Abstract
Background:
Levosimendan is a calcium sensitizer drug causing increased contractility in the myocardium and vasodilation in the vascular system. It is mainly used for the therapy of acute decompensated heart failure. Several studies on animals and humans provided evidence of the cardioprotective properties of levosimendan including preconditioning and anti-apoptotic. In view of these favorable effects, levosimendan has been tested in patients undergoing cardiac surgery for the prevention or treatment of low cardiac output syndrome. However, initial positive results from small studies have not been confirmed in three recent large trials.
Aim:
To summarize levosimendan mechanisms of action and clinical use and to review available evidence on its perioperative use in a cardiac surgery setting.
Methods:
We searched two electronic medical databases for randomized controlled trials studying levosimendan in cardiac surgery patients, ranging from January 2000 to August 2017. Meta-analyses, consensus documents and retrospective studies were also reviewed.
Results:
In the selected interval of time, 54 studies on the use of levosimendan in heart surgery have been performed. Early small size studies and meta-analyses have suggested that perioperative levosimendan infusion could diminish mortality and other adverse outcomes (i.e. intensive care unit stay and need for inotropic support). Instead, three recent large randomized controlled trials (LEVO-CTS, CHEETAH and LICORN) showed no significant survival benefits from levosimendan. However, in LEVO-CTS trial, prophylactic levosimendan administration significantly reduced the incidence of low cardiac output syndrome.
Conclusions:
Based on most recent randomized controlled trials, levosimendan, although effective for the treatment of acute heart failure, can't be recommended as standard therapy for the management of heart surgery patients. Further studies are needed to clarify whether selected subgroups of heart surgery patients may benefit from perioperative levosimendan infusion.
Keywords: Levosimendan, low cardiac output syndrome, cardiac surgery, cardioprotection, inotropic drug, myth or reality
1. INTRODUCTION
In western and developing countries, Ischemic Heart Disease (IHD) is a major problem in healthcare because of its high prevalence and the relevant economic burden it imposes [1, 2]. Despite recent advancements in drugs therapy and percutaneous revascularization strategies, Coronary Artery Bypass Graft (CABG) surgery still remains an indispensable option for many patients with IHD [3]. Moreover, in the last years, due to the aging of populations, the number of patients undergoing heart surgery for valvulopathies has also dramatically increased [4, 5].
Unfortunately, subjects referred to cardiac surgery may develop serious complications including the frequently fatal Low Cardiac Output Syndrome (LCOS) [6, 7]. Pathogenesis of LCOS is variably related to reperfusion injury, inflammation and vasoconstriction in the systemic and pulmonary circulation. In particular, the occurrence of postoperative myocardial dysfunction depends upon transient myocardial stunning that can last up to 48 hours. Traditional inotrope drugs such as catecholamines have been used for the perioperative management of LCOS. However, they can cause arrhythmias, worsen myocardial ischemia through the augment of oxygen consumption and increase in-hospital mortality [8, 9].
It has been argued that prevention and treatment of postoperative LCOS could be obtained by the use of inotropic drugs able to provide cardioprotective effects as well. Levosimendan is a calcium sensitizing and a potassium channel opening drug that acts as a cardiac inotropic agent without increasing myocardial oxygen consumption [10]. The compound also has vasodilatory properties in the vascular system. Interestingly, levosimendan differs from traditional inotropic drugs as it exerts various cardioprotective and preconditioning effects. Even though efficacy and safety of levosimendan in the treatment of acute decompensated Heart Failure (HF) have been widely demonstrated [11-16], its standard perioperative application in the setting of cardiac surgery is still debated. In fact, in contrast to findings of previous small trials and meta-analyses, three recent large randomized trials have produced negative results [17-19].
The aim of the present mini-review is to summarize the available evidences from trials, meta-analyses and consensus documents on levosimendan use in the context of heart surgery, highlighting the findings from more recent studies. A short presentation of the mechanism of action of levosimendan and a brief description of its main clinical use will precede the review.
2. METHODS
Literature search was conducted in PubMed and Google Scholar independently by two authors, ranging from January 2000 to August 2017. Following keywords were chosen and included in combinations: levosimendan, cardiac surgery, heart surgery, low cardiac output syndrome, perioperative, cardioprotection. With regard to searched article types, we decided to include meta-analyses, consensus documents, retrospective studies and Randomized Controlled Trials (RCTs) which recruited patients referred to heart surgery (for CABG or valve surgery) who received levosimendan in the perioperative period. Bibliographies of selected articles and review references were also evaluated in order to identify further relevant manuscripts. Finally, cross-check of manuscripts found by two authors' researches was performed aiming to integrate available evidences on the topic by comparing obtained results. In order to estimate the overlapping area of identified meta-analyses, overlap percentage, covered area and corrected covered area were calculated [20].
3. MECHANISMS OF ACTION OF LEVOSIMENDAN
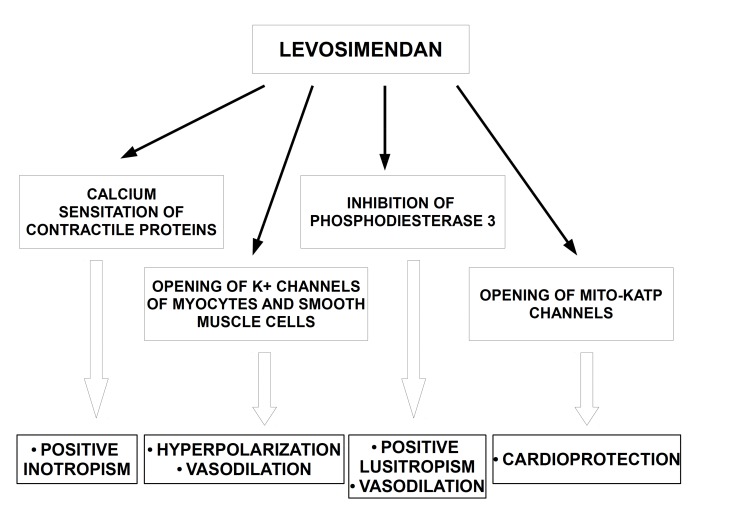
Levosimendan is a class III derivative of pyridazinone-dinitrile which exerts positive inotropic and protective effects on the heart and causes vasodilation in the vascular system (Fig. 1) [10].
Fig. (1).
Schematic diagram showing the main mechanisms of action of levosimendan. Positive inotropic effect of the compound is due to calcium sensitation of heart contractile proteines. The drug exerts also vasodilatory effect on vascular system through the opening of K+ channels of myocytes and smooth muscle cells and via the inhibition of phosphodiesterase 3. Cardioprotective effect of levosimendan is related to opening of mito KATP channels..
In particular, the inotropic action of levosimendan is due to the calcium sensitation of contractile proteins. Indeed, in consequence of levosimendan binding to troponin C (TnC) at N-terminal domain, TnC-Ca2+ complex become more stable and constitution of cross-bridge between actin and myosin accelerates [21]. Elimination half-life of levosimendan has been estimated to be approximately one hour [22]. However, the hemodynamic effects of levosimendan infusion may persist for many hours after its discontinuation, since its active metabolite (OR-1896) has a half-life of about 80 hours [23]. Of note, the drug does not worsen diastolic function and does not affect oxygen demand of the heart [24-26].
Interestingly, levosimendan activates also the opening of ATP-sensitive sarcolemma K+ channels of smooth muscle cells and myocytes and allows their hyperpolarization causing vasodilation [27]. Moreover, the drug increases intracellular cyclic Adenosine Monophosphate (cAMP) and Ca2+, via the selective inhibition of type 3 phosphodiesterase [28].
