Abstract
Policy Points:
Policymakers in the United States should consider expanding pharmacy practice laws to allow pharmacists to vaccinate adolescents as a way to improve geographic access to adolescent vaccines, particularly for human papillomavirus (HPV) vaccine, which has low uptake.
Our state‐level analysis showed that pharmacists are more geographically dispersed than primary care physicians in the US state of Texas.
Including pharmacists among available adolescent vaccine providers would improve the geographic distribution of vaccine providers, especially in areas with an inadequate number of primary care physicians.
Context
The largest disparities in human papillomavirus (HPV) vaccination in the United States are due to geography. One potential way of addressing these disparities is by improving geographic access to HPV vaccination. Two federal panels have recommended including community pharmacists as HPV vaccine providers as a strategy to improve opportunities for HPV vaccination for adolescents. We sought to evaluate whether community pharmacists can improve the number of vaccine providers in areas with primary care physician shortages in the US state of Texas.
Methods
We gathered publicly available physician and pharmacist 2016 workforce data from the Texas Medical Board and Board of Pharmacy. We conducted geospatial analysis of census tracts to analyze the distribution of physicians and pharmacists and how pharmacists change vaccine provider coverage across the state.
Findings
Census tracts with high numbers of physicians per capita tended to be located near one another, in 5 of 5 analyses of Moran's I (median = .04). In contrast, pharmacist rates were not spatially dependent on census tract in any of our analyses. If pharmacists were added to primary care physicians as vaccine providers, 35% of urban census tracts that previously had inadequate coverage would be adequately covered, while 18% of inadequately covered rural census tracts would become adequately covered. Overall, when pharmacists were included with primary care physicians as vaccine providers, vaccine providers per capita increased in 2,413 of the 4,508 urban census tracts (54%), while the rate increased in 223 of 746 rural census tracts (30%).
Conclusions
Pharmacists are more geographically dispersed across census tracts than primary care physicians. As a result, adding pharmacists to the workforce would increase the availability of vaccine providers in areas with inadequate primary care provider coverage.
Keywords: HPV vaccine, access to health care, pharmacists, geographic factors
The largest disparities in human papillomavirus (HPV) vaccination coverage in the United States are geographic rather than related to race, ethnicity, and socioeconomic status, varying dramatically across states and regions and by rurality.1, 2, 3, 4, 5, 6, 7 For instance, HPV vaccine initiation is higher among racial and ethnic minorities and the poor,6 while uptake is lower in rural areas compared to urban ones. A growing body of state‐specific studies has identified substantial variation in vaccine coverage by counties and census tracts.7, 8, 9, 10 Geographic disparities in cancer incidence11, 12 and mortality12 exist alongside lower use of preventive services. As an example, high cervical cancer burden in Appalachia is associated with low HPV vaccination coverage,13 a pattern that is repeated in analyses of state‐level data.14 Spatial targeting of public health interventions may therefore reduce geographic disparities. One way geographic disparities could arise is through the unequal distribution of health care workers available to a population.
The Behavioral Model of Health Services Use describes various individual, community, and societal factors that enable health care utilization such as vaccination, income, education, health insurance coverage, and health systems organization.15 While all these factors interplay to enable patients to obtain HPV vaccinations, a key motivator for easy access to this vaccination service is the availability of health care facilities and providers who administer HPV vaccine. Typically, HPV vaccination research evaluates access to and interactions with primary care providers such as pediatricians and family medicine physicians, as most adolescent vaccinations in the United States are given in practices with these 2 specialties.16, 17 However, a 2010 study found a large portion of families with children have limited access to a primary care physician despite the substantial increase in physicians who see children in the United States.18 As a result, poor primary care coverage in certain areas may be a result of maldistribution of primary care physicians, rather than due to an absolute shortage in the number of primary care physicians. Taken together with evidence of lower health care use among adolescents,19, 20, 21 additional modes of access to HPV vaccination may be warranted as a strategy to improve vaccination coverage.
A recent review of nontraditional settings for HPV vaccination found that pharmacies are particularly well suited to reach US adolescents, given their easy access within US communities, their history administering vaccinations, and their ability to furnish vaccinations in large volumes.22 Between 2014 and 2015, the President's Cancer Panel and the National Vaccine Advisory Committee recommended expanding HPV vaccine provision in pharmacies to help improve access and opportunities for HPV vaccination.23, 24 Fifty states and US territories allow pharmacists to administer HPV vaccine (the exceptions are New Hampshire and New York), but the level of autonomy pharmacists have to vaccinate age‐eligible adolescents varies greatly.25 For instance, pharmacy practice laws may restrict vaccination practices to certain ages25 or by the mechanism through which pharmacists can administer HPV vaccine (eg, independent authority, standing order protocol, or by prescription only).26 Currently, community pharmacists typically do not provide HPV vaccine to age‐eligible adolescents, but if they did begin providing it in those states where it is allowed, they could have a meaningful role in expanding HPV vaccination access. This may be particularly germane in states like Texas where populations are dispersed across large geographic areas, the distribution of primary care physicians has limited their ability to meet population health needs, and pharmacists are allowed to vaccinate adolescents with HPV vaccine as young as 14 through standing order protocol or younger than 14 through physician referrals. The purpose of our study is to characterize the geographic distribution of primary care physicians who typically provide HPV vaccination in 1 US state, Texas, and to evaluate whether adding community pharmacists as vaccine providers would improve access to HPV vaccination services in primary health care shortage areas.
