Abstract
Combined increases in peripheral inflammation and brain glutamate may identify a subtype of depression with distinct neuroimaging signatures. Two contrasting subgroups of depressed subjects—with and without combined elevations in plasma C-reactive protein (CRP) and basal ganglia glutamate (high and low CRP-Glu, respectively) were identified by hierarchical clustering using plasma CRP (indexing peripheral inflammation) and magnetic resonance spectroscopy (MRS)-based measurement of left basal ganglia glutamate. High CRP-Glu group status was associated with greater severity of anhedonia and cognitive and motor slowing. Local- and network-level measures of functional integrity were determined using brain oxygen level-dependent (BOLD)-oscillatory activity and graph theory. Greater decreases in concordance of oscillatory activity between neighboring voxels (Regional Homogeneity ‘ReHo’, p < 0.01) within the MRS volume-of-interest was associated with the High CRP-Glu subgroup. Using brain-wide, CRP-Glu ReHo contrast maps, a covariance network of 41 regions-of-interest (ROIs) with similar ReHo decreases was identified in the High CRP-Glu group and was located to brain structures previously implicated in depression. The 41-ROI network was further decomposed into four subnetworks. ReHo decreases within Subnetwork4—comprised of reward processing regions —was associated with anhedonia. Subnetwork4 ReHo also predicted decreased network integrity, which mediated the link between local ReHo and anhedonia in the Low but not High CRP-Glu group. These findings suggest that decreased ReHo and related disruptions in network integrity may reflect toxic effects of inflammation-induced increases in extrasynaptic glutamate signaling. Moreover, local BOLD oscillatory activity as reflected in ReHo might be a useful measure of target-engagement in the brain for treatment of inflammation-induced behaviors.
Introduction
Research on major depressive disorder (MDD) has been stymied by within- and between-subject variability. One approach to limiting this variability has been to identify patient subgroups based on pathophysiological processes such as increased inflammation1–3. Indeed, a distinct subtype of depression characterized by increased inflammation has been proposed4. In the brain, inflammation triggers a broad array of neurotransmitter, molecular, neural, and behavioral effects5–10. For example, altered glutamate (Glu) neurotransmission in subcortical regions has been a key neurochemical target of inflammation in depression11. Previously published data indicate that increased plasma concentrations of the systemic inflammatory marker c-reactive protein (CRP) among subjects with MDD predicted elevated Glu concentrations in left basal ganglia regions as measured by magnetic resonance spectroscopy (MRS), which in turn predicted greater anhedonia and decreased psychomotor speed, reaction-time, and information processing12. Similar results were found among individuals treated with the inflammatory cytokine interferon-alpha12–14.
As to the mechanisms by which inflammation influences Glu neurotransmission, inflammatory cytokines increase spillover of Glu from the intra- into the extrasynaptic space by decreasing the ability of astrocytes to clear, buffer and contain Glu, while concurrently impairing mechanisms that remove Glu from the extracellular matrix15,16. In addition, glial cells and trafficking macrophages increase surface expression of cystine/glutamate exchanger (Xc)-transporters that extrude Glu into the extrasynaptic space in exchange for cystine11,16,17. Of note, these Xc- transporters release large volumes of Glu in dangerously close proximity to extrasynaptic receptor-binding sites18. Finally, immune activation increases Glu-like molecules such as quinolinic acid, which can promote glial Glu release or impair its reuptake19,20. Taken together, inflammation preferentially increases Glu in extrasynaptic compared to intrasynaptic locations15,18,21–23. Extrasynaptic Glu freely diffuses into the extracellular space and binds to extrasynaptic N-methyl-d-aspartate (NMDA) receptors, leading to chaotic, noisy, incoherent signaling activity in the short-term, and synaptic toxicity by suppressing intracellular survival mechanisms in the long-term18,24–26. Nonetheless, neural activity-based biosignatures associated with inflammation-induced Glu dysregulation are not well-characterized.
A primary objective of this work was to identify and contrast two subgroups of depressed subjects with and without combined elevations in plasma CRP and basal ganglia glutamate and examine their impact on neural activity. Investigating neural activity using oscillatory frequency, amplitude or coherence of spontaneous brain oxygen level-dependent (BOLD)-fluctuations during resting-state functional MRI offers a unique opportunity to examine these associations27–29. Regional Homogeneity (ReHo)—an activity-based metric of concordance in BOLD-signal fluctuations between neighboring voxels—is an effective estimate of local activity coherence at millimetric resolution29–32. Based on the hypothesis that combined increases in inflammation and extrasynaptic Glu can lead to disorderly, incoherent local neural activity, we predicted that concurrent increases in peripheral inflammation and MRS Glu would identify a subgroup of depressed patients with reduced local ReHo in the basal ganglia and potentially other brain regions. Moreover, we hypothesized that decreased ReHo in regions affiliated with reward, salience, and attention would be associated with anhedonia and psychomotor slowing. Finally, using exploratory path analyses, we examined whether altered network-level interactions among the above regions mediate the link between inflammation, Glu, local ReHo, and anhedonia.
Materials and methods
Subjects
Fifty subjects with a diagnosis of MDD confirmed using Structured Clinical Interview for DSM-IV (SCID) and with ages ranging between 21–65 years were screened for inclusion into the study33,34. Bipolar depressed subjects were also included due to shared mechanisms of anhedonia, inflammation and Glu dysregulation35. Exclusion criteria included unstable medical conditions; cognitive, psychotic or substance abuse disorders; and intake of any psychotropic or immune-altering medications. Detailed information on subject recruitment and evaluation is provided in Supplementary Methods. All participants signed informed consent, and the study was approved a priori by the Institutional Review Board of Emory University. Subjects presented in this manuscript represent a subset of those recruited for NCT01426997.
