Abstract
BACKGROUND:
Patients are at increased risk of cardiovascular complications while recovering from sepsis. We aimed to study the temporal change and susceptible periods for cardiovascular complications in patients recovering from sepsis by using a national database.
METHODS:
In this retrospective population-based cohort study, patients with sepsis were identified from the National Health Insurance Research Database in Taiwan. We estimated the risk of myocardial infarction (MI) and stroke following sepsis by comparing a sepsis cohort to a matched population and hospital control cohort. The primary outcome was first occurrence of MI or stroke requiring admission to hospital during the 180-day period following discharge from hospital after sepsis. To delineate the risk profile over time, we plotted the weekly risk of MI and stroke against time using the Cox proportional hazards model. We determined the susceptible period by fitting the 2 phases of time-dependent risk curves with free-knot splines, which highlights the turning point of the risk of MI and stroke after discharge from the hospital.
RESULTS:
We included 42 316 patients with sepsis; stroke developed in 831 of these patients and MI developed in 184 within 180 days of discharge from hospital. Compared with population controls, patients recovering from sepsis had the highest risk for MI or stroke in the first week after discharge (hazard ratio [HR] 4.78, 95% confidence interval [CI] 3.19 to 7.17; risk difference 0.0028, 95% CI 0.0021 to 0.0034), with the risk decreasing rapidly until the 28th day (HR 2.38, 95% CI 1.94 to 2.92; risk difference 0.0045, 95% CI 0.0035 to 0.0056) when the risk stabilized. In a repeated analysis comparing the sepsis cohort with the nonsepsis hospital control cohort, we found an attenuated but still marked elevated risk before day 36 after discharge (HR 1.32, 95% CI 1.15 to 1.52; risk difference 0.0026, 95% CI 0.0013 to 0.0039). The risk of MI or stroke was found to interact with age, with younger patients being associated with a higher risk than older patients (interaction p = 0.0004).
INTERPRETATION:
Compared with the general population with similar characteristics, patients recovering from sepsis had a markedly elevated risk of MI or stroke in the first 4 weeks after discharge from hospital. More close monitoring and pharmacologic prevention may be required for these patients at the specified time.
Sepsis, life-threatening organ dysfunction caused by pathogen-induced systemic inflammation, is a leading cause of death and morbidity worldwide. From 2009 to 2011, sepsis contributed to more than half of all deaths from infectious diseases in Canada.1 In 2011, sepsis was the 12th leading cause of death in Canada and responsible for 1 in 18 deaths.1 In addition to the number of patient deaths attributable to sepsis, recent evidence has shown that there is an increased risk of cardiovascular complications in patients who are recovering from sepsis.2–4 Various mechanisms have been proposed to account for the increased risk of cardiovascular complications after sepsis, including endothelial dysfunction, demand ischemia, disseminated intravascular coagulation, myocardial depression and platelet activation.3,5–9
However, there is a paucity of research on risk of cardiovascular complications during the recovery period for those with a diagnosis of sepsis. We aimed to quantify the temporal change of risks of cardiovascular complications after sepsis by comparing a cohort of patients in hospital who were diagnosed with sepsis with matched community and hospital control cohorts, that included patients without a diagnosis of sepsis. Our second goal was to determine a susceptible period for myocardial infarction (MI) and stroke after sepsis. Third, we aimed to assess the risk of post-sepsis MI and stroke after sepsis in several predefined subpopulations.
Methods
Data source
Taiwan’s national health insurance program is a single-payer mandatory health insurance system that covers more than 98% of the 23 million people residing in Taiwan. Different data subsets of the National Health Insurance Research Database were constructed for research purposes, and research articles on sepsis epidemiology have been published using this database.10,11 To increase the sample size for our analysis, we used the year 2000 version of the Longitudinal Health Insurance Database for this analysis that included a nationally representative sample of 1 million patients. All claims can be linked in chronological order to provide a temporal sequence of all utilizations of health services.
Study design
We conducted a retrospective population-based cohort study comprising all patients who were admitted to a hospital in Taiwan with a diagnosis of sepsis and captured in the Longitudinal Health Insurance Database between Jan. 1, 2000, and Dec. 31, 2011. A timeline for our study can be found in Supplementary Figure 1 of Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.171284/-/DC1). We followed patients with a diagnosis of sepsis who were subsequently discharged from hospital for 4 outcomes: MI or stroke, termination of health insurance coverage, death or the end of the follow-up period. The follow-up period was determined a priori to be 180 days, based on previous reports that the suspected risk of cardiovascular complications is minimal after 180 days.4,12,13
Primary cohort
We included patients with a diagnosis of sepsis who were identified via a validated method of administrative database extraction by searching for signs and symptoms consistent with the latest Sepsis-3 definition.14 Operationally, we defined sepsis as having codes from the clinical modification of the International Classification of Diseases, 9th revision (ICD-9-CM) for both an infectious process plus at least 1 diagnosis of acute organ dysfunction in the database record. ICD-9-CM codes that were used to identify patients with infection are listed in Supplementary List 1 (Appendix 1); those used to identify acute dysfunction are listed in Supplementary List 2 (Appendix 1).
