Abstract
A reference-quality assembly of Fusarium oxysporum f. sp. cepae (Foc), the causative agent of onion basal rot has been generated along with genomes of additional pathogenic and non-pathogenic isolates of onion. Phylogenetic analysis confirmed a single origin of the Foc pathogenic lineage. Genome alignments with other F. oxysporum ff. spp. and non pathogens revealed high levels of syntenic conservation of core chromosomes but little synteny between lineage specific (LS) chromosomes. Four LS contigs in Foc totaling 3.9 Mb were designated as pathogen-specific (PS). A two-fold increase in segmental duplication events was observed between LS regions of the genome compared to within core regions or from LS regions to the core. RNA-seq expression studies identified candidate effectors expressed in planta, consisting of both known effector homologs and novel candidates. FTF1 and a subset of other transcription factors implicated in regulation of effector expression were found to be expressed in planta.
Introduction
The pan-genome of many microbial species, including bacteria, fungi1 and oomycetes2 comprises core genes, often in syntenically conserved, gene-dense regions of the genome and so-called ‘dispensable’ genes, located in gene-sparse regions, flanked by abundant transposable elements3,4. In Fusarium and other fungal phytopathogens these may be specific areas of conserved ‘core’ chromosomes and individual lineage-specific ‘LS’ chromosomes, also known as dispensable or supernumerary chromosomes1.
The fungal pathogen Fusarium oxysporum (Fo) represents a diverse group of formae speciales (ff. spp.) causing crown and root rots as well as vascular wilts5. These ff. spp. are part of the Fo species complex (containing over 150 members6), show narrow host adaptation, and exploit a broad range of niches. Many Fo isolates are non-pathogenic soil saprophytes and some have even been exploited as biocontrol agents7,8.
Bulb onion (Allium cepa L.) is a globally important crop; worldwide production in 2013 was 87Mt9. One of the major constraints to production is Fusarium basal rot (FBR), caused predominantly by F. oxysporum f. sp. cepae (Foc). Symptoms of infection include seedling damping off, root rot and basal rot which spreads up through the bulb scales10. Like many other Fusarium species, Foc produces resilient, long-lived chlamydospores that survive in the soil for many years10,11.
In a recent study, all pathogenic Foc isolates of UK origin were placed in a single clade (divided into 2 sub-clades) based on sequencing of housekeeping genes including EF1-α, whilst non-pathogenic isolates showed much greater diversity and were placed in multiple clades12. This suggests a clonal origin of Foc pathogenicity and is supported by work in Japan where all Fo isolates from bulb onion that were shown to be pathogenic were from the same EF1-α or intergenic spacer region (IGS) clade13. Previous studies based on EF1α14–16, rRNA17, ISSR markers17 and RAPD markers14 have suggested that Foc is more diverse with isolates falling into multiple clades with a potentially polyphyletic origin. However, such studies are rarely associated with large scale pathogenicity testing and many isolates often prove to be non-pathogenic on onions, even though they have been isolated from diseased plants12.
Analysis of tomato plants infected with F. oxysporum f. sp. lycopersici (Fol) has contributed to the identification of 14 F. oxysporum proteins that are Secreted In Xylem sap (SIX 1-14)18 and knockout studies have confirmed that some of these SIX genes (e.g. SIX3, SIX5 and SIX6) contribute to pathogenicity and therefore code for effector proteins19,20. Homologs of SIX genes have also been identified in a wide range of F. oxysporum ff. spp21.
Genome sequencing of Fol isolate 4287 identified LS regions including the ends of chromosomes 1 and 2, as well whole chromosomes 3, 6, 14 and 151. These are hypothesised to have been acquired through horizontal gene transfer1. Of the four LS chromosomes identified in Fol, chromosome 14 has been characterised as being primarily responsible for conferring pathogenicity. LS regions with a total size range of 4–19 Mb have since been identified in a number of other ff. spp. including those infecting cucurbits and legumes and appear to be responsible for host specificity in different ff. spp.21–23.
With the exception of SIX13, all putative SIX effectors identified in Fol 4287, are located on Fol chromosome 14, which is also enriched for secreted proteins and secondary metabolite genes4. This leads to questions over the function of other Fol LS chromosomes that are not strongly implicated in pathogenicity. Due to the difficulties with assembling repeat rich effector-containing regions with short-read technologies, it is still unclear whether other ff. spp. possess four LS chromosomes syntenic to those in Fol or unique complements21. Fo ff. spp. have been shown to possess some effectors that are common to all sequenced Fo isolates (including non-pathogens) as well as effector complements specific to each f. sp23. However, the distribution of these ‘core’ effectors throughout the genome has not yet been determined.
Transcriptional regulation of SIX genes and Fo effector complements is still relatively poorly understood. Nine transcription factors have been identified on Fol LS regions (TF1-9)24. Of these, TF1 (FTF1), a Zn(II)2Cys6-type transcription factor is the best characterised, and regulates SIX gene expression in planta24–26. However, FTF1 contains a number of homologous genes within the Fol genome, including within core chromosomes25. Transcriptional regulation of LS effector genes is dependent upon factors located on core chromosomes, e.g. SGE1 is required for differential expression of Fol effector genes in planta24,27.
SIX genes and other putative effectors are closely associated with miniature inverted-repeat transposable elements (MITEs)4. Two classes of MITEs are associated with SIX genes and are thought to be derived from ancestral transposable elements. Miniature impala (mimp) sequences are found in promoter regions upstream of SIX genes 1–14 and mFot5 sequences are located downstream of SIX genes 2, 4, 5 and 7. Although they are present in the promoter regions of SIX genes, deletion of mimps has not led to differences in SIX gene expression in planta4.
As well as differing between Fo ff. spp., SIX gene complements can also vary within races of individual ff. spp., indicating that there may be loss of genes that do not provide an advantage to pathogenicity. SIX4 has been shown to act as an AVR gene, triggering resistance in tomato through interaction with the I-1 resistance gene28. Fol races 2 and 3 lack SIX4 and have restored virulence on I-1 resistant tomato varieties. It is therefore important to understand the capacity for effector loss in response to selection pressures from deployment of resistant hosts as well as any variation in effector complement in natural populations of pathogenic Fo.
The main aim of this study was to investigate functional specialisation of chromosomes within the Foc genome, using pangenomic comparisons of pathogenic and non-pathogenic isolates and in planta expression studies.
Results
Single molecule sequencing yields a near-complete genome assembly
Three pathogenic Foc (Fus2, 125, A23) and four non-pathogenic Fo isolates (A13, A28, PG, CB3) from onion were selected for whole genome sequencing based upon previous work12. Highly pathogenic isolates were collected from different UK locations, each containing seven SIX genes, whereas the four non-pathogenic isolates had one or no SIX genes. The pathogenic Foc isolate Fus2 has been used in multiple experiments assessing isolate virulence and onion resistance12,15 and was selected for further sequencing using PacBio long-read technology. (Table 1, section A). The Fus2 assembly yielded a 34 contig, 53.4 Mb reference genome while the remaining isolates yielded de novo assemblies of 50–55 Mb in 920–3121 contigs. Gene space within assemblies was shown to be comparable with over 99% of 3725 core Sordariomycete genes (BUSCO) present in all assemblies (Table 1, section A), values comparable to previous Fusarium sequencing projects1.
Table 1.
Summarised assembly (A) and gene model (B) statistics for F. oxysporum (Fo) and F. oxysporum f. sp. cepae (Foc) isolates. Gene models predicted in this study show values predicted by Braker and additional genes predicted by CodingQuarry. Reference values are shown for the published Fo isolate Fo47 and F. oxysporum f. sp. lycopersici (Fol) isolate 4287 genomes.
