Abstract
Hypoxia acts as an important regulator of physiological and pathological processes. Hypoxia inducible factors (HIFs) are the central players involved in the cellular adaptation to hypoxia and are regulated by oxygen sensing EGLN prolyl hydroxylases. Hypoxia affects many aspects of cellular growth through both redox effects and through the stabilization of HIFs. The HIF isoforms likely have differential effects on tumor growth via alteration of metabolism, growth, and self-renewal and are likely highly context-dependent. In some tumors such as renal cell carcinoma, the EGLN/HIF axis appears to drive tumorigenesis, while in many others HIF1 and HIF2 may actually have a tumor suppressive role. An emerging role of HIF biology are its effects on the tumor microenvironment. The EGLN/HIF axis plays a key role in regulating the function of the various components of the tumor microenvironment, which include cancer-associated fibroblasts, endothelial cells, immune cells, and the extracellular matrix (ECM). Here, we discuss hypoxia and the diverse roles of HIFs in the setting of tumorigenesis and the maintenance of the tumor microenvironment as well as possible future directions of the field.
Keywords: Hypoxia, HIF, tumor microenvironment, cellular homeostasis, mouse model
Overview of the Importance of Hypoxia and HIF to Cellular Homeostasis
The cellular response to hypoxia, or low oxygen levels, is an evolutionarily conserved program driven by the transcription factors known as the hypoxia inducible factors (HIFs). Although we often associate hypoxia with ischemic pathophysiologic states such as stroke or cardiovascular disease, our cells must also deal with constant fluctuations in oxygen on a daily basis (Pugh and Ratcliffe, 2003). This is a normal part of cellular and tissue homeostasis. This is exemplified during embryogenesis, when hypoxia is an intermittent consequence of developing prior to established blood vessels, and nutrients and oxygen must diffuse to their target cells. The importance of hypoxia and the cellular response to it was further demonstrated in knockouts of the HIF proteins that were shown to be embryonic lethal due to cardiac and vascular malformations in HIF-1a knockout mice (Kotch et al., 1999) and divergent phenotypes in HIF-2α mice depending on the genetic background of the mice (Patel and Simon, 2008). More recent studies have shown that the hypoxia response pathway regulates critical roles in the liver (Nath and Szabo, 2012), osteoblastic niche cells (Rankin et al., 2012), intestine (Colgan and Taylor, 2010), and the heart (Giordano, 2005; Nakada et al., 2017).
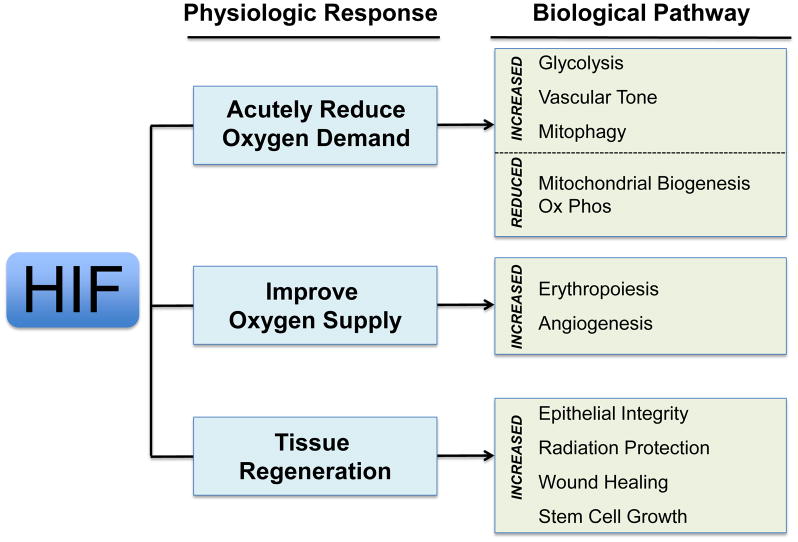
In hypoxic conditions, HIF is rapidly stabilized. When bound to its cognate hypoxia response elements (HREs) within enhancer elements, HIF transcription factors increase the transcription of its target genes. These downstream effectors of HIF teleologically aid in diverse biological processes that aid in survival during hypoxia. For instance, hypoxic cells often shift their metabolism to glycolysis (Semenza et al., 1994), and reduce mitochondrial numbers through mitophagy (Semenza, 2007; Zhang et al., 2007) and decreased biogenesis(LaGory et al., 2015) thus reducing dependence on oxidative metabolism. Furthermore, HIF enhances hematopoiesis (Goldberg et al., 1988; Goldberg et al., 1987), angiogenesis (Shweiki et al., 1992), tissue remodeling (Graham et al., 1999), epithelial permeability (Synnestvedt et al., 2002) and vascular tone (Bodi et al., 1995), which may increase oxygen delivery. Growth is delayed by cycle arrest through the induction of cell cycle inhibitors p21 (Goda et al., 2003) and p27 (Hackenbeck et al., 2009) (Figure 1). Not surprisingly, this powerful coordinated program of gene expression promotes survival in harsh environments. However, this hypoxia program also helps tissues to heal. The preconditioning of tissues against ischemia/hypoxia strengthens and protects them against tremendous physiological insults such as cardiac ischemia (Olenchock et al., 2016), stroke (Karuppagounder and Ratan, 2012; Ratan et al., 2004; Ratan et al., 2007; Reischl et al., 2014), and even lethal radiation damage (Taniguchi et al., 2014).
Figure 1.
