Summary
Arsenic (As) is a poisonous element that causes severe skin lesions and cancer in humans. Rice (Oryza sativa L.) is a major dietary source of As in humans who consume this cereal as a staple food. We hypothesized that increasing As vacuolar sequestration would inhibit its translocation into the grain and reduce the amount of As entering the food chain. We developed transgenic rice plants expressing two different vacuolar As sequestration genes, ScYCF1 and OsABCC1, under the control of the RCc3 promoter in the root cortical and internode phloem cells, along with a bacterial γ‐glutamylcysteine synthetase driven by the maize UBI promoter. The transgenic rice plants exhibited reduced root‐to‐shoot and internode‐to‐grain As translocation, resulting in a 70% reduction in As accumulation in the brown rice without jeopardizing agronomic traits. This technology could be used to reduce As intake, particularly in populations of South East Asia suffering from As toxicity and thereby improve human health.
Keywords: rice, arsenic, vacuolar sequestration, ABC transporter
Introduction
The US Agency for Toxic Substances and Disease Registry (ATSDR) ranked arsenic (As) top among hazardous substances in their 2015 Priority List (http://www.atsdr.cdc.gov/spl). As is widely dispersed around the world; in addition to being a natural component of Earth's lithosphere, As is released into the environment through anthropogenic pollution (Bowell et al., 2014). Rice (Oryza sativa L.) is a major contributor to inorganic As intake for the global population, especially for Asians, who consume rice as a staple food (Meharg, 2004; Meharg et al., 2009; Schoof et al., 1999; Williams et al., 2007; Zhao et al., 2010). Rice cultivated in paddy fields accumulates arsenite and arsenate in its grain through the same uptake and translocation pathways used to acquire silicon and phosphate, respectively. As silicon and phosphate are essential macroelements for rice (Li et al., 2016; Ma et al., 2008; Zhao et al., 2010). Efforts to reduce As intake cannot be based on blocking these pathways but should rather focus on removing As from the environment, including agricultural fields, or reducing As accumulation in the edible parts of the plant (i.e. grain). Removing As from the environment to an extent that would benefit human health is not feasible, because As is widespread and large tracts of agricultural land are contaminated with As (Bowell et al., 2014). Several strategies have been proposed to reduce As accumulation in rice grains, including breeding rice cultivars with low As accumulation, applying silicon fertilizers to limit arsenite uptake and reducing As levels in paddy soil by water management (Arao et al., 2009; Sun et al., 2014; Wang et al., 2016a; Zhao et al., 2010). However, these strategies have limitations. The application of Si fertilizer reduced grain As content by only 20% (Wang et al., 2016a). In addition, water management for aerobic cultivation caused cadmium accumulation in rice grain and reduced rice yield (Arao et al., 2009; Sun et al., 2014). Therefore, developing crops with reduced As levels in their edible parts is the most plausible strategy for decreasing human As intake.
Many studies have reported the mechanisms and genes involved in As uptake, translocation, accumulation and resistance (Punshon et al., 2017; Shi et al., 2016; Wang et al., 2016b; Xu et al., 2017; Yang et al., 2016), but few have examined how rice plants reduce the As content in their grain (Ma et al., 2008; Song et al., 2014). For example, loss‐of‐function rice plants lacking the arsenic efflux transporter Lsi2 showed a reduced As content in the grain (51%–63% that of the wild type (WT)) but also a reduced grain yield (only 40% that of the WT; Ma et al., 2007, 2008). An alternative approach is therefore needed to decrease the As contents of the grain without impairing rice yield.
Vacuolar sequestration has been suggested as a crucial mechanism for the resistance, accumulation and differential distribution of toxic metal(loid)s in plants. The expression of Saccharomyces cerevisiae yeast cadmium factor (ScYCF1), a vacuolar glutathione‐heavy metal(loid) conjugate, enhanced cadmium (Cd) and lead (Pb) resistance and accumulation in Saccharomyces cerevisiae and in plants (Shim et al., 2013; Song et al., 2003). In addition, ScYCF1 was found to transport bis‐glutathione‐As and contribute to As detoxification in yeast (Ghosh et al., 1999). By contrast, a double knockout mutant lacking the two vacuolar ABC transporters that constitute the major phytochelatin‐arsenic complex transporters in Arabidopsis, AtABCC1 and AtABCC2, exhibited a strong As hypersensitive phenotype (Song et al., 2010). OsABCC1, the closest homologue of AtABCC1 and AtABCC2, was found to be important for reducing the As concentration in rice (Song et al., 2014); the grains of the osabcc1 knockout lines accumulated 10 times more As than the WT. OsABCC1 is mainly located in the vacuolar membrane of the nodal phloem companion cells. Together, these results indicated that OsABCC1, expressed in specific cells of the nodes, sequesters As into the vacuole, blocking As translocation to the grains (Song et al., 2014). Overexpression of heavy metal ATPase (HMA3), a heavy metal ATPase regulating Cd sequestration into the vacuole, reduced Cd translocation to the rice shoot, including the grains (Ueno et al., 2010).
In this study, we developed transgenic rice exhibiting reduced As concentration in grain with the strategy of vacuolar As sequestration into cortex and phloem cells of root and internode to inhibit As translocation into grains.
Results and discussion
Development of transgenic rice with OsABCC1, γ‐ECS and ScYCF1
To reduce As accumulation in rice grains, we developed transgenic rice expressing two different vacuolar As sequestration genes, ScYCF1 and OsABCC1. These genes were expressed concomitantly with a bacterial γ‐glutamylcysteine synthetase (γ‐ECS), a key enzyme in glutathione synthesis, to increase the nonprotein thiol (glutathione and phytochelatin) concentration in the cytosol (Ghosh et al., 1999; Song et al., 2014; Zhu et al., 1999). We produced rice lines expressing either V5 tag fused OsABCC1 alone (OsABCC1‐V5, A for short in constructs), OsABCC1‐V5 together with γ‐ECS (AE) or OsABCC1‐V5, γ‐ECS and ScYCF1 (AEY). OsABCC1 and ScYCF1 were driven by the rice root‐specific cDNA clone3 (RCc3) promoter for root cortical cell‐, internode‐ and node‐specific promoter expression (Xu et al., 1995; Figures S1 and 1a), and γ‐ECS was constitutively expressed under the Zea mays ubiquitin (ZmUBI) promoter, a ubiquitous promoter (Figure S2). The corresponding constructs were named RCc3p‐A (RCc3 promoter::OsABCC1‐V5), RCc3p‐AE (RCc3 promoter::OsABCC1‐V5, ZmUBI promoter::γ‐ECS) and RCc3p‐AEY (RCc3 promoter::OsABCC1‐V5, ZmUBI promoter::γ‐ECS, RCc3 promoter::ScYCF1) (Figure 1b). In addition, to determine whether the cell‐specific expression of vacuolar As transporters in the root cortex or the phloem of the node and internode is critical for reducing As accumulation in grains, we prepared the following control transgenic plants expressing OsABCC1 and ScYCF1 using a ZmUBI promoter: UBIp‐A (ZmUBI promoter::OsABCC1‐V5), UBIp‐AE (ZmUBI promoter::OsABCC1‐V5, OsActin2 promoter::γ‐ECS) and UBIp‐AEY (ZmUBI promoter::OsABCC1‐V5, OsActin2 promoter::γ‐ECS, ZmUBI promoter::ScYCF1) (Figure S4a). We first confirmed that all the expression cassettes were successfully inserted into the rice genome and expressed in the transgenic plants (Figures S3a, b, c and S4b, c). We also confirmed that OsABCC1‐V5 protein was produced in the RCc3p‐AEY and UBIp‐AEY lines (Figures S3d and S4d) and that the fused protein was successfully targeted to the vacuolar membrane of the roots of the RCc3p‐AEY plants (Figure 1c).
