Abstract
During the month of Ramadan intermittent fasting (RIF), both dietary and sleep patterns are adversely affected to cope with the rituals of Ramadan. Literature suggests that sleep deprivation and alteration of dietary pattern and nutritional impairment affect the pulmonary structure and function. Pulmonary function during RIF was not explored earlier. The present study aimed to investigate the effects of RIF on pulmonary function tests (PFTs) in healthy young Muslim males. Fifty sedentary nonsmoking healthy young Muslim male individuals of 20 to 25 years of age without any history of pulmonary or other major diseases were recruited by simple random sampling from different parts of Kolkata, India. Participants completed the American Thoracic Society questionnaire to record their personal demographic data, health status, and consent to participate in the study. Expirograph and peak flow meter were used to record the pulmonary function parameters (PFTs). PFTs were within the normal range and did not show any significant variation during the RIF. Body height and body mass depicted significant correlation (p < .05, p < .001) with PFTs. Tidal volume, vital capacity, and peak expiratory flow rate had significant correlation (p < .05, p < .01, p < .001) with age. Simple and multiple regression equations were computed to predict PFTs in the studies population. RIF did not affect the normal range of PFTs in young Muslim males of Kolkata, India. Standard errors of estimate of the computed regression equations were substantially small enough to recommend these equations as norms to predict the PFTs in the studied population.
Keywords: FEV1, FEV1%, FVC, PEFR, Ramadan
Introduction
The major religious period of the Islamic calendar is Ramadan, when healthy postpubescent Muslims fast from sunrise to sunset for 4 weeks. Postpubescent Muslims abstain from all types of liquid or solid nutrient intake in the daytime as well as refrain from all unhealthy or aggressive behavior during this period of purification, internal meditation, and regeneration (Zerguini, Kirkendall, Junge, & Dvorak, 2007). Refrainment from eating and drinking (12-18 hours/day) during Ramadan intermittent fasting (RIF) is different from a normal daily routine (Moosavi, Kabir, Mogbimi, Chebrei, & Mohammed, 2007) and it may cause physiological and psychological perturbations that can have detrimental effects on normal physiological activities of the body. RIF influences substrate availability and utilization which induce acute diurnal dehydration that, in turn, affects physiological functions (Siddiqui, Sabir, & Subhan, 2005; Waterhouse, 2010). Debatable variations in body weight and body composition during Ramadan have also been reported (Meckel, Ismaeel, & Eliakim, 2008). Few studies reported the effects of RIF on performance and fitness parameters in trained and untrained individuals (Aziz, Wahid, Png, & Jesuvadian, 2010; Chaouachi et al., 2009; Roy & Bandyopadhyay, 2015; Siddiqui et al., 2005; Zerguini et al., 2007), whereas other studies reported the relationship of fasting with physiological disorders like diabetes mellitus (Omar & Motata, 1997), thyroid malfunction (Sajid & Aktar, 1991), changes in serum uric acid level (Nomani & Hallak, 1990), and so on. Significant gradual rise in thyroid-stimulating hormone throughout the fasting month has been reported although the mean levels remained within normal limits. Pre-Ramadan levels were reattained well after the end of Ramadan. There was no significant change in T3 and T4 levels (Sajid & Aktar, 1991). An increase in uric acid was reported during the month of Ramadan (Meckel et al., 2008). However, some studies reported a significant decrease in blood glucose level (; Mirzaei, Rahamani-nia, Moghadam, Ziyaolhgh, & Rezaei, 2012; Ziaee et al., 2006), while Mansi (2007) reported a significant increase in the blood glucose level during the month of Ramadan.
Pulmonary function tests (PFTs) are one of the major components of physical fitness (Roy et al., 2014). Spirometry provides the values of different pulmonary function measurements and it is an important and cost effective diagnostic tool that is widely used in pulmonology, public health screening, occupational therapies, and sports medicine (Siddiqui et al., 2005). Literature suggests that sleep deprivation leads to significant deterioration of pulmonary function in healthy people (Phillips, Cooper, & Burke, 1987). Moreover, alteration of dietary pattern and nutritional impairment adversely affects pulmonary structure and function, neural control of breathing, and functioning of the respiratory muscles (Chin & Haponik, 1994; Riley & Thakker-Varia, 1995). Both dietary and sleep patterns are adversely affected during the month of RIF to cope up with the rituals of Ramadan. It is justified to speculate that RIF might have some impact on pulmonary function measurements in fasting Muslims, although the relevant data are scanty. Changes in different parameters of PFTs during RIF have been reported in different populations. Peak expiratory flow rate (PEFR) significantly decreased during RIF in Malaysians (Duncan, Husain, Raman, Cheah, & Ch’ng, 1990), whereas significant increase in post-Ramadan values of forced vital capacity (FVC) and forced expiratory flow rates (FEF75%, [FEF75%-85%]) was reported in Pakistani populations (Siddiqui et al., 2005; Subhan, Siddiqui, Khan, & Sabir, 2006). Significant increase in vital capacity (VC), forced expiratory volume in 1 second (FEV1), FEV1 as a percentage of FVC (FEV1%), PEFR, mid-expiratory flow during 75% of emited volume (MEF75%), mid-expiratory flow during 50% of emited volume (MEF50%) and maximum mid expiratory flow (MMEF) was reported in Iranians during Ramadan as well as in the post-Ramadan period (Moosavi et al., 2007). Similar data on the effects of RIF on pulmonary function measurements are unavailable in Indian Muslim males who undergo fasting during the holy month of Ramadan. Therefore, the present study was aimed to investigate the effects of RIF on PFTs in healthy young Muslim male subjects of Kolkata, India.
