Abstract
Background:
The aim of the study was to evaluate the achievement of ‘no evidence of disease activity’ (NEDA) over a 12-month period in a large multicenter population with relapsing remitting multiple sclerosis (RRMS) treated with delayed-release dimethyl fumarate (DMF) and teriflunomide (TRF) using a propensity-score adjustment.
Methods:
A time-to-event method was used to determine the percentages of patients with RRMS (pwRRMS) in both groups achieving NEDA 3 (no relapses, no 12-week confirmed disability progression, and no new T2/gadolinium-enhancing brain lesions). We described the safety profile of the investigated drugs.
Results:
Of the 587 pwRRMS treated with DMF and the 316 pwRRMS treated with TRF, 468 pwRRMS were successfully paired by propensity score: 234 on DMF and 234 on TRF. The percentages of pwRRMS who achieved NEDA 3 were 80.3% in the DMF group and 77.2% in the TRF group. Serious adverse events occurred in four (1.9%) pwRRMS on DMF and in three (1.3%) pwRRMS on TRF.
Conclusions:
DMF and TRF significantly impacted RRMS disease activity in our study. Serious safety concerns were recorded in less than 2% of the studied population.
Keywords: dimethyl fumarate, efficacy, no evidence of disease activity 3, safety, teriflunomide
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system that most frequently strikes young women.1 In the past 5 years, therapeutic options for treating relapsing remitting MS (RRMS) have impressively broadened and new oral first-line agents have been approved.2,3 In European countries, delayed-release dimethyl fumarate (DMF), also known as gastro-resistant DMF, and teriflunomide (TRF) are used in the treatment of persons with RRMS (pwRRMS).3 Pivotal trials have demonstrated the benefits of DMF and TRF on both clinical symptoms (i.e. number of clinical relapses and disability accrual) and on magnetic resonance imaging (MRI) disease activity, with a generally good tolerability.4–8
No head-to-head clinical trials have compared the efficacy of DMF versus TRF, although a recent number needed to treat (NNT) analysis comparing outcomes from pivotal studies of DMF, TRF, and fingolimod showed that the NNT for key clinical outcomes was similar in the three agents.9–11
In recent years, there has been growing interest in performing high-quality observational cohort studies to collect longitudinal information that is representative of real MS clinical practice, revealing data highly concordant with the evidence obtained from clinical trials.9–11
In this study, we explored the practical utility, efficacy, and safety profile of DMF and TRF over 12 months in a large multicenter population of pwRRMS.
Our main outcome was the proportion of pwRRMS with no evidence of disease activity (NEDA) defined as the absence of relapses, progression of disability, and worsening radiographic findings (NEDA 3).
Methods
This was an independent, multicenter, prospective, observational study conducted in a large Italian population of pwRRMS treated with two oral first-line drugs, DMF and TRF.
All pwRRMS included in the study were treated in accordance with the approved label instructions and the expected standards of good clinical practice. Written informed consents were collected. Treatment protocols, which involved DMF (120 mg twice per day for the first 7 days, then 240 mg twice per day), and TRF (14 mg once per day), are described elsewhere.12,13
Complementary clinical, radiographic (brain MRI lesions), and demographic parameters were acquired retrospectively, up to 12 months before the treatments started.
Data were recorded as part of routine clinical practice at each tertiary participating MS center, with data entry performed at the time of clinical visits. The data entry portal was iMED© software and we followed a rigorous quality assurance procedure.14
pwRRMS were consecutively included in the study at the initiation of DMF or TRF treatment (baseline) and were monitored over 12 months, with data collection performed at baseline and after 12 months following drug initiation. Data were censored at the last follow-up visit.
This study was approved by the Azienda Ospedaliero Universitaria Policlinico Vittorio Emanuele (Catania, Italy) Ethics Committee (n.177/2017/PO).
This study received no financial support for the design, data collection, data analysis, data interpretation, or writing.
The corresponding author had full access to the entire database and had the final responsibility of submitting this manuscript.
Key eligibility criteria included: (a) age 18 years or older; (b) a diagnosis of RRMS according to the McDonald criteria;15 (c) a disability score at enrollment (assessed by the Expanded Disability Status Scale [EDSS])16 of no more than 3.5 (selected to exclude any patients with severe MS); (d) initiating one of the study therapies in the index enrollment window (1 January 2015–1 March 2016) to allow for at least 12 months of follow up; (e) no history of stem-cell transplantation; (f) no ongoing participation in randomized clinical trials.
For all enrolled patients, the following clinical and demographic variables were collected: sex, age, time of first MS symptom, dates of clinical relapses, and disability score assessed by EDSS at commencement of treatment (within 12 months of the start of treatment).
Disability was assessed by Neurostatus-certified MS specialists; any EDSS score recorded within 30 days of a previous relapse was excluded, as described elsewhere.
All pwRRMS willing to authorize the release of their coded medical information to iMED© software signed a consent form. The signed consent form had to be filed in the individual’s MS clinic chart and a copy made available to the pwRRMS.
Outcomes
The primary outcome of the study was to evaluate the proportion of pwRRMS with no evidence of disease activity, as defined by NEDA 3, during the 12-month observational period.
NEDA 3 was defined as a composite that consisted of: (a) the absence of clinical relapses; (b) no confirmed disability progression sustained for 12 weeks (as measured by EDSS); (c) the absence of T1 gadolinium-enhancing (Gad+) brain lesions as well as the absence of any new/newly enlarging T2 brain lesions.
