Abstract
Diabetic nephropathy (DN) is one of the most common microvascular complications associated with diabetes mellitus (DM); the incidence has been predicted to reach 7.7% by 2030 on a global scale. Krüppel-like factor 5 (KLF5) is involved in numerous important biological processes; however, the potential effects of KLF5 on podocytes in patients with diabetic nephrotic (DN) have not yet been investigated. In the present study, synaptopodin expression in podocytes was investigated using an immunofluorescence assay. Following this, the proliferation of podocytes was investigated using an MTT assay. In addition, KLF5 was overexpressed in podocytes, and cell cycle arrest and apoptosis was subsequently investigated using flow cytometry. Western blotting and reverse transcription-quantitative polymerase chain reaction assays were performed to detect the expression levels of genes involved in the cell cycle and apoptosis, and the extracellular signal-regulated protein kinase (ERK)/p38 mitogen-activated protein (MAP) kinase pathway. The results demonstrated that treatment with puromycin aminonucleoside (PAN) suppressed the proliferation of podocytes in a dose- and time-dependent manner, and overexpression of KLF5 induced cell cycle arrest of podocytes regulated by PAN. Furthermore, overexpression of KLF5 was revealed to have inhibited PAN-induced apoptosis of podocytes, and that overexpression of KLF5 suppressed the ERK/p38 MAP kinase pathway in podocytes induced by PAN. Therefore, the results of the present study suggested that KLF5 may represent a potential therapeutic target for treatment of patients with DN.
Keywords: Krüppel-like factor 5, extracellular-regulated protein kinase/mitogen-activated protein kinase pathway, podocytes, puromycin aminonucleoside
Introduction
Diabetic mellitus (DM) is a progressive disease and is usually associated with numerous complications (1–4). The incidence of DM is predicted to reach 7.7% by 2030 on a global scale (5,6). Diabetic nephropathy (DN) is one of the most common microvascular complications associated with DM and has become the second leading cause of end-stage renal disease in China (7). Numerous studies have demonstrated that well-managed blood glucose, blood pressure and blood lipid levels, and medicinal application of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, may postpone disease progression (8,9). The exact mechanisms underlying the pathogenesis of DN remain unclear. A previous study revealed that the onset of DN may be pertinent to oxidative stress in living cells (10). Podocytes are considered to be the most critical component of glomerular permselectivity (11). Changes in podocyte morphology and dysfunction are closely associated with renal diseases (12,13). The apoptosis of podocytes has been regarded as an important factor in the progression of DN (14). Therefore, investigating the molecular mechanism associated with the inhibition of podocyte injury is important for the development of novel therapeutic strategies for the treatment of patients with DN.
Krüppel-like factors (KLFs) are a type of transcriptional regulatory factor. KLFs are associated with cell proliferation, migration, apoptosis and tissue remodeling (15,16). Furthermore, KLFs are associated with the development of numerous diseases, including cardiovascular disease and cancer (17–19). KLF5, a member of the KLF family, can be regulated via phosphorylation, acetylation and ubiquitination following translation (20,21). KLF5 is associated with hypertensive nephropathy and diabetic retinopathy (22). However, the effects of KLF5 on DN are not fully understood.
Puromycin aminonucleoside (PAN) may disrupt the morphology of podocytes, trigger the overproduction of reactive oxygen species and induce nephrosis. Therefore, PAN is frequently used to establish a nephropathy model (23,24). The aim of the present study was to investigate the effect of KLF5 on PAN-induced injury of podocytes and to determine the underlying molecular mechanism. Therefore, the results of the present study may further the understanding of molecular mechanisms associated with DN and provide a potential therapeutic target for DN.
Materials and methods
Cell culture and cell treatment
Podocytes (MPC-5 cells) were acquired from the Bena Culture Collection (Beijing, China). Podocytes were cultured in Ham Nutrient Mix F12-Dulbecco modified Eagle medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 1% penicillin-streptomycin G (Biochrom, Ltd., Cambridge, UK) and 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). Podocytes were seeded in 6-well plates at a concentration of 3×105 cells/ml and subsequently treated with PBS (control), or with PAN (0, 5, 10, 20, 40, 60, 80 and 100 µg/ml) for 0, 6, 12, 24 and 48 h time intervals at 37°C.
Plasmid construction
Mouse KLF5 complementary (c)DNA clone was purchased from GeneCopoeia Inc. (Rockville, MD, USA). KLF5 was amplified with PrimSTAR DNA polymerase (Takara Bio, Inc., Otsu, Japan) using primers with methylation-sensitive EcoRI and XhoI restriction sites. The PCR thermocycling was set as: 95°C, 3 min; 30 cycles of 95°C, 30 sec; 58°C, 30 sec; 72°C, 90 sec; 72°C, 10 min. The primer sequences of KLF5 were as follows: Forward, 5′ATGCCCACGCGGGTGCTGACC3′ and reverse, 5′ATGAAGCGCCACCAGAACTGA3′. PCR products were then digested with EcoRI and XhoI restriction endonucleases and subsequently inserted into the pcDNA 3.1 vector (Invitrogen; Thermo Fisher Scientific, Inc.).