Method
Study Design
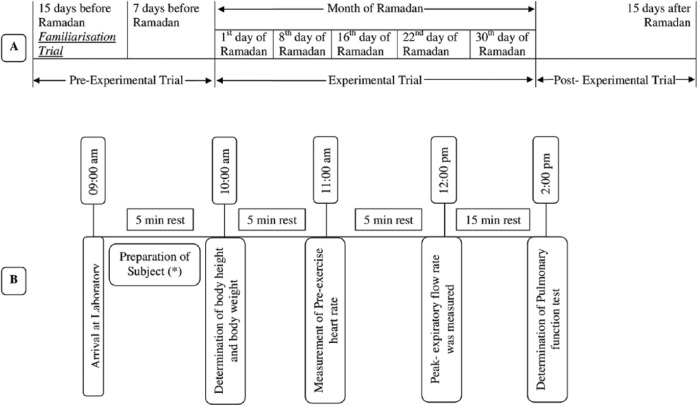
The entire study was conducted in the Sports and Exercise Physiology Laboratory, Department of Physiology, University of Calcutta from June 29 to July 27, 2014. Participants visited the Sports and Exercise Physiology Laboratory for a total of eight occasions which were divided into one familiarization trial, one preexperimental trial, five experimental trials, and one postexperimental trial. The familiarization trial, that is, the first study session, was conducted 15 days before the month of Ramadan to explain and demonstrate to the subjects the experimental requirement as well as to familiarize them with the experimental procedures. Pre-Ramadan data were collected 7 days before the month of Ramadan (preexperimental trial). The data from the five experimental trials were collected on the 1st, 8th, 16th, 22nd, and 30th days of the month of Ramadan. The remaining one session, that is, eighth session or postexperimental trial, was conducted 15 days after the month of Ramadan to record the post-Ramadan values of the PFTs. The entire experimental protocol has been diagrammatically represented in Figure 1.
Figure 1.
Diagrammatic representation of the experimental protocol: (A) experimental design, (B) sequence of tests performed in each session.
PFTs were measured at the same time of the day to avoid any chance of diurnal variation. Participants reported to the laboratory at 9 a.m. The age of each subject was calculated to the nearest year from the date of birth as obtained from their Photo ID issued by the Government of India. Physical parameters, namely, body height and body mass were measured with the subject standing barefoot and wearing minimum clothing on a weighing machine fitted with a height measuring rod (Avery India Ltd., India) with an accuracy of ±0.50 cm and ±0.1 kg, respectively.
Participant Recruitment
Fifty young Muslim untrained male subjects aged 20 to 24 years (M = 21.71 years, SD = 1.76 years) with body height ranging between 159.2 cm and 172.7 cm (M = 167.33 cm, SD = 6.19 cm) who were undergoing RIF were recruited from different parts of Kolkata, India as the experimental group (EG) of this time series study. The control group (CG) composed of 50 untrained Muslim male subjects belonging to same age range (M = 21.63 years, SD = 1.67 years) and having body height ranging between 160.7 cm and 172.8 cm (M = 166.96 cm, SD = 5.80 cm) who were not undergoing fasting during this period and not following the rituals of the month of Ramadan were recruited from the same community. The sample size was calculated using the method of Das and Das (1998), where the input of confidence interval was set as 95%. The study was conducted with 50 subjects in each group, which was greater than the computed sample size of 32 in each group.
The EG abstained from eating and drinking during all the sessions according to the religious recommendations and all of them strictly followed the religious rules of the holy month. Subjects of the CG maintained their normal daily activities including food and water intake but abstained from any heavy work on the days of evaluation as per the objective of the study.
All the subjects were healthy and belonged to a similar socioeconomic status. Subjects belonging to religions other than Muslim, doing regular exercise, and under medication or with any history of health complication were excluded from the study. The experimental protocol was explained and demonstrated to all of the subjects in the familiarization trial to allay their apprehension. Each subject signed the written informed consent form. Subjects completed the American Thoracic Society (1978) questionnaire to record their health status and personal demographic data. The entire study was approved by the Human Ethical Committee, Department of Physiology, University of Calcutta.
Determination of Pulmonary Function Tests
PFTs were recorded on a 9-L closed-circuit-type expirograph (Toshniwal Technologies Pvt. Ltd., India). The parameters measured were tidal volume (TV), VC, FVC, FEV1, FEV1%, mid-expiratory flow rate (FEF25%-75%), and forced expiratory time. The PEFR was recorded with the help of Wright peak flow meter. The expirograph was calibrated daily using a Palmer respiratory hand pump. All the measurements were conducted according to the procedure of Bandyopadhyay, Bhattacharjee, Dalui, and Pal (2013). Subjects were encouraged and motivated to attain maximum inspiratory and expiratory efforts. These tests were recorded at noon before lunch, as expiratory flow rates are highest at noon (Bandyopadhyay, 2011; Bandyopadhyay et al., 2013). For each volunteer, three satisfactory efforts were recorded with at least 3 to 5 minutes rest between the consecutive trials as per the standard norm (Bandyopadhyay, 2011). After a couple of practice runs, at least three trials were conducted of which the highest value was accepted (Bandyopadhyay, 2011). All pulmonary function measurements were expressed at body temperature and pressure saturated with water vapor. In one subject, all the records, that is, anthropometric measurements and recording of pulmonary function measurements were conducted in one sitting on the same day.
Statistical Analysis
Data were expressed as M ± SD. Changes in PFTs over time in both groups were determined by one-way repeated measure analysis of variance. Unpaired student’s t test was performed to test the significance of differences between mean values of CG and EG. Pearson’s product-moment correlation (r) was adopted to test the relationship of physical parameters with the PFTs in both groups. Simple and multiple regression analysis were also adopted to compute the norms for predicting pulmonary function measurements from different physical parameters in the studied population. The level of significance was set at p < .05. The statistical analysis was done by using SPSS 16 software.