Some evidence suggests that people live closer to pharmacists than they do to their primary care providers. The National Association of Chain Drug Stores reported that 93% of US residents live within 5 miles of a community pharmacy.27 The vast majority of pharmacies also have substantial retail operations, which may allow them to be commercially successful in areas where primary care practices would struggle financially. As such, we hypothesized that primary care physicians are more spatially clustered than pharmacists (Hypothesis 1). Furthermore, if pharmacists are more dispersed geographically than primary care physicians, this dispersion may be especially important for high‐need areas, as pharmacists could increase access to vaccination services in areas with poor primary care provider coverage. As such, we also hypothesized that the number of areas with adequate health care provider coverage increases if pharmacists are included with primary care physicians as adolescent vaccine providers (Hypothesis 2). While other important health care professionals such as nurse practitioners and physician assistants also likely provide adolescent vaccines, we were unable to obtain sufficiently accurate practice address location or practice specialty information to include these providers in our study. Therefore, our study focuses on primary care physicians who are likely to administer HPV vaccine and community pharmacists who are legally allowed to provide HPV vaccine to adolescents.
Methods
Data Sources and Procedures
We used publicly available data to determine the geolocation and other characteristics of primary care physicians and pharmacists in Texas.
Primary Care Physicians. Physician workforce data are publicly available from the Texas Medical Board (http://store.tmb.state.tx.us/). Our data set included physicians licensed by December 2016 and contained information about each physician's license status, registration dates, primary and secondary specialties, and practice type and setting. Four physicians (1 pediatrician, 1 gynecologist, and 2 family medicine physicians) advised on the criteria used to identify primary care physicians to include in this data set. We included physicians who had an active practice license, a verifiable practice address in Texas that could be geocoded, and a primary specialty in family medicine, general practice, obstetrics and gynecology, pediatrics, public health and preventive medicine, or urgent care medicine (Figure 1A). The final analytic sample had 12,307 primary care physicians whose sociodemographic characteristics are described in Online Appendix Table 1.
Figure 1A.
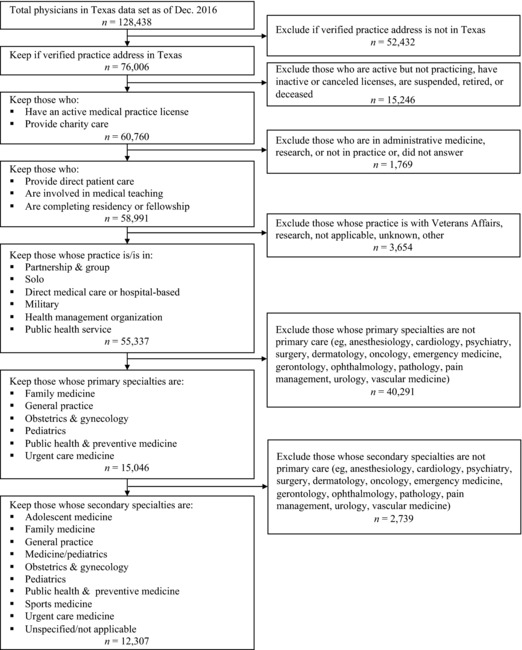
Flow Diagram of Inclusion Criteria for Primary Care Physicians
Community Pharmacists. Pharmacist workforce data are publicly available from the Texas Board of Pharmacy (http://www.pharmacy.texas.gov/dbsearch/tables.asp). Our data set included pharmacists licensed by December 2016 and contained information about each pharmacist's license status, registration dates, and practice setting. Pharmacists were included in the analytic sample if they had an active practice license, had a verifiable practice address in Texas that could be geocoded, and identified a community pharmacy as their employment type (Figure 1B). The final analytic sample had 11,131 pharmacists whose sociodemographic characteristics are described in Online Appendix Table 1.
Figure 1B.
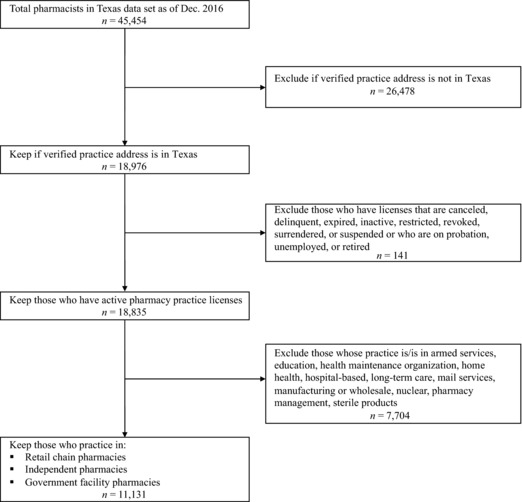
Flow Diagram of Inclusion Criteria for Community Pharmacists
Texas Census Tracts. We collected census tract geographic boundaries and demographic characteristics for Texas from the US Census Bureau via 2016 TIGER/Line® shapefiles (ie, data files that contain geometric location and attribute information on geographic features; http://census.gov/geo/maps-data/data/tiger-data.html). We used the 2010 Decennial Census for Texas population counts for each census tract. We also collected neighborhood sociodemographic characteristics of each census tract using 5‐year estimates from the 2012‐2016 American Community Survey (ACS). Texas has 5,254 census tracts with populations ranging from 0 to 33,201 people (mean = 4,786, standard deviation [SD] = 2,433).
Geocoding Procedures. First, we geocoded the physicians' and pharmacists' locations as points using their given practice addresses. Next, in order to get counts of physicians and pharmacists in each census tract, the points representing the providers were joined to the shapefile containing the census tracts’ geographic boundaries and demographic characteristics. Only points that lay within the boundaries of each areal unit were counted as being contained within that unit. Since practice addresses were verified for both physicians and pharmacists, no individual provider was dropped during the geocoding process. This process was conducted in ESRI ArcGIS 10.5 (Redlands, California).
