Abstract
Oxidative injury of vascular endothelial cells in the initial event of atherosclerosis (AS) in diabetes was assessed in the present study. The antioxidant effect of oleanolic acid (OA) has attracted much attention. In the present study the potential effects of OA on human umbilical vein endothelial cells (HUVECs) were investigated. Cell viability was examined using the CCK-8 assay. The activity of oxidative stress parameters was determined using commercial kits. Flow cytometry analysis was performed to detect the level of reactive oxygen species (ROS), mitochondrial membrane potential (MMP) and cell apoptosis. The expression levels of target genes and proteins were examined by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analysis. It was indicated that cell viability that was suppressed by high glucose was increased by the pretreatment of OA, and nitric oxide (NO) generation, the activities of superoxide dismutase (SOD) and catalase (CAT) were recovered by OA. By contrast, it was observed that OA decreased the MDA content. Notably, the pretreatment of OA alleviated mitochondria damage by reducing the level of ROS and maintaining MMP. In addition, apoptosis that was caused by high glucose was reduced by OA. Pro-apoptotic genes (caspase-3, Fas, Fasl) and anti-apoptotic gene (Bcl-2) expression levels were decreased and increased in the OA groups, respectively. Furthermore, the activity of AKT/endothelial nitric oxide synthase (eNOS) signaling was elevated by OA. Taken together, it was suggested that OA could protect against oxidative stress-induced apoptosis of HUVECs, which was associated with AKT/eNOS signaling pathway.
Keywords: oleanolic acid, oxidative stress, apoptosis, AKT/eNOS, atherosclerosis
Introduction
Atherosclerosis (AS) is a common complication of diabetes. Arteriosclerosis in diabetic patients has higher mortality than that among the non-diabetic (1,2). Hyperglycemia is a primary phenotype of diabetic patients (3). Vascular endothelial cells are critical targets of hyperglycemia injury in diabetes mellitus (4). The injury of vascular endothelial cells is the initial event of AS (5). Moreover, oxidative stress caused by hyperglycemia is a main inducement of vascular endothelial cells injury (6), which is resulted from the imbalance between production and removal of reactive oxygen species (ROS) (7). In normal circumstances, intracellular antioxidant systems, for instance, superoxide dismutase (SOD) and catalase (CAT), maintain the cell redox self-steady state (8). Nevertheless, ROS accumulation will induce lipid peroxidation, resulting in the production of MDA (9,10). Moreover, excessive exposure of ROS can lead to the dysfunction of the vascular endothelial cells (11). Vascular endothelium, a complex endocrine organ (12), could secrete cytoactive factors in response to the change of signals and stress (13). Nitric oxide (NO) plays a critical role in maintaining the vasoconstrictor function, which is a primary endogenous vascular diastolic factor (14). Additionally, NO is helpful to the inhibition of the production of oxygen free radicals (15). It has been documented that oxidative stress and the production or activation of NO were essential for the AS (8).
Furthermore, studies have pointed out that cell apoptosis was closely linked to the ROS accumulation (3,16,17). Importantly, high glucose can promote apoptosis of human umbilical vein endothelial cells (HUVECs) (18). Hence, alleviation of high glucose-induced oxidative stress and apoptosis of endothelial cells may play an important role in the prevention and treatment of AS and other diabetic macrovascular diseases. The PI3K/AKT signaling functions critically in cellular death progression (19). It is well known that the survival of endothelial cells could be maintained by AKT. Moreover, AKT could activate the expression of endothelial nitric oxide synthase (eNOS) (20), which is responsible for the production of NO (21). Therefore, in this study, we paid close attention to the AKT/eNOS signaling for its protective role in endothelial cells.
As previously described (22), oleanolic acid (OA), which is a pentacyclic triterpenoid compound, is widespread in plants. OA has several pharmacological activities. The antitumor effect of OA has been noted previously (23–26). Studies have also reported that OA could exert anti-oxidative effect (27,28). OA is believed to be able to treat diabetes (29,30), and it is reported to be related to arteriosclerosis (31). Thus, it is of research significance to assess the effects of OA on high-glucose induced oxidative stress in HUVECs.
In the present study, we sought to evaluate the effects of OA in HUVECs cultivated in vitro via detecting the oxidative response and apoptosis of HUVECs. The mechanism action of OA was as well investigated. Our research provides reference for developing candidate agent in the treatment of AS in diabetics.
Materials and methods
Cell culture and treatment
HUVECs (ATCC, USA) were maintained in Dulbecco's modified Eagle's medium (DMEM) (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) that contained 10% FBS (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and penicillin/streptomycin (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) at 37°C in an incubator with 5% CO2. The normal blood glucose level ranged from 3.9 and 5.5 mM (32) in non-diabetics. Blood sugar levels increased more than normal range may be an indicator of diabetes. According to some researches on hyperglycemia injury model in vitro (33,34), HUVECs were respectively treated with glucose at 5 and 33 mM for the control and injury model for 48 h. The incubation concentration of OA was determined in reference to a previous study (35). The research groups in this study were classified as follow: Blank group (blank): no glucose treatment; control group (Con): 5 mM glucose treatment; High glucose model group (GC): 33 mM glucose (Sangon Biotech Co., Ltd., Shanghai, China) treatment; positive control group (OA-H/Con): 40 mM OA (purity >98%; Beijing Solarbio Science & Technology Co., Ltd.) (dissolved in ethanol) for incubation for 24 h prior to 5 mM glucose treatment; low OA treatment group (OA-L/GC): Cells were treated with 20 mM OA at 37°C for 24 h prior to 33 mM glucose treatment; high OA treatment group (OA-H/GC): Cells were treated with 40 mM OA at 37°C for 24 h prior to 33 mM glucose treatment.
