Abstract
Introduction:
In our study, we aimed to investigate the association between a traumatic brain injury (TBI) and subsequent erectile dysfunction (ED). This is a population-based study using the claims dataset from The National Health Insurance Research Database.
Methods:
We included 72,642 patients with TBI aged over 20 years, retrospectively, selected from the longitudinal health insurance database during 2000–2010, according to the ICD-9-CM. The control group consisted of 217,872 patients without TBI that were randomly chosen from the database at a ratio of 1:3, with age- and index year matched. Cox proportional hazards analysis was used to estimate the association between the TBI and subsequent ED.
Results:
After a 10-year follow-up, the incidence rate of ED was higher in the TBI patients when compared with the non-TBI control group (24.66 and 19.07 per 100,000, respectively). Patients with TBI had a higher risk of developing ED than the non-TBI cohort after the adjustment of the confounding factors, such as age, comorbidity, residence of urbanization and locations, seasons, level of care, and insured premiums (adjusted hazard ratio (HR) = 2.569, 95% CI [1.890, 3.492], p < .001).
Conclusion:
This is the first study using a comprehensive nationwide database to analyze the association of ED and TBI in the Asian population. After adjusted the confounding factors, patients with TBI have a significantly higher risk of developing ED, especially organic ED, than the general population. This finding might remind clinicians that it’s crucial in early identification and treatment of ED in post-TBI patients.
Keywords: traumatic brain injury, erectile dysfunction, sexuality, epidemiology of men’s health, general health and wellness, sexual dysfunction, sexual disorders
Traumatic brain injury (TBI) is a worldwide public health problem defined as an insult to the brain from external mechanical force which may cause impairment of cognitive and physical functions, and psychological health problems. TBI could be classified as mild, moderate, or severe according to the initial Glasgow Coma Scale (GCS) score or the consciousness (Ghajar, 2000). The incidence of TBI is increasing throughout the world and has been associated with fatalities and long-term disabilities (Roozenbeek, Maas, & Menon, 2013; Stocchetti, 2014). Neuropsychological and neuropsychiatric disorders such as cognitive decline, sleep–wake cycle disturbances, depression, anxiety or posttraumatic stress disorders can also result in disabilities themselves and thus result in a major concern as being the cause of disabilities after TBI (Hibbard, Uysal, Kepler, Bogdany, & Silver, 1998; Moretti et al., 2012; Ouellet, Beaulieu-Bonneau, & Morin, 2015; Zaninotto et al., 2016). Among these problems, sexual dysfunctions and inappropriate sexual behaviors have been reported in the TBI patients that might contribute to an impaired quality of life, and disturbances to the patients with TBI and their caregivers (Ponsford, 2003; Ponsford, Downing, & Stolwyk, 2013; Sander et al., 2013; Turner, Schottle, Krueger, & Briken, 2015; War, Jamuna, & Arivazhagan, 2014).
Erectile dysfunction (ED), or the inability to attain or maintain a penile erection sufficient for successful vaginal intercourse, is a common sexual dysfunction that affects mainly men aged 40 or older (Shamloul & Ghanem, 2013). Previous studies reported that 26%–29% of males had experienced ED aged ≧40 (Hwang, Tsai, Lin, Chiang, & Chang, 2010; Rhoden, Teloken, Sogari, & Vargas Souto, 2002). Diabetes mellitus, hypertension, obesity, limited physical exercise, and lower urinary tract symptoms have been linked to the development of erectile dysfunction (Chaudhary et al., 2016; Clavijo, Miner, & Rajfer, 2014; Kaya, Sikka, & Gur, 2015; Leoni, Fukushima, Rocha, Maifrino, & Rodrigues, 2014; Maiorino, Bellastella, & Esposito, 2015; Phe & Roupret, 2012). Some neurological disorders are frequently associated with ED, including multiple sclerosis, temporal lobe epilepsy, Parkinson’s disease, stroke, Alzheimer’s disease, and spinal cord injury (Shamloul & Ghanem, 2013; Siddiqui et al., 2012). Previous studies have reported that TBI results in problems and disturbances such as inappropriate sexual behaviors and overall sexual dysfunctions in patients after TBI (Hanks, Sander, Millis, Hammond, & Maestas, 2013; Sander, Maestas, Pappadis, Hammond, & Hanks, 2016). Several physical factors such as hypogonadism (Cuesta et al., 2016) and prophylactic antiepileptic drugs (Yang & Wang, 2016) could attribute organic ED, in addition to neuropsychological and neuropsychiatric disorders (Farre, Fora, & Lasheras, 2004; Shamloul & Ghanem, 2013; Yafi et al., 2016). The association between TBI and ED was not studied specifically. This study aimed to clarify the association between TBI and ED in a nationwide, population-based, matched cohort study.
Methods
Data Sources
National Insurance Research Database (NHIRD), a single-payer and universal health coverage system provided by the National Insurance Research Institute since 1995, is composed of the medical documentation from 99.6% of the Taiwanese population (23.67 million in 2016)(Ho Chan, 2010). This study used the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), traditional Chinese version, as the disease diagnosis code and collected the data from NHIRD (Chinese Hospital Association, 2000a).
The National Health Insurance (NHI) Program was launched in Taiwan in 1995, and as of June 2009, included contracts with 97% of the medical providers with approximately 23 million beneficiaries, or more than 99% of the entire population(Ho Chan, 2010). The National Health Insurance Research Database (NHIRD), contains all claims data of the beneficiaries, uses the ICD-9-CM codes to record diagnoses (Chinese Hospital Association, 2000b). All diagnoses of ED in Taiwan are confirmed by board-certified urologists. In Taiwan, urologists adopt the International Society of Impotence Research Nomenclature system for the differential diagnosis and classification organic, psychogenic or mixed ED (Lizza & Rosen, 1999). The NHI administration randomly reviews the records ambulatory care visits and hospital admissions, to verify the accuracy of the diagnoses (NHI Administration). Several studies have demonstrated the accuracy and validity of the diagnoses in the NHIRD (Cheng, Kao, Lin, Lee, & Lai, 2011; Chou, Lin, Lin, Sung, & Kao, 2013; Liang et al., 2011), and, therefore, the NHIRD was used as the data source in this study.
Study Design
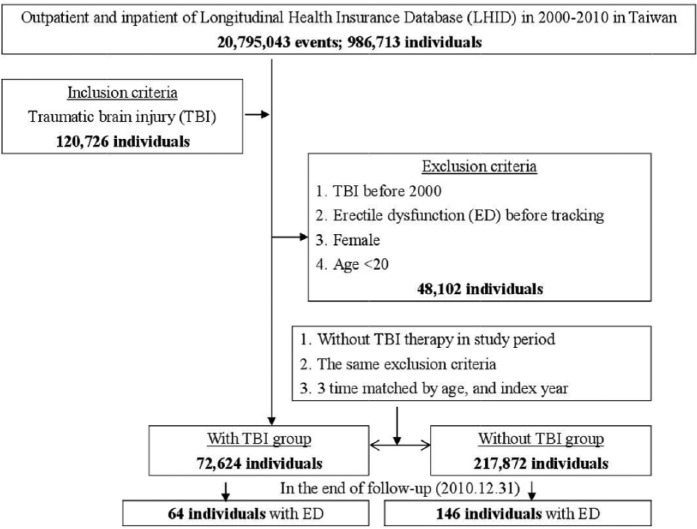
Subjects with TBI aged over 20 were retrospectively selected from the Longitudinal Health Insurance Database (LHID) during 2000 to 2010, according to the ICD as follows: TBI could be categorized as postconcussion syndrome (ICD-9-CM 310.2), fracture of skulls (ICD-9-CM 800-804, 905.0), intracranial injury, excluding those with skull fracture (ICD-9-CM 850-854, 907.0), injury to optic nerve and pathways (ICD-9-CM 950.1, 950.3), head injury (ICD-9-CM 959.01), personal history of traumatic brain injury (959.9; V15.52). The severity of TBI was classified as being treated at an outpatient setting or requiring hospitalization, or with a major trauma Injury Severity Score (ISS) ≥16 (Baker, O’Neill, Haddon, & Long, 1974; Stoner, Heath, Yates, & Frayn, 1980), with references from previous studies (Chien, Chung, Lai, & Chou, 2014; Chien et al., 2017).