Cardioprotective effects of levosimendan are caused by the modulation of several cellular pathways involved in apoptosis, autophagy and prevention of damages from ischemia and reperfusion [29]. A preconditioning mimicking effects of levosimendan has been hypothesized as well [30].
A fundamental cardioprotective mechanism is provided by the opening of mitochondrial ATP-sensitive potassium (mitoKATP) channels. Such action prevents mitochondrial calcium overload and apoptosis of cardiomyocytes [31, 32]. It has been discussed that the increment of potassium influx could contribute in preserving mitochondrial integrity in distress situations also through the interplay with the Na+–H+ exchanger type 1 (NHE-1) which is an important acid excluder in cardiomyocytes [33].
There is evidence that levosimendan even counteracts oxidative stress and favors protective signaling pathways involving Nitric Oxide (NO) action. Benefits of levosimendan on neurohumoral dysfunction have been also demonstrated [34, 35]. Neither tolerance nor rebound phenomena have been observed after long-lasting infusion of levosimendan and after its withdrawal.
Hypokalemia and hypotension are the main adverse effects associated with the use of the drug. While hypotension is related to vasodilating action of the compound, mechanism of hypokalemia is not elucidated yet [36].
4. EFFICACY OF LEVOSIMENDAN IN VARIOUS CLINICAL SETTINGS
The diverse pharmacological actions of levosimendan render it a versatile drug. So, it has been tested in different clinical contexts, including acute HF, cardiogenic shock, septic shock and advanced HF with pulmonary hypertension [Box 1].
The efficacy of levosimendan in the treatment of acute HF has been clearly established in several clinical studies which showed that the drug outweighs traditional inotropes in improving hemodynamics and neurohormonal status [11-16]. In addition, in LIDO and RUSSLAN studies, levosimendan showed also benefit on survival when it was compared to dobutamine and to placebo respectively.
Based on these findings, 2016 guidelines of European Society of Cardiology for treatment of chronic and acute HF recommend levosimendan among inotropic agents in patients with hypotension and/or symptoms/signs of hypoperfusion despite adequate filling status (Class of evidence II b; level of evidence C) [37].
Moreover, the same guidelines support intravenous infusion of levosimendan for acute HF patients in order to reverse the effect of a beta-blockade when beta-blockade itself is suspected to be the cause of hypotension and hypoperfusion (Class of evidence II b; level of evidence C) [37].
In 2016, a review and expert consensus document suggested the usefulness of levosimendan also in acute HF cases complicating Acute Coronary Syndrome (ACS) [38]. Indeed, experts opinion favored levosimendan administration over adrenergic inotropes as a first-line therapy for all ACS- acute HF patients who are taking beta-blockade and/or when urinary output is inadequate despite diuretics.
Levosimendan use for cardiogenic shock is attractive since the compound improves several hemodynamic parameters including cardiac output and stroke volume without an increment of the myocardial oxygen demand. Initial evidence on its administration in cardiogenic shock is encouraging [39]. In fact, in a recent meta-analysis, which examined three trials, overall enrolling 63 patients with cardiogenic shock, levosimendan infusion showed a little, but significant survival advantage when compared to enoximone (Hazard Ratio: 0,33; Confidence Interval 95%: 0,11-0,97) [40].
Theoretically, levosimendan might work well in septic shock because counteracts abnormal calcium handling which is partly responsible for myocardial depression. However, studies which compared levosimendan to dobutamine in septic shock patients showed no significant benefit from levosimendan [41]. Thus, dobutamine is currently considered the first line therapy for septic shock patients with persistent hypoperfusion after fluid loading and use of vasopressors (weak recommendation, quality of evidence: low) [42].
Finally, levosimendan has been recently studied in other clinical settings, as in pulmonary hypertension in advanced HF, in association with Left Ventricular Assist Device (LVAD) and as bridging therapy [43]. However, these observations should be considered preliminary.
5. PERIOPERATIVE LEVOSIMENDAN IN CARDIAC SURGERY
In the past years, numerous studies have suggested that levosimendan could exert a favorable action when it is administered perioperatively in heart surgery patients.
Overall we found 54 relevant studies on perioperative levosimendan in cardiac surgery: 36 RCTs [17-19, 44-76], 6 retrospective studies [77-82], 7 meta-analyses [82-87, 93] and 5 consensus documents [89-93]. Studies were performed in an interval of time ranging from December 2004 to August 2017.
The majority of RCTs compared perioperative levosimendan administration to placebo or other inotropes (mainly dobutamine and milrinone) in heart surgery context. Other studies investigated the optimal time of administration of levosimendan [51, 81, 82]. Some studies involved pediatric patients referred to heart surgery [59, 64, 65, 68, 70].
5.1. Studies Versus Placebo
In 2006 Tritapepe et al. in a pilot trial, examined the effect of the short administration of levosimendan (24 mcg/Kg over 10 min before cardiopulmonary by-pass placement) versus placebo in 24 patients who underwent CABG in elective surgery for stable angina. Interestingly, postoperative Troponine I levels were significantly lower in patients in the levosimendan arm which showed also a higher cardiac index after heart surgery [48]. Three years later, the authors confirmed such observations in a larger randomized double-blind study recruiting one hundred and six patients referred to elective CABG surgery. Of note, in this larger trial, tracheal intubation time, Intensive Care Unit (ICU) stay and need for inotropic support were significantly reduced in patients who received levosimendan infusion [57].
Benefits from levosimendan’s preoperative infusion were observed also in nine patients who underwent LVAD implantation. Indeed, in this small sample retrospective study, a better twelve months survival was observed compared to fifth INTERMACS registry (89% vs. 75%) [80].
In a study which recruited 252 patients with severe left ventricular dysfunction undergoing CABG surgery, 30 days mortality and risk of LCOS were significantly lower in patient assigned to levosimendan arm compared to placebo [66].
In 2014, levosimendan was used as a pretreatment (24 hours infusion) in a randomized study involving 50 patients undergoing off-pump coronary artery bypass grafting (OPCAB) with Left Ventricular Ejection Fraction (LVEF) < 30%. Levosimendan arm was characterized by less need of Cardiopulmonary Bypass (CPB) and Intra-aortic Balloon Pump (IABP) than placebo. Moreover, levosimendan pretreatment was associated with a shorter stay in ICU [73].
5.2. Best Time of Administration
Eris et al. investigated the optimal time of levosimendan administration in a retrospective study of forty patients with left ventricular dysfunction (LVEF less than 30%) referred to CABG. Authors found that infusion of the drug before surgery (0.2 mcg/kg/min 12 hours before the operation) was associated with lower mortality and better improvement of heart function compared to control group and to groups of patients who received levosimendan after induction of anesthesia and during weaning of cardiopulmonary bypass [81].
Similarly, another retrospective analysis conducted in 159 cardiac surgery patients found that early administration of levosimendan reduced mortality and morbidity compared to late start of treatment (one hour after admission in ICU) [82].
In another study of forty-five patients who underwent heart surgery, administration of levosimendan during or after intervention ameliorated stroke volume and cardiac index [51]. In the same study, ICU and hospital stay were significantly shorter in patients who started drug infusion in the operating theater.
5.3. Studies Versus Other Inotropes
Several studies involving heart surgery patients, have investigated the efficacy and safety of the perioperative administration of levosimendan compared to other inotropes. In 2006, Alvarez et al. performed a study of 41 patients with low cardiac output after cardiopulmonary by-pass. Patients were randomly assigned to 24 hours infusion of dobutamine or levosimendan. Authors found that both drugs were able to increase cardiac index, but the increase induced by levosimendan was significantly greater (2.9 l/min per m2 vs. 2.4 l/min per m2 in dobutamine group at 24 h; p<0.05) [49].
In another randomized study including 137 subjects who developed postoperative LCOS, morbidity and mortality were lower in the levosimendan arm compared to dobutamine arm [53]. Of note, patients assigned to levosimendan needed less frequently additional inotropic drugs and intra-aortic balloon counterpulsation.
