Abstract
Background
Hodgkin lymphoma is the most common neoplasm in young adults, with an incidence of 2 to 3 cases per 100 000 persons per year. Risk-adapted chemotherapy and radiotherapy usually lead to cure. Finding ways to lessen the treatment-associated morbidity and mortality is a major goal of current research.
Methods
For the creation of an updated guideline (DKH grant number 111778), a systematic literature search was carried out in medical databases (MEDLINE, CENTRAL) and guideline databases (GIN) (search dates: January 2012 to June 2017).
Results
Results from 10 meta-analyses, 89 randomized and controlled trials, and 81 prospective or retrospective trials were evaluated. The use of positron emission tomography (PET) is strongly recommended in the initial diagnostic evaluation, as well as for the guidance of treatment in advanced stages. In early stages, two cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) and involved-site radiotherapy (IS-RT) at a dose of 20 Gy are recommended. For the treatment of intermediate stages, two cycles of escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) + two cycles of ABVD and 30 Gy IS-RT are recommended. In advanced stages, two cycles of escalated BEACOPP are administered, and then PET is performed for the guidance of further treatment: two further cycles of escalated BEACOPP are recommended if the PET is negative and four further cycles if it is positive, followed by radiotherapy of PET-positive residual tumor tissue. The five-year survival of patients with Hodgkin lymphoma is 95%. In case of disease recurrence, high-dose chemotherapy followed by autologous stem-cell transplantation is performed, and targeted drugs including brentuximab vedotin, nivolumab, and pembrolizuab are used.
Conclusion
The highly favorable long-term prognosis of HL necessitates careful consideration of the intensity of treatment as well as thorough follow-up to enable the detection of late sequelae, such as second tumors or organ damage.
Hodgkin lymphoma (HL) is a malignant disease of the lymphatic system with an incidence of 2 to 3 cases per 100 000 persons per year (1). It is the most common neoplasm in young adults and has two incidence peaks—one in the third decade of life and one after age 55. 5-year overall survival rates of more than 90% can be achieved with combined chemotherapy and radiotherapy. Therefore, finding ways to lessen the treatment-associated morbidity and mortality is now a major goal of scientific research and clinical trials.
The world’s first evidence-based, consensus-derived S3 guideline for HL was issued in 2013 (2). The update of this guideline incorporates new data and their implications for the diagnostic evaluation, treatment, and follow-up of HL patients. Significant changes from the initial guideline include the following:
the use of positron-emission tomography combined with computed tomography (PET/CT) for the diagnostic evaluation and staging of HL and for treatment guidance in advanced stages,
the role and technique of radiotherapy in early and intermediate stages,
treatment recommendations for patients with advanced-stage or recurrent HL,
and recommendations concerning follow-up.
Method
Guideline concept and development
This updated S3 guideline was developed by an interdisciplinary group including clinicians, methodologists, patient representatives, and delegates from 18 medical specialty societies (eSupplement) and the German Hodgkin Study Group (GHSG) under the aegis of the German Society for Hematology and Oncology. The latter society publishes the 1. German Guideline Program in Oncology (Leitlinienprogramm Onkologie, LO), a joint project of the Association of Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften, AWMF), the German Cancer Society (Deutsche Krebsgesellschaft, DKG) and German Cancer Aid (Deutsche Krebshilfe, DKH) (grant no. 111778).
Key questions concerning patient-relevant endpoints were defined, and a systematic literature search was carried out. The retrieved publications were examined for relevance, trials were assessed for methodological quality and potential biases, and data were extracted. To the degree that the trials were clinically homogeneous, effect estimators were determined for direct comparisons with the random-effects model. The quality of the evidence (degree of confidence in the effect estimators) was assessed using the GRADE approach for each of the endpoints that had been defined a priori. The method is extensively described in the eMethods. The involved specialty societies, organizations, experts, and other participants are listed in the eSupplement. The recommendation grades and degrees of confidence in the evidence for each endpoint are listed in the eTable.
eTable. Recommendation grades and the quality of the evidence.
| Recommendation grades | ||
| Grade | Description | Term |
| A | Strong recommendation | „strongly recommended “ |
| B | Recommendation | „should“ |
| 0 | Open recommendation | „may“ |
| Quality of the evidence | ||
| Evidence level | Definition | Symbol |
| High quality | We are very sure that the true effect is near the estimated effect. | + + + + |
| Moderate quality | We have a moderate degree of confidence in the effect estimators: the true effect is probably near the estimated effect, though the possibility remains that the two differ to a relevant degree. | + + + – |
| Low quality | We have limited confidence in the effect estimators: the true effect may well differ to a relevant degree from the estimated effect. | + + – – |
| Very low quality | We have very little confidence in the effect estimators: the true effect probably differs to a relevant degree from the estimated effect. | + – – – |
Results
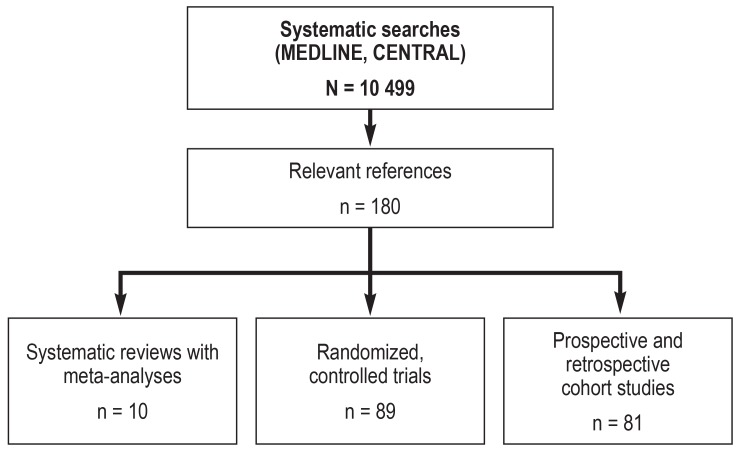
The updated literature searches yielded 10 499 potentially relevant references. The ones selected for use in updating the guideline, because they provided answers to the key questions asked, included 10 systematic reviews with meta-analyses, 89 randomized controlled trials, and 81 prospective or retrospective studies (figure). A total of 14 new recommendations were issued, 77 of the existing recommendations were updated, and 80 were kept unchanged.
Figure.
Results of the systematic literature searches in the MEDLINE and CENTRAL medical databases and the selection of evidence with relevance to the guideline.
The long and short versions of the guideline and the guideline report can be read at www.awmf.org and www.leitlinienprogramm-onkologie.de/leitlinien/. The updated guideline for patients is expected to be published in the autumn of 2018.
Diagnostic evaluation and staging
HL generally becomes symptomatic with the painless enlargement of peripheral lymph nodes. B symptoms (involuntary loss of more than 10% of body weight in 6 months or less; fever above 38°C; night sweats) and other disease-associated symptoms such as alcohol-induced pain or itching may arise as well. For lymphadenopathy of unknown cause that progresses or persists for more than four weeks, further diagnostic evaluation is strongly recommended (expert consensus). To do so, excisional biopsy of an entire lymph node for histopathological examination is strongly recommended (expert consensus).
