Abstract
Background:
ZW10 interactor was recently reported to correlate with human cancers. However, the prognostic value of ZW10 interactor in hepatocellular carcinoma was not reported.
Methods:
The expression level of ZW10 interactor was evaluated by Western blot and immunohistochemistry using tissue microarray. In the present study, we used 5 pairs of hepatocellular carcinoma and peritumoral frozen tissues for Western blot, and 70 paired paraffin-embedded hepatocellular carcinoma and peritumoral tissues as expression pattern cohort (cohort 1), and 280 paraffin-embedded hepatocellular carcinoma tissues were used as prognostic cohort (cohort 2). The integral optic density representing the expression level of ZW10 interactor in each tissue sample, was calculated using Image-Pro Plus. The integral optic density was added to the X-tile software for calculating the outcome-based cut point. Kaplan-Meier and Cox regression were used to evaluate the prognostic values.
Results:
The expression level ZW10 interactor was decreased in hepatocellular carcinoma tissues in 85.7% (60/70) of the cases compared to the corresponding peritumoral tissues evaluated by immunohistochemistry. Similar result was obtained by Western blot analysis using frozen tissue. Expression of ZW10 interactor was closely correlated with age (P = .0001) and liver cirrhosis in cohort 1 and tumor node metastasis (P = .018), tumor size (P = .005), and vascular invasion (P = .022) in cohort 2 based on χ2 analyses. Survival analyses indicated that patients with hepatocellular carcinoma having low ZW10 interactor expression had a shorter overall survival time and time to recurrence compared to cases with high ZW10 interactor expression in the prognostic cohort (P < .0001 for both overall survival and time to recurrence ). Univariate and multivariate Cox analyses indicated that ZW10 interactor was an independent prognostic factor for overall survival (P = .033).
Conclusions:
The present study clearly showed that ZW10 interactor was frequently decreased in hepatocellular carcinoma compared to nontumoral liver tissues, and ZW10 interactor could serve as a potential prognostic marker in patients with hepatocellular carcinoma after surgery.
Keywords: hepatocellular carcinoma, ZW10 interactor, immunohistochemistry, overall survival, time to recurrence
IntroductionHepatocellular carcinoma (HCC) is a common malignant tumor, and it is the fourth leading cause of cancer death in China.1 Although high rates of survival have been reported due to the improvements in HCC treatments, high incidence of tumor recurrence and metastasis, causing the overall prognosis of HCC, remains unsatisfactory.2
Tumor node metastasis (TNM) staging system and the Barcelona Clinic Liver Cancer (BCLC) staging system are the most popular staging systems for the prediction of prognosis of patients with HCC after curative resection.3,4 However, the clinical outcomes varied greatly in patients with the same stage and similar treatment regimens.5 Therefore, novel molecular prognostic biomarkers for HCC, especially those with insights in the same TNM or BCLC stage, are needed for accurate prediction of survival and recurrence.
ZW10 interactor (ZWINT) is part of a separate complex of structural kinetochore components and is critical for recruiting ZW10 to unattached kinetochores.6 ZW10 plays an essential role in mitotic checkpoint and ensures that chromosomes are divided equally between daughter cells,7 and ZW10 gene was mutated in colorectal cancers with chromosomal instability.8 ZW10 interactor has been reported to be associated in several human cancers including breast cancer9 and prostate cancer.10 However, the association between ZWINT and HCC including prognosis has never been reported.
In the present study, we aimed to investigate the potential prognostic ability of ZWINT in HCC. We evaluated ZWINT protein levels in paired HCC and peritumoral tissues using immunohistochemistry (IHC). Next, we detected ZWINT protein levels in another cohort of HCC tissues and determined the prognostic value for patients with HCC after surgery.
Materials and Methods
Patients and Specimens
Formalin-fixed paraffin-embedded (FFPE) pathological specimens (n = 350) from the Eastern Hepatobiliary Surgery Hospital were obtained from December 2005 to December 2008. Among them, 70 cases with paired peritumoral liver tissues comprised an expression pattern cohort (cohort 1 from December 2005 to December 2006) and 280 cases comprised a survival analysis cohort (cohort 2 from January 2007 to December 2008). For Western blot analyses, 5 paired fresh frozen HCC tissues and peritumoral liver tissues were obtained at same hospital in October 2017. Inclusion criteria were as follows: (1) a diagnosis consistent with histological diagnostic criteria of the World Health Organization, (2) pathological diagnosis of hepatocellular lesions, and (3) no preoperative anticancer treatment and no evidence of extrahepatic metastases.11 Each patient provided informed consent, and the Ethics Committee of Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, China (Approval Number: EHBHKY2014-03-006) approved the study. The length of time between surgery and death or the last follow-up examination was used to define overall survival (OS). The date of tumor resection until the detection of tumor recurrence, death, or last observation was calculated for the time to recurrence (TTR).
