Abstract
Pyramidal cells in cortical layers 5 and 6 are the only cells in the cerebral cortex with axons that leave the cortex to influence the thalamus. Layer 6 cells provide modulatory feedback input to all thalamic nuclei. Layer 5 cells provide driving input to higher order thalamic nuclei and do not innervate first order nuclei, which get their driving inputs from subcortical sources. Higher order nuclei innervated by layer 5 cells thus seem to be involved with cortico-thalamo-cortical communication. The layer 5 axons branch to also target additional subcortical structures that mediate interactions with the external environment. These corticofugal pathways represent the only means by which the cortex influences the rest of the neuraxis and thus are essential for proper cortical function and species survival. Here we review current understanding of the corticofugal pathways from layers 5 and 6 and speculate on their functional contributions to neural processing and behavior.
Keywords: thalamus, corticothalamic, corticogeniculate, LGN, pulvinar
Graphical abstract
Pyramidal cells in cortical layers 5 and 6 are the only cells in the cerebral cortex with axons that leave the cortex to influence the thalamus. Layer 6 cells provide modulatory feedback input to all thalamic nuclei, whereas layer 5 cells provide driving input to higher order thalamic nuclei. Here, we review current understanding of the corticofugal pathways and speculate on their functional contributions to neural processing and behavior.
Introduction
The cerebral cortex, with all of its intricate and overwhelming neural circuitry, would be pretty useless except for the fact that it produces projections to subcortical sites allowing it to interact with other parts of the neuraxis and thus affect behavior. These projections emanate from subsets of cells located in layers 5 and 6. Represented here are different corticofugal systems with different functions that are able to operate fairly independently of one another. Furthermore, the vast majority of cells that project subcortically do not appear to project to other cortical areas, and so the corticofugal circuits to at least an extent operate independently of pathways connecting different cortical areas (Petrof, Viaene & Sherman, 2012).
Most layer 6 neurons with axons that leave the cortex project to the thalamus (note: these neurons are distinct from those that project to the claustrum), typically to a thalamic region providing thalamocortical input to the same cortical region from which the layer 6 projection originates (reviewed in Briggs & Usrey, 2008; Sherman & Guillery, 2013). In addition to the extrinsic projection, layer 6 neurons also send local axon collaterals to the layers of cortex that receive thalamic input (Briggs, Kiley, Callaway & Usrey, 2016; Gilbert & Wiesel, 1979; Lund & Boothe, 1975; Usrey & Fitzpatrick, 1996). As a consequence of these connections, layer 6 neurons are in a strategic position to influence feedforward thalamocortical communication and the nature of information the cortical area receives from thalamus. Layer 5 projections are more complex, because these are carried by branching axons that innervate many subcortical sites, often reaching the spinal cord and also often innervating certain thalamic nuclei. These corticofugal projections include numerous targets usually associated with motor control, which are not targets of known projections from layer 6 cells, and thus the layer 5 output represents the only known substrate by which cortex can fairly directly influence behavior. In the sections below, we review the distinction of first order and higher order thalamic nuclei with an emphasis on how they relate to layer 5 and 6 projections. We then examine certain properties of layer 5 and 6 cells and speculate on their functional contributions to neural processing and behavior.
Thalamic Organization: First Order and Higher Order Nuclei
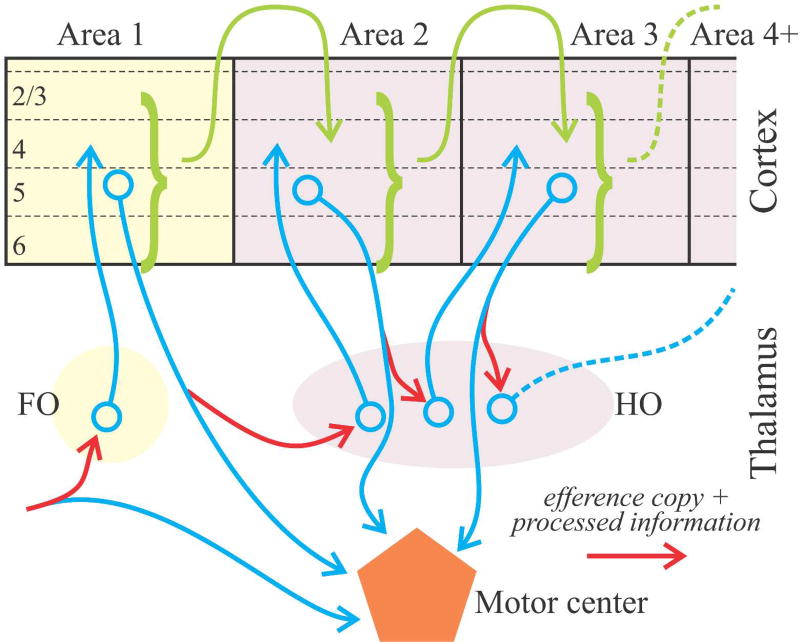
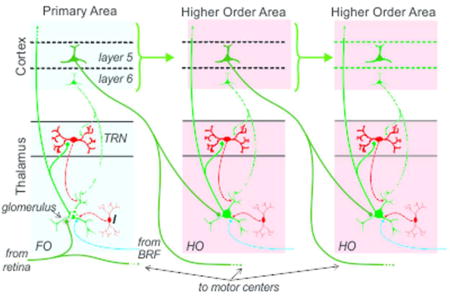
Identifying the driver input to a thalamic nucleus tells us much about the function of that nucleus. Thus the identification of retinal input as the driver of lateral geniculate relay cells tells us that a main function of this thalamic nucleus is the relay of retinal information to cortex. Likewise, we can refer to the ventral posterior nucleus as serving to relay medial lemniscal input to cortex. Using the source of driver input to characterize a key role for a thalamic relay allows us to divide thalamus into first and higher order categories (Figure 1). A first order relay receives its driving input from a subcortical source, such as retinal input to the lateral geniculate nucleus; whereas a higher order relay is driven by input from layer 5 of cortex. This further means that higher order relays serve as central nodes in cortico-thalamo-cortical, or transthalamic, pathways (Figure 1). Note that higher order relays can serve in this capacity not only between primary and secondary cortical areas but also between areas higher in a hierarchy.
Figure 1.
Schematic showing aspects of thalamocortical relationships involving feedforward circuits ascending a hierarchy. Cortical areas are connected both by direct pathways (green arrows) and transthalamic ones involving higher order (HO) thalamic nuclei. Note that the inputs to both first order (FO) and higher order thalamic relays arrive via branching axons (blue and red arrows), with extrathalamic branches innervating a motor center (blue arrows), and the branch innervating thalamus (red arrows) carrying a message that can be interpreted as an efference copy as well as other processed information.