In a study of 60 patients who underwent valve replacement surgery, levosimendan showed to be as effective as dobutamine and better than milrinone for the treatment of LCOS [75]. In this study, levosimendan and dobutamine provided a similar increase of stroke volume.
In a recent study, Kandasamy et al. evaluated the hemodynamic effects of levosimendan and dobutamine in 80 patients with moderate to severe left ventricular dysfunction undergoing OPCAB. Enrolled subjects were randomly assigned to levosimendan (0.1 μg/kg/min) or to dobutamine (5 μg/kg/min). Indices of myocardial contractility (i.e. cardiac index, left and right ventricular stroke work index) significantly increased in levosimendan group, which also showed shorter hospitalization and ICU stay [76].
In a case series of 12 heart surgery patients with LCOS resistant to dobutamine, the addition of levosimendan could reverse LCOS [95].
5.4. Meta-analyses and Consensus Documents
Several meta-analyses of studies investigating the effect of levosimendan administration in context of cardiac surgery confirmed significant benefits from perioperative drug infusion, ranging from reduction of ICU stay to improved survival (Table 1) [83-88]. Interestingly, patients with impaired systolic function benefited the most from levosimendan [85, 87]. Moreover, better nephrological outcomes (i.e. less need for dialysis and a lower rate of development of acute kidney injury) were observed in subjects who received perioperative levosimendan [86-88].
Table 1.
Meta-analyses of studies on perioperative levosimendan in cardiac surgery.
| First Author, Year, [Reference] | Selection Criteria | Number of Studies | Results |
|---|---|---|---|
| Zangrillo A, 2009, [83] | RCTs that reported outcome data of levosimendan in cardiac surgery | 5 RCTs with 139 patients | No difference in mortality Significant decrease in troponin peak release and time to hospital discharge |
| Landoni G, 2010, [84] | RCTs on levosimendan versus control in cardiac surgery patients | 10 RCTs with 440 patients | Reduced postoperative mortality, troponin release and AF |
| Harrison RW, 2013, [85] | RCTs of perioperative levosimendan in patients undergoing cardiac surgery | 14 RCTs with 1,155 patients | Reduction in mortality with levosimendan In subgroup analysis this benefit was confined to the low-EF studies Reductions in the need for dialysis, myocardial injury and postoperative AF with levosimendan |
| Niu ZZ, 2014, [86] | Randomized trials that compared levosimendan versus placebo or any other control in cardiac surgery with data on AKI | 5 RCTs with 529 patients | Levosimendan decreased postoperative incidence of AKI in the levosimendan group |
| Lim JY, 2015, [87] | Studies on levosimendan versus control in adult cardiac surgery | 14 studies (13 RCTs and 1 retrospective study) with 965 patients | Reduced early mortality in patients with reduced LVEF Lower postoperative acute renal failure and shorter ICU stay in levosimendan cohort |
| Zhou C, 2015, [88] | RCTs comparing renal effect of levosimendan vs. placebo or other inotropes in cardiac surgery | 13 RCTs with 1345 patients | Reduced incidence of post-operative AKI and renal replacement therapy in levosimendan group Reduced post-operative mortality, ICU stay and mechanical ventilation duration in levosimendan group |
| Hummel J, 2017, [94] | Studies on levosimendan versus standard inotrope treatments in infants and children (up to 18 years of age) in cardiac surgery for congenital heart disease | 5 RCTs with 212 patients | No significant differences between levosimendan and standard inotrope treatments |
Meta analysis' Overlap Percentage: 64%; Covered Area: 0,300; Corrected Covered Area: 0,188
Note: One RCT included in Landoni's meta-analysis was retracted [see reference 45]
Abbreviations: AF: atrial fibrillation; AKI: acute kidney injury; ICU: intensive care unit; LVEF: left ventricular ejection fraction; RCTs: randomized controlled trials.
Based on such evidence five consensus documents were reviewed (Table 2) [89-93]. The first two consensus documents, interpreting data from a single meta-analysis, concluded that perioperative levosimendan is likely to reduce 30 days mortality. However, the inherent evidence was judged insufficient to recommend routine administration of the drug in perioperative period [89, 90]. In more recent consensus documents, experts’ opinion favored employment of levosimendan infusion for heart surgery patients in perioperative time. Indeed, potential utility of the drug was recognized based on new evidence from trials and meta-analyses, but confirmatory studies were requested [91-93]. In contrast, the use of levosimendan for prevention of LCOS in pediatric patients undergoing surgery for congenital heart disease seems a notable exception. In fact, a systematic review of 5 RCTs showed no clear favorable effect from prophylactic levosimendan on the reduction of postoperative mortality [94].
Table 2.
Consensus documents on the use of perioperative levosimendan in cardiac surgery.
| First Author, Year, [Reference] | Method of Studies Selection | Number of Studies | Conclusions |
|---|---|---|---|
| Landoni G, 2011, [89] | Topics’s suggestions from conference’s participants and from selected Journals with IF. Systematic PubMed search (updated on June 28th 2010) to identify related topics. |
1 meta-analysis of pooled pre- and postoperative levosimendan administration. | Levosimendan seems to reduce 30-days mortality. There is not enough evidence to recommend routine administration. |
| Landoni G, 2012, [90] | PubMed search with no time limits (updated on June 8, 2011) was used to systematically identify all randomized published articles concerning interventions influencing perioperative mortality. | 1 meta-analysis of pooled pre- and postoperative levosimendan administration. | Levosimendan seems to reduce 30-days mortality. There is not enough evidence to recommend routine administration. |
| First Author, Year, [Reference] | Method of Studies Selection | Number of Studies | Conclusions |
| Toller W, 2013, [91] | All randomized trials on the use of levosimendan in cardiac surgery that reported clinically relevant endpoints. | 23 RCTs | Examined evidences suggest a reduced mortality in high-risk cardiac surgery patients and in those with LCOS treated with levosimendan. |
| Toller W, 2015, [92] | Papers and abstracts found on PubMed by searching ([levosimendan OR Simdax] and [surgery OR bypass OR valve OR CABG OR anest*]). Researches were grouped according to the timing of the use of levosimendan and the type of intervention. |
33 studies on preoperative and perioperative use of levosimendan. | In patients undergoing cardiac surgery, levosimendan optimizes hemodynamic of systemic and pulmonary circulation. The drug reduces the need for inotropic agents and mechanical circulatory support and improves kidney and liver function. In hospital and ICU stay are shortened. |
| Landoni G, 2017, [93] | All of the published literature including RCTs which assessed the effects of peiroperative levosimendan on mortality in cardiac surgery patients Systematic PubMed, Scopus and Embase search. No time limits (updated March 6, 2015). |
4 meta-analyses and 1 small RCT. | Potentially useful in patients with depressed LVEF, but confirmatory studies are needed. Agreement of experts' opinion: 88%. |
Abbreviations: CABG: coronary artery bypass grafting; ICU: intensive care unit; IF: impact factor; LCOS: low cardiac output syndrome; LVEF: left ventricular ejection fraction; RCTs: randomized controlled trials.
6. RECENT STUDIES (year 2017) ON LEVOSIMENDAN IN CARDIAC SURGERY
In 2017, results from three large multicenter placebo-controlled trials on levosimendan have been published (Table 3). LEVO-CTS and CHEETAH study involved patients undergoing heart surgery, examining the use of the drug for prophylaxis and treatment of LCOS respectively [17, 18]. LICORN study focused on preoperative drug administration for reducing LCOS in patients undergoing CABG with low LVEF [19].
Table 3.