A total of 280 cases were included in the prognostic cohort and were followed up for 3 months during the first year after surgery and every 6 months thereafter until December 2013. Two physicians who were unaware of the study performed all follow-up examinations. Abdomen ultrasonography, chest X-ray, and a test for serum alpha fetoprotein (AFP) concentration were used to monitor the patients every month during the first year after surgery and every 3 to 6 months thereafter. Magnetic resonance imaging or computed tomography scanning of the abdomen was performed every 6 months or immediately after a recurrence was suspected. The criteria for recurrence were the same as the diagnostic criteria preoperatively.12 Hematoxylin and eosin (HE) staining was used for each FFPE tissue, and staining results were reviewed by 2 experienced hepatopathologists.
Protein Extraction and Western Blotting
Western blot was performed according to previous study.13 Briefly, tissue samples were homogenated in an RIPA buffer (Qiagen, China) with a cocktail of proteinase inhibitors (Roche Applied Science, Switzerland) and a cocktail of phosphatase inhibitors (Roche Applied Science) The protein concentrations were determined using the Bicinchoninic Acid Kit (Pierce). Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Bio-Rad, Hercules, California). The membranes were incubated with primary ZWINT antibodies (HPA005757, 1:100 dilution; Sigma-Aldrich,) overnight at 4°C. After washing the membrane, ZWINT was visualized using ECL development solution.
Immunohistochemistry, Tissue Microarray, and Scoring
Kononen J’s method was used for the construction of tissue microarrays (TMAs).14 Briefly, hematoxylin and eosin (HE)-stained samples were reviewed by 2 experienced pathologists, and the representative cores were premarked in the paraffin blocks. A tissue cylinder with a diameter of 1.5 mm was punched from a marked area of each block. This was incorporated into a recipient paraffin block. Sections that were 4-μm-thick were placed on slides coated with 3-aminopropyltriethoxysilane. Paraffin sections were deparaffinized in xylene and rehydrated through decreasing concentrations of ethanol (100%, 95%, and 85% for 5 minutes each). The antigens were retrieved by microwave irradiation for 5 minutes in citric buffer (pH 6.0) and were then cooled at room temperature for 120 minutes, according to the protocol reported by Jin et al with minor modifications.15 The slides were incubated in 3% H2O2/phosphate-buffered saline to block endogenous peroxidase activity, and nonspecific binding sites were blocked with goat serum. Rabbit polyclonal primary antibodies (HPA005757, 1:200 dilution; Sigma-Aldrich) were used to detect ZWINT. Tissue antigens were visualized with an EnVision Detection kit (GK500705: Gene Tech, Shanghai, China). Counterstaining with hematoxylin was performed for 5 minutes. Negative control slides were created for all assays that had the primary antibodies omitted. The IOD/area of ZWINT was determined as reported previously.16 A Leica CCD camera DFC420 connected to a Leica DM IRE2 microscope (Leica Microsystems Imaging Solutions Let, Cambridge, United Kingdom) was used as the imaging system. High-powered magnification (200×) with Leica QWin Plus v3 software was used to photograph representative fields. The IODs of each image were counted and measured using Image-Pro Plus v6.0 software (Media Cybernetics, Inc, Bethesda, Maryland).
Statistical Analysis
Differences among variables were assessed by χ2 analysis or 2-tailed Student t test. X-tile software for calculating the outcome-based cutoff point.17 Kaplan-Meier analysis was used to assess survival and recurrence. Log-rank tests were used to compare survival of patients between subgroups. Multivariate analyses were performed by multivariate Cox proportional hazard regression model. Data were presented as mean ± standard error of the mean (SEM). Differences were considered to be statistically significant for P < .05. The SPSS statistical software package (SPPS Standard version 13.0; SPSS, Chicago, Illinois) was used for statistical analyses.