It is strongly recommended that a reference pathologist confirms the diagnosis of HL(expert consensus). A diagnostic evaluation for the extent of disease (PET/CT and chest x-ray) and organ function tests and, where appropriate, actions for fertility preservation are strongly recommended to be completed within four weeks of the initial diagnosis (expert consensus).
Bone-marrow puncture is strongly not recommended if a PET/CT has been performed (grade A recommendation) (3). The treatment of HL is adapted to the stage of disease. The criteria determining early-, intermediate- or advanced-stage include not only the clinical stage according to the Ann Arbor classification (table), but also the presence or absence of various risk factors, among which are:
Table. Ann Arbor Staging Classification for Hodgkin Lymphoma.
| Stage I | Involvement of a single lymphatic site or localized involvement of a single extralymphatic organ or site |
| Stage II | Involvement of two or more lymph node regions on the same side of the diaphragm or localized involvement of a single extralymphatic organ or site in association with regional lymph node involvement on the same side of the diaphragm |
| Stage III | Involvement of two or more lymph node regions or of extralymphatic organs on both sides of the diaphragm |
| Stage IV | Non-localized, diffuse or disseminated involvement of one or more extralymphatic organs, with or without associated lymph node involvement |
| Addendum A | B symptoms are absent. |
| Addendum B | B symptoms are present: fever (temperature >38ºC), drenching night sweats, and/or unexplained loss of >10% of body weight within the preceding 6 months. |
mediastinal tumor = 1/3 of the maximal transverse diameter of the chest
extranodal involvement
involvement of = 3 lymph node areas
elevated erythrocyte sedimentation rate (ESR) of = 50 or 30 mm/hr in patients without or with B symptoms.
Early stage
When early-stage HL is initially diagnosed, it is strongly recommended that the patient is treated with combined chemotherapy and radiotherapy (grade A recommendation) (4). After two cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine; grade A recommendation) (5, 6), it is strongly recommended to administer 20 Gy of involved-site radiotherapy (IS-RT) (grade A recommendations, overall survival [OS] 96.6% at 5 years and 94% at 10 years) (5, 7). In individual cases, a PET/CT may be performed after two cycles of ABVD to guide further therapy—e.g., to omit consolidating radiotherapy in young women with mediastinal or axillary involvement who have a negative PET/CT, in the knowledge that the tumor control rate will be lower (expert consensus); there is no obligatory reimbursement for PET/CT in this setting in the German legally mandated health-insurance scheme (4, 8). In such cases, in analogy to the British RAPID study and the European H10F study, consolidating treatment with 1–2 additional cycles of ABVD is indicated (9, 10). 1. The current reimbursement status of PET/CT is described in the Box. Secondary neoplasias arose with an elevated standardized incidence of 2.1 during ten years of follow-up after two cycles of ABVD + 20 Gy involved-field (IF)-RT and were the most common cause of death (2%) (11).
BOX. The Reimbursement of Positron-Emission Tomography Combined with Computed Tomography (PET/CT) in Germany.
The reimbursement of PET/CT by the legally mandated German health insurance carriers is not currently guaranteed for all of the indications recommended in this guideline. In situations where it is not guaranteed, there is a corresponding warning in the guideline. From May 2018 onward, the carriers have covered the costs of interim PET/CT for therapeutic decision-making in advanced Hodgkin lymphoma. Until then, the guideline on methods of patient care for physicians working in the legally mandated insurance system (Richtlinie „Methoden vertragsärztliche Versorgung“) already contained the following statement (Chap. 14 – §1): “PET can be performed as a reimbursable procedure for the following indications, as long as the conditions in §§2 and 3 are met: […] 6. For decision-making with regard to radiotherapy for residual tumors of Hodgkin lymphoma after chemotherapy that have been detected by CT and measure more than 2.5 cm in diameter” (e1). A further paragraph was added on 17 May 2018: “9. For decision-making with regard to the necessary number of cycles of chemotherapy for Hodgkin lymphoma in an advanced stage after two cycles of chemotherapy in conformity with the relevant guidelines” (e2).
Intermediate stage
For patients with intermediate-stage HL a combination of four cycles of chemotherapy and localized radiotherapy is strongly recommended (grade A recommendation) (11– 13). In the HD14 trial of the German Hodgkin Study Group, a significantly higher rate of tumor control was achieved with two cycles of escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) followed by two cycles of ABVD (“2 + 2”) than with four cycles of ABVD (5-year progression-free survival [PFS] 95.3% versus 89.3%; 5-year overall survival [OS] 97.2% versus 96.8%), with comparable acute toxicity (WHO grade III/IV: thrombocytopenia 21.9%, leukocytopenia 79%, infection 7.3%, nervous system 3.2%). Thus, patients up to age 60 should be primarily treated with “2 + 2.” If escalated BEACOPP is contraindicated or refused by the patient, the alternative treatment should be with four cycles of ABVD as the next-best option (grade B recommendations) (11– 14).
After systemic treatment, except in the setting of clinical trials, treatment with 30 Gy of consolidating IS-RT is strongly recommended (grade A recommendation for consolidating radiotherapy; grade A recommendation for IS- rather than IF-RT) (4, 7) (grade A recommendation for 30 Gy after four cycles of ABVD, grade B recommendation for 30 Gy after “2 + 2”) (11, 12).
PET/CT findings should not be used to justify deviations from treatment standards outside of clinical trials (grade B recommendation; PET/CT not reimbursable by the German mandatory health insurance scheme) (8).
In individual cases, in analogy to the recommendation for early-stage patients with a negative PET/CT after systemic therapy, consolidating IS-RT may be dispensed with after weighing of the individual risk for RT-associated morbidity against the increased risk of recurrent HL (9, 10).
Advanced stage
For patients with advanced HL who are = 60 years old treatment with escalated BEACOPP chemotherapy followed by 30 Gy of irradiation of residual PET-positive lymphomas measuring = 1.5 cm after chemotherapy is strongly recommended (grade A recommendation) (15, 16). The total number of chemotherapy cycles depends on the early response after two cycles of escalated BEACOPP. It is strongly recommended to treat patients whose PET/CT at this point already reveals complete metabolic remission with a total of four cycles of escalated BEACOPP (5-year PFS, 92.2%; 5-year OS, 97.7%). Patients who have not yet achieved a metabolic remission on PET/CT are strongly recommended to receive a total of six cycles of escalated BEACOPP (5-year PFS, 88.3%; 5-year OS, 95.5%) (grade A recommendation) (17).
Patients over the age of 60 are more likely to develop severe toxicity and thus BEACOPP-based chemotherapy protocol should not be given (treatment-associated mortality, 14.3% in patients over age 60, versus 0.7–3.8% in patients under age 60 [expert consensus]) (18, 19). As long as polychemotherapy is not contraindicated, these patients should be treated with two cycles of ABVD followed by four to six cycles of AVD (doxorubicin, vinblastine, and dacarbazine) and local treatment of residual lymphomas measuring = 1.5 cm with 30 Gy of radiotherapy (19, 20). Alternatively, they can be given six to eight cycles of PVAG (prednisone, vinblastine, doxorubicin, and gemcitabine) followed by local treatment of residual lymphomas measuring = 1.5 cm with 30 Gy of radiotherapy (expert consensus) (21).