The diverse physiological roles of HIF protein
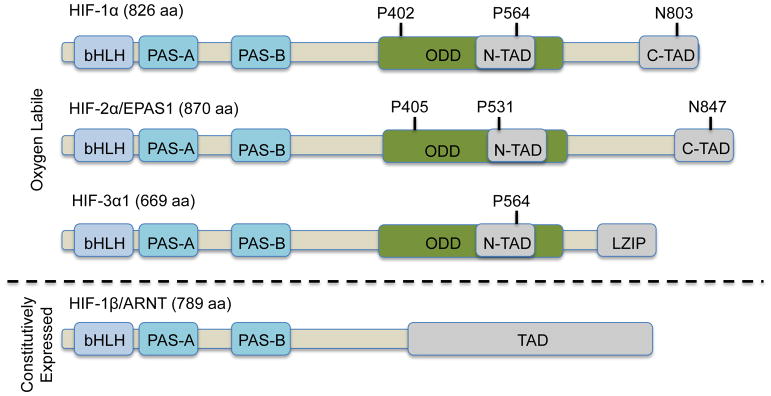
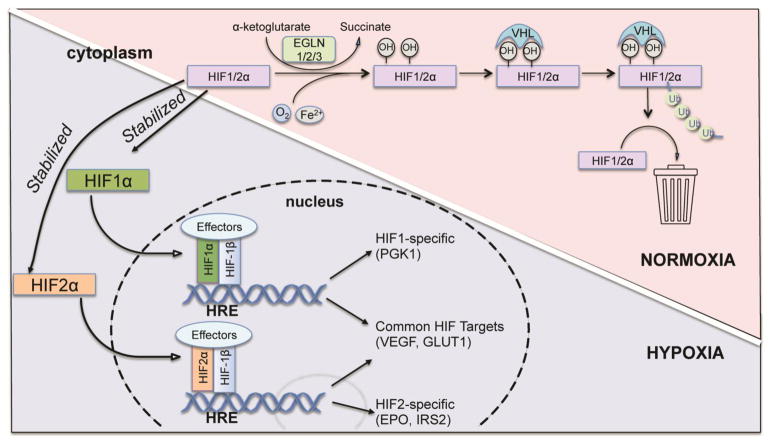
Biochemically, HIF is a heterodimer consisting of one of three alpha (HIF-1α, HIF-2α, HIF-3α) subunits bound to the aryl hydrocarbon nuclear translocator (ARNT), also known as HIF-1β (reviewed in (Huang and Bunn, 2003)). Both HIFα and β are members of the basic helix-loop-helix Per/Arnt/Sim (bHLH-PAS) family of transcription factors (Figure 2). Both HIF-1α and HIF-2α contain O2-dependent degradation (ODD) domain with two different proline sites for hydroxylation in the presence of oxygen. Moreover, HIF-1α and HIF-2α contain both N-terminal transactivation domain (N-TAD) and C-terminal transactivation (C-TAD) while HIF-1β contains only one TAD at the C terminus. In comparison, HIF-3α lacks the C-TAD domain but instead contains the leucine zipper (LZIP) domain in its longest variant. HIF-3α also only contains one proline site in the ODD domain (Heikkila et al., 2011). In the C-TAD domain of HIF-1α and HIF-2α, there is a conserved asparagine residue, which can be hydroxylated by an Fe(II) and O2-dependent enzyme, factor inhibiting HIF (FIH), thus blocking the interaction between HIFα and transcriptional co-factors (Lando et al., 2002). While the beta subunit is continually expressed, the alpha subunit is an oxygen-labile protein whose levels fluctuate with the oxygen tension within the cell such that the alpha subunit is stabilized in hypoxia and destabilized in normoxia (Huang et al., 1998; Salceda and Caro, 1997). Under normal atmospheric oxygen tension, oxygen-dependent prolyl hydroxylases use molecular oxygen along with co-factors iron and 2-oxoglutarate to drive hydroxylation of prolines 402 and 564 of HIF-1α and proline 405 and 531 of HIF-2α (Ivan et al., 2001; Jaakkola et al., 2001). These hydroxyproline moieties then serve as docking sites for the von Hippel Lindau (VHL) protein (Epstein et al., 2001; Hewitson et al., 2003; McNeill et al., 2002) (Figure 3). The VHL protein is the recognition component of an E3 ubiquitin ligase complex that targets HIF for degradation by the 26S proteasome (Kamura et al., 1999; Kibel et al., 1995; Lonergan et al., 1998; Pause et al., 1997; Pause et al., 1999). Thus, prolyl hydroxylases and the VHL protein complex are two key molecules in regulating the levels of HIF, and the hypoxic response. In hypoxic conditions, both HIF-1α and HIF-2α are stabilized and form a heterodimer with HIF-1β, which together binds to HRE sequences residing in the enhancer element of target genes. HIF-1α and HIF-2α regulate some shared as well as unique target genes (Keith et al., 2011) (Figure 3). Both HIF-1α and HIF-2α regulate GLUT1 and VEGF, while HIF-1α specifically regulates PGK1 and LDHA in RCC and mouse ESCs, and HIF-2α regulates EPO and IRS2 in the liver (Wei et al., 2013).
Figure 2.
Components of Human HIF heterodimer.
Figure 3.
Oxygen-dependent regulation of HIFα protein.
There are three oxygen-dependent prolyl hydroxylases in mammals, and they are known by two different naming systems, which can be confusing. For instance, in one method of gene naming, these enzymes are called the prolyl hydroxylase domain-containing (PHD) proteins and the HIF-regulating isoforms are appropriately named PHD1, PHD2, and PHD3 (Fraisl et al., 2009). Alternatively, these HIF prolyl hydroxylases are also named after the C.elegans orthologue egg laying defective nine (EGLN) gene (Kaelin, 2011). However, the gene numbering in the EGLN naming system do not match those in the PHD naming system, which creates significant confusion for those not in the field. For example, EGLN1 is synonymous with PHD2, while EGLN2 is equivalent to PHD1 (Bruick and McKnight, 2001; Jaakkola et al., 2001). Thankfully, EGLN3 simply equates with PHD3. While both naming systems exist in the literature, we have adopted the EGLN nomenclature for several practical reasons. In addition to the confusion brought about by the differences in isoform numbering, PHD proteins share naming similarities to the plant homeodomain (PHD) zinc fingers which are found in many chromatin-modifying proteins (Sanchez and Zhou, 2011). Moreover, a simple literature search for PHD proteins will often return a very large list of papers that includes anyone with a Ph.D. degree. Thus, while both EGLN and PHD designations are correct, we strongly feel that EGLN may be a better way to discuss this important area of biology.
In terms of function, Egln1 appears to be the dominant isoform, since its germline knockout is embryonic lethal (Minamishima et al., 2008). Whole body knockouts of Egln2 and Egln3, on the other hand, were viable and displayed relatively mild phenotypes (Takeda et al., 2008). Egln1 (Chan et al., 2009) and Egln3 (Stiehl et al., 2006) are inducible by hypoxia at the mRNA level and are reliable markers of hypoxia. There does appear to be redundancy in the regulation of HIF by the EGLN isoforms, but studies have suggested that the Egln1 primarily regulates HIF1 (Chan and Giaccia, 2010), while Egln3 regulates HIF2 stability (Bishop et al., 2008). Moreover, there is a growing literature that the Egln proteins have HIF-independent functions that could possibly be even more critical than their roles in regulating HIF stability (Garvalov et al., 2014; Henze et al., 2014).
The Role of HIF in Tumorigenesis
Since hypoxia is important to normal tissues, many have postulated that low oxygen levels contribute to cancer growth and treatment resistance. These assertions have come from an abundant literature that have shown that cancer cells cultured in vitro take on many undesirable traits when placed into hypoxia culture, such as resistance to chemotherapy (Samanta et al., 2014; Warfel and El-Deiry, 2014) and radiation (Harada et al., 2012; Moeller et al., 2004; Zhong et al., 2015), increased proliferation and migration (Chen et al., 2015; Gordan et al., 2007; Liao and Johnson, 2007; Semenza, 2010). However, when in vivo experiments were performed to confirm this phenotype, the experimental results were initially counterintuitive. Rather than being a potent oncoprotein, HIF appears to chiefly function as a tumor suppressor in vivo. For instance, the deletion of HIF2α in lung cancer (Jiang et al., 2009; Mazumdar et al., 2010) or sarcoma models (Nakazawa et al., 2016) demonstrated accelerated tumor growth, suggesting a baseline tumor suppressive effect. This is mediated by the HIF2-dependent suppression of the Scgb3a1(Hin-1) gene in the lung cancer model (Jiang et al., 2009; Mazumdar et al., 2010), and by altered mTORC1 signaling in sarcoma models (Nakazawa et al., 2016). A similar phenotype was observed in mouse models of pancreatic cancer, where HIF1α deletion accelerated tumor growth, also suggesting a basal role in tumor suppression. The mechanism of this tumor suppression by HIF1 is unclear, but may involve the differential activation of immune cells, notably CD45+ immune cell infiltration, and B lymphocytes (Lee et al., 2016). It is notable that one experiment did suggest oncogenic properties of HIF2. When a nondegradable variant of HIF-2α (proline-to-alanine substitutions within the ODD domain) was conditionally overexpressed in a mutant Kras (Kras G12D) model of lung cancer, there was increased tumor burden and decreased survival compared with mice expressing only KrasG12D (Kim et al., 2009). This result appears to be at odds with the previously mentioned reference, however, it should be noted that overexpression of HIF2 in normoxia is not physiologic and may instead reflect off-target biology.
