concentrations
The present association between exposure to RHI and CSF t-tau is consistent with our previous research in the DETECT sample that found a significant positive relationship between RHI and plasma t-tau[5]. Tau is predominantly expressed in neuronal axons and elevated CSF t-tau is hypothesized to a be a marker of neurodegeneration[7,11–13]. An acute mild TBI (concussion) leads to a ‘cascade’ of neurometabolic events due, in part, to diffuse shearing and tearing of axons[44]. CSF t-tau levels have been shown to be increased acutely following concussion or contact sports play, likely representing axonal injury[45,46]. Repeated aggravation of CNS injury from RHI, which involves repeated concussive and subconcussive brain trauma with minimal recovery between head impacts[36], may limit axonal recovery to result in long-term elevations in t-tau. In general, CSF t-tau is a non-specific marker and, given the absence of neuropathological examination in this in vivo study, it is not known if the current relationship between RHI and CSF t-tau is indicative of long-standing t-tau elevation from RHI-associated neuronal injury from the time of play, or later-life t-tau elevation secondary to neurodegeneration from CTE. The lack of differences in t-tau between the former NFL players and controls argues against a neurodegenerative process, as does the lack of relationship between CSF t-tau and neurobehavioral test performance. However, CSF t-tau concentrations have been shown to be only mildly increased in other primary tauopathies (i.e., frontotemporal dementia) when compared to non-demented controls[47]. Overall, the clinical implications of CSF t-tau in the setting of CTE remains inconclusive.
Unlike CSF t-tau, p-tau is considered to be a specific marker of intraneuronal tau pathology. We did not find group differences in p-tau181 and there was not a statistically significant association between RHI and p-tau181. RHI may initiate the physiological events necessary for the eventual development of CTE, but once CTE p-tau pathology manifests, RHI may not be related to the CTE-specific p-tau pathology. Additionally, the presence of CTE neuropathology in this clinical cohort is unknown, and the severity of p-tau pathology may be too limited to result in changed CSF p-tau (or t-tau) levels in these former NFL players. Further, although CSF p-tau181 is an established biomarker for NFT pathology in AD[7] (and FTLD-TDP[ 48]), p-tau231 has been shown to be superior to p-tau181 in the clinical and neuropathological diagnostic prediction of AD[49]. Normal CSF p-tau levels have also been reported in various patient populations with dementia, e.g., Lewy body dementia, frontotemporal dementia[50]. Each neurodegenerative tauopathy, including CTE, may be characterized by distinct p-tau isoforms or species not uniformly detected by CSF assays.
Microglial Activation and Neuronal Injury
In this sample of former NFL players, increased microglial activation (i.e., sTREM2) was associated with t-tau. Increased microglial activation has been found in living and deceased, active and retired, tackle football players[18–21] and is associated with the development of dementia[18]. Remote exposure to RHI was not directly associated with current microglial activation (i.e., sTREM2) in this sample of NFL players who stopped playing football years ago. RHI may initiate microglial activation (as suggested by recent experimental evidence[51]), but this activation may diminish after exposure to RHI has ended, and then increase again when neurodegeneration begins. This is similar to research in AD, which suggests that sTREM2 levels may be dependent on disease stage[28,29,33,35]. That being said, evidence from research among deceased football players with neuropathologically-confirmed CTE suggests that RHI from tackle football may lead to chronic microglial activation, leading to the formation of p-tau as NFTs[18,19]. Mouse models of RHI and CTE have further linked microglial activation with p-tau pathology[52–56], and also found that an induced head impact resulted in microgliosis, as measured by TREM2, and phosphorylated tauopathy[51]. Microglial activation may thus contribute to the pathogenesis of CTE.
Because the current study is cross-sectional, it is plausible that there is a bidirectional relationship between CSF t-tau and sTREM2. Cherry et al. found a bidirectional relationship between p-tau pathology and microglial activation in their sample of 66 deceased tackle football players with CTE[18]. Once neuronal injury from RHI or CTE begins, an additional microglia response may be initiated that could further promote neuronal injury. Regardless of when microglial activation begins (or what causes it), chronic microglial activation can be detrimental due to prolonged exposure to inflammatory cytokines that can result in neuronal toxicity and eventual neurodegeneration[54–58]. Indeed, increased sTREM2 levels strengthened the effect of RHI on t-tau in this sample of former NFL players, and the presence of microglial activation may modify the relationship between RHI and later-life neurological outcomes. Longitudinal studies that follow former football players throughout their life are needed to elucidate directionality in the relationships among RHI, sTREM2, and t-tau.
