Abstract
As an important source of fiber and edible oil, cotton has great economic value. In comparison to their individual studies, association and differentiation between salt and alkaline tolerance has not been focused yet by scientists. We have used next-generation RNA-Seq technique to analyze transcriptional changes under salt and alkaline stresses in cotton. Overall, 25,929 and 6,564 differentially expressed genes (DEGs) were identified in roots and leaves, respectively. Gene functional annotation showed that genes involving ionic homeostasis were significantly up-regulated under NaCl stress and Na2CO3 stress, and genes enriched in starch and sucrose metabolism were up-regulated under NaOH stress and Na2CO3 stress. Furthermore, a synergistic enhancing effect between NaCl and NaOH stress was also observed in this study. Likewise, our studies indicate further that genes related with starch and sucrose metabolism were regulated to respond to the high pH under Na2CO3 stress, inducing plant hormone signal transduction and key enzyme reactive oxygen species (ROS) activity to respond to ionic toxicity and intracellular ionic homeostasis. By analyzing the expression profiles of diverse tissues under different salt and alkaline stresses, this study provides valuable ideas for genetic improvements of cotton tolerance to salt-alkaline stress.
Introduction
Plant growth and development is highly influenced by various biotic and abiotic factors, resulting in a destructive impact on their production. In the north east area of china, soil alkalinity is a major abiotic stress which is responsible for the decline of agricultural production and causes environmental hazard. Soils are considered as Saline-Alkaline soils, upon salinity in soils goes above 0.3%1.
Soil affected by salt are basically classified in three categories: saline soils, alkaline soils and salt-alkaline soils1. Saline soils comprises of excessive amount of neutral salts, which includes NaCl and Na2SO4, as a major part, resulting in salt stress. NaHCO3 and Na2CO3 are responsible for the alkalization of soils by creating a high pH value, with a destructive effect on plants growth2. Stress resulted from alkaline soils causes several issues of osmotic pressure stress, different types of ionic injuries and high pH stress. Plants under salt-alkaline conditions suffer from both salt stress caused by excessive salt ions and alkaline stress caused by high pH3,4.
Salt-alkaline stress causes several damage to plants because of the presence of salt ions and high pH. Previous studies have shown that plants maintain intracellular homeostasis through osmotic adjustment, generating an active oxygen scavenging system and adjusting organic acid under NaCl stress5,6. Genes associated with brassinosteroid biosynthesis were upregulated under alkaline stress (Na2CO3)6,7. Cao et al. found that the expression of Gshdz4 was induced by NaHCO3, indicating that Gshdz4 is only responsible for resisting HCO3−, but not high pH8. The response to NaHCO3 of T. hispida involves multiple physiological and metabolic pathways9. The Aft2 gene plays a negative role, and Na+-ATPase ENA1 is regulated during alkaline pH resistance in Saccharomyces cerevisiae10. Three major mechanisms of plant resistance to salt stress have been found: maintenance of ionic and osmotic homeostasis, detoxification and growth regulation11. High pH can also affect ion balance, root growth4, organic acid accumulation12 and cellular processes13. However, a little is known about the difference between salt and alkaline stress.
As an important source of fiber, vegetable protein and edible oil, cotton has great economic importance14. Soil salt-alkalinity has become an increasingly serious factor in cotton yield because of irrigation and secondary salinization15. So development of salt-alkaline tolerant cotton is absolutely of indispensable importance. In recent years, much attention was paid to salt tolerance in cotton. However, the alkaline tolerance of cotton has not been well studied. Few research has been conducted to show the different responses to salt and alkaline stress in cotton. Recent studies have showed that genome-wide transcriptome analysis of cotton has become popular for studying stress tolerance16,17. Because the genes involved in salt-alkaline response are numerous, salt-alkaline genes can be more easily identified via transcriptome and DEG analyses.
In our study, we used RNA-seq technology to analyze the expression profiles of diverse tissues under different salt and alkaline stresses. Firstly, the phenotypic changes in roots and leaves of cotton under Na2CO3, NaCl, and NaOH stress were observed, and the physiological indexes were measured to demonstrate phenotypic differences. Secondly, we studied the transcriptome changes between the stress groups and the control group. Finally, the difference between salt and alkaline stress was obtained. This study provides new ideas for genetic improvement of cotton tolerance to salt-alkaline stress.
Results
Phenotypic and physiological responses to different salt-alkaline stresses in Gossypium hirsutum L
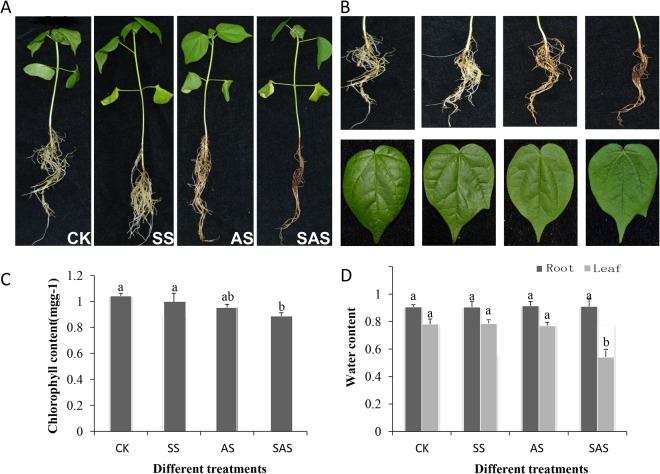
Previous studies have reported that cotton is more sensitive to abiotic stresses at three-leaf stage18. Different morphological has been observed in G. hirsutum Zhong 9807 during its three-leaf stage under various concentrations of Na2CO3 stress. We found that seedlings became obviously different between the treatment and control groups under 50 mM Na2CO3 stress after 12 h (Fig. S1). Two treatments with parallel concentrations of 100 mM and 0.125 mM for NaCl and NaOH respectively were considered to study the comparative effects of CO32−, Na+ and high pH (Table 1). The salt-alkaline tolerance of Zhong 9807 was examined by comparing salt stress (NaCl stress) with two other alkaline stresses, NaOH stress and Na2CO3 stress. The phenotypic changes of the three treatments were shown (Fig. 1A,B). Leaves withered and lost lustre slightly under NaCl stress, while roots have no evident changes in phenotype, suggesting roots may have stronger resistance than leaves. However, slight tarnish in leaves and nigrities were found in roots under NaOH stress. When seedlings were subjected to Na2CO3 stress, roots became seriously withered and nigrescent, leaves tarnished heavily and veins almost darkened. The variance analysis results19 of the chlorophyll content and relative water content (RWC) under Na2CO3 stress (Fig. 1C,D) were significantly different from those of the control group except the RWC in roots.
Table 1.
Stress comparison for 50 mM Na2CO3 with 100 mM NaCl and 0.125 mM NaOH.
| Component | Na2CO3 | NaCl | NaOH |
|---|---|---|---|
| Na+ (mM) | 100 | 100 | 0.125 |
| pH | 11.32 | 7.00 | 11.32 |
Figure 1.
Phenotypic changes of plants under different treatments and measurement of biological indicators. SS: 100 mM NaCl; AS: 0.125 mM NaOH; SAS: 50 mM Na2CO3. (A) Phenotypic changes of plants when Zhong9807 was under the SS, AS and SAS treatments. (B) Phenotypic changes in roots and leaves. (C) Chlorophyll content under different stress treatments. (D) Water content of roots and leaves under different stress treatments.
Transcriptome sequencing and alignment
Using the allotetraploid species Gossypium hirsutum Zhong9807, RNA-Seq analyses were conducted on three biological repetitions of each sample. RNA samples of roots and leaves were collected at 12 h post-salt stress and alkaline stress. Seedlings transplanted to normal conditions were used as controls. 24 qualified libraries were established (Table S1). Raw reads were processed to remove adapter and low-quality reads initially. Clean reads were then mapped to the G. hirsutum referenced genome using TopHat220. Approximately 213.36 Gb of clean reads was obtained. On average, 6.15 Gb of clean reads was obtained from each library. More than 87.02% of reads’ Q-score was Q30, and 78.23–83.44% of the total reads were aligned.