Results
ZWINT Protein was Frequently Decreased in HCC Tissues
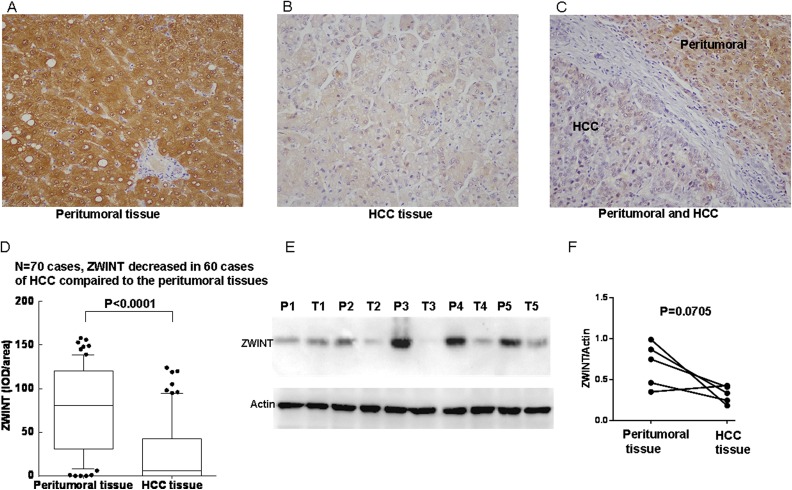
The staining pattern of ZWINT was mainly cytoplasmic and/or nuclear in both HCC tissues and peritumoral tissues according to immunohistochemical analysis. Representative immunohistochemical staining and quantitative analyses were shown that ZWINT protein expression was significantly decreased in HCC tissues compared to the corresponding peritumoral liver tissues (Figure 1A-C). This was evaluated in 70 paired HCC and peritumoral liver tissues (P < .0001), and ZWINT expressions were decreased in 60 cases of HCC tissues and increased in 10 cases of HCC tissues compared to the corresponding peritumoral liver tissues (Figure 1D). For further confirmation, the expression level of ZWINT was analyzed in 5 paired HCC and peritumoral tissue using Western blot. As shown in Figure 1E and F, ZWINT expressions were reduced in HCC tissue (P = .0705).
Figure 1.
Expression level of ZW10 interactor (ZWINT) in hepatocellular carcinoma (HCC) and paired peritumoral liver tissues. Expression of ZWINT in (A) paired peritumoral liver tissue (×200), (B) HCC tissue (×200), (C) HCC tissue and paired peritumoral liver tissue in one section (×200). (D) Expression level of ZWINT was significantly decreased in paired peritumoral liver than those in HCC tissues (n = 70, P < .0001). (E) ZWINT expression was detected by Western blot analyses in 5 paired HCC tissue (T) and peritumoral tissue (P). (F) Quantitation of ZWINT from Western blot analyses shows that ZWINT reduced in HCC tissue (T) compared paired peritumoral liver tissues (P = .0705).
Association Between Clinicopathological Features and ZWINT Expression
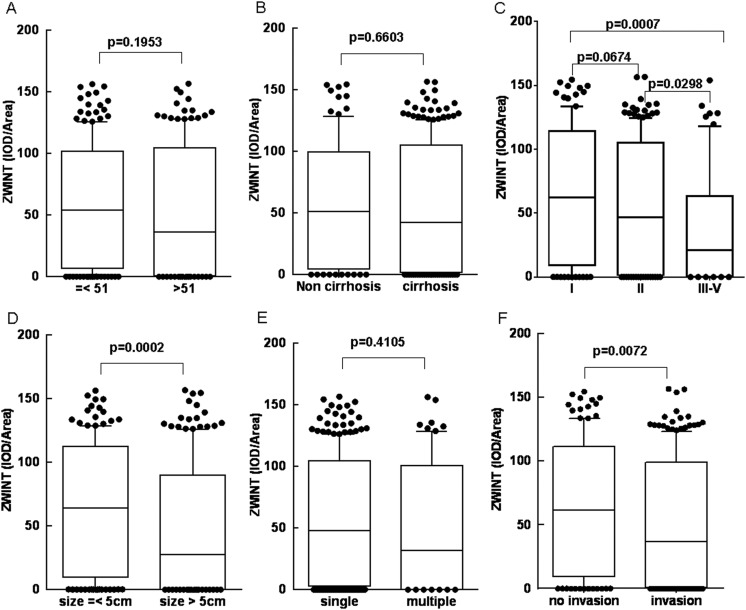
We investigated the relation between clinic–pathological variables and ZWINT expression in cohorts 1 and 2 using χ2 analysis. A significant correlation was found between low or high expression of ZWINT and age (P = .0001), liver cirrhosis (P = .023) in cohort 1 and TNM (P = .018), tumor size (P = .005), tumor number (P = .042), and vascular invasion (P = .022) in cohort 2 (Table 1). Based on χ2 analysis, we further analyzed ZWINT expression (represented as IOD/area) in subgroup of age, liver cirrhosis, TNM, tumor size, tumor number, and vascular invasion. Results showed that ZWINT expression were not showed different in subgroup of age (Figure 2A), liver cirrhosis (Figure 2B), and tumor number (Figure 2E). However, ZWINTs were significantly decreased in TNM III-IV stage compared to TNM I and TNM II stage (P = .0007 and P = .0298; Figure 2C), decreased in large size of tumor (size > 5 cm) than smaller size of tumor (size < 5 cm; P = .0002; Figure 2D), and decreased in invasive tumor than noninvasive tumor (P = .0072; Figure 2F).
