Abstract
Climate change will impact the dynamics of invasive alien plant species (IAPS). However, the ability of IAPS under changing climate to invade mountain ecosystems, particularly the Himalayan region, is less known. This study investigates the current and future habitat of five IAPS of the Himalayan region using MaxEnt and two representative concentration pathways (RCPs). Two invasive species, Ageratum conyzoides and Parthenium hysterophorus, will lose overall suitable area by 2070, while Ageratina adenophora, Chromolaena odorata and Lantana camara will gain suitable areas and all of them will retain most of the current habitat as stable. The southern Himalayan foothills will mostly conserve species ecological niches, while suitability of all the five species will decrease with increasing elevation. Such invasion dynamics in the Himalayan region could have impacts on numerous ecosystems and their biota, ecosystem services and human well-being. Trans-boundary response strategies suitable to the local context of the region could buffer some of the likely invasion impacts.
Electronic supplementary material
The online version of this article (10.1007/s13280-018-1017-z) contains supplementary material, which is available to authorized users.
Keywords: Climate change, Himalayas, Invasive species, MaxEnt, Niche modelling
Introduction
Almost one-sixth of the global land surface is currently highly vulnerable to invasion from alien species, including significant areas of developing economies and global biodiversity hotspots (Early et al. 2016). According to Masters and Norgrove (2010), Invasive alien plant species (IAPS) are “non-indigenous species that adversely affect, economically, environmentally or ecologically, habitats where they have been introduced, either accidentally or deliberately, outside their normal past or present distribution”. Approximately, 0.5–0.7% of global tree and shrub species are currently invasive outside their natural range (Richardson and Rejmanek 2011). In India, Adhikari et al. (2015) reported nineteen out of forty-seven existing eco-regions harbour invasion hotspots. Both climate change and invasive species are the key drivers of biodiversity loss, and if acting together, they could exacerbate the impact on biodiversity and ecosystems as a whole (Mainka and Howard 2010). Many invasive plant populations, in the current context of climate change, are developing adaptations that could lead to exponential population growth in the future (Clements and Ditommaso 2011). IAPS are thus becoming a serious threat to biodiversity and ecosystems, and climate change may increase their distribution range.
Species that can withstand a wide range of environmental conditions show a broader physiological niche and are more likely to be invasive (Higgins and Richardson 2014). Pests and parasites are integral components of a natural ecosystem that help maintain healthy species populations. Torchin and Mitchell (2004) argued that escaping native natural biological enemies, such as pests from their area of origin, is one of the key mechanisms that makes introduced species more prone to proliferate in the novel environment and facilitate them to become a harsh invader. Similarly, Maron et al. (2004) found that adaptive evolution, apart from long-recognized phenotypic plasticity, facilitates the establishment and expansion of invasive species into broad environmental conditions of the invaded range. The high growth rate, wide climatic or environmental tolerance, short generation time, consistent reproduction, small seed size, high dispersal and high capacity for asexual reproduction are some of the specific traits of IAPS that undergo evolutionary change when they invade new areas (Whitney and Gabler 2008). In this context, four distinct pathways, such as transport, colonization, establishment and landscape spread, are essential for an invasive species to successfully complete an invasion (Hellmann et al. 2008).
The ongoing biotic invasion is modifying global natural communities and their ecological characteristics at an unprecedented rate and is being viewed as a major agent for human-driven global change, such as atmospheric and land use changes (Mack et al. 2000; Masters and Norgrove 2010). Disturbances to an ecosystem enhance the diversity and distribution of non-native plants; however, the strength of this enhancement relies on the disturbance type, especially grazing and anthropogenic activities for terrestrial ecosystems (Jauni et al. 2015). Invasive plants could directly exert an influence on the survival, productivity and activity of native biota along with changes in mineral and nutrient contents in plant tissues, and at the community level, to species richness, diversity and soil resources through the interaction between species traits and the biome invaded (Pysek et al. 2012). A recent study (Ren et al. 2017) in the Hindu Kush Himalaya (HKH) that overlaps most of the Himalayas and Tibetan Plateau region revealed a significant increasing trend of the annual mean surface air temperature during the last century. Jayanarayanan et al. (2017) projected an increment of seasonal warming up to 5.4 °C during the winter and 4.9 °C during the summer monsoon in the Karakoram and northwestern region of the Himalaya by the end of this century. Theoretically, greater expansion of the species adapted to low elevation is expected in such high elevation regions with the increasing trend of average temperature, including likely changes in the dynamism and impacts of hot climate plants such as invasive species. Dhar and Reshi (2015) observed invasive species promoting homogenization of the terrestrial ecosystem in Kashmir in the western Himalaya. Likewise, Priyanka and Joshi (2013) reported that most of the southern and western regions of the western Himalaya will be climatically suitable in the future for the spread of Lantana camara due to increased warming. These invasive species have been spreading over the protected areas of the region and creating problems for wildlife habitat and their food availability (Murphy et al. 2013; Aryal et al. 2017). This suggests that the Himalayan region is equally vulnerable to species invasion because of past and future environmental change, such as increased warming.
Non-native invasive species are abundant at lower and mid-elevation regions in mountain ecosystems; however, it is likely that they will spread and become dominant at higher elevations because of ongoing climate change and anthropogenic activities (Alexander et al. 2016). An invasion of alien plant species is possible if the population growth continues in our mountainous areas under a warming climate (Marini et al. 2012). Haider et al. (2010) found that both climatic and habitat conditions affect altitudinal distribution of non-native plants, and hence, climate change can influence the occurrence of these plants directly and indirectly in such terrain conditions. On the other hand, it has also been acknowledged that regions at high elevations are less vulnerable to alien plant invasions with the existing conditions of energy constraints, low propagule pressure and disturbance, even if potential increases in temperature are considered (Marini et al. 2009). For instance, Zhang et al. (2015) reported a decrease in alien species richness with increasing elevation in the Tai and Lao mountain range, and Guo et al. (2017) found similar results in the high elevation protected areas (PAs) of China, which could be an influence of lower lethal temperature and other stressful abiotic environments, for instance, a lower propagule pressure or a less human mediated land use system (Lembrechts et al. 2014). It seems that high elevation landscapes could see more invasions from lowland invasive plants through likely moderate climate and increased human activities in the next few decades.
Some modelling studies on invasion potential are available in the Himalayas (Priyanka and Joshi 2013; Shrestha et al. 2015). However, there is a lack of a comprehensive study focusing on the broad Himalayan region as a whole on the current potential and future predicted distribution of major IAPS of the region. As the literature suggests, such an invasion expansion in mountain ecosystems and along its gradient is important to understand whether invasion phenomenon will occur in the Himalayan region considering the fragile ecosystems it has, the biodiversity therein and the continuing supplement of ecosystem services (ES) for human well-being. Invasive species are largely known to degrade the biota habitat in ecosystems by outcompeting native species through influence on availability of resources such as nutrients and space as well as modification of ecological processes (Grice 2006). The biodiversity-rich and vulnerable region, the Himalaya, is no exception from such invasion. Therefore, this study seeks to use maximum entropy (MaxEnt) (i) to model the invasion dynamics of five IAPS, viz. A. adenophora (Crofton weed), A. conyzoides (Billygoat weed), C. odorata (Siam weed), L. camara (Lantana) and P. hysterophorus (Famine weed), considering current and future (2070) time periods under the IPCC’s RCP4.5 and RCP8.5; (ii) to understand how the five investigated IAPS respond along the altitudinal gradient of the Himalaya under future warming scenarios; and (iii) to see how the five investigated IAPS could affect ongoing conservation efforts, such as existing protected areas in the region. A brief introduction of the five IAPS in Nepal’s context is provided in Supplementary Information S1.