Figure 1.

Development of transgenic rice with reduced As accumulation in the grains using tissue‐specific expression of OsABCC1, γ‐ECS and ScYCF1. (a) Analysis of the tissue‐specific expression of the OsRCc3 promoter by immunostaining OsRCc3 promoter::GUS transgenic plants ( RCc3p::GUS ) using a GUS antibody. The internode section denoted by broken lines is magnified in the inset surrounded by yellow solid lines. Bar = 100 μm. (b) Maps of vectors carrying RCc3p‐A ( RCc3 pro::OsABCC1‐V5), RCc3p‐AE ( RCc3 pro::OsABCC1‐V5, ZmUBI pro::γ‐ECS ) or RCc3p‐AEY ( RCc3 pro::OsABCC1‐V5, ZmUBI pro::γ‐ECS, RCc3 pro::ScYCF1). (c) Subcellular localization of OsABCC1‐V5 in the roots of RCc3p‐AEY plants using a sucrose density gradient analysis. Anti‐V5, anti‐OsABCC1, anti‐V‐ATPase (tonoplast marker), anti‐H+‐ATPase (plasma membrane marker) and Bip (ER marker) were used to assess protein abundance in the different compartments. The signal of OsABCC1‐V5 corresponded to the signals of OsABCC1 and V‐ATPase. (d) Arsenic accumulation in brown rice harvested from T3 transgenic plants transformed with RCc3p‐A, RCc3p‐AE, RCc3p‐AEY, UBIp‐A, UBIp‐AE or UBIp‐AEY. The As concentration was analysed in brown rice from plants grown in soil with a typical basal level of As. The values are means and standard errors (n = 5 plants). Different letters indicate significantly different means (Tukey's multiple comparison analysis, P ≤ 0.05).
Reduced As accumulation in grains from transgenic rice plants expressing OsABCC1 and ScYCF1 under the RCc3 promoter
As concentrations in brown rice were analysed from T3 transgenic rice plants cultivated in normal soil with a typical basal level of As (2.8 ± 0.5 mg/kg dried soil; below the acceptable soil As limits in Korea (25 mg/kg)). As accumulation was reduced in the transgenic lines, including RCc3p‐A, RCc3p‐AE and RCc3p‐AEY. In particular, the brown rice of all RCc3p‐AEY transgenic plants accumulated just 30% of the As levels in WT brown rice (Figure 1d), and As concentrations in the flag leaves of the transgenic lines were highly decreased (Figure S5c). In contrast to the transgenic plants expressing vacuolar As transporters driven by the RCc3 promoter, all single, double and triple overexpressors of OsABCC1, γ‐ECS and ScYCF1 driven by the ubiquitous promoters ZmUBIp and Actin2p had increased or similar levels of As in their brown rice as the WT (Figure 1d). These results indicate that the tissue‐specific expression of the vacuolar transporters had a greater impact on reduced As levels than did their total expression levels. Interestingly, the Cd concentrations in the grains and flag leaves of RCc3p‐AEY were comparable between the WT and transgenic lines (Figure S5a, b).
Reduced As translocation from root to the grains in RCc3p‐AEY plants
All lines exhibited reduced As accumulation in grains and flag leaves compared to the WT and transgenic plants harbouring the UBI‐driven constructs, UBIp‐A, UBIp‐AE and UBIp‐AEY (Figure 1d and Figure S5c). We randomly selected 3–8 transgenic lines to further investigate the mechanisms of As reduction in the brown rice of the RCc3p‐AEY lines. First, we analysed As accumulation in roots and shoots from 4‐week‐old plants treated with 1 μm As(III) for 5 days. The As levels in the roots and shoots of the transgenic rice plants expressing OsABCC1, γ‐ECS and ScYCF1 under the ubiquitous promoters did not differ from those of the WT (Figure S6a, b), consistent with the unaffected As levels in the grains (Figure 1d). By contrast, all the RCc3p‐AE (2 lines) and RCc3p‐AEY (2 lines) transgenic plants consistently exhibited different As levels compared to the WT. All of these lines accumulated more than twofold higher As in their roots, but exhibited strongly reduced As levels in their shoots compared to the WT (Figure S6a, b), indicating that the RCc3p‐AEY plants had reduced root‐to‐shoot As translocation (Figure 2a). We then compared the As distribution in various tissues of RCc3p‐9 transgenic and WT plants at the grain‐filling and vegetative stages. During the grain‐filling stage, the RCc3p‐AEY transgenic plants accumulated 10 times more As in the root than the WT, although the levels of As in the basal tissues of the shoot, such as the basal nodes, internodes and nodes II and III, were similar to those of the WT (Figure 2b). Less As was accumulated in the upper parts of the shoot of these transgenic plants than in the WT, including at node I, the leaves, internode I, the rachis and the brown rice (Figure 2b). To determine whether this was indeed the cause of the As retention in the roots of RCc3p‐AEY plants, we analysed the As concentrations in the xylem sap of rice seedlings treated with As (III) for four hours. Consistent with their As distribution, the xylem sap of the RCc3p‐AEY lines had a lower As concentration than the WT (Figure 2c). However, when treated with organic arsenic dimethylarsinic acid (DMA), the roots and shoots of RCc3p‐AEY transgenic plants had similar As accumulation compared to the WT, demonstrating the substrate specificity of ScYCF1 and OsABCC1 for with As (III) (Figure S7a, b). To verify that DMA is not a major substrate of OsABCC1 and ScYCF1, we performed DMA sensitivity tests using yeast of different genotypes of OsABCC1 and ScYCF1. There is no difference in DMA sensitivity between the ycf1 null mutant and its isogenic wild type, or between SM7 expressing OsABCC1 and SM7 transformed with empty vector (Figure S7c). These results strongly suggest that the two transporters do not recognize DMA as their substrate and that the vacuolar compartmentalization of As in roots inhibits inorganic As translocation to the shoot (Figure 2a, b, c). There was no observable difference in Cd concentration and root‐to‐shoot translocation in any of the transgenic plants tested in this study (Figure S7d, e, f).
Figure 2.
