Abstract
Little is known about the pathogenesis of metabolic syndrome, although Toll-like receptor 4 (TLR4) has been implicated. We investigated whether TLR4 in the intestinal epithelium regulates metabolic syndrome by coordinating interactions between the luminal microbiota and host genes that regulate metabolism. Mice lacking TLR4 in the intestinal epithelium (TLR4ΔIEC), but not mice lacking TLR4 in myeloid cells nor mice lacking TLR4 globally, developed metabolic syndrome; these features were not observed in TLR4ΔIEC mice given antibiotics. Metagenomic analysis of the fecal microbiota revealed differences between TLR4ΔIEC and wild type mice, while meta-transcriptome analysis of the microbiota showed that intestinal TLR4 affected the expression of microbial genes involved in the metabolism of lipids, amino acids, and nucleotides. Genes regulated by peroxisome proliferator-activated receptors (PPARs) and the antimicrobial peptide lysozyme were significantly down-regulated in TLR4ΔIEC mice, suggesting a mechanism by which intestinal TLR4 could exert its effects on the microbiota and metabolic syndrome. Supportingly, antibiotics prevented both downregulation of PPAR genes and the development of metabolic syndrome, while PPAR agonists prevented development of metabolic syndrome in TLR4ΔIEC mice. Thus, intestinal epithelial TLR4 regulates metabolic syndrome through altered host-bacterial signaling, suggesting that microbial or PPAR based strategies might have therapeutic potential for this disease.
Introduction
Metabolic syndrome refers to a cluster of disorders including abdominal obesity, glucose intolerance and hepatic steatosis, and is an important cause of morbidity and mortality1. While the precise causes of metabolic syndrome remain incompletely understood, genetic2, dietary3 and microbial factors4 have each been recognized to play a role in its pathogenesis. The importance of bacteria in the development of metabolic syndrome is supported by the striking observation that the administration of antibiotics prevents its development in mice 5,6, while the transfer of bacteria from obese mice or humans to lean mice induces metabolic syndrome in reciepient mice7,8. From the point of view of the host, polymorphisms in the receptor for Gram-negative bacterial endotoxin, namely Toll-like receptor 4 (TLR4), have been associated with an increased risk for the development of metabolic syndrome and obesity in humans9, and patients with metabolic syndrome show increased TLR4 expression in monocytes10,11. The endotoxin receptor complex consists of TLR4, CD14 and MD-2, and this complex signals in response to either the myeloid differentiation primary response gene 88 (MyD88)-dependent pathway, which is critical for the production of several pro-inflammatory cytokines, or the MyD88-independent pathway, which depends on the TIR domain containing adaptor inducing interferon-beta (TRIF) signal adaptor protein and is crucial for type I interferon production12,13. In mice receiving a high fat diet, TLR4 deficient mice show either reduced14, unaffected15, or increased16 risk for the development of metabolic syndrome compared with wild type counterparts. The apparent discrepancy in the findings regarding the role for TLR4 in the development of metabolic syndrome has proven to be a source of significant controversy in the field, and point to a greater need to understand the impact of host-microbial interactions in its pathogenesis. One possible explanation for the varying results may lie in the fact that TLR4 signaling in various different cells – for example the myeloid cells versus the intestinal epithelial cells – could exert different, and perhaps even opposite, effects on the development of metabolic syndrome.
We now seek to address this controversy by testing the hypothesis that the expression of TLR4 in the intestinal epithelium as opposed to other cell types plays a critical role in the development of metabolic syndrome by coordinating the interaction between the luminal microbiota and genes that regulate metabolically important pathways in the host.
Results
Intestinal epithelial TLR4 expression regulates the development of metabolic syndrome in mice.
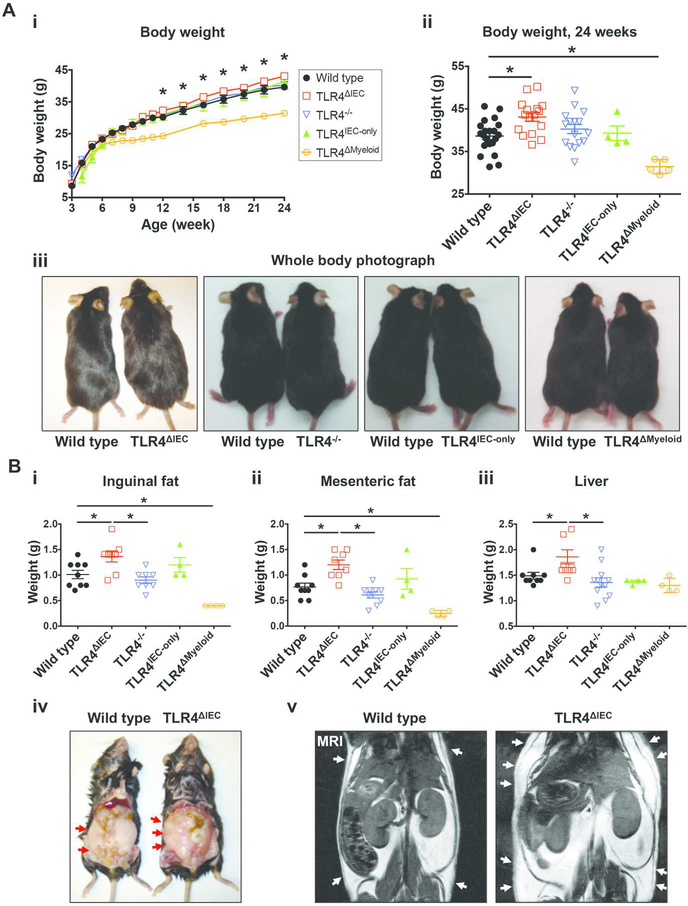
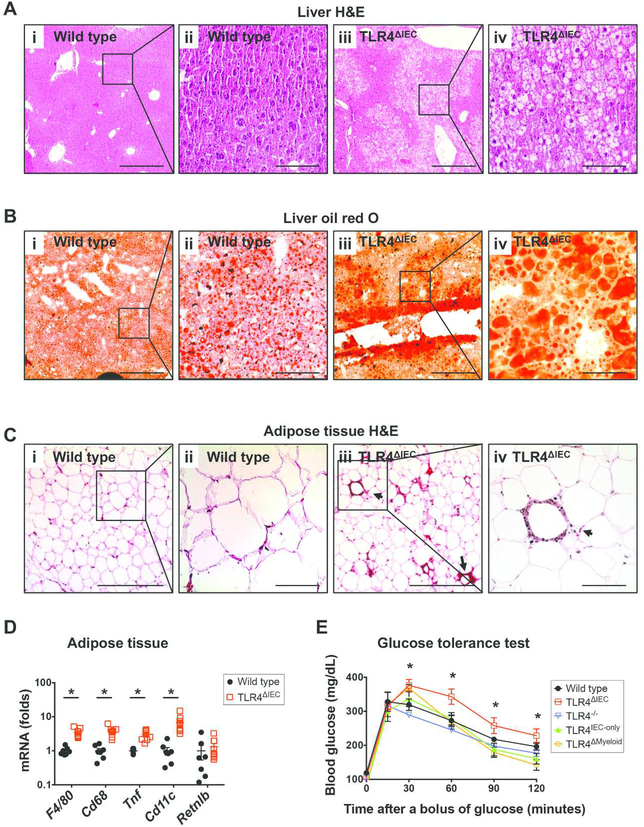
To evaluate the role of intestinal epithelial TLR4 in the development of metabolic syndrome, we first administered standard chow, containing 22% calories as fat, to mice harboring floxed alleles of TLR4 (wild type) or to age- and gender-matched mice in which TLR4 was selectively deleted from the intestinal epithelium (TLR4ΔIEC), from the ages of 3 to 24 weeks. We observed that despite being fed standard chow, when compared with floxed wild type mice, TLR4ΔIEC mice developed a constellation of symptoms consistent with metabolic syndrome1,17, which included significant weight gain (38.64±3.829 g vs 43.08±3.970 g, p<0.05 over 21 weeks) (Figure 1A), and increased weight of adipose tissue and liver (Figure 1B). The differences in weight gain between wild type and TLR4ΔIEC mice strains became noticeable at approximately 12 weeks of age, and became significantly different from wild type mice at 24 weeks of age (Figure 1Ai). The increased body weight in TLR4ΔIEC mice did not depend on food intake (Supplemental Figure 1A), nor hormone levels that regulate appetite since both strains had similar serum leptin and ghrelin expression (Supplemental Figure 1B). Other markers of metabolic syndrome were also observed in the TLR4ΔIEC but not wild type mice, including “hepatocellular ballooning” which indicates the accumulation of fat droplets within hepatocytes (Figure 2A and 2B), a trend towards increased liver triglycerides (Supplemental Figure 1C), the histologic presence of “crown-like structures” within the adipose tissue (Figure 2C) indicating the accumulation of macrophages18, and the increased expression of macrophage markers (F4/80 and Cd68) and the pro-inflammatory M1 phenotype macrophage markers (Tnf and Cd11c), but not the anti-inflammatory M2 phenotype macrophage marker (Retnlb), in the adipose tissue at the age of 24 weeks (Figure 2D). We noted that there was no difference observed in the size of the adipocytes between strains (Supplemental Figure 1D). Despite similar serum insulin (Supplemental Figure 1E), TLR4ΔIEC mice but not wild type counterparts displayed significant insulin resistance, as measured by significant hyperglycemia after the administration of an oral glucose challenge at the age of 24 weeks (Figure 2E). There were no differences in serum cholesterol (Supplemental Figure 1F), serum triglycerides (Supplemental Figure 1G) and serum endotoxin content (Supplemental Figure 1H). Taken together, these findings illustrate that the lack of TLR4 on the intestinal epithelium leads to the development of metabolic syndrome in mice.