Behavioral and cognitive assessments
Depression severity was measured using the Inventory of Depressive Symptoms-Self Reported (IDS-SR)36,37. Anhedonia was measured using a 3-item subscale derived from IDS-SR items #8, 19, and 21 (‘‘anhedonia’’) that has been previously validated in other studies12,38,39. Psychomotor and cognitive performance were assessed using the Finger Tapping Test40; Trails Making Test A (TMT)41; Digit Symbol Substitution Test of Wechsler Adult Intelligence Scale42; Movement and Reaction times—Simple (SMT, SRT, respectively) and Five-Choice Reaction Time (5CMT, 5CRT, respectively)—and “Stockings of Cambridge” (SOC) Tasks of the Cambridge Neuropsychological Test Automated Battery (CANTAB)43,44. Of note, principal components analysis of the SOC yielded performance scores for three dimensions (SOC1, SOC2, SOC3) corresponding to ‘‘mean initial thinking time’’, ‘‘mean moves’’, and ‘‘mean subsequent thinking time’’, respectively (Supplementary Methods).
C-reactive protein
Blood was sampled between 8 and 10:00 a.m. to limit circadian variation, and plasma was collected and stored at −80 °C for batched assay12,39. Hs-CRP was measured by the immunoturbidometric method using the Beckman AU 480 chemistry analyzer and Ultra WR CRP reagent kit (Sekisui Diagnostics, LLC, Lexington, MA, USA). Mean inter- and intra-assay coefficients of variation were reliably < 10%. CRP values were log-transformed for normality.
MRI methods
MRI methods
All scans were acquired using Siemens 3 T Trim-Trio systems (Siemens Medical Systems, Erlangen, Germany).
Acquisition
Anatomical T1 images were obtained using three-dimensional magnetization- prepared rapid gradient-echo with settings of repetition time (TR) = 2300 milliseconds (ms), echo time (TE) = 3.02 ms, time following inversion (TI) = 1100 ms, flip angle = 8o and voxel size 1 × 1 × 1 mm3. Single-voxel MRS data were acquired using a PRESS (point-resolved spectroscopy) technique with TR = 3000 ms, TE = 30 ms, sampling size = 1024, 128 averages. A rectangular voxel sized 20 × 40 × 30 mm3 located on the left basal ganglia was used to obtain single-voxel 1H-MRS12,13. Details of acquisition and voxel location are provided in Supplementary Methods.
Resting-state fMRI images
Resting-state fMRI images were acquired using a Z-saga EPI-pulse sequence for recovering ventral-frontal signal losses regularly seen in gradient-echo BOLD fMRI at 3.4 × 3.4 × 4 mm3 resolution in 30 × 4-mm-thick axial slices with the following parameters: FOV = 220 mm, TR = 2950 ms, TE1/TE2 = 30/67 ms, FA = 90o, scan time = 7.4 min (150 repetitions). Forty-five Subjects were required to look at a fixation cross during scanning.
Post processing MRS data-estimation of absolute Glu concentrations
MRS metabolites were estimated with water-scaling in LC Model using following settings: spectral bandwidth 0.2–4.0 ppm and 2048 complex points46 and corrected for cerebrospinal fluid (CSF) volume fraction using methods published earlier12. Methods used for post processing including voxel tissue composition, spectral quality, and absolute quantitation of metabolites are provided in Supplementary Table 1 and Supplementary Methods.
Analysis of resting-state data
Preprocessing procedure
Signal spike, slice-timing shift, and motion were corrected in AFNI (https://afni.nimh.nih.gov)47, followed by removal of motion and CSF signals within a general linear model (GLM). Local BOLD oscillatory measures were calculated with the residual time-series data derived from the GLM. Of note, white matter (WM) signal was not removed because WM is a key target of inflammation, Glu dysregulation, and depression48,49.
Group comparisons of local BOLD oscillatory data
Local BOLD oscillatory measures—including ReHo, amplitude of low-frequency fluctuations (ALFF) and resting-state fluctuation amplitude (RSFA)—and their fractionated derivatives were obtained and compared. ReHo is a metric that estimates the concordance (statistical similarity of spontaneous neural activity) between a given voxel and its spatially adjacent, neighboring voxels within the context of a given time-series29,32. ALFF is an amplitude-based index of local oscillations in low-frequency range ( < 0.01–0.1 hz)50,51 and RSFA utilizes power spectral analysis to estimate vascular reactivity information in spontaneous BOLD activity28. Oscillatory data were analyzed in two ways: (1) Volume-of-interest (VOI)-based analysis: Averages were calculated across all voxels in the left BG MRS VOI for individual subjects and compared between the groups and (2) Brain-wide analysis of BOLD oscillatory activity that differed in the VOI-based analysis. For each subject, the ReHo data were converted into standard MNI space and spatially smoothed with FWHM = 4 mm. Group comparisons were performed using AFNI “3dttest++”, generating a ReHo-difference map after correcting for multiple-test false-positive errors using Monte Carlo serial simulation followed by p-value thresholds specified in recent publications addressing the issue of multiple comparisons (“Clustsim” option, pvoxel ≤ 0.01, pcluster ≤ 0.05) 52,53.
Network analysis
For each subject, the residual time-series data were temporally filtered (0.01–0.1 Hz), converted into MNI space and spatially smoothed (FWHM = 4 mm). Average time-series data were calculated for regions-of-interest (ROIs). Cross-correlation analysis among time-series data was preformed using MATLAB (MathWorks, Natick, MA, USA), generating a correlation-coefficient matrix which was further transformed into a Fisher z-score matrix. With the z-score matrix, graph-theory-based measures of brain connectivity were estimated using the Brain Connectivity Toolbox in MATLAB (https://sites.google.com/site/bctnet)54,55. Networks were defined as a collection of nodes (ReHo ROIs), and links (edges) between pairs of ROIs. Weighted, undirected network measures were obtained using adjacency and distance matrices within the brain connectivity toolbox. Eleven measures of network integrity including degree (positive and negative connection strengths); internodal clustering (positive and negative clustering coefficients, transitivity, and modularity); assortativity; distance (path length, global efficiency) and eccentricity (diameter and radius) were measured (details in Supplementary Methods). Using graph theory-based metrics enabled computation of integrity at network and subnetwork-level. In addition, ‘‘average connectivity z-scores’’ were also calculated across groups of ROIs analysis by averaging the time-series.
