Toxic lipid oxidation product (LOP) generation in culinary frying oils (CFOs) during high-temperature frying practices: passage into fried foods
LOPs detectable in fried foods are both cytotoxic and genotoxic, and currently a substantial proportion of the human population regularly consumes such toxins in Western diets. This phenomenon presents some considerable and serious public health concerns, i.e., the continuous and sometimes frequent dietary ingestion of foods deep-fried at high temperature in oxidation-prone unsaturated fatty acid (UFA)-rich CFOs potentially increases the risks of humans to a wide variety of chronic, non-communicable human diseases (NCDs), including cardiovascular diseases (1) and cancer (2). Since polyunsaturated fatty acids (PUFAs) are much more susceptible to thermally-induced oxidation (better described as peroxidation) than monounsaturated ones (MUFAs), CFOs rich in them produce the highest levels of hazardous LOPs during frying episodes, which repetitively escalate with the unfortunately common reuse of such frying media (3,4); passage of such thermally-peroxidised, LOP-containing oils into food matrices during shallow- or deep-frying practices renders them available for human consumption. Contrastingly, saturated fatty acids (SFAs) are extremely resistant to peroxidation, and hence SFA-laden frying media such as coconut oil and animal fat (lard) generate little or no LOPs when exposed to authentic or laboratory-simulated high temperature frying practices [usually at ca. 180 °C (3)].
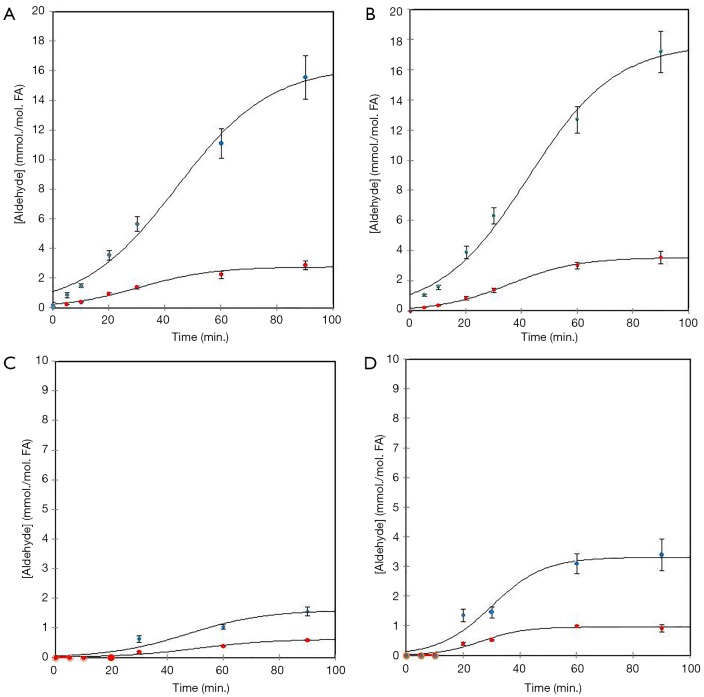
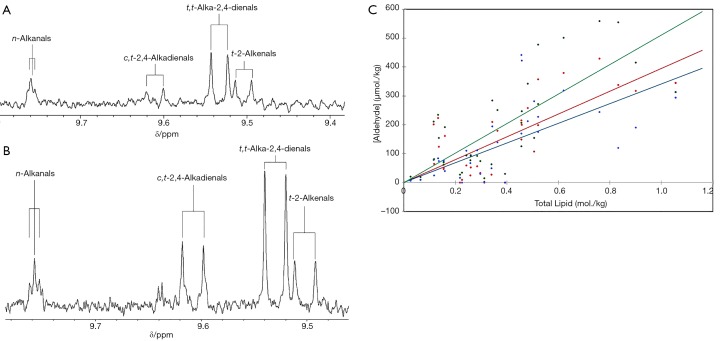
This thermally-promoted lipid peroxidation process involves the autocatalytic free radical-mediated chain generation of lipid hydroperoxides (peroxides), which are sequentially fragmented to a wide series of aldehydes, of which more than 70% comprise the more highly toxic α,β-unsaturated classes (Figure 1). Figure 2A demonstrates the ready penetration of these oil-borne toxins into foods such as potato chips during fast-food restaurant frying episodes. Aldehydic LOPs are also detectable in a range of commercially-available, highly popular packed potato crisp products (Figure 2B). Such toxins predominantly arise from the transfer of thermally-oxidised CFOs containing them into foods throughout shallow- or deep-frying episodes, and our experiments have demonstrated that the aldehyde contents of pre-fried potato chips are strongly related to those of acylglycerols therein (Figure 2C).
