Abstract
Background:
Vascular calcification is known to be a strong risk factor for cardiovascularadverse events and mortality. Atherosclerosis, diabetes, aging, abnormal bone mineral homeostasisand high uremic milieu such as chronic kidney disease are major factors that contribute to theprogression of vascular calcification. Several mechanisms such as the osteoblastic transition of vascularsmooth muscle cells in response to oxidative stress have shed light on the active nature ofvascular calcification, which was once thought to be a passive process. The fine interplay of regulatoryfactors such as PTH, vitamin D3, FGF 23 and klotho reflect the delicate balance between vascularcalcification and bone mineralization. Any disturbance affecting this equilibrium of the bonemineral-vascular axis results in accelerated vascular calcification.
Bisphosphonates share similar mechanism of action as statins, and hence several studies were undertakenin humans to verify if the benefits proven to be obtained in animal models extended tohuman models too. This yielded conflicting outcomes which are outlined in this review. This wasattributed mainly to inadequate sample size and flaws in the study design. Therefore, this benefitcan only be ascertained if studies addressing this are undertaken.
Conclusion:
This review seeks to highlight the pathophysiologic phenomena implicated in vascular and valvular calcification and summarize the literature available regarding the use of bisphosphonates in animal and human models. We also discuss novel treatment approaches for vascular calcification,with emphasis on chronic kidney disease and calciphylaxis.
Keywords: Vascular calcification, bisphosphonates, chronic kidney disease, bone-mineral-vascular axis, statins, osteoblastic transition, calciphylaxis
1. INTRODUCTION
Vascular Calcification (VC) has traditionally been considered to be a passive and degenerative process with increasing age-related burden [1]. It has multiple phenotypes including intimal artery calcification, medial artery calcification, cardiac valve calcification, calciphylaxis and tumor calcinosis [2] (Table 1). Atherosclerosis, diabetes, Chronic Kidney Disease (CKD) and aging are major determinants of VC [2]. There is a higher risk for adverse Cardiovascular (CV) events and mortality in patients with VC [2]. Recent data highlight the analogy of vascular calcification to bone remodeling, which is actively regulated, and is controlled by factors which aid in both, its induction and suppression [3].
Table 1.
Summary of types of VC.
| Type | Risk Factors | Treatment Strategies so Far |
|---|---|---|
| Intimal calcification | Metabolic syndrome, smoking | Statins |
| Medial calcification | Osteoporosis, CKD | Anti resorptive agents, Dialysis, calcimimetics, maintenance of adequate bone turnover |
| Cardiac valve calcification | Aging, HLD, DM, HTN, CKD | Surgery |
| Calcific uremic arteriolopathy | Obesity, ESRD | BP’s, cinacalcet, sodium thiosulfate |
Bisphosphonates have a major impact on preserving Bone Mineral Density (BMD), but there is scarcity of literature on its role in modulating vascular and valvular calcification. It is postulated that bisphosphonates have similar pleiotropic effects as statins [4] and there have been extensive studies with them in animal models of VC with encouraging results [5].
This review seeks to highlight the pathophysiology of vascular calcification and the close interlink with the process of bone mineralization. It will summarize data on bisphosphonates in vascular and valvular calcification with emphasis on high calcification risk milieus, such as CKD.
2. PATHOPHYSIOLOGY OF VASCULAR CALCIFICATION
VC is a systemic process involving vasculature across anatomic sites such as coronary arteries, aorta, dermal arterioles and peripheral arteries [6-9]. The interaction of traditional risk factors such as hypertension, smoking, diabetes, obesity with non-traditional risk factors such as osteoporosis, anemia, malnutrition and uremic states such as CKD result in accelerated VC and cardiovascular disease [10].
Table-1: Summary outlining risk factors and treatment strategies for different types of vascular and valve calcification. Abbreviations: CKD – Chronic Kidney Disease; HLD – Hyperlipidemia; DM – Diabetes Mellitus, HTN – Hypertension, ESRD – End Stage Renal Disease, BP’s – Bisphosphonates.
Chronic Kidney Disease (CKD) is a risk factor for medial calcification which is an independent marker for all-cause mortality and CV mortality [11]. In one study, by Chowdhury, U.K., et al. [12], the prevalence of radial artery calcification in CKD patients was found to be 45 times that of patients without CKD. In another study by Raggi, P., et al. [13], coronary artery calcification scores in ESRD patients were found to be about thrice that of the general population. These findings reflect the systemic nature of VC in high uremic states as CKD with an equal predisposition across the spectrum of small, medium and large sized arteries as well as native cardiac valves emphasizing the accelerated progression of VC in the milieu of CKD.
Systemic mineral composition and bone density is altered early in CKD and is referred to as Chronic Kidney Disease- Mineral Bone Disease (CKD- MBD), which raises the concept of the bone-mineral- vascular axis. Renal function is inversely related to bone mineral disease and resultant calcification. CKD-MBD involves serum calcium and phosphate balance, which is hormonally regulated via Parathyroid Hormone (PTH) and calcitriol, any alteration of which can affect bone mineral homeostasis [14]. Calcific Uremic Arteriolopathy (CUA) or calciphylaxis is an extreme complication of VC in CKD, where dermal arterioles calcify, leading to necrosis and ulceration and is associated with very high morbidity and mortality [15].