ED was classified as psychogenic ED (ICD-9-CM 302.74) and organic ED (ICD-9-CM 607.84). Comorbidities in this study included dementia (ICD-9-CM 290, 294.1-294.2, 331.0), schizophrenia (ICD-9-CM 295), anxiety (ICD-9-CM 300.0, 300.2-300.3, 308.3, 309.31), bipolar disorder (ICD-9-CM 296.0-296.1, 296.4-296.8), depression (ICD-9-CM 296.2-296.3, 300.1, 311), stroke (ICD-9-CM 430-438), coronary artery disease (CAD) (ICD-9-CM 410-414), chronic obstructive pulmonary disease (COPD) (ICD-9-CM 491-492, 496), chronic kidney disease (CKD) (ICD-9-CM 580-589), hypertension (ICD-9-CM 401-405), diabetes mellitus (DM) (ICD-9-CM 250), hyperlipidemia (ICD-9-CM 272), asthma (ICD-9-CM 493), alcohol-related illness (ICD-9-CM 291, 303, 305, 571.0-571.3, 790.3, V11.3), fracture (ICD-9-CM 800-829), dislocation (ICD-9-CM 830-839), sprains and strains (ICD-9-CM 840-849), open wound (ICD-9-CM 870-899), injury to blood vessels (ICD-9-CM 900-904), superficial injury/contusion (ICD-9-CM 910-92), crushing (ICD-9-CM 925-929), foreign body entering through orifice (ICD-9-CM 930-939), burns (ICD-9-CM 940-949), injury to nerves and spinal cord (ICD-9-CM 950-957), poisoning (ICD-9-CM 960-989), with the references to previous studies about ED (Chao, Chen, Wang, Li, & Kao, 2015; Chen, Liang, Lin, Liao, & Kao, 2016; Kao et al., 2016; Wang, Chao, Lin, Tseng, & Kao, 2016; Wu et al., 2016) or TBI (Chi et al., 2016; Wimo et al., 2016; Wu et al., 2017; Yang et al., 2016). This was a retrospective cohort study. Male patients aged >20 years or older newly diagnosed TBI between 1 January 2000 and 31 December 2010 were identified. Patients who had TBI before 2000, ED before tracking, female patients and were younger than 20 years were excluded. The comparison group (patients without TBI) was randomly chosen with the same exclusion criteria from the database at a ratio of 1:3 (Figure 1). Two groups were matched by age and index year.
Figure 1.
The flowchart of study sample selection from National Health Insurance Research Database in Taiwan.
Statistical Analysis
All data were analyzed by the Statistical Product and Service Solutions (SPSS Inc., Chicago, IL). χ2 and t test were used to evaluate the difference of age and comorbidities between the ED and non-ED group. The Fisher exact test for categorical variables was used to statistically examine the differences between the two cohorts. The Cox proportional regression hazard model was used to compare the incidence rate of ED between the TBI and non-TBI control group after the modification of comorbidities. The Kaplan–Meier method and Log-rank test were used to estimate the risk of ED of the two groups. A two-tailed p-value level smaller than .05 was considered significant.
Ethics
This study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The Institutional Review Board of the Tri-Service General Hospital approved this study (TSGH IRB No. 2-105-05-082).
Results
72,642 patients who were diagnosed with TBI, and 217,872 patients without TBI in the control group were included. 126,948 patients (58.5%) were aged from 20 to 64. Demographic statistics and comorbidities are as reported in Table 1. All comorbid medical, psychiatric diseases and injuries were significantly higher in the TBI group than the control group, with the exceptions of hypertension and alcohol-related illness.
Table 1.
Demographic Statistics and Comorbidities with and Without Traumatic Brain Injury.
| Variables | Traumatic brain injury |
p-value | |
|---|---|---|---|
| Yes N (%) | No N (%) | ||
| Total | 72,624 (25) | 217,872 (75) | |
| Age (years) | .999 | ||
| 20–44 | 22,771 (31.35) | 68,313 (31.35) | |
| 45–64 | 19,716 (27.15) | 59,148 (27.15) | |
| ≧65 | 30,137 (41.5) | 90,411 (41.5) | |
| Dementia | 365 (0.47) | 729 (0.33) | <.001 |
| Schizophrenia | 7 (0.01) | 736 (0.34) | <.001 |
| Anxiety | 752 (1.04) | 2,095 (0.96) | .042 |
| Bipolar disorder | 116 (0.16) | 511 (0.23) | <.001 |
| Depression | 1,054 (1.45) | 834 (0.38) | <.001 |
| Stroke | 6,681 (9.20) | 13,150 (6.04) | <.001 |
| CAD | 6,836 (9.41) | 17,183 (7.89) | <.001 |
| COPD | 9,787 (13.48) | 12,768 (5.86) | <.001 |
| CKD | 5,650 (7.78) | 6,604 (3.03) | <.001 |
| Hypertension | 10,478 (14.43) | 31,090 (14.27) | .148 |
| Diabetes (DM) | 8,516 (11.73) | 21,915 (10.06) | <.001 |
| Hyperlipidemia | 951 (1.31) | 5,459 (2.51) | <.001 |
| Asthma | 914 (1.26) | 4,020(1.85) | <.001 |
| Alcohol-related illness | 1,479 (2.04) | 4,274 (1.96) | .108 |
| Fracture | 13,671 (18.82) | 16,839 (7.73) | <.001 |
| Dislocation | 800 (1.10) | 1,464 (0.67) | <.001 |
| Sprains and strains | 891 (1.23) | 1,708 (0.78) | <.001 |
| Open wound | 14,469 (19.92) | 9,628 (4.42) | <.001 |
| Injury to blood vessels | 48 (0.07) | 410 (0.19) | <.001 |
| Superficial injury/contusion | 14,509 (19.98) | 5,103 (2.34) | <.001 |
| Crushing | 117 (0.16) | 1,214(0.56) | <.001 |
| Foreign body entering through orifice | 38 (0.05) | 212 (0.10) | <.001 |
| Burn | 179 (0.25) | 1,485 (0.68) | <.001 |
| Injury to nerves and spinal cord | 964 (1.33) | 1,009 (0.46) | <.001 |
| Poisoning | 364 (0.50) | 1,549 (0.71) | <.001 |
| Season | <.001 | ||
| Spring (March–May) | 18,400 (23.5) | 58,378 (26.79) | |
| Summer (June–August) | 18,052 (24.86) | 52,525 (24.11) | |
| Autumn (September–November) | 17,768 (24.47) | 49,052 (22.51) | |
| Winter (December–February) | 18,404 (25.34) | 57,917 (26.58) | |
| Location | <.001 | ||
| Northern Taiwan | 23,665 (32.59) | 93,246 (42.88) | |
| Middle Taiwan | 23,239 (32.0) | 58,887 (27.03) | |
| Southern Taiwan | 20,766 (28.59) | 51,773 (23.76) | |
| Eastern Taiwan | 4,615 (6.35) | 12,730 (5.84) | |
| Outlets islands | 339 (0.74) | 1,056 (0.48) | |
| Urbanization level | <.001 | ||
| 1 (the highest) | 18,769 (25.84) | 73,574 (33.77) | |
| 2 | 31,648 (43.58) | 96,754 (44.41) | |
| 3 | 6,705 (9.23) | 16,612 (7.62) | |
| 4 (the lowest) | 15,502 (21.35) | 30,932 (12.20) | |
| Level of care | <.001 | ||
| Hospital center | 19,711 (27.14) | 81,193 (37.27) | |
| Regional hospital | 31,319 (43.12) | 74,842 (34.35) | |
| Local hospital | 21,374 (29.43) | 61,157 (28.07) | |
| Physician clinics | 220 (0.30) | 680 (0.31) | |
| Insured premium (NT$) | <.001 | ||
| <18,000 | 71,506 (98.46) | 214,315 (98.37) | |
| 18,000–34,999 | 900 (1.24) | 2,513 (1.15) | |
| ≧35,000 | 218 (0.30) | 1,044 (0.48) | |
Note. p-value (category variable: χ2/Fisher exact test). CAD = coronary artery diseases; COPD = chronic obstructive pulmonary disease; CKD = chronic kidney disease; DM = diabetes mellitus.
