Abstract
The burden of cardiovascular and metabolic diseases is increasing with every year. Although the management of these conditions has improved greatly over the years, it is still far from perfect. With all of this in mind, there is a need for new methods of prophylaxis and treatment. Coenzyme Q10 (CoQ10) is an essential compound of the human body. There is growing evidence that COQ10 is tightly linked to cardiometabolic disorders. Its supplementation can be useful in a variety of chronic and acute disorders. This review analyses the role of COQ10 in hypertension, ischemic heart disease, myocardial infarction, heart failure, viral myocarditis, cardiomyopathies, cardiac toxicity, dyslipidemia, obesity, type 2 diabetes mellitus, metabolic syndrome, cardiac procedures and resuscitation.
Keywords: Coenzyme Q10, hypertension, ischemic heart disease, myocardial infarction, heart failure, viral myocarditis, cardiomyopathies, cardiac toxicity, dyslipidemia, obesity, type 2 diabetes mellitus, metabolic syndrome, cardiac procedures and resuscitation
1. INTRODUCTION
Coenzyme Q10 (CoQ10) is an essential compound of the human body which is synthesized in the mitochondrial inner membrane [1]. The molecule of COQ10 has a highly lipophilic character and the base of its structure belongs to quinone chemical group (Fig. 1). The 10 indicates the number of isoprenyl units, which determines its low polarity and allows its fast diffusion through mitochondrial membrane [2]. It should be taken into consideration that COQ10 exists in 2 forms: oxidized (ubiquinone) and reduced (ubiquinol) [1].
Fig. (1).
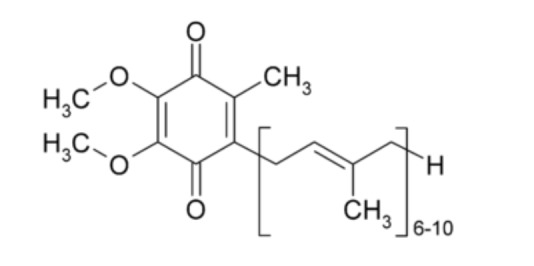
CoQ10 formula.
CoQ10 has many important functions in human body. Firstly, it can be named the key-component of electron transport chain in mitochondria necessary for ATP production [3]. COQ10 transfers electrons from complex 1 to complex 3. Besides that, it plays a role in the protons’ transfer in the inner mitochondrial membrane. This process is called protonmotive Q-cycle [4]. Q-cycle is a series of consecutive reactions of oxidation and reduction of CoQ10, between ubiquinone and ubiquinol forms, which leads to free movement of protons through the lipid bilayer, and in the case of mitochondria through the internal mitochondrial membrane. It should be noted that the Q-cycle is inseparably linked to the respiratory chain of electron transfer.
In addition to its important role in electrons’ transport, COQ10 can act as an intercellular antioxidant, protecting the plasmatic membrane against peroxidation [5]. In a research, supplementation with COQ10 showed an obvious decrease of the lipid hydroperoxides’ concentration in atherosclerotic lesions in apolipoprotein E-deficient mice [6]. As a hydrogen donor, it is more effective than other antioxidants. Besides that COQ10 is able to regenerate the oxidized form of α-tocopherol [4]. We also have to mention that CoQ-dependent NADH-oxidase is a transporter of electrons across the plasma membrane. It plays role in cell growth and differentiation [7]. Likewise, the Q-cycle has generated ubisemiquinone which generates superoxide anion radical by means of reaction with molecular oxygen producing than hydrogen peroxide that influences redox state.
Due to its important place in organisms’ functioning, there are many diseases and degenerative states associated with CoQ10’s deficiency such as diabetes mellitus, cardiovascular disease (including atherosclerosis, hypertension, dyslipidemia), muscular dystrophy, Alzheimer’s disease, Parkinson’s disease and others [8].
Administration of selenium and COQ10 in a group of healthy elderly participants given four years of intervention results in a significantly reduced cardiovascular mortality, which was observed for 10 years [9]. Therefore, this review is aimed to sum up the current possibilities to use COQ10 in a variety of cardiovascular and metabolic conditions with an analysis of its impact on patients’ health and quality of life.
2. FUNCTIONS OF CoQ10 IN HEART DISEASES
In the body, COQ10 is found in all systems of organs (Table 1). The highest concentration of ubiquinone is observed in the tissues of the heart, kidneys, liver and muscles. In its turn, in cells - in the vesicles of the Golgi apparatus, mitochondrial plasma membranes, lysosomes.
Table 1.
Distribution of Ubiquinone and Ubiquinol in tissues.
| Organ |
Ubiquinone
Concentration (µg/g) |
Ubiquinol
Concentration (µg/g) |
Effects | References |
|---|---|---|---|---|
| Heart | 132.0 | 61.0 | Antioxidant Bioenergetic Anti-inflammatory Membrane stabilizer Antiatherogenic |
Aberg et al. [10] Miles et al. [11] |
| Kidneys | 77.0 | 75.0 | ||
| Liver | 63.6 | 95.0 | ||
| Muscle | 39.7 | 65.0 | ||
| Brain | 13.4 | 23.0 | ||
| Pancreas | 32.7 | |||
| Spleen | 24.6 | |||
| Lung | 7.9 | 25.0 | ||
| Thyroidea | 24.7 | |||
| Testis | 10.5 | |||
| Intestine | 11.5 | 95.0 | ||
| Colon | 10.7 | |||
| Ventricle | 11.8 | |||
| Plasma(µmol/ml) | 1.1 | 96.0 |
One of the main causes of death in the world is cardiovascular diseases. Oxidative stress is considered to be an essential player in the development of this group of diseases. In such a way, this leads to the theory that antioxidants’ can lower the risk of cardiovascular disease [12].
Indeed, three out of four patients with heart diseases have low levels of CoQ10. It was noticed that CoQ10’s plasma levels in patients with ischemic heart disease and dilated cardiomyopathy are much lower than in healthy ones. Depending on the severity of heart injury circulating level of COQ10 decreases in direct proportion to disease progression [13]. There are several theories about the role of CoQ10’s mechanism of action in cardiovascular disease.
Firstly, because of its antioxidant effect as it was mentioned above. Ubiquinone should be reduced to ubiquinol to completely show its antioxidative function. It is known, that Reactive Oxygen Species (ROS) can cause serious cellular damage by means of reacting with cell membranes, DNA and protein centers [14]. Besides that, the products of oxidative stress and cytokines may lead to hypertrophy because they trigger the growth of myocytes [15, 16]. Ubiquinol or the reduced form of COQ10 stops the initial process of lipid peroxyl radicals’ formation. That is the reason why COQ10 is considered to be a very potent antioxidant against ROS and free radicals in biological membranes [17].
Secondly, COQ10 plays a great role in the heart’s energetic needs. For example, the process of cardiac contraction, which involves the release of Ca2+ from the sarcoplasmic reticulum and the following activation of the contractile proteins requires energy [18]. There is a theory that myocardial failure may be caused by the reduced production of the energy in mitochondria [13]. However, as it was mentioned before, COQ10 is the main component in the transport of electrons necessary for ATP production.
Besides that, we should mention anti-inflammatory effect, because different cardiovascular diseases, for example, heart failure are related to chronic pro-inflammatory state, supposing increased circulating levels of cytokines and adhesion molecules [19]. There are some new studies that establish anti-inflammatory properties of COQ10 possibly by means of nitric oxide’s regulation, and that mechanism may be effective in heart failure treatment [20, 21]. Thus, the cytokines’ and chemokines’ secretion wouldn’t induce myocardial fibrosis and lead to Heart Failure (HF) development [22]. The main effects of COQ10 administration in different conditions are presented in Table 2.
Table 2.
CoQ10 administration in different conditions.
| Condition | Possible Effects | References |
|---|---|---|
| Hypertension | Scavenging of ROS Vasodilatation Angiotensin effect adjustment Aldosterone level reducing |
[27-29] |
| T2DM | Protection against ROS Antioxidant Fatty acid oxidation enhancement |
[130-132] |
| Metabolic syndrome | Protection against ROS Antioxidant Tissue-protective The increase in triglyceride-rich lipoproteins (VLDL) |
[115, 133] |
| Overall role in cardiovascular disease | Antioxidant Protection against ROS Bioenergetic Anti-inflammatory |
[14, 18, 19] |
3. CoQ10 AND HYPERTENSION
Nowadays, hypertension is one of the major causes of morbidity and mortality worldwide. In 2010, the global prevalence of hypertension was 31% of all adults or 1.39 billion people. Therefore, between 2000 and 2010, there has been an increase of 5.2% in global hypertension prevalence. An interesting fact is that in high-income countries, the number of patients with hypertension decreased by 2.6% but low-income ones increased by 9.9% [23]. It is important to mention that nitric oxide and reactive oxygen species play a significant role in the regulation of blood pressure by means of modulation of the central nervous system [24]. It is known that increased generation of reactive oxygen species and lack of bioavailability of nitric oxygen activate hypertensions’ neurogenic pathogenesis [25].
One of the possible mechanisms for the hypertension development is the superoxide radicals’ production caused by oxidative stress. Superoxide radicals promptly enter in reaction with endothelial nitric oxide and produce peroxynitrite. In such a way, the bioavailability of nitric oxide decreases [26]. At the same time with the nitric oxides’ decrease, the capacity of endothelium to relax underlying smooth muscle disappears and this leads to vasoconstriction and subsequent blood pressure increase. COQ10 in its turn by means of a direct effect on the endothelium provokes vasodilation and lowering of blood pressure [27, 28]. It should be mentioned that though COQ10 sustains nitric oxides’ bioavailability and induces vasodilatation in a patient with hypertension, in healthy people it doesn’t have a vasodilatation effect.