The phenomenon of vascular calcification, which was once thought a passive process as a result of an increased calcium-phosphate product, is now considered an active complex process. The initial step appears to be Vascular Smooth Muscle Cells (VSMC) or other mesenchymal elements (such as pericytes) [16] of the vasculature undergoing a phenotypic shift, such that their pattern of gene expression and behavior resembles that of osteoblasts [17].
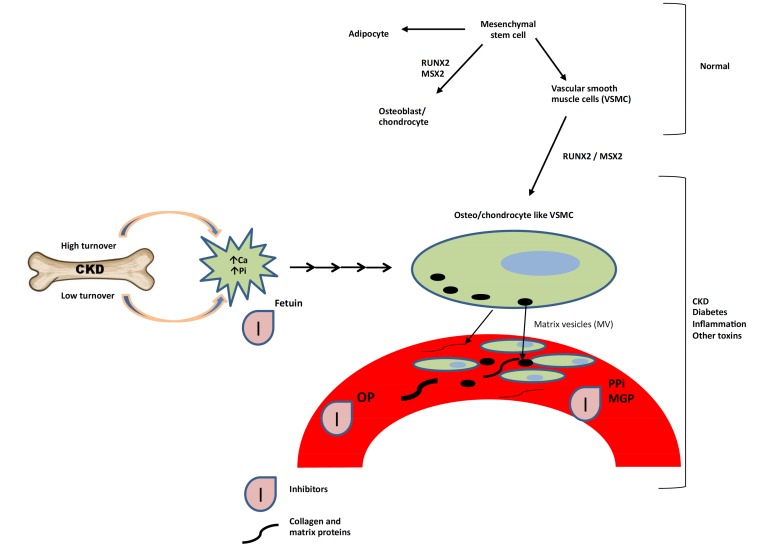
Exposure of cultured VSMCs to adequate levels of oxidative free radicals from hydrogen peroxide induces Runt-related transcription factor 2 (RUNX2; also called core-binding factor alpha-1) and promotes the osteoblastic transition, suggesting that induction of oxidative stress in smooth muscle is instrumental in driving this transition [18, 19]. RUNX2 plays a key role in VC, in boosting expression (directly or indirectly) of a number of other proteins found in osteoblasts—including the transcription factors osterix and msh homeobox-2, Bone Morphogenetic Protein-2 and -4 (BMP-2 and BMP-4), alkaline phosphatase, osteopontin (OPN), osteocalcin, and matrix gamma-carboxyglutamate (Gla) protein (MGP)—that enable or regulate extracellular deposition of hydroxyapatite [18, 20-24]. The differentiation of VSMC’s under the influence of transcription factors mentioned above are depicted in Fig. (1).
Fig. (1).
Factors responsible for induction of oxidative stress and promotion of osteoblastic transition of VSMC’s. Abbreviations: VSMC - vascular smooth muscle cells; CKD – Chronic Kidney Disease; Pi – Inorganic Phosphate; OP- Osteopontin; msx 2 - msh homeobox-2; Ca – Calcium; MGP - Matrix Gla Protein; PPi – Pyrophosphate; RUNX2 - Runt-related transcription factor 2.
3. LINK BETWEEN VASCULAR CALCIFICATION AND BONE MINERAL AXIS: ROLE OF BIOMARKERS:
Elevated serum phosphate is believed to play a major role in driving VC seen in chronic kidney disease, but is also linked to increased risk for VC in subjects with normal renal function [25-31]. Concentrations of phosphate comparable to those typically seen in renal failure patients drive this osteoblastic transition of VSMCs in vitro [25, 26]. A recent study confirms that elevated phosphate exposure promotes oxidative damage of mitochondria resulting in decrease of its trans-membrane potential causing upregulation of markers of apoptosis [32]. Indeed, apoptosis of VSMCs is a typical feature of VC, and is suspected to expedite it by promoting nucleation of hydroxyapatite [17, 33].
The differentiation of VSMC’s is regulated by oxidative free radicals and other such metabolic derangements of which osteogenic modulating proteins are primarily implicated. Osteogenic modulating proteins such as Bone Morphogenetic Proteins (BMP’s) and Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL) are known to promote vascular calcification whereas some others such as Osteoprotegerin (OPG) and Osteopontin (OPN) can be used as reliable markers of VC [34]. VSMCs also express receptors responsive to PTH and estrogenic compounds when cultivated with them in vitro [35].