Nodular lymphocyte-predominant Hodgkin lymphoma
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) accounts for approximately 5% of cases of HL. It tends to progress more slowly than classic HL (cHL) and is usually diagnosed in an early stage. The malignant cells are characterized by obligate expression of CD20 along with CD30-negativity (22).
For patients who have stage IA NLPHL without risk factors treatment with radiotherapy alone is strongly recommended. High rates of PFS and OS can be achieved with 30 Gy IF-RT as the sole treatment (8-year PFS 91.9%, 8-year OS 99%) (23). Nonetheless, as recommended by the International Lymphoma Radiation Oncology Group (ILROG), the guideline states that 30 Gy IS-RT rather than IF-RT should be given (expert consensus) (7). All NLPHL patients who have risk factors or are not in stage IA are strongly recommended to be treated in the same way as patients with cHL (expert consensus).
Patients with histologically confirmed recurrences of NLPHL can be treated with the standard therapy for recurrent cHL, namely high-dose chemotherapy followed by autologous stem-cell transplantation (24). Alternative treatments, such as the administration of anti-CD20 antibodies, conventional chemotherapy, or radiotherapy, can be considered in the light of a number of factors, including prior treatments, the stage at the time of recurrence, and the interval between first-line treatment and the diagnosis of the recurrence (expert consensus) (25).
Recurrent or refractory Hodgkin lymphoma
Up to 20% of patients with HL, depending on their risk group and initial treatment concept, develop either primary progression or recurrent disease. If no older than 60, such patients should be given high-dose BEAM chemotherapy (carmustine, etoposide, cytarabine, and melphalan) and ASCT (freedom from treatment failure at 3 years, 55% with ASCT versus 34% without; grade A recommendation) (26). Before this is done, they should receive salvage therapy with two cycles of DHAP (dexamethasone, high-dose cytarabine, and displatin; response rate 89%, WHO grade III/IV toxicity 48%, grade B recommendation). A PET/CT is strongly recommended to be obtained to assess the treatment response and the risk profile (grade A recommendation) (27). Patients with high-risk recurrences should receive both consolidation chemotherapy (double high-dose and/or brentuximab vedotin) and consolidating radiotherapy (grade B recommendation) (28, 29).
Patients over age 60 may also be treated with high-dose chemotherapy and ASCT if they are in good general condition (expert consensus). Patients for whom ASCT is not feasible are strongly recommended to betreated with brentuximab vedotin, chemotherapy, or radiotherapy (grade A recommendation).
Patients with recurrences after ASCT should be treated with brentuximab vedotin (response rate 75%, WHO grade III/IV toxicity 55%, grade B recommendation) (30). Second recurrences after treatment with brentuximab vedotin should be treated with PD1 antibodies (nivolumab or pembrolizumab), which have been approved for use in this situation (response rates 66.3% and 69%, respectively, WHO grade III/IV toxicity 5% and 2.4%, respectively, grade B recommendation) (31, 32).
In patients with recurrent HL, treatment with myeloablative allogeneic stem-cell transplantation is strongly not recommended because of the high treatment-associated mortality (grade A recommendation). Allogeneic stem-cell transplantation after reduced-dose (non-myeloablative) conditioning may be performed in individual cases to treat recurrences after ASCT in patients who are still in good general condition and who achieved at least partial remission after their last systemic treatment (grade 0 recommendation) (33). The stem cells used for this purpose may be taken from suitable related or unrelated donors; haplo-identical or umbilical-cord stem cells can be used as well (grade 0 recommendation).
Follow-up
Patients with HL are strongly recommended to be followed up regularly: every 3 months in the first year after treatment, every 6 month in the second through fourth years, and annually thereafter. It is strongly recommended for each medical follow-up appointment to include history-taking, physical examination, and a complete blood count (expert consensus). Radiological imaging should not be performed routinely in asymptomatic patients who were in documented remission of HL after the end of treatment (grade B recommendation). If a recurrence is suspected, it is strongly recommended to confirm it or rule it out by appropriate radiological imaging followed by a biopsy (expert consensus).
Patients who were treated with chemotherapy and/or radiotherapy are at risk for the development of a secondary malignancy (40-year cumulative incidence in 3905 patients treated for HL from 1965 to 2000, 48.5%; this is to be compared with the 19% expected incidence of malignancies in the general population [34]) and other late sequelae of treatment (20, 34– 38). Therefore it is strongly recommended to urgently advise them to undergo the recommended tests for the early detection of cancer (expert consensus). Intensified screening for breast cancer is strongly recommended for women who underwent axillary and/or thoracic radiotherapy when they were less than 30 years old, or who underwent radiotherapy with unusually large treatment fields. Intensified screening should be performed eight years after the end of treatment, but not before the patient is 25 years old. It should include magnetic resonance imaging as well as semiannual diagnostic palpation and annual ultrasonography (grade B recommendation).
At each follow-up examination, it is strongly recommended to ask the patient about symptoms that might indicate late sequelae of treatment (grade A recommendation). Asymptomatic patients who received either chemotherapy containing anthracycline or mediastinal radiotherapy at a dose of 20 Gy or more should undergo electrocardiography, echocardiography, and an examination to detect or rule out coronary heart disease (standardized mortality ratio due to a fatal myocardial infarction after chemotherapy with ABVD alone: 7.8 [39]) (grade B recommendation).
Asymptomatic patients who received both chemotherapy containing anthracycline and mediastinal radiotherapy at a dose of 20 Gy or above should undergo electrocardiography and echocardiography five years after treatment and an examination to detect or rule out coronary heart disease ten years after the end of treatment (standardized mortality ratio due to a fatal myocardial infarction after chemotherapy with ABVD in combination with supradiaphragmatic radiotherapy: 12.1 [18] [expert consensus]). It is strongly recommended that the serum level of thyroid-stimulating hormone (TSH) is measured once a year in patients who underwent radiotherapy in the vicinity of the thyroid gland (risk of thyroid dysfunction in 1677 patients after 20 years: 52% [40] [grade A recommendation]).
Patients who underwent chemotherapy containing bleomycin and/or pulmonary or mediastinal radiotherapy should undergo pulmonary function testing, including measurement of the diffusing capacity, 12 months after the end of treatment (grade B recommendation). If gonadal damage is suspected, this should be investigated in consultation with a specialist (grade B recommendation). Patients complaining of chronic fatigue should be referred to a physician or psychologist with experience in the management of this condition (expert consensus).
Overview
The updated evidence- and consensus-based S3 guideline on the diagnosis, treatment, and follow-up of Hodgkin lymphoma contains many changes and new features compared to the original guideline of 2013. In particular, PET/CT is now assigned a more important role in diagnostic evaluation and treatment guidance. More research is needed on this matter, however.