Figure 1. Deletion of the lipopolysaccharide receptor TLR4 from intestinal epithelial cells leads to obesity in mice.
A: i: Determination of body weight between 3 and 24 weeks of wild type (n=33), TLR4ΔIEC (n=31), TLR4−/− (n=31), TLR4IEC-only (n=6) and TLR4ΔMyeloid (n=8) mice who were fed standard chow; ii: body weight at 24 weeks for the wild type (n=21), TLR4ΔIEC (n=16), TLR4−/− (n=16), TLR4IEC-only (n=4) and TLR4ΔMyeloid (n=5) mice is shown. Data are represented as mean ± SEM; *p<0.05 wild type vs TLR4ΔIEC mice at the indicated time point; each symbol represents a separate mouse; iii: representative photographs of wild type, TLR4ΔIEC, TLR4−/−, TLR4IEC-only and TLR4ΔMyeloid at 24 weeks of age is shown revealing the significantly larger size of the TLR4ΔIEC mice compared with wild type mice. B: Weight of inguinal fat (i), mesenteric fat (ii) and liver (iii) of wild type (n=9), TLR4ΔIEC (n=8 to 10 as indicated), TLR4−/− (n=8 to 12 as indicated), TLR4IEC-only (n=4) and TLR4ΔMyeloid (n=4) mice fed standard chow at the age of 24 weeks. Data are represented as mean ± SEM; *p<0.05 for the indicated comparison; each symbol represents a separate mouse; iv-v: representative photograph (iv) and micro-MRI (v) revealing increased abdominal fat in the TLR4ΔIEC as compared with wild type mice at 24 weeks of age; arrows show the deposition of adipose tissue in the subcutaneous tissue.
Figure 2. Determinants of metabolic syndrome in mice lacking TLR4 from the intestinal epithelium.
A: Representative photomicrographs showing liver sections of wild type mice (i and ii) and TLR4ΔIEC mice (iii and iv) fed standard chow at the age of 24 weeks, which were stained with haematoxylin and eosin. Scale bars represent 400-μm and 100-μm in the 10× (i and iii) and 40× (ii and iv) panels, respectively. B: Representative photomicrographs showing liver sections of wild type mice (i and ii) and TLR4ΔIEC mice (iii and iv) fed standard chow at the age of 24 weeks, which were stained with oil red O. Scale bars represent 400-μm and 100-μm in the 10× (i and iii) and 40× (ii and iv) panels, respectively. C: Representative photomicrographs showing white adipose tissue sections of wild type mice (i and ii) and TLR4ΔIEC mice (iii and iv) fed standard chow at the age of 24 weeks, which were stained with haematoxylin and eosin. Arrows point to crown-like structure in the white adipose tissue of TLR4ΔIEC mice, and scale bars represent 400-μm and 100-μm in the 10× (i and iii) and 40× (ii and iv) panels, respectively. D: qPCR showing the expression of the indicated macrophage markers in the white adipose tissues of wild type (n=7) and TLR4ΔIEC (n=9) mice fed standard chow at the age of 24 weeks. All data were normalized to the mRNA expression of Rplp0, and the mRNA expression in wild type mice was set to 1. Data are represented as mean ± SEM; *p<0.05 between groups shown; each symbol represents a separate mouse. E: Oral glucose tolerance test of wild type (n=12), TLR4ΔIEC (n=9), TLR4−/− (n=5), TLR4IEC-only (n=4) and TLR4ΔMyeloid (n=4) mice fed standard chow at the age of 24 weeks. Data are represented as mean ± SEM; *p<0.05 wild type vs. TLR4ΔIEC mice.
Given that many studies examining the development of metabolic syndrome in mice have utilized a high fat diet, whereas the above data were observed in the presence of a “standard” diet, we next administered a high fat diet in which 60% of the total calories are derived from fat to wild type and TLR4ΔIEC mice from the age of 3 to 12 weeks. As shown in Supplemental Figure 2A, on a high fat diet, TLR4ΔIEC mice still gained significantly more weight than wild type mice (40.96±5.061 g vs 35.57±3.423 g, p<0.05 over 9 weeks), and the weight gain was observed at much earlier time points as compared with mice fed which were fed standard chow (3 weeks for a high fat diet vs. 9 weeks for those fed standard chow). Further, although 12-week-old TLR4ΔIEC mice fed standard chow started to show significantly increased body weight (Figure 1Ai), there was no difference between wild type and TLR4ΔIEC mice in glucose tolerance (Supplemental Figure 3). On the contrary, 12-week-old TLR4ΔIEC mice who were fed a high fat diet also showed impaired glucose tolerance as compared with age-matched wild type mice fed high fat diet (Supplemental Figure 2B). Moreover, 12-week-old TLR4ΔIEC mice fed high fat diet showed an increased accumulation of adipose tissue (inguinal fat and mesenteric fat) and increased liver weight (Supplemental Figure 2C), elevated serum cholesterol (Supplemental Figure 2D), but similar serum triglycerides (Supplemental Figure 2E) as compared with wild type mice, supportive of our findings that the lack of TLR4 on the intestinal epithelium results in the development of significant weight gain, insulin resistance and other features of metabolic syndrome regardless the fat content of the diet.
To confirm the role of intestinal epithelial TLR4 expression on metabolism, we next studied two additional TLR4 transgenic mouse strains, namely TLR4IEC-only mice in which TLR4 is expressed only in the intestinal epithelial cells, and TLR4−/− mice that are globally TLR4 deficient. As shown in Figure 1A and 1B, the administration of standard chow containing 22% calories from fat to both TLR4−/− and TLR4IEC-only resulted in similar total body weight, as well as the weight of adipose tissue and liver, compared with wild type mice at 24 weeks of age. Similarly, TLR4−/− and TLR4IEC-only mice displayed equivalent glycemic curves after a bolus of glucose compared with wild type mice (Figure 2E). Of note, although the inguinal and mesenteric fat content is higher in the TLR4ΔIEC mice compared with the wild type and the TLR4−/− mice, there are no differences in the other parameters among all the other strains, and in particular there are no differences in fat depositions between the TLR4ΔIEC and TLR4IEC-only mice.
Given that TLR4ΔIEC mice showed increased weight gain and the development of metabolic syndrome, we next investigated further the observation that TLR4−/− mice did not develop metabolic syndrome, suggesting that the lack of TLR4 on some cells may have opposing effects in the development of metabolic syndrome than its expression on other cells. We therefore next hypothesized that TLR4 expression on myeloid cells plays an opposite role to that on the intestinal epithelium in the regulation of host metabolism. To test this possibility directly, we subjected mice in which TLR4 was selectively deleted from myeloid cells (TLR4ΔMyeloid) mice to the same standard diet above, and observed that at 24 weeks, TLR4ΔMyeloid mice did not develop significant weight gain (Figure 1A and 1B), and had a similar glycemic curve to TLR4−/− mice and wild type mice after a glucose bolus (Figure 2E). These findings indicate that deletion of TLR4 from the intestinal epithelium as opposed to other cell types is required for the development of metabolic syndrome in mice, and we next sought to explore the mechanisms involved by focusing on the intestinal microbiota.