Statistical Analyses
Analyses determined the spatial distribution of physicians and pharmacists and evaluated how pharmacists changed the adequacy of vaccine provider rates in census tracts.
Spatial Clustering of Providers. First, we evaluated the extent of spatial clustering (spatial autocorrelation) of physicians and pharmacists in Texas with Moran's I,28 using census tracts as the units of analysis. Moran's I is a global test statistic that provides a summary of the entire study area of the level of spatial similarity observed among neighboring observations,29 such as the rates of physicians and pharmacists in census tracts. We defined neighboring observations using 3 methods as a means to verify the robustness of our analysis: (1) contiguity neighbor method using a first‐order queen criterion that includes all census tracts that share a border; (2) an inverse‐distance band method with a set threshold and a nearest‐neighbor parameter of 1,30 in which census tracts whose centroids are within 8,047 meters (5 miles) of each other are considered neighbors, and if there are none, then the closest census tract is considered the neighbor; and (3) an inverse‐distance band method in which the software maximized the distance threshold so that all census tracts had at least 1 neighbor.30 We also calculated Moran's I using both Euclidean (ie, ordinary straight‐line distance between 2 points) and Manhattan (ie, distance between 2 points based on strictly horizontal and vertical paths) distances for the inverse‐distance methods, since Euclidean distances tend to underestimate road distances and travel times, while Manhattan distances tend to overestimate both.31 The interpretation of the Moran's I is similar to the Pearson's product‐moment correlation coefficient in that values range from −1 to 1. We used provider rates (ie, providers per capita) in each census tract for the Moran's I statistical test to adjust for the tendencies of areas with larger populations to have more providers.
Change in Providers Per Capita With Pharmacist Inclusion. Next, we analyzed how census tracts’ vaccine providers per capita changed with the inclusion of pharmacists, as a way to determine whether pharmacists can help improve access to HPV vaccination in areas with primary care health professional shortages. Primary care health professional shortage areas (HPSAs) are defined as having 1 or fewer full‐time‐equivalent primary care physician per 3,500 people living in a geographic area.32 Using the previously calculated physician and pharmacist rates, we standardized the vaccine provider rates per 3,500 people. We then added the 2 rates to get an overall provider rate per 3,500 people. To address skewness, we winsorized outlying provider rates to a value of 30 or more providers per 3,500 people (ie, at or above 99th percentile). To evaluate if HPSAs moved to adequate provider coverage with the inclusion of pharmacists, we created 2 dichotomous variables. The first variable indicated whether a census tract had inadequate coverage (coded “0”), if the physician‐to‐population ratio was less than or equal to 1:3,500, or had adequate coverage (coded “1”), if the physician‐to‐population ratio was greater than 1:3,500. The second variable used the same coding scheme, but for physician‐and‐pharmacist‐to‐population ratio less than or equal to 1:3,500 (coded “0”) or greater than 1:3,500 (coded “1”). We then examined the percentage of census tracts that shifted to adequate provider coverage when pharmacists were included in the provider rate.
We next performed several subgroup analyses. First, we conducted paired t‐tests to compare the mean providers per capita, comparing data for physicians only vs physicians and pharmacists. Second, we conducted sign tests to evaluate whether median providers per capita increased when pharmacists were included with physicians. Third, we stratified analyses by urban and rural census tracts using the 2010 Census classification, where tracts with a population greater than 2,500 were designated as urban areas (eg, “urbanized areas” or “urban clusters”).33 We reported providers per capita at interquartile cutoffs to show how pharmacists changed vaccine providers per capita at the 25th, 50th, and 75th percentiles. Finally, we generated choropleth maps (Figures 2 and 3) to visually depict where providers per capita increased in Texas when pharmacists and physicians were included.
Figure 2.
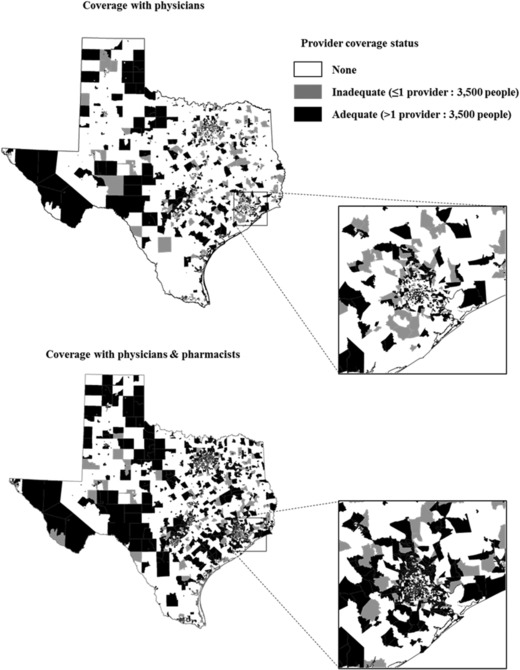
Adequacy of Vaccine Provider Coverage by Census Tract
Figure 3.