Control cohorts
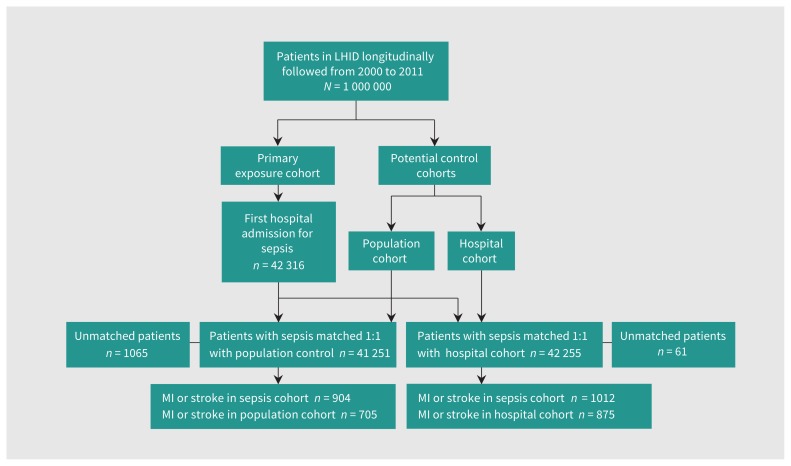
Population control and hospital control cohorts were selected from among patients without a diagnosis of sepsis in the same database, as outlined in Figure 1. We used a 2-stage matching process to identify matched controls.
Figure 1:
Flow diagram of the study population. Note: LHID = Longitudinal Health Insurance Database, MI = myocardial infarction.
First, for each patient with sepsis, we randomly selected 100 controls using the incidence density sampling method and matched them by 5-year age group, sex and quartile Charlson Comorbidity Score (0, 1–2, 3–4 and ≥ 5).15 Next, we calculated the propensity score for admission to hospital with sepsis in the cohort matched for age, gender and comorbidity. We defined propensity score as the probability of admission to hospital for sepsis conditional on the baseline covariates derived by the logistic regression model (Table 1). In the second matching stage, we performed a 1:1 propensity score matching using the greedy algorithm (greedy 5-to-1 digit matching without replacement) to identify a matched pair with a similar propensity score. The standardized differences before and after propensity score matching are in Supplementary Table 1 (Appendix 1).
Table 1:
Baseline characteristics of the matched cohorts
| Characteristic | No. (%)* of participants n = 41 251 | Standardized mean difference | No. (%)* of participants n = 42 255 | Standardized mean difference | ||
|---|---|---|---|---|---|---|
| With sepsis | Matched population control cohort | With sepsis | Matched hospital control cohort | |||
| Male sex | 24 436 (59.2) | 24 436 (59.2) | 0 | 24 976 (59.1) | 24 976 (59.1) | 0 |
| Age, mean ± SD; yr | 67.73 ± 17.59 | 67.95 ± 17.73 | −0.01 | 67.28 ± 17.83 | 66.44 ± 18.43 | 0.05 |
| Preadmission comorbidity | ||||||
| Myocardial infarction | 2437 (5.9) | 2446 (5.9) | −0.0009 | 2360 (5.6) | 1923 (4.5) | 0.04 |
| Congestive heart failure | 10 268 (24.9) | 10 453 (25.3) | −0.01 | 10 204 (24.1) | 7613 (18.0) | 0.15 |
| Peripheral vascular disease | 4718 (11.4) | 5046 (12.2) | −0.02 | 4722 (11.18) | 3650 (8.6) | 0.08 |
| Cerebrovascular disease | 15 631 (37.9) | 15 058 (36.5) | 0.03 | 15 489 (36.7) | 12 294 (29.1) | 0.16 |
| Dementia | 5460 (13.2) | 5112 (12.4) | 0.03 | 5468 (12.9) | 3400 (8.05) | 0.16 |
| Chronic obstructive pulmonary disease | 22 027 (53.4) | 22 460 (54.4) | −0.02 | 22 248 (52.7) | 20 900 (49.5) | 0.06 |
| Rheumatologic disease | 1721 (4.2) | 2048 (5.06) | −0.04 | 1748 (4.1) | 1455 (3.4) | 0.03 |
| Peptic ulcer disease | 20 562 (49.8) | 21 538 (52.2) | −0.05 | 20 646 (48.9) | 20 281 (48.0) | 0.01 |
| Mild liver disease | 16 160 (39.2) | 16 877 (40.9) | −0.04 | 16 191 (38.3) | 15 107 (35.8) | 0.05 |
| Moderate or severe liver disease | 15 495 (37.6) | 15 210 (36.9) | 0.01 | 15 523 (36.7) | 12 797 (30.3) | 0.13 |
| Diabetes without chronic complications | 6947 (16.8) | 6914 (16.8) | 0.002 | 6947 (16.4) | 4673 (11.1) | 0.15 |
| Diabetes with chronic complications | 4191 (10.2) | 4384 (10.6) | −0.02 | 4180 (9.9) | 2796 (6.6) | 0.11 |
| Hemiplegia or paraplegia | 7960 (19.3) | 8176 (19.8) | −0.01 | 7908 (18.7) | 5537 (13.1) | 0.15 |
| Chronic renal disease | 8469 (20.5) | 8060 (19.5) | 0.02 | 8281 (19.6) | 7611 (18.0) | 0.04 |
| Leukemia and lymphoma | 1449 (3.5) | 1032 (2.5) | 0.06 | 1382 (3.3) | 1198 (2.8) | 0.02 |
| Metastatic solid tumour | 2041 (4.9) | 2061 (5.0) | −0.002 | 1900 (4.5) | 1989 (4.7) | −0.01 |
| AIDS or HIV | 63 (0.1) | 63 (0.1) | 0 | 61 (0.1) | 54 (0.1) | 0.004 |
Note: SD = standard deviation.
Unless specified otherwise.
We used the same 2-stage strategy to create the hospital control cohort; the standardized differences before and after matching can be found in Supplementary Table 2 (Appendix 1). The only difference between population and hospital controls in construction of the cohorts is the sampling population. For the population control cohort, all patients selected at the first stage were eligible for sampling during the second stage; however, only patients who were admitted to hospital on the index day for each patient with sepsis were eligible for sampling for the hospital controls.