A relapse was defined as the occurrence of new symptom(s) or the exacerbation of existing symptom(s) persisting for at least 24 h in the absence of concurrent illness or fever, occurring at least 30 days after a previous relapse.17
Confirmed disability progression was defined as an increase in EDSS ⩾ 1.0 point from the baseline EDSS, sustained for 12 weeks or longer. The number of brain MRI lesions on T2, T1, and T1 Gad+ was recorded during the 12-month period before treatment initiation and the 12-month follow-up period.
The secondary outcome was the time of the new first clinical relapse within 12 months.
We collected data to evaluate the real-life safety and tolerability of the two drugs. We examined the frequency of adverse events (AEs) and the proportion of severe AEs (using the European Medical Agency’s definitions of AE and serious adverse event [SAE]). We considered an AE to be any untoward medical occurrence in a subject who had been administered a pharmaceutical product, but without a necessary causal relationship with the treatment.18
An SAE was any AE that resulted in: death, a life-threatening AE, inpatient hospitalization or prolongation of existing hospitalization, a persistent or significant incapacitation or substantial disruption of the ability to conduct normal life functions, a congenital anomaly/birth defect, or any events that may require medical or surgical intervention to prevent one of the outcomes listed in this definition.
Lymphopenia was defined according to the Common Terminology Criteria for Adverse Events definition as a laboratory result indicating a decrease in the number of lymphocytes in a blood specimen. In pwRRMS, it is considered notable when the value drops below 200/mm3.19
Statistical analysis
All patients’ characteristics were reported as frequencies (%) for categorical variables and mean ± standard deviation (SD) or median with interquartile range for continuous variables. Comparisons of baseline characteristics prior to propensity-score matching between the treatment arms were performed using the Wilcoxon rank sum test or the chi-square test, depending on the nature of the variables.
A paired-matched analysis of the two treatments was conducted. The pwRRMS in the DMF group were matched to patients in the TRF group on the basis of propensity scores calculated using a multivariable logistic regression. All of the following were used in the regression analysis as independent variables: age, sex, disease duration, prestudy EDSS scores (measured at 24 months, 12 months, and 6 months), the disease-modifying therapies (DMTs) administered before the beginning of the study (considering both first-line and second-line therapies), and the reason for switching. A 1:1 nearest neighbor matching without replacement was used. The postmatch balance was confirmed by evaluating the standardized mean differences.
The comparison of some outcome variables of interest was carried out with standard tests. Survival analyses were conducted using the Kaplan–Meier method to estimate the time to relapse within 12 months of treatment, and the differences between the two groups of interest were estimated by the logrank test. Results were expressed both as medians and hazard ratios (HR) with corresponding 95% confidence intervals. Statistical significance was achieved at a p value of < 0.05.
The study power was 0.90 (alpha 0.05); null hypothesis: expected NEDA 3 in study drugs 75% ± 15%.
All analyses were performed using the Statistical Analysis System (SAS Institute, Release 9.4), the R package (version 3.3.0), and SPSS version 21 (IBM SPSS Statistics 21, IBM, Armonk, NY, USA).
Results
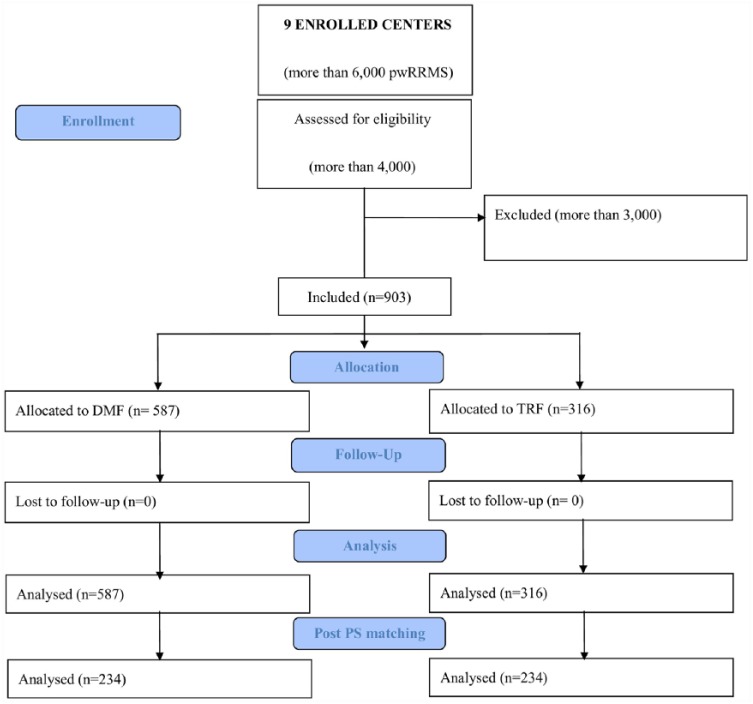
From a total sample of more than 6000 pwRRMS from nine Italian centers, 903 were considered eligible for analyses. Of those, 587 pwRRMS treated with DMF and 316 pwRRMS treated with TRF met the inclusion criteria and were included in this study (Figure 1).
Figure 1.
Flow chart of the study. DMF, dimethyl fumarate; PS, propensity score; pwRRMS, patients with relapsing remitting multiple sclerosis; TRF, teriflunomide.