Cell viability assay
The cell survival rate was examined using Cell Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology, Haimen, China) assay, according to the manufacturer's protocols. To explain further, 1×104/each well were seeded into a 96-well plate and incubated at 37°C for 24 h. CCK-8 (10 µl/well) was added into the 96-well plate, and then the cells were incubated at 37°C for 4 h. Absorbance at 450 nm was detected using a microplate reader (PerkinElmer, Inc., Waltham, MA, USA).
ROS measurement
Intracellular ROS level was detected using fluorescent probe DCFH-DA probe (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Cells were collected and washed by PBS. Next, the cells were maintained with 10 µM DCHF-DA in darkness at 37°C for 30 min. Flow cytometry analysis was carried out to examine the fluorescence signals corresponding to DCHF-DA on flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) with Cell Quest software version 5.1 (BD Biosciences). At least 10,000 events were analyzed in each examination.
Detection for mitochondrial transmembrane potential (MMP)
The Rho 123 accumulation was determined by flow cytometry analysis as previously described (36). Following the treatment above, the cells in 24-well plates (2×105 cells/well) were incubated with 50 mM Rho 123 (Sigma-Aldrich; Merck KGaA) at 37°C for 30 min. The reaction was terminated at 5 min of incubation on ice. Subsequently, the fluorescent of Rho 123 was detected by FACS Calibur flow cytometer (BD Biosciences). Cell Quest software version 5.1 (BD Biosciences) was used to perform the analysis.
Detection of apoptosis
Annexin V-FITC (Sigma-Aldrich; Merck KGaA)/PI (Sigma-Aldrich; Merck KGaA) double-staining for apoptosis analysis was used to determine the aopotosis by flow cytometry analysis. To be more specific, the cells in 6-well plate at a density of 2×106/well were treated as above. Subsequently, the cells were incubated with 5 ul Annexin V-FITC for10 min at room temperature and with 5 µl PI at room temperature for 15 min in the darkness. FACS Calibur flow cytometer (BD Biosciences) and Cell Quest software version 5.1 were used to count the apoptotic cells.
Measurement of NO level
The generation of NO was examined with commercial Nitrate/Nitrite Fluorometric Assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) through nitrate reductase method via its breakdown products, Nitrate/Nitrite, After conducting treatment as designed, the determination was performed following manufacturer's protocols.
Measurement of SOD, CAT, and MDA level
Cells (1×104/well) were seeded into a 96-well plate and incubated at 37°C for 24 h. Then, the cells were centrifuged at 200 g for 5 min at 4°C. After being washed with PBS, the cells were resuspended and collected for the detection of the enzymes activities. The cellular SOD and CAT enzyme were determined using corresponding kits (Beijing Solarbio Science & Technology Co., Ltd.), according to the protocols. The MDA content was detected with thiobarbituric acid substance provided by Malondialdehyde Assay Kit (Beyotime, China).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was exacted using RNAiso Plus (Invitrogen; Thermo Fisher Scientific, Inc.). M-MLV (Promega Corporation, Madison, WI, USA) and oligo (dT) primers (Takara Bio, Inc., Otsu, Japan) were used to synthesis cDNA from RNA (1 µg). The temperature and incubation protocol was set at 25°C for 10 min, at 42°C for 50 min, at 70°C for 10 min. Amplification of the related genes was conducted using LightCycler® 480 SYBR-Green I Master (Roche Diagnostics, Indianapolis, IN, USA). The thermal cycle conditions was as follows: At 95°C, 4 min; at 35 cycles of 95°C, 15 Sec; at 60°C, 45 Sec; at 72°C, 7 min. The expression of target genes was normalized using the expression levels of GAPDH according to 2−ΔΔCq method (37). The primers used were as follows: Fas forward 5′-GTGCTTTGCTTAGGGTTCCC-3′, Fas reverse 5′-AACTTGCACTTCTGGCCATG-3′; Fasl forward 5′-GTCCAACTCAAGGTCCATGC-3′, Fasl reverse 5′-TTGTTGCAAGATTGACCCCG-3′; Bax forward 5′-GTGCCGGAACTGATCAGAAC-3′, Bax reverse 5′-CCAAAGTAGGAGAGGAGGCC-3′; Bcl-2 forward 5′-GCCTTCTTTGAGTTCGGTGG-3′, Bcl-2 reverse 5′-GAAATCAAACAGAGGCCGCA-3′; GAPDH forward: 5′-CACAGTCCATGCCATCACTG-3′; GAPDH reverse: 5′-ATCTCGCTCCTGGAAGATGG-3′;
Western blot analysis
Cells were lysed using 200 µl lysis buffer NP40 (Beyotime Institute of Biotechnology). The concentration of the samples was determined by BCA protein quantitative kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins were separated using 10% SDS-PAGE gels and transferred onto a PVDF membrane (EMD Millipore, Billerica, MA, USA). After being blocked with 5% non-skimmed milk at room temperature for 2 h, the membrane was incubated with primary antibodies as listed: cleaved caspase-3 (ab32042, 1:500; Abcam, Cambridge, UK), Fas (ab82419, 1:1,000), Fasl (ab15285, 1 ug/ml), Bax (ab32503,1:2,000), Bcl-2 (ab32124, 1:1,000), p-AKT (ab812831:5,000), AKT 1/2 (ab182729, 1:5,000), p-eNOS (ab184154, 1:1,000), eNOS (ab76198, 1:1,000), and GAPDH (ab8245, 1:5,000), at 4°C overnight. Then, horseradish peroxidase-conjugated secondary antibody (ab6721, 1:4,000) was added and maintained at room temperature for 1 h. The blot bands were developed using BeyoECL Star (Beyotime Institute of Biotechnology). The gray density was calculated with Quantity One software version 4.6 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analysis was calculated using GraphPad Prism software 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). One-way ANOVA following Tukey's post hoc test was used to compare the difference between groups. The results were shown as means ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
OA protected against high-glucose-induced injury in HUVECs
We first determined the effect of OA on cell viability of HUVECs. The CCK-8 test showed that the cell survival rate of HUVECs decreased obviously in the GC group in comparison to that in the control group, and that the pretreatment of OA improved the cell survival rate in a dose-dependent manner (Fig. 1A). Moreover, the nitric oxide (NO) generation was rescued by the pretreatment of OA (Fig. 1B). We also found that as an indicator of lipid peroxidation, the production of MDA caused by high glucose treatment was inhibited by the pretreatment of OA (Fig. 1C). Similarly, the activities of SOD and CAT were recovered in the pretreatment groups (Fig. 1D and E).