It is considered that COQ10 adjusts the angiotensin effect in sodium retention and decreases the level of aldosterone [29]. This effect was proved in a study where COQ10 was administrated as an adjuvant to usual antihypertensive therapy to keep serum level of COQ10 equal to 2.0 µg/ml [30]. Finally, they got their results and noticed an improvement in functional and clinical condition in 6 months.
In a randomized, double-blind, placebo-controlled study, it was observed that after 12 -week of COQ10 administration, the systolic blood pressure was lowered to normal limits [31]. In another systematic review [32], it was assumed that COQ10 can lower the systolic blood pressure with 11 mm Hg and the diastolic one with 7 mm Hg. In addition, it should be mentioned that in patients with such diseases as type 2 diabetes mellitus and ischemic left ventricular systolic dysfunction, when the blood pressure is normal, administration of COQ10 didn’t modify the blood pressure [33-35]. In other words, the antihypertensive effect of COQ10 is limited only to patients with hypertension and does not decrease systemic pressure in patients without hypertension.
4. ISCHEMIC HEART DISEASE
There are reports that some ethnical groups are more susceptible to ischemic heart disease, possibly due to lower levels of CoQ10. For example, it was noticed that in Indian males, the plasma level of COQ10 is considerately lower than the normal one. It was presumed that due to this fact, they are more susceptible to coronary heart disease [36]. On the contrary, there is the low frequency of ischemic heart disease in Greenlanders. In comparison with Danish population, the Greenlanders have higher serum level of COQ10 = 1.495 nmol/ml (males) and 1.421 nmol/ml (females) (p<0.001). This may be because of the diet, which consists of fish and sea mammals [36].
A study was conducted in which patients with Coronary Artery Disease (CAD) to determine the effect of COQ10 oral administration in dose 100 mg of the endothelium-dependent vasodilatation activity of extracellular superoxide dismutase (ecSOD). The results demonstrated that in COQ10 treated group in comparison with placebo group: ecSOD, endothelium-dependent relaxation was statistically higher [37].
In another study, the amount of COQ10 supplement administrated per day constituted 300 mg. After the beginning of COQ10 supplementation, the extent of anti-inflammatory markers (TNF-α, p=0.039) was significantly lower. In comparison with the placebo group, the levels of vitamin E (p=0.043) and the antioxidant activities of enzymes (p<0.05) were remarkably higher after 12 weeks. Therefore, COQ10 level in plasma had positive correlation with the antioxidant activity of enzymes (p<0.05) and vitamin E (p=0.08) and negative one with interleukin-6 (IL-6) (p=0.027) and TNF-α (p=0.034) [38]. On the other hand, the data shows no relationship between COQ10 serum level and the severity of CAD in patients with angina pectoralis [39].
Lee and coworkers [40] concluded that COQ10 plasma level may have a positive correlation with vitamin B status. In addition, the plasma level of vitamin B-6 and COQ10 in patients with CAD is low. To be more precise, the risk for patients with COQ10 plasma level ≥516.0 nmol/l (0.516 µmol/l) was lower. However, there is a need for further studies for a deep understanding of inter-influence of CoQ10, vitamin B-6 and their coinfluence on CAD.
There are also studies that support a cardioprotective effect of COQ10 where its plasma levels were compared with malondialdehyde level and antioxidant activities of the following enzymes: superoxide dismutase, catalase, glutathione peroxidase [41]. COQ10 plasma level had a positive correlation with glutathione peroxidase and catalase and a negative one with malondialdehyde level and superoxide dismutase. Furthermore, COQ10 administration (150 mg/per day) seems to reduce the IL-6 level in CAD patients. This fact demonstrates its anti-inflammatory properties [42]. It is well known that pro-inflammatory state is a major component of chronic disease and significantly influences their progression.
5. CoQ10 AND MYOCARDIAL INFARCTION
Cardiovascular diseases are the leading cause of death and were accounted for almost the third of all deaths globally in 2013 [43]. Several randomized studies demonstrated beneficial effects of COQ10 in patients with Myocardial Infarction (MI). One of the studies showed a significant increase HDL-C level in serum. Besides the concentrations of intercellular adhesion molecule, 1 and IL-6 in serum were significantly decreased in COQ10 group which underlines the metabolic and anti-inflammatory effects [44]. Another randomized study which involved diabetic patients with CAD supports the findings of the anti-inflammatory effect of COQ10 although didn’t find any improvement of cardiometabolic markers [45]. This may be due to the fact that patients with Type 2 Diabetes Mellitus (T2DM) represent a distinct group of patients with different underlying pathogenetic mechanisms for MI. Finally, another randomized study in patients with MI and hyperlipidemia demonstrated improvement of blood pressure, serum HDL-C as well as LDL-C/HDL-C and TC/HDL-C ratios [46]. Co-administration of COQ10 and L-carnitine along with therapeutic lifestyle change may be a better alternative with a significant impact on the quality of life [47]. The protective effect of COQ10 can be explained by its influence on coagulation. Administration of 100 mg of COQ10 twice daily for 20 days led to a three-fold increase of total serum COQ10 level with a decline in plasma fibronectin (-20.2%), thromboxane B2 (-20.6%), prostacyclin (-23.2%), and endothelin-1 (-17.9%) level as well as inhibition of vitronectin-receptor expression and reduction of platelet size [48, 49]. Animal models have shown similar results with mild antiaggregatory changes in the hemostatic profile [50].
Furthermore, patients with MI who had higher plasma COQ10 concentrations 1 month after primary angioplasty had better left a ventricular performance at 6-months follow-up. In addition, higher plasma COQ10 concentration was associated with lower grade inflammatory and oxidative stress status. The authors, therefore, proposed plasma COQ10 concentration as a prognostic biomarker of left ventricular systolic function after revascularization therapy for MI [51].
Rat models demonstrate that COQ10 injection intravenously 10 min after coronary artery occlusion results in a smaller area of the necrosis, less postinfarction hypertrophy of the left ventricle, greater stroke volume, stroke work, cardiac output, ejection fraction, and contractility, but lower end-diastolic pressure [52]. It seems to improve the survival of myocardial cells during ischemia and limit postinfarction myocardial remodeling [53]. It is important to emphasize that in this model, the plasma concentration of COQ10 was by 87% or more than 2 times higher than in the control group of rats [52, 53].
6. CoQ10 AND HEART FAILURE
HF represents a composite clinical syndrome, which includes a decreased ejection capacity and disturbed cardiac output because of structural or functional disorders of the heart. Globally, every year, millions of people are diagnosed with HF [54]. Besides that, HF has become the most often reason for hospitalization and impairment [55, 56]. According to the statistics, despite the fact of pharmacological development and improvement, deaths from HF exceed 10% per year, but in some settings from 20% to 50% [57]. Oral administration of COQ10 has been observed to raise the endogenous level of COQ10 in plasma [58]. In agreement with studies, the plasma level of COQ10 can be proposed as a predictor of the mortality in HF patients [59].
Besides the functions of COQ10 mentioned before, one of the actions of COQ10 in HF is the inotropic one. It improves cardiac output by the rise of heart’s contractile force [60]. It is supposed that COQ10 improves the oxygen utilization on the cellular level.
In a randomized controlled multicenter trial that evaluated patients with HF that received 100 mg COQ10 3 times daily or placebo, in addition to standard therapy demonstrated lower cardiovascular mortality (9% vs. 16%, p=0.026), all-cause mortality (10% vs. 18%, p=0.018), and incidence of hospital stays for HF (p=0.033). In addition, a significant improvement of NYHA class was found in the COQ10 group after 2 years (p=0.028) [61]. Another Q-Symbio trial demonstrated the inter-influence between COQ10 and HF endpoints during 2 years CoQ10/placebo administration. COQ10 remarkably diminished the long-term endpoint (cardiovascular morbidity) in the group which administrated placebo (the adverse effect was noticed just in 15% patients vs. 26%, p=0.003) [62]. Generally, Q-Symbio studies showed that COQ10 administration along with the standard therapy turned out to be well tolerated and useful in reducing cardiovascular adverse events and HF management [63]. However, the short-term endpoints (biomarker status, functional capacity and symptoms) in patients who administrated COQ10 and placebo were almost the same.
The administration of COQ10 in patients with HF awaiting heart transplant led to a significant improvement in functional status, clinical symptoms, and quality of life. However, there were no objective changes in echo measurements or atrial natriuretic factor and TNF blood levels [64]. A meta-analysis also showed that in HF patients supplementation with COQ10 resulted in a pooled mean net change of 3.67% (95% CI: 1.60%, 5.74%) in the ejection fraction and -0.30 (95% CI: -0.66, 0.06) in the NYHA functional class [65].
Therefore, the conclusion was that the drugs which are used for HF treatment can’t replace coenzymes or vitamins. Coenzymes’ supplement is needed to increase the survival In HF. Weakened bioenergetics and lack of energy which are met in HF could be corrected by COQ10 refill [66].
7. CoQ10 AND ARRHYTHMIAS
The prevalence of the Atrial Fibrillation (AF) and HF are growing worldwide year by year [67]. Atrial fibrillation can be called a typical atrial arrhythmia in patients diagnosed with HF. It is associated with an increase in morbidity and mortality [68, 69].
CoQ10 plays an important role in oxydative phosphorilation, producing ATP and this bioenergetic function is essential for proper heart functioning [70]. Besides that, it has the property to scavenge ROS and antioxidant function [71].
There are many risk factors in the AF development, including the inflammation associated with an increase in the level of circulating cytokines [72]. Besides that, oxidative stress contributes to the accumulation of ROS which depresses the cardiac function [73]. To reduce the inflammation, following drugs are used: angiotensin receptor blockers, statins and others [74].