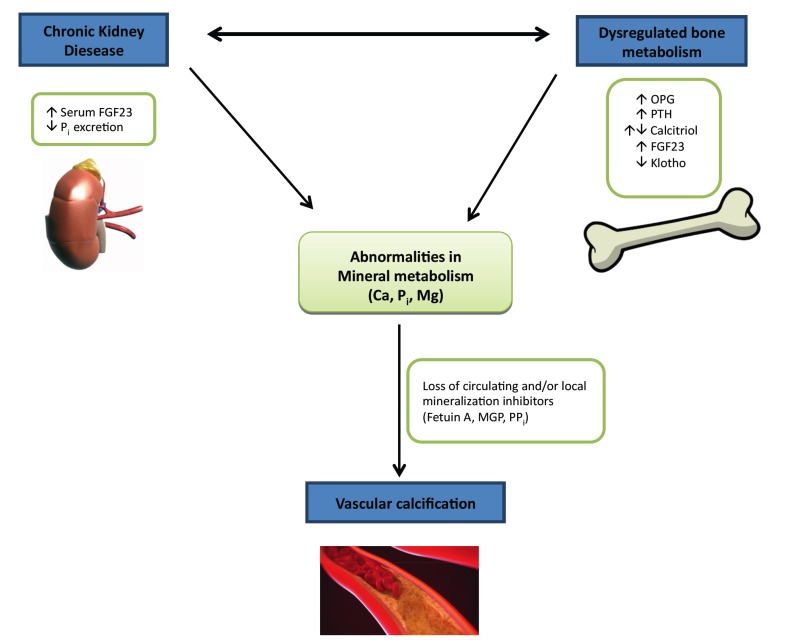
Fibroblast Growth Factor 23 (FGF23) is a hormone derived from bone and controls mineral homeostasis by regulation of serum phosphate [36, 37], Parathyroid Hormone (PTH), and 1,25-(OH)2-vitamin D3 [38]. Klotho is a transmembrane protein encoded by the klotho gene and serves as an obligate co-receptor for FGF 23 [39] that regulates phosphate elimination and vitamin D synthesis by the kidney [40]. Fibroblast Growth Factor 23 (FGF23) and Klotho have been recently known to play a role in ectopic calcification including native cardiac valves and large blood vessels [41-43]. Both Klotho and FGF23 participate in bone mineral homeostasis and regulate ectopic calcification via a fine interaction [44-48]. In Chronic Kidney Disease (CKD) and End-stage Renal Disease (ESRD) the levels of soluble Klotho is dramatically decreased and FGF23 increased. Hence, they have been used as sensitive biomarkers to indicate the state of renal dysfunction and subsequent extra renal manifestations in this group of patients [48, 49]. The balance between VC and bone mineral homeostasis is described in Fig. (2).
Fig. (2).
Factors affecting equilibrium between VC and bone mineral axis. Abbreviations: VC – Vascular Calcification; PTH – Parathyroid Hormone; FGF23 - Fibroblast Growth Factor 23; Pi – Inorganic Phosphate; OPG – Osteoprotegerin; MGP - Matrix Gla Protein; Ca – Calcium; Mg – Magnesium; PPi – Pyrophosphate; Pi – Inorganic Phosphate.
Atherosclerotic calcification is a well-coordinated process, similar to bone formation. Osteopontin, an important factor implicated in bone remodeling, has also been identified in atherosclerotic plaques [50]. Calcified atherosclerotic lesions were also found to contain bone morphogenetic protein-2a, a potent inhibitory factor of vascular calcification and osteoblastic differentiation [51]. Inhibitory factors such as Gla containing proteins (osteocalcin) are suspected to play a key role in mediating calcification of coronary arteries [50].
4. BONE TURN OVER AND VASCULAR CALCIFICATION: THERAPEUTIC TARGETS
Various modalities have been tried for treatment of vascular calcification with different results and success rates. Management strategies in abnormal bone turnover states such as osteoporosis include antiresorptive therapy such as bisphosphonates, novel therapies with monoclonal antibodies directed at RANKL ligand and human parathyroid hormone recombinant protein such as Teriparatide [52]. In CKD patients, a strategy of maintaining adequate bone turnover by ensuring optimum calcium and phosphate balance via phosphate binders such as lanthanum, sevelamer [2] and calcimimetics [53] has been proven effective. Other modalities such as ultrapure dialysate, Vitamin- D receptor agonists, and Vitamin K have also been used in CKD along with antiresorptive strategy of bisphosphonates with good effect in prevention of VC [2, 52].
Statins by far have been shown to reduce vascular thickness most significantly from its effects on prevention of neointimal atherosclerosis [54, 55]. This outcome was seen not only in high risk patients but also in patients with low risk profiles as elicited by the Measuring Effects on Intima-Media Thickness: an Evaluation of Rosuvastatin (METEOR) trial [54]. In a meta-analysis by Bedi U.S., et al., statins have been shown to slow progression of Carotid Intimal-medial Thickness (CIMT) and also proved effective in causing regression of atherosclerosis as measured by CIMT [55]. This was a significant finding as CIMT was later established as an independent risk factor for adverse cardiovascular events [56]. It is thought that the anti-atherogenic effect of statins in attenuating oxidized LDL contributes to this in part [57] along with its effect on lowering LDL cholesterol. This beneficial effect of statins was seen incrementally in a dose dependent manner as evidenced by the results of effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolemia (ASAP) [58] and Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis (ARBITER) trials [59].
Bisphosphonates which are postulated to have similar effects as statins at a cellular level through their inhibition of Farnesyl Pyrophosphate (FPP) and protein prenylation [4] have also been studied in terms of reduction of intimal and medial calcification with mixed results which are outlined below.
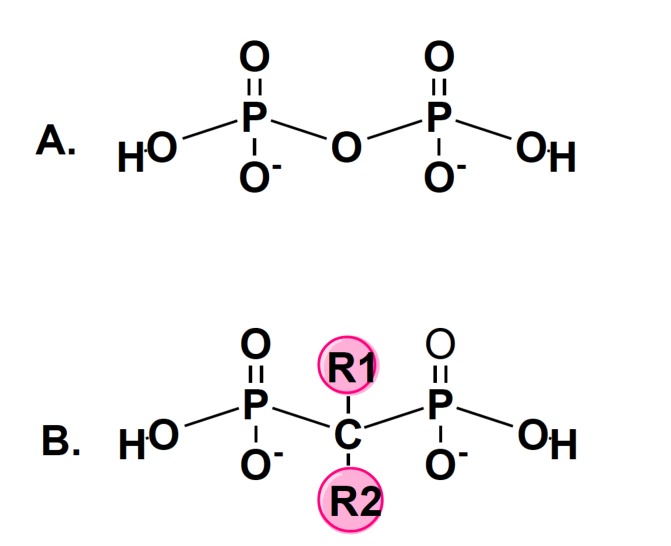
P – Phosphorus; O – Oxygen; H – Hydrogen; C – Carbon; R – Side Chain. In bisphosphonates, the central oxygen atom is replaced with a carbon. All bisphosphonates share a common phosphorus-carbon-phosphorus with two side chains (R1 and R2).