Figure 1.
Generation of cytotoxic and genotoxic aldehydic LOPs in CFOs subjected to laboratory-simulated shallow frying episodes. Sigmoidal time-dependence of mean ± SEM total saturated and α,β-unsaturated aldehyde concentrations (red and blue respectively) generated in (A) corn oil, (B) sunflower oil, (C) coconut oil and (D) butter when exposed to LSSFEs for periods of 0–90 min at 180 °C (1H NMR-distinguishable α,β-unsaturated aldehyde classes comprised trans- and cis-2-alkenals, trans,trans- and cis,trans-alka-2,4-dienals, and 4,5-epoxy- 4-hydroxy- and 4-hydroperoxy-trans-2-alkenals, whereas the saturated aldehydes included both long- and short-chain n-alkanals). 1H NMR-determined concentrations units are expressed as mmol. per mol. of oil total FA contents (concentration axis scales for coconut oil and butter are enhanced two-fold). Each 90 min. heating cycle was replicated n=6 times for all oils investigated, and 1H NMR analysis was performed according to a modification of the method reported in (3). The specified total SFA, MUFA and PUFA contents of these oils were 11%, 28% and 61% for sunflower oil; 14%, 24% and 62% for corn oil; 91%, 7% and 2% for coconut oil; and 52%, 21% and 3.5% (w/w) respectively for butter. The sigmoidal relationships between aldehyde class concentrations and heating time-points are consistent with their autocatalytic generation, and the curves fitted correspond to the relationship [Aldehyde] = pr3/[1 + Exp(-pr1-pr2t)], where pr1, pr2 and pr3 represent constants (the latter the maximal saturation concentration value), and t = LSSFE heating time-point. The much lower concentrations of both total saturated and α,β-unsaturated aldehydes generated in coconut oil and butter proportionately reflects the lower PUFA/higher SFA contents of these frying media. LSSFE, laboratory-simulated shallow frying episode; LOP, lipid oxidation product; CFO, culinary frying oil; FA, fatty acid.
Figure 2.