Figure 1.
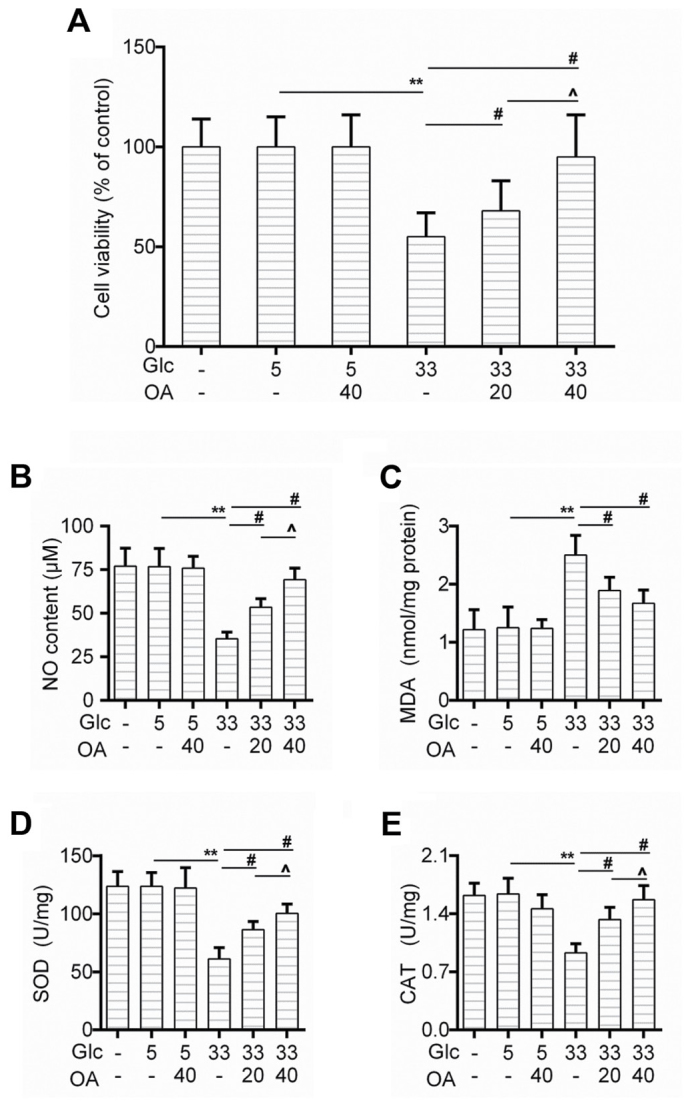
OA protected against high-glucose-induced injury in HUVECs. (A) Cell viability of HUVECs was detected by CCK-8 assay when the cells were treated with high glucose and/or OA. (B-E) Detection of (B) NO content, (C) MDA level, (D) SOD activity and (E) CAT by available commercial kit when the cells were treated with high glucose and/or OA. Blank group (Blank), cuntreated cells; control group (5 mM) (Con/Control), cells were treated with 5 mM glucose; high glucose (33 mM) model group (GC), cells were treated with 33 mM glucose; positive control group (OA-H/Con), cells were treated with 5 mM glucose and 40 µM OA; low OA (20 µM) treatment group (OA-L/GC), cells were treated with 33 mM glucose and 20 µM OA; high OA (40 µM) treatment group (OA-H/GC), cells were treated with 33 mM glucose and 40 µM OA. **P<0.01, #P<0.01 and ^P<0.05 as indicated. OA, oleanolic acid; HUVECs; human umbilical vein endothelial cells; SOD, superoxide dismutase; CAT, catalase.