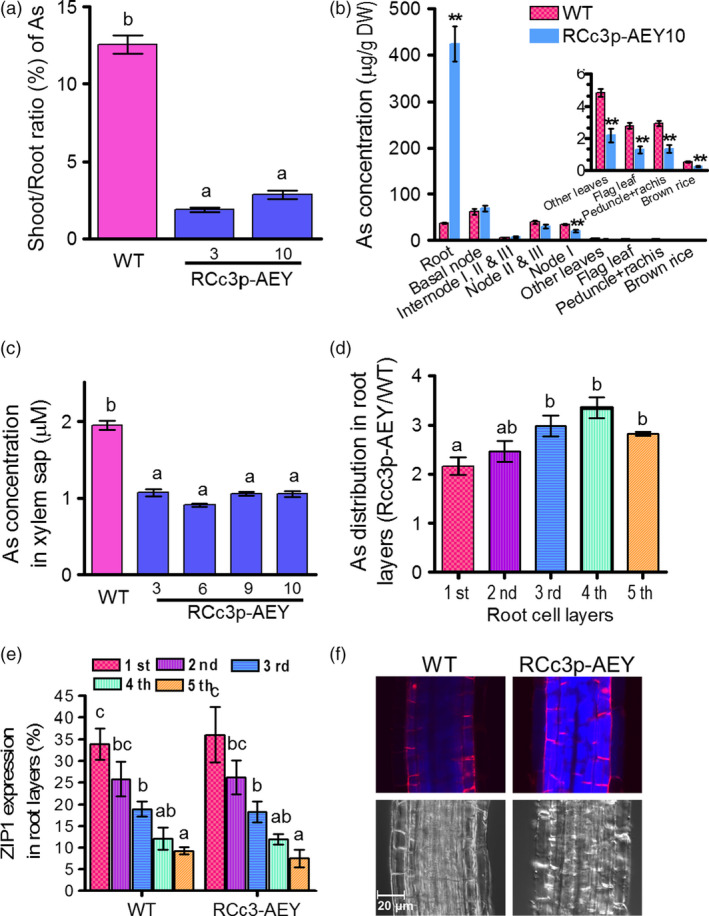
Reduced As translocation into the grains of RCc3p‐AEY plants. (a) Shoot to root As ratio. As contents were measured in the roots and shoots of 4‐week‐old plants treated with 1 μm As(III) for five days, and their shoot to root As ratio was analysed (n = 8 plants). (b) As concentrations of various tissues from four‐month‐old mature rice plants treated with 1 μm As(III) for 10 days. All values are means with corresponding standard errors (n = 5 plants). *P ≤ 0.05, **P ≤ 0.01 (Student's t‐test). (c) As concentration in xylem sap from 4‐week‐old plants treated with 5 μm As(III) for 4 h (n = 8 plants). (d) As distribution in different root layers of RCc3p‐AEY plants. As concentrations were measured in successive layers of root samples. The As concentrations were normalized by total protein content and expressed as the ratios of concentrations in RCc3p‐AEY compared with that in WT (n = 4 batches of 300 roots). (e) Expression pattern of the epidermis marker gene OsZIP1 in the successive layers of root samples analysed in d (n = s4 batches of 300 roots). The result indicates that the first root cell layer corresponds to the epidermis, the second is a mixture of the epidermis and cortex, the third and fourth are derived from the cortex, and the fifth layer is vascular tissue. (f) Accumulation of thiols in roots of 3‐week‐old plants treated with 1 μm As(III) for 3 h. The thiol signals were obtained by staining with 15 μm monobromobimane for 30 min. The red fluorescence indicates the cell wall stained with 20 μm propidium iodide. The values are means and standard errors. In b, c, d and e, the different letters indicate significantly different means (Tukey's multiple comparison analysis, P ≤ 0.05).
An immunohistochemistry analysis of the roots of the RCc3p::GUS plants revealed that the RCc3 promoter mediated high expression of the gene construct in the cortical cell layer of the root, which constitutes most of the rice root volume, and the endodermis, where Lsi2 mediates the efflux of As and promotes its loading into the xylem (Ma et al., 2008) (Figure 1a). To determine whether As is specifically compartmentalized in the root cortical cells of RCc3p‐AEY, we performed several different assays. First, we compared the As concentrations in two root zones, one with live cortical cells (0–3 mm from the tip) and the other with aerenchyma (3–8 mm). Only the root segments of the RCc3p‐AEY plants containing live cortical cells accumulated more As than the WT (Figure S8a). Furthermore, the highest As concentrations were found in the RCc3p‐AEY root samples enriched with cortical layers, prepared using the stepwise grinding method described by Song et al. (2011) (Figure 2d, e). In addition, thiols, which are the major chelators of As and colocalize with As (Moore et al., 2011; Raab et al., 2004), were found to accumulate to high levels in the vacuoles of the root cortex cells in the elongation zone (2–3 mm from the root tip) of the RCc3p‐AEY plants, but not in the WT (Figures 2f, S8b). These results suggest that the vacuolar As compartmentalization in the root cortex, mediated by OsABCC1 and ScYCF1 concomitantly with γ‐ECS, was the major contributing factor to the reduced As translocation to the shoot and grains of the RCc3p‐AEY rice plants.
Reduced As translocation from internode II to the grain of RCc3p‐AEY plants
The node is the most important tissue for As redistribution and transfer into the rice grain (Chen et al., 2015; Moore et al., 2014; Song et al., 2014). A defect in vacuolar As sequestration in phloem companion cells of node I was previously demonstrated to cause a 10‐fold increase in the As accumulation of rice grains (Song et al., 2014). Our immunohistochemistry results (Figure 1a) suggest that the two As transporters introduced into the transgenic rice might also function in the phloem cells of the node and internode. To determine whether the OsABCC1 and YCF1 vacuolar As transporters decreased the translocation of As to the grains from the nodes or the internodes in the RCc3p‐AEY transgenic plants, we performed As feeding assays using stems from upper internode II at the milk stage of grain filling. The RCc3p‐AEY transgenic lines exhibited a highly reduced As concentration in the leaf, flag leaf and grain (Figures 3a, S9a), and accumulated more As than the WT in internode II, which was directly exposed to the As(III) solution (Figure 3a); however, the As distribution was similar in the nodes of both the RCc3p‐AEY and WT lines. These results suggest that As compartmentalization in the nodes of the RCc3p‐AEY plants might not have contributed much to the inhibition of As translocation to the leaves and grains. Endogenous OsABCC1 is likely already expressed in these tissues. The concentration and distribution of rubidium (Rb), the control metal ion used in the feeding assays, was similar in all plants (Figures 3b, S9b). Our results indicate that RCc3p‐AEY rice could efficiently reduce As accumulation in the rice grains by inhibiting the movement of As into the shoot. Furthermore, vacuolar sequestration of As in the root cortex and phloem cells of the internode is an effective strategy for reducing the accumulation of As in grains.
Figure 3.

Reduced As translocation from internode II to the grain. WT and RCc3p‐AEY plants were cultured in 1/2 Kimura medium until the milk stage of grain filling. Plants were cut below internode II and then treated with half‐strength Kimura solution supplemented with 10 μm As(III) and 10 μm Rb(I) for 24 h. After incubation, each organ was harvested to measure As (a) and Rb (b). The values are means and standard errors (n = 7 plants). Different letters indicate significantly different means (Tukey's multiple comparison analysis, P ≤ 0.05).
Grain yield in RCc3p‐AEY transgenic plants
We examined the agronomic traits in RCc30‐AEY transgenic plants cultivated in rice paddy fields to clarify whether hyperaccumulation of As and thiols in the root reduces plant growth and grain yield. We compared the major agronomic traits of four different transgenic lines and WT plants, including plant height, tiller number, panicle length, 1000 grain weight and panicle weight. All of the transgenic lines resembled the WT with respect to these traits (Table 1).