Table 1.
Associations of ZWINT Expression Level With Pathological Characteristics in Cohorts 1 and 2.
| ZWINT in Cohort 1 | ZWINT in Cohort 2 | |||||
|---|---|---|---|---|---|---|
| Variable | Low (n = 28) | High (n = 42) | P Value | Low (n = 46) | High (n = 234) | P Value |
| Sex | .283 | .903 | ||||
| Male | 22 | 37 | 40 | 205 | ||
| Female | 6 | 5 | 6 | 29 | ||
| Age | .0001 | .309 | ||||
| < 51 | 6 | 28 | 21 | 126 | ||
| >51 | 22 | 14 | 25 | 108 | ||
| HBsAg | .826 | .343 | ||||
| Negative | 8 | 11 | 9 | 33 | ||
| Positive | 20 | 31 | 37 | 201 | ||
| Serum AFP | .843 | .190 | ||||
| < 20 ng/mL | 12 | 17 | 13 | 90 | ||
| >20 ng/mL | 16 | 25 | 33 | 144 | ||
| Liver cirrhosis | .023 | .143 | ||||
| No | 5 | 1 | 11 | 82 | ||
| Yes | 23 | 41 | 35 | 152 | ||
| TNM | .018 | |||||
| I | 7 | 15 | .628 | 9 | 84 | |
| II | 13 | 16 | 25 | 121 | ||
| III-IV | 8 | 11 | 12 | 29 | ||
| Child-pugh class | .222 | .587 | ||||
| A | 27 | 37 | 43 | 213 | ||
| B | 1 | 5 | 3 | 21 | ||
| Tumor size | .226 | .005 | ||||
| <5 cm | 8 | 18 | 14 | 124 | ||
| >5 cm | 20 | 24 | 32 | 110 | ||
| Tumor number | .807 | .042 | ||||
| Single | 22 | 34 | 30 | 185 | ||
| Multiple | 6 | 8 | 16 | 49 | ||
| Tumor differentiation | .595 | .330 | ||||
| Well | 1 | 2 | 2 | 25 | ||
| Moderate | 20 | 25 | 44 | 207 | ||
| Poor | 7 | 15 | 0 | 2 | ||
| Vascular invasion | .533 | .022 | ||||
| No | 8 | 15 | 11 | 98 | ||
| Yes | 20 | 27 | 35 | 136 | ||
Abbreviations: AFP, alpha fetoprotein; HBsAg, hepatitis B surface antigen; TNM, tumor-node-metastasis.
Bold-face indicate statistically significant values.
Figure 2.
Expression level of ZW10 interactor (ZWINT) in suptype of age, liver cirrhosis, TNM stage, tumor size, tumor numver, and microvascular invasion of tissues of patients with HCC. Expression of ZWINT does not show difference in (A) age (B) liver cirrhosis, and (E) tumor number and gradually decreased in (C) TNM I, TNM II, and TNM III-IV stage and significantly decreased in (D) tissues larger than 5 cm than those with tumor tissues smaller than 5 cm, (F) and decreased in microvascular invasion group than those in the no microvascular invasion group.