Primary outcome
The primary outcome was first occurrence of MI or stroke that required admission to hospital during the 180-day period following discharge from hospital for the index admission. We used previously validated definitions to identify outcomes. Incident MI was identified by any primary or secondary admission diagnosis containing an ICD-9-CM code of 410.xx together with a prescription for antiplatelet therapy. This search algorithm was validated in the National Health Insurance Research Database with a positive predictive value of 0.84 for MI.16 Incident stroke was identified if ICD-9-CM diagnosis codes 433.xx or 434.xx were entered. A previous validation study found that ICD-9-CM codes 433 and 434 had a positive predictive value of 0.96 and 1.00, respectively, in the database.17
Statistical analysis
Baseline characteristics for participants are presented as frequencies or percentages for categorical variables, and means with standard deviations for continuous variables. We compared categorical variables using the χ2 test for dichotomous variables and continuous variables using the t test. We assessed the association between sepsis and MI and stroke using the multivariate Cox proportional hazards model adjusted for additional confounders not included in the Charlson index (i.e., hypertension, hypercholesterolemia, atrial fibrillation, alcohol abuse, smoking and chronic obstructive pulmonary disease).18 To investigate the effect on public health, we also calculated the risk difference with 95% confidence intervals (CIs) and number needed to harm (NNH) using multiple linear regression analysis.
To explore the susceptible period, we calculated the weekly hazard ratios (HRs) and plotted these against the time after discharge. We determined the turning point of the risk of MI and stroke after discharge from hospital by using the freeknotsplines statistical package in R (https://cran.r-project.org/web/packages/freeknotsplines/), and the full method description can be found in Appendix 1.19 Briefly, we were interested to find out a date where the risk of MI and stroke changed after discharge from hospital, and a single knot was specified. Therefore, in this study, the knot of the spline is the point or the date where the 2 phases of risk connect and can be interpreted as the turning point of the risk of MI and stroke after discharge from the hospital.
To investigate whether patients with different characteristics have a differential risk of developing MI and stroke after sepsis, we stratified the propensity score–matched cohort by age, gender, type of infection, number of organ dysfunctions and medical comorbidities. Patients with sepsis may have high mortality rates in the early period after discharge, which may compete with the observation of MI and stroke after sepsis. We corrected for the competing risk between death and MI and stroke by using inverse probability of treatment weight. For each patient, we assigned a censoring weight based on the inverse of the predicted probability of death.20 We determined the predicted probability of death using logistic regression conditioned on demographics, comorbidities and acute organ dysfunctions. Overall, the inverse probability of treatment weight adjusted for the imbalance in the timing of death between the sepsis cohort and the propensity score–matched control cohort, so that patients with sepsis who died earlier were given a higher weight.21
We performed the statistical analysis with SAS 9.4 (SAS Inc.), except for the fitting of the spline function, which was analyzed using R3.1 statistical software (R Foundation for Statistical Computing). We defined a 2-sided p value of 0.05 as significant for all analyses.