| Organism | Foc | Foc | Foc | Fo | Fo | Fo | Fo | Fo | Fol |
|---|---|---|---|---|---|---|---|---|---|
| Isolate | Fus2 | 125 | A23 | A13 | A28 | CB3 | PG | Fo471 | 42871 |
| (A) Assembly stats: | |||||||||
| Total coverage (fold) | 214 | 63 | 47 | 33 | 53 | 30 | 69 | — | — |
| Technology | PacBio + MiSeq | MiSeq | MiSeq | MiSeq | MiSeq | MiSeq | MiSeq | — | — |
| Assembly size (Mb) | 53.4 | 51.4 | 51.0 | 54.8 | 53.1 | 50.5 | 50.3 | 49.7 | 61.5 |
| Contigs | 34 | 2121 | 1999 | 3121 | 2375 | 1720 | 920 | 124 | 15 + 73* |
| Largest contig (Kb) | 6,434 | 573 | 538 | 806 | 1,221 | 1,006 | 2,304 | 6,199 | 6,855 |
| N50 (Kb) | 414 | 128 | 147 | 167 | 297 | 237 | 413 | 3,844 | 4,590 |
| Sodariomycete genes (BUSCO) | 3687 | 3686 | 3688 | 3692 | 3683 | 3679 | 3684 | 3687 | 3599 |
| % Sodariomycete genes (BUSCO) | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 99 | 97 |
| % Repeatmasked | 10.54 | 7.36 | 6.96 | 9.03 | 8.35 | 5.67 | 6.11 | 5.68 | 16.42 |
| (B) Gene models: | |||||||||
| Total genes | 18855 | 18505 | 18323 | 18790 | 18629 | 17943 | 17830 | 18191 | 20925 |
| Total proteins | 19371 | 18743 | 18557 | 18934 | 18874 | 18178 | 18101 | 24818 | 27347 |
| Braker transcripts | 17823 | 17197 | 17006 | 17986 | 17426 | 16833 | 16811 | — | — |
| CodingQuarry transcripts | 1548 | 1546 | 1551 | 948 | 1448 | 1345 | 1290 | — | — |
| Sodariomycete genes (BUSCO) | 3668 | 3671 | 3663 | 3673 | 3627 | 3663 | 3664 | 3687 | 3577 |
| % Sodariomycete genes (BUSCO) | 98 | 99 | 98 | 99 | 97 | 98 | 98 | 99 | 96 |
| Secreted Genes | 1449 | 1439 | 1431 | 1505 | 1442 | 1433 | 1429 | 1409 | 1493 |
*number of Fol chromosomes + non-scaffolded contigs
Gene annotation in the foc genome
Gene prediction resulted in 17,830–18,855 genes in Fo and Foc assemblies (Table 1, section B), comparable to the 18,191 Fo gene models1. Differences in gene prediction approaches were apparent in the number of predicted proteins, with greater numbers of alternative transcripts predicted in Fo47 and Fol 4287 genomes (Table 1). BUSCO analysis showed a low false negative rate, comparable to that of Fo47 and Fol 4287. Assemblies were submitted as Whole Genome Shotgun projects to DDBJ/ENA/Genbank (Supp. Table 1).
Phylogeny confirms a single clade of onion-infecting Foc
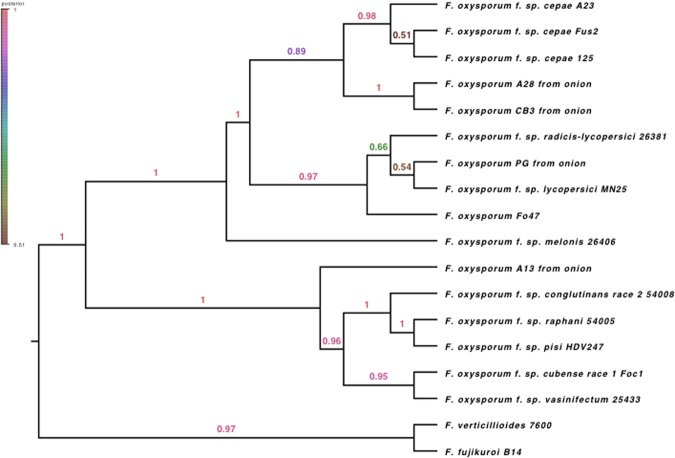
A *BEAST phylogenetic tree of the thirty single copy genes present in all Eukaryotic fungi for all available Fo ff. spp. and non-pathogenic isolates revealed a well-supported clade for all sequenced pathogenic isolates of Foc indicating a monophyletic origin (Fig. 1). Non-pathogenic Fo strains from onion are interspersed throughout the phylogeny and clustered with a range of other ff. spp. For example, isolate PG from onion is in the clade of Fol 4287.
Figure 1.
Bayesian phylogeny of Fusarium oxysporum isolates from onion and other hosts using 30 single copy loci. Pathogenic Foc isolates (Fus2, 125, A23) are monophyletic within the phylogeny while non-pathogenic isolates from onion (A13, A28, CB3, PG), are interspersed throughout the tree.
Genome alignment allows identification of pathogen-specific chromosomes and genomic regions
Alignments of the six de novo assembled genomes to the Foc Fus2 reference genome allowed identification of core and LS regions between the three pathogenic Foc and three non-pathogenic Fo isolates of onion (Table 2). Core contigs represented 46.7 Mb of the Fus2 assembly, while seven LS contigs represented 5.7 Mb of the assembly. Fus2 contig 18 was identified as LS by this analysis; however, later synteny analysis in comparison with Fol showed some conservation of synteny across this contig and it was therefore considered a poorly aligned region of a core chromosome. Of the seven identified LS
Table 2.
Identification of core (C), lineage specific (LS) and pathogen specific (PS) regions in the Fusarium oxysporum f. sp. cepae (Foc) isolate Fus2 genome, through alignment of Foc, F. oxysporum (Fo) and f. sp. lyccopersici (Fol) assemblies. The percentage of non-masked bp covered by aligned sequence is shown for each reference contig.
| Alignment to Fus2 contig | Specificity | Foc | Foc | Foc | Fo | Fo | Fo | Fo | Fo | Fol |
|---|---|---|---|---|---|---|---|---|---|---|
| Fus2 | 125 | A23 | A13 | A28 | CB3 | PG | Fo47 | 4287 | ||
| 1 | C | 100 | 99 | 99 | 96 | 98 | 97 | 98 | 98 | 90 |
| 2 | C | 100 | 99 | 99 | 92 | 97 | 96 | 97 | 98 | 93 |
| 3 | C | 100 | 98 | 99 | 95 | 93 | 96 | 94 | 95 | 92 |
| 4 | C | 99 | 98 | 98 | 91 | 95 | 93 | 78 | 93 | 88 |
| 5 | C | 100 | 99 | 99 | 92 | 94 | 94 | 96 | 94 | 92 |
| 6 | C | 100 | 98 | 98 | 94 | 97 | 97 | 97 | 96 | 92 |
| 7 | C | 100 | 98 | 99 | 89 | 95 | 94 | 93 | 93 | 85 |
| 8 | C | 100 | 98 | 99 | 93 | 93 | 93 | 97 | 96 | 93 |
| 9 | C | 99 | 97 | 97 | 89 | 93 | 94 | 95 | 90 | 85 |
| 10 | LS + PS | 99 | 88 | 87 | 15 | 12 | 11 | 9 | 6 | 24 |
| 11 | C | 99 | 93 | 95 | 80 | 92 | 89 | 88 | 82 | 63 |
| 12 | C | 100 | 98 | 99 | 85 | 89 | 88 | 96 | 94 | 83 |
| 13 | C | 100 | 96 | 97 | 88 | 92 | 91 | 92 | 91 | 88 |
| 14 | LS | 99 | 84 | 86 | 81 | 80 | 29 | 57 | 48 | 30 |
| 15 | C | 100 | 99 | 99 | 91 | 98 | 95 | 96 | 96 | 89 |
| 16 | LS + PS | 93 | 68 | 67 | 15 | 11 | 11 | 12 | 10 | 11 |
| 17 | C | 99 | 97 | 96 | 86 | 86 | 91 | 92 | 91 | 81 |
| 18 | LS* | 99 | 93 | 89 | 60 | 63 | 50 | 51 | 57 | 39 |
| 19 | LS + PS | 93 | 53 | 57 | 19 | 15 | 11 | 12 | 12 | 11 |
| 20 | LS | 98 | 86 | 88 | 87 | 85 | 15 | 10 | 9 | 9 |
| 21 | LS + PS | 91 | 61 | 60 | 9 | 9 | 6 | 4 | 6 | 7 |
| 22 | LS | 100 | 86 | 87 | 80 | 77 | 29 | 46 | 67 | 54 |
*Contig 18 was identified as non-PS LS, but later analysis showed synteny to FOL chromosome 12 and alignment of Fo raw sequencing reads across this region.
contigs, four were only found in the three Foc pathogens, representing 3.9 Mb of the assembly, and were designated as “Pathogen Specific” (PS) contigs. The reciprocal alignments of Foc and non-pathogenic Fo isolates against the Fol genome clearly identified known Fol LS chromosomes (Supp. Table 2). An additional 11 Foc Fus2 contigs, (440 kb in total including mitochondrial sequences) were smaller than 200 kb and were excluded from this analysis.
Single copy orthologous genes allow mapping of Foc contigs to Fol chromosomes
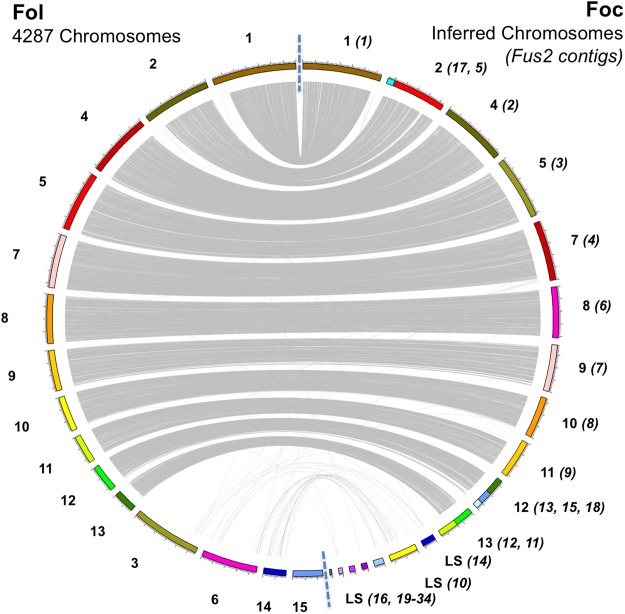
Foc Fus2 contigs were assigned a chromosome ID consistent with the Fol 4287 assembly following synteny analysis of orthologous genes between Fol 4287 and Foc Fus2. Orthology analysis identified genes common between all Fo isolates and those unique to Foc and Fol; 97% proteins were clustered into 15607 orthogroups, 10891 orthogroups were shared between all Fo isolates and represented 151,631 proteins while 7396 orthogroups contained proteins in a 1:1 relationship between Foc Fus2 and Fol 4287. The location of genes encoding these proteins allowed macrosynteny to be assessed between the 15 Fol 4287 chromosomes and the 34 assembled Foc contigs. Of the 34 Foc contigs, 12 were smaller than 200 Kb and were considered too small to be assigned. Of the remaining 22 Foc contigs, 15 were assigned to the 11 core Fol chromosomes (Fig. 2). The remaining 7 Foc contigs did not show clear synteny with Fol chromosomes, supporting their designation as LS in the alignment analysis presented above.