A number of drugs have been approved in the last few years for the treatment of patients with multiply recurrent HL on the basis of findings from prospective trials. Because of the generally favorable long-term prognosis of the disease, follow-up is important. Here, too, findings from further research would be desirable.
Supplementary Material
eMethods
Guideline Concept and Development
This updated S3 guideline on Hodgkin lymphoma was created from September 2014 to December 2017 by an interdisciplinary group including clinicians, methodologists, patient representatives, and delegates from 18 medical specialty societies (eSupplement) and the German Hodgkin Study Group (GHSG) under the aegis of the German Society for Hematology and Oncology. German Guideline Program in Oncology (Leitlinienprogramm Onkologie, LO), a joint project of the Association of Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medzinischen Fachgesellschaften, AWMF), the German Cancer Society (Deutsche Krebsgesellschaft, DKG) and German Cancer Aid (Deutsche Krebshilfe, DKH) (grant no. 111778). The first step was an online survey in which the key questions that were to be updated or newly introduced were identified, assigned priorities, and agreed upon by consensus. The patient-relevant endpoints for each key question were assigned priorities by the panel members and patient representatives in accordance with the GRADE approach (e3).
The systematic literature search began with a search for evidence-based guidelines in the database of the Guideline International Network (www.g-i-n.net). No report on methods (a prerequisite for guideline adaptation) could be found for any of the published guidelines, so previously developed search strategies were updated or new search strategies created. The methodological and content-related inclusion and exclusion criteria were prospectively defined and implemented by a librarian with experience in the medical terminology of MEDLINE and the CENTRAL database of the Cochrane Library. In addition to systematic reviews and randomized controlled trials (RCTs), non-randomized prospective studies, cohort studies, or studies of the accuracy of diagnostic tests were sought for certain key questions. All publications retrieved by the search strategies were independently evaluated by two scientists for their potential relevance to the guideline.
The studies that were included were independently assessed by two review authors for methodological quality and potential biases, and the data were extracted with the aid of a standardized data-extraction form. To the degree that the trials were clinically homogeneous, effect estimators were determined for direct comparisons with the random-effects model.
The quality of the evidence (degree of confidence in the effect estimators) was assessed with the GRADE approach for each of the endpoints that had been defined a priori, and this information was made available to the authors and delegates through the use of the GRADEpro GDT software (https://gradepro.org/). The consensus-finding process was carried out under the guidance and collaboration of two neutral moderators who were trained and experienced in consensus-finding techniques. Recommendations receiving at least 75% of all votes were considered to have been accepted. Recommendations receiving at least 95% of all votes were considered to have been accepted with a strong consensus.
A working group consisting of experts, patient representatives, guideline-method experts, and representatives of the clinical cancer registries and the DKG certification system revised the guideline-based quality indicators using a methodologically standardized process (e4). Four additional quality indicators were derived from the new strong recommendations. These indicators, in addition to five that had already received a consensus in the creation of the initial guideline, can in the future be documented in the national cancer registries in order to enable evaluation of the disease-specific care of adult patients with HL in conformity with the guideline.
eSupplement
Specialty societies, organizations, experts, and participants
Participating specialty societies and organizations
Participants and experts
The numbers in parentheses refer to membership in the specialty societies.
*1 voting delegate
*2 representative
The adresses given here were valid at the time that the guideline was completed.
-
Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie e. V. (DGHO) (1)
(German Society for Hematology and Medical Oncology, leading society)
-
Arbeitsgemeinschaft für Psychoonkologie (PSO) (2)
(Working Group for Psycho-Oncology)
-
Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) (3)
(Working Group for Gynecological Oncology)
-
Arbeitsgemeinschaft Internistische Onkologie (AIO) (4)
(Working Group for Medical Oncology)
-
Arbeitsgemeinschaft Radiologische Onkologie (ARO) (5)
(Working Group for Radiological Oncology)
-
Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e. V. (AWMF) (6)
(Association of Scientific Medical Societies in Germany)
-
Arbeitsgemeinschaft Supportive Maßnahmen in der Onkologie, Rehabilitation und Sozialmedizin (ASORS) (7)
(Working Group for Supportive Measures in Oncology, Rehabilitation, and Social Medicine)
-
Bundesverband Deutscher Pathologen e. V. (BDP) (8)
(National Association of German Pathologists)
Cochrane Haematological Malignancies Group (CHMG) (9)
-
Deutsche Gesellschaft für Endoskopie und Bildgebende Verfahren (DGE-BV) (10)
(German Society of Endoscopy and Imaging Techniques)
-
Deutsche Gesellschaft für Gynäkologie und Geburtshilfe (DGGG) (11)
(German Society of Gynecology and Obstetrics)
-
Deutsche Gesellschaft für Ultraschall in der Medizin (DEGUM) (12)
(German Society for Ultrasound in Medicine)
-
Deutsche Gesellschaft für Innere Medizin (DGIM) (13)
(German Society of Internal Medicine)
-
Deutsche Gesellschaft für Nuklearmedizin (DGN) (14)
(German Society of Nuclear Medicine)
-
Deutsche Gesellschaft für Pathologie (DGP) (15)
(German Society of Pathology)
-
Deutsche Gesellschaft für Radioonkologie (DEGRO) (16)
(German Society of Radio-Oncology)
-
Deutsche Hodgkin Studiengruppe (GHSG) (17)
(German Hodgkin’s Study Group)
-
Deutsche Krebsgesellschaft e. V. (DKG) (18)
(German Cancer Society)
-
Deutsche Krebshilfe e. V. (DKH) (19)