Statistical methods
Agglomerative Hierarchical Cluster Analysis (HCA) using Ward’s linkage method in JMP Pro v13 for Mac (SAS Institute, Cary, NC, USA) was used to classify subjects based on plasma CRP and MRS-based measures of basal ganglia Glu. Both values were log-transformed to scale for uniformity and normality. Cubic Clustering Criterion (CCC—a measure of the cluster numbers providing the best fit) and evaluation of ‘screen’ plots were used to inform the number of groups. Both CCC (–1.891 for 2 cluster solutions vs. −2.88, −2.61, −2.13 for 3, 4, and 5 cluster solutions, respectively) and screen plot revealed a two-cluster solution as the most parsimonious model. A dendrogram depicting the cluster differentiation is provided in Fig. 1b. ‘‘Variable clustering” in JMP Pro (similar to ‘‘varclus’’ in SAS) was also used to identify groupings of variables based on shared brain signatures56. Data were checked for univariate and multivariate normality using standard procedures. Comparisons of background variables were performed using t-tests and chi square, and variables that were different between High and Low CRP-Glu groups were controlled as covariates in further analysis. Canonical, stepwise, discriminant function analysis (DFA) was used to predict membership in High vs. Low CRP-Glu groups using a categorical classifier (x variable = CRP-Glu status) on known continuous variables (‘‘y’’ responses). Adaptive elastic regression models in JMP Pro were used to test predictive associations owing to their ability to shrink covariances, limit collinearity, and decrease overall prediction error. Following variable selection (within elastic net models), the predictors and covariates were imported into standard least squares regression models to test for multiple comparisons (using False Discovery Rate corrected (FDR)-p-values) and to measure statistical power and estimate effect sizes. Effect sizes were measured using Cohen’s d (95% CI)57, and alternate measures (F-statistic, partial eta, omega, r-squared) were converted into ‘‘d’’ for consistency. Path analysis was performed using Structural Equation Modeling (SEM) protocol in STATA (College Park, Texas, USA).
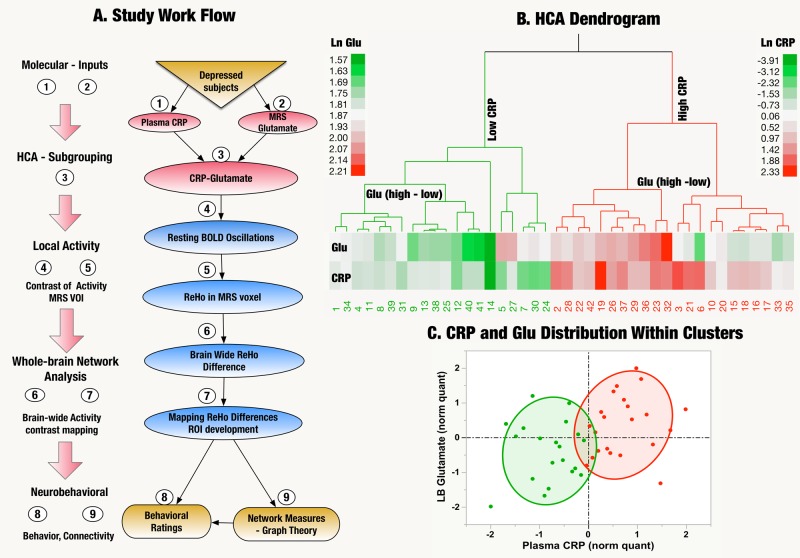
Fig. 1. Overview of analytic pipeline and group assignments.
a Study Work Flow: Data processing and analytic workflow: BOLD brain oxygen level dependent, MRS magnetic resonance spectroscopy, HCA hierarchical cluster analysis, VOI volume-of-interest, CRP plasma C-reactive protein, ReHo regional homogeneity, ROI region-of-interest. b Dendrogram/heatmap of hierarchical clustering analysis (HCA): Using HCA, the sample was divided into High (n = 22) and Low (n = 19) plasma C-reactive protein (CRP)-glutamate (Glu) groups. The accompanying tree-shaped dendrogram and Heatmap illustrate the clustering algorithm. In HCA, the data is arranged hierarchically with log-normalized (Ln) CRP as first node of the hierarchy followed by Ln Left Basal (Glu) as the secondary node. The agglomerative nature of the method used where minor clusters are progressively combined to yield larger clusters is also depicted as branches of the dendrogram. The length of dendrogram lines are proportional to cluster distances. The Legends along the side of the dendrogram provide the range of individual subject measures of the variables used (Ln CRP and Ln LB Glu) color-coded as cool (green)-warm (red) colors. c CRP and Glu distribution: The scatterplot demonstrates the distribution of the association between CRP and LB Glu in the High vs. Low CRP-Glu groups. Shaded 80% confidence-interval ellipses are used to represent High (red) and Low (green) CRP-Glu groups (respectively). Values of CRP and LB Glu in x and y-axis (respectively), were quantile-normalized (norm quant) for easy visual inspection and color-coded for the two groups of interest. The broken lines represent the median values. Using median-split 2/3rd of subjects were classified as having both high CRP/high glutamate in contrast to 1/3rd of subjects with high glutamate who did not fall into the high CRP group (Chi Sq = 4.7, p = 0.03). Thus, the association between CRP and glutamate was neither absolute or invariable with neither qualifying to be a proxy for the other
Results
Sample characteristics
Of the 50 subjects recruited, eight were excluded [six with poor quality MRS data (metabolite variances > 20% or motion artifacts, one due to excessive head motion and one for whom resting-state fMRI was not available]. Data from the remaining 42 patients were included in the analyses. Figure 1a illustrates the analytic pipeline used for the study. Using HCA, two clusters—High (n = 22) vs. Low (n = 20) CRP-Glu groups were identified (Fig. 1b). Both plasma CRP and absolute left basal ganglia Glu were significantly elevated in the High vs. Low CRP-Glu group (p < 0.001 and 0.002, respectively). Of note, the cross-tabulation of subjects classified into ‘‘high’’ and ‘‘low’’ groups using + above/-below median of CRP and glutamate indicated that 66% of individuals with high glutamate fell into the high CRP group, while the remaining 33% of subjects with high glutamate were classified into the low CRP group (Fig. 1c) (Likelihood ratio = 4.76, p = 0.03). Thus, the association between CRP and glutamate was neither absolute or invariable with neither qualifying to be a proxy for the other. The samples were well-matched on all clinical, demographic variables and (right vs. left) handedness other than BMI, which was significantly higher among High vs. Low CRP-Glu subjects (p < 0.001). BMI was entered as a covariate in all subsequent analyses (unless otherwise specified). Only 3/42 subjects were SCID + bipolar depression with 1 and 2 in Low and High CRP-Glu groups, respectively, (Table 1 and Supplementary Table 2).