Cell viability assay
Treated podocytes (2×103 cells/well) were seeded into a 96-well plate. The cells were treated with PAN at different concentrations (0, 5, 10, 20, 40, 60, 80 and 100 µg/ml) and maintained at 37°C for 0, 6, 12, 24 and 48 h time intervals. Following this, 20 µl of MTT solution was added into each well and incubated for 30 min. A total of 150 µl dimethylsulfoxide was subsequently was added into each well. Finally, a microplate reader was used to determine the absorbance at 490 nm. Treatment with 60 µg/ml PAN for 6 h was selected for subsequent analysis as this resulted in decreased cell viability (described below).
Cell transfection
Podocytes were plated in 6-well plates at a density of 1×106 cells per well. Cells were treated with PBS or 60 µg/ml PAN for 6 h at 37°C. KLF5 overexpression (1 ug) or negative control plasmids (pcDNA 3.1; 1 µg) were transfected into podocytes using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. After 12 h following transfection, the cells were used for subsequent experimentation.
RT-qPCR assay
Total RNA was obtained using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). RNA was reversely transcribed to cDNA using a miScript II RT Kit (Qiagen GmbH, Hilden, Germany). The temperature protocol or RT was 25°C for 5 min, 37°C for 60 min, 85°C for 5 min and then held at 4°C. The mRNA expression levels were subsequently determined using the SYBR-Green PCR Master Mix kit (Takara Bio, Inc.) and the ABI 7500 system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling conditions were set as: 5 min pretreatment at 95°C, followed by 28 cycles of 95°C for 15 sec and 60°C for 30 sec, a final extension at 72°C for 10 min. The specific primers used were as follows: KLF5 forward, 5′-TTTCTGTCCCTACCCAGCAG-3′ and reverse, 5′-AGTAAGTGGCCTGTTGTGGA-3′; Bax forward, 5′-GAGCTGCAGAGGATGATTGC-3′ and reverse, 5′-CCAATGTCCAGCCCATGATG-3′; Bcl-2 forward, 5′-GCCTTCTTTGAGTTCGGTGG-3′ and reverse, 5′-GAAATCAAACAGAGGCCGCA-3′; Caspase-3 forward, 5′-TTGCCACCTGTCCAGTTTTG-3′ and reverse, 5′-AGGAGTGAGTGGTCTTGCTC-3′; caspase-8 forward, 5′-TTTCTGTCCCTACCCAGCAG-3′ and reverse, 5′-AGTAAGTGGCCTGTTGTGGA-3′; Caspase-9 forward, 5′-GCCCCATATGATCGAGGACA-3′ and reverse, 5′-CAGAAACGAAGCCAGCATGT-3′; cyclin D1 forward, 5′-GCTGCTCCTGGTGAACAAGC-3′ and reverse, 5′-TTGCGTCTCAGCTCAGGGAC-3′; and c-myc forward, 5′-CCACAGCAAACCTCCTCACA-3′ and reverse, 5′-TCCAACTTGACCCTCTTGGC-3′. GAPDH forward, 5′-GGGTCCCAGCTTAGGTTCAT-3′; GAPDH reverse, 5′-CATTCTCGGCCTTGACTGTG-3′. Data were quantified using the 2−ΔΔCq method (25).
Western blot analysis
Total proteins were prepared using a radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Shanghai, China) containing a protease inhibitor cocktail (P8340; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Protein concentrations were determined using a Bradford Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins (30 µg) were separated via 10% SDS-PAGE gels and then transferred onto polyvinyl difluoride (PVDF) membranes (PerkinElmer, Inc., Waltham, MA, USA). Following this, the membranes were blocked with 5% skimmed milk (BD Biosciences, Franklin Lakes, NJ, USA) at room temperature for 2 h. The membranes were subsequently incubated with the following primary antibodies overnight at 4°C: Anti-GAPDH (1:2,000; cat. no. ab8245; Abcam, Cambridge, UK), anti-KLF5 (1:1,000; cat. no. ab24331; Abcam), anti-B cell lymphoma 2 (Bcl-2) associated X (Bax; 1:1,000; cat. no. ab32503; Abcam), Bcl-2 (1:1,000; cat. no. ab32124; Abcam), anti-caspase-3 (1:1,500; cat. no. ab13586; Abcam), anti-caspase-8 (1:1,500; cat. no. ab25901; Abcam), anti-caspase-9 (1:1,500; cat. no. ab25758; Abcam), anti-cyclin D1 (1:1,000; cat. no. ab134175; Abcam), anti-c-myc (1:1,000; cat. no. ab39688; Abcam), anti-phosphorylated (p)-extracellular signal-regulated protein kinase (ERK)1/2 (1:1,200; cat. no. 4370; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-ERK1/2 (1:1,000; cat. no. ab184699; Abcam), anti-p-p38 (1:1,000; cat. no. ab47363; Abcam), anti-p38 (1:1,000; cat. no. ab170099; Abcam). PVDF membranes were subsequently incubated with a horseradish peroxidase-conjugated secondary antibody (1:5,000; cat. no. sc-2004, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for 1 h. Finally, the proteins were visualized using ECL Western Blotting Substrate (Pierce; Thermo Fisher Scientific, Inc.) in an enhanced chemiluminescence detection system (GE Healthcare, Chicago, IL, USA). The gray value was determined by Quantity One 4.6.2 software (Bio-Rad Laboratories, Inc.).