Ethics approval
This study was approved by the Institutional Review Board of National Taiwan University Hospital, Taipei, Taiwan. Patient consent was waived for this study because we used an electronic database in which data were anonymized.
Results
Cohort assembly and characteristics
From among 1 million patients in the Longitudinal Health Insurance Database (between Jan. 1, 2000, and Dec. 31, 2011) we identified 42 316 patients with sepsis to be included in the primary cohort (Figure 1). All had at least 1 organ dysfunction, 34.6% (n = 14 641) were admitted to the intensive care unit and 22.4% (n = 9469) died within 30 days of admission (Supplementary Table 3, Appendix 1). The 3 most common types of infection were lower respiratory tract (n = 21 739, 51.4%), urinary tract (n = 13 100, 31.0%) and intra-abdominal (n = 2626, 6.2%). The 3 most common types of organ failure were acute respiratory (n = 27 922, 66.0%), cardiovascular or shock (n = 12 288, 29.0%) and acute renal (n = 7129, 16.9%). They were matched to 41 251 participants in the population cohort and 42 255 in the hospital cohort.
Cardiovascular events developed in 1012 participants (84.5 events per 1000 person-years) in the 180-day period after discharge from hospital; stroke developed in 831 participants (69.4 events per 1000 person-years), MI developed in 184 participants (15.4 events per 1000 person-years), and both stroke and MI developed in 3 participants. The preadmission characteristics between the matched patients with sepsis and the controls are summarized in Table 1. After matching, the standardized differences for all baseline covariates were less than 16%.
Risk of myocardial infarction and stroke after sepsis
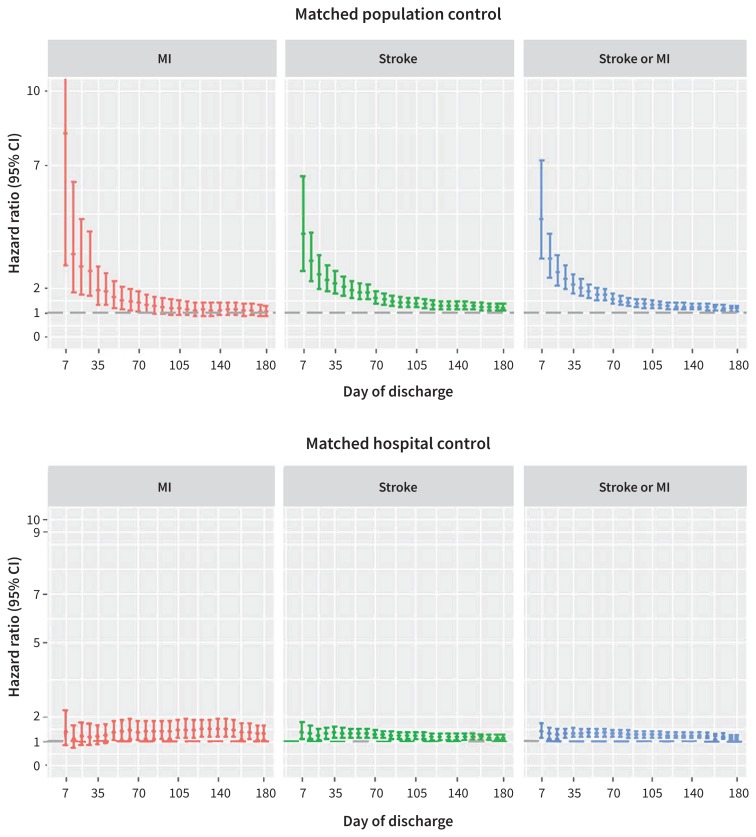
We plotted the time distribution of occurrences of MI and stroke within 180 days after discharge from the hospital in patients with sepsis (Supplementary Figure 1, Appendix 1), with 26.0% (263/1012) of cases of MI and stroke occurring in the first 7 days and 50.6% (512/1012) occurring within 35 days. The weekly HRs for MI and stroke associated with sepsis are shown in Figure 2. Compared with population controls (Figure 2, upper panel), the risk of the composite MI or stroke outcome was highest in the first 7 days (HR 4.78, 95% CI 3.19 to 7.17; risk difference 0.0028, 95% CI 0.0021 to 0.0034) and stabilized 91 days after discharge (HR 1.36, 95% CI 1.20 to 1.54; risk difference 0.0039, 95% CI 0.0024 to 0.0055). The spline-fitting method suggested that day 28 (week 4, HR 2.38, 95% CI 1.94 to 2.92; risk difference 0.0045, 95% CI 0.0035 to 0.0056) was the turning point for the risk of MI and stroke after discharge from the hospital (Supplementary Figure 2 [upper panel], Appendix 1).
