Abstract
Objective:
Far infrared radiation has been widely used in a variety of healthcare institutions and clinical research. Previous studies have shown that far infrared radiation can promote blood circulation and enhance the functioning of the immune system. Many patients receiving peritoneal dialysis have been co-treated with far infrared radiation to reduce the occurrence of inflammation. This study seeks to evaluate the effects of far infrared radiation therapy on inflammation.
Method:
We used the lipopolysaccharide-induced peritonitis mouse model to study the effect of far infrared radiation treatment. Sixteen mice were randomly divided into two groups, a far infrared radiation treatment group (n = 8) and a non-far infrared radiation treatment group (n = 8). Collected blood samples were studied by analyzing the RNA level of peripheral blood mononuclear cells and the plasma protein levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and endothelial nitric oxide synthase (eNOS).
Results:
The administration of far infrared radiation inhibited the RNA levels of interleukin-6 and TNF-α after stimulation by lipopolysaccharide. The far infrared radiation treatment inhibited the endothelial nitric oxide synthase RNA levels at 1 h, but the RNA levels returned close to the baseline level after 2 h. In the control group, the endothelial nitric oxide synthase RNA levels were continuously decreasing. The interleukin-6 concentration in the plasma of the far infrared radiation group showed significant inhibition 30 min after lipopolysaccharide stimulation. The tumor necrosis factor alpha RNA concentration in plasma of the far infrared radiation group was significantly reduced 2 h after lipopolysaccharide stimulation.
Conclusion:
Far infrared radiation therapy can inhibit interleukin-6 and tumor necrosis factor alpha RNA levels of peripheral blood mononuclear cells and recover endothelial nitric oxide synthase expression. These results demonstrate that far infrared radiation therapy might aid in reducing the level of inflammation experienced by patients going through peritoneal dialysis treatment.
Keywords: Peritoneal dialysis, far-infrared radiation, peritonitis
Introduction
Peritoneal dialysis (PD) is a successful therapy, the outcomes of which are equal to those of hemodialysis (HD); however, PD can be administered as a home-based treatment which gives it the added advantage of maintaining the patient’s quality of life while undergoing the treatment. Despite these facts, many patients drop out or transfer to HD therapy because of peritonitis.1,2 Severe and prolonged peritonitis can lead to peritoneal membrane failure and this increases the odds for the subsequent development of encapsulating peritoneal sclerosis.3 Peritonitis can lead to infection-related mortality in PD patients 18% of the time.4 Therefore, it is important to focus attention on prevention and treatment of PD-related peritonitis, including the rapid reduction of inflammation and the conservation of peritoneal membrane functions.
Far infrared radiation (FIR) is a region in the infrared spectrum of electromagnetic radiation with a wavelength of 50.0–1000.0 μm.5 Only in the region of FIR can transfer energy be perceived as pure radiant heat by thermoreceptors in the human skin.6 There are many biological effects of FIR therapy, including the following: inducing accelerated recovery of skeletal muscle function after exercise, increasing arterial and peripheral blood flow in the lymphatic vessels, improving endothelial function and decreasing pain, inflammation, and oxidative stress.5,7–13 This noninvasive and convenient therapy has been shown to be an effective treatment for improving access flow and patency of arteriovenous fistulas (AVFs) in HD patients.14 Studies have also demonstrated that FIR promotes microvascular blood flow and angiogenesis in various animal models.15,16 FIR may produce an anti-inflammatory effect on joints by reversing lipopolysaccharide (LPS)-induced arthritis and relieving inflammation.17,18 It has also been shown to inhibit vascular inflammation by inducing heme oxygenase-1 (HO-1), which in turn increases skin microcirculation.19 The expression of HO-1 and endothelial nitric oxide synthase (eNOS) was induced by nitro-oleic acid, an anti-inflammatory, both in vitro and in vivo that can in turn mediate the anti-inflammatory actions.20 Moreover, eNOS synthase nitric oxide (NO) regulates vascular tone and permeability,21 so it appears to be closely associated with vascular endothelial function.22 Given these positive effects upon the human body, FIR has become a promising treatment option for certain medical conditions.5
There are clinical reports investigating the dose of FIR needed to improve the dialysis treatment in HD or PD patients;14,23–26 however, there are few clinical reports investigating FIR’s effects on peritonitis. In this study, we assessed the biological effects of FIR on the LPS-induced peritonitis mouse model by analyzing the RNA and protein levels of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and endothelial nitric oxide synthase (eNOS).