1H NMR analysis monitoring of toxic aldehydes in fried potato chip servings purchased from fast-food restaurants and commercially-available packaged potato crisp products: dependencies on fried food lipid uptake. (A) Expanded aldehydic-CHO proton regions (9.40–9.84 ppm) of the 1H NMR spectra of deuterochloroform (C2HCl3) extracts of a fried potato chip serving purchased from a fast-food restaurant, which contains resonances assignable to trans-2-alkenals, trans,trans- and cis,trans-alka-2,4-dienals, and n-alkanals as major aldehydic LOPs therein; (B) partial 1H NMR spectrum of a corresponding extract of a sample of commercially-available packeted potato crisps. Typical spectra are shown. Extracts were treated with a sufficient level of the chain-breaking antioxidant 2,5-ditertiary-butylhydroquinone prior to NMR analysis. Abbreviations: t-, t,t- and c,t-, trans-, trans,trans- and cis,trans- respectively; δ, chemical shift value (ppm); (C) plots of concentrations of 1H NMR-determined trans-2-akenals (red), trans,trans-alka-2,4-dienals (blue) and n-alkanals (green) in potato chip servings purchased from fast-food restaurants against their corresponding total lipid content (mol./kg); aldehyde concentration values were 1H NMR-determined as total FA equivalents. These data revealed strong correlations between all three aldehyde class levels and fried potato chip lipid contents (r=0.74, 0.62 and 0.73 for trans-2-akenals, trans,trans-alka-2,4-dienals and n-alkanals respectively; P<10−6 for all classes). One mol/kg lipid corresponds to ca. 30% (w/w) acylglycerol equivalents. The total lipid content of different varieties of fresh, unfried potato tubers is negligible, ca. 0.10% (w/w). As an example, in cases where the unfried food contributes little towards the total lipid content of its final fried product serving (as in potato chips), if the total α,β-unsaturated aldehyde content of a used frying oil is only 10.0 mmol/kg, and the final post-frying food product obtained therefrom contains 10.0% (w/w) of uptaken CFO lipids, then there will be 10%×10.0 mmol/kg =1.0 mmol/kg total α,β-unsaturated aldehydes present. However, this assumption precludes the likelihood of possible chemical reactions of these agents with food biomolecules, particularly Michael and/or Maillard reactions with proteins, peptides and free amino acids. Moreover, it also excludes considerations that the thermally-induced peroxidation of UFAs is promoted by food matrix components during frying practices, especially water and catalytic trace metal ions present therein.
Therefore, the purpose of this Editorial is to fully inform readers of these important toxicological and public health concerns. We also attempt to redress the balance on the public conception that PUFA-rich CFOs employed for frying practices have ‘desirable’ health properties, which are at odds with results from our studies, originally published in 1994–1995 (3). Indeed, many health and nutrition authorities worldwide still continue to erroneously recommend such products as the most ‘healthy’ option available for frying purposes.
Adverse health effects of dietary aldehydic LOPs
Typical biomolecularly- and DNA-reactive α,β-unsaturated aldehydes generated during frying practices featuring PUFA-rich CFOs are absorbed from the gut into the systemic circulation in vivo following oral ingestion (5), and these, together with other toxic LOPs, have been proven to promote a broad spectrum of concentration-dependent cellular stresses. Indeed, their adverse health effects include significant contributions towards the development and perpetuation of atherosclerosis and its cardiovascular disease sequelae, mutagenic and carcinogenic properties, teratogenic and neurotoxic actions, potent pro-inflammatory effects, and gastropathic properties subsequent to dietary ingestion, amongst a wide range of others (4). Moreover, numerous animal model studies have demonstrated the hepatotoxic effects of LOP-laden, thermally-oxidised CFOs, for example (6). These studies provide a convincingly high level of evidence for the adverse public health risks presented by these food toxins. Strikingly, DNA-reactive trans,trans-deca-2,4-dienal, a predominant α,β-unsaturated aldehyde derived from the thermally-induced peroxidation of frying oil linoleoylglycerol species, has been considered as a major priority by the National Cancer Institute (NCI) and National Toxicology Program (NTP) at the National Institutes of Environmental Health Sciences (NIEHS) (7). Moreover, previous investigations have demonstrated a powerful association between female lung adenocarcinoma and exposure to cooking oil fumes (8). The very high levels of aldehydes determined in UFA-containing CFOs (Figure 1) represent only those remaining following exposure to frying episodes—significantly high proportions of many of those generated are volatilised at frying temperatures, and this also poses severe health hazards regarding their inhalation by humans, particularly those employed by fast-food outlets with inadequate ventilation precautions.
Experimental animals exposed long-term to formaldehyde and acetaldehyde (low homologue aldehydes also generated during CFO thermo-oxidation) were found to develop malignant tumours. Furthermore, these toxins exert characteristic carcinogenic effects on a range of organs and tissues (9).