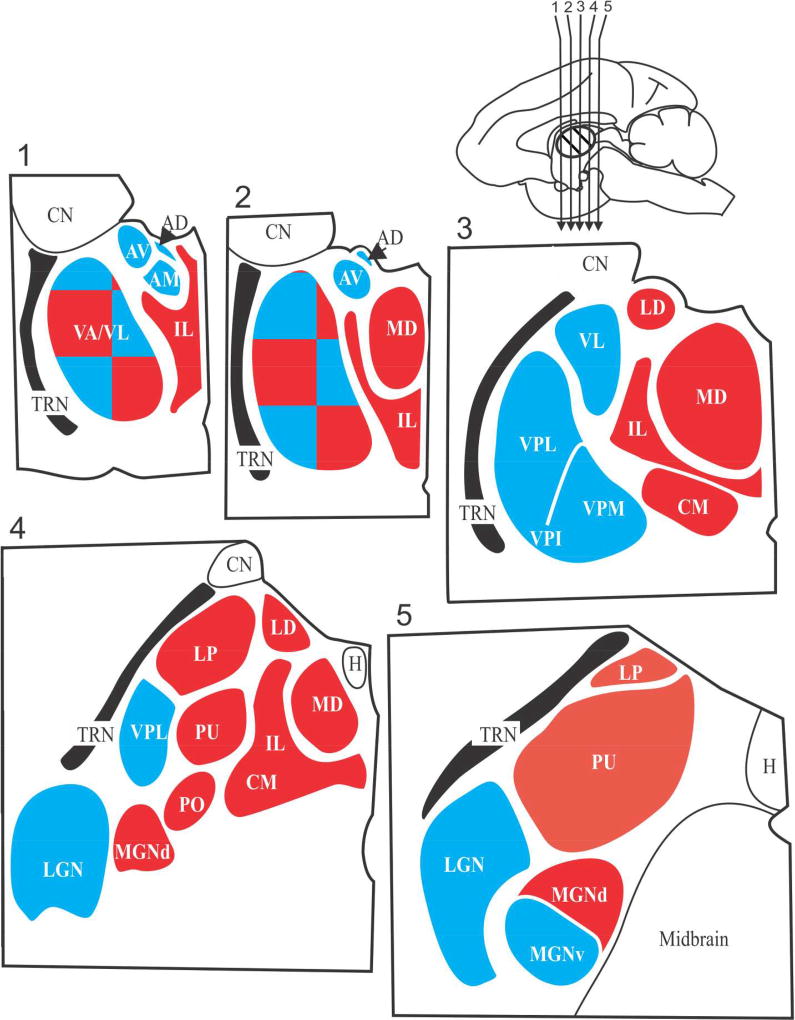
For visual processing, the lateral geniculate nucleus serves as a first order thalamic relay, and the pulvinar, as higher order. For somatosensory processing, the ventral posterior nucleus is first order, the posterior medial nucleus, higher order. For auditory processing, the ventral division of the medial geniculate nucleus is first order, and the dorsal division is higher order. Beyond sensory systems, other thalamic nuclei can also be identified as first or higher order, and this is shown in Figure 2. Most of the thalamus, by volume, is higher order, which means that most of the thalamus, whose function has heretofore been rather mysterious, appears to function as a central participant in transthalamic processing.
Figure 2.
Schematic view of five sections through the thalamus of a monkey. The sections are numbered 1 through 5 and were cut in the coronal planes indicated by the arrows in the upper right mid-sagittal view of the monkey brain. The major thalamic nuclei in one hemisphere for a generalized primate are shown. First order nuclei are shown in blue and higher order nuclei are shown in red; note that VA/VL is a nuclear complex that appears to be a mosaic of first and higher order regions. Abbreviations: AD anterodorsal nucleus; AM anteromedial nucleus, AV anteroventral nucleus; CM, centermedian nucleus; CN caudate nucleus (not a part of the thalamus); H, habenular nucleus; IL, intralaminar (and midline) nuclei; LD, lateral dorsal nucleus; LGN, lateral geniculate nucleus; LP, lateral posterior nucleus; MGN, medial geniculate nucleus; PO, posterior nucleus; PU pulvinar; TRN, thalamic reticular nucleus; VA, ventral anterior nucleus; VL ventral lateral nucleus; VPI, VPL, VPM, are the inferior, the lateral and the medial parts of the ventral posterior nucleus or nuclear group.
Thalamus therefore receives two very different types of cortical input. All thalamic nuclei receive an input from cortical layer 6 that is organized mostly in a feedback pattern and is modulatory in function. In addition some thalamic nuclei (namely, higher order ones), but not others (namely, first order ones), receive a layer 5 input that is not organized as a feedback projection and is driver in function.
Corticofugal projections: Axonal and Synaptic properties dictate function
Although the cells that comprise layer 5 and layer 6 corticothalamic projections all use glutamate as their neurotransmitter, the anatomical and physiological properties of their axons and synapses differ markedly from each other. These distinctions underlie the view that layer 6 projections provide modulatory input to thalamus, whereas layer 5 projections provide driving input to thalamus. Distinctions between layer 5 and layer 6 cells include (reviewed in Sherman, 2016; Sherman & Guillery, 2013):
Layer 5 cells have large and thickly myelinated axons. In contrast, layer 6 cells have thinner and less myelinated axons.
Layer 5 cells have axons with large synaptic terminals that are located on the proximal dendrites of relay cells. In contrast, layer 6 cells have axons with smaller synaptic terminals that are located on the distal dendrites of relay cells.
The postsynaptic receptors of layer 5 axons on relay cells are ionotropic (mainly AMPA and NMDA). In contrast, the postsynaptic receptors of layer 6 axons are both ionotropic and metabotropic.
Activation of layer 5 inputs evoke larger EPSPs than that of layer 6 inputs.
Activation of layer 6 inputs evokes paired-pulse facilitation, which is associated with a low probability of transmitter release initiated by an action potential, whereas that of layer 5 inputs evokes paired-pulse depression, associated with a high probability of release.
Thus, compared to layer 5 inputs to thalamus that produce fast and large synaptic potentials that are effective at driving thalamic activity, layer 6 inputs produce slower and smaller synaptic potentials better suited for modulating thalamic activity. With these distinctions in mind, we now explore the functional properties of the layer 5 and 6 corticofugal systems in greater detail and consider their contributions to neural processing and behavior.
Layer 6 Corticofugal System
As mentioned above, layer 6 corticothalamic neurons provide feedback projections to all thalamic nuclei and local projections to the layers of cortex supplied by those thalamic nuclei, such as layer 4. Based on these connections, layer 6 neurons are in a strategic position to influence the information supplied to their cortical area from the thalamus, since they target both the origin of the thalamic input as well as its targets. Importantly, layer 6 corticothalamic neurons in different cortical areas align with different thalamic nuclei. For instance, layer 6 neurons in primary visual cortex send feedback axons to the lateral geniculate nucleus, which receives feedforward input from the retina, while layer 6 neurons in somatosensory cortex send axons to the ventral posterior nucleus, which receives feedforward input from the head and body via the medial lemniscus. In a similar fashion, layer 6 neurons in auditory cortex send feedback axons to the ventral division of the medial geniculate nucleus, which receives feedforward auditory signals from the inferior colliculus. In each of these cases, the synaptic properties of layer 6 input to thalamus appear similar. With this in mind, we will focus the rest of this discussion primarily on feedback from primary visual cortex to the lateral geniculate nucleus, using it as a model system for understanding corticothalamic feedback.
Overall, the layer 6 axons produce roughly 40–50% of all synapses onto relay cells (Van Horn, Erisir & Sherman, 2000). Anatomically, layer 6 innervates relay cells on distal dendrites, and they do not enter glomeruli (Erisir, Van Horn, Bickford & Sherman, 1997; Wilson, Friedlander & Sherman, 1984). Glomeruli are complex synaptic structures found throughout thalamus. Whereas synapses are usually individually encased in a glial covering, synapses in glomeruli, which contain tens of different synaptic terminals, are not. Instead the entire collection of synapses is covered by glial wrappings (reviewed in Jones, 2007; Sherman & Guillery, 2013). The functional significance of glomeruli is unknown.