Recent large RCTs on perioperative levosimendan in cardiac surgery.
| Study, Year, (First Author) [Reference] | Primary Endpoints | Patients | Results |
|---|---|---|---|
| LEVO-CTS, 2017, (Mehta RH) [17] | 4-component composite of: - Death through day 30 - Renal-replacement therapy through day 30 - Perioperative myocardial infarction through day 5 - Use of a mechanical cardiac assist device through day 5 2-component composite of: - Death through day 30 - Use of a mechanical cardiac assist device through day 5 |
882 patients with LVEF ≤ 35% randomized to levosimendan or placebo before cardiac surgery. | Failure in improving the two primary endpoints. Significantly lower incidence of the LCOS in levosimendan arm. Significantly less need of secondary inotrope use in levosimendan arm. |
| CHEETAH, 2017, (Landoni G) [18] | Mortality at day 30 | 506 patients needing hemodynamic support randomized to levosimendan or placebo after cardiac surgery. | No significant difference between drug arm and placebo arm. |
| LICORN, 2017 (Cholley B) [19] | 3 component composite of: - Prolonged catecholamine infusion - Use of left ventricular mechanical assist device - Renal replacement therapy. |
336 patients with LVEF ≤ 40% randomized to levosimendan or placebo before undergoing isolated or combined CABG. | No significant difference between levosimendan arm and placebo arm. |
Abbreviations: CABG: coronary artery bypass grafting; LCOS: low cardiac output syndrome; LVEF: left ventricular ejection fraction; RCTs: randomized controlled trials.
LEVO-CTS recruited 882 patients with a LVEF ≤ 35% randomized to levosimendan or placebo [17]. Median age of enrolled subjects was 65 years, with women accounting for 19 percent of the total study group. Intravenous infusion of levosimendan started before surgery at a dose of 0.2 μg/kg/min for 1 hour. Then, infusion continued at a dose of 0.1 μg/kg/min for 23 hours. LEVO-CTS had two primary composite outcomes: a four-component and a two-component endpoint. The four component outcome included the following: death through day 30, renal-replacement therapy through day 30, perioperative myocardial infarction through day 5 or use of a mechanical cardiac assist device through day 5. The other two-component endpoint was a composite of death through day 30 or use of a mechanical cardiac assist device through day 5.
Levosimendan failed in improving the two primary endpoints. However, the incidence of the LCOS in levosimendan arm was significantly lower than in placebo arm (OR: 0.62; p = 0.007). Moreover, fewer patients randomized to levosimendan needed secondary inotropic use compared to placebo (OR: 0.71; p = 0.017).
CHEETAH (HSR-LEVO) study enrolled 506 patients needing hemodynamic support after cardiac surgery [18]. The subjects were randomized to placebo or to levosimendan continuous infusion (dose of 0.025 to 0.2 μg/kg/min), for up to 48 hours or until discharge from the ICU. Mortality at 30 days was chosen as primary endpoint but no significant difference emerged between drug and placebo arm so that the trial was stopped for futility. Furthermore, groups did not differ in duration of mechanical ventilation, ICU stay and hospital stay.
LICORN study recruited 336 patients with LVEF ≤ 40% undergoing isolated or combined CABG [19]. The patients were randomized to a 24-hour infusion of levosimendan 0.1 µg/kg/min (n=167) or placebo. Drug’s infusion started after induction of anesthetic. Primary endpoint was a composite of following: prolonged catecholamine infusion, use of left ventricular mechanical assist device or renal replacement therapy. Nevertheless, no significant difference was observed between levosimendan arm and placebo arm in the composite endpoint (absolute risk difference taking into account center effect, -7% [95% CI, -17% to 3%]; p= 0.15).
Overall, results of these recent trials don’t support the standard use of levosimendan in prevention or treatment of LCOS in patients referred to cardiac surgery.
7. DIRECTIONS FOR FUTURE STUDIES
Upcoming studies on perioperative levosimendan in cardiac surgery should try to solve the numerous methodologic uncertainties which likely influenced the results of previous studies. These uncertainties include the optimal doses and time of administration of the drug. Moreover, the best way to define the ventricular dysfunction and the adequate sample size of patients to recruit are not clear yet. Finally, the subgroups of heart surgery patients which could have more benefits from levosimendan remain to be identified.
7.1. Optimal Doses and Time of Administration
Optimal doses of levosimendan, as resulting from the balance between therapeutic and adverse effects, and timing of administration of the drug remain still to be clarified. Authors of LICORN and LEVO-CTS studies have suggested that the negative results of their trials might depend on the use of small doses without the initial bolus. Probably, also starting the drug's infusion very close to heart surgery might have diminished its cardioprotective action [17, 19]. In fact, preconditioning effects of levosimendan could have been limited by the short time of the infusion.
7.2. Definition of Ventricular Dysfunction
It has been argued that drug administration could be effective in improving outcomes of selected patients such as high-risk cardiac surgery patients with left ventricular function impairment. Thus, criteria for the definition of ventricular dysfunction should be enlarged. In fact, focusing only on LVEF value, as in the majority of performed studies, would exclude patients with compromised left ventricular function but preserved LVEF such as those with severe mitral regurgitation in which LVEF can be overestimated. In addition, ultrasonographic calculation of LVEF can be influenced by geometric factors. Future studies investigating efficacy of perioperative levosimendan, might benefit from newer echocardiographic techniques such as strain imaging, apt to identify left ventricular systolic dysfunction and discriminate impairment of longitudinal and circumferential myocardial fibers [96]. More attention should also be played to right ventricular dysfunction. Indeed, levosimendan infusion has proven efficacious and safe in patients with acute right HF. In fact, a recent meta-analysis showed that acute right HF patients treated with levosimendan for 24 hours, had a significant improvement in tricuspid annular plane systolic excursion and a significant reduction in systolic pulmonary artery pressure without an increase of adverse events [97].
7.3. Subgroups of Heart Surgery Patients
Investigations involving mixed populations should be avoided in future researches, since levosimendan could qualify as a useful drug in selected subgroups of heart surgery patients. For example, it is well known that subjects with IHD referred to CABG are not comparable to patients undergoing heart surgery for valvular diseases. Indeed, preliminary data from post-hoc analyses of sub-settings of patients of LEVO-CTS study show a significantly lower mortality in levosimendan group in subjects who underwent only CABG [98].
It seems plausible that efficacy of levosimendan might vary depending upon whether left ventricular dysfunction is due to ischemia or to pressure or volume overload [17]. Thus, to weigh the effect of levosimendan on LCOS incidence, forthcoming well-conducted studies should provide also a systematic hemodynamic evaluation of enrolled patients.
7.4. Bias from Other Inotropes Administration
Bias deriving from the administration of other inotropes should be always considered in future studies on levosimendan in cardiac surgery. Indeed, the inotropic effect of the compound may be reduced by pre-treatment with beta-mimetic drugs [98, 99].
Such bias could occur in CHEETAH trial in which patients received high doses of dobutamine and epinephrine beyond levosimendan itself [18].
7.5. Sample Size and Long-term Survival
Importantly, the sample size needed to detect the effect of levosimendan on post-operative mortality should be defined; authors of LEVO-CTS have indicated that a well-powered trial should recruit 3000 patients [17].
Few studies have investigated long-term survival of patients who received levosimendan in perioperative time, providing negative results at six months [17, 19, 60, 72]. Indeed, the drug is likely to exert its beneficial effects mainly during the perioperative period, but a long-term effect cannot be excluded. Accordingly, it is to be recommended that future studies would consider such endpoint among the outcomes.
CONCLUSION
The evidence that levosimendan in heart surgery patients might ameliorate hemodynamic condition and counteract LCOS seems robust. Such observation has been confirmed in the recent LEVO-CTS trial and appears congruent with findings from early small studies and with the well-recognized efficacy of the drug in counteracting acute decompensated HF. However, the expected reduction of hard outcomes has not been observed in three recent large trials in patients undergoing heart surgery.