The administration of broad spectrum oral antibiotics or co-housing with wild type mice prevented the development of metabolic syndrome in TLR4ΔIEC mice.
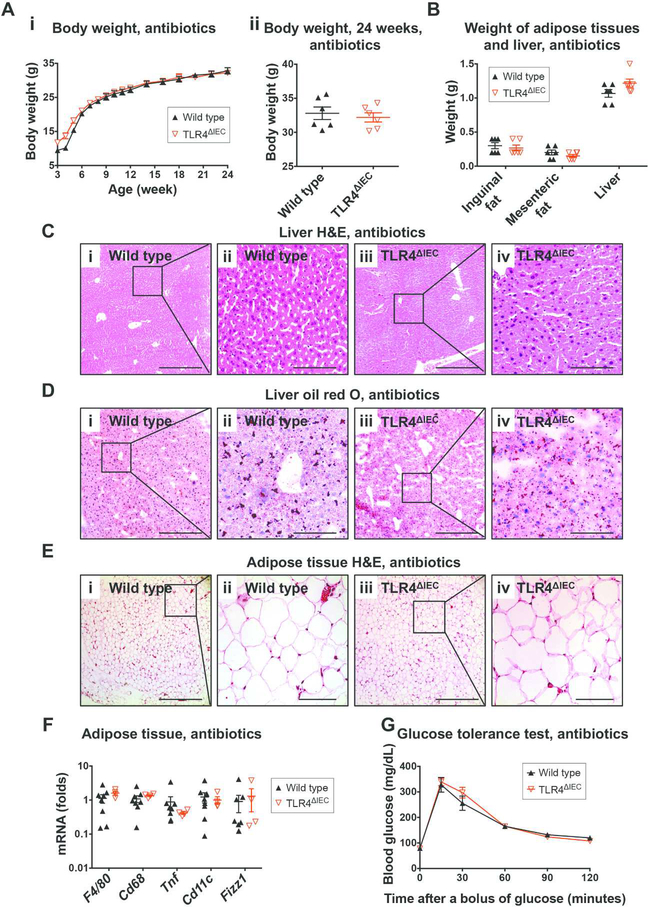
Given that TLR4 is a receptor for bacterial endotoxin, we next hypothesized that the effects of intestinal epithelial TLR4 on the regulation of host metabolism could be mediated in part by an effect on the intestinal microbiota. To test this possibility directly, we orally administered broad spectrum antibiotics to either wild type or TLR4ΔIEC mice which were fed standard chow. The antibiotic treatment was determined to significantly reduce the bacterial load within the intestinal tract as shown in Supplemental Figure 4A, and prevented the previously observed excess weight gain observed in the TLR4ΔIEC strains, which were now found at similar body weights throughout the 24-week period to wild type mice (Figure 3A) and did not show excess weight gain of the adipose tissue and liver (Figure 3B). The administration of antibiotics also prevented the previously observed hepatocellular ballooning (Figure 3C and 3D) and the presence of crown-like structures in the adipose tissue (Figure 3E), and abrogated changes in expression of the macrophage genes in the adipose tissues (Figure 3F). Most strikingly, the administration of oral broad spectrum antibiotics prevented the previously observed glucose intolerance in the in the TLR4ΔIEC mice seen after a glucose bolus (Figure 3G). These findings point to an important role for the microbiota in the development of metabolic syndrome in the TLR4ΔIEC mice. To further evaluate the hypothesis that the effects of intestinal epithelial TLR4 on the regulation of host metabolism could be mediated through effects on the intestinal microbiota, we co-housed TLR4ΔIEC mice and wild type mice together after weaning, and supplied standard chow until 24 weeks of age. Importantly, co-housing resulted in similar body weight gain (Supplemental Figure 4B), similar weight of adipose tissue and liver (Supplemental Figure 4C), and similar glucose tolerance (Supplemental Figure 4D) between TLR4ΔIEC mice and wild type mice, and completey reversed the previously observed metabolic syndrome phenotype. Taken together, these findings support a role for the microbiota in the pathogenesis of metabolic syndrome in the TLR4ΔIEC mice, leading us to next evaluate the effects of TLR4 on the composition and function of the intestinal microbiota in greater detail.
Figure 3. The administration of broad spectrum antibiotics prevented the development of obesity and metabolic syndrome in TLR4ΔIEC mice.
A: The body weight of wild type (n=6) and TLR4ΔIEC (n=6) mice treated with antibiotics from the age of 3 to 24 weeks (i) and at the age of 24 weeks (ii). Data are mean ± SEM; each symbol represents a separate mouse. B: The weight of the inguinal fat, mesenteric fat and liver of wild type (n=6) and TLR4ΔIEC (n=6) mice treated with antibiotics at the age of 24. Data are represented as mean ± SEM; each symbol represents a separate mouse. C: Representative photomicrographs showing liver sections of wild type mice (i and ii) and TLR4ΔIEC mice (iii and iv) treated with antibiotics at the age of 24 weeks, which were stained with haematoxylin and eosin. Scale bars represent 400-μm and 100-μm in the 10× (i and iii) and 40× (ii and iv) panels, respectively. D: Representative photomicrographs showing liver sections of wild type mice (i and ii) and TLR4ΔIEC mice (iii and iv) treated with antibiotics at the age of 24 weeks, which were stained with oil red O. Scale bars represent 400-μm and 100-μm in the 10× (i and iii) and 40× (ii and iv) panels, respectively. E: Representative photomicrographs showing white adipose tissue sections of wild type mice (i and ii) and TLR4ΔIEC mice (iii and iv) treated with antibiotics at the age of 24 weeks, which were stained with haematoxylin and eosin. Scale bars represent 400-μm and 100-μm in the 10× (i and iii) and 40× (ii and iv) panels, respectively. F: qPCR showing the expression of macrophage markers (F4/80 and Cd68), M1 macrophage markers (Tnf and Cd11c) and M2 macrophage marker (Retnlb) in the white adipose tissues of wild type (n=6) and TLR4ΔIEC (n=6) mice treated with antibiotics at the age of 24 weeks. All data were normalized to the mRNA expression of Rplp0, and the mRNA expression in wild type mice was set to 1. Data are represented as mean ± SEM; each symbol represents a separate mouse. G: The oral glucose tolerance test (OGTT) of wild type (n=6) and TLR4ΔIEC (n=6) mice treated with antibiotics at the age of 24 weeks is shown. Data are represented as mean ± SEM; *p<0.05 wild type vs. TLR4ΔIEC mice.
Intestinal epithelial TLR4 influences the composition and function of the intestinal microbiota in mice.
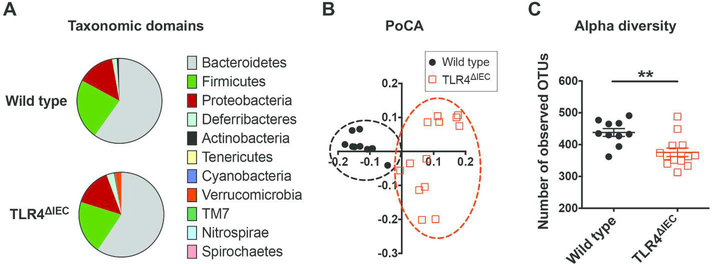
To further explore the role of intestinal epithelial TLR4 on the development of metabolic syndrome, we next subjected stool samples from TLR4ΔIEC and wild type mice to 16S pyrosequencing followed by UniFrac clustering analysis19, and then performed a detailed analysis of the bacterial meta-transcriptome derived from both mouse strains. The composition of the 20 most abundant operational taxonomic units (OTUs) in wild type and TLR4ΔIEC mice are shown in Table 1 and the taxonomic domains are shown in Figure 4A. At the phyla level, the intestinal microbiota of both TLR4ΔIEC and wild type mice were dominated by Bacteroidetes, Firmicutes and Proteobacteria. Although there was an apparent difference in the stool Verrucomicrobia between strains, this was not statistically significant (p=0.09). However, the intestinal microbiota in TLR4ΔIEC mice were revealed to cluster differently from those in wild type mice (Figure 4B), and displayed a significantly lower diversity (Figure 4C). To determine the functional consequences of the loss of TLR4 within the intestinal epithelium on microbial function, we next performed a detailed metatranscriptomic analysis of the stool samples from wild type and TLR4ΔIEC mice. After removing the sequences from mouse origin and thus confining the analysis to bacterial products, 1,001,228 bacterial sequences were identified from the metatranscriptome which were converted to 4,067 unique clusters of orthologous groups (COGs) by running against the COGs protein database. This analysis determined that 246 COGs were found to be differentially expressed between TLR4ΔIEC and wild type mice which were then grouped into the KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways. This investigation revealed a significant alteration in several functional categories that may play a role in the development of metabolic syndrome, including the metabolism of lipid, amino acids, and nucleotides (Table 2), in the bacteria from TLR4ΔIEC as compared with wild type mice, providing insights into the mechanisms by which altered bacterial signaling in response to TLR4 deficiency could lead to the development of metabolic syndrome.