Table 1.
Agronomic traits in RCc3p‐AEY rice plants. The agronomic traits were examined in plants cultivated at paddy fields in 2017. The values are means and standard errors (n = 5 plants). The same letters indicate no significantly different means (Tukey's multiple comparison analysis, P ≤ 0.05)
| Genotypes | Plant height (cm) | Tiller number | Panicle length (cm) | Grain weight (g/1000 grains) | Panicle weight (g/panicle) |
|---|---|---|---|---|---|
| WT | 105.0 ± 1.060a | 11.1 ± 0.167a | 22.6 ± 0.447a | 22.5 ± 0.639a | 4.5 ± 0.128a |
| RCc3p‐AEY‐3 | 104.6 ± 1.037a | 11.0 ± 0.728a | 23.0 ± 0.354a | 22.9 ± 0.761a | 4.5 ± 0.152a |
| RCc3p‐AEY‐6 | 105.4 ± 1.525a | 11.4 ± 0.909a | 22.6 ± 0.671a | 22.1 ± 0.564a | 4.4 ± 0.113a |
| RCc3p‐AEY‐9 | 106.0 ± 1.275a | 11.3 ± 1.085a | 22.6 ± 0.570a | 23.0 ± 0.715a | 4.6 ± 0.143a |
| RCc3p‐AEY‐10 | 104.6 ± 0.908a | 11.1 ± 0.890a | 23.0 ± 0.790a | 22.3 ± 0.604a | 4.4 ± 1.121a |
Conclusion
Rice is a staple food for billions of people in Asia, and its consumption is increasing in Europe (http://airea.net/images/siteimages/EU27-Rice.pdf) and the USA because it is regarded as a healthy, gluten‐free alternative source of carbohydrates (Batres‐Marquez et al., 2009). However, the As content of rice poses a serious problem (Schoof et al., 1999). Particularly alarming are baby foods based on rice, which were considered to be nutritious and safe, but in fact contain relatively high amounts of As and can cause diseases and developmental problems in children (Karagas et al., 2016). Therefore, the As content in rice grains is a critical issue for human health. Here, we successfully developed a strategy to reduce As in rice grains by enhancing As vacuolar sequestration through the transgenic expression of OsABCC1, ScYCF1 and γ‐glutamylcysteine synthetase, while preserving grain yield. The two ABC transporters for As vacuolar compartmentation were driven by OsRCc3p, a tissue‐specific promoter driving expression in the root cortical cell layer, phloem cells of nodes and internodes. Root‐to‐shoot As translocation was greatly reduced in the transgenic plants due to the elevated vacuolar sequestration of As in root cortical cells. This sequestration probably inhibits the radial translocation of As from the cortex to the endodermis, where Lsi2, a major As efflux transporter localized to the proximal side of the endodermal layer, loads As into the xylem (Figure 4). Furthermore, the reduced shoot‐to‐grain translocation of As mediated by the transgenically expressed OsABCC1 and ScYCF1 in the phloem regions of nodes and internodes also contributed to the decreased As accumulation in grain (Figure 4). These results suggest that sequestering As into tissues involved in As translocation is an important step for developing low As rice. However, if these plants are grown in paddy fields containing excess As, their high vacuolar sequestration of As in nongrain tissues leads to hyperaccumulation and consequential cell damage, especially in the root. Therefore, we plan to improve our As‐safe rice by increasing the root's As extrusion activity into the rhizosphere.
Figure 4.

Vacuolar sequestration of As to reduce the amount of As in the grain. WT plants efficiently take up and translocate As into the xylem through the Lsi1 (green colour) and Lsi2 (yellow colour) transporters located in the root exodermis and endodermis. However, RCc3p‐AEY plants sequester As (blue colour) into cortical cell vacuoles via OsABCC1 and ScYCF1 (pink colour). This sequestration inhibits radial translocation of As into the endodermis, where As is translocated into the xylem via Lsi2 and decreases As translocation to the shoot. Furthermore, in phloem cells of the internode, OsABCC1 and ScYCF1 sequester As into vacuoles and decrease As translocation to the grain.
Experimental procedures
Rice culture conditions
Rice seeds (Oryza sativa L.) were germinated on half‐strength Murashige and Skoog (1/2 MS) agar plates. After 10 days, the seedlings were transferred to normal soil with basal levels of As (2.8 ± 0.5 mg/kg dried soil; below the acceptable soil As limits in Korea (25 mg/kg)) or to half‐strength (1/2) Kimura B hydroponic medium (Yoshida, 1976) supplemented with 0.2 mm urea and cultured in a glasshouse at 25–35 °C under natural light or in a growth room at 28 °C under 16 h light/8 h dark conditions. To analyse As accumulation in the rice seedlings, the 4‐week‐old plants grown hydroponically in the growth room were treated with 1 μm As(III) and 0.1 μm Cd(II) for five days or 2 μm DMA(V) for two days. The shoots and roots were washed with ice‐cold water and harvested to measure their As and Cd contents. To investigate the As distribution at the reproductive stage, rice plants at the milk stage of grain development, which had been grown in the hydroponic medium in the glasshouse were treated with 1.0 μm As (III) for 10 days. Samples were prepared of the root, basal stem (1 cm), internodes, nodes, leaves, flag leaf, peduncle, rachis and brown rice to measure As and Cd contents.
Molecular cloning and construct design
To express OsABCC1, ScYCF1 and γ‐ECS genes, the rice RCc3 promoter (RCc3p), Zea mays ubiquitin promoter (ZmUBIp), and rice Actin2 promoters (ACTIN2p) were isolated using PCR. The rice RCc3 promoter (Xu et al., 1995) was amplified with a primer set (RCc3p‐F, RCc3p‐R) and a genomic DNA template from O. sativa (cv. Donging), while ZmUBIp and ACTIN2p were isolated using PCR with gene‐specific primer sets (ZmUBIp‐F and ZmUBIp‐R for ZmUBIp, Act2p‐F and Act2p‐R for ACTIN2p), using the pIPKb002 and pIPKb003 vectors as templates (Himmelbach et al., 2007). Sequences of all primers used are presented in Table S1. All PCR products were cloned using the pGEM‐T Easy Vector (Promega), and their sequence fidelities were confirmed by sequencing. To increase the number of multicloning sites available in the final vectors, restriction enzyme recognition sites were removed from the promoter sequences using site‐directed mutagenesis with gene‐specific primer sets; primers for the removal of the SacI and PmaCI sites in the RCc3 promoter were RCc3p_SacI‐1F, RCc3p_SacI‐1R, RCc3p_SacI‐2F, RCc3p_SacI‐2R, RCc3p_PmaCI‐F and RCc3p_PmaCI‐R; primers the for removal of the SalI, XhoI, SacI and EcoRI sites in ZmUBIp were ZmUBIp_SalI‐F, ZmUBIp_Sal I‐R, ZmUBIp_XhoI‐F, ZmUBIp_XhoI‐R, ZmUBIp_SacI‐F, ZmUBIp_SacI‐R, ZmUBIp_EcoRI‐F and ZmUBIp_EcoRI‐R; primers for the removal of the EcoRI, SacI and BamHI sites in ACTIN2p were Act2p_EcoRI‐F, Act2p_EcoRI‐R, Act2p_SacI‐F, Act2p_SacI‐R, Act2p_BamHI‐F and Act2p_BamHI‐R.
