Abstract
Background
Early gastro-esophageal cancer is staged as m1 to m3 depending on the infiltration of the anatomical layers of the mucosa or, analogously, as sm1 to sm3 depending on the depth of infiltration into the submucosa. The risk of lymph node metastases is low in mucosal carcinoma but increases with the depth of infiltration into the submucosa.
Methods
This review is based on pertinent publications retrieved by a selective search in MEDLINE, PubMed, the Cochrane Library, and the International Standard Randomised Controlled Trial Number (ISRCTN) registry.
Results
New technologies such as narrow-band imaging have improved the endoscopic diagnosis and staging of early gastro-esophageal cancer. The development of endoscopic submucosal dissection has led to a higher R0 resection rate, a lower risk of recurrence, and an increase in the number of endoscopic resections that are performed with curative intent. In squamous-cell carcinoma of the esophagus, surgical oncological esophagectomy is indicated if the cancer infiltrates into the third mucosal layer (T1a, m3) or deeper. In esophageal adenocarcinoma, the prevalence of lymph node metastases is low if the cancer is restricted to the mucosa and increases only when the submucosa is infiltrated. In the current German S3 guideline, endoscopic resection is recommended for intramucosal adenocarcinoma as long as there are no further histopathological risk factors. Lymph node metastasis in gastric carcinoma begins in the deep mucosal infiltration stage (m3). If certain special conditions (“extended criteria”) are met, carcinoma expanding into the first submucosal layer (sm1) can be removed endoscopically. All further stages must be treated with total or subtotal gastrectomy with systematic D2 lymphadenectomy.
Conclusion
Borderline cases between endoscopic and surgical resection of early carcinoma of the esophagus or stomach must be managed with an interdisciplinary treatment algorithm. If there is a risk of lymph node metastasis, surgical oncological resection is indicated. Such resections of gastroesophageal cancer in the locally advanced stage should always be part of a multimodal treatment approach.
The Robert Koch Institute predicts that about 7400 persons (5700 men, 1700 women; incidence rising) will be diagnosed with esophageal cancer and about 14 700 (9100 men, 5600 women; incidence falling) with stomach cancer in Germany in 2018 (1). For many years surgical resection was the only curative treatment for malignancies of the esophagus and stomach, but endoscopic treatment of these cancers is becoming increasingly widespread. The seamless availability of diagnostic endoscopy in the industrialized nations means that malignant tumors are more often being detected at an early stage, rendering local endoscopic treatment technically feasible, provided the oncological situation permits. Two procedures—endoscopic mucosal resection and endoscopic submucosal resection (ESD)—have become established as techniques for removal of early-stage carcinomas. The complication rates of these procedures depend largely on the endoscopist’s experience. For this reason, their use should be restricted to specialized centers.
At the point where the perioperative risk associated with oncologic surgery outweighs the survival advantage, endoscopic tumor resection becomes not only technically feasible but also medically preferable.
Nevertheless, endoscopic resection is often merely a diagnostic procedure. This is particularly the case when histological analysis of the resected tissue shows a high likelihood of metastasis or when complete excision is not possible by endoscopic means. In such cases it is advisable to proceed to surgical resection in the same session. The patient must be informed of this possibility before commencement of endoscopy. Every instance of endoscopic removal of a malignant tumor from the gastrointestinal tract should be followed by interdisciplinary discussions involving pathologists, surgeons, and endoscopists (tumor board) to decide on how best to proceed (repeat endoscopic resection, surgery, follow-up protocol). A second endoscopic resection should take place only in the case of lateral R1 resection, and then only if all the criteria expounded below are met. In the event of deep R1 resection at the basal resection margin, surgical treatment must always follow.
Furthermore, this review sets out to delineate the indications for endoscopic versus surgical tumor resection and describe how borderline cases should be managed. Here too, the decision on the best treatment for a tumor is taken on an individual basis in full consideration of each patient’s specific circumstances. In a patient whose comorbidities greatly increase the risks involved in surgery, for instance, it may be advisable to adjust the boundaries between endoscopic and surgical treatment presented below, or to administer systemic treatment for an early-stage tumor.
Squamous cell carcinoma of the esophagus
Squamous cell carcinoma of the esophagus is completely different from adenocarcinoma with regard to etiopathogenesis, tumor biology, comorbidity, surgical risks, and prognosis (2, 3). The best-known risk factors are chronic alcohol consumption and smoking (3). Mucosal or submucosal esophageal cancer with or without lymph-node metastases (corresponding to Tis or T1 in the 2017 TNM classification of the Union internationale contre le cancer [UICC] [4]) is defined as superficial.