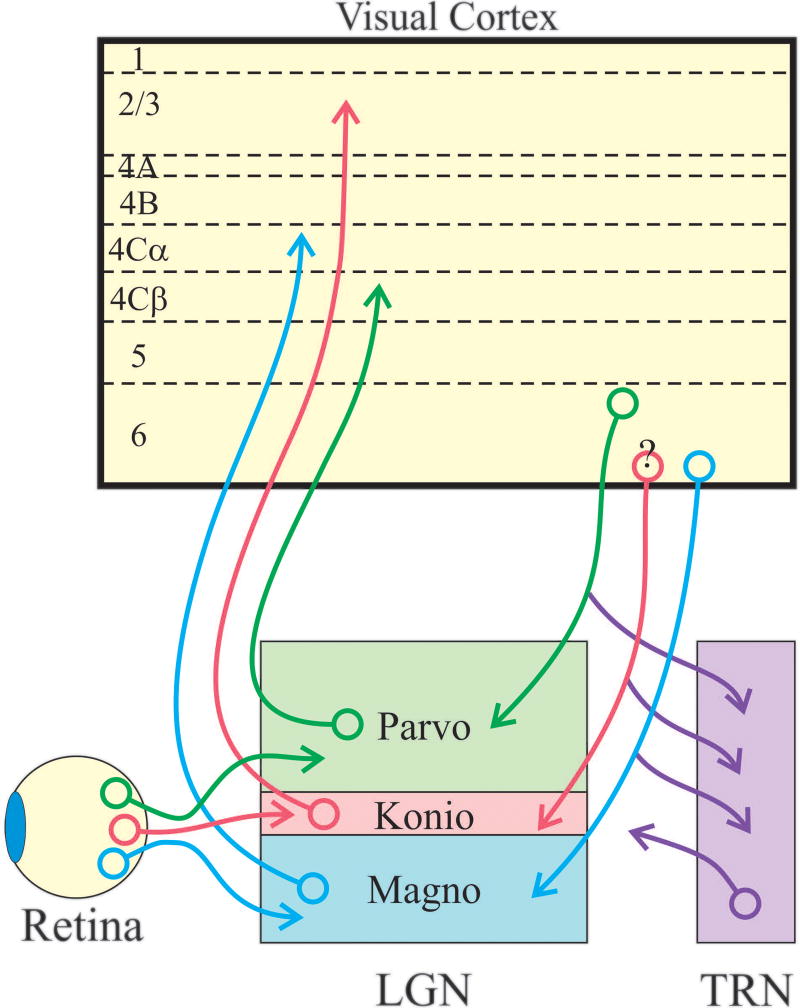
There is a striking relationship between the response properties and projections of corticogeniculate neurons and the feedforward parallel processing streams. In all mammals, information from the retina is relayed to primary visual cortex via parallel processing streams. In macaque monkeys, these streams, known as the parvocellular, magnocellular and koniocellular streams, are particularly prominent (Figure 3; reviewed in Casagrande & Xu, 2004). These involve separate populations of retinal ganglion cells that selectively innervate specific cell types in different laminae of the lateral geniculate nucleus that, in turn, target distinct layers of primary visual cortex. Axons of the magnocellular stream conduct more rapidly than those of the parvocellular stream, which, in turn are faster conducting than those of the koniocellular stream. Neurons in the parvocellular stream have small receptive fields and produce sustained responses to stationary visual stimuli, whereas those in the magnocellular stream have larger receptive fields and produce transient responses. Magnocellular stream neurons also respond better to low-contrast stimuli, better to fast moving stimuli, and have greater extraclassical surround suppression than their parvocellular stream counterparts. Both parvocellular and magnocellular neurons in the lateral geniculate nucleus have axons that terminate in layer 4 of visual cortex, layers 4Cβ and 4Cα, respectively. In contrast, the neurons that comprise the koniocellular stream have axons that bypass layer 4C and terminate in the superficial cortical layers. Compared to parvocellular and magnocellular neurons, we know much less about the physiological properties of koniocellular neurons.
Figure 3.
Organization of feedforward and feedback pathways in the primate. Three major parallel processing streams are established in the retina: the parvocellular, magnocellular, and koniocellular streams, indicated schematically with green, blue and red cell bodies and axons. Retinal ganglion cells belonging to these streams send axons to distinct layers of the lateral geniculate nucleus to synapse with relay neurons that selectively innervate cortical layers 4Cβ, 4Cα, and layers 2 and 3. Although not indicated, relay neurons also often provide sparse input to layers 1 and 6. Layer 6 corticogeniculate neurons are also organized into three major streams. Neurons in upper layer 6 selectively target the parvocellular layers of the lateral geniculate nucleus, while neurons in lower layer 6 target the magnocellular and koniocellular layers (note: although it is unclear in primates whether separate neurons in lower layer 6 target the magnocelluar and koniocellular layers, physiological results from monkeys and anatomical evidence from tree shrews suggests separate populations (Usrey & Fitzpatrick, 1996; Briggs & Usrey, 2009). In addition to making synapses with neurons in the lateral geniculate nucleus, corticogeniculate neurons also send axon collaterals into the thalamic reticular nucleus to synapse with GABAergic neurons that, in turn, project to the lateral geniculate nucleus.
Given the parallel organization of feedforward input to visual cortex, it is noteworthy that, in the monkey, the corticogeniculate feedback pathway is also comprised of stream-specific projections (Figure 3). In particular, separate classes of corticogeniculate neurons have axons selective for the magnocellular, parvocellular, and possibly even the koniocellular layers of the lateral geniculate nucleus (Fitzpatrick, Usrey, Schofield & Einstein, 1994; Ichida & Casagrande, 2002; Ichida, Mavity-Hudson & Casagrande, 2014).
Thus, from an anatomical perspective, corticogeniculate neurons appear to belong to distinct groups with projections that align with the parallel retino-geniculo-cortical pathways. Although less is known about corticogeniculate neurons in other species, evidence from the rat, cat, ferret, and tree shrew supports the view of parallel streams in the corticogeniculate pathway (Bourassa & Deschênes, 1995; Briggs & Usrey, 2005; Tsumoto & Suda, 1980; Usrey & Fitzpatrick, 1996). Returning to the monkey, corticogeniculate neurons with stream-specific connections have visual response properties that also align with their parallel pathways. In particular, corticogeniculate neurons in the lower tier of layer 6, where cells with axons targeting the magnocellular layers of the lateral geniculate nucleus are located (Fitzpatrick, Usrey, Schofield & Einstein, 1994), have fast conducting axons, are more responsive to low-contrast and fast moving stimuli, and show robust extraclassical surround suppression (Briggs & Usrey, 2009). In contrast, corticogeniculate neurons in the upper tier of layer 6, where cells targeting the parvocellular layers are located (Briggs & Usrey, 2009; Fitzpatrick, Usrey, Schofield & Einstein, 1994), have slower conducting axons, are less responsive to low-contrast and fast moving stimuli, and have modest extraclassical suppression (Briggs & Usrey, 2009). As a consequence of this organization, visual stimuli well suited for driving the magnocellular pathway will differentially excite corticogeniculate neurons with axons selective for the magnocellular layers of the lateral geniculate nucleus, while visual stimuli better suited for driving the parvocellular pathway will differentially excite corticogeniculate neurons with axons targeting the parvocellular layers. Thus, corticogeniculate neurons appear well suited to influence the feedforward delivery of visual signals to cortex in a stream-specific fashion.