Table 1 shows the comparison of patients’ baseline characteristics before matching. pwRRMS on TRF were older than pwRRMS on DMF (p < 0.0001). The gender ratio was also different with a predominance of women in the DMF group (p < 0.05).
Table 1.
Comparison of baseline characteristics between the two treatment groups prior to propensity-score matching.
|
Variable
|
DMF
|
TRF
|
p value* |
|---|---|---|---|
| No. Group | 587 | 316 | |
| Age, mean (SD) | 38.6 (10.9) | 46.3 (10.3) | < 0.001 |
| Sex, No. (%) | |||
| Female | 416 (70.9) | 199 (63) | 0.01 |
| Male | 171 (29.1) | 117 (36) | |
| Disease duration (months), mean (SD) | 103.6 (82.6) | 115.7 (86) | 0.03 |
| Reasons switch to study drugs, No. (%) | |||
| Naïve | 168 (28.6) | 70 (22.2) | < 0.001 |
| Tolerability | 294 (50) | 162 (51.3) | |
| Lack of efficacy | 125 (21.3) | 84 (26.6) | |
| Line of therapy pre-switch, No. (%) | |||
| Naïve | 168 (28.6) | 70 (22.2) | < 0.001 |
| First line | 320 (54.5) | 153 (51.2) | |
| Second line | 99 (16.9) | 84 (26.6) | |
| Time on DMT pre-switch (months), mean (SD) | 36.6 (38.4) | 38.6 (45.6) | 0.68 |
| Relapses 24 months before switch, mean (SD) | 0.9 (1.2) | 0.8 (1) | 0.11 |
| Relapses 12 months before switch, mean (SD) | 0.7 (0.8) | 0.6 (0.7) | 0.34 |
| EDSS 12 months before switch | 1.5 (1–3) | 2 (1.5–3.5) | < 0.001 |
| EDSS 6 months before switch | 1.5 (1–3) | 2 (1.5–3.5) | < 0.001 |
| MRI T1 24 months before switch, mean (SD) | 4.4 (8.9) | 3.2 (6) | 0.84 |
| MRI T1 12 months before switch, mean (SD) | 4.5 (9) | 3.6 (7.5) | 0.48 |
| MRI T2 24 months before switch, mean (SD) | 14.9 (31.6) | 13.1 (30.6) | 0.51 |
| MRI T2 12 months before switch, mean (SD) | 15 (21.9) | 12 (16.4) | 0.49 |
| MRI Gad+ 12 months before switch, mean (SD) | 0.5 (1) | 0.4 (1.2) | 0.10 |
| MRI Gad+ 24 months before switch, mean (SD) | 0.2 (0.7) | 0.3 (0.7) | 0.78 |
Results are expressed as mean (SD), median (IQR), and No. (%). *Differences were evaluated via the Wilcoxon rank sum test or the chi-square test.
DMF, dimethyl fumarate; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; Gad+, gadolinium; IQR, interquartile range; MRI, magnetic resonance imaging; SD, standard deviation; TRF, teriflunomide.
We observed that the TRF group had a longer disease duration (⩾ 96 months) than the DMF group (p < 0.05). Furthermore, there were more pwRRMS who had not been previously treated with DMTs in the DMF group compared with the TRF group (p < 0.005). In the two groups, about 54.5% and 51.2% of pwRRMS had been previously treated with first-line therapies; poor tolerability was the most frequent reason for switching (50% and 51.3%, respectively).
The TRF group showed a higher level of disability (EDSS range [min–max] 1.5–3.5 versus 1.0–3.0 for DMF) (p < 0.0001).
The matching procedure improved the balance between the compared groups: no differences in the demographic and clinical characteristics were observed after applying the propensity score (Table 2).
Table 2.
Baseline characteristics of propensity score-matched patients.
|
Variable
|
DMF
|
TRF
|
SD* |
|---|---|---|---|
| No. Group | 234 | 234 | |
| Age, mean (SD) | 45.7 (9.9) | 44.9 (9.2) | 0.12 |
| Sex, No. (%) | |||
| Female | 148 (63.3) | 151 (64.5) | 0.01 |
| Male | 86 (36.8) | 83 (35.5) | |
| Disease duration (months), mean (SD) | 124.4 (85) | 125.8 (85.5) | 0.03 |
| Reasons for switch to oral drug, No. (%) | |||
| Naïve | 24 (10.3) | 25 (10.7) | 0.10 |
| Tolerability | 144 (61.5) | 162 (69.2) | |
| Lack of efficacy | 66 (28.2) | 47 (20) | |
| Line of therapy pre-switch, No. (%) | |||
| Naïve | 24 (10.3) | 25 (10.7) | 0.10 |
| First line | 144 (61.5) | 162 (69.2) | |
| Second line | 66 (28.2) | 47 (20) | |
| Time on DMT pre-switch (months), mean (SD) | 42 (44.4) | 46.8 (42) | 0.11 |
| Relapses 24 months before switch, mean (SD) | 0.8 (1) | 0.9 (1.2) | 0.12 |
| Relapses 12 months before switch, mean (SD) | 0.6 (0.7) | 0.6 (0.8) | 0.08 |
| EDSS 24 months before switch, median (IQR) | 2.5 (1.5–3.5) | 2 (1.5–3.5) | 0.05 |
| EDSS 12 months before switch, median (IQR) | 2.5 (1.5–3.5) | 2 (1.5–3.5) | 0.05 |
| EDSS 6 months before switch, median (IQR) | 2.5 (1.5–3.5) | 2 (1.5–3.5) | 0.04 |
| MRI T1 24 months before switch, mean (SD) | 3.2 (6.3) | 6.5 (11) | 0.35 |
| MRI T1 12 months before switch, mean (SD) | 3.7 (8) | 6.6 (11.9) | 0.28 |
| MRI T2 24 months before switch, mean (SD) | 13.4 (32.9) | 19.9 (39.5) | 0.15 |
| MRI T2 12 months before switch, mean (SD) | 12 (17.2) | 18.8 (25.6) | 0.29 |
| MRI Gad+ 24 months before switch, mean (SD) | 0.4 (1) | 0.36 (1) | 0.06 |
| MRI Gad+ 12 months before switch, mean (SD) | 0.3 (0.6) | 0.2 (0.4) | 0.17 |
Differences were evaluated via the standardized differences (SD). Standardized differences of 0.2, 0.5, and 0.8 represent small, medium, and large differences, respectively. Results are expressed as mean (SD), median (IQR), and No. (%).