Figure 2.
Synteny of chromosomes between Fol and Foc genome assemblies. Relationships are shown through linking single copy orthologous genes, present in both genomes. Core chromosomes can be identified through synteny between Foc and Fol whereas LS regions show reduced synteny. The number of LS chromosomes does not appear to be conserved between assemblies. Fol chromosome 15 harbours no genes in single copy orthogroups.
Effector annotation of the Fus2 genome
Generic effector-prediction approaches based upon identification of secreted proteins with an effector-like structure (small, cysteine rich) as predicted by EffectorP, secreted carbohydrate active enzymes (CAZymes) (Table 3, section A) and identification of secondary metabolite synthesis genes (Table 3, section B) were used to investigate Foc and Fo effector complements. Secreted proteins represented 7.6% of the Fus2 proteo and genes encoding proteins with an effector-like structure approximately 1.9% of the proteome. Overall, comparisons between genomes showed no significant differences between the total numbers of Foc and non-pathogenic Fo genes encoding secreted proteins (t = 0.23, df = 4.8, p-value = 0.83), EffectorP proteins (t = −1.29, df = 4.02, p-value = 0.26), or secreted CAZymes (t = −0.72, df = 4.07, p-value = 0.51).
Table 3.
Effector candidates in Fusarium oxysporum (Fo) and F. oxysporum f. sp. cepae (Foc) genomes. Numbers also shown for publically available Fo isolate Fo47 and F. oxysporum. f. sp. lycopersici (Fol) isolate 4287 genomes. Number of genes encoding putative effector candidates (A), in secondary metabolite clusters (B), or within 2 Kb of a mimp sequence are reported (B) along with numbers of secondary metabolite clusters (C).
| Organism | Foc | Foc | Foc | Fo | Fo | Fo | Fo | Fo | Fol |
|---|---|---|---|---|---|---|---|---|---|
| Isolate | Fus2 | 125 | A23 | A13 | A28 | CB3 | PG | Fo47 | 4287 |
| (A) Effector candidates: | |||||||||
| Secreted & EffectorP | 355 | 357 | 355 | 364 | 346 | 357 | 337 | 291 | 351 |
| Secreted CAZYmes | 386 | 386 | 387 | 397 | 376 | 383 | 381 | 382 | 386 |
| (B) Secondary metabolites: | |||||||||
| Total gene clusters | 50 | 46 | 46 | 49 | 48 | 44 | 45 | 47 | 49 |
| Genes in clusters | 703 | 559 | 561 | 626 | 656 | 604 | 638 | 705 | 701 |
| (C) Mimps | |||||||||
| Mimps in genome | 153 | 140 | 136 | 55 | 35 | 30 | 51 | 25 | 142 + 16* |
| Genes in 2 Kb of Mimp | 155 | 95 | 88 | 49 | 36 | 32 | 43 | 24 | 108 |
| Secreted genes in 2 Kb of Mimp | 31 | 24 | 20 | 9 | 5 | 1 | 3 | 3 | 22 |
*additional mimps located in non-scaffolded Fol contigs.
Mimp distribution in the Fus2 genome and prediction of mimp-associated effector candidates
Mimp sequences are often found in proximity to Fo effectors4. Foc genomes were found to contain 136–153 mimps, significantly more than the 25–55 observed in non-pathogenic Fo isolates (t = −13.29, df = 5.75, p-value < 0.01) (Table 3, section C). Of the 153 mimps within the Foc Fus2 genome, the majority are distributed throughout LS regions, with 120 in newly designated PS regions and 20 in non-PS LS regions. Of the 158 mimps identified in the Fol genome4, 132 are present in Fol LS chromosomes, 10 in Fol core chromosomes and 16 in unplaced Fol contigs.
Foc core chromosomes 11–13 are enriched for secreted proteins and cell wall degrading enzymes
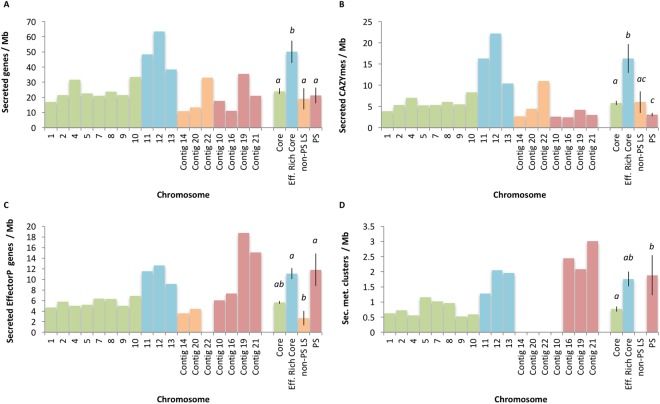
Foc core chromosomes 11–13 were noted to contain many genes with an effector-like structure and secreted CAZymes (Fig. 3). Comparison of the density of genes between core, effector-rich core (chromosomes 11–13), non-PS LS and PS regions of the genome (Fig. 4) showed that secreted genes (SignalP) were present at different densities between core, effector-rich core, LS and PS regions of the Fus2 genome (F(3,14) = 8.072, P < 0.01). Similarly, the density of secreted CAZymes (F(3,14) = 12.04, P < 0.01), EffectorP genes (F(3,14) = 7.506, P < 0.01) and secondary metabolite clusters (F(2,12) = 4.26, P = 0.04) also differed between these regions. Effector-rich core regions were found to contain secreted genes at 50 genes Mb−1; double the density at which these genes were found in other regions of the genome (19–24 genes Mb−1). Similarly, secreted CAZYmes were highly enriched within this region at 16 genes Mb−1 in comparison to 3–6 genes Mb−1 in other regions. The effector-rich core also showed high densities of EffectorP genes and secondary metabolite clusters, although these were not significantly enriched in comparison to core regions (Fig. 4).
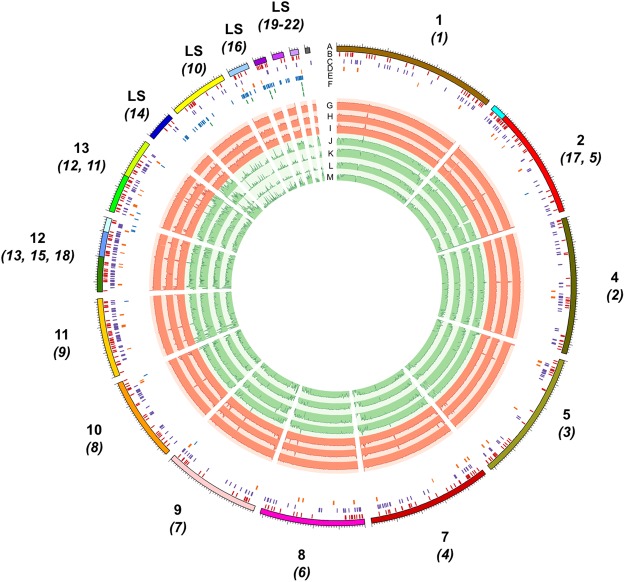
Figure 3.
Visualisation of core chromosomes and 7 assigned LS contigs from Foc isolate Fus2 genome (A). Chromosome designations in relation to Fol are shown, with constituent contents shown in brackets. Locations of predicted effector genes (B), secreted carbohydrate active enzymes (C), secondary metabolite gene clusters (D), mimp sequences (E) and SIX gene homologs (F; shown over three lines) are identified within contigs. Alignment of assemblies from pathogenic Foc isolates Fus2, 125 and A23 (G–I), non-pathogenic isolates A28, PG, CB3 and A13 (J–M).
Figure 4.
Density of genes associated with an effector-associated function in Foc genomic regions including those encoding: secreted proteins (A); secreted carbohydrate active enzymes (CAZYmes) (B); proteins with an effector-like structure (EffectorP) (C); secondary metabolite gene clusters (D). Average gene density (±SE) is also shown by genomic region including significant differences in gene density by region (ANOVA, P < 0.05).