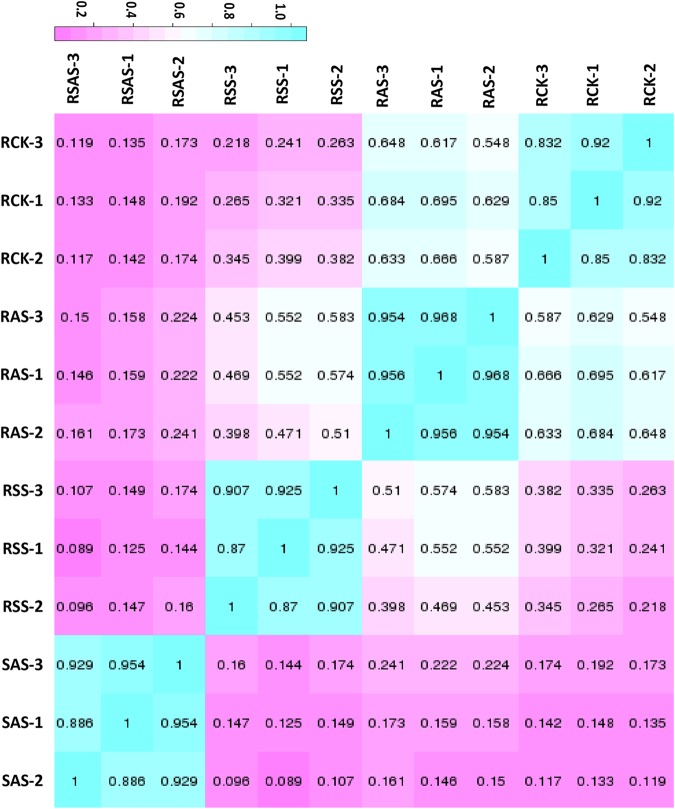
The aligned sequences were assembled with Cufflinks21, which was guided by a annotation genomes of tetraploid species G. hirsutum from Cottongen22. RNA-Seq assays revealed that there were 60,369 unigenes with 95.79% (57,825 unigenes) annotated genes and 9.18% (5,544 unigenes) novel genes (Table 2). For the evaluation of DEGs’ reliability and the filter of abnormal samples, Pearson correlation coefficient (PCC) analysis was conducted. The correlation analysis indicated that under all three salt-alkaline stresses, roots and leaves showed more than 85% similarities except for the genes between RCK-1 and RCK-2 and LSS-1 and LSS-3 (Fig. 2).
Table 2.
Statistics of functional genes with Cufflinks.
| Genes | RSS | RAS | RSAS | LSS | AS | LSAS | Total |
|---|---|---|---|---|---|---|---|
| All gene | 21754 | 13429 | 59143 | 11569 | 8657 | 49257 | 60369 |
| Annotation gene | 18436 | 10122 | 55903 | 8129 | 7672 | 46078 | 54825 |
| Novel gene | 3318 | 3307 | 3240 | 3440 | 985 | 3179 | 5544 |
RSS: Root, 100 mM NaCl; RAS: Root, 0.125 mM NaOH; RSAS: Root, 50 mM Na2CO3. LSS: Leaf, 100 mM NaCl; LAS: Leaf, 0.125 mM NaOH; LSAS:Leaf, 50 mM Na2CO3.
Figure 2.
Heatmap of the correlation of the expression level among root samples. RCK: Root, Control group; RSS: Root, 100 mM NaCl; RAS: Root, 0.125 mM NaOH; RSAS: Root, 50 mM Na2CO3. Numbers in the box refer to Pearson’s correlation coefficient r. The colors of box represent the degree of correlation; blue represents a high degree of correlation and pink represents a low degree of correlation.
The gene expression profile under NaOH stress was almost the same as the situation of control group in roots. The gene expression profile under NaCl and Na2CO3 stresses, however, were different. This result demonstrated that the damage caused by NaOH stress was the slightest one. It also showed the damage caused by Na2CO3 stress was the most serious situation. The expression correlation analysis showed the similar results in leaves and roots (Fig. S2).
GO (Gene Ontology), KEGG (Kyoto Encyclopedia of Genes and Genomes), NR (RefSeq non-redundant proteins), Swiss-Prot, COG (Cluster of Orthologous Groups), KOG (euKaryotic Ortholog Groups), Pfam and eggNOG (evolutionary genealogy of genes: Non-supervised Orthologous Groups) annotation of the novel genes were conducted. Totally, 4,202 and 4,014 novel genes were annotated in roots and leaves, respectively. We found that genes in roots were enriched in “nucleic acid binding” and “RNA-dependent DNA binding” GO terms. The genes in leaves were found enriched in “nucleic acid binding” and “DNA binding” terms. KEGG pathway analysis indicated that genes were enriched in “carbon metabolism”, “amino acid biosynthesis” and “plant hormone signal transduction” pathways (Fig. S3).
Differentially expressed genes analysis in leaves and roots under various salt-alkaline stresses and control group
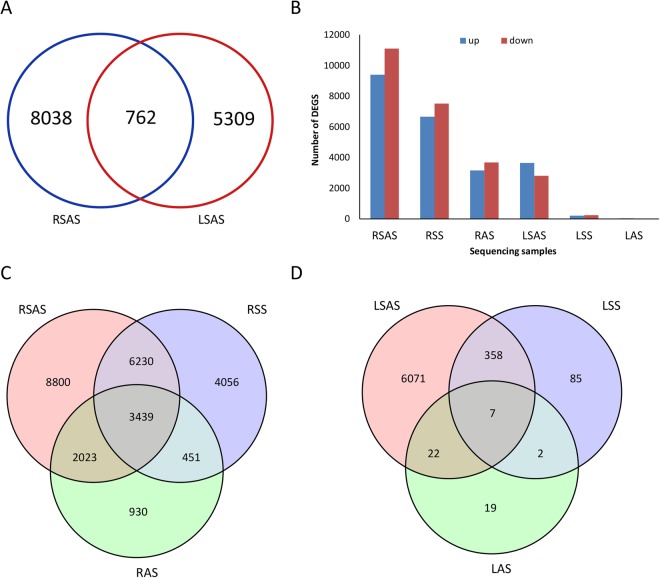
Gene expression levels were estimated by fragments per kilo base of transcript per million fragments mapped (FPKM). Differential expression analysis of treatments and control group was performed using the DESeq. A threshold of Fold Change ≥2 and FDR < 0.01 was used for identifying DEGs. In total, 25,929 DEGs (NaCl: 14,176; NaOH: 6,843; Na2CO3: 20,492) and 6,564 DEGs (NaCl: 452,NaOH: 50, Na2CO3: 6,458) were obtained in roots and leaves, respectively. DEGs numbers under different stresses in roots and leaves were presented as Na2CO3 > NaCl > NaOH stress, which illustrated that Na2CO3 stress induced a significant biological response in the plant as compare to NaCl stress and NaOH stress. In response to salt-alkaline stresses in roots and leaves, majority of genes got down-regulated in roots and up-regulated in leaves (Fig. 3B). These results indicated their tissue-specificity expression in response to stress23.
Figure 3.
Analysis of differentially expressed genes. RSS: Root, 100 mM NaCl; RAS: Root, 0.125 mM NaOH; RSAS: Root, 50 mM Na2CO3. LSS: Leaf, 100 mM NaCl; LAS: Leaf, 0.125 mM NaOH; LSAS:Leaf, 50 mM Na2CO3. (A) Specific DEGs in roots and leaves under SAS. (B) Number of up-regulated and down-regulated DEGs of each sample. (C) Number of DEGs in roots under different stress treatments. (D) Number of DEGs in leaves under different stress treatments.