DMF, dimethyl fumarate; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; Gad+, gadolinium; IQR, interquartile range; MRI, magnetic resonance imaging; SD, standard deviation; TRF, teriflunomide.
The SD for all postmatching characteristics was lower than 0.4. The results of the output variables comparison analysis are shown in Table 3.
Table 3.
Comparison of output variables between the two groups.
|
Variable
|
DMF
|
TRF
|
SD*
|
|---|---|---|---|
| No. Group | No. Group | 234 | 234 |
| NEDA 3, No. (%) | |||
| No disease activity | 181 (77.2) | 188 (80.3) | 0.42 |
| Disease activity | 53 (22.6) | 46 (19.7) | |
| NEDA-relapse, No. (%) | |||
| No disease activity | 209 (89.3) | 210 (89.7) | 0.87 |
| Disease activity | 25 (10.7) | 24 (10.3) | |
| NEDA-EDSS, No. (%) | |||
| No disease activity | 219 (93.6) | 216 (92.3) | 0.29 |
| Disease activity | 15 (6.4) | 18 (7.7) | |
| NEDA-MRI, No. (%) | |||
| No disease activity | 210 (89.7) | 216 (92.3) | 0.94 |
| Disease activity | 24 (10.3) | 18 (7.7) | |
| pw relapses12 months post beginning study drugs, No. (%) | 29 (10.7) | 28 (10.3) | 0.88 |
| EDSS 6 months post beginning study drug, median (IQR) | 2 (1.5–3.5) | 2 (1.5–3.5) | 0.42 |
| EDSS 12 months post treatment, median (IQR) | 1.5 (1.5–3.5) | 2 (1.5–3.5) | 0.28 |
| pw with new T2 lesions 12 months post beginning study drugs, No. (%) | 17 (7.3) | 13 (5.6) | 0.12 |
| pw with new T1 Gad+ lesions post beginning study drugs, No. (%) | 7 (3) | 5 (2.1) | 0.55 |
| MRI T1 12 months post treatment, mean (SD) | 2.4 (4.9) | 7 (11) | 0.10 |
| MRI T2 12 months post treatment, mean (SD) | 7.2 (14.2) | 10.8 (22.9) | 0.04 |
| MRI Gad+ 12 months post treatment, mean (SD) | 0.1 (0.5) | 0.3 (1.2) | 0.29 |
| pw who stopped study drugs, No. (%) | 10 (4.3) | 7 (3) | 0.45 |
| Safety alerts, No. (%) | 62 (26.5) | 28 (12) | < 0.001 |
Differences were evaluated via the Wilcoxon rank sum test or the chi-square test.
Results are expressed as mean (SD), median (IQR) and No. (%).
DMF, dimethyl fumarate; EDSS, Expanded Disability Status Scale; Gad+, gadolinium; IQR, interquartile range; MRI, magnetic resonance imaging; NEDA, no evidence of disease activity; pw, persons with; SD, standard deviation; TRF, teriflunomide.