Thus, at present, routine levosimendan administration cannot be recommended to reduce perioperative mortality in heart surgery [98].
Further study seems necessary to investigate the effect of levosimendan in selected groups of cardiac surgery patients and to establish best doses and timing of drug's infusion.
Finally, the interest of clinicians and researchers in levosimendan is not limited to the cardiac surgery setting given that the drug has been reported to be potentially useful in other clinical contexts [43, 100-103].
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- ACS
Acute Coronary Syndrome
- AF
Atrial Fibrillation
- AKI
Acute Kidney Injury
- ATP
Adenosin Triphosphate
- CABG
Coronary Artery Bypass Grafting
- CHEETAH (HSR-LEVO) study
Levosimendan in High Risk Patients Undergoing Cardiac Surgery
- CPB
Cardio-Pulmonary Bypass
- cAMP
Cyclic Adenosine Monophosphate
- HF
Heart Failure
- IABP
Intra-Aortic Balloon Pump
- ICU
Intensive Care Unit
- IHD
Ischemic Heart Disease
- INTERMACS
Interagency Registry for Mechanically Assisted Circulatory Support
- LCOS
Low Cardiac Output Syndrome
- LEVO-CTS study
Levosimendan in Patients With Left Ventricular Systolic Dysfunction Undergoing Cardiac Surgery On Cardiopulmonary Bypass
- LICORN study
Preoperative Levosimendan in CABG Patients With Poor Left Ventricular Function
- LIDO study
Efficacy and Safety of Intravenous Levosimendan Compared with Dobutamine in Severe Low-output Heart Failure
- LVAD
Left Ventricular Assist Device
- LVEF
Left Ventricular Ejection Fraction
- mitoKATP
Mitochondrial ATPsensitive Potassium
- Min
Minute
- NHE-1
Na+–H+ exchanger Type 1
- NO
Nitric Oxide
- OPCAB
Off-pump Coronary Artery Bypass Grafting
- RCT
Randomized Controlled Trials
- RUSSLANN study
Randomized Study on Safety and Effectiveness of Levosimendan in Patients with Left Ventricular Failure Due to an Acute Myocardial Infarct
- Tn
Troponin
- TnC
Troponin C
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Berry J.D., Dyer A., Cai X., et al. Lifetime risks of cardiovascular disease. N. Engl. J. Med. 2012;366:321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaziano T.A., Bitton A., Anand S., et al. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr. Probl. Cardiol. 2010;35:72–115. doi: 10.1016/j.cpcardiol.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doenst T., Essa Y., Jacoub K., et al. Cardiac surgery 2016 reviewed. Clin. Res. Cardiol. 2017;••• doi: 10.1007/s00392-017-1113-2. [DOI] [PubMed] [Google Scholar]
- 4.d’Arcy J.L., Prendergast B.D., Chambers J.B., et al. Valvular heart disease: the next cardiac epidemic. Heart. 2011;97:91–93. doi: 10.1136/hrt.2010.205096. [DOI] [PubMed] [Google Scholar]
- 5.Iung B., Vahanian A. Epidemiology of valvular heart disease in the adult. Nat. Rev. Cardiol. 2011;8:162–172. doi: 10.1038/nrcardio.2010.202. [DOI] [PubMed] [Google Scholar]
- 6.Rao V., Ivanov J., Weisel R.D., et al. Predictors of low cardiac output syndrome after coronary artery bypass. J. Thorac. Cardiovasc. Surg. 1996;112:38–51. doi: 10.1016/s0022-5223(96)70176-9. [DOI] [PubMed] [Google Scholar]
- 7.Epting C.L., McBride M.E., Wald E.L., et al. Pathophysiology of post-operative low cardiac output syndrome. Curr. Vasc. Pharmacol. 2016;14:14–23. doi: 10.2174/1570161113666151014123718. [DOI] [PubMed] [Google Scholar]
- 8.Shahin J., DeVarennes B., Tse C.W., et al. The relationship between inotrope exposure, six- hour postoperative physiological variables, hospital mortality and renal dysfunction in pa tients undergoing cardiac surgery. Crit. Care. 2011;15:R162. doi: 10.1186/cc10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen D.V., Hansen M.K., Johnsen S.P., et al. Health outcomes with and without use of in otropic therapy in cardiac surgery: results of a propensity score-matched analysis. Anesthesiology. 2014;120:1098–1108. doi: 10.1097/ALN.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 10.Pathak A., Lebrin M., Vaccaro A., et al. Phar macology of levosimendan: inotropic, va sodilatory and cardioprotective effects. J. Clin. Pharm. Ther. 2013;38:341–349. doi: 10.1111/jcpt.12067. [DOI] [PubMed] [Google Scholar]
- 11.Follath F., Cleland J.G., Just H., et al. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe lowoutput heart failure (the LIDO study): a randomised double-blind trial. Lancet. 2002;360:196–202. doi: 10.1016/s0140-6736(02)09455-2. [DOI] [PubMed] [Google Scholar]
- 12.Moiseyev V.S., Poder P., Andrejevs N., et al. Safety and efficacy of a novel calcium sensi tizer, levosimendan, in patients with left ven tricular failure due to an acute myocardial in farction. A randomized, placebo- controlled, double-blind study (RUSSLAN). Eur. Heart J. 2002;23:1422–1432. doi: 10.1053/euhj.2001.3158. [DOI] [PubMed] [Google Scholar]
- 13.Cleland J.G., Freemantle N., Coletta A.P., et al. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROAC TIVE. Eur. J. Heart Fail. 2006;8:105–110. doi: 10.1016/j.ejheart.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Mebazaa A., Nieminen M.S., Packer M., et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SUR VIVE Randomized Trial. JAMA. 2007;297:1883–1891. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 15.Bergh C.H., Andersson B., Dahlstrom U., et al. Intravenous levosimendan vs. dobutamine in acute decompensated heart failure patients on beta-blockers. Eur. J. Heart Fail. 2010;12:404–410. doi: 10.1093/eurjhf/hfq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husebye T., Eritsland J., Müller C., et al. Levosi mendan in acute heart failure following pri mary percutaneous coronary intervention- treated acute ST-elevation myocardial infarc tion. Results from the LEAF trial: a random ized, placebo-controlled study. Eur. J. Heart Fail. 2013;15:565–572. doi: 10.1093/eurjhf/hfs215. [DOI] [PubMed] [Google Scholar]
- 17.Mehta R.H., Leimberger J.D., van Diepen S., et al. Levosimendan in patients with left ventricular dysfunction undergoing cardiac surgery. N. Engl. J. Med. 2017;376:2032–2042. doi: 10.1056/NEJMoa1616218. [DOI] [PubMed] [Google Scholar]
- 18.Landoni G., Lomivorotov V.V., Alvaro G., et al. Levosimendan for hemodynamic support after cardiac surgery. N. Engl. J. Med. 2017;376:2021–2031. doi: 10.1056/NEJMoa1616325. [DOI] [PubMed] [Google Scholar]
- 19.Cholley B., Caruba T., Grosjean S., et al. Effect of levosimendan on low cardiac output syndrome in patients with low ejection fraction undergoing coronary artery bypass grafting with cardiopulmonary bypass: The LICORN Randomized Clinical Trial. JAMA. 2017;318:548–556. doi: 10.1001/jama.2017.9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pieper D., Antoine S.L., Mathes T., et al. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014;67:368–375. doi: 10.1016/j.jclinepi.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Pollesello P., Ovaska M., Kaivola J., et al. Bind ing of a new Ca2+ sensitizer, levosimendan, to recombinant human cardiac troponin C. A molecular modelling, fluorescence probe, and proton nuclear magnetic resonance study. J. Biol. Chem. 1994;269:28584–28590. [PubMed] [Google Scholar]