To some extent, the lack of oncogenic properties of HIF should not be surprising since patients with Von Hippel-Lindau (VHL) disease exhibit only a limited range of cancers. The loss of VHL results in widespread stabilization of HIF in many tissues in these patients, but they only get a limited range of lesions including hemangioblastomas of the central nervous system, retinal angiomas, endolymphatic sac tumors, epididymis or broad ligament cystadenomas, renal cysts and renal cell carcinomas, pheochromocytomas, pancreatic cysts and pancreatic neuroendocrine tumors (Lonser et al., 2003), while clear cell renal carcinoma (ccRCC) and hemangioblastomas are the main causes of death (Maher et al., 1990; Neumann et al., 1992). This is in contradistinction to genetic disorders like Li-Fraumeni which have dysregulated p53 function and are prone to many types of sarcomas and aggressive adenocarcinomas. In addition, inactivation of the VHL gene in embryonic stem cells does not promote teratocarcinoma growth (Mack et al., 2003). It should be made clear, however, that HIF is likely responsible for a narrow range of cancers with the most striking being the aforementioned VHL mutant ccRCC driven by HIF-2α (Kondo et al., 2002). Interestingly, HIF-2α inhibitors have been designed and appear to have a specific activity in this disease (Chen et al., 2016; Cho et al., 2016), but its applicability to other cancers are not clear.
Stromal HIF as a regulator of Cancer Growth
Since the in vivo studies linking HIF and tumorigenesis are relatively weak, there are likely other ways that hypoxia and HIF affect tumor growth. We now understand that the tumor microenvironment plays a strong role in accelerating (Hanahan and Weinberg, 2011; Whiteside, 2008), or possibly constraining tumor growth (Rhim et al., 2014). The role of HIF in the microenvironment is not completely understood. The two-way dialogue between cancer cells and stroma is essential for tumor development and is strongly influenced by hypoxia. The tumor stroma is a fairly ill-defined term for any non-cancerous cell found within a tumor and can be divided into three main categories: cells of mesenchymal origin, cells of hematopoietic origin, and non-cellular components (Pattabiraman and Weinberg, 2014). Cells of mesenchymal origin include fibroblasts, myofibroblasts, endothelial cells, adipocytes, and mesenchymal stem cells. Cells of hematopoietic origin consist of cells of the lymphoid lineage, including T cells, B cells, and natural killer (NK) cells, and those of the myeloid lineage, including macrophage, neutrophils and myeloid-derived suppressor cells (MDSCs). Non-cellular component is mostly extracellular matrix (ECM), which includes proteins, glycoproteins and proteoglycans. Here, we will focus on the cancer-associated fibroblast, endothelial cells, and immune cells.
Cancer-associated fibroblasts (CAFs)
Cancer-associated fibroblasts (CAFs) contains two distinct cell types: cells that are similar to fibroblasts that support normal epithelial tissues and myofibroblasts which are rare in healthy epithelial tissues (Hanahan and Weinberg, 2011). A major feature of activated stromal cells is the formation of contractile stress fibers and expression of α-smooth muscle actin (SMA). Cancer-associated fibroblast secretes a variety of extracellular matrix components, resulting in desmoplastic stroma. Many epithelial tumors exhibit a prominent desmoplastic reaction, including breast, prostate and ovarian cancers. Perhaps the most exaggerated stromal reaction can be found in pancreatic ductal adenocarcinoma (PDAC), which displays extensive desmoplasia that increases intratumoral pressure and blocks intratumoral oxygen transport, rendering the tumors hypoxic (Incio et al., 2016). Moreover, the lack of blood flow from this desmoplastic reaction has been postulated to impede chemotherapy delivery (Koay et al., 2014) and lead to worsened outcomes (Olive et al., 2009). Of note, CAFs within PDAC are often positive for α–SMA-expressing activated pancreatic stellate cells (PSCs), and can account for as much as 80% of the tumor volume (Erkan et al., 2012). Despite the known association of hypoxia, CAF activation, and HIF stabilization within many aggressive solid tumors, the function of HIF within the microenvironment is unclear. One prominent study demonstrated that HIF-1α, but not HIF-2α, activation in human breast cancer stromal cells promotes a shift toward aerobic glycolysis and tumor growth via the paracrine production of lactate that feeds cancer cells (Chiavarina et al., 2012). However, this study merely used in vitro cell culture and in vivo xenograft model, which may not fully recapitulate the in vivo microenvironment, as another study using syngeneic tumor models found that deletion of fibroblast HIF-1α accelerates tumor growth (Kim et al., 2012).
Other similar studies in head and neck CAFs and vulvar CAFs reveal hypoxia or loss of EGLN1 leads to inactivation of CAFs and reduced tumor metastasis (Madsen et al., 2015). But again this study was conducted in in vitro setting. In a spontaneous mammary gland tumor study, global Egln1 haplodeficiency leads to decreased tumor stiffness and metastasis without affecting primary tumor growth, which is due to reduced activation of CAFs (Kuchnio et al., 2015). However, confounding developmental, paracrine, or endocrine events from global Egln1 deletion cannot be completely ruled out. Compartment-specific ablation of Egln1 or Hif1a using a dual recombinase system (Moding et al., 2015) may be a method to overcome this potential methodological deficiency.
Endothelial cells
Tumor vessels, comprised mainly of endothelial cells, mediate tumor perfusion and also secrete regulatory paracrine factors. Unlike normal blood vessels, tumor vessels have abnormal organization, structure, and function. Intratumoral hypoxia is due to inadequate oxygen supply and disorganized network of vasculature. Mounting evidence suggests that hypoxia and HIF play central roles in regulating tumor endothelial cells. HIF-1α and HIF-2α have complementary functions in physiological and pathological angiogenesis. For instance, the loss of HIF-1α in endothelial cells inhibits blood vessel growth and tumor size by decreasing VEGF expression (Tang et al., 2004), whereas HIF-2α null endothelial cells exhibit high vessel density and branching but poor perfusion and low pericyte coverage through delta-like ligand 4/Notch (DII4/Notch) pathway and angiopoietin 2 (Ang2)-mediated pathway. Thus endothelial HIF2α null mice have reduced tumor growth (Skuli et al., 2009; Skuli et al., 2012). HIF also controls small molecule mediators of vascular response other than angiogenic factors, such as nitric oxide (NO), which affects the overall vascular tension of tumor vessels. The loss of endothelial HIF-1α reduces NO synthesis and impairs tumor cell migration through endothelial layers which is a gatekeeper to metastatic cell intravasation, while endothelial HIF-2α appears to play an opposing role (Branco-Price et al., 2012).