Endoscopic treatment
Before endoscopic treatment of a squamous cell carcinoma or its precursor, intraepithelial neoplasia, it is essential to determine how far the tumor has spread. This is a precondition for any attempt at curative treatment. Alongside the necessary systematic work-up, diagnostic endoscopy is needed to determine the precise size and extension of the esophageal lesion. Chromoendoscopy with Lugol solution is helpful in this regard (figure 1). According to one systematic review and meta-analysis, endoscopy with narrow band imaging is also feasible and in fact superior to chromoendoscopy for differentiation from other mucosal lesions (5). Preinterventional endoscopic ultrasonography (EUS) has limitations at this stage of disease (6). EUS may underestimate the extent of early neoplasia, but should nevertheless be carried out to exclude more advanced disease.
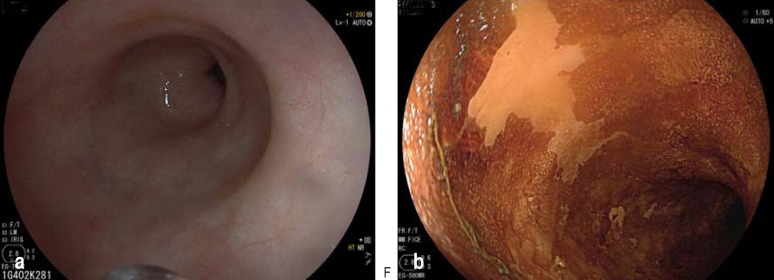
Figure 1.
High-grade intraepithelial neoplasia of the squamous epithelium as precursor of squamous cell carcinoma: a) conventional white light endoscopy; b) chromoendoscopy with Lugol solution
The risk of lymph-node metastases rises with increasing infiltration of deeper layers. For purposes of precise characterization, the depth of infiltration of the mucosa or submucosa is assessed. Depending on which layers within the mucosa are affected, the tumor is classified as m1, m2, or m3. The degree of submucosal infiltration is analogously described as sm1, sm2, or sm3. The risk of lymph-node metastases is low for m1 and m2 mucosal cancers. Further independent factors predicting lymph-node metastasis are tumor size exceeding 2 cm, poor differentiation, and invasion of lymph vessels (7).
Endoscopic resection of squamous cell carcinoma is recommended (8, 9) for m1 and m2 tumors less than 2 cm in size with differentiation grade G1/G2 and no invasion of blood or lymph vessels (L0, V0). Whether the resection takes the form of mucosal or submucosal dissection (Figures 2 and 3) depends on the size of the tumor and the expertise of the center.
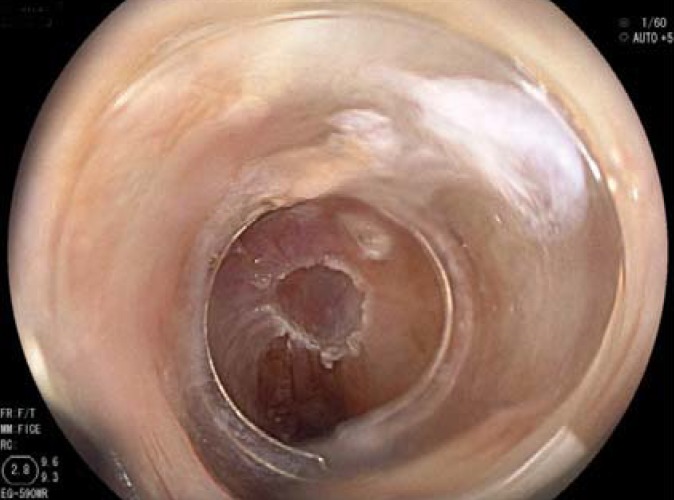
Figure 2.
