Abstract
Context
Hyperuricemia is an independent risk factor for progression of kidney disease.
Objective
To determine whether lowering serum uric acid level (sUA) to below 6 mg/dL (target) improves mild to moderate chronic kidney disease (CKD) and whether CKD stage influences the benefit of lowering sUA to target.
Design
Retrospective epidemiologic cohort study conducted over 8 years. Estimated glomerular filtration rate (eGFR) was required in the 6 months preceding the index date (defined as first occurrence of sUA < 7 mg/dL), and at least 1 sUA and eGFR were required during follow-up. Patients were urate-lowering therapy (ULT) naïve, aged 18 years or older, and had CKD Stages 2 to 4 at baseline. Health Plan enrollment with drug benefit was required. Exclusions included active cancer, dialysis, or other kidney disease.
Main Outcome Measures
A 30% decrease or 30% improvement in eGFR from baseline.
Results
A total of 12,751 patients met inclusion criteria; 2690 patients received ULT during follow-up and 10,061 did not. Target sUA was achieved in 1118 patients (42%) receiving ULT. A 30% improvement in eGFR was likelier in patients who achieved the target (odds ratio [OR] = 1.78, p < 0.001). Pairwise comparison of CKD stages showed a 30% improvement in eGFR in CKD Stage 2 (OR = 2.26, p = 0.017) and Stage 3 (OR = 2.23, p < 0.001) but not Stage 4 (OR = 1.50, p = 0.081).
Conclusion
Patients who achieve an American College of Rheumatology target sUA below 6 mg/dL during ULT have higher rates of eGFR improvement, especially in CKD Stages 2 and 3.
INTRODUCTION
Gout has a substantial and growing impact on the health and well-being of patients around the world. The 2007 to 2008 National Health and Nutrition Examination Survey of self-reported gout showed a prevalence of 3.9% compared with the 1988 to 1994 estimate of 2.7%.1,2 An epidemiologic study from British Columbia notes an increase in both the prevalence (2.4%–3.8%) and incidence (1.71–2.89) of gout from 2000 to 2012.3 This increase in gout comes with substantial costs both to the individual and to the health care system.
Elevated serum uric acid level (sUA), the principal cause of gout, has been shown to be an independent risk factor for progression of kidney disease in both animal and human studies. Large epidemiologic studies from Taiwan4 and Austria5 showed hyperuricemia as an independent risk factor for kidney disease. Studies by Siu et al6 and Kim and colleagues7 demonstrated that when sUA is reduced, the progression of kidney disease can be slowed. A 10-year follow-up study of hyperuricemia and glomerular filtration rates (GFRs) from Iseki et al8 in Okinawa showed that hyperuricemia was a strong independent risk factor for a decline in GFR. Sircar and colleagues,9 in a 6-month, double-blind study in hyperuricemic patients, randomly assigned to receive urate-lowering therapy (ULT) or placebo demonstrated patients receiving ULT improved their GFR by 3.2 mL/min/1.73 compared with the placebo group, which declined by 4.4 mL/min/1.73m2 (p < 0.05). Hyperuricemia has been shown to also have adverse effects on diabetes10 and cardiovascular disease11 and increased overall all-cause mortality.12
In a previous study, we demonstrated that in patients who achieved an American College of Rheumatology (ACR) sUA target of less than 6 mg/dL while receiving ULT had a 37% reduction in the risk of their kidney disease worsening, defined as a 30% decrease in GFR from baseline.13 This current study is designed to determine whether ULT can improve mild to moderate chronic kidney disease (CKD) when patients achieve the ACR target of an sUA below 6 mg/dL (target) and whether baseline CKD stage influences the benefits when the target is attained with ULT.