Over a 10-year follow-up period, the incidence rate of ED was higher in the study subjects when compared with the non-TBI control group (24.66 and 19.07/100,000, respectively). Patients with TBI had a higher risk of developing ED than non-TBI controls (adjusted HR = 2.569, 95% confidence interval (CI) [1.890, 3.492], p < .001), after adjusting the confounding factors such as age, comorbidities, residence of urbanization and locations, seasons, level of care, and insured premiums. The risk was lower in both the 45–64 and ≥ 65 age than in the 20–44 age group (adjusted HR = 0.523, 95% CI [0.360, 0.759], p = .001 and 0.534, 95% CI [0.378, 0.755], p < .001, respectively) (Table 2), and anxiety disorder was associated with an increased risk of ED (adjusted HR = 15.883, p < .001). Some comorbidities, such as stroke, COPD, hypertension, and fracture, were associated with a lower risk of ED (Table 3). The severity of TBI was classified as being treated at an outpatient setting (mild) or requiring hospitalization (moderate), or with a major trauma Injury Severity Score (ISS) of ≥16 (severe). Patient with higher TBI severity also had higher risk of development of ED (adjusted HR = 2.305, 95% CI [1.672, 3.124], p < .001, 2.551, 95% CI [1.884, 3.331], p < .001, and 5.467, 95% CI [2.452, 7.706], p < .001, respectively) (Table 4). The mean follow-up time was 2.49 ± 3.08 year in TBI patients and 3.30 ± 3.32 in non-TBI group (data not reported, no significant difference). The Kaplan–Meier method was used to evaluate cumulative incidence. The cumulative incidence rate of ED in the TBI group was higher than the non-TBI cohort control (0.09% and 0.07 %, respectively, Log-rank p < .001) (Figure 2). There was a significant difference between the two groups after a 7-year follow-up period.
Table 2.
Incidence of Erectile Dysfunction in Different Age and Cox Regression Measured Hazard Ratio for patients with TBI and Non-TBI.
| Traumatic brain injury |
||||||||
|---|---|---|---|---|---|---|---|---|
| Yes |
No |
TBI to non-TBI |
||||||
| Variables | ED | PYs | Rate | ED | PYs | Rate | Crude HR (95% CI) | Adjusted HR (95% CI) |
| Total | 64 | 259,480.24 | 24.66 | 146 | 765,676.72 | 19.07 | 2.234 [1.651, 3.024] | 2.569 [1.89, 3.492] * |
| Age (years) | ||||||||
| 20–44 | 20 | 40,460.60 | 49.43 | 53 | 116,949.23 | 45.32 | Reference | Reference |
| 45–64 | 11 | 60,836.56 | 18.08 | 38 | 211,860.90 | 17.94 | 0.462 [0.321, 0.665] | 0.523(0.36–0.759) * |
| ≧65 | 33 | 178,183.08 | 18.52 | 55 | 436,866.59 | 12.59 | 0.423 [0.0309, 0.58] | 0.534 [0.378, 0.755] * |
Note. ED = erectile dysfunction; TBI = traumatic brain injury; PYs = person-years; rate = incidence rate in per 10,000 person-years; CI = confidence interval; HR = hazard ratio. Adjusted HR: multivariable analysis included sex, age, covariates, and comorbidities (dementia, schizophrenia, anxiety, bipolar disorder, depression, stroke, coronary artery disease, chronic obstructive pulmonary disease, chronic kidney disease, hypertension, diabetes mellitus, hyperlipidemia, asthma, alcohol-related illness, fracture, dislocation, sprains and strains, open wound, injury to blood vessels, superficial injury/contusion, crushing, foreign body entering through orifice, burn, injury to nerves and spinal cord, poisoning).
p ≤ .001.
Table 3.
Risk of Erectile Dysfunction at the End of Follow-Up.
| Variables | Crude HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value |
|---|---|---|---|---|
| Traumatic brain injury | ||||
| Without | Reference | Reference | ||
| With | 2.234 [1.651, 3.024] | <.001 | 2.569 [1.890, 3.492] | <.001 |
| Age (years) | ||||
| 20–44 | Reference | Reference | ||
| 45–64 | 0.462 [0.321, 0.665] | <.001 | 0.523 [0.360, 0.759] | .001 |
| ≧65 | 0.423 [0.309, 0.580] | <.001 | 0.534 [0.378, 0.755] | <.001 |
| Anxiety | ||||
| Without | Reference | Reference | ||
| With | 9.003 [5.552, 14.598] | <.001 | 15.883 [9.326, 27.048] | <.001 |
| Stroke | ||||
| Without | Reference | Reference | ||
| With | 0.357 [0.168, 0.757] | .007 | 0.447 [0.208, 0.959] | .039 |
| COPD | ||||
| Without | Reference | Reference | ||
| With | 0.542 [0.287, 1.023] | .059 | 0.457 [0.212, 0.987] | .046 |
| Hypertension | ||||
| Without | Reference | Reference | ||
| With | 0.400 [0.259, 0.618] | <.001 | 0.489 [0.310, 0.771] | .002 |
| Fracture | ||||
| Without | Reference | Reference | ||
| With | 0.324 [0.120, 0.871] | .025 | 0.278 [0.102, 0.756] | .012 |
Note. COPD = chronic obstructive pulmonary disease; HR, hazard ratio. Adjusted HR: multivariable analysis included sex, age, covariates, and comorbidities (dementia, schizophrenia, anxiety, bipolar disorder, depression, stroke, coronary artery disease, chronic obstructive pulmonary disease, chronic kidney disease, hypertension, diabetes mellitus, hyperlipidemia, asthma, alcohol-related illness, fracture, dislocation, sprains and strains, open wound, injury to blood vessels, superficial injury/contusion, crushing, foreign body entering through orifice, burn, injury to nerves and spinal cord, poisoning).
Table 4.