Materials and methods
Animals
Male ICR mice (8–10 weeks old, weighing 25 ± 3 g) were obtained from BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan) and were acclimated for at least 1 week before use. All mice were housed in a room with a 12-h light/dark cycle and fed ad libitum with free access to clean drinking water at a temperature of 22~24°C and a humidity of 50 ± 5%. Before starting the experiment, mice were randomly divided into two groups (n = 8 mice/group), the FIR treatment group and the control group, which would not receive FIR treatment. All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committees (IACUC).
Peritonitis induction
LPS was purchased from Sigma-Aldrich (St. Louis, MO, USA) and prepared in sterile phosphate-buffered saline (PBS) before use. To induce peritonitis, all mice were given intraperitoneal injections of LPS (100 μg/kg).27,28 The FIR treatment group mice were transferred to a plastic cage under a TY-201 therapy unit (WS Far-Infrared Medical Technology Co., Ltd., Taipei, Taiwan). The top radiator of the unit was positioned 25 cm above the mice. The FIR treated mice received radiation for 15 min every 30 min over the course of 2 h. The control group was housed in the same space as the FIR group, but did not undergo FIR radiation treatment.
The baseline blood samples were collected via the facial vein the day before the experiment began. Other blood samples were also collected via the facial vein every 30 min after LPS injection. The sample collecting tubes contained ethylenediaminetetraacetic acid (EDTA) as an anticoagulant, so that the blood could be separated into plasma and blood cells by centrifuge for further experiments.
RNA quantification
Peripheral blood mononuclear cells (PBMCs) were separated immediately after facial vein collection. The whole blood cells were centrifuged for 30 min at 500 g without stop, at room temperature. The PBMC-containing band was aspirated and stored at −80°C until use. Total RNA was obtained from the blood cells using RNAzol BD reagent (Molecular Research Center, Inc., Cincinnati, OH, USA) according to the manufacturer’s instructions. RNA was quantified by ultraviolet (UV) absorbance at 260 nm. First-strand cDNAs were synthesized from 3 μg of total RNA using a First Strand cDNA Synthesis Kit (Invitrogen, Carlsbad, CA, USA). Real-time polymerase chain reaction (PCR) and quantitative PCR (qPCR) analyses were performed in triplicate in 96-well reaction plates with the Maxima SYBR Green qPCR Master Mix (2X; Thermo Fisher Scientific, Waltham, MA, USA). The amplification was carried out in the ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Primers used for qPCR amplification of IL-6, TNF-α, and eNOS can be seen in Table 1. The results of the mRNA levels were normalized to GAPDH expression in each sample and relative quantification (ΔΔCt) showed expression of target genes.29
Table 1.
List of primers used for real-time qPCR analysis.
| Gene | Primers | Amplicon | |
|---|---|---|---|
| GAPDH | Forward | 5'-TGCACCACCAACTGCTTAG-3' | 187 |
| Reverse | 5'-GGATGCAGGGATGATGTTC-3' | ||
| IL-6 | Forward | 5'-CTCTGGGAAATCGTGGAAAT-3' | 134 |
| Reverse | 5'-CCAGTTTGGTAGCATCCATC-3' | ||
| TNF-α | Forward | 5'-ATGAGAAGTTCCCAAATGGC-3' | 125 |
| Reverse | 5'-CTCCACTTGGTGGTTTGCTA-3' | ||
| eNOS | Forward | 5'-TCCGGAAGGCGTTTGATC-3' | 101 |
| Reverse | 5'-GCCAAATGTGCTGGTCACC-3' | ||
qPCR: quantitative polymerase chain reaction.
Detection of cytokine production
Plasma samples were used to measure IL-6, TNF-α, and eNOS levels using commercially available enzyme-linked immunosorbent assay (ELISA) kits. The IL-6 and TNF-α ELISA kits were purchased from 4Abio (Beijing, China) and the eNOS ELISA kit was purchased from MyBiosource (San Diego, CA, USA). The procedures were performed according to the manufacturers’ instructions. All assays were performed in triplicate. The concentration of each protein was calculated from the standard curve.
Statistics
The data depicted represent eight independent experiments. Triplicate samples were run for all qPCR, with an ELISA for each individual experiment: results are shown with the mean ± SD. Statistical analyses were performed using the two-tailed paired t-test; p values of <0.05 were considered significant, with all statistics using GraphPad Prism 7.0 software.