Table 1.
Twenty most abundant OTUs in the stools of wild type and TLR4ΔIEC mice
| OTU | Test-Statistic | P | FDR P | Bonferroni P | TLR4ΔIEC mean | WT mean | taxonomy |
|---|---|---|---|---|---|---|---|
| 250270 | 18.356813 | 0.000018 | 0.010953 | 0.042384 | 0.8 | 28.4 | Firmicutes, Clostridia, Clostridiales |
| 214919 | 17.787644 | 0.000025 | 0.010953 | 0.057152 | 0.6 | 18.7 | Firmicutes, Bacilli, Turicibacterales, Turicibacteraceae, Turicibacter |
| 449353 | 17.487012 | 0.000029 | 0.010953 | 0.066939 | 0.5 | 12.2 | Firmicutes, Clostridia, Clostridiales, Dehalobacteriaceae, Dehalobacterium |
| 296045 | 17.274160 | 0.000032 | 0.010953 | 0.074871 | 47.1 | 0.1 | Bacteroidetes, Bacteroidia, Bacteroidales, Bacteroidaceae, Bacteroides |
| 193680 | 17.033824 | 0.000037 | 0.010953 | 0.084970 | 0.0 | 6.1 | Firmicutes, Clostridia, Clostridiales, Lachnospiraceae |
| 263518 | 16.988636 | 0.000038 | 0.010953 | 0.087016 | 10.7 | 0.2 | Proteobacteria, Deltaproteobacteria, Desulfovibrionales, Desulfovibrionaceae |
| 4466511 | 16.979866 | 0.000038 | 0.010953 | 0.087419 | 27.5 | 0.2 | Bacteroidetes, Bacteroidia, Bacteroidales, Odoribacteraceae, Odoribacter |
| 1105589 | 16.962352 | 0.000038 | 0.010953 | 0.088229 | 75.3 | 0.2 | Bacteroidetes, Bacteroidia, Bacteroidales, S24–7 |
| 262752 | 16.627907 | 0.000045 | 0.010953 | 0.105234 | 2.0 | 241.8 | Bacteroidetes, Bacteroidia, Bacteroidales, S24–7 |
| 312322 | 16.379482 | 0.000052 | 0.010953 | 0.119966 | 2.4 | 56.6 | Bacteroidetes, Bacteroidia, Bacteroidales, S24–7 |
| 2937207 | 16.371329 | 0.000052 | 0.010953 | 0.120483 | 284.5 | 1.1 | Deferribacteres, Deferribacteres, Deferribacterales, Deferribacteraceae, Mucispirillum, schaedleri |
| 544996 | 15.889564 | 0.000067 | 0.012439 | 0.155380 | 0.6 | 10.4 | Firmicutes, Clostridia, Clostridiales, Ruminococcaceae, Oscillospira |
| 259820 | 15.775570 | 0.000071 | 0.012439 | 0.165029 | 0.1 | 34.3 | Firmicutes, Clostridia, Clostridiales |
| 176868 | 15.595223 | 0.000078 | 0.012439 | 0.181539 | 8.0 | 0.0 | Firmicutes, Clostridia, Clostridiales, Dehalobacteriaceae, Dehalobacterium |
| 835900 | 15.543325 | 0.000081 | 0.012439 | 0.186591 | 112.4 | 0.0 | Bacteroidetes, Bacteroidia, Bacteroidales, Odoribacteraceae, Odoribacter |
| 176952 | 14.922099 | 0.000112 | 0.016204 | 0.259267 | 0.1 | 2.0 | Firmicutes, Clostridia, Clostridiales |
| 259859 | 14.227922 | 0.000162 | 0.021115 | 0.374751 | 1.2 | 32.8 | Bacteroidetes, Bacteroidia, Bacteroidales, S24–7 |
| 186358 | 14.201435 | 0.000164 | 0.021115 | 0.380063 | 3.5 | 0.1 | Bacteroidetes, Bacteroidia, Bacteroidales, Bacteroidaceae, Bacteroides |
| 175485 | 13.881567 | 0.000195 | 0.023712 | 0.450533 | 3.5 | 0.1 | Bacteroidetes, Bacteroidia, Bacteroidales, Bacteroidaceae, Bacteroides |
| 4449524 | 13.549172 | 0.000232 | 0.026888 | 0.537759 | 10.6 | 0.0 | Bacteroidetes, Bacteroidia, Bacteroidales, Prevotellaceae, Prevotella |
Figure 4. The effects of intestinal epithelial TLR4 on the luminal microbiota in mice.
A: The major taxonomic domains of intestinal microbiota of wild type and TLR4ΔIEC mice fed standard chow at the age of 24 weeks. B: UNIFRAG clustering analysis of the intestinal microbiota of wild type (n=10) and TLR4ΔIEC (n=13) mice fed standard chow at the age of 24 weeks. C: The alpha-diversity of the intestinal microbiota of wild type (n=10) and TLR4ΔIEC (n=13) mice fed standard chow at the age of 24 weeks. Data are represented as mean ± SEM; *p<0.05 between wild type and TLR4ΔIEC mice; each symbol represents a separate mouse.
Table 2.
Altered KEGG metabolic pathways of the intestinal microbiota between wild type and TLR4ΔIEC mice
| Class of metabolism pathways | Metabolic pathway name |
|---|---|
| Energy metabolism | Carbon fixation in photosynthetic organisms |
| Carbon fixation pathways in prokaryotes | |
| Methane metabolism | |
| Nitrogen metabolism | |
| Oxidative phosphorylation | |
| Carbohydrate metabolism | Amino sugar and nucleotide sugar metabolism |
| Butanoate metabolism | |
| C5-Branched dibasic acid metabolism | |
| Citrate cycle (TCA cycle) | |
| Glycolysis / Gluconeogenesis | |
| Glyoxylate and dicarboxylate metabolism | |
| Inositol phosphate metabolism | |
| Pentose phosphate pathway | |
| Propanoate metabolism | |
| Pyruvate metabolism | |
| Starch and sucrose metabolism | |
| Lipid metabolism | Ether lipid metabolism |
| Glycerophospholipid metabolism | |
| Amino acid metabolism | Alanine, aspartate and glutamate metabolism |
| Arginine and proline metabolism | |
| Histidine metabolism | |
| Lysine biosynthesis | |
| Valine, leucine and isoleucine biosynthesis | |
| Metabolism of other amino acids | Cyanoamino acid metabolism |
| D-Glutamine and D-glutamate metabolism | |
| Glutathione metabolism | |
| Phosphonate and phosphinate metabolism | |
| Amino acid metabolism | Valine, leucine and isoleucine degradation |
| Purine metabolism | |
| Pyrimidine metabolism | |
| Metabolism of cofactors and vitamins | Biotin metabolism |
| Nicotinate and nicotinamide metabolism | |
| Pantothenate and CoA biosynthesis | |
| Porphyrin and chlorophyll metabolism | |
| Ubiquinone and other terpenoid-quinone biosynthesis | |
| Xenobiotics biodegradation and metabolism | Benzoate degradation |
| Chlorocyclohexane and chlorobenzene degradation | |
| Fluorobenzoate degradation | |
| Nitrotoluene degradation | |
| Polycyclic aromatic hydrocarbon degradation | |
| Toluene degradation | |
| Biosynthesis of other secondary metabolites | Penicillin and cephalosporin biosynthesis |
| Glycan biosynthesis and metabolism | Glycosylphosphatidylinositol(GPI)-anchor biosynthesis |
In seeking to investigate the potential mechanisms by which TLR4 expression in the intestinal epithelium could influence the composition of the microbiota in the first place, we observed that mRNA expression of the anti-microbial peptide lysozyme – which has an established role in regulating the composition of the intestinal microbiota and maintaining intestinal homeostasis20 – was significantly reduced in the small intestine of TLR4ΔIEC mice as compared with wild type mice (Supplemental Figure 5). In addition, the administration of antibiotics did not restore lysozyme expression to wild type levels (Supplemental Figure 5), suggesting a predominant role for intestinal TLR4 in its regulation. These findings led us to next investigate how TLR4mediated host-microbial interactions could lead to the development of metabolic syndrome in mice.