Immunofluorescence (IF) staining
Podocytes were fixed with 4% paraformaldehyde at 4°C for 20 min, and then permeabilized with 0.2% Triton X-100 for 3 min. Following this, cells were washed with PBS and then blocked using 10% goat serum (Thermo Fisher Scientific, Inc.) for 30 min at room temperature. Cells were subsequently incubated with 4 µg/ml anti-synaptopodin antibodies (cat. no. ab220345; Abcam) overnight at 4°C. Following this, cells were washed using PBS and then incubated with Alexa-Fluor® 633-conjugated secondary antibodies (1:500; A-21070, Thermo Fisher Scientific, Inc.) at 37°C for 1 h. Cells were subsequently stained using 4′-6-diamidino-2-phenylindole (Thermo Fisher Scientific, Inc.) for 5 min at room temperature. A fluorescence microscope (magnification, ×200; Olympus Corporation, Tokyo, Japan) was used to visualize the results.
Flow cytometry
Podocytes were treated as described above. Cells (2×105 cells/well) were seeded into 24-well plates and incubated overnight at 37°C. To investigate the cell cycle, cells were centrifuged at 1,000 × g for 5 min at 4°C and subsequently fixed using 70% (v/v) ethanol for 1 h at 4°C. Following this, cells were washed with PBS and then incubated with 500 µl propidium iodide (PI)/RNase buffer (BD Biosciences, Franklin Lakes, NJ, USA) at room temperature for 30 min. Finally, a FACS-Calibur flow cytometer (BD Biosciences) was used to investigate cell cycle distribution, and ModFit LT 2.0 software (Verity Software House, Inc., Topsham, ME, USA) was used to analyze the results. To investigate apoptosis, cells were washed with PBS and then re-suspended with 0.5 ml binding buffer containing 5 µl Annexin V-fluorescein isothiocyanate and PI double stain (BD Biosciences) for 20 min at room temperature in the dark. Following this, the apoptotic rate was determined using a FACS-Calibur flow cytometer (BD Biosciences) and ModFit LT 2.0 software.
Statistical analysis
All data were analyzed by SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) using one-way analysis of variance with Tukey's test. Data are presented as the mean ± standard deviation. All experiments were performed in triplicate. P<0.05 was considered to indicate a statistically significant difference.
Results
PAN inhibits the proliferation of podocytes
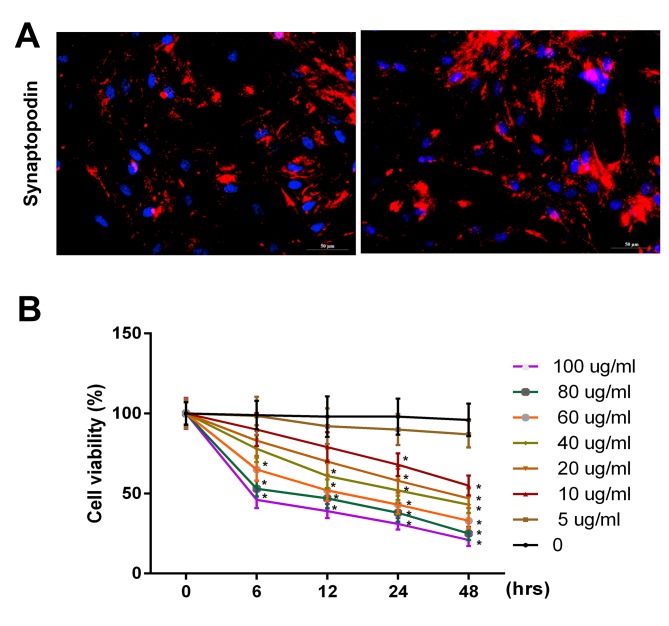
Initially, synaptopodin expression in podocytes was investigated using immunofluorescence. The results revealed that the expression of synaptopodin was positive in podocytes (Fig. 1A). To further investigate the effect of PAN on the proliferation ability of podocytes, podocytes were treated with PBS (control) or PAN (5, 10, 20, 40, 60, 80, and 100 µg/ml) for 0, 6, 12, 24 and 48 h time intervals. The results revealed that the viability of podocytes were markedly inhibited following treatment with PAN in a dose- and time-dependent manner (Fig. 1B; P<0.05). It was observed that the cell viability significantly decreased when the cells were treated with 60 µg/ml PAN for 6 h. Therefore, the present study selected 60 µg/ml PAN to treat the cells for 6 h.
Figure 1.
PAN inhibited the proliferative ability of podocytes in a dose- and time-dependent manner. (A) Synaptopodin expression in podocytes was detected via immunofluorescence assay using a fluorescence microscope (magnification, ×200). (B) Cell viability in podocytes was analyzed using an MTT assay following g treatment with PBS (control), or PAN (5, 10, 20, 40, 60, 80 and 100 µg/ml) for 0, 6, 12, 24 and 48 h time intervals. PAN, puromycin aminonucleoside. *P<0.05 vs. 0 h.
KLF5 is overexpressed in transfected podocytes
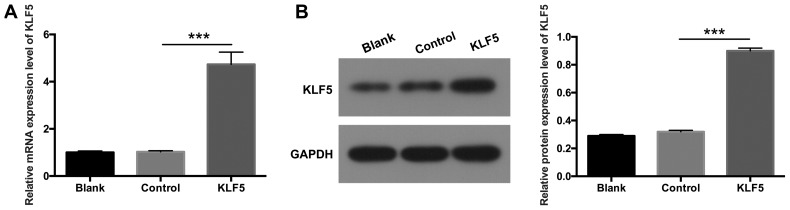
To further investigate the potential role of KLF5 in podocytes, KLF5 was overexpressed in podocytes via transfection with pCDNA3.1-KLF5 plasmids. RT-qPCR and western blot assays were performed to determine the expression levels of KLF5. The results demonstrated that KLF5 expression was significantly increased in podocytes transfected with pCDNA3.1-KLF5 compared with the NC group (Fig. 2; P<0.001), which suggested that transfection to establish the podocyte injury model was successful.