In the study, it was concluded that the use of COQ10 as adjuvant therapy to statins decreased the inflammation level and the levels of inflammatory cytokines. After 6 months of use, the influence on the AF wasn’t shown [75].
A systematic review and meta-analysis of eight clinical trials found that patients with COQ10 treatment were significantly less likely to require inotropic drugs after surgery [OR 95% Confidence Interval (CI) 0.47 (0.27-0.81)], and to develop ventricular arrhythmias after surgery [OR (95% CI) 0.05 (0.01-0.31)].
In a group of patients with HF, there was a significant reduction in the level of malondialdehyde in the COQ10 group. Three patients (6.3%) in the COQ10 group and 12 patients (22.2%) in the control group had episodes of AF after 12 months' treatment (p=0.02) [75]. Thus, it seems that it may have an antiarrhythmic effect.
8. VIRAL MYOCARDITIS
Mice models show that the survival rate is significantly higher in the group of mice with viral myocarditis that received COQ10 than in the control group [76]. Histologic examination showed that the severity of myocarditis was less in the COQ10 group. The inflammatory process induced by the virus was suppressed by the COQ10 treatment. Thus, pretreatment with COQ10 may reduce the severity of viral myocarditis in mice decreasing oxidative stress in the condition [77]. A study in humans demonstrated that there is a beneficial effect of COQ10 and trimetazidine individually, but demonstrated a superior effect of combining the therapies on cardiac left ventricular ejection fraction, and biochemical markers of myocardial damage in acute viral myocarditis [78].
9. CARDIOMYOPATHY
Cardiomyopathy is a debilitating condition, which is associated with a high mortality and poor quality of life. There is extensive evidence from in vitro and animal studies that it is linked to increased oxidative stress [79].
Mice models of diabetic cardiomyopathy demonstrate that COQ10 decreases diabetes-induced left ventricular diastolic dysfunction; cardiomyocyte hypertrophy, fibrosis and apoptosis; expression of the atrial natriuretic peptide, connective tissue growth factor, pro-inflammatory mediators, and β-myosin heavy chain [80, 81].
CoQ10 deficiency is frequently encountered in dilated cardiomyopathy and this may be reversible by the COQ10 administration but the therapeutic effects depend on the basal plasmatic and myocardial levels [82]. It may even attenuate disease progression and preserved left the ventricular function in animal models [83]. In children with dilated cardiomyopathy, it may improve NYHA class, cardiothoracic ratio and shorten ventricular depolarization [84]. In a prospective, randomized, double-blinded, placebo-controlled trial in children with dilated cardiomyopathy, COQ10 administration for 6 months resulted in improvement of diastolic function and a lower mean score for the index of cardiac failure [85].
In patients with hypertrophic cardiomyopathy that were treated with an average of 200 mg/day of CoQ10. All patients noted improvement in symptoms of fatigue and dyspnea with no side effects noted. The mean interventricular septal thickness and mean posterior wall thickness improved significantly. Mitral valve inflow slope by pulsed wave Doppler showed a non-significant trend towards improvement [86]. There is also a significant improvement in NYHA class, and quality of life [87].
10. CoQ10 AND CARDIOTOXICITY
The latest studies hypothesize the role of COQ10 in cardiotoxicity, induced by some drugs.
One of the groups of drugs used in chemotherapy is anthracycline antibiotics. It is usually used in the treatment of hematological cancers: leukemias, lymphomas and in the solid malignancies: carcinomas, sarcomas. One of the strongest and the best-known side effects of anthracycline is cardiotoxicity [88].
Doxorubicin is used for the treatment of early-stage breast cancer. It is known to improve the overall survival. Nonetheless, some patients are likely to develop such side effect as cardiomyopathic disturbances and congestive heart failure. It is suggested that these disturbances may appear by virtue of raised ROS generation. It is known that COQ10 protects mitochondria against ROS. In such a way, it could be introduced in adjuvant therapy to avoid doxorubicin's side effects. On the other hand, there is data that COQ10 did not have any influence on doxorubicin cell toxicity thus there is a need for further studies [89].
Later, it was found, that COQ10 and L-carnitine administration, started within 5 days before doxorubicin use, improved heart’s functions, decreased Troponin-l, Troponin-T, IL-1 and TNF-α levels. It also showed a protection against oxidative stress by reducing levels of nitric oxide and malondialdehyde. Therefore, it seems that COQ10 and L-carnitine administration together may protect the myocardium [90].
In addition, isoproterenol, which is an agonist of β-1and β-2 adrenergic receptors, can induce oxidative stress in the myocardium and produce infarct-like necrosis [91]. Furthermore, it influences on the lipid ratio in the myocardium and this fact can lead to CAD development [92]. Beside this, isoproterenol may stimulate lipid peroxidation and this way disturbs myocardial membrane [93]. COQ10 pretreatment in a dose of 100 mg/kg for 18 days showed a protection against cardiac hypertrophy and cardiotoxicity and lowered lipid peroxidation in rats [94].
11. CoQ10 AND DYSLIPIDEMIA
The 3-hydroxy-3-methylglutaryl Coenzyme A (HMG-CoA) reductase inhibitors are frequently used for the treatment of conditions associated with high levels of circulating cholesterol. Besides, this group of drugs inhibits some antioxidant effectors [95, 96] and vasoactive nitric oxide [95-97]. It should be mentioned that the pathway of cholesterol’ biosynthesis and CoQ10’ is similar (mevalonate pathway). Therefore, HMG-CoA reductase inhibitors block cholesterol synthesis and COQ10 ones by reducing the level of farnesyl pyrophosphate [98]. The depletion of COQ10 is really important in elderly because with time, the endogenous level of COQ10 decreases [99].
Although, in general, statins are safe, the following most frequently occurring side effects were recorded. The most frequent musculoskeletal side effects of statins include increased levels of creatine kinase, myopathy, dermatomyositis, and rhabdomyolysis [100, 101]. Other disorders with the rarer frequency of the musculoskeletal system include arthralgia, myalgia and tendon rupture [102, 103]. In addition, one of the side effects is accelerated cataract progression.CoQ10 deficiency resulting from statin therapy can disrupt cellular energy metabolism and contribute to the development of myopathy and other muscle symptoms [104, 105]. Although a recent meta-analysis of five studies with 226 participants didn’t support these findings. Though it is accurately noted that the human body contains about 2 g of CoQ10, of which 500 mg must be replaced each day by diet and a supplement of one or two grams per day should be evaluated in the future [106, 107]. Finally, there are also other ways to manage statin intolerance [108].
Statins may lower the level of COQ10 up to 40% by means of HMG-CoA reductase blocking. This effect is harmful to patients with heart failure [109]. That fact was proved in many clinical studies [98, 110-115]. In such a way, it was concluded that it is better to administrate COQ10 supplementation simultaneously with statin therapy to avoid myopathic side effects. In the study of 103 patients, it was postulated that statins have a good effect and less side effects being used in combination with COQ10 [116].
12. CoQ10, TYPE 2 DIABETES AND METABOLIC SYNDROME
It is often observed that there is COQ10 deficiency in patients with T2DM, their plasma level is much lower than in the healthy ones [117, 118]. This fact can lead to defensive mechanisms’ decrease in conditions of strong oxidative stress, induced by hyperglycemia [119]. This led to the theory about attenuation of mitochondrial dysfunction by means of supplementation with CoQ10. Thus leading to the idea that it can also influence the glycemia levels [120].
As it was mentioned before, there are two forms of CoQ10: ubiquinone and ubiquinol. In the organism of healthy human, they are in a determined ratio to protect the organism from oxidative stress. Sometimes the ubiquinone-ubiquinol ratio is considered to be the marker of the oxidative stress [121]. Patients with T2DM have a deficiency of ubiquinol, which interacts with reactive species of oxygen and protects the organism. Besides that, the ubiquinone-ubiquinol ratio was much higher in a patient with T2DM after the breakfast and throughout the day, that suggests a heightened oxidative stress on the background of postprandial hyperglycemia [122].
An interesting theory was proposed by Sourris and coworkers, who considered that COQ10 is a precipitating factor for diabetic nephropathy [119]. They explained it with that fact that the level of ubiquinone in the renal cortex and mitochondria in mice was low and was likely to produce diabetic nephropathy [123]. It should be noted that diabetic nephropathy is an important prognostic factor of mortality in patients with diabetes [124].
On the other hand, a recent systematic review and meta-analysis that included seven trials, involving 356 patients demonstrated that COQ10 supplementation had no beneficial effects on glycemic control, lipid profile or blood pressure in patients with diabetes. However, it reduced triglycerides levels. This leads to a reasonable conclusion that there is a need for new well-designed randomized controlled trials that determine the effect of COQ10 on metabolic profile in diabetes as well as an exploration of the dosage effects [125].
Interestingly in randomized trial patients with metabolic syndrome daily intake of 100 mg, COQ10 supplements for 8 weeks had beneficial effects on serum insulin levels, Homeostatic Model Assessment of Insulin Resistance (HOMA-IR), Homeostatic Model Assessment of β-cell Function (HOMA-B) and plasma total antioxidant capacity concentrations [126]. This may also indicate that supplement of COQ10 in patients with metabolic syndrome may be more beneficial than in patients with TDM. For instance, patients with polycystic ovary syndrome have the concomitant metabolic disease and randomized trials demonstrate that COQ10 had beneficial effects on glucose metabolism, serum total- and LDL-cholesterol levels [127].
