Abstract
Intestinal fibroblasts, the main effector cells of intestinal fibrosis, are considered to be a good target for anti-fibrotic therapy. The aim of the present study was to examine the effects of pirfenidone (PFD) on human intestinal fibroblasts (HIFs) stimulated by transforming growth factor (TGF)-β1 and to explore the potential mechanism. Prior to stimulation with TGF-β1 (10 ng/ml), HIFs were treated with or without PFD (1 mg/ml). Cell proliferation was determined by Cell Counting Kit (CCK)-8 and colony formation assays, and cell apoptosis was assessed using flow cytometry and a TUNEL assay. Reverse transcription-quantitative polymerase chain reaction and western blotting were performed to evaluate the mRNA and protein expressions of α-smooth muscle actin (α-SMA), collagen I and fibronectin. The protein expression of TGF-β1/mothers against decapentaplegic homolog (Smad) and phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling pathways was evaluated by western blotting. CCK-8 and colony formation assays demonstrated that PFD significantly inhibited cell proliferation in HIFs stimulated with TGF-β1. Flow cytometry and TUNEL assays revealed that PFD treatment significantly enhanced apoptosis in TGF-β1-stimulated HIFs. In addition, PFD markedly reduced TGF-β1-induced HIF activities, such as myofibroblast differentiation (α-SMA), and collagen production (collagen I and fibronectin). These effects of PFD were mediated by the inhibition of the TGF-β1/Smad and PI3K/AKT signaling pathways. Therefore, the present study demonstrated that PFD reduced TGF-β1-induced fibrogenic activities of HIFs, suggesting that PFD may be a potential therapeutic agent for intestinal fibrosis.
Keywords: pirfenidone, intestinal fibroblasts, proliferation, apoptosis
Introduction
Intestinal fibrosis, a serious complication resulting from inflammatory bowel disease, may result in loss of intestinal compliance, and thus irreversible intestinal stricture (1,2). Unfortunately, available medications have shown disappointing efficacy on prevention and treatment of intestinal fibrosis; endoscopic or surgical intervention remain the most practical treatment option for symptomatic intestinal strictures (2,3). Therefore, it is of great importance to look for effective means for prevention or treatment of intestinal fibrosis.
Activated fibroblasts are the main effector cells in the development of intestinal fibrosis responsible for excessive extracellular matrix (ECM) production, and have been considered as a major therapeutic target for antifibrotic therapy (4). Transforming growth factor β1 (TGF-β1), ‘master cytokine’ in fibrosis (5), plays a crucial role in the pathogenesis of intestinal fibrosis, including fibroblasts proliferation and differentiation into myofibroblasts, and ECM production (6,7). Therefore, TGF-β1-stimulated fibroblasts have been regarded as a good cellular model for evaluating the efficacy of antifibrotic agents (8,9). It has been reported that induction of apoptosis and inhibition of proliferation and profibrogenic effects in fibroblasts have shown potential in the treatment of intestinal fibrosis (10).
Pirfenidone (PFD), a novel anti-fibrotic drug, has shown potent antifibrotic efficacy in many organs, such as lung (11), liver (12), kidney (13), heart (14), and bladder (15). Additionally, it can inhibit cell proliferation and/or activation of different fibroblasts, including human Tenon's fibroblasts (16), rat renal fibroblasts (17), and rat cardiac fibroblasts (14), as well as downregulation of a series of cytokines, including TGF-β1 and connective tissue growth factor (CTGF) (18). Recently, this agent has been approved for clinical use in Europe and Japan due to its safety and efficacy in the treatment of idiopathic pulmonary fibrosis (19,20). In the context of the gastrointestinal tract, PFD has been shown to exert antifibrotic activities in a newly developed mouse model of intestinal fibrosis and a murine colitis model (21,22). In our previous study, we established a rat model of radiation-induced intestinal fibrosis by an exposure of a single dose of 20 Gy to the pelvis, and demonstrated that PFD could attenuate radiation-induced intestinal fibrosis (23).
In the present study, we established ‘fibrotic’ human intestinal fibroblasts (HIFs) through stimulation with TGF-β1 to possibly mimic the in vivo ‘fibrosis’ situation. We explored the effects of PFD on TGF-β1-induced fibrogenic activities of HIFs. The results demonstrated that PFD alleviated TGF-β1-induced HIFs transdifferentiation and collagen production by inhibiting cell proliferation and inducing apoptosis through inhibition of Smad and PI3K/AKT pathway.