The modified ZmUBI promoter was subcloned into the BstXI and EcoRI sites of the pCAMBIA1300, pCAMBIA1302 and pBSK vectors (ZmUBIp1300, ZmUBIp1302, pBSK_ZmUBIp) or BamHI and EcoRI sites of the pBSK vector (pBSK_ZmUBIp‐1). The modified RCc3 promoter was subcloned into the BstXI and EcoRI sites of the pCAMBIA1300, pCAMBIA1302 and pBSK vectors (RCc3p1300, RCc3p1302, pBSK_RCc3p). ACTIN2p was inserted into the NcoI and HindIII sites of the pBSK vector to generate pBSK_Actp. Nos and 35S terminators were isolated from the pCAMBIA1302 vector using PCR and sequence‐specific primer sets (Nos_ter‐SalI and Nos_ter‐EcoRV, Nos_ter‐XbaI and Nos_ter‐BamHI, 35S_ter‐F and 35S_ter‐R) and then subcloned into the pGEM‐T Easy Vector. The Nos terminator was inserted into the SalI and EcoRV sites of the pBSK_RCc3p vector and pBSK_ZmUBIp, or the XbaI and BamHI sites of the pBSK_ZmUBIp‐1 vector (pBSK_ZmUBIp_Noster, pBSK_RCc3p_Noster, pBSK_ZmUBIp‐1_Noster). The 35S terminator was subcloned into the XbaI and PstI sites of the pBSK_Actp vector (pBSK_Actp_35Ster).
Full‐length OsABCC1‐V5 was prepared from the pGK‐OsABCC1 vector (Song et al., 2014), inserted into the BamHI and XbaI sites of ZmUBIp1300, ZmUBIp1302, RCc3p1300 and RCc3p1302 to produce ZmUBIp::OsABCC1‐V5 (UBIp‐A) and RCc3p::OsABCC1‐V5 (RCc3p‐A). Escherichia coli γ‐ECS (Zhu et al., 1999) was isolated from the wild‐type strain (K12) using PCR with a sequence‐specific primer set (γ‐ECS_HindIII‐F, γ‐ECS_SalI‐R) and subcloned into the pGEM‐T Easy Vector for sequencing. The confirmed γ‐ECS gene was inserted into the HindIII and SalI sites of the pBSK_ZmUBIp‐1_Noster and pBSK_Actp_35Ster vectors (pBSK_ZmUBIp‐1_γ‐ECS_Noster and pBSK_Actp_γ‐ECS_35Ster). To combine the OsABCC1‐V5 and γ‐ECS genes in a single vector, the fragments of the 35S terminator‐ACTIN2p‐γ‐ECS (SalI and Ecl136II fragments from the pBSK_Actp_γ‐ECS_35Ster vector) and Nos terminator‐UBI promoter‐γ‐ECS (SalI and Ecl126II from the pBSK_ZmUBIp‐1_γ‐ECS_Noster vector) were inserted into the SalI and PmlI sites of the ZmUBIp1301ABCC1‐V5 and RCc3p1301ABCC1‐V5 vectors, and the RCc3p‐AE (RCc3p::OsABCC1‐V5, ZmUBIp::γ‐ECS) and UBIp‐AE (ZmUBIp::OsABCC1‐V5, ACTIN2p::γ‐ECS) vectors were constructed.
To express ScYCF1 in rice, codon‐optimized ScYCF1 (YCF1) was synthesized and cloned into the EcoRI/XhoI sites of a pGK yeast shuttle vector. YCF1 was subcloned into the BamHI and XbaI sites of ZmUBIp1300 and RCc3p1300 to construct the ZmUBIp::YCF1 and RCc3p::YCF1 vectors or inserted into the EcoRI and XbaI sites of the pBSK_ZmUBIp‐1_Noster and pBSK_RCc3p‐1_Noster vectors to produce pBSK_ZmUBIp‐1_YCF1_Noster and pBSK_RCc3p‐1_YCF1_Noster.
To generate the RCc3p‐AEY (RCc3p::OsABCC1‐V5, ZmUBIp::γ‐ECS, RCc3p::ScYCF1) vector, the two fragments of Nos terminator‐ZmUBIp‐γ‐ECS (XbaI and SalI) from the pBSK_ZmUBIp_γ‐ECS_Noster vector and Noster‐RCc3pYCF1 (SalI and Ecl136 II) from pBSK_RCc3p‐1_YCF1_Noster were inserted into the XbaI and PmlI sites of the ZmUBIp1301ABCC1‐V5 vector. The UBIp‐AEY (ZmUBIp::OsABCC1‐V5, ACTIN2p::γ‐ECS, ZmUBIp::ScYCF1) vector was constructed by ligating three fragments; the 35S terminator‐ACTIN2p‐γ‐ECS (XbaI and SalI) from pBSK_Actp_γ‐ECS_35Ster vector, Noster‐ZmUBI promoter‐YCF1 (SalI and Ecl136II) from pBSK_ZmUBIp‐1_YCF1_Noster vector and the ZmUBIp1301ABCC1‐V5 (XbaI and PmlI) vector.
The coding region of the β‐Glucuronidase (GUS) gene was amplified by PCR with the pBI121 vector as template and the primer set GUS_EcoRI‐F and GUS_XbaI‐R, and then subcloned into the pGEM‐T Easy Vector. This gene was then excised with EcoRI and XbaI and inserted into the EcoRI and XbaI sites of the RCc3p1300 and ZmUBIp1300 vectors, respectively, to generate RCc3p::GUS and ZmUBIp::GUS.
Generation of transgenic rice and expression analysis
The Dongjin cultivar, a currently cultivated japonica rice variety, was used for transformation mediated by Agrobacterium tumefaciens, as described previously (Hiei et al., 1994; Jeon et al., 1999), with the following modifications. Four‐week‐old calli were generated from the scutellum and cocultivated with the Agrobacterium tumefaciens strain GV3101 carrying the binary vectors (RCc3p‐A, RCc3p‐AE, RCc3p‐AEY, UBIp‐A, UBIp‐AE, UBIp‐AEY, RCc3p‐GUS or UBIp‐GUS) on 2N6 medium containing acetosyringone for 2–3 days in darkness at 25 °C. The calli were then washed three times with sterile water containing 100 mg/L cefotaxime, incubated for one day in liquid 2N6 medium supplemented with 50 mg/L hygromycin B and 250 mg/L cefotaxime, washed with sterile water and then incubated at 27 °C on 2N6 solid medium containing 50 mg/L hygromycin B and 250 mg/L cefotaxime for 4–5 weeks. The hygromycin‐resistant calli were transferred onto a preregeneration medium (2N6‐BA) containing 50 mg/L hygromycin B and 250 mg/L cefotaxime, where they were grown for 10 days before being transferred to a regeneration medium containing 25 mg/L hygromycin B and 250 mg/L cefotaxime for 4–6 weeks. The regenerated plants were grown in a 1/2 Kimura B hydroponic medium for 2 weeks and then transferred to soil. The expression of OsRCc3 was investigated in the roots and node I of WT with primer sets (RCc3‐RT‐F and RCc3‐RT‐R, OsActin‐F, and OsActin‐R). To confirm the insertion of the transgenic genes into the rice genome, a genomic PCR was performed using gene‐specific primer sets (mOsABCC1‐F and mOsABCC1‐R for exogenous OsABCC1; mYCF1‐F and mYCF1‐R for YCF1; γECS‐F and γ‐ECS‐R for γ‐ECS). To examine whether the transformed genes were successfully expressed, qRT‐PCR was performed using Thermal Cycler Dice Real Time System (TP‐800, Takara Co., Japan) with gene‐specific primer sets (mOsABCC1‐F, mOsABCC1‐R, mYCF1‐F, mYCF1‐R, γ‐ECS‐F, γ‐ECS‐R, OsActin‐F and OsActin‐R).