(German Cancer Aid)
-
Deutsche Leukämie- und Lymphomhilfe (DLH) (20)
(German Leukemia and Lymphoma Aid)
-
Deutsche Röntgengesellschaft (DRG) (20)
(German Radiological Society)
-
Konferenz Onkologischer Kranken- und Kinderkrankenpflege (KOK) (21)
(Conference of Oncological and Pediatric-Oncological Nursing)
-
Leitlinienprogramm Onkologie (OL) (22)
(1. German Guideline Program in Oncology, GGPO)
Prof. Dr. Gerald Antoch (21*1), Universitätsklinikum Düsseldorf, Institut für Radiologie
PD Dr. Ana Barreiros (10*1), I. Medizinische Klinik und Poliklinik, Mainz
Dr. Christian Baues, Uniklinik Köln, Klinik und Poliklinik für Radioonkologie, Cyberknife- und Strahlentherapie
Dr. Karolin Behringer (17), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Boris Böll (17), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Peter Borchmann (1*1, 17), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Lydia Bothe, Berlin, Ärztliches Zentrum für Qualität in der Medizin
Dr. Paul Bröckelmann (17), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Andreas Buck (14*1), Universitätsklinikum Würzburg, Klinik und Poliklinik für Nuklearmedizin
Carolin Bürkle (17), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Markus Dietlein, Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Christoph Frank Dietrich (10*1), Caritas-Krankenhaus Bad Mergentheim, Medizinische Klinik 2
Prof. Dr. Hans-Theodor Eich (16*1), Universitätsklinikum Münster, Klinik und Poliklinik für Strahlentherapie & Radioonkologie
Prof. Dr. Andreas Engert (1*1, 17), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Hans-Henning Flechtner (2*1), Universitätsklinik für Psychiatrie, Psychotherapie und Psychosomatische Medizin des Kindes- und Jugendalters
Dr. Markus Follmann (22), Leitlinienprogramm Onkologie
Josephine Franke (9), Uniklinik Köln, Klinik I für Innere Medizin
Michael Fuchs, Uniklinik Köln, Klinik I für Innere Medizin
Rainer Göbel (20*2), Deutsche Leukämie- und Lymphom-Hilfe
Marius Goldkuhle (9), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Christian Görg (121*1), Universitätsklinikum Giessen und Marburg
Dr. Teresa Halbsgut, Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Dr. h.c. Martin-Leo Hansmann (8*1; 15*1), Universitätsklinikum Frankfurt, Senckenbergisches Institut für Pathologie
Prof. Dr. Marcus Hentrich, Rotkreuzklinikum München, Innere Medizin III – Hämatologie und Onkologie
Dr. Eva Hilgenfeld, Kompetenz-Centrums Onkologie des GKV-Spitzenverbandes und der MDK-Gemeinschaft
Dr. Ulrike Holtkamp (20*1), Deutsche Leukämie- und Lymphom-Hilfe
Prof. Dr. Jens Huober (3*1), Universitätsklinikum Ulm, Frauenheilkunde und Geburtshilfe
Dr. Patrick Jahn (22*1), Martin-Luther-Universität Halle-Wittenberg, Institut für Gesundheits- und Pflegewissenschaften
Tina Jakob (9), Uniklinik Köln, Klinik I für Innere Medizin
PD Dr. Beate Klimm, Krankenhaus Düren
Prof. Dr. Carsten Kobe, Uniklinik Köln, Klinik I für Innere Medizin
Dr. Stefanie Kreissl (17), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Jan Kriz (16), Universitätsklinikum Münster, Klinik und Poliklinik für Strahlentherapie & Radioonkologie
Thomas Langer (22), Leitlinienprogramm Onkologie
Dr. Birgit Leibbrand (7*1), Salzetalklinik
Dr. Reinhard Lorenz, Universitätsklinikum Würzburg, Klinik und Poliklinik für Nuklearmedizin
Jan Lüneberg (20*2), Deutsche Leukämie- und Lymphom-Hilfe
Ina Monsef (9), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Horst Müller, Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Ralph Naumann (1*1; 4*1; 13*1), St. Marien-Krankenhaus Siegen, Medizinische Klinik III
Dr. Monika Nothacker (6), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften
Dr. Johannes Rosenbrock, Uniklinik Köln, Klinik und Poliklinik für Radioonkologie, Cyberknife- und Strahlentherapie
Prof. Dr. Andreas Rosenwald (8*1; 15*1), Universitätsklinikum Würzburg, Institut für Pathologie
PD Dr. Jens Ulrich Rüffer (2*1), TAKEPART Media + Science GmbH
Dr. Jörn Rüssel (7*2), Uniklinik Halle a. d. Saale, Klinik IV für Innere Medizin
Dr. Stephanie Sasse (17), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Heinz Schmidberger (5*1), Universitätsmedizin der Johannes Gutenberg-Universität Mainz, Klinik und Poliklinik für Radioonkologie und Strahlentherapie
PD Dr. Nicole Skoetz (9), Uniklinik Köln, Klinik I für Innere Medizin
PD Dr. Jörg Stattaus (21*1), Uniklinik Essen, Klinik für Radiologie und Nuklearmedizin
Prof. Dr. Holger Strunk (12*1), Radiologische Universitätsklinik Bonn
Prof. Dr. Bettina Toth (11*1), Universitätsklinik für Gynäkologische Endokrinologie und Reproduktionsmedizin, Innsbruck
Dr. Corinna Trenker (12*2), Universitätsklinikum Giessen und Marburg
PD Dr. Bastian Von Tresckow (17), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Simone Wesselmann (18), Deutsche Krebsgesellschaft
Andrea Will (9), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Christoph Wyen, Uniklinik Köln, Klinik I für Innere Medizin
Key Messages.
Positron-emission tomography combined with computed tomography (PET/CT) plays an increasing role in the diagnosis and staging of Hodgkin lymphoma (HL) and in the guidance of treatment for patients with advanced-stage disease.
Patients = 60 with advanced HL and a negative PET/CT after two cycles of escalated BEACOPP 1. are adequately treated with a total of four cycles.
PET/CT-guided treatment is not standard for patients in early and intermediate stages of HL.
The prognosis of patients with multiply recurrent HL can be improved by treatment with brentuximab vedotin, nivolumab, or pembrolizumab.
The favorable prognosis of HL implies that patient follow-up is very important for the detection and treatment of the late sequelae of treatment for HL.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Dr. Bröckelmann has served as a paid consultant for Takeda. He has received reimbursement of travel expenses and meeting participation fees from Bristol-Myers Squibb (BMS) and Takeda, lecture fees from BMS, and research support (third-party funding) from BMS and Takeda.
Prof. Engert has served as a paid consultant for Takeda and BMS. He has received reimbursement of travel expenses and meeting participation fees from Takeda and BMS, lecture fees from Takeda, BMS and MSD, and research support from Takeda, BMS, and Affimed Therapeutics.
The remaining authors state that they have no conflict of interest.