5. BISPHOSPHONATES
Bisphosphonates are pyrophosphate analogues resistant to enzymatic hydrolysis that are widely used as an antiresorptive drugs to preserve bone mineral density [5]. They have two side-chains (R1 and R2) as demonstrated in Fig. (3). R1 determines its affinity to bone, whereas R2 determines its potency. The presence of nitrogen increases the potency of BPs several folds. Risedronate, pamidronate, ibandronate, alendronate and zoledronate are nitrogen containing BP’s and have been found to be several times more potent than the non-nitrogen containing BPs such as clodronate, etidronate and tiludronate [60, 61].
Fig. (3).
Chemical structure of pyrophosphate (A) and bisphosphonates (B).
5.1. Pharmacokinetics of BPs
The intestinal absorption of bisphosphonates is less than 10% in most instances [62, 63]. This absorption is further limited by food and some other medications, particularly calcium supplements. Hence consumption of oral BP’s on an empty stomach is advised as it enhances its bioavailability. About half of the absorbed BP’s gain access to bone; the rest is excreted almost unchanged, renally [64]. Bisphosphonates accumulate in bone, with a half-life of about 10 years [65] after which it is redistributed.
5.2. Mechanism of Action of BPs
Non-nitrogen containing BP’s act slightly differently from nitrogen containing BP’s. They bind with osteoclasts when they enter bone and limit ATP dependent enzymes and thereby induce osteoclast apoptosis [66]. Nitrogen containing BP’s on the other hand are engulfed by osteoclasts, and other phagocytic cells [67] though their ruffled cell membrane after which they are released, secondary to the low intracellular pH [68]. Here they cause inhibition of osteoclast activity through disruption of the cytoskeleton [69]. At cellular level BPs inhibit the rate limiting enzyme of mevalonate pathway: farnesyl pyrophosphate synthase, an enzyme which is necessary for both the prenylation of proteins and osteoclast ruffled border formation [67].
Bisphosphonates are well known to preserve BMD through its osteoclast inhibitory effects and were used extensively in osteoporosis and in high turnover bone resorption states such as Paget’s disease, Multiple Myeloma and bony metastasis. They were first shown to reduce vascular calcification in animal models [70] and thereafter several studies (mostly observational) were undertaken in humans to investigate if this reduction in vascular calcification translates into reduced mortality which yielded contrasting results.
5.3. Clinical Studies in Animal Models
Price, P.A., et al. conducted one of the first experimental studies in animal models to assess the role of bisphosphonates in VC. They demonstrated that chronic renal failure induced by a combination of low protein diet and a high adenine state resulted in medial VC [71]. This was one of the first animal models with a reliable way of demonstrating uremia induced VC. This group also showed the inhibition of VC with warfarin and toxic doses of Vitamin D in rats with normal renal function with the use of bisphosphonates which was crucial in suggesting a link between VC and BMD. Tamura, K., et al. used the bisphosphonate etidronate in five- sixth’s nephrectomized rats to study the inhibition of VC with encouraging results [72].
5.4. Clinical Trials in Humans
The Multi-Ethnic Study of Atherosclerosis (MESA) trial was one of the first landmark prospective studies regarding the use of bisphosphonates in preventing VC [73]. This trial was unique in that the participants were women and was conducted in ethnically diverse populations and across a wide range of age groups, the mean age being 63 years. The patients included also had a diffuse array of baseline comorbidities. The results were age stratified, the primary end point being cardiovascular calcification. This study demonstrated that bisphosphonates reduced cardiovascular calcification (CV) in patients age > 65 years whereas an increase in CV calcification was noted in the age group < 65 years when compared to the control group of non-bisphosphonate users. This might be in part because the indication for BP use in younger patients is unclear and was thought to be due to more severe forms of secondary osteoporosis. Also, whether the effect on CV calcification can be interpreted as a mortality benefit is unclear as mortality was not the primary end point of this study.
Kranenburg, G., et al. conducted one of the largest meta-analyses to date to ascertain the benefit of bisphosphonates in reducing VC and if it translates into a mortality benefit. A total of 61 articles of RCT’s were used in this study each having a different population and a different research question [74]. Also, different classes of bisphosphonates, dosages, route and duration of treatment were studied. Majority of the studies had placebo administered as control whereas the rest used standard of care. There was significant reduction in aortic wall calcification [75, 76] and all-cause mortality with the largest benefit in patients with osteoporosis and breast cancer. These results were consistent with both nitrogen containing and non-nitrogen containing bisphosphonates. Although this study reflected good outcomes in terms of all-cause mortality, the reduction in vascular calcification did not translate into a reduction in arterial stiffness, CV events and CV mortality. This was partly due to inadequate sample size, duration of treatment and follow up. Also, adverse effects might have reduced the protective effect of bisphosphonates on CV events and mortality. This is evident from a sensitive analysis of pooled data of patients with osteoporosis who had longer follow up which favored bisphosphonates. Hence further studies are required to establish this likely beneficial effect of bisphosphonates on CV mortality.