Results
The values of PFTs recorded during all the sessions indicated that there was insignificant change in the studied pulmonary function parameters during the month of Ramadan (Table 1). There was no significant variation in the PFTs between the EG and the CG. Insignificant difference was also noted in the physical parameters when compared between the groups as well as when compared during different days of RIF separately in the EG and the CG, respectively. The values of correlation coefficient between physical parameters and the PFTs are reported in Table 2. The correlation statistic was performed by using the data that were obtained in both groups during the preexperimental trial, that is, 7 days before the month of Ramadan. Since there was insignificant intergroup variation in PFTs and the values of correlation coefficients in the CG and the EG were very close to each other, correlation coefficients were also computed in the aggregate of all sample (n = 100). VC, FVC, FEV1, FEV1%, and PEFR had significant correlation with age and body height, whereas body mass had significant correlation with FEV1 and FEV1%. Depending on these significant correlations of physical parameters with PFTs in the aggregate of all sample, the simple and multiple regression norms for prediction of PFTs from different physical parameters were computed by using the same preexperimental data of all the subjects. Since body height and age exhibited highest values of correlation coefficient with VC, FVC, FEV1, FEV1%, and PEFR, these two physical parameters were considered as the independent variables to compute the simple and multiple regression norms for prediction of PFTs in the studied population (Figure 2-Figure 7, Table 3).
Table 1.
Values of Pulmonary Function Measurements Before, During, and After the Month of Ramadan in Young Muslim Males of Kolkata, India.
| Pulmonary function measurement | Group | Preexperimental trial (7 days before RIF) | Experimental trials during the month
of Ramadan |
Postexperimental trial (15 days after the RIF) | ||||
|---|---|---|---|---|---|---|---|---|
| 1st Day | 8th Day | 16th Day | 22nd Day | 30th Day | ||||
| Body weight (kg) | C | 63.08 ± 9.46 | 63.90 ± 9.91 | 64.39 ± 10.34 | 63.45 ± 9.31 | 64.97 ± 9.70 | 63.32 ± 10.23 | 62.98 ± 8.89 |
| E | 62.1 ± 11.25 | 62.78 ± 10.19 | 61.98 ± 10.65 | 61.45 ± 10.03 | 61.73 ± 10.82 | 60.24 ± 9.89 | 60.12 ± 9.12 | |
| TV(mL) | C | 571.04 ± 51.06 | 568.97 ± 50.81 | 569.34 ± 51.76 | 567.20 ± 50.64 | 564.64 ± 51.90 | 570.12 ± 50.71 | 565.66 ± 51.38 |
| E | 583.32 ± 59.05 | 568.97 ± 40.81 | 552.34 ± 42.76 | 566.20 ± 40.64 | 558.64 ± 36.90 | 572.12 ± 38.71 | 558.36 ± 42.38 | |
| VC (L) | C | 4.12 ± 0.56 | 4.10 ± 0.52 | 3.98 ± 0.55 | 4.11 ± 0.49 | 4.15 ± 0.53 | 4.17 ± 0.51 | 4.14 ± 0.53 |
| E | 4.10 ± 0.53 | 4.16 ± 0.62 | 4.00 ± 0.56 | 4.14 ± 0.71 | 4.20 ± 0.57 | 4.08 ± 0.63 | 4.09 ± 0.52 | |
| FVC (L) | C | 3.87 ± 0.55 | 4.11 ± 0.67 | 4.01 ± 0.53 | 3.96 ± 0.64 | 4.12 ± 0.63 | 3.82 ± 0.62 | 4.06 ± 0.66 |
| E | 3.83 ± 0.52 | 4.00 ± 0.71 | 3.88 ± 0.71 | 4.12 ± 0.58 | 4.06 ± 0.51 | 3.90 ± 0.53 | 3.95 ± 0.58 | |
| FEV1 (L) | C | 3.53 ± 0.65 | 3.62 ± 0.68 | 3.56 ± 0.63 | 3.62 ± 0.55 | 3.71 ± 0.76 | 3.62 ± 0.64 | 3.58 ± 0.71 |
| E | 3.52 ± 0.62 | 3.76 ± 0.55 | 3.60 ± 0.71 | 3.84 ± 0.67 | 3.66 ± 0.63 | 3.70 ± 0.70 | 3.76 ± 0.56 | |
| FEV1% (%) | C | 90.84 ± 7.04 | 90.00 ± 6.86 | 92.78 ± 6.24 | 93.20 ± 5.62 | 94.56 ± 5.86 | 94.87 ± 6.43 | 94.53 ± 7.08 |
| E | 91.40 ± 6.94 | 94.00 ± 5.74 | 90.26 ± 5.92 | 91.20 ± 6.47 | 92.47 ± 6.52 | 93.06 ± 5.77 | 93.25 ± 5.80 | |
| FEF25%-75% (L/min) | C | 288.62 ± 87.77 | 265.86 ± 56.86 | 277.81 ± 55.32 | 272.00 ± 67.91 | 276.35 ± 70.00 | 278.46 ± 64.72 | 271.74 ± 59.03 |
| E | 278.46 ± 81.23 | 270.45 ± 60.86 | 283.81 ± 61.27 | 269.00 ± 58.39 | 280.71 ± 63.35 | 280.04 ± 57.57 | 280.57 ± 69.07 | |
| FEF75%-85% (L/min) | C | 75.54 ± 11.81 | 74.6 ± 12.17 | 70.16 ± 11.22 | 70.98 ± 12.34 | 73.61 ± 10.81 | 72.53 ± 12.16 | 75.89 ± 11.38 |
| E | 75.86 ± 17.52 | 76.96 ± 16.07 | 72.46 ± 15.28 | 72.71 ± 19.04 | 70.29 ± 20.18 | 73.77 ± 18.56 | 75.00 ± 17.77 | |
| PEFR (L/min) | C | 672.4 ± 60.47 | 675.3 ± 64.51 | 677.4 ± 66.58 | 680.3 ± 67.44 | 678.5 ± 62.34 | 672.3 ± 61.28 | 673.4 ± 69.72 |
| E | 677.8 ± 72.94 | 674.2 ± 72.23 | 682.5 ± 74.65 | 688.9 ± 73.87 | 668.2 ± 72.33 | 676.4 ± 71.46 | 674.9 ± 71.98 | |
Note. RIF = Ramadan intermittent fasting; C = control group; E = experimental group; TV = tidal volume; VC = vital capacity; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; FEV1% = FEV1 as a percentage of FVC; FEF = forced expiratory flow rate; PEFR = peak expiratory flow rate. Values are M ± SD. One-way repeated measure analysis of variance showed insignificant difference.