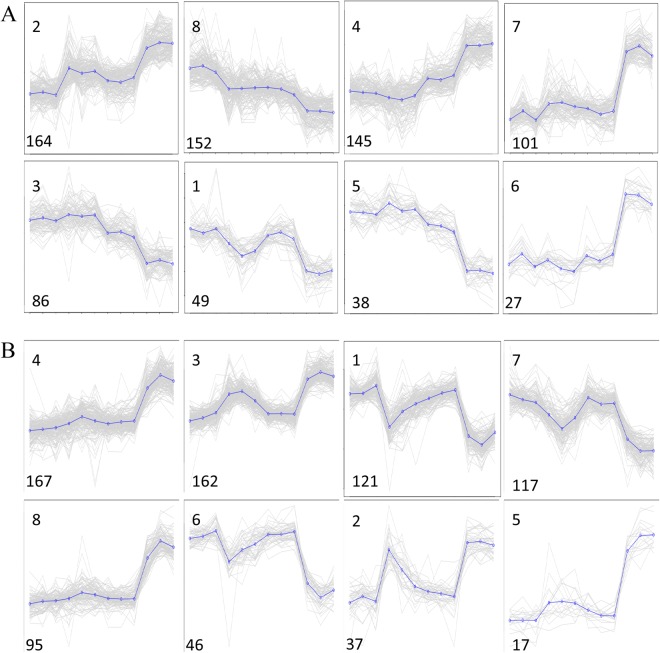
Approximately 8,800 (42.94%) of the total DEGs under Na2CO3 stress were found as root specific and 6,071 (94.00%) were leaf specific DEGs. Furthermore, 4,056 (28.59%) of the total DEGs under NaCl stress were root specific, while 85 (18.8%) DEGs were leaf specific. Moreover, 930 (13.59%) of the total DEGs under NaOH stress were root specific genes and 19 (38.00%) were leaf specific DEGs (Fig. 3C,D). Under Na2CO3 stress, 762 DEGs were commonly identified in roots and leaves (Fig. 3A). To study the expression profiles of these 762 genes under different salt-alkaline stresses, the FPKM of genes was normalized, and K-means cluster analysis with a normalized FPKM was used. These genes were divided into eight clusters. The genes in each cluster had the same expression profile (Fig. 4). Cluster 2 was the largest one in roots (164 genes, 21.52% of 762 genes), followed by cluster 8 (152 genes, 19.94%), cluster 4 (145 genes, 19.02%) and cluster 7 (101 genes, 13.25%). In leaves, cluster 4 was the largest profile (167 genes, 21.91% of 762 genes), followed by cluster 3 (162 genes, 21.26%), cluster 1 (121 genes, 15.88%) and cluster 7 (117 genes, 15.35%). The similar expression profile in roots and leaves was cluster 4, in which DEGs was up-regulated under Na2CO3 stress and unchanged under NaCl and NaOH stress. We mapped common cluster 4 genes within roots and leaves (Table S2) in the GO database. Results are presented in Fig. 5.
Figure 4.
Line graph for the cluster expression of 762 specific DEGs with Na2CO3 tolerance. (A) DEGs in roots. (B) DEGs in leaves. The X-axis shows the different treatments(from left to right: CK:normal condition; SS: 100 mM NaCl; AS: 0.125 mM NaOH; SAS: 50 mM Na2CO3), and the Y-axis shows the standardized FPKM. The number on the bottom right side of cluster panel is cluster number. The number on the bottom left side of cluster panel is genes number of each cluster. Black lines represent the average value of the relative expression level of all genes included in the cluster under different experimental stresses.
Figure 5.
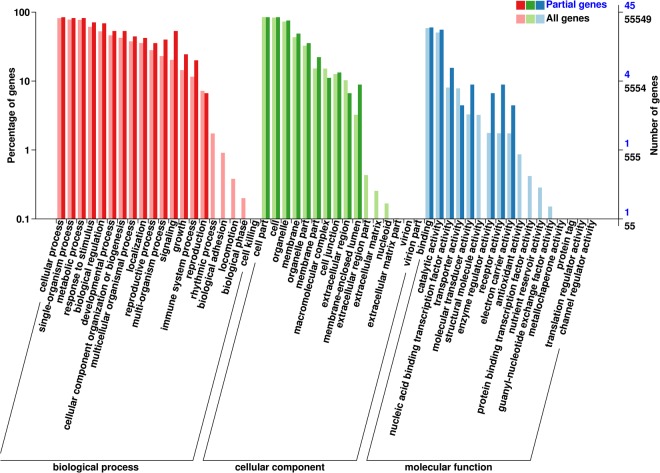
GO classification of cluster4 genes commonly in roots and leaves. X-axis represents the GO terms names: red pillars represent biological process, green pillars represent cellar component, and blue pillars represent molecular function. The deeper colours represent cluster4 genes, the lighter colours represent all genes. The left Y-axis represents percentage of genes, right Y-axis represents number of genes.
GO enrichment analysis of differentially expressed genes
To further investigate the function of DEGs, we mapped all of DEGs to the GO database24. 5,769 (87.89%) and 20,508 (79.09%) DEGs in leaves and roots were annotated, respectively. “Response to stimulus” (GO:0050896) was enriched in both leaves and roots after the results were classified. The enriched DEGs were different among the salt-alkaline stresses (Table 3), which were consistent with the phenotypic changes under different stresses. Under NaCl and NaOH stress, more genes related to “response to stimulus” were identified in roots than in leaves (Table 3), while more genes were recognized in roots as compared to those in leaves under Na2CO3 stress. The results implied that a more complicated physiological process occurred in roots than in leaves when the cotton plants were damaged by both NaCl and NaOH stresses. This was in contrast to the situation when seedlings were under Na2CO3 stress.
Table 3.
Analysis of GO enrichment terms in response to stimulus.
| Term | ID | RSS | LSS | RAS | LAS | RSAS | LSAS |
|---|---|---|---|---|---|---|---|
| divalent metal ion transport | GO:0070838 | 0.106702 29/409 |
0.440725 1/409 |
0.323192 7/409 |
0 | 0.974428 38/409 |
0 86/409 |
| hyperosmotic salinity response | GO:0042538 | 0.000942 154/1857 |
0.49109 3/1857 |
0.468253 26/1857 |
0 | 0.999992 170/1857 |
0 274/1857 |
| cellular cation homeostasis | GO:0030003 | 0.075488 42/601 |
0.208845 2/601 |
0.07003 13/601 |
0 | 0.996858 53/601 |
0 110/601 |
| protein serine/threonine phosphatase activity | GO:0004722 | 0.003649 50/447 |
0 | 0.854654 4/447 |
0 | 0.141608 64/447 |
0.369568 44/447 |
| response to osmotic stress | GO:0006970 | 0.052443 72/787 |
0.02548 4/787 |
0.189197 14/787 |
0 | 0.611997 89/787 |
0.0241 89/787 |
| 347 | 10 | 64 | 0 | 414 | 603 | ||
| total | 1084 | ||||||
RSS: Root, 100 mM NaCl; RAS: Root, 0.125 mM NaOH; RSAS: Root, 50 mM Na2CO3. LSS: Leaf, 100 mM NaCl; LAS: Leaf, 0.125 mM NaOH; LSAS:Leaf, 50 mM Na2CO3. Each box shows the genes numbers and the p-value. The pair of numerals in the left represents number of genes in input list. The pair of numerals in the right represents number of genes in the TM-1 database.