TLR4 expression within the intestinal epithelium regulates the expression of metabolic and inflammatory genes in mice.
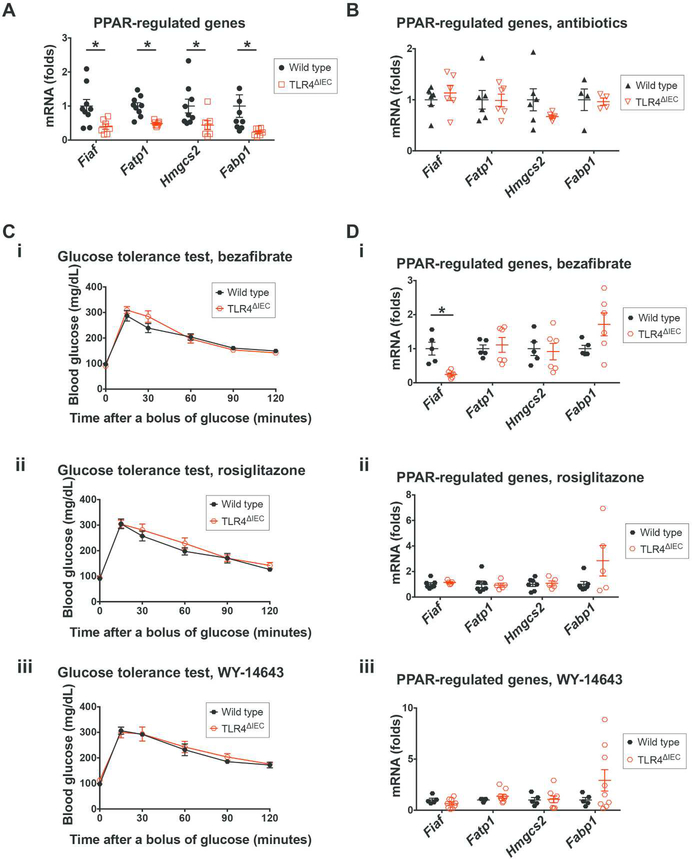
To investigate further how TLR4 expression within the intestinal epithelium could lead to the development of metabolic syndrome, we next performed an unbiased RNA sequencing analysis on intestinal mucosal samples obtained from the terminal ileum of wild type and TLR4ΔIEC mice. This study revealed that key genes within the metabolically relevant PPAR signaling pathway were significantly down-regulated in TLR4ΔIEC mice compared to wild type mice (Table 3 and Supplemental Figure 6A), although the PPAR genes themselves were unchanged (Supplemental Figure 6B). Specific PPAR-regulated genes that were down-regulated included fasting induced adipose factor (Fiaf), fatty acid transport protein 1 (Fatp1), 3-hydroxy-3methylglutaryl-CoA synthase 2 (Hmgcs2) and fatty acid-binding protein 1 (Fabp1) (Figure 5A).
Table 3.
Pathways significantly altered in ileum of wild type and TLR4ΔIEC mice
| Pathway Name | p-value |
|---|---|
| WebGestalt analysis | |
| DNA replication | 0.0087 |
| PPAR signaling pathway | 0.0087 |
| Fructose and mannose metabolism | 0.0391 |
| Vitamin digestion and absorption | 0.0391 |
| Pathway Express analysis | |
| Circadian rhythm | 0.00008 |
| DNA replication | 0.00081 |
| PPAR signaling pathway | 0.00156 |
| Base excision repair | 0.02190 |
| Ingenuity Pathway analysis | |
| Cell Cycle Control of Chromosomal Replication | 0.00004 |
| LPS/IL-1 Mediated Inhibition of RXR Function | 0.00417 |
| Retinol Biosynthesis | 0.00912 |
| Antigen Presentation Pathway | 0.01202 |
| Bupropion Degradation | 0.02042 |
| Estrogen Biosynthesis | 0.02399 |
| Glutathione-mediated Detoxification | 0.02399 |
| PPARα/RXRα Activation | 0.02692 |
| Acetone Degradation I (to Methylglyoxal) | 0.02692 |
| Renal Cell Carcinoma Signaling | 0.03090 |
| Adenosine Nucleotides Degradation II | 0.03981 |
| Glucocorticoid Biosynthesis | 0.03981 |
| Mineralocorticoid Biosynthesis | 0.03981 |
| Interferon Signaling | 0.04266 |
| RAR Activation | 0.04571 |
| Maturity Onset Diabetes of Young (MODY) Signaling | 0.04786 |
Figure 5. Intestinal epithelial TLR4 regulates the expression of PPAR-related genes in the gut while PPAR agonists reversed the glucose intolerance in TLR4ΔIEC mice.
A-B: qPCR showing the expression of PPAR family genes Fiaf, Fatp1, Hmgcs2 and Fabp1 in the ileum of wild type (n=9 and 6, respectively) and TLR4ΔIEC (n=7 and 6, respectively) mice fed standard chow at the age of 24 weeks in the absence (A) or presence (B) of oral antibiotics. All data were normalized to the mRNA expression of Rplp0, and the mRNA expression in wild type mice was set to 1. Data are represented as mean ± SEM; *p<0.05 wild type vs TLR4ΔIEC mice; each symbol represents a separate mouse. C: Blood glucose concentrations during oral glucose tolerance test of wild type (n=5, 7 and 5, respectivley) and TLR4ΔIEC (n=6, 6 and 9, respectively) mice treated with PPAR agonists bezafibrate (i), rosiglitazone (ii) and WY-14643 (iii) at the age of 24 weeks are shown. Data are represented as mean ± SEM, *p<0.05 wild type vs TLR4ΔIEC mice. D: qPCR showing mRNA expression of Fiaf, Fatp1, Hmgcs2 and Fabp1 in the ileum of wild type (n=5, 7 and 5, respectively) and TLR4ΔIEC (n=6, 5 and 9, respectively) mice treatment with PPAR agonists bezafibrate (i), rosiglitazone (ii) and WY-14643 (iii) at the age of 24 weeks were analyzed using qPCR. All data were normalized to the mRNA expression of Rplp0, and the mRNA expression in wild type mice was set to 1. Data are represented as mean ± SEM; each symbol represents a separate mouse. *p<0.05 wild type vs TLR4ΔIEC mice.
In control experiments, the expression of those PPAR-regulated genes were similar between wild type and TLR4ΔMyeloid mice (Supplemental Figure 6C), consistent with the lack of metabolic syndrome observed in the TLR4ΔMyeloid mice. Given that we had identified that the administration of antibiotics prevented the development of metabolic syndrome in TLR4ΔIEC mice, we next sought to assess the effects of antibiotic administration of the expression of these key PPARregulated genes. As shown in Figure 5B, the administration of broad spectrum antibiotics prevented the previously observed differences in expression of Fiaf, Fatp1, Hmgcs2, and Fabp1, in both wild type and TLR4ΔIEC mice after treatment with broad spectrum antibiotics. We next evlauted whether the reduced expression of these key PPAR-regulated metabolic genes in response to TLR4 deletion was required for the development of metabolic syndrome. To do so, we next administrated pharmacologic agonists of the PPAR signaling pathway to wild type and TLR4ΔIEC mice and assessed the consequences on glucose metabolism. Importantly, the oral administration of bezafibrate (which activates all three PPAR subtypes), rosiglitazone (which selectively activates PPAR gamma), or WY-14643 (which activates PPAR alpha, gamma and delta) completely reversed the impaired glucose tolerance in TLR4ΔIEC mice (Figure 5C), and restored the expression of these PPAR-regulated genes, namely Fiaf, Fatp1, Hmgcs2, and Fabp1 (Figure 5D). It is noteworthy that TLR4 deletion did not only regulate PPAR-regulated metabolic genes, as the gene expression profile of TLR4ΔIEC mice revealed a pro-inflammatory phenotype, characterized by up-regulated expression of macrophage markers (F4/80, Cd68 and Mcp1, Supplemental Figure 6D), neutrophil markers (Mpo and Elane, Supplemental Figure 6E), a trend towards increased expression of the pro-inflammatory cytokine interleukin 6 (Il6) in the ileum (Supplemental Figure 6F). These findings are consistent with a potential degree of subtle inflammation in the small intestine of TLR4ΔIEC mice, which could contribute to the development of metabolic syndrome. Taken together, these findings suggest that TLR4 regulates both host and bacterial genes that have a key role in energy metabolism, which acts in concert to govern the development of metabolic syndrome and obesity in mice.