Materials and methods
Reagents
Normal HIFs were purchased from iCell Bioscience Inc., (cas no: HUM-CELL-d022; Shanghai, China). PFD was purchased from Ji'nan Zhuokang Biological Technology Co., Ltd., (cas no: 53179-13-8; Shandong, China). Human recombinant TGF-β1 was purchased from PeproTech, Inc., (cas no: 100-21; Rock Hill, USA). Antibodies against α-smooth muscle actin (α-SMA), Collagen I alpha 1 (COLA1), fibronectin, total-Smad2/3 (t-Smad2/3), phospho-Smad2/3 (p-Smad2/3), phosphoinositide 3-kinase (PI3K), total-AKT (t-AKT), phospho-AKT (p-AKT), Bcl-2, Bad, and GAPDH were purchased from Bioss Antibodies (Beijing, China).
Cell culture and treatment
PFD was dissolved in dimethyl sulphoxide (DMSO) to produce 20 µg/µl stock solution; working solution was obtained by diluting stock solution with Dulbecco's modified minimal essential medium (DMEM)/F12 solution containing 10% fetal bovine serum (the final concentration of DMSO was less than 0.1%). No cytotoxic effect was observed after DMSO treatment. HIFs were grown in DMEM-F12 supplemented with 10% fetal bovine serum (37°C, 5% CO2). All experiments were performed after 3–5 cell passages. Prior to treatment, HIFs were pretreated with PFD (1 mg/ml) for 1 h before subsequent TGF-β1 stimulation (10 ng/ml).
Cytotoxicity and cell proliferation assays
HIFs were pretreated with DMSO and different concentrations of PFD (0, 0.01, 0.05, 0.1, 0.5, 1 mg/ml) for 24 h. Cytotoxicity was assessed with a lactate dehydrogenase (LDH) Cytotoxicity Assay kit (Beyotime Institute of Biotechnology, Jiangsu, China) following the manufacturer's instructions.
Cell proliferation assays
HIFs proliferation was examined using Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) assay according to the manufacturer's instructions. Cells in exponential growth were seeded in 96-well plates, incubated overnight, then starved by serum deprivation for 24 h, and treated with concentrations (0.5 mg/ml) of PFD in 10% FCS DMEM/F12 for 24 h. 10 µl CCK-8 solution (5 mg/ml) was added to each well and incubated at 37°C for 2 h. Then the number of cells was assessed by measuring optical absorbance at 450 nm using a Multiskan Go microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Colony formation assays
HIFs were seeded in 6-well plates at (500 cells per plate) for 24 h before the addition of drugs, cultured for 14 days, fixed with 4% paraformaldehyde for 15 min. Cells were stained with 1% crystal violet for 10 min. The number of colonies with diameters > 1.5 mm were counted.
Flow cytometry of apoptosis
Cell apoptosis was first detected using Annexin V-Phycoerythrin (PE) and 7-amino-actinomycin D (7-ADD) Apoptosis Detection kit I (BD Biosciences, Franklin Lakes, NJ, USA). Briefly, cells were seeded in 6-well plates (4×104 cells per well) and incubated for 24 h, and PFD or vehicle was added and washed twice with PBS. Then, suspension was stained with 5 µl Annexin V-PE and 7-ADD at room temperature for 15 min in the dark. The cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences) within 30 min.
TdT-UTP nick end labeling (TUNEL) assay
TUNEL staining for apoptotic cells was performed using One-Step TUNEL Apoptosis Assay kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) according to the manufacturer's protocol. Nuclei were stained with DAPI. Fluorescence signals were detected with a fluorescence microscope (Olympus Corporation, Tokyo, Japan). Apoptosis index was quantified as the ratio of (number of TUNEL-positive cells)/(number of total cells) ×100%.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from HIFs using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions. cDNA was generated using M-MLV Reverse Transcriptase Product (Promega Corporation, Madison, WI, USA). RT-qPCR was performed in an ABI 7500 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). Primer sequences were as follows: α-SMA: Forward 5′AGAGTTACGAGTTGCCTGATGG3′, Reverse 5′TTGCGGTGGACAATGGAAGG3′. COL-1A1: Forward 5′TGATGGTGCTACTGGTGCTG3′, Reverse 5′CCTCGCTTTCCTTCCTCTCC3′. Fibronectin: Forward 5′GTGGTGTGGTCTACTCTGTGG3′, Reverse 5′TCTGGTCGGCATCATAGTTCTG3′, GAPDH: Forward 5′GGAAGGTGAAGGTCGGAGTC3′, Reverse 5′GGCATTGCTGATGATCTTGAGG3′. qPCR reaction was carried out using SYBR Green reagents (Shanghai Novland Co., Ltd., Shanghai, China) with the following thermocycling conditions: Denaturation at 94°C for 2 min, followed by 94°C for 15 sec, and 62°C for 40 sec for 40 cycles. The relative expression of each targeted gene was determined using the 2−∆∆Cq comparative method (24) and normalized against that of GAPDH. Each sample was run as triplicate and repeated in three independent experiments.