Results
To determine the effect of FIR radiation, the LPS-induced peritonitis mice were analyzed for RNA levels and plasma protein concentrations of IL-6, TNF-α, and eNOS.
RNA levels analysis
Table 2 summarizes the findings of the qPCR and the resulting mRNA levels measured. The ΔCT values of IL-6, TNF-α, and eNOS were obtained by normalizing the CT value to an internal control GAPDH. The fold of baseline values is calculated by the relative quantification ΔΔCt method.29 A continuous rise in the IL-6 RNA levels was observed in the control group, post-LPS-induction, from the time of 30–90 min, and the levels remained high (8.57-fold) for 2 h. Remarkably, the FIR group IL-6 RNA levels only showed a slight increase (2.50-fold) after 1 h and then returned to the baseline level 2 h after LPS-induced stimulation. Previously studies have shown that LPS could induce IL-6 and TNF-α RNA level.30 Interestingly, the RNA levels of TNF-α in the FIR group were not affected by LPS stimulation, but remained steady at the baseline level (Figure 1(b)). However, a significant increase in the TNF-α RNA levels of the control group was found at 30, 60, and 90 min after LPS stimulation. The data collected via these trials seem to indicate that FIR therapy could reduce or inhibit the production of the cytokines, IL-6 and TNF-α, according to the expression level of PBMCs.
Table 2.
Expression levels of IL-6, TNF-α, and eNOS normalized with GAPDH using the comparative CT method.
| Gene | Time | ΔCTa | Mean ΔΔCTb | Fold of baseline | |
|---|---|---|---|---|---|
| IL-6 | Control | Baseline | 10.88 ±1.74 | – | – |
| 0.5 | 8.87 ± 1.64 | −2.02 ± 1.64 | 4.05 | ||
| 1 | 8.39 ± 1.51 | −2.50 ± 1.51 | 5.64 | ||
| 1.5 | 7.68 ± 1.43 | −3.20 ± 1.43 | 9.19 | ||
| 2 | 7.79 ± 1.44 | −3.10 ± 1.44 | 8.57 | ||
| FIR | Baseline | 11.06 ± 2.33 | – | – | |
| 0.5 | 11.00 ± 2.95 | −0.06 ± 2.95 | 1.04 | ||
| 1 | 9.74 ± 1.95 | −1.32 ± 1.95 | 2.50 | ||
| 1.5 | 9.97 ± 2.62 | −1.09 ± 2.62 | 2.13 | ||
| 2 | 11.25 ± 3.51 | 0.20 ± 3.51 | 0.87 | ||
| TNF-α | Control | Baseline | 10.85 ± 2.41 | – | – |
| 0.5 | 10.23 ± 2.72 | −0.58 ± 2.72 | 1.50 | ||
| 1 | 9.86 ± 2.63 | −0.76 ± 2.63 | 1.70 | ||
| 1.5 | 9.86 ± 1.49 | −0.93 ± 1.49 | 1.90 | ||
| 2 | 10.23 ± 1.75 | −0.53 ± 1.75 | 1.44 | ||
| FIR | Baseline | 9.08 ± 2.13 | – | – | |
| 0.5 | 9.50 ± 2.23 | 0.42 ± 2.23 | 0.75 | ||
| 1 | 9.41 ± 2.55 | 0.33 ± 2.55 | 0.80 | ||
| 1.5 | 9.54 ± 2.72 | 0.46 ± 2.72 | 0.73 | ||
| 2 | 9.81 ± 2.08 | 0.73 ± 2.08 | 0.60 | ||
| eNOS | Control | Baseline | 6.84 ± 1.00 | – | – |
| 0.5 | 6.86 ± 0.96 | 0.02 ± 1.96 | 0.98 | ||
| 1 | 6.98 ± 1.48 | 0.15 ± 1.48 | 0.90 | ||
| 1.5 | 7.36 ± 1.03 | 0.79 ± 1.03 | 0.58 | ||
| 2 | 7.95 ± 1.03 | 1.12 ± 1.03 | 0.46 | ||
| FIR | Baseline | 6.71 ± 0.72 | – | – | |
| 0.5 | 7.23 ± 0.65 | 0.52 ± 0.65 | 0.70 | ||
| 1 | 7.34 ± 0.62 | 0.63 ± 0.62 | 0.65 | ||
| 1.5 | 7.14 ± 1.95 | 0.43 ± 1.95 | 0.74 | ||
| 2 | 6.93 ± 1.26 | 0.22 ± 1.26 | 0.86 | ||
The ΔCT value is determined by subtracting the GAPDH CT value from the IL-6, TNF-α and eNOS CT values. Results are expressed as mean ± SEM (n = 8).