Another important component of the endothelial niche is the pericyte, which lines the outside of vascular endothelial cells and is required for a functional vasculature. Tumor-associated vasculature has fewer supporting pericyte cells. Pericyte depletion in early-stage non-hypoxic tumors suppresses neovascularization, tumor growth, and lung metastasis (Cooke et al., 2012). However, in hypoxic tumors, pericyte targeting increases intratumoral hypoxia and lung metastasis through Ang2-mediated pathway (Keskin et al., 2015).
Immune cells
Virtually all adult solid tumors contain infiltrating immune cells including both myeloid cells and tumor infiltrating lymphoid cells (TILs). Myeloid cells include subsets of granulocytes, dendritic cells, tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs). TILs can be divided into two subsets of cells with opposing functions: anti-tumor effector T cells which include CD4+ and CD8+ T cells, and immunosuppressive regulatory T (Treg) cells. HIF induces host immune function, affecting myeloid-derived suppressor cells differentiation and function, regulating lymphocyte development, and driving T cell differentiation and cytotoxicity. HIF-1α is widely expressed in most innate and adaptive immune populations.
Many solid tumors show low oxygen density and abundant macrophage infiltration. Deletion of HIF-1α in macrophages in a progressive murine model of breast cancer results in reduced tumor growth by suppressing tumor-infiltrating T cells (Doedens et al., 2010). Hypoxia significantly increases the expression of PD-L1 on macrophages, dendritic cells and tumor cells through a HIF-1α, but not HIF-2α dependent pathway. Thus blockade of PD-L1 along with inhibition of HIF-1α may provide a novel approach for cancer immunotherapy (Noman et al., 2014). Moreover, hypoxia-induced Semaphorin 3A (Sema3A) contributes to recruitment of macrophages through neuropilin 1 (Nrp1)-plexin signaling (Casazza et al., 2013). Myeloid-derived suppressor cells are another cell source in the tumor microenvironment that can dampen antitumor immunity. Activation of HIF-1α is essential for myeloid cell infiltration and activation in vivo, independent of the function of VEGF (Cramer et al., 2003). Hypoxia via HIF-1α alters the function of MDSC and redirects their differentiation toward tumor-associated macrophages (Corzo et al., 2010). Similarly, mice lacking HIF-2α in myeloid cells displayed reduced tumor associated macrophage (TAM) infiltration in different carcinoma models, which is associated with reduced tumor cell proliferation and progression (Imtiyaz et al., 2010).
T lymphocytes are highly migratory adaptive immune cells that encounter a wide range of oxygen tensions, which are essential for T cell differentiation and function. Naive CD4+ T cells acquire specialized effector functions in response to different cytokines in the microenvironment, which includes Th1 cells, Th2 cells, and Th17 cells. It has been shown that tumor hypoxia promotes the recruitment of Treg cells by releasing chemokines, which in turn, promotes tumor tolerance and angiogenesis (Facciabene et al., 2011). However, there is also contradictory finding suggest otherwise, showing that HIF-1α promotes differentiation of CD4+ Th17 cells while preventing Treg differentiation (Dang et al., 2011). Enhanced HIF activity, due to loss of VHL, modulates the adaptive immune response of CD8+ T cells to persistent infection (Doedens et al., 2013), and maintains the stability and suppressive function of Foxp3(+) T cells and IFN-γ production (Lee et al., 2015). EGLN proteins in T cells suppress pulmonary inflammation, maintain immune tolerance, and may thus create an immunologically permissive environment for tumor colonization in the lung (Clever et al., 2016).
HIF in the metastatic niche
As mentioned previously, hypoxia promotes cell motility. This is interpreted as a model of metastatic progression. Several intriguing mediators of HIF and hypoxia have been postulated. Tumors are stiffer than the surrounding normal tissue, which is induced by ECM deposition and remodeling by resident fibroblasts, and by increased contractility of the epithelium (Frantz et al., 2010). Emerging data indicate the strong link between hypoxia and the composition and the organization of the ECM. It has been shown that HIF-1 directly regulates collagen deposition by activating collagen prolyl hydroxylases, which in turn promotes invasion and metastasis of hypoxic breast cancer cells (Gilkes et al., 2013). Collagen crosslinking is initiated in the extracellular space by the lysyl oxidase (LOX) family of secreted enzymes. LOX is postulated to be secreted directly from hypoxic breast cancer cells to act directly on the extracellular matrix, as well as directly on osteoblasts and osteoclasts, which enhance tumor cell colonization (Cox et al., 2015). In clear cell renal cell carcinoma (ccRCC), stabilized HIF-1 and HIF-2 directly activate the expression of protein kinase GAS6/AXL by binding to the hypoxia-response element in the AXL proximal promoter. GAS6/AXL promotes cellular invasion and metastasis through activating the SRC proto-oncogene (Rankin et al., 2014; Zhou et al., 2016). Recent data also suggest that the HIF/AXL axis may also contribute to response to immunotherapy and radiation (Aguilera et al., 2016).
Implications in Cancer Biology and Therapy
Hypoxia alters the trajectory of cancer and makes tumors more difficult to treat. This is not surprising since the lack of oxygen directly antagonizes chemotherapy and ionizing radiation in damaging DNA. However, we must be careful to assign correlation and causation between effects caused by lack of oxygen and those promoted by HIF (Kaelin, 2017). Hypoxia is often associated with larger and more unfavorable tumors, and HIF is stabilized in hypoxia, but it does not necessarily follow that HIF plays a causative role in the pathophysiology of solid tumors. In fact, many recent studies have shown the opposite: that the EGLN/HIF axis acts as a tumor suppressor in preclinical models and in selected human data.