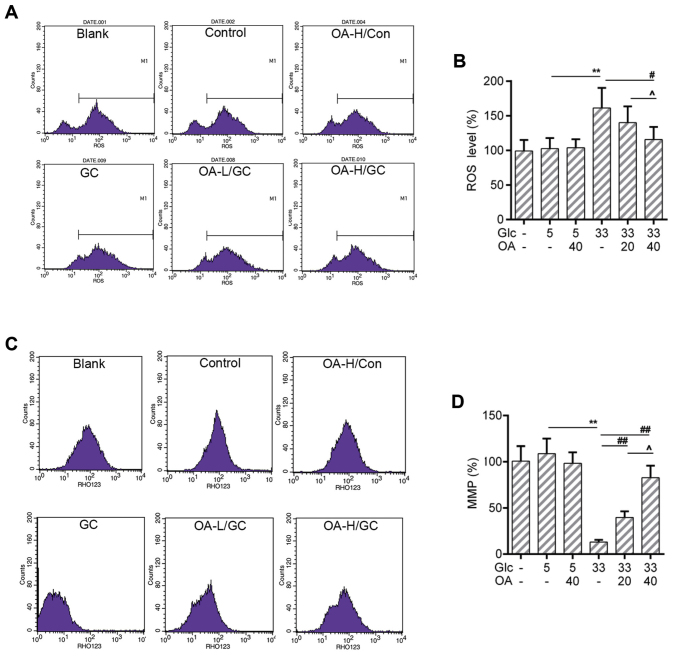
OA depressed the ROS production and mitochondrial membrane potential (MMP) loss in HUVECs
The increased ROS level is one of the sources causing mitochondria damage (38). The loss of MMP is an early hallmark event of mitochondria damage leading to apoptosis (39). Thus, the ROS production and the MMP of HUVECs were measured by flow cytometry analysis. Results showed that the ROS production was triggered by high glucose treatment. Noticeably, the ROS level was lower in the OA pretreatment groups than that in the GC group (Fig. 2A and B). Meanwhile, the loss of MMP caused by high glucose was recovered by the pretreatment of OA (Fig. 2C and D).
Figure 2.
OA prevented oxidative stress in HUVECs. (A and B) Flow cytometry assay for detection of cellular ROS analysis using DCFH-DA dye. (C and D) Flow cytometry analysis for detection of MMP using Rho123. Blank group (Blank), cuntreated cells; control group (5 mM) (Con/Control), cells were treated with 5 mM glucose; high glucose (33 mM) model group (GC), cells were treated with 33 mM glucose; positive control group (OA-H/Con), cells were treated with 5 mM glucose and 40 µM OA; low OA (20 µM) treatment group (OA-L/GC), cells were treated with 33 mM glucose and 20 µM OA; high OA (40 µM) treatment group (OA-H/GC), cells were treated with 33 mM glucose and 40 µM OA. **P<0.01, #P<0.05, ##P<0.01 and ^P<0.05 as indicated. OA, oleanolic acid; HUVECs; human umbilical vein endothelial cells; ROS, reactive oxygen species; MMP, mitochondrial transmembrane potential.
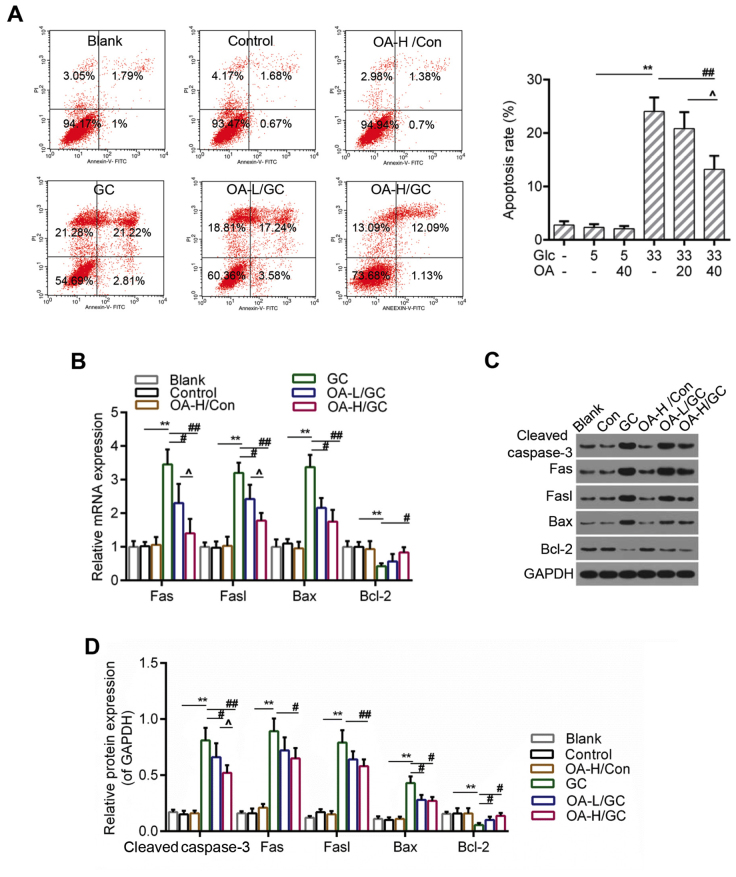
OA inhibited apoptosis caused by oxidative stress in HUVECs
ROS accumulation and the loss of MMP were both considered to be closely associated with apoptosis (40). Therefore, we further detected the apoptosis of HUVECs using flow cytometry analysis. The results indicated that the apoptosis of HUVECs induced by high glucose was apparently inhibited in the OA pretreatment groups (Fig. 3A and B). Moreover, the RT-qPCR and western blot assays showed that the expressions of pro-apoptotic genes including caspase-3, Fas, Fasl and Bax were lower in the OA pretreatment groups than those in the model group. By contrast, the expression of Bcl-2 was decreased in the model group but increased by the pretreatment of OA (Fig. 4C-E).