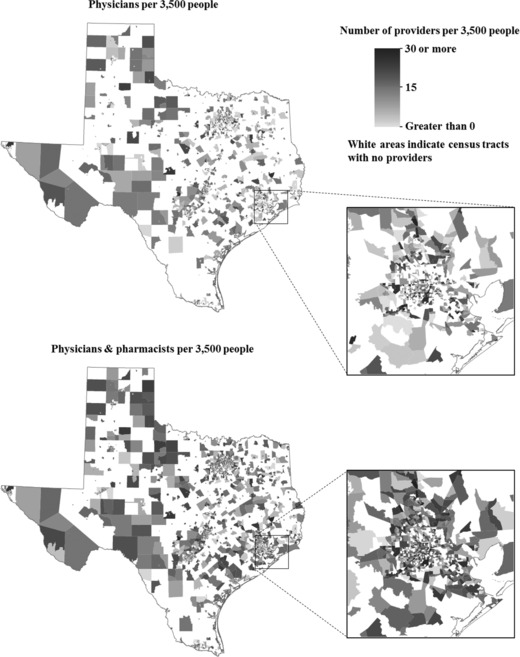
Rate of Vaccine Providers per 3,500 People by Census Tract
In our final set of analyses, we evaluated how vaccine providers per capita varied across census tracts using an overall measure of neighborhood socioeconomic status called the Area Deprivation Index (ADI). G. K. Singh's ADI is a proxy measure of socioeconomic status for a geographic area using 17 indicators grouped into 4 domains: poverty, housing, employment, and education.34 ADI scores for geographic areas have shown validity in predicting patient‐level health outcomes and health care utilization.35 We calculated ADI scores for each census tract in Texas using data from the 2012‐2016 ACS with methods developed by Knighton and colleagues.35 ADI scores in Texas census tracts ranged from −140 (least deprived) to 127 (most deprived; mean = 100, SD = 20). We conducted one‐way analysis of variance to evaluate the mean vaccine providers per capita across census tracts grouped into ADI score quintiles.
The Moran's I test statistic and choropleth map generation were conducted in ESRI ArcGIS 10.5. Data cleaning, manipulation, and statistical tests were conducted in Stata 15.1 (College Station, Texas). All analyses used 2‐tailed statistical tests with a critical = .05.
Results
Spatial Clustering of Providers
Census tracts with high numbers of physicians per capita tended to be located near other census tracts with high physician‐per‐capita ratios (ie, spatial clustering). In analyses at the level of census tracts, physicians per capita exhibited spatial clustering in 5 of 5 analyses (median I = .04; Table 1). Spatial clustering was detectable using the contiguity neighbor method (I = .11, p < .001) and both inverse‐distance methods using Euclidian (approach 1: I = .032, p < .001; approach 2: I = .009, p < .001) and Manhattan distance calculations (approach 1: I = .040, p < .001; approach 2: I = .015, p < .001). However, the ratio of pharmacists per capita by census tract did not indicate any form of spatial dependence using any of the analytic approaches (median I = .00).
Table 1.
Global Test of Spatial Autocorrelation of Physicians and Pharmacists Per Capita: Moran's I a
| Physicians | Pharmacists | |||||||
|---|---|---|---|---|---|---|---|---|
| Euclidean | Manhattan | Euclidean | Manhattan | |||||
| Census Tract | Moran's I | p‐value | Moran's I | p‐value | Moran's I | p‐value | Moran's I | p‐value |
| Contiguity neighborsb | .11 | <.001 | — | — | −.0005 | .50 | — | — |
| Inverse‐distance (approach 1)c | .032 | <.001 | .040 | <.001 | −.0002 | .91 | −.0002 | .17 |
| Inverse‐distance (approach 2)d | .009 | <.001 | .015 | <.001 | −.0004 | .13 | −.0004 | .10 |
The expected value of Moran's I for 5,254 census tracts is −.00019.
Neighbors were assigned using first‐order queen method where census tracts that share a border are neighbors. Contiguity neighbor method does not depend on Euclidean and Manhattan distance calculations.
Census tracts whose centroids were 8,047 meters (5 miles) from other census tracts were considered neighbors. A minimum of 1 identified neighbor was specified in cases where the distance measured from the centroid of a census tract to nearest neighboring census tract was greater than 8,047 meters.
Distance bands were optimized to 84.25 kilometers (Euclidean) and 118.69 kilometers (Manhattan) to ensure at least 1 neighbor for each census tract due to very large census tracts in western Texas.
Provider Rate Change With Pharmacist Inclusion
We next examined how adding pharmacists as vaccine providers would change adequacy of provider coverage, as well as the average number of vaccine providers in each census tract, and whether vaccine coverage improvements varied by rurality and ADI measure.
Adequate Provider Coverage. Adequate provider coverage with only primary care physicians was present in 33% of census tracts (1,720/5,254; Table 2). When pharmacists were included, 55% of census tracts (2,867/5,254) had adequate provider coverage. Thus, among census tracts with inadequate provider coverage, 32% shifted to adequate coverage (1,147/3,534). A visualization of this shift appears in choropleth maps in Figure 2, where black (or gray) areas represent tracts with adequate (or inadequate) provider coverage before and after including pharmacists. Among census tracts that had inadequate or no provider coverage with physicians alone, 35% of urban census tracts became adequate coverage areas with the inclusion of pharmacists (1,055/3,013), while 18% of rural census tracts became adequate coverage areas with the inclusion of pharmacists (92/521).
Table 2.
Vaccine Providers per Census Tract (Rates per 3,500 People)a
| Provider Rate at Each Percentile | |||||
|---|---|---|---|---|---|
| Mean (SD)b | 25th Percentile | 50th Percentile | 75th Percentile | Adequate Vaccine Provider Coverage | |
| Physicians | |||||
| Overall | 1.73 (4.19) | 0 | 0 | 1.59 | 33% (1,720/5,254) |
| Urban | 1.64 (3.83) | 0 | 0 | 1.56 | 33% (1,495/4,508) |
| Rural | 2.31 (5.89) | 0 | 0 | 1.72 | 30% (225/746) |
| Physicians and pharmacists | |||||
| Overall | 3.38 (6.13) | 0 | 1.32 | 4.08 | 55% (2,867/5,254) |
| Urban | 3.23 (5.41) | 0 | 1.39 | 4.05 | 57% (2,550/4,508) |
| Rural | 4.38 (9.33) | 0 | 0 | 4.36 | 42% (317/746) |
Abbreviation: SD, standard deviation.
