Abstract
The aim of the present study was to extract potential sub-pathway biomarkers for spondyloarthropathy (SpA)/ankylosing spondylitis (AS) using a sub-pathway strategy. SpA/AS-relevant data, reference pathways and long non-coding (lnc)RNA-micro (mi)RNA-mRNA interactions were downloaded. The seed pathways based on Kyoto Encyclopedia of Genes and Genomes pathways and the mRNAs in the co-expressed lncRNA-mRNA interactions were extracted. Sub-pathways regulated by lncRNA were selected after establishing condition-specific lncRNA competitively regulated pathways (LCRP) network. Significant sub-pathways were further identified using the attract method. These significant sub-pathways were evaluated in the other independent published AS microarray data (E-GEOD-25101) using in silico validation. In addition, to uncover SpA/AS-relevant lncRNAs, the degree analysis for all nodes in the LCRP network was conducted. A total of 35 lncRNAs, 131 mRNAs and 145 co-expressed interactions were identified. When entering these 131 mRNAs into the reference pathways, 82 seed pathways were extracted, which were transformed into undirected graphs, and the 35 lncRNAs were mapped to the pathway graphs to further establish the condition-specific LCRP network. Based on degree analysis, four hub lncRNAs were selected, including C14orf169, LINC00242, LINC00116 and LINC00482. It was identified that 35 lncRNAs competitively regulating sub-pathways were involved in 56 complete pathways. Among these, the top three sub-pathways were path: 04010_1, which was a subregion of the mitogen-activated protein kinase (MAPK) signaling pathway; path: 04062-1, an important subregion in the chemokine signaling pathway; and path: 04066_2, was a part of HIF-1 signaling pathway. Furthermore, it was validated consistently in the separate microarray data set E-GEOD-25101. Cancer-associated pathways and hub node C14orf169 were identified in validation. Sub-pathways, including the MAPK signaling pathway and chemokine signaling pathway, and hub lncRNA (C14orf169) may serve important roles in SpA/AS.
Keywords: spondyloarthropathy, ankylosing spondylitis, long non-coding RNAs, sub-pathways
Introduction
Spondyloarthropathy (SpA), including ankylosing spondylitis (AS), is a type of inflammatory disorder, which is characterized by uveitis and inflammation of the axial skeleton, and associated to human leukocyte antigen-B27 (1). Initial symptoms of SpA/AS appear in the late teen and early adult years; however, due to a lack of signatures for early diagnosis, treatment is frequently delayed, ultimately leading to disability (2). There is 0.3% incidence rate of AS in people of Asian descent (3). More importantly, the molecular mechanisms driving disease progression are very poorly understood. Therefore, elucidating the pathogenesis of SpA/AS is urgently warranted.
Previously, microarray analyses have become a standard approach for finding the alterations underlying the onset and progression of disease and identifying signatures for diagnosis and response to treatment (4,5). According to literature, numerous microarray studies have been conducted on SpA/AS (6–8). Though these analyses have successfully identified a number of gene biomarkers distinguishing subjects with SpA/AS from healthy subjects, the differentially expressed genes (DEGs) listed in each study have little overlap. Due to the limited performance ability of DEGs, discovering potential pathogenic pathways is crucial, as the pathway biomarkers may enhance the accuracy of detection, relative to individual genes (9,10). Furthermore, long non-coding (lnc)RNAs were demonstrated to competitively regulate biological pathways and exert key functions during the development of bone-associated disease, for example, AS (11,12). Therefore, discovering the pathways competitively regulated by lncRNA may reveal disease pathogenesis and is helpful to expound the biological roles of lncRNAs in disease. In addition, searching for sub-pathways instead of the complete pathways may uncover more meaningful pathways and identify the functions of lncRNAs. The concept of key local subregion was created (13), which was used to successfully identify a number of important sub-pathways. So far, no data on lncRNA-regulated sub-pathways associated with SpA/AS has been reported.
In the present study, to further reveal the mechanisms of the initiation and progression of SpA/AS, a systematical tracking of sub-pathways from the lncRNA competitively regulated pathways (LCRP) based on the combination of lncRNA data and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways was conducted. This method may be beneficial for expounding the functional roles of lncRNAs in SpA/AS.
Materials and methods
Data collection
Microarray data of E-GEOD-41038 (14) were obtained from the ArrayExpress at the European Bioinformatics Institute (www.ebi.ac.uk/arrayexpress/) using the terms ‘ankylosing spondylitis’, ‘spondyloarthritis’ and ‘normal control’ on May 29, 2017. In the E-GEOD-41038, there were 15 knee synovial biopsy tissue samples, including six seronegative SpA, two AS, three osteoarthritis and four normal control biopsies. The platform of E-GEOD-41038 was A-MEXP-1172-Illumina HumanRef-8 v 3.0 Expression BeadChip (www.ebi.ac.uk/arrayexpress/experiments/E-GEOD-41038/). In order to reveal the molecular mechanisms of SpA/AS, we selected 6 seronegative SpA, two AS, and four normal control biopsies for identifying important signatures between SpA/AS and control. The other independent published AS microarray data set (E-GEOD-25101) (15) was used to conduct in silico validation.
Data preprocessing
EXPRESSO function of Affy package (16) was employed to pre-treat the gene expression profile. Specific steps included background adjustment using the robust multiarray average method, normalization via quartile method, perfect match/mismatch match probe correction by means of MAS5.0 and MEDIANPOLISH used to summarize the expression values. Ultimately, 15,593 genes were obtained.