Table 2.
Values of Correlation Coefficients Between Pulmonary Function Measurements and Physical Parameters in Young Muslim Males of Kolkata, India.
| Pulmonary function measurements | Group | Age (years) | Body height (cm) | Body mass (kg) |
|---|---|---|---|---|
| VC (L) | C | .67*** | .89*** | .27 |
| E | .68*** | .80*** | .18 | |
| FVC (L) | C | .60*** | .86*** | .26 |
| E | .62*** | .80*** | .23 | |
| FEV1 (L) | C | .65*** | .88*** | .33* |
| E | .64*** | .83*** | .32* | |
| FEV1% (%) | C | .44** | .55*** | .33* |
| E | .41** | .58*** | .36* | |
| PEFR (L/min) | C | .31* | .70*** | .25 |
| E | .54*** | .85*** | .17 |
Note. VC = vital capacity; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; FEV1% = FEV1 as a percentage of FVC; PEFR = peak expiratory flow rate; C = control group; E = experimental group.
p < .05. **p < .01. ***p < .001.
Figure 2.
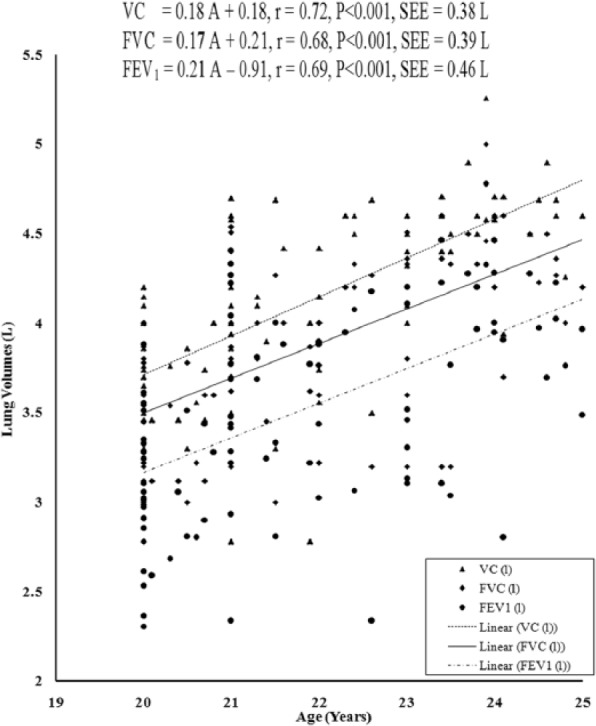
Relationship of VC, FVC, and FEV1 with age in the young Muslim males of Kolkata, India.
Note. VC = vital capacity; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second.
Figure 3.
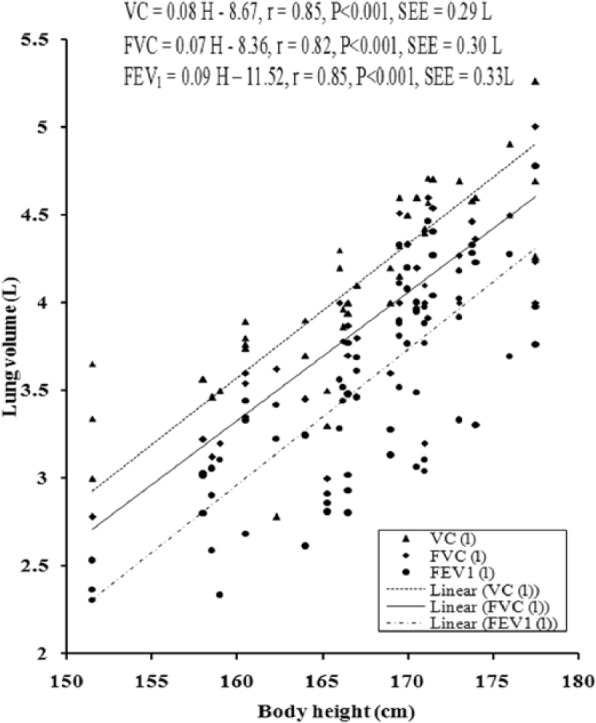
Relationship of VC, FVC, and FEV1 with body height in the young Muslim males of Kolkata, India.
Note. VC = vital capacity; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second.
Figure 4.
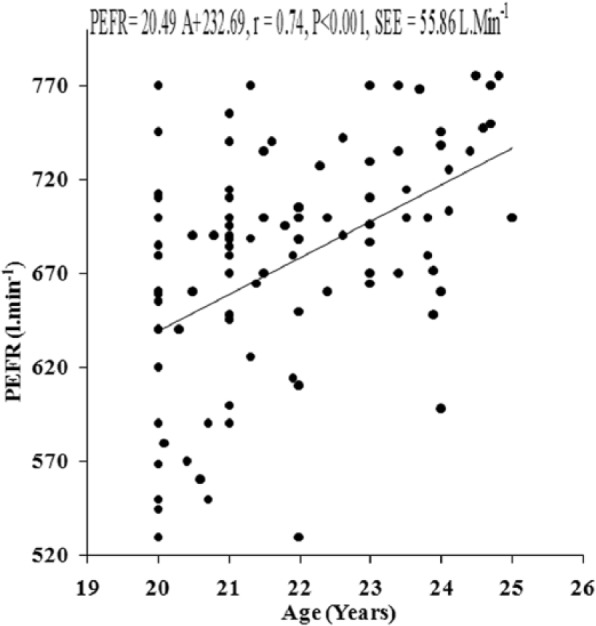
Relationship of PEFR with age in the young Muslim males of Kolkata, India.