The calculation of ΔΔCT involves the subtraction of the baseline ΔCT value. This is a subtraction of an arbitrary constant, so the SD of ΔΔCT is the same as the SD of the ΔCT value.
The fold relative to the baseline was determined by evaluating the expression of the 2-ΔΔCT method.
Figure 1.
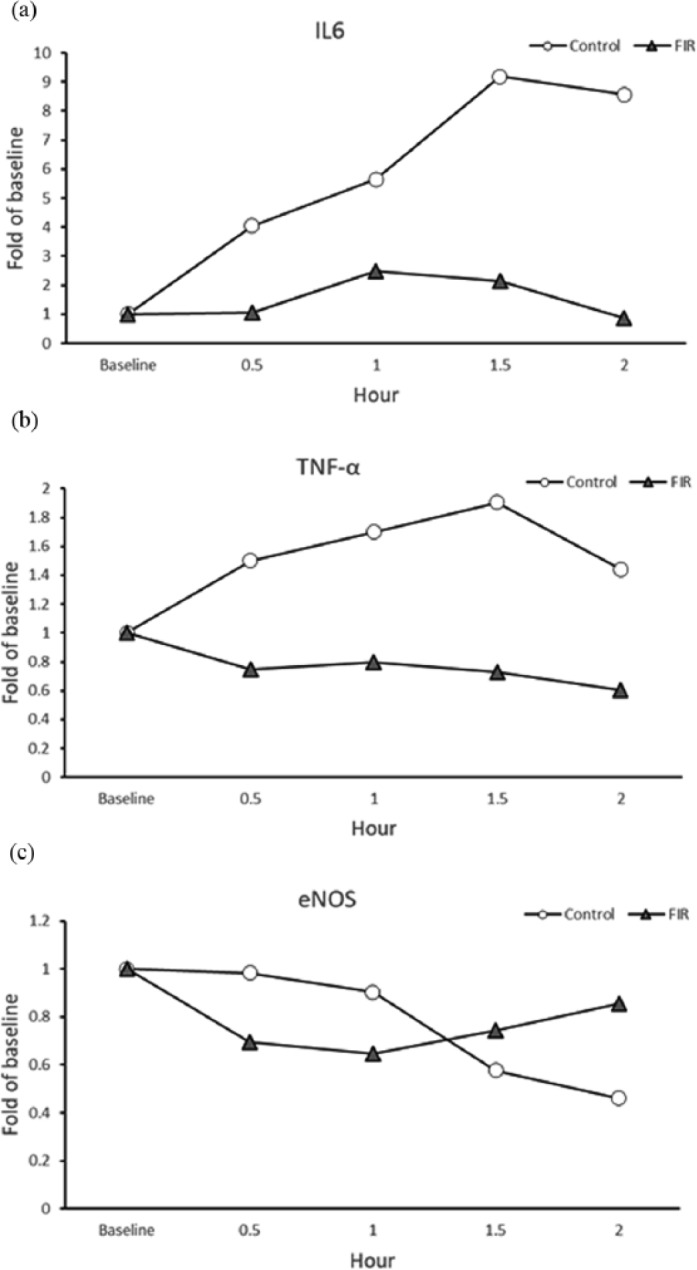
Comparative real-time PCR results were expressed in (a) IL-6, (b) TNF-α, and (c) eNOS relative to baseline levels. The controls and FIR treatment blood samples were collected from LPS-induced peritonitis mice at baseline, 0.5, 1, 1.5, and 2 h post-LPS intraperitoneal injection. The fold relative from baseline was determined by evaluating the expression of the 2-ΔΔCT method.
The RNA levels of eNOS were inhibited 0.65-fold compared with the baseline level of the FIR group at 1 h after the LPS intraperitoneal injection, but the eNOS expression began to appear at 1.5–2 h (Figure 1(c)). The RNA levels of eNOS of the FIR group returned to the baseline level after 2 h, but the RNA levels of eNOS of the control group continuously decreased after LPS stimulation.