Group Differences and Clinical Function
The former NFL players exhibited statistically significant worse clinical test performance than the asymptomatic controls, with the exception of a statistical trend for Trail Making Test Part B. This is likely a manifestation of sample selection as the controls were required to be asymptomatic at the time of telephone screening whereas the former NFL players were required to have self-reported complaints of cognitive, behavior, or mood disturbances. The normative T-scores (and the established cut score of 16 for the CES-D) are a more accurate reflection of the clinical status in this sample of former NFL players. On average, the former NFL players reported clinically meaningful symptoms of depression (based on the average CES-D score of 21). In contrast, scores on the other neuropsychiatric measures and performance on the cognitive tests were all within normal limits (based on normative T-scores).
Although there were no between-group differences in Aβ1–42, lower Aβ1–42 levels were associated with worse episodic memory in the former NFL players, and RHI exposure was not associated with Aβ1–42. Aβ plaques are not a diagnostic neuropathological feature of CTE and, if present, the plaques are often sparse and diffuse, strongly associated with older age, and accelerate the clinical and pathological course of CTE[3,4,10]. The relationship between TBI and Aβ accumulation has been examined by several studies using transgenic mice susceptible to AD pathology (due to mutations of the Aβ precursor protein) subjected to controlled cortical impact[59–61]. Some of these studies provide evidence for reduced or no accumulation of Aβ months after the TBI[59,60]. Interestingly, however, other evidence in transgenic mice with mutations of the amyloid precursor protein suggests that repetitive mild TBI may accelerate Aβ deposition[62], and this is similar to what has been observed in CTE[10]. Future research on Aβ1–42 deposition in the setting of CTE is needed.
The current study did not find group differences for p-tau181 and t-tau (as discussed above), or sTREM2. Total tau and sTREM2 were not associated with clinical function, and higher p-tau181 levels were only associated with greater symptoms of depression (a core clinical feature of CTE)[42]. Again, the presence or severity of CTE pathology in this sample is unknown and there is likely substantial variability across participants in pathology. P-tau181 burden and/or neuronal injury may be below clinical threshold (as evidence by the clinical test performance in the former NFL players) or only a small subset have meaningful pathology[5]. The disease stage of the sample (which is unknown) may have particularly important implications for sTREM2. For example, when disease stage is considered, sTREM2 levels are higher in the early stages of the AD continuum, perhaps even before symptom onset[28,29,33,35]. Interestingly, microglial activation seems to have opposing effects on Aβ and p-tau in AD, as it may facilitate clearance of Aβ[63–65] and promote p-tau pathogenesis[54–56,58].
Limitations
sTREM2 is believed to reflect microglial activation, but the exact biological role(s) of sTREM2 remains unclear[24]. There are multiple TREM2 variants that influence ligand binding and each variant may play a unique role in response to pathology and to the different types of pathologies. Other CSF analytes of neuroinflammation (e.g., CCL11, CD68) not examined in this study could provide additional insight into the dynamic role of neuroinflammation in CTE. In this sample of former NFL players, college level estimates of head impact frequencies were applied for estimation of professional level head impact frequencies because helmet accelerometer data at the professional level have not been published or made public. These methods likely underestimated the extent of RHI in the former NFL players and its relationship with the CSF analytes.
Controls were required to be asymptomatic and without a history of head trauma at the time of telephone screening, thereby limiting the external validity of analyses examining group differences (namely in regards to clinical test performance). The objective of the participant selection method was to obtain a control sample at very low risk for CTE to compare to the former NFL players presumably at high risk for CTE, to facilitate biomarker development. Despite these efforts, the controls had comparable—and even non-significantly higher absolute values of—CSF analyte concentrations compared to former NFL players. The control group was small and demographically homogenous (all but one of the controls were Caucasian). The race disparity between the groups is noteworthy given that in the former NFL players, Caucasian race and older age were associated with higher sTREM2; race further correlated with the p-tau/t-tau ratio and, in the full model, older age correlated with higher CSF t-tau. Because African American race is associated with increased risk for AD, it would have been expected that African Americans have higher sTREM2. There appears to be different TREM2 coding variants that confer risk for AD in African Americans versus Caucasians[66]. In general, race is becoming increasingly recognized as a potential modifier of clinical outcomes related to concussion[67] and exposure to RHI[41]. Future research that recruits larger and demographically-and clinically-diverse (e.g., symptomatic and asymptomatic) controls, in addition to other contact and non-contact sport athletes, is needed to elucidate CSF tau and sTREM2 concentrations in former NFL players.