Same patient as in Figure 1after endoscopic mucosal resection of the affected area
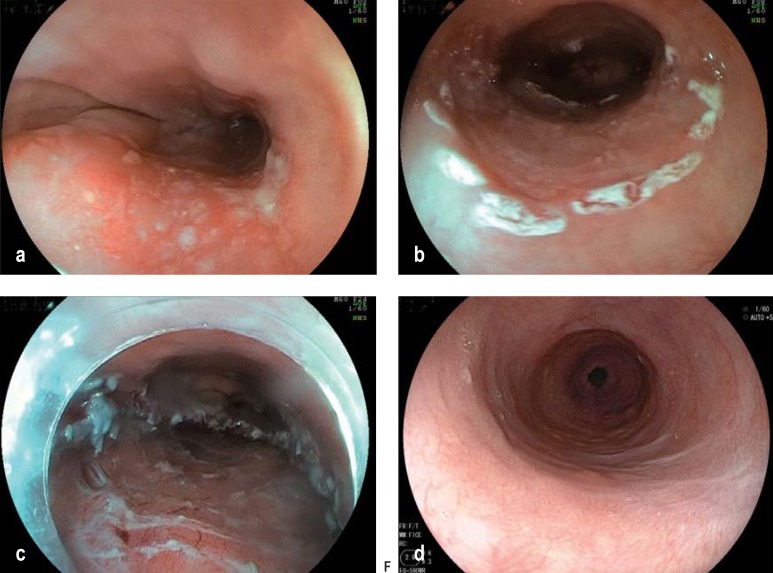
Figure 3.
Endoscopic submucosal dissection (ESD): a) mucosal squamous cell carcinoma before treatment; b) marking of the area for resection; c) resected area after successful ESD; d) scar formation 5 months after ESD
Surgical intervention
To date there are no valid preinterventional diagnostic procedures (endosonography, computed tomography [CT], positron emission tomography/CT) that can be relied upon to identify potential lymph-node metastases of superficial esophageal cancer (mucosal and submucosal infiltration = pT1a, b) with sufficient accuracy.
Surgical studies on resected specimens of superficial squamous cell carcinoma have found that a fairly high rate of lymph-node metastasis can be expected even in mucosal cancer (pT1a) from infiltration depth m3 (10). Stage pT1a, m1 tends not to be associated with lymph-node metastasis, and stage pT1a, m2 only rarely, so these tumors are potential candidates for oncologically adequate endoscopic resection (7, 10–13). From stage pT1a, m3 onward, oncological esophageal resection is indicated. A study of our own patients with oncologically resected pT1 esophageal cancers showed that pV status and tumor size in multivariate analysis were significant predictors of potential lymph-node metastases; the cut-off point for tumor size was 20 mm (14). Oncologically resected submucosal squamous cell carcinomas showed lymph-node involvement in 27% of cases for infiltration depth sm1, 38% for sm2, and as many as 54% for sm3 (15). The prognosis of superficial squamous cell carcinoma of the esophagus is far worse than that of adenocarcinoma (16). The reason is the more aggressive tumor biology exhibited by squamous cell carcinoma. Multivariate analysis in the study by Stein et al. showed that only the histological tumor type and the presence of lymph-node metastases were independent predictors of long-term survival (16). The 5-year survival rate was 83.4% for patients with early adenocarcinoma versus 62.9% for squamous cell carcinoma, and 48.2% for patients with versus 79.5% for those without lymph-node metastases (16).
The outcome in patients treated surgically subsequent to endoscopic therapy of early squamous cell carcinoma of the esophagus (17) led to the recommendation of radical oncological esophageal resection with systematic two-field lymph-node dissection, even if it were technically feasible to perform endoscopic submucosal dissection of pT1b squamous cell carcinoma.
Adenocarcinoma of the esophagus
Chronic exposure of the distal esophagus to acidic gastric contents can bring about Barrett’s esophagus, a change in the mucosal cells, which in turn may lead to esophageal adenocarcinoma (18, 19). The factors predisposing to Barrett’s esophagus are, together with reflux disease and overweight, smoking and male sex. Barrett’s esophagus is more likely to occur after eradication of gastric Helicobacter pylori infection (18– 22). Barrett’s esophagus occurs much more frequently than was previously thought, but progression to adenocarcinoma much less frequently. Population-based studies show that the cancer risk of Barrett’s esophagus is between 0.10% and 0.15% per year and that patients with Barrett’s esophagus rarely die of esophageal adenocarcinoma (23, 24). Compared with the normal population, the relative risk of adenocarcinoma for patients with Barrett’s esophagus is 11.3 (95% confidence interval [8.8; 14.4] (25). Histologically, the most important risk factor for esophageal adenocarcinoma as a result of Barrett’s esophagus is demonstration of intraepithelial neoplasia/dysplasia (25).
Endoscopic treatment
The technique for endoscopic treatment of esophageal adenocarcinoma largely corresponds to that for squamous cell carcinoma. Chromoendoscopy with acetic acid is used to assess tumor spread (figure 4). The risk of lymph node metastases is greater if the tumor extends to the submucosa (26). Endoscopic resection proceeds according to the criteria shown in the Box (9).