- 22.Sandell E.P., Hayha M., Antila S., et al. Pharma cokinetics of levosimendan in healthy volun teers and patients with congestive heart failure. J. Cardiovasc. Pharmacol. 1995;26(Suppl. 1):S57–S62. [PubMed] [Google Scholar]
- 23.Takahashi R., Talukder M.A., Endoh M. In otropic effects of OR-1896, an active metabo lite of levosimendan, on canine ventricular my ocardium. Eur. J. Pharmacol. 2000;400:103–112. doi: 10.1016/s0014-2999(00)00385-x. [DOI] [PubMed] [Google Scholar]
- 24.Haikala H., Nissinen E., Etemadzadeh E., et al. Troponin C-mediated calcium sensitization in duced by levosimendan does not impair relax ation. J. Cardiovasc. Pharmacol. 1995;25:794–801. doi: 10.1097/00005344-199505000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Kaheinen P., Pollesello P., Levijoki J., et al. Ef fects of levosimendan and milrinone on oxygen consumption in isolated guinea-pig heart. J. Cardiovasc. Pharmacol. 2004;43:555–561. doi: 10.1097/00005344-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Michaels A.D., McKeown B., Kostal M., et al. Effects of intravenous levosimendan on human coronary vasomotor regulation, left ven tricular wall stress, and myocardial oxygen up take. Circulation. 2005;111:1504–1509. doi: 10.1161/01.CIR.0000159252.82444.22. [DOI] [PubMed] [Google Scholar]
- 27.Kersten J.R., Montgomery M.W., Pagel P.S., et al. Levosimendan, a new positive inotropic drug, decreases myocardial infarct size via activation of K(ATP) channels. Anesth. Analg. 2000;90:5–11. doi: 10.1097/00000539-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Papp Z., Csapo K., Pollesello P., et al. Pharmacological mechanisms contributing to the clinical efficacy of levosimendan. Cardiovasc. Drug Rev. 2005;23:71–98. doi: 10.1111/j.1527-3466.2005.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 29.Meyer K., Schipke J.D., Klocke R.C., et al. In otropic, vasodilating and preconditioning ac tions of levosimendan in the heart. Thorac. Cardiovasc. Surg. 2008;56:379–385. doi: 10.1055/s-2008-1038729. [DOI] [PubMed] [Google Scholar]
- 30.du Toit E.F., Genis A., Opie L.H., et al. A role for the RISK pathway and K(ATP) channels in pre- and post-conditioning induced by levosi mendan in the isolated guinea pig heart. Br. J. Pharmacol. 2008;154:41–50. doi: 10.1038/bjp.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maytin M., Colucci W.S. Cardioprotection: a new paradigm in the management of acute heart failure syndromes. Am. J. Cardiol. 2005;96:26G–31G. doi: 10.1016/j.amjcard.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Louhelainen M., Vahtola E., Kaheinen P., et al. Effects of levosimendan on cardiac remodeling and cardiomyocyte apoptosis in hypertensive Dahl/Rapp rats. Br. J. Pharmacol. 2007;150:851–861. doi: 10.1038/sj.bjp.0707157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura T., Liu Y., Goto M., et al. Mitochondrial ATP-sensitive K+ channels play a role in cardioprotection by Na+-H+ exchange inhibition against ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2001;37:957–963. doi: 10.1016/s0735-1097(00)01183-9. [DOI] [PubMed] [Google Scholar]
- 34.Parissis J.T., Karavidas A., Bistola V., et al. Effects of levosimendan on flow-mediated vasodilation and soluble adhesion molecules in patients with advanced chronic heart failure. Atherosclerosis. 2008;197:278–282. doi: 10.1016/j.atherosclerosis.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 35.Parissis J.T., Adamopoulos S., Antoniades C., et al. Effects of levosimendan on circulating pro- inflammatory cytokines and soluble apoptosis mediators in patients with decompensated advanced heart failure. Am. J. Cardiol. 2004;93:1309–1312. doi: 10.1016/j.amjcard.2004.01.073. [DOI] [PubMed] [Google Scholar]
- 36.Moreno N., Tavares-Silva M., Lourenc A.P., et al. Levosimendan: The current situation and new prospects. Rev. Port. Cardiol. 2014;33:795–800. doi: 10.1016/j.repc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Ponikowski P., Voors A.A., Anker S.D., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 38.Nieminen M.S., Buerke M., Cohen-Solál A., et al. The role of levosimendan in acute heart failure complicating acute coronary syndrome: A review and expert consensus opinion. Int. J. Cardiol. 2016;218:150–157. doi: 10.1016/j.ijcard.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 39.van Diepen S., Katz J.N., Albert N.M., et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 2017;136(16):e232–e268. doi: 10.1161/CIR.0000000000000525. [DOI] [PubMed] [Google Scholar]
- 40.Unverzagt S, Wachsmuth L, Hirsch K, et al. Inotropic agents and vasodilator strategies for acute myocardial infarction complicated by cardiogenic shock or low cardiac output syndrome. 2014. [DOI] [PubMed]
- 41.Morelli A., De Castro S., Teboul J.L., et al. Effects of levosimendan on systemic and regional hemodynamics in septic myocardial depression. Intensive Care Med. 2005;31:638–644. doi: 10.1007/s00134-005-2619-z. [DOI] [PubMed] [Google Scholar]
- 42.Rhodes A., Evans L.E., Alhazzani W., et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit. Care Med. 2017;45(3):486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 43.Gustafsson F., Guarracino F., Schwinger R.H.G. The inodilator levosimendan as a treatment for acute heart failure in various settings. Eur. Heart J. Suppl. 2017;19(Suppl. C):C2–C7. doi: 10.1093/eurheartj/sux001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barisin S., Husedzinovic I., Sonicki Z., et al. Levosimendan in off-pump coronary artery bypass: a four-times masked controlled study. J. Cardiovasc. Pharmacol. 2004;44(6):703–708. doi: 10.1097/00005344-200412000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Husedzinović I., Barisin S., Bradić N., et al. Levosimendan as a new strategy during off- pump coronary artery bypass grafting: double- blind randomized placebo-controlled trial. Croat. Med. J. 2005;46(6):950–956. [PubMed] [Google Scholar]
- 46.Alvarez J., Taboada M., Rodríguez J., et al. Hemodynamic effects of levosimendan following cardiac surgery. Rev. Esp. Anestesiol. Reanim. 2005;52(7):389–394. [PubMed] [Google Scholar]
- 47.Al-Shawaf E., Ayed A., Vislocky I., et al. Levosimendan or milrinone in the type 2 diabetic patient with low ejection fraction undergoing elective coronary artery surgery. J. Cardiothorac. Vasc. Anesth. 2006;20:353–357. doi: 10.1053/j.jvca.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Tritapepe L., De Santis V., Vitale D., et al. Preconditioning effects of levosimendan in coronary artery bypass grafting—a pilot study. Br. J. Anaesth. 2006;96:694–700. doi: 10.1093/bja/ael082. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez J., Bouzada M., Fernández A.L., et al. Haemodynamic effects of levosimendan compared with dobutamine in patients with low cardiac output after cardiac surgery. Rev. Esp. Cardiol. 2006;59:338–345. [PubMed] [Google Scholar]
- 50.De Hert S.G., Lorsomradee S., Cromheecke S., et al. The effects of levosimendan in cardiac surgery patients with poor left ventricular function. Anesth. Analg. 2007;104(4):766–773. doi: 10.1213/01.ane.0000256863.92050.d3. [Erratum in: Anesth Analg 2007; 104]. [6]. [: 1544. Dosage error in article text]. [DOI] [PubMed] [Google Scholar]