Based on analyses of data for 5,254 census tracts (4,508 urban and 746 rural). Census tracts are designated urban areas if they have at least 2,500 people based on the 2010 Census urban and rural classifications.34
Means are based on provider rates where outliers were winsorized to 30 or more providers per 3,500 people.
Provider Coverage by Rurality. The average number of providers per capita rose when comparing physician‐only rates with physician‐and‐pharmacist rates in both urban and rural census tracts (urban: t = −44.3, p < .001; rural: t = −11.8, p < .001; Table 2). Increases occurred disproportionately, with the largest increases in those tracts that initially had relatively higher provider rates. The 25th percentile provider rates remained unchanged with the inclusion of pharmacists across the urban and rural stratifications. The overall median (50th percentile) rate increased from 0 to 1.32 providers per 3,500 people (p < .001) with the inclusion of pharmacists. Among urban census tracts at the 50th percentile, the vaccine provider per capita rate increased from 0 to 1.39 providers per 3,500 people (p < .001). However, the median rate among the rural tracts remained unchanged with the inclusion of pharmacists. Additionally, the 75th percentile provider rates all increased by more than 2 providers per 3,500 people with the inclusion of pharmacists (Table 2). When pharmacists were included with primary care physicians, vaccine providers per capita increased in 2,413 of the 4,508 urban census tracts (p < .001), while the rate increased in 223 of 746 rural census tracts (p < .001). A visualization of this rate change appears in choropleth maps in Figure 3.
Provider Coverage by Area Deprivation. Mean ADI scores in each quintile ranged from 70.4 (SD = 26) in the least‐deprived census tracts (first quintile) to 116.7 (SD = 3.8) in the most‐deprived census tracts (fifth quintile; Table 3). Vaccine providers per capita varied across ADI quintiles (all p < .001). The rates of physicians and pharmacists followed a similar pattern, where rates were nearly double in the least‐deprived census tracts (first quintile) compared to the rates in census tracts in the other 4 deprivation quintiles. The total rate of vaccine providers doubled in each quintile when pharmacists were included with physicians.
Table 3.
Vaccine Provider Rates by Area Deprivation (Rates per 3,500 People)
| Area Deprivation Index Quintilesa | |||||||
|---|---|---|---|---|---|---|---|
| 1(Least deprived) | 2 | 3 | 4 | 5 (Most deprived) | F‐value | p‐value | |
| ADI score mean (SD) | 70.4 (26) | 97.3 (3) | 105.1 (1.8) | 110.5 (1.4) | 116.7 (3.8) | ||
| Vaccine provider rates b | |||||||
| Physicians | 2.75c | 1.74 | 1.43 | 1.28 | 1.45 | 21.5 | <.001 |
| Pharmacists | 2.26c | 1.70 | 1.56 | 1.38 | 1.40 | 16.7 | <.001 |
| Total rate | 5.01c | 3.43c | 2.99 | 2.66 | 2.84 | 25.8 | <.001 |
Abbreviations: ADI, Area Deprivation Index; SD, standard deviation.
Larger scores indicate more deprivation. Based on analyses of data for 5,254 census tracts.
Vaccine provider rates were winsorized to 30 or more providers per 3,500 people.
Mean provider rates that are statistically different from others are based on Bonferroni multiple comparisons test.
Discussion
Two US federal panels have prioritized the inclusion of community pharmacists as vaccinators to increase opportunities for HPV vaccination for adolescents.23, 24 One way pharmacists may improve vaccination opportunities is by increasing access to adolescent vaccination services in rural and urban areas. Our study findings suggest that community pharmacists could improve health care provider coverage for vaccine delivery above and beyond what primary care physicians alone offer within communities in the US state of Texas. Pharmacists tended to be more geographically dispersed across census tracts than primary care physicians, and as a result giving them the ability to administer HPV vaccine would increase the availability of vaccine providers in areas with inadequate primary care provider coverage. Taken together with pharmacies’ longer operating hours relative to primary care clinics and their ability to provide vaccinations without appointments,36 these findings indicate pharmacies could help increase HPV vaccination in the United States. This is all the more pressing in places like Texas where pharmacists already have the authority to vaccinate age‐eligible adolescents. If states extend pharmacists’ role to include adolescent vaccination, insurance companies and federal programs, including Medicaid and Vaccines for Children, should confer in‐network provider status to pharmacists as vaccine providers.22, 36, 37 It will also be important to address medical organizations’ concerns about pharmacists furnishing adolescent vaccines.38, 39, 40, 41, 42 Pharmacies must also create a sustainable business case for providing adolescent vaccination services, create protocols for dose reporting to primary care providers and state immunization registries, and address vaccine delivery issues within their own practice settings to make them more appealing for parents and adolescents. To the best of our knowledge, our study is the first to directly compare the geographic distribution of primary care physicians with the distribution of community pharmacists, and it provides a preliminary step to further assess how pharmacists can alleviate geographic barriers to HPV vaccination.