METHODS
Setting and Patients
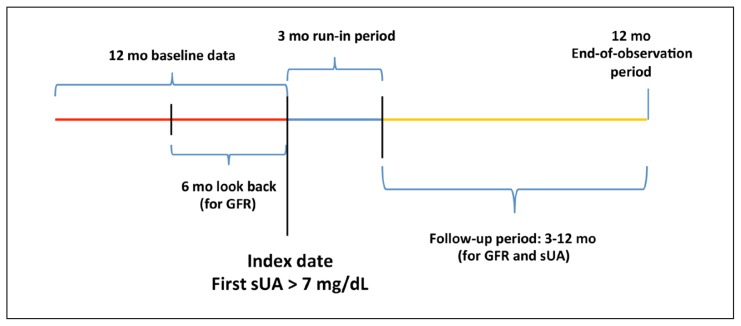
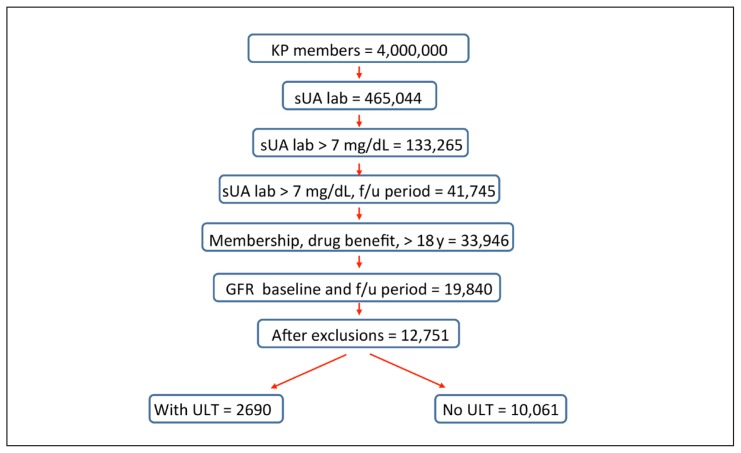
This retrospective cohort study used the Kaiser Permanente (KP) Southern California (KPSC) electronic medical records and administrative databases from January 1, 2008, through July 31, 2014. KPSC has 15 Medical Centers and 225 medical offices serving more than 4 million members. The first instance of an sUA above 7 mg/dL was defined as the index date, not the first diagnosis of gout. To be eligible for study entry, patients were age 18 years or older on the index date and had 12 months of continuous KPSC membership and pharmacy benefits both before and after the index date. The 1-year follow-up period was selected because previous work showed that GFR changes tended to occur within months of ULT initiation.13 All study patients were naïve to ULT for at least 1 year before the index date (Figure 1). Patients with mild to moderate renal impairment at baseline (CKD Stages 2, 3, and 4) were included. Those with CKD Stages 1 and 5 as well as patients with human immunodeficiency virus, active cancer treatment, dialysis, or renal disease including proteinuria and kidney transplant were excluded (Figure 2).
Figure 1.
Study timeline.
GFR = glomerular filtration rate (mL/min); sUA = serum uric acid.
Figure 2.
Inclusions and exclusions for study subjects.
f/u = follow=up; GFR = glomerular filtration rate (mL/min); KP = Kaiser Permanente; lab = laboratory value; sUA = serum uric acid; ULT = urate-lowering therapy.
Outcomes
Study patients had at least 1 estimated GFR (eGFR) measurement in the 6 months before the index date. All subjects had at least 1 sUA and eGFR laboratory test in the period 3 to 12 months after the index date (follow-up period). We excluded eGFR laboratory values within 90 days of the index date from the analysis because this was not enough time for the beneficial effects of ULT to manifest. Outcomes were defined as either a 30% decrease or a 30% improvement in eGFR from baseline.7 The eGFR was calculated from the serum creatinine level using the Modification of Diet in Renal Disease (MDRD) calculation.14
Statistical Analysis
A 30% change in eGFR by ULT status stratified by baseline CKD stage was the primary outcome. Demographic characteristics and comorbidities were categorized and compared between ULT status and CKD stages. The primary outcome of eGFR change was compared between ULT status using χ2 test, and a p value of less than 0.05 (2-sided) was considered statistically significant. Among patients receiving ULT, the odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using multinomial logistic models on the treatment outcome (achieving target or not achieving target). The same analysis was used to further stratify patients by CKD stage. All analyses were performed using SAS Version 9.3 (SAS Institute Inc, Cary, NC). The Southern California Permanente Medical Group institutional review board approved this study.