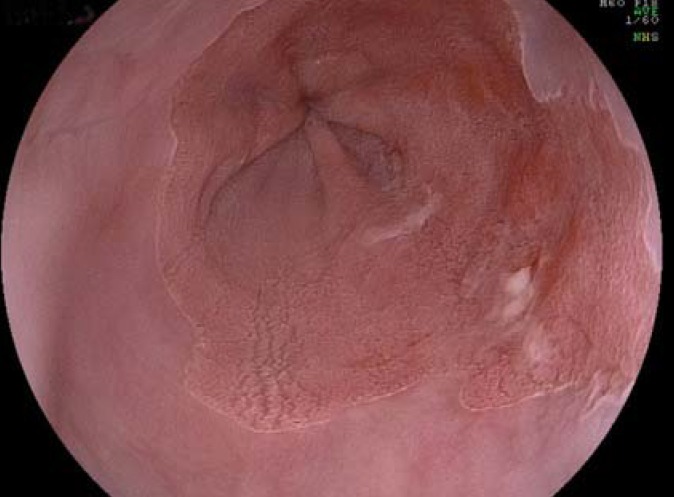
Figure 4.
Chromoendoscopy with acetic acid: early-stage carcinoma in Barrett’s mucosa, chromoendoscopy with 1.5% acetic acid
The therapeutic strategy for esophageal adenocarcinoma often has to include a plan for dealing with precursor intraepithelial neoplasias in existing areas of Barrett’s mucosa. In long-segment Barrett’s esophagus, for instance, Kastelein et al. have reported a rate of 25% per year for progression of high-grade intraepithelial neoplasias to cancer (27). Low-grade intraepithelial neoplasias in Barrett’s esophagus should also be endoscopically eradicated, because all intraepithelial neoplasias pose a risk and the techniques are becoming increasingly widespread and safe (28). Following successful resection of neoplasias in an area of Barrett’s esophagus, the German S3 guideline recommends ablation of the non-neoplastic mucosa in the corresponding area to lower the rate of metachronic neoplasias (9). This thermic removal of the Barrett’s epithelium is accomplished by means of endoscopic radiofrequency application (29). Alternatively, Barrett’s epithelium can be ablated by means of argon plasma coagulation (APC). This more widely available and less costly procedure is used particularly often for shorter segments of Barrett’s esophagus (9).
Surgical intervention
The risk of unanticipated lymph node metastases in patients with Barrett’s adenocarcinoma confined to the mucosa is 1–2%, lower than the reported mortality rate for the operation (30). A nonrandomized controlled comparison of endoscopic resection and oncological esophagectomy in mucosal Barrett’s adenocarcinoma revealed no significant difference with regard to the prognosis (overall survival and disease-free survival) (31). However, the recurrence rate was higher in the endoscopic resection group, so follow-up visits are mandatory (31). On the other hand, morbidity and mortality were higher in the surgical group than after endoscopic resection (31). Surgical resection of Barrett’s esophageal adenocarcinoma following endoscopic resection should always be considered in the presence of the following findings (9, 32– 35):
Infiltration of lymph or blood vessels (L1 or V1)
Poor differentiation (grade = G3)
Submucosal infiltration = 500 µm
Residual tumor at the basal resection margin (R1 basal).
Moreover, surgical treatment is indicated whenever endoscopic resection encounters technical difficulties and/or the endoscopic lifting sign (good raising of the lesion from the underlying tissues) is absent, as well as in the event of repeated recurrences after presumed curative endoscopic resection (36).
The decision whether to opt for endoscopic resection followed by monitoring or proceed immediately to surgical management is not always clear-cut. In surgical specimens of surgically resected superficial Barrett’s adenocarcinoma the boundary between absence and presence of lymphogenic metastasis has been reported to lie between m4 (carcinoma infiltration of the original muscularis mucosae) and sm1, although the borderline for detectable lymph node metastases between these two layers is certainly not sharply defined (36). This is just as true for the distinction between sm1 low risk (good differentiation, L0, V0) and sm1 high risk (poor differentiation, L1, V1). The individual patient’s risk profile with regard to a two-cavity intervention is thus crucial in determining the indications. Problems are also caused by the difficulty of distinguishing between mucosal and submucosal or low-risk and high-risk sm1 Barrett’s adenocarcinomas by means of endosonography or other imaging modalities, so that “diagnostic endoscopic resection” is often the best choice.