Stem As feeding assays
To investigate As translocation from the internodes or nodes to the grains, As feeding assays were performed following a previously published method (Song et al., 2014). The plants of the WT and RCc3p‐AEY lines were grown hydroponically until 10 days after flowering (milk stage of grain development). The stems were cut below node II, and then, internode II was immersed in a 250‐mL flask containing 80 mL 1/2 Kimura medium supplemented with 10 μm As (III) and 10 μm Rb (I) as the control metal for 24 h. Each organ was harvested to measure its As content.
Determination of As and Cd concentrations in the xylem sap
To analyse the translocation of As and Cd from the root to the shoot, 4‐week‐old hydroponically grown rice seedlings were treated with 5.0 μm As (III) and 0.2 μm Cd for 4 h. The shoot was decapitated with a razor blade, and the xylem sap was collected with a micropipette for 45 min. The samples of collected sap were diluted to an equal volume with 5% HNO3.
Histochemical and immunohistochemical localization
To investigate the tissue‐specific RCc3‐ or ZmUBI‐driven expression of exogenous OsABCC1 and ScYCF1 in the transgenic plants, histochemical and immunohistochemical assays were performed in various tissues of RCc3p::GUS and ZmUBIp::GUS plants. Samples of the root, basal node, leaf sheath and leaf blade tissues were prepared from 2‐ to 3‐week‐old plants grown in a 1/2 Kimura B hydroponic medium, while samples of node I and the internode were collected from plants grown in soil during the grain‐filling stage. A GUS histochemical assay was performed as described previously (Jeon et al., 1999). After staining, the samples were dehydrated by sequentially soaking them in an ethanol series (50%, 70%, 90%, 95%, 100%). The hand‐sectioned samples were observed under a dissecting microscope (Olympus).
Immunostaining was performed using a GUS antibody (rabbit IgG antibody fraction; A‐5790, Molecular Probes), as described previously (Deng et al., 2013). Briefly, samples of the root, basal node, internode and node I were fixed using a fixation solution, sectioned to a 150‐μm thickness and treated with anti‐GUS. The fluorescence from the secondary antibody (Alexa Fluor 555 goat anti‐rabbit IgG; A21428, Molecular Probes) was observed using confocal laser‐scanning microscopy (Olympus FV‐1000 or Carl Zeiss LSM700).
Thiol localization in the root
To determine thiol accumulation in the root, monobromobimane (mBB) staining was performed following the published method (Song et al., 2014). Roots were collected from 2‐ to 3‐week‐old plants treated with 1 μm As(III) for 3 h, incubated at room temperature in PBS buffer containing 15 μm mBB and 20 μm propidium iodide (PPI) for 30 min and then washed with PBS buffer. The fluorescence signal from mBB and PPI was observed using a confocal laser microscope (Olympus FV‐1000) and quantified using the ImageJ program (Abramoff et al., 2004).
Immunoblot analysis
Microsome isolation and fractionation were performed according to a method described previously (Huang et al., 2016). The suspended microsomes were fractionated using discontinuous sucrose gradients (20%–60% sucrose in 10 mm Tris‐HCl, pH 7.6, 1 mm EDTA, and 1 mm DTT) by ultracentrifugation at 100 000 g for 18 h, which separated each membrane protein. After the fractionated membranes were recovered by a further ultracentrifugation at 100 000 g for 1 h, equal amounts of the samples (20 μg for the OsABCC1 and V5 detection; 5 μg for the detection of V‐ATPase, H+‐ATPase, and Bip) were incubated at 37 °C for 30 min. The samples were electrophoresed under the SDS‐PAGE gels (6% for OsABCC1 and ABCC1‐V5; 10% for H+‐ATPase, V‐ATPase and Bip) and transferred onto a nitrocellulose blotting membrane (GE Healthcare Life Sciences). The membranes were cross‐reacted with anti‐V5‐conjugated horseradish peroxidase (HRP) (R961‐25; Thermo Fisher Scientific; 5000‐times dilution), anti‐V‐ATPase (AS07213; Agrisera; 5000‐times dilution), anti‐H+‐ATPase (AS07260; Agrisera; 5000‐times dilution) and anti‐Bip (COP‐080017; Cosmo Bio; 5000‐times dilution). Anti‐rabbit antibody conjugated with HRP (Promega; 10 000‐times dilution) was used as a secondary antibody, and the ECL Plus Western Blotting Detection System (32209; Thermo Fisher Scientific) was used to detect the chemiluminescence under an Image Quant LAS 4000 (GE Healthcare Life Sciences).
Assay of As accumulation pattern in root
To compare the As accumulation patterns in the roots of the wild‐type and RCc3p‐AEY lines, a stepwise grinding method using liquid nitrogen was employed to separate the epidermis, cortex and vascular cell layers, as previously described (Song et al., 2011). The samples were analysed for As concentration, protein quantification was performed to normalize As concentration, and a qRT‐PCR analysis was used to determine the source of the cell layers in the root. The epidermis‐specific gene OsZIP1 was used as a marker to determine the cell types of the isolated samples (Ogo et al., 2014). The primers used to detect OsZIP1 expression are listed in Table S1.
As measurement
To analyse As accumulation in grains and other organs, dried tissues were digested with 2–4 mL 65% HNO3 at 150 °C for 10–15 h. The metal(loid) concentrations were determined using an inductively coupled plasma mass spectrometer (ICP‐MS; Perkin Elmer).
Grain yield investigation
To examine the grain yield of the transgenic rice plants, four independent RCc3p‐AEY lines and WT plants were cultivated in rice paddy fields located at the Kyung‐Hee University from May to October 2017. At harvest, the grain yield‐related agronomic traits were analysed.
Statistical analyses
To analyse the statistical significance of the data, two‐tailed Student's t‐tests (significance level of *P < 0.05 or **P < 0.01) were performed using Microsoft Excel, or a one‐way analysis of variance (ANOVA, significance level of P < 0.05) was performed using GraphPad Prism 4.
Supporting information
Figure S1 Promoter‐GUS assays in plants transformed with RCc3 promoter::GUS (RCc3p::GUS) or ZmUBI promoter::GUS (UBIp::GUS).
Figure S2 Promoter‐GUS assays in plants transformed with ZmUBI promoter::GUS (UBIp::GUS).
Figure S3 Development of transgenic plants transformed with RCc3p‐A (RCc3 pro::OsABCC1‐V5), RCc3p‐AE (RCc3 pro::OsABCC1‐V5, ZmUBI pro::γ‐ECS), or RCc3p‐AEY (RCc3 pro::OsABCC1‐V5, ZmUBI pro::γ‐ECS, RCc3 pro::ScYCF1).