It is premature to state that the current findings do or do not provide support for any of the CSF analytes examined as biomarkers for CTE. The development of meaningful fluid biomarkers requires several critical steps[68]. Foremost, this study was without neuropathological examination and the presence of a competing neurodegenerative disease, such as AD, is possible. Biomarker development and validation for CTE will occur through studies that prospectively collect biomarker data (e.g., CSF tau, sTREM2) in living participants who agree to brain donation and then examine the association with post-mortem CTE neuropathology. Such prospective data is unlikely to be available in the near future. In the meantime, PET tau and amyloid imaging may clarify the biomarker utility of CSF t-tau, p-tau, Aβ, and sTREM2 in the setting of CTE. Studies examining biomarker development and validation in CTE using PET tau and amyloid imaging are currently underway.
CONCLUSIONS
In this sample of former NFL players, estimated cumulative exposure to RHI and microglial activation (based on CSF sTREM2) were associated with later-life CSF t-tau concentrations. Microglial activation may be involved in the pathogenesis of long-term neurological consequences associated with exposure to RHI, like CTE. Prospective clinicopathological research, in addition to studies that use PET tau and amyloid imaging are needed to elucidate the clinical and diagnostic implications of CSF t-tau, p-tau181, and sTREM2 in the setting of CTE.
HIGHLIGHTS.
Repetitive head impacts (RHI) were positively associated with CSF total tau in former NFL players
sTREM2 was positively associated with CSF total tau in former NFL players
sTREM2 strengthened the relationship between exposure to RHI and CSF total tau
Acknowledgments
This work was supported by grants from the NIH (P30 AG13846; R01 NS078337; R56 9500304025; U01 NS093334, U01NS086659-01; 1F32NS096803-01; K23AG046377; RF1AG05416; RF1AG054156-01; P30AG10124) and Department of Veterans Affairs (I01-CX001038). This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through BU-CTSI Grant Number 1UL1TR001430. KB holds the Torsten Söderberg professorship at the Royal Swedish Academy of Sciences. the sTREM2 measurements were supported by the Leonard Wolfson Experimental Neurology Centre at UCL. The content is solely the responsibility of the authors. There is no sponsor.
Abbreviations
- CSF
cerebrospinal fluid
- CTE
chronic traumatic encephalopathy
- AD
Alzheimer’s disease
- RHI
repetitive head impacts
- t-tau
total tau
- p-tau
phosphorylated tau
- Aβ
beta-amyloid
- sTREM2
soluble triggering receptor expressed on myeloid cells 2
Footnotes
Potential Conflicts of Interests: RAS is a paid consultant to Eli Lilly (Indianapolis, IN, USA), Avanir Pharmaceuticals (Aliso Viejo, CA), and Biogen (Cambridge, MA, USA). He is a member of the Board of Directors of King-Devick Technologies, Inc. (Chicago, IL, USA), and he receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc. (Lutz, FL, USA). KB has served as a consultant or at advisory boards for Alzheon, BioArctic, Biogen, Eli Lilly, Fujirebio Europe, IBL International, Merck, Novartis, Pfizer, and Roche Diagnostics. HZ has served at advisory boards of Eli Lilly and Roche Diagnostics and has received travel support from Teva. For the remaining authors, there are no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bieniek KF, Ross OA, Cormier KA, et al. Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015;130:877–89. doi: 10.1007/s00401-015-1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern RA, Daneshvar DH, Baugh CM, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81:1122–9. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee AC, Stern RA, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA. 2017;318:360–70. doi: 10.1001/jama.2017.8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alosco ML, Tripodis Y, Jarnagin J, et al. Repetitive head impact exposure and later-life plasma total tau in former National Football League players. Alzheimers Dement (Amst) 2017;7:33–40. doi: 10.1016/j.dadm.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern RA, Tripodis Y, Baugh CM, et al. Preliminary Study of Plasma Exosomal Tau as a Potential Biomarker for Chronic Traumatic Encephalopathy. J Alzheimers Dis. 2016;51:1099–109. doi: 10.3233/JAD-151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–44. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 8.Zetterberg H, Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol. 2016;12:563–74. doi: 10.1038/nrneurol.2016.127. [DOI] [PubMed] [Google Scholar]