Discussion
We now show that TLR4 expression in the intestinal epithelium plays a previously unrecognized role in the development of metabolic syndrome in mice through the regulation of host-bacterial interactions. The conclusion that TLR4 deficiency from the intestinal epithelium leads to the development of metabolic syndrome was reached by studying five different strains of mice, including TLR4ΔIEC mice which developed metabolic syndrome, as well as mice lacking TLR4 on myeloid cells, mice globally deficient in TLR4, mice expressing TLR4 only on the intestinal epithelium and wild type mice which each did not. In terms of understanding how TLR4 signaling in the intestinal epithelial cells regulates body energy metabolism, we now describe a key role for TLR4 in the regulation of the intestinal microbiota, as the administration of antibiotics or co-housing of TLR4ΔIEC mice with wild type mice reversed the induction of metabolic syndrome. We further showed that TLR4 deletion from the intestinal epithelium led to a downregulation of key genes in the regulation of host metabolism, namely PPAR-regulated genes, and that PPAR activation reversed the development of metabolic syndrome. Taken together, these findings illustrate important roles for TLR4 in the regulation of bacteriaepithelium interactions in the development of metabolic syndrome, and suggest the possibility that modulating either the microbiota or host PPAR-regulated genes could offer novel preventive of therapeutic approaches for this disease.
The current study adds to a body of work that has examined the roles of TLR4 in the pathogenesis of metabolic syndrome, around which there has been significant controversy. Researchers have determined that TLR4 inhibition either reduced14,21,22, increased16, or had no effect15 on diet-induced obesity in mice, and attributed these opposing findings to differences in food consumption16, or energy expenditure22 between the knockout and wild type animals. We now report that the lack of TLR4 on the intestinal epithelium as opposed to other cell types is responsible for the development of metabolic syndrome, and that TLR4-dependent differences in the genetic pathways that drive host metabolism play an important mechanistic role. The cellspecific effect of TLR4 on host metabolism in the current study is supported by earlier findings showing that deleting TLR4 from hepatic cells improved insulin resistance23, while deleting TLR4 from myeloid cells either improved21 or had no effect on insulin resistance23. It is reassuring to us that the impaired glucose tolerance in the TLR4ΔIEC mice was consistently observed over a four year period, and was seen in two different animal colonies at two separate institutions, namely at the University of Pittsburgh and Johns Hopkins University.
One of the most intriguing findings of the current work is the observation that the expression of TLR4 on the intestinal epithelium plays a key role not only in the composition but also in the meta-transcriptomic profile of the intestinal microbiota. Explaining, rather than speculating, the role of specific microbial groups would require a more focused investigation, including work with gnotobiotic mice with defined gut microbial communities. The meta-transcriptome analysis of the bacteria in wild type and TLR4ΔIEC mice intestines identified key differences which were primarily linked to carbohydrate metabolism (11 pathways) and energy metabolism (5 pathways) between strains. The observed changes in the microbiota gene expression in the TLR4ΔIEC mice may have effects on host metabolism, as suggested by prior investigators. Specifically, Musso et al have shown that changes in gene expression in gut microbiota metabolism may lead to increased nutrient absorption and bile acid reabsorption leading to metabolic syndrome24, and this topic has been reviewed in recent years25–28. Given that the mammalian intestine has a limited ability to hydrolyze complex polysaccharides, it is noteworthy that we found that 11 genes for carbohydrate metabolism pathways were altered in the microbiota of the TLR4ΔIEC mice which developed metabolic syndrome. These findings are reminiscent of the observations of prior studies showing that the abundance of Firmicutes versus Bacteroidetes has been linked to the development of obesity7 through the differential ability of these strains to break down complex carbohydrates, and thus vary the degree of carbohydrate delivery and absorption by the host29.
While the current findings reveal that TLR4 expression in the intestinal epithelium can impact the composition of the intestinal microbiota, a precise explanation of how this occurs remains lacking. We have previously shown that the deletion of intestinal epithelial TLR4 leads to increased expression of mucin MUC230, which is secreated by goblet cells and forms a mucus barrier which can influence to growth and composition of commensal bacteria in the small intestine31. Further, we now demonstrate that TLR4ΔIEC mice reveal significantly reduced expression of the antimicrobial pepetide lysozyme (Supplemental Figure 5), which is secreated by Paneth cells and plays important roles in maintaining the composition of the intestinal microbiota32. It is possible that the host expression of TLR4 could alter the composition of the microbiota through either effects on goblet cells or lysozyme secretion, and do so in a manner that influences metabolic genes, for reasons that are not obvious. Of note, TRIF−/− mice were shown to also exhibit increased globle cells30, reduced lysozyme-positive Paneth cells33, and more interestingly, impaired glucose tolerance34, supporting this concept, and indicating a potential role for TLR4-TRIF signaling in the development of metabolic syndrome in TLR4ΔIEC mice.
A major finding of the current study centered around the observation that TLR4 expression within the terminal ileum plays a previously unrecognized role in the regulation of key metabolic and inflammatory genes within the intestine of the host, in particular those within the PPAR family35,36. It is noteworthy that in inflammatory diseases, PPAR signaling can decrease the extent of TLR4 signaling in myeloid cells37,38, adipocytes 39, and the intestinal epithelium40, linking the PPAR signaling with altered TLR4 responsiveness. We now extend these prior findings by showing that the reverse can also occur, namely that TLR4 deficiency also reduces PPAR expression. Prior authors have shown that high fat diet-induced obesity is related to an increase in circulating free fatty acids, which can act as endogenous ligands of TLR441 and PPAR42, thus providing a potential explanation as to why TLR4-mutant mice may be protected from obesity and metabolic syndrome41. However, in the current study, in which we show that TLR4ΔIEC mice are at greater risk for metabolic syndrome and obesity, we now posit that in the presence of a non-high-fat diet, in which circulating free fatty acids are not expected to be increased, the genetic consequences of intestinal epithelial TLR4 deficiency predominate, namely a lack of PPAR, which can negatively regulate metabolism in mice43,44. In support of this concept, agonists of PPAR profoundly reversed the effects of TLR4 deficiency on the development of metabolic syndrome in the TLR4ΔIEC mice (Figure 5C).
It is noteworthy that the current findings may allow us to postulate a general signaling mechanism by which bacterial recognition by microbial receptors in the intestinal epithelium leads to alterations in the metabolic profile of the host. Specifically, the work of Gewirtz has revealed that mice lacking the receptor for certain Gram-negative species including Salmonella, namely Toll-like receptor 5, either globally (TLR5−/−)5 or in the intestinal epithelium (TLR5ΔIEC)45, or mice lacking the conserved TLR adaptor proteins namely MyD88 (MyD88−/−)46 or TRIF (TRIF−/−)34, each develop both low-grade intestinal inflammation and metabolic syndrome, supporting the concept that bacterial signaling influences metabolism in the host through MyD88 and/or TRIF. Further studies revealed that mice lacking TLR5 from dendritic cells (TLR5ΔDC)45, or mice lacking MyD88 from dendritic cells (MyD88ΔDC)47 or other myeloid cells (MyD88ΔMyeloid)48 do not show any signs of metabolic syndrome. By contrast, additional studies reveal that the deletion of MyD88 from myeloid cells (MyD88ΔMyeloid) or endothelial cells (MyD88ΔEndo) protected mice from high fat diet-induced metabolic syndrome49. These intriguing studies provide further evidence for the cell-specific nature of TLR signaling in the development of metabolic syndrome, as we now reveal to be the case for TLR4.
In summary, we have now shown that TLR4 signaling in the intestinal epithelium plays a key role in the regulation of metabolic syndrome through the regulation of microbial and host metabolic pathways, and that these effects can be reversed by the administration of PPAR agonists. Our findings suggest a novel link between host metabolism, host genetics and the intestinal microbiota, and indicate that by exploring this relationship in greater detail, we can potentially reverse this complex and highly morbid disease.
