Abstract
Incontinentia pigmenti (IP) is a multisystemic disorder in which pulmonary arterial hypertension (PAH) is a severe and rarely reported association. The prognosis has been poor in reported cases. In our patient, IP was diagnosed during the neonatal period with a combination of cutaneous, ophthalmic, and neurological symptoms. The infant experienced severe collapse with bradycardia during general anesthesia to treat retinal telangiectasia. Echocardiography after resuscitation revealed suprasystemic pulmonary hypertension (PH). Right heart catheterization (RHC) confirmed precapillary PAH not responding to acute vasodilatation test. Lung biopsy was performed to exclude alveolo-capillary dysplasia. Upfront triple therapy with endothelin receptor antagonist, PDE5 inhibitors, and prostacyclin was started. Due to a potential inflammatory mechanism of this acute PAH in the setting of IP, TNF-alpha blockers and steroids were associated. The evolution was favorable with progressive normalization of the pulmonary artery pressure confirmed by RHC after six months. Doses of PAH drugs were tapered down, and finally all PAH treatments could be stopped after 18 months. Subsequent controls including physical exams and echocardiograms did not show signs of PH. This unusual reversible case of pediatric PAH without associated congenital heart disease or portal hypertension highlights the potential reversibility of pediatric PH when an inflammatory mechanism can be suspected. This is the first reported case of non-fatal isolated PAH associated with IP.
Keywords: Pulmonary arterial hypertension, pulmonary hypertension, genetics / genomics / epigenetics, pediatrics, pediatric cardiovascular disease
Introduction
Incontinentia pigmenti (IP; Bloch-Sulzberger syndrome-IP) is a rare X-linked dominant genodermatosis. IP may involve other systems including the skin with evocative successive stages, central nervous system, and the eyes. Only four cases of pulmonary arterial hypertension (PAH) without associated congenital heart disease have been described in IP patients.1–4 All four died before one year of age. Here, we report a new case of severe PAH in a four-month-old female infant with IP that fully resolved with an aggressive unusual combination therapy.
Case report
A full-term female infant was born with normal perinatal adaptation.
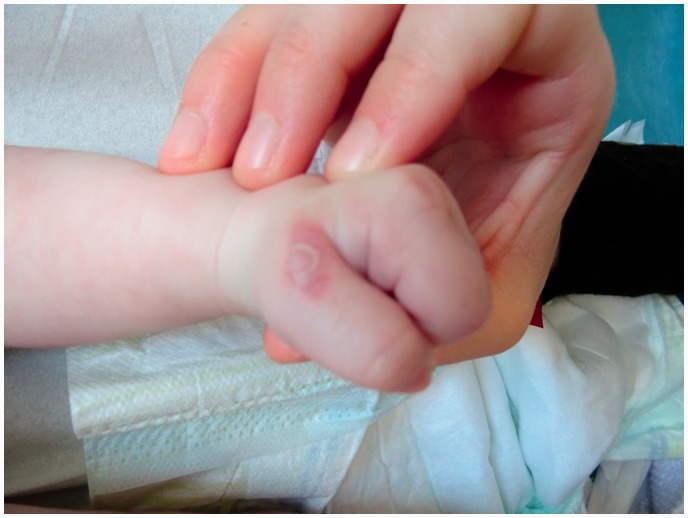
The baby developed seizures at two days of life confirmed as status epilepticus by electroencephalogram. Brain magnetic resonance imaging (MRI) showed innumerable foci of cortical and subcortical ischemic lesions, with restriction of diffusion over the whole right hemisphere and scattered diffusion on the left hemisphere. Electrocardiogram and echocardiography were normal at that time, excluding embolic cause. A few acral inflammatory vesicles on Blaschko lines, highly evocative of the diagnosis of IP, were observed at day 5 (Fig. 1). Skin biopsy was compatible with the diagnosis (keratinocytic necrosis, exocytosis of eosinophilic polynuclear cells, and spongiosis). Screening for the IP gene (NEMO/IKBKG) confirmed the diagnosis showing a classical de novo deletion encompassing exons 4–10 from the IKBKG gene (Inhibitor of Nuclear Factor Kappa B Kinase Subunit Gamma).