Materials and methods
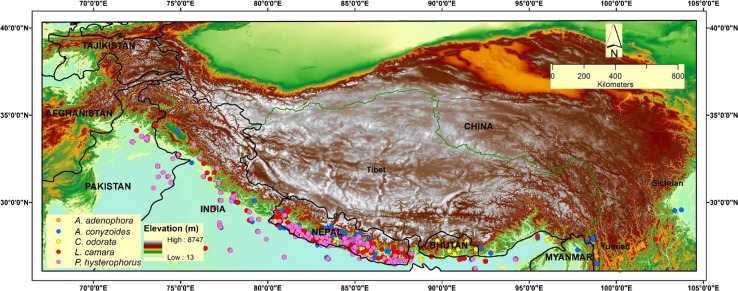
The ecological niches of the five IAPS that are found in the foothills of the Himalayas were modelled using maximum entropy (MaxEnt). MaxEnt is a robust model for invasive species modelling with high accuracy estimations of habitat suitability, even with a limited number of available field data (West et al. 2016), and it has higher stability (Duan et al. 2014). Global occurrence data representing both native and exotic ranges were collected from multiple sources, such as the Global Biodiversity Information Facility (GBIF), National Herbarium and Plant Laboratories (KATH), literature and a personal contact. A total of 23,193 occurrence data were used in the model: A. adenophora (2365), A. conyzoides (3005), C. odorata (3623), L. camara (11 742) and P. hysterophorus (2458). An ecological niche model was first developed for each individual species at a global scale and then distributions specific to our study area (Fig. 1) were clipped. The idea was to create a global robust climatic envelop using the occurrence records of both native and non-native regions (Supplementary Information S2) and then narrow down a niche distribution analysis for the Himalayas. An ecological niche model calibrated on the data of the native region alone could provide a less reliable outcome on the current and future spread of invasive species compared to a model with the distribution data of the entire range (Beaumont et al. 2009). Similarly, Mainali et al. (2015) argued that model accuracy improved drastically if the dataset of all broader geographic space is used in model training rather than relying on data of a certain restricted region. Saurez-Mota et al. (2016) also recommended the use of global distribution records for niche modelling of invasive plants if the species in the target study region had an incipient presence or the record evidence was deemed not sufficient. Henceforth, we used a global dataset of the five IAPS with the assumption that models capture well the environmental and other variables of both native and exotic geographic ranges.
Fig. 1.
Study area showing the occurrence records of the five IAPS and the elevation gradient in the Himalaya
We downloaded 19 grid-based bioclimatic variables from the Worldclim dataset (www.worldclim.org) at 30 arc sec (~ 1 km2) resolution (Supplementary Information S3). The use of such fine resolution climate data is appropriate for regions with complicated topography such as the Himalayas, where climatic conditions change over a short distance. Current global land cover data at 300 m spatial resolution for the study area were obtained from the European Space Agency (http://due.esrin.esa.int/page_globcover.php), while elevation data with a similar resolution to that of bioclimatic variables were obtained from the global multi-resolution terrain elevation data 2010 (http://lta.cr.usgs.gov/GMTED2010). Slope and aspect rasters of the study area were derived from the elevation data, while a population density raster (30 arc sec) was obtained from the Socio-economic Data and Application Centre (http://sedac.ciesin.columbia.edu/data/set/gpw-v4-population-density-adjusted-to-2015-unwpp-country-totals). We analysed a multicollinearity test of climatic (19 bio-climates), ecological (elevation, slope, aspect, land cover) and socio-economic (global population density) variables for each of the five species separately using a Pearson correlation in IBM SPSS Statistic Software version 21, removing all the variables with R2 ≥ 0.75 and keeping variables below this threshold for the MaxEnt analysis. Strong collinearity between the variables in predictive modelling could influence the overall model outcome by placing high emphasis on one or two or more highly correlated variables (Baldwin 2009), resulting in misinterpretation. In this context, Dormann et al. (2012) viewed collinearity as a common feature of an ecological data set and often a problem for parameter estimation by inflating the variance of regression parameters that could lead to incorrect detection of relevant predictors in a statistical model, and therefore one needs to be sure that those environmental predictive variables are orthogonal or mutually independent of each other (Cruz-Cardenas et al. 2014). Binary habitat suitability maps for the present and future (2070) were prepared using the maximum training sensitivity plus the specificity logistic threshold in ArcGIS version 10.2 software package. Maximum training sensitivity plus a specificity threshold approach is a more restrictive and conservative approach to understand habitat suitability. This threshold approach is one of the best methods either for presence/absence data or for presence only data when random points are used (Liu et al. 2005a). Furthermore, a sound principle of threshold selection is based on three criteria, viz. objectivity, equality and discriminability, which is fully satisfied by this approach (Liu et al. 2013).
The MaxEnt model used a 20% random test, five replications and 1000 maximum iterations, whereas other parameters remained at default. These parameters are the software in-built features that a modeller adjusts to calibrate the model for achieving a better model output. The area under the curve (AUC) of the Receiver Operator Characteristic (ROC) plot that is available within MaxEnt was used to measure the discrimination ability of the model i.e. model’s goodness of fit. AUC values range from 0 to 1 and the model with the highest AUC value is considered as the best performer. Of the total, 80% of the occurrence records were allocated to train the model, while 20% were allocated to test the model. The relative contribution of different bioclimatic predictors to the distribution model was evaluated through MaxEnt outcome using percent variable contribution and Jackknife procedures (Elith et al. 2011). The Jackknife procedure identifies the variables with the greatest influence to the overall model. As per Phillips et al. (2006), the Jackknife procedure is an in-built functionality of MaxEnt where each variable is excluded and a model is reconstructed with the remaining ones after which a new model is created using each variable in isolation. Data on existing protected areas (PAs) at the global scale that fell under the definition of the World Database on Protected Areas (WDPA) of UNEP–WCMC (www.protectedplanet.net) and classified and mapped accordingly were downloaded and clipped to study area to see how many PAs are currently and in the future could be invaded by either one or more of the five IAPS.
The Model for Interdisciplinary Research on Climate version 5 (MIROC5) of the global climate model (GCM) was selected to predict the distribution of the five IAPS. This GCM has better simulation of the mean climate, variability, and climate change due to anthropogenic radiative forcing than its past version (for details, please see Watanabe et al. 2010). Further, Mishra et al. (2014) and Sharmila et al. (2015) reported that MIROC5 captures various observed features of future climate very well, especially for the South Asian region, and some studies (Su et al. 2015; Aryal et al. 2016) used this GCM to model species distributions in the high elevation regions, such as the Himalaya and Tibetan Plateau. As per the IPCC (2013), RCP4.5 is the medium future emission scenario that peaks in approximately 2040, with total radiative forcing could reach almost + 4.5 W/m2 (~ 650 ppm CO2 equivalent) by the end of twenty first century and stabilizes thereafter. Similarly, RCP8.5 is an extreme carbon emission scenario that continues to rise throughout the twenty first century, with radiative forcing reaching almost + 8.5 W/m2 (~ 935 ppm CO2 equivalent).
After modelling each species separately, we calculated the likely changes in the suitability areas of each individual species for the future compared to current time period. We then overlaid all five species to see which areas would have impacts from all the five IAPS to show their dynamism in terms of expansion, reduction and stability under the future distribution. Likewise, we categorized the study area into six different elevation belts of 500-m intervals, i.e. B1 (< 1500 m), B2 (1500–2000 m), B3 (2000–2500 m), B4 (2500–3000 m), B5 (3000–3500 m) and B6 (> 3500 m) to see how the five IAPS respond to climate change along the Himalaya gradient. Similarly, we overlaid existing PAs boundary over the merged IAPS suitability maps to look into the current and possible future invasion into PAs of the region. All of the analyses were carried out using spatial analysis tool available in the Environmental System Research Institute (ESRI) ArcGIS 10.2 software.
Results
Distribution models
The four predictor variables with the highest contributions to the model were temperature seasonality (BIO4), annual mean temperature (BIO1), precipitation of coldest quarter (BIO19) and minimum temperature of coldest month (BIO6) for A. adenophora, accounting for 87.4% of the variance; temperature annual range (BIO7), annual precipitation (BIO12), annual mean temperature (BIO1) and population density for A. conyzoides, accounting for 91.8% of the variance; temperature annual range (BIO7), annual precipitation (BIO12), annual mean temperature (BIO1) and population density for C. odorata, accounting for 87.5% of the variance. Similarly, it was annual mean temperature (BIO1), temperature annual range (BIO7), annual precipitation (BIO12) and precipitation of coldest quarter (BIO19) for L. camara, accounting for 88.9% of the variance and annual mean temperature (BIO1), isothermality (BIO3), population density and precipitation of driest month (BIO14) for P. hysterophorus, accounting for 80.1% of the variance (Supplementary Information S4). Overall, BIO1 and BIO12 were common variables for all five IAPS, while the contribution of population density was found to be important for A. conyzoides, C. odorata and P. hysterophorus. The jackknife test also showed that the above variables are important to the model (Supplementary Information S5). Some selected response curves of all the species are presented in Supplementary Information S6, which showed how each environmental variable used for each individual species in the model responded to the predicted suitability of those species, which were found to be well within the ecological range. Similarly, the highest training and test area under the ROC curve (AUC) value as provided by the model for the investigated species were as follows: A. adenophora (0.920, 0.922), A. conyzoides (0.879, 0.879), C. odorata (0.866, 0.857), L. camara (0.749, 0.752) and P. hysterophorus (0.900, 0.899).