- 9.McKee AC, Cairns NJ, Dickson DW, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131:75–86. doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein TD, Montenigro PH, Alvarez VE, et al. Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 2015;130:21–34. doi: 10.1007/s00401-015-1435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack CR, Jr, Bennett DA, Blennow K, et al. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–47. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang JH, Korecka M, Figurski MJ, et al. The Alzheimer’s Disease Neuroimaging Initiative 2 Biomarker Core: A review of progress and plans. Alzheimers Dement. 2015;11:772–91. doi: 10.1016/j.jalz.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsson B, Lautner R, Andreasson U, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–84. doi: 10.1016/S1474-4422(16)00070-3. [DOI] [PubMed] [Google Scholar]
- 14.Zetterberg H, Blennow K. Fluid markers of traumatic brain injury. Mol Cell Neurosci. 2015;66:99–102. doi: 10.1016/j.mcn.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol. 2013;9:201–10. doi: 10.1038/nrneurol.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hesse C, Rosengren L, Andreasen N, et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297:187–90. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- 17.Blaylock RL, Maroon J. Immunoexcitotoxicity as a central mechanism in chronic traumatic encephalopathy-A unifying hypothesis. Surg Neurol Int. 2011;2:107. doi: 10.4103/2152-7806.83391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherry JD, Tripodis Y, Alvarez VE, et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun. 2016;4:112. doi: 10.1186/s40478-016-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherry JD, Stein TD, Tripodis Y, et al. CCL11 is increased in the CNS in chronic traumatic encephalopathy but not in Alzheimer’s disease. PLoS One. 2017;12:e0185541. doi: 10.1371/journal.pone.0185541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coughlin JM, Wang Y, Munro CA, et al. Neuroinflammation and brain atrophy in former NFL players: An in vivo multimodal imaging pilot study. Neurobiol Dis. 2015;74:58–65. doi: 10.1016/j.nbd.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coughlin JM, Wang Y, Minn I, et al. Imaging of Glial Cell Activation and White Matter Integrity in Brains of Active and Recently Retired National Football League Players. JAMA Neurol. 2017;74:67–74. doi: 10.1001/jamaneurol.2016.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccio L, Buonsanti C, Cella M, et al. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain. 2008;131:3081–91. doi: 10.1093/brain/awn217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ulland TK, Song WM, Huang SC, et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell. 2017;170:649–63. e13. doi: 10.1016/j.cell.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jay TR, von Saucken VE, Landreth GE. TREM2 in Neurodegenerative Diseases. Mol Neurodegener. 2017;12:56. doi: 10.1186/s13024-017-0197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerreiro R, Wojtas A, Bras J, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117–27. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonsson T, Stefansson H, Steinberg S, et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368:107–16. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heslegrave A, Heywood W, Paterson R, et al. Increased cerebrospinal fluid soluble TREM2 concentration in Alzheimer’s disease. Mol Neurodegener. 2016;11:3. doi: 10.1186/s13024-016-0071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gispert JD, Suarez-Calvet M, Monte GC, et al. Cerebrospinal fluid sTREM2 levels are associated with gray matter volume increases and reduced diffusivity in early Alzheimer’s disease. Alzheimers Dement. 2016;12:1259–72. doi: 10.1016/j.jalz.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Suarez-Calvet M, Kleinberger G, Araque Caballero MA, et al. sTREM2 cerebrospinal fluid levels are a potential biomarker for microglia activity in early-stage Alzheimer’s disease and associate with neuronal injury markers. EMBO Mol Med. 2016;8:466–76. doi: 10.15252/emmm.201506123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piccio LCC, Bollman B, Cignarella F, Mikesell R. TREM2 regulates microglia activation in response to CNS demyelination. Mult Scler J. 2016;22:54. [Google Scholar]