RESULTS
After inclusion and exclusion criteria were applied to the 133,265 patients with baseline sUA above 7 mg/dL, there were 12,751 remaining who were then analyzed by ULT status (Figure 2). ULT 10,061 did not receive ULT. The racial/ethnic breakdown of the cohort was primarily white but with a large representation of Asian, Hispanic, and African Americans, mirroring the population of Southern California (Table 1). A younger, higher-percentage male population was seen in CKD Stage 2 compared with CKD Stages 3 and 4. Comorbidities for cardiovascular disease, diabetes, dyslipidemia, hypertension, osteoarthritis, and obesity are shown in Table 2. Rheumatoid arthritis was diagnosed in 2% to 4% of the patients. Allopurinol prescriptions accounted for 97% of the ULT prescriptions, with another 2% of patients receiving febuxostat and 1% receiving probenecid. Table 3 shows the impact of ULT on changes in eGFR. The use of ULT had significant impact on the 30% eGFR improved function (13.16% vs 11.47%, p = 0.016) and the 30% eGFR decreased function (6.01% vs 6.10%, p = 0.026) compared with the group not receiving ULT.
Table 1.
Demographics of study cohort a
| Characteristic | ULT (n = 2690) | No ULT (n = 10,061) | CKD Stage 2 (n = 4343) | CKD Stage 3 (n = 6299) | CKD Stage 4 (n = 2109) |
|---|---|---|---|---|---|
| Male sex | 63 | 59 | 68 | 59 | 48 |
| Age, years | |||||
| < 50 | 6 | 10 | 16 | 5 | 6 |
| 50–59 | 16 | 19 | 29 | 13 | 12 |
| 60–70 | 32 | 30 | 34 | 31 | 24 |
| > 70 | 47 | 41 | 22 | 51 | 58 |
| Race | |||||
| White | 53 | 52 | 48 | 54 | 55 |
| Asian | 15 | 13 | 17 | 12 | 9 |
| Hispanic | 17 | 20 | 19 | 18 | 24 |
| Black | 13 | 13 | 13 | 14 | 11 |
| Other | 2 | 2 | 3 | 2 | 1 |
Data are presented as percentages. Some percentages may not total to 100 because of rounding.
CKD = chronic kidney disease; ULT = urate-lowering therapy.
Table 2.
Comorbidities and therapies of study cohorta
| Factors | ULT (n = 2690) | No ULT (n = 10,061) | CKD Stage 2 (n = 4343) | CKD Stage 3 (n = 6299) | CKD Stage 4 (n = 2109) |
|---|---|---|---|---|---|
| CV-related disease | 51 | 46 | 32 | 52 | 63 |
| Diabetes | 50 | 47 | 36 | 51 | 62 |
| Dyslipidemia | 86 | 85 | 78 | 88 | 91 |
| Hypertension | 95 | 92 | 85 | 96 | 98 |
| Obesity (BMI < 30 kg/m2) | 48 | 46 | 49 | 45 | 43 |
| Osteoarthritis | 46 | 42 | 37 | 47 | 43 |
| Rheumatoid arthritis | 4 | 3 | 2 | 3 | 4 |
| Gout diagnosis at index date | 47 | 24 | 33 | 30 | 20 |
| Corticosteroids | 52 | 49 | 48 | 50 | 49 |
| Colchicine | 17 | 9 | 11 | 11 | 9 |
| NSAIDs | 82 | 79 | 83 | 80 | 74 |
| ULT prescription | |||||
| Allopurinol | 97 | NA | 29 | 55 | 16 |
| Febuxostat | 2 | 7 | 57 | 36 | |
| Probenecid | 1 | 52 | 48 | 0 | |
Data are presented as percentages. Some percentages may not total to 100 because patients had more than 1 comorbidity or treatment.
BMI = body mass index; CKD = chronic kidney disease; CV = cardiovascular; NA = not applicable; NSAIDs = nonsteroidal anti-inflammatory drugs; ULT = urate-lowering therapy.