A limited human resistance to dietary/environmental aldehydes appears to promote neurological disorders (including Parkinson’s disease, multiple sclerosis and amyotrophic lateral stress), and the adverse build-up of such reactive aldehydes in the CNS and periphery has been detected in these conditions (10). Intriguingly, autism spectrum disorder (ASD) pathogenesis is fully consistent with the deleterious accumulation of aldehydes in vivo, a process which appears to be induced by key enzyme mutations (10, loc cit).
Although currently there is only limited quantitative toxicological data available for aldehydes in general, recommended maximum human daily intake (MHDI) values for one α,β-unsaturated aldehyde, acrolein (also a significant secondary LOP), by the World Health Organisation (WHO) (11) and the Australian Government Department of Health (AGDH) (12) are 525 and 35 µg, respectively, for an average 70 kg human body weight. However, our estimated mean acrolein mass-adjusted contents of the most predominant trans-2-alkenals, trans,trans-alka-2,4-dienals and n-alkanals for typical 154 g servings of potato chips purchased from fast-food restaurants, including ubiquitous large chain global ones, are as high as 1.1–1.2, 1.0–1.1 and 1.0–1.5 mg respectively, values greater or much greater than the above recommended MHDI values set by the WHO and the AGDH respectively (Table 1), and this for only a single portion of this popular fried food! Moreover, these estimates remain conservative, since 300–400 g servings of such potato chips are certainly not uncommon in Western diets.
Table 1. Estimated mean aldehyde masses ± SEM (mg) and contents (ppm) for a typical fried potato chip serving size of 154 g (n=44 samples).
| Parameter | Acylglycerol hydroperoxide aldehyde precursor | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oleoyl- | Linoleoyl- | Linolenoyl- | ||||||||
| trans-2-Decenal | n-Nonanal | trans-2-Octenal | trans,trans-Deca-2,4-dienal | n-Hexanal | trans-2-Pentenal | trans,trans-Hepta-2,4-dienal | Propanal | |||
| Estimated Aldehyde Mass (mg) | 3.13±0.26 | 3.79±0.53 | 2.54±0.37 | 2.92±0.43 | 2.59±0.37 | 1.75±0.25 | 1.90±0.27 | Trace* | ||
| Acrolein Mass- Adjusted Value (mg) | 1.14±0.09 | 1.47±0.21 | 1.12±0.16 | 1.08±0.16 | 1.45±0.21 | 1.23±0.18 | 1.00±0.14 | Trace* | ||
| Estimated Aldehyde Content (ppm) | 20±1.7 | 25±3.5 | 16.5±2.4 | 19±2.8 | 17±2.4 | 11±1.6 | 12±1.7 | Trace* | ||
| Acrolein Mass-Adjusted Content (ppm) | 7.3±0.6 | 9.7±1.4 | 7.3±1.1 | 7.0±1.0 | 9.5±1.4 | 7.7±1.1 | 6.3±0.9 | Trace* | ||
The aldehydes listed correspond to the most predominant ones generated from the high temperature O2-fueled peroxidation of oleoyl-, linoleoyl- and linoleoylglycerol units (3,4). Acrolein mass-equivalent values in both mg and ppm are also provided in view of the availability of MHDI values for this aldehyde from the WHO (11) and AGDH (12), and this permitted direct comparisons between these values and our estimated mean aldehyde contents available for human ingestion. *, Low concentrations of residual, non-volatilised short-chain alkanals such as propanal and n-butanal were also found in some samples, and these serve as markers of linoleoylglycerol peroxidation in CFOs. Total mean ±95% confidence interval contents of trans-2-alkenals, trans,trans-alka-2,4-dienals and n-alkanals were 130±39, 122±38 and 171±49 µmol.kg−1 respectively (n=44 samples). Potato chip servings were purchased from a total of 31 different fast-food restaurants, including large globally-available chain ones.