Low ZWINT Expression Levels Indicated a Worse Prognosis in Patients With HCC
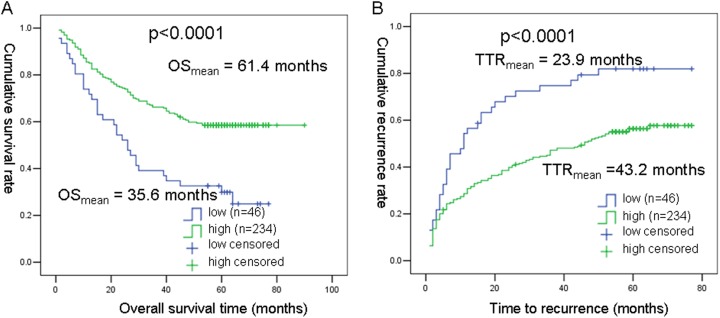
Using X-tile software, we calculated the outcome-based cutoff point as IOD/area = 0.763 for ZWINT. Based on this cutoff point, the relation between ZWINT expression and outcomes of patients with HCC(OS and TTR) for 280 patients with HCC was analyzed with the Kaplan-Meier analyses. As shown in Figure 3, mean OS time was 61.4 months for high ZWINT expression group 35.6 months for low ZWINT expression group (P < .0001). Similarly, TTRs were significantly (P < .0001) shorter in the low ZWINT expression group (23.9 months) compared to that in the high ZWINT expression group (43.2 months).
Figure 3.
Kaplan-Meier curves for overall survival (OS) and time to recurrence (TTR) in patients with HCC (280 cases). A, Probability of postoperative survival of patients showed that mean OS time for patients with HCC expressing low levels of WZINT was 35.6 months and 61.4 months for those expressing high levels of WZINT. B, The mean time to recurrence for patients with HCC expressing low levels of WZINT was 23.9 months compared to those expressing high levels of WZINT was 43.2 months.
Significance of ZWINT in Patients With HCC According to Univariate and Multivariate Analyses
The prognostic significance of ZWINT and clinic–pathologic parameters in 280 cases with HCC was analyzed with univariate and multivariate analyses (Table 2). Our analyses showed that serum AFP (P < .0001), liver cirrhosis (P = .017), TNM stage (P < .0001), tumor size (P < .0001), tumor number (P < .0001), tumor differentiation (P = .025), vascular invasion (P = .004), and ZWINT (P < .0001) were significant prognostic factors for OS in the univariate analysis. Serum AFP (P = .014), liver cirrhosis (P = .013), tumor size (P < .0001), tumor number (P < .0001), and ZWINT (P = .033) were the independent prognostic factors for OS. In addition, serum AFP (P = .001), liver cirrhosis (P = .001), TNM stage (P < .0001), tumor size (P < .0001), tumor number (P < .0001), tumor differentiation (P = .007), vascular invasion (P = .008), and ZWINT (P < .0001) were significant prognostic factors for TTR in the univariate analysis, and serum AFP (P = .047), liver cirrhosis (P < .0001), tumor size (P < .0001), tumor number (P < .0001) were the independent prognostic factors for TTR.
Table 2.
Univariate and Multivariate Analyses of Clinicopathological Factors Associated With OS and TTR.
| Factors | OS | TTR | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate P | Multivariate | Univariate P | Multivariate | |||||
| HR | 95% Cl | P Value | HR | 95% Cl | P Value | |||
| Sex: Male vs Female | .603 | .925 | ||||||
| Age: ≤ 51 vs > 51 | .823 | .489 | ||||||
| HBsAg: positive vs negative | .246 | .102 | ||||||
| serum AFP, ng/mL: ≤ 20 vs > 20 | <.0001 | 1.650 | 1.107-2.460 | .014 | .001 | 1.406 | 1.005 -1.966 | .047 |
| Liver cirrhosis: yes vs no | .017 | 1.663 | 1.111-2.489 | .013 | .001 | 1.897 | 1.327-2.713 | <.0001 |
| TNM: I vs II vs III-IV | <.0001 | <.0001 | ||||||
| Child-pugh: A vs B | .136 | .182 | ||||||
| Tumor size: ≤ 5 cm vs > 5cm | <.0001 | 2.545 | 1.729-3.747 | <.0001 | <.0001 | 2.034 | 1.477-2.801 | <.0001 |
| Tumor number: single vs multiple | <.0001 | 1.997 | 1.380-2.891 | <.0001 | <.0001 | 2.349 | 1.687-3.270 | <.0001 |
| Tumor differentiation: well vs moderate vs poor | .025 | .007 | ||||||
| Vascular invasion: no vs yes | .004 | .008 | ||||||
| ZWINT: low vs High | <.0001 | 0.639 | 0.424-0.964 | .033 | <.0001 | |||
Abbreviations: AFP, alpha fetoprotein; OS, overall survival; TTR, time to recurrence; ZWINT, ZW10 interactor; HR, hazard ratio; CI, confidence interval.