Identification of effector candidates in PS regions
Fus2 PS regions contained 34 secreted EffectorP genes, 11 secreted CAZymes and 4 secondary metabolite gene clusters (Supp. Table 3), of which 14, 4 and 3 were within 2 Kb of a mimp respectively. Non-PS LS regions contained an additional 6 EffectorP genes and 7 Secreted CAZymes; in each case two of these were within 2 Kb of a mimp. Of the four PS secondary metabolite gene clusters, one was a terpene synthesis gene cluster and one was a polyketide synthesis gene cluster, neither of which were within 2 Kb of a mimp.
The Foc genomes were confirmed to carry homologs of the seven Fol SIX genes (SIX3, 5, 7, 9, 10, 12 and 14) through BLAST searches (Supp. Table 4) as reported previously12 with two homologs of SIX3 and SIX9 present. All SIX genes identified within the Foc Fus2 genome are located within the four PS contigs 10, 16, 19 and 21 (Supp. Table 5, Fig. 3). Searches for SIX genes in the non-pathogenic Fo isolates from onion also confirmed that isolate PG contained SIX912.
Investigation into sequence conservation between Foc isolates found no non-synonymous variation between EffectorP genes and secreted CAZymes in PS regions. Similarly, very little variation was seen in these effector candidates in other regions of the genome, with only five EffectorP genes and secreted CAZymes showing variation between Foc isolates (Supp. Table 6).
Effector candidates in LS regions show lower codon usage bias than those located in the core genome
Codon usage was investigated across Foc Fus2 genes in core and LS regions (Supp. Table 7). Mean CBI values for secreted CAZY and secreted EffectorP candidates were 0.385 and 0.336 respectively, significantly higher (t-test, p-value < 10−18) than found for all genes (mean CBI = 0.215). In the Fol genome, Ma et al. (2010) showed a shift in preferred codons between LS and core chromosomes, especially towards GC in their third position1. In Foc there was no change in GC content across these two portions of the genome (core chromosomes, mean 51.7%; LS chromosomes 51.3%). LS regions (mean CBI = 0.145) contained genes with a significantly lower (t-test, p-value < 2.2e-16) average codon usage bias than in the core genome (mean CBI = 0.221). All genes with close proximity (<2 Kb) to mimps showed relaxed codon usage bias and LS secreted genes showed significant (t-test, p-value = 8.84E-03) codon usage bias (mean CBI = 0.284) compared to the rest of the LS genes (mean CBI = 0.145).
Foc LS regions have higher levels of gene duplication than core chromosomes
Many orthogroups on Fol and Foc LS regions contained inparalogs, with homologous genes elsewhere in LS regions (Supp. Fig. 1). Duplicated genes in LS regions showed a bias towards being shared with other LS regions, whereas core Foc regions exhibited lower levels of gene duplication, with a concentration of duplicated genes shared with terminal regions of other chromosomes/contigs. In total, 1,424 gene clusters, representing one or more duplication events were identified. Taking one representative focal gene in each cluster and comparing the chromosomal/contig location between pairs of genes in these clusters, 49% of duplication events were observed to be within or between LS chromosomes/contigs (3,186). In contrast, 28% of duplications were between core and LS (1,822) and 23% within or between core and core (1,517) chromosomes. Overall, 1,235 of 2,115 genes on LS contigs contained a paralog in the genome, corresponding to a lower density of duplicated genes on core than on LS chromosomes (permutation test, p-value < 0.001). Dissection of duplications into tandem and segmental did not reveal any further patterns, with only 69 tandem duplications identified.
Open reading frame density is maintained in Foc LS regions
As in Fol, Foc LS contigs showed increased levels of repetitive and low complexity content in comparison to core regions, with 3–15% repeat-masked in core regions compared with 33–59% in LS regions respectively (Supp. Fig. 2). Gene density of Fol LS regions has previously been shown to be lower than on core regions1 but this was not the case for Foc, where gene density was maintained between core and LS regions (Supp. Fig. 2). Analysis of predicted gene function for Foc LS regions found that Interproscan terms associated with transposon activity were significantly enriched on non-PS LS regions and PS regions (Supp. Table 8, P < 0.05). Terms associated with Helitron helicase transposons were found exclusively on PS regions. Aside from genes with transposon-associated features, PS regions showed enrichment for genes lacking IPR annotations (Supp. Table 8).
Known effectors and effector candidates in PS regions are among the highest expressed genes in planta
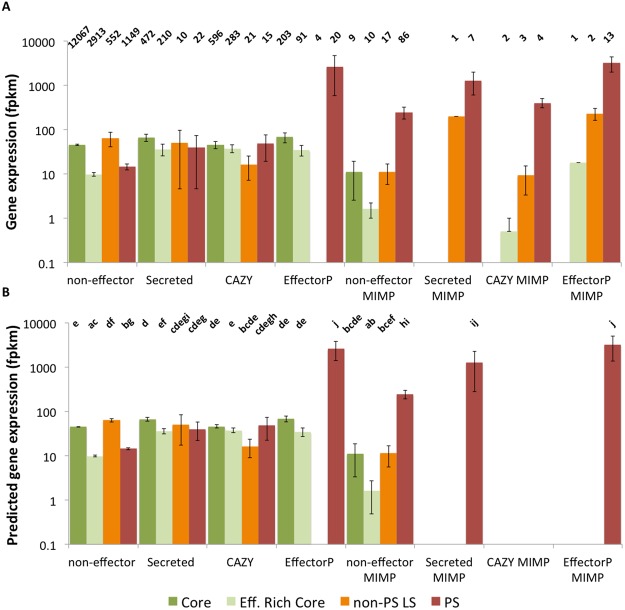
Using an established in vitro onion seedling root infection system, expression of Foc genes in planta was explored at 72 hours post inoculation (hpi), a previously identified timepoint when SIX genes are highly expressed12. Proximity to a mimp was associated with greater expression, with both non-effector candidates and secreted genes within 2 Kb of a mimp showing significantly greater expression than similar non-mimp genes (Fig. 5). Genes with a putative effector status (using the EffectorP pipeline) in PS regions showed high expression in planta, irrespective of proximity to a mimp.
Figure 5.
Observed (A) expression values (mean fpkm) for Foc Fus2 genes expressed during infection of onion seedlings at 72 hpi and predicted expression values from generalized linear modelling (B). Differences in gene expression are observed between effector-type, genomic region and presence of a mimp within 2 Kb of the gene. Number of genes in each category is shown above observed values. Pairwise significances (P < 0.05) are shown above predicted values, as determined by a Tukey test of terms from a negative binomial GLM. Effector categories include genes encoding non-effectors, secreted proteins, secreted carbohydrate active enzymes (CAZY) and secreted proteins with an effector-like structure (EffectorP). Genomic regions shown are core chromosomes 1–10 (Core), effector-enriched core chromosomes 11–13 (effector-enriched core), non-PS LS contigs, and PS contigs. Expression is shown for genes within 2 Kb of a mimp sequence (mimp).
Foc genes showing the highest expression in planta were further investigated. 21 LS Fus2 genes had equal or greater expression than the 50 highest expressed core genes (Supp. Table 9), with 17 of these on PS regions. These 17 highly expressed PS genes included 11 genes predicted as both secreted and within 2 kb of a mimp and represented previously-identified and novel effector candidates.
Foc PS effector candidates, SIX5 and two SIX3 homologs were the three highest expressed genes. Additional SIX gene homologs also showed high expression in planta, with two SIX9 homologs the 7th and 17th highest expressed genes. Other SIX homologs showed lower levels of expression in planta, with SIX10, SIX7 and SIX14 ranking as the 159th, 137th and 3277th highest expressed genes, respectively.
Other PS effector candidates represent putative novel effectors. They did not possess any recognisable interproscan domains and did not have an orthologous gene in the Fol gene models. tBLASTx searches against the PHIbase database did not identify any homologs for these genes (e < 1 × 10−10) and many showed no homology to sequences on NCBI (e < 1 × 10−10). Apart from the 11 genes predicted as both secreted and within 2 Kb of a mimp, six additional genes were present on PS regions and also highly expressed. Five of these, including SIX12, were located within 2 Kb of a mimp and one carried a peptidase domain (IPR001506) indicating that some of these genes may represent additional effector candidates. However, two genes were annotated as membrane-bound proteins discounting them as effector candidates. tBLASTx searches against the PHIbase database did not identify any homologs for these genes (e < 1 × 10−10) and searches against genbank (e < 1 × 10−10) found a homolog to only one gene, a hypothetical protein in Fusarium verticillioides (BLASTn; e = 1 × 10−58) with 83% identity to the query sequence.
Non-PS, LS regions of the genome include genes highly expressed in planta
In general, genes on non-PS, LS regions showed similar patterns of expression to genes in core regions of the genome (Fig. 5). However, four genes on non-PS, LS regions had greater or equal expression to the top 50 expressed genes from core regions of the genome (5th, 8th, 10th and 11th highest expressed genes, Supp. Table 9). These genes were not considered to be effectors (based on mimps, secretion signals, EffectorP or CAZY identification) but were noted to encode three proteins carrying domains associated with formaldehyde activating enzymes (IPR011057, IPR006913) and a polyketide synthase protein (IPR020843) and were found located in close proximity to one another (g15699-g15700, g15704). As such, they may represent a secondary metabolite cluster not identified by Antismash prediction. Further investigation found that genes carrying a formaldehyde-activating domain (IPR006913) were significantly enriched in non-PS LS contigs with eight proteins identified, in contrast to one on PS regions and 15 on core regions (Supp. Table 8b).