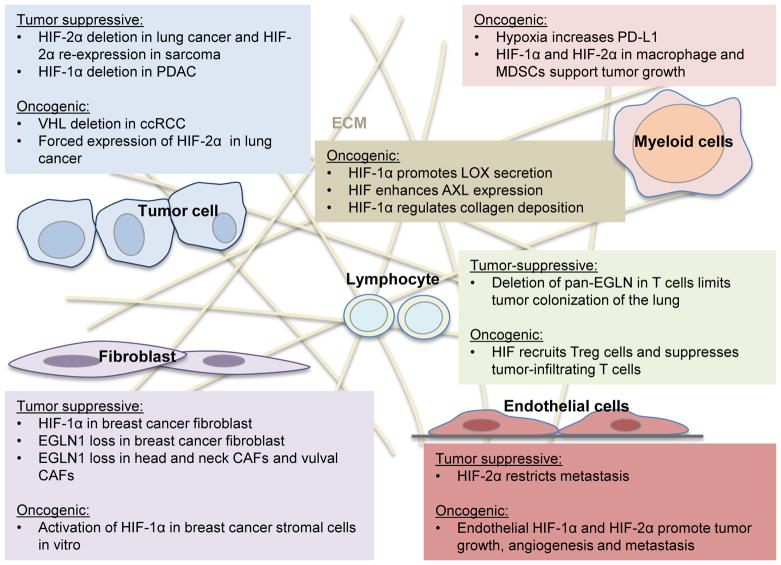
The actions of HIF within stromal cells are unknown and are ripe for further study. We summarize findings of HIF function from many different studies in Figure 4. What is clear is the hypoxic adaptations of tumors also dramatically alter the metabolism and function of stromal cells. (Figure 4). We currently do not know if altering EGLN/HIF expression within the tumor stroma has any functional consequences and further study is needed. However, this problem is methodologically challenging, since there is currently no system that can allow abrogation of HIF function in the stroma without also altering it in the tumor (conventional Cre-lox methodology). Orthotopic transplants into genetically altered mice have confounding developmental issues. Thus, given these methodological constraints, a new system may be needed.
Figure 4.
The oncogenic and tumor suppressive activity of HIF in tumor and stromal cells.
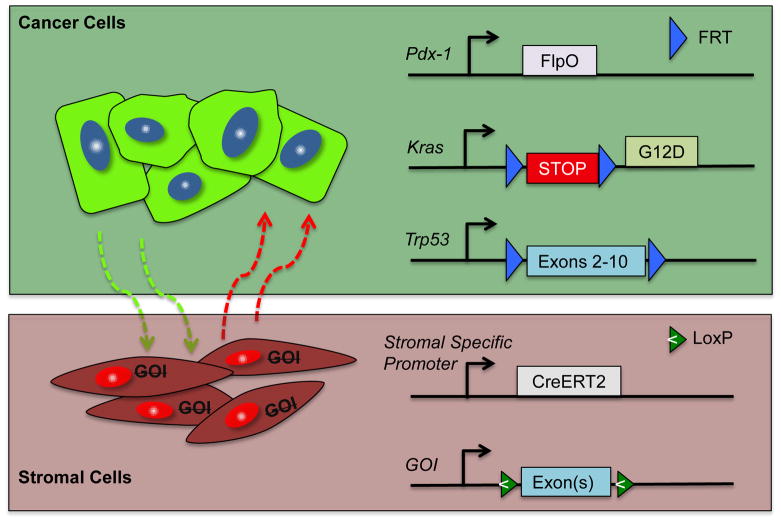
The use of a dual recombinase system might allow for manipulation of the tumor genetics and the stroma separately. We and others (Schonhuber et al., 2014) have developed a system that initiates pancreatic tumorigenesis in a Flp-dependent fashion using a Pdx1-Flp along with a coordinated activation of Kras (G12D) oncogene (FSF-Kras) and a p53 allele (Trp53 FRT/FRT). We can create multi-allelic mice with the capacity to alter the EGLN proteins, HIF proteins, or other key regulators within stromal cells independent of the tumor genetics. This allows complete spatial and temporal control of gene deletion in the stroma or tumor tissues (Figure 4). This same system could afford temporal deletion of genes within the same tumor. Thus, we believe that this powerful system could be used to interrogate each stromal component in a definitive fashion (Figure 5).
Figure 5.
Dual recombinase system for pancreatic cancer. Flp recombinase directs tumorigenesis in the target tissue by a tissue-specific Flp (in this case, Pdx1-FlpO), which activates Kras and knocks out Trp53. This leaves stromal components available to be regulated by the Cre/lox system.
The function of HIF in the tumor and its stroma is of critical importance not only from a basic biology standpoint, but also because we now have the capability to target the function of the HIF transcription factors. For instance, a small molecular inhibitor PT2399 specifically binds to the HIF-2α PAS B domain and causes tumor regression in ccRCC. It is important to understand the possible off-target effects of this drug if it were to be used in other cancer types that do not depend on HIF2 for its growth. Moreover, does HIF1 expressed in CAFs have an effect on stromal formation or treatment resistance? The answers to these questions should be fully understood before pursuing other HIF antagonists for the clinic.
Acknowledgments
Funding
C.M.T. acknowledges funding from CPRIT, V Foundation, the McNair Foundation, and the Sabin Family Fellowships at MD Anderson.
Footnotes
Compliance and ethics
The author(s) declare that they have no conflict of interest.
References
- Bishop T, Gallagher D, Pascual A, Lygate CA, de Bono JP, Nicholls LG, Ortega-Saenz P, Oster H, Wijeyekoon B, Sutherland AI, Grosfeld A, Aragones J, Schneider M, van Geyte K, Teixeira D, Diez-Juan A, Lopez-Barneo J, Channon KM, Maxwell PH, Pugh CW, Davies AM, Carmeliet P, Ratcliffe PJ. Abnormal sympathoadrenal development and systemic hypotension in PHD3−/− mice. Mol Cell Biol. 2008;28:3386–3400. doi: 10.1128/MCB.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodi I, Bishopric NH, Discher DJ, Wu X, Webster KA. Cell-specificity and signaling pathway of endothelin-1 gene regulation by hypoxia. Cardiovas Res. 1995;30:975–984. doi: 10.1016/s0008-6363(95)00164-6. [DOI] [PubMed] [Google Scholar]
- Branco-Price C, Zhang N, Schnelle M, Evans C, Katschinski DM, Liao D, Ellies L, Johnson RS. Endothelial cell HIF-1alpha and HIF-2alpha differentially regulate metastatic success. Cancer cell. 2012;21:52–65. doi: 10.1016/j.ccr.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderachter JA, Tamagnone L, Mazzone M. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Chan DA, Giaccia AJ. PHD2 in tumour angiogenesis. Br J Cancer. 2010;103:1–5. doi: 10.1038/sj.bjc.6605682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DA, Kawahara TL, Sutphin PD, Chang HY, Chi JT, Giaccia AJ. Tumor vasculature is regulated by PHD2-mediated angiogenesis and bone marrow-derived cell recruitment. Cancer Cell. 2009;15:527–538. doi: 10.1016/j.ccr.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhang M, Xing L, Wang Y, Xiao Y, Wu Y. HIF-1alpha contributes to proliferation and invasiveness of neuroblastoma cells via SHH signaling. PLoS One. 2015;10:e0121115. doi: 10.1371/journal.pone.0121115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Hill H, Christie A, Kim MS, Holloman E, Pavia-Jimenez A, Homayoun F, Ma Y, Patel N, Yell P, Hao G, Yousuf Q, Joyce A, Pedrosa I, Geiger H, Zhang H, Chang J, Gardner KH, Bruick RK, Reeves C, Hwang TH, Courtney K, Frenkel E, Sun X, Zojwalla N, Wong T, Rizzi JP, Wallace EM, Josey JA, Xie Y, Xie XJ, Kapur P, McKay RM, Brugarolas J. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016;539:112–117. doi: 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavarina B, Martinez-Outschoorn UE, Whitaker-Menezes D, Howell A, Tanowitz HB, Pestell RG, Sotgia F, Lisanti MP. Metabolic reprogramming and two-compartment tumor metabolism: opposing role(s) of HIF1alpha and HIF2alpha in tumor-associated fibroblasts and human breast cancer cells. Cell cycle. 2012;11:3280–3289. doi: 10.4161/cc.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Du X, Rizzi JP, Liberzon E, Chakraborty AA, Gao W, Carvo I, Signoretti S, Bruick RK, Josey JA, Wallace EM, Kaelin WG. On-target efficacy of a HIF-2alpha antagonist in preclinical kidney cancer models. Nature. 2016;539:107–111. doi: 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, Klebanoff CA, Eil RL, Hickman HD, Yu Z, Pan JH, Palmer DC, Phan AT, Goulding J, Gattinoni L, Goldrath AW, Belkaid Y, Restifo NP. Oxygen Sensing by T Cells Establishes an Immunologically Tolerant Metastatic Niche. Cell. 2016;166:1117–1131. e1114. doi: 10.1016/j.cell.