Table 1.
Demographic and clinical characteristics of the CRP-Glu groups
| Group statisticsMean ± SD | Low CRP-GLU Cluster (n = 20) | High CRP-GLU Cluster (n = 22) | Test, p |
|---|---|---|---|
| Background and clinical | |||
| Age | 38.5 ± 11.0 (122.2) | 37.9 ± 11.3 (127.9) | F(1,40) = 0.3, p = 0.86 |
| Sex: (Females) % | 12 (40%) | 18 (60%) | Chi Sq = 2.44, p = 0.12 |
| Race: African Americans, (%) | 11 (44%) | 14 (56%) | Chi Sq = 0.32, p = 0.57 |
| Education: College grads (%) | 9 (45%) | 8 (36%) | Chi Sq = 0.32, p = 0.57 |
| Smoker (%) | 6 (30%) | 4 (19%) | Chi Sq = 0.81, p = 0.37 |
| BMI: Mean (SD) | 26.63 ± 4.29 | 35.66 ± 7.87 | F(1,40) = 20.7, p < 0.001a |
| Bipolar/unipolar depression | 1/19 | 1/21 | Chi Sq = 0.005, p=0.94 |
| Duration of current episode of depression (months) | 170.90 ± 172.99 | 204.41 ± 153.27 (23491.87) | F(1,40) = 0.44, p = 0.51 |
| Age of onset of depression (Years) | 21.80 ± 12.67 | 16.95 ± 16.95 | F(1,40) = 1.99, p = 0.17 |
| Number of depressive episodes | 1.89 ± 3.02 | 1.38 ± 0.97 | F(1,38) = 0.55, p = 0.46 |
| Number of antidepressants used in current episode | 0.95 ± 2.01 | 0.73 ± 1.16 | F(1,40) = 0.20, p = 0.66 |
| Behavioral | |||
| Anhedonia (IDS3-items) | 4.20 ± 1.93 | 5.50 ± 1.71 | F(1,40) = 5.3, p = 0.03a |
| Response to good/desired events (IDS-SR Item #8) | 1.25 ± 0.85 | 1.82 ± 0.66 | F(1,40) = 5.88, p = 0.02a |
| Leaden paralysis/physical energy (IDS-SR Item #30) | 0.95 ± 0.69 | 1.64 ± 0.95 | F(1,40) = 7.04, p = 0.01a |
| IDS-SR-Total | 34.35 ± 7.5 | 38.2 ± 8.0 | F(1,40) = 2.6, p = 0.12 |
| Cognitive | |||
| Trails (total score) | 28.64 ± 9.73 | 38.69 ± 17.38 | F(1,40) = 5.20, p = 0.03a |
| Sock of Cambridge (initial think time—principal component) | -0.62 ± 0.81 | 0.56 ± 1.80 | F(1,40) = 7.19, p = 0.01a |
| CANTAB 5-choice movement Time (msec) | 468.67 ± 83.49 | 554.27 ± 117.29 | F(1,40) = 7.29, p = 0.01a |
| Neurochemical | |||
| Left basal ganglia glutamate | 6.05 ± 0.68 | 6.93 ± 0.96 | F(1,40) = 11.2, p = 0.002a |
| Immune | |||
| Plasma CRP (mg/L) | 0.39 ± 0.23 (n = 17) | 3.99 ± 2.27 (n = 20) | F(1,35) = 49.6, p < 0.001a |
BMI body mass index, IDS-SR Inventory for Depressive Symptoms-Self-Rated Version, anhedonia 3-item Anhedonia Subscale, CANTAB– Cambridge, CRP c-reactive protein (plasma)
a Significant ANOVA
Behavioral and cognitive variables
DFA with post hoc analysis of variance (ANOVA) indicated that anhedonia [F(1,40) = 5.34, p = 0.03], 5CMT [F(1,40) = 7.29, p = 0.01], TMT [F(1,40) = 5.20, p = 0.03] and SOC1 [F(1,40) = 7.19, p = 0.01] were significantly associated with High CRP-Glu status (Table 1). Analysis of individual IDS-SR items indicated that Item#8, signifying positive valence [“Response of your mood to good or desired events”: F(1,40) = 5.53, p = 0.02] and item#30 [Leaden Paralysis/Physical Energy: F(1,37) = 5.80, p = 0.02] were also associated with High CRP-Glu status. Most the effect sizes were in the ‘‘large’’ range (d > 0.8).
Effect of CRP-Glu status on BOLD-signal metrics in MRS voxel
DFA with post hoc ANOVA identified reductions in ReHo in the left basal ganglia MRS VOI as the local BOLD oscillatory measure associated with High CRP-Glu status [F(1,40) = 10.20, p = 0.003, canonical r = 0.45]; with leave-one-out cross-validation indicating a classification-accuracy of 76.2% (Fig. 2 and Supplementary Table 2). Owing to a lack of similar association, ALFF and RSFA were excluded from further analysis. Testing of 3-way interactions between CRP and glutamate along with covariates (age, sex, race, BMI, smoking) confirmed that neither CRP nor glutamate alone (both p > 0.05) but only (Low-High) CRP-Glu contrast [PE = 0.83 (0.34–1.33), Wald Chi Sq = 10.8, p = 0.001] predicted ReHo. Of note, the same elastic net model also revealed that race [African American-Caucasian American = –0.57(–1.08– –0.06), Wald = 4.82, p = 0.03] had a significant impact on ReHo decreases and hence was controlled as a covariate in all subsequent analyses.