Figure 2.
KLF5 was overexpressed in podocytes. Podocytes were transfected with either empty vectors (control) or pCDNA3.1-KLF5. (A) Relative mRNA expression levels of KLF5 were determined by reverse transcription-quantitative polymerase chain reaction assays. (B) Protein expression levels of KLF5 were analyzed via western blot analysis, and quantitative values were determined based on the gray values. ***P<0.001. KLF5, Krüppel-like factor 5.
Overexpression of KLF5 inhibits cell cycle arrest of PAN-treated podocytes
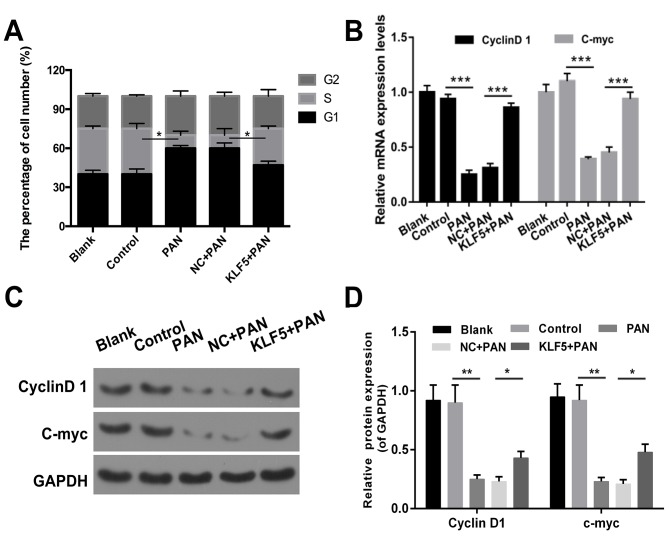
The results revealed that the proportion of cells in the G1 phase was increased in the PAN group compared with the control group (Fig. 3A). The number of cells in the G1 phase was decreased in the KLF5+PAN group compared with the NC+PAN group (Fig. 3A, P<0.05). In addition, the results demonstrated that treatment with PAN promoted cell cycle arrest in podocytes (P<0.05); however, this effect was markedly attenuated following overexpression of KLF5 (Fig. 3A, P<0.05). In addition, the expression levels of cyclin D1 and c-myc were investigated via RT-qPCR and western blot assays, and the results revealed that cells treated with PAN exhibited significantly decreased expression levels of cyclin D1 and c-myc compared with the NC group (P<0.01), whereas cells overexpressing KLF5 exhibited significantly increased expression levels of cyclin D1 and c-myc compared with the NC + PAN group (Fig. 3B-D, P<0.05).
Figure 3.
Overexpression of KLF5 induced cell cycle arrest of podocytes mediated by PAN. Podocytes were treated with either PBS (control), 60 µg/ml PAN, NC + PAN, KLF5 + PAN. (A) Cell cycle distribution in podocytes was investigated using flow cytometry, and the number of cells was quantitatively analyzed. (B) Reverse transcription-quantitative polymerase chain reaction assays were used to determine cyclin D1 and c-myc expression levels in treated podocytes. (C) Western blot assays were performed to determine cyclin D1 and c-myc expression levels in treated podocytes. (D) Cyclin D1 and c-myc protein expression levels were determined according to the gray values. *P<0.05, **P<0.01, ***P<0.001. PAN, puromycin aminonucleoside; KLF5, Krüppel-like factor 5; NC, negative control.
Overexpression of KLF5 suppresses the apoptosis of PAN-treated podocytes
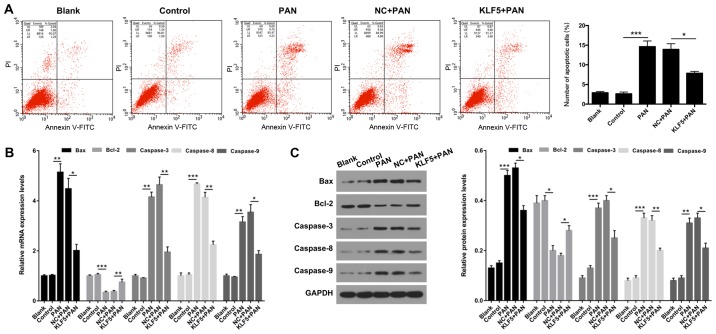
Flow cytometry was performed to determine whether KLF5 has an important role in the apoptosis of podocytes. The results demonstrated that the apoptosis of podocytes was significantly increased in the PAN group compared with the NC group (P<0.001; Fig. 4A); however, this effect was significantly attenuated following overexpression of KLF5 (P<0.05; Fig. 4A). Furthermore, the expression levels of genes associated with apoptosis (Bax, Bcl-2, caspase-3, caspase-8 and caspase-9) were investigated using RT-qPCR and western blot assays. The results revealed that treatment with PAN significantly enhanced the expression levels of Bax, caspase-3, caspase-8 and caspase-9 exhibited by podocytes (P<0.01 and P<0.001; Fig. 4B and C); however, overexpression of KLF5 significantly attenuated this effect (P<0.05 and P<0.01; Fig. 4B and C). In addition, podocytes treated with PAN exhibited significantly decreased Bcl-2 expression, and overexpression of KLF5 significantly attenuated this effect (P<0.05, P<0.01 and P<0.001; Fig. 4B and C).