- 31.Öhrfelt AAM, Malmeström C, Novakova L, Heslegrave A, Blennow K, Lycke J, Zetterberg H. Soluble TREM-2 in cerebrospinal fluid from patients with multiple sclerosis treated with natalizumab or mitoxantrone. Mult Scler. 2016;22:1587–95. doi: 10.1177/1352458515624558. [DOI] [PubMed] [Google Scholar]
- 32.Piccio L, Deming Y, Del-Aguila JL, et al. Cerebrospinal fluid soluble TREM2 is higher in Alzheimer disease and associated with mutation status. Acta Neuropathol. 2016;131:925–33. doi: 10.1007/s00401-016-1533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinberger G, Yamanishi Y, Suarez-Calvet M, et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci Transl Med. 2014;6:243ra86. doi: 10.1126/scitranslmed.3009093. [DOI] [PubMed] [Google Scholar]
- 34.Henjum K, Almdahl IS, Arskog V, et al. Cerebrospinal fluid soluble TREM2 in aging and Alzheimer’s disease. Alzheimers Res Ther. 2016;8:17. doi: 10.1186/s13195-016-0182-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suarez-Calvet M, Araque Caballero MA, Kleinberger G, et al. Early changes in CSF sTREM2 in dominantly inherited Alzheimer’s disease occur after amyloid deposition and neuronal injury. Sci Transl Med. 2016;8:369ra178. doi: 10.1126/scitranslmed.aag1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montenigro PH, Alosco ML, Martin BM, et al. Cumulative Head Impact Exposure Predicts Later-Life Depression, Apathy, Executive Dysfunction, and Cognitive Impairment in Former High School and College Football Players. J Neurotrauma. 2017;34:328–40. doi: 10.1089/neu.2016.4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alosco ML, Jarnagin J, Tripodis Y, et al. Olfactory Function and Associated Clinical Correlates in Former National Football League Players. J Neurotrauma. 2017;34:772–80. doi: 10.1089/neu.2016.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–13. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grossman M, Elman L, McCluskey L, et al. Phosphorylated tau as a candidate biomarker for amyotrophic lateral sclerosis. JAMA Neurol. 2014;71:442–8. doi: 10.1001/jamaneurol.2013.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deters KD, Risacher SL, Kim S, et al. Plasma Tau Association with Brain Atrophy in Mild Cognitive Impairment and Alzheimer’s Disease. J Alzheimers Dis. 2017;58:1245–54. doi: 10.3233/JAD-161114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alosco ML, Koerte IK, Tripodis Y, et al. White matter signal abnormalities in former National Football League players. Alzheimers Dement (Amst) 2018;10:56–65. doi: 10.1016/j.dadm.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montenigro PH, Baugh CM, Daneshvar DH, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. 2014;6:68. doi: 10.1186/s13195-014-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hausman JA. Specification and estimation of simultaneous equation models. Handb Econ. 1983;1:391–448. [Google Scholar]
- 44.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(Suppl 4):S24–33. doi: 10.1227/NEU.0000000000000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neselius S, Brisby H, Theodorsson A, Blennow K, Zetterberg H, Marcusson J. CSF-biomarkers in Olympic boxing: diagnosis and effects of repetitive head trauma. PLoS One. 2012;7:e33606. doi: 10.1371/journal.pone.0033606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zetterberg H, Hietala MA, Jonsson M, et al. Neurochemical aftermath of amateur boxing. Arch Neurol. 2006;63:1277–80. doi: 10.1001/archneur.63.9.1277. [DOI] [PubMed] [Google Scholar]
- 47.Rosso SM, van Herpen E, Pijnenburg YA, et al. Total tau and phosphorylated tau 181 levels in the cerebrospinal fluid of patients with frontotemporal dementia due to P301L and G272V tau mutations. Arch Neurol. 2003;60:1209–13. doi: 10.1001/archneur.60.9.1209. [DOI] [PubMed] [Google Scholar]