It should also be noted that these estimated 154 g potato chip serving aldehyde contents are not dissimilar to those arising from the smoking of a (daily) allocation of 25 tobacco cigarettes, i.e., the α,β-unsaturated and saturated aldehydes crotonaldehyde (1.8–5.7 mg) and n-hexanal (2.5–9.5 mg) respectively (13)!
Toxicological significance of fried food aldehyde concentrations: comparisons to those of acrylamide, monochloropropanediol adducts and trans-fatty acids
Concentrations of aldehydes found in deep-fried potato chip samples collected from food service enterprises are much greater than that of the suspected carcinogen acrylamide (also a fertility and nervous system toxin), and also the fatty acid (FA) and glycidyl FA ester (GFAE) adducts of the known carcinogen 3-monochloropropanediol, in both CFOs and fried food sources. Indeed, concentrations of acrylamide in these potato chip foods are principally below the regulatory limit value established by the European Commission (EC), which is only 0.60 ppm for ready-to-eat fries (14), a level substantially lower than the total of that for each aldehyde class found in this fried food source (11–25 ppm, Table 1). Moreover, a recommendation from the Joint Food and Agriculture Organization/WHO Expert Committee on Food Additives (JECFA) indicates a maximum tolerable daily intake of 2 μg/kg of body weight for monochloropropanediol GFAEs, and the French fry product content of these are only 0.10–0.26 ppm (15), values again considerably lower than those of toxic aldehydes found in such foods In view of the vehemently-voiced toxicological concerns about acrylamide and monochloropropanediols present in CFOs and foods fried therein, why are aldehydes, together with further LOPs detectable in these matrices, not given more attention by relevant health authorities, clinicians, nutritionists, toxicologists and food researchers? We also stress that on a mole-for-mole basis, aldehydes derived from CFO PUFA oxidation during frying practices are substantially more toxic than trans-FAs, and also have the capacity to exert a much broader range of toxic effects than the latter process contaminant.
Influence of high temperature frying practices on the ω-6 to ω-3 PUFA ratios of human diets
The ω-6 to ω-3 PUFA ratios of diets, and more especially CFOs, is currently recommended as a valuable health index [desirable values of 2.0–5.1 have been reported (16)]. Since ω-3 PUFAs, particularly linoleoylglycerols in CFOs (present at relatively high levels in canola oil), oxidise and fragment to secondary LOPs much more readily than ω-6 ones, such ratios will conceivably be greater than those of the unused (control) oils if employed for frying practices, especially repeated ones. Clearly, this will limit this index’s value as a recommended health guideline, particularly if significant amounts of dietary PUFAs consumed are in the form of foods loaded with the CFOs in which they have been fried.
Impact of dietary LOP availability on positive health benefits putatively offered by dietary PUFAs
Additionally, these observations on dietary-available LOPs indicate that the wealth of previous scientific reports available which focus on the beneficial health effects of dietary PUFAs, particularly those featuring dietary feeding trials with human participants or experimental animals, or alternative related epidemiological cohort or meta-analysis ones, should be revisited. Indeed, in virtually all of these studies, the potentially confounding adverse health effects associated with the intake of LOPs such as aldehydes, which were undoubtedly present or even prevalent in the PUFA-containing oils explored (most especially when used for frying purposes), have been neglected or more often completely overlooked.