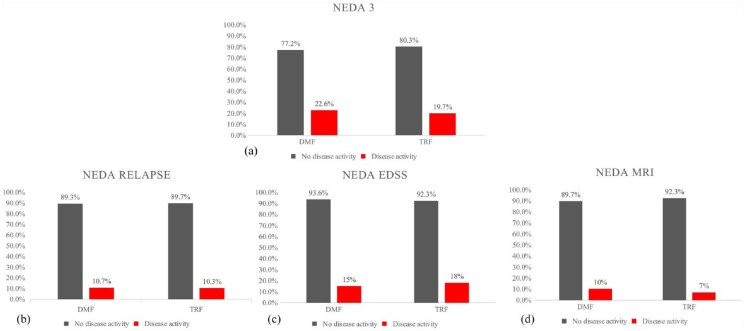
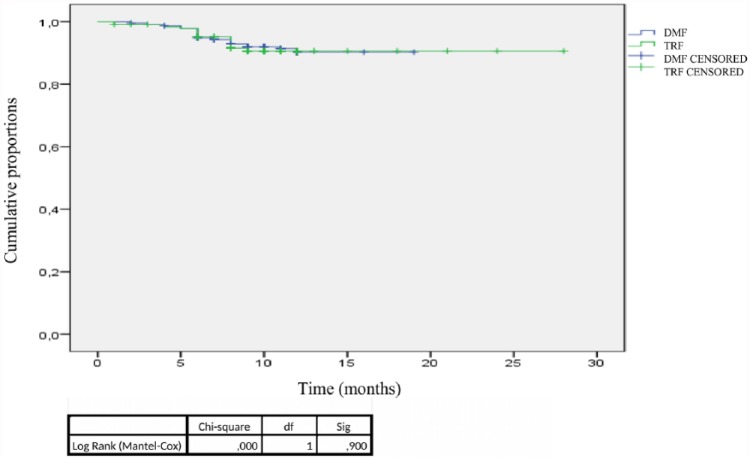
The NEDA 3 status at 12 months showed no differences between the two groups: 188 (80.3%) pwRRMS in the DMF group and 181 (77.2%) pwRRMS in the TRF group met the NEDA 3 criteria (Figure 2). Figure 2 shows that there were no differences between the two groups in the proportion of pwRRMS with NEDA 3 status for all three individual measures (i.e. new relapses, EDSS progression, new MRI lesions) after 12 months of follow up. The Kaplan–Meier survival curves showed no difference between the two groups in the time to reach the first relapse after 12 months of treatment (Figure 3). Among all pwRRMS, 10 (4.3%) in the DMF and 7 (3%) in the TRF group discontinued the study drugs. The reasons for drug discontinuation included a lack of efficacy for six pwRRMS on DMF and for two pwRRMS on TRF; this finding was not statistically significant.
Figure 2.
No evidence of disease activity (NEDA) and proportion of disease activity during 12 months in the two cohorts. (a) The proportion of the cohorts with NEDA and with disease activity is also assessed by individual measures: relapse (b), EDSS progression (c), and MRI (d).
DMF, dimethyl fumarate; EDDS, Expanded Disability Status Scale; MRI, magnetic resonance imaging; TRF, teriflunomide.
Figure 3.
Time to first relapse within 12 months in the two groups.
DF, degrees of freedom; DMF, dimethyl fumarate; TRF, teriflunomide.
Safety
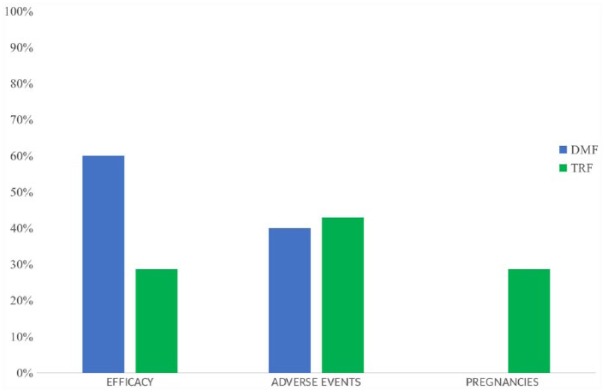
The percentage of pwRRMS who experienced any AE was 26.5% in the DMF group and 12% in the TRF group (p < 0.001). The rate of SAEs was similar between the two groups: 1.3% in the DMF group and 1.9% in the TRF group (Tables 4 and 5). An AE was the reason for drug discontinuation in four pwRRMS on DMF and in three pwRRMS on TRF. Two pwRRMS on TRF stopped therapy for pregnancy and an accelerated elimination procedure with cholestyramine was initiated (Figure 4).20 The cancers observed during the study period included thyroid cancer and melanoma. A medical multidisciplinary team concluded that the cancers were not related to the investigation drug. No pregnancies occurred during the observation period.
Table 4.
Frequency distribution of more common adverse events and serious adverse events in persons with relapsing remitting multiple sclerosis treated with dimethyl fumarate.
| Dimethyl fumarate | 62 (26.5), No. (%) | |
|---|---|---|
| Adverse events | Flushing, itch | 31 (13.3) |
| Diarrhea, colitis | 11 (5.6) | |
| Epigastralgia, nausea | 9 (3.8) | |
| Lymphopenia | 3 (2.1) | |
| Increase of transaminases | 2 (0.9) | |
| Herpes zoster reactivation | 1 (0.4) | |
| Depression | 1 (0.4) | |
| Serious adverse events | Severe lymphopenia | 2 (0.9) |
| Severe diarrhea | 2 (0.9) |
Table 5.
Frequency distribution of more common adverse events and serious adverse events in persons with relapsing remitting multiple sclerosis treated with terifluonomide.
| Terifluonomide | 28 (12), No. (%) | |
|---|---|---|
| Adverse events | Arterial hypertension | 6 (2.6) |
| Transaminases elevation | 5 (2.1) | |
| Alopecia | 4 (1.7) | |
| Nausea, vomiting | 3 (1.3) | |
| Neuropathy | 2 (1.7) | |
| Lymphopenia | 2 (0.9) | |
| Epstein–Barr virus reactivation | 1 (0.4) | |
| Flushing | 1 (0.4) | |
| Articular pain | 1 (0.4) | |
| Serious adverse events | Cancers | 2 (0.9) |
| Severe vomiting | 1 (0.4) |
Figure 4.
Reasons for withdrawal from the two study drug treatments during 12 months.
DMF, dimethyl fumarate; TRF, teriflunomide.