Candidate lncRNA-mRNA interactions
Firstly, lncRNA-micro (mi)RNA interactions were collected from StarBase version 2.0 (17), and the proved mRNA-miRNA interactions were downloaded from the public databases of mirTarBase (18), miRecords (19), TarBase (20) and mir2Disease (21). According to the shared miRNAs of lncRNAs and mRNAs, the candidate lncRNA-mRNA regulated interactions were obtained. For removing unreliable data, the candidate competing mRNAs for each lncRNA were filtered using the following two criteria (22). Criterion one: A hypergeometric test was used to assess the significance of the shared miRNAs, and false discovery rate (FDR) <0.05 was selected as the cut-off threshold. Criterion two: The Jaccard Coefficient of lncRNA-mRNA interactions was calculated and ordered, and the top 20% lncRNA-mRNA interactions were reserved.
Based on the aforementioned two criteria, informative lncRNA-mRNA competitive interactions were identified, which constituted 1,749 mRNAs, 7,693 lncRNA-mRNA associations and 835 lncRNAs.
Constructing the co-expressed lncRNAs-mRNA interactions
In the present study, the Pearson correlation coefficient (PCC) was used to measure the co-expression possibility for any pair of informative lncRNA-mRNA interactions using the matched lncRNA and mRNA expression data, which is reported to measure the correlation between two variables (23). Relying on Fisher's r-to-Z transformation (24), the interaction with r value reaching a significant positive threshold (P<0.05) were kept.
Selecting important sub-pathways
Detecting seed pathways
All KEGG reference pathways were retrieved from the KEGG database. Subsequently, the genes of the co-expressed lncRNAs-mRNA interactions were entered into the reference pathways, which was utilized to correct the P-values using the Benjamini-Hochberg procedure (25). Seed pathways were identified based on the criteria of FDR <0.05.
Establishment of condition-specific LCRP
R packages were used to convert the seed pathways to undirected graphs which held the structure of the original pathways (26). The lncRNAs within the co-expressed lncRNAs-mRNA interactions were entered into the pathway graphs, in which lncRNAs associated with their mediated-mRNAs. Subsequently, the condition-specific LCRP was constructed, which included lncRNA nodes and lncRNA-mRNA regulated edges.
Locating sub-pathways competing regulated by lncRNAs
lncRNAs have been implicated to serve as signature nodes, as they competitively regulate the interested genes. Therefore, the combination of lncRNAs and the topology properties of LCRP is beneficial to effectively locate lncRNA-mediated subregions. Specifically, the shortest path between any two signature nodes was analyzed, on condition that the molecule number between each pair of signature nodes was smaller than the controlled the strength of regulated signals (n), and these signature nodes were combined into one. The molecule number involved in a given pathway more than controlled the sub-pathway size (s) was regarded as candidate sub-pathways mediated by lncRNAs s. Herein, n=1 and s=8 in the present study were utilized to extract the candidate sub-pathways.
Detection of significant sub-pathways using the attract method
To assess whether the candidate sub-pathways were competitively regulated by lncRNAs, these candidate sub-pathways were used to identify the significant sub-pathways using the attract method (27). On the basis of the analysis of variance model, Fisher's test was performed for genes in the candidate sub-pathways and the F-statistic value for gene ‘a’ was counted as follows:
In this formula, N was the total number of sub-pathways; rk represented each cell type; k =1, …, K; y was the mixed effect model; and b stood for the corresponding expression value in each replicate sub-pathway. Subsequently, a t-test was utilized to examine the F-statistics values, and the P-values were obtained. The FDR was applied to adjust the P-values using the Benjamini-Hochberg approach. The significant sub-pathways were identified based on the threshold of FDR <0.05.
Selecting hub lncRNAs in LCRP network
As reported, hub nodes constantly reflect the crucial functions of the network. In a biological network, the degree index was determined as the total count of edges connecting all nodes. Hence, in the present study, the degree distribution of the nodes in the LCRP network were measured and the top 10% lncRNAs with the highest degrees were selected to serve as hub nodes.
In silico validation in the other independent AS microarray data
To predict these important sub-pathways, further AS data were downloaded from the publicly available microarray dataset E-GEOD-25101, which represented 16 patients with AS and 16 normal patients. For verification, all steps and the defined criteria were the same as the aforementioned analysis.
Results
Identifying co-expressed lncRNA-mRNA interactions and seed pathways
In the present study, PCC was used to determine the co-expression possibility for any pair of informative lncRNA-mRNA interactions. Compared with SpA/AS-control, a total of 35 lncRNAs, 131 mRNAs and 145 co-expressed interactions were identified (data not shown). Subsequently, these 131 mRNAs were respectively aligned to the reference pathways to further detect the seed pathways. A total of 82 seed pathways were respectively identified between SpA/AS and control with the FDR set as <0.05 (Table I). Significantly, the top five pathways included pathways in cancer, hepatitis B, prostate cancer, pancreatic cancer and the phosphoinositide 3-kinase (PI3K)-RAC-α serine/threonine-protein kinase (Akt) signaling pathway.
Table I.
List of seed pathways between AS and control.