Figure 4.
Overexpression of KLF5 inhibited PAN-induced apoptosis of podocytes. Podocytes were treated with PBS (control), PAN (60 µg/ml), NC + PAN or KLF5 + PAN. (A) Cell apoptosis was investigated using flow cytometry in treated podocytes, and the number of apoptotic cells were quantitatively analyzed. (B) mRNA expression levels of Bax, Bcl-2, caspase-3, caspase-8 and caspase-9 were determined via by reverse transcription-quantitative polymerase chain reaction assays. (C) Protein expression levels of Bax, Bcl-2, caspase-3, caspase-8 and caspase-9 were investigated using western blot analyses, and quantitative analysis was performed using base gray values and GAPDH. *P<0.05, **P<0.01 and ***P<0.001. PAN, puromycin aminonucleoside; KLF5, Krüppel-like factor 5; NC, negative control; Bcl-2, B cell lymphoma 2; Bax, Bcl-2 associated X; PI, propidium iodide; FITC, fluorescein isothiocyanate.
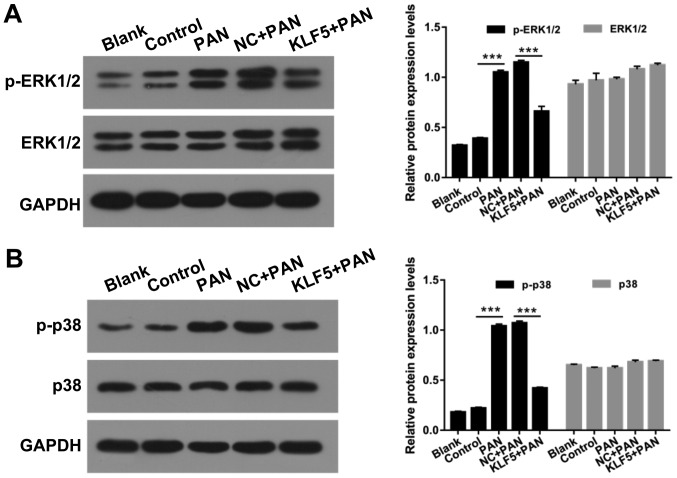
Overexpression of KLF5 suppresses the activation of ERK/p38 mitogen-activated protein (MAP) kinase pathway in PAN-treated podocytes
MAP kinases have important roles in numerous cellular functions and are regulated via independent upstream activation cascades (26). In the present study, the effects of KLF5 and PAN on the ERK/p38 MAP kinase pathway in podocytes were investigated. The protein levels of p-ERK1/2, ERK1/2, p-p38 and p38 in podocytes were investigated using a western blot assay. The results revealed that treatment with PAN significantly enhanced ERK1/2 and p38 phosphorylation compared with the control group; however, overexpression of KLF5 significantly attenuated these effects (P<0.001; Fig. 5).
Figure 5.
Overexpression of KLF5 suppressed the ERK/p38 MAP kinase pathway in podocytes induced by PAN. Podocytes were treated with PBS (control), 60 µg/ml PAN, NC + PAN, KLF5 + PAN. (A) Western blot assays were performed to determine p-ERK1/2 and ERK1/2 protein levels. (B) Western blot assays were performed to determine p-p38 and p38 protein levels. ***P<0.001. PAN, puromycin aminonucleoside; p-, phosphorylated; ERK, extracellular signal-regulated protein kinase; KLF5, Krüppel-like factor 5; NC, negative control.
Discussion
DN is one of the most common complications associated with diabetes and has become the most frequent causative factor resulting in the development of end-stage renal disease (27). The pathogenesis of DN arises from glucose and lipid metabolic disorders, abnormal hemodynamics and oxidative stress (28,29). The results of the present study demonstrated that podocyte injury is an important factor resulting in the development of proteinuria and glomerular sclerosis, and has significant effects on the pathogenesis of DN (30). Considering that synaptopodin is a podocyte marker (31), the expression levels of synaptopodin in podocytes was investigated in the present study, and the results revealed that synaptopodin was highly expressed in podocytes.
Podocytes and the slit diaphragm within foot processes are critical components of the glomerular filtration barrier (32). Associated molecules, including nephrin, podocin, CD2 associated protein and α-actinin-4 have important roles in the development of proteinuria (33). A human podocyte cell line was used in the present study to investigate podocyte injury in vitro. Furthermore, the present study established a podocyte injury model in vitro via administration of PAN. This model provided the possibility to further investigate molecular mechanisms associated with DN (34–36). The results revealed that treatment with PAN markedly inhibited the proliferation of podocytes in a dose- and time-dependent manner.