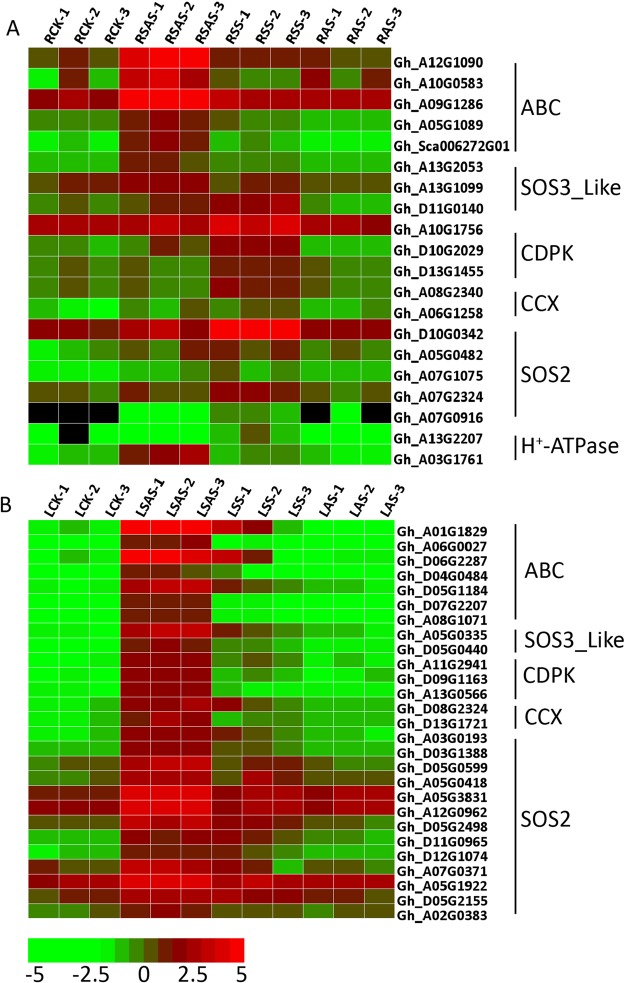
Functional enrichment of the annotated genes was conducted using topGO25, and the results of the enrichment were sorted by p-value numbers. Then the first 20 GO terms with the smallest p-values were chosen. GO enrichment analysis revealed an enrichment of genes involved in plant responses to salt stress, ionic homeostasis, organic substance, hormone signal pathways and osmotic stress in leaves and roots under salt-alkaline stresses (Fig. S4). The results revealed that “divalent metal ion transport”, “hyperosmotic salinity response” and “cellular cation homeostasis” genes had higher expression levels under Na2CO3 stress. “Hyperosmotic salinity response”, “response to osmotic stress” and “protein serine/threonine phosphatase activity” genes reached a higher expression level in NaCl stress, while these genes were non-significantly enriched under Na2CO3 stress. These genes are related to osmotic adjustment and maintenance of intracellular ionic homeostasis, playing vital roles in plant tolerance to salt stress26. According to statistics, DEGs under SS, AS and SAS appeared as following: Na2CO3 > NaCl > NaOH stress (Table S3). Genes related to ion absorption and compartmentalization, such as SOS2, SOS3-Like, CCX, CDPK and ABC transporters, were detected from these genes27–31. These were up-regulated under Na2CO3 and NaCl stress, and were down-regulated under NaOH stress (Fig. 6).
Figure 6.
Heatmap for the co-expression of genes clusters related to ionic homeostasis in roots and leaves under different stresses. RCK: Root, Control group; RSS: Root, 100 mM NaCl; RAS: Root, 0.125 mM NaOH; RSAS: Root, 50 mM Na2CO3, LCK: Leaf, Control group; LSS: Leaf, 100 mM NaCl; LAS: Leaf, 0.125 mM NaOH; LSAS: Leaf, 50 mM Na2CO3. (A) Heat map log2 FC (Fold Change) value of the expression level cluster of roots under different stresses. (B) Heat map log2 FC (Fold Change) value of the expression level cluster of leaves under different stresses. Red = high expression level of genes, and Green = low expression level of genes.
KEGG metabolic pathways annotation of sugar metabolism in responses to different alkaline stresses
Annotation analysis of the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways32 of DEGs contributed to the decoding of gene functions. A total of 1,392 (21.21%) and 5,510 (21.25%) DEGs were annotated in roots and leaves, respectively. The KEGG enrichment pathways (Fig. S5) indicated that the specific DEGs, found under three salt-alkaline stresses in roots and leaves, were widely enriched in the pathways of plant hormone signal transduction33, photosynthesis, peroxidase34,35 and glutathione metabolism36. According to previous studies, these pathways are related to abiotic stresses tolerance.
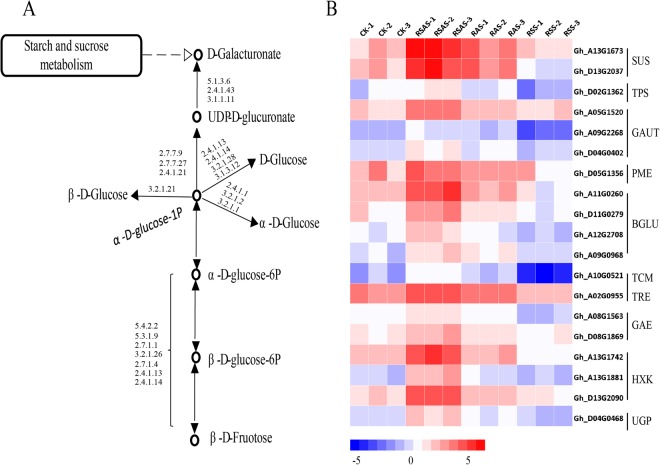
DEGs were significantly enriched in the pathway “starch and sucrose metabolism” (ko00500) under NaOH and Na2CO3 stresses, but not significantly enriched under NaCl stress. The sucrose content of leaves increased significantly when lupin was under NaCl stress37,38. It has reported that exogenous sugar affected the hormone signal transduction, key enzyme metabolism and sucrose metabolism of grape seedlings39. Among DEGs enriched in starch and sucrose metabolism (Figs 7A and S6), we found some genes that had up-regulated under NaOH and Na2CO3 stress and down-regulated under NaCl stress in roots (Fig. 7B). The TPS1-TPS2 (trehalose-6-phosphate synthase) lines displayed a significant increase in drought, freezing, salt and heat tolerance40.
Figure 7.
Analysis of differential genes in pathways of starch and sucrose Metabolism. RCK: Root, Control group; RSS: Root, 100 mM NaCl; RAS: Root, 0.125 mM NaOH; RSAS: Root, 50 mM Na2CO3. (A) A schematic diagram of sugar metabolism. Digits represents regulatory enzyme for specific process. (B) Up-regulated DEGs under Na2CO3 and NaOH treatments and down-regulated DEGs under NaCl treatment.
Transcription factors analysis and annotation
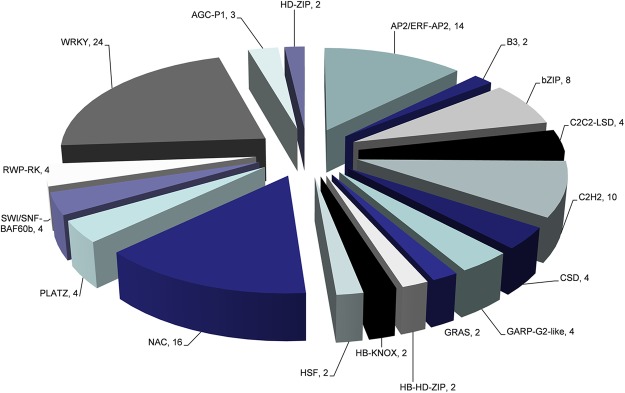
Many studies have reported that transcription factors (TFs) play an important role in stress tolerance41. Transcription factor annotation was performed among 762 specific DEGs (Fig. 3A). These TFs were classified into 18 families and three protein kinases families. Except for bZIP (basic region/leucine zipper)42, NAC (NAM/no apical meristem, ATAF/Arabidopsis transcription activation factor, and CUC/cup-shaped cotyledon)43, MYB (v-myb avian myeloblastosis viral oncogene homolog)44 and ERF (ethylene response elements)45 families were related to salt-alkaline tolerance, C2H2 (Cys2/His2) transcription factor family was also enriched (Fig. 8). C2H2, a zinc finger protein, is related to the osmotic stress tolerance of Arabidopsis thaliana46–48.
Figure 8.
Annotation of transcription factors of specific DEGs tolerance to Na2CO3 stress.
Validation of RNA-Seq data by quantitative real-time PCR
To verify the reliability of sequencing, quantitative real-time PCR (qRT-PCR) was performed using the same RNA samples that were previously used for RNA-seq. A total of 20 genes were randomly selected for qRT-PCR, including 10 up-regulated genes and 10 down-regulated genes. Linear correlation analysis of the data of these two groups was performed. The fold change (FC) of genes between salt-alkaline-stressed tissues and controlled treatments using qRT-PCR were compared to those ones using RNA-Seq. The correlation coefficients between qRT-PCR and RNA-seq were significant in the roots and leaves under different salt-alkaline stresses (Fig. S7).
Discussion
Synergistic enhancing effect of salt stress and alkaline stress
Salt-alkaline situation often results in osmotic stress to plants firstly, following by ionic toxicity and consequently oxidative stress, which leads to an increase in intracellular active oxygen6. Numerous studies on molecular mechanisms of cotton tolerance to salt stress have been conducted by mainly focusing on salt tolerance, but few have investigated the difference between salt tolerance and alkaline tolerance. In comparison with the situation under NaCl and NaOH stresses3,7, maize seedlings suffered more changes under Na2CO3 stress. In this study, significant phenotypic differences were observed in roots and leaves under Na2CO3 stress.