Materials and Methods
Animal strains and experimentation
All mice experiments were approved by the Animal Care and Use Committee of the University of Pittsburgh and by the Johns Hopkins University Animal Care and Use Committee. Mice harboring floxed alleles of TLR4 (wild type), mice in which TLR4 was selectively deleted from the intestinal epithelium (TLR4ΔIEC), mice that are globally TLR4 deficient (TLR4−/−) and mice in which TLR4 was expressed only in the intestinal epithelium (TLR4IEC-only) were generated in our laboratory as recently described30,50. The breeding scheme for the generation of mice in which TLR4 was selectively deleted from the myeloid cells (TLR4ΔMyeloid), and confirmation of the lack of TLR4 in the macrophages, are shown in Supplemental Methods and Supplemental Figure 7. All animals were housed in a specific pathogen free environment on a 12-hour-light/12-hourdark cycle with free access to acidified tap water and standard rodent chow (PicoLab mouse diet 20, 22% kcal% fat) from weaning until 24 weeks. Only male mice were included for analysis, and up to 5 male littermates of each strain were housed in one cage after weaning. For cohousing experiments, up to total 5 male mice of mixed TLR4ΔIEC mice and wild type mice were housed in one cage after weaning. Where indicated, mice were administered a high fat diet in which 60% of the total calories were derived from fat51. In further experiments, mice were administered broad spectrum antibiotics in the drinking water (1 g/L neomycin, 0.5 g/L vancomycin, 1 g/L ampicillin and 1 g/L metronidazole) as the only source of water from the age of 3 to 24 weeks. PPAR agonist-treated mice received 285mg/kg/day bezafibrate, 25 mg/kg/day rosiglitazone, or 140 mg/kg/day WY-14643 orally from the age of 23 to 24 weeks. The total abdominal fat content was determined by micro-MRI, in which isoflurane anesthetized mice were secured to a micro-MRI cradle and advanced into the magnet of a 7Tesla micro-MRI system (Bruker Bio Spin Corporation, Billerica, MA, USA). A rapid acquisition with relaxation enhancement with fat saturation T1 protocol was applied on the abdomen52.
Histologic preparation and oil red-O staining
Adipose tissue and liver were freshly harvested, paraformaldehyde-fixed, and embedded in paraffin. Histological staining was carried out on 4-μm-thick sections which were stained with heamatoxylin and eosin (H&E). Oil red-O staining was performed in fresh liver cryosections which were fixed with 4 % (w/v) paraformaldehyde, and after washing with 60% isopropanol, the cryosections were counterstained with hematoxylin.
RNA isolation, cDNA synthesis, and quantitative PCR (qPCR)
Total RNA was isolated from the epididymal white adipose tissue and ileum of mice of 24-weekold mice using the RNeasy mini kit (Qiagen, Valencia, CA, USA) following the manufacturer’s protocol. Complementary DNA was synthesized from 0.5 μg RNA using M-MLV reverse transcriptase. The qPCR analysis was performed with the Bio-Rad CFX96 Real-Time System (Biorad, Hercules, CA) using the primers listed in Table 4. The relative mRNA expression levels were normalized against the expression of housekeeping gene ribosomal protein lateral stalk subunit P0 (Rplp0) relative to wild type mice.
Table 4.
PCR primers
| Gene | Forward primer (5’ - 3’) | Reverse primer (5’ - 3’) |
|---|---|---|
| Rplp0 | GGCGACCTGGAAGTCCAACT | CCATCAGCACCACAGCCTTC |
| F4/80 | GCTCCTGGGTGCTGGGCATT | TCCCGTACCTGACGGTTGAGCA |
| Cd68 | CTTAAAGAGGGCTTGGGGCA | ACTCGGGCTCTGATGTAGGT |
| Il6 | CCAATTTCCAATGCTCTCCT | ACCACAGTGAGGAATGTCCA |
| Tnf | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| Cd11c | CAAAATCTCCAACCCATGCT | CACCACCAGGGTCTTCAAGT |
| Retnlb | CGTGGAGAATAAGGTCAAGGAAC | CACACCCAGTAGCAGTCATC |
| Fiaf | CTCCGTGGGGACCTTAACTG | AGAGGATAGTAGCGGCCCTT |
| Fatp1 | GTGCCACCAACAAGAAGATTG | CTGCGGTCACGGAAATACA |
| Hmgcs2 | CAAGCTGGAAACAACCAGCC | TCAACCGAGCCAGGGATTTC |
| Fabp1 | GGAAGGACATCAAGGGGGTG | TCACCTTCCAGCTTGACGAC |
| Mcp1 | ATGCAGTTAACGCCCCACTC | CCCATTCCTTCTTGGGGTCA |
| Mpo | GACAGTGTCAGAGATGAAGCTACT | TTGATGCTTTCTCTCCGCTCC |
| Elane | CAGAGGCGTGGAGGTCATTT | GAAGATCCGCTGCACAGAGA |
| Pparα | AAGAACCTGAGGAAGCCGTTCTGTG | GCAGCCACAAACAGGGAAATGTCA |
| Pparg | AAGAACCATCCGATTGAAGC | CCAACAGCTTCTCCTTCTCG |
| Lysozyme | AAGCTGGCTGACTGGGTGTGTTTA | CACTGCAATTGATCCCACAGGCAT |
| LysM | GCAAAACCCCAAGAGCTGTG | CGGTTTTGACAGTGTGCTCG |
Oral glucose tolerance test (OGTT)
Glucose levels were determined via glucometer (Bayer, Whippany, NJ, USA). Oral glucose tolerance test was performed in overnight-fasted 24-week-old mice administered 2g of glucose per kg body weight by oral gavage, and the blood glucose levels were measured via tail puncture at 1 min before and 15, 30, 60, 90 and 120 min after the oral gavage53.
Statistical analysis
Data were analyzed for statistical significance by Mann-Whitney test using GraphPad Prism (GraphPad, La Jolla, CA, USA). A p value of less than 0.05 was considered statistically significant, and data are presented as mean ± SEM as indicated.
Supplementary Material
Acknowledgment:
D.J.H. is supported by R01GM078238 and R01DK083752 from the National Institutes of Health.
Grant support: D.J.H. is supported by R01GM078238 and R01DK083752 from the National Institutes of Health.
Abbreviations:
- TLR4
Toll like receptor 4
- TLR4ΔIEC mice
mice lacking TLR4 in the intestinal epithelium
- TLR4−/− mice
TLR4 deficient mice, TLR4IEC-only mice, mice in which TLR4 is expressed only in the intestinal epithelial cells
- TLR4ΔMyeloid mice
mice lacking TLR4 in leukocytes
- OTUs
operational taxonomic units
- COGs
clusters of orthologous groups
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- Tnf
tumor necrosis factor
- PPAR
peroxisome proliferator-activated receptor
- Fiaf
fasting induced adipose factor
- Fatp1
fatty acid transport protein 1
- Hmgcs2
3-hydroxy-3-methylglutaryl-CoA synthase 2
- Fabp1
fatty acid-binding protein 1
- MyD88
myeloid differentiation primary response gene 88
- TRIF
TIR domain containing adaptor inducing interferon-beta
- Retnlb
Resistin like beta
- Mcp1
monocyte chemotactic protein 1
- Mpo
myeloperoxidase
- Elane
elastase
- Il6
interleukin 6
- Rplp0
ribosomal protein lateral stalk subunit P0
- TLR5
Toll-like receptor 5
- LysM
lysozyme 2
- H&E
heamatoxylin and eosin
- qPCR
quantitative PCR
- OGTT
oral glucose tolerance test
Footnotes
Author Contributions:
Conceptualization, P.L., C.P.S. and D.J.H.; Methodology, P.L., C.P.S. and D.J.H.; Investigation, P.L., C.P.S., Y.Y., H.J., T.P.J., W.B.F., A.V., K.J.B., M.J.M.; Writing – Original Draft, P.L.; Writing – Review & Editing, P.L., C.P.S. and D.J.H.; Funding Acquisition, D.J.H.; Resources, D.J.H.; Supervision, D.J.H..
Disclosures:
The authors have declared that no conflict of interest exists.