KLF-5 is an important member of the KLF protein family, and is located at chromosome 13q21 and encodes a 55 kDa protein that contains 457 amino acids (37). In normal tissue, KLF5 extensively regulates numerous cellular processes, such as proliferation, differentiation, movement, inflammation and pluripotency (38,39). However, the role of KLF5 in DN remains unclear. In the present study, the results revealed that PAN induced cell cycle arrest in podocytes; however, overexpression of KLF5 significantly attenuated this effect via upregulation of cyclin D1 and c-myc expression levels. In addition, a previous study demonstrated that KLF5 inhibited the cell cycle progression of vascular smooth muscle cells via activation of cyclin D1 (40). Furthermore, overexpression of KLF5 was revealed to significantly suppress PAN-mediated apoptosis of podocytes. At a molecular level, the results of the present study revealed that PAN enhanced the expression levels of Bax, caspase-3, caspase-8 and caspase-9; however, treatment with PAN significantly reduced Bcl-2 expression. Furthermore, overexpression of KLF5 significantly decreased Bax, caspase-3, caspase-8 and caspase-9 expression levels, whereas overexpression of KLF5 significantly increased Bcl-2 expression in PAN-treated podocytes. A previous study revealed that treatment with PAN induces apoptosis of glomerular podocytes (41). A further study demonstrated that the downregulation of KLF5 is associated with G1 phase cell cycle arrest (42). The results of the present study were therefore consistent with previous studies. Therefore, it was concluded that overexpression of KLF5 alleviates PAN-mediated podocyte injury.
MAP kinases are serine/threonine-specific protein kinases (43–45). p38 MAP kinase and ERK are factors of the MAP kinase protein family (46,47). The results of the present study suggested that treatment with PAN induced the phosphorylation of ERK1/2 and p38. Overexpression of KLF5 suppressed the phosphorylation of ERK/p38 MAP kinase in PAN-treated podocytes. These results suggested that overexpression of KLF5 protected podocytes from injury via inhibited activation of the ERK1/2 and p38 pathways. However, a previous study demonstrated that KLF5 may promote the activation of ERK1/2 in breast cancer cells (48). This discrepancy may be due to the use of different cells.
Limitations of the present study included that all experiments were performed in vitro, and that the exact mechanism underlying the regulation of KLF5 remains unclear. Thus, future studies should perform further in vivo investigations to determine the role of KLF5 in DN. In conclusion, the results of the present study demonstrated that PAN inhibited the proliferation of podocytes, and that overexpression of KLF5 attenuated PAN-induced cell cycle arrest and apoptosis of podocytes. Furthermore, the results demonstrated that overexpression of KLF5 suppressed the ERK/p38 MAP kinase pathway in PAN-treated podocytes. The present study revealed that a potential therapeutic strategy for the treatment of DN may comprise the upregulation of KLF5 expression.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science Fund project in Shandong Province (grant no. 2017GSF21116).
Availability of data and materials
All data generated and/or analyzed during this study are included in this published article.
Authors' contributions
YL wrote the main manuscript. YL, XH and XS performed the experiments. YL and ZH designed the study. XS and XH performed data analysis. YL, XS and ZH contributed to manuscript revisions. All authors reviewed the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bikbova G, Oshitari T, Tawada A, Yamamoto S. Corneal changes in diabetes mellitus. Curr Diabetes Rev. 2012;8:294–302. doi: 10.2174/157339912800840479. [DOI] [PubMed] [Google Scholar]
- 2.Bril V. Neuromuscular complications of diabetes mellitus. Continuum (Minneap Minn) 20 (3 Neurology of Systemic Disease) 2014:1–544. doi: 10.1212/01.CON.0000450964.30710.a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nentwich MM, Ulbig MW. Diabetic retinopathy-ocular complications of diabetes mellitus. World J Diabetes. 2015;6:489–499. doi: 10.4239/wjd.v6.i3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaul K, Tarr JM, Ahmad SI, Kohner EM, Chibber R. Introduction to diabetes mellitus. Adv Exp Med Biol. 2012;771:1–11. doi: 10.1007/978-1-4614-5441-0_1. [DOI] [PubMed] [Google Scholar]
- 5.Brinks R, Rathmann W. Response to Monesi etal Prevalence, incidence and mortality of diagnosed diabetes: Evidence from an Italian population-based study. Diabet Med. 2012;29:1085–1086; author reply 1085. doi: 10.1111/j.1464-5491.2011.03558.x. [DOI] [PubMed] [Google Scholar]
- 6.Farag YM, Gaballa MR. Diabesity: An overview of a rising epidemic. Nephrol Dial Transplant. 2011;26:28–35. doi: 10.1093/ndt/gfq576. [DOI] [PubMed] [Google Scholar]
- 7.Chao CT, Huang JW, Chiang CK, Chen YC, Fang CC, Hu FC, Chang CC, Yen CJ. Diabetes mellitus, superoxide dismutase and peroxisome proliferator activated receptor gamma polymorphisms modify the outcome of end-stage renal disease patients of Han Chinese origin. Nephrology (Carlton) 2018;23:117–125. doi: 10.1111/nep.12975. [DOI] [PubMed] [Google Scholar]