| Pathways | False discovery rate |
|---|---|
| hsa05200: Pathways in cancer | 1.48×10−23 |
| hsa05161: Hepatitis B | 1.26×10−14 |
| hsa05215: Prostate cancer | 1.93×10−14 |
| hsa05212: Pancreatic cancer | 5.37×10−13 |
| hsa04151: PI3K-Akt signaling pathway | 3.25×10−12 |
| hsa05220: Chronic myeloid leukemia | 3.94×10−11 |
| hsa05214: Glioma | 1.17×10−10 |
| hsa05211: Renal cell carcinoma | 1.43×10−10 |
| hsa05218: Melanoma | 3.77×10−10 |
| hsa05219: Bladder cancer | 1.01×10−9 |
| hsa05222: Small cell lung cancer | 4.47×10−9 |
| hsa04510: Focal adhesion | 3.68×10−8 |
| hsa04066: HIF-1 signaling pathway | 4.21×10−8 |
| hsa04520: Adherens junction | 7.60×10−8 |
| hsa05203: Viral carcinogenesis | 1.96×10−7 |
| hsa04110: Cell cycle | 3.88×10−7 |
| hsa04540: Gap junction | 6.07×10−7 |
| hsa05223: Non-small cell lung cancer | 6.97×10−7 |
| hsa04012: ErbB signaling pathway | 3.96×10−6 |
| hsa05169: Epstein-Barr virus infection | 4.10×10−6 |
| hsa04010: MAPK signaling pathway | 4.37×10−6 |
| hsa04912: GnRH signaling pathway | 6.60×10−6 |
| hsa04320: Dorso-ventral axis formation | 7.68×10−6 |
| hsa04730: Long-term depression | 1.26×10−5 |
| hsa05131: Shigellosis | 2.05×10−5 |
| hsa05166: HTLV-I infection | 2.12×10−5 |
| hsa04115: p53 signaling pathway | 3.21×10−5 |
| hsa05120: Epithelial cell signaling in Helicobacter pylori infection | 3.21×10−5 |
| hsa05213: Endometrial cancer | 4.22×10−5 |
| hsa04910: Insulin signaling pathway | 5.07×10−5 |
| hsa05145: Toxoplasmosis | 6.38×10−5 |
| hsa04722: Neurotrophin signaling pathway | 6.86×10−5 |
| hsa04916: Melanogenesis | 9.53×10−5 |
| hsa04350: TGF-β signaling pathway | 1.05×10−4 |
| hsa05142: Chagas disease (American trypanosomiasis) | 1.20×10−4 |
| hsa05210: Colorectal cancer | 1.33×10−4 |
| hsa04810: Regulation of actin cytoskeleton | 1.58×10−4 |
| hsa05216: Thyroid cancer | 1.67×10−4 |
| hsa04062: Chemokine signaling pathway | 1.81×10−4 |
| hsa04725: Cholinergic synapse | 2.26×10−4 |
| hsa04726: Serotonergic synapse | 2.42×10−4 |
| hsa04664: Fc epsilon RI signaling pathway | 2.87×10−4 |
| hsa04360: Axon guidance | 5.40×10−4 |
| hsa05221: Acute myeloid leukemia | 5.98×10−4 |
| hsa04660: T cell receptor signaling pathway | .6.42×10−4 |
| hsa04728: Dopaminergic synapse | 6.77×10−4 |
| hsa05160: Hepatitis C | 7.56×10−4 |
| hsa04150: mTOR signaling pathway | 7.88×10−4 |
| hsa05132: Salmonella infection | 1.01×10−3 |
| hsa04114: Oocyte meiosis | 1.11×10−3 |
| hsa04064: NF-κB signaling pathway | 1.41×10−3 |
| hsa04144: Endocytosis | 1.88×10−3 |
| hsa04662: B cell receptor signaling pathway | 2.05×10−3 |
| hsa04380: Osteoclast differentiation | 2.85×10−3 |
| hsa05100: Bacterial invasion of epithelial cells | 2.89×10−3 |
| hsa05016: Huntington's disease | 2.95×10−3 |
| hsa04620: Toll-like receptor signaling pathway | 3.37×10−3 |
| hsa05010: Alzheimer's disease | 3.82×10−3 |
| hsa04621: NOD-like receptor signaling pathway | 3.89×10−3 |
| hsa04210: Apoptosis | 5.01×10−3 |
| hsa04370: VEGF signaling pathway | 5.21×10−3 |
| hsa04512: ECM-receptor interaction | 5.30×10−3 |
| hsa05034: Alcoholism | 5.99×10−3 |
| hsa04666: Fc γ R-mediated phagocytosis | 6.59×10−3 |
| hsa04720: Long-term potentiation | 7.75×10−3 |
| hsa04330: Notch signaling pathway | 1.12×10−2 |
| hsa04961: Endocrine and other factor-regulated calcium reabsorption | 1.16×10−2 |
| hsa05162: Measles | 1.18×10−2 |
| hsa04310: Wnt signaling pathway | 1.48×10−2 |
| hsa05164: Influenza A | 1.61×10−2 |
| hsa05152: Tuberculosis | 1.71×10−2 |
| hsa05130: Pathogenic Escherichia coli infection | 1.84×10−2 |
| hsa05168: Herpes simplex infection | 2.10×10−2 |
| hsa04914: Progesterone-mediated oocyte maturation | 2.32×10−2 |
| hsa05020: Prion diseases | 2.83×10−2 |
| hsa04713: Circadian entrainment | 3.34×10−2 |
| hsa04650: Natural killer cell mediated cytotoxicity | 4.11×10−2 |
| hsa04723: Retrograde endocannabinoid signaling | 4.16×10−2 |
| hsa04530: Tight junction | 4.24×10−2 |
| hsa05202: Transcriptional misregulation in cancer | 4.77×10−2 |
| hsa04971: Gastric acid secretion | 4.96×10−2 |
| hsa05133: Pertussis | 4.97×10−2 |
PIK3, phosphatidylinositol 3-kinase; Akt, RAC-α serine/threonine-protein kinase; HIF1, hypoxia-inducible factor 1; ErbB, receptor tyrosine-protein kinase; MAPK, mitogen-activated protein kinase; GnRH, gonadotropin-releasing hormone; HTLV-1, human T-cell lymphotrophic virus type 1; p53, cellular tumor antigen p53; mTOR, serine/threonine-protein kinase mTOR; NF, nuclear factor; NOD, nucleotide oligomerization domain; VEGF, vascular endothelial growth factor; ECM, extracellular matrix.