Figure 3.
OA decreased apoptosis of HUVECs. (A) Flow cytometry analysis for detection of cell apoptosis using Annexin V-FITC/PI staining. (B) Relative quantitative analysis of caspase-3, Fas, Fasl, Bax, and Bcl-2 by RT-qPCR. (C and D) Western blot analysis for caspase-3, Fas, Fasl, Bax, and Bcl-2. GAPDH was assessed as sample loading control. Blank group (Blank), cuntreated cells; control group (5 mM) (Con/Control), cells were treated with 5 mM glucose; high glucose (33 mM) model group (GC), cells were treated with 33 mM glucose; positive control group (OA-H/Con), cells were treated with 5 mM glucose and 40 µM OA; low OA (20 µM) treatment group (OA-L/GC), cells were treated with 33 mM glucose and 20 µM OA; high OA (40 µM) treatment group (OA-H/GC), cells were treated with 33 mM glucose and 40 µM OA. *P<0.05, **P<0.01, #P<0.05, ##P<0.01 and ^P<0.05 as indicated. OA, oleanolic acid; HUVECs; human umbilical vein endothelial cells; FITC, fluorescein isothiocyanate; PI, propidium iodide; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
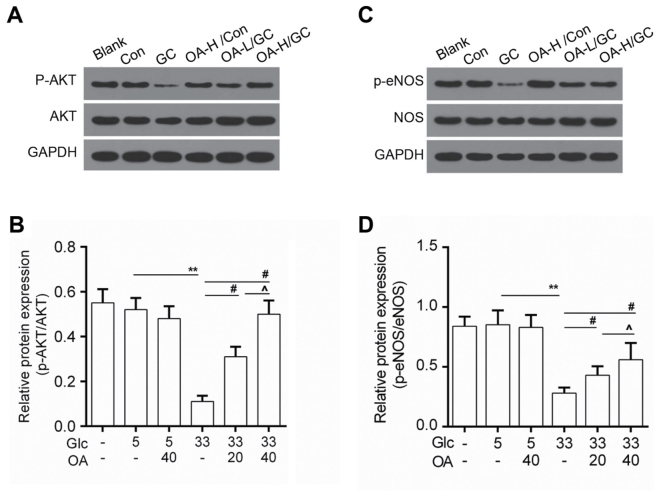
Figure 4.
OA enhanced the activity of AKT/eNOS. (A and B) Western blot analysis for p-AKT and AKT. (C and D) Western blot analysis for the expression of p-eNOS and NOS. Blank group (Blank), cuntreated cells; control group (5 mM) (Con/Control), cells were treated with 5 mM glucose; high glucose (33 mM) model group (GC), cells were treated with 33 mM glucose; positive control group (OA-H/Con), cells were treated with 5 mM glucose and 40 µM OA; low OA (20 µM) treatment group (OA-L/GC), cells were treated with 33 mM glucose and 20 µM OA; high OA (40 µM) treatment group (OA-H/GC), cells were treated with 33 mM glucose and 40 µM OA. **P<0.01, #P<0.05 and ^P<0.05 as indicated. OA, oleanolic acid; eNOS, endothelial nitric oxide synthase.
OA enhanced the activity of AKT/eNOS signaling
To study the underlying mechanisms, the activity of AKT/eNOS signals was determined by Western blot. Data showed that protein level of phosphorylated AKT (p-AKT) was elevated in the OA pretreatment groups (Fig. 4A and B). Meanwhile, we noticed that the phosphorylation of eNOS (p-eNOS) was higher in the OA pretreatment groups than that in the model group (Fig. 4C and D).
Discussion
AS is an arteriosclerotic vascular disease in endothelial cells. Diabetes is one of the major risk factors for the formation of AS (41). Recently, OA has attracted much attention in protecting against AS (42,43).
In the presented study, we found that OA could improve cell viability of HUVECs that was declined by the high glucose treatment, suggesting a protective effect of OA on HUVECs. To explore the role of OA in high-glucose mediated injury of HUVECs, we detected the oxidative response of HUVECs after the treatment with OA. The results showed that OA recovered the NO generation and decreased MDA content. The activities of SOD and CAT were higher in the OA pretreatment groups than those in the model group, indicating that OA had a potential of protecting against the hyperglycemia injury in diabetes via suppressing the oxidative stress. The cell viability is largely dependent on the function of mitochondria (44). Thus, we detected the function of mitochondria by estimating the ROS production and MMP. Interestingly, our results revealed that ROS burst and MMP loss caused by high glucose were alleviated in the OA pretreatment groups. Moreover, apoptosis was reported to be closely related to oxidative stress (45). Therefore, the apoptosis of HUVECs was detected by flow cytometry assay. Results showed that apoptosis of HUVECs caused by high glucose was depressed by OA. Additionally, it is well known that caspase-3 (46), Fas and Fasl (47) act as pro-apoptotic signals. Bcl-2 (48) is considered as an important anti-apoptotic factor. We found that the expressions of caspase-3, Fas and Fasl decreased in the OA pretreatment groups both in the transcriptional and translated levels. Conversely, the expression of Bcl-2 increased in the OA pretreatment groups in comparison to the model group, indicating that OA depressed the cell apoptosis of HUVECs by modulating the expression of apoptosis-related factors.