Note. PEFR = peak expiratory flow rate.
Figure 5.
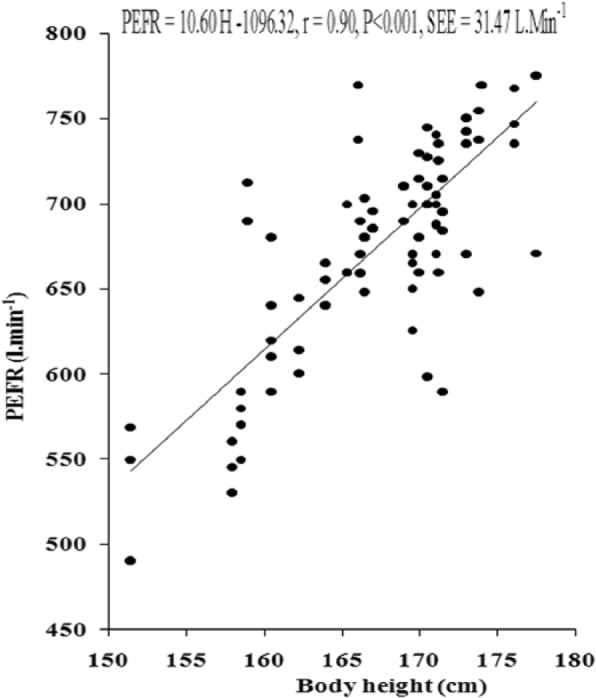
Relationship of PEFR with age in the young Muslim males of Kolkata, India.
Note. PEFR = peak expiratory flow rate.
Figure 6.
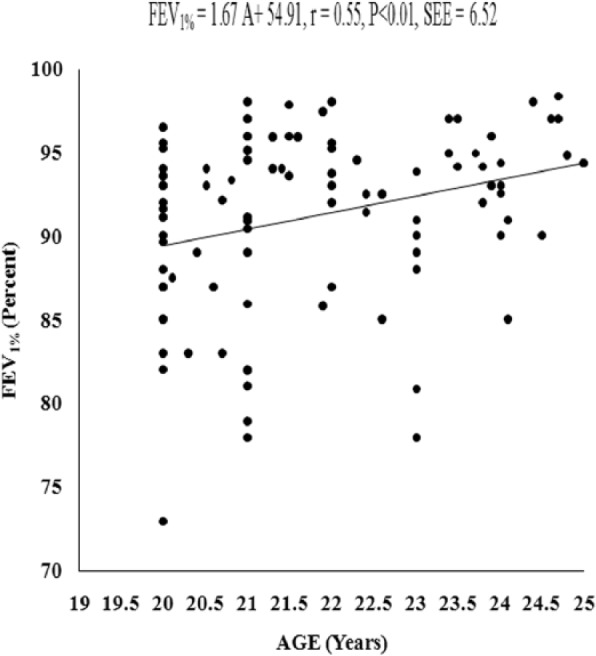
Relationship of FEV1% with age in the young Muslim males of Kolkata, India.
Note. FEV1% = FEV1 as a percentage of forced vital capacity.
Figure 7.
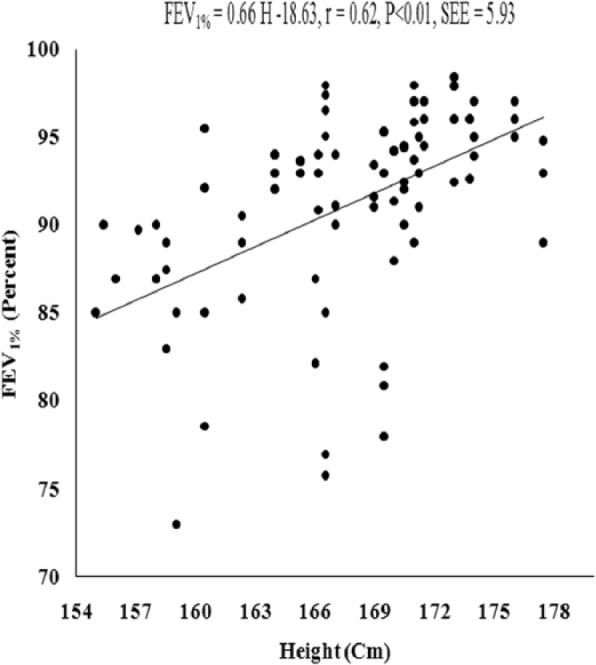
Relationship of FEV1% with body height in the young Muslim males of Kolkata, India.
Note. FEV1% = FEV1 as a percentage of forced vital capacity.
Table 3.
Multiple Regression Norms for the Prediction of Pulmonary Function Measurements in Young Muslim Males of Kolkata, India.
| Pulmonary function measurement | Regression equation | R | R 2 | SEE |
|---|---|---|---|---|
| VC (L) | VC = 0.07 H + 0.05 A − 8.67 | .85* | .7259 | 0.28 |
| FVC (L) | FVC = 0.07 H + 0.03 A − 8.50 | .82* | .6724 | 0.30 |
| FEV1 (L) | FEV1 = 0.08 H + 0.04 A − 9.92 | .85* | .7249 | 0.33 |
| FEV1% (%) | FEV1% = 0.20 H + 2.28 A + 8.15 | .68* | .4761 | 5.12 |
| PEFR (L/min) | PEFR = 11.71 H − 5.37 A − 1165.41 | .82* | .6724 | 40.41 |
Note. VC = vital capacity; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 second; FEV1% = FEV1 as a percentage of FVC; PEFR = peak expiratory flow rate; H = body height; A = age; SEE = standard errors of estimate.
p < .001.