Figure 2:
Risk profile over time of myocardial infarction and stroke in participants after discharge from hospital. Comparison of participants with sepsis with participants without sepsis in the population control group (upper panel). Comparison of participants with sepsis with participants without sepsis in the hospital control group (lower panel). Note: CI = confidence interval, MI = myocardial infarction.
Our analysis of the hospital control cohort found an attenuated risk profile that varied over time (Figure 2, lower panel). The risk of composite MI or stroke was highest in the first week after discharge (HR 1.38, 95% CI 1.10 to 1.73; risk difference 0.0012, 95% CI 0.00034577 to 0.00197) (Figure 2, lower panel), and we observed the knot on day 36 (week 5, HR 1.32, 95% CI 1.15 to 1.52; risk difference 0.0026, 95% CI 0.0013 to 0.0039) (Supplementary Figure 2 [lower panel], Appendix 1).
Overall, the patients with sepsis had a significantly higher risk of MI or stroke after discharge from the hospital compared with either population controls (HR 1.15, 95% CI 1.04 to 1.27; risk difference 0.0023, 95% CI 0.00044 to 0.0042) or hospital controls (HR 1.16, 95% CI 1.06 to 1.27; risk difference 0.0032, 95% CI 0.0012 to 0.0052). The NNH was 427 and 309, respectively (Table 2).
Table 2:
Risk of myocardial infarction and stroke in participants with sepsis compared with those without sepsis in the general population or in hospital, 180 days after discharge from hospital
| Condition | Matched with population control cohort | Matched with hospital control cohort | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR (95% CI) | Risk difference (95% CI) | NNH | HR (95% CI) | Risk difference (95% CI) | NNH | |
| MI | 1.03 (0.83 to 1.27) | 0.00015 (−0.00073 to 0.0010) | 6666 | 1.31 (1.05 to 1.64) | 0.0010 (0.00018 to 0.0019) | 980 |
|
| ||||||
| Stroke | 1.19 (1.07 to 1.34) | 0.0022 (0.00054 to 0.0039) | 448 | 1.12 (1.02 to 1.24) | 0.0021 (0.00023 to 0.0038) | 485 |
|
| ||||||
| Composite* | 1.15 (1.04 to 1.27) | 0.0023 (0.00044 to 0.0042) | 427 | 1.16 (1.06 to 1.27) | 0.0032 (0.0012 to 0.0052) | 309 |
Note: CI = confidence interval, HR = hazard ratio, MI = myocardial infarction, NNH = number needed to harm.
MI or stroke.
Subgroup analysis
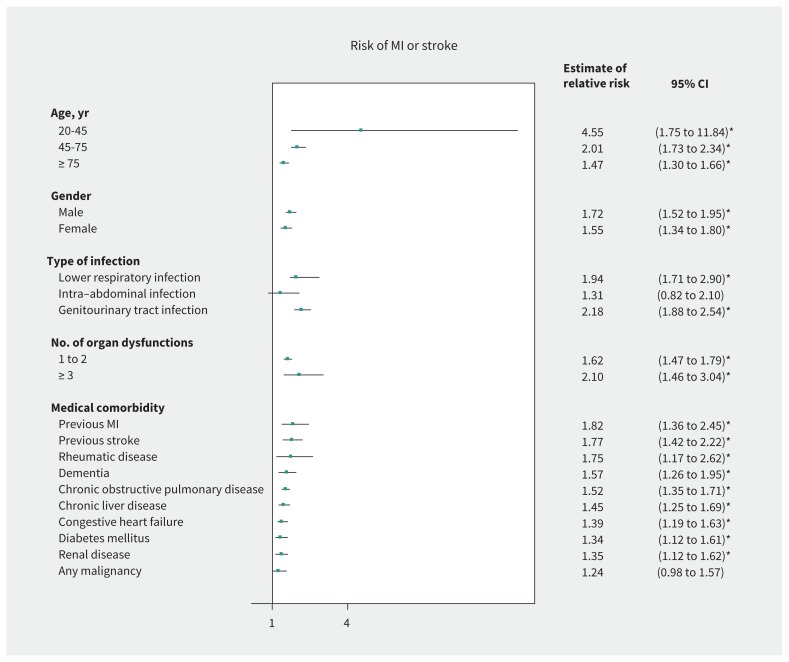
In the stratified analysis using the population control cohort only, the relative increase in risk of MI or stroke was constant across patient subgroups defined by sex and comorbidity burden (Figure 3). However, the relative risk of MI or stroke varied widely between patients in different age categories (interaction p < 0.0004). Compared with patients without a diagnosis of sepsis, younger patients (aged 20–45 yr) with sepsis were associated with higher relative risk (HR 4.55, 95% CI 1.75 to 11.84; risk difference 0.0037, 95% CI 0.0012 to 0.0061) than older patients (aged 75 yr or older; HR 1.47, 95% CI 1.30 to 1.66; risk difference 0.0019, 95% CI −0.0014 to 0.0052). The results on an absolute risk scale for the age categories can be found in Supplementary Table 4 (Appendix 1).