Bisphosphonates were hypothesized to reduce valvular calcification through similar mechanism of action as its effect on vascular calcification. Several studies have been performed to confirm this hypothesis with varying results. One retrospective observational study by including a small patient population of 76 patients aged > 70 years demonstrated a beneficial effect of reducing aortic stenosis significantly in the 8 patients receiving bisphosphonates for > 1 year with an improvement of AVA of 0.1 cm on TTE compared to the 68 who did not [77]. This study was encouraging as it implied the protective effect of bisphosphonates on vascular calcification extended to native cardiac valves as well. But it was limited by its small sample size and its retrospective design. To further elucidate this beneficial relationship of bisphosphonates on valvular calcification, Aksoy, O., et al. undertook a larger retrospective study with a population of 801 female patients [78]. The mean age of the study was 76 years and included 488 bisphosphonate users. The study showed no significant difference in the rate of aortic valve calcification after a median follow up of 1.5 years and there was also no significant improvement in hemodynamics with regards to peak and mean AV gradients.
The results of this study are in contrast with the previous study but it was better powered and had longer follow up. This study was also limited by its retrospective design, and patient compliance. These contrasting results have to be further validated by more extensive studies with an adequate sample size and follow up.
5.5. Role of bisphosphonates in Calciphylaxis
Treatment strategies for calciphylaxis include maintenance of low target values of calcium, phosphate such that a calcium-phosphate product of less than 55 mg2/dL2 is achieved [79]. Medical therapy includes sodium thiosulphate, cinacalcet and bisphosphonates although there is inadequate clinical studies to confirm this, given the rare incidence of this disease entity. Pamidronate was the first bisphosphonate used to successfully treat calciphylaxis, which resulted in resolution of symptoms in about 6 weeks [80]. Other bisphosphonates such as Ibadronate and etidronate have also been used with good results but there are no clinical trials to support this and hence further research is warranted to establish this benefit.
CONCLUSION
Though bisphosphonates have showed promising results in animal models, clinical studies in humans have yielded contrasting results. This is mainly because most of the studies have been observational, the studies that are prospective invariably have inadequate follow up and non-uniform dosing, apart from variable compliance. Randomized controlled trials addressing the question of the role of BP’s in reducing VC are necessary to draw definitive conclusions about their benefits in this condition.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- VC
Vascular Calcification
- CKD
Chronic Kidney Disease
- HLD
Hyperlipidemia
- DM
Diabetes Mellitus
- HTN
Hypertension
- ESRD
End Stage Renal Disease
- BP’s
Bisphosphonates
- BMP
Bone Morphogenetic Protein
- FGF23
Fibroblast Growth Factor 23
- Pi
Inorganic Phosphate
- OPG
Osteoprotegerin
- OPN
Osteopontin
- msh
Muscle Segment Homeobox
- msx 2
msh Homeobox-2
- PTH
Parathyroid Hormone
- Ca
Calcium
- Mg
Magnesium
- MGP
Matrix Gla Protein
- PPi
Pyrophosphate
- RUNX2
Runt-related Transcription Factor 2
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Hayden M.R., Tyagi S.C., Kolb L., Sowers J.R., Khanna R. Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardiovasc. Diabetol. 2005;4:4. doi: 10.1186/1475-2840-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu M., Rementer C., Giachelli C.M. Vascular calcification: An Update on mechanisms and challenges in treatment. Calcif. Tissue Int. 2013;93(4):365–373. doi: 10.1007/s00223-013-9712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bostrom K.I., Rajamannan N.M., Towler D.A. The regulation of valvular and vascular sclerosis by osteogenic morphogens. Circ. Res. 2011;109(5):564–577. doi: 10.1161/CIRCRESAHA.110.234278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luckman S.P., Hughes D.E., Coxon F.P., Graham R., Russell G., Rogers M.J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J. Bone Miner. Res. 1998;13(4):581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 5.Persy V., De Broe M., Ketteler M. Bisphosphonates prevent experimental vascular calcification: Treat the bone to cure the vessels? Kidney Int. 2006;70(9):1537–1538. doi: 10.1038/sj.ki.5001899. [DOI] [PubMed] [Google Scholar]
- 6.Otto C.M., Lind B.K., Kitzman D.W., Gersh B.J., Siscovick D.S. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N. Engl. J. Med. 1999;341(3):142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 7.Eisen A., Tenenbaum A., Koren-Morag N., et al. Calcification of the thoracic aorta as detected by spiral computed tomography among stable angina pectoris patients: Association with cardiovascular events and death. Circulation. 2008;118(13):1328–1334. doi: 10.1161/CIRCULATIONAHA.107.712141. [DOI] [PubMed] [Google Scholar]