Constructing the condition-specific LCRP and identifying sub-pathways
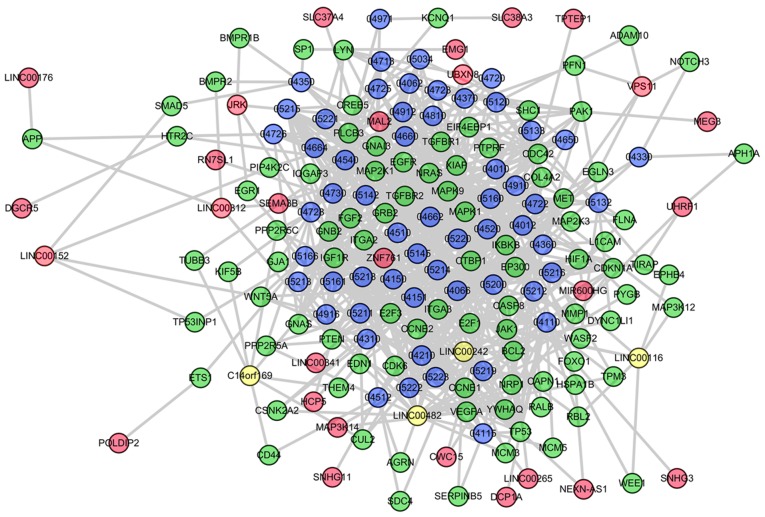
Following the extraction of seed pathways of the two groups, the seed pathways were respectively transformed into undirected graphs, and the 35 lncRNAs in the co-expressed lncRNA-mRNA interactions of SpA/AS were embedded into pathway graphs as nodes by associating with their regulated-mRNAs. An SpA/AS-specific LCRP was established, which covered lncRNA nodes in addition to lncRNA-mRNA edges. Specific LCRPs are presented in Fig. 1. In the SpA/AS-specific network, it was identified that overall, 35 significant lncRNAs competitively regulated sub-pathways involved in 56 complete pathways.
Figure 1.
Condition-specific lncRNA competitively regulates pathway networks based on matched lncRNA and mRNA expression data as well as lncRNA-mRNA interactions. Red, green and blue nodes respectively denote lncRNAs, mRNAs as well as pathways. Yellow nodes represent hub lncRNAs. lncRNA, long non-coding RNA.
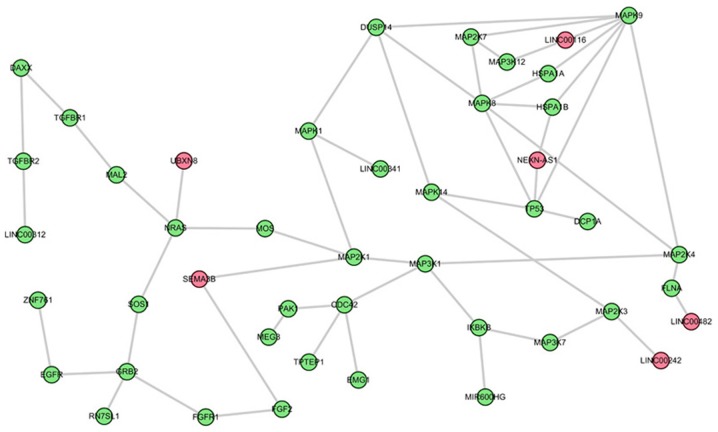
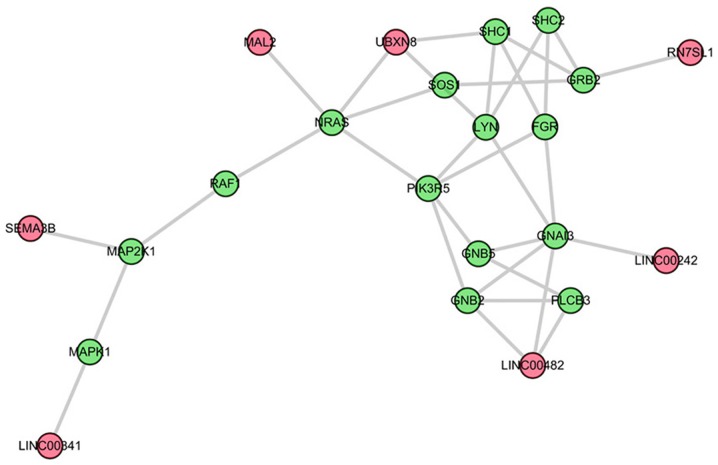
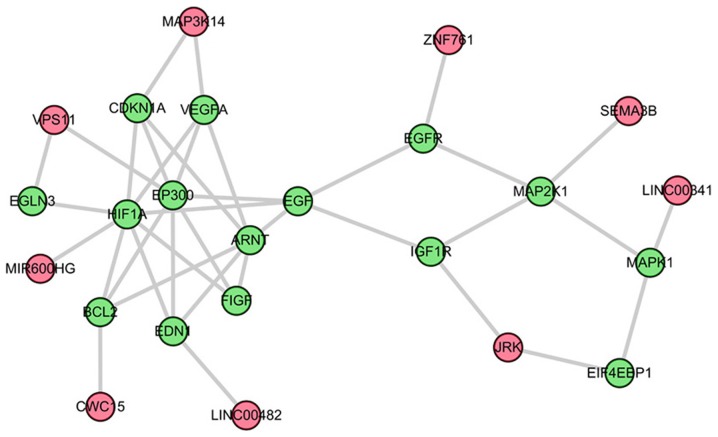
The top three sub-pathways that are competitively regulated by lncRNAs in the comparison between AS and control groups were further analyzed. The first is the most significant sub-pathway path: 04010_1, which was a subregion of mitogen-activated protein kinase (MAPK) signaling pathway (Fig. 2). Based on this module composition, it was observed that this subregion was competitively regulated by six lncRNAs. The second significant sub-pathway was path: 04062-1, an important sub region in the chemokine signaling pathway (Fig. 3). This sub-pathway was regulated by seven lncRNAs. Notably, LINC00482 and UBXN8 regulated three genes. The third sub-pathway, path: 04066_2, was a part of the HIF-1 signaling pathway (Fig. 4).