Finally, the management of T2DM as well as metabolic syndrome is a complex process and includes several drugs. For instance, in a rat model administration of metformin with COQ10 showed a better renoprotective effect than COQ10 or metformin alone [128]. This is also true for other drugs such as sitagliptin [129]. This brings up an important point that COQ10 may potentiate the effects of other drugs by some mechanisms.
13. CARDIAC SURGERY AND PERCUTANEOUS CORONARY INTERVENTION
Cardiac procedures are tightly linked to oxidative stress. The extensive production of reactive oxygen species affects the endogenous antioxidant defense pool. The recovery of antioxidant enzyme activities may be a key goal during the pre- and postoperative periods [134].
Administration of COQ10 increases its concentration in serum, atrial trabeculae, and isolated mitochondria. This results in a more efficient mitochondrial respiration (adenosine diphosphate/oxygen ratio) and decreased mitochondrial MDA content. After 30 minutes of hypoxia in vitro, pectinate trabeculae isolated from patients receiving COQ10 exhibited a greater recovery of developed force compared with those in patients receiving placebo. This leads to the conclusion that preoperative oral COQ10 therapy in patients undergoing cardiac surgery increases myocardial and cardiac mitochondrial COQ10 levels, improves mitochondrial efficiency, and increases myocardial tolerance to in vitro hypoxia-reoxygenation stress [135].
On the other hand, in a swine models of hibernating myocardium with the daily COQ10 administration of 10 mg/kg/day were evaluated with MRI at 4-week following Coronary Artery Bypass Graft Surgery (CABG). COQ10 did not improve contractile reserve or reduce oxidant stress at 4-week post-CABG [136].
In a randomized trial, patients undergoing CABG and/or valve surgery received in double-blinded fashion, while on the waiting list for surgery and one month after surgery, either metabolic therapy (CoQ10, magnesium orotate, lipoic acid, omega-3 fatty acids and selenium) or placebo. The results demonstrated improved redox status, reduced myocardial damage, and shortened length of postoperative hospital stay [137]. Although in this model, the patients received a group of substances and not only CoQ10. Similar studies advocate for a more complex management of the patients, which should include metabolic (CoQ10, alpha- lipoic acid, magnesium orotate and omega-3 fatty acids), physical and mental preparation before cardiac surgery that may improve quality of life, lower systolic blood pressure, reduce levels of oxidative stress and thus has the potential to enhance post-operative recovery [138].
There are also reports that patients who received COQ10 had significantly fewer arrhythmias, lower total inotropic requirement, mediastinal drainage, blood product requirement, and shorter hospital stays [139-141].
Still, there are other studies that did not show improved myocardial protection in patients undergoing coronary revascularization although they were treated with 600 mg of COQ10 12 hours before the procedure [142].
Furthermore, the COQ10 level may play a role in heart rejection after a transplant. COQ10 level and mitochondrial bioenergetic functions of endomyocardial biopsies contribute to the explanation of pathobiochemical mechanisms of rejection thus COQ10 therapy could contribute to the prevention of rejection of the transplanted heart [143]. Myocardial and blood COQ10 concentrations are significantly decreased in the incipient phase of rejection (degree 0-1) and in rejection phase 1 and 2 [144].
Finally, during percutaneous transluminal coronary angioplasty, myocardial ischemia occurring during balloon inflation is brief and regresses completely after balloon deflation. Reperfusion following a short period of acute ischemia does not alter the COQ10 levels and represents a mild oxidative stress [145]. In a randomized, clinical trial, the intervention group of 50 patients received a 300 mg loading dose COQ10 12 hours before the procedure. No significant change was reported in the level of cardiac biomarkers but there was a significant reduction in high sensitive C-reactive peptide level in COQ10 group [146]. This supports the evidence that COQ10 attenuates inflammatory reactions.
14. CARDIAC ARREST AND RESUSCITATION
Animal models have demonstrated that COQ10 may play a crucial role during cardiac arrest and prevent reperfusion complications [147, 148].
In one of the studies, 49 patients were randomly assigned either to hypothermia plus COQ10 or hypothermia plus placebo after Cardiopulmonary Resuscitation (CPR). The three-month survival in the COQ10 group was 68% (17 of 25) compared to 29% (7 of 24) in the placebo group (P=0.0413). Nine COQ10 patients versus five placebo patients survived with a Glasgow Outcome Scale of 4 or 5 [149]. Prospective observational study of post-arrest patients demonstrated that COQ10 levels could be named a statistically significant predictor of poor neurologic outcome and in-hospital mortality [150]. These results are similar to other studies, which demonstrated its role in septic and hemorrhagic shocks [151-153]. This underlines the importance of metabolic resuscitation particularly in case of septic shock but may be useful also in other conditions with severely altered hemodynamics [154].
15. FUTURE DIRECTIONS
Several important directions should be prioritized for future COQ10 research. Firstly, the assessment of the optimal dose of CoQ10. High-performance liquid chromatography gives the possibility to establish the plasma concentration, which is optimal for clinical effect. It also allows determining the normal levels of CoQ10, as well as adjusting the dose of administered CoQ10. Secondary, better-powered studies are needed to assess the COQ10 influence on survival in different subgroups of patients. Finally, the introduction of personalized medicine will allow determining who may benefit from supplements.
CONCLUSION
There are many controversial data on the supplementation of COQ10 in different conditions. The reported dosage of COQ10 differs in a wide range 100-300 mg for CV diseases. Limited data on the amount of COQ10 absorbed in the gastrointestinal tract and its amount in the circulating blood were observed. Rat model demonstrates significant impact at a higher dose when the plasma concentration is increased by more than 80%. Future studies should be aimed at assessment of higher dosage of COQ10 administration as well as evaluation of its pharmacokinetics and pharmacodynamics. Overall, there seems to be a beneficial role of COQ10 co-administration as a supplemental therapy in different cardiac and metabolic conditions. The changes in the antioxidant systems in these conditions support the idea that COQ10 may improve outcome, quality of life and decrease morbidity and mortality. Nevertheless, the findings of some studies are based on preclinical or clinical studies with surrogate endpoints. This subject should be addressed in the future. Finally, more randomized trials should be performed to assess the impact of COQ10 supplementation on survival.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Quinzii C.M., Lopez L.C., Von-Moltke J., et al. Respiratory chain dysfunction and oxidative stress correlate with severity of primary COQ10 deficiency. FASEB J. 2008;22(6):1874–1885. doi: 10.1096/fj.07-100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma A., Fonarow G.C., Butler J., Ezekowitz J.A., Felker G.M. Coenzyme Q10 and heart failure: A state-of-the-art review. Circ Heart Fail. 2016;9(4):e002639. doi: 10.1161/CIRCHEARTFAILURE.115.002639. [DOI] [PubMed] [Google Scholar]
- 3.Deichmann R., Lavie C., Andrews S. Coenzyme Q10 and statin-induced mitochondrial dysfunction. Ochsner J. 2010;10(1):16–21. [PMC free article] [PubMed] [Google Scholar]
- 4.Ayer A.M.P., Stocker R. function and role in heart failure and ischemic heart disease. Annu. Rev. Nutr. 2015;2015(35):175–213. doi: 10.1146/annurev-nutr-071714-034258. [DOI] [PubMed] [Google Scholar]
- 5.Mancuso M., Orsucci D., Volpi L., Calsolaro V., Siciliano G. Coenzyme Q10 in neuromuscular and neurodegenerative disorders. Curr. Drug Targets. 2010;11(1):111–121. doi: 10.2174/138945010790031018. [DOI] [PubMed] [Google Scholar]
- 6.Tsai K.L., Huang Y.H., Kao C.L., et al. A novel mechanism of coenzyme Q10 protects against human endothelial cells from oxidative stress-induced injury by modulating NO-related pathways. J. Nutr. Biochem. 2012;23(5):458–468. doi: 10.1016/j.jnutbio.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Crane F.L., Sun I.L., Clark M.G., Grebing C., Low H. Transplasma-membrane redox systems in growth and development. Biochim. Biophys. Acta. 1985;811(3):233–264. doi: 10.1016/0304-4173(85)90013-8. [DOI] [PubMed] [Google Scholar]