Western blot analysis
Protein extracted from HIFs was quantified by the BCA assay (Beyotime Institute of Biotechnology). Equal quantities of protein (40 µg) were separated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE) and electrotransferred onto a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). The membrane was then blocked in 5% BSA+TBST, incubated with primary antibody against α-SMA, COLA1, fibronectin, Bcl-2, Bad. Smad2/3, p-Smad2/3, PI3K, AKT, and p-AKT, then washed and incubated in horseradish peroxidase (HRP) conjugated secondary antibodies. GAPDH acted as the reference protein for loading control. The signals were detected using an enhanced chemiluminescence kit (Beyotime Institute of Biotechnology). Densitometric analysis was conducted using Image J v.1.43 software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Each experiment was repeated at least three times and data was expressed as the mean ± standard deviation. Comparisons between multiple groups were performed using one-way analysis of variance with the least significant difference post hoc test to analyze the differences between groups. GraphPad Prism v.6.01 software (GraphPad Software, Inc., La Jolla, CA, USA) was used for analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
PFD suppresses cell proliferation in TGF-β1-induced HIFs
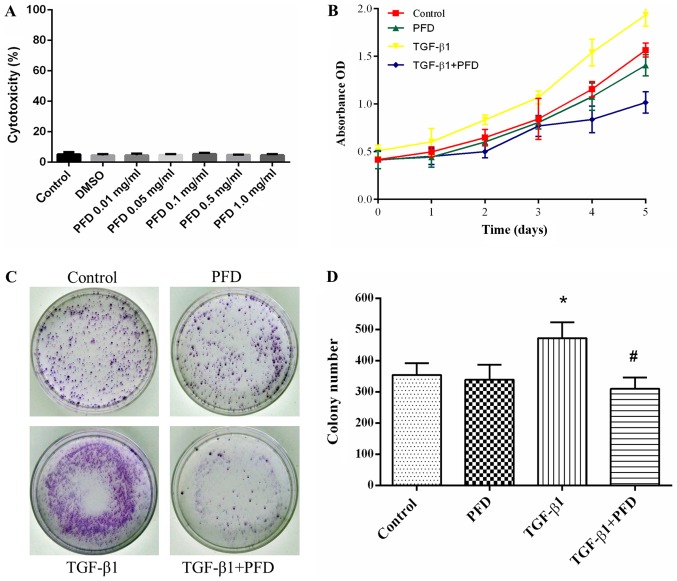
To clarify whether the effects of PFD were mediated by cytotoxicity, LDH cytotoxicity assay was performed after administration of DMSO and various concentration of PFD (0, 0.01, 0.05, 0.1, 0.5, 1 mg/ml). No cytotoxic effect of PFD on HIFs was observed at all tested concentrations (Fig. 1A).
Figure 1.
PFD inhibits cell proliferation in HIFs following TGF-β1 stimulation. (A) HIFs were pretreated with DMSO and different concentrations of PFD (0, 0.01, 0.05, 0.1, 0.5 and 1 mg/ml) for 24 h. Percentage of cytotoxicity was evaluated by a lactate dehydrogenase cytotoxicity assay. (B) HIFs were pretreated with PFD for 1 h prior to stimulation with TGF-β1 (10 ng/ml) for 1, 2, 3, 4 and 5 days. Cell Counting Kit-8 assays revealed a robust proliferative response to TGF-β1 when compared with normal cells, and PFD pretreatment abrogated TGF-β1-induced proliferation in HIFs, restraining cell numbers to basal levels. (C and D) HIFs were pretreated with PFD for 1 h prior to stimulation with TGF-β1 (10 ng/ml) for 14 days. Colony formation assays demonstrated the mean colony number was significantly increased following TGF-β1 stimulation in the colony formation assay, and PFD administration markedly reduced colony growth. Data are presented as the mean ± standard deviation of triplicate experiments. *P<0.05 vs. control group; #P<0.05 vs. TGF-β1 group. PFD, pirfenidone; HIFs, human intestinal fibroblasts; TGF, transforming growth factor; PFD, pirfenidone; OD, optical density.
To determine the effect of PFD on cell proliferation of HIFs, we pretreated HIFs with PFD for 1 h before stimulation with and without TGF-β1 (10 ng/ml) for 1, 2, 3, 4, and 5 days. CCK-8 assay demonstrated that HIFs showed a robust proliferative response to TGF-β1 compared to control group, and PFD pretreatment abrogated TGF-β1-induced proliferation in HIFs, restraining cell numbers to basal levels (Fig. 1B). At 14 days, the colony formation assay demonstrated that the mean colony number was significantly increased after TGF-β1 stimulation, and PFD administration remarkably reduced colony growth (Fig. 1C and D). Together, these results indicated that PFD suppressed cell proliferation in TGF-β1-induced HIFs.