Figure 3:
Risk of MI or stroke in participants recovering from sepsis compared with the population control cohort. Note: CI = confidence interval, MI = myocardial infarction. *Significant result.
Interpretation
In this large nationally representative cohort of 42 316 participants with sepsis, we have shown that those recovering from sepsis had an increased risk of subsequent MI or stroke compared with population controls (HR 1.15, 95% CI 1.04 to 1.27) and hospital controls (HR 1.16, 95% CI 1.06 to 1.27). Day 28 for population controls and day 36 for hospital controls were the turning points for the risk of MI and stroke after discharge from the hospital. In subgroup analysis, we found that the relative risk of MI or stroke after sepsis was constant across patient subgroups defined by sex and comorbidity burden. However, the risk was more prominent in young patients compared with older patients.
Based on 2 long-term cohort studies4,12 and 1 short-term cohort study,13 we hypothesized that 180 days of follow-up was likely to be sufficient for identifying the susceptible period for cardiovascular complications after sepsis. Our result on the critical period (28–36 d after index date of admission) was in line with these previous studies.4,12,13 It has been shown that patients who recovered from sepsis had a 4.48-fold increase in the risk of acute coronary heart diseases within the first year, and the risk attenuated to 1.18-fold after 4 years.12 Similarly, 1 study showed that patients who recovered from sepsis had a 2.33-fold increase in the risk of major adverse cardiovascular events within the first year, and that risk attenuated to 1.22-fold after 5 years.4 Our results are also corroborated by a study that investigated short-term outcomes in patients who recovered from sepsis; the study reported an elevated risk of MI and stroke within 30 days of the onset of sepsis (adjusted relative risk 20.86, 95% CI 15.38 to 28.29), and no differences in cardiovascular risk were seen after 6 months.13 However, in our study, a relatively small difference in the risk of MI and stroke after sepsis was observed for the hospital control group compared with the population control group, which may be explained by the increased risk of MI and stroke in patients in the hospital control group. Patients admitted to hospital may have more conditions associated with MI and stroke, such as systemic infection, incident atrial fibrillation, and stressful events like surgical procedures.
In addition to having an increased risk of MI and stroke, a propensity-matched cohort study suggested that, shortly after discharge, patients who have recovered from sepsis had a higher risk of short-term mortality (< 180 d) than long-term mortality (> 180 d).22 Future studies should observe the potential increased risk of MI or stroke in patients who have recovered from sepsis in the short- and long-term period after discharge.
Previous studies have investigated specific populations that are at an increased risk of postsepsis cardiovascular complications. 4,13 However, these studies lacked generalizability because of the selective hospital-based population and restrictive ICD-9 codes used to identify patients with sepsis. Some previous studies have involved patients with septicemia (ICD-9 CM code 038.9) or bacteremia (positive result for blood cultures) without including organ dysfunction.4,13,23,24 When organ dysfunctions are not included in the criteria for diagnosing sepsis, as many as 70% of patients with sepsis may be missed.25,26 In addition, this approach lacks sensitivity, because the diagnosis of septicemia and bacteremia requires a positive result on microbiological culture, which only occurs in 20% to 40% of patients with sepsis.27,28 We used the combination code strategy to identify patients with sepsis, which has been shown to be the most sensitive among published algorithms and is thought to be close to the Sepsis-3 definition.14,29,30
We found that results of some previous studies were difficult to interpret because they either did not account for competing risk or did not create a propensity score–matched control cohort to account for potential confounding bias. For example, in 1 subgroup analysis study, the authors only accounted for sepsis and did not account for competing risk.4 As a result, they had found paradoxically that the number of organ failures (a proxy for severity of sepsis) was negatively associated with the risk of major adverse cardiac events, which did not agree with clinical observations. 4 They reported that 3 or more organ failures were not associated with an increase in risk of major adverse cardiac events (HR 0.64, 95% CI 0.36 to 1.15), which is in sharp contrast to our findings because we applied the inverse probability of treatment weight to account for the potential competing risk of early death in patients with more severe illness. Nevertheless, a population study in Denmark that accounted for competing risk reported similar trends to our findings for subgroup analysis;13 the increased risk of MI and stroke was found for all subgroups of patients defined by sex and comorbidity burden. Based on our study (Han Chinese) and the study in Denmark13 (European) that reported similar findings for two different ethnic groups, it is likely that these results are generalizable to different populations.