Furthermore, according to previous study, AKT/eNOS contributes to the prevention of apoptosis in endothelial cells (49). Another previous study pointed out that the phosphorylation of AKT/eNOS was decreased by high glucose in HUVECs (50). To illustrate the action mechanism of OA underlying the high glucose-induced HUVECs injury, we examined the activity of AKT/eNOS signaling. The results showed that the pretreatment of OA increased the levels of phosphorylated AKT (p-AKT) and phosphorylated eNOS (p-eNOS) in comparison to model group. A previous study showed that the endothelial injury was alleviated through enhancing the activity of PI3K/Akt/eNOS signaling (51). Although the effects of OA in this study still needed further validation, it provided a new molecular insight for understanding the effects of OA on AS in diabetes. Furthermore, OA could exert anti-tumor effect by inhibiting the activation of Akt signaling (25,52). This was contrary to the activation of Akt signaling by OA in this study. These results seemed to be contradictory; however, they demonstrated the protective effect of OA. The distinct results may be attributed to different cell type and cell context. In addition, due to the complexity of signal transduction, the signal modulation net would be better understood with efforts from further studies.
In summary, OA alleviated the cell injury mediated by high glucose by improving the cell viability, increasing the NO content, decreasing MDA level and rescuing the activities of SOD and CAT. Moreover, the ROS burst and MMP loss caused by high glucose could be mitigated by OA. The apoptosis induced by high glucose was inhibited by OA via modulating the expressions of caspase-3, Fas, Fasl and Bcl-2. Furthermore, the protective effects of OA may be associated with the activation of AKT/eNOS signaling pathway. Collectively, our results provided inspiration of new therapeutic strategies for the treatment of AS on diabetics.
Acknowledgements
Not applicable.
Funding
This work was supported by Health and Family Planning Commission of Sichuan Province Project (grant no. 2016-2017).
Availability of data and materials
All data generated and/or analyzed during this study are included in this published article.
Authors' contributions
WZ and BC made substantial contributions to conception and design of the study. JF, QL and XC contributed to the data acquisition, analysis and interpretation. BC revised the article for important intellectual content. QL provided final approval of the version to be published. XC agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Resnick HE, Howard BV. Diabetes and cardiovascular disease. Annu Rev Med. 2002;53:245–267. doi: 10.1146/annurev.med.53.082901.103904. [DOI] [PubMed] [Google Scholar]
- 2.Lee RT, Schoen FJ, Loree HM, Lark MW, Libby P. Circumferential stress and matrix metalloproteinase 1 in human coronary atherosclerosis. Implications for plaque rupture. Arterioscler Thromb Vasc Biol. 1996;16:1070–1073. doi: 10.1161/01.ATV.16.8.1070. [DOI] [PubMed] [Google Scholar]
- 3.Salmi M, Stolen C, Jousilahti P, Yegutkin GG, Tapanainen P, Janatuinen T, Knip M, Jalkanen S, Salomaa V. Insulin-regulated increase of soluble vascular adhesion protein-1 in diabetes. Am J Pathol. 2002;161:2255–2262. doi: 10.1016/S0002-9440(10)64501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 5.Onat D, Brillon D, Colombo PC, Schmidt AM. Human vascular endothelial cells: A model system for studying vascular inflammation in diabetes and atherosclerosis. Curr Diab Rep. 2011;11:193–202. doi: 10.1007/s11892-011-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Echtay KS, Murphy MP, Smith RA, Talbot DA, Brand MD. Superoxide activates mitochondrial uncoupling protein 2 from the matrix side. Studies using targeted antioxidants. J Biol Chem. 2002;277:47129–47135. doi: 10.1074/jbc.M208262200. [DOI] [PubMed] [Google Scholar]
- 7.Storz G, Imlay JA. Oxidative stress. Curr Opin Microbiol. 1999;2:188–194. doi: 10.1016/S1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 8.Bullon P, Newman HN, Battino M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: A shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol. 2014;64:139–153. doi: 10.1111/j.1600-0757.2012.00455.x. [DOI] [PubMed] [Google Scholar]
- 9.Vornoli A, Pozzo L, Della Croce CM, Gervasi PG, Longo V. Drug metabolism enzymes in a steatotic model of rat treated with a high fat diet and a low dose of streptozotocin. Food Chem Toxicol. 2014;70:54–60. doi: 10.1016/j.fct.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 10.Yang SJ, Je Lee W, Kim EA, Dal Nam K, Hahn HG, Young Choi S, Cho SW. Effects of N-adamantyl-4-methylthiazol-2-amine on hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2014;736:26–34. doi: 10.1016/j.ejphar.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Sakata K, Kondo T, Mizuno N, Shoji M, Yasui H, Yamamori T, Inanami O, Yokoo H, Yoshimura N, Hattori Y. Roles of ROS and PKC-βII in ionizing radiation-induced eNOS activation in human vascular endothelial cells. Vascul Pharmacol. 2015;70:55–65. doi: 10.1016/j.vph.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Zhou H, Wang C. Cytoprotective Effects and Mechanisms of Δ-17 Fatty Acid Desaturase in Injured Human Umbilical Vein Endothelial Cells (HUVECs) Med Sci Monit. 2017;23:1627–1635. doi: 10.12659/MSM.903654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993;22(Suppl 4):S1–S14. doi: 10.1097/00005344-199322004-00002. [DOI] [PubMed] [Google Scholar]