The corticogeniculate feedback pathway targets excitatory relay cells and local GABAergic interneurons in all layers of the lateral geniculate nucleus as well as neurons in the neighboring thalamic reticular nucleus (reviewed in Sherman & Guillery, 2013). Because all neurons in the thalamic reticular nucleus are GABAergic and innervate neurons in the lateral geniculate nucleus, corticogeniculate feedback has the opportunity to influence relay neurons in the lateral geniculate nucleus via direct excitation and disynaptic inhibition. An interesting and unresolved question is whether corticogeniculate axons all branch to innervate both the lateral geniculate nucleus and the thalamic reticular nucleus. Similarly, it is unclear whether all corticogeniculate axons provide input to both relay cells and interneurons in the lateral geniculate nucleus. These are important questions to answer, particular since corticogeniculate synapses with neurons in the lateral geniculate nucleus and the reticular nucleus likely experience varying degrees of rate-dependent dynamics in the strength of synaptic transmission (Crandall, Cruikshank & Connors, 2015).
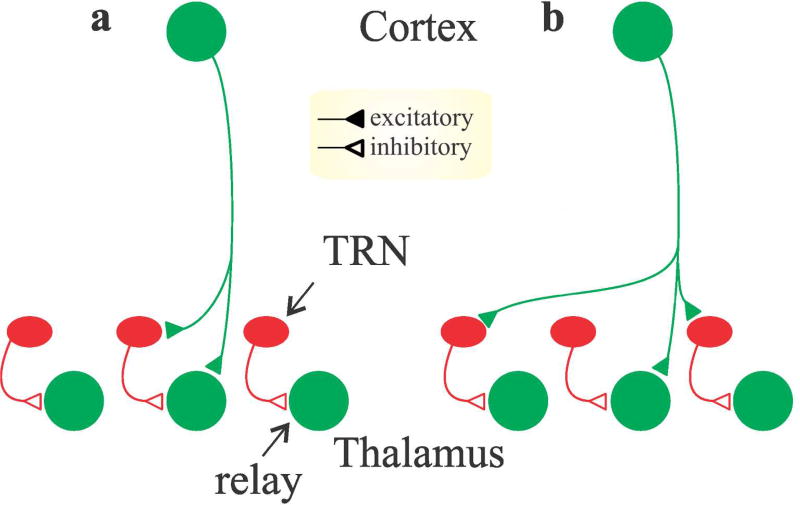
The conventional view of the corticogeniculate feedback is provided by Figure 4A, which shows a corticogeniculate cell providing both direct excitation and disynaptic inhibition (via interneurons or reticular cells) to a relay cell. Recent results suggest corticogeniculate feedback may also provide net excitation or net suppression to neurons in the lateral geniculate nucleus depending on the spatial relationship between the receptive fields of source and target neurons Jones et al., 2012; Wang, Andolina, Lu, Jones & Sillito, 2018). This would result from the schema shown in Figure 4B. In support of this idea, corticogeniculate circuits appear to augment visual responses in lateral geniculate cells when visual stimuli are restricted to the classical receptive fields of geniculate cells and suppress visual responses in the lateral geniculate nucleus when visual stimuli extend into the extraclassical surround (Fisher, Alitto & Usrey; Jones et al., 2012). It is worth noting that this latter effect may be related to a more general view that corticogeniculate circuits are primarily involved in modulating the gain of visual responses in the lateral geniculate nucleus (reviewed in Usrey & Alitto, 2015). Further evidence for such an arrangement exists in the somatosensory thalamus (Lam & Sherman, 2010). Note that the different patterns shown in Figure 4a,b are not mutually exclusive, and this may reflect heterogeneity in details of corticogeniculate circuitry. It is also important to note that the downstream effects of layer 6 influences on cortical neurons are likely to be dynamic, including both excitatory and suppressive effects (Bortone, Olsen & Scanziani, 2014; Crandall, Cruikshank & Connors, 2015; Olsen, Bortone, Adesnik & Scanziani, 2012).
Figure 4.
Schematic circuit diagrams illustrating two possible scenarios for the organization of layer 6 corticogeniculate input to relay neurons and inhibitory interneurons in the thalamus. a. One scenario where corticogeniculate neurons provide monosynaptic excitation to retinotopically aligned relay cells and aligned inhibitory cells (TRN cells or local interneurons) that, in turn, inhibit the same relay cell receiving monosynaptic excitation. b. An alternative scenario where corticogeniculate neurons provide monosynaptic excitation to retinotopically aligned relay cells and non-aligned inhibitory neurons. In this scenario, relay cells receive monosynaptic excitation from retinotopically aligned corticogeniculate neurons (relay cell 2) and disynaptic inhibition from non-aligned corticogeniculate neurons (relay cells 1 and 3).
A topographically ordered center/surround organization of corticogeniculate projections also has implications for effects involving cognitive activities. For instance, corticogeniculate feedback has been postulated to provide a “spotlight” for attentional modulation of thalamic activity. Along these lines, spatial attention has been shown to decrease visual responses in the thalamic reticular nucleus (McAlonan, Brown & Bowman, 2000; McAlonan, Cavanaugh & Wurtz, 2006) and increase activity in the lateral geniculate nucleus; it also increases the strength of synaptic transmission between neurons in the lateral geniculate nucleus and visual cortex (Briggs, Mangun & Usrey, 2013). There is also renewed interest in understanding the relationship between cortical state and visual activity in the lateral geniculate nucleus (McCormick, McGinley & Salkoff, 2015). Along these lines, studies of network interactions identify oscillatory feedback interactions between cortex and the lateral geniculate nucleus in the alpha-frequency band (Bastos, Briggs, Alitto, Mangun & Usrey, 2014). The extent to which these interactions serve to synchronize activity between geniculate cells remains to be determined, as does a functional role for any synchronization.
Layer 5 Corticofugal System
Layer 5 corticofugal cells innervate numerous subcortical targets, including thalamus, basal ganglia, various brainstem sites, and, in some cases, spinal cord. Individual axons of these cells branch repeatedly to innervate many or all of these targets (see below). It is important to note that every cortical area for which appropriate information is available produces such a corticofugal output. By communicating with so many subcortical sites, it is this system and only this system that allows the cortex to communicate with other parts of the neuraxis and thus affect behavior.
Layer 5 contains large pyramidal cells, and these seem to fall into two main groups: one projects subcortically, and the other projects to other cortical areas. Besides their projections subcortically or to other cortical areas, these cells also contribute axonal branches to local circuitry within their cortical regions. Several features distinguish these (reviewed in Sherman, 2014, 2016): 1) the subcortically projecting cells lie more ventrally in layer 5 (often referred to as layer 5b) than do the cortico-cortically projecting cells (said to be in layer 5a); 2) the subcortically projecting cells are the largest pyramidal cells in cortex with apical dendrites usually reaching all the way to layer 1; whereas the other pyramidal cells are smaller with apical dendrites typically failing to reach layer 1; and 3) the subcortically projecting cells often fire in burst mode based on activation of a type of voltage- and time-dependent calcium conductance, a firing mode not seen in the other layer 5 cell group (Larkum, Zhu & Sakmann, 1999; Llano & Sherman, 2009). Our further discussion in this section is limited to the layer 5 cells that project subcortically.