PFD promotes cell apoptosis in TGF-β1-induced HIFs
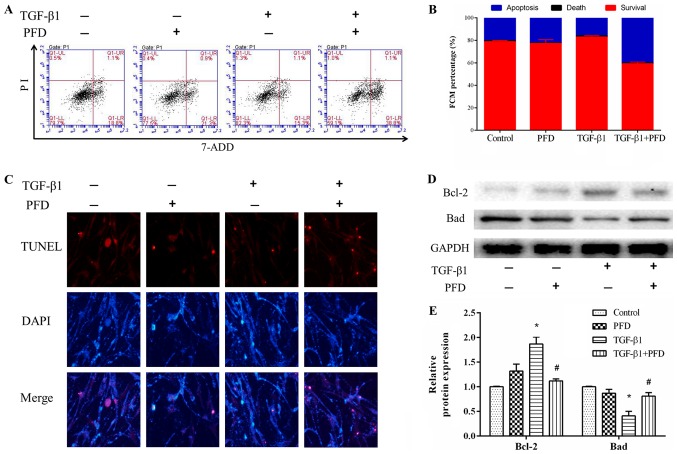
The effect of PFD on cell apoptosis was investigated by using flow cytometry and TUNEL assay. HIFs were pretreated with PFD for 1 h before stimulation with and without TGF-β1 for 48 h. Flow cytometry assay indicated that TGF-β1 treatment induced reduction in cell apoptosis, and pretreatment with PFD reduced TGF-β1-induced reduction in apoptosis (Fig. 2A and B). TUNEL staining showed that TGF-β1 decreased the number of apoptotic cells after stimulation for 48 h, and PFD treatment remarkably increased the number of TUNEL-positive apoptotic cells (Fig. 2C). Collectively, these data suggested that PFD significantly inhibited the anti-apoptotic effects of TGF-β1 on HIFs. To further explore the effect of PFD on apoptosis of HIFs, we determined the protein expression of Bcl-2 and Bad. These results demonstrated that TGF-β1 significantly decreased the expression of pro-apoptotic protein Bad and increased the expression of anti-apoptotic protein Bcl-2, and this effect was reversed by PFD treatment (Fig. 2D and E).
Figure 2.
PFD induces apoptosis in HIFs stimulated with TGF-β1. HIFs were pretreated with PFD for 1 h prior to TGF-β1 stimulation for 48 h. (A and B) Flow cytometry assay indicated that TGF-β1 treatment induced a reduction in cell apoptosis, and pretreatment with PFD reduced the TGF-β1-induced reduction in apoptosis. (C) Representative images of TUNEL assays (n=3 per group). Magnification, ×400. (D) Western blotting and (E) quantitative analysis of Bcl-2 and Bad protein expression. Data are presented as the mean ± standard deviation of triplicate experiments. *P<0.05 vs. control group; #P<0.05 vs. TGF-β1 group. PFD, pirfenidone; HIFs, human intestinal fibroblasts; TGF, transforming growth factor; Bcl-2, B-cell lymphoma 2; Bad, Bcl-2 associated agonist of cell death.
PFD reduces fibroblast differentiation and collagen synthesis in TGF-β1-induced HIFs
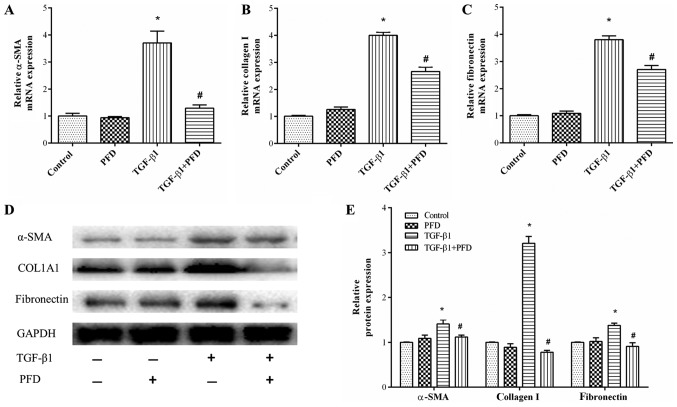
As myofibroblast differentiation is a central event of the pathogenesis of intestinal fibrosis, we determined the mRNA and protein expression of α-SMA, a key marker of myofibroblast differentiation. The results demonstrated that TGF-β1 stimulation significantly elevated α-SMA mRNA and protein expression and PFD treatment resulted in a significant inhibition of TGF-β1-induced expression of α-SMA. However, PFD had no such effects on normal intestinal fibroblasts, as shown in Fig. 3A, D and E.
Figure 3.