- 51.Tasouli A., Papadopoulos K., Antoniou T., et al. Efficacy and safety of perioperative infusion of levosimendan in patients with compromised cardiac function undergoing open-heart surgery: Importance of early use. Eur. J. Cardiothorac. Surg. 2007;32:629–633. doi: 10.1016/j.ejcts.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 52.De Hert SG, Lorsomradee S. A randomized trial evaluating different modalities of levosimendan administration in cardiac surgery patients with myocardial dysfunction. J. Cardiothorac. Vasc. Anesth. 2008;22(5):699–705. doi: 10.1053/j.jvca.2008.02.019. [Retraction in: J Cardiothorac Vasc Anesth 2011; 25: 897]. [DOI] [PubMed] [Google Scholar]
- 53.Levin R.L., Degrange M.A., Porcile R., et al. The calcium sensitizer levosimendan gives superior results to dobutamine in postoperative low cardiac output syndrome. Rev. Esp. Cardiol. 2008;61:471–479. [PubMed] [Google Scholar]
- 54.Järvelä K., Maaranen P., Sisto T., et al. Levosimendan in aortic valve surgery: Cardiac performance and recovery. J. Cardiothorac. Vasc. Anesth. 2008;22(5):693–698. doi: 10.1053/j.jvca.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 55.Levin R., Porcile R., Salvagio F., et al. Levosimendan reduces mortality in postoperative low cardiac output syndrome after coronary surgery. Circulation. 2009;120:S987–S988. [Google Scholar]
- 56.Eriksson H.I., Jalonen J.R., Heikkinen L.O., et al. Levosimendan facilitates weaning from cardiopulmonary bypass in patients undergoing coronary artery bypass grafting with impaired left ventricular function. Ann. Thorac. Surg. 2009;87(2):448–454. doi: 10.1016/j.athoracsur.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 57.Tritapepe L., De Santis V., Vitale D., et al. Levosimendan pre-treatment improves outcomes in patients undergoing coronary artery bypass graft surgery. Br. J. Anaesth. 2009;102:198–204. doi: 10.1093/bja/aen367. [DOI] [PubMed] [Google Scholar]
- 58.Leppikangas H., Järvelä K., Sisto T., et al. Preoperative levosimendan infusion in combined aortic valve and coronary bypass surgery. Br. J. Anaesth. 2011;106(3):298–304. doi: 10.1093/bja/aeq402. [DOI] [PubMed] [Google Scholar]
- 59.Momeni M., Rubay J., Matta A., et al. Levosimendan in congenital cardiac surgery: a randomized, double-blind clinical trial. J. Cardiothorac. Vasc. Anesth. 2011;25(3):419–424. doi: 10.1053/j.jvca.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Lahtinen P., Pitkänen O., Pölönen P., et al. Levosimendan reduces heart failure after cardiac surgery: A prospective, randomized, placebo-controlled trial. Crit. Care Med. 2011;39(10):2263–2270. doi: 10.1097/CCM.0b013e3182227b97. [DOI] [PubMed] [Google Scholar]
- 61.Severi L., Lappa A., Landoni G., et al. Levosimendan versus intra-aortic balloon pump in high-risk cardiac surgery patients. J. Cardiothorac. Vasc. Anesth. 2011;25(4):632–636. doi: 10.1053/j.jvca.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 62.Ristikankare A., Pöyhiä R., Eriksson H., et al. Effects of levosimendan on renal function in patients undergoing coronary artery surgery. J. Cardiothorac. Vasc. Anesth. 2012;26(4):591–595. doi: 10.1053/j.jvca.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 63.Lomivorotov V.V., Boboshko V.A., Efremov S.M., et al. Levosimendan versus an intra-aortic balloon pump in high-risk cardiac patients. J. Cardiothorac. Vasc. Anesth. 2012;26(4):596–603. doi: 10.1053/j.jvca.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 64.Ricci Z., Garisto C., Favia I., et al. Levosimendan infusion in newborns after corrective surgery for congenital heart disease: Randomized controlled trial. Intensive Care Med. 2012;38(7):1198–1204. doi: 10.1007/s00134-012-2564-6. [DOI] [PubMed] [Google Scholar]
- 65.Lechner E., Hofer A., Leitner-Peneder G., et al. Levosimendan versus milrinone in neonates and infants after corrective open-heart surgery: A pilot study. Pediatr. Crit. Care Med. 2012;13(5):542–548. doi: 10.1097/PCC.0b013e3182455571. [DOI] [PubMed] [Google Scholar]
- 66.Levin R., Degrange M., Del Mazo C., et al. Preoperative levosimendan decreases mortality and the development of low cardiac output in high-risk patients with severe left ventricular dysfunction undergoing coronary artery bypass grafting with cardiopulmonary bypass. Exp. Clin. Cardiol. 2012;17:125–130. [PMC free article] [PubMed] [Google Scholar]
- 67.Gandham R., Syamasundar A., Ravulapalli H., et al. A comparison of hemodynamic effects of levosimendan and dobutamine in patients undergoing mitral valve repair / replacement for severe mitral stenosis. Ann. Card. Anaesth. 2013;16(1):11–15. doi: 10.4103/0971-9784.105363. [DOI] [PubMed] [Google Scholar]
- 68.Pellicer A., Riera J., Lopez-Ortego P., et al. Phase 1 study of two inodilators in neonates undergoing cardiovascular surgery. Pediatr. Res. 2013;73(1):95–103. doi: 10.1038/pr.2012.154. [DOI] [PubMed] [Google Scholar]
- 69.Bragadottir G., Redfors B., Ricksten S.E. Effects of levosimendan on glomerular filtration rate, renal blood flow, and renal oxygenation after cardiac surgery with cardiopulmonary bypass: A randomized placebo-controlled study. Crit. Care Med. 2013;41(10):2328–2335. doi: 10.1097/CCM.0b013e31828e946a. [DOI] [PubMed] [Google Scholar]
- 70.Ebade A.A., Khalil M.A., Mohamed A.K. Levosimendan is superior to dobutamine as an inodilator in the treatment of pulmonary hypertension for children undergoing cardiac surgery. J. Anesth. 2013;27:334–339. doi: 10.1007/s00540-012-1537-9. [DOI] [PubMed] [Google Scholar]
- 71.Baysal A., Yanartas M., Dogukan M., et al. Levosimendan improves renal outcome in cardiac surgery: A randomized trial. J. Cardiothorac. Vasc. Anesth. 2014;28(3):586–594. doi: 10.1053/j.jvca.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Erb J., Beutlhauser T., Feldheiser A., et al. Influence of levosimendan on organ dysfunction in patients with severely reduced left ventricular function undergoing cardiac surgery. J. Int. Med. Res. 2014;42(3):750–764. doi: 10.1177/0300060513516293. [DOI] [PubMed] [Google Scholar]
- 73.Shah B., Sharma P., Brahmbhatt A., et al. Study of levosimendan during off-pump coronary artery bypass grafting in patients with LV dysfunction: A double-blind randomized study. Indian J. Pharmacol. 2014;46:29–34. doi: 10.4103/0253-7613.125161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharma P., Malhotra A., Gandhi S., et al. Preoperative levosimendan in ischemic mitral valve repair. Asian Cardiovasc. Thorac. Ann. 2014;22(5):539–545. doi: 10.1177/0218492313499352. [DOI] [PubMed] [Google Scholar]
- 75.Sunny Yunus M. Comparison of levosimendan, milrinone and dobutamine in treating low cardiac output syndrome following valve replacement surgeries with cardiopulmonary bypass. J. Clin. Diagn. Res. 2016;10:UC05–UC08. doi: 10.7860/JCDR/2016/23584.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kandasamy A., Simon H.A., Murthy P., et al. Comparison of levosimendan versus dobutamine in patients with moderate to severe left ventricular dysfunction undergoing off-pump coronary artery bypass grafting: A randomized prospective study. Ann. Card. Anaesth. 2017;20:200–206. doi: 10.4103/aca.ACA_195_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tokuda Y., Grant P.W., Wolfenden H.D., et al. Levosimendan for patients with impaired left ventricular function undergoing cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2006;5(3):322–326. doi: 10.1510/icvts.2005.122390. [DOI] [PubMed] [Google Scholar]