Thalamic innervation
Layer 5 axons innervate relay cells on proximal dendrites often in glomeruli (see above for description of glomeruli) in triadic arrangements, although other inputs are found outside glomeruli in simple contacts onto dendrites (reviewed in Jones, 2007; Sherman & Guillery, 2013). The typical triadic involves three synapses. The three synapses are: 1) the layer 5 terminal contacting a relay cell dendrite, 2) the same layer 5 terminal contacting a GABAergic, dendritic terminal from an interneuron, and 3) the same interneuron terminal contacting the same relay cell dendrite. Thus the dendritic terminal of the interneuron is both presynaptic and postsynaptic. Triads are found throughout thalamus, and for instance, in the lateral geniculate nucleus, the retinal terminal would be found in the same arrangement as the layer 5 terminals as just described. Overall, layer 5 input to relay cells produces only about 2% of all synapses on these cells (Wang, Eisenback & Bickford, 2002).
As noted above, it appears that all thalamic relay cells receive an input from layer 6 cells, an input organized mainly but not exclusively in a feedback manner. In addition, some relay cells receive an input from layer 5 of cortex that is not organized in a feedback manner, meaning that the cortical area of origin differs from the area targeted by the postsynaptic relay cell. Figure 1 shows an example of such circuitry. In this example, the processing is feedforward, which is to say that it is organized to ascend a hierarchical pattern of thalamocortical processing. Also, whereas the layer 6 corticothalamic input is modulatory, that of the layer 5 is driver.
Extrathalamic innervation
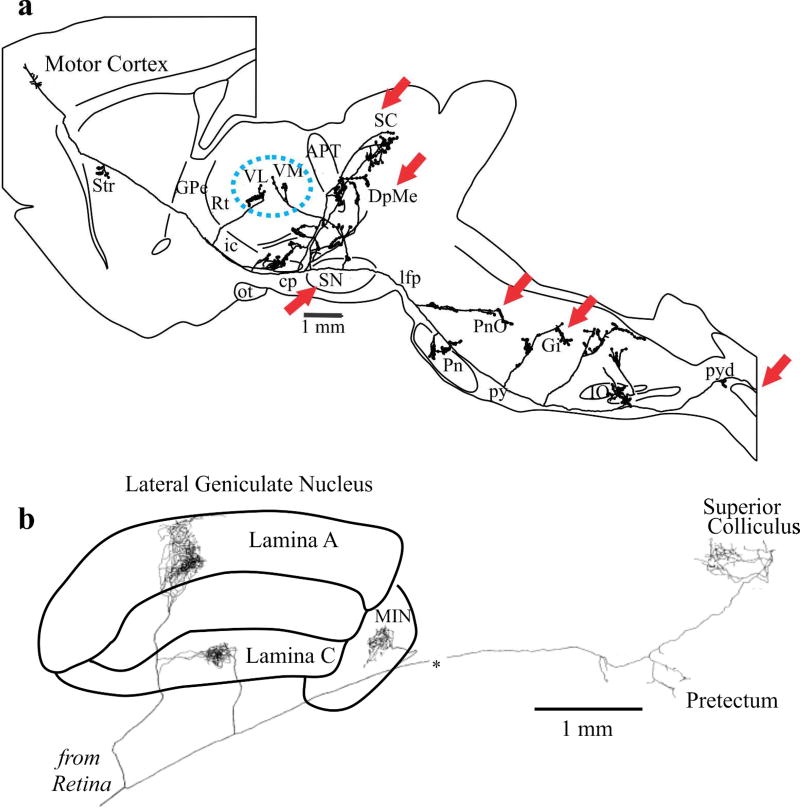
Another notable difference exists between layers 5 and 6 regarding their thalamic innervation. Layer 6 corticothalamic axons innervate only thalamus, including the thalamic reticular formation, whereas the layer 5 innervation of thalamus passes through the thalamic reticular formation without innervating cells there and also involves axons that branch repeatedly to innervate a number of other subcortical sites (reviewed in Sherman, 2014, 2016). Figure 5a shows an example of such a layer 5 corticofugal axon and its multiple targets. One set of branches innervates thalamus (blue dashed oval), representing the start of a transthalamic pathway. Other branches innervate regions identified as bulbospinal control centers (red arrows), and a final branch enters the spinal cord, representing a corticospinal projection. A general feature of these layer 5 corticofugal axons is that many, if not all, branch to innervate higher order thalamus as well as various brainstem and occasionally spinal cord sites associated with motor control (Figures 1 and 5; reviewed in Sherman, 2014, 2016).
Figure 5.
Examples of branching axons of driver inputs to thalamus. a. Example from layer 5 pyramidal tract cell of rat motor cortex; adapted from (Kita & Kita, 2012). b. Example from retinogeniculate axon of cat; adapted from (Uhlrich, Tamamak, Murphy & Sherman, 1995).
Two other points are worth noting. First, the pattern of driver input (from cortical layer 5) to higher order relays involving branching axons that also innervate motor centers is also seen in driver input to first order relays (reviewed in Sherman, 2014, 2016). For instance, retinogeniculate axons branch to also innervate the superior colliculus and pretectal region, areas involved in the control of eye movements, pupil size, accommodation, etc. (Figure 5b and Uhlrich, Tamamaki, Murphy & Sherman, 1995). Second, one feature held in common by the corticothalamic projections from layers 5 and 6 is that the cells of origin do not have axon branches that innervate other cortical areas (Petrof, Viaene & Sherman, 2012), although they do have branches that provide local cortical innervation. Thus the cells in layers 5 and 6 that innervate other cortical areas do not extend subcortical branches, and those that do innervate thalamus and other subcortical sites are not involved in providing direct innervation of other cortical areas. It thus appears that there is at least some degree of independence of direct versus transthalamic corticortical circuitry.
Efference copies: Role of branching axons?
Neurons with axons that branch to innervate multiple structures are found throughout the brain and likely serve multiple purposes. Here, we speculate on the function of branching axons in the corticofugal projections of layer 5 neurons and consider their possible contribution to motor function as efference copies.
Efference copies
When we move our eyes (and we typically scan scenes with saccades three times a second), the sensory stimulus on our retina signals the visual world moving in the direction opposite to the eye movement. But we do not normally perceive the world as spinning about during these eye movements. This is because neural circuits are set up to anticipate these eye movements and eliminate the sensory consequences of them from our perception. Analogous circuits are established with respect to all self-generated movements, not just eye movements. Thus when we palpate an object with our fingers, the sensory stimulation from the act of finger movements is accounted for. Such accounting is needed for the organism to disambiguate sensory stimulation due to self-generated movements from that caused by actual changes in the environment. Note that this process requires a prediction, or “forward model,” of what will occur as a result of the impending action, and that any sensory feedback that can indicate the position of the eyes or finger joints would occur after the movement and be too late for this purpose (Sommer & Wurtz, 2008).
These anticipatory circuits depend on efference copies (also known as “corollary discharges”), which are messages sent from motor areas of the brain back into appropriate sensory processing streams to anticipate impending self-generated behaviors. Excellent recent reviews of efference copies are available (Sommer & Wurtz, 2008; Wolpert & Flanagan, 2010), and so we leave out details of the subject in order to focus on its possible role in thalamocortical processing.