Figure S4 Development of transgenic plants transformed with UBIp‐A (ZmUBI pro::OsABCC1‐V5), UBIp‐AE (ZmUBI pro::OsABCC1‐V5, OsActin2 pro::γ‐ECS), or UBIp‐AEY (ZmUBI pro::OsABCC1‐V5, OsActin2 pro::γ‐ECS, ZmUBI pro::ScYCF1).
Figure S5 Cd and As accumulation in brown rice and flag leaves from T3 transgenic plants grown in soil.
Figure S6 As concentration in roots and shoots of transgenic rice seedlings.
Figure S7 Phenotypic analysis of the transgenic rice seedlings and yeast
strains subjected to the DMA and Cd treatments.
Figure S8 As accumulation and thiols contents in roots.
Figure S9 Reduced As translocation to grains of RCc3p‐AEY plants.
Table S1 Primers used in this study.
Acknowledgements
This work was supported by grants from the BK21 PLUS program funded by the Ministry of Education, Korea (10Z20130012243), the Next‐Generation BioGreen 21 program from Rural Development Administration, Korea (PJ011926 to W‐Y.S.), NRF grants funded by the Korean government (MSIP) (NRF‐2016R1A2B4012478 to W‐Y.S., NRF‐2015R1A2A1A01004294 to Y.L), and a grant for Specially Promoted Research (JSPS KAKENHI Grant Number 16H06296 to J.F.M.). We thank the POCEB at Pohang Techno Park for the use of the ICP‐MS (NexIon300x, Perkin Elmer) facility. The authors declare competing financial interests: ‘Composition for reducing arsenic accumulation’ is the subject of a Korea patent (10‐2017‐0060054 on May 15, 2017) applied by W‐Y.S, Y.L and F.D.
Contributor Information
Youngsook Lee, Email: ylee@postech.ac.kr.
Won‐Yong Song, Email: songwy@postech.ac.kr.
References
- Abramoff, M.D. , Magelhaes, P.J. and Ram, S.J. (2004) Image processing with ImageJ. Biophoton. Int. 11, 36–42. [Google Scholar]
- Arao, T. , Kawasaki, A. , Baba, K. , Mori, S. and Matsumoto, S. (2009) Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ. Sci. Technol. 43, 9361–9367. [DOI] [PubMed] [Google Scholar]
- Batres‐Marquez, S.P. , Jensen, H.H. and Upton, J. (2009) Rice consumption in the United States: recent evidence from food consumption surveys. J. Am. Diet. Assoc. 109, 1719–1727. [DOI] [PubMed] [Google Scholar]
- Bowell, R.J. , Alpers, C.N. , Jamieson, H.E. , Nordstrom, D.K. and Majzlan, J. (2014) The environmental geochemistry of arsenic‐an overview. Rev. Mineral. Geochem. 79, 1–16. [Google Scholar]
- Chen, Y. , Moore, K.L. , Miller, A.J. , McGrath, S.P. , Ma, J.F. and Zhao, F.J. (2015) The role of nodes in arsenic storage and distribution in rice. J. Exp. Bot. 66, 3717–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, F. , Yamaji, N. , Xia, J. and Ma, J.F. (2013) A member of the heavy metal P‐type ATPase OsHMA5 is involved in xylem loading of copper in rice. Plant Physiol. 163, 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, M. , Shen, J. and Rosen, B.P. (1999) Pathways of As(III) detoxification in Saccharomyces cerevisiae . Proc. Natl. Acad. Sci. USA, 96, 5001–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei, Y. , Ohta, S. , Komari, T. and Kumashiro, T. (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T‐DNA. Plant J. 6, 271–282. [DOI] [PubMed] [Google Scholar]
- Himmelbach, A. , Zierold, U. , Hensel, G. , Riechen, J. , Douchkov, D. , Schweizer, P. and Kumlehn, J. (2007) A set of modular binary vectors for transformation of cereals. Plant Physiol. 145, 1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, X.Y. , Deng, F. , Yamaji, N. , Pinson, S.R. , Fujii‐Kashino, M. , Danku, J. , Douglas, A. et al. (2016) A heavy metal P‐type ATPase OsHMA4 prevents copper accumulation in rice grain. Nat. Commun. 7, 12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, J.S. , Chung, Y.Y. , Lee, S. , Yi, G.H. , Oh, B.G. and An, G. (1999) Isolation and characterization of an anther‐specific gene, RA8, from rice (Oryza sativa L.). Plant Mol. Biol. 39, 35–44. [DOI] [PubMed] [Google Scholar]
- Karagas, M.R. , Punshon, T. , Sayarath, V. , Jackson, B.P. , Folt, C.L. and Cottingham, K.L. (2016) Association of rice and rice‐product consumption with arsenic exposure early in life. JAMA Pediatr. 170, 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, N. , Wang, J. and Song, W.Y. (2016) Arsenic uptake and translocation in plants. Plant Cell Physiol. 57, 4–13. [DOI] [PubMed] [Google Scholar]
- Ma, J.F. , Yamaji, N. , Mitani, N. , Tamai, K. , Konishi, S. , Fujiwara, T. , Katsuhara, M. et al. (2007) An efflux transporter of silicon in rice. Nature, 448, 209–212. [DOI] [PubMed] [Google Scholar]
- Ma, J.F. , Yamaji, N. , Mitani, N. , Xu, X.Y. , Su, Y.H. , McGrath, S.P. and Zhao, F.J. (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. USA, 105, 9931–9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meharg, A.A. (2004) Arsenic in rice—understanding a new disaster for South‐East Asia. Trends Plant Sci. 9, 415–417. [DOI] [PubMed] [Google Scholar]
- Meharg, A.A. , Williams, P.N. , Adomako, E. , Lawgali, Y.Y. , Deacon, C. , Villada, A. , Cambell, R.C. et al. (2009) Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ. Sci. Technol. 43, 1612–1617. [DOI] [PubMed] [Google Scholar]
- Moore, K.L. , Schröder, M. , Wu, Z. , Martin, B.G. , Hawes, C.R. , McGrath, S.P. , Hawkesford, M.J. et al. (2011) High‐resolution secondary ion mass spectrometry reveals the contrasting subcellular distribution of arsenic and silicon in rice roots. Plant Physiol. 156, 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, K.L. , Chen, Y. , van de Meene, A.M. , Hughes, L. , Liu, W. , Geraki, T. , Mosselmans, F. et al. (2014) Combined NanoSIMS and synchrotron X‐ray fluorescence reveal distinct cellular and subcellular distribution patterns of trace elements in rice tissues. New Phytol. 201, 104–115. [DOI] [PubMed] [Google Scholar]
- Ogo, Y. , Kakei, Y. , Itai, R.N. , Kobayashi, T. , Nakanishi, H. , Takahashi, H. , Nakazono, M. et al. (2014) Spatial transcriptomes of iron‐deficient and cadmium‐stressed rice. New Phytol. 201, 781–794. [DOI] [PubMed] [Google Scholar]