Figure 2.
Sub-pathway of the MAPK signaling pathway identified on the basis of sub-pathway strategy. Red and green nodes respectively denote long non-coding RNAs and mRNAs. MAPK, mitogen-activated protein kinase.
Figure 3.
Sub-pathway of chemokine signaling pathway identified on the basis of sub-pathway strategy. Red and green nodes respectively denote long non-coding RNAs and mRNAs.
Figure 4.
Sub-pathway of the HIF-1 signaling pathway identified on the basis of sub-pathway strategy. Red and green nodes respectively denote long non-coding RNAs and mRNAs. HIF-1, hypoxia-inducible factor 1.
Dissecting hub lncRNAs in the LCRP network
To dissect key lncRNAs associated with spondyloarthropathy, degree analysis was conducted for all nodes within the LCRP. According to the degree distribution, four hub lncRNAs in SpA/AS-specific LCRP were identified, including LINC00482 (degree=22), LINC00242 (degree=9), C14orf169 (degree=7) and LINC00116 (degree=7). The degree distribution of all lncRNAs in the SpA/AS-specific LCRP network is presented in Table II.
Table II.
Degree distribution of all lncRNAs in the SpA/AS-specific LCRP network.
| LncRNAs | Degree |
|---|---|
| LINC00482 | 22 |
| LINC00242 | 9 |
| C14orf169 | 7 |
| LINC00116 | 7 |
| VPS11 | 5 |
| UBXN8 | 4 |
| LINC00312 | 4 |
| JRK | 4 |
| LINC00152 | 4 |
| ZNF761 | 3 |
| UHRF1 | 3 |
| MIR600HG | 2 |
| SEMA3B | 2 |
| MAL2 | 2 |
| NEXN-AS1 | 2 |
| EMG1 | 2 |
| MAP3K14 | 2 |
| CWC15 | 2 |
| LINC00265 | 2 |
| HCP5 | 2 |
| LINC00341 | 1 |
| RN7SL1 | 1 |
| DCP1A | 1 |
| TPTEP1 | 1 |
| MEG3 | 1 |
| SNHG11 | 1 |
| SLC37A4 | 1 |
| DGCR5 | 1 |
| SLC38A3 | 1 |
| LINC00176 | 1 |
| SNHG3 | 1 |
| POLDIP2 | 1 |
lncRNA, long non-coding RNA; SpA/AS, spondyloarthropathy/ankylosing spondylitis; LCRP, lncRNA competitively regulated pathways.
In silico validation in the other independent AS microarray data
With the attempt to verify the significant sub-pathways identified above, the other AS data from the publicly available microarray dataset E-GEOD-25101 was used.
Following reweighting, a total of 28 lncRNAs, 123 mRNAs and 141 co-expressed interactions were extracted. The 123 mRNAs were entered into the reference pathways to identify the seed pathways. There were 11 seed pathways that differed between subjects with AS and normal subjects, based on the FDR <0.05. These pathways included the PI3K-Akt signaling pathway, focal adhesion, pathways in cancer, pancreatic cancer, cell cycle, influenza A, insulin signaling pathway, p53 signaling pathway, glioma, small cell lung cancer and prostate cancer. Significantly, it was identified that there were three common pathways between the top five pathways in the E-GEOD-41038 and the seed pathways in the E-GEOD-25101, including the PI3K-Akt signaling pathway, pathways in cancer and pancreatic cancer (Table III).
Table III.
Common seed pathways in the top five seed pathways of E-GEOD-41038 and the top five seed pathways in E-GEOD-25101.
| Top five seed pathways in E-GEOD-41038 | Top five seed pathways in E-GEOD-25101 | Common seed pathways |
|---|---|---|
| Pathways in cancer | PI3K-Akt signaling pathway | Pathways in cancer |
| Hepatitis B | Focal adhesion | Pancreatic cancer |
| Prostate cancer | Pathways in cancer | PI3K-Akt signaling pathway |
| Pancreatic cancer | Pancreatic cancer | |
| PI3K-Akt signaling pathway | Cell cycle |
PIK3, phosphatidylinositol 3-kinase; Akt, RAC-α serine/threonine-protein kinase.
Following obtaining the seed pathways, the LCRP was established, which included lncRNA nodes and lncRNA-mRNA edges. Within the LCRP network, a total of 21 significant lncRNAs competitively regulating sub-pathways involved in 11 complete pathways were identified. In further analysis, the top three sub-pathways that were competitively regulated by lncRNAs in the comparison between the AS and normal groups were investigated. The first most significant sub-pathway was path: 04115_1, which was a subregion of the p53 signaling pathway. The second significant sub-pathway was path: 05222_1, an important subregion in small cell lung cancer. The third sub-pathway, path: 05214_1, was involved in glioma. Notably, the top three sub-pathways in the E-GEOD-41038 and E-GEOD-25101 were identified as cancer-associated pathways. Based on the degree distribution, three hub lncRNAs were screened out, including ZNF761, DCP1A and C14orf169. Notably, it was observed that the hub lncRNA C14orf169 was the most common in the E-GEOD-41038 and E-GEOD-25101 (data not shown). These findings demonstrated that the contents of the present study are reliable.