The observed interaction in the relative risk of MI and stroke between patients in different age categories (younger v. older) is consistent with another Taiwanese study, in which the relative risk of MI and stroke in patients with sepsis was more evident among patients in the younger age group than those who were in the older age group.31 Because the baseline incidence of MI and stroke in young people was low, even a small increase in the number of patients with cardiovascular complications after sepsis can translate into a high HR. To provide a complementary view of this comparison, we also examined the effect of sepsis on MI and stroke on an absolute risk scale (Supplementary Table 4, Appendix 1). When risks were presented in terms of NNH, patients aged 20 to 45 years still had a higher absolute risk compared with patients aged 75 years and older (NNH 273 v. 529).
Limitations
Results of this study should be interpreted in light of several limitations. MI and stroke are known risk factors for infection; we cannot completely exclude the possibility of protopathic or reverse causation bias. To limit the risk of protopathic bias, we investigated only incident MI and stroke that developed after the patient was discharged from the index admission to hospital for sepsis. It is unlikely that any latent MI and stoke that occurred before the onset of sepsis would have remained undiagnosed during the index admission to hospital. However, this approach may underestimate the risk of MI and stroke after sepsis that developed during the index hospital admission. Given the observational nature of this study, the efficacy of suggested preventive measures, such as antiplatelet therapy in the critical period, can be confirmed only by a randomized controlled trial (RCT). However, a sufficiently powered RCT would be a challenging task, given the uncommon incidence of MI and stroke after sepsis. As with all administrative databases, detailed in-hospital parameters and laboratory results were not available. Therefore, we could not examine whether classifying patients into different severity scoring systems, such as the Sequential Organ Failure Assessment score, might affect the risk of MI and stroke. However, we conducted a subgroup analysis on the number of organ failures as a proxy to investigate whether severity of sepsis affected the risk of MI and stroke. As with all claims databases, data on lifestyle factors, such as alcohol, smoking and body mass index, were not available. However, we used alcohol, smoking or obesity-related diseases, such as chronic obstructive pulmonary disease and hyperlipidemia, as a proxy for adjustment of lifestyle factors. Finally, this database did not allow us to examine the subtypes of stroke owing to different pathophysiological mechanisms, such as thrombosis, embolism or hypoperfusion. Imaging results would be necessary and were not available in the administrative database.
Conclusion
We delineated the time-varying risk profile of MI and stroke after sepsis in a Taiwanese population. We found that within the first 4 weeks after discharge from hospital was the critical period with a markedly elevated risk of MI and stroke. Further validation of our findings in different populations is needed.
Acknowledgements
The authors thank the staff of the Core Labs in the Department of Medical Research at National Taiwan University Hospital for administrative support, and the Medical Wisdom Consulting Group for technical assistance in statistical analysis.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Contributors Chih-Cheng Lai and Meng-tse Gabriel Lee were joint primary authors. Chien-Chang Lee designed the study, obtained funding, drafted the analytical plan, guided the statistical analysis, interpreted the data and critically revised the manuscript. Chih-Cheng Lai and Meng-tse Gabriel Lee interpreted the results, and wrote the draft manuscript and point-to-point response. Wan-Chien Lee and Tzu-Chun Hsu performed the statistical analysis. Christin Chih-Ting Chao and Si-Huei Lee reviewed the manuscript and provided critical insights on medical content. All of the authors contributed to the design or execution of the study, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was partially supported by Taiwan National Ministry of Science and Technology Grants MOST 104-2314-B-002-039-MY3, MOST 106-2811-B-002-048 and MOST 107-2314-B-002-196; and National Taiwan University Hospital Grants NTUH.106-P04, NTUH.107-P03 and NTUH.107-S3892. No funding bodies had any role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Data sharing: The authors obtained monthly claims data (2001–2011) from the Taiwan National Health Insurance Research Database. This database does not permit external sharing of any data elements.