Relevance to the recent PURE cohort epidemiological study (17)
Finally, since diet represents one of the most important changeable risk factors for NCDs, our findings and public health concerns are also relevant to those of the recently reported PURE study (17), which found that neither grand total, nor total SFA, MUFA and PUFA dietary intakes, were associated with cardiovascular disease and myocardial infarction risks, nor cardiovascular disease mortality. Moreover, evidence for inverse relationships between SFA energy intake and non-cardiovascular disease mortality, stroke risk and total mortality was found, as was an inverse relationship between MUFA intake and total mortality. This latter observation is fully consistent with our observations that MUFA-rich cooking oils generate lower levels of fried food-transferable aldehydes than PUFAs when compared on a conditionally-equivalent frying status basis, and is also in accordance with a pooled analysis of two further cohort investigations which revealed a decreasing dependence of total mortality with increasing dietary MUFA energy intake (17, loc cit). However, unfortunately this PURE study did not consider (I) the percentage of acylglycerol FAs consumed as fried foods, and how this proportion varies at the differing global sites explored; (II) factors exerting effects on the structural nature and concentrations of LOPs available in the edible oils consumed at each site, and therefore their potential contributions towards NCD risks; and (III) the intakes of individual, molecularly-defined FAs within each of the SFA, MUFA and PUFA classes considered—the FA classification system employed in (17) represents a very broad generalised one, and thermally-induced formation of LOP toxins enhances with increasing FA unsaturation status. Additionally, the health-promoting effects of medium-chain saturated FAs overlooked in this study, e.g., the rich sources available in coconut oil, are now highly established and extensively reported (18).
Conclusions
In conclusion, exposure of UFA-containing CFOs, especially PUFA-rich ones, to high temperature frying episodes produces substantial, highly toxicologically-significant concentrations of reactive aldehydes, together with additional LOPs, via a complex series of oxidative recycling bursts (3,4). Migration of thermally-stressed, peroxidised frying oils into foods during standard frying practices renders such LOP toxins available for human consumption (Figure 2), and concentrations of trans-2-alkenals, trans,trans-alka-2,4-dienals and n-alkanals present in potato chips obtained from fast-food retailers and further food outlets are all much greater than those of acrylamide and monochloropropanediol GFAEs detectable, for which a verisimilitude of high level public health concerns have been repeatedly stressed in the scientific literature available. In view of our observations, such LOPs are likely to play pivotal roles regarding the development, progression and incidence of wide range of NCDs, which undoubtedly will promote rising healthcare costs worldwide. Indeed, 30–35% of human cancers arising from environmental sources are attributable to diet alone (19), and it is therefore highly conceivable that dietary LOPs may impact significantly on this incidence level.
Hence, exacting efforts to limit the consumption of foods fried in CFOs with high LOP contents are required. Since CFOs rich in peroxidation-resistant MUFAs, and especially SFAs, produce lower and much lower levels of such LOP toxins during frying episodes respectively, they offer safer, health-friendly alternatives to those laden with PUFAs. However, the future consideration, establishment and ratification of currently-unavailable MHDIs for LOPs of known molecular identities also represent major demands for action. Consumer concerns regarding the nutritional and health properties of their foods strongly support such requirements.
Acknowledgements
BCP is very grateful to De Montfort University, Leicester, UK for the award of a Fees-Waiver PhD Scholarship Bursary.
Provenance: This is an invited Editorial commissioned by Editor-in-Chief Yilei Mao (Department of Liver Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Beijing, China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Iqbal R, Anand S, Ounpuu S, et al. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation 2008;118:1929-37. 10.1161/CIRCULATIONAHA.107.738716 [DOI] [PubMed] [Google Scholar]