- 8.Kondos G.T., Hoff J.A., Sevrukov A., et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107(20):2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 9.Kohsaka S., Jin Z., Rundek T., et al. Impact of mitral annular calcification on cardiovascular events in a multiethnic community. The Northern Manhattan Study. JACC Cardiovasc. Imaging. 2008;1(5):617–623. doi: 10.1016/j.jcmg.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoccali C. Cardiovascular risk in uraemic patients—is it fully explained by classical risk factors? Nephrol. Dial. Transplant. 2000;15(4):454–457. doi: 10.1093/ndt/15.4.454. [DOI] [PubMed] [Google Scholar]
- 11.London G.M., Guérin A.P., Marchais S.J., Métivier F., Pannier B., Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol. Dial. Transplant. 2003;18(9):1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury U.K., Airan B., Mishra P.K., et al. Histopathology and morphometry of radial artery conduits: basic study and clinical application. Ann. Thorac. Surg. 2004;78(5):1614–1621. doi: 10.1016/j.athoracsur.2004.03.105. [DOI] [PubMed] [Google Scholar]
- 13.Raggi P., Boulay A., Chasan-Taber S., et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J. Am. Coll. Cardiol. 2002;39(4):695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 14.Moe S., Drueke T., Cunningham J., et al. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 15.Giachelli C.M. Mechanisms of vascular calcification in uremia. Semin. Nephrol. 2004;24(5):401–402. doi: 10.1016/j.semnephrol.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Doherty M.J., Ashton B.A., Walsh S., Beresford J.N., Grant M.E., Canfield A.E. Vascular pericytes express osteogenic potential in vitro and in vivo. J. Bone Miner. Res. 1998;13(5):828–838. doi: 10.1359/jbmr.1998.13.5.828. [DOI] [PubMed] [Google Scholar]
- 17.Giachelli C.M. Vascular calcification mechanisms. J. Am. Soc. Nephrol. 2004;15(12):2959–2964. doi: 10.1097/01.ASN.0000145894.57533.C4. [DOI] [PubMed] [Google Scholar]
- 18.Byon C.H., Javed A., Dai Q., et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008;283(22):15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H., Lu Q., Huang K. Selenium suppressed hydrogen peroxide-induced vascular smooth muscle cells calcification through inhibiting oxidative stress and ERK activation. J. Cell. Biochem. 2010;111(6):1556–1564. doi: 10.1002/jcb.22887. [DOI] [PubMed] [Google Scholar]
- 20.Xiao G., Jiang D., Ge C., et al. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J. Biol. Chem. 2005;280(35):30689–30696. doi: 10.1074/jbc.M500750200. [DOI] [PubMed] [Google Scholar]
- 21.Ducy P., Zhang R., Geoffroy V., Ridall A.L., Karsenty G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 22.Speer M.Y., Li X., Hiremath P.G., Giachelli C.M. Runx2/Cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J. Cell. Biochem. 2010;110(4):935–947. doi: 10.1002/jcb.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen N.X., Duan D., O’Neill K.D., et al. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int. 2006;70(6):1046–1053. doi: 10.1038/sj.ki.5001663. [DOI] [PubMed] [Google Scholar]
- 24.Liu T.M., Lee E.H. Transcriptional regulatory cascades in Runx2-dependent bone development. Tissue Eng. Part B Rev. 2013;19(3):254–263. doi: 10.1089/ten.teb.2012.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jono S., McKee M.D., Murry C.E., et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000;87(7):E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 26.Giachelli C.M. The emerging role of phosphate in vascular calcification. Kidney Int. 2009;75(9):890–897. doi: 10.1038/ki.2008.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linefsky J.P., O’Brien K.D., Katz R., et al. Association of serum phosphate levels with aortic valve sclerosis and annular calcification: The cardiovascular health study. J. Am. Coll. Cardiol. 2011;58(3):291–297. doi: 10.1016/j.jacc.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adeney K.L., Siscovick D.S., Ix J.H., et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J. Am. Soc. Nephrol. 2009;20(2):381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutierrez O.M. Increased serum phosphate and adverse clinical outcomes: Unraveling mechanisms of disease. Curr. Opin. Nephrol. Hypertens. 2011;20(3):224–228. doi: 10.1097/MNH.0b013e328343ea70. [DOI] [PubMed] [Google Scholar]
- 30.Cancela A.L., Santos R.D., Titan S.M., et al. Phosphorus is associated with coronary artery disease in patients with preserved renal function. PLoS One. 2012;7(5):e36883. doi: 10.1371/journal.pone.0036883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Figueiredo C.P., Rajamannan N.M., Lopes J.B., et al. Serum phosphate and hip bone mineral density as additional factors for high vascular calcification scores in a community-dwelling: The Sao Paulo Ageing & Health Study (SPAH). Bone. 2013;52(1):354–359. doi: 10.1016/j.bone.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Kim H., Kim H.J., Lee K., et al. alpha-Lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J. Cell. Mol. Med. 2012;16(2):273–286. doi: 10.1111/j.1582-4934.2011.