Plasma protein levels analysis
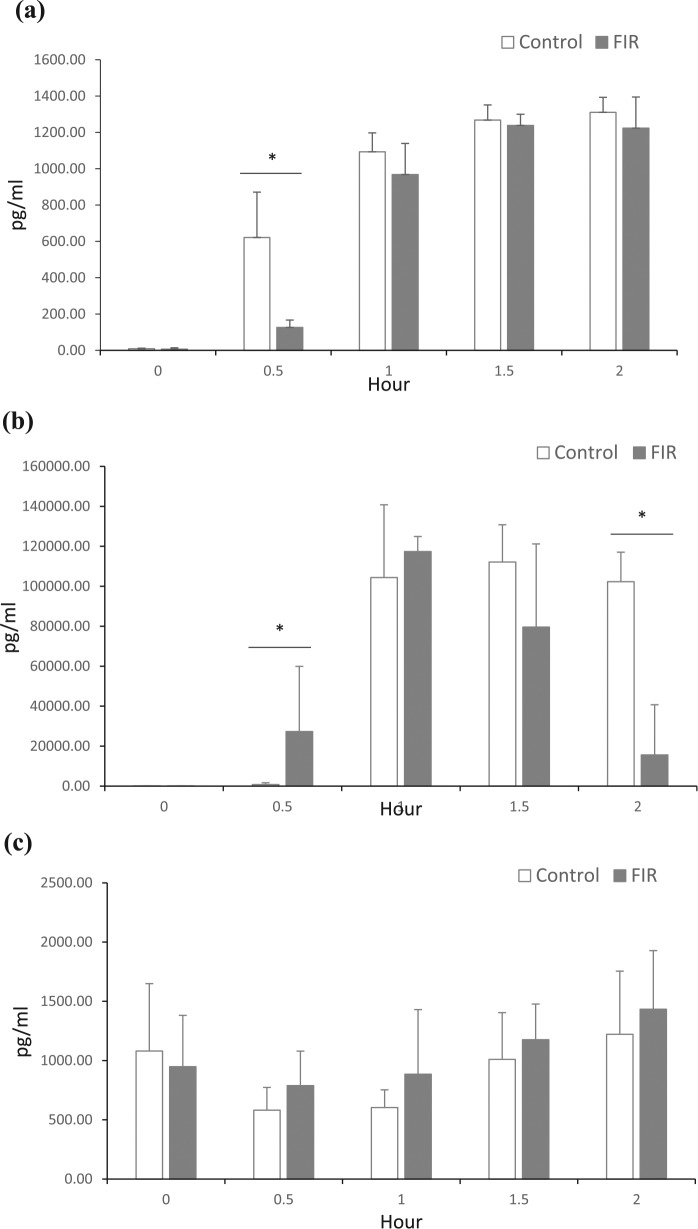
To further examine the effect of FIR on LPS stimulated IL-6, TNF-α, and eNOS response, the concentration of each protein within the plasma was measured with the ELISA method. The FIR treatment group significantly reduced the IL-6 level rise after 30 min compared with the control group (p < 0.05; Figure 2(a)). The concentration of FIR group TNF-α rose faster than the control group after 30 min (Figure 2(b)). The TNF-α levels of both the FIR and the control group reached their maximum level in the plasma from 1 to 2 h after LPS stimulation, while the FIR group’s levels started to decrease at 1.5–2 h. In addition, the TNF-α levels of the FIR group were significantly reduced at 2 h compared with the control group (p < 0.05).
Figure 2.
Protein level responses in LPS-induced peritonitis in the control and FIR treatment group mice. Plasma was collected at baseline, 0.5, 1, 1.5, and 2 h post-LPS intraperitoneal injection and measured by ELISA for (a) IL-6, (b) TNF-α, and (C) eNOS.
Results are expressed as the mean ± SEM from eight mice (n = 8) per time point.
*p < 0.05 indicates statistically significant results between the control and the FIR group.
The FIR treatment did not show a significant effect on plasma concentration of eNOS compared with the control group (Figure 2(c)); yet there were slightly higher levels at 1.5–2 h compared to the baseline measurement.
Discussion
We assessed PBMCs’ RNA levels and serum protein levels for the cytokines, IL-6, TNF-α, and eNOS after stimulation by LPS in two mice populations, a FIR treatment group and a control group, which did not receive FIR treatment. The FIR treatment inhibited IL-6 and TNF-α RNA levels in PBMCs, with only a delayed increased stimulation of the IL-6 protein level in plasma in the first 30 min. IL-6 and TNF-α both play key roles in the inflammatory cascade. The mortality rate of HD patients is significantly proportional to their plasma IL-6 levels.31,32 Therefore, a treatment to inhibit and reduce IL-6 and TNF-α will improve dialysis patient care.