References
- 1.Robert Koch-Institut Krebs in Deutschland. Zentrum für Krebsregisterdaten - www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2017/krebs_in_deutschland_2017.pdf?__blob=publicationFile (last accessed 11 April 2018) [Google Scholar]
- 2.Rancea M, Monsef I, von Tresckow B, Engert A, Skoetz N. High-dose chemotherapy followed by autologous stem cell transplantation for patients with relapsed/refractory Hodgkin lymphoma. Cochrane Database Sys Rev. 2013;6 doi: 10.1002/14651858.CD009411.pub2. CD00941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Galaly TC, d‘Amore F, Mylam KJ, et al. Routine bone marrow biopsy has little or no therapeutic consequence for positron emission tomography/computed tomography-staged treatment-naive patients with Hodgkin lymphoma. J Clin Oncol. 2012;30:4508–4514. doi: 10.1200/JCO.2012.42.4036. [DOI] [PubMed] [Google Scholar]
- 4.Blank O, von Tresckow B, Monsef I, Specht L, Engert A, Skoetz N. Chemotherapy alone versus chemotherapy plus radiotherapy for adults with early stage Hodgkin lymphoma. Cochrane Database Syst Rev. 2017;4 doi: 10.1002/14651858.CD007110.pub3. Cd007110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engert A, Plutschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin’s lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 6.Behringer K, Goergen H, Hitz F, et al. Omission of dacarbazine or bleomycin, or both, from the ABVD regimen in treatment of early-stage favourable Hodgkin’s lymphoma (GHSG HD13): an open-label, randomised, non-inferiority trial. Lancet. 2015;385:1418–1427. doi: 10.1016/S0140-6736(14)61469-0. [DOI] [PubMed] [Google Scholar]
- 7.Specht L, Yahalom J, Illidge T, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG) Int J Radiat Oncol Biol Phys. 2014;89:854–862. doi: 10.1016/j.ijrobp.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Sickinger MT, von Tresckow B, Kobe C, Engert A, Borchmann P, Skoetz N. Positron emission tomography-adapted therapy for first-line treatment in individuals with Hodgkin lymphoma. Cochrane Database Syst Rev. 2015;1 doi: 10.1002/14651858.CD010533.pub2. Cd010533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andre MP, Girinsky T, Federico M, et al. Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 Trial. J Clin Oncol. 2017;35:1786–1794. doi: 10.1200/JCO.2016.68.6394. [DOI] [PubMed] [Google Scholar]
- 10.Radford J, Illidge T, Counsell N, et al. Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med. 2015;17:1598–1607. doi: 10.1056/NEJMoa1408648. [DOI] [PubMed] [Google Scholar]
- 11.Sasse S, Brockelmann PJ, Goergen H, et al. Long-term follow-up of contemporary treatment in early-stage Hodgkin lymphoma: updated analyses of the German Hodgkin Study Group HD7, HD8, HD10, and HD11 Trials. J Clin Oncol. 2017;35:1999–2007. doi: 10.1200/JCO.2016.70.9410. [DOI] [PubMed] [Google Scholar]
- 12.Eich HT, Diehl V, Gorgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199–4206. doi: 10.1200/JCO.2010.29.8018. [DOI] [PubMed] [Google Scholar]
- 13.von Tresckow B, Plutschow A, Fuchs M, et al. Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol. 2012;30:907–13. doi: 10.1200/JCO.2011.38.5807. [DOI] [PubMed] [Google Scholar]
- 14.Skoetz N, Will A, Monsef I, Brillant C, Engert A, von Tresckow B. Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst Rev. 2017;5 doi: 10.1002/14651858.CD007941.pub3. Cd007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379:1791–1799. doi: 10.1016/S0140-6736(11)61940-5. [DOI] [PubMed] [Google Scholar]
- 16.Skoetz N, Trelle S, Rancea M, et al. Effect of initial treatment strategy on survival of patients with advanced-stage Hodgkin’s lymphoma: a systematic review and network meta-analysis. Lancet Oncol. 2013;14:943–952. doi: 10.1016/S1470-2045(13)70341-3. [DOI] [PubMed] [Google Scholar]
- 17.Borchmann P, Goergen H, Kobe C, et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet. 2017;390:2790–2802. doi: 10.1016/S0140-6736(17)32134-7. [DOI] [PubMed] [Google Scholar]
- 18.Wongso D, Fuchs M, Plutschow A, et al. Treatment-related mortality in patients with advanced-stage hodgkin lymphoma: an analysis of the German Hodgkin Study Group. J Clin Oncol. 2013;31:2819–2824. doi: 10.1200/JCO.2012.47.9774. [DOI] [PubMed] [Google Scholar]
- 19.Ballova V, Ruffer JU, Haverkamp H, et al. A prospectively randomized trial carried out by the German Hodgkin Study Group (GHSG) for elderly patients with advanced Hodgkin’s disease comparing BEACOPP baseline and COPP-ABVD (study HD9elderly) Ann Oncol. 2005;16:124–131. doi: 10.1093/annonc/mdi023. [DOI] [PubMed] [Google Scholar]
- 20.Boll B, Goergen H, Behringer K, et al. Bleomycin in older early-stage favorable Hodgkin lymphoma patients: analysis of the German Hodgkin Study Group (GHSG) HD10 and HD13 trials. Blood. 2016;127:2189–2192. doi: 10.1182/blood-2015-11-681064. [DOI] [PubMed] [Google Scholar]
- 21.Boll B, Bredenfeld H, Gorgen H, et al. Phase 2 study of PVAG (prednisone, vinblastine, doxorubicin, gemcitabine) in elderly patients with early unfavorable or advanced stage Hodgkin lymphoma. Blood. 2011;118:6292–6298. doi: 10.1182/blood-2011-07-368167. [DOI] [PubMed] [Google Scholar]
- 22.Eichenauer DA, Engert A. Nodular lymphocyte-predominant Hodgkin lymphoma: a unique disease deserving unique management. Hematology Am Soc Hematol Educ Program. 2017;2017:324–328. doi: 10.1182/asheducation-2017.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eichenauer DA, Plutschow A, Fuchs M, et al. Long-term course of patients with stage IA Nodular Lymphocyte-Predominant Hodgkin Lymphoma: a report from the German Hodgkin Study Group. J Clin Oncol. 2015;33:2857–2862. doi: 10.1200/JCO.2014.60.4363. [DOI] [PubMed] [Google Scholar]
- 24.Akhtar S, Montoto S, Boumendil A, et al. High dose chemotherapy and autologous stem cell transplantation in nodular lymphocyte-predominant Hodgkin lymphoma: A retrospective study by the European Society for Blood and Marrow Transplantation-Lymphoma Working Party. Am J Hematol. 2018;93:40–46. doi: 10.1002/ajh.24927. [DOI] [PubMed] [Google Scholar]
- 25.Eichenauer DA, Pluetschow A, Schroeder L, et al. Relapsed nodular lymphocyte-predominant Hodgkin lymphoma: an analysis from the German Hodgkin Study Group (GHSG) Blood. 2016;128 doi: 10.1182/blood-2018-02-836437. [DOI] [PubMed] [Google Scholar]
- 26.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 27.Brockelmann PJ, Muller H, Casasnovas O, et al. Risk factors and a prognostic score for survival after autologous stem cell transplantation for relapsed or refractory Hodgkin lymphoma. Ann Oncol. 2017;28:1352–1135. doi: 10.1093/annonc/mdx072. [DOI] [PubMed] [Google Scholar]
- 28.Moskowitz AJ, Schoder H, Yahalom J, et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin’s lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 2015;16:284–292. doi: 10.1016/S1470-2045(15)70013-6. [DOI] [PubMed] [Google Scholar]