PFD inhibits HIFs differentiation and the collagen deposition induced by TGF-β1. (A-C) Relative mRNA expression of (A) α-SMA, (B) collagen I and (C) fibronectin. HIFs were pretreated with PFD for 1 h prior to TGF-β1 stimulation for 48 h. (D) Western blotting and (E) quantitative analysis of α-SMA, collagen I and fibronectin protein expression. HIFs were pretreated with PFD for 1 h prior to TGF-β1 stimulation for 48 h. Data are presented as the mean ± standard deviation of triplicate experiments. *P<0.05 vs. control group; #P<0.05 vs. TGF-β1 group. PFD, pirfenidone; HIFs, human intestinal fibroblasts; TGF, transforming growth factor; α-SMA, α-smooth muscle actin; COL1A1, Collagen I α 1.
Collagen accumulation is another characteristic feature of myofibroblasts, which are commonly observed in intestinal fibrosis. To investigate effects of PFD on collagen synthesis, we examined the protein and mRNA expression of collagen I and fibronectin. HIFs were pretreated with PFD for 1 h before TGF-β1 stimulation for 48 h. As shown in Fig. 3B-E, the mRNA expression and protein production of collagen I and fibronectin were enhanced by TGF-β1, and PFD pretreatment substantially abrogated this TGF-β1-mediated collagen I and fibronectin production.
PFD inhibits TGF-β1-simulated phosphorylation of TGF-β1/Smad2/3 and PI3K/AKT signaling pathways in HIFs
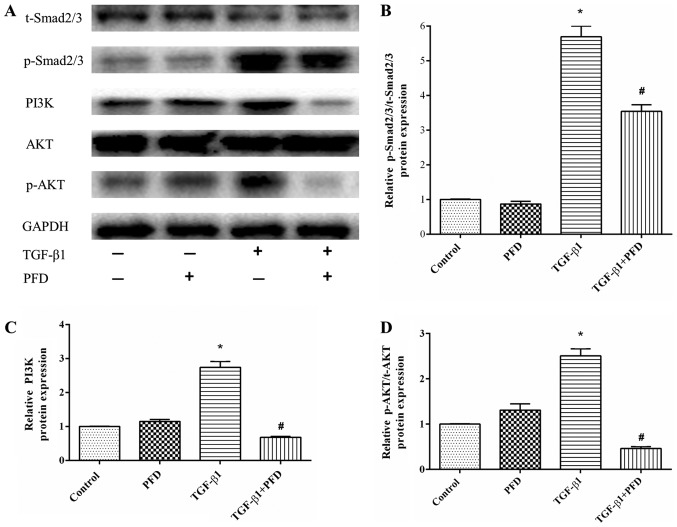
As TGF-β1 is the most crucial mediator in the pathogenesis of intestinal fibrosis, we determined the protein expression in TGF-β1 signaling pathway. In the canonical signaling pathway, TGF-β1 activates down-streaming Smad2/3 pathways to exert its pro-fibrotic effects. To explore the effect of PFD on Smad2/3, we detected the protein expression of p-Smad2/3. HIFs were pretreated with PFD for 1 h before TGF-β1 stimulation for 48 h. As shown in Fig. 4A, the expression of p-Smad2/3 protein was significantly increased after TGF-β1 stimulation (P<0.05), and PFD significantly reduced TGF-β1-induced phosphorylation of Smad2/3 (Fig. 4A and D).
Figure 4.
PFD exerts antifibrotic effects by inhibiting Smad and PI3K/AKT signaling activation in HIFs incubated with TGF-β1. (A) Western blotting analysis of Smad2/3, p-Smad2/3, PI3K, AKT and p-AKT protein expression. HIFs were pretreated with PFD for 1 h prior to TGF-β1 stimulation for 48 h. (B-D) Quantitative analysis of (B) p-Smad2/3 to Smad2/3 ratio, (C) PI3K and (D) p-AKT to t-AKT ratio protein expression. Data are presented as the mean ± standard deviation of triplicate experiments. *P<0.05 vs. control group; #P<0.05 vs. TGF-β1 group. PFD, pirfenidone; HIFs, human intestinal fibroblasts; TGF, transforming growth factor; Smad, mothers against decapentaplegic homolog; p-, phosphorylated; AKT, protein kinase B; PI3K, phosphoinositide 3-kinase; t-, total.
In addition, TGF-β1 can also activate the non-canonical PI3K/AKT pathway to modulate its downstream pro-fibrotic signaling and related protein expression (25,26). As shown in Fig. 4A and B, TGF-β1 significantly increased the protein expression of PI3K and p-AKT, while pretreatment with PFD markedly reduced PI3K and p-AKT expression. These results suggested that PFD inhibited PI3K/AKT activation in response to TGF-β1 in HIFs, which might help to alleviate intestinal fibrosis.