In this study, we found that the FIR treatment inhibited the IL-6 RNA levels of PBMCs and delayed the increased stimulation of IL-6 levels in plasma. We understand that IL-6 is synthesized with local stimulation in the initial stages of inflammation and then moves to the liver through the bloodstream, followed by the rapid induction of several acute phase proteins.33 IL-6 functions as a mediator for notification and sends out a warning signal to the entire body, so that the delayed increase of IL-6 with FIR treatment may ease the acute phase inflammatory response.
TNF-α is involved in systemic inflammation and is one of the mediators of acute phase reaction. It has been reported that PBMCs stimulated by LPS steadily increase the net production of TNF-α.34 The excessive production of TNF-α can trigger detrimental systemic effects by acutely precipitating a syndrome similar to that of septic shock.35 Our study reveals that FIR treatment can reduce TNF-α levels, which may help lower the inflammation response and prevent excessive production of TNF-α. The TNF-α levels of the FIR group increased faster than the control group at 30 min after LPS stimulation, but the FIR group’s TNF-α levels were reduced significantly at 2 h after LPS stimulation. Although few studies have investigated the relationship between FIR treatment and inflammation, one such study demonstrated that the stimulation of HO-1 expression leads to the inhibition of TNF-α expression in HD patients.19 The inhibition of TNF-α activity in PBMCs and plasma concentration within the FIR treatment group reveals some concerning aspects regarding its therapeutic mechanisms.
Similar to the TNF-α levels of the FIR group, the PBMCs` IL-6 RNA level was suppressed with FIR treatment, but the plasma protein level of IL-6 only delayed the increased stimulation during the first 30 min. TNF-α is produced mainly by PBMCs, like macrophages and T cells, but it is also produced by other cell types, such as fibroblasts.36 IL-6 is produced by PBMCs as well as various tissues, so its concentration in plasma is derived not only from PBMCs but also from cells inside their complex cellular and humoral network.37 The reduced and delayed IL-6 levels in PBMCs and plasma found in the FIR treatment group requires further investigation to assess the full impact of FIR treatment on inflammation.
The cytokines, IL-6 and TNF-α, are elevated in most inflammatory states and have been recognized as targets of therapeutic intervention. Our results show the effects of FIR treatment in reducing cytokine levels in the LPS-induced peritonitis mouse model. These results provide therapeutic potential because in continuous ambulatory PD patients, TNF-α and IL-6 are markedly elevated in the acute stage of peritonitis.38,39
In this study, the eNOS protein levels in the plasma did not respond significantly to FIR treatment, but had the subsequent effect of returning to baseline levels of RNA in PBMCs compared to the control group. Long-term FIR therapy is reported to significantly upregulate eNOS mRNA and protein expression as well as serum NO production.15 The production of eNOS facilitates macrophage expression of inducible nitric oxide synthase (iNOS) in response to LPS, which stimulates production of NO, which is involved in antibacterial defense,40 preventing the progression of atherosclerosis.41
The limitations to this study are the physiological inflammatory response and cytokine markers have a relative rather than absolute relationship. Many cytokines, like IL-6, have pleiotropic effects, both proinflammatory and anti-inflammatory, that bring the host back to homeostasis. The results need to be followed up by further investigation in future studies using histological methods and clinical trials.
Conclusion
In continuous ambulatory PD patients, the TNF-α and IL-6 levels markedly increase in the acute stage of peritonitis.38,39 They are elevated in most inflammatory states and have been recognized as targets of therapeutic intervention. Our study found that FIR treatment can inhibit or reduce IL-6 and TNF-α activity, and can stabilize eNOS expression. This study reveals that FIR therapy affects cytokine concentrations in plasma and that FIR therapy has the potential to reduce inflammation and maintain vascular endothelial health and function.
Acknowledgments
The authors thank Hess International Educational Group for editing the manuscript for English grammar and usage. English Editing Certificate Project Number: 10402.
Footnotes
Animal welfare: The present study followed a protocol reviewed and approved by the Institutional Animal Care and Use Committees or Panel (IACUC/IACUP) of Shin-Kong Wu Ho-Su Memorial Hospital (Taipei, Taiwan) for humane animal treatment and complied with relevant legislation. IACUC Approval No: MOST 1070004
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval was not sought for the present study because it is an animal study. The present study followed a protocol reviewed and approved by the Institutional Animal Care and Use Committees or Panel (IACUC/IACUP) of Shin-Kong Wu Ho-Su Memorial Hospital (Taipei, Taiwan) for humane animal treatment and complied with relevant legislation. IACUC Approval No: MOST 1070004.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a Grant (RD1040085-1041A01) from Mackay Medical College.