Discussion
Previously, a number of studies have implied that disruption of cellular pathways competitively mediated by lncRNAs may lead to the onset of disorders (28–30). Therefore, understanding this regulation mechanism may offer novel opportunities for detecting key signatures for disease and for developing novel target therapies. However, the research regarding lncRNA functions involved in SpA/AS is in its infancy. Furthermore, more attention to crucial sub-pathways instead of entire pathways may be more applicable to reveal the roles of lncRNAs in a given disease (13). Additionally, this subregion strategy integrating lncRNA-mRNA data and pathway topologies has a number of advantages. Firstly, lncRNA as a type of novel regulatory layer is covered in the pathway analysis. Secondly, the joint effect of lncRNAs, pathway topologies, in addition to lncRNA competitively regulated genes was comprehensively measured. The sub-pathway method may detect more meaningful pathways. SpA, including AS and non-radiographic SpA, is connected with a significant burden of disease and typically affects patients with AS of working age. Therefore, it is urgently required to identify molecular targets to prevent SpA/AS development, and further improve the prognosis of patients with SpA/AS. In the present study, in order to reveal the etiopathogenesis of SpA/AS, gene expression data E-GEOD-41038 were investigated to identify significant sub-pathways, which may be involved in SpA/AS progression by combining lncRNA-mRNA expression data with pathway topologies using the sub-pathway strategy. A total of 35 significant lncRNAs competitively regulating sub-pathways were involved in 56 complete pathways. The first was the most significant sub-pathway path: 04010_1, which is a subregion of the MAPK signaling pathway. The second significant sub-pathway was path: 04062-1, an important subregion in the chemokine signaling pathway.
The MAPK signaling pathway is known to mediate stress responses and is activated by the proinflammatory cytokines interleukin-1 or tumor necrosis factor-α (31). There are no reports, to the best of the author's knowledge, demonstrating the direct association between the MAPK pathway and SpA/AS. The MAPK signaling pathway has been demonstrated to be highly associated with the functioning of the immune response (32). Furthermore, Chen et al (33) demonstrated that one MAPK pathway serves a key function in the induction of the proinflammatory response, which is involved in SpA. Furthermore, inflammation is suggested to be associated with novel bone formation, which is highly associated with the development of SpA and AS (34,35). Bone formation requires differentiation of osteoblasts (36). Notably, the MAPK pathway is implicated in the regulation of osteoblast differentiation (37,38). Inactivation of the pro-osteogenic MAPK pathway has been reported to inhibit osteoblast differentiation (39). Therefore, it may be inferred that MAPK may serve crucial roles in SpA/AS, partially by regulating the resolution of inflammation and the subsequent new bone formation.
The second sub-pathway was the chemokine signaling pathway in the present analysis. Chemokines are crucial mediators in the inflammatory response, and in parallel, members of the chemokine system serve important roles in AS occurrence and progression (40,41). In addition, Chen et al (33) reported that SpA is associated with certain proinflammatory pathways, for example, the chemokine signaling pathway. Furthermore, Duftner et al (42) reported that type 1 and type 2 chemokines and lymphocytic expression of chemokine receptors serve important roles in AS. Yang et al (43) additionally demonstrated that the chemokine receptor CCR4 is increased in AS. Accordingly, it is speculated that the chemokine signaling pathway may contribute to the progression of SpA/AS, thereby suggesting that this created sub-pathway method is a good approach for biomarker prediction.
C14orf169 was one of the hub lncRNAs in the present study for E-GEOD-41038. In the in silico validation using E-GEOD-25101, C14orf169 was additionally identified as the hub node in the LCRP. The alias of C14orf169 is NO66. NO66 proteins are believed to exhibit enzymatic activity, which regulates gene expression and chromatin remodeling (44). Chromatin remodeling is crucial for controlling Osterix function, which is an osteoblast-specific transcription factor required for osteoblast differentiation and bone formation (45). In accordance with the aforementioned study, a different previous study strongly supported the physiological role of NO66 during osteoblast differentiation (46). C14orf169 may account, at least partially, for the progression of SpA/AS, by regulating bone formation and differentiation.
The present study was the first, to the best of the authors' knowledge, to conduct an analysis on SpA/AS based on a sub-pathway strategy by systematically integrating pathway information with lncRNA-mRNA data. This may be considered the primary strength of the present study. Overall, a number of significant sub-pathways were successfully identified based on this computational method. However, numerous limitations must be taken into consideration in the present study. To begin with, the sample data were recruited from the open access database. The SpA/AS samples used for microarray analysis were not obtained by the present study. Although a number of key sub-pathways and lncRNAs of interest were identified in the present study, it must be considered as an exploratory study at the present time. In addition, the present study only used a bioinformatics approach to select significant sub-pathways to reveal the etiopathogenic process of SpA/AS; however, the association between sub-pathways and SpA/AS has not been validated by experiments. This was the principal limitation. As a result, further independent confirmation studies are required to prove the significance of the present initial findings. Although these drawbacks existed, it was confirmed that the predicted sub-pathways offer researchers valuable resources for providing guidance for focusing research efforts to elucidate disease mechanisms, and detect potential biomarkers for early diagnosis and therapy of SpA/AS. Furthermore, this strategy may be useful for the study of other diseases.