Discussion
Intestinal fibrosis is still considered as a progressive and irreversible process, and currently, no effective antifibrotic therapies are available. Intestinal fibroblasts are the main effector cells of intestinal fibrosis, and considered as a crucial target in treatment of intestinal fibrosis (5). In the present study, PFD inhibited cell proliferation and enhanced apoptosis of HIFs; these effects were accompanied by inhibition of TGF-β1-induced fibrotic activities, including myofibroblast differentiation (α-SMA) and collagen production (collagen I and fibronectin). Furthermore, the results showed that the antifibrotic effects of PFD in vitro may be mediated by via Smad and PI3K/AKT signaling pathway. Collectively, the present results suggested that PFD might be a potential therapeutic agent for intestinal fibrosis.
As the major ECM-producing cells, myofibroblasts play a pivotal role in the development of intestinal fibrosis, and myofibroblasts differentiation has been accepted as a central event in the intestinal fibrosis pathogenesis (4). To possibly mimic the ‘in vivo’ situation, we established a ‘fibrotic’ culture via stimulation with TGF-β1, the ‘master cytokine’ in fibrosis (5). We demonstrated here that TGF-β1 induced myofibroblast differentiation and increased mRNA and protein expression of α-SMA, and such effects were significantly inhibited by PFD pretreatment. This finding was in line with observations in previous researches focused on effects of PFD on cardiac, renal, and hepatic fibroblasts (14,17,12). Activated myofibroblasts were demonstrated to exhibit profibrogenic effects, such as increased proliferative, migratory and secretory activities (4). Herein, we demonstrated that PFD treatment alone did not significantly affect the mRNA and protein of collagen I and fibronectin; conversely, PFD inhibited TGF-β1-induced synthesis of collagen I and fibronectin. Collectively, our data proved that PFD inhibited the TGF-β1-mediated fibrotic process in vitro, which supported effects of PFD observed in hepatic stellate cells, leiomyoma cells, and alveolar epithelial cells (12,27,28).
Excessive fibroblast/myofibroblasts accumulation, which is secondary to increased proliferation and/or inhibited apoptosis, gradually leads to excessive ECM synthesis and deposition and thus progressive distortion of intestinal structure. Given that apoptosis is responsible for mediating the reduction in myofibroblasts number, induction of myofibroblasts apoptosis would have an antifibrotic effect during the resolution of fibrosis (29). Suppression of activation and proliferation and induction of apoptosis could be helpful to reduce activated fibroblasts and their profibrogenic effects. In this context, we examined effects of PFD on the regulation of cell proliferation and apoptosis of HIFs in vitro. PFD inhibited the proliferative activity of HIFs in response to TGF-β1, as demonstrated by CCK-8 assay and colony formation assay. We ruled out that antiproliferative effects were mediated by drug toxicity, as evidenced by LDH cytotoxicity assay. These results were consistent with findings reported previously (9,14,16). Additionally, PFD also induced HIFs cell apoptosis and decreased the number of TUNEL-positive cells after TGF-β1 stimulation, which was accompanied by the increase of Bad protein expression, and the decrease of Bcl-2 protein level. Taken together, these results suggested that PFD reduced the accumulation of intestinal fibroblasts/myofibroblasts by reducing proliferation and increasing apoptosis of HIFs.
TGF-β1 plays a central role in the development of intestinal fibrosis; it can augment the proliferative, migratory, and collagen-producing abilities of intestinal fibroblasts by inducing myofibroblast differentiation (6). TGF-β1 can activate both Smad-dependent and -independent pathways. Smad2/3s are activated after phosphorylation, and the activated form of Smad2/3s is released and translocated into the nucleus, and then induce the subsequent profibrogenic effects (30). The potent antifibrotic properties of PFD including TGF-β1 suppression were demonstrated in many studies (9,14,22,31). In the present work, we analyzed the key participants in TGF-β1/Smad signaling pathway to explore the potential mechanisms responsible for the antifibrotic effects of pirfenidone. PFD treatment was observed to attenuate TGF-β1-induced Smad2/3 phosphorylation, which was consistent with previous results (22,31).
In addition to canonical pathway, TGF-β1-mediated fibrotic process could be mediated through non-canonical signaling pathways. Previous studies have showed that PI3K/AKT signal pathway is involved in the TGF-β1 regulating pathway, and TGF-β1 could induce activation of the PI3K/AKT pathway (25,26). The PI3K/AKT signaling pathway is a prototypic survival pathway, in which AKT is a downstream target and plays an important role in cell growth or survival. When activated, AKT may produce its anti-apoptotic effects via phosphorylation of both anti-apoptotic and pro-apoptotic substrates, such as Bcl-2 and Bad (32). Consistent with previous studies, the up-regulation of PI3K and p-AKT indicated that PI3K/AKT signaling pathway was activated after TGF-β1 treatment, and these changes were accompanied by the increase of Bad expression and the decrease of Bcl-2 expression. In addition, this effect was reversed by PFD treatment, which means that PFD alleviates TGF-β1-induced profibrotic process at least in part by inducing apoptosis of HIFs through PI3K/AKT signaling pathway. Further studies that investigate the underlying mechanism in more detail are warranted.