Current and future projection
The model shows that the suitable habitat of all the five IAPS will change both under RCP4.5 and RCP8.5 by 2070 compared to current distribution (Fig. 1, Table 1). Overall, three species, viz. A. adenophora, C. odorata and L. camara, will see increased suitable area under both RCP’s by 2070, while A. conyzoides and P. hysterophorus will have decreased suitability. The average combined suitable area of all the five species is 578 908, 573 654 and 590 259 km2 for the current, RCP4.5 and RCP8.5 scenarios, respectively. Currently, P. hysterophorus occupies maximum suitable area (33.53% of the total suitable area) followed by A. conyzoides (26.62%), L. camara (14.10%) and C. odorata (13.52%), while A. adenophora occupied the least (12.22%). All the future changes in the suitability of the five species, either an increase or decrease, will be less than 22% for each species. The highest increase in suitability will be seen for A. adenophora, almost 21.88% under RCP8.5, while A. conyzoides will have the highest decrease in suitable area, almost -17% under RCP4.5 compared to current time (Table 1).
Table 1.
Current and future suitability of the five IAPS under the two emission scenarios
| Species | Suitable area (km2) | ||||
|---|---|---|---|---|---|
| Current | RCP4.5 (2070) | % Change | RCP8.5 (2070) | % Change | |
| A. adenophora | 353 986 | 426 496 | 20.48 | 431 439 | 21.88 |
| A. conyzoides | 770 549 | 639 544 | − 17 | 720 774 | − 6.45 |
| C. odorata | 391 386 | 441 639 | 12.84 | 416 016 | 6.29 |
| L. camara | 408 034 | 463 951 | 13.70 | 484 730 | 18.80 |
| P. hysterophorus | 970 589 | 896 641 | − 7.62 | 898 337 | − 7.44 |
Suitability dynamics of individual species
Table 2 and Fig. 2 show how the five IAPS under investigation expand, decrease or remain stable under RCP4.5 and RCP8.5 by 2070 in the Himalayan region. All IAPS will have significant dynamism in terms of suitability with respect to future climate scenarios. The majority of suitable areas for all five species will remain stable under both RCPs compared to the present (Fig. 2). A. conyzoides and L. camara will lose the maximum (19.36%) and minimum (2.66%) of suitable areas, respectively, while A. adenophora and A. conyzoides will have the maximum (20.65%) and minimum (2.85%) gains in suitable area under RPC4.5 by 2070 (Table 2). Similarly, P. hysterophorus and L. camara will lose the maximum (14.66%) and minimum (1.67%) of their suitable area, respectively, while A. adenophora and P. hysterophorus will have the maximum (21.42%) and minimum (7.80%) gain in suitable area under RCP8.5 by 2070 (Table 2). Overall, A. adenophora will have the highest gain in suitability under both RCPs, whereas L. camara will have the smallest reduction in suitable area.
Table 2.
Stable, reduction and expansion suitable areas of the five IAPS by 2070 under RCP4.5 and RCP8.5
| Species | Area under RCP4.5 (km2) | Area under RCP8.5 (km2) | ||||
|---|---|---|---|---|---|---|
| Reduction | Stable | Expansion | Reduction | Stable | Expansion | |
| A adenophora | 19 637 (4.40%) | 334 349 (74.94%) | 92 147 (20.65%) | 19 038 (4.25%) | 334 947 (74.35%) | 96 492 (21.42%) |
| A conyzoides | 153 594 (19.36%) | 616 954 (77.79%) | 22 589 (2.85%) | 122 122 (14.49%) | 648 427 (76.93%) | 72 347 (8.58%) |
| C odorata | 18 578 (4.04%) | 372 808 (81%) | 68 830 (14.96%) | 25 609 (5.80%) | 365 777 (82.82%) | 50 239 (11.37%) |
| L camara | 12 694 (2.66%) | 395 339 (82.94%) | 68 612 (14.39%) | 8 236 (1.67%) | 399 798 (81.10%) | 84 932 (17.23%) |
| P hysterophorus | 144 436 (13.87%) | 826 152 (79.35%) | 70 488 (6.77%) | 154 326 (14.66%) | 816 263 (77.54%) | 82 073 (7.80%) |
Note: The percentages in brackets denote the portion of the total distribution of a species
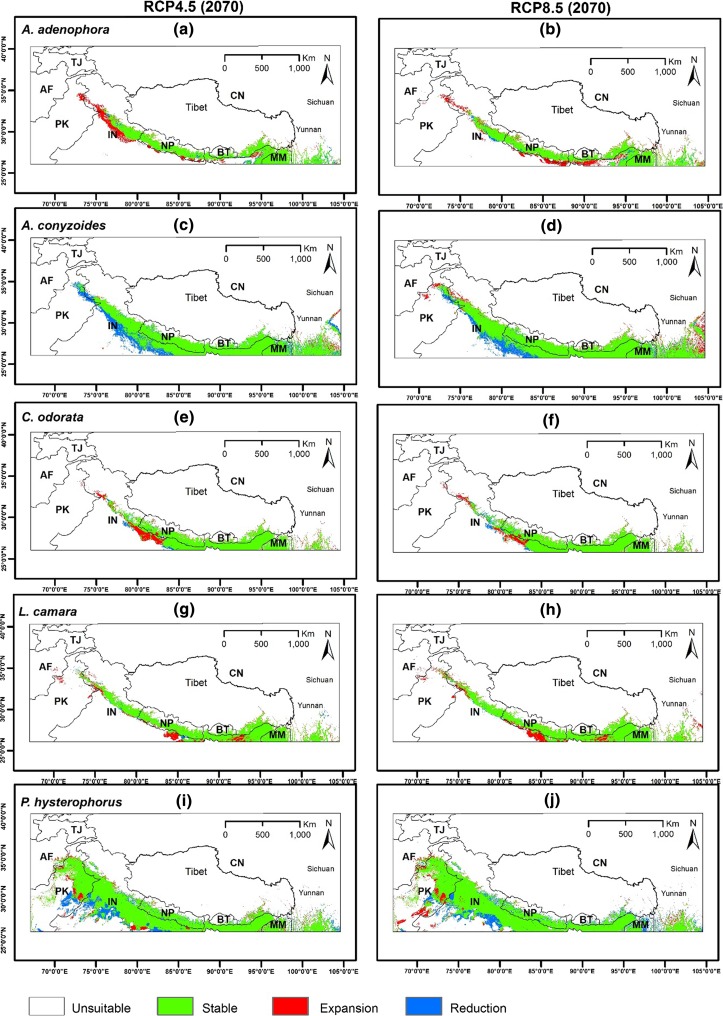
Fig. 2.
Projected distribution maps of five IAPS showing likely stable, expansion and reduction areas under RCP4.5 and RCP8.5 in 2070 with respect to the current time period (AF Afghanistan, TJ Tajikistan, PK Pakistan, IN India, NP Nepal, BT Bhutan, MM Myanmar and CN China)
Ageratina adenophora will expand more in the western Himalaya, especially on the lower belts of Uttarakhand, Himachal Pradesh and Jammu and Kashmir along with some parts of northern Pakistan under RCP4.5 (Fig. 2a). Little expansion can be seen in the lower belt of the central Himalaya and eastern Himalaya in Assam. Minor expansion will occur in the whole northern belt, including the Tibetan Plateau and Yunnan Province of China. However, under RCP8.5 (Fig. 2b), more expansion can be expected in the lower belt of the central and eastern Himalaya, while less expansion in the western Himalaya is evident under RCP4.5. In RCP4.5, the species will have a minor northward expansion throughout the whole region, including Yunnan. For A. conyzoides, some areas will become unsuitable under RCP4.5, especially the lower belt of the western Himalaya and northern India, while Yunnan will see expansion. A reduction of suitability can be seen under RCP8.5, but will also have more expansion both in the western Himalayas and Yunnan province (Fig. 2d). For C. odorata (Fig. 2e), some parts of Jammu and Kashmir will be more suitable, including the border between western Nepal and India as well as Yunnan, under RCP4.5. Northward expansion is visible and similar to that A. adenophora. However, expansion will be less under RCP8.5, though minor northward expansion is still evident. Likewise, L. camara (Fig. 2g) will expand into some parts of Afghanistan and Pakistan, including the lower belt of Himanchal State and Jammu and Kashmir of the western Himalaya, under RCP4.5. Northern India bordering central Nepal and Assam in the eastern Himalaya will see suitable areas for the expansion of L. camara, including the northern elevated range as well as in Yunnan Province. Similar phenomena could be expected for this species under RCP8.5 but with more climatically suitable areas in Yunnan Province. On the contrary, P. hysterophorus (Fig. 2i) will have less climatically suitable areas in the whole northern region at the highest elevations, including Yunnan, under both RCPs compared to the other four species. The southern belt of the Himalaya, mainly Punjab Province of both Pakistan and India, will have less expansion and more contraction in suitability. Northeast Afghanistan will gain some suitable areas under both RCPs, while northern Myanmar will see more unsuitable areas under RCP8.5 by 2070 (Fig. 2j).