Coordinated motor performance of any behaving animal without efference copies is implausible. The critical necessity of organisms to distinguish effects of self-generated movements from environmental changes was recognized at least as far back as the 19th century, and efference copies then were effectively foretold (von Graefe, 1854). It was first experimentally demonstrated independently in fishes and flies (Sperry, 1950; von Holst & Mittelstaedt, 1950), and this indicates that it must occur widely in the animal kingdom and be a core part of our early evolutionary heritage.
It thus logically follows that any message generated anywhere in the central nervous system that leads to a change in motor behavior must have associated with it an efference copy. What are the actual circuits that subserve this function? We suggest that, at least for cortical origins of motor commands, these involve the branching driver innervation of thalamus.
Branching axons and efference copies
One might ask: What is the functional significance of the driver innervation of thalamus involving branching axons? It appears that, at least for action potentials travelling in the orthodromic direction in mammals, there is no branch point failure of propagation except for modest failure at very high frequencies that are generally beyond the physiological range (Goldfinger, 2000; Huguenard, 2000; Zhou & Chiu, 2001). This means that the exact same message, in terms of the pattern of action potentials, travels down all branches of an axon to its multiple targets. This does not mean that the same effect is seen in all postsynaptic neurons, because differences in synaptic properties will lead to different postsynaptic responses to the same afferent messages.
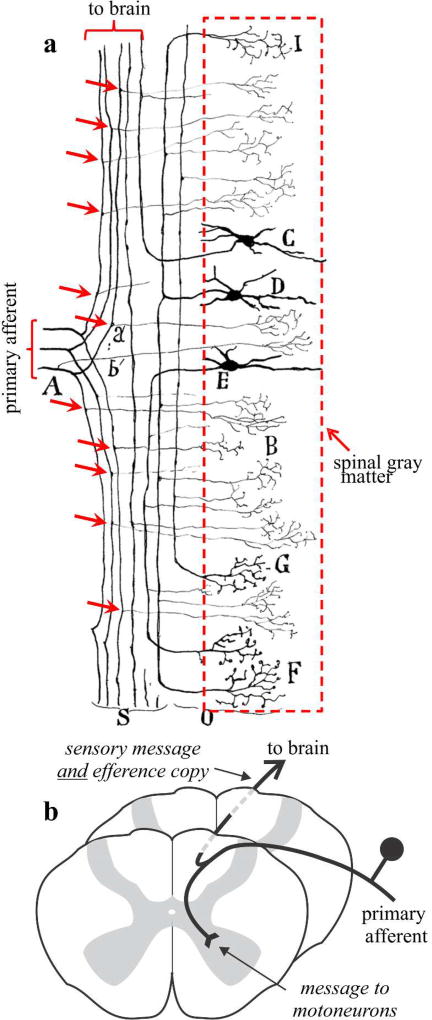
Figure 6 illustrates what this might mean for spinal circuitry, an idea that we extend below to layer 5 corticothalamic circuitry. Figure 6a is a reproduction of an illustration from Cajal (Cajal, 1911). Cajal emphasized the fact that primary afferent axons innervating the spinal cord all branched, one branch innervating the spinal gray matter, and the other ascending to the brain. Figure 6bB shows how a modern textbook might illustrate this point. Because the spinal branch innervates gray matter, we can regard it as carrying a signal that will have significant effects on motoneurons. In other words, it carries a motor message. We normally think of the branch ascending to the brain as carrying a sensory message, such as a change in joint angle or skin indentation. However, because it is a branch, it carries an exact copy of the message aimed at motoneurons; in other words, it carries a copy of a motor message, which is a neat definition of an efference copy.
Figure 6.
Primary spinal afferents are branching axons. a. Cajal illustration (Cajal, 1911) of primary axons entering the spinal cord and branching to innervate the spinal gray matter and brain areas. The red arrows indicate branch points. b. Schematic interpretation of a.
An important additional point to make is that the singular message carried by the ascending branch can have a double purpose to serve both as a sensory signal and as an efference copy. One way to think about it is that the branch may branch further to innervate different neuronal populations, one of which processes the message in terms of its sensory information, and the other, in terms of an efference copy.
An interpretation of the circuitry illustrated in Figures 1 and 5 is that the axon branches innervating thalamus serve as efference copies, because the messages they carry are exact copies of messages targeting motor centers in the brainstem and spinal cord. As is the case with the interpretation given above for Figure 6b, the message carried by these thalamic afferents can serve two purposes: one as information from lower centers, such as visual information from the retina or the results of processing in a lower cortical area, and the other, as an efference copy. This would allow cortical areas higher in a hierarchy to be kept informed about motor commands sent out by lower centers.
Finally, a glance at Figure 1 reveals another challenge to conventional thinking about cortical processing. The conventional view can be summed up as follows: information arrives initially at a thalamic nucleus to be relayed to cortex (think of retinal input to the lateral geniculate nucleus); it is then transferred for further processing from a primary cortical area in multiple steps up a cortical hierarchy of sensory, then sensorimotor, and finally executive motor areas for messages sent to subcortical centers to influence behavior. A key point here is that once information reaches cortex, the processing thereof stays entirely within cortex until the final executive stage, and such processing has no role for what we have defined as higher order thalamic nuclei. The suggestion offered by Figure 1 is that every area, including primary sensory areas, has a layer 5 output that can influence behavior, and thus differences between “sensory” and “motor” cortex are more quantitative than qualitative, because all cortical areas have a motor output. We thus suggest that the terminology of “sensory” and “motor” (and “association”) cortex in this context is misleading.
Evolutionary considerations of efference copies
Because of the importance and ubiquity of efference copies in the animal kingdom, we can explore certain plausible scenarios of their evolution starting with primitive vertebrates for our example. Such a primitive vertebrate ancestor would likely operate mainly on spinal circuitry for control of body movements, and the associated efference copies could plausibly be represented as in Figure 6b. However, as higher brainstem centers for control of spinal circuitry evolve, such as the rubrospinal, tectospinal, and reticulospinal tracts, new efferent copy circuits would necessarily have evolved with these. As evolution proceeded to it latest stage for motor control, namely with the evolution of thalamus and cortex, efference copy circuits must have concurrently evolved. If this scenario is correct, this would mean that our central nervous system has multiple levels of efference copies arranged in a sort of hierarchical order reflecting their evolutionary history. And that related to cortex is served by the pattern of driver inputs to thalamus. This creates a problem that deserves further investigation: How does the brain deal with the possibility of multiple efferent copies associated with motor commands, some of which may not be associated with actual behavior?
Concluding Remarks
The only pathways out of cortex to other parts of the neuraxis emanate from layers 5 and 6, and their cells of origin appear not to send axon collaterals to other cortical areas, although they do exhibit axons that provide very local innervation. The projections from layers 5 and 6 are both glutamatergic but otherwise quite different in terms of their structure as well as function. Key differences in structure and function have been described above.
Layer 6 function
Functionally, layer 6 projections appear to act as a modulator, like cholinergic, noradrenergic, etc., modulatory inputs to thalamus. All of these modulatory inputs activate metabotropic receptors, which seems a key aspect of their ability to modulate, but the layer 6 modulatory input is uniquely topographic in connections. The layer 6 projection is not only feedback in the sense that the innervation is to the same thalamic cell group that innervates the region of cortex in which the layer 6 cells reside, but there is even further feedback specificity here seen in the corticogeniculate projection: the parallel ascending koniocellular, magnocellular, and parvocellular streams each has their unique set of corticogeniculate afferents.