Fig. 1.
Skin involvement typical of IP: rash with warty inflammatory lesions on the lines of Blaschko.
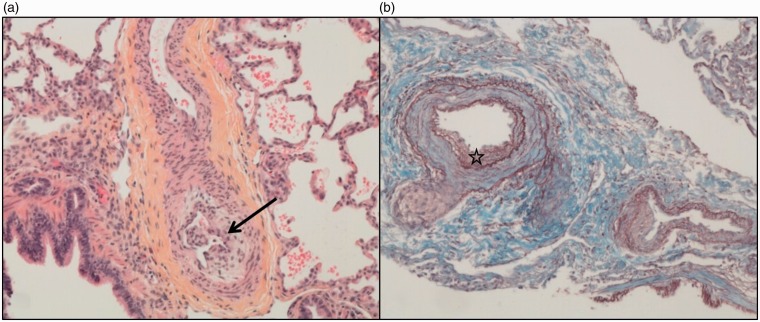
At the age of four months, the patient suffered a severe collapse during general anesthesia for photocoagulation of an IP retinal vasculopathy. Echocardiography performed after resuscitation showed severe pulmonary hypertension (PH) with major right ventricular dysfunction and low cardiac output. Right heart catheterization (RHC) performed after stabilization confirmed more than systemic pulmonary arterial hypertension (PAH) with pulmonary arterial pressure (PAP) 78/30–51 mmHg (pulmonary vascular resistance = 12.3 Wood Units m2) without response to acute vasodilation testing with nitric oxide. Chest computed tomography (CT) excluded parenchymal lung disease and pulmonary veins anomalies. Lung biopsy was performed to exclude alveolo-capillary dysplasia. It showed focal arterial and arteriolar abnormalities with intimal thickening suggestive of PAH (Fig. 2). Neither vasculitis nor inflammatory processes were noted. No mutation was found in a panel of usually tested PAH genes nor in developmental vascular lung diseases genes. Systemic hypereosinophila was noticed (1%, 0.5 G/L).
Fig. 2.
(a) Marked intimal thickening (arrow) due to cellular intimal proliferation in a pulmonary artery (H&E, ×100). (b) Duplicated internal elastic lamina (star; orcein staining of elastic fibers, ×100).
Due to PAH severity, upfront PAH triple therapy with intravenous epoprostenol, bosentan, and sildenafil was immediately started. Because of the suspected involvement of the NFκB pathway, anti-inflammatory steroid treatment with intravenous bolus of methylprednisolone followed by oral long-term steroids and subcutaneous weekly etanercept injections were associated.
After six months of triple PAH therapy and progressive tapering of steroids, PAP and right ventricular function had normalized on echocardiography.
A new RHC confirmed normalization of PAP and cardiac output. She was then progressively weaned from PAH therapy and immunomodulatory therapy. PAPs remained normal during treatment weaning. Finally, six months after all PAH and immunosuppressive treatments had been stopped, frequent clinical cardiovascular examination of the infant did not reveal any anomaly and she had normal PAP on echocardiography and normal NT-proBNP.
Discussion
PH is an exceptional and severe complication of IP only described in infants with different types of PH described in the reported cases.1–6
PH in two patients was caused by anatomical anomalies. The first patient presented with stenosis in the bilateral pulmonary artery branches and survived.6 The outcome of pulmonary artery branch stenosis is highly variable but might be favorable with slow progression and preserved right ventricular function. The second patient had partial anomalous pulmonary venous return responsible for left-to-right shunt.5 The reversibility of flow-related PH after repair in this defect is usual.
The other cases of PH associated with IP were described as PAH.1–4 In the absence of treatable causes such as operable left-to-right cardiac shunts, portal hypertension, PAH is irreversible. Indeed, the four reported cases of PAH in IP had a fatal course. Death was related to intractable PAH in all reported cases. In these previous cases, PAH treatment was mainly sildenafil. Only one patient received prednisolone.