Discussion
The present study was conducted to enumerate the effects of RIF on pulmonary function parameters and the pre-Ramadan values were considered as the baseline values for valid comparison with during Ramadan and post-Ramadan values. The study was further focused to compare the data with other national and international counterparts as well as to compute the population-specific regression norms for the prediction of PFTs in the studied community. It was observed that pulmonary function measurements were not influenced by the religious rituals of Ramadan in the studied population. The difference in PFTs between the CG and the EG in each trial was statistically insignificant. One-way repeated measure analysis of variance indicated that the difference in PFTs recorded before, during, and after the month of Ramadan were statistically insignificant in both the EG and the CG. Similar finding was also reported in earlier studies (Duncan et al., 1990; Subhan et al., 2006). Significant difference in FVC during RIF was reported in a Pakistani population (Siddiqui et al., 2005) and this finding contradicted the observation of the present study.
The present investigation depicted insignificant change in body mass during the month of Ramadan as also reported in earlier studies (Duncan et al., 1990; Sweileh, Schnitzler, Hunter, & Davis, 1992). Several factors attribute to the loss of body mass, for example, duration of fasting, diet, environmental condition, level of activity performed by the fasting individual, the season in which the Ramadan falls, level of dehydration, and so on (Siddiqui et al., 2005). Since there was no alteration in the body mass during the course of the current study, it might be postulated that these factors were also within the physiological limit during the study. Coexistence of significant variation in body mass (pre-Ramadan = 70.48 ± 2.20 kg, during Ramadan = 69.96 ± 2.22 kg, and post-Ramadan = 70.87 ± 2.14 kg) and FVC (pre-Ramadan = 4.61 ± 0.12 kg, during Ramadan = 4.70 ± 0.13 kg, and post-Ramadan = 4.54 ± 0.13 kg) during RIF has been reported in a Pakistani population (Siddiqui et al., 2005). It is a limitation that hydration status was not measured during the course of the present investigation. It has been reported that hypohydration causes significant increase in respiratory flow rates because loss of water from the bronchovascular sheath and airway mucosa potentially decreased the airway resistance (Subhan et al., 2006). Conflicting results regarding the effects of RIF on flow rates have been reported in previous studies (Moosavi et al., 2007; Subhan et al., 2006). One study reported significant change in FEF75% and FEF75%-85% following RIF, while FEF25% and FEF50% depicted insignificant variation following the month of Ramadan (Subhan et al., 2006). The other study reported insignificant change in MEF75% after the month of Ramadan, while MEF50%, MEF75%, and MMEF depicted significant increase following the month of RIF. In light of these literatures, it may be hypothesized that in the present study, the hydration status of the subjects was not disturbed and insignificant alteration in the body weight may have had some physiological impact to maintain the PFTs during the RIF.
Although it is a limitation of the current study that the anatomical dead space was not measured, an earlier report suggested that Ramadan fasting had a significant impact on the ratio of dead space to TV in a Malay population (Duncan et al., 1990). It has been reported that a larger variation in the environmental factors might cause significant changes in the lung function (Siddiqui et al., 2005). Environmental temperature and relative humidity varied within a very narrow span during the course of the present study and that might be a reason for the insignificant change in the PFTs in the currently studied population.
Comparison of the pulmonary function measurements of the present study with those of foreign populations and those of populations from other provinces in India is difficult due to variations in the anthropometric profiles that largely affect the lung function measurements (Bandyopadhyay, 2011). Due to such an obstacle, according to Bandyopadhyay et al. (2013), the values of PFTs reported in other studies were standardized with age and height for a valid comparison.
The values of FVC, FEV1, and FEV1% obtained in both the groups were higher than Malaysian males (Singh, Singh, & Sirisinghe, 1994). The pulmonary function measurements of the present investigation were higher than Nepalese males (Morris, Koski, & Johnson, 1971). Comparison of the data with other Indian literatures indicated that the present values were higher than the Bengalis and other populations from different provinces of India (De & De, 1939; Rao, Gupta, Saha, & Devi, 1961). Such differences might be attributed to the variation in habitat, ethnicity, dietary practice, and sociodemographic nature (Bandyopadhyay et al., 2013; Chatterjee & Mandal, 1991).
PFT values in the present study were also higher than the Western Indians (Gupta, Gupta, & Ajmera, 1979) but comparable with the Northwestern part of India (Kamat, Thiruvengadam, & Rao, 1967). Techniques and instruments employed in those studies were different than the present one. De and De (1939) investigated PFTs in sitting posture, while Rao et al. (1961) investigated those in supine posture. The lower pulmonary function values in those studies might be due to the variation in posture since a considerable reduction in lung volumes would take place when the subjects moved from a sitting to a recumbent position (Geubelle & Giffin, 1962, as cited in Jain & Ramaiah, 1967).
FVC scores of the present investigation are lower than the age-matched Europeans (Quanjer, 1983; Roca, Sanchis, & Augusti-Vidal, 1986), Americans (Cotes, Saunders, Adam, Anderson, & Hall, 1973), and Senegalese (Dufetel, Pigearias, Lonsdorfer, Derossi, & Faltot, 1989). The precise reason for these interethnic differences is uncertain although it has been attributed to both genetic and environmental factors (Chatterjee, Saha, & Chatterjee, 1988). Anthropometric variations might explain some of these differences as the physical stature of Westerners on the average are somewhat larger than that of the Asians (Bandyopadhyay, 2011). The specific reason for the existence of difference in pulmonary function variables in different healthy populations is uncertain, although they may be attributable to sociodemographic factors, for example, ethnicity, habitat, anthropometric characteristics, and so on.
The majority of the literature indicated significant positive correlation of body height with FVC, FEV1, and PEFR as also observed in the present investigation. Comparison of the present anthropometric data with previously reported literatures from India and abroad revealed that variation in FVC, FEV1, and PEFR of the presently studied population differed proportionally with the body height of the concerned population. This finding corroborated with the finding of Bandyopadhyay (2011) and Bandyopadhyay et al. (2013).