- 8.Garrido-Maraver J., Cordero M.D., Oropesa-Avila M., et al. Coenzyme Q10 therapy. Mol. Syndromol. 2014;5(3-4):187–197. doi: 10.1159/000360101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alehagen U., Aaseth J., Johansson P. Reduced cardiovascular mortality 10 years after supplementation with selenium and coenzyme Q10 for four years: Follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly citizens. PLoS One. 2015;10(12):e0141641. doi: 10.1371/journal.pone.0141641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aberg F., Appelkvist E.L., Dallner G., Ernster L. Distribution and redox state of ubiquinones in rat and human tissues. Arch. Biochem. Biophys. 1992;295(2):230–234. doi: 10.1016/0003-9861(92)90511-t. [DOI] [PubMed] [Google Scholar]
- 11.Miles M.V., Horn P.S., Morrison J.A., Tang P.H., DeGrauw T., Pesce A.J. Plasma coenzyme Q10 reference intervals, but not redox status, are affected by gender and race in self-reported healthy adults. Clin. Chim. Acta. 2003;332(1-2):123–132. doi: 10.1016/s0009-8981(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 12.Singh U., Devaraj S., Jialal I. Coenzyme Q10 supplementation and heart failure. Nutr. Rev. 2007;65(6 Pt 1):286–293. doi: 10.1301/nr.2007.jun.286-293. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A., Kaur H., Devi P., Mohan V. Role of coenzyme Q10 (CoQ10) in cardiac disease, hypertension and Meniere-like syndrome. Pharmacol. Ther. 2009;124(3):259–268. doi: 10.1016/j.pharmthera.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Bergamini C., Cicoira M., Rossi A., Vassanelli C. Oxidative stress and hyperuricaemia: Pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. Eur. J. Heart Fail. 2009;11(5):444–452. doi: 10.1093/eurjhf/hfp042. [DOI] [PubMed] [Google Scholar]
- 15.Lim J.Y., Park S.J., Hwang H.Y., et al. TGF-beta1 induces cardiac hypertrophic responses via PKC-dependent ATF-2 activation. J. Mol. Cell. Cardiol. 2005;39(4):627–636. doi: 10.1016/j.yjmcc.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Nakagami H., Takemoto M., Liao J.K. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 2003;35(7):851–859. doi: 10.1016/s0022-2828(03)00145-7. [DOI] [PubMed] [Google Scholar]
- 17.Turunen M., Olsson J., Dallner G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta. 2004;1660(1-2):171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Kayo C.Y., Carsten M.E. In: Cellular Aspects of Smooth Muscle Function. Pres C.U., editor. Cambridge University Press; 2005. [Google Scholar]
- 19.Yang Y.K., Wang L.P., Chen L., et al. Coenzyme Q10 treatment of cardiovascular disorders of ageing including heart failure, hypertension and endothelial dysfunction. Clin. Chim. Acta. 2015;450:83–89. doi: 10.1016/j.cca.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Jung H.J., Park E.H., Lim C.J. Evaluation of anti-angiogenic, anti-inflammatory and antinociceptive activity of coenzyme Q(10) in experimental animals. J. Pharm. Pharmacol. 2009;61(10):1391–1395. doi: 10.1211/jpp/61.10.0017. [DOI] [PubMed] [Google Scholar]
- 21.Swarnakar N.K., Jain A.K., Singh R.P., Godugu C., Das M., Jain S. Oral bioavailability, therapeutic efficacy and reactive oxygen species scavenging properties of coenzyme Q10-loaded polymeric nanoparticles. Biomaterials. 2011;32(28):6860–6874. doi: 10.1016/j.biomaterials.2011.05.079. [DOI] [PubMed] [Google Scholar]
- 22.Kai H., Kuwahara F., Tokuda K., Imaizumi T. Diastolic dysfunction in hypertensive hearts: Roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens. Res. 2005;28(6):483–490. doi: 10.1291/hypres.28.483. [DOI] [PubMed] [Google Scholar]
- 23.Bloch M.J. Worldwide prevalence of hypertension exceeds 1.3 billion. J. Am. Soc. Hypertens. 2016;10(10):753–754. doi: 10.1016/j.jash.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Hirooka Y., Kishi T., Sakai K., Takeshita A., Sunagawa K. Imbalance of central nitric oxide and reactive oxygen species in the regulation of sympathetic activity and neural mechanisms of hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300(4):R818–R826. doi: 10.1152/ajpregu.00426.2010. [DOI] [PubMed] [Google Scholar]
- 25.Hirooka Y. Oxidative stress in the cardiovascular center has a pivotal role in the sympathetic activation in hypertension. Hypertens. Res. 2011;34(4):407–412. doi: 10.1038/hr.2011.14. [DOI] [PubMed] [Google Scholar]
- 26.Grunfeld S., Hamilton C.A., Mesaros S., et al. Role of superoxide in the depressed nitric oxide production by the endothelium of genetically hypertensive rats. Hypertension. 1995;26(6 Pt 1):854–857. doi: 10.1161/01.hyp.26.6.854. [DOI] [PubMed] [Google Scholar]
- 27.Digiesi V., Cantini F., Oradei A., et al. Coenzyme Q10 in essential hypertension. Mol. Aspects Med. 1994;15(Suppl.):s257–s263. doi: 10.1016/0098-2997(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 28.Ignarro L.J. Biological actions and properties of endothelium-derived nitric oxide formed and released from artery and vein. Circ. Res. 1989;65(1):1–21. doi: 10.1161/01.res.65.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Fabre L.F., Jr, Banks R.C., McIsaac W.M., Farrell G. Effects of ubiquinone and related substances on secretion of aldosterone and cortisol. Am. J. Physiol. 1965;208:1275–1280. doi: 10.1152/ajplegacy.1965.208.6.1275. [DOI] [PubMed] [Google Scholar]
- 30.Langsjoen P., Langsjoen P., Willis R., Folkers K. Treatment of essential hypertension with coenzyme Q10. Mol. Aspects Med. 1994;15(Suppl.):S265–S272. doi: 10.1016/0098-2997(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 31.Burke B.E., Neuenschwander R., Olson R.D. Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in isolated systolic hypertension. South. Med. J. 2001;94(11):1112–1117. doi: 10.1097/00007611-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Ho M.J., Bellusci A., Wright J.M. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database Syst. Rev. 2009;2009(4):CD007435. doi: 10.1002/14651858.CD007435.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Dai Y.L., Luk T.H., Yiu K.H., et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: a randomized controlled trial. Atherosclerosis. 2011;216(2):395–401. doi: 10.1016/j.atherosclerosis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Lim S.C., Lekshminarayanan R., Goh S.K., et al. The effect of coenzyme Q10 on microcirculatory endothelial function of subjects with type 2 diabetes mellitus. Atherosclerosis. 2008;196(2):966–969. doi: 10.1016/j.atherosclerosis.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton S.J., Chew G.T., Watts G.F. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009;32(5):810–812. doi: 10.2337/dc08-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedersen H.S., Mortensen S.A., Rohde M., et al. High serum coenzyme Q10, positively correlated with age, selenium and cholesterol, in Inuit of Greenland. A pilot study. Biofactors. 1999;9(2-4):319–323. doi: 10.1002/biof.5520090230. [DOI] [PubMed] [Google Scholar]
- 37.Tiano L., Belardinelli R., Carnevali P., Principi F., Seddaiu G., Littarru G.P. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: A double-blind, randomized controlled study. Eur. Heart J. 2007;28(18):2249–2255. doi: 10.1093/eurheartj/ehm267. [DOI] [PubMed] [Google Scholar]
- 38.Lee B.J., Tseng Y.F., Yen C.H., Lin P.T. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: A randomized, placebo-controlled trial. Nutr. J. 2013;12(1):142. doi: 10.1186/1475-2891-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buyukkaya E., Evliyaoglu O., Islamoglu Y., et al. The relationship between coenzyme Q10 and severity of coronary artery disease. Med. Glas. 2013;10(2):229–233. [PubMed] [Google Scholar]
- 40.Lee B.J., Yen C.H., Hsu H.C., Lin J.Y., Hsia S., Lin P.T. A significant correlation between the plasma levels of coenzyme Q10 and vitamin B-6 and a reduced risk of coronary artery disease. Nutr. Res. 2012;32(10):751–756. doi: 10.1016/j.nutres.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Lee B.J., Lin Y.C., Huang Y.C., Ko Y.W., Hsia S., Lin P.T. The relationship between coenzyme Q10, oxidative stress, and antioxidant enzymes activities and coronary artery disease. Sci. World J. 2012;2012:792756. doi: 10.1100/2012/792756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee B.J., Huang Y.C., Chen S.J., Lin P.T. Effects of coenzyme Q10 supplementation on inflammatory markers (high-sensitivity C-reactive protein, interleukin-6, and homocysteine) in patients with coronary artery disease. Nutrition. 2012;28(7-8):767–772. doi: 10.1016/j.nut.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohseni M., Vafa M., Zarrati M., Shidfar F., Hajimiresmail S.J., Rahimi Forushani A. Beneficial effects of coenzyme Q10 supplementation on lipid profile and intereukin-6 and intercellular adhesion molecule-1 reduction, preliminary results of a double-blind trial in acute myocardial infarction. Int. J. Prev. Med. 2015;6:73. doi: 10.4103/2008-7802.162461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirhashemi S.M., Najafi V., Raygan F., Asemi Z. The effects of coenzyme Q10 supplementation on cardiometabolic markers in overweight type 2 diabetic patients with stable myocardial infarction: A randomized, double-blind, placebo-controlled trial. ARYA Atheroscler. 2016;12(4):158–165. [PMC free article] [PubMed] [Google Scholar]