RNA-seq analysis of roots and leaves under NaCl, NaOH and Na2CO3 stresses was performed to investigate different molecular mechanisms under salt and alkaline stress. The expression profiles induced by Na2CO3 and NaCl in Puccinellia tenuiflora3 and maize7 were relatively pronounced, while those induced by NaOH were relatively unchanged. Results indicated that NaOH stress have triggered few biological responses, probably related to the neutralization of pH by organic acid, such as ascorbic acid accumulation4, alleviating serious damage. Significant biological responses occur under NaCl stress, because plants cannot eliminate ions immediately and may constantly suffer from osmotic stress and ionic toxicity6,49. More biological responses may occur in plants if there is both high pH and salt stress under Na2CO3 stress. In our study, the DEGs numbers are presented as: Na2CO3 > NaCl > NaOH stress which showed in Fig. 3. It indicates that there is a complicated and synergistic effect between NaCl and NaOH stresses. It also explains that NaCl and NaOH stresses together exhibit significant damages to plants.
Ion homeostasis in response to Na+ stress
Plant under salt-alkaline stress may first generate a series of substances for osmotic adjustment and then regulate relevant genes expression to maintain ionic homeostasis and balance the cellular osmotic potential6,49. According to GO function enrichment of DEGs, we found that the GO enrichment terms “hyperosmotic salinity response”, “response to osmotic stress”, “protein serine/threonine phosphatase activity”, “divalent metal ion transport”, and “cellular cation homeostasis” genes, which are related to osmotic adjustment and maintenance of intracellular ion homeostasis, were enriched under NaCl and Na2CO3 stresses, but not under NaOH stress27,30. Hence, we paid close attention to these genes related to osmotic adjustmentand ion homeostasis. As shown in Fig. S4, there may be a special mechanism of Na+ stress tolerance.
The ABC (ATP-binding cassette) transporters use the energy produced by hydrolysis of ATP for the transportation of micromolecules and play a vital role in ion homeostasis50. In our study, several ABC transporter genes were up-regulated under NaCl and Na2CO3 stresses and down-regulated under NaOH stress. We found that the ABCB21 (Gh_A12G1090), ABCG36 (Gh_A10G0583, Gh_A05G1089, and Gh_Sca006272G01), and ABC2 (Gh_A09G1286) genes in the roots of Gossypium hirsutum were up-regulated under Na+ stress. Kamimoto et al. demonstrated that Arabidopsis ABCB21 imported/exported auxin51. Auxin (IAA) regulated NAC transcription factors of NTM2 expression under salt stress52. In our study, we found that the NAC family was enriched under Na2CO3 stress. Kim et al. found that AtPDR8 (ABCG36) was an efflux pump of Cd2+ 53. AtPDR12 mediates the uptake of ABA (abscisic acid) in Arabidopsis54. ABA stimulates calcium-dependent protein kinases (CDPKs) and serine/threonine phosphatase activity (SOS2).The level of SOS2 and SOS3-Like (also known as CBLs) proteins were both up-regulated in roots and leaves under Na+ stress. The protein SOS2 can regulate Na+/K+ transport proteins and CBLs worked as a calcium sensor in plant salt tolerance55. cpk mutation is salt-insensitive56. CBLs and CDPKs can alter the transcriptional profile, such as the transcription factors (MYB, WRKY, and bZIP), which are regulated and expression of the downstream genes is changed6. MYBs regulated the genes of the anthocyanin pathway in Rosaceae57. Kim et al. introduced a double knockout mutant of bZIP17 and bZIP28 to analyze the function of bZIP1758. They found that mutant plants exhibited multiple developmental defects including scarce root elongation. These TFs were significantly up-regulated under Na+ stress in roots and leaves. We also found that CCX-related genes were up-regulated. Chen et al. speculated that AtCCX1 is vital for Na+ resistance and serves as a Na+/K+ exchanger in vacuoles.
These results indicate that ionic homeostasis may not be affected by the high pH induced by NaOH stress. However, high pH may increase osmotic stress together with Na+ and produce a synergistic enhancing effect of NaCl and NaOH stresses. The reason is that Na+ easily affects the dynamic equilibrium of the cytomembrane under environment with high pH4,59,60. The thorough mechanism of the synergistic enhancing effect needs further study.
Oxygen deficiency in response to high pH
For further detection of the differences between salt and alkaline stresses, KEGG pathways enrichment analysis of DEGs was performed. The results demonstrate that under NaOH and Na2CO3 stresses, DEGs were found significantly enriched in “starch and sucrose metabolism” pathways, but non-significantly under NaCl stress. It has been noted in results that genes enriched with this pathway may have some relationship with high pH. It has been reported in previous studies that the cytosolic pH of maize roots decreases from 7.5 to 6.5 under oxygen deprivation61. Annabelle et al. found that the transcription level of Sus2 (sucrose synthase) was ABA-dependent and specifically induced by O2 deficiency62. We found that genes encoding Sus were up-regulated under high pH stress (under both NaOH and Na2CO3 stresses, but not NaCl stress). Hence, we speculated that high pH results insufficient oxygen level in plant cells, followed by induction of Sus genes and increased transformation of glucose into sucrose β-glucosidase (BGLU) was accumulated in the ER (endoplasmic reticulum) body, which plays a vital role in the defense system of plants63. β-glucosidase is a substrate of glucose production, and BGLU genes were up-regulated. Plants use HXK (hexokinase) as a glucose sensor in response to a changing environment63. Mu et al. showed that trehalose-6-phosphate synthase (TPS) genes are stress-related in cotton64. Nelson et al. found that AtTPS1 is probably a member of the HXK1-dependent Glc-signaling pathway65, and that O2 deficiency is regulated by the expression of Ugp66. Studies have reported that the use of exogenous sugar may affect hormone signal transduction, key enzyme metabolism and sucrose metabolism of grape seedlings39. Genes enriched in “starch and sucrose metabolism” pathways are up-regulated under NaOH and Na2CO3 stress and down-regulated under NaCl stress. These genes then participate in plant hormone signal transduction and affect the activities of some key enzymes (such as ROS, ATPase and RuBisCO). Galacturonosyltransferases (GalATs/GAUT) are required for the synthesis of pectin67. Pectin methylesterase (PME) catalyzes pectin deesterification, releasing acid pectin and methanol, which cause cell wall changes68. The Subcellular locations of GAUT, PME and UDP-D-glucuronate 4-epimerase (GAE) are Golgi69, suggesting that PME, GAUT and GAE were related to synthesis of cell wall.
The expression of these genes indicates that high pH firstly causes oxygen deprivation stress to cotton plants. Lacking of oxygen leads to anaerobic respiration of plants and produce ethylene which causes roots rotten70. In addition, there were nigrities in roots under NaOH and Na2CO3 stresses and in leaves under Na2CO3 stress. Hence, we speculated the phenotypic changes under high pH were related to the oxygen deprivation signal pathway. Consequently, glucose synthesis and decomposition, TPS synthesis and other sugar metabolisms are involved in defending against oxygen deprivation. What is more, genes related to the synthesis of pectin were up-related. Pectin is an important cell wall polysaccharide that allows primary cell wall extension and plant growth68. We inferred that high pH induce genes that recoded proteins of cell wall synthesis (PME, GAUT and GAE) were up-regulated to strengthen cell wall and defend high pH damage.
Conclusion
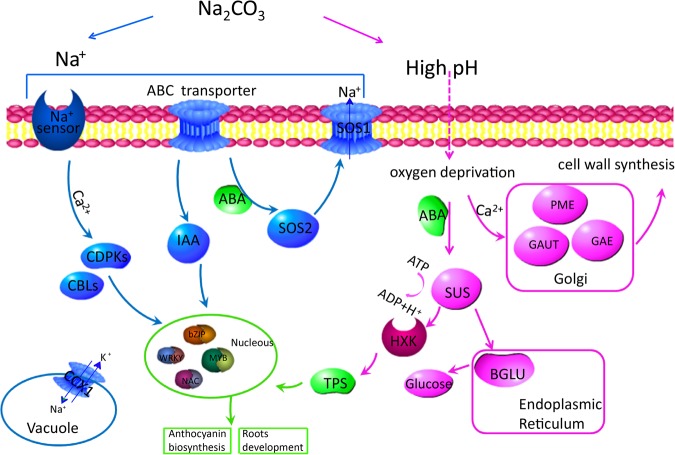
This paper described the possible mechanism response to Na2CO3 tolerance in cotton. In general, most of the genes and proteins related to Na+ stress (treated with NaCl) and high pH (treated with NaOH) are also involved in the pathways against Na2CO3 stress (Fig. 9). We speculate that cotton may regulate the metabolism of starch and sucrose due to the toxicity of high pH, which results in the specifical expression of some genes under Na2CO3 and NaOH stresses. High pH leads to oxygen deprivation stress, which causes cotton organs nigrities and rottenness. In addition, high pH also increases the synthesis of pectin-related enzymes, which strengthens cell wall to defense damage of high pH. In the process of the hydrolysis of ATPase, extra H+ produced help to neutralize OH− within the cytoplasm.