Table 3.
Changes in glomerular filtration rate based on use of urate-lowering therapy (ULT)
| Therapy | Change in glomerular filtration rate | |||
|---|---|---|---|---|
| Decrease 30% | −30% to +30% | Improve 30% | Total | |
| No ULT (% of row total) | 605 (6.01) | 8302 (82.52) | 1154 (11.47) | 10,061 |
| ULT (% of row total) | 164 (6.1)a | 2172 (80.74) | 354 (13.16)b | 2690 |
| Total (% of row total) | 769 (6.03) | 10,474 (82.14) | 1508 (11.83) | 12,751 |
p = 0.026.
p = 0.016.
− = decrease; + = increase.
Table 4 shows the changes in eGFR in patients receiving ULT for the entire cohort and for the individual stages. There were 2690 patients receiving ULT, of which 1118 achieved the target sUA and 1572 did not. Patients at target, with mild to moderate CKD showed improvement (OR = 1.78; 95 CI = 1.42.–2.23; p = 0.001), compared with patients receiving ULT not at target. Individual CKD Stages 2, 3, and 4 were evaluated for nonprogression of CKD (eGFR decreased by more than 30%) and CKD improvement (eGFR improved by more than 30%). In patients with sUA below 6 mg/dL, eGFR improvement by 30% or more was seen in those with CKD Stage 2 (OR = 2.26; 95% CI = 1.16–4.41; p = 0.017) and CKD Stage 3 (OR = 2.23; 95% CI = 1.65–3.00; p ≤ 0.001), but not in CKD Stage 4 (OR = 1.50; 95% CI = 0.95–2.37; p = 0.081).
Table 4.
Impact of serum uric acid target by 30% change in glomerular filtration rate
| Category | At target, % | Not at target, % | Difference | Odds ratio (95% CI) | p value |
|---|---|---|---|---|---|
| Patients with < 30% decline in glomerular filtration rate | |||||
| CKD Stage 2 | 4.24 | 5.58 | −1.34 | 0.75 (0.39–1.45) | 0.390 |
| CKD Stage 3 | 3.21 | 6.29 | −3.08 | 0.49 (0.29–0.83) | 0.008 |
| CKD Stage 4 | 15.0 | 10.56 | 4.44 | 1.49 (0.82–2.72) | 0.189 |
| Total | 5.0 | 6.9 | −1.9 | 0.71 (0.51–1.00) | 0.048 |
| Patients with < 30% improvement in glomerular filtration rate | |||||
| CKD Stage 2 | 7.06 | 3.26 | 3.8 | 2.26 (1.16–4.41) | 0.017 |
| CKD Stage 3 | 19.87 | 10.02 | 9.85 | 2.23 (1.65–3.00) | < 0.001 |
| CKD Stage 4 | 30.0 | 22.18 | 7.82 | 1.50 (0.95–2.37) | 0.081 |
| Total | 17.1 | 10.4 | 6.7 | 1.78 (1.42–2.23) | < 0.001 |
CI = confidence interval; CKD = chronic kidney disease.
DISCUSSION
Hyperuricemia has been conclusively linked to decline in renal function. Hyperuricemic mouse models demonstrate the underlying pathophysiology responsible for kidney damage with glomerular changes including vascular wall thickening, tubulointerstitial inflammation, fibrosis, and arterial hypertension.15,16 Hyperuricemia can cause endothelial changes and vascular pathologies leading to renal insufficiency.17 Studies using mouse models show that allopurinol has the potential of reversing kidney disease when the sUA is normalized.16,17 Whereas the guidelines for treating symptomatic gout are well defined, the treatment of asymptomatic hyperuricemia remains controversial. In human subjects, Obermayr et al5 showed that risk of incident kidney disease increased with sUA greater than 8 (OR = 1.74; CI = 1.45–2.09) and was more likely when the sUA is above 9 (OR = 3.12; CI = 2.29–4.25).5 A study by Uchida and colleagues18 also showed that hyperuricemia can lead to end-stage renal disease using 3 different propensity scores. Hyperuricemia, independent of gout, has recognized comorbidities of kidney, cardiac, cerebral disease, and an increase in all-cause mortality.11,12,19 A recent multicenter international review by Sivera et al20 did not recommend treating asymptomatic hyperuricemia, citing articles that showed no significant differences in GFR, serum creatinine level, or proteinuria. A double-blind, placebo-controlled study by Sircar et al,9 not included in the review by Sivera and colleagues, showed a significant improvement in GFR with ULT. Our study also shows the potential benefit of normalizing the sUA in hyperuricemic patients with CKD. The beneficial effect is not uniform across the CKD spectrum; patients with CKD Stages 2 and 3 appear to have the greatest benefit when ACR target sUA is reached.