Primary care physicians spatially clustered at the level of census tracts while pharmacists did not, providing support for Hypothesis 1. Our findings were robust to the different methods employed. Economic processes may partially explain the different spatial patterns observed between the distribution of physicians and pharmacists. First, economies of scale, whereby the cost of rendering services reduces as the number of services increases, may incentivize physicians to group together in larger practices, geographically clustering them. A recent study of primary care found that larger practices had smaller ratios of nonphysician staff (including administrative staff, nurses, and physician assistants) per physician, likely because physicians can share this resource.43 Second, as primary care remuneration structures shift from fee‐for‐service to value‐based, primary care practices may be compelled to be more integrated with other providers in order to address health care needs of patients and meet quality metrics set forth for compensation.44 Third, physicians have stronger network ties to other physicians who share similar patient panel characteristics.45, 46 Since medical practices tend to provide a limited number of services by virtue of practice specialization (ie, practices may tend not to overlap in scope), it would be reasonable to believe that they would gain financially by being able to refer patients to one another as a way to increase their patient caseload to achieve economies of scale. As such, physicians who create formal referral networks are likely to be geographically close to one another.45
Compared to physicians in our study, the relative geographic dispersion of pharmacists may be primarily facilitated by economies of scope, whereby the cost of rendering services at pharmacies decreases with an increase in the diversity of services provided.47 Pharmacies often have retail operations, achieving profitability by selling a variety of products and services. This retail emphasis in turn could incentivize pharmacy businesses, and thus the location of pharmacists who practice there, to be geographically dispersed to avoid competition with one another and to be located closer to where consumers work and live for easier access. Additionally, the diversification of products and services provided at a pharmacy business, particularly in retail chain operations, can allow such businesses to remain fiscally solvent despite potential losses that can occur due to poor reimbursement from insurance providers for pharmacy services. While economies of both scale and scope provide compelling hypotheses for how spatial patterns arise for these 2 health care provider types, there is a real paucity of research that could provide an empirical basis for these assertions, particularly for the pharmacy workforce. Additionally, economies of scale and scope are 2 processes that are not mutually exclusive, and both physician and pharmacy practices may pursue both methods to achieve economic efficiency. Future studies can explore economies of scale and scope as motivators behind the spatial clustering process of these 2 provider types and may help predict how physicians and pharmacists pick practice locations.
Pharmacists were also able to improve health care provider coverage, consistent with Hypothesis 2. While both urban and rural areas appeared to have an increase in provider coverage when pharmacists were included, this effect was more pronounced in urban areas, where nearly twice as many urban census tracts moved to adequate provider coverage compared to rural census tracts. Additionally, when comparing across the interquartile cutoffs, we saw larger increases in vaccine providers per capita when pharmacists were included in both urban and rural areas that already had some physicians, most notably when comparing the 50th and 75th interquartile cutoffs. One reason for this pattern of increased provider adequacy in certain census tracts may be a consequence of an ecological Matthew effect, where areas that already have economic advantage (eg, at least some amount of access to primary care providers) will continue to accumulate other resources at a faster rate (eg, the availability of pharmacists in those areas), widening disparities with disadvantaged areas that do not exhibit the same growth.48
A previous study found that residents in metropolitan areas in 23 US states were more likely to have geographic access to physicians compared to residents in rural areas, using 3 measures of access: physician‐to‐population ratios, distance traveled, and caseload per physician.49 Similarly in our study, pharmacists, like physicians, tend to provide care in areas with larger population growth and community wealth. However, as geographic markets become saturated, the retail model that increasingly drives pharmacy businesses may encourage them to spill over to markets with lower demands such as rural areas, called the “sand pile” hypothesis,49 as Rosenthal and colleagues found when modeling geographic access to physicians over time. Additionally, pharmacists would increase the number of vaccine providers overall, regardless of the socioeconomic makeup of communities in Texas, and thus increase the availability of potential vaccine providers. Their inclusion does not appear to change equity in provider rates across neighborhoods’ socioeconomic status.
However, strategies can be implemented to help address the preferential location of physicians and pharmacists in census tracts that are urban and lower deprivation, which can alleviate disparities seen in rural areas and areas of higher socioeconomic deprivation. For instance, policymakers can look to bolster economic incentives that are known to promote the movement of health care providers to medically underserved communities as one means to improve access to necessary primary care services like vaccinations.50, 51 Additionally, US programs have tried other alternative vaccine delivery models such as school‐located vaccination, which has been highly successful in countries like Australia, Bhutan, Rwanda, and Sweden.52 However, the effectiveness and sustainability of school‐located vaccination in the United States will be limited until reimbursement mechanisms are in place to bill for the cost and administration of vaccines; implementation challenges related to working with school districts with competing priorities and limited resources are overcome; and low demand from families is addressed.52
Strengths of our study include use of a comprehensive and accurate list of primary care physicians and community pharmacists from the Texas Medical Board and Board of Pharmacy. We also used geospatial analysis to understand the patterns of these 2 provider types, a novel method in evaluating health care workforce that takes into account the spatial dependence of our observations. Notwithstanding, our study findings should be interpreted in light of several limitations. Our study assumed that all included primary care physicians and community pharmacists either provide or have the potential to provide HPV vaccine, while in reality many of these providers may not be furnishing this vaccination service. Additionally, we were unable to model the geographic distribution of nurse practitioners and physician assistants who also have a role in adolescent vaccination. As such, pharmacists may have a smaller impact in improving the adequacy of vaccine provider coverage. Our study is also focused on health care access in the United States, so the findings and implications we present should be interpreted with care when comparing HPV vaccination strategies in other countries. Finally, while we adjusted the number of providers in each census tract by population as a method of measuring adequate provider coverage, several other ways exist for measuring potential and realized access to providers, for example, by using distance lived to providers, public transit access, caseload per provider, and other sociodemographic indicators (eg, cultural, language, or financial) that do not derive health care access barriers to distance alone. Examining these alternative approaches is an important area for future research.
Conclusion
Community pharmacists could help to meaningfully improve the adequacy of health care providers who can administer HPV vaccination due to their substantial reach and availability in communities.22, 36 Future workforce studies should account for individual and community factors that may be associated with provider locations. Additionally, future studies that correlate provider workforce availability with vaccination coverage can help elucidate how geographic patterns in HPV vaccination may occur and can also help identify areas for targeted public health interventions to address vaccination disparities. This may further the policy case to include pharmacists as adolescent vaccine providers, especially if future studies find evidence that pharmacists are well positioned to furnish care for medically underserved or vulnerable populations.