Figure 9.
Model of the regulatory networks in response to Na+ stress and high pH. The left blue part of the networks is related to Na+ stress, the right red part of the networks is related to high pH. The crossed green part is related to both Na+ stress and high pH.
Besides, genes and proteins related with ion homeostasis under Na+ stress were also found in our study, such as protein kinases, transcription and transporters (Fig. 9). And these genes and proteins have been reported in previous studies. MYBs that regulate genes of the anthocyanin pathway were up-regulated under Na2CO3 stress, which always cause leaves to turn red or orange in apple71. bZIPs play a important role in roots development under salt stress58.
The mechanism of salt and alkaline tolerance in cotton are complicated. Further study is still needed, even though several genes have been transported into cotton and other plants. Here our study provides some candidate genes particularly responsing to high pH and Na+ stresses. For instance, Hexokinase (HXK) acted as a sugar sensor in eukaryotic cells72, being found to be up-regulated under high pH stress, which indicates that genes encoding HXK may be related with high pH stress.
Materials and Methods
Plant materials and salt-alkaline stresses conditions
G. hirsutum cultivars Zhong9807 was used for this study. Seeds were sown in sand soil pots.The sand was washed cleanly and sterilized at 121 °C for 8 h. Four seedlings in each pot were cultivated in a 28 °C/14 h light and 25 °C/10 h dark cycle with a light intensity of 150 μmol m−2 s−1and 75% relative humidity for approximately 30 days. Seedlings containing three true leaves and one heart-shaped leaf were washed out carefully and transplanted into conical flasks containing 0, 25, 50, 75, and 100 mM Na2CO3 solution for 0, 6, 12, and 24 h to observe phenotypic changes. Then, 50 mM Na2CO3 over 12 h was chosen as the applicable stress concentration and time. In addition, for salinity stress treatment, seedlings were transferred into ddH2O supplemented with 100 mM NaCl (pH = 7.0). For alkaline stress treatments, seedlings were transplanted into ddH2O supplemented with 0.125 mM NaOH (pH = 11.32) and 50 mM Na2CO3 (pH = 11.32). Seedlings transplanted into normal ddH2O were used as controls. After exposure for 12 h, antepenultimate leaf and whole root samples were collected. Each sample was tested three time. Samples were frozen in liquid nitrogen and stored at −80 °C for physiological measurement and transcriptome analysis.
Measurement of relative water content and chlorophyll content
The relative water content (RWC) of roots and leaves was calculated following the method described previously73. The total chlorophyll content of leaves was measured spectrophotometrically using the method of Arnon74, and variance analysis proceeded as described by Anders19.
cDNA libraries preparation and RNA-seq
Total RNA of roots and leaves was extracted. RNA degradation and contamination was monitored on 1% agarose gels. RNA purity was checked using the NanoPhotometer spectrophotometer (IMPLEN, CA, USA). RNA concentration was measured using Qubit RNA Assay Kit in Qubit 2.0 Flurometer (Life Technologies, CA, USA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA). Qualified RNA samples were randomly digested with fragmentation buffer. Library preparation for RNA-Seq was performed using the Employed NEB Next Ultra TM RNA Library Prep Kit (NEB, USA). Library quality was assessed on the Agilent Bioanalyzer 2100 system. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq PE Cluster Kit v4-cBot-HS (Illumia) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina Hiseq. 2500 platform and paired-end reads were generated.
Quality control, alignment and differential expression genes analysis
Raw data in the fastq format was first processed through in-house perl scripts. The adaptor sequences and low-quality sequence reads were removed from the data sets. Raw sequences were transformed into clean reads after data processing. These clean reads were mapped to the G.hirsutum genome using TopHat2 tool20. These mapped reads were spliced using Cufflinks software21 based on the reference genome sequence. Quantification of the gene expression levels were estimated as fragments per kilobase of transcript per million fragments mapped (FKPM)75. Differential expression analysis of two groups was performed using the DESeq R package (1.10.1). DESeq provides statistical routines for determining differential expression in digital gene expression data using a model based on the negative binomial distribution. The resulting P values were adjusted using Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes with an adjusted P-value < 0.05 were found using DESeq76 and were assigned as differentially expressed. Consequently, DEGs were obtained of three biological conditions. Fold Change ≥2 and FDR < 0.01 were taken as the thresholds for determining whether a gene had differential expression.
Gene ontology and gene pathway enrichment analysis
Gene Ontology (GO) enrichment analysis of the DEGs was implemented using the GOseq R packages based on the Wallenius non-central hyper-geometric distribution, which can adjust for gene-length bias in DEGs24. KEGG32 is a database resource for understanding high-level functions and utilities of the biological systems, such as the cell, organism and ecosystem, from molecular-level information, especially large-scale molecular datasets generated by genome sequencing and other high-throughput experimental technologies (http://www.genome.jp/kegg/). We used KOBAS77 software to test the statistical enrichment of differential expression of genes in KEGG pathways.
qRT-PCR verification of RNA-seq data
qRT-PCR was carried out using the same samples. 20 genes were chosen randomly (Table S4), including 10 up-regulated and 10 down-regulated genes from the roots and leaves according to the FPKM. qRT-PCR was performed using the Applied Biosystems@7500 Fast instrument and TransStart Top Green qPCR SuperMix. Reactions were performed with three technological and biological repetitions: 0.4 μL of each primer (10 μM/μL), 0.4 μL of passive reference Dye and 10 μL of Top Green qPCR Supermix at a final volume of 20 μL. The profile for amplification was as follows: 5 min at 95 °C, followed by 40 cycles amplification of 95 °C for 15 s, then 20 s at 58 °C, and 30 s at 72 °C. The ΔΔCt method was used to calculate the relative fold change for each sample78. The GhHis3 house-keeping gene was used as a control. The correlation coefficients between qRT-PCR and RNA-seq was performed.
Electronic supplementary material
Acknowledgements
This work was supported by grants from National key research and development program (2016YFD0101006).
Author Contributions
Wuwei Ye conceived and designed the experiments. Binglei Zhang, Xiugui Chen and Xuke Lu performed the experiments, prepared the materials and conducted the manuscript. Na Shu, Xiaoge Wangand Xiaomin Yang participated in data analyses. Thanks Shuai Wang, Junjuan Wang, Delong Wang, LixueGuo for providing some significant advices. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31668-z.
References
- 1.Novo, F. G. & Bouzas, F. G. Water and nature. The berth of life. Water Crisis: Myth or Reality? 235–252 (2006).