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke VG, LeBleu VS, Keskin D, Khan Z, O'Connell JT, Teng Y, Duncan MB, Xie L, Maeda G, Vong S, Sugimoto H, Rocha RM, Damascena A, Brentani RR, Kalluri R. Pericyte depletion results in hypoxia-associated epithelial-to-mesenchymal transition and metastasis mediated by met signaling pathway. Cancer Cell. 2012;21:66–81. doi: 10.1016/j.ccr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, Rumney RM, Schoof EM, Perryman L, Hoye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR, Huggins ID, Lang G, Linding R, Gartland A, Erler JT. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang EV, Barbi J, Yang HY, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen HR, Luo W, Zeller K, Shimoda L, Topalian SL, Semenza GL, Dang CV, Pardoll DM, Pan F. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nature immunology. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L, Coukos G. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- Fraisl P, Aragones J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov. 2009;8:139–152. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. Journal of cell science. 2010;123:4195–4200. doi: 10.1242/jcs.023820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvalov BK, Foss F, Henze AT, Bethani I, Graf-Hochst S, Singh D, Filatova A, Dopeso H, Seidel S, Damm M, Acker-Palmer A, Acker T. PHD3 regulates EGFR internalization and signalling in tumours. Nat Commun. 2014;5:5577. doi: 10.1038/ncomms6577. [DOI] [PubMed] [Google Scholar]
- Gilkes DM, Chaturvedi P, Bajpai S, Wong CC, Wei H, Pitcairn S, Hubbi ME, Wirtz D, Semenza GL. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer research. 2013;73:3285–3296. doi: 10.1158/0008-5472.CAN-12-3963. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda N, Ryan HE, Khadivi B, McNulty W, Rickert RC, Johnson RS. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol. 2003;23:359–369. doi: 10.1128/MCB.23.1.359-369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MA, Dunning SP, Bunn HF. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science. 1988;242:1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- Goldberg MA, Glass GA, Cunningham JM, Bunn HF. The regulated expression of erythropoietin by two human hepatoma cell lines. Proc Natl Acad Sci USA. 1987;84:7972–7976. doi: 10.1073/pnas.84.22.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CH, Forsdike J, Fitzgerald CJ, Macdonald-Goodfellow S. Hypoxia-mediated stimulation of carcinoma cell invasiveness via upregulation of urokinase receptor expression. Int J Cancer. 1999;80:617–623. doi: 10.1002/(sici)1097-0215(19990209)80:4<617::aid-ijc22>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Hackenbeck T, Knaup KX, Schietke R, Schodel J, Willam C, Wu X, Warnecke C, Eckardt KU, Wiesener MS. HIF-1 or HIF-2 induction is sufficient to achieve cell cycle arrest in NIH3T3 mouse fibroblasts independent from hypoxia. Cell Cycle. 2009;8:1386–1395. doi: 10.4161/cc.8.9.8306. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Harada H, Inoue M, Itasaka S, Hirota K, Morinibu A, Shinomiya K, Zeng L, Ou G, Zhu Y, Yoshimura M, McKenna WG, Muschel RJ, Hiraoka M. Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate towards tumour blood vessels. Nat Commun. 2012;3:783. doi: 10.1038/ncomms1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze AT, Garvalov BK, Seidel S, Cuesta AM, Ritter M, Filatova A, Foss F, Dopeso H, Essmann CL, Maxwell PH, Reifenberger G, Carmeliet P, Acker-Palmer A, Acker T. Loss of PHD3 allows tumours to overcome hypoxic growth inhibition and sustain proliferation through EGFR. Nat Commun. 2014;5:5582. doi: 10.1038/ncomms6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson KS, McNeill LA, Elkins JM, Schofield CJ. The role of iron and 2-oxoglutarate oxygenases in signalling. Biochem Soc Trans. 2003;31:510–515. doi: 10.1042/bst0310510. [DOI] [PubMed] [Google Scholar]
- Huang LE, Bunn HF. Hypoxia-inducible factor and its biomedical relevance. J Biol Chem. 2003;278:19575–19578. doi: 10.1074/jbc.R200030200. [DOI] [PubMed] [Google Scholar]
- Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incio J, Liu H, Suboj P, Chin SM, Chen IX, Pinter M, Ng MR, Nia HT, Grahovac J, Kao S, Babykutty S, Huang Y, Jung K, Rahbari NN, Han X, Chauhan VP, Martin JD, Kahn J, Huang P, Desphande V, Michaelson J, Michelakos TP, Ferrone CR, Soares R, Boucher Y, Fukumura D, Jain RK. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016;6:852–869. doi: 10.1158/2159-8290.CD-15-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jiang HL, Xu CX, Kim YK, Arote R, Jere D, Lim HT, Cho MH, Cho CS. The suppression of lung tumorigenesis by aerosol-delivered folate-chitosan-graft-polyethylenimine/Akt1 shRNA complexes through the Akt signaling pathway. Biomaterials. 2009;30:5844–5852. doi: 10.1016/j.biomaterials.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr Cancer and altered metabolism: potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases. Cold Spring Harb Symp Quant Biol. 2011;76:335–345. doi: 10.1101/sqb.2011.76.010975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG., Jr Common pitfalls in preclinical cancer target validation. Nat Rev Cancer. 2017 doi: 10.1038/nrc.2017.32. [DOI] [PubMed] [Google Scholar]