Incidence of Erectile Dysfunction by Injury Severity Using Cox Regression Model.
| Traumatic brain injury |
||||||||
|---|---|---|---|---|---|---|---|---|
| Yes |
No |
|||||||
| Injury severity | ED | PYs | Rate | ED | PYs | Rate | Adjusted HR (95% CI) | p- value |
| Total | 64 | 259,480.24 | 24.66 | 146 | 765,676.72 | 19.07 | 2.569 [1.890, 3.492] | <.001 |
| Outpatient (mild) | 35 | 152,418.07 | 22.96 | 146 | 765,676.72 | 19.07 | 2.305 [1.672, 3.124] | <.001 |
| Inpatient (moderate) | 22 | 93,607.72 | 23.50 | 146 | 765,676.72 | 19.07 | 2.551 [1.884, 3.331] | <.001 |
| ISS ≧16 (severe) | 7 | 13,454.45 | 52.03 | 146 | 765,676.72 | 19.07 | 5.467 [2.452, 7.706] | <.001 |
Note. ED, erectile dysfunction; PYs, person-years; rate, incidence rate in per 10,000 person-years; CI, confidence interval; HR = hazard ratio. Adjusted HR: multivariable analysis included sex, age, covariates, and comorbidities (dementia, schizophrenia, anxiety, bipolar disorder, depression, stroke, coronary artery disease, chronic obstructive pulmonary disease, chronic kidney disease, hypertension, diabetes mellitus, hyperlipidemia, asthma, alcohol-related illness, fracture, dislocation, sprains and strains, open wound, injury to blood vessels, superficial injury/contusion, crushing, foreign body entering through orifice, burn, injury to nerves and spinal cord, poisoning).
Figure 2.

The Kaplan–Meier method for cumulative risk of erectile dysfunction among male aged 20 and over stratified by traumatic brain injury with Log-rank test.
In the subgroup analysis of the comorbidity, compared to the non-TBI control, the TBI patients without dementia, schizophrenia, anxiety, bipolar disorder, depression, CKD, hyperlipidemia, asthma, alcohol-related illness, or all injuries, were associated with more risk of ED. (Table 5). Furthermore, among the psychogenic and organic ED, the patients with TBI were associated with a significantly increased risk of organic ED than in the control group (adjusted HR=2.373, 95% CI [2.028, 3.854], p < 0.001). Subjects with postconcussion syndrome, fracture of skulls, intracranial injury, excluding those with skull fracture, and unspecified head injury were associated with overall ED and psychogenic ED, and subjects with personal history of traumatic brain injury were associated with overall ED (Table 6).
Table 5.
Factors of Erectile Dysfunction at the End of Follow-Up.
| Traumatic brain injury |
||
|---|---|---|
| Variables | Adjusted HR | p-value |
| Total | 2.569 (1.890–3.492) | <.001 |
| Age (years) | ||
| 20–44 | 1.765 (1.030–3.024) | .039 |
| 45–64 | 2.046 (1.019–4.108) | .044 |
| ≧65 | 4.183 (2.656–6.588) | <.001 |
| Dementia | ||
| Without | 2.548 (1.870–3.471) | <.001 |
| With | 38.034 (0.152–98.358) | .156 |
| Schizophrenia | ||
| Without | 2.569 (1.890–3.492) | <.001 |
| With | – | – |
| Anxiety | ||
| Without | 2.924 (2.132–4.011) | <.001 |
| With | 0.295 (0.038–2.299) | .244 |
| Bipolar disorder | ||
| Without | 2.569 (1.890–3.492) | <.001 |
| With | – | – |
| Depression | ||
| Without | 2.592 (1.903–3.530) | <.001 |
| With | 0.436 (0.012–8.993) | .603 |
| Stroke | ||
| Without | 2.435 (1.775–3.340) | <.001 |
| With | 11.334 (1.771–72.553) | .010 |
| CAD | ||
| Without | 2.495 (1.815–3.429) | <.001 |
| With | 3.787 (1.134–12.641) | .030 |
| COPD | ||
| Without | 2.354 (1.710–3.240) | <.001 |
| With | 13.834 (3.219–59.462) | <.001 |
| CKD | ||
| Without | 2.598 (1.894–3.563) | <.001 |
| With | 2.760 (0.748–10.188) | .128 |
| Hypertension | ||
| Without | 2.424 (1.749–3.360) | <.001 |
| With | 4.811 (1.928–12.002) | .001 |
| Diabetes mellitus | ||
| Without | 2.506 (1.862–3.619) | <.001 |
| With | 2.609 (1.164–5.847) | .020 |
| Hyperlipidemia | ||
| Without | 2.582 (1.898–3.511) | <.001 |
| With | 0.000 | .985 |
| Asthma | ||
| Without | 2.555 (1.875–3.481) | <.001 |
| With | 0.749 (0.020–7.407) | .875 |
| Alcohol-related illness | ||
| Without | 2.523 (1.849–3.442) | <.001 |
| With | 11.295 (0.732–76.442) | .084 |
| Fractures | ||
| Without | 2.563 (1.879–3.495) | <.001 |
| With | 3.557 (0.405–31.220) | .252 |
| Dislocation | ||
| Without | 2.557 (1.879–3.480) | <.001 |
| With | – | – |
| Sprains and strains | ||
| Without | 2.509 (1.840–3.421) | <.001 |
| With | – | – |
| Open wound | ||
| Without | 2.538 (1.861–3.459) | <.001 |
| With | 4.737 (0.002–9.940) | .624 |
| Injury to blood vessels | ||
| Without | 2.569 (1.890–3.492) | <.001 |
| With | – | – |
| Superficial injury/contusion | ||
| Without | 2.584 (1.896–3.520) | <.001 |
| With | 2.019 (0.181–22.485) | .568 |
| Crushing | ||
| Without | 2.569 (1.890–3.492) | <.001 |
| With | – | – |
| Foreign body entering through orifice | ||
| Without | 2.569 (1.890–3.492) | <.001 |
| With | – | – |
| Burn | ||
| Without | 2.569 (1.890–3.492) | <.001 |
| With | – | – |
| Injury to nerves and spinal cord | ||
| Without | 2.569 (1.890–3.492) | <.001 |
| With | – | – |
| Poisoning | ||
| Without | 2.569 (1.890–3.492) | <.001 |
| With | – | – |
Note. PYs, person-years; adjusted HR, adjusted hazard ratio, adjusted for all the variables above; CI, confidence interval, CAD, coronary artery diseases; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease.
Table 6.
Incidence of Organic and Psychogenic Erectile Dysfunction by Cox Regression Model.