References
- 1.Navaneelan T, Alam S, Peters P, et al. Deaths involving sepsis in Canada. Ottawa: Statistics Canada; 2016. Cat no 82-624-X201600114308. [Google Scholar]
- 2.Jafarzadeh SR, Thomas BS, Warren DK, et al. Longitudinal study of the effects of bacteremia and sepsis on 5-year risk of cardiovascular events. Clin Infect Dis 2016;63:495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakihana Y, Ito T, Nakahara M, et al. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care 2016;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ou SM, Chu H, Chao PW, et al. Long-term mortality and major adverse cardiovascular events in sepsis survivors: a nationwide population-based study. Am J Respir Crit Care Med 2016;194:209–17. [DOI] [PubMed] [Google Scholar]
- 5.Merx MW, Weber C. Sepsis and the heart. Circulation 2007;116:793–802. [DOI] [PubMed] [Google Scholar]
- 6.Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care 2009;15:392–7. [DOI] [PubMed] [Google Scholar]
- 7.Kilickap M, Goksuluk H, Candemir B, et al. Evaluation of acute infection-induced endothelial dysfunction and its potential mediators. Acta Cardiol 2011; 66:581–7. [DOI] [PubMed] [Google Scholar]
- 8.Vallance P, Collier J, Bhagat K. Infection, inflammation, and infarction: does acute endothelial dysfunction provide a link? Lancet 1997;349:1391–2. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto K, Tamura T, Sawatsubashi Y. Sepsis and disseminated intravascular coagulation. J Intensive Care 2016;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen H-N, Lu C-L, Yang H-H. Epidemiologic trend of severe sepsis in Taiwan from 1997 through 2006. Chest 2010;138:298–304. [DOI] [PubMed] [Google Scholar]
- 11.Lee C-C, Yo C-H, Lee M-tG, et al. Adult sepsis – a nationwide study of trends and outcomes in a population of 23 million people. J Infect 2017;75:409–19. [DOI] [PubMed] [Google Scholar]
- 12.Wang HE, Moore JX, Donnelly JP, et al. Risk of acute coronary heart disease after sepsis hospitalization in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort. Clin Infect Dis 2017;65:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalager-Pedersen M, Sogaard M, Schonheyder HC, et al. Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study. Circulation 2014;129:1387–96. [DOI] [PubMed] [Google Scholar]
- 14.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–10. [DOI] [PubMed] [Google Scholar]
- 15.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- 16.Cheng CL, Lee CH, Chen PS, et al. Validation of acute myocardial infarction cases in the National Health Insurance Research Database in Taiwan. J Epidemiol 2014;24:500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011;20:236–42. [DOI] [PubMed] [Google Scholar]
- 18.Gayat E, Resche-Rigon M, Mary JY, et al. Propensity score applied to survival data analysis through proportional hazards models: a Monte Carlo study. Pharm Stat 2012;11:222–9. [DOI] [PubMed] [Google Scholar]
- 19.Spiriti S, Eubank R, Smith PW, et al. Knot selection for least-squares and penalized splines. J Stat Comput Simul 2013;83:1020–36. [Google Scholar]
- 20.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004;75:45–9. [DOI] [PubMed] [Google Scholar]
- 21.Bolch CA, Chu H, Jarosek S, et al. Inverse probability of treatment-weighted competing risks analysis: an application on long-term risk of urinary adverse events after prostate cancer treatments. BMC Med Res Methodol 2017;17:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prescott HC, Osterholzer JJ, Langa KM, et al. Late mortality after sepsis: propensity matched cohort study. BMJ 2016;353:i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JT, Chung WT, Lin JD, et al. Increased risk of stroke after septicaemia: a population-based longitudinal study in Taiwan. PLoS One 2014;9:e89386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shih CJ, Chao PW, Ou SM, et al. Long-term risk of cardiovascular events in patients with chronic kidney disease who have survived sepsis: a nationwide cohort study. J Am Heart Assoc 2017;6:e004613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilhelms SB, Huss FR, Granath G, et al. Assessment of incidence of severe sepsis in Sweden using different ways of abstracting International Classification of Diseases codes: difficulties with methods and interpretation of results. Crit Care Med 2010;38:1442–9. [DOI] [PubMed] [Google Scholar]
- 26.Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013;41:1167–74. [DOI] [PubMed] [Google Scholar]
- 27.Serody JS, Berrey MM, Albritton K, et al. Utility of obtaining blood cultures in febrile neutropenic patients undergoing bone marrow transplantation. Bone Marrow Transplant 2000;26:533–8. [DOI] [PubMed] [Google Scholar]
- 28.Glerant JC, Hellmuth D, Schmit JL, et al. Utility of blood cultures in community-acquired pneumonia requiring hospitalization: influence of antibiotic treatment before admission. Respir Med 1999;93:208–12. [DOI] [PubMed] [Google Scholar]
- 29.Iwashyna TJ, Angus DC. Declining case fatality rates for severe sepsis: good data bring good news with ambiguous implications. JAMA 2014;311:1295–7. [DOI] [PubMed] [Google Scholar]
- 30.Iwashyna TJ, Odden A, Rohde J, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care 2014;52:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shih CJ, Chao PW, Ou SM, et al. Long-term risk of cardiovascular events in patients with chronic kidney disease who have survived sepsis: a nationwide cohort study. J Am Heart Assoc 2017;6:e004613. [DOI] [PMC free article] [PubMed] [Google Scholar]