The 2012 ACR guidelines suggest initiation of ULT in the setting of tophi, frequent gout attacks, or CKD Stage 2 or worse.21 Guideline protocol states that patients should have sUA monitoring every 2 to 5 weeks during ULT initiation and every 6 months after the target sUA is achieved. The recent American College of Physicians recommendation takes a different approach, favoring treating acute attacks over the ACR treat-to-target approach.22 Compliance issues in gout treatment are substantial as reported by Riedel et al23 in an allopurinol compliance study; the diagnosis of gout was made in 42.6% of their 9482 study patients, of which 56% of the patients were compliant with their allopurinol regimen during the 2-year follow-up period and 44% were noncompliant. Even with strong evidence for the treatment of gout, it often remains underdiagnosed and undertreated.24,25
Untreated or poorly treated gout leads to increases in direct health care costs and indirect costs of absenteeism in the workplace. Actual cost estimates for gout vary widely because of the degree of disease burden and different methods. Patients incur direct costs up to $25,000, with higher costs seen in patients with higher sUA and tophi. In addition to the direct costs, indirect costs have a major impact through lost wages.26 A US study estimated that patients with gout annually missed 4.6 days of work and were less productive at work than were individuals without gout.27,28 There is a linear relationship between disease burden, as measured by presence of tophi and number of gout flares, and overall health-related quality of life.29 Wood et al30 compared patients who were controlled with patients who were inadequately controlled, defined by sUA greater or less than 6 mg/dL, respectively, and counted the number of gout flares over 12 months. Patients whose sUA was under control had better quality of life, better productivity, fewer work absences, and less impairment while working than did patients with inadequate control.
One innovative study from KP Northern California used a protocol-driven pharmacist intervention compared with usual care. The primary measure was each group’s ability to achieve the ACR sUA target of 6 mg/dL during a 26-week period.31 Patients in the intervention group lowered their sUA, on average, by 1.5 mg/dL, with 35% of the patients achieving the ACR target compared with 13% in the control group (risk ratio = 2.8; 95% CI = 1.1–7.1; p = 0.03). One conclusion from this study emphasized the importance of periodic contact with the patient. Contact including request for laboratory testing, office visits, phone calls, and letters seemed to improve overall compliance and lessen the risk of adverse consequences of hyperuricemia and gout. Factors contributing to improved control include active management, typically by a rheumatologist, and dose escalation of ULT leading to fewer gout flares.32
In this study we extended the work of our first study to examine whether lowering sUA could improve kidney function and whether that improvement was influenced by CKD stage. We did not include “normal” patients with no signs of renal disease (Stage 1 CKD) or those who had “pre-end-stage renal disease” (Stage 5 CKD). We have noted in previous work that the GFR changes with ULT tend to occur within the first year in patients with more advanced disease, whereas asymptomatic patients would not be expected to show changes for years. In addition, patients with CKD Stage 5 would require only a small change in eGFR to trigger the designation of an outcome. For example, a patient with an eGFR of 15 mL/min would require a change of only 4.5 mL/min, which is well within the daily variation and imprecision of MDRD testing in advanced kidney disease.33
This study has several notable strengths. It reflects the real world because the patients are from KPSC’s 4.2-million-member enrollment. Furthermore, KPSC’s integrated electronic medical system includes clinic notes, hospitalizations, pharmacy, and laboratory data. We used an eGFR change of 30% that is based on the literature34 and a desire to establish a sufficiently high bar to not overstate the effects of therapy.