- 14.Mezzetti A. Pharmacological modulation of plaque instability. Lupus. 2005;14:769–772. doi: 10.1191/0961203305lu2218oa. [DOI] [PubMed] [Google Scholar]
- 15.Haluzik M, Nedvídková J, Skrha J. The influence of NO synthase inhibitor and free oxygen radicals scavenger-methylene blue-on streptozotocin-induced diabetes in rats. Physiol Res. 1998;47:337–341. [PubMed] [Google Scholar]
- 16.Szuster-Ciesielska A, Stachura A, Słotwińska M, Kamińska T, Sniezko R, Paduch R, Abramczyk D, Filar J, Kandefer-Szerszen M. The inhibitory effect of zinc on cadmium-induced cell apoptosis and reactive oxygen species (ROS) production in cell cultures. Toxicology. 2000;145:159–171. doi: 10.1016/S0300-483X(00)00144-X. [DOI] [PubMed] [Google Scholar]
- 17.Antherieu S, Ledirac N, Luzy AP, Lenormand P, Caron JC, Rahmani R. Endosulfan decreases cell growth and apoptosis in human HaCaT keratinocytes: partial ROS-dependent ERK1/2 mechanism. J Cell Physiol. 2007;213:177–186. doi: 10.1002/jcp.21108. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner-Parzer SM, Wagner L, Pettermann M, Grillari J, Gessl A, Waldhäusl W. High-glucose-triggered apoptosis in cultured endothelial cells. Diabetes. 1995;44:1323–1327. doi: 10.2337/diab.44.11.1323. [DOI] [PubMed] [Google Scholar]
- 19.Fulda S. Synthetic lethality by co-targeting mitochondrial apoptosis and PI3K/Akt/mTOR signaling. Mitochondrion. 2014;19:85–87. doi: 10.1016/j.mito.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Michell BJ, Griffiths JE, Mitchelhill KI, Rodriguez-Crespo I, Tiganis T, Bozinovski S, de Montellano PRO, Kemp BE, Pearson RB. The Akt kinase signals directly to endothelial nitric oxide synthase. Curr Biol. 1999;9:845–848. doi: 10.1016/S0960-9822(99)80371-6. [DOI] [PubMed] [Google Scholar]
- 21.Wiest R, Das S, Cadelina G, Garcia-Tsao G, Milstien S, Groszmann RJ. Bacterial translocation in cirrhotic rats stimulates eNOS-derived NO production and impairs mesenteric vascular contractility. J Clin Invest. 1999;104:1223–1233. doi: 10.1172/JCI7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollier J, Goossens A. Oleanolic acid. Phytochemistry. 2012;77:10–15. doi: 10.1016/j.phytochem.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 23.Wei J, Liu H, Liu M, Wu N, Zhao J, Xiao L, Han L, Chu E, Lin X. Oleanolic acid potentiates the antitumor activity of 5-fluorouracil in pancreatic cancer cells. Oncol Rep. 2012;28:1339–1345. doi: 10.3892/or.2012.1921. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Liu H, Zhang L, Guo T, Wang P, Geng M, Li Y. Synthesis and antitumor activities of naturally occurring oleanolic acid triterpenoid saponins and their derivatives. Eur J Med Chem. 2013;64:1–15. doi: 10.1016/j.ejmech.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Mu DW, Guo HQ, Zhou GB, Li JY, Su B. Oleanolic acid suppresses the proliferation of human bladder cancer by Akt/mTOR/S6K and ERK1/2 signaling. Int J Clin Exp Pathol. 2015;8:13864–13870. [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, Wei L, Shen A, Chu J, Lin J, Peng J. Oleanolic acid modulates multiple intracellular targets to inhibit colorectal cancer growth. Int J Oncol. 2015;47:2247–2254. doi: 10.3892/ijo.2015.3198. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Aragón S, de las Heras B, Sanchez-Reus MI, Benedi J. Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes. Exp Toxicol Pathol. 2001;53:199–206. doi: 10.1078/0940-2993-00185. [DOI] [PubMed] [Google Scholar]
- 28.Xin W, Rui L, Wei Z, Xiaodi Z, Nai L, Zhao W, Wenli L, Xujun Q, Chunxu H. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol Cell Endocrinol. 2013;376:70–80. doi: 10.1016/j.mce.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Chen Y, Abdelkader D, Hassan W, Sun H, Liu J. Combination therapy with oleanolic acid and metformin as a synergistic treatment for diabetes. J Diabetes Res. 2015;2015:973287. doi: 10.1155/2015/973287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mukundwa A, Langa SO, Mukaratirwa S, Masola B. In vivo effects of diabetes, insulin and oleanolic acid on enzymes of glycogen metabolism in the skin of streptozotocin-induced diabetic male Sprague-Dawley rats. Biochem Biophys Res Commun. 2016;471:315–319. doi: 10.1016/j.bbrc.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Han D, Zhang X, Zhang J, Guo X, Zheng Y, Sui S, Zheng J. Oleanolic acid suppresses vascular smooth muscle cell proliferation by increasing lincRNA-p21 expression. Oncol Lett. 2016;12:3519–3522. doi: 10.3892/ol.2016.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engelgau MM, Narayan KM, Herman WH. Screening for type 2 diabetes. Diabetes Care. 2000;23:1563–1580. doi: 10.2337/diacare.23.10.1563. [DOI] [PubMed] [Google Scholar]