- Kamura T, Koepp DM, Conrad MN, Skowyra D, Moreland RJ, Iliopoulos O, Lane WS, Kaelin WG, Jr, Elledge SJ, Conaway RC, Harper JW, Conaway JW. Rbx1, a component of the VHL tumor suppressor complex and SCF ubiquitin ligase. Science. 1999;284:657–661. doi: 10.1126/science.284.5414.657. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Ratan RR. Hypoxia-inducible factor prolyl hydroxylase inhibition: robust new target or another big bust for stroke therapeutics? J Cereb Blood Flow Metab. 2012;32:1347–1361. doi: 10.1038/jcbfm.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keskin D, Kim J, Cooke VG, Wu CC, Sugimoto H, Gu C, De Palma M, Kalluri R, LeBleu VS. Targeting vascular pericytes in hypoxic tumors increases lung metastasis via angiopoietin-2. Cell reports. 2015;10:1066–1081. doi: 10.1016/j.celrep.2015.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kibel A, Iliopoulos O, DeCaprio JA, Kaelin WG., Jr Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995;269:1444–1446. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- Kim JW, Evans C, Weidemann A, Takeda N, Lee YS, Stockmann C, Branco-Price C, Brandberg F, Leone G, Ostrowski MC, Johnson RS. Loss of fibroblast HIF-1alpha accelerates tumorigenesis. Cancer Res. 2012;72:3187–3195. doi: 10.1158/0008-5472.CAN-12-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Perera S, Zhou B, Carretero J, Yeh JJ, Heathcote SA, Jackson AL, Nikolinakos P, Ospina B, Naumov G, Brandstetter KA, Weigman VJ, Zaghlul S, Hayes DN, Padera RF, Heymach JV, Kung AL, Sharpless NE, Kaelin WG, Jr, Wong KK. HIF2alpha cooperates with RAS to promote lung tumorigenesis in mice. J Clin Invest. 2009;119:2160–2170. doi: 10.1172/JCI38443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koay EJ, Truty MJ, Cristini V, Thomas RM, Chen R, Chatterjee D, Kang Ya, Bhosale PR, Tamm EP, Crane CH, Javle M, Katz MH, Gottumukkala VN, Rozner MA, Shen H, Lee JE, Wang H, Chen Y, Plunkett W, Abbruzzese JL, Wolff RA, Varadhachary GR, Ferrari M, Fleming JB. Transport properties of pancreatic cancer describe gemcitabine delivery and response. The Journal of Clinical Investigation. 2014;124:1525–1536. doi: 10.1172/JCI73455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol. 1999;209:254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- Kuchnio A, Moens S, Bruning U, Kuchnio K, Cruys B, Thienpont B, Broux M, Ungureanu AA, Leite de Oliveira R, Bruyere F, Cuervo H, Manderveld A, Carton A, Hernandez-Fernaud JR, Zanivan S, Bartic C, Foidart JM, Noel A, Vinckier S, Lambrechts D, Dewerchin M, Mazzone M, Carmeliet P. The Cancer Cell Oxygen Sensor PHD2 Promotes Metastasis via Activation of Cancer-Associated Fibroblasts. Cell Rep. 2015;12:992–1005. doi: 10.1016/j.celrep.2015.07.010. [DOI] [PubMed] [Google Scholar]
- LaGory EL, Wu C, Taniguchi CM, Ding CK, Chi JT, von Eyben R, Scott DA, Richardson AD, Giaccia AJ. Suppression of PGC-1alpha Is Critical for Reprogramming Oxidative Metabolism in Renal Cell Carcinoma. Cell Rep. 2015;12:116–127. doi: 10.1016/j.celrep.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Elly C, Park Y, Liu YC. E3 Ubiquitin Ligase VHL Regulates Hypoxia-Inducible Factor-1alpha to Maintain Regulatory T Cell Stability and Suppressive Capacity. Immunity. 2015;42:1062–1074. doi: 10.1016/j.immuni.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KE, Spata M, Bayne LJ, Buza EL, Durham AC, Allman D, Vonderheide RH, Simon MC. Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia. Cancer Discov. 2016;6:256–269. doi: 10.1158/2159-8290.CD-15-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev. 2007;26:281–290. doi: 10.1007/s10555-007-9066-y. [DOI] [PubMed] [Google Scholar]
- Lonergan KM, Iliopoulos O, Ohh M, Kamura T, Conaway RC, Conaway JW, Kaelin WG., Jr Regulation of hypoxia-inducible mRNAs by the von Hippel-Lindau tumor suppressor protein requires binding to complexes containing elongins B/C and Cul2. Mol Cell Biol. 1998;18:732–741. doi: 10.1128/mcb.18.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- Mack FA, Rathmell WK, Arsham AM, Gnarra J, Keith B, Simon MC. Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth. Cancer Cell. 2003;3:75–88. doi: 10.1016/s1535-6108(02)00240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen CD, Pedersen JT, Venning FA, Singh LB, Moeendarbary E, Charras G, Cox TR, Sahai E, Erler JT. Hypoxia and loss of PHD2 inactivate stromal fibroblasts to decrease tumour stiffness and metastasis. EMBO Rep. 2015;16:1394–1408. doi: 10.15252/embr.201540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, Ferguson-Smith MA. Clinical features and natural history of von Hippel-Lindau disease. The Quarterly journal of medicine. 1990;77:1151–1163. doi: 10.1093/qjmed/77.2.1151. [DOI] [PubMed] [Google Scholar]
- Mazumdar J, Hickey MM, Pant DK, Durham AC, Sweet-Cordero A, Vachani A, Jacks T, Chodosh LA, Kissil JL, Simon MC, Keith B. HIF-2alpha deletion promotes Kras-driven lung tumor development. Proc Natl Acad Sci U S A. 2010;107:14182–14187. doi: 10.1073/pnas.1001296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill LA, Hewitson KS, Gleadle JM, Horsfall LE, Oldham NJ, Maxwell PH, Pugh CW, Ratcliffe PJ, Schofield CJ. The use of dioxygen by HIF prolyl hydroxylase (PHD1) Bioorg Med Chem Lett. 2002;12:1547–1550. doi: 10.1016/s0960-894x(02)00219-6. [DOI] [PubMed] [Google Scholar]
- Minamishima YA, Moslehi J, Bardeesy N, Cullen D, Bronson RT, Kaelin WG., Jr Somatic inactivation of the PHD2 prolyl hydroxylase causes polycythemia and congestive heart failure. Blood. 2008;111:3236–3244. doi: 10.1182/blood-2007-10-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moding EJ, Castle KD, Perez BA, Oh P, Min HD, Norris H, Ma Y, Cardona DM, Lee CL, Kirsch DG. Tumor cells, but not endothelial cells, mediate eradication of primary sarcomas by stereotactic body radiation therapy. Sci Transl Med. 2015;7:278ra234. doi: 10.1126/scitranslmed.aaa4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Nakada Y, Canseco DC, Thet S, Abdisalaam S, Asaithamby A, Santos CX, Shah AM, Zhang H, Faber JE, Kinter MT, Szweda LI, Xing C, Hu Z, Deberardinis RJ, Schiattarella G, Hill JA, Oz O, Lu Z, Zhang CC, Kimura W, Sadek HA. Hypoxia induces heart regeneration in adult mice. Nature. 2017;541:222–227. doi: 10.1038/nature20173. [DOI] [PubMed] [Google Scholar]