ORCID iD: Yuanmay Chang  https://orcid.org/0000-0001-9537-4344
https://orcid.org/0000-0001-9537-4344
References
- 1. McDonald S, Chang S, Excell L. Peritoneal dialysis [appendix 1] ANZDATA Registry report 2007. Adelaide, SA, Australia: Australia and New Zealand Dialysis and Transplant Registry. [Google Scholar]
- 2. Mactier R. Peritonitis is still the achilles’ heel of peritoneal dialysis. Perit Dial Int 2009; 29: 262–266. [PubMed] [Google Scholar]
- 3. Kawanishi H, Kawaguchi Y, Fukui H, et al. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis 2004; 44: 729–737. [PubMed] [Google Scholar]
- 4. Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30: 393–423. [DOI] [PubMed] [Google Scholar]
- 5. Vatansever F, Hamblin MR. Far infrared radiation (FIR): its biological effects and medical applications. Photonics Lasers Med 2012; 4: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Plaghki L, Decruynaere C, Van Dooren P, et al. The fine tuning of pain thresholds: a sophisticated double alarm system. PLoS ONE 2010; 5: e10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hausswirth C, Louis J, Bieuzen F, et al. Effects of whole-body cryotherapy vs. far-infrared vs. passive modalities on recovery from exercise-induced muscle damage in highly-trained runners. PLoS ONE 2011; 6: e27749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beever R. Far-infrared saunas for treatment of cardiovascular risk factors: summary of published evidence. Can Fam Physician 2009; 55: 691–696. [PMC free article] [PubMed] [Google Scholar]
- 9. Inoue S, Takemoto M, Chishaki A, et al. Leg heating using far infra-red radiation in patients with chronic heart failure acutely improves the hemodynamics, vascular endothelial function, and oxidative stress. Intern Med 2012; 51: 2263–2270. [DOI] [PubMed] [Google Scholar]
- 10. Kihara T, Biro S, Imamura M, et al. Repeated sauna treatment improves vascular endothelial and cardiac function in patients with chronic heart failure. J Am Coll Cardiol 2002; 39: 754–759. [DOI] [PubMed] [Google Scholar]
- 11. Akasaki Y, Miyata M, Eto H, et al. Repeated thermal therapy up-regulates endothelial nitric oxide synthase and augments angiogenesis in a mouse model of hindlimb ischemia. Circ J 2006; 70: 463–470. [DOI] [PubMed] [Google Scholar]
- 12. Ise N, Katsuura T, Kikuchi Y, et al. Effect of far-infrared radiation on forearm skin blood flow. Ann Physiol Anthropol 1987; 6: 31–32. [DOI] [PubMed] [Google Scholar]
- 13. Imamura M, Biro S, Kihara T, et al. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol 2001; 38: 1083–1088. [DOI] [PubMed] [Google Scholar]
- 14. Lin CC, Chang CF, Lai MY, et al. Far-infrared therapy: a novel treatment to improve access blood flow and unassisted patency of arteriovenous fistula in hemodialysis patients. J Am Soc Nephrol 2007; 18: 985–992. [DOI] [PubMed] [Google Scholar]
- 15. Ikeda Y, Biro S, Kamogawa Y, et al. Repeated sauna therapy increases arterial endothelial nitric oxide synthase expression and nitric oxide production in cardiomyopathic hamsters. Circ J 2005; 69: 722–729. [DOI] [PubMed] [Google Scholar]
- 16. Yu SY, Chiu JH, Yang SD, et al. Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol Photoimmunol Photomed 2006; 22: 78–86. [DOI] [PubMed] [Google Scholar]
- 17. Podolin PL, Bolognese BJ, Foley JJ, et al. A potent and selective nonpeptide antagonist of CXCR2 inhibits acute and chronic models of arthritis in the rabbit. J Immunol 2002; 169: 6435–6444 [DOI] [PubMed] [Google Scholar]
- 18. Leung TK, Chen CH, Lai CH, et al. Bone and joint protection ability of ceramic material with biological effects. Chin J Physiol 2012; 55: 47–54. [DOI] [PubMed] [Google Scholar]
- 19. Lin CC, Liu XM, Peyton K, et al. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler Thromb Vasc Biol 2008; 28: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khoo NK, Rudolph V, Cole MP, et al. Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids. Free Radic Biol Med 2010; 48: 230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kone BC. Nitric oxide in renal health and disease. Am J Kidney Dis 1997; 30: 311–333. [DOI] [PubMed] [Google Scholar]