- Punshon, T. , Jackson, B.P. , Meharg, A.A. , Warczack, T. , Scheckel, K. and Guerinot, M.L. (2017) Understanding arsenic dynamics in agronomic systems to predict and prevent uptake by crop plants. Sci. Total Environ. 581–582, 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab, A. , Feldmann, J. and Meharg, A.A. (2004) The nature of arsenic–phytochelatin complexes in Holcus lanatus and Pteris cretica . Plant Physiol. 134, 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof, R.A. , Yost, L.J. , Eickhoff, J. , Crecelius, E.A. , Cragin, D.W. , Meacher, D.M. and Menzel, D.B. (1999) A market basket survey of inorganic arsenic in food. Food Chem. Toxicol. 37, 839–846. [DOI] [PubMed] [Google Scholar]
- Shi, S. , Wang, T. , Chen, Z. , Tang, Z. , Wu, Z. , Salt, D.E. , Chao, D.Y. et al. (2016) OsHAC1;1 and OsHAC1;2 Function as arsenate reductases and regulate arsenic accumulation. Plant Physiol. 172, 1708–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim, D. , Kim, S. , Choi, Y.I. , Song, W.Y. , Park, J. , Youk, E.S. , Jeong, S.C. et al. (2013) Transgenic poplar trees expressing yeast cadmium factor 1 exhibit the characteristics necessary for the phytoremediation of mine tailing soil. Chemosphere, 90, 1478–1486. [DOI] [PubMed] [Google Scholar]
- Song, W.Y. , Sohn, E.J. , Martinoia, E. , Lee, Y.J. , Yang, Y.Y. , Jasinski, M. , Forestier, C. et al. (2003) Engineering tolerance and accumulation of lead and cadmium in transgenic plants. Nat. Biotechnol. 21, 914–919. [DOI] [PubMed] [Google Scholar]
- Song, W.Y. , Park, J. , Mendoza‐Cózatl, D.G. , Suter‐Grotemeyer, M. , Shim, D. , Hörtensteiner, S. , Geisle, M. et al. (2010) Arsenic tolerance in Arabidopsis is mediated by two ABCC‐type phytochelatin transporters. Proc. Natl. Acad. Sci. USA, 107, 21187–21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W.Y. , Choi, K.S. , de Alexis, A. , Martinoia, E. and Lee, Y. (2011) Brassica juncea plant cadmium resistance 1 protein (BjPCR1) facilitates the radial transport of calcium in the root. Proc. Natl. Acad. Sci. USA, 108, 19808–19813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, W.Y. , Yamaki, T. , Yamaji, N. , Ko, D. , Jung, K.H. , Fujii‐Kashino, M. , An, G. et al. (2014) A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl. Acad. Sci. USA, 111, 15699–15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, L. , Zheng, M. , Liu, H. , Peng, S. , Huang, J. , Cui, K. and Nie, L. (2014) Water management practices affect arsenic and cadmium accumulation in rice grains. Scientific World J. 24, 596438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno, D. , Yamaji, N. , Kono, I. , Haung, C.F. , Ando, T. , Yano, M. , Ma, J.F. (2010) Gene limiting cadmium accumulation in rice. Proc. Natl. Acad. Sci. USA, 107, 16500–16505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H.Y. , Wen, S.L. , Chen, P. , Zhang, L. , Cen, K. and Sun, G.X. (2016a) Mitigation of cadmium and arsenic in rice grain by applying different silicon fertilizers in contaminated fields. Environ. Sci. Pollut. Res. Int. 23, 3781–3788. [DOI] [PubMed] [Google Scholar]
- Wang, P. , Zhang, W. , Mao, C. , Xu, G. and Zhao, F.J. (2016b) The role of OsPT8 in arsenate uptake and varietal difference in arsenate tolerance in rice. J. Exp. Bot. 67, 6051–6059. [DOI] [PubMed] [Google Scholar]
- Williams, P.N. , Deacon, C. , Raab, A. , Figuerola, J. , Green, A.J. , Feldmann, J. and Meharg, A.A. (2007) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ. Sci. Technol. 41, 6854–6859. [DOI] [PubMed] [Google Scholar]
- Xu, Y. , Buchholz, W.G. , DeRose, R.T. and Hall, T.C. (1995) Characterization of a rice gene family encoding root‐specific proteins. Plant Mol. Biol. 27, 237–248. [DOI] [PubMed] [Google Scholar]
- Xu, J. , Shi, S. , Wang, L. , Tang, Z. , Lv, T. , Zhu, X. , Ding, X. et al. (2017) OsHAC4 is critical for arsenate tolerance and regulates arsenic accumulation in rice. New Phytol. 215, 1090–1101 10.1111/nph.14572. [DOI] [PubMed] [Google Scholar]
- Yang, J. , Gao, M.X. , Hu, H. , Ding, X.M. , Lin, H.W. , Wang, L. , Xu, J.M. et al. (2016) OsCLT1, a CRT‐like transporter 1, is required for glutathione homeostasis and arsenic tolerance in rice. New Phytol. 211, 658–670. [DOI] [PubMed] [Google Scholar]
- Yoshida, S. (1976) Routine procedure for growing rice plants in culture solution. In Laboratory Manual for Physiological Studies of Rice ( Yoshida, S. , Forno, D.A. , Cook, J.H. and Gomez, K.A. eds.), pp. 61–66. Philippines: International rice research institute. [Google Scholar]
- Zhao, F.J. , McGrath, S.P. and Meharg, A.A. (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu. Rev. Plant Biol. 61, 535–559. [DOI] [PubMed] [Google Scholar]
- Zhu, Y.L. , Pilon‐Smits, E.A. , Weber, S.U. , Jouanin, L. and Terry, N. (1999) Cadmium tolerance and accumulation in Indian mustard is enhanced by overexpressing γ‐glutamylcysteine synthetase. Plant Physiol. 121, 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Promoter‐GUS assays in plants transformed with RCc3 promoter::GUS (RCc3p::GUS) or ZmUBI promoter::GUS (UBIp::GUS).
Figure S2 Promoter‐GUS assays in plants transformed with ZmUBI promoter::GUS (UBIp::GUS).
Figure S3 Development of transgenic plants transformed with RCc3p‐A (RCc3 pro::OsABCC1‐V5), RCc3p‐AE (RCc3 pro::OsABCC1‐V5, ZmUBI pro::γ‐ECS), or RCc3p‐AEY (RCc3 pro::OsABCC1‐V5, ZmUBI pro::γ‐ECS, RCc3 pro::ScYCF1).
Figure S4 Development of transgenic plants transformed with UBIp‐A (ZmUBI pro::OsABCC1‐V5), UBIp‐AE (ZmUBI pro::OsABCC1‐V5, OsActin2 pro::γ‐ECS), or UBIp‐AEY (ZmUBI pro::OsABCC1‐V5, OsActin2 pro::γ‐ECS, ZmUBI pro::ScYCF1).
Figure S5 Cd and As accumulation in brown rice and flag leaves from T3 transgenic plants grown in soil.
Figure S6 As concentration in roots and shoots of transgenic rice seedlings.
Figure S7 Phenotypic analysis of the transgenic rice seedlings and yeast
strains subjected to the DMA and Cd treatments.
Figure S8 As accumulation and thiols contents in roots.
Figure S9 Reduced As translocation to grains of RCc3p‐AEY plants.
Table S1 Primers used in this study.