- 33.Liu J, Deng W, Fan L, Tian L, Jin L, Jin Z, Guo Q, Xu Y, Li N. The role of radix hedysari polysaccharide on the human umbilical vein endothelial cells (HUVECs) induced by high glucose. Eur J Intern Med. 2012;23:287–292. doi: 10.1016/j.ejim.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Zhong ZY, Tang Y. Upregulation of periostin prevents high glucose-induced mitochondrial apoptosis in human umbilical vein endothelial cells via activation of Nrf2/HO-1 signaling. Cell Physiol Biochem. 2016;39:71–80. doi: 10.1159/000445606. [DOI] [PubMed] [Google Scholar]
- 35.Tsai SJ, Yin MC. Antioxidative and anti-inflammatory protection of oleanolic acid and ursolic acid in PC12 cells. J Food Sci. 2008;73:H174–H178. doi: 10.1111/j.1750-3841.2008.00864.x. [DOI] [PubMed] [Google Scholar]
- 36.Ludescher C, Thaler J, Drach D, Drach J, Spitaler M, Gattringer C, Huber H, Hofmann J. Detection of activity of P-glycoprotein in human tumour samples using rhodamine 123. Br J Haematol. 1992;82:161–168. doi: 10.1111/j.1365-2141.1992.tb04608.x. [DOI] [PubMed] [Google Scholar]
- 37.Arocho A, Chen B, Ladanyi M, Pan Q. Validation of the 2-DeltaDeltaCt calculation as an alternate method of data analysis for quantitative PCR of BCR-ABL P210 transcripts. Diagn Mol Pathol. 2006;15:56–61. doi: 10.1097/00019606-200603000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Indo HP, Davidson M, Yen HC, Suenaga S, Tomita K, Nishii T, Higuchi M, Koga Y, Ozawa T, Majima HJ. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion. 2007;7:106–118. doi: 10.1016/j.mito.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 39.Ly JD, Grubb DR, Lawen A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis. 2003;8:115–128. doi: 10.1023/A:1022945107762. [DOI] [PubMed] [Google Scholar]
- 40.Singh AP, Sarkar S, Tripathi M, Rajender S. Mucuna pruriens and its major constituent L-DOPA recover spermatogenic loss by combating ROS, loss of mitochondrial membrane potential and apoptosis. PLoS One. 2013;8:e54655. doi: 10.1371/journal.pone.0054655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 42.Pan Y, Zhou F, Song Z, Huang H, Chen Y, Shen Y, Jia Y, Chen J. Oleanolic acid protects against pathogenesis of atherosclerosis, possibly via FXR-mediated angiotensin (Ang)-(1–7) upregulation. Biomed Pharmacother. 2018;97:1694–1700. doi: 10.1016/j.biopha.2017.11.151. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Q, Hao R, Wang W, Gao H, Wang C. SIRT1/Atg5/autophagy are involved in the antiatherosclerosis effects of ursolic acid. Mol Cell Biochem. 2016;420:171–184. doi: 10.1007/s11010-016-2787-x. [DOI] [PubMed] [Google Scholar]
- 44.Benard G, Karbowski M. Mitochondrial fusion and division: Regulation and role in cell viability. Semin Cell Dev Biol. 2009;20:365–374. doi: 10.1016/j.semcdb.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 46.Salvesen GS. Caspases: Opening the boxes and interpreting the arrows. Cell Death Differ. 2002;9:3–5. doi: 10.1038/sj.cdd.4400963. [DOI] [PubMed] [Google Scholar]
- 47.Wajant H. The Fas signaling pathway: More than a paradigm. Science. 2002;296:1635–1636. doi: 10.1126/science.1071553. [DOI] [PubMed] [Google Scholar]
- 48.Vaux DL, Cory S, Adams JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- 49.Park CW, Kim HW, Lim JH, Yoo KD, Chung S, Shin SJ, Chung HW, Lee SJ, Chae CB, Kim YS, Chang YS. Vascular endothelial growth factor inhibition by dRK6 causes endothelial apoptosis, fibrosis, and inflammation in the heart via the Akt/eNOS axis in db/db mice. Diabetes. 2009;58:2666–2676. doi: 10.2337/db09-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J, Lei MX, Xie XY, Liu L, She YM, Mo J, Wang S. Rosiglitazone inhibits high glucose-induced apoptosis in human umbilical vein endothelial cells through the PI3K/Akt/eNOS pathway. Can J Physiol Pharmacol. 2009;87:549–555. doi: 10.1139/Y09-040. [DOI] [PubMed] [Google Scholar]
- 51.Ou HC, Lee WJ, Lee SD, Huang CY, Chiu TH, Tsai KL, Hsu WC, Sheu WH. Ellagic acid protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol Appl Pharmacol. 2010;248:134–143. doi: 10.1016/j.taap.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 52.Li X, Song Y, Peng Z, Zhu H, Chen L, Xiao Y, Xing Y. Oleanolic acid inhibits cell survival and proliferation of prostate cancer cells in vitro and in vivo through the PI3K/Akt pathway. Tumour Biol. 2016;37:7599–7613. doi: 10.1007/s13277-015-4655-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and/or analyzed during this study are included in this published article.