Helitrons may play a role in re-arrangement of PS regions
Non-canonical Helitrons appear to be a key feature of Fo pathogenicity chromosomes29. In our analysis only Foc PS regions, were enriched for Helitron helicase-like domains (IPR025476) (Supp. Table 8, panel A) while non-PS LS regions of the genome were not (Supp. Table 8, panel B), with no annotations found in genes on core regions. A total of 35 genes were identified as helitron/helicase-like transposable elements (IPR025476, PF14214) in the Foc genome, with one on an unplaced contig (contig 33) and the rest located on PS regions. Furthermore, these transposable elements showed evidence of expression in planta, with six of the 35 genes having a mean fpkm >5. These results support recent identification of helitrons in F. oxysporum ff. spp.29, and indicate that a Helitron-based mechanism of genomic rearrangement is characteristic of PS regions, rather than core regions, or non-pathogen associated LS regions of the Foc genome.
Transcription factor analysis in LS regions
To investigate the diversity of transcription factors on Foc LS regions, Interproscan functional annotations were searched for transcription factor domains. This led to the identification of 34 putative transcription factors (Supp. Table 10). Of these, 17 transcription factors were located on non-PS LS regions, of which 12 showed evidence of expression in planta, including a homolog of TF4 (Supp. Table 11b). The remaining 17/34 putative TF’s were located on PS regions, and included homologs to previously identified Fol transcription factors TF1, TF3, TF8 and TF9 (Supp. Table 11a). Five PS TF’s showed evidence of expression in planta at 72 hpi including a homolog to TF1 (FTF1). With the exception of TF3, each of the previously described TF1-9 genes had a homolog in the core genome, that showed evidence of expression in planta.
Foc carries a distinct complement of FTF1 genes
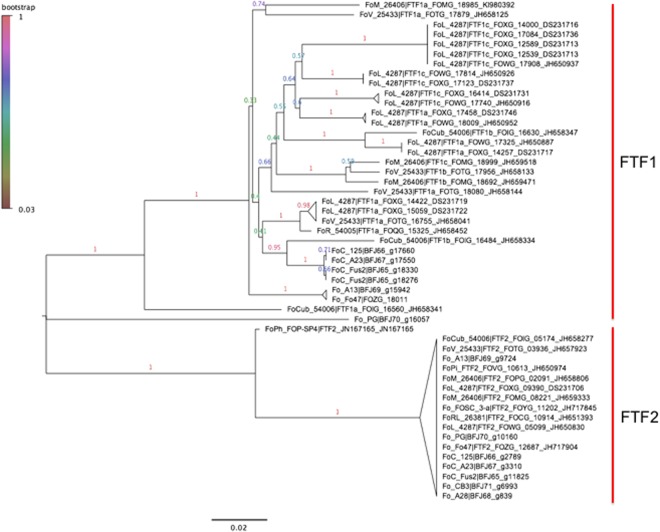
Due to the association of TF1 (FTF1) with SIX gene expression in several Fo ff. spp.24, the FTF gene family was further investigated for all the sequenced Fo isolates from onion. A single orthogroup was found to contain all FTF genes previously described in Fo strains, Fo47 and Fol-428725 as well as the BLAST homologs of FTF genes in Foc and Fo. Alignment and phylogenetic analysis of these genes allowed separation of FTF genes in FTF1 and FTF2 families (Fig. 6). All Foc isolates were found to carry a single copy of FTF2, which was located in the core genome of Fus2. Two copies of FTF1 were found in Fus2, which were both present in PS contig 19. The two other Foc isolates (125, A23) carried an identical FTF1 gene in a single copy. Interestingly, FTF1 homologs were identified in non-pathogenic Fo isolates A13, PG and Fo47, although these gene sequences were distinct from those in Foc (Fig. 6).
Figure 6.
Neighbour joining phylogeny of FTF gene sequences from Foc, Fol, f. sp. pisi (FoPi), radicis-lycopersici (FoRL), cubense (Focub), vasinifectum (FoV), melonis (FoM), conglutinans (Foco) and phaseoli (FoPh). Foc FTF1 homologs are distinct from those from other Fo ff. spp. and in non pathogenic isolates PG and A13. Branches are labelled by bootstrap support from 1000 replicates.
Discussion
In this study, through the use of single molecule sequencing, the structural conservation of lineage-specific (LS) chromosomes has been revealed in the onion basal rot pathogen F. oxysporum f. sp. cepae through comparison with F. oxysporum f. sp. lycopersci. Using a comparative-genomic approach, this work has revealed for the first time that LS chromosomes can be subdivided into pathogen-specific (PS) and nonspecific chromosomes that are specifically associated with Foc. RNAseq has shown that known effector candidates, conserved in other Fusarium ff. spp., as well as novel effector candidates are expressed in planta, paving the way for future functional studies and effector-informed resistance breeding approaches.
Previous studies have indicated that many other ff. spp., including Fol, have a polyphyletic origin30 with some exhibiting clear patterns of ‘race’ evolution through stepwise loss of effectors31. All pathogenic Foc isolates formed a single clade, consistent with a single origin of pathogenicity on onion12. Examination of other non-pathogenic Fo isolates from onion showed that these were interspersed throughout the phylogenetic tree, with two isolates forming a sister clade to Foc but other strains, grouping with diverse pathogenic ff. spp. Foc may therefore represent one of the minority of Fo ff. spp. with a monophyletic origin. Our work has revealed a unique complement of ‘SIX’ gene homologues as well as other candidate effectors that are specific to Foc and are completely conserved across more than 40 isolates from Australia, Chile, Colombia, Czech Republic, Netherlands, South Africa, Spain, UK and USA12.
Despite the clear conservation of synteny between Foc and Fol core chromosomes, synteny was not conserved between Foc and Fol LS regions. Interestingly, the LS regions identified in Foc appeared to be much smaller than in Fol, with a total of 5.7 Mb identified in Foc versus 14 Mb in Fol. Designation of 3.9 Mb of Foc LS contigs as PS regions supports previous findings that Foc SIX3 is located on a ~4 Mb chromosome13, indicating that a single pathogenicity chromosome is present in Foc.
Subdivision of LS regions of the Foc genome into PS and non-PS regions showed for the first time that non-pathogenic isolates contain certain LS elements and their presence is independent of the position of the isolate in the phylogenetic tree. For example, contig 14 present in all pathogenic Foc strains, appears to be conserved in non-pathogenic Fo isolates A13 and A28 (distantly separated in the phylogeny), but not in isolate CB3, which is most closely related to A28. This suggests that there may be genetic exchange between divergent lineages of non-pathogens in their LS chromosome complement. The functional significance of these non-pathogen associated LS chromosomes is a topic for further investigation.
Structural analysis revealed chromosome-specific patterns of gene duplication, with most gene duplications being segmental and within LS regions. This was consistent with the breakdown of synteny observed between Foc and Fol LS regions. Transposon activity has been shown to drive evolution of LS regions in other fungal pathogens32. The finding that Helitron-containing transposons are restricted to PS chromosomes suggests that they may be important for the high degree of rearrangements within PS chromosomes. This may be evolutionarily advantageous in a clonal organism as it may facilitate rapid more adaptation, not only through a higher rate of large-scale genomic deletions (which may be adaptive when evading host recognition) but also in preventing the accumulation of linked deleterious mutations from interfering with selection, the so-called Hill-Robertson effect33. One question which requires further study, is how helitron helicase transposons are limited to PS chromosomes given there is some evidence for expression.
Similar numbers of effector candidates were identified in both pathogenic and non-pathogenic Fo isolates from onion, mainly due to the large number of secreted proteins present in the core genome and on accessory chromosomes, irrespective of pathogenicity. Despite being conserved in Fo, Fol chromosome 12 has been reported as conditionally dispensable34. A concentration of effector-like genes on the homologous Foc chromosome indicates a functional distinction between Foc effector-rich core chromosomes and the remaining core genome. Foc contains a large complement of core effectors, with enrichment on core chromosomes 11, 12 and 13 (Fig. 4). However, these core candidate effector genes were not highly expressed in planta. LS contigs present in non-pathogenic isolates from onion contained far fewer mimp sequences, at a level similar to the core genome, far below the levels seen in PS contigs, again highlighting the specific differences between PS chromosomes and the rest of the LS and core genomes.
Candidate effector genes on Foc PS regions showed high levels of expression in planta; the highest expressed genes were homologs to known SIX (most notably SIX3 and SIX5) genes but also included novel effector candidates. It will be important to test whether the I-2 resistance gene, identified in tomato recognises the Foc variants of SIX335. Additional non-PS LS regions were also identified; these also possessed high numbers of mimps, and included highly expressed genes.
Transcription factors have previously been identified in the PS chromosome 14 of Fol (13, representing nine gene families; TF1-9)24. Foc was found to carry homologs to each of these previously identified TF1-9 genes distributed throughout the core and LS regions, but with no homolog of TF3 in the core genome24. However, similar to the different complements of SIX genes between Fo ff. spp., the pattern of TF1-9 genes present on PS regions in Foc was distinct from Fol. Identification of homologs to known TFs regulating pathogenicity indicates a conservation of transcriptional regulation between Fol and Foc. The role of novel TF candidates on PS regions requires further investigation, including their conservation through comparisons with other Fo ff. spp.