It is further noteworthy that the layer 6 axons also branch to innervate layer 4 cells, which are the main target of thalamocortical afferents. This means that the layer 6 corticothalamic neurons can affect thalamocortical transmission both at its source, via its innervation of thalamic cells, as well as at its target in cortex. The bottom line is that the layer 6 feedback allows cortex considerable control over its thalamic input.
Layer 5 function
Layer 5 cells provide a driving input to thalamic relay cells, meaning that they seem to represent the main information relayed by thalamic cells to their cortical targets. As such, the layer 5 output is seen as the beginning of a cortico-thalamo-cortical, or transthalamic, circuit to allow information to pass between cortical areas in parallel to direct corticocortical circuits (reviewed in Sherman & Guillery, 2013).
Given two pathways to connect cortical circuits, one might ask: What is different between the direct and transthalamic circuits? There is one striking anatomical difference that needs to be considered: direct corticocortical axons have no subcortical branches, so the information they carry stays strictly within cortex, whereas the layer 5 cells of the transthalamic circuit branch to innervate extrathalamic targets, meaning that the information these axons carry is shared with much of the subcortical neuraxis. We have speculated above, based on this, that, because some of the extrathalamic targets seem to be motor centers that represent the means by which cortex can influence behavior, and because the nature of branching axons means that these motor messages are copies to higher order thalamic neurons for relay to cortex, these message can be read in part as copies of these motor messages. In other words, these copies are efference copies for further cortical processing.
Final questions
There is still much to be learned about these corticofugal projection systems. We finish with what we regard as key questions that require further study.
Do individual neurons in the thalamic reticular nucleus receive stream-specific or mixed-stream input from layer 6 feedback neurons? Likewise, are the projections of thalamic reticular neurons specific for cell classes in the lateral geniculate nucleus?
How does layer 6 feedback influence thalamic activity at the population level? For example, does layer 6 feedback influence rhythmic/oscillatory activity between neurons?
Given that individual layer 6 axons branch to target the thalamus and the cortical layers that receive thalamic input, what are the effects of the input to layer 4 on thalamocortical communication?
Is layer 6 feedback a route for cognitive processes to influence the thalamus?
Are layer 6 feedback projections to higher order thalamic nuclei comprised of parallel streams similar to those found in the projections to first-order thalamic nuclei?
How common is the pattern of parallel direct and transthalamic pathways connecting cortical areas? Or, are some cortical areas connected by just one or the other?
Why is one of the paths filtered through the thalamus? One possible answer might have to do with the fact that thalamic relays can operate like a gate and be shut down when the relays cells are sufficiently inhibited. This may be especially true of higher order thalamic relays Bokor, Frere, Eyre, Slezia, Ulbert, Luthi & Acsády, 2005; Lavallée, Urbain, Dufresne, Bokor, Acsády & Deschênes, 2005).
What is different in the information carried by the direct versus transthalamic pathways? Our suggestion that one difference is that the latter pathways contains a signal that can serve as an efference copy is merely a hypothesis without much empirical support, but it is an attempt to explain the anatomical fact of axonal branching for many, most, or all of driver inputs to thalamus, a fact that does require an explanation.
To restate a question raised above: How does the brain deal with the possibility of multiple efferent copies associated with motor commands, some of which may not be associated with actual behavior?
Do feedback transthalamic pathways exist? There is no evidence yet for this, but the possibility should be explored that, just like feedforward corticocortical processing, feedback circuits might involve both direct and transthalamic pathways.
Acknowledgments
This work was supported by NIH grants EY013588 and EY012576 (WMU) and EY022338, NS094184, and DC00879 (SMS).
Footnotes
The authors declare no competing financial interests.
References
- Bastos AM, Briggs F, Alitto HJ, Mangun GR, Usrey WM. Simultaneous recordings from the primary visual cortex and lateral geniculate nucleus reveal rhythmic interactions and a cortical source for gamma-band oscillations. Journal of Neuroscience. 2014;34(22):7639–7644. doi: 10.1523/JNEUROSCI.4216-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokor H, Frere SGA, Eyre MD, Slezia A, Ulbert I, Luthi A, Acsády L. Selective GABAergic control of higher-order thalamic relays. Neuron. 2005;45(6):929–940. doi: 10.1016/j.neuron.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Bortone DS, Olsen SR, Scanziani M. Translaminar inhibitory cells recruited by layer 6 corticothalamic neurons suppress visual cortex. Neuron. 2014;82(2):474–485. doi: 10.1016/j.neuron.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa J, Deschênes M. Corticothalamic projections from the primary visual cortex in rats: A single fiber study using biocytin as an anterograde tracer. Neuroscience. 1995;66:253–263. doi: 10.1016/0306-4522(95)00009-8. [DOI] [PubMed] [Google Scholar]
- Briggs F, Kiley CW, Callaway EM, Usrey WM. Morphological substrates for parallel streams of corticogeniculate feedback originating in both V1 and V2 of the macaque monkey. Neuron. 2016 doi: 10.1016/j.neuron.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Mangun GR, Usrey WM. Attention enhances synaptic efficacy and the signal-to-noise ratio in neural circuits. Nature. 2013;499(7459):476–480. doi: 10.1038/nature12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Temporal properties of feedforward and feedback pathways between the thalamus and visual cortex in the ferret. Thalamus. Relat Syst. 2005;3(2):133–139. doi: 10.1017/S1472928807000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Emerging views of corticothalamic function. Current Opinion in Neurobiology. 2008;18(4):403–407. doi: 10.1016/j.conb.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Parallel processing in the corticogeniculate pathway of the macaque monkey. Neuron. 2009;62(1):135–146. doi: 10.1016/j.neuron.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SRy. Histologie du Système Nerveaux de l'Homme et des Vertébrés. Paris: Maloine; 1911. [Google Scholar]
- Casagrande VA, Xu X. Parallel visual pathways: a comparative perspective. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Cambridge, MA: MIT Press; 2004. pp. 494–506. [Google Scholar]
- Crandall SR, Cruikshank SJ, Connors BW. A corticothalamic switch: controlling the thalamus with dynamic synapses. Neuron. 2015;86(3):768–782. doi: 10.1016/j.neuron.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erisir A, Van Horn SC, Bickford ME, Sherman SM. Immunocytochemistry and distribution of parabrachial terminals in the lateral geniculate nucleus of the cat: A comparison with corticogeniculate terminals. Journal of Comparative Neurology. 1997;377(4):535–549. [PubMed] [Google Scholar]
- Fisher TG, Alitto HJ, Usrey WM. Retinal and Non-Retinal Contributions to Extraclassical Surround Suppression in the Lateral Geniculate Nucleus. Journal of Neuroscience. 2017;37(1):226–235. doi: 10.1523/JNEUROSCI.1577-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D, Usrey WM, Schofield BR, Einstein G. The sublaminar organization of corticogeniculate neurons in layer 6 of macaque striate cortex. Visual Neuroscience. 1994;11:307–315. doi: 10.1017/s0952523800001656. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979;280:120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- Goldfinger MD. Computation of high safety factor impulse propagation at axonal branch points. Neuro Report. 2000;11(3):449–456. doi: 10.1097/00001756-200002280-00005. [DOI] [PubMed] [Google Scholar]