Fig. 2. Local Activity Contrast in the MRS Voxel-of- Interest (VOI).
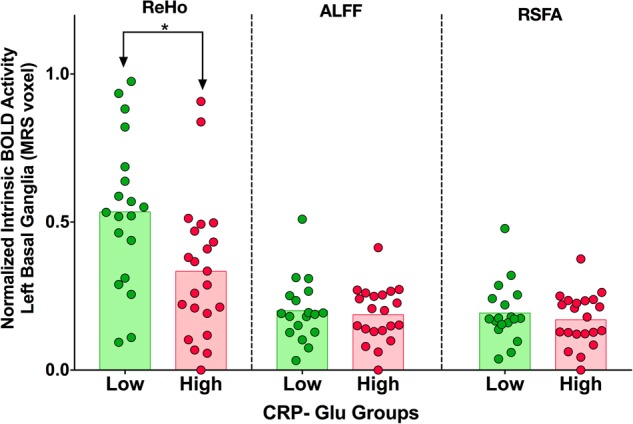
Scatterplot with bar demonstrating differences in plasma CRP and glutamate in the left basal ganglia between High vs. Low CRP-Glu groups. The upper end of the bar is the mean and shaded regions represent range between mean and 0. Green shade represents Low CRP-Glu and red shades represent High CRP-Glu. Comparison of group means of the three bold oxygen level-dependent (BOLD) indices between (Low-High) CRP-Glu groups revealed that Regional Homogeneity (ReHo) was the only metric of BOLD oscillatory activity that significantly differed between groups: (ReHo). Table 2 provides a detailed summary of individual means and SD
Whole-brain ReHo and ReHo subnetwork partitioning
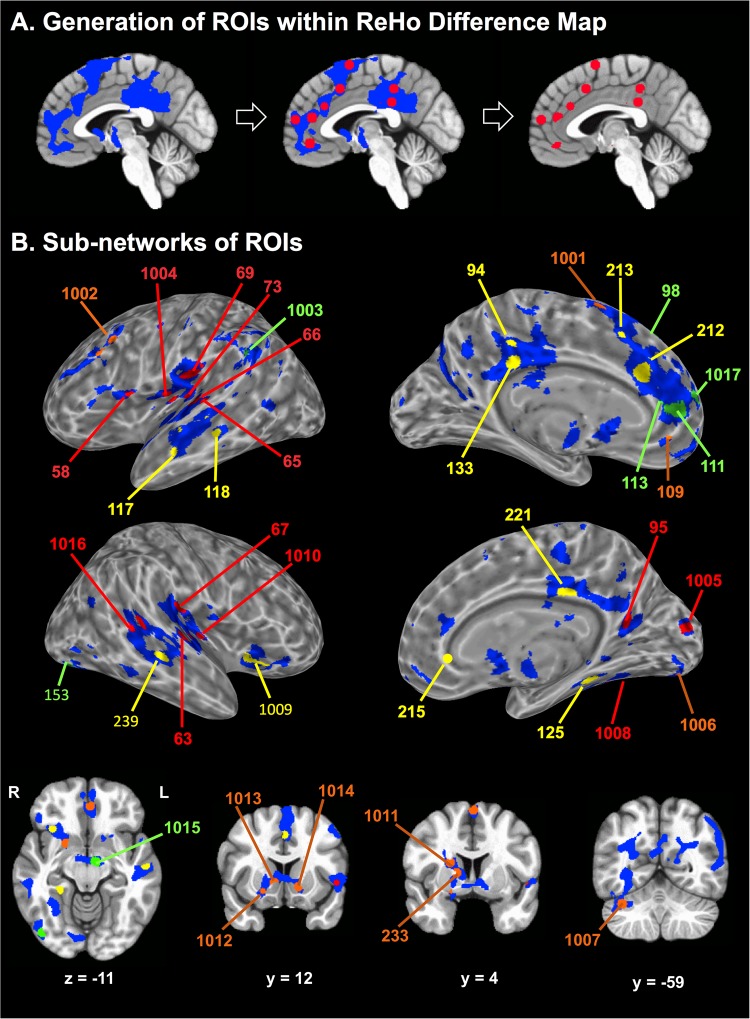
To reliably locate whole-brain ReHo changes within functionally defined space, 10 mm spheres (#283) were placed on previously published functional coordinates56,58,59, and the spheres demonstrating > 50% overlap with ReHo-difference map were included as ROIs. A total of 41 ROIs—27 from the first set of 264 ROIs58 and an additional set of 17 ROIs curated from depression-related studies56,59 that fulfilled the “overlap’’ criteria were included for further analysis (Fig. 3). Variable clustering approaches were used to decompose the large number of ROIs (#41) into four subnetworks of ROIs. A detailed description of the ROIs including their coordinates and subnetwork affiliations are provided in Supplementary Table 3. ROI masks were applied to calculate ‘‘mean ReHo” of each subnetwork for each individual and grouped for further analyses.
Fig. 3. Regions-of-interest (ROIs) within the ReHo-difference map.
a Generation of ROIs (right: red spheres) within the ReHo-difference map (left: blue patches): Overlapped portions between the ReHo-difference map and 10 mm diameter spheres (middle: red circles on blue regions). The background image is a sagittal slice of the MNI-152 brain atlas. b Four sub-networks (SBNs) of ROIs shown on the cortical surface (top, middle) and slices (bottom) of the MNI-152 brain. The ROI numbers and their corresponding brain structures are identified in Supplementary Table 3
Subnetwork4 ReHo and behavioral associations
Subnetwork4 ReHo was the only predictor of anhedonia [PE = –20.8, t = –3.76, FDR p < 0.001, d = 1.22 (0.55–1.84)] and IDS-SR-positive valence Item#8 [PE = –18.1, t = –3.05, FDR p = 0.004, d = 1.03 (0.33–1.63]. Of note, Subnetwork4 included several ROIs within canonical reward and salience networks including ventromedial prefrontal (vMPFC), and dorsal (DS) and ventral striatal (VS) regions (Fig. 3 and Supplementary Table 3)56,59,60. None of the other three subnetworks demonstrated significant associations with anhedonia, although Subnetwork1 ReHo was predictor of psychomotor processing speed as measured by TMT [PE = –0.41, t = –2.71, p = 0.01, d = 0.85 (0.19–1.46) and Subnetwork2 ReHo was predictor of IDS-SR leaden paralysis Item#30 [PE = –0.35, t = –2.38, p < 0.02, d = 0.74 (0.19–1.34)].