One of the major objectives of characterising the genomic basis of pathogenicity is to inform resistance breeding approaches using information about the effector complement of the pathogen36. The durability of a single resistance gene is dependent upon the necessity of the detected effector for the infection process and the adaptive potential of the effector gene. Effectors can either mutate to evade recognition by an R gene, such as SIX3 (AVR2) in Fol race3, or be lost such as SIX4 in Fol races 2 and 328. It is only through the characterisation of effector function, combined with a population genetics approach, that an assessment can be made about the long-term utility of any R gene based resistance in breeding, or the breeding approach needed to effectively deploy the available resistance.
It is therefore important that future work addresses the functional essentiality of effectors, the global diversity in the Foc population, the ability for effectors to mutate and evade recognition and the extent of R gene based resistance in onion.
Methods
DNA extraction, library preparation and sequencing
DNA was extracted from freeze-dried mycelium for the three Foc (Fus2, 125, A23) and four Fo isolates (A13, A28, PG, CB3) using the Macherey-Nagel Nucleospin Plant II kit (Fisher 11912262). DNA was sheared using the Covaris M220 with microTUBE-50 (Covaris 520166) and size selected using the Blue Pippin (Sage Science). Illumina libraries were constructed using either Illumina TruSeq LT kit (FC-121-2001), or with a PCR-free method using NEBNext End Repair (E6050S), NEBNext dA-tailing (E6053S) and Blunt T/A ligase (M0367S) New England Biolabs modules. Libraries were sequenced using Illumina Miseq v2 2 × 250 bp PE (MS-102-2003) or v3 2 × 300 bp PE (MS-102-3003). Pacbio libraries were prepared by the Earlham Institute UK according to manufacturer specifications and sequenced to achieve approximately 65 times coverage using P6-C4 chemistry. The sequencing of our standard highly pathogenic Foc isolate Fus2 resulted in 69 times and 145 times coverage from PacBio and MiSeq reads, respectively; 30–69 times coverage was generated for the six remaining Fo isolates using MiSeq sequencing.
Genome assembly
PacBio reads for Foc isolate Fus2 were assembled using Canu and polished using Illumina MiSeq reads in Pilon to correct erroneous SNPs and InDels37,38. De novo assembly of MiSeq data for the remaining six genomes was performed using Spades v.3.5.039. In all cases Quast40 was used to summarise assembly statistics and BUSCO41 used to assess completeness of gene space within the assembly. Assemblies were edited in accordance with results from the NCBI contamination screen (run as part of submission to Genbank in November 2016) with contigs split, trimmed or excluded as required. RepeatModeler, RepeatMasker and transposonPSI were used to identify repetitive and low complexity regions (http://www.repeatmasker.org, http://transposonpsi.sourceforge.net). In addition to generating de novo assemblies, Illumina sequencing reads were mapped to the PacBio Fus2 assembly. Alignment was performed using Bowtie2 v.2.2.4 before bedtools-intersect was used to determine number of reads aligning across 100 Kb windows in Fus2 contigs42,43.
Whole-genome phylogenetic analysis
A thirty gene phylogenetic tree was constructed using selected single copy genes present in the BUSCO ver. 1.22 Eukaryota fungi list for all the Fo isolates sequenced in this study as well as additional publically available Fusarium spp. genomes41. Additional genomes were: (non-pathogenic) F. oxysporum (FO_Fo47_V1), Fo f. sp. lycopersici (FO_MN25_V1), Fo f. sp. conglutinans (FO_PHW808_V1), Fo f. sp. cubense (Foc1_1.0), Fo f. sp. melonis (FO_melonis_V1), Fo f. sp. pisi (FO_HDV247_V1), Fo f. sp. radicis-lycopersici (FO_CL57_V1), Fo f. sp. raphani (FO_PHW815_V1), Fo f. sp. vasinfectum (FO_Cotton_V1), F. fujikuroi (assembly EF1), F. verticillioides (ASM14955v1) available from EnsemblGenomes Fungi database44. CDS sequences of single copy genes conserved across Fungi identified by BUSCO ver. 1.2241 that were found to be complete and single copy in all the genome assemblies from this study were used as input in preparing the BEAST phylogenies. In total, 652 such genes were identified and aligned with MAFFT ver. 7.22245. The alignments were inspected visually with MEGA746, trimmed and 30 genes in the top 5% highest nucleotide diversity selected for further phylogenetic analysis. A best-fit sequence evolution model for each gene was determined with PartitionFinder ver. 1.1.147 using BIC (Bayesian Information Criterion). The gene trees derived from the set of 30 single copy genes were investigated with multi-locus analysis in *BEAST ver. 2.4.248. For each replicate *BEAST run, 300 million MCMC iterations, sampled every 10,000 chains, were run due to the size of the dataset. The molecular clock was set to strict due to intra-specific sampling and consequent expectation of lower inter-branch rate variation49. A Yule prior was placed on species tree and population size model set to follow linear growth with a constant root. The first 10% of results was discarded as burn-in, and run convergence and stationarity were inspected in Tracer ver 1.6 (http://tree.bio.ed.ac.uk/software/tracer/) to confirm that ESS scores for all estimated parameters reached at least stable 200. This was followed by generation of maximum clade credibility tree with median heights with BEAST’s TreeAnnotator and visualisation of the species trees in FigTree ver. 1.4 (http://tree.bio.ed.ac.uk/software/figtree/). Run convergence was established by performing three independent runs and checking the variability of results across runs; a single representative run is reported here.
Identification of LS regions
LS regions were identified in the Foc Fus2 genome and the previously characterised Fol 4287 assembly using MUMmer v3.23 (PROmer –mum, delta-filter -g)50. Repeat-masked assemblies of non-pathogenic Fo, Foc and Fol isolates were aligned against the repeat-masked reference genome51. The percentage of unmasked bp covered by aligned sequence was calculated for each reference contig and a threshold of 30% identity was set as the boundary at which a contig was described as present or absent in a strain.
In planta RNAseq
RNAseq data was used to aid gene prediction and assess expression of effector candidates. Onion seedlings were inoculated with either the standard pathogenic Foc Fus2 or the non-pathogenic Fo47 isolate using a sterile, square petri dish system as previously reported12. Three replicate plates were set up for each isolate and RNA was extracted from pooled root samples taken from five plants at 72 hpi. Library preparation was carried out using a TruSeq RNA Sample Prep kit V2 (Illumina) and RNA sequencing carried out using an Illumina HiSeq machine with 100 bp paired end reads. Samples were multiplexed over two runs to give approximately 50 million reads per sample.
Gene prediction
RNAseq reads were aligned to de novo assembled genomes using Bowtie2 v.2.2.4 and Tophat v.2.1.0 to aid training of gene prediction programs42,52. An initial RNAseq alignment was used to estimate “mean insert size” and “fragment length distribution” of RNAseq reads and Tophat alignments re-run using these parameters. Gene prediction was performed on softmasked genomes using Braker1 v.253, a pipeline for automated training and gene prediction of AUGUSTUS 3.154. Additional gene models were called in intergenic regions using CodingQuarry v.255. Braker1 was run using the “fungal” flag and CodingQuarry was run using “pathogen” flag.
Orthology analysis
Orthology was identified between predicted proteins from the Foc and Fo isolates sequenced in this study, the publicly available genomes for F. oxysporum isolate Fo47 and Fol isolate 42871. OrthoMCL v.2.0.956 was run with an inflation value of 5 on the combined set of 193,973 predicted proteins from Foc (Fus2, 125, A23), Fo (Fo47, A13, A28, PG, CB3) and Fol (4287) genomes. Venn diagrams visualising genes common between Foc and Fo were plotted using the R package VennDiagram57.
Distribution of duplicated genes
CDS sequences (one representative longest transcript) of the Foc Fus2 genome were searched against each other using BLASTN, using an E-value threshold of e-10. In order to focus on only recent, lineage-specific duplication events, the BLAST results were filtered to retain only hits with 90% minimum percent identity and minimum 80% subject coverage, which are more stringent criteria than previously applied in similar analyses58,59. The BLAST output was subsequently parsed using DAGChainer60 in order to identify the reciprocal BLAST hits. Dependent on the position of the genes on the chromosomes, duplications were classified as either tandem (proximal) or segmental (distal). Two parameters: max. distance and number of intervening genes were tested to help classify the duplications as either tandem or segmental and the density of duplication events on chromosomes was plotted using karyoploteR ver. 0.99.8 R package (http://bioconductor.org/packages/devel/bioc/html/karyoploteR.html). Enrichment of duplications in different regions of the genome was tested using permutation tests with 1000 iterations using the regioneR ver. 1.6 R package61. In the initial analysis, more than half of identified duplications contained genes with transposon-related InterProScan domains (IPR000477, IPR012337, IPR018289, IPR006600, IPR000477, IPR025476, IPR008906, as well as keywords “transpos*” and “integrase”) and these were subsequently removed from the analysis.