- 46.Mohseni M., Vafa M.R., Hajimiresmail S.J., et al. Effects of coenzyme Q10 supplementation on serum lipoproteins, plasma fibrinogen, and blood pressure in patients with hyperlipidemia and myocardial infarction. Iran. Red Crescent Med. J. 2014;16(10):e16433. doi: 10.5812/ircmj.16433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharifi M.H., Eftekhari M.H., Ostovan M.A., Rezaianazadeh A. Effects of a therapeutic lifestyle change diet and supplementation with Q10 plus L-carnitine on quality of life in patients with myocardial infarction: A randomized clinical trial. J. Cardiovasc. Thorac. Res. 2017;9(1):21–28. doi: 10.15171/jcvtr.2017.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serebruany V.L., Gurbel P.A., Ordonez J.V., et al. Could coenzyme Q10 affect hemostasis by inhibiting platelet vitronectin (CD51/CD61) receptor? Mol. Aspects Med. 1997;18(Suppl.):S189–S194. doi: 10.1016/s0098-2997(97)00012-5. [DOI] [PubMed] [Google Scholar]
- 49.Serebruany V.L., Ordonez J.V., Herzog W.R., et al. Dietary coenzyme Q10 supplementation alters platelet size and inhibits human vitronectin (CD51/CD61) receptor expression. J. Cardiovasc. Pharmacol. 1997;29(1):16–22. doi: 10.1097/00005344-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Serebruany V.L., Herzog W.R., Atamas S.P., et al. Hemostatic changes after dietary coenzyme Q10 supplementation in swine. J. Cardiovasc. Pharmacol. 1996;28(2):175–181. doi: 10.1097/00005344-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Huang C.H., Kuo C.L., Huang C.S., et al. High plasma coenzyme Q10 concentration is correlated with good left ventricular performance after primary angioplasty in patients with acute myocardial infarction. Medicine (Baltimore) 2016;95(31):e4501. doi: 10.1097/MD.0000000000004501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ivanov A.V., Gorodetskaya E.A., Kalenikova E.I., Medvedev O.S. Single intravenous injection of coenzyme Q10 protects the myocardium after irreversible ischemia. Bull. Exp. Biol. Med. 2013;155(6):771–774. doi: 10.1007/s10517-013-2249-3. [DOI] [PubMed] [Google Scholar]
- 53.Kalenikova E.I., Gorodetskaya E.A., Kolokolchikova E.G., Shashurin D.A., Medvedev O.S. Chronic administration of coenzyme Q10 limits postinfarct myocardial remodeling in rats. Biochemistry. 2007;72(3):332–338. doi: 10.1134/s0006297907030121. [DOI] [PubMed] [Google Scholar]
- 54.Lei L., Liu Y. Efficacy of coenzyme Q10 in patients with cardiac failure: A meta-analysis of clinical trials. BMC Cardiovasc. Disord. 2017;17(1):196. doi: 10.1186/s12872-017-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Javed U., Deedwania P.C. Beta-adrenergic blockers for chronic heart failure. Cardiol. Rev. 2009;17(6):287–292. doi: 10.1097/CRD.0b013e3181bdf63e. [DOI] [PubMed] [Google Scholar]
- 56.Massie B.M., Shah N.B. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am. Heart J. 1997;133(6):703–712. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 57.Mahmood S.S., Wang T.J. The epidemiology of congestive heart failure: The Framingham Heart Study perspective. Glob. Heart. 2013;8(1):77–82. doi: 10.1016/j.gheart.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niklowitz P., Sonnenschein A., Janetzky B., Andler W., Menke T. Enrichment of coenzyme Q10 in plasma and blood cells: Defense against oxidative damage. Int. J. Biol. Sci. 2007;3(4):257–262. doi: 10.7150/ijbs.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molyneux S.L., Florkowski C.M., George P.M., et al. Coenzyme Q10: an independent predictor of mortality in chronic heart failure. J. Am. Coll. Cardiol. 2008;52(18):1435–1441. doi: 10.1016/j.jacc.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 60.Greenberg S., Frishman W.H. Co-enzyme Q10: A new drug for cardiovascular disease. J. Clin. Pharmacol. 1990;30(7):596–608. doi: 10.1002/j.1552-4604.1990.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 61.Mortensen S.A., Rosenfeldt F., Kumar A., et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014;2(6):641–649. doi: 10.1016/j.jchf.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 62.DiNicolantonio J.J., Bhutani J., McCarty M.F., O’Keefe J.H. Coenzyme Q10 for the treatment of heart failure: A review of the literature. Open Heart. 2015;2(1):e000326. doi: 10.1136/openhrt-2015-000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jafari M., Masood Mousavi S., Asgharzadeh A., Yazdani N. Coenzyme Q10 in the treatment of heart failure: A systematic review of systematic reviews. Indian Heart J. doi: 10.1016/j.ihj.2018.01.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berman M., Erman A., Ben-Gal T., et al. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: A randomized, placebo-controlled study. Clin. Cardiol. 2004;27(5):295–299. doi: 10.1002/clc.4960270512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fotino A.D., Thompson-Paul A.M., Bazzano L.A. Effect of coenzyme Q(1)(0) supplementation on heart failure: A meta-analysis. Am. J. Clin. Nutr. 2013;97(2):268–275. doi: 10.3945/ajcn.112.040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mortensen S.A. Overview on coenzyme Q10 as adjunctive therapy in chronic heart failure. Rationale, design and end-points of “Q-symbio”--a multinational trial. Biofactors. 2003;18(1-4):79–89. doi: 10.1002/biof.5520180210. [DOI] [PubMed] [Google Scholar]
- 67.Wang T.J., Larson M.G., Levy D., et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation. 2003;107(23):2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 68.Maisel W.H., Stevenson L.W. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 2003;91(6A):2D–8D. doi: 10.1016/s0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 69.Hynes B.J., Luck J.C., Wolbrette D.L., et al. Atrial fibrillation in patients with heart failure. Curr. Opin. Cardiol. 2003;18(1):32–38. doi: 10.1097/00001573-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 70.Keith M., Geranmayegan A., Sole M.J., et al. Increased oxidative stress in patients with congestive heart failure. J. Am. Coll. Cardiol. 1998;31(6):1352–1356. doi: 10.1016/s0735-1097(98)00101-6. [DOI] [PubMed] [Google Scholar]
- 71.Baggio E., Gandini R., Plancher A.C., Passeri M., Carmosino G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure. COQ10 Drug Surveillance Investigators. Mol. Aspects Med. 1994;15(Suppl.):s287–s294. doi: 10.1016/0098-2997(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 72.Korantzopoulos P., Kolettis T.M., Kountouris E. Inflammation and anti-inflammatory interventions in atrial fibrillation. Int. J. Cardiol. 2005;104(3):361–362. doi: 10.1016/j.ijcard.2005.04.037. [DOI] [PubMed] [Google Scholar]
- 73.Tanaka T., Tsutamoto T., Nishiyama K., et al. Impact of oxidative stress on plasma adiponectin in patients with chronic heart failure. Circ. J. 2008;72(4):563–568. doi: 10.1253/circj.72.563. [DOI] [PubMed] [Google Scholar]
- 74.Fazio G., Amoroso G.R., Barbaro G., Novo G., Novo S. The role of statins in preventing the progression of congestive heart failure in patients with metabolic syndrome. Curr. Pharm. Des. 2008;14(25):2605–2612. doi: 10.2174/138161208786071218. [DOI] [PubMed] [Google Scholar]
- 75.Zhao Q., Kebbati A.H., Zhang Y., Tang Y., Okello E., Huang C. Effect of coenzyme Q10 on the incidence of atrial fibrillation in patients with heart failure. J. Investig. Med. 2015;63(5):735–739. doi: 10.1097/JIM.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 76.Kishimoto C., Tamaki S., Matsumori A., Tomioka N., Kawai C. The protection of coenzyme Q10 against experimental viral myocarditis in mice. Jpn. Circ. J. 1984;48(12):1358–1361. doi: 10.1253/jcj.48.1358. [DOI] [PubMed] [Google Scholar]
- 77.Kishimoto C., Tomioka N., Nakayama Y., Miyamoto M. Anti-oxidant effects of coenzyme Q10 on experimental viral myocarditis in mice. J. Cardiovasc. Pharmacol. 2003;42(5):588–592. doi: 10.1097/00005344-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Shao L., Ma A., Figtree G., Zhang P. Combination therapy with coenzyme Q10 and trimetazidine in patients with acute viral myocarditis. J. Cardiovasc. Pharmacol. 2016;68(2):150–154. doi: 10.1097/FJC.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 79.Senes M., Erbay A.R., Yilmaz F.M., et al. Coenzyme Q10 and high-sensitivity C-reactive protein in ischemic and idiopathic dilated cardiomyopathy. Clin. Chem. Lab. Med. 2008;46(3):382–386. doi: 10.1515/CCLM.2008.061. [DOI] [PubMed] [Google Scholar]
- 80.De Blasio M.J., Huynh K., Qin C., et al. Therapeutic targeting of oxidative stress with coenzyme Q10 counteracts exaggerated diabetic cardiomyopathy in a mouse model of diabetes with diminished PI3K(p110alpha) signaling. Free Radic. Biol. Med. 2015;87:137–147. doi: 10.1016/j.freeradbiomed.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 81.Huynh K., Kiriazis H., Du X.J., et al. Targeting the upregulation of reactive oxygen species subsequent to hyperglycemia prevents type 1 diabetic cardiomyopathy in mice. Free Radic. Biol. Med. 2013;60:307–317. doi: 10.1016/j.freeradbiomed.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 82.Manzoli U., Rossi E., Littarru G.P., et al. Coenzyme Q10 in dilated cardiomyopathy. Int. J. Tissue React. 1990;12(3):173–178. [PubMed] [Google Scholar]