Discussion
In our population, the treatment with TRF and DMF resulted in the control of disease activity (assessed by NEDA 3 and time to the new first clinical relapse) at 12 months of follow up. DMF and TRF are licensed as first-line therapy options in RRMS; their approval has changed the treatment of early RRMS. In pivotal trials, both showed similar efficacy in controlling the clinical and MRI disease activity of RRMS with a generally good tolerability and safety profile.4–7,21
No head-to-head clinical trials have compared the efficacy of DMF versus TRF as measured by NEDA.
A recent multicomparative analysis of MS outcomes in pwRRMS treated with DMF compared with propensity-matched fingolimod, interferon, glatiramer acetate, and TRF treated patients showed that DMF resulted in a similar risk of experiencing a new relapse to that of a matched cohort of fingolimod-treated patients. Conversely, DMF was associated with a statistically significant reduction in risk of relapse relative to interferon, glatiramer acetate, and TRF.22
Similarly, at the American Academy of Neurology’s 2017 meeting, no difference in risk of relapse between fingolimod and DMF was described (HR 0.995; p = 0.94), while TRF was associated with a significantly higher risk of experiencing relapse (HR 1.302; p < 0.01). Similar findings were observed in the subgroups stratified by receipt of DMTs in the prior year (newly treated patients versus patients previously treated with a different agent). The results were adjusted for demographics, baseline comorbidities, MS symptoms, prior DMT use, and pre-enrollment annualized relapse rate.23
Finally, a comparative effectiveness research analysis from a large health insurance claims database was performed to estimate the relative risk of relapse between MS patients treated with fingolimod or TRF compared with DMF after adjusting for demographics, baseline comorbidities, MS symptoms, prior MS drug use, and pre-index annualized relapse rate. It showed that TRF was associated with a 30% (HR 1.302; p < 0.01) increase in relative risk of MS relapse compared with DMF.9
In summary, our data are informative regarding treatment decisions in pwRRMS. Prior to matching, the data showed that the proportion of men and older patients was greater in the TRF group than in the DMF group. This may reflect Italian clinical practice pattern, and the concern about prescribing TRF to women of childbearing potential. Recent data on the outcomes of 83 pregnancies among TRF-treated women are mitigating such concerns.24
Interestingly, more than 50% of pwRRMS in our population had experienced another treatment prior to the initiation of DMF or TRF. This may reflect the rapid shift from injectables (due to AEs, poorer compliance, and the unwillingness of pwRRMS to undergo injections) upon the introduction of oral agents in Italy.
The majority of pwRRMS in our study had mild functional disability (EDSS < 3.0). The disability levels of our pwRRMS mirror those populations enrolled in the pivotal trials of the investigated drugs. Moreover, in Italian clinical practice, pwRRMS with highly active disease and with worsening disability are usually stratified to second-line therapies such as fingolimod, natalizumab, or alemtuzumab.
After the propensity-score adjustment, the data showed the efficacy of DMF and TRF in impacting RRMS activity.
Recent postmarketing studies have investigated the efficacy and safety of DMF and TRF in RRMS.25–27 An observational study performed in Kuwait which evaluated 119 pwRRMS on DMF found 89.9% of pwRRMS to be free from relapses, 85% of pwRRMS to be free from new brain MRI findings, and 93% of pwRRMS to be free from disability accrual (assessed by EDSS) after a mean observational period of 20 months. No composite measure such as NEDA was evaluated.28
A recent retrospective study examining 102 pwRRMS treated with TRF showed that approximately 10% of pwRRMS experienced relapses during the treatment period; 40% of these relapses occurred during the first 6 months of therapy. Of 74 pwRRMS treated with TRF, the EDSS remained constant in approximately 67% of this population during 15 (±5.3) months of follow up. Of 30 pwRRMS treated with TRF, 47% of pwRRMS experienced new T2 brain lesions on MRI.29
In our study, pwRRMS on TRF experienced more progression of MRI activity than those treated with DMF at the time of follow up. A correlation between MRI T2 lesion load and MS disease duration and disability level has previously been described.
One limitation to our study was the relatively short follow-up period. Detecting significant changes in EDSS during this short period is challenging, due to the intrinsic bias of such outcomes measure.30 Therefore, our findings on disability accrual and also on disease activity (clinical and/or radiological) need confirmation over a longer observation time. Moreover, the mean age of our cohort was more than 40 years, and age is a well-known modulator of a therapy’s efficacy.31 Moreover, the propensity-score model cannot adjust for unexpected bias such as physicians’ recommendations in medical treatment decisions.
In our study, the percentage of AEs was higher in the TRF group compared with the DMF group. Despite this, the proportion of SAEs leading to discontinuation of the study drug was similar between the two groups.
The most commonly reported AEs for the DMF group included flushing, diarrhea, and abdominal pain. These findings are similar to previously reported data.13
pwRRMS treated with DMF in our study experienced fewer SAEs than previously reported in registered trials in which 12% of patients who received DMF were found to have SAEs.7
In the DMF group in our study, lymphopenia was reported in five patients and necessitated discontinuation of the drug in two of them. Notably, the pwRRMS who discontinued therapy for lymphopenia had a longer disease duration (mean of 12 years) and had received more than one prior therapy. These data suggest that certain individuals may have a poorer tolerability of drugs, thus requiring multiple changes in DMTs in RRMS. We plan to further investigate this possibility in extended follow-up studies.