Suitability along elevation belts
The percentage change in climatic suitability with respect to different elevation belts in the Himalayan region for the present and 2070 under RCP4.5 and RCP8.5 is provided in Table 3. On average, almost 78% of the current suitable habitats of the five IAPS lies below 1500 m (B1). Approximately 89% and 64% of the current suitable habitats of C. odorata and A. adenophora lie below 1500 m, respectively. From this, it is obvious that all the investigated species categorically belong to tropical and sub-tropical climates. However, there is a sharp decline in potential suitable areas with increasing elevation. A. adenophora and L. camara have almost 20% suitable area at B2 (1500–2000 m), while the remaining three species have only approximately 8% of suitability on average, and this suitability declines further at higher elevations. The future suitability of all the five IAPS under both RCPs follows the same pattern; however, they differ in the expansion and reduction of such suitability. For instance, the suitability of A. adenophora and L. camara continues to decline in both RCPs beyond B3 (2000–2500 m), while the rest of the species shows a mixed trend of both expansion and contraction. Such dynamism also differs if we consider the same elevation belts between the present and RCP4.5 and RCP8.5 in 2070.
Table 3.
Percentage change in current and future suitability areas of the five IAPS with elevation belts and two emission scenarios
| Elevation Belts (m) | CC scenario | < 1500 (B1) | 1500–2000 (B2) | 2000–2500 (B3) | 2500–3000 (B4) | 3000–3500 (B5) | > 3500 (B6) |
|---|---|---|---|---|---|---|---|
| A. adenophora | Current % | 63.897 | 22.449 | 11.966 | 1.588 | 0.082 | 0.018 |
| RCP4.5 (% change) | 2.899 | − 2.705 | − 0.920 | 0.539 | 0.169 | 0.018 | |
| RCP8.5 (% change) | − 1.027 | − 2.002 | 1.036 | 1.523 | 0.423 | 0.048 | |
| A. conyzoides | Current % | 72.084 | 11.253 | 9.410 | 4.461 | 2.646 | 0.146 |
| RCP4.5 (% change) | − 3.372 | 1.979 | 0.889 | 0.612 | − 0.163 | 0.055 | |
| RCP8.5 (% change) | − 6.082 | 2.034 | 2.920 | 2.044 | − 0.969 | 0.052 | |
| C. odorata | Current % | 89.485 | 8.477 | 1.570 | 0.429 | 0.032 | 0.006 |
| RCP4.5 (% change) | − 2.252 | 0.261 | 1.329 | 0.648 | 0.015 | − 0.001 | |
| RCP8.5 (% change) | − 0.875 | 0.138 | 0.944 | − 0.191 | − 0.015 | − 0.002 | |
| L. camara | Current % | 75.640 | 18.705 | 5.434 | 0.190 | 0.027 | 0.004 |
| RCP4.5 (% change) | − 1.181 | − 0.296 | 1.385 | 0.075 | 0.011 | 0.006 | |
| RCP8.5 (% change) | − 0.475 | − 0.780 | 1.117 | 0.123 | 0.011 | 0.005 | |
| P. hysterophorus | Current % | 87.245 | 5.826 | 5.510 | 0.601 | 0.378 | 0.440 |
| RCP4.5 (% change) | − 0.338 | 0.790 | − 0.474 | 0.569 | − 0.246 | − 0.301 | |
| RCP8.5 (% change) | 0.831 | 0.048 | − 1.432 | 1.150 | − 0.184 | − 0.413 |
Note: Corresponding areas in km2 with respect to elevation belts for each climate scenario are provided in Supplementary Information S7
Invasion of IAPS in the protected areas
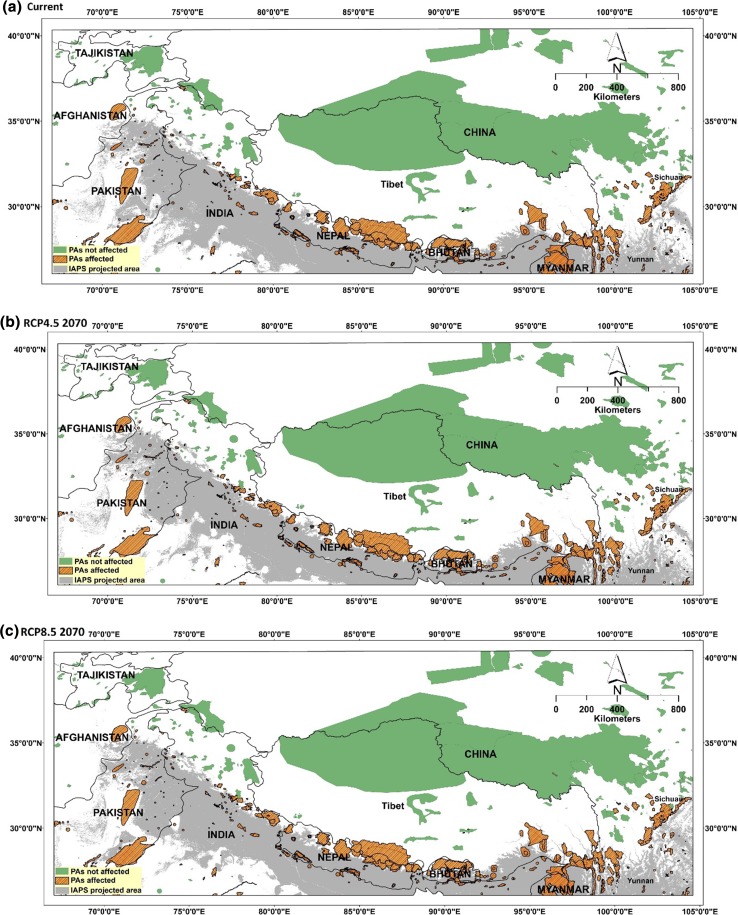
A total of 493 protected areas (PAs), based on the classification and mapping of the World Database on Protected Areas (WDPA) of UNEP–WCMC, exist in the study area (Fig. 3). Our results show that no new PAs in the study area will be invaded by 2070 under both RCPs, since most of them (338 PAs, which is ~ 69%) are already being invaded in whole or part by either one or more of the five investigated IAPS. This includes almost all the PAs in Nepal and Bhutan, as well as one from Afghanistan, 78 from China, 114 from India, 5 from Myanmar and 83 from Pakistan. However, the areal extent of the five IAPS will either increase or decrease within the boundaries of the PAs in the future (under both RCPs by 2070).
Fig. 3.
Combined invasion of the five IAPS (grey coloured) for the present, RCP4.5 and RCP8.5 for the year 2070 and vulnerable protected areas (light brown coloured) in the study area
For example, the future suitability expansion of A. adenophora will mostly be to PAs of north-western India, while C. odorata could affect some PAs of northern India (Figs. 2, 3). Similarly, the L. camara habitat expansion could invade PAs up to northern Pakistan and eastern India (Fig. 2, 3).
Discussion
This study investigated current potential and future predicted habitat suitability of the five IAPS in the Himalayan region using the MaxEnt ecological niche modelling software and also looked at possible impacts on biodiversity-rich PAs. The current potential habitat distribution of all five IAPS highly matched with the existing occurrence records used in the model analysis (Fig. 1) and supports the findings of Xu et al. (2012) and Chen et al. (2017), who also reported that the distribution of invasive species in China is highly concentrated in Yunnan, eastern and coastal provinces, while areas in western regions, such as the Tibetan Plateau, have the least. Our models obtained AUC values that ranged from almost 0.75 for L. camara to 0.92 for A. adenophora, which are within the acceptable range for models to be considered robust. Swets (1988), Pearce and Ferrier (2000) and Elith (2000) suggested that AUC values above 0.75 are potentially useful and acceptable for interpreting a niche model output. In this context, Evangelista et al. (2008) showed that predictive ecological niche models perform poorly for generalist plants such as invasive species that can withstand broad environmental conditions that are not easily defined by data, independent variables or model design compared to geographically restricted specialist plants. Thus, we considered our model performance sufficient for interpreting the overall result of the five IAPS in the Himalayas. The annual mean temperature and annual precipitation were found to be the most influential variables for most of the IAPS, along with population density, compared to topographical variables such as elevation, slope and aspect in describing their distribution. Zhu et al. (2007) reported temperature and precipitation as the major variables influencing the spread of A. adenophora in China. Human population density and economic activity were strongly correlated with invasive species richness in 31 provinces of China (Liu et al. 2005b), which has been reported as a similar case to the central Himalaya (Bhattarai et al. 2014; Shrestha et al. 2015), suggesting that both of these factors are the keys behind the spread of invasion, as shown by our models.