| Traumatic brain injury |
||||||||
|---|---|---|---|---|---|---|---|---|
| Yes |
No |
|||||||
| Variables | ED | PYs | Rate | ED | PYs | Rate | Adjusted HR (95% CI) | p- value |
| All TBI | ||||||||
| Overall ED | 64 | 259,480.24 | 24.66 | 146 | 765,676.72 | 19.07 | 2.569 [1.890, 3.492] | <.001 |
| Psychogenic ED | 4 | 259,480.24 | 1.54 | 17 | 765,676.72 | 2.22 | 0.196 [0.011, 4.278] | .472 |
| Organic ED | 60 | 259,480.24 | 23.12 | 60 | 765,676.72 | 7.84 | 2.373 [2.028, 3.845] | <.001 |
| Postconcussional syndrome | ||||||||
| Overall ED | 2 | 10,362.40 | 19.30 | 146 | 765,676.72 | 19.07 | 2.010 [1.479, 2.733] | <.001 |
| Psychogenic ED | 0 | 10,362.40 | 0.00 | 17 | 765,676.72 | 2.22 | 0.000 | .952 |
| Organic ED | 2 | 10,362.40 | 19.30 | 60 | 765,676.72 | 7.84 | 1.981 [1.693, 3.209] | <.001 |
| Fracture of skulls | ||||||||
| Overall ED | 39 | 160,135.65 | 24.35 | 146 | 765,676.72 | 19.07 | 2.587 [1.866, 3.448] | <.001 |
| Psychogenic ED | 3 | 160,135.65 | 1.87 | 17 | 765,676.72 | 2.22 | 0.238 [0.013, 5.199] | .402 |
| Organic ED | 36 | 160,135.65 | 22.48 | 60 | 765,676.72 | 7.84 | 2.307 [1.972, 3.738] | <.001 |
| Intracranial injury, excluding those with skull fracture | ||||||||
| Overall ED | 19 | 77,798.02 | 24.42 | 146 | 765,676.72 | 19.07 | 2.544 [1.871, 3.458] | <.001 |
| Psychogenic ED | 0 | 77,798.02 | 0.00 | 17 | 765,676.72 | 2.22 | 0.000 | .897 |
| Organic ED | 19 | 77,798.02 | 24.42 | 60 | 765,676.72 | 7.84 | 2.506 [2.142, 4.061] | <.001 |
| Injury to optic nerve and pathways | ||||||||
| Total ED | 0 | 0.00 | – | 146 | 765,676.72 | 19.07 | – | – |
| Psychogenic ED | 0 | 0.00 | – | 17 | 765,676.72 | 2.22 | – | – |
| Organic ED | 0 | 0.00 | – | 60 | 765,676.72 | 7.84 | – | – |
| Unspecified head injury | ||||||||
| Total ED | 3 | 10,578.64 | 28.36 | 146 | 765,676.72 | 19.07 | 2.954 [2.178, 4.015) | <.001 |
| Psychogenic ED | 0 | 10,578.64 | 0.00 | 17 | 765,676.72 | 2.22 | 0.000 | .938 |
| Organic ED | 3 | 10,578.64 | 28.36 | 60 | 765,676.72 | 7.84 | 2.910 [2.487, 4.716] | .001 |
| Personal history of traumatic brain injury | ||||||||
| Total ED | 1 | 605.53 | 165.14 | 146 | 765,676.72 | 19.07 | 17.021 [12.675, 23.381] | .013 |
| Psychogenic ED | 1 | 605.53 | 165.14 | 17 | 765,676.72 | 2.22 | 20.997 [10.178, 58.300] | .024 |
| Organic ED | 0 | 605.53 | 0.00 | 60 | 765,676.72 | 7.84 | 0.000 | .879 |
Note. ED, erectile dysfunction; PYs, person-years; rate, incidence rate in per 10,000 person-years; CI, confidence interval; HR = hazard ratio. Adjusted HR: multivariable analysis included age and confounding factors.
Discussion
Association Between TBI and ED
There are some researches using the population-based NHIRD to study the association between ED and medical illness (Chao et al., 2015; Chen et al., 2016; Kao et al., 2016; Michalowsky et al., 2016; Wang et al., 2016; Wimo et al., 2016; Wu et al., 2016). This is the first study to use a nationwide database to analyze the association of ED and TBI in Taiwan. 217,872 individuals matched by age and index year for comparison were enrolled. The TBI patients were more likely to have ED when compared with the non-TBI controls (adjusted HR 2.569, 95% CI [1.890, 3.492], p < .001) after adjusting the confounding factors such as age, comorbidities, residence of urbanization and locations, seasons, level of care, and insured premiums. Those patients particularly aged 65 years or older revealed a higher risk of development of the subsequent ED. Kaplan–Meier analysis revealed that the TBI group had a significantly higher rate of ED than the control groups at the 7-year follow-up period.
TBI has made a profound impact on the sexuality of the patients and studies: inappropriate sexual behaviors are related to TBI, and about 30% of sexual dysfunctions in patients after TBI (Hanks et al., 2013; Sander et al., 2016). But there were only two previous case reports about brain injuries and subsequent ED, one was about the ED related to hypopituitarism after brain injury (Lane, 2010), and sexual therapies for two patients with ED after TBI (Simpson, McCann, & Lowy, 2016). This study depicts the association between TBI and ED and could remind clinicians on this important topic. Since the severity of TBI was classified as being treated at an outpatient setting or requiring hospitalization, or with a major trauma ISS ≥ 16, TBI patients with ISS ≥ 16 or hospitalization were associated with higher risk of the development of ED.
ED can be classified as psychogenic, organic, or mixed psychogenic and organic depending on its mechanism. Organic ED may be resulted from neurogenic, endocrinological, vasculogenic, drug-related, systemic diseases, or local penile factors(Shamloul & Ghanem, 2013). Psychogenic ED should be considered in patients with physical and mental health problems, psychological trauma, relationship problems, partner dissatisfaction, family or social pressures, and depression(Pastuszak, 2014). In mixed ED, Patient presents both organic and psychogenic factors. The ICD-9-CM codes do not include mixed ED, and patients with mixed ED were coded as organic ED (Chao et al., 2015). In this study, patients with TBI were associated with a higher rate of organic ED than in the control group (adjusted HR=2.373, 95% CI [2.028, 3.854], p < .001).
The rate of ED was about 26 cases per 1,000 men annually in a study in the United States (Johannes et al., 2000), and 65.6 cases per 1,000 men annually in Brazil (Johannes et al., 2000). The incidence rate of ED was higher in TBI patients when compared with the non-TBI control group (24.66 and 19.07 per 100,000, respectively), or 0.09% (64 in 72,624) in the TBI subjects and 0.07% (146 in 217,872) in the non-TBI control. The incidence rate is similar to other studies using NHIRD for ED (Kao et al., 2016; Shen, Weng, Wang, & Tien, 2014). The differences of prevalence rates among these studies might be related to the studies design. In this study, the incidence was from the patients who sought medical help instead of using questionnaires.
The possible mechanisms between the TBI and organic ED are complex and may be associated with a variable multifactor. First, damage to the brain parenchyma can result from neurogenic ED. Several studies reported posttraumatic hypopituitarism occurred in a range from 21.3% to 31% after TBI. Prevalence of hypogonadism was estimated at about 1.9% to 17.1% (Alavi, Tan, Menon, Simpson, & Hutchinson, 2016; Schneider, Kreitschmann-Andermahr, Ghigo, Stalla, & Agha, 2007; Silva et al., 2015). Patients with symptoms of hypogonadism (that included ED) after TBI were even more predictive of hypopituitarism than other symptoms (58% vs 16%, P <.0001) (Cuesta et al., 2016). Medications, such as antiepileptic drugs used to prevent posttraumatic seizure, can also attribute organic ED (Yang & Wang, 2016). Psychiatric factors, such as anxiety in this study, might be associated with increased risk of development of ED, and this finding is similar with previous studies (Farre et al., 2004; Wyllie, 2005). In this study, the subjects with stroke, COPD, hypertension, and fracture, were associated with a lower risk of ED. Further studies may need to understand the underlying mechanisms. Limitation capacity of overall physical activities in these diseases, including sexual activities, might decrease the need for sexual activities and thus the limited help-seeking behaviors for ED treatment in the NHIRD, which is a claims database. Further study is needed to clarify the underlying reasons for the associations between these diseases and decreased risk of ED.
In the subgroup analysis, compared to the non-TBI control, the TBI patients without dementia, schizophrenia, anxiety, bipolar disorder, depression, CKD, hyperlipidemia, asthma, alcohol-related illness, or all injuries, were associated with more risk of ED. In the enrollee subgroup without anxiety, TBI patients were associated with higher risk of ED, in comparison to non-TBI patients, but for the enrollee subgroup with anxiety, TBI patients were not associated with higher risk of ED, in comparison to non-TBI patients. It means that anxiety plays an important role in the development of ED, as aforementioned, the effects of TBI become not significant for the subgroup with anxiety. The reasons why subgroups without dementia, schizophrenia, bipolar disorder, depression, stroke, CAD, CKD, hyperlipidemia, asthma, alcohol-related illness, or all injuries were associated with increased risk of development of ED, remain unknown.