- 83.Momomura S., Serizawa T., Ohtani Y., Iizuka M., Sugimoto T. Coenzyme Q10 attenuates the progression of cardiomyopathy in hamsters. Jpn. Heart J. 1991;32(1):101–110. doi: 10.1536/ihj.32.101. [DOI] [PubMed] [Google Scholar]
- 84.Soongswang J., Sangtawesin C., Durongpisitkul K., et al. The effect of coenzyme Q10 on idiopathic chronic dilated cardiomyopathy in children. Pediatr. Cardiol. 2005;26(4):361–366. doi: 10.1007/s00246-004-0742-1. [DOI] [PubMed] [Google Scholar]
- 85.Kocharian A., Shabanian R., Rafiei-Khorgami M., Kiani A., Heidari-Bateni G. Coenzyme Q10 improves diastolic function in children with idiopathic dilated cardiomyopathy. Cardiol. Young. 2009;19(5):501–506. doi: 10.1017/S1047951109990795. [DOI] [PubMed] [Google Scholar]
- 86.Langsjoen P.H., Langsjoen A., Willis R., Folkers K. Treatment of hypertrophic cardiomyopathy with coenzyme Q10. Mol. Aspects Med. 1997;18(Suppl.):S145–S151. doi: 10.1016/s0098-2997(97)00006-x. [DOI] [PubMed] [Google Scholar]
- 87.Adarsh K., Kaur H., Mohan V. Coenzyme Q10 (CoQ10) in isolated diastolic heart failure in hypertrophic cardiomyopathy (HCM). Biofactors. 2008;32(1-4):145–149. doi: 10.1002/biof.5520320117. [DOI] [PubMed] [Google Scholar]
- 88.Conklin K.A. Coenzyme q10 for prevention of anthracycline-induced cardiotoxicity. Integr. Cancer Ther. 2005;4(2):110–130. doi: 10.1177/1534735405276191. [DOI] [PubMed] [Google Scholar]
- 89.Greenlee H., Shaw J., Lau Y.I., Naini A., Maurer M. Lack of effect of coenzyme q10 on doxorubicin cytotoxicity in breast cancer cell cultures. Integr. Cancer Ther. 2012;11(3):243–250. doi: 10.1177/1534735412439749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mustafa H.N., Hegazy G.A., Awdan S.A.E., AbdelBaset M. Protective role of COQ10 or L-carnitine on the integrity of the myocardium in doxorubicin induced toxicity. Tissue Cell. 2017;49(3):410–426. doi: 10.1016/j.tice.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 91.Prince P.S., Rajadurai M. Preventive effect of Aegle marmelos leaf extract on isoprenaline-induced myocardial infarction in rats: Biochemical evidence. J. Pharm. Pharmacol. 2005;57(10):1353–1357. doi: 10.1211/jpp.57.10.0015. [DOI] [PubMed] [Google Scholar]
- 92.Sathish V., Ebenezar K.K., Devaki T. Synergistic effect of Nicorandil and Amlodipine on tissue defense system during experimental myocardial infarction in rats. Mol. Cell. Biochem. 2003;243(1-2):133–138. doi: 10.1023/a:1021612230000. [DOI] [PubMed] [Google Scholar]
- 93.Battino M., Ferri E., Gorini A., et al. Natural distribution and occurrence of coenzyme Q homologues. Membr. Biochem. 1990;9(3):179–190. doi: 10.3109/09687689009025839. [DOI] [PubMed] [Google Scholar]
- 94.Ghule A.E., Kulkarni C.P., Bodhankar S.L., Pandit V.A. Effect of pretreatment with coenzyme Q10 on isoproterenol-induced cardiotoxicity and cardiac hypertrophy in rats. Curr. Ther. Res. Clin. Exp. 2009;70(6):460–471. doi: 10.1016/j.curtheres.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hong H., Zeng J.S., Kreulen D.L., Kaufman D.I., Chen A.F. Atorvastatin protects against cerebral infarction via inhibition of NADPH oxidase-derived superoxide in ischemic stroke. Am. J. Physiol. Heart Circ. Physiol. 2006;291(5):H2210–H2215. doi: 10.1152/ajpheart.01270.2005. [DOI] [PubMed] [Google Scholar]
- 96.Makabe S., Takahashi Y., Watanabe H., Murakami M., Ohba T., Ito H. Fluvastatin protects vascular smooth muscle cells against oxidative stress through the Nrf2-dependent antioxidant pathway. Atherosclerosis. 2010;213(2):377–384. doi: 10.1016/j.atherosclerosis.2010.07.059. [DOI] [PubMed] [Google Scholar]
- 97.Asahi M., Huang Z., Thomas S., et al. Protective effects of statins involving both eNOS and tPA in focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2005;25(6):722–729. doi: 10.1038/sj.jcbfm.9600070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Folkers K., Langsjoen P., Willis R., et al. Lovastatin decreases coenzyme Q levels in humans. Proc. Natl. Acad. Sci. USA. 1990;87(22):8931–8934. doi: 10.1073/pnas.87.22.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pepe S., Marasco S.F., Haas S.J., Sheeran F.L., Krum H., Rosenfeldt F.L. Coenzyme Q10 in cardiovascular disease. Mitochondrion. 2007;7(Suppl.):S154–S167. doi: 10.1016/j.mito.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 100.Manoukian A.A., Bhagavan N.V., Hayashi T., Nestor T.A., Rios C., Scottolini A.G. Rhabdomyolysis secondary to lovastatin therapy. Clin. Chem. 1990;36(12):2145–2147. [PubMed] [Google Scholar]
- 101.Delbosc S., Morena M., Djouad F., Ledoucen C., Descomps B., Cristol J.P. Statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, are able to reduce superoxide anion production by NADPH oxidase in THP-1-derived monocytes. J. Cardiovasc. Pharmacol. 2002;40(4):611–617. doi: 10.1097/00005344-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 102.Silva M.A., Swanson A.C., Gandhi P.J., Tataronis G.R. Statin-related adverse events: A meta-analysis. Clin. Ther. 2006;28(1):26–35. doi: 10.1016/j.clinthera.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 103.Golomb B.A., Evans M.A. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs. 2008;8(6):373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thompson P.D., Clarkson P., Karas R.H. Statin-associated myopathy. JAMA. 2003;289(13):1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 105.Zita C., Overvad K., Mortensen S.A., Sindberg C.D., Moesgaard S., Hunter D.A. Serum coenzyme Q10 concentrations in healthy men supplemented with 30 mg or 100 mg coenzyme Q10 for two months in a randomised controlled study. Biofactors. 2003;18(1-4):185–193. doi: 10.1002/biof.5520180221. [DOI] [PubMed] [Google Scholar]
- 106.Banach M., Serban C., Sahebkar A., et al. Effects of coenzyme Q10 on statin-induced myopathy: A meta-analysis of randomized controlled trials. Mayo Clin. Proc. 2015;90(1):24–34. doi: 10.1016/j.mayocp.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 107.Braillon A. Coenzyme Q10 and statin-induced myopathy--II. Mayo Clin. Proc. 2015;90(3):420. doi: 10.1016/j.mayocp.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 108.Abd T.T., Jacobson T.A. Statin-induced myopathy: A review and update. Expert Opin. Drug Saf. 2011;10(3):373–387. doi: 10.1517/14740338.2011.540568. [DOI] [PubMed] [Google Scholar]
- 109.Ghirlanda G., Oradei A., Manto A., et al. Evidence of plasma CoQ10-lowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study. J. Clin. Pharmacol. 1993;33(3):226–229. doi: 10.1002/j.1552-4604.1993.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 110.Langsjoen P.H., Langsjoen A.M. The clinical use of HMG CoA-reductase inhibitors and the associated depletion of coenzyme Q10. A review of animal and human publications. Biofactors. 2003;18(1-4):101–111. doi: 10.1002/biof.5520180212. [DOI] [PubMed] [Google Scholar]
- 111.Silver M.A., Langsjoen P.H., Szabo S., Patil H., Zelinger A. Statin cardiomyopathy? A potential role for Co-Enzyme Q10 therapy for statin-induced changes in diastolic LV performance: Description of a clinical protocol. Biofactors. 2003;18(1-4):125–127. doi: 10.1002/biof.5520180214. [DOI] [PubMed] [Google Scholar]
- 112.Mortensen S.A., Rosenfeldt F., Kumar A., et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014;2(6):641–649. doi: 10.1016/j.jchf.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 113.Silver M.A., Langsjoen P.H., Szabo S., Patil H., Zelinger A. Effect of atorvastatin on left ventricular diastolic function and ability of coenzyme Q10 to reverse that dysfunction. Am. J. Cardiol. 2004;94(10):1306–1310. doi: 10.1016/j.amjcard.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 114.Stocker R., Pollicino C., Gay C.A., et al. Neither plasma coenzyme Q10 concentration, nor its decline during pravastatin therapy, is linked to recurrent cardiovascular disease events: A prospective case-control study from the LIPID study. Atherosclerosis. 2006;187(1):198–204. doi: 10.1016/j.atherosclerosis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 115.Hargreaves I.P., Duncan A.J., Heales S.J., Land J.M. The effect of HMG-CoA reductase inhibitors on coenzyme Q10: Possible biochemical/clinical implications. Drug Saf. 2005;28(8):659–676. doi: 10.2165/00002018-200528080-00002. [DOI] [PubMed] [Google Scholar]
- 116.Kumar A., Kaur H., Mohan V. 2005. [Google Scholar]
- 117.Ates O., Bilen H., Keles S., et al. Plasma coenzyme Q10 levels in type 2 diabetic patients with retinopathy. Int. J. Ophthalmol. 2013;6(5):675–679. doi: 10.3980/j.issn.2222-3959.2013.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.El-ghoroury E.A., Raslan H.M., Badawy E.A., et al. Malondialdehyde and coenzyme Q10 in platelets and serum in type 2 diabetes mellitus: correlation with glycemic control. Blood Coagul. Fibrinolysis. 2009;20(4):248–251. doi: 10.1097/mbc.0b013e3283254549. [DOI] [PubMed] [Google Scholar]