Of the five species, three species, viz. A. adenophora, C. odorata and L. camara, will have overall increased suitability under both RCPs compared to the present, while A. conyzoides and P. hysterophorus will have decreased suitability overall. This change in suitability in the future depends not only on the temperature or precipitation variables that the MaxEnt model used but also on many non-climatic factors as well as the individual plant’s morphological and physiological advancement and its ability to cope with an adverse climate. All five IAPS are characteristically known as broad ecological and environmentally tolerant species that possess the inherent features necessary to maintain their growth and survival in adverse conditions. It is for this reason that it is difficult for us to explain why some species will expand and others may contract their suitable areas in the next couple of decades. Bezeng et al. (2017), for instance, modelled 162 non-native trees and shrubs of South Africa and found that over half of them will have decreased climatic suitability by 2070. Fandohan et al. (2015) also reported a decrease in the suitability of C. odorata in the protected areas (PAs) of four West African countries by 2070. Similarly, the changes in either expansion or reduction of such suitable area are less than twenty two percent, while most of the area remains stable for all species (Table 2, Fig. 2). This suggests that all the modelled IAPS will conserve most of their current ecological niche even with future changes in climate in the Himalayan region. Some species will expand towards the south, especially in the central and eastern foothills of the Himalaya. This is obvious, as Goncalves et al. (2014), citing spread of L. camara in India, reported that invasive species should not always be expected to conserve their strict niche, and therefore, areas in all directions may be at risk of a potential future invasion. Invasive species exhibit more phenotypic plasticity, an evolutionary mechanism, than native species occurring in the same region (Daehler 2003), signifying their ability to move into suitable climatic areas that enhance their growth and development. For instance, Zhao et al. (2012) reported that phenotypic plasticity is the only factor that helps the invasive weed A. adenophora to succeed in different climatic and geographic contexts in Yunnan, China. The southward expansion of L. camara in northern India on the border of Nepal and Assam shown by our model can be further justified because these areas are elevated terrain between 500 and 1300 m that could be under cold stress currently and whose climate may warm by 2070 to better suit the species requirement.
Our model shows less northward expansion overall from all of the five IAPS, with almost none towards the Tibetan Plateau, while there is some expansion towards the western Himalayan region (Fig. 2). As mentioned, it should be noted first that all the modelled species are of tropical and sub-tropical origin, with lower growth form that could not withstand the harsh climate of the high Himalaya and Tibetan Plateau, even though some climatic modification is projected there. For instance, alien invasive plants can have a strong response in the currently warm, urbanized and low elevation regions rather than the areas with cold and high elevations that have a low human population and less disturbance (Marini et al. 2009). In the case of China, Lin et al. (2007) argued that provinces located in southern China and the coastal areas of eastern China, which saw a major boost in economic activities in the last quarter of 20th century and are now more economically developed, have higher abundances of invasive species compared to provinces in inland and western China, such as our study area, that contributed as much to the expansion of invasive species as climatic factors.
Similarly, the B1 (< 1500 m) belt has high suitability for all the species, which tends to decrease with increasing elevation under both RCPs by 2070 (Table 3). McDougall et al. (2011) and Alexander et al. (2011) reiterated that invasion risk in the mountain ecosystem is comparatively less than other ecosystems. Low propagule availability, the absence of species exposed to wider environmental conditions, low anthropogenic disturbances and low invasibility of natural communities are reasons that the alpine ecosystem currently has a restricted number and intensity of invasive species (Alexander et al. 2016). Abiotic stressful areas, such as the Himalayas and Tibetan Plateau, are less prone to invasion than more moderate environments. Zefferman et al. (2015) explained the low invasibility of harsh areas through twin hypotheses: (i) propagule limitation hypothesis—suggests invasion on such sites is limited by the low arrival rates of propagules than in moderate habitat found at lower elevations; and (ii) invasion resistance hypothesis—suggests that such sites bear abiotic stressful conditions even in the future and also have increased biotic resistance from resident organisms. Our findings are consistent with (i) Andersen et al. (2015) and Averett et al. (2016), who reported a decreasing distribution of invasive species with increasing elevation in the Wallowa Mountains of the USA; (ii) Becker et al. (2005) and Seipel et al. (2016), for similar observations in the Swiss Alps; and (iii) Barni et al. (2012) in a mountain ecosystem in the Italian Alps. They argued that an invasion into such highlands will proceed at rather a slow pace due to the adverse climate in the context of future warming. Joshi et al. (2006) reported that lower winter temperatures or high frost levels constrained the vertical distribution of invasive plant species such as C. odorata towards the northern boundary of the Nepal Himalaya, mainly due to their impacts on photosynthetic activity.
The problems known so far as a result of the invasion of alien species should act as a lesson for us to take actions even before we are able to unearth all of their effects (McNeely 2001). Impacts from invasive species to ecosystems and their biota is pervasive at a global scale, which in turn affects ecosystem services (ES) upon which millions of people derive or sustain their livelihood. Our result shows the possible expansion of suitable areas of the five IAPS in different ecosystems ranging from tropical and sub-tropical to temperate in the elevated northern region (Fig. 2, Table 3). Similarly, Fig. 3 depicts that almost 69% of the PAs established within the study area are currently invaded and will remain so in the future by the five investigated IAPS that are capable of invading forests, shrub land, grassland, pasture and agroecosystems. This will have irreversible impacts on the habitat and food availability for many endangered wildlife species. The whole Himalayan region is a hotspot of biodiversity with numerous fragile ecosystems along the northern boundary. For instance, Chettri et al. (2008) reported that 32% of the PAs within the Hindu Kush Region fall under a global biodiversity hotspot, while 62% are under the Global 200 eco-regions. PAs in China occupy almost 15% of the total land area, with a high concentration found in the western region, including Tibet and Qinghai (Xu et al. 2017). Kannan et al. (2013) reported that the majority of PAs in India, i.e. 102 national parks and 515 wildlife sanctuaries spatially distributed from lowlands to highlands, are currently invaded by invasive species, notably with Lantana and Eupatorium spp., while future impacts are largely unknown. Even the southern tropical PAs are the critical habitat of numerous endangered species, such as rhinoceros, royal Bengal tiger and many indigenous flora, for which invasion is considered highly destructive. The provisioning ES, such as non-timber forest products (NTFPs) on which the livelihoods of mountain people depend, could be heavily hit from such invasions in the sub-temperate and temperate regions.
Similarly, as discussed, a niche model performs comparatively poorly for generalist plants, such as the ones that have been modelled in this paper. MaxEnt is based on climate, and many non-climatic factors such as biotic interactions, soil types, as well as dispersal mode and abilities that are also responsible for the future dynamism of invasive species by restricting or expanding their ranges are not included in the modelling process. Clements and Ditommaso (2011) argued that current modelling approaches should consider potential evolutionary changes to correctly predict the invasive range expansion because these plants are capable of rapid genetic changes to boost their invasive ability into newly invaded areas with a modified ecosystem. Urban et al. (2016) recently emphasized the use of biological mechanisms of species in the model to increase the prediction accuracy and make the model uncertainties explicit. There are uncertainties inherent in the existing model projection and future emission scenarios itself (Stott and Kettleborough 2002; Knutti and Sedlacek 2013). Similarly, Jones (2012) reported that high current niche model accuracy, especially in the case of invasive species, may not point to high future accuracy, mainly because they are not at equilibrium with the environment and therefore suggested to use it more cautiously.
The five IAPS investigated in this paper are of tropical origin, not temperate or alpine origin, and the growth form and physiological and morphological characteristics totally differ compared to plants from high elevations; therefore, we could not expect a massive northward and high elevation expansion with a future warming climate in the region, as suggested for cold-adapted plant species in other studies (Song et al. 2004; Benito et al. 2011; Xiaodan et al. 2011; Lamsal et al. 2017). Human activities, such as agriculture and urban development, might move upward in the mountain region under current global warming (Price 2006). Marini et al. (2012) reported human population pressure as a major driver of alien plant invasions in the European Alps in their distribution compared to climatic conditions. As the literature suggests, such activities in high lands cause areas to become suitable for invasion, which is also the case for the Himalayas. In addition, Zhang et al. (2015) found that the invasive species that reach higher elevation of mountain forests in Sangdong Province of China are those that have successfully invaded low elevations and filtered out to areas with successive worsening climate and decreasing anthropogenic propagule pressure. Similar findings have been reported from the Canary Islands of Spain (Haider et al. 2010), where most of the non-native plants of higher altitudinal ranges were the ones introduced in lowlands and preadapted to local climates. All the IAPS considered in this study were introduced and are currently well adapted to the lowlands of the Himalaya foothills and therefore are likely to expand along the high elevation gradient, though at a very low pace.