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proudfoot D., Skepper J.N., Hegyi L., Bennett M.R., Shanahan C.M., Weissberg P.L. Apoptosis regulates human vascular calcification in vitro: Evidence for initiation of vascular calcification by apoptotic bodies. Circ. Res. 2000;87(11):1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 34.Liberman M., Pesaro A.E.P., Carmo L.S., Serrano C.V. Vascular calcification: Pathophysiology and clinical implications. Einstein (Sao Paulo) 2013;11(3):376–382. doi: 10.1590/S1679-45082013000300021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somjen D., Weisman Y., Kohen F., et al. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111(13):1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 36.Gattineni J., Baum M. Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatr. Nephrol. 2010;25(4):591–601. doi: 10.1007/s00467-009-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ADHR-Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000;26(3):345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 38.Krajisnik T., Bjorklund P., Marsell R., et al. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J. Endocrinol. 2007;195(1):125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 39.Kuro-o M. Klotho and the aging process. Korean J. Intern. Med. (Korean. Assoc. Intern. Med.) 2011;26(2):113–122. doi: 10.3904/kjim.2011.26.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuro-o M. Klotho as a regulator of fibroblast growth factor signaling and phosphate/calcium metabolism. Curr. Opin. Nephrol. Hypertens. 2006;15(4):437–441. doi: 10.1097/01.mnh.0000232885.81142.83. [DOI] [PubMed] [Google Scholar]
- 41.Hu M.C., Shi M., Zhang J., et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011;22(1):124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuro-o M., Matsumura Y., Aizawa H., et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 43.Nakatani T., Sarraj B., Ohnishi M., et al. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23) -mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23(2):433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bian A., Xing C., Hu M.C. Alpha Klotho and phosphate homeostasis. J. Endocrinol. Invest. 2014;37(11):1121–1126. doi: 10.1007/s40618-014-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuro-o M. Klotho, phosphate and FGF-23 in ageing and disturbed mineral metabolism. Nat. Rev. Nephrol. 2013;9(11):650–660. doi: 10.1038/nrneph.2013.111. [DOI] [PubMed] [Google Scholar]
- 46.Myrvang H. Basic research: Role of renal Klotho in mineral metabolism. Nat. Rev. Nephrol. 2012;8(10):553. doi: 10.1038/nrneph.2012.174. [DOI] [PubMed] [Google Scholar]
- 47.Huang C.L., Moe O.W. Klotho: A novel regulator of calcium and phosphorus homeostasis. Pflugers Arch. 2011;462(2):185–193. doi: 10.1007/s00424-011-0950-5. [DOI] [PubMed] [Google Scholar]
- 48.Gutierrez O.M. Fibroblast growth factor 23 and disordered vitamin D metabolism in chronic kidney disease: Updating the “trade-off” hypothesis. Clin. J. Am. Soc. Nephrol. 2010;5(9):1710–1716. doi: 10.2215/CJN.02640310. [DOI] [PubMed] [Google Scholar]
- 49.Scholze A., Liu Y., Pedersen L., et al. Soluble alpha-klotho and its relation to kidney function and fibroblast growth factor-23. J. Clin. Endocrinol. Metab. 2014;99(5):E855–E861. doi: 10.1210/jc.2013-4171. [DOI] [PubMed] [Google Scholar]
- 50.Gadeau A.P., Chaulet H., Daret D., Kockx M., Daniel-Lamaziere J.M., Desgranges C. Time course of osteopontin, osteocalcin, and osteonectin accumulation and calcification after acute vessel wall injury. J. Histochem. Cytochem. 2001;49(1):79–86. doi: 10.1177/002215540104900108. [DOI] [PubMed] [Google Scholar]
- 51.Li X., Yang H-Y., Giachelli C.M. BMP-2 Promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199(2):271–277. doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen N.C., Hsu C.Y., Chen C.L. The strategy to prevent and regress the vascular calcification in dialysis patients. BioMed Res. Int. 2017;2017:11. doi: 10.1155/2017/9035193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Floege J., Raggi P., Block G.A., et al. Study design and subject baseline characteristics in the ADVANCE Study: effects of cinacalcet on vascular calcification in haemodialysis patients. Nephrol. Dial. Transplant. 2010;25(6):1916–1923. doi: 10.1093/ndt/gfp762. [DOI] [PubMed] [Google Scholar]
- 54.Crouse J.R., Raichlen J.S., Riley W.A., et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: The meteor trial. JAMA. 2007;297(12):1344–1353. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 55.Bedi U.S., Singh M., Singh P.P., et al. Effects of statins on progression of carotid atherosclerosis as measured by carotid intimal—medial thickness: A meta-analysis of randomized controlled trials. J. Cardiovasc. Pharmacol. Ther. 2010;15(3):268–273. doi: 10.1177/1074248410369110. [DOI] [PubMed] [Google Scholar]
- 56.Stein JH, Korcarz CE, Hurst RT, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. 2008. [DOI] [PubMed]
- 57.Aviram M., Dankner G., Cogan U., Hochgraf E., Brook J.G. Lovastatin inhibits low-density lipoprotein oxidation and alters its fluidity and uptake by macrophages: In vitro and in vivo studies. Metabolism. 1992;41(3):229–235. doi: 10.1016/0026-0495(92)90263-a. [DOI] [PubMed] [Google Scholar]
- 58.Smilde T.J., van Wissen S., Awollersheim H., Trip M.D., Kastelein J., Stalenhoef A.F. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolemia (ASAP): A prospective, randomised, double-blind trial. Lancet. 2001;357(9256):577–581. doi: 10.1016/s0140-6736(00)04053-8. [DOI] [PubMed] [Google Scholar]