References
- 1.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006; 23(5): 469–480. [DOI] [PubMed] [Google Scholar]
- 2.Brown AE, Walker M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr Cardiol Rep 2016; 18(8): 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kovatcheva-Datchary P, Arora T. Nutrition, the gut microbiome and the metabolic syndrome. Best Pract Res Clin Gastroenterol 2013; 27(1): 59–72. [DOI] [PubMed] [Google Scholar]
- 4.Parekh PJ, Balart LA, Johnson DA. The Influence of the Gut Microbiome on Obesity, Metabolic Syndrome and Gastrointestinal Disease. Clin Transl Gastroenterol 2015; 6: e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 2010; 328(5975): 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Luccia B, Crescenzo R, Mazzoli A, Cigliano L, Venditti P, Walser JC et al. Rescue of Fructose-Induced Metabolic Syndrome by Antibiotics or Faecal Transplantation in a Rat Model of Obesity. PLoS One 2015; 10(8): e0134893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444(7122): 1022–1023. [DOI] [PubMed] [Google Scholar]
- 8.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 2013; 341(6150): 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinhardt AP, Aranguren F, Tellechea ML, Gomez Rosso LA, Brites FD, Martinez-Larrad MT et al. A functional nonsynonymous toll-like receptor 4 gene polymorphism is associated with metabolic syndrome, surrogates of insulin resistance, and syndromes of lipid accumulation. Metabolism 2010; 59(5): 711–717. [DOI] [PubMed] [Google Scholar]
- 10.Jialal I, Huet BA, Kaur H, Chien A, Devaraj S. Increased toll-like receptor activity in patients with metabolic syndrome. Diabetes Care 2012; 35(4): 900–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardy OT, Kim A, Ciccarelli C, Hayman LL, Wiecha J. Increased Toll-like receptor (TLR) mRNA expression in monocytes is a feature of metabolic syndrome in adolescents. Pediatr Obes 2013; 8(1): e19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gay NJ, Symmons MF, Gangloff M, Bryant CE. Assembly and localization of Toll-like receptor signalling complexes. Nat Rev Immunol 2014; 14(8): 546–558. [DOI] [PubMed] [Google Scholar]
- 13.O’Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol 2013; 13(6): 453–460. [DOI] [PubMed] [Google Scholar]
- 14.Pierre N, Deldicque L, Barbe C, Naslain D, Cani PD, Francaux M. Toll-like receptor 4 knockout mice are protected against endoplasmic reticulum stress induced by a high-fat diet. PLoS One 2013; 8(5): e65061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 2007; 100(11): 1589–1596. [DOI] [PubMed] [Google Scholar]
- 16.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006; 116(11): 3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanwar P, Kowdley KV. The Metabolic Syndrome and Its Influence on Nonalcoholic Steatohepatitis. Clin Liver Dis 2016; 20(2): 225–243. [DOI] [PubMed] [Google Scholar]
- 18.Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y et al. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009; 58(11): 2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 2005; 71(12): 8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 2011; 9(5): 356–368. [DOI] [PubMed] [Google Scholar]
- 21.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G et al. Hematopoietic cellspecific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab 2009; 10(5): 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes 2007; 56(8): 1986–1998. [DOI] [PubMed] [Google Scholar]
- 23.Jia L, Vianna CR, Fukuda M, Berglund ED, Liu C, Tao C et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nature communications 2014; 5: 3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med 2011; 62: 361–380. [DOI] [PubMed] [Google Scholar]
- 25.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012; 489(7415): 242–249. [DOI] [PubMed] [Google Scholar]
- 26.Sonnenburg JL, Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016; 535(7610): 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W et al. Host-gut microbiota metabolic interactions. Science 2012; 336(6086): 1262–1267. [DOI] [PubMed] [Google Scholar]
- 28.Hooper LV. Bacterial contributions to mammalian gut development. Trends Microbiol 2004; 12(3): 129–134. [DOI] [PubMed] [Google Scholar]
- 29.Li M, Gu D, Xu N, Lei F, Du L, Zhang Y et al. Gut carbohydrate metabolism instead of fat metabolism regulated by gut microbes mediates high-fat diet-induced obesity. Benef Microbes 2014; 5(3): 335–344. [DOI] [PubMed] [Google Scholar]
- 30.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 2012; 143(3): 708–718 e701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson ME, Ambort D, Pelaseyed T, Schutte A, Gustafsson JK, Ermund A et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci 2011; 68(22): 36353641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wittkopf N, Gunther C, Martini E, Waldner M, Amann KU, Neurath MF et al. Lack of intestinal epithelial atg7 affects paneth cell granule formation but does not compromise immune homeostasis in the gut. Clin Dev Immunol 2012; 2012: 278059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stockinger S, Duerr CU, Fulde M, Dolowschiak T, Pott J, Yang I et al. TRIF signaling drives homeostatic intestinal epithelial antimicrobial peptide expression. J Immunol 2014; 193(8): 42234234. [DOI] [PubMed] [Google Scholar]
- 34.Hutton MJ, Soukhatcheva G, Johnson JD, Verchere CB. Role of the TLR signaling molecule TRIF in beta-cell function and glucose homeostasis. Islets 2010; 2(2): 104–111. [DOI] [PubMed] [Google Scholar]
- 35.Pineda Torra I, Gervois P, Staels B. Peroxisome proliferator-activated receptor alpha in metabolic disease, inflammation, atherosclerosis and aging. Current opinion in lipidology 1999; 10(2): 151159. [DOI] [PubMed] [Google Scholar]
- 36.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nature medicine 2004; 10(4): 355–361. [DOI] [PubMed] [Google Scholar]
- 37.Dasu MR, Park S, Devaraj S, Jialal I. Pioglitazone inhibits Toll-like receptor expression and activity in human monocytes and db/db mice. Endocrinology 2009; 150(8): 3457–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appel S, Mirakaj V, Bringmann A, Weck MM, Grunebach F, Brossart P. PPAR-gamma agonists inhibit toll-like receptor-mediated activation of dendritic cells via the MAP kinase and NFkappaB pathways. Blood 2005; 106(12): 3888–3894. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Calvo R, Serrano L, Coll T, Moullan N, Sanchez RM, Merlos M et al. Activation of peroxisome proliferator-activated receptor beta/delta inhibits lipopolysaccharide-induced cytokine production in adipocytes by lowering nuclear factor-kappaB activity via extracellular signal-related kinase 1/2. Diabetes 2008; 57(8): 2149–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eun CS, Han DS, Lee SH, Paik CH, Chung YW, Lee J et al. Attenuation of colonic inflammation by PPARgamma in intestinal epithelial cells: effect on Toll-like receptor pathway. Dig Dis Sci 2006; 51(4): 693–697. [DOI] [PubMed] [Google Scholar]
- 41.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007; 56(7): 1761–1772. [DOI] [PubMed] [Google Scholar]
- 42.Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator activated receptors alpha and gamma. Proceedings of the National Academy of Sciences of the United States of America 1997; 94(9): 4318–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costet P, Legendre C, More J, Edgar A, Galtier P, Pineau T. Peroxisome proliferator-activated receptor alpha-isoform deficiency leads to progressive dyslipidemia with sexually dimorphic obesity and steatosis. The Journal of biological chemistry 1998; 273(45): 29577–29585. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Matthews J, Zhou L, Pelton P, Liang Y, Xu J et al. Improvement of dyslipidemia, insulin sensitivity, and energy balance by a peroxisome proliferator-activated receptor alpha agonist. Metabolism: clinical and experimental 2008; 57(11): 1516–1525. [DOI] [PubMed] [Google Scholar]
- 45.Chassaing B, Ley RE, Gewirtz AT. Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice. Gastroenterology 2014; 147(6): 1363–1377 e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hosoi T, Yokoyama S, Matsuo S, Akira S, Ozawa K. Myeloid differentiation factor 88 (MyD88)deficiency increases risk of diabetes in mice. PLoS One 2010; 5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanian M, Ozcan L, Ghorpade DS, Ferrante AW Jr., Tabas I. Suppression of Adaptive Immune Cell Activation Does Not Alter Innate Immune Adipose Inflammation or Insulin Resistance in Obesity. PLoS One 2015; 10(8): e0135842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Everard A, Geurts L, Caesar R, Van Hul M, Matamoros S, Duparc T et al. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat Commun 2014; 5: 5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu M, Zhou H, Zhao J, Xiao N, Roychowdhury S, Schmitt D et al. MyD88-dependent interplay between myeloid and endothelial cells in the initiation and progression of obesity-associated inflammatory diseases. J Exp Med 2014; 211(5): 887–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Afrazi A, Branca MF, Sodhi CP, Good M, Yamaguchi Y, Egan CE et al. Toll-like receptor 4mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem 2014; 289(14): 9584–9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Heek M, Compton DS, France CF, Tedesco RP, Fawzi AB, Graziano MP et al. Diet-induced obese mice develop peripheral, but not central, resistance to leptin. J Clin Invest 1997; 99(3): 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson DH, Narayan S, Wilson DL, Flask CA. Body composition analysis of obesity and hepatic steatosis in mice by relaxation compensated fat fraction (RCFF) MRI. J Magn Reson Imaging 2012; 35(4): 837–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beguinot F, Nigro C. Measurement of glucose homeostasis in vivo: glucose and insulin tolerance tests. Methods Mol Biol 2012; 933: 219–228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.