In our own efforts to establish a model for prediction, we found that the predictors of lymphogenic metastasis were ranked as follows: grade 3 differentiation; sm3 infiltration; lymphovascular (L1) and microvascular (V1) infiltration; sm2 and sm1 infiltration (37). The risk of lymph-node metastases and lymphovascular invasion increases with tumor infiltration into deeper submucosal layers (14, 38, 39).
Gastric carcinoma
Gastric carcinoma is among the five most commonly occurring cancers in both men and women in Europe (40), and ranks equally highly for mortality (40). In international comparisons of age-standardized incidence and mortality, German men rank second and German women fifth (1).
Endoscopic treatment
As with esophageal carcinoma, there are defined criteria to follow when deciding on the indications for endoscopic treatment (table). For stomach cancer, however, one has to distinguish between absolute and extended indications for endoscopic resection (e1– e4). The extended criteria should be used only in clinical studies.
Table. Treatment options in mucosal and submucosal stomach cancer (from e3).
| Infiltration | Mucosal (T1a) | Submucosal (T1b) | ||||
| Histology | Nonulcerated | Ulcerated | sm1 | sm2 | ||
| Diameter | ≤ 2 cm | >2 cm | ≤ 3 cm | >3 cm | ≤ 3 cm | Any diameter |
| Intestinal type | ER | Extended indications ER |
Extended indications ER |
D2 gastrectomy | Extended indications ESD |
D2 gastrectomy |
| Diffuse type | Consider gastrectomy | D2 gastrectomy | D2 gastrectomy | D2 gastrectomy | D2 gastrectomy | D2 gastrectomy |
ER, endoscopic resection; ESD, endoscopic submucosal dissection
Resection of early tumors by means of endoscopic submucosal dissection yields a higher en-bloc resection rate, a higher rate of complete resection, and a lower recurrence rate than endoscopic mucosal resection (e5).
Surgical intervention
For stomach cancer, lymph-node metastasis begins with infiltration of the deep mucosal layer, m3 (e6), where the risk of spread to the lymph nodes has been stated as 13% (e6). In the case of the extended indications for endoscopic submucosal dissection, the cut-off point is infiltration of the submucosa to a depth of no more than 300 µm (e7). All other submucosal stages (sm1 and Laurén diffuse type, tumor diameter = 3 cm; sm2, Laurén intestinal and diffuse type, any diameter; and all sm3 tumors) necessitate oncological gastrectomy with systematic D2 lymphadenectomy (e3). The proximal safety margin—in accordance with the German S3 guideline on stomach cancer—should be 5 cm for Laurén intestinal type tumors and 8 cm for the diffuse type (e3, e8).
The following risk factors have been identified for lymph-node metastasis in the mucosal type: tumor size, undifferentiated tumor type, lymphogenic and perineural invasion, and tumor ulceration (e9).
The overall risk of lymphogenic metastasis in the presence of submucosal tumor infiltration is reported as circa 25% (e10). The likelihood of lymph-node metastases is not necessarily correlated with the depth of infiltration.
Problems arise when the endoscopic resection is piecemeal rather than en-bloc. It is then harder to assess whether R0 resection has been achieved. Piecemeal resection increases the risk of local recurrence, the reported rate of which ranges from 2% to 35% (e11). Recurrences after endoscopic submucosal dissection must be distinguished from synchronic and metachronic lesions.
Submucosal stomach cancer in a high-risk surgical candidate can, as a “compromise,” be treated with laparoendoscopic (full-thickness wall) resection (e12). On oncological criteria this seems to represent a useful, low-risk option.
BOX.
Indications for endoscopic resection of esophageal adenocarcinoma (9)
-
Mucosal cancer
L0
V0
No ulceration
Grade G1 or G2
-
Submucosal cancer
pT1sm1; <500 µm depth of infiltration
<20 mm
L0
V0
No ulceration
Grade G1 or G2
Key messages.
Both endoscopic mucosal resection and endoscopic submucosal dissection have become established as techniques for removal of early-stage carcinomas from the upper gastrointestinal tract.
The methods and indications for curative endoscopic treatment versus surgery are laid down in national and international guidelines. These reflect the risk of lymphogenic metastasis and/or recurrence, on the basis of defined tumor infiltration depths and further histopathological characteristics.