Study limitations include characteristics of all epidemiologic studies, including the inability to control laboratory acquisition and compliance issues, making this essentially a usual-care study across our entire population. We “lost” more than 100,000 potential patients because they did not obtain GFR or sUA tests during the follow-up period. Table 2 shows only 47% of the study patients had a gout diagnosis at the index date. The study design used the first incidence of laboratory test of sUA above 7 mg/dL for the diagnosis of gout, rather than the initiation of ULT. This would account for the seemingly low number of patients with gout in the study. Proteinuria is a frequent feature of kidney disease, but because it was not a determinant for therapy, it was not included as a comorbidity factor. Additionally, we were not able to control for use of over-the-counter nonsteroidal anti-inflammatory drugs or differences in diet because these items are generally not contained in the clinical record. It is known from other work that nonsteroidal anti-inflammatory drugs are nearly universally used either by prescription or over the counter.35,36 Only 3% of the study patients used an ULT other than allopurinol, 2% for febuxostat and 1% for probenecid. There were not sufficient numbers of patients in these subgroups for separate analysis. As more patients use febuxostat in the future, a study will be needed to see if allopurinol benefit also holds for febuxostat. All patients came from a single medical group, albeit a very large one with more than 4.2 million members that mirrors Southern California in its diversity by age, race, and ethnicity. Finally, our laboratory uses the MDRD equation for estimation of GFR instead of the newer CKD Epidemiology Collaboration equation, potentially underestimating the actual GFR.37
Our study shows that in patients with mild to moderate CKD, achieving the ACR target sUA of less than 6 mg/dL may have a beneficial effect on their kidneys. The OR of 30% improvement when the sUA is below 6 mg/dL for the entire cohort was 1.78 (95% CI = 1.42–2.23; p < 0.001), with the primary benefit coming from the CKD Stage 3 subset with an OR of 2.23 (95% CI = 1.65–3.00; p < 0.001). Goicoechea et al38 prospectively followed up 113 patients for 2 years with assessments at 6, 12, and 24 months. Changes in sUA, inflammatory markers, and cardiovascular events and eGFR were apparent by 6 months.38 This information and our previous study results suggest that improvement can occur within a few months (unpublished data); therefore, we elected to limit our follow-up period to 1 year.
CONCLUSION
Our study shows the benefit to the kidney of treating hyperuricemia to the ACR sUA target of below 6 mg/dL, with the greatest beneficial effect in patients with CKD Stages 2 and 3. This study joins the growing body of research findings suggesting that ULT should be considered in patients with hyperuricemia and CKD.39
Gout
Causes of the gout are: … hereditary disease, high living and exercises … overabundance of wine and venery. Bacchus pater, Venus mater, ira obstetrix arthriditis.