Network correlates of ReHo changes
Clustered cell plots of correlations in functional connectivity (Fisher z-scores) between all 41 ReHo-ROIs in High and Low CRP-Glu groups are demonstrated in Fig. 4a, b. Significant differences were noted in correlation- and covariance-based comparisons of Fisher-z-connectivity matrix between High and Low CRP-Glu groups [Jennrich’s Chi Sq(820) = 2235.65 and Box Chi Sq(861) = 2645.61, respectively, both p < 0.001]. A MANOVA model with post hoc Benjamini-Hochberg correction61 identified significant but modest reductions in “global efficiency” (reflecting greater network dysconnectivity) of a putative network combining all 41 ROIs measured in the High vs. Low CRP-Glu group [mean (95% CI) = 0.22 (0.20–0.24) vs. 0.25 (0.23–0.27), t(34) = -2.10, p < 0.04, d = 0.66 (0.03–1.280)].
Fig. 4. Decreased ReHo is associated with decreased network integrity.
a, b. Clustered correlation maps of grouped Fisher-z-connectivity scores: Heatmap demonstrating clustered cell plot of correlations in functional connectivity (Fisher z-scores) between all 41 ReHo-ROIs in the Low CRP-Glu (a) and in the High CRP-Glu grouping (b). Correlations in functional connectivity (Fisher z-transformed scores) between all 41 ReHo ROI-seeds are depicted as ranging from red ( + 1) to blue (–1). c, d. Path Analysis of ReHo, Network Measures and Anhedonia: Path Analysis of ReHo, Network Measures and Anhedonia: A significant effect for the path extending from Subnetwork4 ReHo → network eccentricity (diameter, radius) → anhedonia was seen only in the Low [diameter path coef (95% CI) = 0.14 (0.33–0.24), z = 2.58, p = 0.01] (c) but not in High CRP-Glu groups (both p > 0.05) (d)
Disruptions in Subnetwork4 architecture associated with ReHo changes
Multivariate ANCOVA followed by post hoc Benjamini-Hochberg correction indicated that Subnetwork4 mean ReHo positively predicted global efficiency and node strength (p-corr = 0.009 and 0.02, respectively) and negatively predicted network diameter, path length and radius (p-corr = 0.01, 0.01, and 0.03, respectively) in the groups combined (Supplementary Table 4). Subnetwork4 assortativity (indicating decreasing network resilience) was also associated with psychomotor slowing indexed by prolongation of 5CMT [PE = 0.47, t = 3.46, FDR p = 0.004, d = 0.77 (0.09–1.40)]. Network eccentricity measures, i.e., diameter and radius (reflecting greater nodal segregation) positively predicted severity of scores on IDS-SR item#30 “paralysis” [PE = 1.01, t = 3.55, FDR p < 0.001, d = 1.18 (0.60–1.84) and PE -1.02, t = –3.6, FDR p < 0.001, d = 1.24 (0.55–1.88) for diameter and radius, respectively].
Exploratory path analysis linking CRP-Glu, ReHo, network metrics, and behavior
A path analysis based on Structural Equation Modeling was used to examine links among ReHo, network metrics and anhedonia. The pathway from Subnetwork4 ReHo → Subnetwork4 measure → anhedonia was examined independently in the combined (both), and the High and Low CRP-Glu groups separately (Fig. 4c, d). A significant effect for the path extending from Subnetwork4 ReHo → network eccentricity (diameter, radius) → anhedonia was seen only in the Low [diameter path coef (95% CI) = 0.14 (0.33–0.24), z = 2.58, p = 0.01, and radius = [0.13 (0.006–0.26, z = 2.06, p = 0.04)] but not among High or combined CRP-Glu groups (both p > 0.05). SEM models replacing diameter with radius yielded identical results, while other measures associated with Subnetwork4 ReHo (global efficiency, network strength, path length) did not demonstrate statistically meaningful mediation effects.
Discussion
A distinct subtype of depressive behaviors including anhedonia and psychomotor slowing was revealed by CRP-Glu status. Behavior is an emergent property of coherent network activity patterns, and symptoms represent a failure of this function. It is unclear, if the lower limit of a critical minimum threshold of ordered, coherent local activity necessary to maintain network homeostasis is breached in the High (but not Low) CRP-Glu group. As noted earlier, a disrupted balance between intra- vs. extrasynaptic signaling is likely to underlie the reduction in local homogeneity in the High CRP-Glu group 18,24,62.
Stressed astroglial cells functioning under the duress of immune stimulation might lie at the epicenter of this network imbalance. Astroglial cells exist in assemblies that act as hubs that connect closely located but functionally divergent circuits (e.g., reward vs. aversion) by cross-synaptic dissemination of tonic, coherent, “near threshold” stimulation via their gliotransmission capabilities63. The association between this tonic, coherent activity and local homogeneity is unclear but worth exploring. MRS primarily measures intracellular (astroglial) glutamate pool and hence increases in these signals might reflect increased cycling of glutamate or excessive intracellular pooling due to progressively declining astrocytic function64,65. Of note, our previous MRS data in depressed patients indicate increased basal ganglia myo-inositol (a putative marker of astroglial function) in association with increased CRP, possibly indicating a relationship between inflammation and astroglial dysfunction 12.