- 8.Tagawa A, Yasuda M, Kume S, Yamahara K, Nakazawa J, Chin-Kanasaki M, Araki H, Araki S, Koya D, Asanuma K, et al. Impaired podocyte autophagy exacerbates proteinuria in diabetic nephropathy. Diabetes. 2016;65:755–767. doi: 10.2337/db15-0473. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda-Yamahara M, Kume S, Tagawa A, Maegawa H, Uzu T. Emerging role of podocyte autophagy in the progression of diabetic nephropathy. Autophagy. 2015;11:2385–2386. doi: 10.1080/15548627.2015.1115173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamiyama M, Urushihara M, Morikawa T, Konishi Y, Imanishi M, Nishiyama A, Kobori H. Oxidative stress/angiotensinogen/renin-angiotensin system axis in patients with diabetic nephropathy. Int J Mol Sci. 2013;14:23045–23062. doi: 10.3390/ijms141123045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weening JJ, Rennke HG. Glomerular permeability and polyanion in adriamycin nephrosis in the rat. Kidney Int. 1983;24:152–159. doi: 10.1038/ki.1983.139. [DOI] [PubMed] [Google Scholar]
- 12.Amann K, Nichols C, Tornig J, Schwarz U, Zeier M, Mall G, Ritz E. Effect of ramipril, nifedipine, and moxonidine on glomerular morphology and podocyte structure in experimental renal failure. Nephrol Dial Transplant. 1996;11:1003–1011. doi: 10.1093/ndt/11.6.1003. [DOI] [PubMed] [Google Scholar]
- 13.Mifsud SA, Allen TJ, Bertram JF, Hulthen UL, Kelly DJ, Cooper ME, Wilkinson-Berka JL, Gilbert RE. Podocyte foot process broadening in experimental diabetic nephropathy: Amelioration with renin-angiotensin blockade. Diabetologia. 2001;44:878–882. doi: 10.1007/s001250100561. [DOI] [PubMed] [Google Scholar]
- 14.Kim D, Lim S, Park M, Choi J, Kim J, Han H, Yoon K, Kim K, Lim J, Park S. Ubiquitination-dependent CARM1 degradation facilitates Notch1-mediated podocyte apoptosis in diabetic nephropathy. Cell Signal. 2014;26:1774–1782. doi: 10.1016/j.cellsig.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Courboulin A, Tremblay VL, Barrier M, Meloche J, Jacob MH, Chapolard M, Bisserier M, Paulin R, Lambert C, Provencher S, Bonnet S. Krüppel-like Factor 5 contributes to pulmonary artery smooth muscle proliferation and resistance to apoptosis in human pulmonary arterial hypertension. Respir Res. 2011;12:128. doi: 10.1186/1465-9921-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tetreault MP, Yang Y, Katz JP. Krüppel-like factors in cancer. Nat Rev Cancer. 2013;13:701–713. doi: 10.1038/nrc3582. [DOI] [PubMed] [Google Scholar]
- 17.Courboulin A, Tremblay VL, Barrier M, Meloche J, Jacob MH, Chapolard M, Bisserier M, Paulin R, Lambert C, Provencher S, Bonnet S. Krüppel-like factor 5 contributes to pulmonary artery smooth muscle proliferation and resistance to apoptosis in human pulmonary arterial hypertension. Respir Res. 2011;12:128. doi: 10.1186/1465-9921-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limame R, Op de Beeck K, Lardon F, De Wever O, Pauwels P. Krüppel-like factors in cancer progression: Three fingers on the steering wheel. Oncotarget. 2014;5:29–48. doi: 10.18632/oncotarget.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin KJ, Hamblin M, Fan Y, Zhang J, Chen YE. Krüppel-like factors in the central nervous system: Novel mediators in stroke. Metab Brain Dis. 2015;30:401–410. doi: 10.1007/s11011-013-9468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Zhou Z, Guo P, Dong JT. Proteasomal degradation of the KLF5 transcription factor through a ubiquitin-independent pathway. FEBS Lett. 2007;581:1124–1130. doi: 10.1016/j.febslet.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Dong JT, Chen C. Essential role of KLF5 transcription factor in cell proliferation and differentiation and its implications for human diseases. Cell Mol Life Sci. 2009;66:2691–2706. doi: 10.1007/s00018-009-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agardh E, Lundstig A, Perfilyev A, Volkov P, Freiburghaus T, Lindholm E, Rönn T, Agardh CD, Ling C. Genome-wide analysis of DNA methylation in subjects with type 1 diabetes identifies epigenetic modifications associated with proliferative diabetic retinopathy. BMC Med. 2015;13:182. doi: 10.1186/s12916-015-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kakimoto T, Okada K, Fujitaka K, Nishio M, Kato T, Fukunari A, Utsumi H. Quantitative analysis of markers of podocyte injury in the rat puromycin aminonucleoside nephropathy model. Exp Toxicol Pathol. 2015;67:171–177. doi: 10.1016/j.etp.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Trachtman H, Del Pizzo R, Futterweit S, Levine D, Rao PS, Valderrama E, Sturman JA. Taurine attenuates renal disease in chronic puromycin aminonucleoside nephropathy. Am J Physiol. 1992;262:F117–F123. doi: 10.1152/ajprenal.1992.262.1.F117. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Baĭramov RB, Abdullaeva RT. The impact of early gastric cancer diagnosis on indices of survival in patients after radical surgical intervention. Klin Khir. 2013:1–21. [PubMed] [Google Scholar]