The present study demonstrated that pirfenidone, an orally potent antifibrotic agent, could alleviate TGF-β1-induced fibrogenic activities, such as myofibroblast differentiation (α-SMA) and collagen production (collagen I and fibronectin), as wells as pro-proliferative and anti-apoptotic effects of TGF-β1 on HIFs. In addition, the antifibrotic effects of PFD on HIFs may be mediated by inhibiting phosphorylation of Smad2/3 and AKT pathways.
Acknowledgements
The authors would like to thank the Fujian Medical University Stem Cell Research Institute (Fujian, China) for their technical assistance.
Funding
The present study was supported by National Clinical Key Specialty Construction Project (General Surgery) of China (grant no. 2012-649) and Guiding Key Project of Social Development by the Fujian Provincial Science and Technology Department (grant no. 2015Y0058).
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contribution
YS and PC conceived and designed the study. YS and YZ performed the experiments and were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Rieder F, Fiocchi C. Intestinal fibrosis in IBD-a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228–235. doi: 10.1038/nrgastro.2009.31. [DOI] [PubMed] [Google Scholar]
- 2.Stacey R, Green JT. Radiation-induced small bowel disease: Latest developments and clinical guidance. Ther Adv Chronic Dis. 2014;5:15–29. doi: 10.1177/2040622313510730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamama S, Delanian S, Monceau V, Vozenin MC. Therapeutic management of intestinal fibrosis induced by radiation therapy: From molecular profiling to new intervention strategies et vice et versa. Fibrogenesis Tissue Repair. 2012;5(Suppl 1):S13. doi: 10.1186/1755-1536-5-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: Novel roles and mediators. Front Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeisberg M, Kalluri R. Cellular mechanisms of tissue fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am J Physiol Cell Physiol. 2013;304:C216–C225. doi: 10.1152/ajpcell.00328.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speca S, Giusti I, Rieder F, Latella G. Cellular and molecular mechanisms of intestinal fibrosis. World J Gastroenterol. 2012;18:3635–3661. doi: 10.3748/wjg.v18.i28.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: The master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Liu H, Liang Y, Peng P, Ma X, Zhang X. DKK3 regulates cell proliferation, apoptosis and collagen synthesis in keloid fibroblasts via TGF-β1/Smad signaling pathway. Biomed Pharmacother. 2017;91:174–180. doi: 10.1016/j.biopha.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 9.Conte E, Gili E, Fagone E, Fruciano M, Iemmolo M, Vancheri C. Effect of pirfenidone on proliferation, TGF-β-induced myofibroblast differentiation and fibrogenic activity of primary human lung fibroblasts. Eur J Pharm Sci. 2014;58:13–19. doi: 10.1016/j.ejps.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Abe Y, Murano M, Murano N, Morita E, Inoue T, Kawakami K, Ishida K, Kuramoto T, Kakimoto K, Okada T, et al. Simvastatin attenuates intestinal fibrosis independent of the anti-inflammatory effect by promoting fibroblast/myofibroblast apoptosis in the regeneration/healing process from TNBS-induced colitis. Dig Dis Sci. 2012;57:335–344. doi: 10.1007/s10620-011-1879-4. [DOI] [PubMed] [Google Scholar]
- 11.Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, Taguchi Y, Nagai S, Itoh H, Ohi M, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 12.Di Sario A, Bendia E, Svegliati Baroni G, Ridolfi F, Casini A, Ceni E, Saccomanno S, Marzioni M, Trozzi L, Sterpetti P, et al. Effect of pirfenidone on rat hepatic stellate cell proliferation and collagen production. J Hepatol. 2002;37:584–591. doi: 10.1016/S0168-8278(02)00245-3. [DOI] [PubMed] [Google Scholar]
- 13.Miric G, Dallemagne C, Endre Z, Margolin S, Taylor SM, Brown L. Reversal of cardiac and renal fibrosis by pirfenidone and spironolactone in streptozotocin-diabetic rat. Br J Pharmacol. 2001;133:687–694. doi: 10.1038/sj.bjp.0704131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Q, Liu X, Bai Y, Cui C, Li J, Li Y, Hu S, Wei Y. In vitro effects of pirfenidone on cardiac fibroblasts: Proliferation, myofibroblast differentiation, migration and cytokine secretion. PLoS One. 2011;6:e28134. doi: 10.1371/journal.pone.0028134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duan L, Qi J, Huang T, Gu X, Xu D, Kong X, Qian XQ. Pirfenidone attenuates bladder fibrosis and mitigates deterioration of bladder function in a rat model of partial bladder outlet obstruction. Mol Med Rep. 2015;12:3639–3647. doi: 10.3892/mmr.2015.3814. [DOI] [PubMed] [Google Scholar]