- 59.Taylor A.J., Kent S.M., Flaherty P.J., Coyle L.C., Markwood T.T., Vernalis M.N. ARBITER: Arterial biology for the investigation of the treatment effects of reducing cholesterol. Circulation. 2002;106(16):2055–2060. doi: 10.1161/01.cir.0000034508.55617.65. [DOI] [PubMed] [Google Scholar]
- 60.Russell R.G. Bisphosphonates: The first 40 years. Bone. 2011;49(1):2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 61.Li B., Ling Chau J.F., Wang X., Leong W.F. Bisphosphonates, specific inhibitors of osteoclast function and a class of drugs for osteoporosis therapy. J. Cell. Biochem. 2011;112(5):1229–1242. doi: 10.1002/jcb.23049. [DOI] [PubMed] [Google Scholar]
- 62.Razzaque M.S., Lanske B. The emerging role of the fibroblast growth factor-23-klotho axis in renal regulation of phosphate homeostasis. J. Endocrinol. 2007;194(1):1–10. doi: 10.1677/JOE-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Makela S., Saha H., Ala-Houhala I., Liukko-Sipi S., Ylitalo P. Steady state pharmacokinetics and dose equivalents of oral clodronate in renal failure. Int. J. Clin. Pharmacol. Ther. 2011;49(2):128–136. doi: 10.5414/cpp49128. [DOI] [PubMed] [Google Scholar]
- 64.Troehler U., Bonjour J.P., Fleisch H. Renal secretion of diphosphonates in rats. Kidney Int. 1975;8(1):6–13. doi: 10.1038/ki.1975.70. [DOI] [PubMed] [Google Scholar]
- 65.Lin J.H. Bisphosphonates: A review of their pharmacokinetic properties. Bone. 1996;18(2):75–85. doi: 10.1016/8756-3282(95)00445-9. [DOI] [PubMed] [Google Scholar]
- 66.Frith J.C., Monkkonen J., Blackburn G.M., Russell R.G., Rogers M.J. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J. Bone Miner. Res. 1997;12(9):1358–1367. doi: 10.1359/jbmr.1997.12.9.1358. [DOI] [PubMed] [Google Scholar]
- 67.Ott S.M. Pharmacology of bisphosphonates in patients with chronic kidney disease. Semin. Dial. 2015;28(4):363–369. doi: 10.1111/sdi.12388. [DOI] [PubMed] [Google Scholar]
- 68.Coxon F.P., Thompson K., Roelofs A.J., Ebetino F.H., Rogers M.J. Visualizing mineral binding and uptake of bisphosphonate by osteoclasts and non-resorbing cells. Bone. 2008;42(5):848–860. doi: 10.1016/j.bone.2007.12.225. [DOI] [PubMed] [Google Scholar]
- 69.Hampson G., Fogelman I. Clinical role of bisphosphonate therapy. Int. J. Womens Health. 2012;4:455–469. doi: 10.2147/IJWH.S24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schenk R., Merz W.A., Muhlbauer R., Russell R.G., Fleisch H. Effect of ethane-1-hydroxy-1,1-diphosphonate (EHDP) and dichlorome-thylene diphosphonate (Cl 2 MDP) on the calcification and resorption of cartilage and bone in the tibial epiphysis and metaphysis of rats. Calcif. Tissue Res. 1973;11(3):196–214. doi: 10.1007/BF02547219. [DOI] [PubMed] [Google Scholar]
- 71.Price P.A., Roublick A.M., Williamson M.K. Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int. 2006;70(9):1577–1583. doi: 10.1038/sj.ki.5001841. [DOI] [PubMed] [Google Scholar]
- 72.Tamura K., Suzuki Y., Hashiba H., Tamura H., Aizawa S., Kogo H. Effect of etidronate on aortic calcification and bone metabolism in calcitriol-treated rats with subtotal nephrectomy. J. Pharmacol. Sci. 2005;99(1):89–94. doi: 10.1254/jphs.fpj05019x. [DOI] [PubMed] [Google Scholar]
- 73.Elmariah S., Delaney J.A., O’Brien K.D., et al. Bisphosphonate use and prevalence of valvular and vascular calcification in women MESA (The Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 2010;56(21):1752–1759. doi: 10.1016/j.jacc.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kranenburg G., Bartstra J.W., Weijmans M., et al. Bisphosphonates for cardiovascular risk reduction: A systematic review and meta-analysis. Atherosclerosis. 2016;252:106–115. doi: 10.1016/j.atherosclerosis.2016.06.039. [DOI] [PubMed] [Google Scholar]
- 75.Kawahara T., Nishikawa M., Kawahara C., Inazu T., Sakai K., Suzuki G. Atorvastatin, etidronate, or both in patients at high risk for atherosclerotic aortic plaques: A randomized, controlled trial. Circulation. 2013;127(23):2327–2335. doi: 10.1161/CIRCULATIONAHA.113.001534. [DOI] [PubMed] [Google Scholar]
- 76.Toussaint N. Cardiovascular disease and vascular calcification in renal failure. Intern. Med. J. 2010;40:56–57. [Google Scholar]
- 77.Innasimuthu A.L., Katz W.E. Effect of bisphosphonates on the progression of degenerative aortic stenosis. Echocardiography. 2011;28(1):1–7. doi: 10.1111/j.1540-8175.2010.01256.x. [DOI] [PubMed] [Google Scholar]
- 78.Aksoy O., Cam A., Goel S.S., et al. Do bisphosphonates slow the progression of aortic stenosis? J. Am. Coll. Cardiol. 2012;59(16):1452–1459. doi: 10.1016/j.jacc.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 79.Vedvyas C., Winterfield L.S., Vleugels R.A. Calciphylaxis: A systematic review of existing and emerging therapies. J. Am. Acad. Dermatol. 2012;67(6):e253–e260. doi: 10.1016/j.jaad.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 80.Phanish M.K., Kallarackal G., Ravanan R., Lawson T.M., Baboolal K. Tumoral calcinosis associated with pyrexia and systemic inflammatory response in a haemodialysis patient: Successful treatment using intravenous pamidronate. Nephrol. Dial. Transplant. 2000;15(10):1691–1693. doi: 10.1093/ndt/15.10.1691. [DOI] [PubMed] [Google Scholar]