Invasive species pose a significant global problem, causing detrimental impacts to all states, to their ecosystems and diversity, and thus deserve a significant global response (McNeely 2001). IAPS in South Asia are a comparatively a less studied topic, though this region is highly vulnerable to invasion due to the high mosaic of biodiversity hotspots and low human response capacity. The fate of high elevation mountain ecosystems, such as the Himalayan region, is even unknown. Zhang et al. (2015) suggested that the monitoring and management of IAPS should first target low elevation areas to prevent their invasion into high elevation regions, which could be the case for the Himalayas. Likewise, Funk et al. (2014) reported the impact of IAPS on ecosystem services that directly hinders economic and ecological benefit to human beings. These effects should be considered in an inter-governmental policy dialogue to assure continuous and timely investment in control activities.
Conclusion
This study reveals that the five IAPS will mostly conserve their existing ecological niches in the future, with lesser expansion towards the northern high elevation regions of the Himalayas, because the high mountains and harsh climate could potentially serve as barriers. Therefore, our findings are timely and are thus expected to help resource managers, including those in PAs, in preparing a baseline database and initiating the formulation of future response strategies in the Himalayan region against anticipated invasion risks arising from these five IAPS. The newly identified areas at high risk as a result of transboundary scale IAPS expansion can be delineated for strategic control measures to prevent their spread. A good and realistic transboundary strategic response policy that is suitable to local context of each country, for example, indigenous biological and cultural weed control measures, could be a viable option to buffer against the current and future detrimental impacts of invasion into the fragile ecosystems of the region that harbour numerous endangered biota.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
We would like to thank the National Herbarium and Plant Laboratories (KATH), Godavari, Nepal for providing an opportunity to study and record spatial distribution datasets of selected invasive species through their archived herbarium sheets. We also appreciate Dr. Bharat Babu Shrestha, Dr. Chudamani Joshi and Mr. Rajesh Malla for making available some distribution dataset of selected invasive species. We are equally grateful to two anonymous reviewers for providing insightful comments on the earlier versions of this manuscript.
Biographies
Pramod Lamsal
is a Ph.D. candidate at The University of New England, Australia. His research interests include climate change impact and species distribution modelling, remote sensing, geo-spatial modelling and ecosystem services.
Lalit Kumar
is a Professor at The University of New England, Australia. His research interests include climate change impacts on biodiversity and Small Island states, mainly in the Pacific and the Himalayan region, remote sensing, spatial modelling, spatial ecology and species distribution modelling.
Achyut Aryal
is an honorary research fellow at The University of Sydney/Federation University of Australia, Australia; Toi Ohomai Institute of Technology, New Zealand, and research associate at Human-Wildlife Interaction Research Group, Massey University, New Zealand. His research interest includes conservation biology, wildlife ecology and climate change.
Kishor Atreya
is an agricultural and environmental health expert at Asia Network for Sustainable Agriculture and Bioresources (ANSAB), Nepal. His research interests include environment-human health/livelihood nexus; biodiversity, ecosystem services and climate change; health risk assessment; social cost–benefit analysis; and economic valuation of environmental resources/pollution.
Contributor Information
Pramod Lamsal, Phone: +61 406346240, Email: plamsal@myune.edu.au, Email: pramod_lamsal@yahoo.com.
Lalit Kumar, Email: lkumar@une.edu.au.
Achyut Aryal, Email: savefauna@gmail.com.
Kishor Atreya, Email: k.atreya@gmail.com.
References
- Adhikari D, Tiwary R, Barik SK. Modelling hotspots for invasive alien plants in India. PLoS ONE. 2015;10:e0134665. doi: 10.1371/journal.pone.0134665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JM, Lembrechts JJ, Cavieres LA, Daehler C, Haider S, Kueffer C, Liu G, McDougall K, et al. Plant invasion into mountains and alpine ecosystems: Current status and future challenges. Alpine Botany. 2016;126:89–103. doi: 10.1007/s00035-016-0172-8. [DOI] [Google Scholar]
- Alexander JM, Kueffer C, Daehler CC, Edwards PJ, Pauchard A, Seipel T, MIREN Consortium Assembly of nonnative floras along elevational gradients explained by directional ecological filtering. Proceedings of the National Academy of Sciences. 2011;108:656–667. doi: 10.1073/pnas.1013136108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen KM, Naylor BJ, Endress BA, Parks CG. Contrasting distribution patterns of invasive and naturalized non-native species along environmental gradients in a semi-arid montane ecosystem. Applied Vegetation Science. 2015;18:683–693. doi: 10.1111/avsc.12185. [DOI] [Google Scholar]
- Aryal A, Shrestha UB, Ji W, Ale SB, Shreshta S, Ingty T, Maraseni T, Cokcfield G, Raubenheimer D. Predicting the distribution of predator (snow leopard) and prey (blue sheep) under climate change in the Himalaya. Ecology and Evolution. 2016;6:4065–4075. doi: 10.1002/ece3.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal A, Acharya KP, Shrestha UB, Dhakal M, Raubenhiemer D, Wright W. Global lessons from successful rhinoceros conservation in Nepal. Conservation Biology. 2017;31:1494–1497. doi: 10.1111/cobi.12894. [DOI] [PubMed] [Google Scholar]
- Averett JP, McCune B, Parks CG, Naylor BJ, DelCurto T, Mata-Gonzalez R. Non-native plant invasion along elevation and canopy closure gradients in a middle rocky Mountain ecosystem. PLoS ONE. 2016;11:e0147826. doi: 10.1371/journal.pone.0147826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin RA. Use of maximum entropy modelling in wildlife research. Entropy. 2009;11:854–866. doi: 10.3390/e11040854. [DOI] [Google Scholar]
- Barni E, Bacaro G, Falzoi S, Spanna F, Siniscalco C. Establishing climatic constraints shaping the distribution of alien plant species along the elevation gradient in the Alps. Plant Ecology. 2012;213:757–767. doi: 10.1007/s11258-012-0039-z. [DOI] [Google Scholar]
- Beaumont LJ, Gallagher RV, Thuiller W, Downey PO, Leishman MR, Hughes L. Different climate envelopes among invasive populations may lead to underestimations of current and future biological invasions. Diversity and Distribution. 2009;15:409–420. doi: 10.1111/j.1472-4642.2008.00547.x. [DOI] [Google Scholar]
- Becker T, Dietz H, Billeter R, Buschmann H, Edwards PJ. Altitudinal distribution of alien plant species in the Swiss Alps. Perspective in Plant Ecology, Evolution and Systematics. 2005;7:173–183. doi: 10.1016/j.ppees.2005.09.006. [DOI] [Google Scholar]
- Benito B, Lorite J, Penas J. Simulating potential effects of climatic warming on altitudinal patterns of key species in Mediterranean-alpine ecosystems. Climatic Change. 2011;108:471–483. doi: 10.1007/s10584-010-0015-3. [DOI] [Google Scholar]
- Bezeng BS, Morales-Castilla I, van der Bank M, Yessoufou K, Daru BH, Davies TJ. Climate change may reduce the spread of non-native species. Ecosphere. 2017;8:e01694. doi: 10.1002/ecs2.1694. [DOI] [Google Scholar]
- Bhattarai KR, Inger E, Maren SCS. Biodiversity and invasibility: Distribution patterns of invasive plant species in the Himalayas, Nepal. Journal of Mountain Science. 2014;11:688–696. doi: 10.1007/s11629-013-2821-3. [DOI] [Google Scholar]
- Chen C, Wang QH, Wu JY, Huang D, Zhang WH, Zhao N, Li XF, Wang LX. Historical introduction, geographical distribution, and biological characteristics of alien plants in China. Biodiversity and Conservation. 2017;26:353–381. doi: 10.1007/s10531-016-1246-z. [DOI] [Google Scholar]
- Chettri N, Shakya B, Thapa R, Sharma E. Status of a protected area system in the Hindu Kush-Himalayas: An analysis of PA coverage. The International Journal of Biodiversity Science and Management. 2008;4:164–178. doi: 10.3843/Biodiv.4.3:4. [DOI] [Google Scholar]
- Clements DR, Ditommaso A. Climate change and weed adaptation: Can evolution of invasive plants lead to greater range expansion than forecasted? Weed Research. 2011;51:227–240. doi: 10.1111/j.1365-3180.2011.00850.x. [DOI] [Google Scholar]