- 2.Stott-Miller M, Neuhouser ML, Stanford JL. Consumption of deep-fried foods and risk of prostate cancer. Prostate 2013;73:960-9. 10.1002/pros.22643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haywood RM, Claxson AW, Hawkes GE, et al. Detection of aldehydes and their conjugated hydroperoxydiene precursors in thermally-stressed culinary oils and fats: investigations using high resolution proton NMR spectroscopy. Free Radic Res 1995;22:441-82. 10.3109/10715769509147552 [DOI] [PubMed] [Google Scholar]
- 4.Grootveld M, Silwood C, Addis PB, et al. Health effects of oxidised heated oils. Foodservice Res Internat 2006;13:41-55. 10.1111/j.1745-4506.2001.tb00028.x [DOI] [Google Scholar]
- 5.Grootveld M, Atherton MD, Sheerin AN, et al. In vivo absorption, metabolism, and urinary excretion of alpha,beta-unsaturated aldehydes in experimental animals. Relevance to the development of cardiovascular diseases by the dietary ingestion of thermally stressed polyunsaturate-rich culinary oils. J Clin Invest 1998;101:1210-8. 10.1172/JCI1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perumalla Venkata R, Subramanyam R. Evaluation of the deleterious health effects of consumption of repeatedly heated vegetable oil. Toxicol Rep 2016;3:636-43. 10.1016/j.toxrep.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Toxicology Program, P. H. S., National Institutes of Health, U.S. Department of Health and Human Services (1993). 2,4-Decadienal CAS No. 25152–84–5. Testing Status of Agents at NTP. Available online: https://ntp.niehs.nih.gov/ntp/htdocs/st_rpts/tox076.pdf
- 8.Ko YC, Cheng LS, Lee CH, et al. Chinese food cooking and lung cancer in women nonsmokers. Am J Epidemiol 2000;151:140-7. 10.1093/oxfordjournals.aje.a010181 [DOI] [PubMed] [Google Scholar]
- 9.Soffritti M, Belpoggi F, Lambertini L, et al. Results of long-term experimental studies on the carcinogenicity of formaldehyde and acetaldehyde in rats. Ann N Y Acad Sci 2002;982:87-105. 10.1111/j.1749-6632.2002.tb04926.x [DOI] [PubMed] [Google Scholar]
- 10.Matveychuk D, Dursun SM, Wood PL, et al. Reactive aldehydes and neurodegenerative disorders. Bull Clin Psychopharmacol 2011;21:277-88. 10.5455/bcp.19691231040000 [DOI] [Google Scholar]
- 11.Gomes DR, Meek ME. Concise International Chemical Assessment Document 43 ACROLEIN. Geneva: World Health Organization, 2002. [Google Scholar]
- 12.Acceptable Daily Intakes for Agricultural and Veterinary Chemicals: Current as of 31 March 2016, Australian Government Department of Health (Commonwealth of Australia, 2016). The Office of Chemical Safety, Department of Health, MDP 71, GPO Box 9848, CANBERRA ACT 2601 (2016). Available online: https://jr.chemwatch.net/galleria/LEGSREGS/40-1-11-356-61-3-AA-20160601.pdf
- 13.van Andel I, Sleijffers A, Schenk E, et al. RIVM report 340630002/2006. Adverse health effects of cigarette smoke: aldehydes crotonaldehyde, butyraldehyde, hexanal, and malonaldehyde. Available online: https://www.rivm.nl/bibliotheek/rapporten/340630002.pdf
- 14.Powers SJ, Mottram DS, Curtis A, et al. Acrylamide levels in potato crisps in Europe from 2002 to 2016. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2017;34:2085-100. 10.1080/19440049.2017.1379101 [DOI] [PubMed] [Google Scholar]
- 15.Zelinková Z, Doležal M, Velíšek J. 3-Chloropropane-1,2-diol fatty acid esters in potato products. Czech J Food Sci 2009;27:S421-4. 10.17221/1065-CJFS [DOI] [Google Scholar]
- 16.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 2002;56:365-79. 10.1016/S0753-3322(02)00253-6 [DOI] [PubMed] [Google Scholar]
- 17.Dehghan M, Mente A, Zhang X, et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017;390:2050-62. 10.1016/S0140-6736(17)32252-3 [DOI] [PubMed] [Google Scholar]
- 18.Bengmark S. Choose right carbohydrates and right fats (RCRF) - keys to optimal health. Hepatobiliary Surg Nutr 2017;6:429-33. 10.21037/hbsn.2017.12.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colditz GA, Sellers TA, Trapido E. Epidemiology - identifying the causes and preventability of cancer? Nat Rev Cancer 2006;6:75-83. 10.1038/nrc1784 [DOI] [PubMed] [Google Scholar]