Bold-face indicate statistically significant values.
Discussion
The risk of prognosis can be defined with the molecular classification. The investigation of molecular biomarkers can also help with the development of novel therapeutic targets for the treatment for patients with HCC.18 Although progress in predictive biomarkers in clinical and experimental oncology and clinical treatment strategies 19 have been made in the recent years,20–22 the prognosis in patients with HCC is still not very optimistic.
ZW10 interactor was also reported to be increased in castration-resistant prostate cancers,10 and aromatase inhibitor treatment is associated with changes in expression of ZWINT.23 ZW10 interactor was reported by Endoh as 1 factor of the8 genes for prognostic model of pulmonary adenocarcinoma, and prognostic model was determined by quantitative real-time reverse transcriptase polymerase chain reaction data.24 High expression of ZWINT was associated with worse OS in patients with ovarian cancer.25 In general, several recent studies have documented an involvement of ZWINT in cancer behavior and prognosis as described earlier. However, the status of ZWINT and its potential prognostic impact on HCC have not been explored so far. In this present study, we investigated the expression levels of ZWINT in paired HCC and noncancer liver tissues first by TMA-based immunohistochemistry. Our results demonstrated that ZWINT protein expression was decreased in HCC tissues compared to the corresponding peritumoral liver tissues. Subsequently, the prognostic value of ZWINT in HCC tissue was also investigated. In survival analyses based on Kaplan-Meier analyses, we observed an association between ZWINT protein expression and clinical outcomes in patients with HCC after surgery. Exactly, patients with low expression of ZWINT had shorter OS and recurrence times compared to those with high expression ZWINT. The multivariate analyses showed that ZWINT was an independent predictor for OS patients with HCC after surgery. Although our study suggests that ZWINT has a predictive role in the prognosis of patients with HCC, this may require a multicenter, prospective study to further confirm this phenomenon.
The present study is the first report that explored the expression status of ZWINT in HCC tumor and peritumoral liver tissues. It also was the first to show the prognostic value of ZWINT in HCC tissues. Our study suggested that ZWINT could be used as a new prognostic biomarker for HCC based on immunohistochemical analysis.
Abbreviations
- AFP
alpha fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- BCA
Bicinchoninic Acid
- FFPE
formalin-fixed paraffin-embedded
- HCC
hepatocellular carcinoma
- IHC
immunohistochemistry
- OS
overall survivor
- TMA
tissue microarrays
- TNM
tumor-node-metastasis
- TTR
time-to-recurrence
- ZWINT
ZW10 interactor
Footnotes
Author Notes: Xiao-Yu Yang is a first author and Bin Wu is a second author and Xiao-Yu Yang and Bin Wu equally contributed to this work
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was supported by the Shanghai Natural Science Foundation (No.: 14ZR1409300) and Glorious Funds from Chinese foundation for hepatitis prevention and control (No: GHF2012209).