Several other physical or psychosocial factors could contribute to the impaired sexuality, including ED (Bivona et al., 2016; Sander & Maestas, 2014). In patients with TBI, damage to the parts of the brain, hormonal imbalance, medication side effects, fatigue, spasticity pain, weakness, slowed or uncoordinated movements, and balance problems, may make it difficult to have vaginal intercourse (Shamloul & Ghanem, 2013; Yafi et al., 2016). Self-esteem problems, changes in thinking and communication abilities, anxiety, depression, and changes in relationships and social activities could also result in similar difficulties (Bivona et al., 2016; Downing, Stolwyk, & Ponsford, 2003; Ponsford et al., 2013). The pathogenesis of TBI and ED has not yet been clarified.
The TBI-subjects of age of 45–64 and ≧ 65, in comparison to the TBI-subjects of age 20–44 (as the reference), were associated with lower adjusted HR for risk of ED. One study found that 45% Asians with sexual problems sought no medical help for lack of perception of their problems, embarrassment, or lack of access (Nicolosi, Glasser, Kim, Marumo, & Laumann, 2005). The study results of age effects varied on help-seeking behaviors for ED or other sexual problems: The mean age in our study was younger in one study for Chinese patients with ED, in comparison to a Western study (43.4 vs 50.4 years), but another study in the United States reveals that a significant effect of age was seen in men at age 60–69 years (OR 5.2, p ≤ .01) (Laumann, Glasser, Neves, & Moreira, 2009), compared with the referent group aged 40–49 years. Therefore, help-seeking behaviors might be one contributory factor, and further studies are needed to clarify the age distribution and help-seeking behaviors for TBI patients with ED.
Limitations
Although this study is based on the large population data and the result revealed the association between TBI and subsequent ED, there are still some limitations: First, diagnoses of TBI were identified by the ICD-9-CM codes, and authors could only separate those with psychogenic ED from those with organic ED by the ICD-9-CM codes. Furthermore, the contents of the widely used, multidimensional self-report instrument for the evaluation of male sexual function, international index of erectile function (IIEF) were not included in this claims dataset study (Rosen, Cappelleri, & Gendrano, 2002). Second, even though this might be the first study on the topic about the association between TBI and ED, clinicians should pay attention to the fact that the NRIRD lacked personal information such as body mass index, exercise habits, smoking, and alcohol consumption, which may also be associated with ED, which might reduce the utility of study results. Third, patients with ED may seek private therapy instead of visiting a hospital. Due to the culture difference and embarrassment, the diagnosis can be underestimated. Fourth, the treatment setting, or ISS ≥ 16 to categorize the severity of TBI, instead of initial Glasgow Coma Scale (GCS) score and functional outcomes which were not included in the NHIRD.
Conclusion
This study identified the association between TBI and the risk in subsequent ED. After adjusting the confounding factors such as age, comorbidity, residence of urbanization and locations, seasons, level of care, and insured premiums, patients with TBI are associated with a high risk of developing subsequent ED especially organic ED, in comparison to the controls. This finding might remind clinicians that it’s crucial in early identification and treatment of ED in post-TBI patients. Further research is required to establish this underlying mechanism.
Acknowledgments
Authors appreciate San-Yuan Huang, MD, PhD, Chin-Bin Yeh, MD, PhD, Ru-Band-Lu, MD, Yu-Ching Chou, PhD, and Wei-Shan Chiang, MSc for their help in inspiration, guidance, and initial proof-reading.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Tri-Service General Hospital Research Foundation (TSGH-C105-130, TSGH-C105-130, and TSGH-C106-002).
ORCID iDs: Kun-Ting Hong  http://orcid.org/0000-0002-6918-7550
http://orcid.org/0000-0002-6918-7550
References
- Alavi S. A., Tan C. L., Menon D. K., Simpson H. L., Hutchinson P. J. (2016). Incidence of pituitary dysfunction following traumatic brain injury: A prospective study from a regional neurosurgical centre. British Journal of Neurosurgery, 30(3), 302–306. doi: 10.3109/02688697.2015.1109060 [DOI] [PubMed] [Google Scholar]
- Baker S. P., O’Neill B., Haddon W., Jr., Long W. B. (1974). The injury severity score: A method for describing patients with multiple injuries and evaluating emergency care. The Journal of Trauma, Injury: Infection, and Critical Care, 14(3), 187–196. [PubMed] [Google Scholar]
- Bivona U., Antonucci G., Contrada M., Rizza F., Leoni F., Zasler N. D., Formisano R. (2016). A biopsychosocial analysis of sexuality in adult males and their partners after severe traumatic brain injury. Brain Injury, 30(9), 1082–1095. doi: 10.3109/02699052.2016.1165867 [DOI] [PubMed] [Google Scholar]
- Chao C. H., Chen H. J., Wang H. Y., Li T. C., Kao C. H. (2015). Increased risk of organic erectile dysfunction in patients with chronic fatigue syndrome: A nationwide population-based cohort study. Andrology, 3(4), 666–671. doi: 10.1111/andr.12052 [DOI] [PubMed] [Google Scholar]
- Chaudhary R. K., Shamsi B. H., Chen H. M., Tan T., Tang K. F., Xing J. P. (2016). Risk factors for erectile dysfunction in patients with cardiovascular disease. Journal of International Medical Research, 44(3), 718–727. doi: 10.1177/0300060515621637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. F., Liang S. J., Lin C. L., Liao W. C., Kao C. H. (2016). Sleep disorders increase risk of subsequent erectile dysfunction in individuals without sleep apnea: A nationwide population-base cohort study. Sleep Medicine, 17, 64–68. doi: 10.1016/j.sleep.2015.05.018 [DOI] [PubMed] [Google Scholar]
- Cheng C. L., Kao Y. H., Lin S. J., Lee C. H., Lai M. L. (2011). Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiology and Drug Safety, 20(3), 236–242. doi: 10.1002/pds.2087 [DOI] [PubMed] [Google Scholar]
- Chi Y. C., Wu H. L., Chu C. P., Huang M. C., Lee P. C., Chen Y. Y. (2016). Traumatic brain injury and affective disorder: A nationwide cohort study in Taiwan, 2000–2010. Journal of Affective Disorders, 191, 56–61. doi: 10.1016/j.jad.2015.11.035 [DOI] [PubMed] [Google Scholar]
- Chien W. C., Chung C. H., Lai C. H., Chou C. H. (2014). A retrospective population-based study of injury types among elderly in Taiwan. International Journal of Injury Control and Safety Promotion, 21(1), 3–8. doi: 10.1080/17457300.2012.717084 [DOI] [PubMed] [Google Scholar]
- Chien W. C., Chung C. H., Lin F. H., Yeh C. B., Huang S. Y., Lu R. B., . . . Tzeng N. S. (2017). The risk of injury in adults with attention-deficit hyperactivity disorder: A nationwide, matched-cohort, population-based study in Taiwan. Research in Developmental Disabilities, 65, 57–73. doi: 10.1016/j.ridd.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Chinese Hospital Association. (2000. a). ICD-9-CM English-Chinese dictionary. Taipei: Chinese Hospital Association Press. [Google Scholar]