- Cruz-Cardenas G, Lopez-Malta L, Villasenor JL, Ortiz E. Potential species distribution modelling and the use of principal component analysis as predictor variables. Revista Mexicana de Biodiversidad. 2014;85:189–199. doi: 10.7550/rmb.36723. [DOI] [Google Scholar]
- Daehler CC. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annual Review of Ecology and Systematics. 2003;34:183–211. doi: 10.1146/annurev.ecolsys.34.011802.132403. [DOI] [Google Scholar]
- Dhar PA, Reshi ZA. Do alien plant invasions cause biotic homogenization of terrestrial ecosystems in the Kashmir Valley, India? Tropical Ecology. 2015;56:111–123. [Google Scholar]
- Dromann CF, Elith J, Bahcer S, Buchmann C, Carl G, Carre G, Marquez JRG, Gruber B, et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2012;36:27–46. doi: 10.1111/j.1600-0587.2012.07348.x. [DOI] [Google Scholar]
- Duan RY, Kong XQ, Huang MY, Fan WY, Wang ZG. The predictive performance and stability of six species distribution models. PLoS ONE. 2014;9:e112764. doi: 10.1371/journal.pone.0112764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early RBA, Bradley JS, Dukes JJ, Lawler JD, Olden DM, Blumenthal P, Gonzalez ED Grosholz, et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nature Communication. 2016;7:12485. doi: 10.1038/ncomms12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elith J. Quantitative methods for modelling species habitat: Comparative performance and an application to Australian plants. In: Ferson S, Burgman M, editors. Quantitative methods for conservation biology. New York: Springer; 2000. pp. 39–58. [Google Scholar]
- Elith J, Phillips SJ, Hastie T, Dudik M, Chee YE, Yates CJ. A statistical explanation of Maxent for ecologist. Diversity and Distribution. 2011;17:43–57. doi: 10.1111/j.1472-4642.2010.00725.x. [DOI] [Google Scholar]
- Evangelista PH, Kumar S, Stohlgren TJ, Jarnevich CS, Crall AW, Norman JB, Barnett DT. Modelling invasion for a habitat generalist and a specialist plant species. Diversity and Distribution. 2008;14:808–817. doi: 10.1111/j.1472-4642.2008.00486.x. [DOI] [Google Scholar]
- Fandohan AB, Oduor AM, Sodé AI, Wu L, Cuni-Sanchez A, Assédé E, Gouwakinnou GN. Modeling vulnerability of protected areas to invasion by Chromolaena odorata under current and future climates. Ecosystem Health and Sustainability. 2015;1:20. doi: 10.1890/EHS15-0003.1. [DOI] [Google Scholar]
- Funk JL, Matzek V, Bernhardt M, Johnson D. Broadening the case for invasive species management to include impacts on ecosystem services. BioScience. 2014;64:58–63. doi: 10.1093/biosci/bit004. [DOI] [Google Scholar]
- Goncalves E, Herrera I, Duarte M, Bustamante RO, Lampo M, Velasquez G, Sharma GP, Garcia-Rangel S. Global invasion of Lantana camara: Has the climatic niche been conserved across continents? PLoS ONE. 2014;9:e111468. doi: 10.1371/journal.pone.0111468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Mazer SJ, Xu X, Luo X, Huang K, Xu X, et al. Biological invasions in nature reserves in China. In: Wan F, et al., editors. Biological invasions and its management in China. New York: Springer; 2017. pp. 125–147. [Google Scholar]
- Grice AC. The impacts of invasive plant species on the biodiversity of Australian rangelands. The Rangeland Journal. 2006;28:27–35. doi: 10.1071/RJ06014. [DOI] [Google Scholar]
- Haider S, Alexander J, Dietz H, Trepl L, Edwards PJ, Kueffer C. The role of bioclimatic origin, residence time and habitat context in shaping non-native plant distributions along altitudinal gradient. Biological Invasion. 2010;12:4003–4018. doi: 10.1007/s10530-010-9815-7. [DOI] [Google Scholar]
- Hellmann JJ, Byers JE, Bierwagen BG, Dukes JS. Five potential impacts of climate change for invasive species. Conservation Biology. 2008;22:534–543. doi: 10.1111/j.1523-1739.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- Higgins SI, Richardson DM. Invasive plants have broader physiological niches. Proceeding of the National Academy of Sciences. 2014;111:10610–10614. doi: 10.1073/pnas.1406075111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. 2013. Summary for Policymakers. In: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds.). Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- Jauni M, Gripenberg S, Ramula S. Non-native plant species benefits from disturbance: A meta-analysis. Oikos. 2015;124:122–129. doi: 10.1111/oik.01416. [DOI] [Google Scholar]
- Jayanarayanan S, Krishnan R, Shrestha AB, Rajbhandari R, Guo RY. Downscaled climate change projections for the Hindu Kush Himalayan region using CORDEX South Asia regional climate models. Advances in Climate Change Research. 2017;8:185–198. doi: 10.1016/j.accre.2017.08.003. [DOI] [Google Scholar]
- Jones CC. Challenges in predicting the future distribution of invasive plant species. Forest Ecology and Management. 2012;284:69–77. doi: 10.1016/j.foreco.2012.07.024. [DOI] [Google Scholar]
- Joshi C., J. de Leeuw, and A.K. Skidmore. 2006. Upscaling species invasion patterns from local to regional for forest ecosystem management. ISPRS mid-term symposium: “Remote sensing: from pixel to processes”, Commission VI, WG VI/7; 8-11 May 2006, the Netherlands.
- Kannan R, Shackleton CM, Shaanker RU. Playing with the forest: invasive alien plants, policy and protected areas in India. Current Science. 2013;104:1159–1165. [Google Scholar]
- Knutti R, Sedlacek J. Robustness and uncertainties in the new CMIP5 climate model projections. Nature Climate Change. 2013;3:369–373. doi: 10.1038/nclimate1716. [DOI] [Google Scholar]
- Lamsal P, Kumar L, Shabani F, Atreya K. The greening of the Himalaya and Tibetan Plateau under climate change. Global and Planetary Change. 2017;159:77–92. doi: 10.1016/j.gloplacha.2017.09.010. [DOI] [Google Scholar]
- Lembrechts JJ, Milbau A, Nijs I. Alien roadside species more easily invade alpine than lowland plant communities in a subarctic mountain ecosystem. PLoS ONE. 2014;9:e89664. doi: 10.1371/journal.pone.0089664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Zhou G, Cheng X, Xu R. Fast economic development accelarates biological invasion in China. PLoS ONE. 2007;11:e1208. doi: 10.1371/journal.pone.0001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Berry PM, Dawson TP, Pearson RG. Selecting thresholds of occurrence in the prediction of species distribution. Ecograhpy. 2005;28:385–393. doi: 10.1111/j.0906-7590.2005.03957.x. [DOI] [Google Scholar]
- Liu C, White M, Newell G. Selecting thresholds for the prediction of species occurrence with presence only data. Journal of Biogeography. 2013;40:778–789. doi: 10.1111/jbi.12058. [DOI] [Google Scholar]
- Liu J, Liang SC, Liu FH, Wang RQ, Dong M. Invasive alien plant species in China: Regional distribution pattern. Diversity and Distribution. 2005;11:341–347. doi: 10.1111/j.1366-9516.2005.00162.x. [DOI] [Google Scholar]
- Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. Biotic invasion: Causes, epidemiology, global consequences, and control. Ecological Applications. 2000;10:689–710. doi: 10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2. [DOI] [Google Scholar]
- Mainali KP, Warren DL, Dhileepan K, McConnachie A, Strathie L, Hassan G, Karki D, Shrestha BB, Parmesan C. Projecting future expansion of invasive species: Comparing and improving methodologies for species distribution methods. Global Change Biology. 2015;21:4464–4480. doi: 10.1111/gcb.13038. [DOI] [PubMed] [Google Scholar]
- Mainka SA, Howard GW. Climate change and invasive species: Double jeopardy. Integrative Zoology. 2010;5:102–111. doi: 10.1111/j.1749-4877.2010.00193.x. [DOI] [PubMed] [Google Scholar]
- Marini L, Battisti A, Bona E, Federici G, Martini F, Pautasso M, Hulme PE. Alien and native plant life-forms respond differently to human and climate pressures. Global Ecology and Biogeography. 2012;21:534–544. doi: 10.1111/j.1466-8238.2011.00702.x. [DOI] [Google Scholar]
- Marini L, Gaston KJ, Prosser F, Hulme PE. Contrasting response of native and alien plant species richness to environmental energy and human impact along alpine elevation gradient. Global Ecology and Biogeography. 2009;18:652–661. doi: 10.1111/j.1466-8238.2009.00484.x. [DOI] [Google Scholar]
- Maron JL, Vila M, Bommarco R, Elmendorf S, Beardsley P. Rapid Evolution of an Invasive Plant. Ecological Monographs. 2004;72:261–280. doi: 10.1890/03-4027. [DOI] [Google Scholar]
- Masters, G., and L. Norgrove. 2010. Climate change and invasive alien species. CABI Working Paper 1, 30 pp.