In conclusion, sub-pathways, including the MAPK signaling pathway and chemokine signaling pathway, may be potential biomarkers for SpA/AS therapy. The identified sub-pathways and lncRNAs may provide valuable diagnostic and therapeutic targets for SpA/AS.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MD and TJG performed the experiments, analyzed the data and drafted the manuscript. CYW and BHC conceptualized the study design and critically revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Garg N, van den Bosch F, Deodhar A. The concept of spondyloarthritis: Where are we now? Best Pract Res Clin Rheumatol. 2014;28:663–672. doi: 10.1016/j.berh.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Park R, Kim TH, Ji JD. Gene expression profile in patients with axial spondyloarthritis: Meta-analysis of publicly accessible microarray datasets. J Rheum Dis. 2016;23:363–372. doi: 10.4078/jrd.2016.23.6.363. [DOI] [Google Scholar]
- 3.Guo YY, Yang LL, Cui HD, Zhao S, Zhang N. Coexisting ankylosing spondylitis and rheumatoid arthritis: A case report with literature review. Chin Med J (Engl) 2011;124:3430–3432. [PubMed] [Google Scholar]
- 4.Bauer JW, Bilgic H, Baechler EC. Gene-expression profiling in rheumatic disease: Tools and therapeutic potential. Nat Rev Rheumatol. 2009;5:257–265. doi: 10.1038/nrrheum.2009.50. [DOI] [PubMed] [Google Scholar]
- 5.Häupl T, Stuhlmüller B, Grützkau A, Radbruch A, Burmester GR. Does gene expression analysis inform us in rheumatoid arthritis? Ann Rheum Dis. 2010;69:37–42. doi: 10.1136/ard.2009.119487. [DOI] [PubMed] [Google Scholar]
- 6.Duan R, Leo P, Bradbury L, Brown MA, Thomas G. Gene expression profiling reveals a downregulation in immune-associated genes in patients with AS. Ann Rheum Dis. 2010;69:1724. doi: 10.1136/ard.2009.111690. [DOI] [PubMed] [Google Scholar]
- 7.Sharma SM, Choi D, Planck SR, Harrington CA, Austin CR, Lewis JA, Diebel TN, Martin TM, Smith JR, Rosenbaum JT. Insights in to the pathogenesis of axial spondyloarthropathy based on gene expression profiles. Arthritis Res Ther. 2009;11:R168. doi: 10.1186/ar2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assassi S, Reveille JD, Arnett FC, Weisman MH, Ward MM, Agarwal SK, Gourh P, Bhula J, Sharif R, Sampat K, et al. Whole-blood gene expression profiling in ankylosing spondylitis shows upregulation of toll-like receptor 4 and 5. J Rheumatol. 2011;38:87–98. doi: 10.3899/jrheum.100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Agarwal P. A pathway-based view of human diseases and disease relationships. PLoS One. 2009;4:e4346. doi: 10.1371/journal.pone.0004346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ulitsky I, Krishnamurthy A, Karp RM, Shamir R. DEGAS: De novo discovery of dysregulated pathways in human diseases. PLoS One. 2010;5:e13367. doi: 10.1371/journal.pone.0013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Chai W, Zhang G, Ni M, Chen J, Dong J, Zhou Y, Hao L, Bai Y, Wang Y. Down-regulation of lncRNA-AK001085 and its influences on the diagnosis of ankylosing spondylitis. Med Sci Monit. 2017;23:11–16. doi: 10.12659/MSM.898915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sui W, Li H, He H, Xue W, Zhao X, Dai Y. Microarray analysis of long non-coding RNA expression in ankylosing spondylitis. doi: 10.15761/IMM.1000172. [DOI] [Google Scholar]
- 13.Lin S, Li T, Zhu D, Ma C, Wang Y, He L, Zhu C, Xing Q. The association between GAD1 gene polymorphisms and cerebral palsy in Chinese infants. Tsitol Genet. 2013;47:22–27. [PubMed] [Google Scholar]
- 14.Thomas GP, Ran D, Pettit AR, Helen W, Simranpreet K, Malcolm S, Brown MA. Expression profiling in spondyloarthropathy synovial biopsies highlights changes in expression of inflammatory genes in conjunction with tissue remodelling genes. BMC Musculoskel Disord. 2013;14:354. doi: 10.1186/1471-2474-14-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pimentelsantos FM, Ligeiro D, Matos M, Mourão AF, Costa J, Santos H, Barcelos A, Godinho F, Pinto P, Cruz M, et al. Whole blood transcriptional profiling in ankylosing spondylitis identifies novel candidate genes that might contribute to the inflammatory and tissue-destructive disease aspects. Arthritis Res Ther. 2011;13:R57. doi: 10.1186/ar3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautier L, Cope L, Bolstad BM, Irizarry RA. Affy-analysis of Affymetrix GeneChip data at the probe level. Bioinformatics. 2004;20:307–315. doi: 10.1093/bioinformatics/btg405. [DOI] [PubMed] [Google Scholar]
- 17.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database Issue):D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu SD, Lin FM, Wu WY, Liang C, Huang WC, Chan WL, Tsai WT, Chen GZ, Lee CJ, Chiu CM, et al. Mirtarbase: A database curates experimentally validated microrna-target interactions. Nucleic Acids Res. 2011;39(Database Issue):D163–D169. doi: 10.1093/nar/gkq1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao F, Zuo Z, Cai G, Kang S, Gao X, Li T. Mirecords: An integrated resource for microrna-target interactions. Nucleic Acids Res. 2009;37(Database Issue):D105–D110. doi: 10.1093/nar/gkn851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vergoulis T, Vlachos IS, Alexiou P, Georgakilas G, Maragkakis M, Reczko M, Gerangelos S, Koziris N, Dalamagas T, Hatzigeorgiou AG. TarBase 6.0: Capturing the exponential growth of miRNA targets with experimental support. Nucleic Acids Res. 2011;40(Database Issue):D222–D229. doi: 10.1093/nar/gkr1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Q, Wang Y, Hao Y, Juan L, Teng M, Zhang X, Li M, Wang G, Liu Y. miR2Disease: A manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009;37(Database Issue):D98–D104. doi: 10.1093/nar/gkn714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi X, Xu Y, Zhang C, Feng L, Sun Z, Han J, Su F, Zhang Y, Li C, Li X. Subpathway-LNCE: Identify dysfunctional subpathways competitively regulated by lncRNAs through integrating lncRNA-mRNA expression profile and pathway topologies. Oncotarget. 2016;7:69857–69870. doi: 10.18632/oncotarget.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nahler G. Pearson correlation coefficient. Dictionary of Pharmaceutical Medicine. 2010:132. [Google Scholar]