- 27.Krolewski AS, Skupien J, Rossing P, Warram JH. Fast renal decline to end-stage renal disease: An unrecognized feature of nephropathy in diabetes. Kidney Int. 2017;91:1300–1311. doi: 10.1016/j.kint.2016.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kowluru RA, Mishra M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta. 2015;1852:2474–2483. doi: 10.1016/j.bbadis.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Aoki Y, Yazaki K, Shirotori K, Yanagisawa Y, Oguchi H, Kiyosawa K, Furuta S. Stiffening of connective tissue in elderly diabetic patients: Relevance to diabetic nephropathy and oxidative stress. Diabetologia. 1993;36:79–83. doi: 10.1007/BF00399098. [DOI] [PubMed] [Google Scholar]
- 30.Yasuno K, Kamiie J, Shirota K. Analysis of ultrastructural glomerular basement membrane lesions and podocytes associated with proteinuria and sclerosis in Osborne-Mendel rats with progressive glomerulonephropathy. J Vet Sci. 2013;14:223–226. doi: 10.4142/jvs.2013.14.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, Kistler A, Faridi MH, Meyer JO, Tryniszewska B, Mehta D, Yue L, Dryer S, Reiser J. Synaptopodin limits TRPC6 podocyte surface expression and attenuates proteinuria. J Am Soc Nephrol. 2016;27:3308–3319. doi: 10.1681/ASN.2015080896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kriz W, Lemley KV. Potential relevance of shear stress for slit diaphragm and podocyte function. Kidney Int. 2017;91:1283–1286. doi: 10.1016/j.kint.2017.02.032. [DOI] [PubMed] [Google Scholar]
- 33.Cara-Fuentes G, Clapp WL, Johnson RJ, Garin EH. Pathogenesis of proteinuria in idiopathic minimal change disease: Molecular mechanisms. Pediatr Nephrol. 2016;31:2179–2189. doi: 10.1007/s00467-016-3379-4. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Zhang X, Li X, Ding F, Ding J. The role of survivin in podocyte injury induced by puromycin aminonucleoside. Int J Mol Sci. 2014;15:6657–6673. doi: 10.3390/ijms15046657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu SY, Qi R. Role of bad in podocyte apoptosis induced by puromycin aminonucleoside. Transplant Proc. 2013;45:569–573. doi: 10.1016/j.transproceed.2012.07.160. [DOI] [PubMed] [Google Scholar]
- 36.Zennaro C, Rastaldi MP, Pascolo L, Stebel M, Trevisan E, Artero M, Tiribelli C, Di Maso V, Carraro M. Podocyte expression of membrane transporters involved in puromycin aminonucleoside-mediated injury. PLoS One. 2013;8:e66159. doi: 10.1371/journal.pone.0066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marrero-Rodríguez D, Taniguchi-Ponciano K, Jimenez-Vega F, Romero-Morelos P, Mendoza-Rodríguez M, Mantilla A, Rodriguez-Esquivel M, Hernandez D, Hernandez A, Gomez-Gutierrez G, et al. Krüppel-like factor 5 as potential molecular marker in cervical cancer and the KLF family profile expression. Tumour Biol. 2014;35:11399–11407. doi: 10.1007/s13277-014-2380-4. [DOI] [PubMed] [Google Scholar]
- 38.Sousa MI, Rodrigues AS, Pereira S, Perestrelo T, Correia M, Ramalho-Santos J. Mitochondrial mechanisms of metabolic reprogramming in proliferating cells. Curr Med Chem. 2015;22:2493–2504. doi: 10.2174/0929867322666150514095718. [DOI] [PubMed] [Google Scholar]
- 39.Ci X, Xing C, Zhang B, Zhang Z, Ni JJ, Zhou W, Dong JT. KLF5 inhibits angiogenesis in PTEN-deficient prostate cancer by attenuating AKT activation and subsequent HIF1α accumulation. Mol Cancer. 2015;14:91. doi: 10.1186/s12943-015-0365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amirak E, Zakkar M, Evans PC, Kemp PR. Perfusion of veins at arterial pressure increases the expression of KLF5 and cell cycle genes in smooth muscle cells. Biochem Biophys Res Commun. 2010;391:818–823. doi: 10.1016/j.bbrc.2009.11.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao H, Shi W, Liu S, Wang W, Zhang B, Zhang Y, Xu L, Liang X, Liang Y. 1,25-Dihydroxyvitamin D(3) prevents puromycin aminonucleoside-induced apoptosis of glomerular podocytes by activating the phosphatidylinositol 3-kinase/Akt-signaling pathway. Am J Nephrol. 2009;30:34–43. doi: 10.1159/000200769. [DOI] [PubMed] [Google Scholar]
- 42.Qin WW, Zhang R, Chen RA, Li GH, Ji YR, Liu L, Wang T. MicroRNA-145 induces cell cycle arrest in G1 phase by directly targeting KLF5 in colon cancer. Int J Clin Exp Pathol. 2016;9:5197–5209. [Google Scholar]
- 43.Gaestel M. MAPK-activated protein kinases (MKs): Novel insights and challenges. Front Cell Dev Biol. 2016;3:88. doi: 10.3389/fcell.2015.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hettenhausen C, Schuman MC, Wu J. MAPK signaling: A key element in plant defense response to insects. Insect Sci. 2015;22:157–164. doi: 10.1111/1744-7917.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Callaghan C, Fanning LJ, Barry OP. p38δ MAPK: Emerging roles of a neglected isoform. Int J Cell Biol. 2014;2014:272689. doi: 10.1155/2014/272689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q, Wang J, Duan MT, Han SP, Zeng XY, Wang JY. NF-κB, ERK, p38 MAPK and JNK contribute to the initiation and/or maintenance of mechanical allodynia induced by tumor necrosis factor-alpha in the red nucleus. Brain Res Bull. 2013;99:132–139. doi: 10.1016/j.brainresbull.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 48.Liu R, Zheng HQ, Zhou Z, Dong JT, Chen C. KLF5 promotes breast cell survival partially through fibroblast growth factor-binding protein 1-pERK-mediated dual specificity MKP-1 protein phosphorylation and stabilization. J Biol Chem. 2009;284:16791–16798. doi: 10.1074/jbc.M808919200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated and/or analyzed during this study are included in this published article.