Decisions on how to proceed in borderline cases between endoscopy and surgery require a differentiated interdisciplinary treatment algorithm together with careful assessment of each individual patient’s risk profile including comorbidities.
In early-stage carcinomas it is often expedient to start with endoscopic resection, which may turn out to have been merely a diagnostic intervention. Options for further management include endoscopic monitoring, endoscopic reintervention, and surgical resection.
Acknowledgments
Translated from the original German by David Roseveare
Footnotes
Conflict of interest statement Prof. Hoffmeister has received payments for lectures and the preparation of scientific meetings from the Falk Foundation.
Prof. Gockel has received payments for lectures from the Falk Foundation.
References
- 1.Zentrum für Krebsregisterdaten, Gesellschaft der epidemiologischen Krebsregisterdaten (eds.) Robert Koch-Institut. Berlin: 2017. Krebs in Deutschland für 2013/2014. 11. Ausgabe. [Google Scholar]
- 2.Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729–735. doi: 10.1111/j.1440-1746.2009.05824.x. [DOI] [PubMed] [Google Scholar]
- 3.Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol. 2007;17:38–44. doi: 10.1016/j.semradonc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Brierley JDG, Gospodarowicz MK, Wittekind C, editors. Wiley Blackwell. 8. Oxford: 2017. TNM classification of malignant tumours. [Google Scholar]
- 5.Morita FH, Bernardo WM, Ide E, et al. Narrow band imaging versus lugol chromoendoscopy to diagnose squamous cell carcinoma of the esophagus: a systematic review and meta-analysis. BMC Cancer. 2017;17 doi: 10.1186/s12885-016-3011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ning B, Abdelfatah MM, Othman MO. Endoscopic submucosal dissection and endoscopic mucosal resection for early stage esophageal cancer. Ann Cardiothorac Surg. 2017;6:88–98. doi: 10.21037/acs.2017.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Du J, Li H, Luo J, Chen L, Wang W. Clinicopathologic analysis of lymph node status in superficial esophageal squamous carcinoma. World J Surg Oncol. 2016;14 doi: 10.1186/s12957-016-1016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2015;47:829–854. doi: 10.1055/s-0034-1392882. [DOI] [PubMed] [Google Scholar]
- 9.Porschen R, Buck A, Fischbach W, et al. S3-Leitlinie Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus (Langversion 10 - September 2015, AWMF-Registernummer: 021/023OL) Z Gastroenterol. 2015;53:1288–1347. doi: 10.1055/s-0041-107381. [DOI] [PubMed] [Google Scholar]
- 10.Takubo K, Aida J, Sawabe M, et al. Early squamous cell carcinoma of the oesophagus: the Japanese viewpoint. Histopathology. 2007;51:733–742. doi: 10.1111/j.1365-2559.2007.02766.x. [DOI] [PubMed] [Google Scholar]
- 11.Endo M, Yoshino K, Kawano T, Nagai K, Inoue H. Clinicopathologic analysis of lymph node metastasis in surgically resected superficial cancer of the thoracic esophagus. Dis Esophagus. 2000;13:125–129. doi: 10.1046/j.1442-2050.2000.00100.x. [DOI] [PubMed] [Google Scholar]
- 12.Eguchi T, Nakanishi Y, Shimoda T, et al. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. Mod Pathol. 2006;19:475–480. doi: 10.1038/modpathol.3800557. [DOI] [PubMed] [Google Scholar]
- 13.Matsubara T, Ueda M, Abe T, Akimori T, Kokudo N, Takahashi T. Unique distribution patterns of metastatic lymph nodes in patients with superficial carcinoma of the thoracic oesophagus. Br J Surg. 1999;86:669–673. doi: 10.1046/j.1365-2168.1999.01067.x. [DOI] [PubMed] [Google Scholar]
- 14.Gockel I, Domeyer M, Sgourakis GG, et al. Prediction model of lymph node metastasis in superficial esophageal adenocarcinoma and squamous cell cancer including D2-40 immunostaining. J Surg Oncol. 2009;100:191–198. doi: 10.1002/jso.21336. [DOI] [PubMed] [Google Scholar]
- 15.Gockel I, Sgourakis G, Lyros O, et al. Risk of lymph node metastasis in submucosal esophageal cancer: a review of surgically resected patients. Expert Rev Gastroenterol Hepatol. 2011;5:371–384. doi: 10.1586/egh.11.33. [DOI] [PubMed] [Google Scholar]