- 2.Shi DC, Wang DL. Effects of various salt-alkaline mixed stresses on Aneurolepidium chinense (Trin.) Kitag. Plant Soil. 2005;271:15–26. doi: 10.1007/s11104-004-1307-z. [DOI] [Google Scholar]
- 3.Zhang X, Wei L, Wang Z, Wang T. Physiological and molecular features of Puccinellia tenuiflora tolerating salt and alkaline-salt stress. Journal of integrative plant biology. 2013;55:262–276. doi: 10.1111/jipb.12013. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Q, et al. Na2CO3-responsive mechanisms in halophyte Puccinellia tenuiflora roots revealed by physiological and proteomic analyses. Scientific reports. 2016;6:32717. doi: 10.1038/srep32717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson NJ, Urwin PE. The interaction of plant biotic and abiotic stresses: from genes to the field. J Exp Bot. 2012;63:3523–3543. doi: 10.1093/jxb/ers100. [DOI] [PubMed] [Google Scholar]
- 6.Deinlein U, et al. Plant salt-tolerance mechanisms. Trends in Plant Science. 2014;19:371–379. doi: 10.1016/j.tplants.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang LM, et al. Early Transcriptomic Adaptation to Na2CO3 Stress Altered the Expression of a Quarter of the Total Genes in the Maize Genome and Exhibited Shared and Distinctive Profiles with NaCl and High pH Stresses. Journal of integrative plant biology. 2013;55:1147–1165. doi: 10.1111/jipb.12100. [DOI] [PubMed] [Google Scholar]
- 8.Cao L, et al. A novel Glycine soja homeodomain-leucine zipper (HD-Zip) I gene, Gshdz4, positively regulates bicarbonate tolerance and responds to osmotic stress in Arabidopsis. BMC plant biology. 2016;16:184. doi: 10.1186/s12870-016-0872-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao CQ, et al. Expression profiling of salinity-alkali stress responses by large-scale expressed sequence tag analysis in Tamarix hispid. Plant molecular biology. 2008;66:245–258. doi: 10.1007/s11103-007-9266-4. [DOI] [PubMed] [Google Scholar]
- 10.Xu N, et al. Aft2, a novel transcription regulator, is required for iron metabolism, oxidative stress, surface adhesion and hyphal development in Candida albicans. Plos one. 2013;8:e62367. doi: 10.1371/journal.pone.0062367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, et al. Comparative effects of salt and alkali stresses on organic acid accumulation and ionic balance of seabuckthorn (Hippophae rhamnoides L.) Industrial Crops & Products. 2009;30:351–358. doi: 10.1016/j.indcrop.2009.06.007. [DOI] [Google Scholar]
- 13.Wu FL, et al. The pH-responsive transcription factor PacC regulates mycelial growth, fruiting body development, and ganoderic acid biosynthesis in Ganoderma lucidum. Mycologia. 2016;108:1104–1113. doi: 10.3852/16-020. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, et al. Targeted mutagenesis in cotton (Gossypium hirsutum L.) using the CRISPR/Cas9 system. Scientific reports. 2017;7:44304. doi: 10.1038/srep44304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, P. Y., Wu, J. H. & Qian, H. Regulation of secondary soil salinization in semi-arid regions: a simulation research in the Nanshantaizi area along the Silk Road, northwest China. Environ Earth Sci75 (2016).
- 16.Cnudde F, et al. Changes in gene expression during male meiosis in Petunia hybrida. Chromosome research. 2006;14:919–932. doi: 10.1007/s10577-006-1099-5. [DOI] [PubMed] [Google Scholar]
- 17.Natasha L, et al. Combining next-generation pyrosequencing with microarray for large scale expression analysis in non-model species. BMC Genomics. 2009;10:555. doi: 10.1186/1471-2164-10-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang GW, Lu HL, Zhang L, Chen BL, Zhou ZG. Salt tolerance evaluation of cotton (Gossypium hirsutum) at its germinating and seedling stages and selection of related indices. The journal of applied ecology. 2011;22:2045–2053. [PubMed] [Google Scholar]
- 19.Anders K. Resolution of Students t-tests, ANOVA and analysis of variance components from intermediary data. Biochemia medica. 2017;27:253–258. doi: 10.11613/BM.2017.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D, et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nature Protocols. 2012;7:562. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T, et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat Biotechnol. 2015;33:531–537. doi: 10.1038/nbt.3207. [DOI] [PubMed] [Google Scholar]
- 23.Majdi M, Malekzadeh-Mashhady A, Maroufi A, Crocoll C. Tissue-specific gene-expression patterns of genes associated with thymol/carvacrol biosynthesis in thyme (Thymus vulgaris L.) and their differential changes upon treatment with abiotic elicitors. Plant physiology and biochemistry: PPB. 2017;115:152–162. doi: 10.1016/j.plaphy.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Young MD, Wakefield MJ, Smyth GK, Alicia O. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11:1–12. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexa, A. & Rahnenfuhrer, J. topGO: Enrichment analysis for Gene Ontology. R Package Version (2006).
- 26.Zhang JL, Shi H. Physiological and molecular mechanisms of plant salt tolerance. Photosynthesis research. 2013;115:1–22. doi: 10.1007/s11120-013-9813-6. [DOI] [PubMed] [Google Scholar]
- 27.Quan R, et al. EIN3 and SOS2 synergistically modulate plant salt tolerance. Scientific reports. 2017;7:44637. doi: 10.1038/srep44637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin H, Du W, Yang Y, Schumaker KS, Guo Y. A calcium-independent activation of the Arabidopsis SOS2-like protein kinase24 by its interacting SOS3-like calcium binding protein 1. Plant physiology. 2014;164:2197–2206. doi: 10.1104/pp.113.232272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt S, Pfluger K, Kogl S, Spanheimer R, Muller V. The salt-induced ABC transporter Ota of the methanogenic archaeon Methanosarcina mazei Go1 is a glycine betaine transporter. FEMS microbiology letters. 2007;277:44–49. doi: 10.1111/j.1574-6968.2007.00938.x. [DOI] [PubMed] [Google Scholar]
- 30.Chen ZY, Wu YJ, Di LJ, Wang GD, Shen YF. The AtCCX1 transporter mediates salinity tolerance in both Arabidopsis and yeast (Retracted article. See vol. 110, pg. 317, 2012) Plant Cell Tiss Org. 2012;109:91–99. doi: 10.1007/s11240-011-0077-6. [DOI] [Google Scholar]
- 31.Asano T, et al. Functional characterisation of OsCPK21, a calcium-dependent protein kinase that confers salt tolerance in rice. Plant molecular biology. 2011;75:179–191. doi: 10.1007/s11103-010-9717-1. [DOI] [PubMed] [Google Scholar]
- 32.Kanehisa M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Research. 2008;36:D480–D484. doi: 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryu H, Cho YG. Plant hormones in salt stress tolerance. J Plant Biol. 2015;58:147–155. doi: 10.1007/s12374-015-0103-z. [DOI] [Google Scholar]
- 34.Meloni DA, Oliva MA, Martinez CA, Cambraia J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot. 2003;49:69–76. doi: 10.1016/S0098-8472(02)00058-8. [DOI] [Google Scholar]
- 35.Lopez F, Vansuyt G, CasseDelbart F, Fourcroy P. Ascorbate peroxidase activity, not the mRNA level, is enhanced in salt-stressed Raphanus sativus plants. Physiologia plantarum. 1996;97:13–20. doi: 10.1111/j.1399-3054.1996.tb00472.x. [DOI] [Google Scholar]
- 36.Dong, Y. T. et al. Glutathione S-Transferase Gene Family in Gossypium raimondii and G. arboreum: Comparative Genomic Study and their Expression under Salt Stress. Frontiers in plant science 7 (2016). [DOI] [PMC free article] [PubMed]
- 37.Fernandes FM, Arrabaca MC, Carvalho LMM. Sucrose metabolism in Lupinus albus L. under salt stress. Biol Plantarum. 2004;48:317–319. doi: 10.1023/B:BIOP.0000033465.59361.d2. [DOI] [Google Scholar]
- 38.Gong X, Liu ML, Wang C, Zhang LJ, Liu W. Sucrose Transporter Gene AtSUC4 Regulates Sucrose Distribution and Metabolism in Response to Salt Stress in Arabidopsis Thaliana. Adv Mater Res-Switz. 2013;726–731:217–221. doi: 10.4028/www.scientific.net/AMR.726-731.217. [DOI] [Google Scholar]
- 39.Mao, J. et al. Different exogenous sugars affect the hormone signal pathway and sugar metabolism in “Red Globe” (Vitis vinifera L.) plantlets grown in vitro as shown by transcriptomic analysis. Planta (2017). [DOI] [PubMed]
- 40.Miranda JA, et al. A bifunctional TPS-TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis. Planta. 2007;226:1411–1421. doi: 10.1007/s00425-007-0579-y. [DOI] [PubMed] [Google Scholar]