VC, FVC, and FEV1, FEV1% exhibited significant correlation with body height. However, only FEV1 and FEV1% showed significant correlation with body mass in both the EG and the CG in young Muslim males. In other studies (Bandyopadhyay, Basak, Tripathy, & Bandyopadhyay, 2006; Chatterjee et al., 1988; Singh et al., 1994), age exhibited significant negative correlation with PFTs, whereas in the present investigation age shows significant positive relationship with dynamic pulmonary function measurements in both groups of the studied population. This finding might be attributed to younger age group and narrow age range of the present population. Chatterjee et al. (1988) proposed that age-related decline in pulmonary function measurements might be due to progressive loss of elastic recoil with aging even in the absence of impairment by cigarette smoking and pulmonary diseases. Such age-related changes might not have appeared in the presently studied population that belongs to a substantially narrow age range of a younger age group. That was probably the reason for obtaining inconsistent relationships of pulmonary function measurements with age in both the groups of the present investigation.
PEFR, which is considered as one of the most significant parameters to indicate one’s pulmonary function status (Bandyopadhyay, 2011), exhibited significant positive correlation with all the physical parameters in both groups of young Muslim males in the present study. As far as the flow rates are concerned, the FEF25%-75% and FEF75%-85% did not show any significant correlation with the physical parameters.
The mean values of PFTs did not show any significant difference between the CG and the EG. The values of correlation coefficients obtained in both the groups were also very close to each other. Hence, the data of the preexperimental trial (7 days before Ramadan month) of both the groups were taken together to compute the simple and multiple regression equations which could be used as norms for the prediction of VC, FVC, FEV1, FEV1%, and PEFR from body height and age in the studied population (Figures 2-7, Table 3). The standard errors of estimate of the computed simple and multiple regression equations were substantially small enough to tentatively propose these equations as applicable norms to predict PFTs in the studied population for practical use in epidemiological studies and also in clinical settings. However, official or final recommendation of these norms needs further study in a larger sample size with cross validation of the data by applying suitable statistical methods.
From the present investigation, it can be concluded that the PFTs of young Muslims of Kolkata were within the normal range. RIF did not affect their normal pulmonary function measurements. The standard errors of estimate of the computed equations were substantially small and therefore these equations were tentatively proposed as applicable norms to predict the PFTs in the studied population. Future studies may be conducted to explore the effects of dietary abnormalities, hydration status, sleep deprivation, and nutritional status on the pulmonary function parameters during the month of RIF. Consequence of changes in blood parameters, if any, on the pulmonary function measurements may also be attempted in future researches.
Study limitations are as follows:
Hydration status, sleep pattern, food habit, and energy intake were not monitored during the course of the study.
Anatomical and physiological dead space were not measured in this study.
Biochemical and routine examination of blood parameter were not conducted during the period of the present investigation.
Footnotes
Authors’ Note: Authors are indebted to the subjects who have wholeheartedly extended their support by participating in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- American Thoracic Society. (1978). Standard questionnaires on respiratory symptoms, tests of pulmonary function, and chest radiographs. American Review Respiratory Disease, 118, 10-23. [Google Scholar]
- Aziz A. R., Wahid M. F., Png W., Jesuvadian C. V. (2010). Effects of Ramadan fasting on 60 min of endurance running performance in moderately trained men. British Journal of Sports Medicine, 44, 516-521. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A. (2011). Pulmonary function studies in young healthy Malaysians of Kelantan, Malaysia. Indian Journal of Medical Research, 134, 653-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A., Basak A. K., Tripathy S., Bandyopadhyay P. (2006). Peak expiratory flow rate in female brick field workers of West Bengal, India. Ergonomics SA, 18, 22-27. [Google Scholar]
- Bandyopadhyay A., Bhattacharjee I., Dalui R., Pal S. (2013). Pulmonary function of healthy non-smoking male university students of Kolkata, India—Revisited. Malaysian Journal of Medical Sciences, 20, 17-24. [PMC free article] [PubMed] [Google Scholar]
- Chaouachi A., Coutts A. J., Chamari K., Wong del P., Chaouachi M., Chtara M., . . . Amri M. (2009). Effect of Ramadan intermittent fasting on aerobic and anaerobic performance and perception of fatigue in male elite judo athletes. Journal of Strength and Conditioning Research, 23, 2702-2709. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Mandal A. (1991). Pulmonary function studies in health school boys of West Bengal. Japanese Journal of Physiology, 41, 797-808. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Saha D., Chatterjee B. P. (1988). Pulmonary function studies in healthy non-smoking men of Calcutta. Annals of Human Biology, 15, 365-374. [DOI] [PubMed] [Google Scholar]
- Chin R. J., Haponik E. F. (1994). Nutrition, respiratory function, and disease. In Shils M. E., Olson J. A., Shike M. (Eds.), Modern nutrition in health and disease (8th ed., pp. 1374-1390). Philadelphia, PA: Lea & Febiger. [Google Scholar]
- Cotes J. E., Saunders M. J., Adam J. E. R., Anderson H. R., Hall A. M. (1973). Lung function in coastal and high land New Guineans: Comparison with Europeans. Thorax, 28, 320-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D., Das A. (1998). Statistics in biology and psychology. Kolkata, West Bengal, India: Academic. [Google Scholar]