Supporting information
Online Appendix Table 1. Texas Primary Care Physician (n = 12,307) and Community Pharmacist (n = 11,131) Characteristicsa
Funding/Support
Shah's time was partially supported by a National Research Service Award Post‐Doctoral Traineeship from the Agency for Healthcare Research and Quality sponsored by the Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill (Grant No. T32 HS000032). The funder played no role in (1) the study design; (2) the collection, analysis, and interpretation of data; (3) the writing of the report; or (4) the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Conflict of Interest Disclosures: Brewer and Trogdon have received independent grants from Merck. Brewer has served on paid advisory boards for Merck.
References
- 1. Pruitt SL, Schootman M. Geographic disparity, area poverty, and human papillomavirus vaccination. Am J Prev Med. 2010;38(5):525‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reagan‐Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(29):784‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Du P, Camacho F, McCall‐Hosenfeld J, Lengerich E, Meyers CM, Christensen ND. Human papillomavirus vaccination among adults and children in 5 US states. J Public Health Manag Prac. 2015;21(6):573‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henry KA, Stroup AM, Warner EL, Kepka D. Geographic factors and human papillomavirus (HPV) vaccination initiation among adolescent girls in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(2):309‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reagan‐Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(33):850‐858. [DOI] [PubMed] [Google Scholar]
- 6. Walker TY, Elam‐Evans LD, Singleton JA, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2016. MMWR Morb Mortal Wkly Rep. 2017;66(33):874‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rutten LJF, Wilson PM, Jacobson DJ, et al. A population‐based study of sociodemographic and geographic variation in HPV vaccination. Cancer Epidemiol Biomarkers Prev. 2017;26(4):533‐540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eberth JM, Hossain MM, Tiro JA, Zhang X, Holt JB, Vernon SW. Human papillomavirus vaccine coverage among females aged 11 to 17 in Texas counties: an application of multilevel, small area estimation. Womens Health Issues. 2013;23(2):e131‐e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moss JL, McCarthy SH, Gilkey MB, Brewer NT. Application of the Carolina Framework for cervical cancer prevention. Gynecol Oncol. 2014;132(Suppl. 1):S33‐S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trogdon JG, Ahn T. Geospatial patterns in human papillomavirus vaccination uptake: evidence from uninsured and publicly insured children in North Carolina. Cancer Epidemiol Biomarkers Prev. 2015;24(3):595‐602. [DOI] [PubMed] [Google Scholar]
- 11. Tsui J, Rodriguez HP, Gee GC, Escobedo LA, Kominski GF, Bastani R. Are HPV vaccination services accessible to high‐risk communities? A spatial analysis of HPV‐associated cancer and Chlamydia rates and safety‐net clinics. Cancer Causes Control. 2013;24(12):2089‐2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horner M‐J, Altekruse SF, Zou Z, Wideroff L, Katki HA, Stinchcomb DG. US geographic distribution of prevaccine era cervical cancer screening, incidence, stage, and mortality. Cancer Epidemiol Biomarkers Prev. 2011;20(4):591‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reiter PL, Katz ML, Paskett ED. HPV vaccination among adolescent females from Appalachia: implications for cervical cancer disparities. Cancer Epidemiol Biomarkers Prev. 2012;21(12):2220‐2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moss JL, Reiter PL, Brewer NT. Correlates of human papillomavirus (HPV) vaccine coverage: a state‐level analysis. Sex Transm Dis. 2015;42(2):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersen R, Newman JF. Societal and individual determinants of medical care utilization in the United States. Milbank Q. 2005;83(4). 10.1111/j.1468-0009.2005.00428.x. [DOI] [PubMed] [Google Scholar]
- 16. Stokley S, Curtis C, Jeyarajah J, Harrington T, Gee J, Markowitz LE. Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2013—United States. MMWR Morb Mortal Wkly Rept. 2013;62(29):591. [PMC free article] [PubMed] [Google Scholar]
- 17. Liu G, Kong L, Du P. HPV vaccine completion and dose adherence among commercially insured females aged 9 through 26 years in the US. Papillomavirus Res. 2016;2:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shipman SA, Lan J, Chang C‐H, Goodman DC. Geographic maldistribution of primary care for children. Pediatrics. 2011;127(1):19‐27. 10.1542/peds.2010-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rand CM, Shone LP, Albertin C, Auinger P, Klein JD, Szilagyi PG. National health care visit patterns of adolescents: implications for delivery of new adolescent vaccines. Arch Pediatr Adolesc Med. 2007;161(3):252‐259. [DOI] [PubMed] [Google Scholar]
- 20. Irwin CE, Adams SH, Park MJ, Newacheck PW. Preventive care for adolescents: few get visits and fewer get services. Pediatrics. 2009;123(4):e565‐e572. [DOI] [PubMed] [Google Scholar]
- 21. Tsai Y, Zhou F, Wortley P, Shefer A, Stokley S. Trends and characteristics of preventive care visits among commercially insured adolescents, 2003–2010. J Pediatr. 2014;164(3):625‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shah PD, Gilkey MB, Pepper JK, Gottlieb SL, Brewer NT. Promising alternative settings for HPV vaccination of US adolescents. Expert Rev Vaccines. 2014;13(2):235‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rimer B, Harper H, Witte O. Accelerating HPV Vaccine Uptake: Urgency for Action to Prevent Cancer; A Report to the President of the United States from the President's Cancer Panel. Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]
- 24. National Vaccine Advisory Committee . Recommendations to Address Low HPV Vaccination Coverage Rates in the United States. Washington, DC: Department of Health and Human Services; 2015. [Google Scholar]
- 25. American Pharmacists Association . Pharmacist Administered Vaccines: Types of Vaccines Authorized to Administer. Washington, DC: American Pharmacists Association; 2015. http://www.pharmacist.com/sites/default/files/files/Pharmacist_IZ_Authority_1_31_15.pdf. Accessed February 18, 2016. [Google Scholar]
- 26. Brewer NT, Chung JK, Baker HM, Rothholz MC, Smith JS. Pharmacist authority to provide HPV vaccine: novel partners in cervical cancer prevention. Gynecol Oncol. 2014;132(Suppl. 1):S3‐S8. [DOI] [PubMed] [Google Scholar]
- 27. National Association of Chain Drug Stores . 2011–2012 Chain Pharmacy Industry Profile illustrates pharmacy value. Alexandria, VA: National Association of Chain Drug Stores; 2011. [Google Scholar]
- 28. Moran PA. Notes on continuous stochastic phenomena. Biometrika. 1950;37(1/2):17‐23. [PubMed] [Google Scholar]
- 29. Waller LA, Gotway CA. Applied Spatial Statistics for Public Health Data. Vol 368 New York, NY: John Wiley & Sons; 2004. [Google Scholar]
- 30. ESRI . Modeling spatial relationships. ArcGIS Pro website. http://pro.arcgis.com/en/pro-app/tool-reference/spatial-statistics/modeling-spatial-relationships.htm. Published 2017. Accessed April 28, 2017.