- Chinese Hospital Association. (2000. b). ICD-9-CM English-Chinese dictionary. Taipei: Chinese Hospital Association Press. [Google Scholar]
- Chou I. C., Lin H. C., Lin C. C., Sung F. C., Kao C. H. (2013). Tourette syndrome and risk of depression: A population-based cohort study in Taiwan. Journal of Developmental & Behavioral Pediatrics, 34(3), 181–185. doi: 10.1097/DBP.0b013e3182829f2b [DOI] [PubMed] [Google Scholar]
- Clavijo R. I., Miner M. M., Rajfer J. (2014). Erectile dysfunction and essential hypertension: The same aging-related disorder? Reviews in Urology, 16(4), 167–171. [PMC free article] [PubMed] [Google Scholar]
- Cuesta M., Hannon M. J., Crowley R. K., Behan L. A., Tormey W., Rawluk D., . . . Thompson C. J. (2016). Symptoms of gonadal dysfunction are more predictive of hypopituitarism than nonspecific symptoms in screening for pituitary dysfunction following moderate or severe traumatic brain injury. Clinical Endocrinology, 84(1), 92–98. doi: 10.1111/cen.12874 [DOI] [PubMed] [Google Scholar]
- Downing M. G., Stolwyk R., Ponsford J. L. (2013). Sexual changes in individuals with traumatic brain injury: A control comparison. Journal of Head Trauma Rehabilitation, 28(3), 171–178. doi: 10.1097/HTR.0b013e31828b4f63 [DOI] [PubMed] [Google Scholar]
- Farre J. M., Fora F., Lasheras M. G. (2004). Specific aspects of erectile dysfunction in psychiatry. International Journal of Impotence Research, 16(Suppl 2), S46–S49. doi: 10.1038/sj.ijir.3901243 [DOI] [PubMed] [Google Scholar]
- Ghajar J. (2000). Traumatic brain injury. The Lancet, 356(9233), 923–929. doi: 10.1016/s0140-6736(00)02689-1 [DOI] [PubMed] [Google Scholar]
- Hanks R. A., Sander A. M., Millis S. R., Hammond F. M., Maestas K. L. (2013). Changes in sexual functioning from 6 to 12 months following traumatic brain injury: A prospective TBI model system multicenter study. Journal of Head Trauma Rehabilitation, 28(3), 179–185. doi: 10.1097/HTR.0b013e31828b4fae [DOI] [PubMed] [Google Scholar]
- Hibbard M. R., Uysal S., Kepler K., Bogdany J., Silver J. (1998). Axis I psychopathology in individuals with traumatic brain injury. Journal of Head Trauma Rehabilitation, 13(4), 24–39. [DOI] [PubMed] [Google Scholar]
- Ho Chan W. S. H. (2010). Taiwan’s healthcare report 2010. The EPMA Journal, 1(4), 563–585. doi: 10.1007/s13167-010-0056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T. I., Tsai T. F., Lin Y. C., Chiang H. S., Chang L. S. (2010). A survey of erectile dysfunction in Taiwan: Use of the erection hardness score and quality of erection questionnaire. The Journal of Sexual Medicine, 7(8), 2817–2824. doi: 10.1111/j.1743-6109.2010.01837.x [DOI] [PubMed] [Google Scholar]
- Johannes C. B., Araujo A. B., Feldman H. A., Derby C. A., Kleinman K. P., McKinlay J. B. (2000). Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts male aging study. The Journal of Urology, 163(2), 460–463. [PubMed] [Google Scholar]
- Kao C. C., Lin C. L., Huang W. Y., Cha T. L., Lin T. Y., Shen C. H., Kao C. H. (2016). Association between inflammatory bowel disease and erectile dysfunction: A nationwide population-based study. Inflammatory Bowel Diseases, 22(5), 1065–1070. doi: 10.1097/mib.0000000000000695 [DOI] [PubMed] [Google Scholar]
- Kaya E., Sikka S. C., Gur S. (2015). A comprehensive review of metabolic syndrome affecting erectile dysfunction. The Journal of Sexual Medicine, 12(4), 856–875. doi: 10.1111/jsm.12828 [DOI] [PubMed] [Google Scholar]
- Lane J. (2010). Hypopituitarism after brain injury. British Journal of Neurosurgery, 24(1), 8. doi: 10.3109/02688690903550240 [DOI] [PubMed] [Google Scholar]
- Laumann E. O., Glasser D. B., Neves R. C., Moreira E. D., Jr. (2009). A population-based survey of sexual activity, sexual problems and associated help-seeking behavior patterns in mature adults in the United States of America. International Journal of Impotence Research, 21(3), 171–178. doi: 10.1038/ijir.2009.7 [DOI] [PubMed] [Google Scholar]
- Leoni L. A., Fukushima A. R., Rocha L. Y., Maifrino L. B., Rodrigues B. (2014). Physical activity on endothelial and erectile dysfunction: A literature review. Aging Male, 17(3), 125–130. doi: 10.3109/13685538.2014.923836 [DOI] [PubMed] [Google Scholar]
- Liang J. A., Sun L. M., Muo C. H., Sung F. C., Chang S. N., Kao C. H. (2011). The analysis of depression and subsequent cancer risk in Taiwan. Cancer Epidemiology Biomarkers & Prevention, 20(3), 473–475. doi: 10.1158/1055-9965.epi-10-1280 [DOI] [PubMed] [Google Scholar]
- Lizza E. F., Rosen R. C. (1999). Definition and classification of erectile dysfunction: Report of the nomenclature committee of the international society of impotence research. International Journal of Impotence Research, 11(3), 141–143. [DOI] [PubMed] [Google Scholar]
- Maiorino M. I., Bellastella G., Esposito K. (2015). Lifestyle modifications and erectile dysfunction: What can be expected? Asian Journal of Andrology, 17(1), 5–10. doi: 10.4103/1008-682x.137687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalowsky B., Eichler T., Thyrian J. R., Hertel J., Wucherer D., Hoffmann W., Flessa S. (2016). Healthcare resource utilization and cost in dementia: Are there differences between patients screened positive for dementia with and those without a formal diagnosis of dementia in primary care in Germany? International Psychogeriatrics, 28(3), 359–369. doi: 10.1017/s1041610215001453 [DOI] [PubMed] [Google Scholar]
- Moretti L., Cristofori I., Weaver S. M., Chau A., Portelli J. N., Grafman J. (2012). Cognitive decline in older adults with a history of traumatic brain injury. The Lancet Neurology, 11(12), 1103–1112. doi: 10.1016/s1474-4422(12)70226-0 [DOI] [PubMed] [Google Scholar]
- National Health Insurance Administration. National health insurance regulations. Retrieved June 23, 2016, from http://www.who.int/mediacentre/news/releases/2012/dementia_20120411/en/index.htm
- Nicolosi A., Glasser D. B., Kim S. C., Marumo K., Laumann E. O. (2005). Sexual behaviour and dysfunction and help-seeking patterns in adults aged 40–80 years in the urban population of Asian countries. BJU International, 95(4), 609–614. doi: 10.1111/j.1464-410X.2005.05348.x [DOI] [PubMed] [Google Scholar]
- Ouellet M. C., Beaulieu-Bonneau S., Morin C. M. (2015). Sleep-wake disturbances after traumatic brain injury. The Lancet Neurology, 14(7), 746–757. doi: 10.1016/s1474-4422(15)00068-x [DOI] [PubMed] [Google Scholar]
- Pastuszak A. W. (2014). Current diagnosis and management of erectile dysfunction. Current Sexual Health Reports, 6(3), 164–176. doi: 10.1007/s11930-014-0023-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phe V., Roupret M. (2012). Erectile dysfunction and diabetes: A review of the current evidence-based medicine and a synthesis of the main available therapies. Diabetes & Metabolism, 38(1), 1–13. doi: 10.1016/j.diabet.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Ponsford J. (2003). Sexual changes associated with traumatic brain injury. Neuropsychological Rehabilitation, 13(1–2), 275–289. doi: 10.1080/09602010244000363 [DOI] [PubMed] [Google Scholar]