ORCID iD: Ai-Jun Li, MD, PhD  http://orcid.org/0000-0002-1697-4446
http://orcid.org/0000-0002-1697-4446
Reference
- 1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in china, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 2. Portolani N, Coniglio A, Ghidoni S, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243(2):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edge SB BD CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed New York: Springer; 2010. [Google Scholar]
- 4. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the bclc staging classification. Seminars Liver Dis. 1999;19(3):329–338. [DOI] [PubMed] [Google Scholar]
- 5. Mao YP, Xie FY, Liu LZ, et al. Re-evaluation of 6th edition of ajcc staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73(5):1326–1334. [DOI] [PubMed] [Google Scholar]
- 6. Starr DA, Saffery R, Li Z, et al. Hzwint-1, a novel human kinetochore component that interacts with hzw10. J Cell Sci. 2000;113(Pt 11):1939–1950. [DOI] [PubMed] [Google Scholar]
- 7. Wang H, Hu X, Ding X, et al. Human zwint-1 specifies localization of zeste white 10 to kinetochores and is essential for mitotic checkpoint signaling. J Biol Chem. 2004;279(52):54590–54598. [DOI] [PubMed] [Google Scholar]
- 8. Wang Z, Cummins JM, Shen D, et al. Three classes of genes mutated in colorectal cancers with chromosomal instability. Cancer Res. 2004;64(9):2998–3001. [DOI] [PubMed] [Google Scholar]
- 9. Brendle A, Brandt A, Johansson R, et al. Single nucleotide polymorphisms in chromosomal instability genes and risk and clinical outcome of breast cancer: a swedish prospective case-control study. Eur J Cancer. 2009;45(3):435–442. [DOI] [PubMed] [Google Scholar]
- 10. Urbanucci A, Sahu B, Seppala J, et al. Overexpression of androgen receptor enhances the binding of the receptor to the chromatin in prostate cancer. Oncogene. 2012;31(17):2153–2163. [DOI] [PubMed] [Google Scholar]
- 11. Jin GZ, Yu WL, Dong H, et al. Suox is a promising diagnostic and prognostic biomarker for hepatocellular carcinoma. J Hepatol. 2013;59(3):510–517. [DOI] [PubMed] [Google Scholar]
- 12. Tan N, Liu Q, Liu X, et al. Low expression of b-cell-associated protein 31 in human primary hepatocellular carcinoma correlates with poor prognosis. Histopathology. 2016;68(2):221–229. [DOI] [PubMed] [Google Scholar]
- 13. Jin H, Wang C, Jin G, et al. Regulator of calcineurin 1 gene isoform 4, down-regulated in hepatocellular carcinoma, prevents proliferation, migration, and invasive activity of cancer cells and metastasis of orthotopic tumors by inhibiting nuclear translocation of nfat1. Gastroenterology. 2017;153(3):799–811.e733. [DOI] [PubMed] [Google Scholar]
- 14. Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–847. [DOI] [PubMed] [Google Scholar]
- 15. Jin GZ, Li Y, Cong WM, et al. Itraq-2dlc-esi-ms/ms based identification of a new set of immunohistochemical biomarkers for classification of dysplastic nodules and small hepatocellular carcinoma. J Proteome Res. 2011;10(8):3418–3428. [DOI] [PubMed] [Google Scholar]
- 16. Li Y, Wang W, Jia X, et al. A targeted multiple antigenic peptide vaccine augments the immune response to self tgf-beta1 and suppresses ongoing hepatic fibrosis. Arch Immunol Ther Exp. 2015;63(4):305–315. [DOI] [PubMed] [Google Scholar]
- 17. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. [DOI] [PubMed] [Google Scholar]
- 18. Yang XR, Xu Y, Yu B, et al. Cd24 is a novel predictor for poor prognosis of hepatocellular carcinoma after surgery. Clin Cancer Res. 2009;15(17):5518–5527. [DOI] [PubMed] [Google Scholar]
- 19. San Miguel C, Fundora Y, Triguero J, et al. Immunosuppression strategies in the treatment of hepatocellular carcinoma in virgen de las nieves university hospital. Transplant proc. 2015;47(8):2371–2373. [DOI] [PubMed] [Google Scholar]
- 20. Hwang HW, Ha SY, Bang H, Park CK. Atad2 as a poor prognostic marker for hepatocellular carcinoma after curative resection. Cancer Res Treat. 2015;47(4):853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seo SI, Kim HS, Kim WJ, et al. Diagnostic value of pivka-ii and alpha-fetoprotein in hepatitis b virus-associated hepatocellular carcinoma. World J Gastroenterol. 2015;21(13):3928–3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shen Q, Fan J, Yang XR, et al. Serum dkk1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncology. 2012;13:817–826. [DOI] [PubMed] [Google Scholar]
- 23. Miller WR. Clinical, pathological, proliferative and molecular responses associated with neoadjuvant aromatase inhibitor treatment in breast cancer. J Steroid Biochem Mol Biol. 2010;118(4-5):273–276. [DOI] [PubMed] [Google Scholar]
- 24. Endoh H, Tomida S, Yatabe Y, et al. Prognostic model of pulmonary adenocarcinoma by expression profiling of eight genes as determined by quantitative real-time reverse transcriptase polymerase chain reaction. J Clin Oncol. 2004;22(5):811–819. [DOI] [PubMed] [Google Scholar]
- 25. Xu Z, Zhou Y, Cao Y, Dinh TL, Wan J, Zhao M. Identification of candidate biomarkers and analysis of prognostic values in ovarian cancer by integrated bioinformatics analysis. Med Oncol. 2016;33(11):130. [DOI] [PubMed] [Google Scholar]