- 31. Shahid R, Bertazzon S, Knudtson ML, Ghali WA. Comparison of distance measures in spatial analytical modeling for health service planning. BMC Health Serv Res. 2009;9(1):200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Primary care health professional shortage areas (HPSAs) . Kaiser Family Foundation website. http://kff.org/other/state-indicator/primary-care-health-professional-shortage-areas-hpsas/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Published 2017. Accessed April 5, 2017.
- 33. 2010 Census urban and rural classification and urban area criteria . US Census Bureau website. https://www.census.gov/geo/reference/ua/urban-rural-2010.html. Published 2015. Accessed April 5, 2017.
- 34. Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. Am J Public Health. 2003;93(7):1137‐1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. eGEMs. 2016;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goad J, Bach A. The role of community pharmacy‐based vaccination in the USA: current practice and future directions. Integr Pharm Res Pract. 2015;4:67‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Trogdon JG, Shafer PR, Shah PD, Calo WA. Are state laws granting pharmacists authority to vaccinate associated with HPV vaccination rates among adolescents? Vaccine. 2016;34(38):4514‐4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McIntosh J, Sturpe DA, Khanna N. Human papillomavirus vaccine and cervical cancer prevention: practice and policy implications for pharmacists. J Am Pharm Assoc. 2008;48(1):e1‐e16. [DOI] [PubMed] [Google Scholar]
- 39. Keely JL. Pharmacist scope of practice. Ann Intern Med. 2002;136(1):79‐85. [DOI] [PubMed] [Google Scholar]
- 40. Middleman AB, Rosenthal SL, Rickert VI, Neinstein L, Fishbein DB, D'Angelo L. Adolescent immunizations: a position paper of the Society for Adolescent Medicine. J Adolesc Health. 2006;38(3):321‐327. [DOI] [PubMed] [Google Scholar]
- 41. Hammer LD, Curry E, Harlor A, et al. Increasing immunization coverage. Pediatrics. 2010;125(6):1295‐1304. [DOI] [PubMed] [Google Scholar]
- 42. Academy chapters use AAFP resource to fight pharmacist‐administered inoculations legislation . American Academy of Family Physicians website. http://www.aafp.org/news/government-medicine/20110622pharmbrief.html. Published 2011. Accessed February 18, 2016.
- 43. Peikes DN, Reid RJ, Day TJ, et al. Staffing patterns of primary care practices in the comprehensive primary care initiative. Ann Fam Med. 2014;12(2):142‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Porter ME, Pabo EA, Lee TH. Redesigning primary care: a strategic vision to improve value by organizing around patients’ needs. Health Aff (Millwood). 2013;32(3):516‐525. [DOI] [PubMed] [Google Scholar]
- 45. Landon BE, Keating NL, Barnett ML, et al. Variation in patient‐sharing networks of physicians across the United States. JAMA. 2012;308(3):265‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barnett ML, Landon BE, O'Malley AJ, Keating NL, Christakis NA. Mapping physician networks with self‐reported and administrative data. Health Serv Res. 2011;46(5):1592‐1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Panzar JC, Willig RD. Economies of scope. Am Econ Rev. 1981;71(2):268‐272. [Google Scholar]
- 48. Perc M. The Matthew effect in empirical data. J R Soc Interface. 2014;11(98). 10.1098/rsif.2014.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rosenthal MB, Zaslavsky A, Newhouse JP. The geographic distribution of physicians revisited. Health Serv Res. 2005;40(6p1):1931‐1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bärnighausen T, Bloom DE. Designing financial‐incentive programmes for return of medical service in underserved areas: seven management functions. Hum Resour Health. 2009;7(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bärnighausen T, Bloom DE. Financial incentives for return of service in underserved areas: a systematic review. BMC Health Serv Res. 2009;9(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kempe A, Allison MA, Daley MF. Can school‐located vaccination have a major impact on human papillomavirus vaccination rates in the United States? Acad Pediatr. 2018;18(2S):S101‐S105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Appendix Table 1. Texas Primary Care Physician (n = 12,307) and Community Pharmacist (n = 11,131) Characteristicsa