- 16.Stein HJ, Feith M, Bruecher BL, Naehrig J, Sarbia M, Siewert JR. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg. 2005;242:566–573. doi: 10.1097/01.sla.0000184211.75970.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motoyama S, Jin M, Matsuhashi T, et al. Outcomes of patients receiving additional esophagectomy after endoscopic resection for clinically mucosal, but pathologically submucosal, squamous cell carcinoma of the esophagus. Surg Today. 2013;43:638–642. doi: 10.1007/s00595-012-0295-5. [DOI] [PubMed] [Google Scholar]
- 18.Herregods TV, Bredenoord AJ, Smout AJ. Pathophysiology of gastroesophageal reflux disease: new understanding in a new era. Neurogastroenterol Motil. 2015;27:1202–1213. doi: 10.1111/nmo.12611. [DOI] [PubMed] [Google Scholar]
- 19.Kaz AM, Grady WM, Stachler MD, Bass AJ. Genetic and epigenetic alterations in Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterol Clin North Am. 2015;44:473–489. doi: 10.1016/j.gtc.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadaleto BF, Herbella FA, Patti MG. Gastroesophageal reflux disease in the obese: pathophysiology and treatment. Surgery. 2016;159:475–486. doi: 10.1016/j.surg.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 21.Pohl H, Pech O, Arash H, et al. Length of Barrett‘s oesophagus and cancer risk: implications from a large sample of patients with early oesophageal adenocarcinoma. Gut. 2016;65:196–201. doi: 10.1136/gutjnl-2015-309220. [DOI] [PubMed] [Google Scholar]
- 22.Watari J, Tomita T, Oshima T, Fukui H, Miwa H. Relationship between helicobacter pylori status and the development of reflux esophagitis or Barrett’s esophagus. Nihon Rinsho. 2013;71:1453–1461. [PubMed] [Google Scholar]
- 23.de Jonge PJ, van Blankenstein M, Grady WM, Kuipers EJ. Barrett‘s oesophagus: epidemiology, cancer risk and implications for management. Gut. 2014;63:191–202. doi: 10.1136/gutjnl-2013-305490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labenz J, Koop H, Tannapfel A, Kiesslich R, Holscher AH. The epidemiology, diagnosis, and treatment of Barrett‘s carcinoma. Dtsch Arztebl Int. 2015;112:224–234. doi: 10.3238/arztebl.2015.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hvid-Jensen F, Pedersen L, Drewes AM, Sorensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett‘s esophagus. N Engl J Med. 2011;365:1375–1383. doi: 10.1056/NEJMoa1103042. [DOI] [PubMed] [Google Scholar]
- 26.Boys JA, Worrell SG, Chandrasoma P, et al. Can the risk of lymph node metastases be gauged in endoscopically resected submucosal esophageal adenocarcinomas? A multi-center study. J Gastrointest Surg. 2016;20:6–12. doi: 10.1007/s11605-015-2950-9. [DOI] [PubMed] [Google Scholar]
- 27.Kastelein F, van Olphen S, Steyerberg EW, et al. Surveillance in patients with long-segment Barrett‘s oesophagus: a cost-effectiveness analysis. Gut. 2015;64:864–871. doi: 10.1136/gutjnl-2014-307197. [DOI] [PubMed] [Google Scholar]
- 28.Phoa KN, van Vilsteren FG, Weusten BL, et al. Radiofrequency ablation vs endoscopic surveillance for patients with Barrett esophagus and low-grade dysplasia: a randomized clinical trial. JAMA. 2014;311:1209–1217. doi: 10.1001/jama.2014.2511. [DOI] [PubMed] [Google Scholar]
- 29.Desai M, Saligram S, Gupta N, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett‘s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc. 2017;85:482–495. doi: 10.1016/j.gie.2016.09.022. e4. [DOI] [PubMed] [Google Scholar]
- 30.Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett‘s esophagus: a systematic review. Am J Gastroenterol. 2012;107:850–863. doi: 10.1038/ajg.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pech O, Bollschweiler E, Manner H, Leers J, Ell C, Holscher AH. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett‘s esophagus at two high-volume centers. Ann Surg. 2011;254:67–72. doi: 10.1097/SLA.0b013e31821d4bf6. [DOI] [PubMed] [Google Scholar]
- 32.Alvarez Herrero L, Pouw RE, van Vilsteren FG, et al. Risk of lymph node metastasis associated with deeper invasion by early adenocarcinoma of the esophagus and cardia: study based on endoscopic resection specimens. Endoscopy. 2010;42:1030–1036. doi: 10.1055/s-0030-1255858. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald RC, di Pietro M, Ragunath K, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett‘s oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 34.Manner H, May A, Pech O, et al. Early Barrett‘s carcinoma with „low-risk“ submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol. 2008;103:2589–2597. doi: 10.1111/j.1572-0241.2008.02083.x. [DOI] [PubMed] [Google Scholar]