- Huguenard JR. Reliability of axonal propagation: the spike doesn't stop here. Proceedings of the National Academy of Science. 2000;97(17):9349–9350. doi: 10.1073/pnas.97.17.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida JM, Casagrande VA. Organization of the feedback pathway from striate cortex (V1) to the lateral geniculate nucleus (LGN) in the owl monkey (Aotus trivirgatus) Journal of Comparative Neurology. 2002;454(3):272–283. doi: 10.1002/cne.10441. [DOI] [PubMed] [Google Scholar]
- Ichida JM, Mavity-Hudson JA, Casagrande VA. Distinct patterns of corticogeniculate feedback to different layers of the lateral geniculate nucleus. Eye Brain. 2014;6(Suppl 1):57–73. doi: 10.2147/EB.S64281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The Thalamus: Second Edition. Cambridge, U.K.: Cambridge University Press; 2007. [Google Scholar]
- Jones HE, Andolina IM, Ahmed B, Shipp SD, Clements JT, Grieve KL, Cudeiro J, Salt TE, Sillito AM. Differential feedback modulation of center and surround mechanisms in parvocellular cells in the visual thalamus. Journal of Neuroscience. 2012;32(45):15946–15951. doi: 10.1523/JNEUROSCI.0831-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Kita H. The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. Journal of Neuroscience. 2012;32(17):5990–5999. doi: 10.1523/JNEUROSCI.5717-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Sherman SM. Functional organization of the somatosensory cortical layer 6 feedback to the thalamus. Cerebral Cortex. 2010;20(1):13–24. doi: 10.1093/cercor/bhp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- Lavallée P, Urbain N, Dufresne C, Bokor H, Acsády L, Deschênes M. Feedforward inhibitory control of sensory information in higher-order thalamic nuclei. Journal of Neuroscience. 2005;25(33):7489–7498. doi: 10.1523/JNEUROSCI.2301-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Differences in intrinsic properties and local network connectivity of identified layer 5 and layer 6 adult mouse auditory corticothalamic neurons support a dual corticothalamic projection hypothesis. Cerebral Cortex. 2009;19(12):2810–2826. doi: 10.1093/cercor/bhp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JS, Boothe RG. Interlaminar connections and pyramidal neuron organisation in the visual cortex, area 17, of the Macaque monkey. Journal of Comparative Neurology. 1975;159:304–334. [Google Scholar]
- McAlonan K, Brown VJ, Bowman EM. Thalamic reticular nucleus activation reflects attentional gating during classical conditioning. Journal of Neuroscience. 2000;20(23):8897–8901. doi: 10.1523/JNEUROSCI.20-23-08897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. Journal of Neuroscience. 2006;26(16):4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, McGinley MJ, Salkoff DB. Brain state dependent activity in the cortex and thalamus. Current Opinion in Neurobiology. 2015;31:133–140. doi: 10.1016/j.conb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SR, Bortone DS, Adesnik H, Scanziani M. Gain control by layer six in cortical circuits of vision. Nature. 2012;483(7387):47–52. doi: 10.1038/nature10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof I, Viaene AN, Sherman SM. Two populations of corticothalamic and interareal corticocortical cells in the subgranular layers of the mouse primary sensory cortices. Journal of Comparative Neurology. 2012;520:1678–1686. doi: 10.1002/cne.23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. The function of metabotropic glutamate receptors in thalamus and cortex. Neuroscientist. 2014;20(2):136–49. doi: 10.1177/1073858413478490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. Thalamus plays a central role in ongoing cortical functioning. Nature Neuroscience. 2016;19(4):533–541. doi: 10.1038/nn.4269. [DOI] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Thalamocortical Processing: Understanding the Messages that Link the Cortex to the World. Cambridge, MA: MIT Press; 2013. [Google Scholar]
- Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annual Review of Neuroscience. 2008;31:317–338. doi: 10.1146/annurev.neuro.31.060407.125627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry RW. Neural basis of the spontaneous optokinetic response produced by visual inversion. Journal of Comparative Neurology. 1950;43:482–489. doi: 10.1037/h0055479. [DOI] [PubMed] [Google Scholar]
- Tsumoto T, Suda K. Three groups of cortico-geniculate neurons and their distribution in binocular and monocular segments of cat striate cortex. Journal of Comparative Neurology. 1980;193:223–236. doi: 10.1002/cne.901930115. [DOI] [PubMed] [Google Scholar]
- Uhlrich DJ, Tamamaki N, Murphy PC, Sherman SM. Effects of brainstem parabrachial activation on receptive field properties of cells in the cat's lateral geniculate nucleus. Journal of Neurophysiology. 1995;73:2428–2447. doi: 10.1152/jn.1995.73.6.2428. [DOI] [PubMed] [Google Scholar]
- Usrey WM, Alitto HJ. Visual functions of the thalamus. Annual Review of Vision Science. 2015;1:351–371. doi: 10.1146/annurev-vision-082114-035920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Fitzpatrick D. Specificity in the axonal connections of layer VI neurons in tree shrew striate cortex: Evidence for distinct granular and supragranular systems. Journal of Neuroscience. 1996;16(3):1203–1218. doi: 10.1523/JNEUROSCI.16-03-01203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usrey WM, Fitzpatrick D. Specificity in the axonal connections of layer VI neurons in tree shrew striate cortex: Evidence for distinct granular and supragranular systems. Journal of Neuroscience. 1996;16(3):1203–1218. doi: 10.1523/JNEUROSCI.16-03-01203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn SC, Erisir A, Sherman SM. The relative distribution of synapses in the A-laminae of the lateral geniculate nucleus of the cat. Journal of Comparative Neurology. 2000;416:509–520. [PubMed] [Google Scholar]
- von Graefe A. Beitr ge zur Physiologie und Pathologie der schiefen Augenmuskeln. Archive fur Opthlalmologie. 1854;1(1):1–81. [Google Scholar]
- von Holst E, Mittelstaedt H. The reafference principle. Interaction between the central nervous system and the periphery. In: Martin TbR., editor. Selected Papers of Erich von Holst: The Behavioural Physiology of Animals and Man. Vol. 1. Coral Gables: University of Miami Press; 1950. pp. 139–173. [Google Scholar]
- Wang S, Eisenback MA, Bickford ME. Relative distribution of synapses in the pulvinar nucleus of the cat: Implications regarding the "driver/modulator" theory of thalamic function. Journal of Comparative Neurology. 2002;454(4):482–494. doi: 10.1002/cne.10453. [DOI] [PubMed] [Google Scholar]
- Wang W, Andolina IM, Lu Y, Jones HE, Sillito AM. Focal gain control of thalamic visual receptive fields by layer 6 corticothalamic feedback. Cerbral Cortex. 2018;28(1):267–280. doi: 10.1093/cercor/bhw376. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Friedlander MJ, Sherman SM. Fine structural morphology of identified X- and Y-cells in the cat's lateral geniculate nucleus. Proceedings of the Royal Society of London B. 1984;221:411–436. doi: 10.1098/rspb.1984.0042. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR. Motor learning. Current Biology. 2010;20(11):R467–R472. doi: 10.1016/j.cub.2010.04.035. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chiu SY. Computer model for action potential propagation through branch point in myelinated nerves. Journal of Neurophysiology. 2001;85(1):197–210. doi: 10.1152/jn.2001.85.1.197. [DOI] [PubMed] [Google Scholar]