- 41.Gruber V, et al. Identification of transcription factors involved in root apex responses to salt stress in Medicago truncatula. Mol Genet Genomics. 2009;281:55–66. doi: 10.1007/s00438-008-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia MNM, et al. Characterization of StABF1, a stress-responsive bZIP transcription factor from Solanum tuberosum L. that is phosphorylated by StCDPK2 in vitro. Planta. 2012;235:761–778. doi: 10.1007/s00425-011-1540-7. [DOI] [PubMed] [Google Scholar]
- 43.Lu, X. et al. Three stress-responsive NAC transcription factors from Populus euphratica differentially regulate salt and drought tolerance in transgenic plants. Physiologia plantarum (2017). [DOI] [PubMed]
- 44.Xu R, et al. Salt-induced transcription factor MYB74 is regulated by the RNA-directed DNA methylation pathway in Arabidopsis. J Exp Bot. 2015;66:5997–6008. doi: 10.1093/jxb/erv312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, et al. Genome-Wide Identification of AP2/ERF Transcription Factors in Cauliflower and Expression Profiling of the ERF Family under Salt and Drought Stresses. Frontiers in plant science. 2017;8:946. doi: 10.3389/fpls.2017.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kielbowicz-Matuk A. Involvement of plant C2H2-type zinc finger transcription factors in stress responses. Plant Science. 2012;185:78–85. doi: 10.1016/j.plantsci.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 47.Muthamilarasan M, et al. C2H2 type of zinc finger transcription factors in foxtail millet define response to abiotic stresses. Funct Integr Genomic. 2014;14:531–543. doi: 10.1007/s10142-014-0383-2. [DOI] [PubMed] [Google Scholar]
- 48.Ma X, Liang W, Gu P, Huang Z. Salt tolerance function of the novel C2H2-type zinc finger protein TaZNF in wheat. Plant physiology and biochemistry: PPB. 2016;106:129–140. doi: 10.1016/j.plaphy.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 49.Gorham J, Jones RGW, Mcdonnell E. Some mechanisms of salt tolerance in crop plants. Plant & Soil. 1985;89:15–40. doi: 10.1007/BF02182231. [DOI] [Google Scholar]
- 50.Wilkens S. Structure and mechanism of ABC transporters. F1000prime Rep. 2015;7:14. doi: 10.12703/P7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamimoto Y, et al. Arabidopsis ABCB21 is a facultative auxin importer/exporter regulated by cytoplasmic auxin concentration. Plant & cell physiology. 2012;53:2090–2100. doi: 10.1093/pcp/pcs149. [DOI] [PubMed] [Google Scholar]
- 52.Jung JH, Park CM. Auxin modulation of salt stress signaling in Arabidopsis seed germination. Plant Signal Behav. 2011;6:1198–1200. doi: 10.4161/psb.6.8.15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant. J. Plant Journal. 2007;50:207–218. doi: 10.1111/j.1365-313X.2007.03044.x. [DOI] [PubMed] [Google Scholar]
- 54.Kang J, et al. PDR-type ABC transporter mediates cellular uptake of the phytohormone abscisic acid. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2355–2360. doi: 10.1073/pnas.0909222107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quan R, et al. SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell. 2007;19:1415–1431. doi: 10.1105/tpc.106.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu SY, et al. Two Calcium-Dependent Protein Kinases, CPK4 and CPK11, Regulate Abscisic Acid Signal Transduction in Arabidopsis. Plant Cell. 2007;19:3019–3036. doi: 10.1105/tpc.107.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin-Wang K, et al. An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC plant biology. 2010;10:50. doi: 10.1186/1471-2229-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim, J. S., Yamaguchi-Shinozaki, K. & Shinozaki, K. ER-Anchored Transcription Factors bZIP17 and bZIP28 Regulate Root Elongation. Plant physiology, 01414–02017 (2018). [DOI] [PMC free article] [PubMed]
- 59.Billini M, Stamatakis K, Sophianopoulou V. Two members of a network of putative Na+/H+ antiporters are involved in salt and pH tolerance of the freshwater cyanobacterium Synechococcus elongatus. Journal of bacteriology. 2008;190:6318–6329. doi: 10.1128/JB.00696-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wutipraditkul N, et al. Halotolerant cyanobacterium Aphanothece halophytica contains NapA-type Na+/H+ antiporters with novel ion specificity that are involved in salt tolerance at alkaline pH. Applied and environmental microbiology. 2005;71:4176–4184. doi: 10.1128/AEM.71.8.4176-4184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roberts JK, Callis J, Wemmer D, Walbot V, Jardetzky O. Mechanism of Cytoplasmic pH Regulation in Hypoxic Maize Root Tips and Its Role in Survival under Hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Déjardin A, Sokolov LN, Kleczkowski LA. Sugar/osmoticum levels modulate differential abscisic acid-independent expression of two stress-responsive sucrose synthase genes in Arabidopsis. The Biochemical journal. 1999;344(Pt 2):503. [PMC free article] [PubMed] [Google Scholar]
- 63.Matsushima R, Kondo M, Nishimura M, Hara-Nishimura I. A novel ER-derived compartment, the ER body, selectively accumulates a beta-glucosidase with an ER-retention signal in Arabidopsis. The Plant Journal. 2003;33:493–502. doi: 10.1046/j.1365-313X.2003.01636.x. [DOI] [PubMed] [Google Scholar]
- 64.Mu M, et al. Erratum to: Genome-wide Identification and analysis of the stress-resistance function of the TPS (Trehalose-6-Phosphate Synthase) gene family in cotton. BMC genetics. 2016;17:1–1. doi: 10.1186/s12881-015-0265-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leyman B, Thevelein JM, Iturriaga G. The Arabidopsis Trehalose-6-P Synthase AtTPS1 Gene Is a Regulator of Glucose, Abscisic Acid, and Stress Signaling. Plant physiology. 2004;136:3649–3659. doi: 10.1104/pp.104.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ciereszko I, Johansson H, Kleczkowski LA. Sucrose and light regulation of a cold-inducible UDP-glucose pyrophosphorylase gene via a hexokinase-independent and abscisic acid-insensitive pathway in Arabidopsis. Biochem J. 2001;354:67–72. doi: 10.1042/bj3540067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sterling JD, et al. Functional identification of an Arabidopsis pectin biosynthetic homogalacturonan galacturonosyltransferase. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:5236–5241. doi: 10.1073/pnas.0600120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klok EJ, et al. Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell. 2002;14:2481–2494. doi: 10.1105/tpc.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng Y, Liao C, Zhao S, Wang C, Guo Y. The Glycosyltransferase QUA1 Regulates Chloroplast-Associated Calcium Signaling During Salt and Drought Stress in Arabidopsis. Plant & cell physiology. 2017;58:329–341. doi: 10.1093/pcp/pcw192. [DOI] [PubMed] [Google Scholar]
- 70.Hole DJ, Cobb BG, Hole PS, Drew MC. Enhancement of Anaerobic Respiration in Root Tips of Zea mays following Low-Oxygen (Hypoxic) Acclimation. Plant physiology. 1992;99:213–218. doi: 10.1104/pp.99.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ban Y, et al. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant & cell physiology. 2007;48:958–970. doi: 10.1093/pcp/pcm066. [DOI] [PubMed] [Google Scholar]
- 72.Claeyssen E, Wally O, Matton DP, Morse D, Rivoal J. Cloning, expression, purification, and properties of a putative plasma membrane hexokinase from Solanum chacoense. Protein Expression & Purification. 2006;47:329–339. doi: 10.1016/j.pep.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 73.Giaccari A, et al. Relative contribution of glycogenolysis and gluconeogenesis to hepatic glucose production in control and diabetic rats. A re-examination in the presence of euglycaemia. Diabetologia. 1998;41:307–314. doi: 10.1007/s001250050908. [DOI] [PubMed] [Google Scholar]
- 74.Arnon DI. Copper Enzymes in Isolated Chloroplasts. Polyphenoloxidase in Beta Vulgaris. Plant physiology. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Florea L, Li S, Salzberg SL. Thousands of exon skipping events differentiate among splicing patterns in sixteen human tissues. F1000research. 2013;2:188. doi: 10.12688/f1000research.2-188.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang L, Feng Z, Wang X, Wang X, Zhang X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- 77.Mao X, Tao C, Olyarchuk JG, Wei L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005;21:3787–3793. doi: 10.1093/bioinformatics/bti430. [DOI] [PubMed] [Google Scholar]
- 78.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.