- De P., De B. N. (1939). The vital capacity of Bengalis. Indian Medical Gazette, 74, 409-414. [PMC free article] [PubMed] [Google Scholar]
- Dufetel P., Pigearias B., Lonsdorfer J., Derossi D. C., Faltot P. J. (1989). Spirometric reference values in Senegalese black adults. European Respiratory Journal, 2, 252-258. [PubMed] [Google Scholar]
- Duncan M. T., Husain R., Raman A., Cheah S. H., Ch’ng S. L. (1990). Ventilatory function in Malay Muslims during normal activity and the Ramadan fast. Singapore Medical Journal, 31, 543-547. [PubMed] [Google Scholar]
- Geubelle F., Giffin K. (1962). Respiratory studies in children. IV: Lung volumes and body positions in healthy children. Acta Paediatrica, 51, 255-260. [DOI] [PubMed] [Google Scholar]
- Gupta P., Gupta S., Ajmera P. L. (1979). Lung function test in Rajasthani subjects. Indian Journal of Physiology and Pharmacology, 23, 8-14. [PubMed] [Google Scholar]
- Jain S. K., Ramaiah T. J. (1967). Spirometric studies in healthy women 15-40 years age. Indian Journal of Chest Diseases, 9, 1-12. [PubMed] [Google Scholar]
- Kamat S. R., Thiruvengadam K. V., Rao T. L. (1967). A study of pulmonary function among Indians and assessment of the Wright peak flow meter in relation to spirometry for field test. American Review Respiratory Disease, 96, 707-716. [DOI] [PubMed] [Google Scholar]
- Mansi K. M. S. (2007). Study the effects of Ramadan fasting on the serum glucose and lipid profile among healthy Jordanian students. American Journal of Applied Sciences, 8, 565-569. [Google Scholar]
- Meckel Y., Ismaeel A., Eliakim A. (2008). The effect of the Ramadan fast on physical performance and dietary habits in adolescent soccer players. European Journal of Applied Physiology, 102, 651-657. [DOI] [PubMed] [Google Scholar]
- Mirzaei B., Rahamani-nia F., Moghadam M. G., Ziyaolhgh S. J., Rezaei A. (2012). The effect of Ramadan fasting on biochemical and performance in collegiate wrestlers. Iranian Journal of Basic Medical Sciences, 15, 1215-1220. [PMC free article] [PubMed] [Google Scholar]
- Moosavi S. A., Kabir A., Mogbimi A., Chebrei A., Mohammed R. B. (2007). Evaluation of the effect of Islamic fasting on lung volumes and capacities in the healthy persons. Saudi Medical Journal, 28, 1666-1670. [PubMed] [Google Scholar]
- Morris J. F., Koski A., Johnson L. C. (1971). Spirometric standards for healthy nonsmoking adults. American Review of Respiratory Disease, 103, 57-67. [DOI] [PubMed] [Google Scholar]
- Nomani M. Z., Hallak M. H. (1990). Effect of Ramadan fasting on plasma uric acid and body weight healthy men. Journal of the American Dietetic Association, 90, 1435-1436. [PubMed] [Google Scholar]
- Omar M. A., Motata A. A. (1997). Fasting in Ramadan and diabetic patient. Diabetes Care, 20, 1925-1926. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Cooper K. R., Burke T. V. (1987). The effect of sleep loss on breathing in chronic obstructive pulmonary disease. Chest, 91, 29-32. [DOI] [PubMed] [Google Scholar]
- Quanjer P. H. (1983). Standardised lung function testing. Bulletin Européen de Physiopathologie Respiratoire, 19(Suppl. 5), 1-95. [PubMed] [Google Scholar]
- Rao M. N., Gupta A. S., Saha P. N., Devi S. A. (1961). Physiological norms in Indians: Pulmonary capacities in health. Indian Journal of Medical Research, 38, 1-104. [PubMed] [Google Scholar]
- Riley D. J., Thakker-Varia S. (1995). Effect of diet on lung structure, connective tissue metabolism and gene expression. Journal of Nutrition, 125, 1657-1660. [DOI] [PubMed] [Google Scholar]
- Roca J., Sanchis J., Augusti-Vidal A. (1986). Spirometric reference values from a Mediterranean population. Bulletin Européen de Physiopathologie Respiratoire, 22, 217-224. [PubMed] [Google Scholar]
- Roy A. S., Bandyopadhyay A. (2015). Effect of Ramadan intermittent fasting of selective fitness profile parameters on young untrained Muslim men. BMJ Open Sport & Exercise Medicine, e000020. doi: 10.1136/bmjsem-2015-000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. S., Bhattacharjee I., Dalui R., Pal S., Bandyopadhyay A. (2014). Gender difference on the effects of body mass index in prediction of spirometric reference values in healthy young Indian adults. International Journal of Clinical and Experimental Physiology, 1, 73-75. [Google Scholar]
- Sajid K. M., Aktar M. (1991). Ramadan fasting and thyroid hormone profile. Journal of Pakistan Medical Association, 41, 213-216. [PubMed] [Google Scholar]
- Siddiqui Q. A., Sabir S., Subhan M. M. (2005). The effect of Ramadan fasting on spirometry in healthy subjects. Respirology, 10, 525-528. [DOI] [PubMed] [Google Scholar]
- Singh R., Singh H. J., Sirisinghe R. G. (1994). Spirometric volumes in Malaysian males. Southeast Asian Journal of Tropical Medicine and Public Health, 25, 341-348. [PubMed] [Google Scholar]
- Subhan M. M. F., Siddiqui Q. A., Khan N. M., Sabir S. (2006). Does Ramadan fasting affects expiratory flow rates in healthy subjects? Saudi Medical Journal, 27, 1656-1660. [PubMed] [Google Scholar]
- Sweileh N., Schnitzler A., Hunter G. R., Davis B. (1992). Body composition and energy metabolism in resting and exercising Muslims during Ramadan fast. Journal of Sports Medicine and Physical Fitness, 32, 156-163. [PubMed] [Google Scholar]
- Waterhouse J. (2010). Effects of Ramadan on physical performance: Chronobiological considerations. British Journal of Sports Medicine, 44, 509-515. [DOI] [PubMed] [Google Scholar]
- Zerguini Y., Kirkendall D., Junge A., Dvorak J. (2007). Impact of Ramadan on physical performance in professional soccer players. British Journal of Sports Medicine, 4, 398-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaee V., Razaei M., Ahmadinejad Z., Shaikh H., Yousefi R., Yarmohammadi L., . . . Behjati M. J. (2006). The changes of metabolic profile and weight during Ramadan fasting. Singapore Medical Journal, 5, 409-414. [PubMed] [Google Scholar]