- 29.Sibon D, Morschhauser F, Resche-Rigon M, et al. Single or tandem autologous stem-cell transplantation for first-relapsed or refractory Hodgkin lymphoma: 10-year follow-up of the prospective H96 trial by the LYSA/SFGM-TC study group. Haematologica. 2016;101:474–481. doi: 10.3324/haematol.2015.136408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30:2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17:1283–1289. doi: 10.1016/S1470-2045(16)30167-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen R, Zinzani PL, Fanale MA, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017;35:2125–2132. doi: 10.1200/JCO.2016.72.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sureda A, Canals C, Arranz R, et al. Allogeneic stem cell transplantation after reduced intensity conditioning in patients with relapsed or refractory Hodgkin’s lymphoma Results of the HDR-ALLO study—a prospective clinical trial by the Grupo Espanol de Linfomas/Trasplante de Medula Osea (GEL/TAMO) and the Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2012;97:310–317. doi: 10.3324/haematol.2011.045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015;373:2499–2511. doi: 10.1056/NEJMoa1505949. [DOI] [PubMed] [Google Scholar]
- 35.Behringer K, Goergen H, Muller H, et al. Cancer-related fatigue in patients with and survivors of Hodgkin lymphoma: The impact on treatment outcome and social reintegration. J Clin Oncol. 2016;34:4329–4337. doi: 10.1200/JCO.2016.67.7450. [DOI] [PubMed] [Google Scholar]
- 36.Kreissl S, Mueller H, Goergen H, et al. Cancer-related fatigue in patients with and survivors of Hodgkin’s lymphoma: a longitudinal study of the German Hodgkin Study Group. Lancet Oncol. 2016;17:1453–1462. doi: 10.1016/S1470-2045(16)30093-6. [DOI] [PubMed] [Google Scholar]
- 37.Behringer K, Mueller H, Goergen H, et al. Gonadal function and fertility in survivors after Hodgkin lymphoma treatment within the German Hodgkin Study Group HD13 to HD15 trials. J Clin Oncol. 2013;31:231–239. doi: 10.1200/JCO.2012.44.3721. [DOI] [PubMed] [Google Scholar]
- 38.Franklin J, Eichenauer DA, Becker I, Monsef I, Engert A. Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis. Cochrane Database Syst Rev. 2017;9 doi: 10.1002/14651858.CD008814.pub2. CD008814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst. 2007;99:206–214. doi: 10.1093/jnci/djk029. [DOI] [PubMed] [Google Scholar]
- 40.Hancock SL, Cox RS, McDougall IR. Thyroid diseases after treatment of Hodgkin’s disease. N Engl J Med. 1991;325:599–605. doi: 10.1056/NEJM199108293250902. [DOI] [PubMed] [Google Scholar]
- E1.Gemeinsamer Bundesausschuss. Richtlinie des Gemeinsamen Bundesausschusses zu Untersuchungs- und Behandlungsmethoden der vertragsärztlichen Versorgung (Richtlinie Methoden vertragsärztliche Versorgung) www.g-ba.de/downloads/62-492-1527/MVV-RL_2017-11-17_iK-2018-02-01.pdf (last accessed 11 April 2018) [Google Scholar]
- E2.Gemeinsamer Bundesausschuss. Richtlinie Methoden vertragsärztliche Versorgung: Positronenemissionstomographie (PET); Computertomographie (CT) zum Interim-Staging bei fortgeschrittenen Hodgkin-Lymphomen (Beschlusstext) www.g-ba.de/downloads/39-261-3333/2018-05-17_MVV-RL-PET_Interim-Staging_fortg_Hodgkin-Lymphome.pdf (last accessed 18 June 2018) [Google Scholar]
- E3.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2 Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- E4.Follmann M, Barlag H, Klinkhammer-Schalke M, et al. Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): Entwicklung von leitlinienbasierten Qualitätsindikatoren Methodenpapier für das Leitlinienprogramm Onkologie. www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Methodik/QIEP_OL_2017.pdf (last accessed 11 April 2018) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Guideline Concept and Development
This updated S3 guideline on Hodgkin lymphoma was created from September 2014 to December 2017 by an interdisciplinary group including clinicians, methodologists, patient representatives, and delegates from 18 medical specialty societies (eSupplement) and the German Hodgkin Study Group (GHSG) under the aegis of the German Society for Hematology and Oncology. German Guideline Program in Oncology (Leitlinienprogramm Onkologie, LO), a joint project of the Association of Scientific Medical Societies in Germany (Arbeitsgemeinschaft der Wissenschaftlichen Medzinischen Fachgesellschaften, AWMF), the German Cancer Society (Deutsche Krebsgesellschaft, DKG) and German Cancer Aid (Deutsche Krebshilfe, DKH) (grant no. 111778). The first step was an online survey in which the key questions that were to be updated or newly introduced were identified, assigned priorities, and agreed upon by consensus. The patient-relevant endpoints for each key question were assigned priorities by the panel members and patient representatives in accordance with the GRADE approach (e3).
The systematic literature search began with a search for evidence-based guidelines in the database of the Guideline International Network (www.g-i-n.net). No report on methods (a prerequisite for guideline adaptation) could be found for any of the published guidelines, so previously developed search strategies were updated or new search strategies created. The methodological and content-related inclusion and exclusion criteria were prospectively defined and implemented by a librarian with experience in the medical terminology of MEDLINE and the CENTRAL database of the Cochrane Library. In addition to systematic reviews and randomized controlled trials (RCTs), non-randomized prospective studies, cohort studies, or studies of the accuracy of diagnostic tests were sought for certain key questions. All publications retrieved by the search strategies were independently evaluated by two scientists for their potential relevance to the guideline.
The studies that were included were independently assessed by two review authors for methodological quality and potential biases, and the data were extracted with the aid of a standardized data-extraction form. To the degree that the trials were clinically homogeneous, effect estimators were determined for direct comparisons with the random-effects model.
The quality of the evidence (degree of confidence in the effect estimators) was assessed with the GRADE approach for each of the endpoints that had been defined a priori, and this information was made available to the authors and delegates through the use of the GRADEpro GDT software (https://gradepro.org/). The consensus-finding process was carried out under the guidance and collaboration of two neutral moderators who were trained and experienced in consensus-finding techniques. Recommendations receiving at least 75% of all votes were considered to have been accepted. Recommendations receiving at least 95% of all votes were considered to have been accepted with a strong consensus.
A working group consisting of experts, patient representatives, guideline-method experts, and representatives of the clinical cancer registries and the DKG certification system revised the guideline-based quality indicators using a methodologically standardized process (e4). Four additional quality indicators were derived from the new strong recommendations. These indicators, in addition to five that had already received a consensus in the creation of the initial guideline, can in the future be documented in the national cancer registries in order to enable evaluation of the disease-specific care of adult patients with HL in conformity with the guideline.
eSupplement
Specialty societies, organizations, experts, and participants
Participating specialty societies and organizations
Participants and experts
The numbers in parentheses refer to membership in the specialty societies.
*1 voting delegate
*2 representative
The adresses given here were valid at the time that the guideline was completed.