- 35.Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol. 2013;11:630–635. doi: 10.1016/j.cgh.2012.12.040. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz D, Origer J, Pauthner M, et al. Prognostic risk factors of early esophageal adenocarcinomas. Ann Surg. 2014;259:469–476. doi: 10.1097/SLA.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 37.Sgourakis G, Gockel I, Lang H. Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: a systematic review. World J Gastroenterol. 2013;19:1424–1423. doi: 10.3748/wjg.v19.i9.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holscher AH, Bollschweiler E, Schroder W, Metzger R, Gutschow C, Drebber U. Prognostic impact of upper, middle, and lower third mucosal or submucosal infiltration in early esophageal cancer. Ann Surg. 2011;254:802–807. doi: 10.1097/SLA.0b013e3182369128. [DOI] [PubMed] [Google Scholar]
- 39.Zemler B, May A, Ell C, Stolte M. Early Barrett‘s carcinoma: the depth of infiltration of the tumour correlates with the degree of differentiation, the incidence of lymphatic vessel and venous invasion. Virchows Arch. 2010;456:609–614. doi: 10.1007/s00428-010-0925-5. [DOI] [PubMed] [Google Scholar]
- 40.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- E1.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4) Gastric Cancer. 2017;20:1–19. doi: 10.1007/s10120-016-0622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Kim YI, Lee JH, Kook MC, et al. Lymph node metastasis risk according to the depth of invasion in early gastric cancers confined to the mucosal layer. Gastric Cancer. 2016;19:860–868. doi: 10.1007/s10120-015-0535-7. [DOI] [PubMed] [Google Scholar]
- E3.Moehler M, Al-Batran SE, Andus T, et al. German S3-guideline “Diagnosis and treatment of esophagogastric cancer”. Z Gastroenterol. 2011;49:461–531. doi: 10.1055/s-0031-1273201. [DOI] [PubMed] [Google Scholar]
- E4.Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3–15. doi: 10.1111/den.12518. [DOI] [PubMed] [Google Scholar]
- E5.Facciorusso A, Antonino M, Di Maso M, Muscatiello N. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: a meta-analysis. World J Gastrointest Endosc. 2014;6:555–563. doi: 10.4253/wjge.v6.i11.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Holscher AH, Drebber U, Monig SP, Schulte C, Vallbohmer D, Bollschweiler E. Early gastric cancer: lymph node metastasis starts with deep mucosal infiltration. Ann Surg. 2009;250:791–797. doi: 10.1097/SLA.0b013e3181bdd3e4. [DOI] [PubMed] [Google Scholar]
- E7.Eom BW, Yu JS, Ryu KW, et al. Optimal submucosal invasion of early gastric cancer for endoscopic resection. Ann Surg Oncol. 2015;22:1806–1812. doi: 10.1245/s10434-014-4308-z. [DOI] [PubMed] [Google Scholar]
- E8.Laurén P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- E9.Choi KK, Bae JM, Kim SM, et al. The risk of lymph node metastases in 3951 surgically resected mucosal gastric cancers: implications for endoscopic resection. Gastrointest Endosc. 2016;83:896–901. doi: 10.1016/j.gie.2015.08.051. [DOI] [PubMed] [Google Scholar]
- E10.Kang HJ, Kim DH, Jeon TY, et al. Lymph node metastasis from intestinal-type early gastric cancer: experience in a single institution and reassessment of the extended criteria for endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:508–515. doi: 10.1016/j.gie.2010.03.1077. [DOI] [PubMed] [Google Scholar]
- E11.Ishikawa S, Togashi A, Inoue M, et al. Indications for EMR/ESD in cases of early gastric cancer: relationship between histological type, depth of wall invasion, and lymph node metastasis. Gastric Cancer. 2007;10:35–38. doi: 10.1007/s10120-006-0407-2. [DOI] [PubMed] [Google Scholar]
- E12.Hiki N, Nunobe S, Matsuda T, Hirasawa T, Yamamoto Y, Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig Endosc. 2015;27:197–204. doi: 10.1111/den.12404. [DOI] [PubMed] [Google Scholar]