- Nakazawa MS, Eisinger-Mathason TS, Sadri N, Ochocki JD, Gade TP, Amin RK, Simon MC. Epigenetic re-expression of HIF-2alpha suppresses soft tissue sarcoma growth. Nat Commun. 2016;7:10539. doi: 10.1038/ncomms10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology. 2012;55:622–633. doi: 10.1002/hep.25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann HP, Eggert HR, Scheremet R, Schumacher M, Mohadjer M, Wakhloo AK, Volk B, Hettmannsperger U, Riegler P, Schollmeyer P, et al. Central nervous system lesions in von Hippel-Lindau syndrome. Journal of neurology, neurosurgery, and psychiatry. 1992;55:898–901. doi: 10.1136/jnnp.55.10.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211:781–790. doi: 10.1084/jem.20131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olenchock BA, Moslehi J, Baik AH, Davidson SM, Williams J, Gibson WJ, Chakraborty AA, Pierce KA, Miller CM, Hanse EA, Kelekar A, Sullivan LB, Wagers AJ, Clish CB, Vander Heiden MG, Kaelin WG., Jr EGLN1 Inhibition and Rerouting of alpha-Ketoglutarate Suffice for Remote Ischemic Protection. Cell. 2016;164:884–895. doi: 10.1016/j.cell.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, DeNicola G, Feig C, Combs C, Winter SP, Ireland H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog Signaling Enhances Delivery of Chemotherapy in a Mouse Model of Pancreatic Cancer. Science (New York, NY) 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15:628–634. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nature reviews Drug discovery. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause A, Lee S, Worrell RA, Chen DY, Burgess WH, Linehan WM, Klausner RD. The von Hippel-Lindau tumor-suppressor gene product forms a stable complex with human CUL-2, a member of the Cdc53 family of proteins. Proc Natl Acad Sci USA. 1997;94:2156–2161. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pause A, Peterson B, Schaffar G, Stearman R, Klausner RD. Studying interactions of four proteins in the yeast two-hybrid system: structural resemblance of the pVHL/elongin BC/hCUL- 2 complex with the ubiquitin ligase complex SKP1/cullin/F-box protein. Proc Natl Acad Sci USA. 1999;96:9533–9538. doi: 10.1073/pnas.96.17.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Fuh KC, Castellini L, Viswanathan K, Finger EC, Diep AN, LaGory EL, Kariolis MS, Chan A, Lindgren D, Axelson H, Miao YR, Krieg AJ, Giaccia AJ. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci U S A. 2014;111:13373–13378. doi: 10.1073/pnas.1404848111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Wu C, Khatri R, Wilson TL, Andersen R, Araldi E, Rankin AL, Yuan J, Kuo CJ, Schipani E, Giaccia AJ. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012;149:63–74. doi: 10.1016/j.cell.2012.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratan RR, Siddiq A, Aminova L, Lange PS, Langley B, Ayoub I, Gensert J, Chavez J. Translation of ischemic preconditioning to the patient: prolyl hydroxylase inhibition and hypoxia inducible factor-1 as novel targets for stroke therapy. Stroke. 2004;35:2687–2689. doi: 10.1161/01.STR.0000143216.85349.9e. [DOI] [PubMed] [Google Scholar]
- Ratan RR, Siddiq A, Smirnova N, Karpisheva K, Haskew-Layton R, McConoughey S, Langley B, Estevez A, Huerta PT, Volpe B, Roy S, Sen CK, Gazaryan I, Cho S, Fink M, LaManna J. Harnessing hypoxic adaptation to prevent, treat, and repair stroke. J Mol Med (Berl) 2007;85:1331–1338. doi: 10.1007/s00109-007-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reischl S, Li L, Walkinshaw G, Flippin LA, Marti HH, Kunze R. Inhibition of HIF prolyl-4-hydroxylases by FG-4497 reduces brain tissue injury and edema formation during ischemic stroke. PLoS One. 2014;9:e84767. doi: 10.1371/journal.pone.0084767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive KP, Stanger BZ. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salceda S, Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- Samanta D, Gilkes DM, Chaturvedi P, Xiang L, Semenza GL. Hypoxia-inducible factors are required for chemotherapy resistance of breast cancer stem cells. Proc Natl Acad Sci U S A. 2014;111:E5429–5438. doi: 10.1073/pnas.1421438111. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sanchez R, Zhou MM. The PHD finger: a versatile epigenome reader. Trends Biochem Sci. 2011;36:364–372. doi: 10.1016/j.tibs.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhuber N, Seidler B, Schuck K, Veltkamp C, Schachtler C, Zukowska M, Eser S, Feyerabend TB, Paul MC, Eser P, Klein S, Lowy AM, Banerjee R, Yang F, Lee CL, Moding EJ, Kirsch DG, Scheideler A, Alessi DR, Varela I, Bradley A, Kind A, Schnieke AE, Rodewald HR, Rad R, Schmid RM, Schneider G, Saur D. A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nat Med. 2014;20:1340–1347. doi: 10.1038/nm.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. Journal of Biological Chemistry. 1994;269:23757–23767. [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, Runge A, Liu L, Kim MN, Liang J, Schenkel S, Yodh AG, Keith B, Simon MC. Endothelial HIF-2alpha regulates murine pathological angiogenesis and revascularization processes. J Clin Invest. 2012;122:1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehl DP, Wirthner R, Koditz J, Spielmann P, Camenisch G, Wenger RH. Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem. 2006;281:23482–23491. doi: 10.1074/jbc.M601719200. [DOI] [PubMed] [Google Scholar]
- Synnestvedt K, Furuta G, Comerford K, Louis N, Karhausen J, Eltzschig H, Hansen K, Thompson L, Colgan S. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Aguila HL, Parikh NS, Li X, Lamothe K, Duan LJ, Takeda H, Lee FS, Fong GH. Regulation of adult erythropoiesis by prolyl hydroxylase domain proteins. Blood. 2008;111:3229–3235. doi: 10.1182/blood-2007-09-114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Taniguchi CM, Miao YR, Diep AN, Wu C, Rankin EB, Atwood TF, Xing L, Giaccia AJ. PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci Transl Med. 2014;6:236ra264. doi: 10.1126/scitranslmed.3008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfel NA, El-Deiry WS. HIF-1 signaling in drug resistance to chemotherapy. Curr Med Chem. 2014;21:3021–3028. doi: 10.2174/0929867321666140414101056. [DOI] [PubMed] [Google Scholar]
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Zhong R, Xu H, Chen G, Zhao G, Gao Y, Liu X, Ma S, Dong L. The role of hypoxia-inducible factor-1alpha in radiation-induced autophagic cell death in breast cancer cells. Tumour Biol. 2015;36:7077–7083. doi: 10.1007/s13277-015-3425-z. [DOI] [PubMed] [Google Scholar]
- Zhou L, Liu XD, Sun M, Zhang X, German P, Bai S, Ding Z, Tannir N, Wood CG, Matin SF, Karam JA, Tamboli P, Sircar K, Rao P, Rankin EB, Laird DA, Hoang AG, Walker CL, Giaccia AJ, Jonasch E. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35:2687–2697. doi: 10.1038/onc.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]