Strengths of this study include use of multi-modal imaging techniques to compare key local and long-distance metrics of functional brain activity in a well-characterized, unmedicated group of subjects. Nevertheless, several limitations of the study need to be considered. Based on the aims and hypotheses, attention was focused on MRS glutamate signals from the left basal ganglia region—excluding other ROIs and other metabolites. However, a group comparison of the various other MRS metabolites did not reveal any group differences (Supplementary Table 2). Use of methods that estimate glutamine, lactate and glutamine-glutamate cycling such as 13carbon, hyperpolarized or ultra-high field MRS might have enabled a better appreciation of the underlying bioenergetic changes and helped discriminate glutamate dysregulation induced by astrocytic vs. neuronal pathologies. In addition, MRS provides limited spatial resolution that does not enable precise information about synaptic location or activity. However, MRS glutamate signals have consistently predicted neural activity in task- and transcranial magnetic stimulation (TMS)-activation based functional magnetic resonance imaging (fMRI) paradigms as well as functional connectivity in the resting-state66–68. Questions on mathematical modeling used to obtain ReHo (especially its non-linear origins) have been raised and clarified30,32,50,69. The adjusted power after control of covariates for the primary effects was robust (CRP-Glu prediction of MRS VOI ReHo = 0.81 and Subnetwork4 ReHo prediction of anhedonia = 0.94). Nevertheless, the sample size was relatively small, and inferences drawn from the study could also be limited by its cross-sectional design. We have provided effect size estimates with uncertainty (95% CI), applied bias-correction, corrected for multiple comparisons and wherever possible. Plasma CRP rather than other cytokines or CSF inflammatory markers was chosen for this study due to its overall stability, sensitivity and practical utility. Furthermore, as noted in another report from our group; plasma CRP demonstrated robust correlations with several other plasma inflammatory markers as well as CSF CRP justifying the focus on peripheral CRP 70.
In summary, the findings point to disruption of local and long-distance functional brain activity in a subgroup of patients with both high inflammation and basal ganglia glutamate. The study illustrates the possibility of combining blood and neuroimaging-based biomarkers relevant to the pathophysiology of depression to identify biologically homogenous subtypes for research and personalized treatments. If replicated, ReHo might also emerge as a measure of target-engagement in the brain to test efficacy of glial stabilizers (riluzole), glutamate modulators (memantine, lamotrigine) or anti-inflammatory agents (infliximab), especially in conditions where connectivity and network metrics are extensively compromised.
Electronic supplementary material
Acknowledgements
This study was supported by grants R01MH H107033 & K23MH091254 (Dr. Haroon), R01MH112076 (Drs. Haroon & Miller), R01MH087604, R25MH101079 (Dr. Miller), and R01MH109637 (Dr. Felger) from the National Institute of Mental Health. In addition, the study was supported in part by PHS Grants UL1TR000454 and KL2TR000455 from the Clinical and Translational Science Award program, and by the NIH/NCI under award number P30CA138292. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. This work was supported in part by funding from a Shared Instrumentation Grant (S10) grant 1S10OD016413–01 to the Emory University Center for Systems Imaging Core. We would like to acknowledge the help rendered by the staff and management of Emory University Center for Systems Imaging Core.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41398-018-0241-4).
References
- 1.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 2016;17:497–511. doi: 10.1038/nrn.2016.69. [DOI] [PubMed] [Google Scholar]
- 3.Wong ML, et al. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry. 2016;21:797–805. doi: 10.1038/mp.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller AH, Haroon E, Felger JC. Therapeutic implications of brain-immune interactions: treatment in translation. Neuropsychopharmacology. 2017;42:334–359. doi: 10.1038/npp.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenberger NI, et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol. Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison NA, et al. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol. Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remus J. L., Dantzer R. Inflammation models of depression in rodents: Relevance to psychotropic drug discovery. Int. J. Neuropsychopharmacol.19, (2016) [DOI] [PMC free article] [PubMed]
- 8.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savitz J, et al. Activation of the kynurenine pathway is associated with striatal volume in major depressive disorder. Psychoneuroendocrinology. 2015;62:54–58. doi: 10.1016/j.psyneuen.2015.07.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menard C, et al. Social stress induces neurovascular pathology promoting depression. Nat. Neurosci. 2017;20:1752–1760. doi: 10.1038/s41593-017-0010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haroon E, Miller AH, Sanacora G. Inflammation, glutamate, and glia: A trio of trouble in mood disorders. Neuropsychopharmacology. 2017;42:193–215. doi: 10.1038/npp.2016.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haroon E, et al. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol. Psychiatry. 2016;21:1351–1357. doi: 10.1038/mp.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haroon E, et al. IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology. 2014;39:1777–1785. doi: 10.1038/npp.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haroon E, et al. Age-related increases in basal ganglia glutamate are associated with TNF, reduced motivation and decreased psychomotor speed during IFN-alpha treatment: Preliminary findings. Brain Behav. Immun. 2015;46:17–22. doi: 10.1016/j.bbi.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haroon E, Miller AH. Inflammation effects on brain glutamate in depression: Mechanistic considerations and treatment implications. Curr. Top. Behav. Neurosci. 2017;31:173–198. doi: 10.1007/7854_2016_40. [DOI] [PubMed] [Google Scholar]
- 16.Dantzer R, Walker AK. Is there a role for glutamate-mediated excitotoxicity in inflammation-induced depression? J. Neural Transm. (Vienna) 2014;121:925–932. doi: 10.1007/s00702-014-1187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewerenz J, et al. The cystine/glutamate antiporter system x(c)(-) in health and disease: from molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013;18:522–555. doi: 10.1089/ars.2011.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piani D, Fontana A. Involvement of the cystine transport system xc- in the macrophage-induced glutamate-dependent cytotoxicity to neurons. J. Immunol. 1994;152:3578–3585. [PubMed] [Google Scholar]
- 22.Gras G, et al. EAAT expression by macrophages and microglia: still more questions than answers. Amino acids. 2012;42:221–229. doi: 10.1007/s00726-011-0866-6. [DOI] [PubMed] [Google Scholar]