The ENDORSE trial, an ongoing 12-year extension study of DEFINE/CONFIRM evaluating the long-term safety, efficacy, and overall risk–benefit profile of DMF in patients with RRMS, maintains a favorable outlook on the long-term use of this drug despite the occurrence of AEs.26
In our study, the most commonly reported AEs associated with the use of TRF included elevated transaminases, alopecia, and increased blood pressure.
Recent reports on safety outcomes from up to 9 years of treatment with TRF in an extension (NCT00803049) of the pivotal phase III Teriflunomide Multiple Sclerosis Oral (TEMSO) trial showed that approximately 11% of pwRRMS discontinued TRF treatment due to AEs. Around 20% of pwRRMS treated with this agent experienced SAEs. There were three deaths in the study, unrelated to TRF.8
Limitations of our study included the absence of blinding, which may have introduced detection and reporting bias. However, the follow-up protocols used in our study were largely comparable, and as such, the magnitude of such a bias is expected to be minimal. Although the data entry at our center typically occurs in real time or soon thereafter, some variables such as relapse-related information may be susceptible to recall bias. Finally, despite evidence showing the positive predictive value of NEDA 3 as a prognostic indicator at 7 years, the accuracy of this data point as representative of true disease activity may be called into question by some.30
This study compared treatment outcomes over 12 months and evaluated disability accumulation events over 6–12 months, which are highly indicative of long-term disability outcomes.
Although the analyses of these observational data do not serve as a substitute for randomized clinical trials, our study does provide practical evidence representative of clinical care in tertiary MS centers and allows for valuable insights into the challenges of therapeutic management of RRMS.
Acknowledgments
We would like to thank BioMed Proofreading® for language editing. We are also grateful to Reload Onlus for supporting our projects.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: ED, GC, GB, AG, and MB received personal fees from Biogen and Sanofi. ED, GC, GB, AG, PV, and MB received travel funding from Bayer Biogen and Merck. AZ received travel funding from Bayer Schering and Sanofi Genzyme outside the submitted work. PV received personal fees from Biogen, Sanofi, Novartis, and Roche. SC and GS received personal fees for speaking activities from Bayer Biogen, Merck, Novartis, and Teva. PR received travel expenses or honoraria for consultancy from Merck Serono, Biogen idec, Novartis, Sanofi Genzyme, and Teva. RBB served on the advisory board for Almirall and received grants for congress participation from Sanofi and Merck. LMEG and CP served on advisory boards for Bayer, Biogen, Celgene, Merck, Novartis, Roche, Sanofi, and Teva. LMEG and CP also received personal fees for speaking activities at congresses or sponsored symposia. GT, MZ, and FP served on advisory boards for Bayer, Biogen, Celgene, Merck, Novartis, Roche, Sanofi, Teva, and Almirall. GT, MZ, and FP also received personal fees for speaking activities at congresses or sponsored symposia.
ORCID iD: Francesco Patti  https://orcid.org/0000-0002-6923-0846
https://orcid.org/0000-0002-6923-0846
Contributor Information
Emanuele D’Amico, Department G.F. Ingrassia, Multiple Sclerosis Center, University of Catania, Italy.
Aurora Zanghì, Department G.F. Ingrassia, Multiple Sclerosis Center, University of Catania, Italy.
Graziella Callari, Institute Foundation G. Giglio, Cefalù, Italy.
Giovanna Borriello, S. Andrea Hospital, Sapienza Rome University, Rome, Italy.
Antonio Gallo, I Neurologic Clinic, Federico II University of Naples, Italy.
Giusi Graziano, Giovanni Paolo II, IRCCS Cancer Institute, Bari, Italy.
Paola Valentino, Azienda Ospedaliera Universitaria Mater Domini, Catanzaro, Italy.
Maria Buccafusca, Azienda Ospedaliera Universitaria G. Martino, Messina, Italy.
Salvatore Cottone, Azienda Ospedaliera Ospedali Riuniti Villa Sofia-Cervello, Palermo, Italy.
Giuseppe Salemi, Policlinico Paolo Giaccone, Palermo, Italy.
Paolo Ragonese, Policlinico Paolo Giaccone, Palermo, Italy.
Roberto Bruno Bossio, Azienda Sanitaria Provinciale di Cosenza, U. O. di Neurologia, Cosenza, Italy.
Renato Docimo, I Neurologic Clinic, Federico II University of Naples, Italy.
Luigi Maria Edoardo Grimaldi, Institute Foundation G. Giglio, Cefalù, Italy.
Carlo Pozzilli, S. Andrea Hospital, Sapienza Rome University, Rome, Italy.
Gioacchino Tedeschi, I Neurologic Clinic, Federico II University of Naples, Italy.
Mario Zappia, Department G.F. Ingrassia, Multiple Sclerosis Center, University of Catania, Italy.
Francesco Patti, Department G. Ingrassia, Policlinico G. Rodolico, V. Santa Sofia 78, 95123, Catania, Italy.