Functional annotation and effector prediction
Draft functional annotations were determined for gene models using InterProScan-5.18–57.062 and through identifying homology between predicted proteins and those contained in the July 2016 release of the SwissProt database63 (using BLASTP (E-value > 1 × 10−100). Interproscan terms were used to test for enrichment of functional domains within PS and non-PS LS regions. Abundance of each interproscan term was tested using Fisher’s exact test, comparing number of genes carrying the annotation to those without. Benjamini Hochberg correction was applied for multiple testing.
Putative secreted proteins were identified through prediction of signal peptides using SignalP v.4.1 and removing those predicted to contain transmembrane domains using TMHMM v.2.064,65. Additional programs were used to provide additional sources of evidence for effectors and pathogenicity factors. EffectorP v1.0 was used to screen secreted proteins for characteristics of length, net charge and amino acid content typical of fungal effectors66. Secreted proteins were also screened for carbohydrate active enzymes using HMM models from the CAZY database67 and HMMER368. Regions of the genome containing secondary metabolite gene clusters were identified using the Antismash 3.0 webserver69. Locations of gene clusters were parsed to gff3 format before genes intersecting these regions were identified using Bedtools.
Genes within 2 Kb of a mimp sequence were identified using the consensus sequence for the mimp 3′ inverted repeat4. This was searched against assembled genomes using Perl regular expressions /CAGTGGG.GCAA[TA]AA/ and /TT[TA]TTGC.CCCACTG/. Genes within 2 Kb of these mimp sequences were marked as candidates for being under the influence of a mimp-containing promoter.
Differences in total numbers of genes encoding secreted proteins, secreted CAZYmes and secreted EffectorP proteins between Foc and Fo isolates were assessed using t-tests in R. Differences in density of genes encoding secreted proteins in different regions of the Fus2 genome were tested using ANOVA in R, with pairwise differences between regions assessed using t-tests with bonferroni correction. Identical analysis were performed to assess density of secreted CAZYmes, secreted EffectorP proteins and density of secondary metabolite clusters by genomic region.
Codon bias amongst putative pathogenicity-related genes
Multivariate correspondence analysis of codon usage to detect presence of codon bias was first investigated using codonW ver. 1.3 (http://codonw.sourceforge.net/). Prior to codonW runs, Foc Fus2 coding sequences (one longest representative sequence per gene) were filtered to remove genes with potentially unusual codon usage stemming from their foreign origin which could indicate mis-annotated false positives; transposon genes (see above) but also genes with no domain annotation.
After identifying preferred codons, differences in codon usage between different classes of putative effector genes situated on lineage-specific (LS) and core chromosomes were investigated. Two statistics summarising gene codon usage bias were calculated in addition to RSCU and ENc; frequency of optimal codons (Fop) and Codon Bias Index (CBI). Codon usage can be influenced by the selection on the sequence GC content so overall GC content of each gene (GC) and GC content in the third position of synonymous codons (GC3s) were also calculated by codonW. Pairwise correlation between all codon bias metrics, expression level, GC, GC3s content were investigated with Spearman’s rank correlation, and differences in codon usage bias between different subsets of genes compared with a t-test. All the statistical analyses were carried out in R.
Gene expression
Expression values for predicted genes were determined using Cufflinks to quantify fpkm values of RNAseq reads aligned to the genome during gene prediction. A mean fpkm value was taken for each gene from the three technical replicates. Expression of genes belonging to different effector categories and in different regions of the genome was investigated using negative binomial generalised linear model (glm), with log transformation using the glm function in R and other base functions57. A final model tested terms for region (Core, Effector-Rich Core, non-PS LS and PS) gene type (Secreted, CAZyme, EffectorP, non-effector) and whether the gene was within 2 Kb of a mimp (Yes, No). Combinations of terms were combined into a single input factor into the glm. This allowed removal of four combinations that were not present in the dataset, as no CAZyme, EffectorP or Secreted genes were within 2 Kb of a mimp and found on core chromosomes, also no secreted genes were within 2 Kb of a mimp and were located on effector rich core regions.
Data Availability
For Fusarium isolates and RNA seq data contact Dr. John Clarkson. For further information about bioinformatics and sequencing, contact Dr. Richard Harrison. All DNA sequence data is deposited in Genbank (see Supplementary Table 1) for accession numbers.
Electronic supplementary material
Acknowledgements
The authors wish to thank Hazera Seeds, specifically, Reinout de Heer, Wessel van Leeuwen, Ningwen Zhang, Tosca Ferber and Hans van den Biggelaar for support and advice and David Cole and Cathryn Lambourne for useful discussion. We are grateful to BBSRC (BB/K020870/1 and BB/K020730/1) and AHDB (CP 116) for funding. This article is dedicated to Dr Dez Barbara who was instrumental in the early development of this research.
Author Contributions
R.J.H. and J.C. devised the study. A.A. carried out genome assembly, annotation, synteny analysis and RNAseq analysis was carried out by S.O., L.B. and A.A., A.T. and A.J. carried out the RNA seq experiment, M.K.S. carried out segmental and tandem duplication analysis, variation analysis and codon usage bias studies, B.P.J.G. extracted DNA for PacBio sequencing, H.B. and F.W. carried out Illumina library preparation and sequencing. A.A., A.T., M.K.S., H.J.B., J.C. and R.J.H. wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-30335-7.
References
- 1.Ma L-J, et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature. 2010;464:367–373. doi: 10.1038/nature08850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong S, Raffaele S, Kamoun S. The two-speed genomes of filamentous pathogens: waltz with plants. Curr Opin Genet Dev. 2015;35:57–65. doi: 10.1016/j.gde.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Medini D, Donati C, Tettelin H, Masignani V, Rappuoli R. The microbial pan-genome. Curr Opin Genet Dev. 2005;15:589–594. doi: 10.1016/j.gde.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt SM, et al. MITEs in the promoters of effector genes allow prediction of novel virulence genes in Fusarium oxysporum. BMC Genomics. 2013;14:119. doi: 10.1186/1471-2164-14-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leslie, J. F. & Summerell, B. A. The Fusarium Laboratory Manual. (Oxford: Blackwell Publishing, 2006).
- 6.Baayen RP, et al. Gene Genealogies and AFLP Analyses in the Fusarium oxysporum Complex Identify Monophyletic and Nonmonophyletic Formae Speciales Causing Wilt and Rot Disease. Phytopathology. 2000;90:891–900. doi: 10.1094/PHYTO.2000.90.8.891. [DOI] [PubMed] [Google Scholar]
- 7.Aimé S, Alabouvette C, Steinberg C, Olivain C. The endophytic strain Fusarium oxysporum Fo47: a good candidate for priming the defense responses in tomato roots. Mol Plant Microbe Interact. 2013;26:918–926. doi: 10.1094/MPMI-12-12-0290-R. [DOI] [PubMed] [Google Scholar]
- 8.Teetor-Barsch GH, Roberts DW. Entomogenous Fusarium species. Mycopathologia. 1983;84:3–16. doi: 10.1007/BF00436991. [DOI] [PubMed] [Google Scholar]
- 9.FAOSTAT. Food and Agricultural Organization of the United Nations- Production Statistics. at http://faostat.fao.org/site/339/default.aspx (2012).