- 23.Rothstein JD. Excitotoxicity hypothesis. Neurology. 1996;47:S19–25. doi: 10.1212/WNL.47.4_Suppl_2.19S. [DOI] [PubMed] [Google Scholar]
- 24.Dorsett CR, et al. Glutamate neurotransmission in rodent models of traumatic brain injury. J. Neurotrauma. 2016;34:263–272. doi: 10.1089/neu.2015.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCullumsmith RE, Sanacora G. Regulation of extrasynaptic glutamate levels as a pathophysiological mechanism in disorders of motivation and addiction. Neuropsychopharmacology. 2015;40:254–255. doi: 10.1038/npp.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okubo Y, Iino M. Visualization of glutamate as a volume transmitter. J. Physiol. 2011;589(Pt 3):481–488. doi: 10.1113/jphysiol.2010.199539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10:165–170. doi: 10.1002/(SICI)1099-1492(199706/08)10:4/5<165::AID-NBM454>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Kannurpatti SS, Biswal BB. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. Neuroimage. 2008;40:1567–1574. doi: 10.1016/j.neuroimage.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, et al. Alterations of resting-state fMRI measurements in individuals with cervical dystonia. Hum. Brain. Mapp. 2017;38:4098–4108. doi: 10.1002/hbm.23651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang L, Zuo XN. Regional homogeneity: A multimodal, multiscale neuroimaging marker of the human connectome. Neuroscientist. 2016;22:486–505. doi: 10.1177/1073858415595004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, et al. Regional homogeneity associated with overgeneral autobiographical memory of first-episode treatment-naive patients with major depressive disorder in the orbitofrontal cortex: A resting-state fMRI study. J. Affect Disord. 2017;209:163–168. doi: 10.1016/j.jad.2016.11.044. [DOI] [PubMed] [Google Scholar]
- 32.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 33.APA. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC, USA.: American Psychiatric Press; 2000. [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV. Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- 35.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr. Opin. Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rush AJ, et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol. Psychiatry. 2003;54:573–583. doi: 10.1016/S0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 37.Trivedi MH, et al. The inventory of depressive symptomatology, clinician rating (IDS-C) and self-report (IDS-SR), and the quick inventory of depressive symptomatology, clinician rating (QIDS-C) and self-report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol. Med. 2004;34:73–82. doi: 10.1017/S0033291703001107. [DOI] [PubMed] [Google Scholar]
- 38.Ameli R, et al. SHAPS-C: the Snaith-Hamilton pleasure scale modified for clinician administration. PeerJ. 2014;2:e429. doi: 10.7717/peerj.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Felger JC, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry. 2016;21:1358–1365. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collyer CE, Broadbent HA, Church RM. Preferred rates of repetitive tapping and categorical time production. Percept. Psychophys. 1994;55:443–453. doi: 10.3758/BF03205301. [DOI] [PubMed] [Google Scholar]
- 41.Reitan RM. The relation of the trail making test to organic brain damage. J. Consult. Psychol. 1955;19:393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 42.Wechsler D. Wechsler Adult Intelligence Test. 4th edn. San Antonio, TX, USA: Pearson; 2008. [Google Scholar]
- 43.Robbins TW, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. J. Int. Neuropsychol. Soc. 1998;4:474–490. doi: 10.1017/S1355617798455073. [DOI] [PubMed] [Google Scholar]
- 44.Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: discussion paper. J. R. Soc. Med. 1992;85:399–402. [PMC free article] [PubMed] [Google Scholar]
- 45.Heberlein KA, Hu X. Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-shot z-shim: Z-SAGA. Magn. Reson. Med. 2004;51:212–216. doi: 10.1002/mrm.10680. [DOI] [PubMed] [Google Scholar]
- 46.Provencher SK. LCModel and LCMgui User’s Manual. pp. 1–174 (LCMODEL). Steven K. Provencher (2016).
- 47.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 48.Alexopoulos G, Morimoto S. The inflammation hypothesis in geriatric depression. Int. J. Geriatr. Psychiatry. 2011;26:1109–1118. doi: 10.1002/gps.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benedetti F, et al. Inflammatory cytokines influence measures of white matter integrity in Bipolar Disorder. J. Affect Disord. 2016;202:1–9. doi: 10.1016/j.jad.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 50.Zang YF, et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain. Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Zou QH, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl Acad. Sci. USA. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI clustering in AFNI: False-positive rates redux. Brain Connect. 2017;7:152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Sporns O. Networks of the Brain. Cambridge, MA: The MIT Press; 2011. [Google Scholar]
- 56.Drysdale AT, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Hillsdale, N.J.: L. Erlbaum Associates; 1988. [Google Scholar]
- 58.Power JD, et al. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.). 1995;57:289–300. [Google Scholar]
- 62.Shan D, Yates S, Roberts RC, McCullumsmith RE. Update on the neurobiology of schizophrenia: a role for extracellular microdomains. Minerva Psichiatr. 2012;53:233–249. [PMC free article] [PubMed] [Google Scholar]
- 63.Rossi D. Astrocyte physiopathology: At the crossroads of intercellular networking, inflammation and cell death. Prog. Neurobiol. 2015;130:86–120. doi: 10.1016/j.pneurobio.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Mason GF, Krystal JH. MR spectroscopy: its potential role for drug development for the treatment of psychiatric diseases. NMR Biomed. 2006;19:690–701. doi: 10.1002/nbm.1080. [DOI] [PubMed] [Google Scholar]
- 65.Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24:943–957. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horn DI, et al. Glutamatergic and resting-state functional connectivity correlates of severity in major depression - the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci. 2010;4:1–10. doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rowland LM, et al. Frontal glutamate and gamma-aminobutyric acid levels and their associations with mismatch negativity and digit sequencing task performance in schizophrenia. JAMA Psychiatry. 2016;73:166–174. doi: 10.1001/jamapsychiatry.2015.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walter M, et al. The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch. Gen. Psychiatry. 2009;66:478–486. doi: 10.1001/archgenpsychiatry.2009.39. [DOI] [PubMed] [Google Scholar]
- 69.Zhang B, et al. Altered functional connectivity density in major depressive disorder at rest. Eur. Arch. Psychiatry Clin. Neurosci. 2016;266:239–248. doi: 10.1007/s00406-015-0614-0. [DOI] [PubMed] [Google Scholar]
- 70.Felger, JC. et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 10.1038/s41380-018-0096-3 (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.