- 22. Su JB. Vascular endothelial dysfunction and pharmacological treatment. World J Cardiol 2015; 7: 719–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin CC, Chung MY, Yang WC, et al. Length polymorphisms of heme oxygenase-1 determine the effect of far-infrared therapy on the function of arteriovenous fistula in hemodialysis patients: a novel physicogenomic study. Nephrol Dial Transplant 2013; 28: 1284–1293. [DOI] [PubMed] [Google Scholar]
- 24. Choi SJ, Cho EH, Jo HM, et al. Clinical utility of far-infrared therapy for improvement of vascular access blood flow and pain control in hemodialysis patients. Kidney Res Clin Pract 2016; 35: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin C-H, Lee L-S, Su L-H, et al. Thermal therapy in dialysis patients—a randomized trial. Am J Chin Med 2011; 39: 839–851. [DOI] [PubMed] [Google Scholar]
- 26. Lai CC, Fang HC, Mar GY, et al. Post-angioplasty far infrared radiation therapy improves 1-year angioplasty-free hemodialysis access patency of recurrent obstructive lesions. Eur J Vasc Endovasc Surg 2013; 46: 726–732. [DOI] [PubMed] [Google Scholar]
- 27. Hansen MK, Nguyen KT, Fleshner M, et al. Effects of vagotomy on serum endotoxin, cytokines, and corticosterone after intraperitoneal lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol 2000; 278: R331–R336. [DOI] [PubMed] [Google Scholar]
- 28. Veloso CC, Cabral LDM, Bitencourt AD, et al. Anti-inflammatory and antinociceptive effects of the hydroethanolic extract of the flowers of Pyrostegia venusta in mice. Rev Bras Farmacogn 2012; 22: 162–168. [Google Scholar]
- 29. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 30. Song R, Kim J, Yu D, et al. Kinetics of IL-6 and TNF-alpha changes in a canine model of sepsis induced by endotoxin. Vet Immunol Immunopathol 2012; 146: 143–149. [DOI] [PubMed] [Google Scholar]
- 31. Hasuike Y, Nonoguchi H, Ito K, et al. Interleukin-6 is a predictor of mortality in stable hemodialysis patients. Am J Nephrol 2009; 30: 389–398. [DOI] [PubMed] [Google Scholar]
- 32. Navasa M, Follo A, Filella X, et al. Tumor necrosis factor and interleukin-6 in spontaneous bacterial peritonitis in cirrhosis: relationship with the development of renal impairment and mortality. Hepatology 1998; 27: 1227–1232. [DOI] [PubMed] [Google Scholar]
- 33. Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990; 265: 621–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jansky L, Reymanova P, Kopecky J. Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS or infected by Borrelia. Physiol Res 2003; 52: 593–598. [PubMed] [Google Scholar]
- 35. Tracey KJ, Beutler B, Lowry SF, et al. Shock and tissue injury induced by recombinant human cachectin. Science 1986; 234: 470–474. [DOI] [PubMed] [Google Scholar]
- 36. Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol 1992; 10: 411–452. [DOI] [PubMed] [Google Scholar]
- 37. De Groote D, Zangerle PF, Gevaert Y, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine 1992; 4: 239–248. [DOI] [PubMed] [Google Scholar]
- 38. Brauner A, Hylander B, Wretlind B. Tumor necrosis factor-α, interleukin-1β, and interleukin-1 receptor antagonist in dialysate and serum from patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 1996; 27: 402–408. [DOI] [PubMed] [Google Scholar]
- 39. Brauner A, Hylander B, Wretlind B. Interleukin-6 and Interleukin-8 in dialysate and serum from patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 1993; 22: 430–435. [DOI] [PubMed] [Google Scholar]
- 40. Connelly L, Madhani M, Hobbs AJ. Resistance to endotoxic shock in endothelial nitric-oxide synthase (eNOS) knock-out mice: a pro-inflammatory role for eNOS-derived no in vivo. J Biol Chem 2005; 280: 10040–10046. [DOI] [PubMed] [Google Scholar]
- 41. Anggard E. Nitric oxide: mediator, murderer, and medicine. Lancet 1994; 343: 1199–1206. [DOI] [PubMed] [Google Scholar]