— Sir William Rowley, KB, c 1690–1768, Admiral of the Fleet, British Royal Navy Officer
Acknowledgment
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011 Oct;63(10):3136–41. doi: 10.1002/art.30520. [DOI] [PubMed] [Google Scholar]
- 2.Degli Esposti L, Desideri G, Saragoni S, Buda S, Pontremoli R, Borghi C. Hyperuricemia is associated with increased hospitalization risk and healthcare costs: Evidence from an administrative database in Italy. Nutr Metab Cardiovasc Dis. 2016 Oct;26(10):951–61. doi: 10.1016/j.numecd.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Rai SK, Aviña-Zubieta JA, McCormick N, et al. The rising prevalence and incidence of gout in British Columbia, Canada: Population-based trends from 2000 to 2012. Semin Arthritis Rheum. 2017 Feb;46(4):451–6. doi: 10.1016/j.semarthrit.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004 Oct;44(4):642–50. doi: 10.1053/j.ajkd.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R. Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol. 2008 Dec;19(12):2407–13. doi: 10.1681/asn.2008010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006 Jan;47(1):51–9. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Shin S, Kim K, Choi S, Lee K. Effect of urate-lowering therapy on renal disease progression in hyperuricemic patients with chronic kidney disease. J Rheumatol. 2015 Nov;42(11):2143–8. doi: 10.3899/jrheum.150067. [DOI] [PubMed] [Google Scholar]
- 8.Iseki K, Iseki C, Kinjo K. Changes in serum uric acid have a reciprocal effect on eGFR change: A 10-year follow-up study of community-based screening in Okinawa, Japan. Hyperten Res. 2013 Jul;36(7):650–4. doi: 10.1038/hr.2013.11. [DOI] [PubMed] [Google Scholar]
- 9.Sircar D, Chatterjee S, Waikhom R, et al. Efficacy of febuxostat for slowing the GFR decline in patients with CKD and asymptomatic hyperuricemia: A 6-month, double-blind, randomized, placebo-controlled trial. Am J Kidney Dis. 2015 Dec;66(6):945–50. doi: 10.1053/j.ajkd.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Qin T, Zhou X, Wang J, et al. Hyperuricemia and the prognosis of hypertensive patients: A systemic review and meta-analysis. J Clin Hypertens. 2016 Dec;18(12):1–11. doi: 10.1111/jch.12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu WC, Hung CC, Chen SC, et al. Association of hyperuricemia with renal outcomes, cardiovascular disease, and mortality. Clin J Am Soc Nephrol. 2012 Apr;7(4):541–8. doi: 10.2215/cjn.09420911. [DOI] [PubMed] [Google Scholar]
- 12.Chen JH, Lan JL, Cheng CF, et al. Effect of urate-lowering therapy on all-cause and cardiovascular mortality in hyperuricemic patients without gout: A case-matched cohort study. PLoS One. 2015 Dec 18;10(12):e0145193. doi: 10.1371/journal.pone.0145193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. J Rheumatol. 2014 May;41(5):955–62. doi: 10.3899/jrheum.131159. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 15.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001 Nov;38(5):1101–6. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Lozada LG1, Tapia E, Santamaría J, et al. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005 Jan;67(1):237–47. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 17.Neogi T, George J, Rekhraj S, Struthers AD, Choi H, Terkeltaub RA. Are either or both hyperuricemia and xanthine oxidase directly toxic to the vasculature? A critical appraisal. Arthritis Rheum. 2012 Feb;64(2):327–38. doi: 10.1002/art.33369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uchida S, Chang WX, Ota T, et al. Targeting uric acid and the inhibition of progression to end-stage renal disease—a propensity score analysis. PLoS One. 2015 Dec 23;10(12):e0145506. doi: 10.1371/journal.pone.0145506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu CY, Hu HY, Chou YJ, et al. High serum uric acid levels are associated with all-cause and cardiovascular, but not cancer, mortality in elderly adults. J Am Geriatr Soc. 2015 Sep;63(9):1829–36. doi: 10.1111/jgs.13607. [DOI] [PubMed] [Google Scholar]
- 20.Sivera F, Andrés M, Carmona L, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: Integrating systemic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis. 2014;73(2):328–35. doi: 10.1136/annrheumdis-2013-203325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res. 2012;64(10):1431–46. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qaseem A, Harris RP, Forciea MA Clinical Guidelines Committee of the American College of Physicians. Management of acute and recurrent gout: A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017 Jan 3;166(1):58–68. doi: 10.7326/M16-0570. [DOI] [PubMed] [Google Scholar]