- 24.Best DJ, Roberts DE. Algorithm AS 89: The upper tail probabilities of Spearman's Rho. J Royal Stat Soc Series C (Appl Stat) 1975;24:377–379. [Google Scholar]
- 25.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 26.Li C, Han J, Yao Q, Zou C, Xu Y, Zhang C, Shang D, Zhou L, Zou C, Sun Z, et al. Subpathway-GM: Identification of metabolic subpathways via joint power of interesting genes and metabolites and their topologies within pathways. Nucleic Acids Res. 2013;41:e101. doi: 10.1093/nar/gkt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mar JC, Matigian NA, Quackenbush J, Wells CA. Attract: A method for identifying core pathways that define cellular phenotypes. PLoS One. 2011;6:e25445. doi: 10.1371/journal.pone.0025445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu AL, Fan MP, Liu DQ. Dysfunctional subpathways of osteoarthritis identified through combining lncRNA-mRNA expression profile with pathway topologies. Int J Clin Exp Med. 2018;11:1260–1269. [Google Scholar]
- 29.Chen X, Dong H, Liu S, Yu L, Yan D, Yao X, Sun W, Han D, Gao G. Long noncoding RNA MHENCR promotes melanoma progression via regulating miR-425/489-mediated PI3K-Akt pathway. Am J Transl Res. 2017;9:90–102. [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, He L, Du Y, Zhu P, Huang G, Luo J, Yan X, Ye B, Li C, Xia P, et al. The long noncoding RNA lncTCF7 promotes self-renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. 2015;16:413–425. doi: 10.1016/j.stem.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Freshney NW, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-X. [DOI] [PubMed] [Google Scholar]
- 32.Lee MS, Kim YJ. Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem. 2007;76:447–480. doi: 10.1146/annurev.biochem.76.060605.122847. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T, Liu Y, Tupper T, Ouyang J, Li J, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;483:613–617. doi: 10.1038/nature10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lories RJ, Luyten FP, de Vlam K. Progress in spondylarthritis. Mechanisms of new bone formation in spondyloarthritis. Arthritis Res Ther. 2009;11:221. doi: 10.1186/ar2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen SJ, Chiowchanwisawakit P, Lambert RG, Østergaard M, Maksymowych WP. Resolution of inflammation following treatment of ankylosing spondylitis is associated with new bone formation. J Rheumatol. 2011;38:1349–1354. doi: 10.3899/jrheum.100925. [DOI] [PubMed] [Google Scholar]
- 36.Lee EJ, Lee EJ, Chung YH, Song DH, Hong S, Lee CK, Yoo B, Kim TH, Park YS, Kim SH, et al. High level of interleukin-32 gamma in the joint of ankylosing spondylitis is associated with osteoblast differentiation. Arthritis Res Ther. 2015;17:350. doi: 10.1186/s13075-015-0870-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ge C, Xiao G, Jiang D, Franceschi RT. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J Cell Biol. 2007;176:709–718. doi: 10.1083/jcb.200610046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y, Chan E, Wang SX, Li B. Activation of p38 mitogen-activated protein kinase is required for osteoblast differentiation. Endocrinology. 2003;144:2068–2074. doi: 10.1210/en.2002-220863. [DOI] [PubMed] [Google Scholar]
- 39.Redlich K, Smolen JS. Inflammatory bone loss: Pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11:234–250. doi: 10.1038/nrd3669. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Zhao Q, Wang G, Yang C, Xu Y, Li Y, Yang P. Circulating levels of Th1 and Th2 chemokines in patients with ankylosing spondylitis. Cytokine. 2016;81:10–14. doi: 10.1016/j.cyto.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Tao K, Tang X, Wang B, Li RJ, Zhang BQ, Lin JH, Li H. Distinct expression of chemokine-like factor 1 in synovium of osteoarthritis, rheumatoid arthritis and ankylosing spondylitis. J Huazhong Univ Sci Technology Med Sci. 2016;36:70–76. doi: 10.1007/s11596-016-1544-4. [DOI] [PubMed] [Google Scholar]
- 42.Duftner C, Dejaco C, Kullich W, Klauser A, Goldberger C, Falkenbach A, Schirmer M. Preferential type 1 chemokine receptors and cytokine production of CD28- T cells in ankylosing spondylitis. Ann Rheum Dis. 2006;65:647–653. doi: 10.1136/ard.2005.042085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang PT, Kasai H, Zhao LJ, Xiao WG, Tanabe F, Ito M. Increased CCR4 expression on circulating CD4(+) T cells in ankylosing spondylitis, rheumatoid arthritis and systemic lupus erythematosus. Clin Exp Immunol. 2004;138:342–347. doi: 10.1111/j.1365-2249.2004.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eilbracht J, Reichenzeller M, Hergt M, Schnölzer M, Heid H, Stöhr M, Franke WW, Schmidt-Zachmann MS. NO66, a highly conserved dual location protein in the nucleolus and in a special type of synchronously replicating chromatin. Mol Biol Cell. 2004;15:1816–1832. doi: 10.1091/mbc.e03-08-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 46.Sinha KM, Yasuda H, Coombes MM, Dent SY, De Crombrugghe B. Regulation of the osteoblast-specific transcription factor Osterix by NO66, a Jumonji family histone demethylase. EMBO J. 2010;29:68–79. doi: 10.1038/emboj.2009.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.