-
Deutsche Gesellschaft für Hämatologie und Medizinische Onkologie e. V. (DGHO) (1)
(German Society for Hematology and Medical Oncology, leading society)
-
Arbeitsgemeinschaft für Psychoonkologie (PSO) (2)
(Working Group for Psycho-Oncology)
-
Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) (3)
(Working Group for Gynecological Oncology)
-
Arbeitsgemeinschaft Internistische Onkologie (AIO) (4)
(Working Group for Medical Oncology)
-
Arbeitsgemeinschaft Radiologische Onkologie (ARO) (5)
(Working Group for Radiological Oncology)
-
Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e. V. (AWMF) (6)
(Association of Scientific Medical Societies in Germany)
-
Arbeitsgemeinschaft Supportive Maßnahmen in der Onkologie, Rehabilitation und Sozialmedizin (ASORS) (7)
(Working Group for Supportive Measures in Oncology, Rehabilitation, and Social Medicine)
-
Bundesverband Deutscher Pathologen e. V. (BDP) (8)
(National Association of German Pathologists)
Cochrane Haematological Malignancies Group (CHMG) (9)
-
Deutsche Gesellschaft für Endoskopie und Bildgebende Verfahren (DGE-BV) (10)
(German Society of Endoscopy and Imaging Techniques)
-
Deutsche Gesellschaft für Gynäkologie und Geburtshilfe (DGGG) (11)
(German Society of Gynecology and Obstetrics)
-
Deutsche Gesellschaft für Ultraschall in der Medizin (DEGUM) (12)
(German Society for Ultrasound in Medicine)
-
Deutsche Gesellschaft für Innere Medizin (DGIM) (13)
(German Society of Internal Medicine)
-
Deutsche Gesellschaft für Nuklearmedizin (DGN) (14)
(German Society of Nuclear Medicine)
-
Deutsche Gesellschaft für Pathologie (DGP) (15)
(German Society of Pathology)
-
Deutsche Gesellschaft für Radioonkologie (DEGRO) (16)
(German Society of Radio-Oncology)
-
Deutsche Hodgkin Studiengruppe (GHSG) (17)
(German Hodgkin’s Study Group)
-
Deutsche Krebsgesellschaft e. V. (DKG) (18)
(German Cancer Society)
-
Deutsche Krebshilfe e. V. (DKH) (19)
(German Cancer Aid)
-
Deutsche Leukämie- und Lymphomhilfe (DLH) (20)
(German Leukemia and Lymphoma Aid)
-
Deutsche Röntgengesellschaft (DRG) (20)
(German Radiological Society)
-
Konferenz Onkologischer Kranken- und Kinderkrankenpflege (KOK) (21)
(Conference of Oncological and Pediatric-Oncological Nursing)
-
Leitlinienprogramm Onkologie (OL) (22)
(1. German Guideline Program in Oncology, GGPO)
Prof. Dr. Gerald Antoch (21*1), Universitätsklinikum Düsseldorf, Institut für Radiologie
PD Dr. Ana Barreiros (10*1), I. Medizinische Klinik und Poliklinik, Mainz
Dr. Christian Baues, Uniklinik Köln, Klinik und Poliklinik für Radioonkologie, Cyberknife- und Strahlentherapie
Dr. Karolin Behringer (17), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Boris Böll (17), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Peter Borchmann (1*1, 17), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Lydia Bothe, Berlin, Ärztliches Zentrum für Qualität in der Medizin
Dr. Paul Bröckelmann (17), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Andreas Buck (14*1), Universitätsklinikum Würzburg, Klinik und Poliklinik für Nuklearmedizin
Carolin Bürkle (17), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Markus Dietlein, Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Christoph Frank Dietrich (10*1), Caritas-Krankenhaus Bad Mergentheim, Medizinische Klinik 2
Prof. Dr. Hans-Theodor Eich (16*1), Universitätsklinikum Münster, Klinik und Poliklinik für Strahlentherapie & Radioonkologie
Prof. Dr. Andreas Engert (1*1, 17), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Hans-Henning Flechtner (2*1), Universitätsklinik für Psychiatrie, Psychotherapie und Psychosomatische Medizin des Kindes- und Jugendalters
Dr. Markus Follmann (22), Leitlinienprogramm Onkologie
Josephine Franke (9), Uniklinik Köln, Klinik I für Innere Medizin
Michael Fuchs, Uniklinik Köln, Klinik I für Innere Medizin
Rainer Göbel (20*2), Deutsche Leukämie- und Lymphom-Hilfe
Marius Goldkuhle (9), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Christian Görg (121*1), Universitätsklinikum Giessen und Marburg
Dr. Teresa Halbsgut, Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Dr. h.c. Martin-Leo Hansmann (8*1; 15*1), Universitätsklinikum Frankfurt, Senckenbergisches Institut für Pathologie
Prof. Dr. Marcus Hentrich, Rotkreuzklinikum München, Innere Medizin III – Hämatologie und Onkologie
Dr. Eva Hilgenfeld, Kompetenz-Centrums Onkologie des GKV-Spitzenverbandes und der MDK-Gemeinschaft
Dr. Ulrike Holtkamp (20*1), Deutsche Leukämie- und Lymphom-Hilfe
Prof. Dr. Jens Huober (3*1), Universitätsklinikum Ulm, Frauenheilkunde und Geburtshilfe
Dr. Patrick Jahn (22*1), Martin-Luther-Universität Halle-Wittenberg, Institut für Gesundheits- und Pflegewissenschaften
Tina Jakob (9), Uniklinik Köln, Klinik I für Innere Medizin
PD Dr. Beate Klimm, Krankenhaus Düren
Prof. Dr. Carsten Kobe, Uniklinik Köln, Klinik I für Innere Medizin
Dr. Stefanie Kreissl (17), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Jan Kriz (16), Universitätsklinikum Münster, Klinik und Poliklinik für Strahlentherapie & Radioonkologie
Thomas Langer (22), Leitlinienprogramm Onkologie
Dr. Birgit Leibbrand (7*1), Salzetalklinik
Dr. Reinhard Lorenz, Universitätsklinikum Würzburg, Klinik und Poliklinik für Nuklearmedizin
Jan Lüneberg (20*2), Deutsche Leukämie- und Lymphom-Hilfe
Ina Monsef (9), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Horst Müller, Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Ralph Naumann (1*1; 4*1; 13*1), St. Marien-Krankenhaus Siegen, Medizinische Klinik III
Dr. Monika Nothacker (6), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften
Dr. Johannes Rosenbrock, Uniklinik Köln, Klinik und Poliklinik für Radioonkologie, Cyberknife- und Strahlentherapie
Prof. Dr. Andreas Rosenwald (8*1; 15*1), Universitätsklinikum Würzburg, Institut für Pathologie
PD Dr. Jens Ulrich Rüffer (2*1), TAKEPART Media + Science GmbH
Dr. Jörn Rüssel (7*2), Uniklinik Halle a. d. Saale, Klinik IV für Innere Medizin
Dr. Stephanie Sasse (17), Uniklinik Köln, Klinik I für Innere Medizin
Prof. Dr. Heinz Schmidberger (5*1), Universitätsmedizin der Johannes Gutenberg-Universität Mainz, Klinik und Poliklinik für Radioonkologie und Strahlentherapie
PD Dr. Nicole Skoetz (9), Uniklinik Köln, Klinik I für Innere Medizin
PD Dr. Jörg Stattaus (21*1), Uniklinik Essen, Klinik für Radiologie und Nuklearmedizin
Prof. Dr. Holger Strunk (12*1), Radiologische Universitätsklinik Bonn
Prof. Dr. Bettina Toth (11*1), Universitätsklinik für Gynäkologische Endokrinologie und Reproduktionsmedizin, Innsbruck
Dr. Corinna Trenker (12*2), Universitätsklinikum Giessen und Marburg
PD Dr. Bastian Von Tresckow (17), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Simone Wesselmann (18), Deutsche Krebsgesellschaft
Andrea Will (9), Uniklinik Köln, Klinik I für Innere Medizin
Dr. Christoph Wyen, Uniklinik Köln, Klinik I für Innere Medizin