Our patient survived with aggressive treatment. While the pathophysiology of this rare complication in IP remains unknown, this may suggest that PAH develops in IP during the first weeks of life and has a transient evolution as has been described in the case of brain vasculitis in neonates.7
We decided to treat this female infant with a triple combination of PAH drugs because of the initial severity of PAH revealed by cardiac arrest. We also utilized immunomodulatory drugs as inflammatory mechanisms are supposed to play an important role in IP.
Indeed, NEMO is essential for Nuclear factor-kappa B (NFκB) activation, which is involved in the regulation of epidermal homeostasis, the control of the expression of many genes with important function in inflammation, immune responses, cell proliferation, and apoptosis. NFκB activation is defective in IP cells. IP cells are highly sensitive to TNF-induced apoptosis due to the inactivation of the NFκB pathway. Nenci et al.9 in 2006 used a mouse model showing that the genetic ablation of tumor necrosis factor receptor 1 (TNFRI) rescued the skin phenotype, demonstrating that TNF signaling is essential for skin lesion pathogenesis in IP.
In NEMO-deficient animals, the brain lesions are characterized by a rarefaction of cerebral capillaries and hypoperfusion of the central nervous system.7
Finally, inflammation has a central role in IP and participates to the progression of PAH.8,10 It can be suggested that anti-TNF alpha could have played a role through the pro-inflammatory NFκB pathway in our patient.
It is not possible to disentangle the respective roles of PAH drugs and anti-inflammatory treatment in the favorable course of the disease in this patient. The transient evolution of the vascular involvement at least in the brain and lungs in IP remains unexplained but the mechanisms involved should be further explored as they might be key to better treat these patients and potentially other children with PAH.
Conclusion
PAH is an exceptional complication of IP with poor reported outcomes. This case also suggests that aggressive therapy combining vasodilatators and anti-inflammatory treatment might change the course of this devastating complication in IP.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Yasuda K, Minami N, Yoshikawa Y, et al. Fatal pulmonary arterial hypertension in an infant girl with incontinentia pigmenti. Pediatr Int 2016; 58: 394–396. [DOI] [PubMed] [Google Scholar]
- 2.Godambe S, McNamara P, Rajguru M, et al. Unusual neonatal presentation of incontinentia pigmenti with persisten pulmonary hypertension of the newborn: a case report. J Perinatol 2005; 25: 289–292. [DOI] [PubMed] [Google Scholar]
- 3.Hayes IM, Varigos G, Upjohn EJ, et al. Unilateral acheiria and fatal primary pulmonary hypertension in a girl with incontinentia pigmenti. Am J Med Genet A 2005; 135: 302–303. [DOI] [PubMed] [Google Scholar]
- 4.Triki C, Devictor D, Kah S, et al. [Cerebral complications of incontinentia pigmenti. A clinicopathological study of a case]. Rev Neurol (Paris) 1992; 148: 773–776. [PubMed] [Google Scholar]
- 5.Miteva L, Nikolova A. Incontinentia pigmenti: a case associated with cardiovascular anomalies. Pediatr Dermatol 2001; 18: 54–56. [DOI] [PubMed] [Google Scholar]
- 6.Alshenqiti A, Nashabat M, AlGhoraibi H, et al. Pulmonary hypertension and vasculopathy in incontinentia pigmenti: a case report. Ther Clin Risk Manag 2017; 13: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller K, Courtois G, Ursini MV, et al. New insight into the pathogenesis of cerebral small-vessel diseases. Stroke 2017; 48: 520–527. [DOI] [PubMed] [Google Scholar]
- 8.Sawada H, Mitani Y, Maruyama J, et al. A nuclear factor-kappaB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Chest 2007; 132: 1265–1274. [DOI] [PubMed] [Google Scholar]
- 9.Nenci A, Huth M, Funteh A, et al. Skin lesion development in a mouse model of incontinentia pigmenti is triggered by NEMO deficiency in epidermal keratinocytes and requires TNF signaling. Hum Mol Genet 2006; 15: 531–542. [DOI] [PubMed] [Google Scholar]
- 10.Soon E, Crosby A, Southwood M, et al. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production. A gateway to pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192: 859–872. [DOI] [PMC free article] [PubMed] [Google Scholar]