References
- 1. Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med 2018; 378(2): 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. D’Amico E, Leone C, Caserta C, et al. Oral drugs in multiple sclerosis therapy: an overview and a critical appraisal. Expert Rev Neurother 2015; 15(7): 803–824. [DOI] [PubMed] [Google Scholar]
- 3. Ingwersen J, Aktas O, Hartung HP. Advances in and algorithms for the treatment of relapsing-remitting multiple sclerosis. Neurotherapeutics 2016; 13(1): 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arnold DL, Gold R, Kappos L, et al. Effects of delayed-release dimethyl fumarate on MRI measures in the phase 3 DEFINE study. J Neurol 2014; 261(9): 1794–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Neurol 2014; 13(3): 247–256. [DOI] [PubMed] [Google Scholar]
- 6. Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367(12): 1087–1097. [DOI] [PubMed] [Google Scholar]
- 7. Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367(12): 1098–1107. [DOI] [PubMed] [Google Scholar]
- 8. O’Connor P, Comi G, Freedman MS, et al. Long-term safety and efficacy of teriflunomide: nine-year follow-up of the randomized TEMSO study. Neurology 2016; 86(10): 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boster A, Nicholas J, Wu N, et al. Comparative effectiveness research of disease-modifying therapies for the management of multiple sclerosis: analysis of a ge Health Insurance Claims Database. Neurol Ther 2017; 6(1): 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deleu D, Mesraoua B, Canibano B, et al. Oral disease-modifying therapies for multiple sclerosis in the Middle Eastern and North African (MENA) region: an overview. Curr Med Res Opin 2018; 18: 1–12. [DOI] [PubMed] [Google Scholar]
- 11. Freedman MS, Montalban X, Miller AE, et al. Comparing outcomes from clinical studies of oral disease-modifying therapies (dimethyl fumarate, fingolimod, and teriflunomide) in relapsing MS: assessing absolute differences using a number needed to treat analysis. Mult Scler Relat Disord 2016; 10: 204–212. [DOI] [PubMed] [Google Scholar]
- 12. Aubagio (teriflunomide) tablets, http://products.sanofi.eu/aubagio (accessed April, 30 2018).
- 13. Tecfidera (dimethyl fumarate), http://www.ema.europa.eu/docs/it_IT/document_library/EPAR_-_Product_Information/human/002601/WC500162069.pdf (accessed April, 30 2018).
- 14. What is MSBase. MSBase registry website, https://www.msbase.org (accessed April 30).
- 15. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50(1): 121–127. [DOI] [PubMed] [Google Scholar]
- 16. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33(11): 1444–1452. [DOI] [PubMed] [Google Scholar]
- 17. Havrdova E, Galetta S, Stefoski D, et al. Freedom from disease activity in multiple sclerosis. Neurology 2010; 74(Suppl. 3): S3–S7. [DOI] [PubMed] [Google Scholar]
- 18. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/09/WC500172402.pdf (accessed April, 30 2018).
- 19. U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE) version 4.0, May 28, 2010. National Institutes of Health National Cancer Institute; (accessed April, 30 2018). [Google Scholar]
- 20. Sanofi Genzyme. https://www.aubagiohcp.com/content/pdf/drug_elimination_guide.pdf (accessed April, 30 2018).
- 21. Miller DH, Fox RJ, Phillips JT, et al. Effects of delayed-release dimethyl fumarate on MRI measures in the phase 3 CONFIRM study. Neurology 2015; 84(11): 1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spelman T, Kalincik T, Trojano M, et al. Comparative analysis of MS outcomes in dimethyl fumarate-treated patients relative to propensity matched fingolimod, interferon, glatiramer acetate, or teriflunomide. Mult Scler J 2016; 22: 602–603. [Google Scholar]
- 23. Nicholas J, Boster A, Wu N, et al. Comparative effectiveness of delayed-release dimethyl fumarate versus fingolimod and teriflunomide on risk of relapse. Neurology 2017; 88(16): P6-375. [Google Scholar]
- 24. Kieseier BC, Benamor M. Pregnancy outcomes following maternal and paternal exposure to teriflunomide during treatment for relapsing-remitting multiple sclerosis. Neurol Ther 2014; 3(2): 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Everage NJ, Prada C, Liu S, et al. Safety and efficacy of delayed-release dimethyl fumarate in multiple sclerosis patients treated in routine medical practice: interim analysis of ESTEEM (P6.333). Neurology 2017; 88(16): P6.33. [Google Scholar]
- 26. Gold R, Arnold DL, Bar-Or A, et al. Long-term effects of delayed-release dimethyl fumarate in multiple sclerosis: interim analysis of ENDORSE, a randomized extension study. Mult Scler 2017; 23(2): 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O’Connor P, Comi G, Freedman MS, et al. Long-term safety and efficacy of teriflunomide: nine-year follow-up of the randomized TEMSO study. Neurology 2016; 86(10): 920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alroughani R, Ahmed SF, Behbehani R, et al. Effectiveness and safety of dimethyl fumarate treatment in relapsing multiple sclerosis patients: real-world evidence. Neurol Ther 2017; 6(2): 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elkjaer ML, Molnar T, Illes Z. Teriflunomide for multiple sclerosis in real-world setting. Acta Neurol Scand 2017; 136: 447–453. DOI: 10.1111/ane.12755. [DOI] [PubMed] [Google Scholar]
- 30. Rotstein DL, Healy BC, Malik MT, et al. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 2015; 72(2): 152–158. [DOI] [PubMed] [Google Scholar]
- 31. Weideman AM, Tapia-Maltos MA, Johnson K, et al. Meta-analysis of the Age-Dependent Efficacy of Multiple Sclerosis Treatments. Front Neurol. 2017. November 10; 8: 577. doi: 10.3389/fneur.2017.00577. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]