- 119.Sourris K.C., Harcourt B.E., Tang P.H., et al. Ubiquinone (coenzyme Q10) prevents renal mitochondrial dysfunction in an experimental model of type 2 diabetes. Free Radic. Biol. Med. 2012;52(3):716–723. doi: 10.1016/j.freeradbiomed.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 120.Alam M.A., Rahman M.M. Mitochondrial dysfunction in obesity: Potential benefit and mechanism of Co-enzyme Q10 supplementation in metabolic syndrome. J. Diabetes Metab. Disord. 2014;13:60. doi: 10.1186/2251-6581-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamashita S., Yamamoto Y. Simultaneous detection of ubiquinol and ubiquinone in human plasma as a marker of oxidative stress. Anal. Biochem. 1997;250(1):66–73. doi: 10.1006/abio.1997.2187. [DOI] [PubMed] [Google Scholar]
- 122.Hasegawa G., Yamamoto Y., Zhi J.G., et al. Daily profile of plasma %CoQ10 level, a biomarker of oxidative stress, in patients with diabetes manifesting postprandial hyperglycaemia. Acta Diabetol. 2005;42(4):179–181. doi: 10.1007/s00592-005-0199-6. [DOI] [PubMed] [Google Scholar]
- 123.Shen Q., Pierce J.D. Supplementation of coenzyme Q10 among patients with type 2 diabetes mellitus. Health Care (Don Mills) 2015;3(2):296–309. doi: 10.3390/healthcare3020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gall M-A., Borch-Johnsen K., Hougaard P., Nielsen F.S., Parving H-H. Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes. 1995;44(11):1303–1309. doi: 10.2337/diab.44.11.1303. [DOI] [PubMed] [Google Scholar]
- 125.Suksomboon N., Poolsup N., Juanak N. Effects of coenzyme Q10 supplementation on metabolic profile in diabetes: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 2015;40(4):413–418. doi: 10.1111/jcpt.12280. [DOI] [PubMed] [Google Scholar]
- 126.Raygan F., Rezavandi Z., Dadkhah Tehrani S., Farrokhian A., Asemi Z. The effects of coenzyme Q10 administration on glucose homeostasis parameters, lipid profiles, biomarkers of inflammation and oxidative stress in patients with metabolic syndrome. Eur. J. Nutr. 2016;55(8):2357–2364. doi: 10.1007/s00394-015-1042-7. [DOI] [PubMed] [Google Scholar]
- 127.Samimi M., Zarezade Mehrizi M., Foroozanfard F., et al. The effects of coenzyme Q10 supplementation on glucose metabolism and lipid profiles in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Endocrinol. (Oxf.) 2017;86(4):560–566. doi: 10.1111/cen.13288. [DOI] [PubMed] [Google Scholar]
- 128.Maheshwari R.A., Balaraman R., Sen A.K., Seth A.K. Effect of coenzyme Q10 alone and its combination with metformin on streptozotocin-nicotinamide-induced diabetic nephropathy in rats. Indian J. Pharmacol. 2014;46(6):627–632. doi: 10.4103/0253-7613.144924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Maheshwari R., Balaraman R., Sen A.K., Shukla D., Seth A. Effect of concomitant administration of coenzyme Q10 with sitagliptin on experimentally induced diabetic nephropathy in rats. Ren. Fail. 2017;39(1):130–139. doi: 10.1080/0886022X.2016.1254659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Littarru G.P., Tiano L. Clinical aspects of coenzyme Q10: An update. Nutrition. 2010;26(3):250–254. doi: 10.1016/j.nut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 131.Quiles J.L., Ochoa J.J., Battino M., et al. Life-long supplementation with a low dosage of coenzyme Q10 in the rat: effects on antioxidant status and DNA damage. Biofactors. 2005;25(1-4):73–86. doi: 10.1002/biof.5520250109. [DOI] [PubMed] [Google Scholar]
- 132.Lee S.K., Lee J.O., Kim J.H., et al. Coenzyme Q10 increases the fatty acid oxidation through AMPK-mediated PPARalpha induction in 3T3-L1 preadipocytes. Cell. Signal. 2012;24(12):2329–2336. doi: 10.1016/j.cellsig.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 133.Mohr D., Stocker R. Radical-mediated oxidation of isolated human very-low-density lipoprotein. Arterioscler. Thromb. 1994;14(7):1186–1192. doi: 10.1161/01.atv.14.7.1186. [DOI] [PubMed] [Google Scholar]
- 134.Pechan I., Olejarova I., Danova K., et al. Antioxidant status of patients after on-pump and off-pump coronary artery bypass grafting. Bratisl. Lek Listy. 2004;105(2):45–50. [PubMed] [Google Scholar]
- 135.Rosenfeldt F., Marasco S., Lyon W., et al. Coenzyme Q10 therapy before cardiac surgery improves mitochondrial function and in vitro contractility of myocardial tissue. J. Thorac. Cardiovasc. Surg. 2005;129(1):25–32. doi: 10.1016/j.jtcvs.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 136.Hocum Stone L., Butterick T.A., Duffy C., et al. Cardiac strain in a swine model of regional hibernating myocardium: Effects of COQ10 on contractile reserve following bypass surgery. J. Cardiovasc. Transl. Res. 2016;9(4):368–373. doi: 10.1007/s12265-016-9696-y. [DOI] [PubMed] [Google Scholar]
- 137.Leong J.Y., van der Merwe J., Pepe S., et al. Perioperative metabolic therapy improves redox status and outcomes in cardiac surgery patients: a randomised trial. Heart Lung Circ. 2010;19(10):584–591. doi: 10.1016/j.hlc.2010.06.659. [DOI] [PubMed] [Google Scholar]
- 138.Hadj A., Esmore D., Rowland M., et al. Pre-operative preparation for cardiac surgery utilising a combination of metabolic, physical and mental therapy. Heart Lung Circ. 2006;15(3):172–181. doi: 10.1016/j.hlc.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 139.Makhija N., Sendasgupta C., Kiran U., et al. The role of oral coenzyme Q10 in patients undergoing coronary artery bypass graft surgery. J. Cardiothorac. Vasc. Anesth. 2008;22(6):832–839. doi: 10.1053/j.jvca.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 140.Chello M., Mastroroberto P., Romano R., et al. Protection by coenzyme Q10 from myocardial reperfusion injury during coronary artery bypass grafting. Ann. Thorac. Surg. 1994;58(5):1427–1432. doi: 10.1016/0003-4975(94)91928-3. [DOI] [PubMed] [Google Scholar]
- 141.Tanaka J., Tominaga R., Yoshitoshi M., et al. Coenzyme Q10: The prophylactic effect on low cardiac output following cardiac valve replacement. Ann. Thorac. Surg. 1982;33(2):145–151. doi: 10.1016/s0003-4975(10)61900-5. [DOI] [PubMed] [Google Scholar]
- 142.Taggart D.P., Jenkins M., Hooper J., et al. Effects of short-term supplementation with coenzyme Q10 on myocardial protection during cardiac operations. Ann. Thorac. Surg. 1996;61(3):829–833. doi: 10.1016/0003-4975(95)01120-X. [DOI] [PubMed] [Google Scholar]
- 143.Gvozdjakova A., Kucharska J., Mizera S., et al. Coenzyme Q10 depletion and mitochondrial energy disturbances in rejection development in patients after heart transplantation. Biofactors. 1999;9(2-4):301–306. doi: 10.1002/biof.5520090227. [DOI] [PubMed] [Google Scholar]
- 144.Kucharska J., Gvozdjakova A., Mizera S., et al. Participation of coenzyme Q10 in the rejection development of the transplanted heart: A clinical study. Physiol. Res. 1998;47(6):399–404. [PubMed] [Google Scholar]
- 145.Tomasetti M., Alleva R., Piva R., et al. Evaluation of ischemia-reperfusion damage during coronary angioplasty. Electrocardiographic assessment and biochemical modifications in blood from the coronary sinus. Ital. Heart J. 2000;1(3):216–220. [PubMed] [Google Scholar]
- 146.Aslanabadi N., Safaie N., Asgharzadeh Y., et al. The randomized clinical trial of coenzyme Q10 for the prevention of periprocedural myocardial injury following elective percutaneous coronary intervention. Cardiovasc. Ther. 2016;34(4):254–260. doi: 10.1111/1755-5922.12195. [DOI] [PubMed] [Google Scholar]
- 147.Ren Z., Ding W., Su Z., et al. Mechanisms of brain injury with deep hypothermic circulatory arrest and protective effects of coenzyme Q10. J. Thorac. Cardiovasc. Surg. 1994;108(1):126–133. [PubMed] [Google Scholar]
- 148.Mori F., Mohri H. Effects of coenzyme Q10 added to a potassium cardioplegic solution for myocardial protection during ischemic cardiac arrest. Ann. Thorac. Surg. 1985;39(1):30–36. doi: 10.1016/s0003-4975(10)62519-2. [DOI] [PubMed] [Google Scholar]
- 149.Damian M.S., Ellenberg D., Gildemeister R., et al. Coenzyme Q10 combined with mild hypothermia after cardiac arrest: A preliminary study. Circulation. 2004;110(19):3011–3016. doi: 10.1161/01.CIR.0000146894.45533.C2. [DOI] [PubMed] [Google Scholar]
- 150.Cocchi M.N., Giberson B., Berg K., et al. Coenzyme Q10 levels are low and associated with increased mortality in post-cardiac arrest patients. Resuscitation. 2012;83(8):991–995. doi: 10.1016/j.resuscitation.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Donnino M.W., Mortensen S.J., Andersen L.W., et al. Ubiquinol (reduced Coenzyme Q10) in patients with severe sepsis or septic shock: A randomized, double-blind, placebo-controlled, pilot trial. Crit. Care. 2015;19:275. doi: 10.1186/s13054-015-0989-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Donnino M.W., Cocchi M.N., Salciccioli J.D., et al. Coenzyme Q10 levels are low and may be associated with the inflammatory cascade in septic shock. Crit. Care. 2011;15(4):R189. doi: 10.1186/cc10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shen Q., Holloway N., Thimmesch A., Wood J.G., Clancy R.L., Pierce J.D. Ubiquinol decreases hemorrhagic shock/resuscitation-induced microvascular inflammation in rat mesenteric microcirculation. Physiol. Rep. 2014;2(11):1. doi: 10.14814/phy2.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leite H.P., de Lima L.F. Metabolic resuscitation in sepsis: a necessary step beyond the hemodynamic? J. Thorac. Dis. 2016;8(7):E552–E557. doi: 10.21037/jtd.2016.05.37. [DOI] [PMC free article] [PubMed] [Google Scholar]