- 10.Cramer C. Breeding and genetics of Fusarium basal rot resistance in onion. Euphytica. 2000;115:159–166. doi: 10.1023/A:1004071907642. [DOI] [Google Scholar]
- 11.Brayford D. IMI descriptions of fungi and bacteria set 127. Mycopathologia. 1996;133:35–63. doi: 10.1007/BF00437097. [DOI] [PubMed] [Google Scholar]
- 12.Taylor A, et al. Identification of pathogenicity-related genes in Fusarium oxysporum f. sp. cepae. Mol Plant Pathol. 2016;17:1032–1047. doi: 10.1111/mpp.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki K, Nakahara K, Tanaka S, Shigyo M, Ito S. Genetic and Pathogenic Variability of Fusarium oxysporum f. sp. cepae Isolated from Onion and Welsh Onion in Japan. Phytopathology. 2015;105:525–532. doi: 10.1094/PHYTO-06-14-0164-R. [DOI] [PubMed] [Google Scholar]
- 14.Southwood MJ, Viljoen A, Mostert G, McLeod A. Molecular identification of two vegetative compatibility groups of Fusarium oxysporum f. sp. cepae. Phytopathology. 2012;102:204–213. doi: 10.1094/PHYTO-04-11-0107. [DOI] [PubMed] [Google Scholar]
- 15.Taylor A, et al. Identification of differential resistance to six Fusarium oxysporum f. sp.cepae isolates in commercial onion cultivars through the development of a rapid seedling assay. Plant Pathol. 2013;62:103–111. doi: 10.1111/j.1365-3059.2012.02624.x. [DOI] [Google Scholar]
- 16.Southwood MJ, Viljoen A, Mostert L, Rose LJ, McLeod A. Phylogenetic and Biological Characterization of Fusarium oxysporum Isolates Associated with Onion in South Africa. Plant Dis. 2012;96:1250–1261. doi: 10.1094/PDIS-10-11-0820-RE. [DOI] [PubMed] [Google Scholar]
- 17.Bayraktar H, Dolar FS. Molecular Identification and Genetic Diversity of Fusarium species Associated with Onion Fields in Turkey. J Phytopathol. 2011;159:28–34. doi: 10.1111/j.1439-0434.2010.01715.x. [DOI] [Google Scholar]
- 18.Houterman PM, et al. The mixed xylem sap proteome of Fusarium oxysporum-infected tomato plants. Mol Plant Pathol. 2007;8:215–221. doi: 10.1111/j.1364-3703.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- 19.Gawehns F, et al. The effector repertoire of Fusarium oxysporum determines the tomato xylem proteome composition following infection. Front Plant Sci. 2015;6:967. doi: 10.3389/fpls.2015.00967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma, L. et al. The AVR2 – SIX5 gene pair is required to activate I-2 -mediated immunity in tomato. 5 (2015). [DOI] [PubMed]
- 21.Williams AH, et al. Comparative genomics and prediction of conditionally dispensable sequences in legume-infecting Fusarium oxysporum formae speciales facilitates identification of candidate effectors. BMC Genomics. 2016;17:191. doi: 10.1186/s12864-016-2486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt SM, et al. Comparative genomics of Fusarium oxysporum f. sp. melonis reveals the secreted protein recognized by the Fom-2 resistance gene in melon. New Phytol. 2016;209:307–318. doi: 10.1111/nph.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Dam P, et al. Effector profiles distinguish formae speciales of Fusarium oxysporum. Environ Microbiol. 2016;18:4087–4102. doi: 10.1111/1462-2920.13445. [DOI] [PubMed] [Google Scholar]
- 24.van der Does HC, et al. Transcription Factors Encoded on Core and Accessory Chromosomes of Fusarium oxysporum Induce Expression of Effector Genes. PLoS Genet. 2016;12:e1006401. doi: 10.1371/journal.pgen.1006401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niño-Sánchez J, et al. The FTF gene family regulates virulence and expression of SIX effectors in Fusarium oxysporum. Mol Plant Pathol. 2016;17:1124–1139. doi: 10.1111/mpp.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos B, et al. The gene coding for a new transcription factor (ftf1) of Fusarium oxysporum is only expressed during infection of common bean. Fungal Genet Biol. 2007;44:864–876. doi: 10.1016/j.fgb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Michielse CB, et al. The nuclear protein Sge1 of Fusarium oxysporum is required for parasitic growth. PLoS Pathog. 2009;5:e1000637. doi: 10.1371/journal.ppat.1000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Houterman PM, Cornelissen BJC, Rep M. Suppression of plant resistance gene-based immunity by a fungal effector. PLoS Pathog. 2008;4:e1000061. doi: 10.1371/journal.ppat.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chellapan BV, van Dam P, Rep M, Cornelissen BJC, Fokkens L. Non-canonical Helitrons in Fusarium oxysporum. Mob DNA. 2016;7:27. doi: 10.1186/s13100-016-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epstein L, et al. Races of the Celery Pathogen Fusarium oxysporum f. sp. apii Are Polyphyletic. Phytopathology. 2017;107:463–473. doi: 10.1094/PHYTO-04-16-0174-R. [DOI] [PubMed] [Google Scholar]
- 31.Biju, V. C., Fokkens, L., Houterman, P. M., Rep, M. & Cornelissen, B. J. C. Multiple Evolutionary Trajectories Have Led to the Emergence of Races in Fusarium oxysporum f. sp. lycopersici. Appl Environ Microbiol83 (2017). [DOI] [PMC free article] [PubMed]
- 32.Faino L, et al. Transposons passively and actively contribute to evolution of the two-speed genome of a fungal pathogen. Genome Res. 2016;26:1091–1100. doi: 10.1101/gr.204974.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barton NH. Genetic linkage and natural selection. Philos Trans R Soc Lond, B, Biol Sci. 2010;365:2559–2569. doi: 10.1098/rstb.2010.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlaardingerbroek I, et al. Exchange of core chromosomes and horizontal transfer of lineage-specific chromosomes in Fusarium oxysporum. Environ Microbiol. 2016;18:3702–3713. doi: 10.1111/1462-2920.13281. [DOI] [PubMed] [Google Scholar]
- 35.Ma L, et al. The AVR2-SIX5 gene pair is required to activate I-2-mediated immunity in tomato. New Phytol. 2015;208:507–518. doi: 10.1111/nph.13455. [DOI] [PubMed] [Google Scholar]
- 36.Vleeshouwers VGAA, Oliver RP. Effectors as tools in disease resistance breeding against biotrophic, hemibiotrophic, and necrotrophic plant pathogens. Mol Plant Microbe Interact. 2014;27:196–206. doi: 10.1094/MPMI-10-13-0313-IA. [DOI] [PubMed] [Google Scholar]
- 37.Koren, S., Walenz, B. P., Berlin, K., Miller, J. R. & Phillippy, A. M. Canu: scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. bioRxiv (2016). [DOI] [PMC free article] [PubMed]
- 38.Walker BJ, et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE. 2014;9:e112963. doi: 10.1371/journal.pone.0112963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bankevich A, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gurevich A, Saveliev V, Vyahhi N, Tesler G. QUAST: quality assessment tool for genome assemblies. Bioinformatics. 2013;29:1072–1075. doi: 10.1093/bioinformatics/btt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31:3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- 42.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kersey PJ, et al. Ensembl Genomes 2016: more genomes, more complexity. Nucleic Acids Res. 2016;44:D574–80. doi: 10.1093/nar/gkv1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lanfear R, Calcott B, Ho SYW, Guindon S. Partitionfinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 2012;29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- 48.Drummond, A. J. & Bouckaert, R. R. Bayesian Evolutionary Analysis with BEAST. (Cambridge University Press), 10.1017/CBO9781139095112 (2015).
- 49.Brown RP, Yang Z. Rate variation and estimation of divergence times using strict and relaxed clocks. BMC Evol Biol. 2011;11:271. doi: 10.1186/1471-2148-11-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delcher AL, Phillippy A, Carlton J, Salzberg SL. Fast algorithms for large-scale genome alignment and comparison. Nucleic Acids Res. 2002;30:2478–2483. doi: 10.1093/nar/30.11.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smit, AFA, Hubley, R & Green, P. RepeatMasker Open-4.0. at, http://www.repeatmasker.org (2015).
- 52.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoff KJ, Lange S, Lomsadze A, Borodovsky M, Stanke M. BRAKER1: Unsupervised RNA-Seq-Based Genome Annotation with GeneMark-ET and AUGUSTUS. Bioinformatics. 2016;32:767–769. doi: 10.1093/bioinformatics/btv661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stanke M, Morgenstern B. AUGUSTUS: a web server for gene prediction in eukaryotes that allows user-defined constraints. Nucleic Acids Res. 2005;33:W465–7. doi: 10.1093/nar/gki458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Testa AC, Hane JK, Ellwood SR, Oliver RP. CodingQuarry: highly accurate hidden Markov model gene prediction in fungal genomes using RNA-seq transcripts. BMC Genomics. 2015;16:170. doi: 10.1186/s12864-015-1344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li L, Stoeckert CJJ, Roos DS. OrthoMCL: Identification of Ortholog Groups for Eukaryotic Genomes. Genome Research. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Team, R. C. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URLhttps://www.R-project.org/ (2015).
- 58.Jiang S-Y, González JM, Ramachandran S. Comparative genomic and transcriptomic analysis of tandemly and segmentally duplicated genes in rice. PLoS ONE. 2013;8:e63551. doi: 10.1371/journal.pone.0063551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Camacho C, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haas BJ, Delcher AL, Wortman JR, Salzberg SL. DAGchainer: a tool for mining segmental genome duplications and synteny. Bioinformatics. 2004;20:3643–3646. doi: 10.1093/bioinformatics/bth397. [DOI] [PubMed] [Google Scholar]
- 61.Gel B, et al. regioneR: an R/Bioconductor package for the association analysis of genomic regions based on permutation tests. Bioinformatics. 2016;32:289–291. doi: 10.1093/bioinformatics/btv562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones P, et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Apweiler R, et al. UniProt: the Universal Protein knowledgebase. Nucleic Acids Res. 2004;32:D115–9. doi: 10.1093/nar/gkh131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 65.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 66.Sperschneider J, et al. EffectorP: predicting fungal effector proteins from secretomes using machine learning. New Phytol. 2016;210:743–761. doi: 10.1111/nph.13794. [DOI] [PubMed] [Google Scholar]
- 67.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–5. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mistry J, Finn RD, Eddy SR, Bateman A, Punta M. Challenges in homology search: HMMER3 and convergent evolution of coiled-coil regions. Nucleic Acids Res. 2013;41:e121. doi: 10.1093/nar/gkt263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weber T, et al. antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015;43:W237–43. doi: 10.1093/nar/gkv437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For Fusarium isolates and RNA seq data contact Dr. John Clarkson. For further information about bioinformatics and sequencing, contact Dr. Richard Harrison. All DNA sequence data is deposited in Genbank (see Supplementary Table 1) for accession numbers.