- 23.Riedel AA, Nelson M, Joseph-Ridge N, Wallace K, MacDonald P, Becker M. Compliance with allopurinol therapy among managed care enrollees with gout: A retrospective analysis of administrative claims. J Rheumatol. 2004 Aug;31(8):1575–81. [PubMed] [Google Scholar]
- 24.Sarawate CA, Brewer KK, Yang W, et al. Gout medication treatment patterns and adherence to standards of care from a managed care perspective. Mayo Clin Proc. 2006 Jul;81(7):925–34. doi: 10.4065/81.7.925. [DOI] [PubMed] [Google Scholar]
- 25.Singh JA, Hodges JS, Asch SM. Opportunities for improving medication use and monitoring in gout. Ann Rheum Dis. 2009 Aug;68(8):1265–70. doi: 10.1136/ard.2008.092619. [DOI] [PubMed] [Google Scholar]
- 26.Rai SK, Burns LC, De Vera MA, Haji A, Giustini D, Choi HK. The economic burden of gout: A systemic review. Semin Arthritis Rheum. 2015 Aug;45(1):75–80. doi: 10.1016/j.semarthrit.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Wertheimer A, Morlock R, Becker MA. A revised estimate of the burden of illness of gout. Curr Ther Res Clin Exp. 2013 Dec;75:1–4. doi: 10.1016/j.curtheres.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleinman NL, Brook RA, Patel PA, et al. The impact of gout on work absence and productivity Value Health 2007. July–August104231–7. 10.1111/j.1524-4733.2007.00173.x [DOI] [PubMed] [Google Scholar]
- 29.Khanna PP, Nuki G, Bardin T, et al. Tophi and frequent gout flares are associated with impairments to quality of life, productivity, and increased healthcare resource use: Results from a cross-sectional survey. Health Qual Life Outcomes. 2012 Sep 22;10:117. doi: 10.1186/1477-7525-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood R, Fermer S, Ramachandran S, Baumgartner S, Morlock R. Patients with gout treated with conventional urate-lowering therapy: Association with disease control, health-related quality of life, and work productivity. J Rheumatol. 2016 Oct;43(10):41–8. doi: 10.3899/jrheum.151199. [DOI] [PubMed] [Google Scholar]
- 31.Goldfien R, Pressman A, Jacobson A, Ng M, Avins A. A pharmacist-staffed, virtual gout management clinic for achieving target serum uric acid levels: A randomized clinical trial. Perm J. 2016 Summer;20(3):18–23. doi: 10.7812/TPP/15-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rashid N, Levy GD, Wu YL, Zheng C, Koblick R, Cheetham TC. Patient and clinical characteristics associated with gout flares in an integrated healthcare system. Rheumatol Int. 2015 Nov;35(11):1799–807. doi: 10.1007/s00296-015-3284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szummer K, Evans M, Carrero JJ, et al. Comparison of the Chronic Kidney Disease Epidemiology Collaboration, the Modification of Diet in Renal Disease study and the Cockcroft-Gault equation in patients with heart failure. Open Heart. 2017 Jun 8;4(2):e000568. doi: 10.1136/openhrt-2016-000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang WX, Asakawa S, Toyoki D, et al. Predictors and the subsequent risk of end-stage renal disease—usefulness of 30% decline in estimated GFR over 2 years. PLoS One. 2015 Jul 15;10(7):e0132927. doi: 10.1371/journal.pone.0132927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malone DC, Hutchins DS, Haupert H, et al. Assessment of potential drug-drug interactions with a prescription claims database. Am J Health Syst Pharm. 2005 Oct 1;62(19):1983–91. doi: 10.2146/ajhp040567. doi: 10.2146/ajhp040567. [DOI] [PubMed] [Google Scholar]
- 36.Cheetham TC, Levy G, Niu F, Bixler F. Gastrointestinal safety of nonsteroidal antiinflammatory drugs and selective cyclooxygenase-2 inhibitors in patients on warfarin. Ann Pharmacother. 2009 Nov;43(11):1765–73. doi: 10.1345/aph.1m284. [DOI] [PubMed] [Google Scholar]
- 37.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010 Apr;55(4):622–7. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goicoechea M, de Vinuesa SG, Verdalles U, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol. 2010 Aug;5(8):1388–93. doi: 10.2215/cjn.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy G, Cheetham TC. Is it time to start treating asymptomatic hyperuricemia? Am J Kidney Dis. 2015 Dec;66(6):933–5. doi: 10.1053/j.ajkd.2015.09.002. [DOI] [PubMed] [Google Scholar]