- 48.Hu WT, Watts K, Grossman M, et al. Reduced CSF p-Tau181 to Tau ratio is a biomarker for FTLD-TDP. Neurology. 2013;81:1945–52. doi: 10.1212/01.wnl.0000436625.63650.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spiegel J, Pirraglia E, Osorio RS, et al. Greater specificity for cerebrospinal fluid P-tau231 over P-tau181 in the differentiation of healthy controls from Alzheimer’s disease. J Alzheimers Dis. 2016;49:93–100. doi: 10.3233/JAD-150167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hampel H, Buerger K, Zinkowski R, et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- 51.Tagge CA, Fisher AM, Minaeva OV, Gaudreau-Balderrama A, Moncaster JA, Zhang XL, Wojnarowicz MW, Casey N, Lu H, Kokiko-Cochran ON, Saman S, Ericsson M, Onos KD, Veksler R, Senatorov VV, Kondo A, Zhou XZ, Miry O, Vose LR, Gopaul KR, Upreti C, Nowinski CJ, Cantu RC, Alvarez VE, Knorad J, Hamilton JA, Hua N, Tripodis Y, Anderson AT, Howell GR, Kaufer D, Hall GF, Lu KP, Ransohoff RM, Cleveland RO, Kowall NW, Huber BR, Stein TD, Lamb BT, Moss WC, Friedman A, Stanton PK, McKee AC, Goldstein LE. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain. 2018 doi: 10.1093/brain/awx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kondo A, Shahpasand K, Mannix R, et al. Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature. 2015;523:431–6. doi: 10.1038/nature14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petraglia AL, Plog BA, Dayawansa S, et al. The pathophysiology underlying repetitive mild traumatic brain injury in a novel mouse model of chronic traumatic encephalopathy. Surg Neurol Int. 2014;5:184. doi: 10.4103/2152-7806.147566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee DC, Rizer J, Selenica ML, et al. LPS- induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. J Neuroinflammation. 2010;7:56. doi: 10.1186/1742-2094-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:8843–53. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605–11. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 58.Metcalfe MJ, Figueiredo-Pereira ME. Relationship between tau pathology and neuroinflammation in Alzheimer’s disease. Mt Sinai J Med. 2010;77:50–8. doi: 10.1002/msj.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakagawa Y, Nakamura M, McIntosh TK, et al. Traumatic brain injury in young, amyloid-beta peptide overexpressing transgenic mice induces marked ipsilateral hippocampal atrophy and diminished Abeta deposition during aging. J Comp Neurol. 1999;411:390–8. [PubMed] [Google Scholar]
- 60.Nakagawa Y, Reed L, Nakamura M, et al. Brain trauma in aged transgenic mice induces regression of established abeta deposits. Exp Neurol. 2000;163:244–52. doi: 10.1006/exnr.2000.7375. [DOI] [PubMed] [Google Scholar]
- 61.Smith DH, Nakamura M, McIntosh TK, et al. Brain trauma induces massive hippocampal neuron death linked to a surge in beta-amyloid levels in mice overexpressing mutant amyloid precursor protein. Am J Pathol. 1998;153:1005–10. doi: 10.1016/s0002-9440(10)65643-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uryu K, Laurer H, McIntosh T, et al. Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci. 2002;22:446–54. doi: 10.1523/JNEUROSCI.22-02-00446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee CYD, Daggett A, Gu X, et al. Elevated TREM2 Gene Dosage Reprograms Microglia Responsivity and Ameliorates Pathological Phenotypes in Alzheimer’s Disease Models. Neuron. 2018;97:1032–48. e5. doi: 10.1016/j.neuron.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Udeochu J, Sayed FA, Gan L. TREM2 and Amyloid Beta: A Love-Hate Relationship. Neuron. 2018;97:991–3. doi: 10.1016/j.neuron.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y, Wu X, Li X, et al. TREM2 Is a Receptor for beta-Amyloid that Mediates Microglial Function. Neuron. 2018;97:1023–31. e7. doi: 10.1016/j.neuron.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin SC, Carrasquillo MM, Benitez BA, et al. TREM2 is associated with increased risk for Alzheimer’s disease in African Americans. Mol Neurodegener. 2015;10:19. doi: 10.1186/s13024-015-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Houck Z, Asken B, Clugston J, Perlstein W, Bauer R. Socioeconomic Status and Race Outperform Concussion History and Sport Participation in Predicting Collegiate Athlete Baseline Neurocognitive Scores. J Int Neuropsychol Soc. 2018;24:1–10. doi: 10.1017/S1355617717000716. [DOI] [PubMed] [Google Scholar]
- 68.Teunissen CE, Otto M, Engelborghs S, et al. White paper by the Society for CSF Analysis and Clinical Neurochemistry: Overcoming barriers in biomarker development and clinical translation. Alzheimers Res Ther. 2018;10:30. doi: 10.1186/s13195-018-0359-x. [DOI] [PMC free article] [PubMed] [Google Scholar]