- McDougall KL, Khuroo AA, Loope LL, Parks CG, Pauchard A, Reshi ZA, Rushworth I, Keuffer C. Plant invasions in mountains: global lessons for better management. Mountain Research and Development. 2011;31:380–387. doi: 10.1659/MRD-JOURNAL-D-11-00082.1. [DOI] [Google Scholar]
- McNeely J. Invasive species: a costly catastrophe for native biodiversity. Land Use and Water Resources Research. 2001;1:1–10. [Google Scholar]
- Mishra V, Kumar D, Ganguly AR, Sanjay J, Majumdar M, Krishnan R, Shah RP. Reliability of regional and global climate models to simulate precipitation extremes over India. Journal of Geophysical Research. 2014;119:9301–9323. [Google Scholar]
- Murphy ST, Subedi N, Jnawali SR, Lamichane BR. Invasive mikania in Chitwan National Park, Nepal: The threat to the greater one-horned rhinoceros (Rhinoceros unicornis) and factors driving the invasion. Oryx. 2013;47:361–368. doi: 10.1017/S003060531200124X. [DOI] [Google Scholar]
- Pearce J, Ferrier S. An evaluation of alternative algorithms for fitting species distribution models using logistic regression. Ecological Modelling. 2000;128:127–147. doi: 10.1016/S0304-3800(99)00227-6. [DOI] [Google Scholar]
- Phillips S, Anderson R, Schapire R. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190:231–259. doi: 10.1016/j.ecolmodel.2005.03.026. [DOI] [Google Scholar]
- Price ME. Global change in mountain regions. Dunkow: Sapiens Publishing; 2006. [Google Scholar]
- Priyanka N, Joshi PK. Effects of climate change on invasion potential distribution of Lantana camara. Journal of Earth Science and Climate Change. 2013;4:164. [Google Scholar]
- Pysek P, Jarosik V, Hulme PE, Pergl J, Hejda M, Schaffner U, Vila M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Global Change Biology. 2012;18:1725–1737. doi: 10.1111/j.1365-2486.2011.02636.x. [DOI] [Google Scholar]
- Ren YY, Ren GY, Sun XB, Shrestha AB, You QL, Zhan YJ, Rajbhandari R, Zhang PF, et al. Observed changes in surface air temperature and precipitation in the Hindu Kush Himalayan region over the last 100-plus years. Advances in Climate Change Research. 2017;8:148–156. doi: 10.1016/j.accre.2017.08.001. [DOI] [Google Scholar]
- Richardson DM, Rejmanek M. Trees and shrubs as invasive alien species—a global review. Diversity and Distribution. 2011;17:788–809. doi: 10.1111/j.1472-4642.2011.00782.x. [DOI] [Google Scholar]
- Saurez-Mota ME, Oritz E, Villasenor JL, Espinosa-Garcia FJ. Ecological niche modeling of invasive plant species according to invasion status and management needs: The case of Chromolaena odorata (Asteraceae) in South Africa. Polish Journal of Ecology. 2016;64:369–383. doi: 10.3161/15052249PJE2016.64.3.007. [DOI] [Google Scholar]
- Seipel T, Alexander JM, Edwards PJ, Kueffer C. Range limits and population dynamics of non native plants spreading along elevation gradient. Perspective in Plant Ecology, Evolution and Systematics. 2016;20:46–55. doi: 10.1016/j.ppees.2016.04.001. [DOI] [Google Scholar]
- Sharmila S, Joseph S, Sahai AK, Abhilash S, Chattopadhyay R. Future projection of Indian summer monsoon variability under climate change scenario: An assessment from CMIP5 climate models. Global and Planet Change. 2015;124:62–78. doi: 10.1016/j.gloplacha.2014.11.004. [DOI] [Google Scholar]
- Shrestha BB, Shabbir A, Adkins SW. Parthenium hysterophorus in Nepal: A review of its weed status and possibilities for management. Weed Research. 2015;55:132–144. doi: 10.1111/wre.12133. [DOI] [Google Scholar]
- Song M, Zhou C, Ouyang H. Distributions of dominant tree species on the Tibetan Plateau under current and future climate scenarios. Mountain Research and Development. 2004;24:166–173. doi: 10.1659/0276-4741(2004)024[0166:DODTSO]2.0.CO;2. [DOI] [Google Scholar]
- Stott PA, Kettleborough JA. Origin and estimates of uncertainty in predictions of twenty first century temperature rise. Nature. 2002;416:723–726. doi: 10.1038/416723a. [DOI] [PubMed] [Google Scholar]
- Su J, Aryal A, Nan Z, Ji W. Climate change-induced range expansion of a subterranean rodent: Implications for rangeland management in Qinghai-Tibetan Plateau. PLoS ONE. 2015;10:e0138969. doi: 10.1371/journal.pone.0138969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swets K. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- Torchin ME, Mitchell CE. Parasites, pathogens, and invasion by plants and animals. Frontiers in Ecology and Environment. 2004;2:183–190. doi: 10.1890/1540-9295(2004)002[0183:PPAIBP]2.0.CO;2. [DOI] [Google Scholar]
- Urban MC, Bocedi G, Hendry AP, Mihoub JB, Péer G, Singer A, Bridle JR, Crozier LG, et al. Improving the forecast for biodiversity under climate change. Science. 2016;353:aad8464. doi: 10.1126/science.aaf4802. [DOI] [PubMed] [Google Scholar]
- Watanabe, M., T. Suzuki, R. O'ishi, Y. Komuro, S. Watanabe, S. Emori, T. Takemura, M. Chikira, et al. 2010. Improved climatic simulation by MIROC5: Mean states, variability, and climatic sensitivity. Journal of Climate 23: 6312–6335.
- West AM, Kumar S, Brown CS, Stohlgren TJ, Bromberg J. Field validation of an invasive species Maxent model. Ecological Informatics. 2016;36:126–134. doi: 10.1016/j.ecoinf.2016.11.001. [DOI] [Google Scholar]
- Whitney KD, Gabler CA. Rapid evolution in invasive species, ‘invasive traits’, and receipient communities: Challenges for predicting invasive potential. Diversity and Distribution. 2008;14:569–580. doi: 10.1111/j.1472-4642.2008.00473.x. [DOI] [Google Scholar]
- Xiaodan W, Genwei C, Xianghao Z. Assessing potential impacts of climatic change on subalpine forests on the eastern Tibetan Plateau. Climatic Change. 2011;108:225–241. doi: 10.1007/s10584-010-0008-2. [DOI] [Google Scholar]
- Xu H, Qiang S, Genovesi P, Ding H, Wu J, Meng L, Han Z, Miao J, et al. An inventory of invasive alien species in China. NeoBiota. 2012;15:1–26. doi: 10.3897/neobiota.15.3575. [DOI] [Google Scholar]
- Xu W, Xiao Y, Zhang J, Yang W, Zhang L, Hull V, Wang Z, Zheng H, et al. Strengthening protected areas for biodiversity and ecosystem services in China. Proceedings of the National Academy of Sciences. 2017;114:1601–1606. doi: 10.1073/pnas.1620503114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zefferman E, Stevens JT, Charles GK, Dunbar-Irwin M, Emam T, Fick S, Morales LV, Wolf KM, et al. Plant communities in harsh sites are less invaded: A summary of observations and proposed explanations. AoB PLANTS. 2015;7:plv056. doi: 10.1093/aobpla/plv056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Yin D, Huang D, Du N, Liu J, Guo W, Wang R. Altitudinal patterns illustrate the invasion mechanisms of alien plants in temperate mountain forests of northern China. Forest Ecology and Management. 2015;351:1–8. doi: 10.1016/j.foreco.2015.05.004. [DOI] [Google Scholar]
- Zhao X, Liu W, Zhou M. Lack of local adaptation of invasive crofton weed (Ageratina adenophora) in different climatic areas of Yunnan province, China. Journal of Plant Ecology. 2012;6:316–322. doi: 10.1093/jpe/rts036. [DOI] [Google Scholar]
- Zhu L, Sun OJ, Sang W, Li Z, Ma K. Predicting the spatial distribution of an invasive plant species (Eupatorium adenophorum) in China. Landscape Ecology. 2007;22:1143–1154. doi: 10.1007/s10980-007-9096-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.