- Ponsford J. L., Downing M. G., Stolwyk R. (2013). Factors associated with sexuality following traumatic brain injury. Journal of Head Trauma Rehabilitation, 28(3), 195–201. doi: 10.1097/HTR.0b013e31828b4f7b [DOI] [PubMed] [Google Scholar]
- Rhoden E. L., Teloken C., Sogari P. R., Vargas Souto C. A. (2002). The use of the simplified International Index of Erectile Function (IIEF-5) as a diagnostic tool to study the prevalence of erectile dysfunction. International Journal of Impotence Research, 14(4), 245–250. doi: 10.1038/sj.ijir.3900859 [DOI] [PubMed] [Google Scholar]
- Roozenbeek B., Maas A. I., Menon D. K. (2013). Changing patterns in the epidemiology of traumatic brain injury. Nature Reviews Neurology, 9(4), 231–236. doi: 10.1038/nrneurol.2013.22 [DOI] [PubMed] [Google Scholar]
- Rosen R. C., Cappelleri J. C., Gendrano N. (2002). The International Index of Erectile Function (IIEF): A state-of-the-science review. International Journal of Impotence Research, 14(4), 226–244. doi: 10.1038/sj.ijir.3900857 [DOI] [PubMed] [Google Scholar]
- Sander A. M., Maestas K. (2014). Sexuality after traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 95(9), 1801–1802. doi: 10.1016/j.apmr.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Sander A. M., Maestas K. L., Nick T. G., Pappadis M. R., Hammond F. M., Hanks R. A., Ripley D. L. (2013). Predictors of sexual functioning and satisfaction 1 year following traumatic brain injury: A TBI model systems multicenter study. Journal of Head Trauma Rehabilitation, 28(3), 186–194. doi: 10.1097/HTR.0b013e31828b4f91 [DOI] [PubMed] [Google Scholar]
- Sander A. M., Maestas K. L., Pappadis M. R., Hammond F. M., Hanks R. A. (2016). Multicenter study of sexual functioning in spouses/partners of persons with traumatic brain injury. Archives of Physical Medicine and Rehabilitation, 97(5), 753–759. doi: 10.1016/j.apmr.2016.01.009 [DOI] [PubMed] [Google Scholar]
- Schneider H. J., Kreitschmann-Andermahr I., Ghigo E., Stalla G. K., Agha A. (2007). Hypothalamo pituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: A systematic review. JAMA, 298(12), 1429–1438. doi: 10.1001/jama.298.12.1429 [DOI] [PubMed] [Google Scholar]
- Shamloul R., Ghanem H. (2013). Erectile dysfunction. The Lancet, 381(9861), 153–165. doi: 10.1016/s0140-6736(12)60520-0 [DOI] [PubMed] [Google Scholar]
- Shen Y. C., Weng S. F., Wang J. J., Tien K. J. (2014). Erectile dysfunction and risk of end stage renal disease requiring dialysis: A nationwide population-based study. PLoS One, 9(7), e102055. doi: 10.1371/journal.pone.0102055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui M. A., Peng B., Shanmugam N., Yeo W., Fook-Chong S., Tat J. C. L., . . . Yue W. M. (2012). Erectile dysfunction in young surgically treated patients with lumbar spine disease: A prospective follow-up study. Spine (Phila Pa 1976), 37(9), 797–801. doi: 10.1097/BRS.0b013e318232601c [DOI] [PubMed] [Google Scholar]
- Silva P. P., Bhatnagar S., Herman S. D., Zafonte R., Klibanski A., Miller K. K., Tritos N. A. (2015). Predictors of hypopituitarism in patients with traumatic brain injury. Journal of Neurotrauma, 32(22), 1789–1795. doi: 10.1089/neu.2015.3998 [DOI] [PubMed] [Google Scholar]
- Simpson G. K., McCann B., Lowy M. (2016). Treating male sexual dysfunction after traumatic brain injury: Two case reports. NeuroRehabilitation, 38(3), 281–289. doi: 10.3233/nre-161319 [DOI] [PubMed] [Google Scholar]
- Stocchetti N. (2014). Traumatic brain injury: Problems and opportunities. The Lancet Neurology, 13(1), 14–16. doi: 10.1016/s1474-4422(13)70280-1 [DOI] [PubMed] [Google Scholar]
- Stoner H. B., Heath D. F., Yates D. W., Frayn K. N. (1980). Measuring the severity of injury. Journal of Research Social Medicine, 73(1), 19–22. [PMC free article] [PubMed] [Google Scholar]
- Turner D., Schottle D., Krueger R., Briken P. (2015). Sexual behavior and its correlates after traumatic brain injury. Current Opinion in Psychiatry, 28(2), 180–187. doi: 10.1097/yco.0000000000000144 [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Chao C. H., Lin C. L., Tseng C. H., Kao C. H. (2016). Increased subsequent risk of erectile dysfunction among middle and old age males with chronic osteomyelitis: A nationwide population-based cohort study. International Journal of Impotence Research, 28(4), 143–147. doi: 10.1038/ijir.2016.17 [DOI] [PubMed] [Google Scholar]
- War F. A., Jamuna R., Arivazhagan A. (2014). Cognitive and sexual functions in patients with traumatic brain injury. Asian Journal of Neurosurgery, 9(1), 29–32. doi: 10.4103/1793-5482.131061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimo A., Guerchet M., Ali G. C., Wu Y. T., Prina A. M., Winblad B., . . . Prince M. (2016). The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s & Dementia, 13(1), 1–7. doi: 10.1016/j.jalz.2016.07.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. L., Kor C. T., Chiu P. F., Tsai C. C., Lian I. B., Yang T. H., . . . Chang C. C. (2017). Long-term renal outcomes in patients with traumatic brain injury: A nationwide population-based cohort study. PLoS One, 12(2), e0171999. doi: 10.1371/journal.pone.0171999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. H., Chuang E., Chuang T. Y., Lin C. L., Lin M. C., Yen D. J., Kao C. H. (2016). A Nationwide population-based cohort study of migraine and organic-psychogenic erectile dysfunction. Medicine (Baltimore), 95(10), e3065. doi: 10.1097/md.0000000000003065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie M. G. (2005). The underlying pathophysiology and causes of erectile dysfunction. Clinical Cornerstone, 7(1), 19–27. [DOI] [PubMed] [Google Scholar]
- Yafi F. A., Jenkins L., Albersen M., Corona G., Isidori A. M., Goldfarb S., . . . Hellstrom W. J. G. (2016). Erectile dysfunction. Nature Reviews Disease Primers, 2, 16003. doi: 10.1038/nrdp.2016.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. H., Chen P. C., Wang T. C., Kuo T. Y., Cheng C. Y., Yang Y. H. (2016). Endocrine dysfunction following traumatic brain injury: A 5-year follow-up nationwide-based study. Scientific Reports, 6(1), 32987. doi: 10.1038/srep32987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang X. (2016). Sexual dysfunction related to antiepileptic drugs in patients with epilepsy. Expert Opinion Drug Safety, 15(1), 31–42. doi: 10.1517/14740338.2016.1112376 [DOI] [PubMed] [Google Scholar]
- Zaninotto A. L., Vicentini J. E., Fregni F., Rodrigues P. A., Botelho C., de Lucia M. C., Paiva W. S. (2016). Updates and current perspectives of psychiatric assessments after traumatic brain injury: A systematic review. Front Psychiatry, 7, 95. doi: 10.3389/fpsyt.2016.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]