- 78.Lehmann A., Kiessling A.H., Isgro F., et al. Levosimendan in patients with acute myocardial ischaemia undergoing emergency surgical revascularization. Eur. J. Anaesthesiol. 2008;25(3):224–229. doi: 10.1017/S0265021507002761. [DOI] [PubMed] [Google Scholar]
- 79.Brezina A., Riha H., Pirk J. Prophylactic application of levosimendan in cardiac surgical patients with severe left ventricle dysfunction. Exp. Clin. Cardiol. 2009;14:e31–e34. [PMC free article] [PubMed] [Google Scholar]
- 80.Theiss H.D., Grabmaier U., Kreissl N., et al. Preconditioning with levosimendan before implantation of left ventricular assist devices. Artif. Organs. 2014;38:231–234. doi: 10.1111/aor.12150. [DOI] [PubMed] [Google Scholar]
- 81.Eris C., Yavuz S., Toktas F., et al. Preoperative usages of levosimendan in patients undergoing coronary artery bypass grafting. Int. J. Clin. Exp. Med. 2014;7:219–229. [PMC free article] [PubMed] [Google Scholar]
- 82.Treskatsch S., Balzer F., Geyer T., et al. Early levosimendan administration is associated with decreased mortality after cardiac surgery. J. Crit. Care. 2015;859:e1–e6. doi: 10.1016/j.jcrc.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 83.Zangrillo A., Biondi-Zoccai G., Mizzi A., et al. Levosimendan reduces cardiac troponin release after cardiac surgery: A meta-analysis of randomized controlled studies. J. Cardiothorac. Vasc. Anesth. 2009;23:474–478. doi: 10.1053/j.jvca.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 84.Landoni G., Mizzi A., Biondi-Zoccai G., et al. Reducing mortality in cardiac surgery with levosimendan: A meta-analysis of randomized controlled trials. J. Cardiothorac. Vasc. Anesth. 2010;24:51–57. doi: 10.1053/j.jvca.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 85.Harrison R.W., Hasselblad V., Mehta R.H., et al. Effect of levosimendan on survival and adverse events after cardiac surgery: a meta-analysis. J. Cardiothorac. Vasc. Anesth. 2013;27:1224–1232. doi: 10.1053/j.jvca.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 86.Niu Z.Z., Wu S.M., Sun W.Y., et al. Perioperative levosimendan therapy is associated with a lower incidence of acute kidney injury after cardiac surgery: A meta-analysis. J. Cardiovasc. Pharmacol. 2014;63:107–112. doi: 10.1097/FJC.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 87.Lim J.Y., Deo S.V., Rababa’h A., et al. Levosimendan reduces mortality in adults with left ventricular dysfunction undergoing cardiac surgery: A systematic review and meta‐analysis. J. Card. Surg. 2015;30:547–554. doi: 10.1111/jocs.12562. [DOI] [PubMed] [Google Scholar]
- 88.Zhou C., Gong J., Chen D., et al. Levosimendan for prevention of acute kidney injury after cardiac surgery: A meta-analysis of randomized controlled trials. Am. J. Kidney Dis. 2016;67(3):408–416. doi: 10.1053/j.ajkd.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 89.Landoni G., Augoustides J.G., Guarracino F., et al. Mortality reduction in cardiac anesthesia and intensive care: Results of the first International Consensus Conference. Acta Anaesthesiol. Scand. 2011;55(3):259–266. doi: 10.1111/j.1399-6576.2010.02381.x. [DOI] [PubMed] [Google Scholar]
- 90.Landoni G., Rodseth R.N., Santini F., et al. Randomized evidence for reduction of perioperative mortality. J. Cardiothorac. Vasc. Anesth. 2012;26(5):764–772. doi: 10.1053/j.jvca.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 91.Toller W., Algotsson L., Guarracino F., et al. Perioperative use of levosimendan: Best practice in operative settings. J. Cardiothorac. Vasc. Anesth. 2013;27:361–366. doi: 10.1053/j.jvca.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 92.Toller W., Heringlake M., Guarracino F., et al. Preoperative and perioperative use of levosimendan in cardiac surgery: European expert opinion. Int. J. Cardiol. 2015;184:323–336. doi: 10.1016/j.ijcard.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 93.Landoni G., Pisano A., Lomivorotov V., et al. Randomized evidence for reduction of perioperative mortality: An updated consensus process. J. Cardiothorac. Vasc. Anesth. 2017;31:719–730. doi: 10.1053/j.jvca.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 94.Hummel J., Rücker G., Stiller B. Prophylactic levosimendan for the prevention of low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease. Cochrane Database Syst. Rev. 2017;8:CD011312. doi: 10.1002/14651858.CD011312.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Malliotakis P., Xenikakis T., Linardakis M., et al. Haemodynamic effects of levosimendan for low cardiac output after cardiac surgery: A case series. Hellenic J. Cardiol. 2007;48:80–88. [PubMed] [Google Scholar]
- 96.Stokke T.M., Hasselberg N.E., Smedsrud M.K., et al. Geometry as a confounder when assessing ventricular systolic function: Comparison between ejection fraction and strain. J. Am. Coll. Cardiol. 2017;70:942–954. doi: 10.1016/j.jacc.2017.06.046. [DOI] [PubMed] [Google Scholar]
- 97.Qiu J., Jia L., Hao Y., et al. Efficacy and safety of levosimendan in patients with acute right heart failure: A meta-analysis. Life Sci. 2017;184:30–36. doi: 10.1016/j.lfs.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 98.Guarracino F., Heringlake M., Cholley B., et al. Use of levosimendan in cardiac surgery: An update after the LEVO-CTS, CHEETAH, and LICORN trials in the light of clinical practice. J. Cardiovasc. Pharmacol. 2018;71(1):1–9. doi: 10.1097/FJC.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Desai A.S., Jarcho J.A. Levosimendan for the low cardiac output syndrome after cardiac surgery. N. Engl. J. Med. 2017;376:2076–2078. doi: 10.1056/NEJMe1705455. [DOI] [PubMed] [Google Scholar]
- 100.Wang X., Li S. Effect of small-dose levosimendan on mortality rates and organ functions in Chinese elderly patients with sepsis. Clin. Interv. Aging. 2017;12:917–921. doi: 10.2147/CIA.S136355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bhattacharjee S., Soni K.D., Maitra S., et al. Levosimendan does not provide mortality benefit over dobutamine in adult patients with septic shock: A meta-analysis of randomized controlled trials. J. Clin. Anesth. 2017;39:67–72. doi: 10.1016/j.jclinane.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 102.Pölzl G., Altenberger J., Baholli L., et al. Repetitive use of levosimendan in advanced heart failure: Need for stronger evidence in a field in dire need of a useful therapy. Int. J. Cardiol. 2017;243:389–395. doi: 10.1016/j.ijcard.2017.05.081. [DOI] [PubMed] [Google Scholar]
- 103.Pollesello P., Parissis J., Kivikko M., et al. Levosimendan meta-analyses: Is there a pattern in the effect on mortality? Int. J. Cardiol. 2016;209:77–83. doi: 10.1016/j.ijcard.2016.02.014. [DOI] [PubMed] [Google Scholar]