- 16.Lin X, Yu M, Wu K, Yuan H, Zhong H. Effects of pirfenidone on proliferation, migration, and collagen contraction of human Tenon's fibroblasts in vitro. Invest Ophthalmol Vis Sci. 2009;50:3763–3770. doi: 10.1167/iovs.08-2815. [DOI] [PubMed] [Google Scholar]
- 17.Hewitson TD, Kelynack KJ, Tait MG, Martic M, Jones CL, Margolin SB, Becker GJ. Pirfenidone reduces in vitro rat renal fibroblast activation and mitogenesis. J Nephrol. 2001;14:453–460. [PubMed] [Google Scholar]
- 18.Chan AL, Rafii R, Louie S, Albertson TE. Therapeutic update in idiopathic pulmonary fibrosis. Clin Rev Allergy Immunol. 2013;44:65–74. doi: 10.1007/s12016-010-8244-9. [DOI] [PubMed] [Google Scholar]
- 19.Cottin V. The role of pirfenidone in the treatment of idiopathic pulmonary fibrosis. Respir Res. 2013;14(Suppl 1):S5. doi: 10.1186/1465-9921-14-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda Y, Tsujino K, Kijima T, Kumanogoh A. Efficacy and safety of pirfenidone for idiopathic pulmonary fibrosis. Patient Prefer Adherence. 2014;8:361–370. doi: 10.2147/PPA.S37233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meier R, Lutz C, Cosín-Roger J, Fagagnini S, Bollmann G, Hünerwadel A, Mamie C, Lang S, Tchouboukov A, Weber FE, et al. Decreased fibrogenesis after treatment with pirfenidone in a newly developed mouse model of intestinal fibrosis. Inflamm Bowel Dis. 2016;22:569–582. doi: 10.1097/MIB.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 22.Li G, Ren J, Hu Q, Deng Y, Chen G, Guo K, Li R, Li Y, Wu L, Wang G, et al. Oral pirfenidone protects against fibrosis by inhibiting fibroblast proliferation and TGF-β signaling in a murine colitis model. Biochem Pharmacol. 2016;117:57–67. doi: 10.1016/j.bcp.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Sun YW, Zhang YY, Ke XJ, Wu XJ, Chen ZF, Chi P. Pirfenidone prevents radiation-induced intestinal fibrosis in rats by inhibiting fibroblast proliferation and differentiation and suppressing the TGF-β1/Smad/CTGF signaling pathway. Eur J Pharmacol. 2018;822:199–206. doi: 10.1016/j.ejphar.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Ni BB, Li B, Yang YH, Chen JW, Chen K, Jiang SD, Jiang LS. The effect of transforming growth factor β1 on the crosstalk between autophagy and apoptosis in the annulus fibrosus cells under serum deprivation. Cytokine. 2014;70:87–96. doi: 10.1016/j.cyto.2014.07.249. [DOI] [PubMed] [Google Scholar]
- 26.Wu L, Zhang Q, Mo W, Feng J, Li S, Li J, Liu T, Xu S, Wang W, Lu X, et al. Quercetin prevents hepatic fibrosis by inhibiting hepatic stellate cell activation and reducing autophagy via the TGF-β1/Smads and PI3K/Akt pathways. Sci Rep. 2017;7:9289. doi: 10.1038/s41598-017-09673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takano M, Yamamoto C, Yamaguchi K, Kawami M, Yumoto R. Analysis of TGF-β1- and drug-induced epithelial-mesenchymal transition in cultured alveolar epithelial cell line RLE/Abca3. Drug Metab Pharmacokinet. 2015;30:111–118. doi: 10.1016/j.dmpk.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Lee BS, Margolin SB, Nowak RA. Pirfenidone: A novel pharmacological agent that inhibits leiomyoma cell proliferation and collagen production. J Clin Endocrinol Metab. 1998;83:219–223. doi: 10.1210/jcem.83.1.4503. [DOI] [PubMed] [Google Scholar]
- 29.Luna J, Masamunt MC, Lawrance IC, Sans M. Mesenchymal cell proliferation and programmed cell death: Key players in fibrogenesis and new targets for therapeutic intervention. Am J Physiol Gastrointest Liver Physiol. 2011;300:G703–G708. doi: 10.1152/ajpgi.00504.2010. [DOI] [PubMed] [Google Scholar]
- 30.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 31.Choi K, Lee K, Ryu SW, Im M, Kook KH, Choi C. Pirfenidone inhibits transforming growth factor-β1-induced fibrogenesis by blocking nuclear translocation of Smads in human retinal pigment epithelial cell line ARPE-19. Mol Vis. 2012;18:1010–1020. [PMC free article] [PubMed] [Google Scholar]
- 32.Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]